Abstract
Compared to the general population, patients with chronic pancreatitis have an up to 12-fold higher risk of developing pancreatic cancer. The aim of our study was the identification of potential proteomic biomarkers to contribute to the detection of pancreatic cancer among patients with chronic pancreatitis. We initially performed a proteomic screening analysis of 105 analytes on plasma pools. To validate this finding, we quantitatively determined leptin concentrations in individual plasma samples using the ELISA technique. Additionally, we explored the plasma expression of CEACAM1, an important regulator of leptin expression in various cancer cells using the same method. The preliminary semi-quantitative proteomic analysis identified leptin as the only protein with substantially higher expression in patients with pancreatic cancer compared to those with chronic pancreatitis. Subsequently, by quantitative ELISA, we determined a higher median leptin concentration in the plasma of patients with pancreatic cancer compared to those with chronic pancreatitis. The statistical significance was maintained regardless of other variables like BMI or gender. Additionally, we explored the plasma expression of CEACAM1, an important regulator of leptin expression in various cancer cells, in order to provide insights into the complex mechanisms underlying pancreatic cancer and chronic pancreatits. CEACAM1 concentrations were higher in the plasma of the patients with pancreatic cancer than in those with chronic pancreatitis. However, we did not find a statistically significant correlation between leptin and CEACAM1 expression variation in the two study groups, with CEACAM1 concentration also dependent on other parameters such as BMI, gender, and serum triglyceride level. In conclusion, leptin seems to be a biomarker that can contribute to differentiate patients with pancreatic cancer from patients with chronic pancreatitis.
Keywords: Pancreatic cancer, Chronic pancreatitis, Proteomic biomarkers, Leptin, CEACAM1
Highlights
-
•
Leptin seems to be a biomarker that can contribute to differentiate patients with pancreatic cancer from patients with chronic pancreatitis.
-
•
The identification of elevated serum leptin levels in patients with chronic pancreatitis should raise suspicion of malignant transformation and prompt further diagnostic management.
-
•
CEACAM1 concentrations were higher in plasma of the patients with pancreatic cancer than in those with chronic pancreatitis.
-
•
Leptin demonstrated a correlation with pancreatic cancer in humans that was less confounded by other factors compared to CEACAM1.
Simple summary
The relationship between chronic pancreatitis and an increased risk of developing pancreatic cancer is well known. Considering the challenges associated with diagnosing pancreatic cancer in the context of chronic pancreatitis, our study aimed to identify new biomarkers that could aid in the differentiation of these two diseases. We initially conducted a proteomic screening analysis of 105 analytes in a plasma pool, identifying a single protein, leptin, with a significantly increased expression in the plasma of patients with pancreatic cancer versus patients with chronic pancreatitis. To validate this finding, we quantitatively determined leptin concentrations in individual plasma samples using the ELISA technique. Additionally, we explored the plasma expression of CEACAM1, an important regulator of leptin expression in various cancer cells, in order to provide insights into the complex mechanisms underlying pancreatic cancer and chronic pancreatitis.
1. Introduction
Pancreatic cancer is one of the most aggressive cancers, with the global burden of this disease doubling in the last 25 years [1]. This can be explained by the population ageing globally but also by the increase in the prevalence of some risk factors for this condition, such as smoking, alcohol consumption, obesity, or diabetes [1]. With a total of 495,773 cases globally in 2020, pancreatic cancer came in at the 12th position among newly diagnosed cancer cases in both sexes, according to GLOBOCAN [2]. Of these, 47.1 % of patients were recorded in Asia, 28.3 % in Europe, 12.6 % in Northern America, 7.5 % in Latin America and 3.4 % in Africa [1]. This malignant condition represented approximately 3 % of all cancer cases and 8 % of all cancer deaths in the United States in 2020 [3]. Regarding the mortality rate, GLOBOCAN 2020 places pancreatic cancer in 7th place in the ranking of deaths from cancer, with a total of 466,003 deaths globally [2]. Geographic variability of the death rate associated with this malignant disease was also identified, with 48.1 % of deaths occurring in Asia, 28.4 % in Europe, 11.4 % in North America, 7.7 % in Latin America, and 3.6 % in Africa [2]. Despite the progress in managing patients with malignant diseases, the 5-year survival rate of patients with pancreatic cancer does not exceed 9 % [1,4]. The negative prognosis is mainly due to the late diagnosis and the poor response to oncological treatments [5]. Nowadays, the percentage of patients diagnosed early, when the tumor is surgically resectable, does not exceed 20 % [1].
Chronic pancreatitis is a condition associated with an increased risk of pancreatic cancer [5]. Kirkegard et al. reported an approximately threefold increase in the risk of pancreatic cancer among patients with chronic pancreatitis compared to the general population [6]. Also, the same authors highlighted the possibility of delaying the diagnosis of pancreatic cancer due to the initial misclassification of pancreatic cancer as chronic pancreatitis [6]. This can be explained by the similarity between the clinical signs and the radiological and biochemical changes induced by these conditions [6]. In another study that followed 471,992 subjects, approximately 5 % of patients were initially misdiagnosed with chronic pancreatitis, and the diagnosis of pancreatic cancer was delayed by > 2 months in more than half of cases [7]. Muller et al. reported a 12-fold increased relative risk of pancreatic cancer detection after a diagnosis of chronic pancreatitis compared to the general population [8]. Data from specialized literature suggest a series of mechanisms that can be implicated in the malignant transformation of metaplastic acinar cells: oxidative stress, activation of some inflammatory pathways (for example, Cyclooxygenase-2), activation of natural-factor kappa-B (NF-kB) and STAT3 pathways [9].
In order to improve the prognosis of patients with pancreatic cancer, research in recent years has focused on the identification of biomarkers that may contribute to the early detection of this neoplasia. The progress recorded in the field of molecular diagnostics, including the detection of different proteins, circulating tumor deoxyribonucleic acid (ctDNA), or micro ribonucleic acids (microRNAs), have proven to be promising for the early diagnosis of pancreatic cancer [10].
Another critical issue in the management of patients with this malignant disease is the high occurrence of phenotypes resistant to chemotherapy [11]. Cytotoxic therapies are therefore linked to significantly suboptimal clinical outcomes and a substantial likelihood of severe adverse effects [11]. The suboptimal outcomes could be attributed to a distinctive tumor microenvironment (TME) [12]. In recent years, research has documented potential beneficial results associated with the use of novel drug classes, including small molecule inhibitors and immunotherapy, in patients with pancreatic cancer [12,13]. A significant barrier to immunotherapy in these patients is the desmoplastic stroma, which blocks T cell infiltration and the abnormally high number of immunosuppressive cells in the TME [12]. However, a murine study illustrated that survival can be substantially enhanced through the administration of FAK VS 4718, a selective inhibitor of focal adhesion kinase (FAK), which effectively diminishes tumor fibrosis and decreases the number of immunosuppressive cells infiltrating the tumor [12]. Additionally, the utilization of FAK inhibitor VS 4718 was linked to an enhanced response to immunotherapy including T cells or programmed death-1(PD-1) antagonists [12]. The identification of prognostic biomarkers facilitates the development of selection scores for targeted therapies for these patients. Furthermore, the identification of novel therapeutic targets may result from a better understanding of the pathogenic mechanisms involved in the development of pancreatic cancer [11,14].
Our study aims to identify potential non-invasive biomarkers with a role in the early diagnosis of pancreatic cancer among patients with chronic pancreatitis. Thus, we performed an initial proteomic screening, on plasma pools, in patients with pancreatic cancer vs. patients with chronic pancreatitis. Following this analysis, we identified a single plasma protein with significantly increased expression in patients with pancreatic cancer vs. chronic pancreatitis, respectively, leptin. Subsequently, we followed the validation of this result through the quantitative determination of leptin concentrations in individual plasma samples using the ELISA technique. We explored also the plasma expression of carcinoembryonic antigen-related cell adhesion molecule 1 (CEACAM1) by quantitative ELISA since positive correlation between this biomarker's concentration and body mass index, comparable to leptin, were reported previously [15,16]. Moreover, it appears that this biomarker regulates leptin expression by interfering with certain signaling pathways that are also implicated in oncogenesis.
2. Materials and methods
2.1. Patients
We conducted a prospective, experimental, non-randomized study in which we included 30 patients with pancreatic cancer (group A) and 30 patients with chronic pancreatitis (group B). This study was approved by the ethics committee of the Emergency Clinical Hospital of Bucharest (no. 3929/12.04.2021) and conducted according to the principles of the Declaration of Helsinki. All the subjects included in the study signed a written informed consent, agreeing to the processing of their personal data and the collection of blood samples. The diagnosis of pancreatic cancer or chronic pancreatitis was established through histopathological examination. The biological samples were obtained by endoscopic ultrasound-guided fine needle biopsy (EUS-FNB). The research was conducted over a three-year period.
2.2. Collection of blood samples
Blood samples were collected from each patient included in the study. We used ethylenediamine tetraacetic acid (EDTA) -coated test tubes for blood collection. Within 2 h, the blood samples were transported to the Stefan S. Nicolau Institute of Virology, where they underwent centrifugation. Thus, two aliquots of plasma were collected and freezed from each subject blood sample to minimize repeated freeze-thaw cycles for independent experiments.
2.3. Screening stage of proteomic analysis on plasma pools
In the screening stage of the proteomic analysis, the Proteome Profiler Human XL Cytokine Array Kit (ARY022B, R&D Systems, Abingdon, UK), based on the dot-blot technique, was used for the simultaneous semi-quantitative determination of 105 analytes (cytokines, chemokines, angiogenesis markers, growth factors, and other soluble proteins) in a plasma pool of samples from patients with pancreatic cancer and a pool of patients with chronic pancreatitis, respectively.
Brifely, according to the protocol recommended by the manufacturer, after a prior blocking step to avoid non-specific protein binding, each nitrocellulose membranes with specific capture antibodies against 105 distinct analytes, along with control antibodies dot-spoted in duplicates on each membrane, was incubated with 100 μl of undiluted plasma at 4 °C overnight. The membranes were subsequently washed and incubated for 1 h at room temperature with biotinylated detection antibodies. After a further washing step, the streptavidin-HRP conjugate was added, followed by the chemiluminescent substrate for HRP. The chemiluminescent signals generated per spot, corresponding to the quantity of bound proteins, were recorded by the Micro Chemi 4.2 device (DNR BioImaging Systems, Israel). Signal intensity (pixel density) was quantified using ImageJ 1.42 (National Institute of Health, Bethesda, MD, USA) image analysis software. The mean intensity of the signals from the corresponding pair of spots was calculated for each analyte. The background signal was subtracted, and a normalization to the mean intensity of the signals generated by the reference spots on the membranes (positive control) was eventually performed.
The mean pixel density value of the controls was set to 1. The relative change between samples, expressed as fold change (FC), was calculated for each protein. The relative variation of plasma protein expression in patients with pancreatic cancer versus patients with chronic pancreatitis was calculated using Microsoft Excel software.
2.4. Validation at the level of individual plasma samples
For the result validation on individual samples, we used quantitative ELISA assays. Human Leptin ELISA kit (KAC2281, Invitrogen, USA) and Human CEACAM1 Simple Step ELISA kit (ab215540, Abcam, UK) were used according to the manufacturer's indications. Briefly, after adding samples and leptin/CEACAM1 standards into the wells, a biotinylated secondary antibody was added. The excess of the secondary antibody-biotin was then removed, and streptavidin-peroxidase enzyme was added to complete the detection sandwich. After a second incubation and washing to remove the unbound enzyme-biotinylated antibody, a chromogenic peroxidase substrate solution (tetramethylbenzidine- TMB) was added. The absorbance was recorded using TriStar's LB 942 Multimode Reader (Berthold Technologies, Germany) and the concentration of human leptin/CEACAM1 was calculated using the standard curve equation.
2.5. Statistical analysis
Statistical analysis was performed with GraphPad Prism 9 software. Values exceeding 1.5 FC were defined as significantly increased values, and those below 0.5 FC were defined as significantly lower values. To compare the median values of leptin/CEACAM1 concentration in patients with pancreatic cancer vs. those with chronic pancreatitis, we used the Mann-Whitney test. The application of this test was determined by the need to compare the average plasma concentrations of different biomarkers with a non-Gaussian distribution between two groups of patients. Subsequently, we utilized Spearman's test to verify the correlation between leptin and CEACAM1 concentrations. We opted to assess the confidence interval of the Spearman correlation coefficient due to the fact that the number of variables examined exceeded 10.
We used a simple linear regression to identify the clinical and paraclinical factors that influenced the plasma expression of leptin, respectively, CEACAM1. Subsequently, the significant predictors identified in the simple model were employed in multiple linear regression. The model was constructed using a retrograde selection algorithm for the predictors. The level of significance α was 0.05, so p-values lower than 0.05 were considered statistically significant.
3. Results
The average age of patients with pancreatic cancer was slightly higher than that of patients with chronic pancreatitis (65 years vs. 52.5 years). Similar average body mass index (BMI) values were observed between the two patient groups (24.55 in pancreatic cancer patients vs. 28.15 in chronic pancreatitis patients) (Table 1). Approximately one-third of the patients, regardless of the diagnosis of pancreatic cancer or chronic pancreatitis, had diabetes mellitus. Also, both smoking and alcohol consumption have been shown to be frequent in patients with chronic pancreatitis and those with pancreatic cancer (Table 1).
Table 1.
Epidemiological, clinical, and paraclinical data in patients with pancreatic cancer vs. chronic pancreatitis.
| Variable | Pancreatic cancer N = 30 |
Chronic pancreatitis N = 30 |
p-value1 |
|---|---|---|---|
| Age, Mean | 65 | 52.5 | <0.001 |
| BMI, Mean | 24.55 | 28.15 | 0.017 |
| Gender, n (%) | |||
| M | 12 (40) | 22 (73.3 %) | |
| F | 18 (60) | 8 (26.6) | 0.08 |
| Diabetes mellitus, n (%) | |||
| Yes | 12 (40) | 8 (26.6) | |
| No | 18 (60) | 22 (73.33) | 0.123 |
| Smoking, n (%) | |||
| Yes | 17 (56.6) | 22 (73.3) | |
| No | 13 (43.4) | 8 (26.7) | 0.723 |
| Alcohol consumption, n (%) | |||
| Yes | 17 (56.6) | 19 (63.3) | |
| No | 13 (43.3) | 11 (36.6) | 0.560 |
| Cholesterol, Mean | 175.9 | 185.3 | 0.475 |
| Triglycerides, Mean | 129.0 | 171.0 | 0.065 |
Welch Two Sample t-test; Pearsons's Chi-squared test.
In the screening stage of proteomic analysis, we evaluated semi-quantitatively, simultaneously, a total of 105 cytokines, chemokines, angiogenesis markers, growth factors, and other soluble proteins. The comparative analysis of plasma protein expression in patients with pancreatic cancer and chronic pancreatitis identified:
-
•
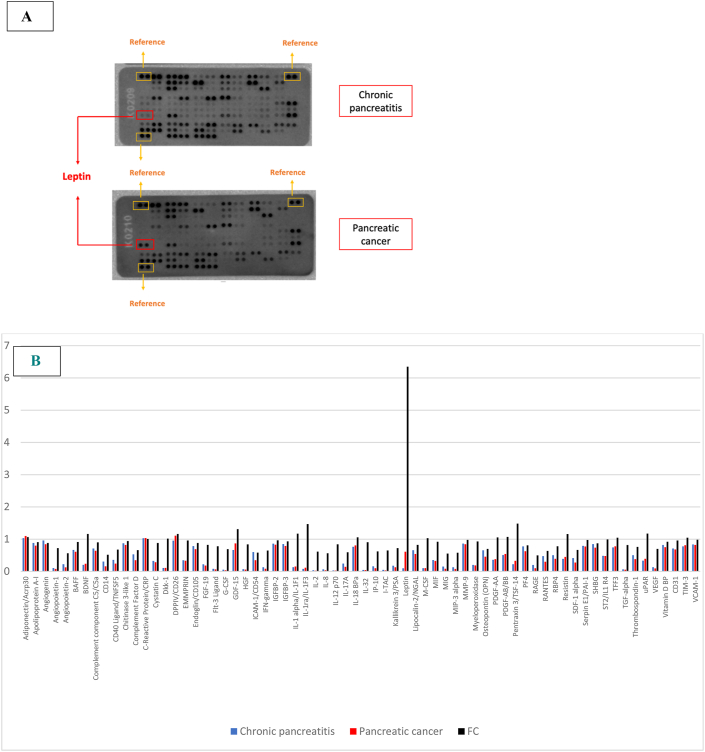
1 plasma protein whose expression exceeded 1.5 FC: leptin (x 6.3 FC) (Fig. 1).
-
•
37 plasma proteins with expression below 0.5 FC: CD30 (also known as Tumor Necrosis Factor Receptor Superfamily Member 8 or Ki-1), Cripto-1, epidermal growth factor (EGF), epithelial neutrophil-activating protein 78 (ENA-78), Fas Ligand, fibroblast growth factor (FGF) basic, FGF-7, granulocyte-macrophage colony-stimulating factor (GM-CSF), growth-regulated oncogene alpha (GRO-alpha), growth hormone (GH), interleukin-1 beta (IL-1 beta/IL-1F2), IL-3, IL-4, IL-5, IL-6, IL-10, IL-11, IL-13, IL-15, IL-16, IL-19, IL-22, IL-23, IL-24, IL-27, IL-31, IL-33, IL-34, leukemia inhibitory factor (LIF), monocyte chemotactic protein-1 (MCP-1), MCP-3, macrophage inflammatory protein- 1 (MIP-1 alpha/MIP-1 beta), MIP-3 beta, relaxin-2, thymus and activation regulated chemokine (TARC), transferrin receptor (TfR), tumor necrosis factor alpha (TNF-alpha).
Fig. 1.
A. Representation of the plasma protein profile in patients with pancreatic cancer vs. chronic pancreatitis analysed on multianalyte protein dot-spoted arrays. The spots marked in the red box demonstrate the highest differences in densitometric signal between array membranes. Reference spots, marked in yellow boxes, were used to normalize the signal. B. Profiles of mean spot pixel density for pancreatic cancer plasma pool (red) or chronic pancreatitis plasma pool (blue) after background subtraction and normalization to the reference spots signal, and relative change between protein expression in plasma pools of patients with pancreatic cancer versus plasma pools of patients with chronic pancreatitis samples, expressed as fold change (FC, black). In the graph are represented only the analysts that had FC > 0.5.
Subsequently, we used quantitative ELISA technique on individual plasma samples from each patient included in the study to validate leptin, the adipokine with a 6.3 FC between patients with pancreatic cancer and chronic pancreatitis. In order to provide insight into the complex mechanisms underlying pancreatic cancer and chronic pancreatitis, we also investigated the plasma expression of CEACAM1, an important regulator of leptin expression in different cancer cells. CEACAM1, one member of the human carcinoembryonic antigen (CEA) family, is a glycoprotein that recently has been reported with increased expression in patients with pancreatic cancer, suggesting the usefulness of this proteomic biomarker in the detection of malignant pancreatic tumors [11].
Quantitative ELISA allowed us to determine each patient's leptin and CEACAM1 concentrations. We calculated leptin and CEACAM1 concentrations for the samples based on standard curve concentrations.
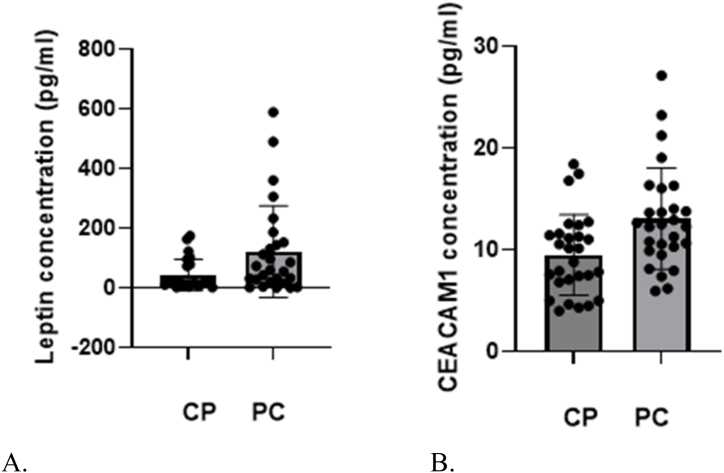
Due to the non-Gaussian distribution of the data, the Mann-Whitney test was used to determine the median values of leptin and CEACAM1 concentration in patients with pancreatic cancer versus those with chronic pancreatitis (Table 2, Fig. 2A and B). Our results showed that the median concentration of leptin was significantly higher in cancer patients than in those with chronic pancreatitis. (59.41 pg/ml vs. 17.96 pg/ml; p = 0.0405). Also, the median values of CEACAM1 were found to be higher in patients with pancreatic cancer (12.75 pg/ml vs. 9.89 pg/ml; p = 0.0282). The difference in expression levels between the two patient groups for both biomarkers were statistically significant.
Tabel 2.
Median concentration of leptin in patients with pancreatic cancer vs. patients with chronic pancreatitis.
| Biomarker | Pancreatic cancer, n = 30 | Chronic pancreatitis, n = 30 | P- valuea |
|---|---|---|---|
| Leptin, Median | 59.41 | 17.96 | 0.0405 |
| CEACAM1, Median | 12.75 | 9.89 | 0.0282 |
Mann-Whitney test.
Fig. 2.
A. Median values of leptin concentration in patients with chronic pancreatitis (CP) versus those with pancreatic cancer (PC).
BMedian values of CEACAM1 concentration in patients with chronic pancreatitis (CP) versus those with pancreatic cancer (PC).
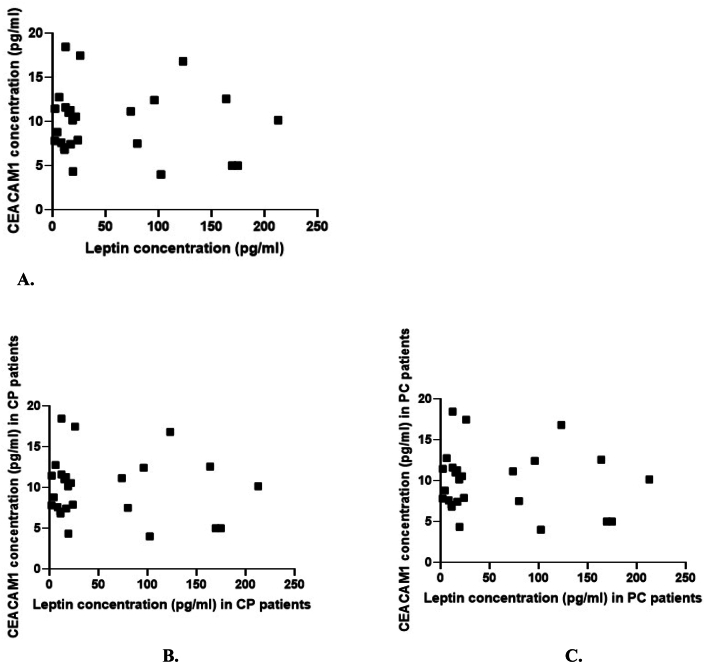
We used the Spearman test to determine whether there is a correlation between the variation of leptin plasma expression and the variation of CEACAM1 plasma expression in the two study groups (Table 3, Fig. 3A, B and 3C). However, no statistically significant correlation was found between the plasma levels of the two biomarkers (Table 3).
Table 3.
Correlation between the variation of leptin plasma expression and the variation of CEACAM1 plasma expression in patients with pancreatic cancer vs. chronic pancreatitis.
| Correlation | ra | 95 % CIb | P value |
|---|---|---|---|
| Leptin vs. CEACAM1 | 0.072 | −0.20–0.34 | 0.60 |
| Leptin in chronic pancreatitis vs. CEACAM1 in chronic pancreatitis | −0.087 | −0.46–0.32 | 0.66 |
| Leptin in pancreatic cancer vs. CEACAM 1 in pancreatic cancer |
−0.011 | −0.39–0.38 | 0.95 |
Spearman test.
r = Spearman correlation coefficient.
CI = Confidence interval.
Fig. 3.
A. Correlation between the variation of leptin plasma expression and the variation of CEACAM1 plasma expression.
B. Correlation between the variation of leptin plasma expression and the variation of CEACAM1 plasma expression in patients with pancreatic cancer (PC).
C. Correlation between the variation of leptin plasma expression and the variation of CEACAM1 plasma expression in patients with chronic pancreatitis (CP).
In the following stage, we investigated various epidemiological, clinical, and paraclinical parameters to identify the potential determinants that could affect the levels of the two biomarkers in the blood. Therfore, we conducted a simple linear regression analysis with the concentration of leptin/CEACAM1 as the dependent variable and the other study-monitored parameters as the independent variables. Then, we selected the variables that showed statistical significance in the simple model and included them in a multiple linear regression analysis. (Table 4, Table 5).
Table 4.
Simple and multiple linear regression to determine the factors influencing plasma leptin concentrations.
| Predictors | N | Simple linear regression |
Multiple linear regression |
||
|---|---|---|---|---|---|
| 95 % CIa | p-value | 95 % CIa | p-value | ||
| Group | 60 | ||||
| Pancreatic cancer | – | ||||
| Chronic pancreatitis | 1.20–1.51 | 0.001 | 3.66–142.9 | 0.039 | |
| Age | 60 | 53.75–62.52 | 0.597 | ||
| Gender | 60 | 1.11–1.43 | 0.037 | −27.95–108.1 | 0.242 |
| BMI | 60 | 23.33–26.20 | 0.010 | −1.01–13.82 | 0.08 |
| Smoking | 60 | ||||
| Yes | |||||
| No | 1.05–1.35 | 0.096 | |||
| Alcohol consumption | 60 | ||||
| Yes | |||||
| No | 1.27–1.60 | 0.053 | |||
| Diabetes mellitus | 60 | ||||
| Yes | |||||
| No | 1.45–1.79 | 0.934 | |||
| Cholesterol | 60 | 163.6–198.2 | 0.648 | ||
| Triglycerides | 60 | 130.4–193.2 | 0.622 | ||
CI = Confidence interval.
Table 5.
Simple and multiple linear regression to determine the factors that influence plasma CEACAM1 concentrations.
| Predictors | N | Simple linear regression |
Multiple linear regression |
||
|---|---|---|---|---|---|
| 95 % CI1 | p-value | 95 % CIa | p-value | ||
| Group | 60 | ||||
| Pancreatic cancer | – | ||||
| Chronic pancreatitis | 0.82 to 1.50 | 0.028 | 24.67 to 460.1 | 0.029 | |
| Age | 60 | 43.02 to 60.26 | 0.067 | ||
| Gender | 60 | 1.40 to 2.07 | 0.025 | −588.1 to −157.3 | 0.001 |
| BMI | 60 | 20.02 to 26.06 | 0.041 | 8.73 to 54.27 | 0.0077 |
| Smoking | 60 | ||||
| Yes | |||||
| No | 1.04 to 1.70 | 0.598 | |||
| Alcohol consumption | 60 | ||||
| Yes | |||||
| No | 1.02 to 1.72 | 0.270 | |||
| Diabetes mellitus | 60 | ||||
| Yes | |||||
| No | 1.54 to 2.21 | 0.142 | |||
| Cholesterol | 60 | 120.5 to 190.6 | 0.148 | ||
| Triglycerides | 60 | −15.19 to 94.59 | <0.0001 | 1.11 to 3.39 | 0.0002 |
CI = Confidence interval.
We found a significant association between the diagnosis of pancreatic cancer compared to chronic pancreatitis (p = 0.001), gender (p = 0.037) and BMI (p = 0.01) and the concentrations of leptin in the plasma. (Table 4). Thus, plasma leptin concentrations have been shown to be higher in patients with pancreatic cancer but also in overweight patients, and all these correlations were statistically significant. Also, alcohol consumption appears to have a minor impact on plasma leptin concentration. Furthermore, we evaluated additional variables, such as age, smoking status, diabetes diagnosis, and serum cholesterol and triglyceride levels. However, no statistically significant correlations were found between these parameters and leptin concentration (Table 4). According to the multiple linear regression analysis results, only the diagnosis of pancreatic cancer versus chronic pancreatitis had a statistically significant association with the level of leptin expression (Table 4).
Regarding CEACAM1 plasma expression, simple linear regression revealed positive associations between this biomarker, diagnosis of pancreatic cancer versus chronic pancreatitis, gender, BMI and serum triglycerides level (Table 5). Additionally, after multiple linear regression, each of these correlations remained statistically significant (Table 5).
The expression of CEACAM1 is significantly higher in patients with pancreatic cancer compared to those with chronic pancreatitis, as determined by the analysis of these results. However, other factors, including BMI, gender, and serum triglyceride level, additionally influence the plasma level of CEACAM1.
4. Discussion
In our study, semi-quantitative screening proteomic analysis using the Proteome Profiler Human XL Cytokine Array Kit (ARY022B, R&D Systems, Abingdon, UK) identified only one protein with significantly increased values in patients with pancreatic cancer versus patients with chronic pancreatitis, namely leptin.
We quantitatively determined leptin plasma concentrations in individual plasma samples to validate these results. The mean concentration of leptin in patients with pancreatic cancer was significantly higher than that in patients with chronic pancreatitis (59.41 pg/ml vs. 17.96 pg/ml; 0.0405). The simple linear regression identified among the factors correlated with plasma leptin concentrations, the diagnosis of pancreatic cancer vs. chronic pancreatitis, gender and BMI. In the multiple linear regression, only diagnosis retained their statistical significance in terms of their impact on plasma leptin concentration. Our findings indicate that leptin may have a role in identifying pancreatic cancer in individuals with chronic pancreatitis. However, it is important to note that our study focused on patients with advanced pancreatic cancer, and therefore, we cannot draw conclusions about the biomarker's potential in early detection of this condition. It is widely recognized that early detection of pancreatic cancer remains challenging, as only a small percentage (10–20 %) of patients are diagnosed during the initial stages of the disease [5].
Hori et al. demonstrated an association between obesity and increased leptin expression in the pancreatic tissue in a study that used hamsters. They found a two-fold increase in the serum concentration of leptin and an increased risk of pancreatic cancer [17]. White et al. observed, in a study on mice, a direct correlation between leptin levels and the development of pancreatic cancer, suggesting body weight's role in accelerating tumor growth [18]. Our study validates these results in humans by identifying an initial correlation between BMI values, diagnosis of pancreatic cancer and plasma leptin concentration. In multiple linear regression, however, only one correlation remained statistically significant: the one between the diagnosis of pancreatic cancer and the increase in serum leptin levels. In this category of patients, the plasma expression of leptin seems to depend more on the cancer diagnosis than on BMI.
There is growing proof that leptin and other pro-inflammatory molecules released by adipocytes play a role in cancer development [19]. Thus, leptin has been shown to stimulate pancreatic cancer progression through various signaling pathways, while adiponectin could exert a protective effect [19]. Leptin is an adipokine encoded by LEP, synthesized in adipose tissue, with a role in regulating appetite, glucose homeostasis, and body weight [20,21]. Leptin acts through a transmembrane receptor, the obesity receptor (Ob-R) [21]. It is distributed in the hypothalamus, choroid plexuses, cardiovascular system, kidney, liver, pancreas, and other organs [19]. According to the structure, there are six isoforms of Ob-R, which are divided into three classes: long, short, and secretory [22]. A long isoform, active Ob-Rb, is expressed mainly in the hypothalamus and participates in energy homeostasis [21]. Ob-Rb is also present in all innate and adaptive immune system cells [21]. Short isoforms (Ob-Ra, Ob-Rc, Ob-Rd, and Ob-Re) can bind Janus kinases (JAK kinases) as well as activate signal transduction cascades [21]. The effects of the activation of the short isoforms differ from those of the activation of the long isoforms, their main role is related to the internalization and degradation of leptin [23]. Soluble isoforms can bind serum leptin and inhibit signal transduction pathways or serve as transport proteins for leptin to membrane receptors [21]. The Ob-R receptor's expression has also been identified in many gastrointestinal tissues and tumor cell lines [24,25]. Certain malignancies can express both, leptin and the Ob-R receptor, thereby permitting autocrine signaling [26,27]. The involvement of leptin in oncogenesis can be explained by effects such as promoting cell proliferation, migration, and invasion, improving tumor vascularization, or inhibiting tumor cell apoptosis [24]. Moreover, leptin can inhibit regulatory T cell activity, influencing gastrointestinal cancer's immune surveillance [26,27].
The JAK-STAT pathway plays a major role in leptin signal transduction through membrane receptors [28]. Leptin regulates MMP-13 by mediating the JAK2/STAT3 signaling pathway [29]. The MMP family has been shown to be involved in the dysregulation of extracellular matrix composition (e.g., fibronectin and basement membrane), with consequent promotion of tumor metastasis [29]. In addition, the positive relationship between the expression levels of Ob-R and MMP-13 and their correlation with lymphatic metastasis in pancreatic cancer patients has been demonstrated [29]. Mendonsa et al. identified short and long isoforms of Ob-R in pancreatic tumor tissue [30]. These authors demonstrated the hypothesis that leptin activates the PI3K/AKT pathway, promoting the migration of pancreatic tumor cells. In addition, application of the AKT signaling pathway inhibitor, LY294001, to pancreatic tumor cells led to a significant reversal of the effect of leptin on tumor cell proliferation [31]. In contrast, applying the AKT pathway activator, IGF-1, activated leptin [31]. Thus, the role of leptin in promoting the proliferation and migration of pancreatic tumor cells is suggested by activating the PI3K/AKT signaling pathway [20,30,31]. Other studies have suggested the involvement of the leptin-Notch signaling axis in the progression of pancreatic tumor cells. Harbuzariu et al. investigated the hypothesis of the involvement of the leptin-Notch axis in pancreatic oncogenesis by using pancreatic cancer cell lines (BxPC-3, MiaPaCa-2, Panc-1, and AsPC-1) [32]. Tumor cells secreted leptin and expressed the Ob-R receptor, suggesting an autocrine/paracrine signaling loop of leptin that could influence tumor progression [32]. Notch receptors and their ligands were highly expressed in leptin-treated cultures, while Notch expression levels were significantly reduced after administration of Notch inhibitory factor and leptin inhibitory factor, indicating a Notch-leptin interrelationship in pancreatic cell proliferation [32]. In conclusion, the leptin-Notch axis could be a new therapeutic target, especially in obese patients with pancreatic cancer [32].
In the final stage of our investigation, we compared the plasma expression of CEACAM1 in patients with pancreatic cancer and chronic pancreatitis to validate the results on leptin, and also the results reported previously by other groups that suggested its importance as a biomarker foe pancreatic cancer [33]. Our results showed that this biomarker's concentration was dependent on the diagnosis and BMI, gender, and serum triglyceride concentration. This may explain why there is no correlation between leptin expression (which is only dependent on the diagnosis) and CEACAM1 expression.
CEACAMs belong to the glycosylphosphatidylinositol-linked immunoglobulin family and are found on the numerous cell types, including endothelial and epithelial cells of various organs [33]. These molecules are involved in the regulation of the cell cycle and cell adhesion, intercellular and intracellular signaling, inflammation, angiogenesis, the progression of tumor processes, and metastasis [33]. Wei et al. reported the overexpression of decoy receptor-3 (DcR3) not only in the plasma of patients with pancreatic cancer but also in tumor tissue [34]. DcR3 enhanced the phosphorylation of signal transducers and activators of transcription 1 (STAT1), which stimulated the expression and activity of interferon regulatory factor 1 (IRF1) [34]. DcR3/STAT1/IRF1 created a positive feedback loop that increased CEACAM1 transcriptional activity in pancreatic cancer [34]. Pinkert et al. recently demonstrated the role of CEACAM6 in the suppression of antitumor responses such as T-cell-mediated killing or cytokine secretion [35]. The immunosuppressive activity of CEACAM6 is mediated by binding to CEACAM1 by activated tumor-specific T-cells [35].
Simeone et al. identified CEACAM expression in 91 % of patients with pancreatic cancer and in 66 % of patients with chronic pancreatitis [15]. The median value of CEACAM1 concentration was significantly higher in patients with pancreatic cancer versus those with chronic pancreatitis (29 ng/ml vs. 5 ng/ml) [15]. Our study validates these results by identifying higher median values of CEACAM1 in patients with pancreatic cancer compared to those with chronic pancreatitis (11.29 pg/ml vs. 7.8 pg/ml). The difference between the concentrations is smaller in our study but statistically significant (0.015). Another study published more recently reports a difference of 4.8 pg/ml between the median values of CEACAM concentration in patients with pancreatic cancer and chronic pancreatitis, supporting our results [36].
Some data support the idea that leptin and CEACAM1 are involved in the occurrence of obesity and some secondary comorbidities, such as insulin resistance or non-alcoholic steatohepatitis [16]. CEACAM 1 undergoes phosphorylation by the insulin receptor, which is activated in response to rising levels of this hormone [37]. By binding to Shc during phosphorylation, CEACAM 1 sequesters Shc and downregulates the mitogenic action of insulin [37]. Reduced expression or mutation of CEACAM1 in the liver reduces insulin clearance in the portal circulation, resulting in hyperinsulinemia, insulin resistance (via downregulation of the insulin receptor), and increased hepatic lipogenesis [38]. In addition, increased hepatic lipogenesis results in the redistribution of lipids to white adipose tissue, leading to an increase in visceral adiposity [38]. This results in hyperleptinemia, which, combined with hyperinsulinemia, increases food intake and energy imbalance, thereby aggravating obesity [37]. There are also data suggesting that, in the context of CEACAM 1 inhibition, Shc stabilization can promote oncogenesis [37]. In our study, simple linear regression identified correlations between BMI and both biomarkers. However, after conducting multiple linear regression, the correlation between BMI and CEACAM1 remained statistically significant, while the correlation between BMI and leptin did not. Additionally, our study found a correlation between triglyceride levels and the expression of CEACAM1. These findings are supported by data from the literature [38]. CEACAM1 expression in mice was associated with insulin resistance and an increased risk of hepatic steatosis and non-alcoholic steatohepatitis by increasing serum triglyceride and total cholesterol levels [37]. This effect is now thought to be related to increased fatty acid synthase (FAS) activity and leptin resistance [39]. The potential implication of this signaling pathway in malignancies is underscored, given that FAS is a recognized oncogene [37].
The novelty of our study lies in the comparative evaluation of leptin and CEACAM1 plasma concentrations, specifically in patients with pancreatic cancer versus patients with chronic pancreatitis. It appears that in our study, leptin demonstrated a correlation with pancreatic cancer in humans that was less confounded by other factors compared to CEACAM1. While there is data supporting the involvement of both these biomarkers in pancreatic carcinogenesis, their role in distinguishing pancreatic cancer from chronic pancreatitis remains insufficiently unknown. One limitation of our study is that all patients with pancreatic cancer were diagnosed with stage III or IV disease, which limits our ability to evaluate the biomarkers' performance in early-stage pancreatic cancer. Further research is required to evaluate the changes in serum leptin concentrations between chronic pancreatitis and the early stages of pancreatic cancer. Additionally, it is important to acknowledge that our study cohort was relatively small, and larger studies are needed to confirm our findings. A well-known challenge faced by research in this field is the low incidence of pancreatic adenocarcinoma and the need for large national and international collaborations to obtain a sufficient number of biological samples necessary for the identification of new biomarkers [40]. Thus, future collaborative studies on larger cohorts of patients are needed to validate these results.
On the other hand, Pereira et al. emphasize in a meta-analysis published in 2021 the heterogeneity of pancreatic tumors and the need to develop biomarker panels to increase their diagnostic sensitivity, specificity, and accuracy [41]. Therfore, we emphasize the need to study the efficiency of leptin, as a component of a panel of biomarkers, in the early detection of pancreatic cancer among patients with chronic pancreatitis.
5. Conclusions
Due to the low incidence of pancreatic cancer, implementating screening strategies in the general population is not considered cost-effective. Identifying non-invasive, inexpensive biomarkers, such as leptin, that have a potential role in detecting pancreatic cancer could greatly benefit patients with this disease. By utilizing such biomarkers, the early detection and timely intervention in pancreatic cancer cases may lead to improved outcomes for affected individuals.
Starting from an extensive proteomic analysis of 105 chemokines, cytokines, growth factors, angiogenesis markers, and other proteins, we identified leptin as a potential biomarker useful in the differential diagnosis of pancreatic cancer with chronic pancreatitis. We validated these results by using quantitative ELISA technique on individual plasma samples, showing higher plasma concentrations in patients with pancreatic cancer versus those with chronic pancreatitis. Our study also demonstrates the increase in CEACAM1 concentrations in patients with pancreatic cancer compared to chronic pancreatitis. However, the dependence of CEACAM1 expression on multiple parameters, such as diagnosis, gender, BMI, or serum triglyceride levels, and the dependence of leptin concentration solely on diagnosis, strengthen the hypothesis of leptin's utility in the differential diagnosis of the two diseases. Thus, we suggest the effectiveness of monitoring leptin serum levels in patients with chronic pancreatitis in order to identify pancreatic cancer.
Our study advances the hypothesis suggesting that the identification of elevated serum leptin levels in patients with chronic pancreatitis should raise suspicion of malignant transformation and prompt further diagnostic management. Early detection of pancreatic cancer is the final objective, as it holds the potential to facilitate curative surgical resection.
Consent for publication
Not applicable.
Ethics approval
This study was approved by the ethics committee of the Emergency Clinical Hospital of Bucharest (no. 3929/12.04.2021) and conducted according to the principles of the Declaration of Helsinki.
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
CRediT authorship contribution statement
Gina Gheorghe: Writing – original draft, Project administration, Methodology, Investigation, Data curation, Conceptualization. Carmen Cristina Diaconu: Writing – review & editing, Visualization, Validation, Supervision, Project administration, Funding acquisition, Conceptualization. Cristina Mambet: Validation, Methodology, Investigation, Conceptualization. Coralia Bleotu: Writing – review & editing, Supervision, Methodology, Investigation. Vlad Alexandru Ionescu: Writing – original draft, Software, Resources, Methodology, Formal analysis, Data curation, Conceptualization. Camelia Cristina Diaconu: Writing – review & editing, Visualization, Validation, Supervision, Project administration, Funding acquisition, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors would like to express their gratitude to Department of Cellular and Mollecular Pathology, Stefan S. Nicolau Institute of Virology, Romanian Academy, whose support made it possible to conduct the experiments described in this study.
References
- 1.Klein A.P. Pancreatic cancer epidemiology: understanding the role of lifestyle and inherited risk factors. Nat. Rev. Gastroenterol. Hepatol. 2021;18:493–502. doi: 10.1038/s41575-021-00457-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Globocan 2020. https://gco.iarc.fr/today/data/factsheets/cancers/13-Pancreas-fact-sheet.pdf
- 3.Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70:7–30. doi: 10.3322/caac.21590. [DOI] [PubMed] [Google Scholar]
- 4.Rawla P., Sunkara T., Gaduputi V. Epidemiology of pancreatic cancer: global trends, etiology and risk factors. World J. Oncol. 2019;10:10–27. doi: 10.14740/wjon1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gheorghe G., Ionescu V.A., Moldovan H., Diaconu C.C. Clinical and biological data in patients with pancreatic cancer vs. Chronic pancreatitis—a single center comparative analysis. Diagnostics. 2023;13:369. doi: 10.3390/diagnostics13030369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kirkegard J., Mortensen F.V., Cronin-Fenton D. Chronic pancreatitis and pancreatic cancer risk: a systematic review and meta-analysis. Am. J. Gastroenterol. 2017;112:1366–1372. doi: 10.1038/ajg.2017.218. [DOI] [PubMed] [Google Scholar]
- 7.Munigala S., Kanwal F., Xian H., Agarwal B. New Diagnosis of chronic Pancreatitis: risk of missing an underlying pancreatic cancer. Am. J. Gastroenterol. 2014;109:1824–1830. doi: 10.1038/ajg.2014.318. [DOI] [PubMed] [Google Scholar]
- 8.Muller N., Sarantitis I., Rouanet M., de Mestier L., Halloran C., Greenhalf W., Férec C., Masson E., Ruszniewski P., Lévy P., et al. Natural history of SPINK1 germline mutation related-pancreatitis. EBioMedicine. 2019;48:581–591. doi: 10.1016/j.ebiom.2019.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Le Cosquer G., Maulat C., Bournet B., Cordelier P., Buscail E., Buscail L. Pancreatic cancer in chronic pancreatitis: pathogenesis and diagnostic approach. Cancers. 2023;15:761. doi: 10.3390/cancers15030761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gheorghe G., Bungau S., Ilie M., Behl T., Vesa C.M., Brisc C., Bacalbasa N., Turi V., Costache R.S., Diaconu C.C. Early diagnosis of pancreatic cancer: the key for survival. Diagnostics. 2020;10:869. doi: 10.3390/diagnostics10110869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Principe D.R., Underwood P.W., Korc M., Trevino J.G., Munshi H.G., Rana A. The current treatment paradigm for pancreatic ductal adenocarcinoma and barriers to therapeutic efficacy. Front. Oncol. 2012;11 doi: 10.3389/fonc.2021.688377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jiang H., Hegde S., Knolhoff B.L., Zhu Y., Herndon J.M., Meyer M.A., Nywening T., Hawkins W.G., Shapiro I.M., Weaver D.T., Pachter J.A., Wang-Gillam A., DeNardo D.G. Targeting focal adhesion kinase renders pancreatic cancers responsive to checkpoint immunotherapy. Nat. Med. 2016;22(2016):851–860. doi: 10.1038/nm.4123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sun J., Russell C.C., Scarlett C.J., McCluskey A. Small molecule inhibitors in pancreatic cancer. RSC Med. Chem. 2020;11:164–183. doi: 10.1039/c9md00447eI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pecoraro C., De Franco M., Carbone D., Bassani D., Pavan M., Cascioferro S., Parrino B., Cirrincione G., Dall'Acqua S., Moro S., Gandin V., Diana P. 1,2,4-Amino-triazine derivatives as pyruvate dehydrogenase kinase inhibitors: synthesis and pharmacological evaluation. Eur. J. Med. Chem. 2023;249 doi: 10.1016/j.ejmech.2023.115134. [DOI] [PubMed] [Google Scholar]
- 15.Simeone S.M., Ji B., Banerjee M., Arumugam T., Li D., Anderson M., Bamberger S.M., Greenson J., Brand R., Ramachandran V., et al. CEACAM1, a novel serum biomarker for pancreatic cancer. Pancreas. 2007;34:436–443. doi: 10.1097/MPA.0b013e3180333ae3. [DOI] [PubMed] [Google Scholar]
- 16.Najjar S., Russo L. CEACAM1 loss links inflammation to Insulin Resistance in obesity and Non-alcoholic Steatohepatitis (NASH) Semin. Immunopathol. 2013;36:55–71. doi: 10.1007/s00281-013-0407-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hori M., Kitahashi T., Imai T., Ishigamori R., Takasu S., Mutoh M., Sugimura T., Wakabayashi K., Takahashi M. Enhancement of carcinogenesis and fatty infiltration in the pancreas in N-nitrosobis(2-oxopropyl)amine-treated hamsters by high-fat diet. Pancreas. 2011;408:1234–1240. doi: 10.1097/MPA.0b013e318220e742. [DOI] [PubMed] [Google Scholar]
- 18.White P.B., True E.M., Ziegler K.M., Wang S.S., Swartz-Basile D.A., Pitt H.A., Zyromski Insulin N.J. Leptin, and tumoral adipocytes promote murine pancreatic cancer growth. J. Gastrointest. Surg. 2010;14:1888–1893. doi: 10.1007/s11605-010-1349-x. [DOI] [PubMed] [Google Scholar]
- 19.Stolzenberg-Solomon R.Z., Newton C.C., Silverman D.T., Pollak M., Nogueira L.M., Weinstein S.J., Albanes D., Mannisto S., Jacobs E.J. Circulating leptin and risk of pancreatic cancer: a pooled analysis from 3 cohorts. Am. J. Epidemiol. 2015;182:187–197. doi: 10.1093/aje/kwv041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marroquí L., Gonzalez A., Ñeco P., Caballero-Garrido E., Vieira E., Ripoll C., Nadal A., Quesada I. Role of leptin in the pancreatic β-cell: effects and signaling pathways. J. Mol. Endocrinol. 2012;491:R9–R17. doi: 10.1530/JME-12-0025. [DOI] [PubMed] [Google Scholar]
- 21.Wang Q., Wang H., Ding Y., Wan M., Xu M. The role of adipokines in pancreatic cancer. Front. Oncol. 2022;12 doi: 10.3389/fonc.2022.926230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goggins M. Molecular markers of early pancreatic cancer. J. Clin. Oncol. 2005;23:4524–4531. doi: 10.1200/JCO.2005.19.711. [DOI] [PubMed] [Google Scholar]
- 23.Chan J.L., Bluher S., Yannakouris N., Suchard M.A., Kratzsch J., Mantzoros C.S. Regulation of circulating soluble leptin receptor levels by gender, adiposity, sex steroids, and leptin: observational and interventional studies in humans. Diabetes. 2002;51:2105–2112. doi: 10.2337/diabetes.51.7.2105. [DOI] [PubMed] [Google Scholar]
- 24.Fruhbeck G. Intracellular signaling pathways activated by leptin. Biochem. J. 2006;393:7–20. doi: 10.1042/BJ20051578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alemán J.O., Eusebi L.H., Ricciardiello L., Patidar K., Sanyal A.J., Holt P.R. Mechanisms of obesity-induced gastrointestinal neoplasia. Gastroenterology. 2014;1462:357–373. doi: 10.1053/j.gastro.2013.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hoda M.R., Keely S.J., Bertelsen L.S., Junger W.G., Dharmasena D., Barrett K.E. Leptin acts as a mitogenic and antiapoptotic factor for colonic cancer cells. Br. J. Surg. 2007;94:346–354. doi: 10.1002/bjs.5530. [DOI] [PubMed] [Google Scholar]
- 27.Sennello J.A., Fayad R., Morris A.M., Eckel R.H., Asilmaz E., Montez J., Friedman J.M., Dinarello C.A., Fantuzzi G. Regulation of T cell-mediated hepatic inflammation by adiponectin and leptin. Endocrinology. 2005;146:2157–2164. doi: 10.1210/en.2004-1572. [DOI] [PubMed] [Google Scholar]
- 28.Deng T., Lyon C.J., Bergin S., Caligiuri M.A., Hsueh W.A. Obesity, inflammation, and cancer. Annu. Rev. Pathol. 2016;11:421–449. doi: 10.1146/annurev-pathol-012615-044359. [DOI] [PubMed] [Google Scholar]
- 29.Fan Y., Gan Y., Shen Y., Cai X., Song Y., Zhao F., Yao M., Gu T., Tu H. Leptin signaling enhances cell invasion and promotes the metastasis of human pancreatic cancer via increasing MMP-13 production. Oncotarget. 2015;6:16120–16134. doi: 10.18632/oncotarget.3878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mendonsa A.M., Chalfant M.C., Gorden L.D., VanSaun M.N. Modulation of the leptin receptor mediates tumor growth and migration of pancreatic cancer cells. PLoS One. 2015;10 doi: 10.1371/journal.pone.0126686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xu Y., Tan M., Tian X., Zhang J., Zhang J., Chen J., Xu W., Sheng H. Leptin receptor mediates the proliferation and glucose metabolism of pancreatic cancer cells via AKT pathway activation. Mol. Med. Rep. 2022;21:945–952. doi: 10.3892/mmr.2019.10855. [DOI] [PubMed] [Google Scholar]
- 32.Harbuzariu A., Rampoldi A., Daley-Brown D.S., Candelaria P., Harmon T.L., Lipsey C.C., Beech D.J., Quarshie A., Ilies G.O., Gonzalez-Perez R.R. Leptin-notch signaling Axis is involved in pancreatic cancer progression. Oncotarget. 2017;8:7740–7752. doi: 10.18632/oncotarget.13946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zinczuk J., Zareba K., Romaniuk W., Kaminska D., Niziol M., Baszun M., Kedra B., Guzinska-Ustymowicz K., Pryczynicz A. Expression of chosen carcinoembryonic-related cell adhesion molecules in pancreatic intraepithelial neoplasia (PanIN) associated with chronic pancreatitis and pancreatic ductal adenocarcinoma (PDAC) Int. J. Med. Sci. 2019;16:583–592. doi: 10.7150/ijms.32751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wei Y., Chen X., Yang J., Yao J., Yin N., Zhang Z., Li D., Zhu D., Zhou J. DcR3 promotes proliferation and invasion of pancreatic cancer via a DcR3/STAT1/IRF1 feedback loop. Am. J. Cancer Res. 2019;9:2618–2633. [PMC free article] [PubMed] [Google Scholar]
- 35.Pinkert J., Boehm H.H., Trautwein M., Doecke W.D., Wessel F., Ge Y., Gutierrez E.M., Carretero Freiberg R., Gritzan U., et al. T cell-mediated elimination of cancer cells by blocking CEACAM6–CEACAM1 interaction. OncoImmunology. 2022;11 doi: 10.1080/2162402X.2021.2008110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Song J., Sokoll L.J., Pasay J.J., Rubin A.L., Li H., Bach D.M., Chan D.W., Zhang Z. Identification of serum biomarker panels for the early detection of pancreatic cancer. Cancer Epidemiol. Biomarkers Prev. 2018;28:174–182. doi: 10.1158/1055-9965.EPI-18-0483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dankner M., Gray-Owen S.D., Huang Y.H., Blumberg R.S., Beauchemin N. CEACAM1 as a multi-porpose target for cancer immunotherapy. OncoImmunology. 2017;6 doi: 10.1080/2162402X.2017.1328336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Heinrich G., Muturi H.T., Rezaei K., Al-Share Q.Y., DeAngelis A.M., Bowman T.A., Ghadieh H.E., Ghanem S.S., Zhang D., Garofalo R.S., et al. Reduced hepatic Carcinoembryonic antigen-related cell adhesion molecule 1 level in obesity. Front. Endocrinol. 2017;8:54. doi: 10.3389/fendo.2017.00054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Heinrich G., Russo L., Castaneda T.R., Pfeiffer V., Ghadieh H.E., Ghanem S.S., Wu J., Faulkner L.D., Ergun S., McInerney M.F., et al. Leptin resistance contributes to obesity in mice with null mutation of carcinoembryonic antigen cell adhesion molecule 1. J. Biol. Chem. 2016;291:11124–11132. doi: 10.1074/jbc.M116.716431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sogawa K., Takano S., Iida F., Satoh M., Tsuchida S., Kawashima Y., Yoshitomi H., Sanda A A., Kodera Y., Takizawa H., et al. Identification of a novel serum biomarker for pancreatic cancer, C4b-binding protein α-chain (C4BPA) by quantitative proteomic analysis using tandem mass tags. Br. J. Cancer. 2016;115:949–956. doi: 10.1038/bjc.2016.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pereira S.P., Oldfield L., Ney A., Hart P.A., Keane M.G., Pandol S.J., Li D., Greenhalf W., Jeon C.Y., Koay E.J., et al. Early detection of pancreatic cancer. Lancet Gastroenterol Hepatol. 2021;5:698–710. doi: 10.1016/S2468-1253(19)30416-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.