Abstract
The systemic coordination of accumulation of plasma membrane aquaporins (PIP) was investigated in this study in relation to mycorrhized maize response to a rapid development of severe drought followed by rewatering. In non-mycorrhizal roots, drought led to a drop in PIP abundance, followed by a transient increase under rewatering, whereas leaves showed an opposite pattern. In contrast, mycorrhiza contributed to maintenance of high and stable levels of PIPs in both plant organs after an initial increase, prolonged over the irrigation period. Isoelectric focusing electrophoresis resolved up to 13 aquaporin complexes with highly reproducible pl positions across leaf and root samples, symbiotic and non-symbiotic, stressed or not. Mass spectrometry recognized in leaves and roots a different ratio of PIP1 and PIP2 subunits within 2D spots that accumulated the most. Regardless of symbiotic status, drought regulation of aquaporins in roots was manifested as the prevalence of complexes that comprise almost exclusively PIP2 monomers. In contrast, the leaf response involved enrichment in PIP1s. PIP1s are thought to enhance water transport, facilitate CO2 diffusion but also affect stomatal movements. These features, together with elevated aquaporin levels, might explain a stress tolerance mechanism observed in mycorrhizal plants, resulting in faster recovery of stomatal water conductance and CO2 assimilation rate after drought.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-72828-8.
Keywords: Arbuscular mycorrhiza, Drought tolerance, Heteromerization, Plasma membrane aquaporins, Protein abundance, Proteomics, Rhizophagus irregularis, Zea mays
Subject terms: Proteins, Plant physiology, Arbuscular mycorrhiza
Introduction
Arbuscular mycorrhizal (AM) symbiosis occurs between soil fungi—representing the division Glomeromycota—and a large majority of vascular land plants. The host plant improves water and nutrient uptake thanks to an extensive network of mycelium that both penetrates the root cortex cell layer and can reach soil zones inaccessible to the roots1,2.
AM symbiosis improves host performance under a variety of abiotic stresses, such as drought or salinity3–6. An often suggested explanation for this effect is improved plant nutrition; however, mycorrhizal fungi can modify host water relations in a way entirely unrelated to increased acquisition of mineral compounds7. Published meta-analysis data provide evidence that extraradical water uptake is an equally important fungal benefit that can influence plant fitness8. Therefore, it is postulated to untangle experimentally these two alternative components, to identify more directly the factors that promote the hydraulic conductance of roots, leaf gas exchange parameters, and thus maintenance of the root-leaf water potential gradient. In addition to the effect on plant stress-related genes, AM symbiosis modifies the expression of aquaporins7,9–11, which seems to be under the control of complex hormonal signalization12–14.
Plasma membrane aquaporins (PIP proteins) are tetrameric water channels that maintain transmembrane water permeability, providing an alternative to apoplastic lateral water distribution15,16. Transport of xylem water is separated from the apoplastic radial pathway by the hydrophobic barrier of the Casparian strips in roots and the tightly packed ring of bundle sheath cells that envelop the vascular system in leaves15,17. Cell-to-cell water flow through aquaporins and plasmodesmata is one of the postulated mechanisms that allows water to pass these ‘control zones’. Therefore, one of the proposed hypotheses explaining the limitations of long-distance water transport and transpiration involves controlling radial water flow by regulation of aquaporin expression and activity17–20.
On this account, when discussing issues related to water availability in mycorrhizal interactions, the level of functionality of plant (and fungal) aquaporins becomes an issue of central importance10,21–24. It seems that the synergistic interaction of fungal and plant aquaporins at the interface between the cell membrane and arbuscular structures is the most effective model of regulation, thus contributing to symbiotic drought tolerance9,25.
The activity of plant aquaporins may be substantially influenced by the proportion of isoforms that make up their tetrameric structure. Oligomerization occurs through the interaction of α-helices of neighbouring monomers and an extracellular A loop, which contributes to the stabilization of the tetramer26–28.
Plant PIP proteins can be divided into two large groups, PIP1 and PIP2, based on their sequence and water channel activity. PIP2 homotetramers show high water channel activity when expressed in Xenopus oocytes or in yeast. PIP1 homotetramers produced in such systems are usually inactive or have very low activity; however, this effect may be eliminated by coexpression with PIP2 homologues29,30. This confirmed the hypothesis that heterotetramer formation may play an important role in the correct location of water channels on the surface of plant cells31. This rule also applies to maize (Zea mays), where an increase in permeability coefficient has been shown to occur if ZmPIP1;2 is coexpressed with different ZmPIP2 isoforms30.
Interestingly, coexpression of PIP1s and PIP2s results in increased membrane permeability, greater than when only PIP2-PIP2 homocomplexes are formed30,32. The tissue colocalization of the PIP1-PIP2 transcripts and their similar abundance pattern in response to different stress conditions support the hypothesis that the interaction between these proteins is a key mechanism enabling plants to maintain the water status. The generation of the associations of PIP1s and PIP2s must be regulated at the transcriptional and post-translational levels. However, transcriptional variation affects the abundance of proteins, which consequently determines the ratio of PIP1 to PIP2 present in the cell membrane, thus dictating the functionality of PIP tetramers32.
Published data on the expression of plant PIP proteins show that the PIP1 and PIP2 isoforms are always present together in plasma membranes. Protein immunoprecipitation experiments have provided evidence for the physical interactions of ZmPIP1;2 and ZmPIP2;1 also in maize roots and cell suspensions31. Differences in the expression level of each member of the subfamily determine the appearance of functional PIP1-PIP2 or PIP2-PIP2 tetramers32–35. However, the stoichiometry within heterocomplexes consisting of proteins of each group may differ significantly in tissues from distant organs, such as roots and leaves. Therefore, studies are needed to reveal aquaporin interactions in plant cells and to determine the physiological relevance of these processes, also in the context of mycorrhizal cooperation.
In our previous study36, mycorrhizal (AM) plants showed a much faster reversal of drought-induced leaf senescence, stomatal water conductance, and CO2 fixation rate than their non-mycorrhizal (NM) counterparts. In the present study we test the hypothesis that rapid recovery of AM maize from deep water deficits is linked to symbiotic regulation of maize aquaporins. In the following, we argue that the ratio of PIP1 and PIP2 monomers that form aquaporin heterocomplexes, corresponds to the patterns of their tissue-specific accumulation under given hydration conditions and their abundance can be altered by the presence of mycorrhiza.
Materials and methods
Conditions of plant culture
Plant cultures (Zea mays L., hybrid Opoka, Plant Breeding Smolice Ltd., Poland) and seedling inoculation with Rhizophagus irregularis spores, obtained from monoxenic root cultures37 were carried out in a high fertilized soil-free semi hydroponic system according to the procedure described previously36 until silking (63 BBCH stage, 12 weeks after sowing). The plant culture was carried out in such a way to ensure that long-term mycorrhizae vitality remained undisturbed and that symbiotic plants did not differ from non-mycorrhized counterparts in terms of shoot size and plant nutritional status. At silking time-point the plants were subjected to fast (as compared to our previous research36) and reversible water-stress procedure described below. All research under this study was conducted according to Polish national law and did not require any additional permits.
We designed a rapid development of water stress to minimize leaf senescence progression related to drought-altered nutrients availability. Well-watered plants were removed from pots and transferred, leaving the root system exposed, to the cabinet with low air humidity (30%). The mixture of coconut fiber and sand in which the roots remained immersed helped to obtain severe but reversible drought effects in as little as 7 days. Soil drought was imposed by stopping irrigation for 7 days to achieve a severe drop in plant water potential ( leaves < -1.5 MPa), followed by renewed fertilizer irrigation for 5 days until complete rehydration of plant tissues.
Before stress imposition, both symbiotic variants did not differ significantly in nitrogen and phosphorus content within each leaf shoot position investigated. This was due to the high-fertilized cultivation system used, discussed in our previous work36, in which the symbiotic state does not affect the growth and the nutritional status of the plants (Supplementary Fig. 1). Using this cultivation setup, the level of mycorrhizal colonization was achieved in the present study at the similar level as previously, reaching stable, more than 55%, arbuscular abundance in colonized parts of root fragments at the time of experiments (12 weeks after sowing, not shown).
Measurements of plant-physiological parameters
The midday leaf and root water potential, the light-saturated leaf gas exchange capacity (stomatal water conductance; gs, transpiration rate, Tmax and photosynthetic rate; Amax), chlorophyll fluorescence kinetics (fluorescence decrease ratio, Rfd and maximum PSII quantum yield in a dark-adapted state, Fv/Fm) and leaf nitrogen management index (NBI) were measured as described in36. The evaluation of leaf physiology was carried out in middle leaves (the ear leaf and the leaf above the cob).
Isolation of the cell microsomal fraction
The samples were taken from combined middle leaves (ear leaf and leaf above), or the mix of secondary feeder roots (diameter less than 1.5 mm), collected from four plants removed on consecutive days of water treatment. A modification of the methods of Abas and Luschnig38 and Santoni39 was developed: 5 g of plant tissue was homogenized with 12.5 ml of buffer: 100 mM Tris-HCl pH 7,5, 25% saccharose (w/w), 5% glycerol (v/v), 10 mM EDTA pH 8, 10 mM EGTA pH 8, 5 mM KCl, 1 mM DTT, 0,2% casein, 1% PIC (Protease Inhibitor Cocktail, Merck) and the sediment of buffer-saturated polyvinylpolypyrrolidone. The homogenate was filtered through Miracloth (Millipore) and centrifuged for 10 min at 10,000 g at 4 °C. The supernatant was diluted with water to obtain 12–13% saccharose at final volume. After centrifugation at 100,000 g for 1 h at 4 °C, the microsomal sediment was washed, centrifuged again, and resuspended in the buffer: 20 mM Tris HCl pH 7.5, 5 mM EDTA, 5 mM EGTA, 1 mM PMSF, 1% PIC. Protein concentration was determined using the Bradford40 method.
Immunoidentification of gel-separated proteins
SDS-PAGE electrophoresis was performed on 11% polyacrylamide gels according to the TGX Stain-Free method (BioRad), with addition of 4 M urea. 10 µg of protein samples were mixed in a 1:1,6 ratio (v/v) with solubilization buffer (96 mM Tris-HCl pH 6.8, 9.6 M urea, 24% saccharose, 3.2% SDS, 5% β-mercaptohetanol), and denatured at 60 °C for 10 min. The semidry blotter system (Merck) was then used to transfer proteins to Immobilon-P membrane, according to Millipore instructions.
Immunochemical identification was carried out with anti-PIP1;1–3 (Agrisera AS09 489) and anti-PIP2;1–7 (Agrisera, AS12 2110) antibodies. The membrane was blocked overnight at 4 °C in 3% BSA in PBS and then incubated for 1 h in RT with PIP1;1–3 antibody at 1:1000 dilution and with PIP2;1–7 antibody at 1:3000 dilution in PBS-T buffer containing 1% BSA. The membrane was then incubated for 1 h in RT with the secondary antibodies (Agrisera, AS09 6), at a 1:20000 dilution in 1 x PBS-T. Antigens were detected with Lumi-Light Western Blotting Kit (Roche).
For estimation of PIP monomers composition, the spots were taken from untreated gels, according to coordinates determined by immunodetection on parallel gels, and then subjected to tandem mass spectrometry procedure.
Solubilisation of integral membrane proteins and IEF-SDS-PAGE
The microsomes were treated with Brij-58 detergent to delipidate and remove surface-associated proteins41. 120 µg of protein, 240 µl 2% Brij-58 and 4 µl PIC were gently mixed for 30 min at 4 °C. After overnight precipitation at -26 °C with 10% (w/v) TCA in acetone and 0.07% (v/v) β-mercaptoethanol the proteins were pelleted for 15 min at 20,000 g and then washed three times with pure acetone and 0,07% β-mercaptohetanol. Then, acetone was removed and the precipitate was dried for 10 min. The solubilization of integral membrane proteins was carried out by suspending 120 µg of protein in 120 µl of IEF buffer containing: 7 M urea, 2 M thiourea, 2% ASB-14, 65 mM DTT, 1% PIC and 1% IPG 3–10 buffer (Bio-Rad).
Isoelectrofocusing was carried out at 20 °C in PROTEAN 12TM IEF Cell (BioRad) using IPG strips with immobilized 3–10 pH gradient (BioRad), first for 1.5 h at 300 V, then for 1.5 h in the 300–3500 V gradient and next at 3500 V until 20,000 Vh was reached. After IEF, the strips were equilibrated according to our modification of the McDonough and Marbán42 method. IPG strips were placed for 15 min in 10 ml of pH equilibrating buffer: 65 mM DTT, 6 M urea, 30% glycerol, 6% SDS, 50 mM Tris-HCl pH 6,8 and then alkylated for 15 min in a buffer containing 2,5% iodoacetamide instead of 65 mM DTT. For protein separation according to their molecular masses, the strips were subjected to Stain-Free SDS-PAGE as above.
Immunoprecipitation of PIP1-PIP2 aquaporin complexes
To identify physical interactions of aquaporin subunits, proteins were isolated by immunoprecipitation, according to our modification of the method described in Zelazny, et al.31. 250 µg of microsomes were solubilized by adding 2% ASB-14 and 0.1% TX-100 detergents suspended in 300 µl of TBS in the presence of 1.7% PIC. The sample was incubated for 2 h at 4 °C with stirring and then centrifuged for 10 min at 110,000 g. The supernatant was collected and diluted 1:1 with TBS to obtain the final 1% concentration of ASB detergent. The samples were agitated overnight at 4 °C with 1 µL of PIP2;1–7 antibodies. Antigen-antibody complexes were bound to Protein A-Sepharose 4B bed (Merck), washed and then eluted by incubation with 1.6x sample buffer (20mM Tris pH 6.8, urea 6 M, SDS 2%) at 60 °C for 10 min. The supernatant after the following centrifugation was quenched with 2 ml of cold acetone with 0.07% β-mercaptohetanol and precipitated as described above. The obtained pellet was dried in a fume hood for 30 min and then dissolved in 100 µl buffer: 4 M urea, 2 M thiourea, 2% ASB-14, 65 mM DTT. The final acetone concentration was 7 M to avoid the risk of precipitation of urea present in the sample buffer.
Protein identification by tandem mass spectrometry
Protein spots were excised from the gel under sterile conditions and analysed by liquid chromatography (Nano-Acquity, Waters) coupled to the mass spectrometer (Orbitrap Velos or Orbitrap Elite) in the Laboratory of Mass Spectrometry, Institute of Biochemistry and Biophysics, Polish Academy of Sciences (Warsaw, Poland) as previously described by Kubala, et al.43.
Raw data files were pre-processed with Mascot Distiller software (version 2.5, MatrixScience). The data obtained was compared with the database of data deposited in the amino acid sequences of green plant proteins or Z. mays deposited in the NCBI database using the Mascot program (Mascot Server 2.4.1, Matrixscience). The following parameters were used for comparison: enzymatic specificity: trypsin or semitrypsin, precision of mass measurement: ± 30 ppm, precision of the measurement accuracy: ± 0.1 Da, allowed number of deficiencies: 1, modification of amino acid residues: oxidation, phosphorylation, carbamidomethylation. The basis for acceptance of the peptides was the score value threshold calculated by the Mascot program44. The mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium via the PRIDE45 partner repository with the dataset identifier PXD046819 and 10.6019/PXD046819.
The abundance of MS identified PIP1 and PIP2 isoforms were drawn from Exponentially Modified Protein Abundance Index (emPAI) - the index estimating protein abundances from peptide counts in a single LC-MS/MS experiment, implemented within MASCOT software. EmPAI is defined as 10PAI minus one, where PAI has been defined as the ratio of the number of observed peptides to the number of observable peptides. This is a strictly probabilistic method however based on empirical approximations from the large-scale proteome profiling experiments46,47.
Statistics
The experimental data are presented as means of a specified number of replicates (n) ± SEM. Statistical analysis was carried out using the Student’s t-test, for groups with normal distribution (Shapiro-Wilk test), one-way or two-way analysis of variance (ANOVA) and Tukey-Kramer multiple comparison test. Statistically significant results were those for which the level of statistical significance value (p) reached: p ≤ 0,05 (*), p < 0,01 (**) or p ≤ 0,001 (***).
Results
Physiological response of roots and leaves to fast drought development and rewatering
For the purposes of the present study, we strived to observe the response of the plants to the soil water deficit as directly as possible, in relation to the intraradical mycorrhizal water supply. At the same time, we intended to make the observations unaffected by imbalance of nutrient availability between NM and AM plants. For this reason, we used a previously designed highly fertilized semi-hydroponic system36 to establish AM (with Rhizophagus irregularis) and NM cultures of similar size and nutritional status.
As a result of the fast development of water stress, the leaf and root water potential was strongly reduced in all plants, so mycorrhiza did not help to avoid dehydration under the conditions applied. The physiological response of leaves and the change in water potential allowed us to discriminate three phases of the water treatment: optimal watering (S0), short heavy stress (S7), and a recovery period of five days (R3 and R5) (Fig. 1).
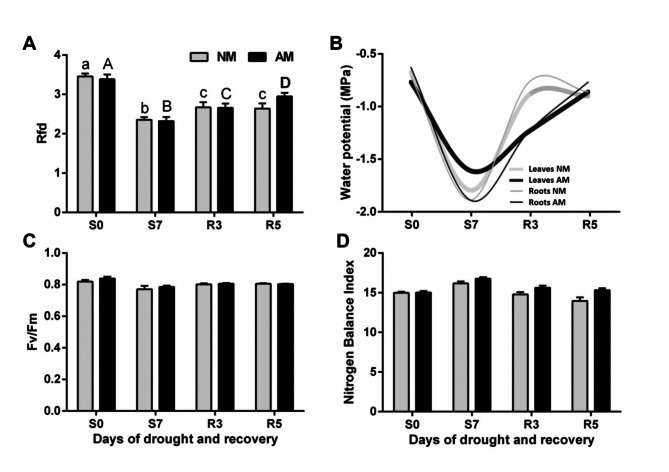
Fig. 1.
Effect of fast drought development (S0, S7) and rehydration (R3, R5) on plant PSII quantum conversion efficiency [(A) Rfd and (C) Fv/Fm], (B) water potential, and (D) leaf nitrogen balance index (NBI) of mycorrhizal (AM) and non-mycorrhizal (NM) maize. The bars represent average values taken from the middle leaves (ear leaf and the leaf above) in the upper half of leaf length. The error bars show the standard error of the mean (NBI: n = 100, i.e. 5 plants x 2 leaves x 10 measurement points; RFd and Fv/Fm: n = 14, i.e. 7 plants x 2 leaves). No statistically significant differences between mean values for NM and AM plants were found. Lowercase or uppercase letters indicate significant differences (p < 0.05) among stress time points for plants of the same symbiotic status (one-way ANOVA).
The tissue dehydration state was determined on the basis of root and leaf water potential. Initially, this measure was at a similar level in both tissues (-0.6 MPa). After stopping watering, the root water potential in both AM and NM plants decreased within 7 days to -1.9 MPa, while AM leaves maintained a slightly higher level (-1.6 MPa) than NM leaves (-1.8 MPa). Within the following 5 days of renewed irrigation, these values returned to the initial level, but this process appeared to be markedly slower in AM plants (Fig. 1).
As we intended, the rapid development of drought in both AM and NM plants caused no signs of impaired nitrogen management (Fig. 1). Moreover, it eliminated the symbiotic effect on both NBI and PSII quantum conversion indices (Rfd and Fv/Fm). Only Rfd, the ratio measured under saturating light and therefore believed to be proportional to total photosynthetic efficiency, showed drought sensitivity (Fig. 1).
Measurements of leaf gas exchange parameters exposed a compensatory effect of mycorrhiza because a faster restoration of photosynthetic efficiency occurred in the course of rewatering (Table 1). Under drought (S7), the rate of CO2 assimilation (Amax), stomatal conductance (gs), and transpiration (Tmax) strongly decreased, regardless of the symbiotic state. However, on day 5 of rehydration (R5), these activities were fully restored in AM plants, while NM plants did not reach more than 50% of the initial efficiency.
Table 1.
Evaluation of light-saturated leaf gas exchange efficiency under mycorrhiza and water treatment.
| S0 | S7 | R5 | |||||
|---|---|---|---|---|---|---|---|
| Mean ± SD | p | Mean ± SD | p | Mean ± SD | p | ||
| Amax | NM | 14.8 ± 1.9 | * | 0.8 ± 0.4 | 7.6 ± 2.7 | * | |
| AM | 15.5 ± 2.4 | 1.2 ± 0.5 | 16.0 ± 3.9 | ||||
| gs | NM | 0.09 ± 0.02 | * | 0.03 ± 0.02 | 0.05 ± 0.04 | * | |
| AM | 0.12 ± 0.01 | 0.03 ± 0.02 | 0.08 ± 0.03 | ||||
| Tmax | NM | 3.43 ± 0.76 | * | 1.62 ± 0.9 | 1.37 ± 0.57 | * | |
| AM | 3.86 ± 0.21 | 1.74 ± 0.7 | 2.71 ± 0.87 | ||||
| WUE | NM | 4.56 ± 1.49 | 0.74 ± 0.3 | 5.55 ± 1.30 | |||
| AM | 3.96 ± 0.44 | 0.81 ± 0.3 | 5.90 ± 1.45 | ||||
These results indicate that high fertilization before stress and the imposing of fast soil drought could eliminate fungal adjustments in leaf physiology during the progression of the plant water deficit but left a variation in the Amax and gs parameters at the time of rewatering.
Light-saturated CO2 assimilation rate (Amax, µmol m− 2 s− 1), water exchange parameters (gs, Tmax, mol m− 2 s− 1), and water use efficiency (WUE, Amax/ Tmax, µmol m− 1) of mycorrhizal (AM) and non-mycorrhizal (NM) plants, measured in the main phases of the water treatment: optimal watering (S0), short heavy stress (day 7 of drought, S7) and day 5 of rehydration (R5). Asterisks indicate significant differences (p < 0.05) between AM and NM plants on particular days of water treatment (Student’s t-test, n = 8, i.e. 4 plants x 2 leaves).
Immunodetection and MS-MS identification of dimeric and monomeric aquaporin forms
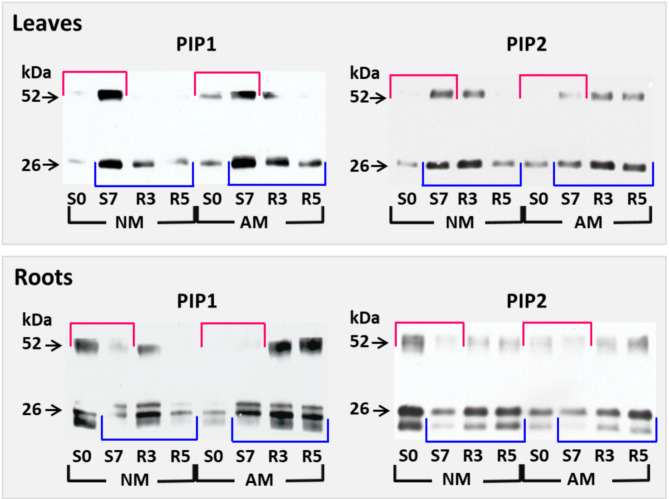
The PIP proteins were immunodetected with two antibodies specific to a wide range of PIP1 or PIP2 isoforms (see section ‘Materials and Methods’). Apart from monomeric 26-kDa forms, almost every SDS-PAGE separation revealed 52-kDa PIP dimers (Fig. 2). The anti-PIP1 and anti-PIP2 antibodies recognized in root samples an additional 22-kDa monomeric band (Figs. 2 and 4). Comments in Supplementary Fig. 3 explain the possible reasons for the appearance of the additional monomeric band. The isoelectric focusing-mass spectrometry (IEF-MS) analyses confirmed (Supplementary Fig. 3 and Supplementary Table 1) that this protein is an expected target for anti-PIP2 antibodies (Fig. 4, spot no. 8 of 8.5 pI and spot no. 12 of 9.2 pI). In line with this, both monomeric forms and dimer band were used for quantification as densitometric values presented in Fig. 3.
Fig. 2.
Representative western blots showing PIP1 and PIP2 aquaporin abundance in maize leaves and roots at the beginning and end of drought development (S0, S7) and during rehydration (R3, R5), in relation to mycorrhiza presence (AM) or absence (NM). Red boxes indicate the accumulation changes caused by drought, while blue boxes indicate the rehydration-induced changes. Immunostaining with PIP1-specific antibodies revealed dimeric (52-kDa) and monomeric (26-kDa) forms. The anti-PIP1s and anti-PIP2s antibodies exposed additional 22 kDa monomers in roots preparations, identified as aquaporins by MS/MS approach. Extracts were taken from combined middle leaves (ear leaf and the leaf above), or a mix of secondary feeder roots, collected from four plants for each stress point. The molecular marker was cut-of prior to incubation of membranes with antibodies and stained using Coomassie Blue, hence only cropped images are presented. For further information see Supplementary data.
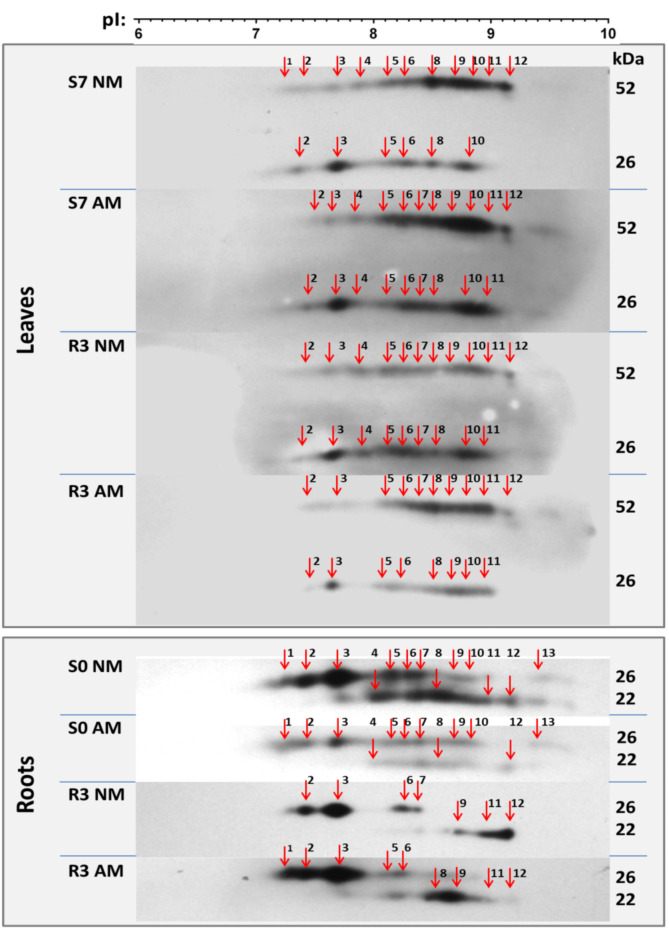
Fig. 4.
Immuno-identification of aquaporin complexes reacting with anti-PIP2 antibodies. The microsomal samples were solubilized and separated by IEF-SDS PAGE. Western blot staining revealed 13 IEF spots of very reproducible pI positions across leaf and root samples taken from different stress points and mycorrhizal (AM) or non-mycorrhizal (NM) plants. Samples represent fully irrigated variants (S0), day 7 of drought (S7), and day 3 of rehydration (R3). Other stress points were omitted due to strong down-regulation of PIP protein levels (see Figs. 2 and 3). IEF was performe d on a linear gradient pH 3–10. For other details, see description under Fig. 2. The molecular marker was cut-of prior to incubation of membranes with antibodies and stained using Coomassie Blue, hence only cropped images are presented. For further information see Supplementary data.
Fig. 3.
Leaf and root protein accumulation patterns for PIP1 or PIP2 aquaporins at subsequent time points of drought development (S0, S7) and rehydration (R3, R5), in relation to mycorrhiza presence (AM) or absence (NM). Densitometric values after immunodetection were normalized to densitometry of Stain Free proteins staining taken from the same blots. Mean values (n = 4) and standard error from two technical replicates from each of two independent cultures are presented. In the case of leaf preparations, the accumulation level was referred to (S7) NM, and for roots to (S0) NM, which represented the highest densitometric values.
Accumulation time course of maize PIP1s and PIP2s in response to mycorrhiza and water treatment
The establishment of the above-described cultivation regime allowed us to proceed with analyses aimed at demonstrating whether the overall level of aquaporin accumulation in root and leaf cells is subject to change in correlation with the symbiotic status and rapidly changing irrigation conditions. Of particular interest was the rehydration period, during which mycorrhiza was found to have a compensatory effect on transpiration and photosynthetic efficiency.
Aquaporin immunodetection under drought treatment (Fig. 2, top marked in red and recovery patterns underlined in blue) was further quantified by densitometry and averaged (Fig. 3). In NM plants, opposite patterns of root and leaf aquaporin accumulation were observed in response to water treatment. In roots, both PIP1s and PIP2s were highly abundant under full hydration (S0), while during drought (S7) their level dropped to about 50% and 25%, respectively, for PIP1s and PIP2s. In leaves, the opposite was the case: under watering, the accumulation of aquaporins was low, whereas protein levels increased 4–5-fold as severe drought developed.
Lowercase or uppercase letters indicate significant differences (p < 0.05) among stress time points for plants of the same symbiotic status. Asterisks indicate significant differences (p < 0.05) between AM and NM plants on particular days of water treatment (two-way ANOVA with Tukey’s post-hoc test, *** p < 0.001).
Unlike NM plants, mycorrhiza maintained high and stable levels of PIP1s and PIP2s in the leaves and PIP1s in the roots not only during drought but also during the first days of rehydration. Note that the higher level of aquaporin accumulation in AM plants during the rehydration period is well correlated with doubled water stomatal conductance, compared to NM counterparts shown in Table 1.
IEF-MS identification of tissue-specific heteromeric composition of PIP aquaporins and their stress-induced accumulation patterns
The aim of this stage of the study was to verify the hypothesis that aquaporins form different heterocomplexes, whose accumulation is specifically regulated depending on the presence of mycorrhiza and the phase of water treatment. We assumed that these complexes are composed of PIP1-PIP2 or PIP2-PIP2 dimers of tissue-specific composition. This stage involved separation of PIP oligomers by two-dimensional electrophoresis (IEF-SDS PAGE). The main assumption and condition for the success of this approach is that functional aquaporin dimers formed in vivo retain their integrity during the IEF step of two-way electrophoresis, due to disulphide bridges that connect monomers covalently26,48.
Aquaporins are membrane-spanning proteins and therefore are more challenging to analyse by proteomic methods than soluble proteins because of their hydrophobic properties. However, we have managed to develop a solubilization procedure that allows effective separation of functional PIP dimers from the microsomal fraction.
IEF procedure offers the possibility of reducing a complex mixture of PIP proteins isolated from plant tissues into fractions differing in pI value. IEF spots distribution is the reflection of differences in post-translational modifications (see comments on possible effect of phosphorylation on spot distribution in the supplementary data file). We obtained these fractions in the form of 13 IEF spots of very reproducible pI positions across leaf and root samples taken from different stress time points and symbiotic variants (Fig. 4). This indicated the presence of PIP complexes in both roots and leaves with very similar enrichment in post-translational modifications.
Samples taken from fully irrigated variants (S0), from day 7 of drought (S7), and from day 3 of rehydration (R3) were selected for this purpose. Drought (S7) or initial irrigation period (S0) was accompanied by a strong down-regulation of the level of PIP proteins in the roots or leaves, respectively. Therefore, these variants were omitted from the analysis presented.
In root samples taken during full hydration (S0), the regulation of aquaporins did not appear to favour any of the IEF isoforms, although the level of accumulation of individual complexes was much higher in non-mycorrhizal plants. Notably, we did not analyse samples from the drought period (S7), however, in the rehydration period, we observed selective persistence of oligomers in the range of pI 7.2–7.7, representing the association PIP2-PIP2, parallel to the almost complete removal of some complexes in the range of pI 8.5–9.2.
In leaf samples, regardless of the symbiotic status and irrigation level, the highest abundance was attributed to the IEF complexes located at the pI positions 8.1 to 9.2. When analysing independent SDS-IEF replicates (not shown), it was not possible to identify IEF complexes whose accumulation would be prominent in response to drought or irrigation restoration or symbiotic variants.
The next step was indication of PIP1 and PIP2 subunits composition for individual IEFresolved spots. This was performed by tandem mass spectrometry (ESI-MS/MS) on the representative PIP-rich root (S0 NM) and leaf (S7 AM) preparations, further enriched by immunoprecipitation with antibodies specific to a broad spectrum of PIP2-type aquaporins. The enrichment by immunoprecipitation led to a high MS identification efficiency of aquaporins (high total Mascot scores). A complete list of MS-recognized PIP isoforms is presented in Supplementary Table 3. To determine the approximate ratio of PIP1 to PIP2 for individual 2D spots, we used the emPAI value readings implemented in the MS/MS method (Figs. 5 and 6 and Supplementary Table 3).
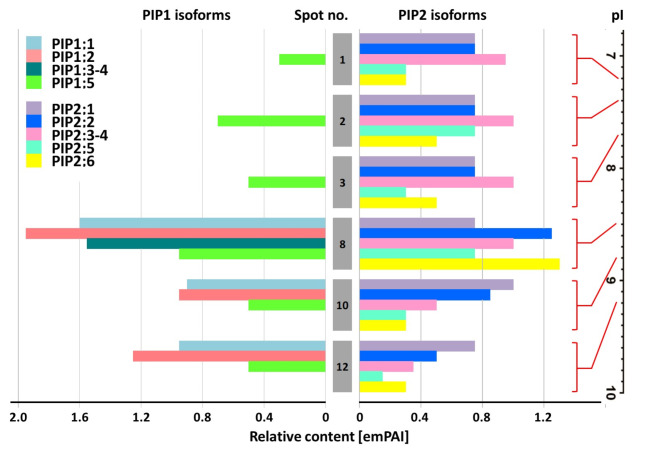
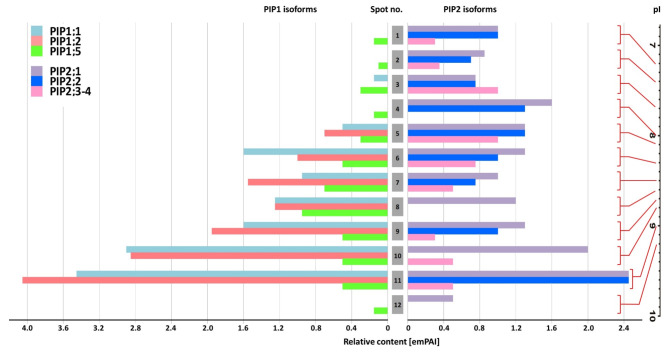
Fig. 5.
Semiquantitative estimation of PIP1-type and PIP2-type monomer associations within aquaporin heterocomplexes isolated from roots. The native protein-protein interactions were confirmed by immunoprecipitation with PIP2-specific antibodies. The representative PIP-rich preparation ‘S0 NM’ was applied, which also featured the full spectrum of 2D spots (see Fig. 4). Protein abundance was expressed as emPAI values taken from mass spectrometry analysis within IEF-SDS PAGE resolved complexes. Protein spots were taken from untreated gels, according to coordinates determined by immunodetection of protein spots on parallel gels after separation and subjected to MS-MS analysis. The numbering corresponds to IEF spots (and thus the pI values) pointed by arrows in Fig. 4.
Fig. 6.
Semiquantitative estimation of PIP1-type and PIP2-type monomer associations within aquaporin heterocomplexes isolated from leaves. The native protein-protein interactions were confirmed by immunoprecipitation with PIP2-specific antibodies. The representative PIP-rich preparation ‘S7 AM’ for leaves was applied, which also featured the full spectrum of 2D spots (see Fig. 4). Protein abundance was expressed as emPAI values taken from mass spectrometry analysis within complexes resolved by IEF-SDS PAGE. Protein spots were taken from untreated gels, according to coordinates determined by immunodetection of protein spots on parallel gels after separation and subjected to MS-MS analysis. The numbering corresponds to IEF spots (and thus the pI values) pointed by arrows in Fig. 4.
This approach makes it possible not only to identify PIP isoforms but also to determine with a high probability their relative (semiquantitative) ratio in IEF fractions46,47. Due to some limitations related to the high homology within PIP1 or PIP2 subgroups, the proportions were approximated based on the emPAI ratio of two arbitrarily chosen isoforms: PIP1;2 and PIP2;1. These isoforms were also commonly found in the 2D spots identified in this study and had high emPAI values. The detailed results of these comparisons are shown in Supplementary Table 2 (see also comments therein). The MS identification showed that the ratio of PIP1 and PIP2 monomers was more or less equal in spots located at the pI positions 8.1 to 9.2, while in the less basic oligomers, the proportion of PIP1s remained significantly lower, to zero.
Supplementary Fig. 5 shows that although immunoprecipitation gave low yield for some IEF spots from roots, all other pl positions of the proteins persisted in gels. Therefore, we assumed that PIP1/PIP2 composition identified in IEF spots after immunoprecipitation would be representative also for 2D spots in Fig. 3 with analogous pI values.
Discussion
According to our previous experiences, mycorrhiza in highly fertilized maize pot cultures does not help to prevent leaf senescence development under long progression of severe drought, and fungal contribution appears to be narrowed only to the period of recovery36. For current use, the desired modification was to obtain a rapid development of a severe but fully reversible drop in plant water potential (see details in ‘Results’ subsection ‘Physiological response of roots and leaves’). In result, AM-altered plant nutrition—the often suggested explanation of drought tolerance in mycorrhizal plants—seems to be of little importance in our experimental setup. This conclusion is apparently confirmed by similar growth before imposing stress (Supplementary Fig. 1) and by the lack of signs of fungal adjustment in leaf physiology during the progression of plant water deficit (Fig. 1; Table 1). Nevertheless, similar to what we saw earlier36, AM plants recovered faster from water stress than their non-mycorrhizal counterparts with respect to water and CO2 stomatal conductance (Table 1). This is the hallmark of better tolerance to drought and a common phenomenon, as follows from numerous mycorrhizal reports (see reviews7,49).
Our attempt to evaluate the correlation of this effect with aquaporin accumulation patterns was based on the hypothesis of a causal relationship between drought-regulated expression of these proteins and the intensity of long-distance water transport17–20. Given the specificity of PIP isoforms in leaf tissues, they may not only transport water but also function as CO2 distribution channels in mesophyll tissues and contribute to stomatal regulation50. According to data on the specific AQPs functions in maize stomatal complexes, more than 80% of PIPs expression in these cells is associated with PIP1-type isoforms51, while the presence of some PIP2s transcripts, such as ZmPIP2;5, suggests H2O2 channel activity necessary for PIP-mediated regulation of stomatal closure52.
Moreover, the influx of water from xylem to mesophyll can be mediated by PIP1;2, PIP2;1, and PIP2;5 isoforms, which help to bypass the apoplastic barriers in bundle sheath cells53,54 Besides, stomatal movement depends on the adjustment of turgor pressure which is facilitated by water diffusion to guard cells, which is facilitated by plasma membrane aquaporins53,54.
Complementary accumulation patterns of root and leaf PIP aquaporins under rapidly changing hydration conditions and the effect of mycorrhiza
Regulation of PIPs in NM plants
As summarized in Table 2, the fast-developing drought stress was accompanied in non-mycorrhizal plants by a correspondingly strong increase in leaf accumulation and a drop in root accumulation of both PIP1s and PIP2s. Considering the down-regulation of PIPs in NM roots, it seems to be a response to the risk of osmotic water loss from cortical cells, caused by a drop in soil water content. Moreover, such a response may play a critical role in the entire plant water budget. Vandeleur, et al.55 reached a similar conclusion from the rapid reduction in the hydraulic conductance of roots induced by the lowering of transpiration demands. Such a fast systemic regulation is most plausibly explained by PIPs activation and is probably mediated by shoot-to-root xylem hydraulic signals. Aquaporin activity has been found to respond to hydraulic pressure changes and these water channels also have to be gated by hydraulic pressure stimuli56. Although a correlation between PIP protein accumulation and the time course of root hydraulic conductivity has not always been observed, the lowering of root PIP abundance has already been reported for water-stressed plants, including maize (see a review19). These inconsistencies could arise from different strategies to overcome drought in different species or varieties, which may involve aquaporins for anisohydric or isohydric behaviour18,20.
Table 2.
Schematic overview of the regulation of PIP aquaporin abundance in roots and leaves in relation to a given sequence of water availability and symbiotic status.
| Roots | Leaves |
|---|---|
| Abundance of PIP1s & PIP2s under optimal irrigation | |
| Higher in NM than in AM | Nearly equal in NM and AM |
| Regulatory patterns under water stress and recovery | |
| Incremental accumulation in AM plants | |
| PIP1s: S0 ↗ S7 ↗ R3 → R5 | PIP1s: S0 ↗ S7 → R3 → R5 |
| PIP2s: S0 → S7 ↗ R3 → R5 | PIP2s: S0 ↗ S7 → R3 → R5 |
| Sinusoidal accumulation in NM plants | |
| PIP1s: S0 ↘ S7 ↗ R3 ↘ R5 | PIP1s: S0 ↗ S7 → R3 ↘ R5 |
| PIP2s: S0 ↘ S7 ↗ R3 ↘ R5 | PIP2s: S0 ↗ S7 → R3 ↘ R5 |
PIP1s and PIP2s refer to two aquaporin subfamilies detected on immunoblots. The patterns of their abundance are based on the evaluation shown in Figs. 2 and 4. AM—mycorrhizal plants: NM—non-mycorrhizal plants. Three phases of the water treatment: optimal watering (S0), short heavy stress (day 7 of drought, S7) and recovery period (days 3 and 5: R3 and R5). Symbols ↘ and ↗ and → denote decrease, increase, and no change in PIP abundance.
As reported here, the accumulation of aquaporins in leaves could counteract the risk of lowering the hydraulic conductance of the leaf and consequently reducing CO2 assimilation due to stomata closure18,20. Leaf pathways other than transcellular, mainly apoplastic, could be dominant outside the cells surrounding xylem (with ample water supply)57. However, aquaporin-driven enhancement of external xylem water flow is also reported58; therefore, under soil drought induced in our study, it appears to be partitioned to a greater extent by cell-to-cell water flow.
Regulation of PIPs in AM plants
The risks and factors described above could also affect AM plants but, as summarized in Table 2, the symbiotic regulation of PIPs followed a drought temporal pattern clearly different from that recorded in NM plants: (i) AM root samples taken under fully hydrated conditions contained about 25–35% less aquaporins than NM roots; (ii) drought had a much lower impact on PIP accumulation in AM roots, compared to a significant drop in NM roots; (iii) AM roots showed an incremental accumulation of PIPs against the whole sequence of water availability, in contrast to the sinusoidal pattern found in NM roots; and (iv) after rewatering, AM leaves retained an elevated amount of PIP proteins longer than NM leaves.
Considering features (ii) and (iii) of AM plants, their roots appeared to be capable of engaging a positive feedback mechanism, while the response of NM plants was based on a negative feedback scheme. According to feature (iv), a similar positive regulation of PIPs distinguishes the leaf response of AM plants. Although PIPs also accumulated in NM leaves during the drought phase, mycorrhiza maintained this effect when irrigation was restored, apparently contributing to a faster recovery of leaf gas exchange.
Differential physical interactions of PIP1 and PIP2 monomers within heterocomplexes of leaf and root aquaporins
The discovery that PIP1 transport to the plasma membrane depends on structural PIP1-PIP2 interactions, provided new insight into regulation of PIP-type aquaporins30,31. The conformational interaction between PIP1-type and PIP2-type monomers also plays an important role in the resulting water and CO2 conduction efficiency30,32,59–61. Recently, Yaneff, et al.62 proposed to consider PIP1–PIP2 pairs as specific functional units under joint regulation, both at the transcriptional and post-translational levels, when a plant is exposed to a certain stress or change in the environment.
These data suggest that the regulation of aquaporin activity in response to abiotic stresses with an osmotic component may include an additional post-transcriptional level of complexity involving tetramer formation by different subunit associations63. Nevertheless, to date, no attempt has been made to determine the effect of drought on the variation in monomers’ association within aquaporin oligomers and their accumulation when a mycorrhizal partner is involved in water conduction mechanisms.
The aquaporin PIP1 or PIP2 monomers form dimeric complexes that are difficult to break down by SDS PAGE method, due to disulphide bridges located at highly conserved cysteine residues in the A loop that connects monomers26,48. This feature allowed us to design the procedure to reveal the presence of functional aquaporin dimers under the assumption that the stability of disulphide bridges is preserved during the isoelectric focusing step of two-way electrophoresis. We assumed that we could identify these dimers if they differed in pI values due to the different associations of the PIP1 and/or PIP2 monomers and the associated post-translational modification, such as phosphorylation.
As a result, up to 13 IEF resolved aquaporin complexes were immunodetected with highly reproducible pl positions across leaf and root samples taken from different stress points and symbiotic variants (Fig. 3). This indicated the presence of PIP complexes in both roots and leaves, with very similar enrichment in post-translational modifications. To study the interaction of PIP1 and PIP2 isoforms, immunoprecipitation with anti-PIP2 antibodies and IEF separation combined with MS/MS analysis were then performed. Interestingly, highly reproducible pl positions were also observed in gels after immunoprecipitation (see examples in Supplementary Fig. 5). Therefore, we assumed that the PIP1/PIP2 composition identified by MS in IEF spots after enrichment by immunoprecipitation would be similar also for 2D spots in Fig. 3 with analogous pI values.
Post-translational modification by the addition of phosphate induces a pI shift of PIP aquaporins, and thus may explain the separation of secondary spots in IEF gels in addition to unmodified isoforms64. The hypothesis of the involvement of phosphorylation in symbiotic PIP regulation found support in the work of Quiroga, et al.24, who showed that phosphorylation of aquaporins, rather than an increase in their synthesis, is a mechanism correlated with the increase in root cell water transport capacity in the presence of mycorrhiza. Nevertheless, we did not achieve sufficient efficiency in identifying phosphorylated residues to formulate conclusions about the post-translational modifications of individual 2D spots in response to the imposed water conditions (see comments on possible effect of phosphorylation on IEF spot distribution in the supplementary data file).
The pI positions of the most abundant 2D spots appeared to be different in root and leaf preparations. Moreover, PIP1/PIP2 composition of these complexes also greatly differed, as recognized by semiquantitative MS/MS analyses (Figs. 5 and 6, Supplementary Table 3). It refers to the fact that root-abundant oligomers separated in the pI range 7.2–7.7 showed a very low abundance of PIP1-type isoforms. Such a disproportion between PIP1 and PIP2 isoforms was not present in leaf-abundant complexes located at pI positions 8.1 to 9.2.
The compositional conclusions were expressed as emPAI values by comparing the levels of only two isoforms, PIP1;2 and PIP2;1, whose protein sequence differences qualified the method (see Supplementary Tables 2 and comments therein and in section ‘Results’). The selection of these isoforms was supported by other reports suggesting ZmPIP1;2-ZmPIP2;1 as a functional unit under joint regulation in maize tissues31,62 and above mentioned co-occurrence in mechanisms facilitating water and CO2 diffusion to leaf cells61,62.
Under full irrigation, the root samples presented the full spectrum of diversity of IEF isoforms, regardless of the symbiotic state. On the contrary, the root response after resumption of irrigation was dominated by oligomers with a high or almost exclusive content of PIP2-type monomers (Supplementary Table 2). In leaves, the highest accumulation was attributed to oligomers with an increased PIP1 content, reaching amounts equal to those of PIP2 isoforms (Supplementary Table 2). These heterocomplexes appeared to be uniformly regulated, both in response to drought and rehydration, and also regardless of the plant symbiotic status. It is known that PIP1 and PIP2 co-expression and physical interaction contribute to increased trans-membrane water flow30,32,59,65. According to experimental and theoretical models, the expected effect of PIP1 enrichment, regardless of its proportion, is a two-fold increase in water conduction60. Table 2 summarises the patterns of aquaporin abundance, showing that in AM plants their accumulation persisted during the first days of irrigation in both roots and leaves, while in NM tissues their levels decreased. This indicates an increased ability to restore water and CO2 conduction, which, in turn, correlates well with increased leaf gas exchange parameters in AM plants during recovery (Table 1).
Furthermore, some PIP1 aquaporins could facilitate CO2 diffusion across mesophyll membranes and also play a key role in controlling stomatal movements61. A different proportion of PIP1 and PIP2 aquaporins in the tetramer structure exerts opposing effects on mesophyll conductance to CO2 and water. It raises a very interesting hypothesis that leaf aquaporins act as a factor establishing a balance between these antagonistic pathways of stomatal diffusion66,67. However, as our results suggest, apart from combining PIP1 and PIP2 monomers in different proportions, an additional level of regulation may be the change in accumulation of the individual tetramers formed in this way (Fig. 7). Considering contribution of PIP1s to water and CO2 diffusion to mesophyll and stomatal cells we suggest that elevated content of PIP1s shown in leaves may be crucial to sustain high rates of photosynthesis.
Fig. 7.
The proposed systemic coordination scheme for the regulation of PIP aquaporin in response to re-watering after drought. On the left, leaf gas exchange and overall PIPs abundance are shown according to the results obtained. On the right, we illustrate enrichment in PIP1 or PIP2 monomers, specific to the most abundant aquaporin heteromers present in leaf or root samples. The tissue-specific composition was approximated based on the emPAI ratio of PIP1;2 to PIP2;1 isoform estimated by MS/MS. Mycorrhizal regulation during rehydration manifested in roots primarily as accumulation of PIP2-rich oligomers. Such a composition can result in lower water transmembrane conductivity than that involving PIP1, as predicted by experimental and theoretical models. This may allow a more effective counterbalance against the risk of root water loss. Considering enrichment of leaf aquaporins in PIP1s subunits and their contribution to water and CO2 diffusion and controlling stomatal movements, this feature may be crucial to sustain high rates of photosynthesis. PIP1 enrichment was accompanied in AM leaves by aquaporin accumulation prolonged over the irrigation period, in contrast to NM plants. This feature indicates a ‘risk-taking’ behaviour that could act as positive feedback on stomatal gas exchange capacity, which, together with stable aquaporin levels in the roots, might explain the faster recovery of the photosynthetic rate observed in AM plants.
Conclusions on AM-altered PIP accumulation
Ectomycorrhizal and arbuscular hyphae are supposed to increase root hydraulic conductance mainly through apoplastic transport68. However, mycorrhizas have also been shown to affect cell-to-cell water flow by regulating plant aquaporin expression and/or activity21–24. Barzana et al.68 postulated an improved ability of AM plants to switch between apoplastic and transcellular water transport pathways according to the water demands of the shoot, allowing for a more flexible response to water deficiency conditions. The incremental pattern of accumulation of PIPs in AM plants against rapidly changing water conditions, revealed in our study, supports this hypothesis. As we showed, this effect can be further enhanced by the drought-induced accumulation of leaf aquaporin oligomers with an increased PIP1s to PIP2s ratio, which is believed to result in greater water transmembrane transport32,59,60, but also to facilitate CO2 mesophyll diffusion66,67 but also affect stomata movements61.
This transpiration-promoting mechanism puts plants at risk of water loss and therefore reflects an anisohydric behaviour recognized in different species or varieties as helpful in overcoming drought. Anisohydric strategy is defined as favouring gas exchange at the cost of lowering leaf water potential and is now explained by the involvement of root and shoot PIPs, which ultimately promotes hydraulic conductivity throughout the plant18,20,69.
In this study, the risk of water loss in AM roots was controlled during drought by negative regulation of aquaporins in roots. During recovery, in turn, when increased transpiration creates higher water flow demands, it apparently required roots to counterbalance by accumulation of complexes comprising almost exclusively PIP2 monomers. The accumulation of PIP2-rich oligomers, especially intense in AM plants, in conjunction with the mobilization of PIP1 subunits in leaves, could be considered as the systemic mechanism of mycorrhizal stress tolerance, which could act as positive feedback on stomatal gas exchange capacity and explain the faster recovery of the photosynthetic rate observed in AM plants (Fig. 7).
Supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We would like to thank Maksymilian Chmielewski for help with depositing mass spectrometry proteomics data in the PRIDE database.
Author contributions
W.P. conceived the original research plan, E.P.-L. & W.P. designed and performed the experiments; E.P.-L. analyzed the data and created the figures and tables; E.P.-L. was responsible for writing first draft, W.P. was responsible for reviewing and editing.
Funding
The publication was co-financed within the National Science Centre, Poland (Project Number 2011/01/B/NZ9/00362 to WP) and the European Union under the European Social Fund (Project Number DFS.VI.052.4.17.6 to EP-L).
Data availability
The mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium via the PRIDE partner repository with the dataset identifier PXD046819 and doi:10.6019/PXD046819.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Ewelina Paluch-Lubawa, Email: e.paluch@amu.edu.pl.
Władysław Polcyn, Email: polcyn@amu.edu.pl.
References
- 1.Azcón-Aguilar, C. & Barea, J. Nutrient cycling in the mycorrhizosphere. J. Soil. Sci. Plant. Nutr.15, 372–396 (2015). [Google Scholar]
- 2.Smith, S. E. & Read, D. J. Mycorrhizal Symbiosis (Academic, 2010).
- 3.Chitarra, W. et al. Insights on the impact of arbuscular mycorrhizal symbiosis on tomato tolerance to water stress. Plant. Physiol.171, 1009–1023 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lenoir, I. & Fontaine, J. Lounès-Hadj Sahraoui, A. Arbuscular mycorrhizal fungal responses to abiotic stresses: A review. Phytochemistry. 123, 4–15. 10.1016/j.phytochem.2016.01.002 (2016). [DOI] [PubMed] [Google Scholar]
- 5.Ruiz-Lozano, J. M. et al. Arbuscular mycorrhizal symbiosis induces strigolactone biosynthesis under drought and improves drought tolerance in lettuce and tomato. Plant. Cell. Environ.39, 441–452. 10.1111/pce.12631 (2016). [DOI] [PubMed] [Google Scholar]
- 6.Quiroga, G., Erice, G., Aroca, R., Chaumont, F. & Ruiz-Lozano, J. M. Enhanced drought stress tolerance by the arbuscular mycorrhizal symbiosis in a drought-sensitive maize cultivar is related to a broader and differential regulation of host plant aquaporins than in a drought-tolerant cultivar. Front. Plant. Sci.8 (2017). [DOI] [PMC free article] [PubMed]
- 7.Augé, R. M. Water relations, drought and vesicular-arbuscular mycorrhizal symbiosis. Mycorrhiza. 11, 3–42 (2001). [Google Scholar]
- 8.Delavaux, C. S., Smith-Ramesh, L. M. & Kuebbing, S. E. Beyond nutrients: a meta-analysis of the diverse effects of arbuscular mycorrhizal fungi on plants and soils. Ecology. 98, 2111–2119. 10.1002/ecy.1892 (2017). [DOI] [PubMed] [Google Scholar]
- 9.Cheng, S. et al. Elucidating the mechanisms underlying enhanced drought tolerance in plants mediated by arbuscular mycorrhizal fungi. Front. Microbiol.12 (2021). [DOI] [PMC free article] [PubMed]
- 10.Paluch-Lubawa, E., Prosicka, B. & Polcyn, W. Expression patterns of maize PIP aquaporins in middle or upper leaves correlate with their different physiological responses to drought and mycorrhiza. Front. Plant. Sci.13 (2022). [DOI] [PMC free article] [PubMed]
- 11.Ruiz-Lozano, J., Porcel, R., Bárzana, G., Azcón, R. & Aroca, R. Contribution of arbuscular mycorrhizal symbiosis to plant drought tolerance: State of the art. Plant. Responses Drought Stress, 335–362 (2012).
- 12.Ruiz-Lozano, J. M. et al. Using the Maize Nested Association Mapping (NAM) population to partition arbuscular mycorrhizal effects on drought stress tolerance into hormonal and hydraulic components. Int. J. Mol. Sci.23 (2022). [DOI] [PMC free article] [PubMed]
- 13.Sánchez-Romera, B. et al. Involvement of the def-1 mutation in the response of tomato plants to Arbuscular Mycorrhizal Symbiosis Under Well-Watered and Drought conditions. Plant. Cell. Physiol.59, 248–261 (2018). [DOI] [PubMed] [Google Scholar]
- 14.Zhang, F., Ni, Q., Zou, Y., Wu, Q. & Huang, Y. Preliminary study on the mechanism of AMF in enhancing the drought tolerance of plants. J. Fungal Res.15, 8–13 (2017). [Google Scholar]
- 15.Kreszies, T., Schreiber, L. & Ranathunge, K. Suberized transport barriers in Arabidopsis, barley and rice roots: from the model plant to crop species. J. Plant Physiol.227, 75–83. 10.1016/j.jplph.2018.02.002 (2018). [DOI] [PubMed] [Google Scholar]
- 16.Paluch, E., Lubawy, J. & Polcyn, W. Akwaporyny roślinne-funkcje i czynniki regulujące ich aktywność. Postepy Biol. Komorki42 (2015).
- 17.Sade, N. & Moshelion, M. In Plant Aquaporins185–206 (Springer, 2017).
- 18.Moshelion, M., Halperin, O., Wallach, R., Oren, R. & Way, D. A. Role of aquaporins in determining transpiration and photosynthesis in water-stressed plants: crop water‐use efficiency, growth and yield. Plant. Cell. Environ.38, 1785–1793 (2015). [DOI] [PubMed] [Google Scholar]
- 19.Aroca, R., Porcel, R. & Ruiz-Lozano, J. M. Regulation of root water uptake under abiotic stress conditions. J. Exp. Bot.63, 43–57. 10.1093/jxb/err266 (2012). [DOI] [PubMed] [Google Scholar]
- 20.Maurel, C., Verdoucq, L. & Rodrigues, O. Aquaporins and plant transpiration. Plant. Cell. Environ.39, 2580–2587. 10.1111/pce.12814 (2016). [DOI] [PubMed] [Google Scholar]
- 21.Aroca, R., Porcel, R. & Ruiz-Lozano, J. M. How does Arbuscular mycorrhizal symbiosis regulate root hydraulic properties and plasma membrane aquaporins in Phaseolus vulgaris under drought, cold or salinity stresses? New Phytol.173, 808–816. 10.1111/j.1469-8137.2006.01961.x (2007). [DOI] [PubMed] [Google Scholar]
- 22.Porcel, R., Aroca, R., Azcon, R. & Ruiz-Lozano, J. M. PIP aquaporin gene expression in arbuscular mycorrhizal Glycine max and Lactuca sativa plants in relation to drought stress tolerance. Plant Mol. Biol.60, 389–404. 10.1007/s11103-005-4210-y (2006). [DOI] [PubMed] [Google Scholar]
- 23.Ruiz-Lozano, J. M., del Mar Alguacil, M., Bárzana, G., Vernieri, P. & Aroca, R. Exogenous ABA accentuates the differences in root hydraulic properties between mycorrhizal and non mycorrhizal maize plants through regulation of PIP aquaporins. Plant Mol. Biol.70, 565 (2009). [DOI] [PubMed] [Google Scholar]
- 24.Quiroga, G. et al. The arbuscular mycorrhizal symbiosis regulates aquaporins activity and improves root cell water permeability in maize plants subjected to water stress. Plant. Cell. Environ.42, 2274–2290. 10.1111/pce.13551 (2019). [DOI] [PubMed] [Google Scholar]
- 25.Cheng, H. Q. et al. PlantAquaporinResponsestoMycorrhizalSymbiosisunder Abiotic Stress (2020).
- 26.Bienert, G. P. et al. A conserved cysteine residue is involved in disulfide bond formation between plant plasma membrane aquaporin monomers. Biochem. J.445, 101–111 (2012). [DOI] [PubMed] [Google Scholar]
- 27.Fotiadis, D. et al. Structural characterization of two aquaporins isolated from native spinach leaf plasma membranes. J. Biol. Chem.276, 1707–1714 (2001). [DOI] [PubMed] [Google Scholar]
- 28.Murata, K. et al. Structural determinants of water permeation through aquaporin-1. Nature. 407, 599–605 (2000). [DOI] [PubMed] [Google Scholar]
- 29.Suga, S. & Maeshima, M. Water channel activity of radish plasma membrane aquaporins heterologously expressed in yeast and their modification by site-directed mutagenesis. Plant. Cell. Physiol.45, 823–830. 10.1093/pcp/pch120 (2004). [DOI] [PubMed] [Google Scholar]
- 30.Fetter, K., Van Wilder, V., Moshelion, M. & Chaumont, F. Interactions between plasma membrane aquaporins modulate their water channel activity. Plant. Cell.16, 215–228 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zelazny, E. et al. FRET imaging in living maize cells reveals that plasma membrane aquaporins interact to regulate their subcellular localization. Proc. Natl. Acad. Sci. U S A. 104, 12359–12364 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jozefkowicz, C., Berny, M. C., Chaumont, F. & Alleva, K. Heteromerization of plant aquaporins. Plant aquaporins: from transport to signaling, 29–46 (2017).
- 33.Alexandersson, E. et al. Transcriptional regulation of aquaporins in accessions of Arabidopsis in response to drought stress. Plant. Journal: Cell. Mol. Biology. 61, 650–660. 10.1111/j.1365-313X.2009.04087.x (2010). [DOI] [PubMed] [Google Scholar]
- 34.Boursiac, Y. et al. Early effects of salinity on water transport in Arabidopsis roots. Molecular and cellular features of aquaporin expression. Plant. Physiol.139, 790–805. 10.1104/pp.105.065029 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fouquet, R., Leon, C., Ollat, N. & Barrieu, F. Identification of grapevine aquaporins and expression analysis in developing berries. Plant Cell Rep.27, 1541–1550. 10.1007/s00299-008-0566-1 (2008). [DOI] [PubMed] [Google Scholar]
- 36.Polcyn, W., Paluch-Lubawa, E., Lehmann, T. & Mikula, R. Arbuscular Mycorrhiza in highly fertilized maize cultures alleviates short-term Drought effects but does not improve fodder yield and quality. Front. Plant. Sci.10, 496. 10.3389/fpls.2019.00496 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Adholeya, A., Tiwari, P. & Singh, R. in In vitro culture of mycorrhizas 315–338Springer, (2005).
- 38.Abas, L. & Luschnig, C. Maximum yields of microsomal-type membranes from small amounts of plant material without requiring ultracentrifugation. Anal. Biochem.401, 217–227 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Santoni, V. Plant plasma membrane protein extraction and solubilization for proteomic analysis. Methods Mol. Biol.355, 93–109. 10.1385/1-59745-227-0 (2007). [DOI] [PubMed] [Google Scholar]
- 40.Bradford, M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem.72, 248–254. 10.1006/abio.1976.9999 (1976). [DOI] [PubMed] [Google Scholar]
- 41.Alexandersson, E. et al. Purification and proteomic analysis of plant plasma membranes. Methods Mol. Biol.432, 161–173 (2008). [DOI] [PubMed] [Google Scholar]
- 42.McDonough, J. & Marbán, E. Optimization of IPG strip equilibration for the basic membrane protein mABC1. Proteomics. 5, 2892–2895 (2005). [DOI] [PubMed] [Google Scholar]
- 43.Kubala, S. et al. Deciphering priming-induced improvement of rapeseed (Brassica napus L.) germination through an integrated transcriptomic and proteomic approach. Plant. Sci.231, 94–113 (2015). [DOI] [PubMed] [Google Scholar]
- 44.Perkins, D. N., Pappin, D. J., Creasy, D. M. & Cottrell, J. S. Probability-based protein identification by searching sequence databases using mass spectrometry data. ELECTROPHORESIS: Int. J.20, 3551–3567 (1999). [DOI] [PubMed] [Google Scholar]
- 45.Perez-Riverol, Y. et al. The PRIDE database resources in 2022: A hub for mass spectrometry-based proteomics evidences. Nucleic Acids Res.50, D543–D552 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ishihama, Y. et al. Exponentially modified protein abundance index (emPAI) for estimation of absolute protein amount in proteomics by the number of sequenced peptides per protein. Mol. Cell. Proteomics. 4, 1265–1272 (2005). [DOI] [PubMed] [Google Scholar]
- 47.Kudlicki, A. The optimal exponent base for emPAI is 6.5. PLoS One. 7, 5 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jozefkowicz, C. et al. Loop A is critical for the functional interaction of two Beta vulgaris PIP aquaporins. PLoS ONE. 8, e57993 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Augé, R. M., Toler, H. D. & Saxton, A. M. Arbuscular mycorrhizal symbiosis alters stomatal conductance of host plants more under drought than under amply watered conditions: A meta-analysis. Mycorrhiza. 25, 13–24 (2015). [DOI] [PubMed] [Google Scholar]
- 50.Nunes, T. D. G., Zhang, D. & Raissig, M. T. Form, development and function of grass stomata. Plant J.101, 780–799. 10.1111/tpj.14552 (2020). [DOI] [PubMed] [Google Scholar]
- 51.Heinen, R. B. et al. Expression and characterization of plasma membrane aquaporins in stomatal complexes of Zea mays. Plant Mol. Biol.86, 335–350. 10.1007/s11103-014-0232-7 (2014). [DOI] [PubMed] [Google Scholar]
- 52.Ding, L. et al. The plasma membrane aquaporin ZmPIP2;5 enhances the sensitivity of stomatal closure to water deficit. Plant. Cell. Environ.45, 1146–1156. 10.1111/pce.14276 (2022). [DOI] [PubMed] [Google Scholar]
- 53.Heinen, R. B., Ye, Q. & Chaumont, F. Role of aquaporins in leaf physiology. J. Exp. Bot.60, 2971–2985 (2009). [DOI] [PubMed] [Google Scholar]
- 54.Yaaran, A. & Moshelion, M. Role of aquaporins in a Composite Model of Water Transport in the Leaf. Int. J. Mol. Sci.17 (2016). [DOI] [PMC free article] [PubMed]
- 55.Vandeleur, R. K. et al. Rapid shoot-to-root signalling regulates root hydraulic conductance via aquaporins. Plant. Cell. Environ.37, 520–538. 10.1111/pce.12175 (2014). [DOI] [PubMed] [Google Scholar]
- 56.Schenk, H. J., Jansen, S. & Holtta, T. Positive pressure in xylem and its role in hydraulic function. New Phytol.230, 27–45. 10.1111/nph.17085 (2021). [DOI] [PubMed] [Google Scholar]
- 57.Buckley, T. N., John, G. P., Scoffoni, C. & Sack, L. How does Leaf Anatomy Influence Water Transport outside the Xylem? Plant. Physiol.168, 1616–1635. 10.1104/pp.15.00731 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Brodribb, T. J. & Buckley, T. N. In the Leaf: A Platform for Performing Photosynthesis81–96 (Springer, 2018).
- 59.Jozefkowicz, C. et al. PIP water transport and its pH dependence are regulated by tetramer stoichiometry. Biophys. J.110, 1312–1321 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yaneff, A. et al. Heteromerization of PIP aquaporins affects their intrinsic permeability. Proc. Natl. Acad. Sci.111, 231–236 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ding, L. & Chaumont, F. Are aquaporins expressed in Stomatal complexes Promising targets to Enhance Stomatal dynamics? Front. Plant. Sci.11 (2020). [DOI] [PMC free article] [PubMed]
- 62.Yaneff, A., Vitali, V. & Amodeo, G. PIP1 aquaporins: intrinsic water channels or PIP2 aquaporin modulators? FEBS Lett.589, 3508–3515. 10.1016/j.febslet.2015.10.018 (2015). [DOI] [PubMed] [Google Scholar]
- 63.Yepes-Molina, L., Barzana, G. & Carvajal, M. Controversial regulation of Gene expression and Protein Transduction of Aquaporins under Drought and salinity stress. Plants (Basel). 910.3390/plants9121662 (2020). [DOI] [PMC free article] [PubMed]
- 64.Santoni, V., Vinh, J., Pflieger, D., Sommerer, N. & Maurel, C. A proteomic study reveals novel insights into the diversity of aquaporin forms expressed in the plasma membrane of plant roots. Biochem. J.373, 289–296 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Berny, M. C., Gilis, D., Rooman, M. & Chaumont, F. Single mutations in the transmembrane domains of maize plasma membrane aquaporins affect the activity of monomers within a heterotetramer. Mol. Plant. 9, 986–1003 (2016). [DOI] [PubMed] [Google Scholar]
- 66.Flexas, J. et al. Mesophyll diffusion conductance to CO2: an unappreciated central player in photosynthesis. Plant Sci.193, 70–84 (2012). [DOI] [PubMed] [Google Scholar]
- 67.Flexas, J. et al. In the leaf: A Platform for Performing Photosynthesis 163–208 (Springer, 2018).
- 68.Bárzana, G. et al. Arbuscular mycorrhizal symbiosis increases relative apoplastic water flow in roots of the host plant under both well-watered and drought stress conditions. Ann. Bot.109, 1009–1017 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Scoffoni, C., Sack, L. & Ort, D. The causes and consequences of leaf hydraulic decline with dehydration. J. Exp. Bot.68, 4479–4496. 10.1093/jxb/erx252 (2017). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium via the PRIDE partner repository with the dataset identifier PXD046819 and doi:10.6019/PXD046819.