Abstract
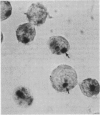
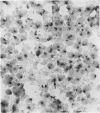
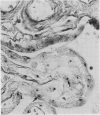
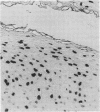
A sensitive in situ hybridisation technique, using a biotin-streptavidin-polyalkaline phosphatase complex detection system, was successfully applied to smears of fresh cultured cells, frozen sections, and formalin fixed paraffin processed tissue: the procedure was successful for DNA-DNA hybridizations using a variety of DNA probes. The detection method is rapid, reliable, and economical producing a purplish-blue precipitate at the site of hybridisation and clearly visible by low power light microscopy.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bentley D. L., Rabbitts T. H. Human V kappa immunoglobulin gene number: implications for the origin of antibody diversity. Cell. 1981 Jun;24(3):613–623. doi: 10.1016/0092-8674(81)90088-x. [DOI] [PubMed] [Google Scholar]
- Brigati D. J., Myerson D., Leary J. J., Spalholz B., Travis S. Z., Fong C. K., Hsiung G. D., Ward D. C. Detection of viral genomes in cultured cells and paraffin-embedded tissue sections using biotin-labeled hybridization probes. Virology. 1983 Apr 15;126(1):32–50. doi: 10.1016/0042-6822(83)90460-9. [DOI] [PubMed] [Google Scholar]
- Burns J., Chan V. T., Jonasson J. A., Fleming K. A., Taylor S., McGee J. O. Sensitive system for visualising biotinylated DNA probes hybridised in situ: rapid sex determination of intact cells. J Clin Pathol. 1985 Oct;38(10):1085–1092. doi: 10.1136/jcp.38.10.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke H. J., Schmidtke J., Gosden J. R. Characterisation of a human Y chromosome repeated sequence and related sequences in higher primates. Chromosoma. 1982;87(5):491–502. doi: 10.1007/BF00333470. [DOI] [PubMed] [Google Scholar]
- Dürst M., Gissmann L., Ikenberg H., zur Hausen H. A papillomavirus DNA from a cervical carcinoma and its prevalence in cancer biopsy samples from different geographic regions. Proc Natl Acad Sci U S A. 1983 Jun;80(12):3812–3815. doi: 10.1073/pnas.80.12.3812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gissmann L., Diehl V., Schultz-Coulon H. J., zur Hausen H. Molecular cloning and characterization of human papilloma virus DNA derived from a laryngeal papilloma. J Virol. 1982 Oct;44(1):393–400. doi: 10.1128/jvi.44.1.393-400.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta J., Gendelman H. E., Naghashfar Z., Gupta P., Rosenshein N., Sawada E., Woodruff J. D., Shah K. Specific identification of human papillomavirus type in cervical smears and paraffin sections by in situ hybridization with radioactive probes: a preliminary communication. Int J Gynecol Pathol. 1985;4(3):211–218. doi: 10.1097/00004347-198509000-00005. [DOI] [PubMed] [Google Scholar]
- Rathjen F. G., Schachner M. Immunocytological and biochemical characterization of a new neuronal cell surface component (L1 antigen) which is involved in cell adhesion. EMBO J. 1984 Jan;3(1):1–10. doi: 10.1002/j.1460-2075.1984.tb01753.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unger E. R., Budgeon L. R., Myerson D., Brigati D. J. Viral diagnosis by in situ hybridization. Description of a rapid simplified colorimetric method. Am J Surg Pathol. 1986 Jan;10(1):1–8. doi: 10.1097/00000478-198601000-00001. [DOI] [PubMed] [Google Scholar]
- de Villiers E. M., Gissmann L., zur Hausen H. Molecular cloning of viral DNA from human genital warts. J Virol. 1981 Dec;40(3):932–935. doi: 10.1128/jvi.40.3.932-935.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]