Abstract
Microglia are macrophage cells residing in the brain, where they exert a key role in neuronal protection. Through the gut–brain axis, metabolites produced by gut commensal microbes can influence brain functions, including microglial activity. The nuclear factor erythroid 2-related factor 2 (NRF2) is a key regulator of the oxidative stress response in microglia, controlling the expression of cytoprotective genes. Lactobacilli-derived cell-free supernatants (CFSs) are postbiotics that have shown antioxidant and immunomodulatory effects in several in vitro and in vivo studies. This study aimed to explore the effects of lactobacilli CFSs on modulating microglial responses against oxidative stress and inflammation. HMC3 microglia were exposed to lipopolysaccaride (LPS), as an inflammatory trigger, before and after administration of CFSs from three human gut probiotic species. The NRF2 nuclear protein activation and the expression of NRF2-controlled antioxidant genes were investigated by immunoassay and quantitative RT-PCR, respectively. Furthermore, the level of pro- and anti-inflammatory cytokines was evaluated by immunoassay. All CFSs induced a significant increase of NRF2 nuclear activity in basal conditions and upon inflammation. The transcription of antioxidant genes, namely heme oxygenase 1, superoxide dismutase (SOD), glutathione-S transferase, glutathione peroxidase, and catalase also increased, especially after inflammatory stimulus. Besides, higher SOD1 activity was detected relative to inflamed microglia. In addition, CFSs pre-treatment of microglia attenuated pro-inflammatory TNF-α levels while increasing anti-inflammatory IL-10 levels. These findings confirmed that gut microorganisms’ metabolites can play a relevant role in adjuvating the microglia cellular response against neuroinflammation and oxidative stress, which are known to cause neurodegenerative diseases.
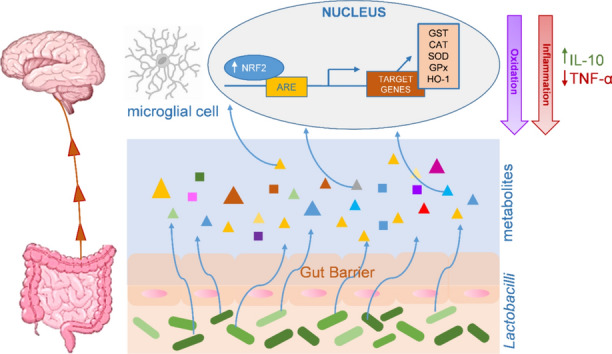
Graphical Abstract
Gut-brain crosstalk: molecular point of view. Metabolites contained in the supernatant derived from Lactobacilli can cross the gut barrier and reach the central nervous system, where they are taken up by microglial cells. They induce the activation of the NRF2 pathway and the production of inflammatory mediators. This interaction attenuates two important events: oxidation (with high levels of NRF2) and inflammation (with high levels of IL-10 and low levels of TNF-α).
Supplementary Information
The online version contains supplementary material available at 10.1007/s10571-024-01494-1.
Keywords: Lactobacilli CFS, LPS, Gut–brain axis, NRF2, Postbiotics, Cytokines
Introduction
Microglia are macrophages residing in the central nervous system (CNS), where they perform immune surveillance and control synaptic remodeling and neurogenesis (Ransohoff and El Khoury 2015; Abdel-Haq et al. 2019). As immunocompetent cells, microglia continuously survey the surrounding parenchyma and monitor signals arising from brain injuries or potential pathogens, hence being highly sensitive to both local and exogenous stimuli, including those coming from the gut (Abdel-Haq et al. 2019).
The gut microbiota comprises a vast and diverse microbial community that has a profound impact on human health. This complex ecosystem is considered a virtual organ that improves digestion of nutrients, benefits host metabolism, strengthens gut mucosal barrier, and modulates innate and adaptive immune responses (Evans et al. 2013). The gut microbiota influences also the physiology of organs and systems outside the gastro-intestinal tract. Indeed, through a complex network of interactions referred to as the gut–brain axis, it is known to modulate several facets of the CNS, including maturation and activation of microglia (Abdel-Haq et al. 2019; Carabotti et al. 2015). Altered composition of the gut microbiota, i.e., dysbiosis, may be detrimental to host health, being often associated to chronic diseases. For instances, intestinal dysbiosis have been observed in patients suffering from multiple sclerosis and other inflammatory neurological disorders (Abdel-Haq et al. 2019; Fettig and Osborne 2021; Dinan and Dinan 2022), whose pathogenesis is known to be associated to microglia dysfunctions. In fact, it is known that both in physiological and pathological conditions, microglial cells can be regulated by compounds, such as short-chain fatty acids (SCFAs) or lipopolysaccarides (LPS) originating from commensal intestinal bacteria (Erny et al. 2015). In response to these signals, microglia are able to activate their specific functions, bringing improvements or causing decompensations in pathological cases. For instance, the sudden activation of microglia by LPS was observed in a condition linked to synaptic disorders and long-term cognitive deficits (Jung et al. 2023). Once activated, microglia undergo morphological and functional switches, i.e., from a resting state, they can polarize toward a pro-inflammatory or anti-inflammatory phenotype (Cherry et al. 2014; Hu et al. 2015; Blandini 2013). Indeed, upon stimulation, in order to perform scavenging as well as tissue repair activities, microglia may acquire phagocytic functions, produce reactive oxygen species, secrete neurotrophic factors and produce a variety of inflammatory mediators (Blandini 2013).
One of the major regulators of the response to oxidative damage in microglia is the nuclear factor erythroid 2-related factor 2 (NRF2). Under physiological conditions, NRF2 is localized in the cytosol, where it binds to Kelch-like ECH-associated protein (KEAP1), which regulates its proteasome-mediated degradation (Ngo and Duennwald 2022). Following oxidative stress, NRF2 dissociates from KEAP1, escapes proteasomal degradation, and translocates into the nucleus, thus activating the transcription of a pool of genes encoding enzymes, such as heme oxygenase 1 (HO-1), superoxide dismutase (SOD), glutathione-S transferase (GST), glutathione peroxidase (GPx), and catalase (CAT) (Zhang et al. 2004; Yamamoto et al. 2008; Zhang and Hannink 2003), which are responsible for various detoxification and antioxidant defense processes. Besides, NRF2 plays an important role in regulating the anti-inflammatory response in microglia (Huang et al. 2015; Li et al. 2015).
Psychobiotics are probiotics that confer health benefits on the activities of the CNS, including cognitive functions (Bermúdez-Humarán et al. 2019). The communication axis between the gut and the brain involves the enteric nervous system (ENS), which, through the production of various neurotransmitters, such as acetylcholine and serotonin, mediates possible environmental changes, contributing to immune defenses (Sarkar et al. 2016; O’malley et al. 2010). Among the most studied psychobiotics, it has been observed that lactobacilli and bifidobacteria can reduce the inflammatory state of some neurological disorders through the secretion of metabolites, such as SCFAs (Tankou et al. 2018; Kouchaki et al. 2017). Indeed, postbiotics, defined as non-viable probiotic cells, including their components and metabolites, have been shown to possess similar health-promoting characteristics to the probiotics from which they originate (Salminen et al. 2021; Aguilar-Toalá et al. 2021).
Although emerging studies have revealed the potential anti-inflammatory role of probiotic-derived cell-free culture supernatants (CFSs) (De Marco et al. 2018; Rocchetti et al. 2023; Frick et al. 2007; Ren et al. 2020; Bermudez-Brito et al. 2013; 2015), data on their anti-inflammatory and antioxidant properties on human brain and on microglial cells are missing. In the present study, we evaluated whether probiotic-derived metabolites modulate the responses of microglia. To this aim, HMC3 immortalized human microglial cells were treated with CFSs from three probiotic species which are known to colonize the human intestine. Then, levels of activated NRF2 antioxidant system, its target genes, and inflammatory markers were evaluated under conditions simulating a pro-inflammatory environment.
Materials and Methods
Reagents
Minimum essential medium (MEM), fetal bovine serum (FBS), and Dulbecco’s phosphate-buffered saline (DPBS) were from Corning (Manassas, VA, USA); trypsin–EDTA, penicillin, streptomycin, amphotericin B, and L-glutamine were from Euroclone (Carlsbad, CA, USA); 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazoliumbromide (MTT), dimethyl sulfoxide (DMSO), and lipopolysaccharides (LPS) from Escherichia coli O127:B8 were purchased from Sigma-Aldrich (St Louis, MO,USA); de Man-Rogosa-Sharpe (MRS) broth was from Oxoid (Basingstoke, UK).
Bacterial Cultivation and Preparation of Cell-Free Supernatants
The following bacterial strains were from the Spanish Culture Collection (Colección Espaňola de Cultivos Tipo, CECT, Paterna, Spain): Limosilactobacillus reuteri NCFB 2589 (CECT 925) (Lr 13), Lacticaseibacillus rhamnosus NCIMB 8010 (CECT 278) (Lrh 19), Lactiplantibacillus plantarum (CECT 8328) (Lp 10). Bacteria were cultivated in MRS medium at 37 °C. Their cell-free supernatants (CFSs) were obtained by centrifugation (5,000 × rpm, 10 min) and filtration (0.45 µm) of stationary phase cultures (with an estimated concentration of 2–5 × 108 colony-forming units [CFU] per mL).
Cell Culture
The Human Microglia Clone 3 (HMC3), a line derived from human embryonic microglial cells (Janabi et al. 1995), were cultured in MEM supplemented with 10% FBS, 100-U/mL penicillin/streptomycin, and 100-U/mL amphotericin B, at 37 °C in a humidified incubator, under 5% CO2.
MTT Assay
Based on our earlier work (Rocchetti et al. 2023), HMC3 cells were treated with 5% or 10% (v/v) CFSs from L. plantarum, L. reuteri, and L. rhamnosus to evaluate their cytotoxicity. Cells were seeded (2 × 104 / well) into a 96-well culture plate and cultured for 24 h (Rocchetti et al. 2023). After 24 h, the culture medium was removed and microglia cells were incubated with 0.5-mg/mL MTT for 4 h at 37 °C. Then, formazan crystals were dissolved with DMSO and the absorbance at 540 nm was immediately measured. CFS-untreated cells were used as positive control, defining 100% viability. Based on the toxicity revealed by the MTT test (table S1), a concentration of 5% (v/v) CFS, i.e., allowing a viability above or equal to 80%, was regarded as safe, and thus selected to treat the HMC3 cells (see below).
Treatment of Microglia Cells with Bacterial CFSs
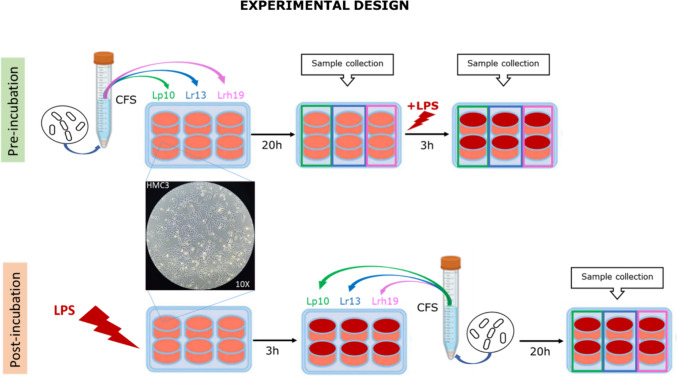
HMC3 cells (3 × 105 cells/mL) were seeded into a 6-well tissue culture plate and cultured at 37 °C until confluence was reached. Two conditions were set-up in order to evaluate the capacity of CFSs to modulate inflammation and redox status (Scheme 1). In the pre-incubation condition, HMC3 cells were pre-treated with CFSs 5% (v/v) for 20 h. Then, the medium was removed and cells were stimulated for additional 3 h with 1 µg/mL LPS. In the post-incubation condition, HMC3 were stimulated first with LPS (1 µg/mL) for 3 h. Then, the culture medium was removed and cells were incubated with CFSs for additional 20 h. The negative and positive controls were represented by CFS-untreated HMC3 not incubated and incubated with LPS, respectively. After each treatment, cells and /or their conditioned medium were processed for subsequent analysis.
Scheme 1.
Experimental design of the treatment of human microglia cells (HMC3) with bacterial CFSs. Samples from different treatments were collected and processed for ELISA, transcriptional analyses, and enzymatic activity
Transcriptional Analysis of Genes Involved in Oxidative Stress
Total RNA was isolated from treated and control HMC3 cells using the RNeasy Kit (Zymo Research, Orange, CA, USA). Purity and concentration of the RNA samples were determined by NanoDrop™ instrument (Thermo Fisher Scientific, Waltham, MA, USA). cDNA was synthesized from 500 ng of total RNA, using the iScript cDNA Synthesis Kit (Applied Biosystems, Waltham, MA, USA). cDNAs were stored at − 80 °C until analysis. To analyze the transcriptional level of genes encoding superoxide dismutase (SOD), catalase (CAT), glutathione-S transferase (GST), glutathione peroxidase (GPx), heme oxygenase 1 (HO-1), and interleukin-1β (IL-1β), a quantitative RT-PCR was performed in a real-time instrument (Applied Biosystems, USA), using SsoAdvanced™ Universal SYBR Green Supermix (Bio-Rad Laboratories, Hercules, CA, USA). The oligonucleotide primers (Sigma-Aldrich, USA) derived from Origene™ Technologies (table S2) were used at a concentration of 0.25 µM. PCR were performed using the following temperature profile: initial denaturation 95 °C for 30 s and 45 cycles of 95 °C for 10 s, 60 °C for 60 s, and 72 °C for 30 s. The PCR specificity was determined through melting curve analysis. The housekeeping genes encoding β-actin and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were used to normalize the expression level of target genes using the ΔΔCt method.
Quantification of HMC3-Secreted Cytokines and Nuclear Level of NRF2
To analyze the level of secreted IL-10, TNF-α and IL-8, supernatants from HMC3 cultures were collected and stored at -80 °C until further analysis. The concentration of secreted cytokines was determined by ELISA assay (codes A103966, A78896 and A1476, Antibodies, Cambridge, UK,) according to the manufacturer’s guidelines.
In order to measure NRF2 nuclear activity levels, pellets from microglia cell cultures were obtained by centrifugation (1,100 × rpm, 10 min), and total nuclear proteins were isolated using a nuclear extraction kit (ab113474-Abcam, Cambridge, UK), according to manufacturer’s instructions. Total protein concentration was measured using Pierce™BCA Protein Assay kit (Thermo Fisher Scientific), and the nuclear activation of NRF2 was determined by a colorimetric assay (ab207223-Abcam) according to the manufacturer’s guidelines. The NRF2 levels were normalized to those of the control HMC3 cells (O.D. ratio).
SOD Activity
Cytoplasmic SOD1 and mitochondrial SOD2 activities were assessed using the SOD colorimetric Activity Kit (Arbor Assays, Ann Arbor, MI, USA) according to manufacturer’s guidelines.
Statistical Analysis
Statistical analysis of experimental data was performed using multiple unpaired, non-parametric Mann–Whitney t-tests and ANOVA followed by Tukey’s post hoc test, to compare the averages of two or more groups, respectively. Variables means and standard deviations (SD) were calculated, for each experimental condition, from at least three independent biologic replicates. All calculations were performed using StatView software package SAS (v. 5.0). p < 0.05 was considered statistically significant.
Results
CFSs Increase NRF2 Nuclear Activity and Transcription of Antioxidant Genes
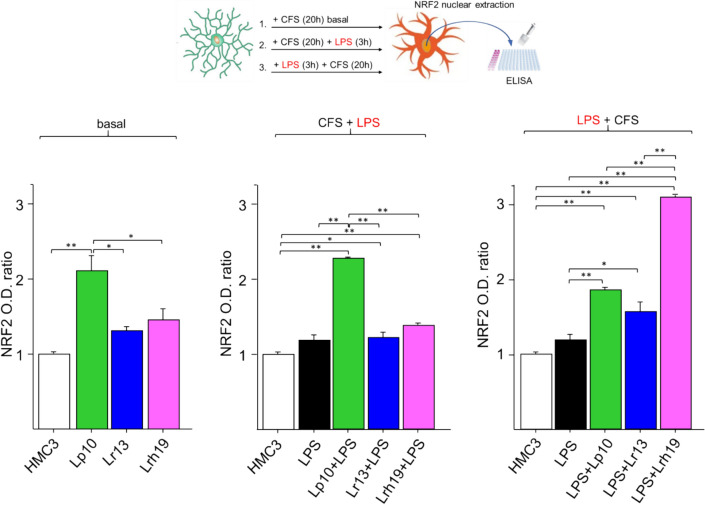
In order to evaluate the antioxidant properties of lactobacilli-derived metabolites, we monitored NRF2 nuclear activation in microglia incubated for 20 h with the CFSs from each of the investigated species (Fig. 1). CFSs from all tested lactobacilli tended to increase NRF2 level under basal conditions (i.e., in the absence of LPS stimulation) relative to untreated microglia, with Lp10 CFS inducing a statistically significant increase compared to untreated control cells (p < 0.01). Significantly increased NRF2 nuclear activity (p < 0.01) was also observed in microglia pre-treated with Lp10 CFS and then subjected to LPS treatment (CFS + LPS). When microglia were first treated with LPS and then with CFSs (LPS + CFS), the highest NRF2 level resulted from Lrh19 CFS treatment, although all tested CFSs significantly augmented NFR2 nuclear localization (p < 0.01) relative to untreated and inflamed HMC3 cells.
Fig. 1.
Nuclear activity of NRF2. NRF2 activity was assessed by an ELISA-based colorimetric assay on nuclear extracts from untreated microglia (open bar), LPS-treated cells (solid bars), and cells treated for 20 h with CFS from each bacterial strain (Lp10, Lr13, Lhr19) without (basal), before (CFS + LPS), or following (LPS + CFS) 3-h LPS stimulation. NRF2 activation was normalized to that detected in untreated control cells and expressed as optical density (O.D. 450 nm) ratio. The results represent the mean ± SD from three independent experiments. Statistically significant differences were determined by one-way ANOVA and Tukey’s post hoc test, *p < 0.05, **p < 0.01. CFSs were used at 5% (v/v) concentration; LPS was used at the concentration of 1 µg/mL
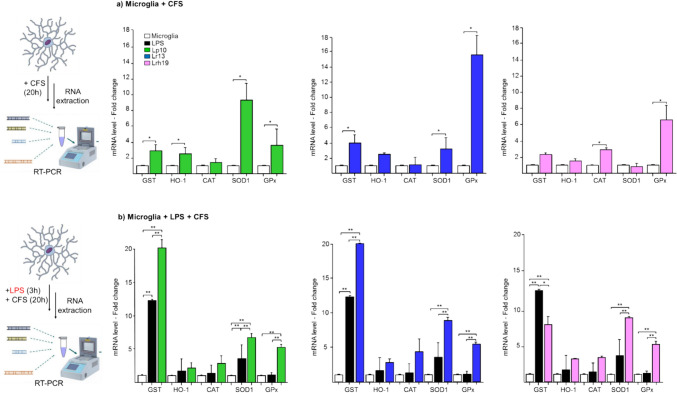
In order to better ascertain the potential involvement of NRF2 as part of the effects of lactobacilli CFSs, the transcriptional level of genes directly controlled by NRF2 was analyzed (Fig. 2). In the absence of LPS treatment, CFSs from all three probiotic species significantly increased the expression of glutathione peroxidase (GPx) gene, while glutathione-S transferase (GST) transcription was significantly increased only by Lp10 and Lr13 (Fig. 2a). In particular, Lr13 CFS induced the highest expression of both genes. The superoxide dismutase (SOD1) gene mRNA level was significantly increased upon treatment with Lp10 and Lr13 CFSs, with Lp10 inducing its highest expression. Conversely, only Lrh19 induced a significant up-regulation of the catalase (CAT) gene (Fig. 2a). Incubation of LPS-inflamed microglia with CFS from all three probiotic species resulted in a significant beneficial modulation of most of the antioxidant genes (Fig. 2b). All CFSs induced a statistically significant increase of GPx and SOD1 expression (p < 0.01), both relative to untreated control and to LPS-stimulated cells (Fig. 2b). The expression of CAT and HO tended to be increased by all CFSs, albeit without statistical significance. GST gene was greatly induced by CFS from Lp10 and Lr13, with statistically relevant increase compared to both untreated and LPS-treated cells (p < 0.01). Compared to untreated control, LPS stimulation alone significantly upregulated (p < 0.01) only GST gene. The transcriptional levels of these genes were also analyzed in microglia pre-treated with CFS and then inflamed with LPS; however, they were found to be not significantly different from the control (data not shown).
Fig. 2.
Transcriptional level of NRF2-controlled genes. a Relative transcriptional level in untreated microglia cells (white bars) and upon incubation for 20 h with CFS from Lp10 (green bars), Lr13 (blue bars), and Lrh19 (pink bars). b Relative transcriptional level in untreated microglia (white bars), in LPS-treated microglia (solid bars), and in LPS-treated microglia following 20-h incubation with CFS from each of the indicated Lactobacilli strains (same color code as in A). mRNA levels were determined by qRT-PCR and by normalizing to that of untreated control cells. Mean and SD from three independent experiments. Statistically significant differences were determined by Mann–Whitney t tests or by ANOVA followed by Tukey’s post hoc test, as appropriate. *p < 0.05, **p < 0.01. GST (glutathione-S transferase), HO-1 (heme oxygenase 1), CAT (catalase), SOD (superoxide dismutase), GPx (glutathione peroxidase). CFSs were used at 5% (v/v) concentration; LPS was used at the concentration of 1 µg/mL
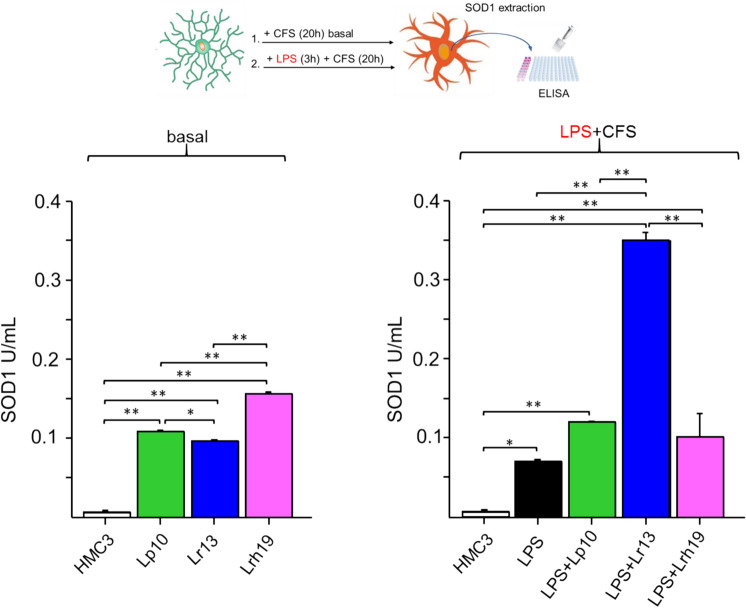
CFSs Enhance SOD Activity
In an attempt to verify CFSs-dependent anti-oxidative effect on microglial cells, we measured the enzymatic activity of SOD1, whose gene is controlled by NRF2 and whose transcription was found to be significantly modulated by all CFSs in the post-incubation condition. SOD1 activity was determined in microglia cells treated for 20 h with CFSs (Fig. 3). In agreement with gene expression data, all CFSs significantly augmented SOD1 activity in the absence of LPS stimulation compared to untreated microglia. In particular, Lrh19 highly induced the activity of SOD1 in basal conditions, although it seemed not to modulate its gene expression (Fig. 2a). In line with gene expression analysis (Fig. 2b), following stimulation with LPS, incubation with CFSs, and particularly Lr13 CFS significantly raised SOD1 activity. Since ROS-scavenging activity is carried out also by SOD2, and such enzyme is known to be induced in activated microglia (Ishihara et al. 2015), we quantified its activity, finding it enhanced upon treatment with all CFSs, both in basal- and in LPS-treated cells, especially for Lr13 (Supplementary Fig. S1).
Fig. 3.
Activity of SOD1. SOD1 activity was assessed in untreated microglia (open bar), in LPS-treated cells (solid bar), and in cells treated for 20 h with 5% (v/v) CFS from each bacterial strain (Lp10, green bars; Lr13, blue bars; Lhr19, pink bars) in the presence of absence of 1 µg/mL LPS (LPS + CFS). Results expressed in U/mL represent the mean ± SD from three independent experiments. Statistically significant differences were assessed by one-way ANOVA followed by Tukey’s post hoc test. *p < 0.05; **p < 0.01
CFSs Modulate the Level of Secreted Inflammatory Mediators
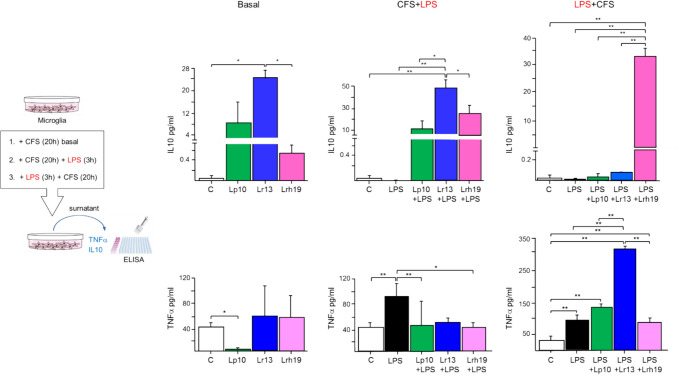
In order to assess the inflammatory properties of CFSs, we monitored the secretion of TNF-α and IL-10 from microglia incubated with CFSs alone, incubated with CFSs and subsequently stimulated with LPS or treated with LPS and subsequently incubated with CFSs (Fig. 4).
Fig. 4.
Level of secreted cytokines. The concentrations (pg/mL) of IL-10 (upper panel) and TNF-α (lower panel) were determined by ELISA in untreated microglia cells (control, white bars) and in microglia treated for 20 h with CFS from the different bacterial species (basal); in 3-h LPS-treated microglia (solid bars) and in 3-h LPS-treated microglia pre-incubated with CFS for 20 h (CFS + LPS); and in 3-h LPS-treated microglia (solid bars) and in 20-h CFS-incubated microglia following 3-h LPS stimulation (LPS + CFS). The results represent mean ± SD from three independent experiments; statistically significant differences were determined by one-way ANOVA followed by Tukey’s post hoc test. *p < 0.05; **p < 0.01. CFSs were used at 5% (v/v) concentration; LPS was used at the concentration of 1 µg/mL
In the absence of LPS, i.e., under basal condition, treatment with CFSs tended to increase the level of the anti-inflammatory cytokine IL-10 (Fig. 4). Such increase was statistically significant for Lr13 CFS, thus showing the best anti-inflammatory effect on basal conditions. The addition of LPS to CFSs-treated microglia (CFS + LPS) increased even more the level of secreted IL-10, compared to unstimulated and LPS-treated microglia, suggesting anti-inflammatory properties of all CFSs, more evident for Lr13. When microglia were pre-stimulated with LPS and then incubated with CFS (LPS + CFS), only Lrh 19 CFS determined significantly higher levels of secreted IL-10 (Fig. 4).
When evaluating the basal level of the pro-inflammatory molecule TNF-α, incubation with Lp10 CFS led to its significant reduction (p < 0.05), compared to control (Fig. 4). When a pro-inflammatory stimulus was added to cells pre-incubated with CFSs, all CFSs attenuated TNF-α production compared to LPS-activated microglia, although this effect was statistically significant only for CFS from two strains. On the contrary, the subsequent addition of CFSs to LPS-activated microglia exacerbated the pro-inflammatory response, increasing TNF-α secretion for Lp10 and significantly for Lr13 (Fig. 4).
When considering the expression of other pro-inflammatory cytokines, namely IL-1β mRNA level and IL-8 protein level (Supplementary Fig. S2), the capacity to down-regulate these signals under basal conditions was significant only for strain Lr13, while the preventive and post-incubation anti-inflammatory effect was confirmed for CFSs from all strains, although only in relation to secreted IL-8.
Discussion
Microglia play homeostatic and reparative functions within the CNS; however, their defense reaction can be inadequate, putting the brain microenvironment at risk of neuroinflammation (Koutsilieri et al. 2002), which can lead to neurodegeneration (Zhang et al. 2023). The immune activity of microglia can be stimulated by a plethora of physiological and stress stimuli. Thanks to gut–brain interaction pathways, brain cells are exposed to microbial metabolites originating in the intestine. Several compounds deriving from the human gut microbiota are recognized as neuroprotective, because they exhibit anti-inflammatory and anti-oxidative effects in the context of different neurodegenerative diseases (Wang et al. 2022). CFSs contain a wide range of compounds secreted during bacterial growth: organic acids, fatty acids, esters, alcohols, phenolics, peptides, and specific secondary metabolites (Ramos et al. 2015; Mani López et al. 2022). As CFS are mixtures of diverse bioactive compounds, they exhibit various biologic activities. Lactobacilli CFSs were previously shown to modulate inflammatory and oxidative responses both in vitro (De Marco et al. 2018; Kwun et al. 2024; Dubey et al. 2021; Hao et al. 2023; Qadi et al. 2023; Chakamian et al. 2023) and in vivo (Dubey et al. 2021; Xu et al. 2022). The antioxidant effect of such type of postbiotics, specifically impacting NRF2 signaling and related downstream genes, has been reported in some recent studies (Karaca et al. 2022; Şirin 2023; Gholami et al. 2023; Zhang et al. 2022); however, data on human microglia are lacking.
To examine the anti-inflammatory and antioxidant properties of CFSs on human microglial cells, we adopted in vitro experimental conditions that could simulate neuroinflammation, i.e., we activated HMC3 cells with LPS, which are known to induce inflammation and oxidative stress in microglia (Hanisch 2002; Block and Hong 2005). Indeed, LPS act as immunogens inducing a microglial pro-inflammatory phenotype (Cherry et al. 2014; Hu et al. 2015; Block and Hong 2005). We focused on three species of lactobacilli, i.e., L. plantarum, L. reuteri and L. rhamnosus, since such probiotics can colonize the human gut and were previously shown to exert beneficial effects in animal models of neuroinflammation (Zolfaghari et al. 2021), cognitive (Xu et al. 2022), and neurological (Wu et al. 2022) dysfunctions. Moreover, these bacterial species possess the qualified presumption of safety (QPS) (EFSA BIOHAZ Panel 2024) and their therapeutic properties have been documented by several studies (Yadav et al. 2020), including clinical trials (https://clinicaltrials.gov/; https://www.who.int/ictrp/en/), also related to neurological disorders (Wiegers et al. 2022). We tested bacterial metabolites rather than live bacterial cells, indeed, compared to probiotics, postbiotics are safer, allow more stable formulations, and are tailorable for specific needs (Nataraj et al. 2020). Moreover, some tissues and organs, such as brain, are more likely to be influenced by microbial molecules rather than viable probiotic cells (Erny et al. 2015; Cosola et al. 2018).
In the present study, we analyzed the effects of CFSs on both naive and LPS-activated HMC3 cells. To quantify NRF2 activity, we employed a kit that assays its DNA-binding capacity: such method has been adopted in some recent papers studying NRF2 signaling and oxidative stress (Clementi et al. 2020; Brasil et al. 2021). Our results indicate that all tested CFSs exert a positive modulation on NRF2 antioxidant pathway by increasing NRF2 nuclear activation, both under basal condition and in LPS-treated cells, with particularly relevant effects by specific probiotics, i.e., L. plantarum Lp10 and L. rhamnosus Lrh19. These findings are in line with recently published data, demonstrating that L. plantarum CFS improved the antioxidant capacity of the hippocampus by increasing NRF2 and SOD2 levels in an animal model of cognitive dysfunction (Xu et al. 2022).
NRF2 is a key regulator of the cellular response to inflammatory and oxidative stress (Huang et al. 2015; Tonelli et al. 2018). The protective role of NRF2 pathway seems particularly relevant in the context of neuronal damage, and has potential for the clinical management of neurodegenerative and neuroinflammation-associated diseases (Nakano-Kobayashi et al. 2020; Qu et al. 2020). Studies revealed that NRF2 knockout mice had neurological and cognitive deficiencies (Branca et al. 2017; Sigfridsson et al. 2020). In accordance with the observed increase in NRF2 activity in the nuclear fractions, gene expression analyses, in our study, revealed an up-regulation of several antioxidant enzymes controlled by NRF2, in CFSs-treated microglia, with or without LPS. Interestingly, LPS alone induces a slight nuclear increase of NRF2 activity which, in turn, should trigger the transcriptional levels of some antioxidant enzymes in a sort of cytoprotective mechanism (Chang et al. 2001; Qin et al. 2004; Pawate et al. 2004). Notably, it was observed that LPS treatment of murine BV2 microglia cells also activates NRF2 pathway and antioxidant enzymes (Li et al. 2015; Barber et al. 2023). Here, we found that treatments of LPS-activated microglia with CFSs leads to a further increase of nuclear NRF2 protein level and up-regulation of antioxidant enzymes mRNAs compared to LPS alone: this could enhance the overall ROS-scavenging capacity of the cell, thus entailing protective properties of the tested CFSs.
We found that CFSs increased SOD1 activity in microglia, thus further supporting their antioxidant and protective effects. SOD1 plays a critical role in neuroprotection (Polazzi et al. 2013). This enzyme is particularly studied in relation to brain pathophysiology. In fact, its genetic mutations are known to cause many familial forms of neurodegenerative diseases (Valentine et al. 2005; Moezzi et al. 2022). Both SOD1 and SOD2 are induced in the CNS, under inflammatory conditions (Ishihara et al. 2015; Polazzi et al. 2013; Barber et al. 2023). Previously, it was reported that activated microglia strongly express SOD2 (Ishihara et al. 2015). Intriguingly, we found that even SOD2 activity increased upon CFSs treatment (Supplementary Fig. 1), hence further corroborating the protective effects of the tested postbiotics and Lr13 particularly.
Besides activating antioxidant response, NRF2 also promotes anti-inflammatory pathways (Nakano-Kobayashi et al. 2020; Chen et al. 2006; Xu et al. 2015; Li et al. 2015). In fact, NRF2 nuclear translocation inhibits the redox-sensitive NF-kB pathway, preventing TNF-α synthesis (Huang et al. 2015; Cuadrado et al. 2014; Jin et al. 2008). TNF-α is a pleiotropic cytokine with a key role in the pathogenesis of neurodegenerative diseases (Sriram et al. 2006; Amor et al. 2010; Vincenzi et al. 2021); hence, its modulation could be critical for therapeutic purposes (Frankola et al. 2011; Amin et al. 2022). Moreover, this mediator is associated to NRF2 signaling by an autocrine loop (Rushworth et al. 2011). Earlier studies demonstrated that CFSs from lactobacilli down-regulate pro-inflammatory cytokines such as TNF-α in vivo and in vitro (De Marco et al. 2018; Cristofori et al. 2021; Peña and Versalovic 2003). In the present study, we found that pre-treatment of microglia with CFSs reversed the production of pro-inflammatory TNF-α (significantly for CFSs from Lp10 and Lrh19), while increasing the secretion of anti-inflammatory IL-10, especially when the inflammatory stimulus was added. Interestingly, it was found that the release of IL-10 from microglia induced the synaptic formation in early brain development (Lim et al. 2013); thus, probiotic CFSs could be applied in this direction by future in vitro study on neuronal cells. The pattern of expression we observed for other pro-inflammatory cytokines (i.e., IL-1β and IL-8) suggests that one of the investigated strain (Lr13) can have an overall anti-inflammatory action. These findings point to the possible use of a mixture of CFSs from the different strains to contrast microglia inflammation by their synergistic effect on cytokines modulation.
To our knowledge, this is the first attempt to investigate the anti-inflammatory and antioxidant effect of lactobacilli CFSs on human microglia cells. Just recently, bacterial conditioned media (BCM, i.e., equivalent to CFSs) from lactobacilli were tested on murine microglia cells and found to down-regulate oxidative stress and inflammation in vitro (Bulacios et al. 2023).
In conclusion, our data suggest that lactobacilli CFSs could act at the brain level to prevent and protect microglial cells from inflammation and oxidative stress, through a positive modulation of the NRF2 pathway. These results corroborate the interplay between gut and brain and the importance of this crosstalk in neurodegenerative disease. These results may represent a basis for translational studies on human brain, using the CSFs directly in ex vivo biopsies from human brain surgeries (Milior et al. 2020) and evaluating their effect on microglia–neuron interactions. Moreover, analyzing human brain and feces samples, it could be possible to link the differences in the composition and metabolites of the intestinal microbiota in patients suffering from neurodegenerative diseases, often associated with neuroinflammation. This will confirm the possibility that microbiota mediators can influence the physio-pathology of the human brain, opening to new therapeutic strategies based on postbiotics administrations.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We would like to remember prof. Sergio Minucci that in the meanwhile unfortunately passed away and that continuously supported our research. We would like to thank prof. Massimo Venditti for his little financial support.
Author Contributions
Study conception and design: MDC, MTR, DF, AG. Material preparation, data collection and analysis: MDC, MTR, DF. Sample collection: PR, CA. The first draft of the manuscript was written by MDC, MTR, DF. Contributions to the manuscript writing and revision: AG, GS, GM, RMG, FS, PP, LG, CA. All authors read and approved the final manuscript.
Funding
Open access funding provided by Università di Foggia within the CRUI-CARE Agreement. This work was supported by funding received from the authors: RMG is funded by PON “RICERCA E INNOVAZIONE” 2014–2020–Innovazione (D.M. 10 AGOSTO 2021, N. 1062); FS, PP, and LG are funded by “Complementary National Plan PNC-I.1 “Research initiatives for innovative technologies and pathways in the health and welfare sector” D.D. 931 of 06/06/2022, DARE – DigitAl lifelong pRevEntion initiative, code PNC0000002, CUP: B53C22006420001”; DF and GS were partially funded by PON project “Conservabilità, qualità e sicurezza dei prodotti ortofrutticoli ad alto contenuto di servizio” POFACS – CUP B74I20000120005; DF received fundings from University of Foggia, bando PRA-HE 2021; and LG and PP received funds from the Italian Ministry of University and Research for the project “ONFOOD-Research and innovation network on food and nutrition Sustainability, Safety and Security – Working ON Foods” DD 1550, 11/10/2022. The funders had no role in paper design, data collection, data analysis, interpretation, and writing of the paper.
Data Availability
No datasets were generated or analysed during the current study.
Declarations
Conflict of interest
The authors have no conflicts of interest to declare.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Mariagiovanna Di Chiano and Maria Teresa Rocchetti have contributed equally to this work.
References
- Abdel-Haq R, Schlachetzki JCM, Glass CK et al (2019) Microbiome-microglia connections via the gut-brain axis. J Exp Med 216(1):41–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguilar-Toalá JE, Arioli S, Behare P et al (2021) Postbiotics - when simplification fails to clarify. Nat Rev Gastroenterol Hepatol 18(11):825–826 [DOI] [PubMed] [Google Scholar]
- Amin R, Quispe C, Docea AO et al (2022) The role of Tumour Necrosis Factor in neuroinflammation associated with Parkinson’s disease and targeted therapies. Neurochem Int 158:105376 [DOI] [PubMed] [Google Scholar]
- Amor S, Puentes F, Baker D et al (2010) Inflammation in neurodegenerative diseases. Immunology 129(2):154–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber K, Mendonca P, Evans JA et al (2023) Antioxidant and anti-inflammatory mechanisms of cardamonin through Nrf2 activation and NF-kB suppression in LPS-activated BV-2 microglial cells. Int J Mol Sci 24(13):10872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bermudez-Brito M, Muñoz-Quezada S, Gomez-Llorente C et al (2013) Cell-free culture supernatant of Bifidobacterium breve CNCM I-4035 decreases pro-inflammatory cytokines in human dendritic cells challenged with Salmonella typhi through TLR activation. PLoS ONE 8(3):e59370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bermudez-Brito M, Muñoz-Quezada S, Gómez-Llorente C et al (2015) Lactobacillus paracasei CNCM I-4034 and Its culture supernatant modulate salmonella-induced inflammation in a novel transwell co-culture of human intestinal-like dendritic and Caco-2 cells. BMC Microbiol 15(1):79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bermúdez-Humarán LG, Salinas E, Ortiz GG et al (2019) From probiotics to psychobiotics: live beneficial bacteria which act on the brain-gut axis. Nutrients 11(4):890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blandini F (2013) Neural and immune mechanisms in the pathogenesis of Parkinson’s disease. J Neuroimmune Pharmacol 8(1):189–201 [DOI] [PubMed] [Google Scholar]
- Block ML, Hong JS (2005) Microglia and inflammation-mediated neurodegeneration: multiple triggers with a common mechanism. Prog Neurobiol 76(2):77–98 [DOI] [PubMed] [Google Scholar]
- Branca C, Ferreira E, Nguyen TV et al (2017) Genetic reduction of Nrf2 exacerbates cognitive deficits in a mouse model of Alzheimer’s disease. Hum Mol Genet 26(24):4823–4835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brasil FB, Bertolini Gobbo RC, Souza de Almeida FJ et al (2021) The signaling pathway PI3K/Akt/Nrf2/HO-1 plays a role in the mitochondrial protection promoted by astaxanthin in the SH-SY5Y cells exposed to hydrogen peroxide. Neurochem Int 146:105024 [DOI] [PubMed] [Google Scholar]
- Bulacios GA, Cataldo PG, Naja JR et al (2023) Improvement of key molecular events linked to Alzheimer’s disease pathology using postbiotics. ACS Omega 8(50):48042–48049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carabotti M, Scirocco A, Maselli MA et al (2015) The gut-brain axis: interactions between enteric microbiota, central and enteric nervous systems. Ann Gastroenterol 28(2):203–209 [PMC free article] [PubMed] [Google Scholar]
- Chakamian K, Robat-Jazi B, Naser Moghadasi A et al (2023) Immunosuppressive effects of two probiotics, Lactobacillus paracasei DSM 13434 and Lactobacillus plantarum DSM 15312, on CD4+ T cells of multiple sclerosis patients. Iran J Allergy Asthma Immunol 22(1):34–45 [DOI] [PubMed] [Google Scholar]
- Chang SC, Kao MC, Fu MT et al (2001) Modulation of NO and cytokines in microglial cells by Cu/Zn-superoxide dismutase. Free Radical Biol Med 31(9):1084–1089 [DOI] [PubMed] [Google Scholar]
- Chen XL, Dodd G, Thomas S et al (2006) Activation of Nrf2/ARE pathway protects endothelial cells from oxidant injury and inhibits inflammatory gene expression. Am J Physiol 290(5):H1862-1870 [DOI] [PubMed] [Google Scholar]
- Cherry JD, Olschowka JA, O’Banion MK et al (2014) Neuroinflammation and M2 microglia: the good, the bad, and the inflamed. J Neuroinflammation 11:98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clementi ME, Sampaolese B, Sciandra F et al (2020) Punicalagin protects human retinal pigment epithelium cells from ultraviolet radiation-induced oxidative damage by activating Nrf2/HO-1 signaling pathway and reducing apoptosis. Antioxidants 9(6):473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosola C, Rocchetti MT, Cupisti A et al (2018) Microbiota metabolites: pivotal players of cardiovascular damage in chronic kidney disease. Pharmacol Res 130:132–142 [DOI] [PubMed] [Google Scholar]
- Cristofori F, Dargenio VN, Dargenio C et al (2021) Anti-inflammatory and immunomodulatory effects of probiotics in gut inflammation: a door to the body. Front Immunol 12:578386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuadrado A, Martín-Moldes Z, Ye J et al (2014) Transcription factors NRF2 and NF-κB are coordinated effectors of the rho family, GTP-binding protein RAC1 during Inflammation. J Biol Chem 289(22):15244–15258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Marco S, Sichetti M, Muradyan D et al (2018) Probiotic cell-free supernatants exhibited anti-inflammatory and antioxidant activity on human gut epithelial cells and macrophages stimulated with LPS. Evid Based Complement Altern Med 2018:1756308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinan K, Dinan TG (2022) Gut microbes and neuropathology: is there a causal nexus? Pathogens 11(7):796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubey AK, Podia M et al (2021) Insight into the beneficial role of Lactiplantibacillus plantarum supernatant against bacterial infections, oxidative stress, and wound healing in A549 cells and BALB/c mice. Front Pharmacol 12:728614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA Biohaz Panel, Koutsoumanis K, Allende A, Alvarez-Ordonez A et al (2024) Updated list of QPS-recommended microorganisms for safety risk assessments carried out by EFSA. Zenodo. 10.5281/zenodo.10534041
- Erny D, Hrabě de Angelis AL, Jaitin D et al (2015) Host microbiota constantly control maturation and function of microglia in the CNS. Nat Neurosci 18(7):965–977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans JM, Morris LS, Marchesi JR et al (2013) The gut microbiome: the role of a virtual organ in the endocrinology of the host. J Endocrinol 218(3):R37-47 [DOI] [PubMed] [Google Scholar]
- Fettig NM, Osborne LC (2021) Direct and indirect effects of microbiota-derived metabolites on neuroinflammation in multiple sclerosis. Microbes Infect 23(6–7):104814 [DOI] [PubMed] [Google Scholar]
- Frankola KA, Greig NH, Luo W et al (2011) Targeting TNF-α to elucidate and ameliorate neuroinflammation in neurodegenerative diseases. CNS Neurol Disord 10(3):391–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frick JS, Schenk K, Quitadamo M et al (2007) Lactobacillus fermentum attenuates the proinflammatory effect of Yersinia enterocolitica on human epithelial cells. Inflamm Bowel Dis 13(1):83–90 [DOI] [PubMed] [Google Scholar]
- Gholami A, Montazeri-Najafabady N, Ashoori Y et al (2023) The ameliorating effect of Limosilactobacillus fermentum and its supernatant postbiotic on cisplatin-induced chronic kidney disease in an animal model. BMC Complement Med Therapies 23(1):243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanisch UK (2002) Microglia as a source and target of cytokines. Glia 40(2):140–155 [DOI] [PubMed] [Google Scholar]
- Hao R, Liu Q, Wang L et al (2023) Anti-inflammatory effect of Lactiplantibacillus plantarum T1 cell-free supernatants through suppression of oxidative stress and NF-κB- and MAPK-signaling pathways. Appl Environ Microbiol 89(10):e0060823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X, Leak RK, Shi Y et al (2015) Microglial and macrophage polarization—new prospects for brain repair. Nat Rev Neurol 11(1):56–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang CS, Lin AH, Yang TC et al (2015) Shikonin inhibits oxidized LDL-induced monocyte adhesion by suppressing NFκB activation via up-regulation of PI3K/Akt/Nrf2-dependent antioxidation in EA.Hy926 endothelial cells. Biochem Pharmacol 93(3):352–361 [DOI] [PubMed] [Google Scholar]
- Ishihara Y, Takemoto T, Itoh K et al (2015) Dual role of superoxide dismutase 2 induced in activated microglia: oxidative stress tolerance and convergence of inflammatory responses. J Biol Chem 290(37):22805–22817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janabi N, Peudenier S, Héron B et al (1995) Establishment of human microglial cell lines after transfection of primary cultures of embryonic microglial cells with the SV40 large T antigen. Neurosci Lett 195(2):105–108 [DOI] [PubMed] [Google Scholar]
- Jin W, Wang H, Yan W (2008) Disruption of Nrf2 enhances upregulation of nuclear factor-kappaB activity, proinflammatory cytokines, and intercellular adhesion molecule-1 in the brain after traumatic brain Injury. Mediators Inflamm 2008:725174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung J, Lee D, You H et al (2023) LPS induces microglial activation and GABAergic Synaptic deficits in the hippocampus accompanied by prolonged cognitive impairment. Sci Rep 13(1):6547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karaca B, Yilmaz M, Gursoy UK et al (2022) Targeting Nrf2 with probiotics and postbiotics in the treatment of periodontitis. Biomolecules 12(5):729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouchaki E, Tamtaji OR, Salami M et al (2017) Clinical and metabolic response to probiotic supplementation in patients with multiple sclerosis: a randomized, double-Blind. Placebo-Control Trial Clin Nutr 36(5):1245–1249 [DOI] [PubMed] [Google Scholar]
- Koutsilieri E, Scheller C, Tribl F et al (2002) Degeneration of neuronal cells due to oxidative stress-microglial contribution. Parkinsonism Relat Disord 8(6):401–406 [DOI] [PubMed] [Google Scholar]
- Kwun SY, Yoon JA, Kim GY et al (2024) Isolation of a potential probiotic Levilactobacillus brevis and evaluation of its exopolysaccharide for antioxidant and α-glucosidase inhibitory activities. J Microbiol Biotechnol 34(1):167–175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Lv O, Zhou F et al (2015) Linalool inhibits LPS-induced inflammation in BV2 microglia cells by activating Nrf2. Neurochem Res 40(7):1520–1525 [DOI] [PubMed] [Google Scholar]
- Lim SH, Park E, You B et al (2013) Neuronal synapse formation induced by microglia and interleukin 10. PLoS ONE 8(11):e81218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mani-López E, Arrioja-Bretón D, López-Malo A (2022) The impacts of antimicrobial and antifungal activity of cell-free supernatants from lactic acid bacteria in vitro and foods. Compreh Rev Food Sci Food Saf 21(1):604–641 [DOI] [PubMed] [Google Scholar]
- Milior G, Morin-Brureau M, Chali F et al (2020) Distinct P2Y receptors mediate extension and retraction of microglial processes in epileptic and peritumoral human tissue. J Neurosci 40(7):1373–1388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moezzi D, Dong Y, Jain RW et al (2022) Expression of antioxidant enzymes in lesions of multiple sclerosis and its models. Sci Rep 12(1):12761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano-Kobayashi A, Fukumoto A, Morizane A et al (2020) Therapeutics potentiating microglial P21-Nrf2 axis can rescue neurodegeneration caused by neuroinflammation. Sci Adv 6(46):1428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nataraj BH, Ali SA, Behare PV et al (2020) Postbiotics-parabiotics: the new horizons in microbial biotherapy and functional foods. Microb Cell Fact 19(1):168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngo V, Duennwald ML (2022) Nrf2 and oxidative stress: a general overview of mechanisms and implications in human disease. Antioxidants 11(12):2345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’malley D, Julio-Pieper M, Gibney SM et al (2010) Differential stress-induced alterations of colonic corticotropin-releasing factor receptors in the Wistar Kyoto Rat. Neurogastroenterol Motil 22(3):301–311 [DOI] [PubMed] [Google Scholar]
- Pawate S, Shen Q, Fan F (2004) Redox regulation of glial inflammatory response to lipopolysaccharide and interferongamma. J Neurosci Res 77(4):540–551 [DOI] [PubMed] [Google Scholar]
- Peña JA, Versalovic J (2003) Lactobacillus rhamnosus GG decreases TNF-alpha production in lipopolysaccharide-activated murine macrophages by a contact-independent mechanism. Cell Microbiol 5(4):277–285 [DOI] [PubMed] [Google Scholar]
- Polazzi E, Mengoni I, Caprini M (2013) Copper-zinc Superoxide Dismutase (SOD1) is released by microglial cells and confers neuroprotection against 6-OHDA neurotoxicity. Neurosignals 21(1–2):112–128 [DOI] [PubMed] [Google Scholar]
- Qadi WSM, Mediani A, Kasim ZM et al (2023) Biological characterization and metabolic variations among cell-free supernatants produced by selected plant-based lactic acid bacteria. Metabolites 13(7):849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin L, Liu Y, Wang T et al (2004) NADPH oxidase mediates lipopolysaccharide-induced neurotoxicity and proinflammatory gene expression in activated microglia. J Biol Chem 279(2):1415–1421 [DOI] [PubMed] [Google Scholar]
- Qu Z, Sun J, Zhang W et al (2020) Transcription factor NRF2 as a promising therapeutic target for Alzheimer’s disease. Free Radical Biol Med 159:87–102 [DOI] [PubMed] [Google Scholar]
- Ramos AN, Sesto Cabral ME, Arena ME et al (2015) Compounds from Lactobacillus plantarum culture supernatants with potential pro-healing and anti-pathogenic properties in skin chronic wounds. Pharm Biol 53(3):350–358 [DOI] [PubMed] [Google Scholar]
- Ransohoff RM, El Khoury J (2015) Microglia in health and disease. Cold Spring Harb Perspect Biol 8(1):a020560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren C, Cheng L et al (2020) Lactic acid bacteria secrete toll like receptor 2 stimulating and macrophage immunomodulating bioactive factors. Journal of Functional Foods 66:103783 [Google Scholar]
- Rocchetti MT, Russo P, De Simone N et al (2023) Immunomodulatory activity on human macrophages by cell-free supernatants to explore the probiotic and postbiotic potential of Lactiplantibacillus plantarum strains of plant origin. Probiot Antimicrob Proteins. 10.1007/s12602-023-10084-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rushworth SA, Shah S, MacEwan DJ (2011) TNF mediates the sustained activation of Nrf2 in human monocytes. J Immunol 187(2):702–707 [DOI] [PubMed] [Google Scholar]
- Salminen S, Collado MC, Endo A et al (2021) The international scientific association of probiotics and prebiotics (ISAPP) consensus statement on the definition and scope of postbiotics. Nat Rev Gastroenterol Hepatol 18(9):649–667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar A, Lehto SM, Harty S et al (2016) Psychobiotics and the manipulation of bacteria–gut–brain signals. Trends Neurosci 39(11):763–781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigfridsson E, Marangoni M, Hardingham GE et al (2020) Deficiency of Nrf2 exacerbates white matter damage and microglia/macrophage levels in a mouse model of vascular cognitive impairment. J Neuroinflammation 17(1):367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Şirin S (2023) Lactic acid bacteria-derived exopolysaccharides mitigate the oxidative response via the NRF2-KEAP1 pathway in PC12 cells. Curr Issues Mol Biol 45(10):8071–8090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sriram K, Matheson JM, Benkovic SA et al (2006) Deficiency of TNF receptors suppresses microglial activation and alters the susceptibility of brain regions to MPTP-induced neurotoxicity: role of TNF-alpha. FASEB J 20(6):670–682 [DOI] [PubMed] [Google Scholar]
- Tankou SK, Regev K, Healy BC et al (2018) A probiotic modulates the microbiome and immunity in multiple sclerosis. Ann Neurol 83(6):1147–1161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonelli C, Chio IIC, Tuveson DA et al (2018) Transcriptional regulation by Nrf2. Antioxid Redox Signal 29(17):1727–1745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentine JS, Doucette PA, Zittin Potter S et al (2005) Copper-zinc superoxide dismutase and amyotrophic lateral sclerosis. Annu Rev Biochem 74:563–593 [DOI] [PubMed] [Google Scholar]
- Vincenzi A, Goettert MI, Volken de Souza CF (2021) An evaluation of the effects of probiotics on tumoral necrosis factor (TNF-α) signaling and gene expression. Cytokine Growth Factor Rev 57:27–38 [DOI] [PubMed] [Google Scholar]
- Wang Y, Zhang Z, Li B et al (2022) New insights into the gut microbiota in neurodegenerative diseases from the perspective of redox homeostasis. Antioxidants 11(11):2287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiegers C, Veerman MA, Brummer RJ et al (2022) Reviewing the state of the art of probiotics as clinical modalities for brain-gut-microbiota axis associated disorders. Front Microbiol 13:1053958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Wang Y, Hu A et al (2022) Lactobacillus plantarum-derived postbiotics prevent salmonella-induced neurological dysfunctions by modulating gut-brain axis in mice. Front Nutr 9:946096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Li H, Hou X et al (2015) Punicalagin induces Nrf2/HO-1 expression via upregulation of PI3K/AKT pathway and inhibits LPS-induced oxidative stress in RAW2647 macrophages. Mediat Inflamm 2015:e380218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z, Zhang J, Wu J et al (2022) Lactobacillus Plantarum ST-III culture supernatant ameliorates alcohol-induced cognitive dysfunction by reducing endoplasmic reticulum stress and oxidative stress. Front Neurosci 16:976358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav M, Mandeep SP (2020) Probiotics of diverse origin and their therapeutic applications: a review. J Am Coll Nutr 39(5):469–479 [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Suzuki T, Kobayashi A et al (2008) Physiological significance of reactive cysteine residues of keap1 in determining Nrf2 activity. Mol Cell Biol 28(8):2758–2770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang DD, Hannink M (2003) Distinct cysteine residues in Keap1 are required for Keap1-dependent ubiquitination of Nrf2 and for stabilization of Nrf2 by chemopreventive agents and oxidative stress. Mol Cell Biol 23(22):8137–8151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang DD, Lo SC, Cross JV et al (2004) Keap1 Is a redox-regulated substrate adaptor protein for a Cul3-dependent ubiquitin ligase complex. Mol Cell Biol 24(24):10941–10953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Xiao D, Mao Q et al (2023) Role of neuroinflammation in neurodegeneration development. Signal Transduct Target Ther 8(1):267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Zhao J, Jiang Y et al (2022) Bacillus amyloliquefaciens lysate ameliorates photoaging of human skin fibroblasts through NRF2/KEAP1 and TGF-β/SMAD signaling pathways. Appl Sci 12(18):9151 [Google Scholar]
- Zolfaghari SI, Rabbani Khorasgani M, Noorbakhshnia M (2021) The Effects of Lactobacilli (L. Rhamnosus, L. Reuteri, L. Plantarum) on LPS-induced memory impairment and changes in CaMKII-α and TNF-α genes expression in the hippocampus of rat. Physiol Behav 229:113224 [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- EFSA Biohaz Panel, Koutsoumanis K, Allende A, Alvarez-Ordonez A et al (2024) Updated list of QPS-recommended microorganisms for safety risk assessments carried out by EFSA. Zenodo. 10.5281/zenodo.10534041
Supplementary Materials
Data Availability Statement
No datasets were generated or analysed during the current study.