Abstract
Objective
To estimate the association between food needs and diabetes outcomes.
Research design and methods
Longitudinal cohort study, using a target trial emulation approach. 96,792 adults with type 2 diabetes mellitus who underwent food need assessment in a network of community-based health centers were followed up to 36 months after initial assessment. We used targeted minimum loss estimation to estimate the association between not experiencing food needs, compared with experiencing food needs, and hemoglobin a1c (HbA1c), systolic and diastolic blood pressure (SBP and DBP), and LDL cholesterol. The study period was June 24th, 2016 to April 30th, 2023.
Results
We estimated that not experiencing food needs, compared with experiencing food needs, would be associated with 0.12 percentage points lower (95% Confidence Interval [CI] −0.16% to −0.09%, p = < 0.0001) mean HbA1c at 12 months. We further estimated that not experiencing food needs would be associated with a 12-month SBP that was 0.67 mm Hg lower (95%CI -0.97 to −0.38 mm Hg, p < .0001), DBP 0.21 mm Hg lower (95%CI -0.38 to −0.04 mm Hg, p = .01). There was no association with lower LDL cholesterol. Results were similar at other timepoints, with associations for HbA1c, SBP, and DBP of similar magnitude, and no difference in LDL cholesterol.
Conclusions
We estimated that not experiencing food needs may be associated with modestly better diabetes outcomes. These findings support testing interventions that address food needs as part of their mechanism of action.
Keywords: Diabetes mellitus, Food insecurity, Socioeconomic factors, Population health, Health equity, Social determinants of health
Highlights
-
•
The impact of addressing food needs on diabetes outcomes is unclear.
-
•
We estimated the impact of addressing food needs on diabetes outcomes.
-
•
We estimated improvements in hemoglobin a1c, systolic and diastolic blood pressure.
-
•
We did not estimate any improvement in low-density lipoprotein cholesterol.
Food insecurity, lack of consistent access to the food needed for an active, healthy life, is a major threat to public health (Rabbitt et al.; Seligman & Berkowitz, 2019). Food insecurity is associated with poor health outcomes through several pathways, including worse diet quality, forcing trade-offs between food and other goods and services needed for health (like medications), and increasing psychological distress (Leung & Tester, 2018; Myers, 2020; Orr et al., 2019; Palakshappa et al., 2021; Te et al., 2021). The harms of food insecurity are particularly clear for type 2 diabetes mellitus (T2DM). In T2DM, food insecurity is associated with worse metabolic control, and increased hypoglycemia, microvascular and macrovascular diabetes complications, emergency department visits, and hospitalizations (Berkowitz et al., 2013, 2018a; Crews et al., 2014, 2022; Dean et al., 2020; Gibson, 2019; Johnson et al., 2021; Seligman et al., 2012; Te et al., 2021; Volpp et al., 2023; Weinstein et al., 2022).
As understanding of the prevalence and harms of food insecurity has expanded, interventions to address it are of increasing clinical interest (Mozaffarian et al., 2024; Volpp et al., 2023). The provision of such interventions is facilitated by the recent rise in assessing food insecurity and other health-related social needs as part of clinical care (National Academies of Sciences). Although it is not feasible for clinical providers to administer comprehensive food insecurity measures used for research and epidemiologic surveillance (Arteaga & Wilde, 2023), brief ‘food needs’ assessments, which have adequate sensitivity and specificity for indicating food insecurity, are commonly used in many healthcare settings (De et al., 2023; Hager et al., 2010).
Despite their potential, many questions regarding clinical food need interventions are still unanswered. In part this is because randomized clinical trials – understood as the gold standard for testing intervention effects – have important limitations in their ability to test such interventions’ impacts. They can be slow and expensive to conduct, are often limited to small, non-generalizable samples, and are ill-equipped to identify specific routes of intervention impact when interventions may affect multiple concurrent pathways (Berkowitz et al., 2023; Carls et al., 2017; Najafzadeh & Schneeweiss, 2017; PCORI Methodology Standards, 2015).
Observational studies, like the one conducted here, can address some of these limitations while concurrently generating data to inform trial design (for example, by suggesting what effect sizes may be plausible in a given timeframe) (Hernán et al., 2022). Moreover, observational study samples may better reflect real-world populations than those in trials, which can have enrollment bias. Observational studies can also focus on specific pathways of interest, which is difficult to study in multi-component interventions such as those commonly used to address food needs (PCORI Methodology Standards, 2015).
Of course, observational data must be used carefully. One well known issue relates to unmeasured confounding (Hernán & Robins, 2020). But another important issue relates to study design—some observational study designs create selection and other biases that present threats to validity even in the absence of unmeasured confounding (Hernán et al., 2022; Hoffman et al., 2022; Matthews et al., 2022). To help prevent these issues, a target trial emulation framework (that is, thinking of an observational study as if trying to carry out a randomized trial) can be helpful (Hernán et al., 2022; Hoffman et al., 2022; Madenci et al., 2021; Matthews et al., 2022).
This study used observational data to generate evidence that both complements and informs studies of food need interventions. To help avoid common biases of other observational studies, we used a target trial emulation framework to conduct an observational study designed to estimate how a hypothetical intervention addressing food needs might affect outcomes in a sample of adults with T2DM seen in community health centers.
1. Methods
We conducted a target trial emulation study of the association between food needs and outcomes for adults with T2DM. The institutional review board at the University of North Carolina at Chapel Hill provided approval for these analyses. The purpose of this study was to evaluate the association between food needs and diabetes outcomes, and this study does not evaluate actual interventions that may have been provided.
In designing the target trial emulation, investigators need to determine which treatment strategies to emulate. One way to address food needs is to prevent them from occurring. Our primary analyses attempt to emulate this (described in more detail below). However, another way to address them is to ‘treat’ them once they occur. We also attempted to emulate this strategy in a set of sensitivity analyses, described in more detail in the statistical analysis section.
1.1. Setting and study sample
This study used data from a network of community-based health centers located across the U.S. that share an electronic health record (EHR) through membership in OCHIN, Inc. (not an acronym) (Gold et al., 2017, 2018). We drew on prior work to identify a cohort of adults with T2DM who received an assessment for food needs at least once in the study period (Gold et al., 2018; Nichols et al., 2015, 2016). Data were observed from June 24th, 2016 to April 30th, 2023; data analysis occurred through June 2024.
1.2. Components of target trial protocol
This study applied a target trial emulation approach to help understand the connection between food needs and diabetes outcomes (Hernán et al., 2022; Hoffman et al., 2022; Madenci et al., 2021). A protocol for this hypothetical target trial is presented as Appendix Table 1.
We included adults (age 18 years or older at time of initial food need assessment) with T2DM, determined by a previously validated algorithm (Nichols et al., 2016), who received a food need assessment at least once in a network clinic between June 24th, 2016 and April 30th, 2023 (Appendix Table 2). (Gold et al., 2018) To make the sample as generalizable as possible, we included data from all individuals who met these criteria.
We defined two situations of interest (within the terminology of target trial emulation, these situations are referred to as ‘intervention strategies’): ‘always report a food need’ and ‘always report not having a food need.’ These can be thought of as testing a hypothetical intervention that effectively prevents food needs from occurring, compared with a scenario in which an intervention caused individuals to experience persistent food needs. An individual was considered ‘assigned’ to the ‘have a food need’ strategy if they reported a food need at their first food need assessment, and ‘assigned’ to the ‘do not have a food need’ strategy if they reported not having a food need at their first assessment.
Food need assessment data were taken from the OCHIN EHR, where the results of a food need assessment are recorded (Bensken et al., 2023; Gold et al., 2018; Nguyen et al., 2023). In the EHR's social determinants interface, users can choose from several widely-used tools. The tools most often used are the Protocol for Responding to and Assessing Patient's Assets, Risks, and Experience (PRAPARE) tool(CSL STYLE ERROR: reference with no printed form.) and the Accountable Health Communities (AHC) Health-Related Social Needs Tool. (CMS) PRAPARE was developed by the National Association of Community Health Centers for use in community health centers and has high penetrance. (CSL STYLE ERROR: reference with no printed form.) The AHC tool was developed by CMS, and its food items are identical to those of the widely used Hunger Vital Sign, which derives from the USDA Household Food Security Survey Module (Hager et al., 2010; CMS; Bickel et al.). Prior studies have found that several tools assess food needs similarly well, allowing for determination of food need status that is homogenous across instruments used. (Risk Screening Tools Review) The primary outcome was hemoglobin A1c (HbA1c). Secondary outcomes were systolic blood pressure (SBP), diastolic blood pressure (DBP), and low-density lipoprotein (LDL) cholesterol. For most individuals with diabetes, the recommendation would be for HbA1c to be <7.0% or <8.0% (depending on comorbidities, preferences, and the treatments needed to achieve that level), the blood pressure treatment goal would be < 130/80 mm Hg, and the LDL goal would be < 70 mg/dL, although these recommendations need to be individualized (American Diabetes Association Professional Practice Committee, 2023, 2024).
The date of the first food need assessment was designated ‘time zero’, or the date the individual became ‘eligible for’ and was ‘enrolled in’ the hypothetical intervention (Appendix Fig. 1). The primary time point for outcome analysis was 12 months after the date of food need assessment. Secondary time points were 6, 18, 24, 30, and 36 months after the date of first food need assessment.
We sought to estimate the difference in mean values of the outcome that result from contrasting the hypothetical scenario in which an intervention prevented the entire cohort from having a food need with the hypothetical scenario in which the entire cohort experienced a persistent food need.
In an actual clinical trial, randomization is meant to provide unconditional exchangeability between treatment groups. This observational study needed to account for potential confounding between the experience of food needs and diabetes outcomes, to help support conditional exchangeability. To do so, we considered several covariates known from prior work to be associated with both food needs and study outcomes (Rabbitt et al.; Gundersen & Ziliak, 2015; Berkowitz et al., 2024). These covariates were designated as time-invariant or time-varying. Time-invariant covariates were: date of first food need assessment; age at first food need assessment; sex (we use the term ‘sex’ in this paper while acknowledging that the EHR data available often do not distinguish well between sex and gender identity (Bates et al., 2022)); race and ethnicity (socially constructed variables included as they may indicate the experience of racism); primary language; and an ICD-10 code based comorbidity index (Sun et al., 2017) meant to indicate the burden of other conditions participants experienced, calculated at the time of first food need assessment. Time-varying covariates were: health insurance, income expressed as a percentage of the federal poverty threshold (to account for both household size and inflation), the social vulnerability index of the census tract of residence (given that area-level factors can impact diabetes outcomes) (CDC/ATSDR Social Vulnerability Index, 2024), and the analysis outcome in the preceding periods (e.g., HbA1c prior to food need assessments for analyses where HbA1c is the outcome). A directed acyclic graph (DAG) illustrates the hypothesized relationship between the included variables under which our analyses were conducted (Appendix Fig. 2).
If on a subsequent food needs assessment individuals reported a status not concordant with their ‘assigned’ strategy, they were censored from that time point forward (i.e., if a person ‘assigned’ to have a food need based on their first food need assessment then reported that they did not have a food need in a subsequent assessment, or if a person ‘assigned’ to not have a food need then reported that they did have a food need, their data were censored from the time of the later assessment onwards) (Appendix Table 3). (Hernán et al., 2016, 2022; Hernán & Robins, 2017) Censoring data at the time of deviation from the hypothetical protocol is necessary to prevent bias that could otherwise be introduced by excluding those individuals from the analysis entirely or not accounting for the fact that their data are no longer consistent with their ‘assigned’ treatment strategy (Hernán et al., 2016; Hernán & Robins, 2017). Individuals were considered lost to follow-up, and censored, if they did not report whether or not they had a food need at least every 24 months, or did not have an outcome measurement within 6 months prior to the timepoint of a particular analysis (e.g., for the 12 month analysis of HbA1c, individuals were censored if they did not have an HbA1c measurement at some point in the preceding 6 months).
Missing data for variables not related to censoring as described above were handled using multiple imputation by chained equations, with 50 imputation datasets (Appendix Table 4). (Buuren et al., 2023; White et al., 2011)
1.3. Statistical analysis
To estimate the differences in means as described above, we used targeted minimum loss estimation (TMLE) (Laan & Rose, 2013, p. 700; van der and van der Laan, 2010; Díaz et al., 2021). TMLE is a multiply-robust approach that is well suited for these analyses given the possibility of treatment confounder feedback (e.g., when a confounder in one period affects a treatment that in turn can affect the confounder in a subsequent period) (Hernán & Robins, 2020; Laan & Rose, 2013). For instance, if income in one period affects the risk of experiencing a food need in that period, but experiencing a food need affects income in the subsequent period (e.g., by worsening health and impairing the ability to work), then conventional regression approaches (such as linear mixed models) will be biased as they must either adjust for income even though it is a mediator, or not adjust for income even though it is a confounder (Hernán & Robins, 2020; Johnson et al., 2021; Weinstein et al., 2022). Methods such as TMLE do not have this limitation (Hernán & Robins, 2020; Laan & Rose, 2013).
In brief, TMLE, at each timepoint, first fits an outcome regression model and standardizes outcome estimates over the distribution of observed covariates (similar to parametric g-formula analyses) (Laan & Rose, 2013,; KHstats). It then estimates the treatment mechanism (i.e., the probability of ‘treatment’ conditional on covariates, also called the ‘propensity score’) and finally uses estimates from that propensity score model to update the outcome estimates made in the first step, by using what is termed the ‘clever covariate’ to fluctuate initial estimates (Laan & Rose, 2013; van der Laan, 2010). TMLE also estimates the censoring mechanism along with the treatment mechanism, which helps account for possibly informative censoring, such as differential loss to follow-up (similar to inverse probability of censoring weights) (van der Laan & Rose). It does this analogously to its approach for estimating the treatment mechanism, using observed variables to estimate the probability of remaining uncensored, incorporating these estimates into the ‘clever covariate,’ and using the ‘clever covariate’ to update the initial estimates to account for (possibly informative) censoring (Laan & Rose, 2013).
Our primary approach in conducting TMLE used generalized linear models. However, TMLE can also be conducted using an ensemble of supervised machine learning algorithms that may better account for interactions and nonlinearities in the data, and reduce the impact of model misspecification on results (Laan & Rose, 2013; van der et al., 2007). This is sometimes called a ‘SuperLearner’ approach (van der Laan et al., 2007). We conducted TMLE using a SuperLearner approach in sensitivity analyses, using an ensemble of generalized linear models, multivariable adaptive regression splines, and gradient boosted tree learners.
As noted above, one way to address food needs is to prevent them from occurring, as modeled in our main analyses, while another is to ‘treat’ them once they occur. To investigate this second approach, we conducted another set of sensitivity analyses to assess associations between a hypothetical intervention that treated food needs after individuals experienced them and diabetes outcomes. To do this, we created a sub-cohort from our primary analytic cohort consisting of those individuals who initially reported a food need and then reported whether or not they still had a food need within 12 months of their initial assessment. We ‘assigned’ those who again reported a food need to an interventional strategy of ‘continue to report a food need’ and those who reported not having a food need at follow-up to an interventional strategy of ‘continue to report not having a food need’. This can be thought of as testing a hypothetical intervention that effectively treats food needs once they occur, compared with a scenario in which individuals persistently experience food needs. We then estimated the difference in outcome means at the 12-month timepoint in this cohort, following the same hypothetical trial protocol and analytic approach as for the main analyses.
Analyses were performed in SAS version 9.4 and R version 4.3.1. Multiple imputation was conducted using the mice package, and TMLE analyses were conducted using the lmtp package (Díaz et al., 2021; Hoffman et al., 2023; Williams & Díaz, 2022). Results across 50 multiple imputation datasets were combined using Rubin's rules (Little & Rubin, 2002).
1.4. Data availability
The datasets generated during and analyzed during the current study are not publicly available due to the data use agreements under which the study was conducted.
2. Results
2.1. Characteristics of the study sample
96,792 adults with T2DM were assessed for a food need at least once and were included in the study. They lived in 41 states and were seen in 1268 unique departments of healthcare facilities. The cohort was 56.3% female, the mean age at time of first food need assessment was 55.1 years (SD: 13.7 years), and the mean income expressed as a percentage of the federal poverty threshold was 123.5% (SD: 308.4%) (Table 1). 24,767 (25.6%) individuals reported a food need at initial screening.
Table 1.
Demographic characteristics of cohort.
| Characteristic |
Overall |
Did Not Report Food Need |
Reported Food Need |
P-value |
|---|---|---|---|---|
| N = 96,792 |
N = 72,025 |
N = 24,767 |
||
| Mean (SD) or % (N) | Mean (SD) or % (N) | Mean (SD) or % (N) | ||
| Age at Initial Food Need Assessment, Years | 55.15 (13.68) | 55.77 (14.05) | 53.33 (12.36) | <0.001 |
| Female | 56.34% (54,516) | 56.10% (40,396) | 57.04% (14,120) | 0.01 |
| Racial Identity | <0.001 | |||
| American Indian/Alaska Native | 1.11% (1079) | 1.04% (752) | 1.32% (327) | |
| Asian | 4.78% (4624) | 5.36% (3858) | 3.09% (766) | |
| Black | 26.03% (25,198) | 25.31% (18,227) | 28.15% (6971) | |
| Multiple | 0.89% (857) | 0.73% (524) | 1.34% (333) | |
| Native Hawaiian or Other Pacific Islander | 0.77% (750) | 0.73% (529) | 0.89% (221) | |
| Not Reported | 7.90% (7647) | 7.75% (5582) | 8.34% (2065) | |
| White | 58.51% (56,637) | 59.08% (42,553) | 56.87% (14,084) | |
| Hispanic Ethnicity | 33.99% (31,698) | 34.45% (23,889) | 32.65% (7809) | <0.001 |
| Primary Language Other Than English | 33.86% (32,749) | 35.26% (25,381) | 29.79% (7368) | <0.001 |
| Comorbidity Index | 0.23 (0.93) | 0.22 (0.95) | 0.27 (0.89) | <0.001 |
| Health Insurance | <0.001 | |||
| Medicaid | 35.19% (33,513) | 32.72% (23,184) | 42.40% (10,329) | |
| Medicare | 25.72% (24,490) | 27.06% (19,172) | 21.83% (5318) | |
| Other Public | 2.03% (1934) | 2.01% (1423) | 2.10% (511) | |
| Private | 18.51% (17,626) | 20.77% (14,716) | 11.95% (2910) | |
| Uninsured | 18.55% (17,659) | 17.45% (12,366) | 21.73% (5293) | |
| Household Income as Percentage of Federal Poverty Threshold | 123.50 (308.37) | 133.46 (328.81) | 96.35 (242.06) | <0.001 |
| Social Vulnerability Index at Census Tract Level | 0.69 (0.24) | 0.69 (0.24) | 0.71 (0.23) | <0.001 |
| Mean HbA1c in Year Prior to Food Need Assessment, % | 7.66 (1.95) | 7.60 (1.89) | 7.82 (2.09) | <0.001 |
| Mean Systolic Blood Pressure in Year Prior to Food Need Assessment, mm Hg | 129.78 (13.49) | 129.75 (13.44) | 129.87 (13.64) | 0.80 |
| Mean Diastolic Blood Pressure in Year Prior to Food Need Assessment, mm Hg | 78.05 (8.01) | 77.79 (7.93) | 78.78 (8.18) | <0.001 |
| Mean LDL Cholesterol in Year Prior to Food Need Assessment, mg/dL | 101.11 (35.17) | 100.61 (34.93) | 102.52 (35.79) | <0.001 |
Greater comorbidity index scores indicate greater comorbidity; greater social vulnerability index scores indicates greater risk; HbA1c = hemoglobin a1c; LDL = low density lipoprotein.
The unadjusted mean HbA1c in the year prior to food need assessment was 7.82% in individuals who reported a food need, and 7.60% in individuals who did not (p < .001). Similarly, comparing individuals who did versus did not report a food need, unadjusted mean SBP in the year prior to assessment was 129.9 vs. 129.8 mm Hg (p = .80), unadjusted mean DBP was 78.8 vs. 77.8 mm Hg (p < .001), and unadjusted mean LDL was 102.5 vs. 100.6 mg/dL (p < .001).
2.2. Target trial emulation
We estimated that not experiencing food needs, compared with experiencing food needs, would be associated with a 0.12 percentage points lower mean HbA1c at 12 months (95% Confidence Interval [CI] -0.16% to -0.09%, p = < 0.0001) (Table 2, Fig. 1, Appendix Table 5). The observed HbA1c standard deviation of 1.9% implies that an actual clinical trial would need approximately 7560 individuals (3780 per arm) to have 80% power of detecting a difference of this magnitude or greater.
Table 2.
Differences in diabetes outcomes associated with not experiencing, compared with experiencing, food needs among all individuals assessed for food needs.
| Timepoint |
Hemoglobin A1c |
Systolic Blood Pressure |
Diastolic Blood Pressure |
LDL Cholesterol |
||||
|---|---|---|---|---|---|---|---|---|
| Estimated Mean Difference, % (95% CI) | p-value | Estimated Mean Difference, mm Hg (95% CI) | p-value | Estimated Mean Difference, mm Hg (95% CI) | p-value | Estimated Mean Difference, mg/dL (95% CI) | p-value | |
| 6 Months | −0.13 (−0.17 to −0.09) | <0.0001 | −0.44 (−0.68 to −0.20) | 0.0003 | −0.26 (−0.40 to −0.11) | 0.0004 | −0.30 (−1.40 to 0.81) | 0.60 |
| 12 Months | −0.12 (−0.16 to −0.09) | <0.0001 | −0.67 (−0.97 to −0.38) | <0.0001 | −0.21 (−0.38 to −0.04) | 0.01 | −0.60 (−1.80 to 0.60) | 0.33 |
| 18 Months | −0.15 (−0.19 to −0.10) | <0.0001 | −0.63 (−0.98 to −0.29) | 0.0003 | −0.39 (−0.59 to −0.19) | 0.0002 | −0.40 (−1.66 to 0.86) | 0.54 |
| 24 Months | −0.19 (−0.24 to −0.14) | <0.0001 | −1.04 (−1.40 to −0.68) | <0.0001 | −0.48 (−0.69 to −0.27) | <0.0001 | −1.13 (−2.41 to 0.15) | 0.08 |
| 30 Months | −0.21 (−0.27 to −0.15) | <0.0001 | −0.51 (−1.01 to 0.00) | 0.0498 | −0.50 (−0.82 to −0.18) | 0.002 | 0.65 (−0.71 to 2.01) | 0.35 |
| 36 Months | −0.20 (−0.28 to −0.11) | <0.0001 | −0.23 (−1.06 to 0.61) | 0.60 | −0.56 (−1.01 to −0.11) | 0.02 | 1.21 (−0.84 to 3.26) | 0.25 |
Estimated mean difference compares the estimated difference in outcomes between counterfactual scenarios in which individuals did not versus did experience a food need from the time of first food need assessment to the specified time point, using a longitudinal targeted minimum loss estimation approach. A negative value indicates an association with estimated benefit.
LDL = Low Density Lipoprotein.
CI = Confidence Interval.
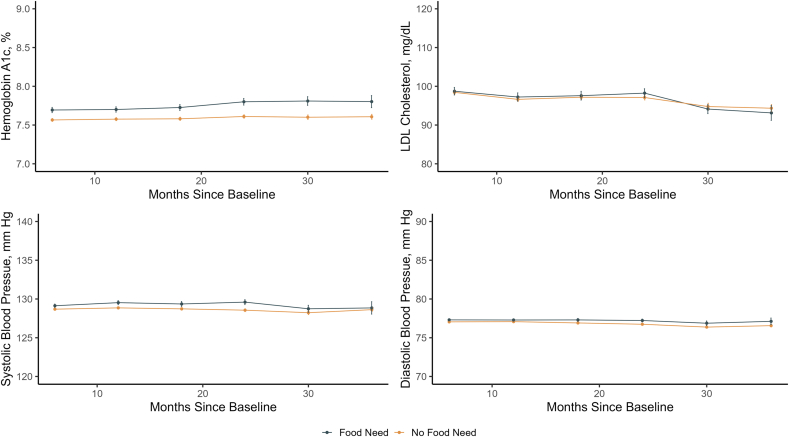
Fig. 1.
Legend: Estimated of Mean Hemoglobin A1c, Systolic Blood Pressure, Diastolic Blood Pressure, and Low Density Lipoprotein (LDL) Cholesterol comparing counterfactual scenarios in which individuals did versus did not experience a food need between baseline and the specified time points.
For other study outcomes at 12 months, the differences were: SBP 0.67 mm Hg lower (95%CI -0.97 to -0.38 mm Hg, p < .0001), DBP 0.21 mm Hg lower (95%CI -0.38 to -0.04 mm Hg, p = .01), and LDL 0.60 mg/dL lower (95%CI -1.80 to 0.60 mg/dL, p = .33). Results were similar at other timepoints, typically with differences of similar magnitude associated with not experiencing food needs for HbA1c, SBP, and DBP, and no difference for LDL cholesterol. In sensitivity analyses using the SuperLearner approach to TMLE, results were quite similar (Appendix Table 6).
The demographics of the cohort of individuals who initially reported a food need and had a re-assessment within 12 months are reported in Appendix Table 7. Target trial emulation analyses in this cohort yielded results similar to the main analyses (Table 3).
Table 3.
Differences in diabetes outcomes at 12 Months associated with not experiencing, compared with experiencing, food needs among individuals who initially reported a food need.
| Hemoglobin A1c, % (95% CI) | Systolic Blood Pressure, mm Hg (95% CI) | Diastolic Blood Pressure, mm Hg (95% CI) | LDL Cholesterol, mg/dL (95% CI) | |
|---|---|---|---|---|
| Mean if No Food Need | 7.63 (7.54–7.72) | 128.94 (128.17–129.71) | 77.00 (76.56–77.44) | 94.88 (92.07–97.68) |
| Mean if Food Need | 7.76 (7.66–7.86) | 129.85 (129.08–130.63) | 77.65 (77.19–78.10) | 95.36 (92.58–98.15) |
| Estimated Mean Difference | −0.13 (−0.26 to 0.00) | −0.91 (−1.97 to 0.15) | −0.65 (−1.26 to −0.04) | −0.49 (−4.37 to 3.40) |
Estimated mean difference compares the estimated difference in outcomes between counterfactual scenarios in which individuals did not versus did experience a food need from the time of first food need assessment to the specified time point, using a longitudinal targeted minimum loss estimation approach. A negative value indicates an association with estimated benefit.
LDL = Low Density Lipoprotein.
CI = Confidence Interval.
3. Discussion
In this observational study among adults with T2DM seen in community health centers, we estimated that not experiencing food needs, compared with experiencing persistent unmet food needs, would be associated with lower HbA1c, SBP, and DBP at most timepoints, and would not be associated with lower LDL. Despite the statistical significance of the associations, the magnitude of the difference was small and may not be clinically meaningful. Sensitivity analyses estimating associations with ‘treating’ food needs once they occurred, rather than preventing them, yielded similar results.
The results of this study are consistent with and expand on those of previous studies. Several studies, albeit not specific to T2DM, have found that SNAP (the Supplemental Nutrition Assistance Program), which effectively relieves food insecurity, is associated with reduced healthcare utilization, suggesting a beneficial impact on health (Berkowitz et al., 2017, 2021). Specific to T2DM, prior work, predominately cross-sectional, found that food insecurity, food needs, and other forms of food hardships are associated with worse HbA1c (Berkowitz et al., 2013; Seligman et al., 2012; Te Vazquez et al., 2021). However, some randomized trials of food interventions did not find significant improvements for T2DM outcomes (Bryce et al., 2021; Doyle et al., 2023; Seligman et al., 2018). The effect estimates in those studies had confidence intervals that included the estimates seen here (Bryce et al., 2021; Doyle et al., 2023; Seligman et al., 2018). Moreover, our results suggest that an actual trial would need a sample size of approximately 7560 patients to have 80% power for detecting HbA1c differences of the magnitude seen here. As this is larger than many randomized food need intervention trials to date, it may be that studies with null results were underpowered to detect real, but modest, differences.
Differences in HbA1c and blood pressure outcomes were apparent at the 6- and 12-month timeframes, with little change in those differences at later timepoints. Many randomized trials of interventions to address food needs use 6-to 12-month timeframes, and our results provide support for doing so. Finally, we did not find associations between food needs and LDL cholesterol outcomes, also consistent with prior interventional work (Berkowitz et al., 2019).
The estimated associations between addressing food needs and HbA1c and blood pressure seen in this study were smaller than those seen in some other observational studies (Berkowitz et al., 2013, 2018b; Seligman et al., 2011, 2012). There may be several explanations for this. First, this study adopted a target trial emulation approach that may have addressed forms of bias affecting other observational studies (Hernán et al., 2022; Hoffman et al., 2022; Matthews et al., 2022). Second, in focusing on community-based health centers, which on average serve lower income populations than other clinic types, we may have removed some confounding due to income or other socioeconomic factors more effectively than prior studies. Third, this study defined its exposures using data on pragmatic food need assessments as performed in clinics, rather than longer food insecurity instruments used in research. This is justified as these measures are used in practice to identify food needs and determine intervention eligibility, but it may also have resulted in misclassification bias relative to using longer instruments.
This study has important implications and suggests directions for future investigation. The analyses examined a hypothetical interventional strategy in the abstract, rather than a specific intervention, to focus on one particular pathway to poor health for the purposes of epidemiological investigation. Real-world interventions often have more than one mechanism of action. For example, medically tailored meals can address food needs, improve diet quality, and provide social support—all of which may have clinical benefits (Berkowitz et al., 2020). Thus, real-world interventions may have larger benefits than estimated here. These findings do support the idea, however, of addressing multiple pathways to maximize clinical impact, which is central to many ‘food is medicine’ interventions that seek to combine food provision with clinical support (Mozaffarian et al., 2024; Volpp et al., 2023).
Study results should be considered in light of several limitations. First, this observational study may be subject to bias owing to unmeasured confounding. Although we did adjust for measured time varying and time invariant confounders and used a design that helps account for unmeasured time invariant confounding, unmeasured time varying confounding is still possible. Given the possibility of unmeasured time-varying confounding, the associations we report may not be causal. Second, the study sample drew from patients seen in community-based health centers that shared a common EHR, and thus may not be nationally representative. However, it is a large sample of diverse clinics located across many states, and the focus on individuals seen in community-based health centers is important as they are disproportionately burdened by food needs. Third, some data collection occurred during the COVID pandemic, but we adjusted for the time at which observations were made, and it is not clear that this would have differential impact on the groups studied. Fourth, we did not have data on use of programs such as SNAP (the Supplemental Nutrition Assistance Program). Finally, the study goal was to better understand the relationship between food needs and clinical outcomes, rather than to evaluate a specific intervention. We believe this is scientifically useful, but note that these results do not predict what any particular intervention will achieve. We plan additional work evaluating the impact of specific interventions that address food needs. These limitations were balanced by several strengths. We observed a large number of individuals over a relatively long timeframe and used both study design and analytical approaches that helped minimize the impact of confounding and selection bias on study results.
4. Conclusions
Overall, we found evidence suggesting that not experiencing food needs among adults with T2DM may be associated with significantly, albeit modestly, better diabetes outcomes. These findings support testing interventions that address adverse social conditions, like food needs, as one part of their putative mechanism of action. These findings also help define what sample sizes and timeframes may be useful for detecting intervention effects in clinical trials. As it is clear that adverse social conditions impact health for people with T2DM, learning how to improve health for people in these circumstances is critical. This study makes progress in understanding how to do so.
Funding
This work was funded in part by R01DK125406. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Role of the funder
The funding organizations had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Ethical statement
The institutional review board at the University of North Carolina at Chapel Hill provided approval for these analyses.
CRediT authorship contribution statement
Seth A. Berkowitz: Writing – original draft, Supervision, Formal analysis, Conceptualization. Aileen Ochoa: Writing – review & editing, Data curation. Jenna M. Donovan: Writing – review & editing, Data curation. Jenine Dankovchik: Writing – review & editing, Data curation. Myklynn LaPoint: Writing – review & editing, Project administration, Data curation. Marlena L. Kuhn: Writing – review & editing, Data curation. Suzanne Morrissey: Writing – review & editing, Project administration. Mufeng Gao: Writing – review & editing, Methodology, Data curation. Michael G. Hudgens: Writing – review & editing, Methodology. Sanjay Basu: Writing – review & editing, Methodology, Conceptualization. Rachel Gold: Writing – review & editing, Supervision, Project administration, Methodology, Funding acquisition, Conceptualization.
Declaration of competing interest
SAB reports research grants from NIH, North Carolina Department of Health and Human Services, Blue Cross Blue Shield of North Carolina, Feeding America, the American Diabetes Association, and the American Heart Association, and personal fees from the Aspen Institute, Rockefeller Foundation, Gretchen Swanson Center for Nutrition, and Kaiser Permanente, outside of the submitted work. ML reports research grant support from North Carolina Department of Health and Human Services. SM reports research support from NIH. All other authors declare nothing to report.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ssmph.2024.101709.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
Data availability
The data that have been used are confidential.
References
- American Diabetes Association Professional Practice Committee 6. Glycemic goals and hypoglycemia: Standards of care in diabetes—2024. Diabetes Care. 2023;47(Supplement_1):S111–S125. doi: 10.2337/dc24-S006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Diabetes Association Professional Practice Committee 10. Cardiovascular disease and risk management: Standards of care in diabetes-2024. Diabetes Care. 2024;47(Suppl 1):S179–S218. doi: 10.2337/dc24-S010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arteaga I., Wilde P.E. Measuring food security in the United States for more than 25 years: History, methods, findings, and opportunities. Journal of the Academy of Nutrition and Dietetics. 2023;123(10):S5–S19. doi: 10.1016/j.jand.2023.01.007. [DOI] [PubMed] [Google Scholar]
- Bates N., Chin M., Becker T., editors. Measuring sex, gender identity, and sexual orientation [internet] National Academies Press; Washington, D.C.: 2022. https://www.nap.edu/catalog/26424 [cited 2024 Mar 28]. Available from: [PubMed] [Google Scholar]
- Bensken W.P., McGrath B.M., Gold R., Cottrell E.K. Area-level social determinants of health and individual-level social risks: Assessing predictive ability and biases in social risk screening. J Clin Transl Sci. 2023;7(1) doi: 10.1017/cts.2023.680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkowitz S.A., Baggett T.P., Wexler D.J., Huskey K.W., Wee C.C. Food insecurity and metabolic control among U.S. adults with diabetes. Diabetes Care. 2013;36(10):3093–3099. doi: 10.2337/dc13-0570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkowitz S.A., Basu S., Hanmer J. Eliminating food insecurity in the USA: A target trial emulation using observational data to estimate effects on health-related quality of life. Journal of General Internal Medicine. 2023;38(10):2308–2317. doi: 10.1007/s11606-023-08095-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkowitz S.A., Delahanty L.M., Terranova J., Steiner B., Ruazol M.P., Singh R., et al. Medically tailored meal delivery for diabetes patients with food insecurity: A randomized cross-over trial. Journal of General Internal Medicine. 2019;34(3):396–404. doi: 10.1007/s11606-018-4716-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkowitz S.A., Karter A.J., Corbie-Smith G., Seligman H.K., Ackroyd S.A., Barnard L.S., et al. Food insecurity, food “deserts,” and glycemic control in patients with diabetes: A longitudinal analysis. Diabetes Care. 2018:dc171981. doi: 10.2337/dc17-1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkowitz S.A., Palakshappa D., Rigdon J., Seligman H.K., Basu S. Supplemental nutrition assistance program participation and health care use in older adults : A cohort study. Annals of Internal Medicine. 2021 doi: 10.7326/M21-1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkowitz S.A., Seligman H.K., Meigs J.B., Basu S. Food insecurity, healthcare utilization, and high cost: A longitudinal cohort study. American Journal of Managed Care. 2018;24(9):399–404. [PMC free article] [PubMed] [Google Scholar]
- Berkowitz S.A., Seligman H.K., Palakshappa D. Understanding food insecurity risk in the United States: A longitudinal analysis. SSM - Popul Health. 2024;25 doi: 10.1016/j.ssmph.2023.101569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkowitz S.A., Seligman H.K., Rigdon J., Meigs J.B., Basu S. Supplemental nutrition assistance program (SNAP) participation and health care expenditures among low-income adults. JAMA Internal Medicine. 2017;177(11):1642–1649. doi: 10.1001/jamainternmed.2017.4841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkowitz S.A., Shahid N.N., Terranova J., Steiner B., Ruazol M.P., Singh R., et al. “I was able to eat what I am supposed to eat”-- patient reflections on a medically-tailored meal intervention: A qualitative analysis. BMC Endocrine Disorders. 2020;20(1):10. doi: 10.1186/s12902-020-0491-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickel G, Nord M, Price C, Hamilton W, Cook J. Guide to Measuring Household Food Security, Revised 2000. [Internet]. U.S. Department of Agriculture, Food and Nutrition Service, Alexandria, VA; 2000 [cited 2018 Sep 17]. Available from: https://fns-prod.azureedge.net/sites/default/files/FSGuide.pdf.
- Bryce R., Wolfson J.A., Cohen A., Milgrom N., Garcia D., Steele A., et al. A pilot randomized controlled trial of a fruit and vegetable prescription program at a federally qualified health center in low income uncontrolled diabetics. Prev Med Rep. 2021;23 doi: 10.1016/j.pmedr.2021.101410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buuren S van, Groothuis-Oudshoorn K., Vink G., Schouten R., Robitzsch A., Rockenschaub P., et al. mice: Multivariate imputation by chained equations [internet] 2023. https://cran.r-project.org/web/packages/mice/index.html [cited 2024 Mar 8]. Available from:
- Carls G.S., Tuttle E., Tan R.D., Huynh J., Yee J., Edelman S.V., et al. Understanding the gap between efficacy in randomized controlled trials and effectiveness in real-world use of GLP-1RA and DPP4 therapies in patients with type 2 diabetes. Diabetes Care. 2017;10 doi: 10.2337/dc16-2725. [DOI] [PubMed] [Google Scholar]
- CDC/ATSDR social vulnerability index (SVI) [internet] 2024. https://www.atsdr.cdc.gov/placeandhealth/svi/index.html [cited 2024 Mar 28]
- CMS. The Accountable Health Communities Health-Related Social Needs Screening Tool [Internet]. [cited 2019 Sep 28]. Available from: https://innovation.cms.gov/Files/worksheets/ahcm-screeningtool.pdf.
- Crews D.C., Kuczmarski M.F., Grubbs V., Hedgeman E., Shahinian V.B., Evans M.K., et al. Effect of food insecurity on chronic kidney disease in lower-income Americans. American Journal of Nephrology. 2014;39(1):27–35. doi: 10.1159/000357595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews D., Patzer R., Cervantes L., Knight R., Purnell T., Powe N., et al. Designing interventions addressing structural racism to reduce kidney health disparities: A report from an NIDDK workshop. J Am Soc Nephrol JASN. 2022 doi: 10.1681/ASN.2022080890. ASN.2022080890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Marchis E.H., Aceves B.A., Brown E.M., Loomba V., Molina M.F., Gottlieb L.M. Assessing implementation of social screening within US health care settings: A systematic scoping review. J Am Board Fam Med JABFM. 2023:220401R1. doi: 10.3122/jabfm.2022.220401R1. jabfm.2022. [DOI] [PubMed] [Google Scholar]
- Dean E.B., French M.T., Mortensen K. Food insecurity, health care utilization, and health care expenditures. Health Services Research. 2020;55(Suppl 2):883–893. doi: 10.1111/1475-6773.13283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Díaz I., Williams N., Hoffman K.L., Schenck E.J. Nonparametric causal effects based on longitudinal modified treatment policies. Journal of the American Statistical Association. 2021;0(0):1–16. [Google Scholar]
- Doyle J., Alsan M., Skelley N., Lu Y., Cawley J. Effect of an intensive food-as-medicine program on health and health care use: A randomized clinical trial. JAMA Internal Medicine. 2023 doi: 10.1001/jamainternmed.2023.6670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson D.M. Food insecurity, eye care receipt, and diabetic retinopathy among US adults with diabetes: Implications for primary care. Journal of General Internal Medicine. 2019 doi: 10.1007/s11606-019-04992-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold R., Bunce A., Cowburn S., Dambrun K., Dearing M., Middendorf M., et al. Adoption of social determinants of health EHR tools by community health centers. The Annals of Family Medicine. 2018;16(5):399–407. doi: 10.1370/afm.2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold R., Cottrell E., Bunce A., Middendorf M., Hollombe C., Cowburn S., et al. Developing electronic health record (EHR) strategies related to health center patients' social determinants of health. The Journal of the American Board of Family Medicine. 2017;30(4):428–447. doi: 10.3122/jabfm.2017.04.170046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundersen C., Ziliak J.P. Food insecurity and health outcomes. Health Aff Proj Hope. 2015;34(11):1830–1839. doi: 10.1377/hlthaff.2015.0645. [DOI] [PubMed] [Google Scholar]
- Hager E.R., Quigg A.M., Black M.M., Coleman S.M., Heeren T., Rose-Jacobs R., et al. Development and validity of a 2-item screen to identify families at risk for food insecurity. Pediatrics. 2010;126(1):e26–e32. doi: 10.1542/peds.2009-3146. [DOI] [PubMed] [Google Scholar]
- Hernán M.A., Robins J.M. Per-protocol analyses of pragmatic trials. New England Journal of Medicine. 2017;377(14):1391–1398. doi: 10.1056/NEJMsm1605385. [DOI] [PubMed] [Google Scholar]
- Hernán M.A., Robins J.M. Chapman & Hall/CRC; Boca Raton, FL: 2020. Causal inference: What if. [Google Scholar]
- Hernán M.A., Sauer B.C., Hernández-Díaz S., Platt R., Shrier I. Specifying a target trial prevents immortal time bias and other self-inflicted injuries in observational analyses. Journal of Clinical Epidemiology. 2016;79:70–75. doi: 10.1016/j.jclinepi.2016.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernán M.A., Wang W., Leaf D.E. Target trial emulation: A framework for causal inference from observational data. JAMA [internet] 2022. [cited 2022 Dec 12] [DOI] [PubMed]
- Hoffman K.L., Salazar-Barreto D., Rudolph K.E., Díaz I. Introducing longitudinal modified treatment policies: A unified framework for studying complex exposures [internet]. arXiv. 2023. http://arxiv.org/abs/2304.09460 [cited 2023 May 26]
- Hoffman K.L., Schenck E.J., Satlin M.J., Whalen W., Pan D., Williams N., et al. Comparison of a target trial emulation framework vs cox regression to estimate the association of corticosteroids with COVID-19 mortality. JAMA Network Open. 2022;5(10) doi: 10.1001/jamanetworkopen.2022.34425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson K.T., Palakshappa D., Basu S., Seligman H., Berkowitz S.A. Examining the bidirectional relationship between food insecurity and healthcare spending. Health Services Research. 2021 doi: 10.1111/1475-6773.13641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risk Screening Tools Review [Internet]. [cited 2019 Sep 12]. Available from: https://sdh-tools-review.kpwashingtonresearch.org/.
- KHstats - Visual Guides for Causal Inference [Internet]. [cited 2024 Mar 8]. Available from: https://www.khstats.com/art/illustrations_viz.
- Laan MJ van der, Rose S. 2011th ed. Springer; New York, NY: 2013. Targeted learning: Causal inference for observational and experimental data. [Google Scholar]
- Leung C.W., Tester J.M. The association between food insecurity and diet quality varies by race/ethnicity: An analysis of national health and nutrition examination Survey 2011-2014 results. Journal of the Academy of Nutrition and Dietetics. 2018 doi: 10.1016/j.jand.2018.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little D.B., Rubin R. 2nd ed. Wiley-Interscience; 2002. Statistical analysis with missing data. [Google Scholar]
- Madenci A.L., Wanis K.N., Cooper Z., Haneuse S., Subramanian S.V., Hofman A., et al. Strengthening health services research using target trial emulation: An application to volume-outcomes studies. American Journal of Epidemiology. 2021;190(11):2453–2460. doi: 10.1093/aje/kwab170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews A.A., Danaei G., Islam N., Kurth T. Target trial emulation: Applying principles of randomised trials to observational studies. BMJ. 2022;378 doi: 10.1136/bmj-2022-071108. [DOI] [PubMed] [Google Scholar]
- Mozaffarian D., Aspry K.E., Garfield K., Kris-Etherton P., Seligman H., Velarde G.P., et al. “Food is medicine” strategies for nutrition security and cardiometabolic health equity: JACC state-of-the-art review. Journal of the American College of Cardiology. 2024;83(8):843–864. doi: 10.1016/j.jacc.2023.12.023. [DOI] [PubMed] [Google Scholar]
- Myers C.A. Food insecurity and psychological distress: A review of the recent literature. Curr Nutr Rep. 2020;9(2):107–118. doi: 10.1007/s13668-020-00309-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Najafzadeh M., Schneeweiss S. From trial to target populations - calibrating real-world data. New England Journal of Medicine. 2017;376(13):1203–1205. doi: 10.1056/NEJMp1614720. [DOI] [PubMed] [Google Scholar]
- National Academies of Sciences Integrating social care into the delivery of health care: Moving upstream to improve the nation’s health [internet] 2019. https://www.nap.edu/catalog/25467/integrating-social-care-into-the-delivery-of-health-care-moving [cited 2019 Sep 27] [PubMed]
- Nguyen C.J., Gold R., Mohammed A., Krancari M., Hoopes M., Morrissey S., et al. Food insecurity screening in primary care: Patterns during the COVID-19 pandemic by encounter modality. American Journal of Preventive Medicine. 2023;65(3):467–475. doi: 10.1016/j.amepre.2023.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols G.A., McBurnie M., Paul L., Potter J.E., McCann S., Mayer K., et al. The high prevalence of diabetes in a large cohort of patients drawn from safety net clinics. Preventing Chronic Disease. 2016;13:E78. doi: 10.5888/pcd13.160056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols G.A., Schroeder E.B., Karter A.J., Gregg E.W., Desai J., Lawrence J.M., et al. Trends in diabetes incidence among 7 million insured adults, 2006-2011: The SUPREME-DM project. American Journal of Epidemiology. 2015;181(1):32–39. doi: 10.1093/aje/kwu255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr C.J., Keyserling T.C., Ammerman A.S., Berkowitz S.A. Diet quality trends among adults with diabetes by socioeconomic status in the U.S.: 1999-2014. BMC Endocrine Disorders. 2019;19(1):54. doi: 10.1186/s12902-019-0382-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palakshappa D., Ip E.H., Berkowitz S.A., Bertoni A.G., Foley K.L., Miller D.P., et al. Pathways by which food insecurity is associated with atherosclerotic cardiovascular disease risk. Journal of American Heart Association. 2021;10(22) doi: 10.1161/JAHA.121.021901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PCORI Methodology Standards Standards for studies of complex interventions [internet] 2015. https://www.pcori.org/research-results/about-our-research/research-methodology/pcori-methodology-standards#Complex [cited 2018 Dec 6]
- PRAPARE. NACHC. [cited 2019 Aug 20]. Available from: http://www.nachc.org/research-and-data/prapare/.
- Rabbitt MP, Hales LJ, Burke MP, Coleman-Jensen A. Household Food Security in the United States in 2022 [Internet]. [cited 2024 Mar 8]. Available from: http://www.ers.usda.gov/publications/pub-details/?pubid=107702.
- Seligman H.K., Berkowitz S.A. Aligning programs and policies to support food security and public health goals in the United States. Annual Review of Public Health. 2019;40:319–337. doi: 10.1146/annurev-publhealth-040218-044132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seligman H.K., Jacobs E.A., Lopez A., Sarkar U., Tschann J., Fernandez A. Food insecurity and hypoglycemia among safety net patients with diabetes. Archives of Internal Medicine. 2011;171(13):1204–1206. doi: 10.1001/archinternmed.2011.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seligman H.K., Jacobs E.A., López A., Tschann J., Fernandez A. Food insecurity and glycemic control among low-income patients with type 2 diabetes. Diabetes Care. 2012;35(2):233–238. doi: 10.2337/dc11-1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seligman H.K., Smith M., Rosenmoss S., Marshall M.B., Waxman E. Comprehensive diabetes self-management support from food banks: A randomized controlled trial. American Journal of Public Health. 2018;19:e1–e8. doi: 10.2105/AJPH.2018.304528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J.W., Rogers J.R., Her Q., Welch E.C., Panozzo C.A., Toh S., et al. Adaptation and validation of the combined comorbidity score for ICD-10-CM. Medical Care. 2017;55(12):1046–1051. doi: 10.1097/MLR.0000000000000824. [DOI] [PubMed] [Google Scholar]
- Te Vazquez J., Feng S.N., Orr C.J., Berkowitz S.A. Food insecurity and cardiometabolic conditions: A review of recent research. Curr Nutr Rep. 2021 doi: 10.1007/s13668-021-00364-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Laan M.J. Targeted maximum likelihood based causal inference: Part I. International Journal of Biostatistics. 2010;6(2) doi: 10.2202/1557-4679.1211. Article 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Laan M.J., Polley E.C., Hubbard A.E. Super learner. Statistical Applications in Genetics and Molecular Biology. 2007;6 doi: 10.2202/1544-6115.1309. [DOI] [PubMed] [Google Scholar]
- Volpp K.G., Berkowitz S.A., Sharma S.V., Anderson C.A.M., Brewer L.C., Elkind M.S.V., et al. Food is medicine: A presidential advisory from the American Heart association. Circulation. 2023 doi: 10.1161/CIR.0000000000001182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein J.M., Kahkoska A.R., Berkowitz S.A. Food insecurity, missed workdays, and hospitalizations among working-age US adults with diabetes. Health Aff Proj Hope. 2022;41(7):1045–1052. doi: 10.1377/hlthaff.2021.01744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White I.R., Royston P., Wood A.M. Multiple imputation using chained equations: Issues and guidance for practice. Statistics in Medicine. 2011;30(4):377–399. doi: 10.1002/sim.4067. [DOI] [PubMed] [Google Scholar]
- Williams N., Díaz [aut I, cph. lmtp: Non-parametric causal effects of feasible interventions based on modified treatment policies [internet] 2022. https://CRAN.R-project.org/package=lmtp [cited 2022 Sep 6]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and analyzed during the current study are not publicly available due to the data use agreements under which the study was conducted.
The data that have been used are confidential.