Abstract
The management of solid waste poses a worldwide obstacle in the pursuit of a sustainable society. This issue has intensified with the increase in waste production caused by rapid population expansion, industrialization, and urbanization. The continuously growing volume of municipal solid waste, particularly the substantial volume of organic waste, along with improper disposal practices, results in the release of greenhouse gases and other harmful airborne substances which simultaneously causes health risks and socioeconomic concerns. This article examines various waste-to-energy (energy production in the form of heat and electricity) concepts as well as waste-to-materials (various value-added materials including biofuel, biochemical, char, bio-oil, soil fertilizer, etc.) methods of converting municipal solid waste into environmentally friendly fuels, which appear to be economically feasible and attractive. It starts with a thorough analysis of the characteristics of municipal solid waste followed by the generation procedure. The study provides an overview of different thermochemical conversion methods including incineration, pyrolysis, co-pyrolysis, liquefaction, hydrothermal carbonization, gasification, combustion for transformation of municipal solid waste, and their recent advancement. The review comprehensively discussed the pros and cons of each method highlighting their strength, weakness, opportunities, and threats to transforming MSW. The current state of municipal solid waste management, including effective dumping and deviation, is comprehensively assessed, along with the prospects and challenges involved. Energy justice concepts and fuzzy logic tool is used to address the selection criteria for choosing the best waste treatment techniques. Moreover, several recommendations are offered to enhance the existing solid waste management system. This review could assist scholars, researchers, authorities, and stakeholders in making informed decisions regarding MSW management.
Keywords: Municipal solid waste, Bioenergy, Waste management, Thermochemical treatment, Circular economy
Graphical abstract
Highlights
-
•
Globally, enormous volumes of diverse municipal solid waste (MSW) are produced.
-
•
Waste to Energy conversion techniques can efficiently convert MSW and reduce its' volume.
-
•
Thermochemical conversion methods are promising to for conversion of MSW into biofuels.
-
•
The economic aspects and potential for energy production of MSW are evaluated.
Abbreviation
- AHP =
Analytic Hierarchy Process
- ANP =
Analytic Network Process
- APC =
Air Pollution Control
- BWM =
Best Worst Method
- CGE =
Cold Gasification Efficiency
- EcC =
Economic Cost
- EnC =
Environmental Cost
- FAHP =
Fuzzy Analytic Hierarchy Process
- FDM =
Fuzzy Delphi method
- GIS =
Geographic Information Systems
- HTC =
Hydrothermal carbonization
- HTL =
Hydrothermal liquefaction
- IPCC =
Intergovernmental Panel on Climate Change
- IVFMCDM =
Interval-valued fuzzy group multi-criteria decision-making
- IVFS =
Interval-valued fuzzy set
- LHV =
Lower Heating Value
- MAP =
Microwave-assisted pyrolysis
- MSW =
Municipal solid waste
- MSWM =
Municipal solid waste management
- RDF =
Refuse-derived fuel combustion
- SAW =
Simple additive weighting
- SDG =
Sustainable development goal
- SMCDM =
Stratified MCDM
- WtE =
Waste to Energy
1. Introduction
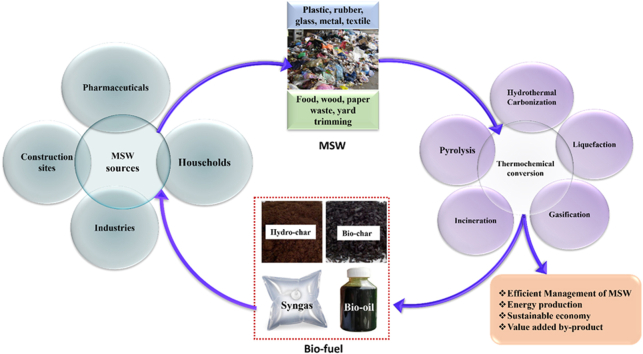
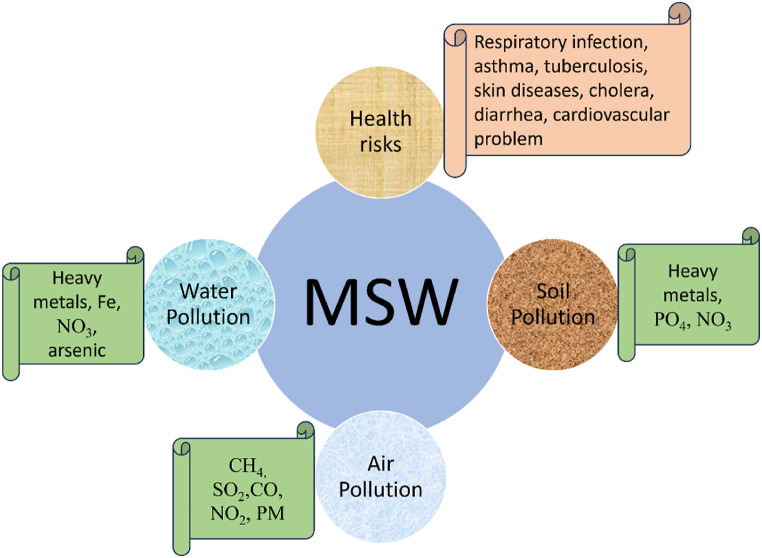
Increasing population growth rate, globalization, and rapid economic progress cause faster proliferation of municipal solid waste (MSW) which is becoming a great challenge to the ecological system [1]. MSW is generated every day from households, medical stores, garments, educational institutes, printing presses, groceries, shopping malls, etc. due to the inevitable livelihood needs. Nowadays per capita waste generation is around 0.74 kg/day. World generation of MSW was around 2.01 billion tonnes annually in 2016 due to different human activities and it is predicted that this will increase to 3.40 billion tonnes resulting in 2.6 billion tonnes of CO2-eq greenhouse gas emission (GHG) by 2050 [2]. The growing rate of MSW generation is creating complexity in the waste disposal system of society. Management of this huge volume of MSW is a solemn concern in terms of environmental sustainability. Proper waste management and disposal are crucial for any nation to maintain hygiene and support sustainable development. Wealthier nations tend to focus on advanced incineration and landfill methods for waste disposal. Landfilling, burning, composting, recycling and open dumping are the common management practices that cause soil, air, and water pollution; diseases spreading becoming a medium of pathogen; and blockage of drainage systems [3]. Among these, open dumping is the most common method, accounting for 38.16 % of waste disposal globally, except in prosperous regions like North America. Composting is less popular as a waste disposal method. East Asia and the Pacific region lead in using incineration for waste treatment, while North America and the Latin America and Caribbean region primarily rely on landfills, with shares of 54.3 % and 68.5 %, respectively. All of these waste disposal technologies also cause several problems including human toxicity, land use, ecotoxicity, ozone layer depletion, and natural resource damage [1,4,5]. Improper or open disposal of waste creates several environmental crises, threatens community safety, causes societal and economic issues, air pollution, soil contamination, water contamination (both surface and ground) human health as well as financial aspects [6]. Fig. 1 depicts the environmental and health risks of improper disposal of MSW. Poranek et al. [7] reported excessive GHG emissions cause temperature rise resulting in ice melting, climate change, and climatic disasters. Wiedinmyer et al. [8] mentioned that around 40 % of MSW is burned throughout the world every year. Landfilling of waste is a hazardous technique of waste disposal as it causes air and water pollution along with land use [9,10].
Fig. 1.
Effects of MSW mismanagement.
The proper design of waste disposal and management systems is crucial. In countries with lower economic status, the complexity of municipal solid waste management is heightened due to rapid urbanization and population expansion [11]. Problems with waste management belong more in developing countries than in developed ones. Research revealed that handling these wastes will result in a sharp rise in greenhouse gas emissions. In many countries, the lack of attention and priority given to municipal solid waste has grown to be a significant problem that presents ecological danger and difficulties. This has prompted researchers to consider municipal solid waste as a resource that needs to be managed properly [12].
Alteration of disposal methods with energy conversion technology will lead to a way of energy generation from MSW. There are several ways to convert MSW into bioenergy such as thermal, thermochemical, biological, biochemical, etc. These conversion techniques are also known as waste to energy (WtE) routes. Currently, there are more than 1700 WtE factories throughout the world. Asia Pacific has the largest share of them at 62 %, followed by Europe at 33 % and North America at 4.5 % [13]. Thermochemical processes require heat and energy to convert MSW into energy facilitating minimal resource use, lowering environmental emissions, and contributing to renewable energy sources. While the biological processes requires the involvement of various fungi, bacteria or other microorganism for the decomposition of organic fraction of MSW. Composting, vermicomposting, landfilling, anaerobic digestion are included in biochemical conversion process. It generally requires long processing time, high investment cost with very low operational cost. On contrary, thermochemical conversion technologies are efficient and appropriate waste valorization and energy production methods [14] to produce different solid (bio-char), liquid (bio-oil), gaseous (syngas) bio-fuel, and heat. Thermochemical transformation of MSW to generate energy can reduce dependency on non-renewable sources by replacing fossil fuels with biofuel and can be a reliable energy source. Santos et al. [15] reported transformation of solid waste biomass into energy can cover around 25 % of renewable energy demand by 2040. Transformation of MSW into energy may reduce air and water pollution, and use of conventional energy and natural resources [16] and helps to achieve Sustainable Development Goal (SDG) 6 (Clean Water and Sanitation), 11 (Sustainable Cities and Communities), 12 (Responsible Consumption and Production) and 13 (Climate Action) [17]. Thus, ensuring energy security it can be a supplier of green energy and fulfill SDG goal 7 (Affordable and Clean Energy).
Several studies reported that thermochemical conversion technologies namely incineration [18,19], pyrolysis [20], liquefaction [21] gasification [22], and hydrothermal carbonization [3,23] are emerging technologies for energy production from MSW, resulting in solid, liquid, and gaseous products.
Comprehensive studies about different thermochemical conversions of MSW comparing the environmental and economic feasibility are still limited. There are several reviews available on MSW and most of which focuses on direct conversion especially incineration, analysis of public private partnership, various conversion techniques. However, there is still a lack of knowledge on technology transfer. Selection of proper thermochemical conversion technology for heretogeneous MSW can make MSW management a part of cyclic economy and provide pollution free environment worldwide [24]. Integration of two or more thermochemical technologies depends on MSW characteristics, cost-effectiveness, and environmental consequences of these processes [25]. Thus, the aims of this study includes: the characterization of MSW along with its source of generation and exploring the factors that affect municipal solid waste management. The purpose of this study is to analyze recent advancements in various technologies including incineration, pyrolysis, liquefaction, gasification, and hydrothermal carbonization for the generation of energy from MSW; investigate the criteria to figure out the most eco-friendly and economically feasible thermochemical conversion technology based on energy justice and fuzzy logic concepts; and analyze the potentiality of thermochemical conversion to achieve circular economy in waste management technology.
2. Composition and source of MSW
Municipal solid waste (MSW) is a mixture of different organic and inorganic wastes from various sources, including industrial facilities, offices, residential areas, retail establishments, schools, etc. [26]. Diverse categories, such as newspapers, cardboard, furniture, fresh products, organic matter, and discarded food items encompass these materials. Additionally, non-renewable materials like plastics constitute a substantial component of MSW. This waste stream also comprises items that are no longer needed or have been discarded due to routine activities.
It is crucial to distinguish MSW from other waste categories. Medical waste, radioactive materials, and industrial byproducts, for instance, fall outside the scope of MSW and require separate disposal processes [27]. Various physical states, including solid, liquid, semi-solid, and gaseous forms, are manifested by MSW, all stemming from human activities.
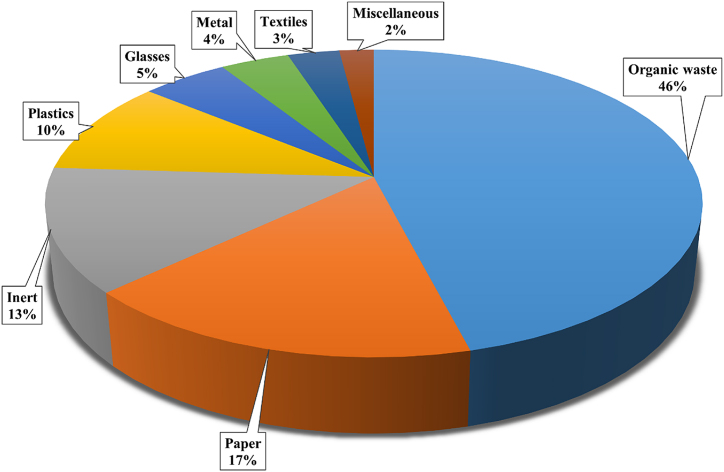
Worldwide distribution of MSW components in urban areas is demonstrated in the pie chart (Fig. 2) where MSW can be divided into 07 primary categories where organic waste (46%) is the predominant component followed by paper, constituting 17 % of the waste stream [28]. In another way, MSW can be categorized into composted and non-composted. Among these, organic wastes (food waste, yard trimmings, and wood) are composted readily and contribute to sustainable waste management practices and bio-energy production [29]. Conversely, inorganic waste (metals, plastics, and glass) can be subjected to recycling processes, which may reduce the utilization of natural resources and energy consumption.
Fig. 2.
Compositions of municipal solid waste.
The proportion of each component in MSW varies based on regional context. This variation is mainly due to the degree of urbanization, industrialization, waste valorization technique, and economy of any area. Handling and application of suitable technology for MSW valorization into energy largely depends on MSW composition. This information is also essential for using solid waste in power plants in any region [30] as it varies from region to region. Moreover, potential environmental emissions also vary based on the MSW feedstock's properties. This constitutional difference in MSW has become an obstacle to the adoption of appropriate waste-to-energy (WtE) conversion technology.
Depending on the heating value and components of MSW, scientists and engineers may determine its use as a fuel. In several developing countries, MSW exhibits varying heating values, influenced by waste composition. For example, in Bangladesh, MSW is characterized by a calorific value of 717 kcal/kg, while in India, it ranges from 800 to 1100 kcal/kg. In contrast, developed countries generate MSW with higher heating values due to lower water content and higher percentages of carbon in the solid portion of MSW. Japan's MSW has calorific values ranging from 2000 to 2200 kcal/kg, the UK's from 2200 to 3000 kcal/kg, and South Korea's from 2600 to 3000 kcal/kg [31].
Human activities in urban and rural areas also affect the types and sources of MSW [32]. A study showed that around 55%–80 % of MSW is generated from households and 10%–30 % from the different commercial areas including markets, industries, etc. in developing countries [33]. In waste management strategies, the management of MSW has assumed significant importance in recent years. This is facilitated through a series of well-coordinated processes, including waste collection, segregation, temporary storage, transportation, and ultimate disposal. Notably, waste reduction initiatives are a linchpin in the minimization of the environmental impact of MSW [34].
Sorting and segregation of MSW are contingent upon factors like its origin, size, quantity, quality, and inherent characteristics. The composition of MSW also varies due to the divergent methods employed for waste collection. As a general trend, a notable proportion of plastic materials is often found in municipal solid waste, and a high ash content characterizes it.
In urban areas, MSW accumulation in any specific location and segregation of municipal solid waste are typically carried out by local authorities. Around 70 % of the total investment is attributed to MSW collection and the rest of the budget is for transporting it to any specific location for disposal [35]. Due to the substantial investment required for the construction and operation of waste disposal facilities, methods like landfilling are currently preferred under current circumstances [36].
Furthermore, the management of MSW involves a spectrum of methods beyond mere disposal. WtE technology has become prominent for extracting value from MSW while reducing landfill dependence. These technologies involve the production of electricity and heat from MSW, contributing to sustainable energy production [37].
3. Thermochemical technologies for converting MSW to energy
3.1. Incineration
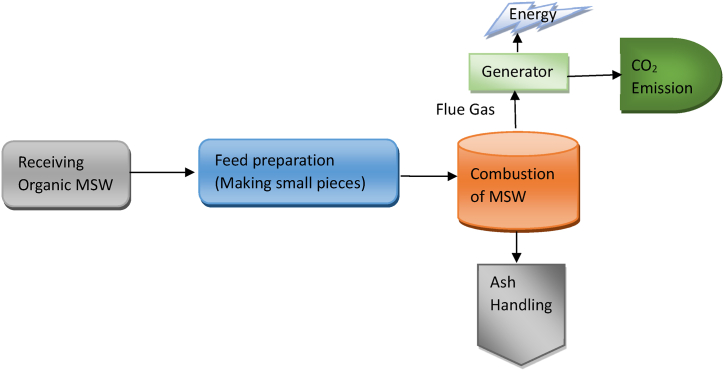
Incineration is a thermal WtE technique to produce heat by burning waste materials under a controlled oxygen supply [38]. It's one of the most attractive WtE techniques as it reduces waste volume to a greater extent (95–96 %) and destroys harmful bacteria and chemicals [39]. Besides incineration, several metropolises use compaction to compile rubbish at their landfills. Previous studies revealed incineration as one of the best-suited WtE conversion technologies and employed in different countries successfully around the globe [40] as it reduces dependency on non-renewable fossil fuels. The heat produced during the incineration of waste material can be used to produce electricity and thus contribute in reducing energy scarcity. Along with the heat, other by-products such as flue gas (combination of CO2, N2O, and SO2) and ash are also produced [41], and management of those by-products is a great issue for the incineration process. MSW incineration has been favored over landfills, which compete with nearby residential and agricultural land areas and have an unpleasant stench.
Typically, incineration takes place between 850 °C and 1650 °C temperature and facilitates the conversion of combustible constituents primarily into heat [38]. The macroscopic and microscopic analytical qualities of MSW have an impact on the amount of heat produced during the incineration process. The macroscopic features include heterogeneity, density, ash fusion temperature, moisture content, heating value, elemental constituent, feedstock particle size, and proximal analysis. Thermal analysis, chemical kinetic research, and mineral materials are examples of the microscopic qualities. The degree of thermal deterioration, the incineration temperature, the moisture content, and the thermal conductivity all have a significant impact on the thermal parameters of MSW, particularly specific heat, specific gravity, and volatile emissions. Methane, vapor, hydrogen, oxides of carbon, some simple and complex hydrocarbons, and some aromatic hydrocarbons are among the volatile matter emissions from incineration. Moreover, the yield of heat generated from the incineration of MSW is affected by incineration temperature, method of oxygen supply, presence of toxic substances, type of incinerator, etc. A list of incinerators for MSW used worldwide is listed in Table 1.
Table 1.
List of incinerators used for MSW incineration.
| Type of incinerator | Type of waste | Working principle | Advantages | References |
|---|---|---|---|---|
| Rotary Kiln | Industrial waste and MSW | Thermally decomposed rotational waste in a chamber with heat | Lower emission of nitrogen oxides and breakdown of harmful chemicals | [41] |
| Moving Grate | MSW | Waste is carried in the combustion chamber using a porous descending grate and air is supplied from the bottom and above the grate. | Ensure complete combustion. | [41] |
| Fluidized Bed | MSW and hazardous waste | Pretreated small waste particles are suspended in the air which is supplied from beneath | Increase transfer of heat and thus combustion efficiency. | [41] |
| Mass-burn incinerator | All types of waste except hazardous chemicals and large furniture | Waste is mixed properly in a storage pit and then moved over the grate where combustion occurs with a controlled oxygen supply. The heat produced is used to generate stems which produce electricity operating the turbine. | Operation is simple and easy. A huge quantity of waste can be incinerated. No need for pre-processing waste before incineration. | [39] |
| Modular incinerator | All types of waste except hazardous chemicals and large furniture | Similar to mass-burn incinerator | Facilitate incineration of small quantities waste, easy set-up and less equipment required compared to mass-burn incinerator | [39] |
Al-Ghouti et al. [42] reported that incineration is applied to manage MSW due to its capacity to reduce the volume of MSW by 70%–90 %, centralize MSW management, demolition of pathogens, use limited land, and diversified use of heat. They also reported the classification of incineration depending on process facilities namely: mass burning and refuse-drive fuel. Mass burning doesn't require sorting whereas refuse-drive fuel needs sorting before burning MSW. According to Chen et al. [19], incineration may replace landfilling with energy recovery which can produce stem and thus electricity. They also reported that some oxides of silicon, calcium, aluminum, and magnesium are generated along with toxic compounds during incineration which can be used as construction materials.
The emission of greenhouse gases, heavy metals, toxic compounds like furan, dioxin, and by-products of incineration both fly ash and bottom ash is a great concern of MSW incineration [43]. Cudjoe and Acquah [44] conducted environmental emission assessment during incineration in different African countries and found an increase in global warming potential in 2025 compared to 2012 due to emissions during incineration. Implications of appropriate air pollution control (APC) systems can reduce emissions. Cho et al. [43] reported the use of appropriate APC and refuse-drive fuel incineration processes for MSW incineration can reduce the emission of heavy metals and toxic compounds. Nidoni [41] reported ash produced during MSW incineration is a great construction material and flue gas oxidation produces elementary sulfur, and nitrogen which can be used in different chemical reactions. In addition, fly ash finds application in the following fields: agriculture (e.g., soil amendment), geotechnical (e.g., road pavement and embankment), and building materials (e.g., cement, ceramics, glass, and composites). Additionally, it can be used as a sorbent and also for sludge conditioning [45,46]. Fig. 3 represents a typical process of MSW incineration.
Fig. 3.
Typical process of Incineration.
Further benefits of incineration include lower capital and operating costs, reduced human drudgery, and a high throughput rate. The full thermal decomposition of biohazards, or the remains of dead creatures, and mineralizes organic matter which produces comparatively less harmful by-products and these are two more benefits of MSW incineration. High moisture content and organic matter load in MSW can significantly reduce its combustibility, and energy recovery rate resulting in poor performance.
3.2. Pyrolysis
The integration of renewable energy sources is a crucial aspect of promoting sustainable development. MSW holds significant potential as a renewable energy source, especially when coupled with advanced technologies like pyrolysis. Pyrolysis is considered as a ground-breaking and straightforward method for converting MSW into biofuel, offering a promising avenue for energy production [47]. Pyrolysis stands out as a thermochemical technology employed to transform biomass into energy and chemical products without the presence of oxygen, encompassing solid, liquid, and gaseous biofuel.
Pyrolysis is categorized into three major classes: slow, fast, and flash pyrolysis based on retention time, and temperature of pyrolysis focusing on either maximizing liquid (bio-oil) or solid (biochar) yields. Table 2 depicts the operating conditions of different types of pyrolysis. Slow pyrolysis stands out due to its longer retention time, lower heating rate, and moderate temperatures resulting in increased char yields on the other hand flash, and fast pyrolysis are conducted at high heating rates, temperature, and with brief reaction times resulting in higher bio-oil yield [48]. Lignocellulosic biomass and biochar naturally contain alkali and alkaline earth metals, potentially influencing the pyrolysis reaction. MSW being heterogeneous, contains some alkali, sulfur, and different inorganic constituents. These metals, combined with sulfur, play crucial roles in ash melting, fly ash generation, aerosol emission, and reactor corrosion when employed as feedstock [49]. Intense pyrolysis temperatures cause biomass and other organics to undergo dehydration and depolymerization, generating volatile components. Upon condensation, these volatiles cool rapidly, forming bio-oil. The quality and quantity of liquid biofuel from pyrolysis depend on the quenching method and residence time of volatile vapors [50]. Bio-oils, oxygenated liquid products, are the results of the thermal breakdown of different complex organic compounds found in biomass or MSW. The pyrolysis of cellulose and hemicellulose yields acids, alcohols, aldehydes, esters, ethers, furans, ketones, sugars, and mixed oxygenates [51].
Table 2.
Operating conditions for different types of pyrolysis.
| Parameters | Slow Pyrolysis | Fast Pyrolysis | Flash Pyrolysis | Reference |
|---|---|---|---|---|
| Temperature (◦C) | 550–950 | 850–1250 | 900–1200 | [52] |
| Heating rate (◦C/s) | 0.1–1.0 | 10–200 | 1000 | |
| Particle size (mm) | 5–50 | <1 | <0.5 | |
| Residence time (s) | 300–550 | 0.5–10 | <1 |
The trio of valuable products resulting from pyrolysis enhances versatility and sustainability of MSW conversion significantly. Initial in the lineup is the creation of biochar, a solid residue abundant in carbon and fortified with nutrients. This is achieved by subjecting MSW in between 300 and 700 °C temperature range in an oxygen deficient environment. The process not only reduces waste but also transforms organic materials into a carbon-rich substance with enhanced nutrient content, fostering environmental and agricultural sustainability [53]. Next in line is the production of bio-oil, a liquid fuel akin to conventional crude oil, usually derived by heating MSW in a pyrolysis reactor under oxygen-absent conditions at temperatures ranging from 400 to 600 °C. This bio-oil stands as a versatile asset, serving purposes such as electricity generation, heat production, and the synthesis of various fuels and chemicals [54]. Concluding the trio is syngas, a valuable energy resource comprised of hydrogen, carbon monoxide, carbon dioxide, methane, and a trace amount of higher hydrocarbon. Formation of syngas generally happens at 700–900 °C in an oxygen-deprived environment [55] which can be applied in generating electricity, heating, and as a precursor for an array of fuels.
Critical parameters such as feedstock type and size, retention time, heating rate, applied pressure and temperature, and type of catalyst affect the pyrolysis process. Primary degradation, occurring at 200–300 °C, breaks down complex biomass structures especially hemicellulose [56], producing volatiles, char, and bio-oil. Volatiles are gases, char is solid residue, and bio-oil, a viscous liquid, can be upgraded using techniques like hydrotreating. Application of temperature above 500 °C involves thermal cracking, generating gases like light hydrocarbons, and boosting overall gas and liquid production. Optimization of parameters allows tailoring the process for desired end products, including bioenergy, biofuels, and bio-based compounds. Primary reactions involve breaking down lignin, cellulose, and hemicellulose, while secondary reactions decompose intermediates and transform major products into smaller molecules and char [57,58].
Numerous pyrolysis reactors have been innovatively designed to extract bio-oil from a variety of waste materials. These reactor variations encompass a stationary bed reactor, a gravity-driven reactor, a fluidized bed reactor, an induction-heating reactor, and a catalytic fixed bed reactor. Each of these reactor types offers some unique features and advantages in the conversion of various wastes into valuable bio-oil, contributing to the versatility and efficiency of the overall pyrolysis technology. Among these reactor types, the fixed bed reactor is being used widely because of its simplicity in construction, cost-effectiveness, and low maintenance requirements [59]. The quality of end products and efficacy of pyrolysis are largely affected by the selection of pyrolysis type. Diverse reactor designs offer unique advantages in this context. Recent progress in reactor technology is directed towards refining the energy balance and elevating the quality of pyrolysis products. These advancements address operational challenges, striving to optimize efficiency across a range of scales [60].
The multifaceted benefits of pyrolysis in MSW conversion extend beyond waste management, positioning it as a low-carbon technology. The carbon sequestration potential of biochar, combined with the clean energy output from syngas and bio-oil, contributes significantly in lowering greenhouse gas emissions. Moreover, pyrolysis outperforms traditional waste disposal methods by efficiently recovering more energy from MSW, thereby, alleviating the burden on landfills and providing a diverse spectrum of valuable resources aligned with industrial and agricultural needs [61].
Depending on the heat source pyrolysis is categorized into conventional, microwave, solar energy, and plasma pyrolysis, respectively. Conventional and solar energy pyrolysis use electric heaters (burners) and solar energy, respectively. These may require a longer time, uneven temperature distribution, and increased energy requirement. In case of microwave-assisted pyrolysis, microwave radiation act as a the heat source to break down waste polymer to produce the end product of pyrolysis [62]. It has benefits over conventional pyrolysis as it facilitates faster chemical reactions with a control and selective heating system, higher volume reduction of waste material, and a comparatively economical process [[63], [64]]. Zhang et al. [65] stated during microwave-assisted pyrolysis (MAP) of biomass increase in microwave power causes higher temperatures which results in higher production of bio-oils. The performance of microwave-assisted pyrolysis varies with feedstock moisture content, microwave radiation, temperature rate, condition of microwave absorber, retention time, and density [66]. Greater volume reduction and significant oil production are possible using MAP of MSW. Gedam and Regupathi [67] reported MAP of MSW occurs spontaneously when the power supply is greater than 450W. Without absorber MAP of MSW is not possible below this temperature. However, the use of graphite, charcoal, and iron facilitates MAP lower than the power rating of 450 W. Beneroso et al. [68] also reported MAP as a suitable method for MSW to energy conversion. They found that using char as a microwave absorber during MAP at 150–450 W results in hydrogen-rich syngas which is economically feasible. Li et al. [64] found MAP of MSW produces 74.88 % combustible gas having 36.02 MJ/m3 consuming 0.58 KWh/Kg to 0.7 KWh/Kg energy.
Plasma pyrolysis is a sustainable thermochemical process in which a plasma arc converts waste polymer into molecules and then in high-temperature syngas is produced in anaerobic condition. It is an eco-friendly process in which above 99 % organic matter reduction and waste transformation occur in less than 1 s [69]. Dharmaraj et al. [70] also noted plasma pyrolysis as a suitable advanced technology for medical waste management. Tang et al. [71] reported the superiority of plasma pyrolysis over conventional pyrolysis. Although there is a research gap for plasma pyrolysis of MSW, Bhatt et al. [72] recommended plasma pyrolysis for MSW management.
Catalytic pyrolysis increases the isomerization rate decreases reaction time and improves the quality of oil but improvement in oil quality relays on the type of catalyst [73]. Almohamadi et al. [74] examined the effect of catalysts on the catalytic pyrolysis of MSW and found addition of magnesium oxide, magnesium oxide with aluminum oxide, and magnesium oxide with activated carbon during catalytic pyrolysis increases syngas production during pyrolysis. They also found that only aluminum oxide has no effect on the end product but addition of magnesium oxide with aluminum oxide improves the heating value of bio-oil from pyrolysis. Li et al. [75] reported catalyst formulation, porosity, and exposed surface of species affect MSW catalytic pyrolysis. They also reported use of zeolites as a catalyst during MSW catalytic pyrolysis causes comparatively lower production of liquid products, attapulgite enhances the production of gaseous products, dolomite has an inhibitory effect on bio-oil production, and metal-based catalysts are often used worldwide for MSW catalytic pyrolysis. Abdullah [76] investigated catalytic pyrolysis of MSW for fuel (hydrocarbon) production and found it can reduce retention time, require higher temperature, and improve features of bio-oil compared to indigenous pyrolysis as it converts around 80 % plastic to bio-oil having the same quality as diesel.
Despite its promising potential, challenges hinder the widespread adoption of pyrolysis for MSW conversion. Substantial upfront costs associated with pyrolysis reactors and equipment pose financial obstacles, thus, commercial-scale implementation is still in its early stages which requires further development. Addressing air pollution control is imperative, necessitating effective filtration and purification methods for potential pollutants in pyrolysis products. Additionally, creating a robust market for pyrolysis products, including biochar and syngas, demands coordinated efforts in education, promotion, and standards development. Overcoming these challenges is crucial to unlocking the full sustainable potential of pyrolysis in MSW conversion.
Despite existing issues, including initial costs and air pollution control, ongoing research, robust government support, collaborative initiatives, and market development endeavors are anticipated to propel pyrolysis into a pivotal role for reliable and feasible MSW management along with energy production. Looking ahead to the future of pyrolysis for MSW conversion, continuous research efforts persist, with numerous enterprises advancing commercial-scale pyrolysis reactors. As technology costs decrease and markets for pyrolysis products mature, the potential for pyrolysis to play a pivotal role in the conversion of MSW into energy and valuable resources becomes increasingly evident.
3.3. Liquefaction
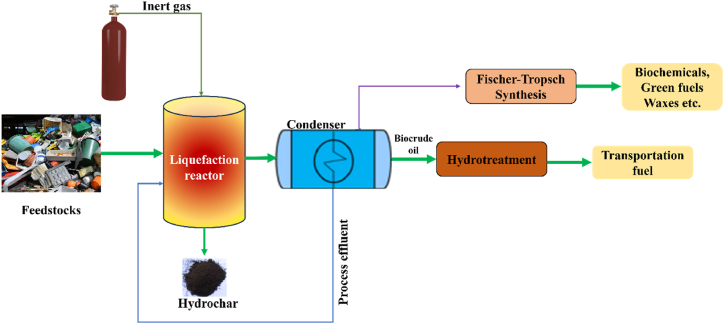
The utilization of liquefaction as a WtE technique to produce quality bio-oil with high energy value has gathered attention worldwide. This process operates under an elevated pressure range of 4–20 MPa with 200–370 °C temperature. The controlled application of heat and pressure within these parameters facilitates the conversion of different feedstocks into liquid fuels [77]. Beyond bio-oil, liquefaction yields some valuable end products (adhesives, resins, bio-polyols, and polyurethane foams) [78]. Fig. 4 illustrates the layout of the thermochemical liquefaction process. The liquefaction reactions can be outlined through the following fundamental routes [79].
-
(i)
Hydrolysis of biomass
 smaller monomers.
smaller monomers. -
(ii)
Smaller monomers
 smaller compounds (by cleavage and decarboxylation).
smaller compounds (by cleavage and decarboxylation). -
(iii)
Recombination of the smaller fragments
 new compounds (condensation, polymerization).
new compounds (condensation, polymerization).
Fig. 4.
The process representation of the liquefication of biomass.
The incorporation of catalysts in the liquefaction process plays a pivotal role in influencing its dynamics. Catalysts not only have the potential to lower reaction temperatures but also enhance reaction kinetics, leading to improved end products. Alkali catalysts, including hydroxides of sodium and potassium and carbonate of sodium and potassium, demonstrate the ability to suppress char formation, consequently elevating bio-oil yields [80]. On the contrary, acidic catalysts, such as H2SO4, H3PO4, and p-toluenesulfonic acid, contribute to a reduction in process temperature and reaction time, as highlighted in research by Ref. [48].
Hydrothermal liquefaction (HTL) is a kind of liquefaction where water acts as the reaction medium. HTL is carried out within 250–374 °C temperature and 4–25 MPa pressure, as reported by Mathanker et al. [81]. Water in its subcritical or supercritical state stands out as a promising solvent for HTL due to its abundance supply and economic feasibility. This method is superior for conversion of aquatic biomass (algae) and other feedstocks with high moisture content (moisturized MSW, cattle manure, swedge sludge) than any other thermochemical technologies.
Solvent plays a major role in liquefaction to break down feedstock into simple organic compounds [79] and that's why ethanol and water (subcritical) are used as solvents. Among two phases (tar phase and water phase) in liquefaction process, water phase consists of water that can be reused as solvent after recycling [82].
Since the degree of polymer bond cleavage during plastic liquefaction depends on solvent, the proper selection of solvent is essential. In a study by Serrano et al. [83], various solvents were investigated for the accelerated liquefaction of high-density polyethylene through free radical mechanisms. The literature suggests that low solvent concentrations in liquefaction may induce free radical mechanisms for cracking, akin to supercritical water gasification [84].
While studies about direct liquefaction of MSW are limited, extensive studies has been conducted on the hydrothermal liquefaction, and co-liquefaction of individual MSW components. Wang et al. [85] investigated hydrothermal liquefaction of selective MSW components and wheat straw uncovering a synergistic effect at an optimal mixing ratio for the highest oil and gas yields.
In the study by Mosteiro-Romero et al. [86], hydrothermal liquefaction of wood in subcritical water at 250–350 °C temperatures and 15–30 MPa pressure, with varying holding times revealed that with rising temperatures, there was an increase in both liquid and gas yields, concurrently accompanied by higher carbon content in the char. Elevating the reaction temperature (190–240 °C) and incorporating acidic catalysts positively influenced liquid yields. Similarly, Liang et al. [87] found around 67%–80 % of liquid products from the liquefaction of different cereal crop residues. To enhance liquefaction reactions and process selectivity, high H2 pressures (2–10 MPa) and catalysts were deemed essential.
Isa et al. [77] examined miscanthus hydrothermal liquefaction in both subcritical and supercritical conditions where water acted as a hydrogen donor solvent, and found approximately 0.5 % hydrogen donation in the product conversion process. The study revealed that around 90 % of biomass conversion to high-quality bio-oil is possible through HTL. They also reported HTL above 400 °C with a higher water ratio decreased oxygen content to 12–16 %. A comparative analysis of the performance of solvents in biomass liquefaction, concerning the bio-oil quality, is presented in Table 3.
Table 3.
Comparison for the performances of the hydrogen donor solvents in liquefaction of biomass on oxygen content of the produced bio-oil.
| Sample | Reaction conditions | Catalyst | Solvent | Oxygen content (%) | Ref |
|---|---|---|---|---|---|
| Bio-oil from pre-treated bagasse |
|
KOH | Water | 14.50 | [88] |
| Bio-oil from miscanthus |
|
Supercritical water | 14 | [82] | |
| Bio-oil from miscanthus |
|
Tetralin | 12 | [89] | |
| Bio-oil from rice husk |
|
NaOH | Ethanol | 29.63 | [90] |
| Bio-oil from rice straw |
|
Ethanol | 16.1 | [91] | |
| Bio-oil from bagasse |
|
MgMnO2 | Supercritical water | 23.26 | [92] |
*T = temperature, t = residence time, B/S = biomass to solvent ratio.
3.4. Hydrothermal carbonization (HTC)
In the realm of managing MSW, there is a worldwide inclination to boost the percentage of recycled waste for the creation of secondary raw materials or energy. Concurrently, efforts are being exerted to broaden the possibilities of WtE initiatives through the application of contemporary technologies, including drying, gasification, pyrolysis, or torrefaction, specifically tailored for handling organic waste. Nevertheless, these endeavors encounter several challenges associated with the varied composition, low density, and elevated moisture content prevalent in municipal solid waste [93]. In recent times, HTC has gained recognition as a highly promising technique for recycling MSW, intending to generate a substance referred to as hydrochar. It aims to reduce greenhouse gas emissions while generating residual materials possessing inherent value. Hydrothermal carbonization relies on a basic chemical mechanism, specifically the separation of water from carbohydrates, which is commonly referred to as dehydration.
A basic energy assessment of this process reveals its exothermic nature, with energy being released during the reaction [94]. During HTC, the raw material undergoes heating in subcritical water, typically between 180 and 350 °C temperature, and experiences autogenous pressures. This leads to the breakdown of the feedstock through a set of concurrent reactions, which encompass hydrolysis, dehydration, decarboxylation, aromatization, and recondensation [95]. Recent studies on HTC of MSW are tabulated in Table 4.
Table 4.
Recent studies on Hydrothermal carbonization of MSW into biochar.
HTC is affecetd by several parameters that impact production and quality of end products. These include temperature, pressure, retention time, feedstock composition, pH, catalysts, water-to-substrate ratio, heating rate, feedstock particle size, and mixing. Higher temperatures and pressures generally lead to increased reaction rates and carbonization efficiency. Physio-chemical composition of the feedstock, including moisture content affects the yield and quality of the hydrochar. pH levels, presence of catalysts, and water-to-feedstock ratio also influence reaction kinetics and hydrochar properties. Proper control and optimization of these factors are essential for achieving desired outcomes in HTC processes [101].
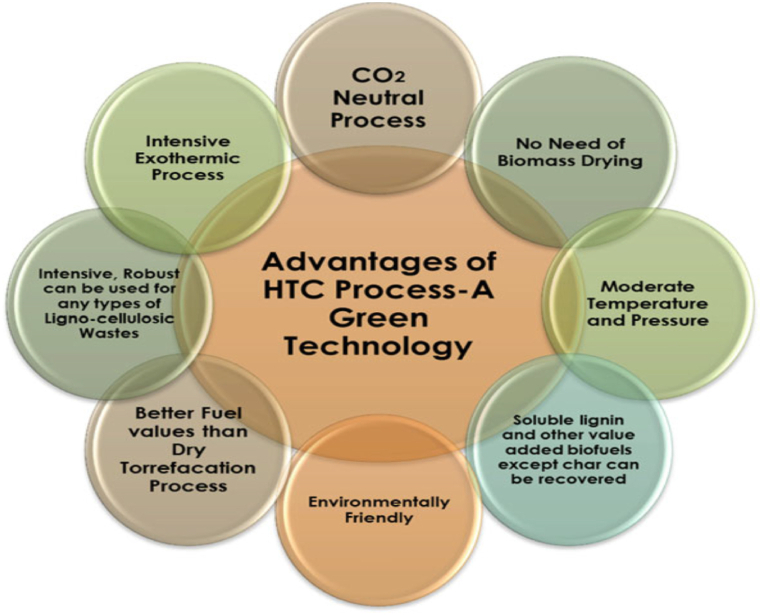
HTC has shown to be a successful and economically feasible process in the past for converting solid waste and biomass into high-value end products [102]. Compared to alternative thermochemical methods, HTC necessitates minimal investment and operational expenses, along with simpler safety solutions. Moreover, its shorter residence time results in decreased equipment volumes. HTC enables precise control over the physio-chemical properties of the feedstock, which is crucial for subsequent biochemical processes, especially in cases of highly heterogeneous solid waste. This aspect is particularly significant when managing incoming solid waste with varying characteristics. HTC is recognized as a supplementary approach for treating MSW and is crucial for increasing WtE conversion efficiency [103]. Fig. 5 depicts various advantages of HTC over other thermochemical conversion technologies.
Fig. 5.
Advantages of hydrothermal carbonization process.
3.5. Gasification
Gasification is another thermochemical WtE conversion technology which enables alteration of carbon-based organic materials into synthesis gas (syngas), a mixture of H2, and CO. The end product also contains carbon dioxide (CO2), methane (CH4), and traces amount of ethylene (C2H4), acetylene (C2H2), and ethane (C2H6). This exhibiting one of the lowest exergy rates among various hydrocarbon fuels [79].
The energy content of syngas lies in between 4 and 50 MJ/Nm3, which is approximately 33 % of that found in natural gas [104]. Syngas production from MSW through gasification facilitates leveraging of existing natural gas infrastructure, enabling easy, and economic distribution without retrofitting. Gasification's high operational temperatures allow for heat recovery, and the resulting slag, primarily inorganic, finds application in road construction. Gasification is more advantageous than incineration as it facilitates the valorization of inorganic portion of MSW [105]. Gasification offers a broader range of products including heat and syngas, while incineration primarily generates heat.
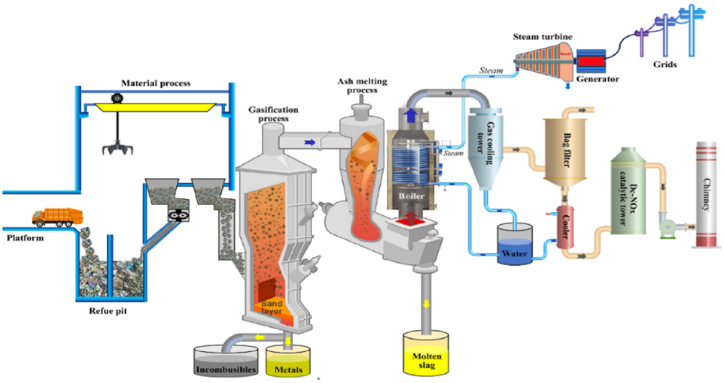
In smaller-scale setups, integrating gasifiers with internal combustion engine can boost energy production while keeping pollutant emissions minimal [106]. As gasification technology advances, operators now have a wide array of gasifier options to choose based on their specific operational requirements and desired performance outcomes. For a more vivid representation of the technology, Fig. 6 demonstrates a model of a MSW gasification system for energy generation.
Fig. 6.
Diagram of commercial gasification of MSW for integrated energy system [37].
Gasification process is becoming attractive for clean hydrogen generation, possessing a remarkable calorific value of 141.7 MJ/kg. Gasification process can be conducted under various mediums such as air, steam, or water. In hydrothermal gasification, water plays a critical role as both the reaction medium and reactant. When water surpasses its critical temperature (TC ≥ 374.1 °C) and critical pressure (PC ≥ 22.1 MPa), it becomes supercritical water. Conversely, subcritical water refers to the fluid phase of water existing below the critical temperature (TC < 374.1 °C) and pressure (PC < 22.1 MPa) [107]. Supercritical water's thermo-physical properties create optimal conditions for the oxidation of organic wastes, making it an efficient medium for hydrothermal gasification processes.
During the gasification process of organic wastes, the initial stage involves heating biomass to approximately 500 °C, causing water vapor and volatile components emission from feedstock. These compounds include polysaccharides such as cellulose, hemicellulose, and starch, as well as lignin, lipids, fats, proteins, and pectin. Hemicellulose degrades between 200 and 300 °C, cellulose between 250 and 350 °C, and lignin within the range of 200–500 °C [108].
In the subsequent phases of gasification, products of the initial phase undergo in the second stage in between 500 and 600 °C temperature and produce phenolics, aromatics, aliphatics, aldehydes, and olefins from the breakdown of biomass constituents. Transitioning into the third stage (600–900 °C), intermediate degradation products further undergo cracking reactions, leading to the production of permanent gases like hydrogen (H2), carbon monoxide (CO), carbon dioxide (CO2), methane (CH4), and higher hydrocarbons (C2+). Concurrently, tertiary products such as benzene, naphthalene, anthracene, pyrene, and phenanthrene are formed during this phase [105]. Different operating conditions including percentage of water content in the feedstock, the reaction temperature and reaction medium and performance of gasification process shown in Table 5. Lower Heating Value (LHV), hydrogen (H2) content, carbon monoxide (CO) content, and Cold Gasification Efficiency (CGE) provide insights into the energy content and syngas components obtained from different waste materials under varied operating conditions. Upon observation, it is apparent that alterations in temperature result in distinct variations in the percentages of H2 and CO. At lower temperatures, such as 600 °C and 650 °C, a higher concentration of CO is evident in the syngas produced, reaching values of 42 % and 30 %, respectively [109]. However, an increase in temperature to 700 °C exhibits a substantial rise in the H2 composition, showcasing a sharp increase to 43 %, concurrently demonstrating a decrease in CO concentration to 42 % [110].
Table 5.
Operating condition and performance of waste gasification Unit.
| Feed | Operating conditions | Initial Moisture content (wt%) | Lower heating value (LHV) (MJ/Nm3) | H2 concentration (%) | CO concentration (%) | Cold gasification efficiency (CGE) (%) | Ref |
|---|---|---|---|---|---|---|---|
| MSW |
|
10.0 | 5.4 | 16.0 | 24.0 | 62 | [110] |
| 30 | 4.8 | 12 | 25 | 48 | |||
| 50 | 4.2 | 8.5 | 26 | 38 | |||
| MSW |
|
48 | 5.8 | 43 | 42 | – | [104] |
| 48 | 3.7 | 42 | 26 | – | |||
| Poly-ethylene |
|
0.02 | 7.1 | 35 | 25 | 40 | [111] |
| Dry Tyres |
|
– | – | 15 | 8.1 | – | [112] |
| MSW |
|
50.9 | – | 24 | 30 | 54 | [113] |
| MSW |
|
51.7 | – | 28 | 25 | 88 | [114] |
| MSW |
|
– | 7 | 6.2 | 9.73 | 53 | [109] |
| SRF |
|
26.8 | 6.1 | 25.5 | 19.4 | 54.2 | |
| MSW |
|
7.6 | 5.43 | 6.9 | 18.8 | 40.3 | [115] |
| Pin sawdust |
|
11.7 | 4.97 | 8.8 | 16.7 | 37 | [115] |
| Coffee husk |
|
10.1 | 15.47 | 15.1 | 26.01 | – | [116] |
Hydrothermal gasification, in comparison to thermochemical gasification, presents several advantages. It facilitates quicker hydrolysis, improved solubility of feedstock, rapid degradation of organic matter, and increased carbon conversion, even at relatively lower temperatures [93]. This method yields syngas with increased proportions of hydrogen (H2), exhibits lower generation of solid by-products, and diminishes polymerization possibilities of intermediate components [117].
Hydrothermal gasification (supercritical condition) of raw sludge and cattle manure reduces cost. This method yields an increased volume of hydrogen-rich syngas at high temperatures and pressures, extended retention times, and lower feed concentrations. Various catalysts, such as alkali Lewis acids [118] and transition metals, enhance the process by accelerating hydrogenation, methanation, and water-gas shift reactions.
He et al. [119] examined how temperature and calcined dolomite catalyst influenced steam gasification of a mixed MSW blend. Elevated temperatures (850–950 °C) with calcined dolomite increased hydrogen (H2) and carbon monoxide (CO) yields, achieving maximum H2 concentration (53.3 mol%), H2 yield (38.6 mol/kg), and syngas production (36.4–70.2 mol%). The resulting char at 950 °C showed high ash content (84 %), low H2 levels (0.4 wt%), and reduced carbon (4.1 wt%) due to dehydrogenation and carbon gasification.
The improvement of waste gasification technology requires specific enhancements, notably in the careful selection of the gasifying agent, a point emphasized by Adnan et al. [80]. This choice significantly affects the yield, component selection, and the calorific value of syngas. Advancements in research and technology for improving gasification efficacy in this area offer a promising avenue for energy recovery, either integrated with a synthetic chemistry foundation or within a waste material-based biorefinery.
4. Criteria for choosing the best waste management techniques
Reduce, reuse, recycle, recovery (WtE), and waste disposal with treatment are the ways to manage MSW. The government should make a significant decision in selecting the most appropriate method. Gaining local population consensus is essential for implementing any solid waste management plan. In making this decision, several factors must be taken into account including economic, technical, political, institutional, socio-cultural, and legal aspects related to the environmental conditions at the proposed site for the treatment plant. Additionally, the decision-making process typically involves a diverse group of stakeholders, including local residents, administrators, engineers, scholars, and environmentalists, each with their own unique perspectives and concerns. Typically, the factors influencing the selection of a treatment plant include Environmental Cost (EnC) and Economic Cost (EcC). EnC is assessed based on the environmental burden caused by greenhouse gas (GHG) emissions and the resulting climate change from different treatment methods. On the other hand, EcC is determined by factors such as plant operation costs, labor costs, and other related expenses. For instance, a management approach that reduces management expenses and environmental effects would be readily embraced by the community [120]. A decision support framework is essential for helping decision-makers establish a sustainable MSW management system. Authorities need reliable tools to model the best decision alternatives that meet the area's specific conditions and select the most suitable option using an efficient optimization technique [121].
Energy justice plays a key role in determining and planning the appropriate waste disposal methods, ensuring that waste management goals are met by aligning with the socio-economic aspects of energy [122]. This approach applies moral and social principles to ensure fair decision-making in energy production, distribution, and consumption, promoting the equitable distribution of the costs and benefits associated with energy systems. The energy justice concept can be used as a decision-making tool for stakeholders, policymakers, and planners in making more informed energy choices, leading to a balanced and impartial approach to addressing the challenges of climate change, energy poverty, and energy security known as energy trilemma [123]. The energy trilemma is further divided into four criteria: 2A, Rights, Social Aspects, and Environmental Issues and 10 sub-criteria as tabulated in Table 6.
Table 6.
Deciding criteria of MSW management method.
| Criteria | Name | Sub-criteria | Perception |
|---|---|---|---|
| 1 | 2A |
|
The criteria emphasize the distribution of energy in a specific region, optimizing operational costs to make it accessible to the majority, and creating job opportunities. |
| 2 | Rights |
|
This criterion highlights the importance of ensuring that everyone has the right to use energy safely, without health risks, and that energy is distributed equitably among the population and acknowledges the rights of future generations. |
| 3 | Social Aspects |
|
It focuses on ensuring transparency in providing information to the public, educating people about the new technology and its potential impacts on the social and cultural development of the region, gaining social acceptance for each scenario, and considering vulnerable groups in the decision-making process. |
| 4 | Environmental Issues |
|
This involves addressing issues like soil, air, and water pollution, climate change, and the release of harmful substances. |
Moreover, fuzzy logic-based model can be used for decision-making in selecting a suitable thermochemical waste conversion technology. Fuzzy set theory can be applied to minimize uncertainty and enhance numerical quantification. Fuzzy logic offers a framework that effectively handles imprecise and vague information, commonly found in environmental data and decision-making processes [124]. The complexity of waste characteristics, as MSW is heterogeneous with varying compositions and properties; decision-making under uncertainty, considering fluctuating waste generation rates and varying market conditions for by-products; and the optimization of thermochemical conversion processes (such as pyrolysis, gasification, and liquefaction) by evaluating multiple criteria like efficiency, cost, and environmental impact. For example, Fuzzy Delphi method (FDM) to screen key criteria, the Fuzzy Analytic Hierarchy Process (FAHP) to assess their importance, and Fuzzy TOPSIS to rank alternatives, leading to a reliable and efficient decision-making process. Different methods have compared MSW treatment technologies using multiple evaluation criteria to help users select the best scenario. Interval-valued fuzzy group multi-criteria decision-making (IVFMCDM) has also been widely used due to having several advantages such as it combines grey relational analysis with interval-valued fuzzy set (IVFS) theory, which improves handling of uncertainties and imprecise information over traditional fuzzy set theory; it lets stakeholders rate alternatives using linguistic variables instead of exact data; and it asks various stakeholder groups about their preferences and opinions during the decision-making process and uses their input to determine evaluation weights [125]. Additionally, multi-attributes group decision-making (MAGDM) approach offers a framework and approach to facilitate collaborative decision-making, select criteria, determine weights, and identify the most appropriate AI solutions for achieving energy efficiency objectives in buildings [126].
Arikan et al. [127] proposed using TOPSIS, PROMETHEE, and fuzzy TOPSIS methods to select the best solid waste disposal methods, with ordered storing and burning identified as the top alternatives among 18 criteria and 10 options. Coban et al. [128] used TOPSIS, PROMETHEE I, and II to identify effective municipal solid waste management (MSWM) techniques, selecting recycling and landfilling as the top methods from eight alternatives based on seven criteria. Roy et al. [129] applied the credibility TOPSIS method in a triangular fuzzy environment to identify the best MSWM approach, selecting Refuse-derived fuel (RDF) combustion over direct incineration as the top option among four approaches based on ten criteria. Rahimi et al. [130] conducted a study to identify the best landfill site near Mahallat city, Iran, using a triangular fuzzy approach such as Geographic Information Systems (GIS), MULTIMOORA, and the Best Worst Method (BWM) to evaluate 11 criteria across 14 potential sites. Torkayesh et al. [131] proposed a sustainable waste disposal technology using the Stratified MCDM (SMCDM) technique, evaluating six alternatives landfilling, anaerobic digestion, incineration, pyrolysis, plasma, and gasification based on nine criteria. Rahman et al. [132] applied the Analytic Hierarchy Process (AHP) model to prioritize anaerobic digestion, pyrolysis, and plasma gasification as waste-to-energy technologies for Dhaka, Bangladesh, based on technological, environmental, and financial factors. Li et al. [133] proposed an MSWM scenario using BWM to determine the weights of 12 criteria. Eight scenarios were scored on a 0–100 scale, with the combination of landfilling and incineration with energy recovery identified as the best disposal option. Paul et al. [134] used cubic Pythagorean fuzzy, comparatively new techniques and found pyrolysis as the most efficient way to dispose of MSW after analysis of thirteen important criteria listed using the CuP-EDAS methodology.
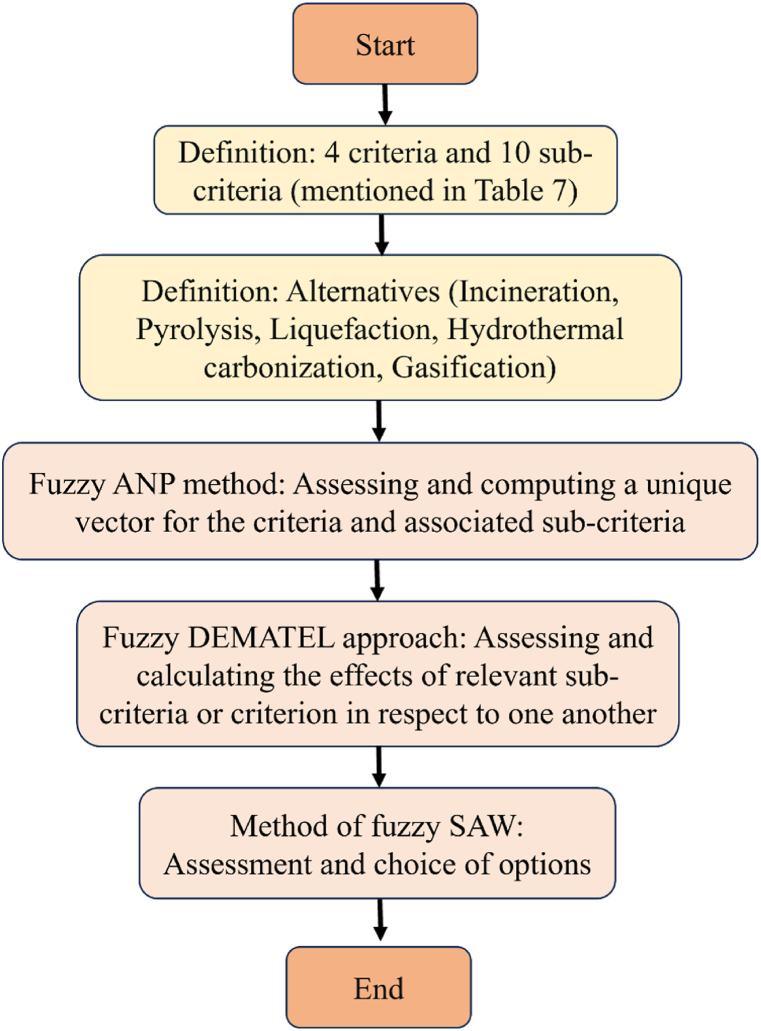
However, the combination of the energy justice concept and fuzzy logic tool can be applied for choosing best conversion method that fulfill all the criteria mentioned. Fetanat et al. [122] used an integrated multi-criteria decision-making model consisting of fuzzy DEMATEL method, the analytic network process (ANP) and the simple additive weighting (SAW) approach, and also considered energy justice criteria to choose the options among four alternatives including anaerobic digestion, pyrolysis, gasification, and incineration. A flow diagram of decision making problem is presented in Fig. 7.
Fig. 7.
Problem-solving flowchart.
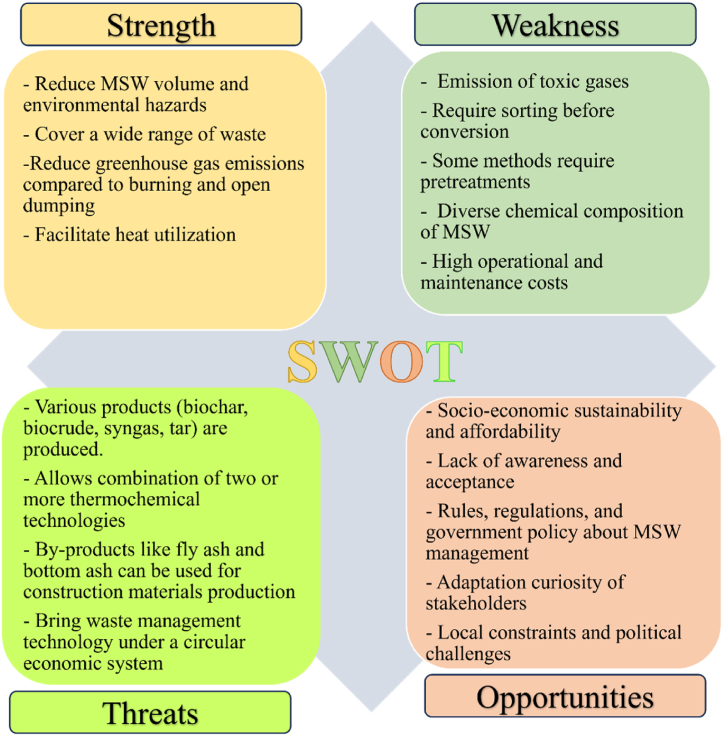
Furthermore, a SWOT analysis is presented in Fig. 8 highlighting the strengths, weaknesses, opportunities and threats of thermochemical conversion techniques of MSW while Table 7 depicts the merits and demerits of each conversion technology described in section 3. However, decision-makers must consider all aspects of various criteria when choosing a waste disposal method to ensure the environment remains healthy and preserved [122]. Each waste disposal method has unique characteristics, and the most suitable option should be selected based on specific needs and feasibility.
Fig. 8.
SWOT analysis of thermochemical process of MSW.
Table 7.
Merits and demerits of various thermochemical conversion techniques.
| Thermochemical conversion | Advantages | Disadvantages | References |
|---|---|---|---|
| Incineration |
|
|
[7,19] |
| Pyrolysis |
|
|
[9,135,136] |
| Liquefaction |
|
|
[81,137] |
| Hydrothermal carbonization (HTC) |
|
|
[3,138] |
| Gasification |
|
|
[9] |
5. Management of MSW for circular economy
Management of waste should be conducted in such a way that environmental emissions due to waste disposal can be minimized. In the case of waste disposal, the Intergovernmental Panel on Climate Change (IPCC) also recommends an economy with minimal waste generation, WtE conversion, and reuse of waste [139] which leads to a circular economic system. Circular economy offers various opportunities by lowering the quantity of MSW dumped in landfills and validating the quantity used for recycling and such a regenerative system can decrease waste residues, byproducts, gas emissions, energy leakage, and the tapering of the energy and material loop. Effectively designed and targeted MSW processing methods combined with streamlined marketing procedures for the final goods and byproducts could help eliminate cash flow problems and remove the financial barriers associated with waste management.
Wajda [140] reported waste management based on the circular economic concept reduces exposure of waste directly to the environment, reduce the use of natural resources, and increase income sources. Sobolewski et al. [141] reported the thermochemical method of MSW conversion into energy as a great method for circular economy. Filho et al. [142] and Sajid et al. [143] reported gasification of MSW is a means of circular economy due to energy production and overall GHG emission reduction for MSW. Sobolewski et al. [141] mentioned incineration as a less convenient method compared to other thermochemical method due to GHG and heavy metal emission. But incineration also plays a great role in the implementation of a circular economic system as gas produced during incineration can be used in energy production and by-product ash from incineration can be used instead of cement and concrete material. Poranek et al. [7] also reported the use of bottom ash from incineration leads to this thermochemical waste treatment technology in circular economic systems. Ischia and Fiori [144] stated hydrothermal carbonization fulfills the conditions of circular economic system development by converting organic wastes into energy and useable materials. Pyrolysis oil can be used as input materials in petrochemicals to generate new polymers and thus help to achieve a circular economic system [135]. Park et al. [145] found pyrolysis of plastic in a university area reduces cost and GHG emissions during pyrolysis compared to traditional management which is in line with the concept of waste management in the circular economy model. So, pyrolysis of MSW may also reduce GHG and the cost of waste disposal achieving circularity in this system. Reduction, recycling, reuse, and recovery are key criteria that can result in a win-win situation for communities and waste management systems and municipalities.
Although sustainability practices have been proposed, there are still many areas where waste management is inadequate. Lifecycle assessment (LCA) is a crucial method for assessing ecological sustainability by analyzing resource use and environmental impacts. LCA is employed to evaluate the environmental impact of products throughout their entire lifecycle, as it helps determine their overall environmental footprint.
6. Challenges and future prospects
Sorting MSW is important because heterogeneity in the chemical composition of MSW poses a big challenge for thermochemical conversion of MSW. Variation of materials in MSW is a great threat to the selection of single thermochemical technology for waste management. The presence of non-combustible products like glass and metal had a negative effect on thermochemical conversion like pyrolysis [146]. The syngas composition from MSW gasification differs depending on the composition of MSW [15]. Rules and regulations of government, economic feasibility, social barriers, insufficient technology, and shortage of skilled labor turned into main challenges for the establishment of thermochemical biomass conversion as sustainable technology [9]. Proper use of by-products and residue during thermochemical conversion should be increased as by-products of thermochemical conversion hinder marketing the application of this technology for MSW management. The primary reasons for the failure of waste-to-energy conversion technology include improper waste material collection, a lack of public awareness and responsibility, insufficient resources for collection and segregation, the volume of waste generated, inadequate financial support, and the absence of effective policies. Other factors that contribute to the failure of waste to energy conversion technology include inadequate planning of waste management, characterization of municipal solid waste, rules and regulations not being implemented and followed, and improper coordination between the federal and state governments.
Flexible control of process parameters may lead this thermochemical conversion of waste to energy toward its sustainability. In the case of thermochemical conversion, the end product depends on process parameters and conditions during operation. Studies reveal that only change in temperature during pyrolysis affects the proportion of oil, gas, and biochar production during pyrolysis [136]. So, by controlling process parameters, it is possible to get end-product based demand. Proper waste management policy may promote thermochemical WtE technologies [147]. Yao et al. [137] reported the thermochemical conversion of waste facilitates the recovery of heavy metal during energy production which is also beneficial. Increasing awareness about thermochemical conversion technologies and marketing of products of thermochemical conversion of MSW will help in spreading of these technologies.
7. Conclusions and recommendations
7.1. Conclusions
Global trash generation has increased dramatically and is predicted to continue expanding due to both population and economic growth which pose various challenges to the environment, air, water, and humans. Nevertheless, MSW contains a variety of contaminants that may be valuable and have multiple uses. Reduce, reuse, and recycle are the primary waste management strategies of MSW at the present situation. The landfill offers an inexpensive and straightforward method of disposing of waste, but if it is not managed properly, it affects the environment adversely. Conversion of MSW into energy is a reliable pathway to solve current energy shortage and reduction in ecological impact for open dumping of it. Production of energy and useable by-products such as char, bio-oil, tar for various purpose make the thermochemical conversion of MSW as sustainable technology for developing circular economy in terms of production and consumption of energy. Moreover, proper management of by-products obtained from thermochemical technologies may also enhance the commercialization of these technologies for MSW management. While gasification produces H2 rich syngas, pyrolysis and liquefaction of MSW usually result in energy-dense bio-oil that may be utilized to make various chemicals, biodiesel, and so on. In addition to those techniques, HTC produces char, a carbon-rich substance that can be used to improve soil, sequester carbon, adsorb carbon, or create customized materials with a variety of uses. The process parameters of temperature, heating rate, pressure, reaction time, feed concentration, type, and amount of catalyst all have an impact on the product quality and yield of all thermochemical processes.
However, most of the thermochemical conversion techniques of MSW are hampered by their high operating cost (involving cost for waste collection, sorting, and equipment) and poor waste utilization. These high operational costs can be reduced by implementing more efficient designs, increasing plant capacity, generating revenue from selling valuable by-products, adopting lean manufacturing processes, and using integrated energy systems. The key takeaway is to utilize waste comprehensively, maximizing the production of secondary products with minimal energy loss, making more efficient investments, and reducing pollution footprints. As there is a variation in the final product of each thermochemical technology that's why proper conversion technology should be select based on target end products. Proper utilization of end products and by-products of all MSW thermochemical conversion technology can bring the system under a circular economic system.
However, WtE technologies also have some drawbacks. For example, incineration causes air pollution and release of toxic volatiles during pyrolysis of MSW can affect human health. To popularize WtE technology of MSW, several techniques need to be implemented including through analysis for methodology, developing the standard, educating people, providing financial support to research and development, etc. Moreover, strong working relationships between the various management partners are necessary to guarantee the MSW management system's long-term sustainability. The findings of this study might help stake-holders to select proper thermochemical conversion technology for MSW management based on the composition of available MSW and proper use of final products. It will also help policymakers to decide on commercialization of any of these thermochemical technologies based on regional MSW composition and integrate this waste management technique into a circular economic system. Furthermore, subsequent research endeavors ought to aim at surmounting the constraints posed by the thermochemical conversion technology of MSW.
7.2. Recommendations
Some recommendations for adapting sustainable conversion techniques of MSW to energy are given below.
-
•
Public awareness about the necessity of proper MSW management can popularize thermochemical conversion technologies. If stakeholders know the benefits of harnessing energy from MSW, they may promote this technology and thus it will become a sustainable energy source.
-
•
The government should take the necessary steps to promote the thermochemical conversion of MSW by applying new rules and regulations on energy production. Different subsidy programs for adapting these technologies should be added to their energy policies.
-
•
Non-government organizations should also work to increase awareness about the importance of MSW management. They can offer training about the operation and maintenance of plants during thermochemical conversion. Incentives can be offered by different private organizations. They can also help to construct plants by offering money, which stakeholders will pay back through installments.
-
•
Physio-chemical characteristics, waste segregation, type, and origin of MSW affect the selection of suitable technology. Teaching common people about these will increase the adaptation of thermochemical technologies as it will enhance energy production.
-
•
Managing by-products is a burning issue during the adaptation of these technologies. Short training, manual, and arranging programs on proper management of these by-products should be arranged by different government and non-governmental organizations.
-
•
The importance of waste-to-energy conversion, and MSW management should be included in the academic curriculum which will help the young generation to know its importance and steps to become entrepreneurs in the energy sector. Extensive research should be carried out in universities and research organizations on these technologies to bring their output to the field level. In this case, government and non-government organizations should provide funds to conduct these research works.
-
•
During the construction, operation, and maintenance of MSW management plants authority should involve local people, especially women. Involving local people may help to overcome social barriers and local constraints as it will become an income source for them.
-
•
Land for MSW thermochemical conversion plants should be selected carefully concerning human and animal health, and ecological conditions. Toxic gases or products from this plant can harm the lives of the environment.
CRediT authorship contribution statement
Tumpa R. Sarker: Writing – review & editing, Writing – original draft, Supervision, Methodology, Investigation, Data curation, Conceptualization. Mst. Lucky Khatun: Writing – original draft, Methodology, Investigation. Dilshad Z. Ethen: Writing – original draft, Conceptualization. Md. Rostom Ali: Writing – review & editing. Md. Shariful Islam: Methodology. Sagor Chowdhury: Methodology. Kazi Shakibur Rahman: Methodology. Nafis Sadique Sayem: Methodology. Rahman Samsur Akm: Funding acquisition.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Saravanan A., Kumar P.S., Nhung T.C., Ramesh B., Srinivasan S., Rangasamy G. A review on biological methodologies in municipal solid waste management and landfilling: resource and energy recovery. Chemosphere. 2022;309 doi: 10.1016/j.chemosphere.2022.136630. [DOI] [PubMed] [Google Scholar]
- 2.Kaza S., Yao L., Bhada-Tata P., Van Woerden F. Urban Development, Urban Development; 2018. What a Waste 2.0: A Global Snapshot of Solid Waste Management to 2050. [Google Scholar]
- 3.Raja W., Brahmbhatt J. Conversion of municipal solid waste into hydrochar using hydrothermal carbonization. J. Emerg. Technol. Innov. Res. 2021;8:e593–e599. [Google Scholar]
- 4.Natarajan R., Al Fazari F., Al Saadi A. Municipal waste water treatment by natural coagulant assisted electrochemical technique—parametric effects. Environ. Technol. Innov. 2018;10:71–77. [Google Scholar]
- 5.Varjani S., Shahbeig H., Popat K., Patel Z., Vyas S., V Shah A., Barceló D., Ngo H.H., Sonne C., Lam S.S., Aghbashlo M., Tabatabaei M. Sustainable management of municipal solid waste through waste-to-energy technologies. Bioresour. Technol. 2022;355 doi: 10.1016/j.biortech.2022.127247. [DOI] [PubMed] [Google Scholar]
- 6.Xiao S., Dong H., Geng Y., Francisco M.J., Pan H., Wu F. An overview of the municipal solid waste management modes and innovations in Shanghai, China. Environ. Sci. Pollut. Res. 2020;27:29943–29953. doi: 10.1007/s11356-020-09398-5. [DOI] [PubMed] [Google Scholar]
- 7.Poranek N., Łaźniewska-Piekarczyk B., Czajkowski A., Pikoń K. Circular economy for municipal solid waste incineration bottom ash (MSWIBA) management in mortars with CSA and CEM I, MSWIBA glassy phase, and DTG. Energies. 2022;15:135. doi: 10.3390/en15010135. [DOI] [Google Scholar]
- 8.Wiedinmyer C., Yokelson R.J., Gullett B.K. Global emissions of trace gases, particulate matter, and hazardous air pollutants from open burning of domestic waste. Environ. Sci. Technol. 2014;48:9523–9539. doi: 10.1021/es502250z. [DOI] [PubMed] [Google Scholar]
- 9.Lawińska O., Korombel A., Zajemska M. Pyrolysis-based municipal solid waste management in Poland—SWOT analysis. Energies. 2022;15:510. [Google Scholar]
- 10.Ozbay G., Jones M., Gadde M., Isah S., Attarwala T. Design and operation of effective landfills with minimal effects on the environment and human health. J. Environ. Public Health. 2021;2021:1–13. doi: 10.1155/2021/6921607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu J., Li R., Shi Y., Deng Y. Life cycle assessment-based optimization approaches for sustainable disposal of municipal solid waste. Sustain. Cities Soc. 2022;79 doi: 10.1016/J.SCS.2021.103665. [DOI] [Google Scholar]
- 12.Pandit A., Nakagawa Y., Timilsina R.R., Kotani K., Saijo T. Taking the perspectives of future generations as an effective method for achieving sustainable waste management. Sustain. Prod. Consum. 2021;27:1526–1536. doi: 10.1016/J.SPC.2021.03.019. [DOI] [Google Scholar]
- 13.Chand Malav L., Yadav K.K., Gupta N., Kumar S., Sharma G.K., Krishnan S., Rezania S., Kamyab H., Pham Q.B., Yadav S., Bhattacharyya S., Yadav V.K., Bach Q.V. A review on municipal solid waste as a renewable source for waste-to-energy project in India: current practices, challenges, and future opportunities. J. Clean. Prod. 2020;277 doi: 10.1016/j.jclepro.2020.123227. [DOI] [Google Scholar]
- 14.Shahnazari A., Rafiee M., Rohani A., Bhushan Nagar B., Ebrahiminik M.A., Aghkhani M.H. Identification of effective factors to select energy recovery technologies from municipal solid waste using multi-criteria decision making (MCDM): a review of thermochemical technologies. Sustain. Energy Technol. Assessments. 2020;40 doi: 10.1016/j.seta.2020.100737. [DOI] [Google Scholar]
- 15.Santos S.M., Assis A.C., Gomes L., Nobre C., Brito P. Waste gasification technologies: a brief overview. Waste. 2023;1:140–165. doi: 10.3390/waste1010011. [DOI] [Google Scholar]
- 16.Amen R., Hameed J., Albashar G., Kamran H.W., Shah M.U.H., Zaman M.K.U., Mukhtar A., Saqib S., Ch S.I., Ibrahim M., Ullah S., Al-Sehemi A.G., Ahmad S.R., Klemeš J.J., Bokhari A., Asif S. Modelling the higher heating value of municipal solid waste for assessment of waste-to-energy potential: a sustainable case study. J. Clean. Prod. 2021;287 [Google Scholar]
- 17.United Nations The 17 goals, glob. Goals Sustain. Dev. 2018:15–18. https://sdgs.un.org/goals [Google Scholar]
- 18.Singh P., Boora A., Kumar Gupta A. A review on utilizing municipal solid waste incineration (MSWIA) in construction activates. IOP Conf. Ser. Earth Environ. Sci. 2023 doi: 10.1088/1755-1315/1110/1/012042. [DOI] [Google Scholar]
- 19.Chen D., Zhang Y., Xu Y., Nie Q., Yang Z., Sheng W., Qian G. Municipal solid waste incineration residues recycled for typical construction materials- a review. RSC Adv. 2022;12:6279–6291. doi: 10.1039/d1ra08050d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.He M., Xiao B., Liu S., Hu Z., Guo X., Luo S., Yang F. Syngas production from pyrolysis of municipal solid waste (MSW) with dolomite as downstream catalysts. J. Anal. Appl. Pyrolysis. 2010;87 [Google Scholar]
- 21.Matuszewska A., Owczuk M., Biernat K. Current trends in waste plastics' liquefaction into fuel fraction: a review. Energies. 2022;15 doi: 10.3390/en15082719. [DOI] [Google Scholar]
- 22.Wei J., Guo Q., Ding L., Kunio Yoshikawa G.Y. Synergy mechanism analysis of petroleum coke and municipal solid waste (MSW)-Derived hydrochar Co-gasification. Appl. Energy. 2017;206:1354–1363. [Google Scholar]
- 23.Berge N.D., Ro K.S., Mao J., Flora J.R.V., Chappell M.A., Bae S. Hydrothermal carbonization of municipal waste streams. Environ. Sci. Technol. 2011;45:5696–5703. doi: 10.1021/es2004528. [DOI] [PubMed] [Google Scholar]
- 24.Munawar M.A., Li P., Ma Q., Haque M.A., Chen W.-T. Chapter five - thermochemical conversions of municipal solid waste into fuels and chemicals. Adv. Bioenergy. 2023;8:239–305. [Google Scholar]
- 25.Nandhini R., Berslin D., Sivaprakash B., Rajamohan N., Vo D.V.N. Thermochemical conversion of municipal solid waste into energy and hydrogen: a review. Environ. Chem. Lett. 2022;20:1645–1669. doi: 10.1007/s10311-022-01410-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sondh S., Upadhyay D.S., Patel S., Patel R.N. A strategic review on municipal solid waste (living solid waste) management system focusing on policies, selection criteria and techniques for waste-to-value. J. Clean. Prod. 2022;356 [Google Scholar]
- 27.Deus R.M., Mele F.D., Bezerra B.S., Battistelle R.A.G. A municipal solid waste indicator for environmental impact: assessment and identification of best management practices. J. Clean. Prod. 2020;242 doi: 10.1016/j.jclepro.2019.118433. [DOI] [Google Scholar]
- 28.Asamoah B., Nikiema J., Gebrezgabher S., Odonkor E., Njenga M. A Review on Production, Marketing and Use of Fuel Briquettes. 2016. [Google Scholar]
- 29.Sarker T.R., Ethen D.Z., Islam S., Asha H.H., Ali M.R. Transformation of municipal solid waste to biofuel and bio ‑ chemicals – a review. Int. J. Environ. Sci. Technol. 2024 doi: 10.1007/s13762-024-05975-0. [DOI] [Google Scholar]
- 30.Saidan M.N., Drais A.A., Al-Manaseer E. Solid waste composition analysis and recycling evaluation: zaatari Syrian refugees camp, Jordan. Waste Manag. 2017;61 doi: 10.1016/j.wasman.2016.12.026. [DOI] [PubMed] [Google Scholar]
- 31.Kumar A., Samadder S.R. A review on technological options of waste to energy for effective management of municipal solid waste. Waste Manag. 2017;69:407–422. doi: 10.1016/j.wasman.2017.08.046. [DOI] [PubMed] [Google Scholar]
- 32.Rajmohan K.S., Chandrasekaran R., Varjani S. A review on occurrence of pesticides in environment and current technologies for their remediation and management. Indian J. Microbiol. 2020:125–138. doi: 10.1007/s12088-019-00841-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nagpure A.S. Assessment of quantity and composition of illegal dumped municipal solid waste (MSW) in Delhi. Resour. Conserv. Recycl. 2019;141:54–60. doi: 10.1016/j.resconrec.2018.10.012. [DOI] [Google Scholar]
- 34.Quina M.J., Bontempi E., Bogush A., Schlumberger S., Weibel G., Braga R., Funari V., Hyks J., Rasmussen E., Lederer J. Technologies for the management of MSW incineration ashes from gas cleaning: new perspectives on recovery of secondary raw materials and circular economy. Sci. Total Environ. 2018;635:526–542. doi: 10.1016/j.scitotenv.2018.04.150. [DOI] [PubMed] [Google Scholar]
- 35.Laner D., Crest M., Scharff H., Morris J.W.F., Barlaz M.A. A review of approaches for the long-term management of municipal solid waste landfills. Waste Manag. 2012;32:498–512. doi: 10.1016/j.wasman.2011.11.010. [DOI] [PubMed] [Google Scholar]
- 36.Raghab S.M., Abd El Meguid A.M., Hegazi H.A. Treatment of leachate from municipal solid waste landfill. HBRC J. 2013;9:187–192. doi: 10.1016/j.hbrcj.2013.05.007. [DOI] [Google Scholar]
- 37.Hoang A.T., Varbanov P.S., Nižetić S., Sirohi R., Pandey A., Luque R., Ng K.H., Pham V.V. Perspective review on Municipal Solid Waste-to-energy route: characteristics, management strategy, and role in circular economy. J. Clean. Prod. 2022;359 doi: 10.1016/j.jclepro.2022.131897. [DOI] [Google Scholar]
- 38.Patil A.A., Kulkarni A.A., Patil B.B. Waste to energy by incineration. J. Comput. Technol. 2014;3:12–15. [Google Scholar]
- 39.Karim M., Corazzini B. The current status of MSW disposal and energy production: a brief review of waste incineration. MOJ Ecol. Environ. Sci. 2019;4:34–37. doi: 10.15406/mojes.2019.04.00129. [DOI] [Google Scholar]
- 40.Hu Y., Cheng H., Tao S. The growing importance of waste-to-energy (WTE) incineration in China's anthropogenic mercury emissions: emission inventories and reduction strategies. Renew. Sustain. Energy Rev. 2018;97:119–137. [Google Scholar]
- 41.Nidoni P.G. Incineration process for solid waste management and effective utilization of by Products. Int. Res. J. Eng. Technol. 2017;4:378–382. [Google Scholar]
- 42.Al-Ghouti M.A., Khan M., Nasser M.S., Al-Saad K., Heng O.E. Recent advances and applications of municipal solid wastes bottom and fly ashes: insights into sustainable management and Conservation of resources. Environ. Technol. Innov. 2021;21 doi: 10.1016/j.eti.2020.101267. [DOI] [Google Scholar]
- 43.Cho B.H., Nam B.H., An J., Youn H. Municipal solid waste incineration (MSWI) ashes as construction materials-a review. Materials. 2020;13:1–30. doi: 10.3390/ma13143143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cudjoe D., Acquah P.M. Environmental impact analysis of municipal solid waste incineration in african countries. Chemosphere. 2021;265 doi: 10.1016/j.chemosphere.2020.129186. [DOI] [PubMed] [Google Scholar]
- 45.Belviso C. State-of-the-art applications of fly ash from coal and biomass: a focus on zeolite synthesis processes and issues. Prog. Energy Combust. Sci. 2018;65:109–135. doi: 10.1016/j.pecs.2017.10.004. [DOI] [Google Scholar]
- 46.Xu G., Shi X. Characteristics and applications of fly ash as a sustainable construction material: a state-of-the-art review. Resour. Conserv. Recycl. 2018;136:95–109. doi: 10.1016/j.resconrec.2018.04.010. [DOI] [Google Scholar]
- 47.Hasan M.M., Rasul M.G., Khan M.M.K., Ashwath N., Jahirul M.I. Energy recovery from municipal solid waste using pyrolysis technology: a review on current status and developments. Renew. Sustain. Energy Rev. 2021;145 doi: 10.1016/j.rser.2021.111073. [DOI] [Google Scholar]
- 48.Parakh P.D., Nanda S., Kozinski J.A. Eco-friendly transformation of waste biomass to biofuels, curr. Biochem. Eng. 2020;6:120–134. doi: 10.2174/2212711906999200425235946. [DOI] [Google Scholar]
- 49.Nanda S., Dalai A.K., Berruti F., Kozinski J.A. Biochar as an exceptional bioresource for energy, agronomy, carbon sequestration, activated carbon and specialty materials. Waste and Biomass Valorization. 2016;7:201–235. doi: 10.1007/s12649-015-9459-z. [DOI] [Google Scholar]
- 50.Nanda S., Sarker T.R., Kang K., Li D. 2023. Perspectives on Thermochemical Recycling of End-Of-Life Plastic Wastes to Alternative Fuels; pp. 1–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lu X., Gu X. A review on lignin pyrolysis: pyrolytic behavior, mechanism, and relevant upgrading for improving process efficiency. BioMed Central. 2022 doi: 10.1186/s13068-022-02203-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tripathi M., Sahu J.N., Ganesan P. Effect of process parameters on production of biochar from biomass waste through pyrolysis: a review. Renew. Sustain. Energy Rev. 2016;55:467–481. doi: 10.1016/j.rser.2015.10.122. [DOI] [Google Scholar]
- 53.Osman A.I., Farghali M., Ihara I., Elgarahy A.M., Ayyad A., Mehta N., Ng K.H., Abd El-Monaem E.M., Eltaweil A.S., Hosny M., Hamed S.M., Fawzy S., Yap P.S., Rooney D.W. Springer International Publishing; 2023. Materials, Fuels, Upgrading, Economy, and Life Cycle Assessment of the Pyrolysis of Algal and Lignocellulosic Biomass: a Review. [DOI] [Google Scholar]
- 54.Onay O., Koçkar O.M. Pyrolysis of rapeseed in a free fall reactor for production of bio-oil. Fuel. 2006;85:1921–1928. doi: 10.1016/j.fuel.2006.03.009. [DOI] [Google Scholar]
- 55.Zhao X., Joseph B., Kuhn J., Ozcan S. Biogas reforming to syngas: a review. iScience. 2020;23 doi: 10.1016/j.isci.2020.101082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sarker T.R., Nanda S., Dalai A.K., Meda V. A review of torrefaction technology for upgrading lignocellulosic biomass to solid biofuels. Bioenergy Res. 2021;14:645–669. doi: 10.1007/s12155-020-10236-2. [DOI] [Google Scholar]
- 57.Kaur R., Gera P., Jha M.K. Study on effects of different operating parameters on the pyrolysis of biomass: a review. J. Biofuels Bioenergy. 2015;1:135. doi: 10.5958/2454-8618.2015.00015.2. [DOI] [Google Scholar]
- 58.Sarker T.R., Pattnaik F., Nanda S., Dalai A.K., Meda V., Naik S. Hydrothermal pretreatment technologies for lignocellulosic biomass: a review of steam explosion and subcritical water hydrolysis. Chemosphere. 2021;284 doi: 10.1016/j.chemosphere.2021.131372. [DOI] [PubMed] [Google Scholar]
- 59.Musale H.K., Bhattacharyulu Y.C., Bhoyar R.K., Student P.G., Professor A. " design consideration of pyrolysis reactor for production of bio-oil ". Int. J. Eng. Trends Technol. 2013;5:83–85. [Google Scholar]
- 60.Zaman C.Z., Pal K., Yehye W.A., Sagadevan S., Shah S.T., Adebisi G.A., Marliana E., Rafique R.F., Bin Johan R. Pyrolysis: a sustainable way to generate energy from waste. Pyrolysis. 2017 doi: 10.5772/intechopen.69036. [DOI] [Google Scholar]
- 61.Kumar A., Bhattacharya T., Shaikh W.A., Roy A., Chakraborty S., Vithanage M., Biswas J.K. Springer Nature Singapore; 2023. Multifaceted Applications of Biochar in Environmental Management: a Bibliometric Profile. [DOI] [Google Scholar]
- 62.Sarker T.R., Azargohar R., Dalai A.K., Meda V. Enhancement of fuel and physicochemical properties of canola residues via microwave torrefaction. Energy Rep. 2021;7:6338–6353. doi: 10.2139/ssrn.3900345. [DOI] [Google Scholar]
- 63.Sarker T.R., Nanda S., Meda V. Microwave torrefaction: an emerging technology to manufacture solid fuels. Emerg. Biofuels. 2024:179–207. doi: 10.1016/B978-0-323-99547-4.00010-1. [DOI] [Google Scholar]
- 64.Li X., Yan J., Yang H., Peng T., Yang Q. 2011 Int. Conf. Mater. Renew. Energy Environ. 2011. Study on processing technology for microwave pyrolysis of municipal solid waste; pp. 336–340. [Google Scholar]
- 65.Zhang Y., Chen P., Liu S., Fan L., Zhou N., Min M., Cheng Y., Peng P., Anderson E., Wang Y., Wan Y., Liu Y., Li B., Ruan R. Pyrolysis. 2017. Microwave‐assisted pyrolysis of biomass for bio‐oil production. [DOI] [Google Scholar]
- 66.Ethaib S., Omar R., Kamal S.M.M., Biak D.R.A., Zubaidi S.L. Microwave-assisted pyrolysis of biomass waste: a mini review. Processes. 2020;8:1190. doi: 10.3390/pr8091190. [DOI] [Google Scholar]
- 67.Gedam V.V., Regupathi I. Pyrolysis of municipal solid waste for syngas production by microwave irradiation. Nat. Resour. Res. 2012;21:75–82. doi: 10.1007/s11053-011-9161-1. [DOI] [Google Scholar]
- 68.Beneroso D., Bermúdez J.M., Arenillas A., Menéndez J.A. Influence of the microwave absorbent and moisture content on the microwave pyrolysis of an organic municipal solid waste. J. Anal. Appl. Pyrolysis. 2014;105:234–240. doi: 10.1016/j.jaap.2013.11.009. [DOI] [Google Scholar]
- 69.Dave P.N., Joshi A.K. Plasma pyrolysis and gasification of plastics waste - a review. J. Sci. Ind. Res. (India). 2010;69:177–179. [Google Scholar]
- 70.Dharmaraj S., Ashokkumar V., Pandiyan R., Munawaroh H.S.H., Chew K.W., Chen W.-H., Ngamcharussrivichai C. Pyrolysis: an effective technique for degradation of COVID-19 medical wastes. Chemosphere. 2020;275 doi: 10.1016/j.chemosphere.2021.130092. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 71.Tang L., Huang H., Hao H., Zhao K. Development of plasma pyrolysis/gasification systems for energy efficient and environmentally sound waste disposal. J. Electrostat. 2013;71:839–847. doi: 10.1016/j.elstat.2013.06.007. [DOI] [Google Scholar]
- 72.Bhatt K.P., Patel S., Upadhyay D.S., Patel R.N. A critical review on solid waste treatment using plasma pyrolysis technology. Chem. Eng. Process. - Process Intensif. 2022;177 doi: 10.1016/j.cep.2022.108989. [DOI] [Google Scholar]
- 73.Pal S., Kumar A., Sharma A.K., Ghodke P.K., Pandey S., Patel A. Recent advances in catalytic pyrolysis of municipal plastic waste for the production of hydrocarbon fuels. Processes. 2022;10:1497. [Google Scholar]
- 74.Almohamadi H., Aljabri A., Mahmoud E.R.I., Khan S.Z. Catalytic pyrolysis of municipal solid waste : effects of pyrolysis parameters. Bull. Chem. React. Eng. Catal. 2021;16:342–352. doi: 10.9767/bcrec.16.2.10499.342-352. [DOI] [Google Scholar]
- 75.Li Q., Faramarzi A., Zhang S., Wang Y., Hu X., Gholizadeh M. Progress in catalytic pyrolysis of municipal solid waste. Energy Convers. Manag. 2020;226 doi: 10.1016/j.enconman.2020.113525. [DOI] [Google Scholar]
- 76.Abdullah C. Xi’an Jiaotong University; 2023. Study of Catalytic Pyrolysis of Municipal Solid Waste to Hydrocarbon Fuel. [Google Scholar]
- 77.Isa K.M., Abdullah T.A.T., Ali U.F.M. Hydrogen donor solvents in liquefaction of biomass: a review. Renew. Sustain. Energy Rev. 2018 doi: 10.1016/j.rser.2017.04.006. [DOI] [Google Scholar]
- 78.Jiang Y., Dennehy C., Lawlor P.G., Hu Z., McCabe M., Cormican P., Zhan X., Gardiner G.E. Inhibition of volatile fatty acids on methane production kinetics during dry co-digestion of food waste and pig manure. Waste Manag. 2018;79:302–311. doi: 10.1016/j.wasman.2018.07.049. [DOI] [PubMed] [Google Scholar]
- 79.Gollakota A.R.K., Kishore N., Gu S. A review on hydrothermal liquefaction of biomass. Renew. Sustain. Energy Rev. 2018 doi: 10.1016/j.rser.2017.05.178. [DOI] [Google Scholar]
- 80.Adnan M.A., Hossain M.M., Kibria M.G. Converting waste into fuel via integrated thermal and electrochemical routes: an analysis of thermodynamic approach on thermal conversion. Appl. Energy. 2022;311 [Google Scholar]
- 81.Mathanker A., Das S., Pudasainee D., Khan M., Kumar A., Gupta R. A review of hydrothermal liquefaction of biomass for biofuels production with a special focus on the effect of process parameters, Co-solvents, and extraction solvents. Energies. 2021;14:4916. [Google Scholar]
- 82.Hu Y., Feng S., Bassi A., Xu C. (Charles. Improvement in bio-crude yield and quality through Co-liquefaction of algal biomass and sawdust in ethanol-water mixed solvent and recycling of the aqueous by-product as a reaction medium. Energy Convers. Manag. 2018;171:618–625. doi: 10.1016/j.enconman.2018.06.023. [DOI] [Google Scholar]
- 83.Serrano D.P., Aguado J., Vicente G., Sánchez N. Effects of hydrogen-donating solvents on the thermal degradation of HDPE. J. Anal. Appl. Pyrolysis. 2007;78:194–199. doi: 10.1016/j.jaap.2006.07.001. [DOI] [Google Scholar]
- 84.Ahamed Kameel N.I., Wan Daud W.M.A., Abdul Patah M.F., Mohd Zulkifli N.W. Influence of reaction parameters on thermal liquefaction of plastic wastes into oil: a review. Energy Convers. Manag. X. 2022;14 doi: 10.1016/j.ecmx.2022.100196. [DOI] [Google Scholar]
- 85.Wang B., Huang Y., Zhang J. Hydrothermal liquefaction of lignite, wheat straw and plastic waste in sub-critical water for oil: product distribution. J. Anal. Appl. Pyrolysis. 2014;110:382–389. doi: 10.1016/j.jaap.2014.10.004. [DOI] [Google Scholar]
- 86.Mosteiro-Romero M., Vogel F., Wokaun A. Liquefaction of wood in hot compressed water Part 1 – experimental results. Chem. Eng. Sci. 2014;109 doi: 10.1016/j.ces.2013.12.038. [DOI] [Google Scholar]
- 87.Liang L., Mao Z., Li Y., Wan C., Wang T., Zhang L., Zhang L. Liquefaction of crop residues for polyol production. Bioresources. 2006;1 doi: 10.15376/biores.1.2.248-256. [DOI] [Google Scholar]
- 88.Bi Z., Zhang J., Peterson E., Zhu Z., Xia C., Liang Y., Wiltowski T. Biocrude from pretreated sorghum bagasse through catalytic hydrothermal liquefaction. Fuel. 2017;188:112–120. doi: 10.1016/j.fuel.2016.10.039. [DOI] [Google Scholar]
- 89.Deng H., Meredith W., Uguna C.N., Snape C.E. Impact of solvent type and condition on biomass liquefaction to produce heavy oils in high yield with low oxygen contents. J. Anal. Appl. Pyrolysis. 2015;113:340–348. doi: 10.1016/j.jaap.2015.02.015. [DOI] [Google Scholar]
- 90.Huang H.J., Yuan X.Z., Zeng G.M., Liu Y., Li H., Yin J., Wang X.L. Thermochemical liquefaction of rice husk for bio-oil production with sub- and supercritical ethanol as solvent. J. Anal. Appl. Pyrolysis. 2013;102:60–67. doi: 10.1016/j.jaap.2013.04.002. [DOI] [Google Scholar]
- 91.Jun Huang H., zhong Yuan X., na Zhu H., Li H., Liu Y., li Wang X., ming Zeng G. Comparative studies of thermochemical liquefaction characteristics of microalgae, lignocellulosic biomass and sewage sludge. Energy. 2013;56:52–60. doi: 10.1016/j.energy.2013.04.065. [DOI] [Google Scholar]
- 92.Long J., Li Y., Zhang X., Tang L., Song C., Wang F. Comparative investigation on hydrothermal and alkali catalytic liquefaction of bagasse: process efficiency and product properties. Fuel. 2016;186:685–693. doi: 10.1016/j.fuel.2016.09.016. [DOI] [Google Scholar]
- 93.Sarker T.R., Meda V., Dalai A.K. Catalytic hydrothermal gasification of aqueous effluents obtained from microwave torrefaction of agricultural residue. J. Environ. Chem. Eng. 2022;10 doi: 10.1016/j.jece.2022.108835. [DOI] [Google Scholar]
- 94.Titirici M.M., Thomas A., Antonietti M. Back in the black: hydrothermal carbonization of plant material as an efficient chemical process to treat the CO2 problem? New J. Chem. 2007;31:787–789. doi: 10.1039/b616045j. [DOI] [Google Scholar]
- 95.Libra J.A., Ro K.S., Kammann C., Funke A., Berge N.D., Neubauer Y., Titirici M.M., Fühner C., Bens O., Kern J., Emmerich K.H. Hydrothermal carbonization of biomass residuals: a comparative review of the chemistry, processes and applications of wet and dry pyrolysis. Biofuels. 2011;2:71–106. doi: 10.4155/bfs.10.81. [DOI] [Google Scholar]
- 96.Sevilla M., Fuertes A.B. The production of carbon materials by hydrothermal carbonization of cellulose. Carbon N. Y. 2009;47:2281–2289. doi: 10.1016/j.carbon.2009.04.026. [DOI] [Google Scholar]
- 97.Putra H.E., Djaenudin D., Damanhuri E., Dewi K., Pasek A.D. Hydrothermal carbonization kinetics of lignocellulosic municipal solid waste. J. Ecol. Eng. 2021;22:188–198. doi: 10.12911/22998993/132659. [DOI] [Google Scholar]
- 98.Vlaskin M.S., Vladimirov G.N. Hydrothermal carbonization of organic components from municipal solid waste. Theor. Found. Chem. Eng. 2018;52:996–1003. doi: 10.1134/S0040579518050421. [DOI] [Google Scholar]
- 99.Lin Y., Ge Y., Xiao H., He Q., Wang W., Chen B. Investigation of hydrothermal co-carbonization of waste textile with waste wood, waste paper and waste food from typical municipal solid wastes. Energy. 2020;210 doi: 10.1016/j.energy.2020.118606. [DOI] [Google Scholar]
- 100.Pawlak-Kruczek H., Niedzwiecki L., Sieradzka M., Mlonka-Mędrala A., Baranowski M., Serafin-Tkaczuk M., Magdziarz A. Hydrothermal carbonization of agricultural and municipal solid waste digestates – structure and energetic properties of the solid products. Fuel. 2020;275 doi: 10.1016/j.fuel.2020.117837. [DOI] [Google Scholar]
- 101.Barua P., Hossain N. Waste to energy: an overview by global perspective. 2021. [DOI]
- 102.Román S., Libra J., Berge N., Sabio E., Ro K., Li L., Ledesma B., Alvarez A., Bae S. Hydrothermal carbonization: modeling, final properties design and applications: a review. Energies. 2018;11:1–28. doi: 10.3390/en11010216. [DOI] [Google Scholar]
- 103.Basso D., Weiss-Hortala E., Patuzzi F., Castello D., Baratieri M., Fiori L. Hydrothermal carbonization of off-specification compost: a byproduct of the organic municipal solid waste treatment. Bioresour. Technol. 2015;182:217–224. doi: 10.1016/j.biortech.2015.01.118. [DOI] [PubMed] [Google Scholar]
- 104.Chen C., Jin Y.Q., Yan J.H., Chi Y. Simulation of municipal solid waste gasification in two different types of fixed bed reactors. Fuel. 2013;103:58–63. doi: 10.1016/j.fuel.2011.06.075. [DOI] [Google Scholar]
- 105.Yong Z.J., Bashir M.J.K., Ng C.A., Sethupathi S., Lim J.W., Show P.L. Sustainable waste-to-energy development in Malaysia: appraisal of environmental , financial , and municipal solid waste. Processes. 2019;7 [Google Scholar]
- 106.Teixeira S., Monteiro E., Silva V., Rouboa A. Prospective application of municipal solid wastes for energy production in Portugal. Energy Pol. 2014;71:159–168. doi: 10.1016/j.enpol.2014.04.002. [DOI] [Google Scholar]
- 107.Sarker T.R., Nanda S., Dalai A.K. Parametric studies on hydrothermal gasification of biomass pellets using Box-Behnken experimental design to produce fuel gas and hydrochar. J. Clean. Prod. 2023;388 doi: 10.1016/j.jclepro.2022.135804. [DOI] [Google Scholar]
- 108.Sarker T.R., Azargohar R., Dalai A.K., Venkatesh M. Physicochemical and fuel characteristics of torrefied agricultural residues for sustainable fuel production. Energy Fuel. 2020;34:14169–14181. doi: 10.1021/acs.energyfuels.0c02121. [DOI] [Google Scholar]
- 109.Basu P. 2010. Biomass Gasification and Pyrolysis Practical Design and Theory. [Google Scholar]
- 110.Niu M., Huang Y., Jin B., Wang X. Simulation of syngas production from municipal solid waste gasification in a bubbling fluidized bed using aspen plus. Ind. Eng. Chem. Res. 2013;52:14768–14775. doi: 10.1021/ie400026b. [DOI] [Google Scholar]
- 111.Al Amoodi N., Kannan P., Al Shoaibi A., Srinivasakannan C. Aspen plus simulation of polyethylene gasification under equilibrium conditions. Chem. Eng. Commun. 2013;200:977–992. doi: 10.1080/00986445.2012.715108. [DOI] [Google Scholar]
- 112.Mitta N.R., Ferrer-Nadal S., Lazovic A.M., Parales J.F., Velo E., Puigjaner L. 16th Eur. Symp. Comput. Aided Process Eng. 9th Int. Symp. Process Syst. Eng. 2006. Modelling and simulation of a tyre gasification plant for synthesis gas production; pp. 1771–1776. [DOI] [Google Scholar]
- 113.Ramzan N., Ashraf A., Naveed S., Malik A. Simulation of hybrid biomass gasification using aspen plus: a comparative performance analysis for food, municipal solid and poultry waste. Biomass Bioenergy. 2011;35:3962–3969. doi: 10.1016/j.biombioe.2011.06.005. [DOI] [Google Scholar]
- 114.Shehzad A., Bashir M.J.K., Sethupathi S. System analysis for synthesis gas (syngas) production in Pakistan from municipal solid W gasification using a circulating fluidized bed gasifier. Renew. Sustain. Energy Rev. 2016;60:1302–1311. doi: 10.1016/j.rser.2016.03.042. [DOI] [Google Scholar]
- 115.Cao Y., Fu L., Mofrad A. Combined-gasification of biomass and municipal solid waste in a fluidized bed gasifier. J. Energy Inst. 2019 doi: 10.1016/j.joei.2019.01.006. [DOI] [Google Scholar]
- 116.Wilson L., John G.R., Mhilu C.F., Yang W., Blasiak W. Coffee husks gasification using high temperature air/steam agent. Fuel Process. Technol. 2010;91:1330–1337. doi: 10.1016/j.fuproc.2010.05.003. [DOI] [Google Scholar]
- 117.Okolie J.A., Nanda S., Dalai A.K., Kozinski J.A., Dalai A.K. Optimization and modeling of process parameters during hydrothermal gasification of biomass model compounds to generate hydrogen-rich gas products. Int. J. Hydrogen Energy. 2019;45:18275–18288. doi: 10.1016/j.ijhydene.2019.05.132. [DOI] [Google Scholar]
- 118.Gong M., Nanda S., Romero M.J., Zhu W., Kozinski J.A. Subcritical and supercritical water gasification of humic acid as a model compound of humic substances in sewage sludge. J. Supercrit. Fluids. 2017;119:130–138. doi: 10.1016/j.supflu.2016.08.018. [DOI] [Google Scholar]
- 119.He M., Hu Z., Xiao B., Li J., Guo X., Luo S., Yang F., Feng Y., Yang G., Liu S. Hydrogen-rich gas from catalytic steam gsification of municipal solid waste (MSW): influence of catalyst and temperature on yield and product composition. Int. J. Hydrogen Energy. 2009;34:195–203. doi: 10.1016/j.ijhydene.2008.09.070. [DOI] [Google Scholar]
- 120.De Feo G., Panza D., Belgiorno V. 2005. Public Opinion and Residents Concerns, Perception and Attitudes toward MSW Treatment and Disposal Plants. [Google Scholar]
- 121.Govind Kharat M., Murthy S., Jaisingh Kamble S., Raut R.D., Kamble S.S., Govind Kharat M. Fuzzy multi-criteria decision analysis for environmentally conscious solid waste treatment and disposal technology selection. Technol. Soc. 2019;57:20–29. doi: 10.1016/j.techsoc.2018.12.005. [DOI] [Google Scholar]
- 122.Fetanat A., Mofid H., Mehrannia M., Shafipour G. Informing energy justice based decision-making framework for waste-to-energy technologies selection in sustainable waste management: a case of Iran. J. Clean. Prod. 2019;228:1377–1390. doi: 10.1016/j.jclepro.2019.04.215. [DOI] [Google Scholar]
- 123.McCauley D. Energy justice Re-balancing the trilemma of security. Poverty and Climate Change. 2018 doi: 10.1007/978-3-031-31785-9_1. [DOI] [Google Scholar]
- 124.Paul T.K., Jana C., Pal M. Multi-criteria group decision-making method in disposal of municipal solid waste based on cubic Pythagorean fuzzy EDAS approach with incomplete weight information. Appl. Soft Comput. 2023;144 doi: 10.1016/j.asoc.2023.110515. [DOI] [Google Scholar]
- 125.Wang Z., Ren J., Goodsite M.E., Xu G. Waste-to-energy, municipal solid waste treatment, and best available technology: comprehensive evaluation by an interval-valued fuzzy multi-criteria decision making method. J. Clean. Prod. 2018;172:887–899. doi: 10.1016/j.jclepro.2017.10.184. [DOI] [Google Scholar]
- 126.Abbasi S.N., Ashraf S., Akram M., Jana C., Simic V., Pamucar D. A Pythagorean fuzzy Z-number-based neutrality aggregation model for AI-enabled energy efficiency management. Appl. Soft Comput. 2024;161:1–12. doi: 10.1016/j.asoc.2024.111753. [DOI] [Google Scholar]
- 127.Arıkan E., Şimşit-Kalender Z.T., Vayvay Ö. Solid waste disposal methodology selection using multi-criteria decision making methods and an application in Turkey. J. Clean. Prod. 2017;142:403–412. doi: 10.1016/j.jclepro.2015.10.054. [DOI] [Google Scholar]
- 128.Coban A., Ertis I.F., Cavdaroglu N.A. Municipal solid waste management via multi-criteria decision making methods: a case study in Istanbul, Turkey. J. Clean. Prod. 2018;180:159–167. doi: 10.1016/j.jclepro.2018.01.130. [DOI] [Google Scholar]
- 129.Roy J., Adhikary K., Kar S. Springer Singapore; 2019. Advances in Waste Management. [DOI] [Google Scholar]
- 130.Rahimi S., Hafezalkotob A., Monavari S.M., Hafezalkotob A., Rahimi R. Sustainable landfill site selection for municipal solid waste based on a hybrid decision-making approach: fuzzy group BWM-MULTIMOORA-GIS. J. Clean. Prod. 2020;248 doi: 10.1016/j.jclepro.2019.119186. [DOI] [Google Scholar]
- 131.Torkayesh A.E., Malmir B., Rajabi Asadabadi M. Sustainable waste disposal technology selection: the stratified best-worst multi-criteria decision-making method. Waste Manag. 2021;122:100–112. doi: 10.1016/j.wasman.2020.12.040. [DOI] [PubMed] [Google Scholar]
- 132.Rahman S.M.S., Azeem A., Ahammed F. Selection of an appropriate waste-to-energy conversion technology for Dhaka City, Bangladesh. Int. J. Sustain. Eng. 2017;10:99–104. doi: 10.1080/19397038.2016.1270368. [DOI] [Google Scholar]
- 133.Li Z., Jia X., Jin H., Ma L., Xu C., Wei H. Determining optimal municipal solid waste management scenario based on best-worst method. J. Environ. Eng. Landsc. Manag. 2021;29:150–161. doi: 10.3846/jeelm.2021.14843. [DOI] [Google Scholar]
- 134.Thengane S.K., Kung K.S., Gomez-Barea A., Ghoniem A.F. Advances in biomass torrefaction: parameters, models, reactors, applications, deployment, and market. Prog. Energy Combust. Sci. 2022;93 doi: 10.1016/j.pecs.2022.101040. [DOI] [Google Scholar]
- 135.Kulas D.G. 2022. Towards a Circular Economy: Liquid-Fed Fast Pyrolysis of Waste Polyolefin Plastics. [Google Scholar]
- 136.Chen D., Yin L., Wang H., He P. Pyrolysis technologies for municipal solid waste: a review. Waste Manag. 2014;34:2466–2486. doi: 10.1016/j.wasman.2014.08.004. [DOI] [PubMed] [Google Scholar]
- 137.Yao Y., Ramu C., Procher A., Littlejohns J., Hill J.M., Butler J.W. Potential for thermo-chemical conversion of solid waste in Canada to fuel. Heat, and Electricity, Waste. 2023;1:689–710. doi: 10.3390/waste1030041. [DOI] [Google Scholar]
- 138.Sharma H.B., Panigrahi S., Dubey B.K. Food waste hydrothermal carbonization: study on the effects of reaction severities, pelletization and framework development using approaches of the circular economy. Bioresour. Technol. 2021;333 doi: 10.1016/j.biortech.2021.125187. [DOI] [PubMed] [Google Scholar]
- 139.Mhatre P., Panchal R., Singh A., Bibyan S. A systematic literature review on the circular economy initiatives in the European union. Sustain. Prod. Consum. 2021;26:187–202. [Google Scholar]
- 140.Wajda A. Int. Multidiscip. Sci. GeoConference SGEM; Sofia: 2018. Management of wastes from energy industry in the frame of circular economy on the example of microspheres. [Google Scholar]
- 141.Sobolewski A., Chmielniak T., Bigda J., Billig T., Fryza R., Popowicz J. Closing of carbon cycle by waste gasification for circular economy implementation in Poland. Energies. 2022;15:4983. doi: 10.3390/en15144983. [DOI] [Google Scholar]
- 142.Filho D.M., Coelho S.T., Perecin D. Opportunities and challenges of gasification of municipal solid waste (MSW) in Brazil. Energies. 2022;15:2735. doi: 10.3390/en15082735. [DOI] [Google Scholar]
- 143.Sajid M., Raheem A., Ullah N., Asim M., Ur Rehman M.S., Ali N. Gasification of municipal solid waste: progress, challenges, and prospects. Renew. Sustain. Energy Rev. 2022;168 doi: 10.1016/j.rser.2022.112815. [DOI] [Google Scholar]
- 144.Ischia G., Fiori L. Hydrothermal carbonization of organic waste and biomass: a review on process, reactor, and plant modeling. Waste and Biomass Valorization. 2020;12:2797–2824. doi: 10.1007/s12649-020-01255-3. [DOI] [Google Scholar]
- 145.Park H., Kim K., Yu M., Yun Z., Lee S. Economic analysis of the circular economy based on waste plastic pyrolysis oil: a case of the university campus. Environ. Dev. Sustain. 2023 doi: 10.1007/s10668-023-02963-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Sipra A.T., Gao N., Sarwar H. Municipal solid waste (MSW) pyrolysis for bio-fuel production: a review of effects of MSW components and catalysts. Fuel Process. Technol. 2018;175:131–147. doi: 10.1016/j.fuproc.2018.02.012. [DOI] [Google Scholar]
- 147.Makarichi L., Kan R., Jutidamrongphan W., Techato K.A. Suitability of municipal solid waste in african Cities for thermochemical waste-to-energy conversion: the case of harare metropolitan city, Zimbabwe. Waste Manag. Res. 2019;37:83–94. doi: 10.1177/0734242X18804029. [DOI] [PubMed] [Google Scholar]