Abstract
The present study investigates the temporal effects of flaxseed supplementation on boar semen quality, antioxidant status, and in-vivo fertility under high-temperature humidity index (THI) conditions in a sub-tropical climate. Twelve Hampshire crossbreed boars were randomly assigned to control and treatment groups, with the treatment group receiving flaxseed oil supplementation. Semen samples were collected and analyzed for semen quality parameters, sperm kinematics, and antioxidant status. Fertility outcomes were assessed through in-vivo mating trials. Flaxseed supplementation resulted in time dependent significant improvements in semen volume, sperm concentration, total and progressive sperm motility, sperm quality parameters, and antioxidant status. Fertility outcomes, including farrowing rates and litter sizes, were also enhanced in the flaxseed-supplemented group. These findings highlight the potential of flaxseed supplementation to improve boar fertility under high ambient stress conditions, with implications for optimizing reproductive performance in swine production systems.
Keywords: Boar fertility, Flaxseed supplementation, Semen quality, Sperm kinematics, Antioxidant status, Sub-tropical climate
Subject terms: Reproductive disorders, Infertility
Introduction
Pigs are more susceptible to environmental heat stress because of anatomical and physiological reasons. The thermo-neutral zone of pig is 18 °C to 25 °C only. In adult pigs above 110 kg body weight, the thermo-neutral zone is further reduced below 21 °C hence, they are more susceptible to environmental heat stress1,2. With increasing global temperature, the risk of heat stress for pig is also growing. Climate induced heat stress affects behavioral, physiological, immunological and reproductive functions of pigs3. These changes become more evident in adverse climate where intensity and frequency of heat stress is more. In tropical and sub-tropical climate, high humidity and high temperature is a major constraint for pig production and reproduction3,4. Under such climate, the long duration of high temperature humidity index period severely jeopardize the pig welfare and reproduction. It has been reported earlier that high temperature humidity index negatively affects the boars’ libido, semen quality and in-vivo fertility5. Heat stress during summer season is known to decrease the reproductive efficiency of boars6. The boar’s inefficient capacity to sweat, and the extensive use of temperate pigs’ breeds in sub-tropical conditions, can negatively affect the boar’s fertility7. High heat stress to boar disrupts their testis’s thermoregulation and thereby adversely affects the spermatogenesis.
Moreover, the boar scrotum is not pendulous7,8 and boar spermatozoa tend to be more susceptible to temperature shock9. Stone10 reported that spermatogenesis in boars is impaired when ambient temperatures rise above 29 °C. Heat stress in boars has been shown to result in lower semen volume, reduced sperm concentration3,11 lower motility and higher rates of abnormal spermatozoa, interference in testosterone production, reduced libido and decreased in-vivo fertility3,4,12. It was earlier reported that heat stress induces DNA damage in boar spermatozoa during spermatogenesis which may contribute significantly to seasonal pregnancy failure and reduced litter size in sows13. In pigs, Didion et al.14 have proposed that spermatozoa with greater than 6% DNA fragmentation results into decreased farrowing rates and average number of piglets born. Furthermore, it was reported that there is reduced antioxidant capacity of the boar seminal plasma during the summer period3,5,15.
Besides, the boar sperm are more susceptible to damage by oxidative stress and lipid peroxidation16 because of their structural composition which becomes more pronounced during summer season when the ambient temperature is high17,18. Boar sperm membrane has a unique composition with low cholesterol to phospholipids ratio and therefore more susceptible to cold shock during liquid preservation. In today’s swine reproduction, artificial insemination with liquid preserved boar semen has central role in advancing the genetic gain and increasing the productivity19.
However, heat stress induced sperm damage and low keeping quality during summer months in sub-tropical climate is a significant constraint to fully reap the benefit of artificial insemination in swine reproduction20. It was reported in earlier study that boar semen quality and antioxidant status is high in autumn–winter as compared to summer season21. In tropical and sub-tropical climate, the long duration and high severity of heat stress further reduces the optimal utilization of superior genetic boar in artificial insemination programme3–5. Considering the impact of climate change and predicted heat waves, feasible strategies (managemental and nutritional supplementation) to mitigate the impact of heat stress in boars are necessary particularly in tropical climate. Recently, use of flaxseed supplementation has gained importance to mitigate the heat stress in sows and boars. Flaxseed contains 53 per cent linolenic acid which is a omega-3 fatty acid22. There are only few studied documenting the effect of flaxseed oil supplementation to boar on semen quality under tropical and sub-tropical climate3–5. Previous studies are mostly laboratory based and few have small in-vivo fertility trials. However, there is no report available on temporal effect of flaxseed oil supplementation to boar on semen quality, antioxidant status and in-vivo fertility. Therefore, the objectives of this study were (i) to determine the temporal effect of supplementing flaxseed oil on sexual behavior and semen quality parameters (SQPs) of boar sperm in humid sub-tropical climate, (ii) to evaluate effect of flaxseed oil feeding on antioxidant status and in-vivo fertility of the boar (Table 1).
Table 1.
Ingredients and composition of the basal diet used in the experiment.
| SI No | Ingredients | Proportion (%) | CP (%) | ME (Kcal/kg) |
|---|---|---|---|---|
| 1 | Maize | 62.5 | 5.938 | 2262.5 |
| 2 | Wheat Bran | 14.5 | 2.103 | 348 |
| 3 | Groundnut Cake | 11 | 4.95 | 363 |
| 4 | Rice Bran | 10 | 1.6 | 270 |
| 5 | Vitamin Mineral premixa | 1.5 | 0 | 0 |
| 6 | Salt | 0.5 | 0 | 0 |
| 7 | Total | 100.00 | 14.59 | 3243.5 |
a –composition (per 500 g): Ca, 23%; P, 17%; Cu, 525 mg; Co, 100 mg; I, 125 mg; Mn, 1000 mg; Fe, 1700 mg; S, 60 mg; Zn, 400 mg; Selenium, 350 mcg; Mg, 500; Niacin, 500 mg; Methionine, 2100 mg; Lysin, 2500 mg; Choline, 250 mg; vitamin A, 1,70,000 i.u.; vitamin B1, 8 mg; vitamin B2, 100 mg; vitamin B6, 10 mg; vitamin B12, 50 mg; vitamin D3, 60,000 i.u.; and vitamin K, 100 mg.
Results
Effects of flaxseed supplementation to boar on sexual behavior and semen quality parameters at day 0
There was significant effect of feeding flaxseed oil on reaction time and false mount from 2nd and 8th week post feeding, respectively (Table 2). Similarly, sperm viability, sperm acrosomal integrity and HOST reactive spermatozoa were significantly higher in flaxseed group from 8th weeks of flaxseed oil feeding. Sperm abnormality decreased significantly (P < 0.05) in flaxseed oil group at 6th week post treatment till 16th week.
Table 2.
Temporal effect of feeding linseed oil on some libido traits and semen quality parameters of boars at day 0 (mean ± SEM).
| Parameters | Groups | -2wk | -1wk | 0wk | 1wk | 2wk | 4wk | 6wk | 8wk | 10wk | 12wk | 14wk | 16wk |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Reaction time (min) | Group-I | 5 ± 0.00a | 5 ± 0.26a | 5 ± 0.00a | 4.83 ± 0.4a | 5 ± 0.00a | 5 ± 0.00a | 4.33 ± 0.49a | 4 ± 0.45a | 4 ± 0.45a | 4 ± 0.45a | 3.83 ± 0.4a | 3.83 ± 0.4a |
| Group-II | 4.5 ± 0.34a | 4 ± 0.45a | 4.33 ± 0.21b | 4 ± 0.45a | 3.83 ± 0.17b | 2.33 ± 0.21b | 2 ± 0.00b | 2.33 ± 0.21b | 2.17 ± 0.17b | 2.17 ± 0.17b | 2.33 ± 0.21b | 2.33 ± 0.21b | |
| False mount (numbers) | Group-I | 2.33 ± 0.21a | 2.33 ± 0.21a | 2.17 ± 0.17a | 2.33 ± 0.42a | 2.33 ± 0.33a | 2.00 ± 0.26a | 2.33 ± 0.33a | 2.17 ± 0.17a | 2.17 ± 0.17a | 2.17 ± 0.17a | 2.33 ± 0.21a | 2.33 ± 0.21a |
| Group-II | 2.83 ± 0.31a | 2.50 ± 0.22a | 2.33 ± 0.21a | 2.33 ± 0.21a | 2.17 ± 0.17a | 1.83 ± 0.31a | 1.83 ± 0.17a | 1.67 ± 0.21b | 1.50 ± 0.22b | 1.67 ± 0.21b | 1.50 ± 0.22b | 1.00 ± 0.26b | |
| Viability (%) | Group-I | 81.50 ± 0.5a | 82.83 ± 0.48a | 82.67 ± 0.71a | 82.67 ± 0.71a | 83.50 ± 0.50a | 82.67 ± 0.80a | 81.67 ± 0.33a | 82.17 ± 0.31b | 82.17 ± 0.75b | 81.67 ± 1.45b | 81 ± 0.52b | 82.17 ± 0.83b |
| Group-II | 81.50 ± 0.81a | 82.33 ± 0.67a | 83.50 ± 1.34a | 84 ± 0.63a | 84.67 ± 0.99a | 84.83 ± 0.70a | 82.17 ± 1.17a | 90.67 ± 1.38a | 92.50 ± 1.06a | 92.33 ± 0.67a | 93.17 ± 0.75a | 92.33 ± 0.76a | |
| Abnormality (%) | Group-I | 17.67 ± 0.56a | 17.17 ± 0.75a | 16.17 ± 0.65a | 15.67 ± 0.67a | 15.67 ± 0.49a | 16.33 ± 0.99a | 16.67 ± 0.61a | 16.50 ± 0.56a | 16 ± 0.52a | 15.50 ± 0.43a | 15.83 ± 0.40a | 16.67 ± 0.42a |
| Group-II | 16.67 ± 0.56a | 15.83 ± 0.65a | 16.17 ± 0.70a | 16.67 ± 0.76a | 16.33 ± 1.05a | 15.50 ± 0.56a | 14.67 ± 0.21b | 12.83 ± 0.60b | 11 ± 0.52b | 12.17 ± 0.60b | 11.33 ± 0.49b | 11.17 ± 0.83b | |
| Acrosomal integrity (%) | Group-I | 77.33 ± 0.56a | 77.17 ± 0.70a | 77.50 ± 0.34a | 77.50 ± 0.67a | 76 ± 0.82a | 74.67 ± 0.56b | 77 ± 0.63a | 76.17 ± 0.60b | 76.83 ± 0.70b | 77.17 ± 0.87b | 76.33 ± 0.88b | 76.50 ± 1.02b |
| Group-II | 77 ± 0.82a | 77 ± 0.68a | 76.83 ± 0.60a | 77 ± 0.68a | 78 ± 0.58a | 76.83 ± 0.65a | 79.17 ± 0.91a | 84.50 ± 0.34a | 85.83 ± 0.48a | 85.67 ± 0.67a | 84.17 ± 0.54a | 86.17 ± 0.54a | |
| Hypo-osmotic swelling test (%) | Group-I | 75.17 ± 0.75a | 74.67 ± 0.21a | 75.67 ± 0.49a | 74.83 ± 0.83a | 74.67 ± 0.80a | 73.17 ± 0.70b | 76 ± 0.68a | 74 ± 0.63b | 75 ± 0.82b | 74.17 ± 0.48b | 74.50 ± 0.50b | 74.17 ± 0.79b |
| Group-II | 74.67 ± 0.33a | 74.67 ± 0.21a | 74.50 ± 0.81a | 75.17 ± 0.17a | 74 ± 0.68a | 76.17 ± 0.83a | 75 ± 1.34a | 80.33 ± 0.67a | 80.67 ± 0.49a | 80.50 ± 0.67a | 79.33 ± 0.61a | 80.83 ± 0.54a |
Values with different letters in superscripts in a column within a parameters express significant (P < 0.05) differences. n = 72 samples per group (12 ejaculates per boar).
Effects of flaxseed supplementation to boar on computer assisted semen analysis at day 0
Velocity attributes viz. VAP and STR% increased significantly (P < 0.05) in flaxseed group at 8th weeks post feeding till 16th week (Table 3). VSL, VCL, ALH and BCF increased significantly (P < 0.05) at 6th weeks of flaxseed oil feeding as compared to control group. LIN % increased significantly (P < 0.05) in group-II at 10th week post feeding. Total sperm motility (Fig. 5) and progressive sperm motility (Fig. 6) was significantly increased (P < 0.05) in treatment group at 6th week post feeding till 16th week.
Table 3.
Temporal effect of feeding linseed oil on boar semen quality parameters (CASA) at day 0 (mean ± SEM).
| Parameters | Groups | -2wk | -1wk | 0wk | 1wk | 2wk | 4wk | 6wk | 8wk | 10wk | 12wk | 14wk | 16wk |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| VAP (µm/sec) | Group-I | 65.50 ± 0.81a | 63.67 ± 1.28a | 60.67 ± 1.86a | 61.50 ± 2.20a | 62.67 ± 1.89a | 64.17 ± 1.30a | 62.67 ± 1.89a | 64.83 ± 0.79 b | 65 ± 1.00b | 62.50 ± 2.14b | 64 ± 0.68b | 65.50 ± 0.92b |
| Group-II | 61.67 ± 1.17b | 63.67 ± 1.28a | 61.17 ± 1.40a | 60.67 ± 0.99a | 62.83 ± 0.95a | 64.17 ± 0.79a | 66.17 ± 1.11a | 79.67 ± 1.63a | 78.83 ± 1.62a | 78 ± 1.51a | 77.83 ± 1.90a | 78.83 ± 1.60a | |
| VSL(µm/sec) | Group-I | 52.17 ± 1.33a | 49.33 ± 2.39a | 49.83 ± 1.62a | 54 ± 2.59a | 53 ± 1.86a | 55.50 ± 1.73a | 53.67 ± 1.61b | 51.17 ± 2.21b | 53.83 ± 0.87b | 52 ± 2.10b | 51.17 ± 1.87b | 55.83 ± 1.99b |
| Group-II | 52.17 ± 2.10a | 49.33 ± 1.82a | 54 ± 1.51a | 53.67 ± 1.84a | 54.67 ± 2.11a | 56.17 ± 0.83a | 60.33 ± 1.89a | 71.83 ± 0.91a | 72.50 ± 0.89a | 72.67 ± 0.80a | 72.67 ± 0.84a | 70.83 ± 1.54a | |
| VCL (µm/sec) | Group-I | 123.83 ± 1.89a | 126 ± 1.26a | 127.83 ± 3.62a | 128.83 ± 3.42a | 12.33 ± 2.01a | 125.17 ± 1.47a | 124 ± 3.27b | 124 ± 1.88b | 126.83 ± 3.75b | 128 ± 4.07b | 120.67 ± 1.98b | 129.67 ± 2.72b |
| Group-II | 128.33 ± 2.95a | 125 ± 2.31a | 124.17 ± 3.42a | 123.67 ± 2.16a | 118.67 ± 2.75a | 123.17 ± 4.24a | 139.33 ± 4.18a | 158 ± 2.83a | 154 ± 2.07a | 152.33 ± 2.76a | 152 ± 2.5a | 153.67 ± 2.51a | |
| ALH (µm) | Group-I | 5.67 ± 0.49a | 5.33 ± 0.56a | 5.50 ± 0.34a | 5.50 ± 0.50a | 5.33 ± 0.42a | 6.17 ± 0.75a | 4.83 ± 0.40b | 5.50 ± 0.56b | 5.33 ± 0.33b | 5.17 ± 0.60b | 5.67 ± 0.21b | 5.83 ± 0.40b |
| Group-II | 5.33 ± 0.42a | 4.83 ± 0.17a | 5.17 ± 0.48a | 5.17 ± 0.40a | 5.50 ± 0.67a | 4.83 ± 0.48a | 6.50 ± 0.56a | 8.33 ± 0.33a | 8.17 ± 0.31a | 8 ± 0.37a | 8.17 ± 0.31a | 8 ± 0.37a | |
| BCF (Hz) | Group-I | 30 ± 1.71a | 31.17 ± 0.91a | 31.50 ± 1.06a | 31.33 ± 1.4a | 31.50 ± 1.20a | 30.67 ± 1.74a | 28.33 ± 1.43b | 28.33 ± 1.52b | 29.67 ± 1.12b | 30.17 ± 1.08b | 30.83 ± 1.49b | 32.17 ± 0.40b |
| Group-II | 30.67 ± 0.88a | 30.83 ± 1.35a | 29.83 ± 0.65a | 29.83 ± 1.08a | 30.17 ± 1.83a | 27.50 ± 0.92a | 41.50 ± 0.50a | 40.50 ± 0.50a | 42.17 ± 1.14a | 42 ± 1.53a | 41 ± 0.52a | 40.83 ± 1.35a | |
| STR (%) | Group-I | 79.68 ± 2.01a | 77.62 ± 3.90a | 82.34 ± 2.69a | 87.75 ± 2.36a | 84.79 ± 3.05a | 86.66 ± 3.04a | 86.07 ± 3.82a | 79.02 ± 3.69b | 82.98 ± 2.34b | 83.59 ± 3.87b | 79.94 ± 2.70b | 85.39 ± 3.53a |
| Group-II | 84.88 ± 4.23a | 77.61 ± 3.04a | 88.51 ± 3.08a | 88.50 ± 2.91a | 87.24 ± 4.19a | 87.60 ± 1.68a | 91.34 ± 3.31a | 90.33 ± 1.97a | 92.15 ± 2.10a | 93.33 ± 2.01a | 93.56 ± 1.89a | 89.99 ± 2.40a | |
| LIN (%) | Group-I | 42.24 ± 1.60a | 39.16 ± 1.92a | 39.15 ± 1.68a | 43.61 ± 2.97a | 43.43 ± 1.90a | 44.35 ± 1.31a | 43.44 ± 1.80a | 41.38 ± 2.17a | 42.63 ± 1.43b | 40.70 ± 1.47b | 42.52 ± 1.97b | 43.25 ± 2.14a |
| Group-II | 40.85 ± 2.29a | 39.47 ± 1.25a | 43.75 ± 2.17a | 43.45 ± 1.58a | 46.26 ± 2.33a | 45.86 ± 1.61a | 43.36 ± 1.08a | 45.56 ± 1.22a | 47.14 ± 1.09a | 47.80 ± 1.17a | 47.90 ± 1.24a | 46.13 ± 1.07a |
Values with different letters in superscripts in the same row express differences which were significant (P < 0.05). VAP: Average Path Velocity, VSL: Straight line Velocity, VCL: Curve linear Velocity, ALH: Amplitude of lateral head displacement, BCF: Beat cross frequency, STR: Straightness, LIN: Linearity. n = 72 samples per group (12 ejaculates per boar).
Fig. 5.
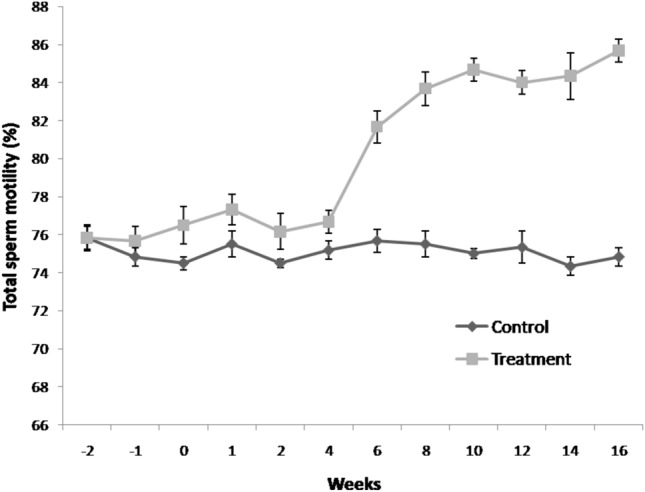
Temporal effect of feeding linseed oil on total sperm motility of boar at day 0 (mean ± SEM). n = 72 samples per group (12 ejaculates per boar). Control (group-I) and Treatment (group-II) were fed vegetable oil and flaxseed oil for 16 weeks, respectively.
Fig. 6.
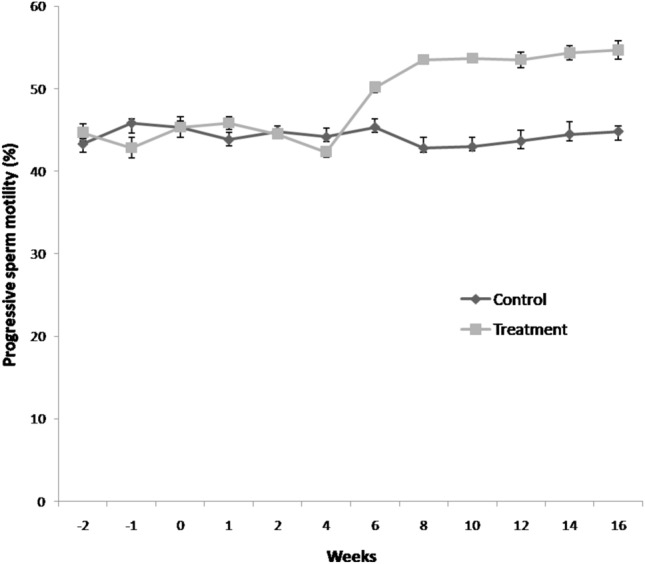
Temporal effect of feeding linseed oil on progressive sperm motility of boar at day 0 (mean ± SEM). n = 72 samples per group (12 ejaculates per boar). Control (group-I) and Treatment (group-II) were fed vegetable oil and flaxseed oil for 16 weeks, respectively.
Effects of flaxseed supplementation to boar on semen volume and sperm concentration
Semen volume increased significantly (P < 0.05) in group-II (flaxseed) from 2nd week post feeding till 16th week as compared to the control group (Fig. 3). Sperm concentration increased significantly (P < 0.05) from 6th week onward in flaxseed group as compared to control group (Fig. 4). In overall, flaxseed oil supplementation resulted in significant enhancement of boar semen production and improvement in semen quality parameters during summer months.
Fig. 3.
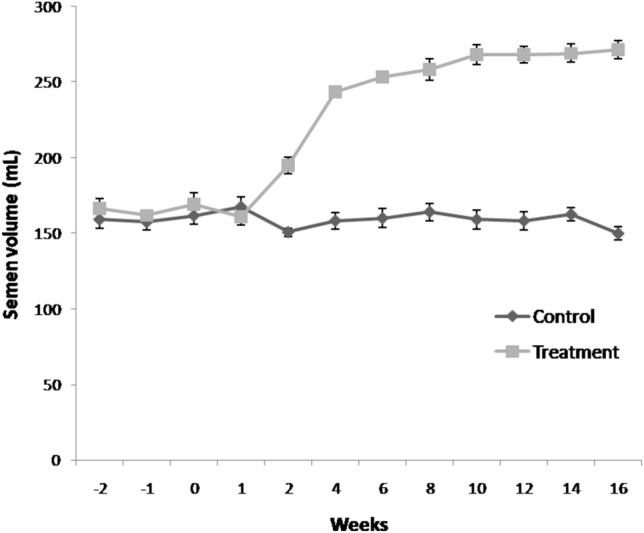
Temporal effect of feeding linseed oil on boar semen volume (mean ± SEM). n = 72 samples per group (12 ejaculates per boar). Control (group-I) and Treatment (group-II) were fed vegetable oil and flaxseed oil for 16 weeks, respectively.
Fig. 4.
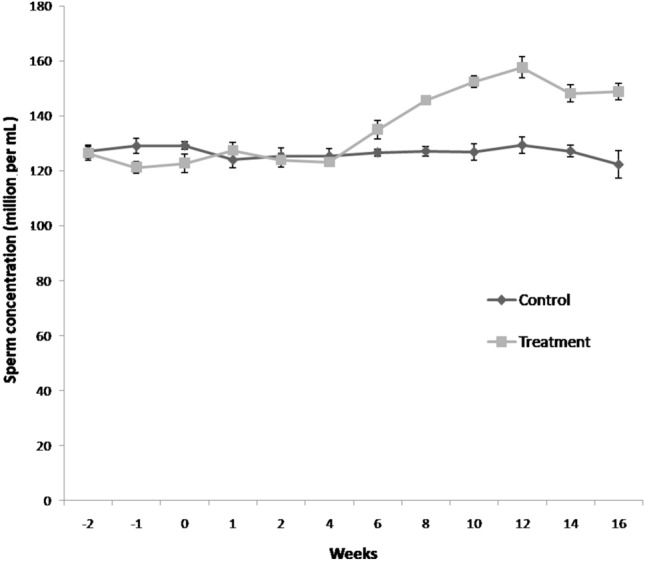
Temporal effect of feeding linseed oil on sperm concentration of boar (mean ± SEM). n = 72 samples per group (12 ejaculates per boar). Control (group-I) and Treatment (group-II) were fed vegetable oil and flaxseed oil for 16 weeks, respectively.
Effects of flaxseed supplementation to boar on semen quality parameters in liquid stored boar semen after 72 h of storage
After liquid storage of semen for 72 h, sperm livability and acrosomal integrity were significantly (P < 0.01) higher in flaxseed group (group-II) as compared to control group (group-I) from 6th weeks onward (Table 4). Abnormal spermatozoa were significantly lesser (P < 0.01) in flaxseed group as compared to control group from 6th weeks onward. HOST positive sperm were significantly (P < 0.01) increased in flaxseed group as compared to control group from 8th weeks onward (Table 4).
Table 4.
Temporal effect of feeding linseed oil on boar semen quality parameters after 72 h of storage (mean ± SEM).
| Parameters | Groups | -2wk | -1wk | 0wk | 1wk | 2wk | 4wk | 6wk | 8wk | 10wk | 12wk | 14wk | 16wk |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Viability (%) | Group-I | 76.33 ± 0.61a | 75 ± 0.45a | 74.33 ± 0.21a | 74.67 ± 0.95a | 73.33 ± 0.61a | 73 ± 0.77a | 74.33 ± 0.49b | 73.83 ± 0.60b | 73.83 ± 0.1.08b | 74.50 ± 0.22b | 73.50 ± 0.50b | 73.67 ± 0.56b |
| Group-II | 73.67 ± 1.12a | 74.67 ± 0.92a | 73.33 ± 1.09a | 73.17 ± 0.70a | 72.17 ± 0.91a | 72.83 ± 0.70a | 77.67 ± 0.67a | 81.67 ± 0.21a | 82 ± 0.63a | 83.17 ± 0.75a | 81.83 ± 1.38a | 81.17 ± 0.65a | |
| Abnormality (%) | Group-I | 19.33 ± 0.71a | 19.33 ± 1.45a | 19.33 ± 1.23a | 19.33 ± 0.88b | 19.83 ± 0.54a | 20.33 ± 1.09a | 20.83 ± 0.83a | 22.17 ± 0.54a | 20.83 ± 0.70a | 20 ± 0.45b | 20.33 ± 0.61a | 20 ± 0.73a |
| Group-II | 20.83 ± 0.48a | 19.83 ± 0.65a | 21 ± 0.52a | 22.50 ± 0.62a | 20.33 ± 0.71a | 20.50 ± 0.56a | 15.83 ± 0.87b | 16 ± 0.58b | 15.50 ± 0.56b | 14.50 ± 0.34a | 15.33 ± 0.33b | 16 ± 0.63b | |
| Acrosomal integrity (%) | Group-I | 70.67 ± 0.67a | 71.67 ± 0.67a | 70.50 ± 0.81a | 72 ± 1.57a | 71.17 ± 1.08a | 70 ± 1.24a | 71.67 ± 0.92a | 70.50 ± 1.15b | 71.83 ± 0.70b | 71.83 ± 0.54b | 71 ± 0.37b | 71.50 ± 0.43b |
| Group-II | 70.50 ± 0.62a | 72.17 ± 0.40a | 71.83 ± 0.48a | 72.50 ± 0.34a | 73.17 ± 0.70a | 72.67 ± 0.42a | 71.67 ± 0.56a | 80.50 ± 0.43a | 80.83 ± 0.60a | 81 ± 0.63a | 80.17 ± 0.75a | 79.67 ± 0.76a | |
| Hypo-osmotic swelling test (%) | Group-I | 64.83 ± 0.98a | 63.17 ± 0.60a | 63.33 ± 0.61a | 63.50 ± 0.56b | 63.33 ± 0.67a | 64 ± 0.89a | 63.50 ± 0.67a | 63.50 ± 0.43b | 64.33 ± 0.92b | 62.67 ± 0.49b | 64 ± 1.00b | 63.67 ± 0.88b |
| Group-II | 65 ± 0.26a | 63.83 ± 0.83a | 64.83 ± 0.79a | 65.50 ± 0.56a | 65.33 ± 0.61a | 64.33 ± 0.61a | 65 ± 0.93a | 73 ± 0.63a | 74.33 ± 0.61a | 73.67 ± 0.61a | 73.33 ± 0.92a | 74.17 ± 0.48a |
Values with different letters in superscripts in the same row express differences which were significant (P < 0.05). n = 72 samples per group (12 ejaculates per boar).
Effects of flaxseed supplementation to boar on computer assisted semen analysis of liquid stored boar semen after 72 h of storage
After 72 h of storage, total sperm motility and progressive sperm motility were significantly increased (P < 0.05) in treatment group at 6th week post feeding till 16th week (Table 5). Similarly velocity attributes viz. VAP, VSL, VCL, ALH and BCF were significantly (P < 0.01) higher in flaxseed group as compared to control group at 6th week onward of flaxseed oil feeding.
Table 5.
Temporal effect of feeding linseed oil on boar semen quality parameters (CASA) after 72 h of storage (mean ± SEM).
| Parameters | Groups | -2wk | -1wk | 0wk | 1wk | 2wk | 4wk | 6wk | 8wk | 10wk | 12wk | 14wk | 16wk |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total motility (%) | Group-I | 63.83 ± 0.48b | 64.67 ± 0.67a | 64.50 ± 0.76a | 64.17 ± 0.79a | 64.17 ± 0.60a | 64.50 ± 0.67a | 65.50 ± 0.67b | 64.67 ± 0.56b | 65.83 ± 0.60b | 64.50 ± 0.92b | 62.67 ± 0.67b | 64.17 ± 0.54b |
| Group-II | 66.17 ± 0.48a | 63.67 ± 0.49a | 64.67 ± 0.84a | 65.17 ± 0.48a | 65 ± 1.03a | 64.17 ± 0.70a | 72 ± 1.06a | 74.50 ± 0.22a | 75.17 ± 0.31a | 74.33 ± 0.56a | 74.33 ± 0.21a | 76 ± 0.77a | |
| Progressive motility (%) | Group-I | 33.83 ± 0.65a | 34 ± 0.68a | 35.17 ± 0.79a | 34.17 ± 0.70a | 35 ± 0.63a | 33.83 ± 0.98a | 34 ± 0.63b | 34.50 ± 1.20b | 34.17 ± 0.60b | 33.33 ± 0.71b | 33.83 ± 1.19b | 35 ± 1.03b |
| Group-II | 36 ± 1.13a | 34.67 ± 0.42a | 34 ± 1.13a | 32.50 ± 0.56a | 33.33 ± 0.61a | 33.67 ± 0.76a | 40 ± 0.58a | 42.17 ± 0.54a | 43 ± 0.52a | 44 ± 1.00a | 44.83 ± 0.83a | 43.33 ± 0.76a | |
| VAP (µm/sec) | Group-I | 55.6 ± 0.76a | 57 ± 0.68a | 55.83 ± 0.75a | 56 ± 0.89a | 55 ± 0.68a | 54.83 ± 0.91a | 57 ± 0.82b | 53.83 ± 0.95b | 58.33 ± 0.92b | 56.50 ± 0.72b | 55.17 ± 0.83b | 56 ± 1.00b |
| Group-II | 55.17 ± 0.79a | 54 ± 0.86b | 54.83 ± 0.70a | 53.67 ± 0.92a | 54 ± 0.68a | 55 ± 1.21a | 62.67 ± 0.71a | 67 ± 0.77a | 70.67 ± 1.78a | 68.67 ± 1.15a | 67.67 ± 1.05a | 69.83 ± 1.83a | |
| VSL(µm/sec) | Group-I | 43.67 ± 1.65a | 43.17 ± 2.15a | 43.17 ± 1.45a | 42.67 ± 1.26a | 45.67 ± 2.42a | 44.83 ± 2.47a | 43.33 ± 2.36b | 43.33 ± 2.08b | 42.33 ± 0.95b | 42.17 ± 1.68b | 41.67 ± 2.49b | 43 ± 1.90b |
| Group-II | 43.33 ± 0.92a | 44 ± 1.03a | 43.83 ± 1.01a | 43.50 ± 1.06a | 43.17 ± 1.08a | 42.83 ± 1.08a | 55.33 ± 0.61a | 58.50 ± 1.41a | 57.83 ± 2.63a | 59.50 ± 1.48a | 58 ± 0.93a | 58 ± 1.21a | |
| VCL (µm/sec) | Group-I | 105 ± 3.57a | 103.67 ± 5.78a | 106 ± 4.74a | 102.83 ± 3.99a | 97.50 ± 4.03a | 98.83 ± 2.15a | 96 ± 1.57b | 97.50 ± 2.40b | 101.67 ± 3.11b | 102.67 ± 2.55b | 101.67 ± 3.04b | 104.50 ± 4.26b |
| Group-II | 101.67 ± 2.84a | 104.17 ± 3.76a | 107.33 ± 3.32a | 105.83 ± 4.00a | 103 ± 3.85a | 97.83 ± 2.50a | 138.50 ± 1.31a | 140.33 ± 1.43a | 137.83 ± 3.25a | 140.67 ± 2.32a | 145.67 ± 3.90a | 137.67 ± 3.43a | |
| ALH (µm) | Group-I | 4.17 ± 0.40a | 4.17 ± 0.17a | 4.17 ± 0.40a | 3.50 ± 0.43a | 4.83 ± 0.17a | 4.33 ± 0.33a | 3.83 ± 0.40b | 3.83 ± 0.60b | 4.17 ± 0.31b | 3.50 ± 0.43b | 4.17 ± 0.48b | 3.83 ± 0.40b |
| Group-II | 4.50 ± 0.56a | 4.67 ± 0.33a | 4.17 ± 0.31a | 4.50 ± 0.22a | 5 ± 0.58a | 4.50 ± 0.22a | 6 ± 0.37a | 6 ± 0.52a | 6.0 ± 0.52a | 5.00 ± 0.26a | 6 ± 0.52a | 6 ± 0.37a | |
| BCF (Hz) | Group-I | 25 ± 0.45a | 24.50 ± 0.43a | 24.67 ± 0.76a | 24.83 ± 0.17a | 25.50 ± 0.89a | 24.50 ± 0.34a | 24.83 ± 0.48b | 24.33 ± 0.42b | 25.50 ± 0.92b | 25.17 ± 0.65b | 25.83 ± 0.60b | 25.67 ± 0.80b |
| Group-II | 25.33 ± 0.71a | 24.50 ± 0.22a | 24.17 ± 0.31a | 24 ± 0.26b | 25 ± 0.52a | 24.50 ± 0.22a | 33.17 ± 0.79a | 33.83 ± 0.60a | 34 ± 0.82a | 32 ± 0.73a | 32.17 ± 1.62a | 32.67 ± 1.31a | |
| STR (%) | Group-I | 78.67 ± 3.84a | 75.91 ± 4.27a | 77.43 ± 3.05a | 76.35 ± 2.88a | 80.03 ± 3.75a | 82.12 ± 5.43a | 76.17 ± 4.66b | 80.90 ± 4.95a | 72.69 ± 2.17a | 74.59 ± 2.58b | 75.40 ± 3.95b | 76.83 ± 3.26a |
| Group-II | 78.59 ± 1.66a | 81.56 ± 2.08a | 80.05 ± 2.41a | 81.06 ± 1.38a | 79.95 ± 1.80a | 78.24 ± 3.50a | 88.38 ± 1.68a | 87.41 ± 2.60a | 82.18 ± 4.53a | 86.73 ± 2.24a | 85.75 ± 1.15a | 83.36 ± 2.90a | |
| LIN (%) | Group-I | 42.05 ± 2.93a | 42.37 ± 3.37a | 41.11 ± 2.12a | 41.80 ± 1.93a | 47.67 ± 1.46a | 45.64 ± 3.16a | 45.15 ± 2.34a | 44.49 ± 2.11a | 41.83 ± 1.56a | 41.09 ± 1.35a | 41.12 ± 2.60a | 41.37 ± 1.99a |
| Group-II | 42.71 ± 0.98a | 42.45 ± 1.53a | 40.93 ± 0.92a | 41.32 ± 1.50a | 42.28 ± 2.20a | 43.89 ± 1.39a | 39.95 ± 0.30a | 41.72 ± 1.19a | 42.08 ± 2.12a | 42.33 ± 1.08a | 40 ± 1.48a | 42.34 ± 1.84a |
Values with different letters in superscripts in the same row express significant differences (P < 0.05). VAP: Average Path Velocity, VSL: Straight line Velocity, VCL: Curve linear Velocity, ALH: Amplitude of lateral head displacement, BCF: Beat cross frequency, STR: Straightness, LIN: Linearity. n = 72 samples per group (12 ejaculates per boar).
Effects of flaxseed supplementation to boar on antioxidant status in seminal plasma and spermatozoa
Seminal plasma GPx and TAC were significantly (P < 0.01) elevated following flaxseed oil supplementation as compared to control group from first week onwards (Table 6). Seminal plasma MDA decreased significantly (P < 0.01) in flaxseed group as compared to control group from first week post feeding till 16th weeks. However, spermatozoa MDA level decreased significantly in flaxseed group from 6th weeks onward only.
Table 6.
Temporal effect of feeding linseed oil on boar semen antioxidant parameters and lipid peroxidation at fresh stage (mean ± SEM).
| Parameters | Groups | -2wk | -1wk | 0wk | 1wk | 2wk | 4wk | 6wk | 8wk | 10wk | 12wk | 14wk | 16wk |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Seminal plasma GPx (nmol/min/mL) | Group-I | 76.83 ± 1.35a | 79 ± 1.71a | 78.17 ± 2.10a | 75.67 ± 0.76b | 77 ± 1.61b | 76.50 ± 1.73b | 77.83 ± 1.99b | 76.17 ± 1.58b | 75.17 ± 1.72b | 80.67 ± 2.20b | 76.67 ± 1.28b | 76.50 ± 2.32b |
| Group-II | 77.50 ± 1.23a | 78.67 ± 1.78a | 81.83 ± 0.75a | 88.67 ± 1.76a | 108 ± 2.08a | 113 ± 0.82a | 110 ± 1.39a | 112 ± 2.80a | 107 ± 1.26a | 111.50 ± 1.93a | 112.50 ± 1.23a | 109.67 ± 2.64a | |
| Seminal plasma TAC (mmol/L) | Group-I | 0.28 ± 0.01a | 0.29 ± 0.01a | 0.28 ± 0.01b | 0.28 ± 0.01b | 0.29 ± 0.01b | 0.31 ± 0.01b | 0.30 ± 0.01b | 0.29 ± 0.01b | 0.30 ± 0.01b | 0.30 ± 0.01b | 0.29 ± 0.01b | 0.28 ± 0.01b |
| Group-II | 0.29 ± 0.01a | 0.30 ± 0.01a | 0.32 ± 0.01a | 0.32 ± 0.01a | 0.38 ± 0.00a | 0.38 ± 0.01a | 0.38 ± 0.01a | 0.36 ± 0.01a | 0.38 ± 0.01a | 0.38 ± 0.01a | 0.37 ± 0.01a | 0.38 ± 0.01a | |
| Seminal plasma MDA (nmol/mL) | Group-I | 1.67 ± 0.02a | 1.64 ± 0.03a | 1.68 ± 0.02a | 1.70 ± 0.04a | 1.67 ± 0.03a | 1.63 ± 0.03a | 1.71 ± 0.02a | 1.68 ± 0.02a | 1.64 ± 0.03a | 1.69 ± 0.03b | 1.66 ± 0.02a | 1.74 ± 0.02a |
| Group-II | 1.72 ± 0.01a | 1.67 ± 0.02a | 1.63 ± 0.02a | 1.55 ± 0.01b | 1.43 ± 0.02b | 1.34 ± 0.02b | 1.37 ± 0.03b | 1.36 ± 0.03b | 1.33 ± 0.03b | 1.36 ± 0.05a | 1.33 ± 0.04b | 1.37 ± 0.02b | |
| Spermatozoa MDA (nmol/108 sperm cells) | Group-I | 3.62 ± 0.08a | 3.73 ± 0.05a | 3.72 ± 0.06a | 3.68 ± 0.05a | 3.70 ± 0.05a | 3.66 ± 0.07a | 3.68 ± 0.06a | 3.58 ± 0.05a | 3.70 ± 0.09a | 3.66 ± 0.04a | 3.67 ± 0.07a | 3.69 ± 0.06a |
| Group-II | 3.66 ± 0.03a | 3.66 ± 0.03a | 3.63 ± 0.02a | 3.66 ± 0.02a | 3.64 ± 0.01a | 3.65 ± 0.06a | 2.94 ± 0.11b | 2.89 ± 0.02b | 3.08 ± 0.03b | 3 ± 0.05a | 3.06 ± 0.04b | 2.95 ± 0.05b |
Values with different letters in superscripts in the same row express significant differences (P < 0.05). GPx: Glutathione peroxidase, TAC: Total antioxidant capacity, MDA: Malondialdehyde. n = 72 samples per group (12 ejaculates per boar).
Effects of flaxseed supplementation to boar on in-vivo fertility
Significantly (P < 0.05) higher farrowing rate was recorded with semen of boars supplemented with flaxseed oil as compared to control group (Table 7). Litter size at birth and litter size at weaning was significantly (P < 0.05) higher in flaxseed group (group-II) as compared to control group (group-I).
Table 7.
Effect of feeding linseed oil to boar on in-vivo fertility (mean ± SEM).
| Sows inseminated(n) | Animal farrowed (n) | Farrowing rate (%) | Litter size at birth (mean ± SEM) | Litter size at weaning (mean ± SEM) | Pre-weaning mortality (%) | |
|---|---|---|---|---|---|---|
| Group-I | 198 | 140 | 70.70b | 9.85 ± 0.14b | 8.44 ± 0.10b | 14.34 |
| Group-II | 140 | 115 | 82.14a | 10.96 ± 0.18a | 9.71 ± 0.14a | 12.21 |
Values with different letters in superscripts in the same column express significant differences (P < 0.05). n = 198 pluriparous sows in the control group and 140 pluriparous sows in treatment group.
Discussion
It has previously been reported that boar semen is affected by season, temperature and photoperiod which in turns impact reproductive performance4,7,31–33. During the course of the present experiment, boars were under very high environmental stress as evident by high temperature humidity index which caused poor semen quality in control group. It was reported that high temperature-humidity index compromises sperm quality and fertility of Holstein bulls34 and boars5,11,35. A greater incidence of sperm morphological abnormalities, reduced sperm motility and compromised fertility of boars was reported at higher ambient temperatures36.
The present experiment, recorded for the first time the temporal effect of flaxseed oil supplementation to the boar diets on semen quality and reproductive performance in sub-tropical climate under high environmental stress condition. The results of the present study indicate that flaxseed supplementation had temporal effects (positive) on the semen quality parameters and sperm kinematics at fresh stage as well as after 72 h of liquid storage. Besides, feeding flaxseed oil further improves the antioxidant status of the boar as well as enhances the in-vivo fertility.
In the present study, significant effect of feeding flaxseed oil on reaction time and false mount from 2nd and 8th week post feeding, respectively was recorded. Similarly, sperm quality parameters and CASA attributes were significantly improved from 8th weeks of flaxseed oil feeding at the fresh stage as well as after 72 h of storage at 17 °C. However, sperm abnormality decreased significantly in flaxseed oil group at 6th week post treatment. In the present study, significantly higher semen volume was obtained from second week onward where as increased sperm concentration was recorded from sixth week onward of the flaxseed oil supplementation. Increased boar semen volume and also of sperm concentration by flaxseed supplementation during high environmental stress period will produce more functional sperm per ejaculates. This will in turn increase the reproductive efficiency of genetic superior boars by inseminating more sows with a single collection3. According to the results of the present study, the flaxseed oil supplementation to boar has to be started at least six weeks prior to summer season to optimize the production of good quality fertile sperm. Liu et al.25 reported positive effect of PUFAs supplementation to boars at six weeks after treatment. In the literature, there is an inconsistent report regarding effects of PUFA supplementation to boar on its semen quality and fertility. However, the present study recorded a clear temporal response of feeding flaxseed oil and improvement in semen quality parameters in boars during high ambient stress. M.J.Estinne et al.37 reported increased sperm concentration of boar after dietary supplementation with omega-3 fatty acid there was no improvement in spem morphology. Similarly, Maldjian et al.38 reported that PUFAs through tuna fish oil supplementation to boar diets increased sperm output. In another study, it was observed that dietary ratio of n-6:n-3 is important to have positive outcome and 6.6:1 ratio was found to improve progressive sperm motility in boars25. Castellano et al.39 reported improvement in liquid stored semen quality of boars after tuna fish oil supplementation but no effect was observed on cryopreserved semen. It was also reported that supplementation of flax meal to the boars' diet improved the quality of fresh as well as cryopreserved boar semen40. In another study, supplementing bull diets with flaxseed oil significantly increased total motility, progressive motility and motion characteristics of cryopreserved sperm, however, no effect was observed at the fresh stage41. In stallion, dietary supplementation of linseed oil plus antioxidants improved cooled–stored stallion semen quality, however, it did not improve cryopreserved semen42. The difference with the other studies might be because of differences in climatic condition, intensity of environmental stress, breed (genetic selection for heat tolerant AI-boars), duration of feeding and source of omega-3 fatty acid4,11,43. Supplementation with dietary linseed oil during peri-puberty is known to stimulate steroidogenesis and testis development in rams44.
The improvement in semen quality parameters and fertility after the flaxseed oil supplementation may be because of the lowering of lipid peroxidation in spermatozoa40 as observed in present study also. Yan et al.45, observed a positive effect of supplementation with n-3/n-6 PUFAs with ratio of 1:1 in boars on testicular development and spermatogenesis. Polyunsaturated fatty acids affect the hypothalamic-pituitary–gonadal axis which in turn improves the endocrine profiles, testicular development and spermatogenesis. In rat, higher n-3 fatty acid in diet increased the concentrations of GnRH, FSH, LH and testosterone45 which in turn increased fertile sperm production. It was also postulated that n-3 fatty acids integrates into the spermatozoa membrane and alter the sperm membrane lipid profile and thereby improves sperm quality and fertility46. In agreement with our findings, Perumal et al.47 observed significant improvement in mithun bull’s semen under the similar climatic condition of high THI after flaxseed oil supplementation. It was suggested that dietary flaxseed oil (high in n-3 PUFA) supplementation possibly improved cholesterol to phospholipid ratio of sperm plasma membranes, altered the affinity and expression of gonadotropin receptors and upregulated the testosterone synthesis mechanism47. It can be suggested that PUFAs present in flaxseed oil are involved in the flagellar movement of sperm which in turn increases sperm motility and fertility47. Similar results were reported in other species like buffalo48,49 stallion42 ram44 and cattle50. In addition, feeding flaxseed oil might have improved the semen quality by increasing the antioxidant status of the boars’ semen and spermatozoa under the stressful condition of high THI.
The antioxidant defense of the boar semen and sperm in control group was in compromised state during high THI period. In case of treatment group, flaxseed oil supplementation to boar significantly alleviate oxidative stress during high ambient temperature humidity index period thereby improved the semen quality and fertility. Seminal plasma GPx, TAC and MDA were improved following flaxseed oil supplementation from first week onwards. However, spermatozoa MDA level decreased significantly in flaxseed group from 6th weeks onward only. The finding suggests that antioxidant milieu of seminal plasma improved at the earliest followed by that of spermatozoa post flaxseed oil supplementation. Mammalian sperm are prone to oxidative stress owing to their limited antioxidant capacity and this in turn adversely affects sperm survival, impair their functions such as motility, membrane integrity and fertilizing ability51. Among farm animals, boar sperm are more prone to oxidative stress, because of the high proportion of PUFAs in the membrane and lower cholesterol to phospholipid ratio, which can be easily oxidized leading to lipid peroxidation52. Oxidative damage in sperm occurs due to increase production of reactive oxygen substances (ROS) and/or a decreased natural antioxidant defense system more particularly in adverse climatic condition like high ambient temperature53. Spermatozoa have limited antioxidant defenses to protect itself from oxidative damage of ROS. Heat stress may induces DNA strand breaks, fragmentation of spermatozoa DNA and spermatozoa chromatin packaging defects, germ cell apoptosis in testis, incomplete epididymal spermatozoa maturation, and increased exposure to ROS54. The negative impact of heat stress on spermatozoa DNA integrity is coupled with its downstream effect on early embryo development13. However, seminal plasma protects the spermatozoa from oxidative damage and keeps ROS-levels within the physiological range, compatible with the functional life of the sperm55. In agreement with our findings, Perumal et al.47 reported reduced oxidative damage in mithun spermatozoa after flaxseed oil supplementation in subtropical climatic conditions. Dietary n-6:n-3 fatty acid in 6.6 ratio significantly improved antioxidant status in boar serum, sperm and seminal plasma25. Strzezek et al.3,5,56 reported similar results in boar seminal plasma. Diet enriched with transgenic flax improves the quality of fresh and conserved boar semen by lowering the lipid peroxidation in boar spermatozoa40. Flaxseed oil may have protected spermatozoa because of its antioxidant and/or free radical quenching properties. This property of flaxseed oil is because of its constituent bioactive ingredients such as omega-3 fatty acids and lignans57. Lignans has antioxidant property in quenching the free radical and it inhibits peroxyl-radical-mediated damage of DNA58. It was reported that the presence of polyphenols and vitamin E may also have contributed to its antioxidant property or it stimulates the activities of antioxidant enzymes47. However, further study is needed to better understand flaxseed oil role in improving the antioxidant defense mechanism of semen and spermatozoa.
Despite all of the above discussed finding, till now there is no single boar semen quality test which can be used as a prospective indicator of their in-vivo fertility59. Therefore, in-vivo fertility study was done in this experiment to validate the hypothesis that flaxseed oil improves boar sperm fertility. In-vivo fertility results revealed higher fertility of semen of boars supplemented with flaxseed oil under high THI. Farrowing rate improved significantly (increased by 17%) with semen of boars supplemented with flaxseed oil as compared to control group. This was an interesting finding as it recorded a greater quantitative difference between flaxseed supplemented and control group. Also, the more numbers of sows inseminated conclusively validate the positive effect of flaxseed oil supplementation on improved boar fertility during high environmental stress period. Similarly, litter size at birth and litter size at weaning was significantly higher in flaxseed group. The pre-weaning mortality was statistically similar in both groups. In our previous study, we reported improvement in in-vivo fertility but not in litter size at birth after flaxseed oil supplementation, however, that study included very small numbers of sows3,4. Increased in-vivo fertility following flaxseed oil supplementation could be due to improved sperm quality parameters, antioxidant status and fertilizing ability both at the fresh stage as well as after 72 h of storage. With increased in antioxidant capacity, spermatozoa from boars supplemented with flaxseed oil would be more adapted to survive in the oviductal environment until the moment of fertilisation. The number of total embryos that a sow can produce after an ovulation does not depend on the total number of ovulated oocytes, but rather on the number of oocytes that are optimally fecundated31. Therefore, sperm of flaxseed supplemented boars would have a greater fertilizing ability, thus increasing the number of fully functional embryos yielded. The positive correlation between sperm quality and field fertility has been reported previously60,61. Singh et al.3,4 reported significantly higher in-vivo fertility of sows breeding by AI using semen from flaxseed oil-fed boar. However, in another study, in-vivo fertility of boars did not improve after cod liver oil supplementation as a source of PUFAs62. Excessive reactive oxygen species production in boar semen more particularly during environmental stress compromise sperm membrane functions, effuses cholesterol from sperm membrane, damage DNA and mitochondrial membrane potential, thereby, resulting in poor fertility outcome31,63. Supplementation of flaxseed oil might have inhibited all these processes in spermatozoa and thereby improved in-vivo fertility. Although the findings of the study have implications for improving the pig production system in sub-tropical climate, however, it applicability in other climatic condition needs to be evaluated further. Besides, long term study are needed to assess the sustainability of the observed improvements and to evaluate any potential adverse effects associated with prolonged flaxseed supplementation.
In conclusion, the present study has demonstrated for the first time that dietary supplementation of flaxseed oil to boar improved the semen quality parameters and sperm kinematics in time dependent manner under high environmental stress condition. It also improved total antioxidant capacity and minimized lipid peroxidation in seminal plasma as well as in sperm. Besides, flaxseed supplementation significantly improved in in-vivo fertility (in terms of farrowing rate and litter size) of semen of boar fed flaxseed oil. Therefore, it can be concluded that flaxseed oil may be incorporated in boar’s diet before six weeks of adverse climate or season for improvement of semen quality and fertility in a sub-tropical climate. Nevertheless, further large studies in other breeds are warranted for delineation of the exact mechanism by which flaxseed oil supplementation improves sperm quality and fertility.
Methods
Location of study and climatic parameters
The study was approved by Institute Animal Ethics Committee of ICAR Research Complex for North Eastern Hill Region, Umiam, Meghalaya, India (2100/GO/RBi/L/20/CPCSEA dated 19.05.2020) and all methods were performed in accordance with the relevant national guidelines and regulations. The study was conducted in compliance with ARRIVE guidelines. No anaesthetic agent was used in the present study. After completion of experiment, animals were continously used in the farm for routine breeding programme. The experiment was conducted at Pig Research Farm of ICAR Research Complex for NEH Region, Nagaland Centre, Medziphema, Nagaland, India from 13th May to 22nd September. The research farm is located at latitude of 25°45' N, the longitude of 93°50' E and altitude of 281 m above mean sea level. The climate of the region is hot-humid sub-tropical with annual rainfall varies from 1500 to 2000 mm. Temperature and humidity data were collected by an automatic weather station of Gramin Krishi Mausam Seva of India Metrological Department (IMD) located at ICAR Research farm close to Pig Research Farm. The following equation was used to calculate the THI3,23 where T is the temperature in degrees Celsius and RH is relative humidity.
The weakly mean THI was calculated as the mean of the daily mean THI values. During the study period, the minimum and maximum temperature humidity index was 77.21 and 91.99, respectively (Fig. 1). The THI indicates that boars were under severe heat stress as reported earlier by Singh et al.3,5.
Fig. 1.
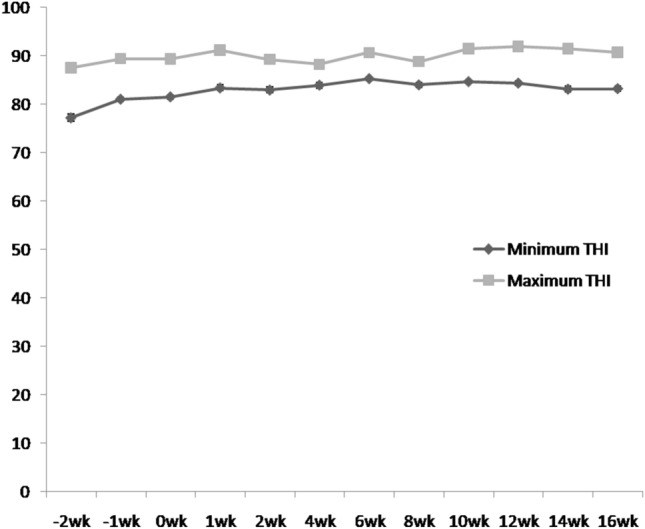
Maximum and minimum temperature humidity index (THI) during the study period (mean ± SEM).
Animals
Twelve Hampshire crossbreed (50% Hampshire and 50% Gunghroo) boars aged 18 to 24 months were included in this study. The average body weights of the animals were 150.16 ± 3.43 and 149.66 ± 3.67 kg in control and treatment group, respectively. Boars were housed in individual concrete pens (9 m2 area) with the provision of the open area (4 m2 area); however, no cooling facilities were present; therefore, temperatures were similar inside and outside of the pen. Experimental animals were maintained under uniform feeding, lighting, housing and other standard managemental practices as per the farm schedule.
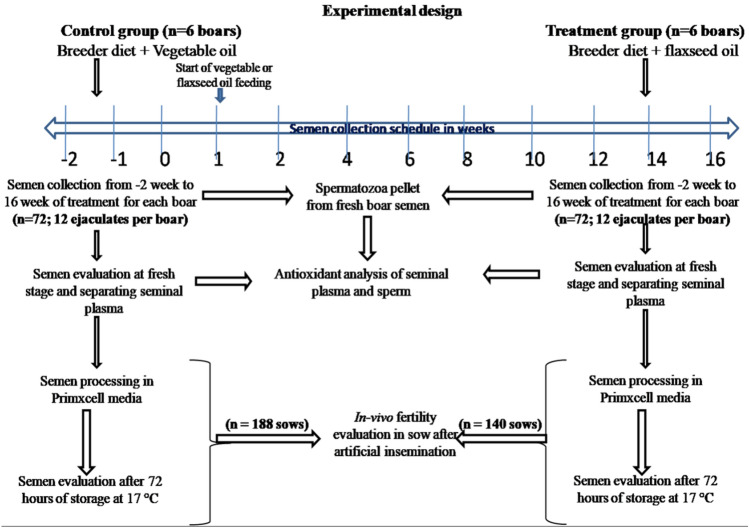
Experimental design
Boars were randomly divided into control (Group-I) and treatment (Group-II) with six boars in each group (Fig. 2). Animals were fed a corn and groundnut cake diet (Table 1) and the boars were restricted to 3.0 kg feed/day (3). The diet composition was based on the nutritional requirements according to NRC24. The boars were provided ad libitum access to drinking water. In control and treatment group, vegetable oil (canola) and flaxseed oil was top-dressed at the rate of 3.0 per cent (90 mL) in basal diets for each animal on daily basis3,4. Flaxseed or vegetable oil was given in morning and evening feeding (45 ml each time) for 16 weeks. Semen samples were collected from three weeks prior to start of treatment till 16th weeks of feeding. Semen ejaculates were collected weekly (from three weeks prior to start of treatment to two weeks post treatment) and bi-weekly from second weeks post treatment (Figs. 3, 4).
Fig. 2.
Experimental design of feeding of flaxseed oil to boar and semen quality, antioxidant and in-vivo fertility evaluation.
Semen collection and processing for evaluating the effect of flaxseed supplementation
Semen samples were collected by the gloved-hand technique. In total, 144 ejaculates (72 ejaculates in control and 72 ejaculates in treatment group) were collected for the study (12 ejaculates per boar). Ejaculates were collected into a pre-warmed (37 °C) thermous flask. Only sperm rich fractions of the ejaculates were collected. Ejaculates were transported to laboratory for further examination and processing within five minutes of collection. After examination at fresh stage, ejaculates were diluted in isothermal Primxcell (IMV, France) media. Dilution was done so that each 80 ml semen pouch (GTB Bag manual, IMV, France) contains 3 billion motile spermatozoa. Thereafter semen pouches were stored at 17 °C in BOD incubator for 72 h. After 72 h of storage, semen pouches were warmed at 35 °C for examination. To prevent human bias in the study, the individual who collected the boar semen was not informed of feeding practices and he was not involved in laboratory semen analysis (Fig. 5).
Evaluation of sexual behavior of boars
Reaction time was recorded in min as the time from entering collection room to the first attempt to mount the artificial sow by boar. False mounts were counted as mounting artificial sow but dismounting before semen collection by boar (Fig. 6).
Evaluation of flaxseed supplementation on semen quality parameters
Semen volume was measured using a graduated cylinder. Sperm concentration was determined using haemocytometer methods25. The semen sample was diluted with 1% PBS-buffered formalin. The diluted semen was placed on a hemacytometer with the sperm counted in five squares of one chamber. Sperm heads in five squares (each square contained sixteen smaller squares) were counted in each chamber, and the counts on both sides were averaged. Semen analyses for SQPs (liveability, abnormality, acrosomal integrity, and hypo-osmotic swelling test (HOST)) were done at fresh stage and after 72 h of storage at 17 °C. The percent live spermatozoa were determined by adopting differential staining technique using Eosin-Nigrosin stain26. Spermatozoa were examined for the following abnormal morphologies: abnormal heads, abnormal tails, abnormal midpieces, detached heads, coiled tails, presence of cytoplasmic droplets27. The acrosome integrity of spermatozoa was assessed using Giemsa stain28. At least 200 spermatozoa were counted and acrosomes were considered to be intact if the entire acrosomal cap was present as viewed under oil immersion microscopy at 1000 X magnification. Spermatozoa plasma membrane integrity was evaluated by hypo-osmotic swelling test (HOST) as per29. Semen sample (100 µl) was incubated with 900 µl hypo-osmotic solution (7.35 g sodium citrate, 13.5 g fructose, in 1 L distilled water) at 37 °C for 45 min. After 45 min of incubation, eosin was added to the mixture and the mixture was spread on a warm glass slide. Dried slides were examined using light microscopy under 400 X magnification for the curled and swollen tails of 200 sperm cells in five nonconsecutive microscopic fields. Bent tails indicated that sperm cells had an intact plasma membrane.
Computer assisted semen analysis
Computer assisted semen analysis was done at fresh stage and after 72 h of storage at 17 °C as per the method described by Perumal et al.30 and Singh et al.4. Sperm kinematic parameters viz. total motility, progressive motility, average path velocity (VAP), straight line velocity (VSL), curve linear velocity (VCL), amplitude of lateral head displacement (ALH), beat cross frequency (BCF), straightness (STR) and linearity (LIN), were evaluated by Hamilton Thorne Sperm Analyser (HTM-IVOS, version IVOS 11, Hamilton Thorne Research, USA). Semen was diluted in Primxcell extender and 4 µL of this extended semen sample was placed into a pre-warmed (37 °C) chamber of disposable Leja slide (IMV, France) and was permitted to settle on the heating plate (38 °C) just prior to analysis. Five microscopic fields were analyzed for each ejaculate, in duplicates and results were presented based on the analysis of 250–400 total cells per sample.
Antioxidant analysis in seminal plasma and spermatozoa
To see the effect of flaxseed oil feeding on the antioxidant status of animals, antioxidants were estimated in seminal plasma (GPx, TAC and MDA) and spermatozoa (MDA). After collection, the semen sample of each boar was immediately centrifuged at 1000 X g/min for 15 min. Seminal plasma was stored in 2.0 mL Eppendorf (EP) tubes at -20 °C for further analysis. The sperm pellets obtained after centrifugation were re-suspended in 0.85% NaCl and washed twice by centrifugation. Aliquots of spermatozoa samples containing 3 × 108 sperm were used for the analyses of malondialdehyde (MDA). Absorbances of samples were measured by Thermo Scientific Multiskan GO Microplate Spectrophotometer, USA.
Glutathione Peroxidase (GPx) assay
Glutathione peroxidase was estimated by Cayman's Glutathione Peroxidase assay kit (703,102, Cayman Chemical Co., USA) as per the manufacturer's guidelines. This assay measures GPx activity indirectly by a coupled reaction with glutathione reductase. Oxidized glutathione, produced upon reduction of hydroperoxide by GPx, is recycled to its reduced state by glutathione reductase and NADPH. The oxidation of NADPH to NADP + is accompanied by a decrease in absorbance at 340 nm. The rate of decrease in the absorbance at 340 nm is directly proportional to the GPx activity in the sample. The absorbance was measured at 340 nm to estimate the GPx in the sample. GPx activity was expressed in nmol/min/mL. The intra and inter-assay coefficient of variation were 5.7% (n = 77) and 7.2% (n = 77), respectively.
Total antioxidant capacity (TAC) assay
Total antioxidant capacity (TAC) level (mM) was estimated using Caymen’s Antioxidant Assay Kit (Catalogue: 709,001, Cayman Chemical Co., USA) following the manufacture’s protocol. A Trolox standard curve was used to quantitate the antioxidant capacity of the sample, measured in millimolar Trolox equivalents. Absorbance was read at 750 nm. TAC concentration was expressed in mmol/L. The intra and inter-assay coefficient of variation were 3.4% (n = 84) and 3% (n = 20), respectively.
Malondialdehyde (MDA) assay
The concentration of malondialdehyde (MDA) was determined using Caymen’s thiobarbituric acid reactive substances (TBARS) assay kit (Catalogue: 10,009,055, Cayman Chemical Co., USA). Briefly, 100 µL of reconstituted MDA standard and 100 µL of samples were added in the respective five mL vial. SDS solution (100 µL) was added to each vial and swirled to mix. After that, four mL colour reagent was added forcefully and then vials were placed in boiling water for one hour. After boiling, vials were immediately placed in an ice bath for 10 min to stop the reaction. The vials were centrifuged for 10 min at 1600 X g at 4 °C and 150 µL supernatant (in duplicate) from each vial was transferred to the colorimetric plate. The absorbance was read at 540 nm. The seminal plasma MDA concentration was expressed in nmol/mL.The intra and inter-assay coefficient of variation were 5.5% (n = 10) and 5.9% (n = 8), respectively.
Malondialdehyde (MDA) assay in spermatozoa
The concentration of malondialdehyde (MDA) was determined using Caymen’s thiobarbituric acid reactive substances (TBARS) assay kit (Catalogue: 10,009,055, Cayman Chemical Co., USA) as briefed in 2.7.3. The spermatozoa MDA content was expressed as nmol/108 sperm cells.
In-vivo fertility of semen
In-vivo fertility of semen was evaluated by artificially inseminating pluriparous sow (198 with the semen of control group and 140 with the semen of treatment group on detection of oestrus as per the standard practice. To prevent human bias in the fertility outcome, the individual who did the AI in sows was not informed of feeding protocols. For in-vivo fertility, semen of control and treatment group was used from 6th weeks after start of treatment. All AIs were done within 72 h of semen collection. Farrowing rate was recorded as numbers of sows farrowed as compared to numbers of sows inseminated. Litter size at birth was recorded as actual numbers of live piglets farrowed per pig. Litter size at weaning was recorded as the number of piglets weaned at 42 days post farrowing per pig. Pre-weaning mortality was calculated as numbers of piglets weaned at 42 days compared to numbers of live piglets born. Piglets were given iron injection on 4th and 14th day of life as per standard management practices at farm.
Statistical analyses
Statistical analysis was performed using IBM Statistical Package for the Social Sciences (SPSS) v26 (SPSS Inc.; Chicago, Illinois, USA). The dataset was checked for normality and homogeneity of variances throughy Shapiro–Wilk and Levene tests, respectively. The data were analyzed using independent student t test. Farrowing rate was compared between the two groups by Chi-Square test. Square root transformation was applied to litter size and then compared by ANOVA. Results are presented as mean ± SEM with each ejaculate was considered as an independent observation, and differences were considered significant at P < 0.05.
Acknowledgements
The authors are thankful for the financial assistance received under the project “Effect of feeding linseed oil on fertility of pig and poultry under sub-tropical condition of Nagaland and Umiam” (IXX14140) of ICAR Research Complex for NEH Region, Umiam, Meghalaya, India and ICAR-All India Coordinated Research Project-Pig, New Delhi.
Author contributions
M.S. conceptualized and designed the experiment, conducted the laboratory assessments, analysed the data and contributed to write the manuscript. R.T.M. conducted the laboratory and field assessment, performed AI trials. D.K. designed the nutritional aspect of experiment and contributed to write the manuscript. R.K., J.K.C, S.K., J.K.C and S.D. analysed the data and contributed to write the manuscript. H.K. and V.K.M. designed the experiment and contributed to write the manuscript. All authors gave their final approval of the manuscript.
Data availability
All data generated or analysed during this study are included in this published article. For more information, queries may be directed to corresponding author.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Lugar, D. W. Heat stress and in utero heat stress: effects on boar reproduction and the efficacy of nutritional mitigation. Open Access Dissertations. 2009. https://docs.lib.purdue.edu/open_access_dissertations/2009. (2018)
- 2.Vermeer, H. M. & Aarnink, A. J. A. Review on heat stress in pigs on farm. Wageningen Livestock Research, The Netherlands January 2023. 10.5281/zenodo.7620726 (2023).
- 3.Singh, M. et al. Dietary flaxseed oil improve boar semen quality, antioxidant status and in-vivo fertility in humid sub-tropical region of North East India. Theriogenology159, 123–131. 10.1016/j.theriogenology.2020.10.023 (2021). [DOI] [PubMed] [Google Scholar]
- 4.Singh, M. et al. Linseed oil in boar's diet improved in vivo fertility and antioxidant status. Reprod. Dom. Anim. 1–12. (2022). [DOI] [PubMed]
- 5.Singh, M. et al. Linseed oil in boar’s diet during high temperature humidity index (THI) period improves sperm quality characteristics, antioxidant status and fatty acid composition of sperm under hot humid sub-tropical climate. Theriogenology189, 127–136. 10.1016/j.theriogenology.2022.06.012 (2022). [DOI] [PubMed] [Google Scholar]
- 6.Auvigne, V. et al. Seasonal infertility in sows: A five year field study to analyze the relative roles of heat stress and photoperiod. Theriogenology74(1), 60–66. 10.1016/J.THERIOGENOLOGY.2009.12.019 (2010). [DOI] [PubMed] [Google Scholar]
- 7.Peña, S. T. et al. Tropical summer induces DNA fragmentation in boar spermatozoa: implications for evaluating seasonal infertility. Reprod. Fertil. Dev.31, 590–601. 10.1071/RD18159 (2019). [DOI] [PubMed] [Google Scholar]
- 8.Knox, R. ‘The Anatomy and Physiology of Sperm Production in Boars’. (Department of Animal Sciences, University of Illinois: Urbana, IL.) Available at http://www.ansci.wisc.edu/jjp1/pig_case/html/library/boara&p.pdf [accessed on 01 September 2023] (2003).
- 9.Einarsson, S., Brandt, Y., Lundeheim, N. & Madej, A. Stress and its influence on reproduction in pigs: A review. Acta Vet. Scand.50, 48 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stone, B. A. Heat induced infertility of boars: The inter-relationship between depressed sperm output and fertility and an estimation of the critical air temperature above which sperm output is impaired. Anim. Reprod. Sci.4, 283–299 (1982). [Google Scholar]
- 11.Argenti, L. E. et al. Effects of season on boar semen parameters and antioxidant enzymes in the south subtropical region in Brazil. Andrologia10.1111/and.12951 (2018). [DOI] [PubMed] [Google Scholar]
- 12.Barranco, I. et al. Season of ejaculate collection influences the freezability of boar spermatozoa. Cryobiology67, 299–304 (2013). [DOI] [PubMed] [Google Scholar]
- 13.Peña, S. J. T., Gummow, B., Parker, A. J. & Paris, D. B. B. P. Revisiting summer infertility in the pig: Could heat stress-induced sperm DNA damage negatively affect early embryo development?. Anim. Prod. Sci.57, 1975–1983 (2017). [Google Scholar]
- 14.Didion, B. A., Kasperson, K. M., Wixon, R. L. & Evenson, D. P. Boar fertility and sperm chromatin structure status: A retrospective report. J. Androl.30, 655–660 (2009). [DOI] [PubMed] [Google Scholar]
- 15.Strzeżek, J. et al. Effect of depletion tests (DT) on the composition of boar semen. Theriogenology54, 949–963. 10.1016/S0093-691X(00)00404-0 (2000). [DOI] [PubMed] [Google Scholar]
- 16.Aitken, R. J. & Drevet, J. R. The importance of oxidative stress in determining the functionality of mammalian spermatozoa: A two-edged sword. Antioxidants10.3390/antiox9020111 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ciereszko, A., Ottobre, J. S. & Glogowski, J. Effects of season and breed on sperm acrosin activity and semen quality of boars. Animal Reprod. Sci.64, 89–96 (2000). [DOI] [PubMed] [Google Scholar]
- 18.Murase, T., Imaeda, N., Yamada, H. & Miyazawa, K. Seasonal changes in semen characteristics, composition of seminal plasma and frequency of acrosome reaction induced by calcium and calcium ionophore A23187 in Large White boars. J. Reprod. Dev.53, 853–865 (2007). [DOI] [PubMed] [Google Scholar]
- 19.Singh, M. et al. Effect of artificial insemination in comparison to natural mating on the reproductive performance and profitability of smallholder pig production system in Indian Himalaya. Front. Sustain. Food Syst.10.3389/fsufs.2022.1067878 (2022).35586613 [Google Scholar]
- 20.Hidalgo, D. M., García, B. M., Marín, L. J. G., Bragadoa, M. J. & Fernández, L. G. Boar spermatozoa proteomic profile varies in sperm collected during the summer and winter. Anim. Reprod. Sci.10.1016/j.anireprosci.2020.106513R (2020). [DOI] [PubMed] [Google Scholar]
- 21.Fraser, L., Strzeżek, J., Filipowicz, K., Mogielnicka-Brzozowska, M. & Zasiadczyk, L. Age and seasonal-dependent variations in the biochemical composition of boar semen. Theriogenology10.1016/j.theriogenology.2016.02.035 (2016). [DOI] [PubMed] [Google Scholar]
- 22.Reese, D. E. Omega-3 fatty acids and swine reproduction–A review. Nebraska Swine Report 30–33 (2003).
- 23.Luceño, N. L. et al. Exposing dairy bulls to high temperature-humidity index during spermatogenesis compromises subsequent embryo development in vitro. Theriogenology141, 16–25 (2020). [DOI] [PubMed] [Google Scholar]
- 24.NRC. Nutrient Requirements of Swine (11th rev. ed.). National Academy Press (2012).
- 25.Liu, Q. et al. Effects of dietary n-6: n-3 fatty acid ratio and vitamin E on semen quality, fatty acid composition and antioxidant status in boars. Anim. Reprod. Sci.162, 11–19 (2015). [DOI] [PubMed] [Google Scholar]
- 26.Lasley, J. F. & Bogart, R. A comparative study of epididymal and ejaculated spermatozoa of boar. J. Anim. Sci.3, 360–370 (1942). [Google Scholar]
- 27.Shipley, C. F. Breeding soundness examination of the boar. Swine Health Prod.7, 117–120 (1999). [Google Scholar]
- 28.Watson, P. F. Use of Giemsa stain to detect changes in the acrosome of frozen ram spermatozoa. Vet. Rec.97, 12–15 (1975). [DOI] [PubMed] [Google Scholar]
- 29.Jeyendran, R. S., Vander Ven, H. H., Parez-Pelaez, M., Crabo, B. G. & Zaneweld, L. J. D. Development of an assay to assess the functional integrity of the human membrane and its relationship to other semen characteristics. J. Reprod. Fertil.70, 219–228 (1984). [DOI] [PubMed] [Google Scholar]
- 30.Perumal, P., Chang, S., Baruah, K. K. & Srivastava, N. Administration of slow release exogenous melatonin modulates oxidative stress profiles and in vitro fertilizing ability of the cryopreserved mithun (Bos frontalis) spermatozoa. Theriogenology120, 79–90 (2018). [DOI] [PubMed] [Google Scholar]
- 31.Yeste, M. et al. Specific LED-based red light photo-stimulation procedures improve overall sperm function and reproductive performance of boar ejaculates. Sci. Rep.6, 22569. 10.1038/srep22569 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zasiadcyzk, L., Fraser, L., Kordan, W. & Wasilewska, K. Individual and seasonal variations in the quality of fractionated boar ejaculates. Theriogenology83, 1287–1303 (2015). [DOI] [PubMed] [Google Scholar]
- 33.Iida, R. & Koketsu, Y. Interactions between climatic and production factors on returns of female pigs to service during summer in Japanese commercial breeding herds. Theriogenology80, 487–493 (2013). [DOI] [PubMed] [Google Scholar]
- 34.Luceño, N. L. et al. High temperature-humidity index compromises sperm quality and fertility of Holstein bulls in temperate climates. J. Dairy Sci.10.3168/jds.2019-18089 (2020). [DOI] [PubMed] [Google Scholar]
- 35.Vargas, J. C., Kerns, K. & Rothschild, M. F. Lunar and climatic effects on boar ejaculate traits. Anim. Reprod. Sci.193, 117–125 (2018). [DOI] [PubMed] [Google Scholar]
- 36.Flowers, W. L. Factors affecting the efficient production of boar sperm. Reprod. Domest. Anim.50, 25–30 (2015). [DOI] [PubMed] [Google Scholar]
- 37.Estienne, M. J., Harper, A. F. & Crawford, R. J. Dietary supplementation with a source of omega-3 fatty acids increases sperm number and the duration of ejaculation in boars. Theriogenology70, 70–76 (2008). [DOI] [PubMed] [Google Scholar]
- 38.Maldjian, A. et al. Changes in sperm quality and lipid composition during cryopreservation of boar semen. Theriogenology63, 411–421 (2005). [DOI] [PubMed] [Google Scholar]
- 39.Castellano, C. A., Audet, I., Bailey, J. L., Laforest, J. P. & Matte, J. J. Dietary omega-3 fatty acids (fish oils) have limited effects on boar semen stored at 17 °C or cryopreserved. Theriogenology74, 1482–1490 (2010). [DOI] [PubMed] [Google Scholar]
- 40.Bielas, W. et al. Diet enriched with new varieties of transgenic flax improves quality of fresh and conserved boar semen. Reprod. Biol.13S, 22–64 (2013). [Google Scholar]
- 41.Khoshniat, M. T. et al. Dietary omega-3 fatty acids from linseed oil improve quality of post-thaw but not fresh sperm in Holstein bulls. Cryobiology93, 102–108 (2020). [DOI] [PubMed] [Google Scholar]
- 42.Lausigk, Y. S. & Aurich, C. Influences of a diet supplemented with linseed oil and antioxidants on quality of equine semen after cooling and cryopreservation during winter. Theriogenology81, 966–973 (2014). [DOI] [PubMed] [Google Scholar]
- 43.Yeste, M., Barrerab, X., Coll, D. & Bonet, S. The effects on boar sperm quality of dietary supplementation with omega-3 polyunsaturated fatty acids differ among porcine breeds. Theriogenology76, 184–196 (2011). [DOI] [PubMed] [Google Scholar]
- 44.Li, W. et al. Supplementation with dietary linseed oil during peri-puberty stimulates steroidogenesis and testis development in rams. Theriogenology102, 10–15 (2017). [DOI] [PubMed] [Google Scholar]
- 45.Yan, L. et al. Effect of different dietary omega-3/ omega-6 fatty acid ratios on reproduction in male rats. Lipids Health Dis.12, 33 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mitre, R., Cheminade, C., Allaume, P., Legrand, P. & Legrand, A. B. Oral intake of shark liver oil modifies lipid composition and improves motility and velocity of boar spermatozoa. Theriogenology62, 1557–1566 (2004). [DOI] [PubMed] [Google Scholar]
- 47.Perumal, P., Chang, S., Khate, K., Vupru, K. & Bag, S. Flaxseed oil modulates semen production and its quality profiles, freezability, testicular biometrics and endocrinological profiles in mithun. Theriogenology136, 47–59 (2019). [DOI] [PubMed] [Google Scholar]
- 48.Shah, S. M. H., Ali, S., Zubair, M., Jamil, H. & Ahmad, N. Effect of supplementation of feed with Flaxseed (Linum usitatissimum) oil on libido and semen quality of Nilli-Ravi buffalo bulls. J. Anim. Sci. Tech.58, 25 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tran, L. V. et al. Effect of omega-3 and omega-6 polyunsaturated fatty acid enriched diet on plasma IGF-1 and testosterone concentration, puberty and semen quality in male buffalo. Anim. Reprod. Sci.173, 63–72 (2016). [DOI] [PubMed] [Google Scholar]
- 50.Khan, H. et al. Dietary Flaxseed supplementation effect on bovine semen quality parameters. Veterinaria3, 9–13 (2015). [Google Scholar]
- 51.Aitken, R. J., Smith, T. B., Jobling, M. S., Baker, M. A. & De Iuliis, G. N. Oxidative stress and male reproductive health. Asian J. Androl.16, 31–38 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Brouwers, J. F., Silva, P. F. & Gadella, B. M. New assays for detection and localization of endogenous lipid peroxidation products in living boar sperm after BTS dilution or after freeze-thawing. Theriogenology63, 458–469 (2005). [DOI] [PubMed] [Google Scholar]
- 53.Guthrie, H. D. & Welch, G. R. Effects of reactive oxygen species on sperm function. Theriogenology78, 1700–170856 (2012). [DOI] [PubMed] [Google Scholar]
- 54.Aitken, R. J., Bronson, R., Smith, T. B. & De Iuliis, G. N. The source and significance of DNA damage in human spermatozoa; a commentary on diagnostic strategies and straw man fallacies. Mol. Hum. Reprod.19, 475–485 (2013). [DOI] [PubMed] [Google Scholar]
- 55.Barranco, I. et al. High total antioxidant capacity of the porcine seminal plasma (SP-TAC) relates to sperm survival and fertility. Sci. Rep.10.1038/srep18538 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Strzezek, J., Fraser, L., Kuklinska, M., Dziekonska, A. & Lecewicz, M. Effects of dietary supplementation with polyunsaturated fatty acids and antioxidants on biochemical characteristics of boar semen. Reprod. Biol.4, 271–287 (2004). [PubMed] [Google Scholar]
- 57.Burdge, G. C. & Calder, P. C. Conversion of alpha-linolenic acid to longer-chain polyunsaturated fatty acids in human adults. Reprod. Nutr. Dev.45, 581–589 (2005). [DOI] [PubMed] [Google Scholar]
- 58.Hu, C., Yuan, Y. V. & Kitts, D. D. Antioxidant activities of the flaxseed lignan secoisolariciresinol diglucoside, its aglycone secoisolariciresinol and the mammalian lignans enterodiol and enterolactone in-vitro. Food. Chem. Toxicol.45(11), 2219–2227 (2007). [DOI] [PubMed] [Google Scholar]
- 59.Popwell, J. M. & Flowers, W. L. Variability in relationships between semen quality and estimates of in vivo and in vitro fertility in boars. Anim. Reprod. Sci.81, 97–113 (2004). [DOI] [PubMed] [Google Scholar]
- 60.Waberski, D., Petrunkina, A. & Töpfer-Petersen, E. Can external quality control improve pig AI efficiency?. Theriogenology70, 1346–1351 (2008). [DOI] [PubMed] [Google Scholar]
- 61.Tsakmakidis, I. A., Lymberopoulos, A. G. & Khalifa, T. A. A. Relationship between sperm quality traits and field-fertility of porcine semen. J. Vet. Sci.11, 151–154 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Paulenz, H., Taugbol, O., Hofmo, P. O. & Saarem, K. A preliminary study on the effect of dietary supplementation with cod liver oil on the polyunsaturated fatty acid composition of boar semen. Vet. Res. Commun.19, 273–284 (1995). [DOI] [PubMed] [Google Scholar]
- 63.Awda, B. J., Mackenzie-Bell, M. & Buhr, M. M. Reactive oxygen species and boar sperm function. Biol. Reprod.81, 553–561 (2009). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analysed during this study are included in this published article. For more information, queries may be directed to corresponding author.