Abstract
The diverse physiography of the Portuguese land and marine territory, spanning from continental Europe to the Atlantic archipelagos, has made it an important repository of biodiversity throughout the Pleistocene glacial cycles, leading to a remarkable diversity of species and ecosystems. This rich biodiversity is under threat from anthropogenic drivers, such as climate change, invasive species, land use changes, overexploitation, or pathogen (re)emergence. The inventory, characterisation, and study of biodiversity at inter- and intra-specific levels using genomics is crucial to promote its preservation and recovery by informing biodiversity conservation policies, management measures, and research. The participation of researchers from Portuguese institutions in the European Reference Genome Atlas (ERGA) initiative and its pilot effort to generate reference genomes for European biodiversity has reinforced the establishment of Biogenome Portugal. This nascent institutional network will connect the national community of researchers in genomics. Here, we describe the Portuguese contribution to ERGA’s pilot effort, which will generate high-quality reference genomes of six species from Portugal that are endemic, iconic, and/or endangered and include plants, insects, and vertebrates (fish, birds, and mammals) from mainland Portugal or the Azores islands. In addition, we outline the objectives of Biogenome Portugal, which aims to (i) promote scientific collaboration, (ii) contribute to advanced training, (iii) stimulate the participation of institutions and researchers based in Portugal in international biodiversity genomics initiatives, and (iv) contribute to the transfer of knowledge to stakeholders and engaging the public to preserve biodiversity. This initiative will strengthen biodiversity genomics research in Portugal and fuel the genomic inventory of Portuguese eukaryotic species. Such efforts will be critical to the conservation of the country’s rich biodiversity and will contribute to ERGA’s goal of generating reference genomes for European species.
Subject terms: Genomics, Biodiversity
Introduction
The remarkable diversity of life on Earth is essential for maintaining ecosystem stability, fostering ecological interactions among species, regulating the climate, and providing fundamental resources that sustain human well-being and promote the development of society. Today, Earth’s biodiversity, ecological and evolutionary heritage, and the ecosystem services they provide are under intense and extensive strain as a result of many direct and indirect anthropogenic activities. These major activities include habitat destruction and fragmentation caused by agricultural expansion and other forms of land conversion, overexploitation of natural resources, overharvesting, illegal killing and trading of wild species, climate changes, environmental pollution, and global spread of exotic species1–5. These pressures not only result in the decline and extinction of populations, species, and ecosystems, but also have cascading effects on various dimensions of biodiversity, including genetic, functional, or phenological diversity and the interactions within biodiversity networks. These impacts harm the health of natural populations and their survival and reduce their potential to adapt to environmental challenges6. Characterising and understanding in detail the genetic variation underlying biodiversity at all levels provides essential modern tools to delineate sound and efficient strategies to mitigate the impacts of human activities, design management plans for conservation and restoration, and support data-driven biodiversity policy development7–10. Genomics, anchored on high-quality reference genomes, offers unparalleled precision in identifying genetic diversity, population structure, and adaptive potential, which are critical for predicting species’ responses to environmental changes, enhancing breeding programmes, and ensuring the genetic health of populations11,12. Regional biodiversity genomics initiatives that promote research and applications under the umbrella of international coalitions, can leverage shared resources and expertise, facilitating the development of robust, scalable solutions to global conservation challenges.
Europe hosts a remarkable biodiversity richness despite its temperate climate and historically anthropogenic landscapes13, and has an important biodiversity hotspot along the Mediterranean basin3,14. Most of the continental territory of Portugal, located in southwestern Europe in the Iberian Peninsula, is part of the Mediterranean biodiversity hotspot14. The country also includes the volcanic Atlantic archipelagos of Azores, Madeira, and Selvagens (Fig. 1). Even though Portugal is the 19th largest European country (land area ~92,000 km2), it holds the 5th largest marine territory (~1.7 million km2)15. As a result of its unique geographic location, climate, and geophysical features, Portugal comprises a variety of landscapes and ecosystems and harbours a large diversity of species. The Iberian Peninsula provided refugia for species throughout the glacial periods and remains one of the most important repositories of biodiversity in Europe, both at inter- and intraspecific level16,17. In addition, the geographic isolation of the archipelagos of Azores, Madeira, and Selvagens, which together with the Canary and Cabo Verde archipelagos form the biogeographical unit of Macaronesia, has led to the divergence and speciation of many lineages that resulted in many endemisms18. According to the Portuguese National Strategy for the Conservation of Nature and Biodiversity 203019, Portugal holds ~35,000 animal and plant species, representing a relevant proportion of these species groups present in Europe. The International Union for Conservation of Nature’s Red List of Threatened Species (IUCN Red List database—www.iucnredlist.org; 27 April 2023) lists ~800 of the assessed species as endemic to the continental mainland or archipelagos of Portugal. There are 414 protected areas in Portugal encompassing 22.28% of the land and 2.46% of marine water, and 404 species and 102 habitats are protected under European Union law (Biodiversity Information System for Europe: biodiversity.europa.eu/countries/portugal). The ENCNB 2030 recognises the importance of a systematic inventory and characterisation of biodiversity at all levels—ecosystems, species and genetic diversity—to properly anchor conservation strategies and genomics offers unprecedented power to understand biodiversity at the inter- and intraspecific levels11,12. Thus, gathering scientific expertise in the area, coordinating research efforts, and promoting genomic studies of native Portuguese species can make an invaluable contribution to preserving the rich biodiversity of Portugal and its ecosystem services. Here, we describe the Portuguese participation in ERGA, our contribution to ERGA’s pilot project, and how these efforts have motivated building a national network on biodiversity genomics. A Portuguese version of the article can be found at: 10.5281/zenodo.8119533.
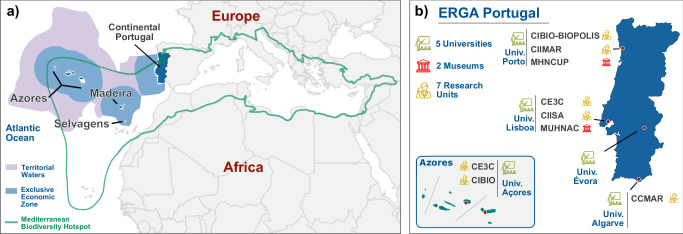
Fig. 1. Geographic distribution and institutional affiliations of ERGA-Portugal members across the Portuguese territory.
a Spatial context of the Portuguese territory, including the two Autonomous Regions (Azores and Madeira). b Distribution of the Portugal-based members of ERGA (ERGA-Portugal), including their host institutions (31 May 2023). Maps made with Natural Earth (www.naturalearthdata.com).
ERGA-Portugal community and the Pilot effort
The European Reference Genome Atlas (ERGA; www.erga-biodiversity.eu/) is an ambitious and ground-breaking initiative that aims to revolutionise our understanding of biodiversity by creating a comprehensive genomic resource for European species. ERGA is implemented under the umbrella of the Earth Biogenome Project (EBP; www.earthbiogenome.org/), the global network of networks that aims to catalogue the eukaryotic life diversity of Earth through genome sequencing. ERGA gathers researchers, scientists, and biodiversity enthusiasts to collect and sequence the genomes of species across Europe, providing a valuable tool for studying and preserving biodiversity, understanding evolutionary processes, and addressing pressing environmental challenges. The network leverages the power of genomics to unlock the secrets of European biodiversity and foster collaborative efforts towards its conservation and sustainable management.
ERGA includes more than 50 members in Portugal, distributed across seven research units, five universities, and two natural history museums (31st May 2023; Fig. 1). This community of members based in Portugal is called ERGA-Portugal, and covers a wide range of expertise that is relevant for biodiversity genomics research, from taxonomy and systematics across diverse biological groups (e.g., microorganisms, plants, insects, fish, amphibians, birds or mammals), to comparative and population genomics and bioinformatics. This community communicates through open meetings, mailing lists, and message-sharing channels to facilitate the exchange of biodiversity genomic expertise across Portugal. Many Portuguese community members are also active participants in ERGA’s scientific committees.
To contribute to the establishment and mission of ERGA, ERGA-Portugal participated in its pilot test20. This pilot aimed at testing and developing the biodiversity genomic networks across Europe through the sequencing of several European species, with the contribution of expert ERGA members and European institutions and without a centralised source of funding. In Portugal, the goal of this project was to consolidate the ERGA-Portugal community and initiate collective efforts to generate reference genomes for Portuguese species. This pilot effort in Portugal focused on endemic, endangered and iconic species from several taxonomic groups and different ecosystems in both the mainland and the Azores islands. From an initial list of 53 species proposed by members of ERGA-Portugal, 11 were prioritised based on ERGA’s feasibility criteria defined by the Sampling and Sample Processing (SSP) Committee21. These species were then ranked based on an anonymous online survey open to all members of ERGA-Portugal at the time. The six species with the highest score were included in the pilot effort. This shortlist included one fish, one mammal, one bird, one invertebrate, and two plants (Fig. 2).
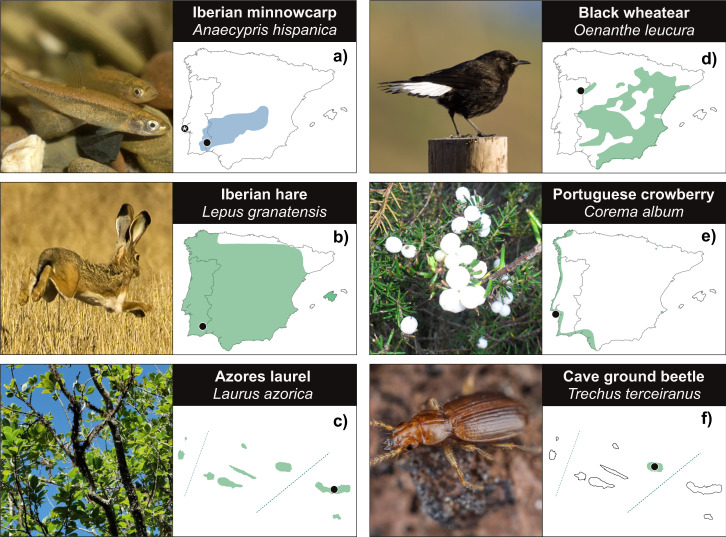
Fig. 2. Distribution and sampling localities of ERGA-Portugal species sequenced in ERGA’s pilot effort across Portuguese territories.
Approximate distributions and sampling localities of ERGA-Portugal species sequenced in ERGA’s pilot effort: a Iberian minnowcarp—Anaecypris hispanica; b Iberian hare—Lepus granatensis; c Azores laurel—Laurus azorica; d Black wheatear—Oenanthe leucura; e Portuguese crowberry—Corema album; f Cave ground beetle—Trechus terceiranus. Picture credits: a Carlos Carrapato; b Pedro Moreira; c Mónica Moura; d Ricardo Jorge Lopes; e Manuela Sim-Sim; f Javier Torrent (Azorean Biodiversity Group). Species distributions are coloured according to terrestrial (green) or aquatic (blue) territories. Sampling localities are marked with a black dot except for the Iberian minnowcarp (a) that is double marked, as it was sampled in the Aquário Vasco da Gama—Lisbon (black dot with a white star inside), but it is originally from a population of River Chança. All specimens were sampled within Portuguese territory, including both mainland Portugal and the Azores. Distributions were adjusted from the IUCN Red List of Threatened Species Website (https://www.iucnredlist.org), except for the Portuguese crowberry, which was based on information from the Anthos geographical information system for Spanish Flora (www.anthos.es).
Each species was championed by a genome team, led by one or two species ambassadors, and included ERGA-Portugal members and other ERGA members with transdisciplinary skills, who were responsible for successfully implementing each step for generating a reference genome. These steps included sampling, taxonomic identification, vouchering, laboratory work, sequencing, assembly, annotation, and downstream analysis. These six genome teams, detailed below for each species, promoted new national and international collaborations. The implementation of this project also promoted interactions with national authorities, for example, to obtain permits for capture, storage, and export of samples, in coordination with the Portuguese National Authority on Nature Conservation (Institute for Nature Conservation and Forests—ICNF), and the Azorean Regional Directorate for the Environment and Climate Change (DRAAC). For mainland Portugal, the capture and collection of samples of wildlife specimens were authorised by the ICNF, while for the Autonomous regions of the Azores, permission was given by the DRAAC. For the Azores, collections complied with the Access and Benefit Sharing provisions codified in the Nagoya Protocol (Convention on Biological Diversity, 2010). For all species, the sampling steps followed the guidelines provided by the ERGA SSP committee21. Sampling was optimised to ensure that high molecular weight (HMW) DNA and RNA could be obtained from at least one individual and ideally from multiple tissues (see details for each species below). The immediate preservation and transport of tissues in liquid nitrogen were prioritised, and tissues were subsequently maintained at −80 °C conditions. All specimens were sampled within Portuguese territory, in the continental mainland or in the Azores.
ERGA-Portugal pilot species
Iberian minnowcarp—Anaecypris hispanica (Steindachner, 1866)
Common name in Portuguese: saramugo
The Iberian minnowcarp (Anaecypris hispanica) is a strictly freshwater fish with a short lifespan that inhabits a restricted geographical area in two river basins of the Southern Iberian Peninsula22,23, a region that is severely affected by the effects of global climate change. This endemic species is considered to be the most endangered strictly freshwater fish in Iberian rivers, and is listed as Endangered on the IUCN Red List24 and as Critically Endangered on the Portuguese Red List25. These fish live in vulnerable freshwater ecosystems and are exposed to multiple threats, including increased temperatures and propensity for drought, pollution, habitat fragmentation, dams, intensive water use, and invasive species proliferation22,25. The Iberian minnowcarp is considered an iconic species as it is the only living member of the genus Anaecypris, representing a phylogenetically unique old lineage that persisted in the Iberian Peninsula26.
Sampling and sample processing
An adult female was collected by hand net from an open-air breeding tank at Aquário Vasco da Gama (Cruz Quebrada-Dafundo, Portugal), where an ex-situ conservation programme is ongoing with captive-born fish derived from 37 individuals from the River Chança (Guadiana river basin, Portugal). The individual was sampled, euthanised, and dissected on the same day, according to permits from the ICNF (Permit P-026382/2021) and animal welfare regulations of the host institution (ORBEA-MARE 02/2021). The head of the fish was flash-frozen and used for biobanking. Fin clips were used for barcoding and preserved in ethanol. Fin clips were also used to derive cell lines, which were flash-frozen and can be used for karyotyping and further genetic studies in the future. Tissues were collected immediately and flash-frozen in liquid nitrogen. Liver, spleen, muscle, eyes, and ovary tissues were collected.
Sequencing
Aliquots of these tissues were shipped to the Max Planck Institute of Molecular Cell Biology and Genetics (MPI-CBG, Germany), where the remaining steps for DNA extraction and sequencing were performed. The HMW DNA extraction and library preparation for PacBio HiFi were done using the spleen, whereas Hi-C library preparation for Illumina was done using muscle. RNA-Sequencing and Iso-Seq libraries from eye and gonad tissues were produced and sequenced.
Reference genome status
Sequencing data was used to obtain a genome assembly, which was done and completed by the genome team members from MPI-CBG (Germany) using the Vertebrate Genomes Project (VGP) standards (vertebrategenomesproject.org/). A manually curated assembly has been completed, and the annotation of the genome is ongoing in a collaborative effort involving researchers from ERGA-Portugal, as well as institutions and national computational resources.
Objectives
A high-quality reference genome will enable reconstruct the evolutionary history of this monospecific genus, elucidating its relationship with other fish lineages. Previously, phylogenetic and population genetic studies have been limited by the lack of comprehensive genomic data, hindering the understanding of its adaptation potential and population structure. Given that the Iberian minnowcarp is fragmented into small populations, inhabiting a region highly impacted by drought and water scarcity27, the availability of its reference genome will be crucial for understanding adaptation to intermittent environments, informing conservation management, and predicting responses to increasing water temperatures. The Iberian minnowcarp is included in ongoing ex situ conservation programmes. Hence these genomic resources will allow quantifying the loss of genetic diversity due to consecutive generations bred from small initial stocks of wild breeders, as well as optimising and monitoring future ex situ efforts.
Iberian hare—Lepus granatensis (Rosenhauer, 1856)
Common name in Portuguese: lebre Ibérica
The Iberian hare (Lepus granatensis), also known as Granada hare, is a lagomorph species endemic to the Iberian Peninsula, and the only hare species present in Portugal28. It is an iconic species of major ecological and economic importance in the Iberian ecosystems, acting as an important prey to apex predators and a relevant small game species in Portugal and Spain. Genetic studies have promoted this species as an outstanding model to understand diverse evolutionary questions, such as the impact of glacial cycles in repeated changes in species ranges, and the influence of introgressive hybridisation on their adaptive potential29. It is currently classified as Least Concern in the IUCN Red List30, but a marked decline over the last decade led to its classification as Vulnerable in the last review of the Red Book of Mammals of Portugal31. The rate of population decline has increased due to the emergence of a natural recombinant derived from the rabbit Myxoma virus, the hare Myxoma virus (ha-MYXV)32–34.
Sampling and sample processing
One male individual Iberian hare was sampled in Mértola, southern Portugal, during the regular permitted hunting season, duly authorised by ICNF (Permit 012/2022/CAPT). Tissues were collected shortly after the animal’s death and flash-frozen in liquid nitrogen. Tissues from several organs were collected during the necropsy and kept at −80 °C for biobanking: liver, kidney, spleen, lung, and testis. The whole body will be prepared for deposition at the Natural History and Science Museum of the University of Porto (MHNCUP_MAM 0812).
Sequencing
Tissue samples were shipped to the University of Antwerp for Omni-C and RNA-Sequencing library preparation, and to the University of Florence for PacBio DNA library preparation. Final Omni-C libraries were generated using kidney tissue, while RNA sequencing libraries were produced for liver, kidney, spleen, lung, and testis tissues. These libraries, along with PacBio DNA libraries, were sequenced at the University of Florence. Long-read DNA sequencing was performed in HiFi mode in a PacBio Sequel IIe platform using five 8 million ZMW SMRT cells.
Reference genome status
Sequencing data was used to produce a genome assembly following the gold standard instructions implemented in the VGP Galaxy Pipeline35,36. The genome manual curation and annotation is ongoing, and involves ERGA-Portugal members, as well as institutions and national computational resources.
Objectives
Generating a high-quality reference genome will significantly improve genomic studies in this species, addressing previous limitations in understanding its speciation and adaptation processes29. This enhancement in genomic resources will benefit not only research on this species but also on other related species and systems. Furthermore, it will anchor research aiming to quantify the impact of emerging pathogens on the naïve host hare populations, which can be used to inform conservation measures and manage disease risks effectively.
Azores laurel—Laurus azorica (Seub.) Franco
Common name in Portuguese: louro-da-terra and louro-bravo
The Azores laurel (Laurus azorica) is a micro or mesophanerophyte dioecious perennial tree endemic to the Azores Islands. It can be commonly found in submontane Laurus forests37 but also occupies habitats such as coastal scrublands, among other native Azorean vegetation37. It is listed as Least Concern in the IUCN Red List38, yet it is facing habitat loss due to competition with invasive species and alteration of habitats resulting from the implementation of production forests and pastures39–42. The genus Laurus L. (Lauraceae) is currently restricted to isolated refugia in the southern Black Sea area, Mediterranean Basin, Northern Africa, and the Macaronesian archipelagos. Its taxonomic classification remains uncertain, with some recognising the existence of two species, Laurus azorica (Seub.) Franco, endemic to the Azores and Laurus nobilis L. However, while some molecular studies contradict this distinction43, others even recognise a third species, Laurus novo-canariensis Rivas Mart., Lousã, Fern.Prieto, E.Dias, J.C.Costa & C.Aguiar44.
Sampling and sample processing
Fresh branches with healthy leaves were collected using gardening scissors from one mature male individual in the Azores islands, specifically, São Miguel Island, in Lombadas. The branches were loosely wrapped in moist absorbent paper, placed inside an open plastic bag, and sent on the same day through express mail to the University of Lisbon, where they were kept at −20 °C until DNA extraction. The corresponding voucher duplicate was dried using standard herbarium procedures and stored in the AZB herbarium at the Biology Department of the Faculty of Sciences and Technology of the University of the Azores, with voucher AZB4382, and collector code LZ001. A second sampling was conducted during March 2023 and followed the same methodology. The Nagoya certificate has CCIR number 61/2021/DRCTD.
Sequencing
The HMW DNA extraction was performed at the Department of Plant Biology of the Faculty of Sciences of the University of Lisbon (FCUL), using a modified cetyltrimethylammonium bromide (CTAB) protocol45 (later modified by ref. 46) adjusted based on the exchange of protocols within the ERGA community. DNA extraction was challenging, as extractions did not pass the quality control for PacBio Hi-Fi library preparation and sequencing. Leaf tissue was thus shipped in dry ice to the University of Florence, where the libraries for Pac-Bio were prepared and sequenced.
Reference genome status
The assembly of the genome has started, and involves ERGA-Portugal members, as well as institutions and national computational resources.
Objectives
A high-quality reference genome will allow performing phylogenetic and population genetic studies, which have been constrained by the absence of comprehensive genomic data. This will facilitate the reconstruction of the evolutionary history of the Laurus genus, contributing to clarifying its diversity and taxonomy. This genomic resource will resolve ambiguities in species classification and support conservation strategies by identifying genetic variations critical for species maintenance.
Black wheatear–Oenanthe leucura (Gmelin, 1789)
Common name in Portuguese: chasco-preto
The black wheatear (Oenanthe leucura) is a passerine bird species confined to North Africa and southwest Europe, a region where global environmental changes (namely desertification and agricultural abandonment) are impacting the distribution of many bird species47–49. Black wheatears are no exception, with findings supporting that some edge populations have already vanished (France) whilst others continue to decrease (Western populations in Portugal and Spain). Despite the decline and fragmentation of these populations, an isolated population located in northern Portugal (~100 pairs) still subsists, confined mainly to cultivated lands (primarily vineyards and olive orchards) of the Portuguese section of the Douro River, internationally known for Port wine. For this reason, despite being classified globally as Least Concern by the IUCN Red List, Portuguese populations are classified as Critically Endangered in the Portuguese Red List25,50.
Sampling and sample processing
Due to the small size of the black wheatear population in Portugal, the sacrifice of a bird would be unjustified and, therefore, ICNF authorised the collection of blood samples from live birds (Permit 720/2021/REC). Three individuals (one female and two males) were caught using spring traps at the Douro Valley, near the Tua River mouth. Each bird was ringed, photographed, and measured, and a blood sample (~100 microliters) was taken by venepuncture at the ulnar vein with a microcapillary. Each blood sample was immediately stored in liquid nitrogen and transferred to a −80 °C freezer on the same day.
Sequencing
Samples were sent to the ERGA partners in dry ice, to generate Omni-C (University of Antwerp) and PacBio HiFi data (University of Florence) from the female (C96097) and RNA-Sequencing (University of Antwerp) from one of the males (E29638). All DNA libraries were sequenced at the University of Florence. Long-read DNA sequencing was performed using two 8 million ZMW SMRT cells.
Reference genome status
Genome assembly was performed following the gold standard instructions implemented in the VGP Galaxy Pipeline35,36. The manual curation and genome annotation are ongoing, involving ERGA-Portugal members, as well as institutions and national computational resources.
Objectives
A high-quality reference genome, combined with population genomics data, will overcome previous limitations in characterising the population structure and history. This population is closely associated with anthropogenic agricultural lands in the Douro Valley, yet geographically isolated from other Iberian populations that occur mostly in natural habitats. This isolation and fragmentation could have irreversible effects on the survival of these populations, and their conservation may require the implementation of specific conservation measures. The reference genome will establish the baseline for genomic analysis of the Portuguese populations, providing information on individuals’ relatedness and dispersal capacity, which are essential for implementing specific conservation measures.
Portuguese crowberry—Corema album (L.) D. Don
Common name in Portuguese: camarina and camarinha
The Portuguese crowberry (Corema album) is a dioecious perennial woody shrub endemic to the Atlantic coast of the Iberian Peninsula (ssp. album), and to the Azores Islands (ssp. azoricum). In the Iberian Peninsula it inhabits coastal areas from Galicia to Gibraltar, and is an important species in sand dune habitats, which are highly valuable for conservation purposes51. The dynamic nature of these coastal ecosystems provides a vast variety of habitats with unique floristic and animal richness. However, these dune systems face increasing disturbances as they support various economic and leisure activities, associated with the growth of the coastal population52. Because of habitat loss, C. album has been classified as Vulnerable on the Red List of Andalusia, Spain53. In the Azores Islands, it inhabits volcanic lava and ash fields54. The fruits of the Portuguese crowberry are edible, producing bioactive compounds that have been associated with chemoprotective activity and potential health benefits54–56.
Sampling and sample processing
Two male and two female adult plants from the same population were sampled during two field trips to Azeitão, near Arrábida Natural Park. Young expanding leaves and fruits were collected after 48 hours of dark treatment (plants covered with light-opaque paper sheets with a few holes that allowed air flow), according to permits from ICNF (21PTLX00657D). Samples from leaves and fruits for DNA and RNA extraction were flash-frozen at −20 °C and −80 °C, respectively. Voucher specimens were deposited at the Herbarium (LISU 270092) of the “Museu Nacional de História Natural e da Ciência”, Universidade de Lisboa.
Sequencing
The HMW DNA extraction was very challenging. An initial extraction was performed at the Department of Plant Biology of FCUL using a modified CTAB protocol45 (later modified by ref. 46), which resulted in low DNA quality even after purification. Taking advantage of the ERGA network, we used a nuclei isolation protocol adapted from the ARIMA-HiC kit, shared by Narjes Yousefi (pers. comm.), a member of ERGA. This resulted in higher quality and quantity of extracted DNA, but it still did not pass the quality control for PacBio Hi-Fi library preparation and sequencing at the University of Florence. After several attempts, to prevent DNA degradation during shipping, leaf tissue was directly shipped in dry ice so that DNA extraction could be performed at the University of Florence, where the libraries for Pac-Bio were prepared and sequenced.
Reference genome status
The genome assembly is complete, and annotation is ongoing involving ERGA-Portugal members and Biogenome Portugal institutions.
Objectives
Building a high-quality annotated reference genome will support ongoing projects on Portuguese crowberry and related plant species from coastal areas. Previous studies have been limited by the lack of detailed genomic information, restricting the understanding of evolutionary adaptations to coastal environments under global change scenarios. Moreover, the annotated reference genome will provide a valuable resource for uncovering the genetic basis of the production of Portuguese crowberry’s bioactive compounds associated with health benefits, opening new avenues for both conservation and biotechnological applications.
Cave ground beetle—Trechus terceiranus57
Common name in Portuguese: escaravelho-cavernícola-da-Ilha-Terceira
The cave ground beetle Trechus terceiranus (Coleoptera) is endemic to Terceira Island in the Azores and is restricted to subterranean habitats, including lava tubes. These tiny beetles have up to 4.3 mm in length, do not fly, and have several morphological traits associated with their life in subterranean habitats57. This species is listed as Vulnerable in the IUCN Red List58. Interestingly, several species of the genus Trechus occur in the Azores islands, most of which inhabit subterranean habitats (seven species), but two occur in surface habitats. One epigean species, T. terrabravensis Borges, Serrano & Amorim, 2004, co-exists on Terceira Island with T. terceiranus.
Sampling and sample processing
Six adult individuals were sampled in a subterranean habitat (volcanic pit, lava tube) at 45–70 m deep, using pitfall traps. Sampling was done according to permits issued by the Regional Government of the Azores (IRCC 23/2021/DRCTD). Adult individuals were flash-frozen with liquid nitrogen. Dried vouchers were stored at the Dalberto Teixeira Pombo entomological collection at the University of the Azores, Terceira (DTP-MF1091).
Sequencing
The frozen whole organisms were shipped in dry ice to the University of Lisbon, from where they were subsequently sent to Centro Nacional de Análisis Genómico Barcelona for RNA extraction and sequencing, and to the University of Florence where DNA extraction was attempted. Due to constraints in obtaining high-quality HMW DNA for PacBio sequencing, additional samples were sent in order to repeat the extraction to obtain enough DNA amount and quality for sequencing. All DNA libraries were sequenced at the University of Florence.
Reference genome status
The assembly of the genome is currently ongoing, and involves ERGA-Portugal members, as well as institutions and national computational resources.
Objectives
A high-quality reference genome will be crucial to pursue comparative genomics, phylogenetic and population genetic studies, overcoming past limitations in understanding the evolutionary history of this group. This genomic resource will facilitate the implementation of adequate conservation strategies and enable the detection of genes involved in adaptation to subterranean habitats. It will also allow researchers to determine the genetic architecture of traits related to these habitats, such as eye development, pigmentation, and biological clock regulation, providing a comprehensive understanding of the species’ unique adaptations.
Building a national network for biodiversity genomics: Biogenome Portugal
Objectives and structure of Biogenome Portugal
While ERGA and ERGA-Portugal are a community of researchers, their collaboration in activities and in the pilot test has played a significant role in forming a national institutional network for biodiversity genomics, called Biogenome Portugal (BGP). This network is being formally established with the objective of fostering scientific collaboration, facilitating the exchange of expertise and infrastructure, organising advanced training programmes, promoting Portugal’s active participation in international biodiversity genomics initiatives, and contributing to knowledge transfer and outreach efforts. The network will comprise members with a diverse range of expertise, encompassing fields such as genomics, ecology, taxonomy, evolution, and more, spanning various taxonomic groups.
By bringing together experts from different fields, the BGP network aims to facilitate the exchange of ideas, data and resources that will lead to the development of new research projects centred on generating and using high-quality reference genomes for Portuguese species and addressing a plethora of biodiversity applications. Such collaboration also implies sharing institutional genomic and computational infrastructures and articulating with the national network of infrastructures related to BGP’s areas of activity. Cooperation among members is crucial for promoting the exchange of expertise, a key objective of the network. By sharing knowledge and skills, the network members will strengthen their collective expertise and coordinate efforts. Activities towards the transfer of skills and expertise include organising advanced training in the field of biodiversity genomics. Through these initiatives, BGP aims to foster the training of specialised human resources able to accompany the fast development of genomic data analysis tools. To broaden its scope, the network intends to facilitate the involvement of individual researchers, even if their institution is not formally affiliated with the network.
BGP also aims to disseminate the results of its activities to stakeholders and the general public, thus promoting knowledge transfer and outreach. These activities are important not only to enrich scientific literacy about the importance of genomics for biodiversity conservation but also to promote and encourage the use of genomic research findings across stakeholders.
Finally, BGP also aims to coordinate and synchronise Portuguese participation in global biodiversity genomics initiatives. By reinforcing and sharing the opportunities for institutional representation in internationally funded projects, BGP can promote the contribution of Portuguese expertise to important regional, continental, or worldwide initiatives.
Articulation with the Portuguese National Strategy for the Conservation of Nature and Biodiversity 2030
The Portuguese National Strategy for the Conservation of Nature and Biodiversity19 is the main legal Portuguese document that is used to frame all national policies until 2030. It recognises the importance of Portugal’s national biodiversity at both the European and global scale and acknowledges how the nation’s biogeographic specificities have led to high levels of endemic and relict species, each with a unique evolutionary history and genetic composition, which is important to preserve. The national strategy has several main objectives, including planning and executing action promoting the conservation and recovery of species and habitats at the national level and promoting the conservation of plant and animals’ genetic diversity. These objectives align with the aims of the nascent BGP. The network can provide the knowledge-based capacities necessary for the conservation and sustainable use of the national marine, terrestrial, and freshwater genetic resources. BGP can reinforce a science-based approach for species conservation action plans and guide complex conservation strategies, including in situ and ex situ conservation.
Fostering national research infrastructures
In 2014, the National Public Agency for Science, Technology, and Innovation (Fundação para a Ciência e a Tecnologia, FCT) created a National Roadmap for Research Infrastructures of Strategic Interest. This roadmap aimed at promoting cooperation, research excellence, and internationalisation to strengthen national scientific infrastructures. A total of 56 Research Infrastructures (RIs) have been supported during the first funding cycle in key areas, such as the Environment, Health and Food, and Social and Cultural Innovation, among others. BGP identified several RIs of interest, intersecting its area of intervention. Among these are GenomePT (the National Laboratory for Genome Sequencing and Analysis), Biodata.pt (ELIXIR PT—Portuguese Distributed Infrastructure for Biological Data), PORBIOTA (Portuguese E-Infrastructure for Information and Research on Biodiversity), EMBRC.PT (European Marine Biological Resource Centre—Portugal), and PRISC (Portuguese Research Infrastructure for Scientific Collections). Future action implies fostering the establishment of strategic cooperation with the active infrastructures, which can be facilitated by the current presence of several institutions of BGP in the RIs, pending the continuation of the current roadmap.
Engaging the community: establishing training programmes, supporting the generation of additional reference genomes, and expanding funding opportunities
The analysis of reference genomes at scale requires standardised procedures for the sampling and storing of the biological material, as well as sequencing and analysis of the genomic data. Researchers working to analyse and utilise these cutting-edge genomic resources need specific training, to maintain quality standards and streamline procedures. The development of bioinformatic pipelines by the larger ERGA community using open-source platforms such as Galaxy59 allows quick implementation of analyses across research centres. Within the BGP network, an online training programme is being implemented to cover different topics related to genomic analyses, such as genome and transcriptome assembly, annotation, and comparative and population genomics. This programme aims to expand and facilitate training to enhance expertise in genomic analyses across the Portuguese research community. The first introductory course to Galaxy and VGP assembly pipeline took place in the first semester of 2023.
Further expertise sharing will come from the expansion of reference genome projects under the BGP umbrella, which meets the technical and quality standards set by ERGA. For example, two reference genomes of Anthozoan species belonging to octocorals are being generated under the BGP initiative: the pink sea fan (Eunicella verrucosa) and the dead man’s fingers (Alcyonium digitatum). Octocoral genomes remain poorly studied, with only ~10 reference genomes available from the >3000 described species. These two species belong to a pool of octocorals for which the sequencing of reference genomes was supported by EASI-Genomics (H2020 824110; Project ID 10240, CoGeCo). The two species are widely distributed along the Portuguese coast in sublittoral rocky habitats, and sequencing, genome assembly, and annotations are underway.
Attracting national and international funding will be crucial to support the implementation of BGP’s activities. Working as a network will lead to stronger research proposals and more successful grant applications and increase collective participation in international consortia to foster biodiversity genomics cutting-edge research and knowledge transfer. This strategy will allow consolidating, strengthening, and expanding the BGP network and its impact on fundamental and applied science and innovation.
Producing high-quality chromosome-level assemblies of Portuguese species
The alignment of BGP with ERGA can foster the multiplication of opportunities for sequencing projects for Portuguese biodiversity. This aligns with ERGA's aim of “propagating guidelines for state-of-the-art genome establishment through training and knowledge transfer”. The genomes produced can, therefore, take advantage from ERGA's standardised sampling methods, molecular protocols, and bioinformatics pipelines for sequencing, genome assembly, and annotation, and can be included under the ERGA umbrella. While promoting the visibility and accessibility of the genomes to the network and beyond, those genomes will contribute to the Reference Genome Atlas of European biodiversity. Such parallel initiatives under the BGP and ERGA umbrella, coordinated with ERGA projects, will expedite the production of a genomic inventory for all Portuguese eukaryotic species. Those efforts are invaluable in enabling the use of genomic applications to preserve and protect the country’s rich biodiversity.
Acknowledgements
We thank Fernando Racimo, Svein-Ole Mikalsen and an anonymous reviewer for the initial open peer review and recommendation at Peer Community In Genomics (preprint and recommendation: 10.24072/pci.genomics.100257). Work supported by the European Union's Horizon 2020 Research and Innovation Programme under the Grant Agreement Number 857251. This work was also supported by National Funds through FCT-Fundação para a Ciência e a Tecnologia in the scope of the project UIDP/50027/2020. Strategic funding from FCT to CE3C and BioISI Research Units (UIDB/00329/2020 and UIDB/04046/2020) and to the associate laboratory CHANGE (LA/P/0121/2020) are gratefully acknowledged. Financing by Azorean government regional funds, SRCCTD/DRCTD, project ERGA TAXA AÇORES (M2.2.A/PROJ.INT/A/001/2021—ERGA taxa Açores). I.R.A. was funded by national funds through FCT, under the Norma Transitória 10.54499/DL57/2016/CP1375/CT0003. J.M.-F. acknowledges support from FCT—contract 2021.00150.CEECIND and project grant HybridChange (10.54499/PTDC/BIA-EVL/1307/2020) via Portuguese national funds and GenomePT for supporting CIBIO’s genomic infrastructure. P.C.A. acknowledges support by NORTE-01-0246-FEDER-000063, supported by Norte Portugal Regional Programme. A.J.A. was funded by 2021.02058.CEECIND; PTDC/CVT-CVT/28798/2017. P.F.C. was partially supported by National Funds through FCT, I.P. CEECIND/01799/2017. T. Brown was supported by DFG (INST 269/768-1). FCT/MEC supports the research contract to da L.P.S. (CEECIND/02064/2017). R.J.L. was supported by an FCT—Transitory Norm contract (DL57/2016/CP1440/CT0006). P.A.V.B. was funded by project FCT-UIDB/00329/2020-2024 (Thematic Line 1—integrated ecological assessment of environmental change on biodiversity). S.L.M. is supported by an FCT PhD studentship (SFRH/BD/145153/2019). J.M. Moreno is supported by an FCT PhD studentship (SFRH/BD/143199/2019). This study received Portuguese national funds from FCT through project UIDB/04326/2020. H.S. was supported by the Flemish University Research Fund (BOF) and the Research Foundation—Flanders (FWO) through grant G047521N. A.M. was supported by a Postdoctoral Researcher Fellowship of the Fonds de la Recherche Scientifique—FNRS. Portuguese National Funds, through FCT, support the research contract to A.C. (2020.00823.CEECIND/CP1601/CT0003) and R.F. (contract 2020.00275.CEECIND; grant Invcontinuum—PTDC/BIA-EVL/1614/2021). C.R.F. thanks the support of CE3C through an assistant researcher contract (Fciência.ID contract #366) and FCT for Portuguese National Funds attributed to CE3C within the strategic project UID/BIA/00329/2020; C. R.F. also thanks FPUL for a contract of invited assistant professor. Operational Programme (NORTE2020), under the PORTUGAL 2020 Partnership Agreement, through the European Regional. V.C.S. acknowledges support from FCT project grant HYBRIDOMICS (10.54499/PTDC/BIA-EVL/4345/2021), via Portuguese national funds. CIIMAR acknowledges Strategic Funding UIDB/04423/2020, UIDP/04423/2020 and LA/P/0101/2020 through national funds provided by FCT. We gratefully acknowledge the generous support of the Max Planck Society for this research. Sequencing of Iberian minnowcarp was carried out at the DRESDEN-concept Genome Center, supported by the DFG Research Infrastructure Programme (Project 407482635) and part of the Next Generation Sequencing Competence Network NGS-CN (Project 423957469). Additional support was obtained from Horizon Europe under the Biodiversity, Circular Economy and Environment (REA.B.3); co-funded by the Swiss State Secretariat for Education, Research and Innovation (SERI) under contract number 22.00173; and by the UK Research and Innovation (UKRI) under the Department for Business, Energy and Industrial Strategy’s Horizon Europe Guarantee Scheme. ERGA Hubs: Illumina and PacBio HiFi sequencing (RNA + DNA) was supported by the sequencing facility of the Department of Biology, University of Florence through the Departments of Excellence programme funded by the Italian Ministry for University and Research. We thank the Antwerp University Hospital Center of Medical Genetics and Jarl Bastianen for access to sequencing library quality control equipment. The Hi-C library preparation for Trechus terceiranus is being done at the Metazoa Phylogenomics Lab, Institute of Evolutionary Biology (CSIC-University Pompeu Fabra) through a collaboration with Judit Salces-Ortiz, Nuria Escudero, and Rosa Fernández. ERGA infrastructure: We acknowledge access to the storage resources at the Barcelona Supercomputing Center, which are partially funded by the European Union H2020-INFRAEOSC-2018-2020 programme through the DICE project (Grant Agreement no. 101017207). We would like to thank Alisha Ahamed, Josephine Burgin, Joana Paupério, Jeena Rajan, and Guy Cochrane from the European Nucleotide Archive (ENA) for their support regarding data coordination and submission of genomic data. Commercial Partners: We would like to acknowledge and thank all supplier partners who have kindly donated kits and reagents to the ERGA pilot Library Preparation Hubs to support species without funding to produce the generation of high-quality genomes and annotations. This support has been key to embedding a culture of diversity, equity, inclusion, and justice in the Pilot Project. Specifically, we want to thank Dovetail Genomics, Part of Cantata Bio LLC, especially Mark Daly, Thomas Swale, and Lily Shuie; Arima Genomics; PacBio; Integrated DNA Technologies (IDT); MagBio Genomics Europe GmbH; Zymo Research; Agilent Technologies; Fisher Scientific Spain; Illumina Inc.
Author contributions
Shared first authorship: J.P.M., P.C.A., I.R.A., R.J.L., M. M., E.W.M., M. S.-S. and C.S.-S. Shared senior authorship: V.C.S. and J.M.-F. Species ambassador or executive coordination: J.P.M., P.C.A., I.R.A., R.J.L., M. M., E.W.M., M. S.-S., C.S.-S., and J.M.-F. Genome Team Member: J.P.M., P.C.A., I.R.A., R.J.L., M. M., E.W.M., M. S.-S., C.S.-S., M.J.A., P.A.V.B., T.B., M.C., C.Ca., L.M.P.C., C.Ci., L.P.d.S., G.D, M.A.D., L.F., G.F., F.G., M.L.G., A.I., H.G.L., C.M., A.M.M.C., S.L.M., J.M.M., M.M., A.M., C.N., F.P., R.M.C.R., R.R., G.R., H.S., P.J. E., H.T., S.V., S.W., V.C.S., and J.M.-F. ERGA-Portugal member: J.P.M., P.C.A., I.R.A., R.J.L., M. M., M. S.-S., C.S.-S., M.J.A., M.C., L.M.P.C., H.T., S.V., M.A., A.J.A., A. A., P.F.C., A.V.M.C., R.C., L.F.C.C., A.C., M.V.C., G.E.T., P.J. E., R.F., C.R.F., J.B.L., B.L., S.M., O.S.P., G.A.P., J.P., T.L.S., E.A.S., F.P.-M., V.C.S. and J.M.-F. ERGA-Portugal coordination: V.C.S. and J.M.-F. Write manuscript: J.P.M., I.R.A., R.J.L., M.M., M.S.-S., C.S.-S., H.T., G.E.T., C.R.F., J.B.L., O.S.P., V.C.S. and J.M.-F. Revise manuscript: J.P.M., P.C.A., I.R.A., R.J.L., M. M., M. S.-S., C.S.-S., C.Ci., A.M.M.C., A.M., H.T., M.A., A.J.A., A. A., A.V.M.C., R.C., L.F.C.C., A.C., M.V.C., G.E.T., T.B., P.J.E., R.F., C.R.F., J.B.L., B.L., S.M., O.S.P., T.L.S., E.A.S., F.P.-M., V.C.S. and J.M.-F. Read and approve the manuscript: all authors.
Competing interests
A.M.M.C. serves as associate editor of this journal and had no role in the peer-review or decision to publish this manuscript. A.M.M.C. declares no financial competing interests. The remaining authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
João P. Marques, Email: joao.marques@cibio.up.pt
José Melo-Ferreira, Email: jmeloferreira@cibio.up.pt.
Vítor C. Sousa, Email: vmsousa@ciencias.ulisboa.pt
References
- 1.Ceballos, G., Ehrlich, P. R. & Dirzo, R. Biological annihilation via the ongoing sixth mass extinction signaled by vertebrate population losses and declines. Proc. Natl. Acad. Sci.114, E6089–E6096 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cowie, R. H., Bouchet, P. & Fontaine, B. The sixth mass extinction: fact, fiction or speculation? Biol. Rev.97, 640–663 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Habel, J. C. et al. Final countdown for biodiversity hotspots. Conserv. Lett.12, e12668 (2019). [Google Scholar]
- 4.Karger, D. N., Kessler, M., Lehnert, M., & Jetz, W. Limited protection and ongoing loss of tropical cloud forest biodiversity and ecosystems worldwide. Nat. Ecol. Evol.5, 6 (2021). [DOI] [PubMed]
- 5.O’Hara, C. C., Frazier, M. & Halpern, B. S. At-risk marine biodiversity faces extensive, expanding, and intensifying human impacts. Science372, 84–87 (2021). [DOI] [PubMed] [Google Scholar]
- 6.Dauphin, B. et al. Genomic vulnerability to rapid climate warming in a tree species with a long generation time. Glob. Chang. Biol.27, 1181–1195 (2021). [DOI] [PubMed] [Google Scholar]
- 7.Browne, L., Wright, J. W., Fitz-Gibbon, S., Gugger, P. F. & Sork, V. L. Adaptational lag to temperature in valley oak (Quercus lobata) can be mitigated by genome-informed assisted gene flow. Proc. Natl Acad. Sci.116, 25179–25185 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hohenlohe, P. A., Funk, W. C. & Rajora, O. P. Population genomics for wildlife conservation and management. Mol. Ecol.30, 62–82 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ralls, K., Sunnucks, P., Lacy, R. C. & Frankham, R. Genetic rescue: a critique of the evidence supports maximizing genetic diversity rather than minimizing the introduction of putatively harmful genetic variation. Biol. Conserv.251, 108784 (2020). [Google Scholar]
- 10.Segelbacher, G. et al. New developments in the field of genomic technologies and their relevance to conservation management. Conserv. Genet.23, 217–242 (2022). [Google Scholar]
- 11.Formenti, G. et al. The era of reference genomes in conservation genomics. Trends Ecol. Evol.37, 197–202 (2022). [DOI] [PubMed] [Google Scholar]
- 12.Theissinger, K. et al. How genomics can help biodiversity conservation. Trends Genet. 10.1016/j.tig.2023.01.005 (2023). [DOI] [PubMed]
- 13.Ette, J.-S. & Geburek, T. Why European biodiversity reporting is not reliable. Ambio50, 929–941 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Myers, N., Mittermeier, R. A., Mittermeier, C. G., da Fonseca, G. A. B., & Kent, J. Biodiversity hotspots for conservation priorities. Nature403, 6772 (2000). [DOI] [PubMed]
- 15.Pauly, D., Zeller, D., & Palomares, M. L. D. Sea around us concepts, design and data. www.seaaroundus.org (2020).
- 16.Gómez, A., & Lunt, D. H. Refugia within Refugia: patterns of phylogeographic concordance in the Iberian peninsula. In: S. Weiss & N. Ferrand (Eds.), Phylogeography of Southern European Refugia: Evolutionary perspectives on the origins and conservation of European biodiversity (pp. 155–188). Springer Netherlands. 10.1007/1-4020-4904-8_5 (2007).
- 17.Hewitt, G. M. Genetic consequences of climatic oscillations in the Quaternary. Philos. Trans. R. Soc. B: Biol. Sci.359, 183–195 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Florencio, M. et al. Macaronesia as a fruitful arena for ecology, evolution, and conservation biology. Front. Ecol. Evol.9, 718169 (2021). [Google Scholar]
- 19.ENCNB 2030—Portuguese national strategy for the conservation of nature and biodiversity. Available at: https://dre.pt/dre/detalhe/resolucao-conselho-ministros/55-2018-115226936 (2018).
- 20.Mc Cartney, A. M. et al. The European Reference Genome Atlas: piloting a decentralised approach to equitable biodiversity genomics (p. 2023.09.25.559365). bioRxiv. 10.1101/2023.09.25.559365 (2023). [DOI] [PMC free article] [PubMed]
- 21.Böhne, A. et al Contextualising samples: Supporting reference genomes for European biodiversity through sample and associated metadata collection (p. 2023.06.28.546652). bioRxiv. 10.1101/2023.06.28.546652 (2023). [DOI] [PMC free article] [PubMed]
- 22.Cardoso, A. Updating Anaecypris hispanica distribution and conservation status in Portugal. FiSHMED Journal. https://www.fishmedjournal.sibic.org/FiSHMED.2022.001/ (2023).
- 23.De Miguel, R. et al. On the occurrence of Anaecypris hispanica, an extremely endangered Iberian endemism, in the Guadalquivir River basin. J. Fish. Biol.76, 1454–1465 (2010). [DOI] [PubMed] [Google Scholar]
- 24.Crivelli, A. J. IUCN Red list of threatened species: Anaecypris hispanica. IUCN Red List of Threatened Species. https://www.iucnredlist.org/en (2006).
- 25.Cabral, M. J. et al. Livro Vermelho dos Vertebrados de Portugal. Instituto da Conservação da Natureza. http://dspace.uevora.pt/rdpc/handle/10174/6006 (2005).
- 26.Perea, S. et al. Phylogenetic relationships and biogeographical patterns in Circum-Mediterranean subfamily Leuciscinae (Teleostei, Cyprinidae) inferred from both mitochondrial and nuclear data. BMC Evol. Biol.10, 265 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sousa-Santos, C. et al. Metapopulations in temporary streams—the role of drought–flood cycles in promoting high genetic diversity in a critically endangered freshwater fish and its consequences for the future. Mol. Phylogenet. Evol.80, 281–296 (2014). [DOI] [PubMed] [Google Scholar]
- 28.Alves, P. C., Acevedo, P., & Melo-Ferreira, J. Iberian Hare Lepus granatensis Rosenhauer, 1856. In: K. Hackländer & F. E. Zachos (Eds.) Handbook of the Mammals of Europe (pp. 1–23). Springer International Publishing. 10.1007/978-3-319-65038-8_8-1 (2023).
- 29.Seixas, F. A., Boursot, P. & Melo-Ferreira, J. The genomic impact of historical hybridization with massive mitochondrial DNA introgression. Genome Biol.19, 91 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Soriguer, R., & Carro, F. IUCN Red List of Threatened Species: Lepus granatensis. IUCN Red List of Threatened Species. https://www.iucnredlist.org/en (2018).
- 31.Mathias, M. L., et al. (2023). Livro Vermelho dos Mamíferos de Portugal Continental. FCiências.ID / ICNF.
- 32.Águeda-Pinto, A. et al. Genetic Characterization of a Recombinant Myxoma virus in the Iberian Hare (Lepus granatensis). Viruses11, 530 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cardoso, B. et al. Effect of Myxoma Virus Species Jump on Iberian Hare Populations. Emerg. Infect. Dis.30, 1293–1296 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dalton, K. P. et al. Myxoma virus jumps species to the Iberian hare. Transbound. Emerg. Dis.66, 2218–2226 (2019). [DOI] [PubMed] [Google Scholar]
- 35.Batut, B. et al. Community-Driven Data Analysis Training for Biology. Cell Syst.6, 752–758.e1 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Larivière, D. et al. Scalable, accessible and reproducible reference genome assembly and evaluation in Galaxy. Nat. Biotechnol.42, 367–370 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Elias, R. B., et al. Natural zonal vegetation of the Azores Islands: Characterization and potential distribution. Phytocoenologia, 107–123. 10.1127/phyto/2016/0132 (2016).
- 38.Silva, L., & Beech, E. (2016). IUCN Red List of Threatened Species: Laurus azorica. https://www.iucnredlist.org/en
- 39.Borges Silva, L. et al. Biomass valorization in the management of woody plant invaders: the case of Pittosporum undulatum in the Azores. Biomass Bioenergy109, 155–165 (2018). [Google Scholar]
- 40.Dutra Silva, L., Brito de Azevedo, E., Vieira Reis, F., Bento Elias, R., & Silva, L. Limitations of species distribution models based on available climate change data: a case study in the Azorean forest. Forests10, 575 (2019).
- 41.Hortal, J., Borges, P. A. V., Jiménez-Valverde, A., de Azevedo, E. B. & Silva, L. Assessing the areas under risk of invasion within islands through potential distribution modelling: The case of Pittosporum undulatum in São Miguel, Azores. J. Nat. Conserv.18, 247–257 (2010). [Google Scholar]
- 42.Lourenço, P., Medeiros, V., Gil, A. & Silva, L. Distribution, habitat and biomass of Pittosporum undulatum, the most important woody plant invader in the Azores Archipelago. For. Ecol. Manag.262, 178–187 (2011). [Google Scholar]
- 43.Rodríguez-Sánchez, F., Guzmán, B., Valido, A., Vargas, P. & Arroyo, J. Late Neogene history of the laurel tree (Laurus L., Lauraceae) based on phylogeographical analyses of Mediterranean and Macaronesian populations. J. Biogeogr.36, 1270–1281 (2009). [Google Scholar]
- 44.Rivas-Martínez, S., et al. Vascular plant communities of Spain and Portugal: Addenda to the syntaxonomical checklist of 2001. 2. Itinera Geobotanica (España). https://scholar.google.com/scholar_lookup?title=Vascular+plant+communities+of+Spain+and+Portugal%3A+addenda+to+the+syntaxonomical+checklist+of+2001.+2&author=Rivas-Mart%C3%ADnez%2C+S.+%28Universidad+Complutense+de+Madrid.+Facultad+de+Farmacia%29&publication_year=2002 (2002).
- 45.Doyle, J. J. & Doyle, J. L. A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem. Bull.19, 11–15 (1987). [Google Scholar]
- 46.Weising, K., Nybom, H., Pfenninger, M., Wolff, K., & Meyer, W. DNA Fingerprinting in Plants and Fungi. CRC Press, Boca Raton, 338 pp. Taylor & Francis. https://books.google.pt/books?id=AtkHlIks64YC (1994).
- 47.Fernández-Nogueira, D., & Corbelle-Rico, E. Land Use Changes in Iberian Peninsula 1990–2012. Land, 7, Article 3. 10.3390/land7030099 (2018).
- 48.Fernández-Nogueira, D., & Corbelle-Rico, E. Determinants of Land Use/Cover Change in the Iberian Peninsula (1990–2012) at Municipal Level. Land, 9, Article 1. 10.3390/land9010005 (2020).
- 49.Fusco, J. et al. Land use changes threaten bird taxonomic and functional diversity across the mediterranean basin: a spatial analysis to prioritize monitoring for conservation. Front. Ecol. Evol.9, https://www.frontiersin.org/articles/10.3389/fevo.2021.612356 (2021).
- 50.Almeida J, Godinho C, Leitão D, Lopes RJ. Lista Vermelha das Aves de Portugal Continental. SPEA, ICNF, LabOR/UÉ, CIBIO/BIOPOLIS, Portugal (2022).
- 51.Council Directive 92/43/EEC of 21 May 1992 on the conservation of natural habitats and of wild fauna and flora, CONSIL, 206 OJ L. http://data.europa.eu/eli/dir/1992/43/oj/eng (1992).
- 52.Antunes, C. et al. Understanding plant drought resistance in a Mediterranean coastal sand dune ecosystem: Differences between native and exotic invasive species. J. Plant Ecol.11, 26–38 (2018). [Google Scholar]
- 53.Cabezudo, B. et al. Lista Roja de la flora vascular de Andalucía. Consejería de Medio Ambiente, Junta de Andalucía, Sevilla (2005).
- 54.de Oliveira, P. B. & Dale, A. Corema album (L.) D. Don, the white crowberry—a new crop. J. Berry Res.2, 123–133 (2012). [Google Scholar]
- 55.Jacinto, J. et al. Quality attributes of cultivated white crowberries (Corema album (L.) D. Don) from a multi-origin clonal field. Euphytica217, 40 (2021). [Google Scholar]
- 56.Zunzunegui, M. et al. Ecophysiology, growth timing and reproductive effort of three sexual forms of Corema album (Empetraceae). Plant Ecol.183, 35–46 (2006). [Google Scholar]
- 57.Machado, A. Two new cavernicolous species of the genus Trechus Clairv. From the Azores (Coleoptera, Carabidae). Bocagiana119, 1–8 (1988). [Google Scholar]
- 58.Borges, P.A.V. & Amorim, I.R. Trechus terceiranus. The IUCN Red List of Threatened Species. 10.2305/IUCN.UK.2018-1.RLTS.T97125072A99166594.en (2018).
- 59.The Galaxy Community. The Galaxy platform for accessible, reproducible and collaborative biomedical analyses: 2022 update. Nucleic Acids Res.50, W345–W351 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]