Abstract
Fluorescent brightening agent OB-1 (OB-1) is often used in plastic goods because of its non-toxic nature, chemical stability, remarkable heat resistance, and light stability. Raw OB-1 is challenging to use in textiles using the exhaustion method. This study used a novel method using raw OB-1 powder to whiten polyester fabric in water and decamethylcyclopentasiloxane (D5). The Taguchi approach investigated the interaction between whitening process parameters such as temperature, OB-1 mass, water: D5 ratio, and treatment time with four levels. The study shows that the temperature and water: D5 ratio during the whitening process significantly affect the whiteness of polyester fabric (P < 0.05), with contribution percentages of 74.2 % and 25.2 %. Subsequently, various analytical techniques were employed, including FTIR, SEM, TGA, and XRD, to characterise the whitened fabric. The findings imply that using water: D5 medium was effective in whitening polyester fabric without causing major alterations to the structure of the PET fabric. The study also examined the fastness of washing and crocking to determine their whitening stability. Overall, polyester fabric whitened with water and D5 medium exhibited satisfactory whitening performance and might be a potential scope for use on a larger scale in developing the sustainable textile industry.
Keywords: Whiteness, Fluorescent whitening agent, Polyester, D5, Taguchi method
Graphical abstract
Highlights
-
•
Raw fluorescent brighter agent OB-1 successfully whitens PET fibre by wet process.
-
•
Whitening PET fibre in a mixture of water and D5 media.
-
•
Cleaner whitening process without using massive amount of surfactant.
-
•
Whitening performance of the PET fibre with high fastness to washing and rubbing.
1. Introduction
Textiles made from polyester (PET) fibre are widely used in various applications due to their versatility, durability, and ease of use [1]. However, the conventional whitening process for PET fibre involves the use of chemicals that can have harmful effects on the environment. Chemical bleaching [2], which aims to decolourise the colourants in fibres by oxidising them to smaller chemical groups or reducing them to saturated factors to progress whiteness, often leads to the degradation of fibres and a decrease in strength. To address this environmental issue, researchers have explored alternative methods for whitening textiles that avoid the limitations of chemical bleaching agents. One such alternative is fluorescent whitening treatment, which involves using compounds that absorb ultraviolet light (330–380 nm) and re-emit it as visible light (400–450 nm) [3,4]. This process effectively increases the whiteness and brightness of the fabric, providing an eco-friendly and sustainable approach to PET fabric whitening.
Fluorescent brightener whitening agent OB-1 (OB-1, Fig. 1a) is a highly effective oxazole whitening agent. It is non-toxic, chemically stable, heat-resistant, and light-stable, making it a popular choice for use in plastic products [5,6]. In textiles, OB-1 is particularly suitable for the dope whitening of PET fibre due to its high washing fastness and whitening effect. However, wet processing can be challenging for PET fibre due to its low solubility and poor dispersion in water. OB-1 molecules are highly planar, which makes them prone to aggregation through van der Waals interaction forces [7,8]. To apply OB-1 in PET fabric using an exhaust method, the raw powder is milled with surfactants to reduce the particle size of OB-1, and the treated particles of OB-1 successfully whiten PET fabric [9]. However, after whitening treatment, preparing the milled particles with many surfactants hints at potential water pollution issues. Therefore, finding an alternative, sustainable wet process for OB-1 whitening PET fabric is highly desirable.
Fig. 1.
Chemical structures of (a) OB-1 and (b) D5.
Textile experts constantly seek innovative methods to minimise the environmental impact of their industry. Decamethylcyclopentasiloxane (D5, Fig. 1b) has emerged as a new treatment medium with easy recycling [10], finding acceptance in the textile industry, such as dyeing of PET [[11], [12], [13], [14]], nylon [[15], [16], [17]], cotton [[18], [19], [20]], wool [[20], [21], [22]], and so on. The development of this non-aqueous medium represents a significant advancement within the textile industry. In a non-aqueous medium using D5, the whitening of PET fabric with raw fluorescent brightener OB-1, i.e., without milling with massive surfactants, shows great potential for significantly enhancing exhaustion and fixation rates. Incorporating water alongside D5 is noteworthy as it aligns with sustainability principles by reducing reliance on traditional solvents with negative environmental impacts [23]. This innovative approach aims to achieve a cleaner whitening process for PET fabrics, effectively addressing both the performance requirements of the textile industry and the ever-growing demand for environmentally conscious practices.
The conventional view holds that optimisation methods that make a single adjustment while holding all parameters constant are inefficient and wasteful. Design of experiments (DOE) is a method that takes a methodical approach to finding the connection between process inputs and outputs in this context [24]. One may classify DOE methods as either full factorial or Taguchi experimental designs. All conceivable permutations of the parameters are considered and studied in complete factorial design. In contrast, Taguchi's experimental design research considers the levels explicitly selected for assessment. The use of an orthogonal array (OA) architecture gives the Taguchi method its reputation as a durable technology [25]. With the help of the OA, we can quantitatively determine the optimal parameters and levels while cutting down on the time, money, and effort needed for experiments. The Taguchi method finds the best circumstances with the most important elements by analysing the signal-to-noise (S/N) ratio and using analysis of variance (ANOVA) tables to find the statistical significance. Following this, confirmation tests were used to ensure that experimental designs were feasible [26]. Although the whitening process has not been investigated, some researchers have used the Taguchi experimental design technique to enhance the quality of textile fibre dyeing [[27], [28], [29]].
This study delves into the green whitening of PET fabric, exploring the interactions between fluorescent brightener OB-1 and the mixture of D5 and water media. The present study reports for the first time that OB-1 can be used to whiten PET fabrics in a mixture medium consisting of D5 and water. The current study used an exhaust method to whiten PET fabrics. An optimisation of the parameters of the whitening process, such as the duration of the process (0–70 min), the temperature (90–130 °C), the weight of the OB-1 (0.0025–0.0375 % o.w.f (on weight of fabric)), as well as the water to D5 ratio (1:99 to 10:90), was conducted. The replicability of this work was ensured by validating the sixteen Taguchi orthogonal arrays (L16). A variety of analytical techniques, such as FTIR, SEM, TGA, XRD, and fastness to washing and crocking, were applied to determine the characterisation of the whitened fabrics.
2. Experimental
2.1. Materials
Raw fluorescent whitening agent OB-1 powders were provided by Hubei Hongxin Chemical Co., Ltd, China. A scoured polyester knitted fabric (bilateral loop pile structure, with a horizontal density of 65 courses/5 cm, a vertical density of 110 wales/5 cm, and a surface density of 130 g/m2) was supplied by TST Group Holding Limited, China. A non-ionic detergent (Luton 500) was purchased from Dalton UK Company, China. Decamethylcyclopentasiloxane (D5) was obtained from Jiangxi Bluestar Xinghuo Silicones Corporation, China. An ethanol solvent (CH3CH2OH, ≥99.7 %) was purchased from China National Pharmaceutical Group Corporation, China.
2.2. Whitening process
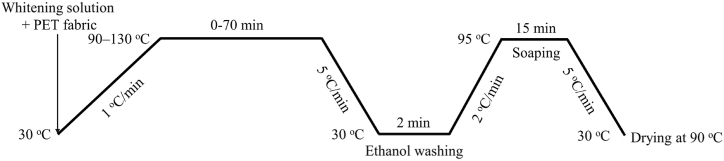
The weight percentage of OB-1, ranging from 0.0025 to 0.0375 % based on the fabric weight (o.w.f), was incorporated into a blended medium consisting of water and D5. The ratio of water to D5 in this mixture was varied from 0:100 to 10:90 (by volume) to formulate the whitening solution. Subsequently, the PET knitted fabric was immersed in the whitening solution with a liquor-to-mass ratio (LR) of 25:1. The whitening treatment was carried out in an Infrared-heating dyeing machine (HB-HW × 24, Ronggui Huibao dyeing and finishing machinery factory, Foshan, China), according to the whitening process shown in Fig. 2. The temperature of the whitening treatment was increased to the target temperature (90–130 °C) from 30 °C with a 1 °C/min heating rate and maintained at the target temperature for a required period. Next, the whitening temperature was dramatically decreased to 30 °C with a 5 °C/min rate. The whitened PET fabric was squeezed and washed in a pure ethanol solvent at 30 °C for 2 min in the dyeing machine to remove the adsorption of D5 medium, followed by a soaping process in a solution containing 2 g/L of non-ionic detergent with an LR of 50:1 at 95 °C for 15 min in the dyeing machine as well. Finally, the soaped fabric was dried at 90 °C in an oven.
Fig. 2.
Whitening process of PET fabric with OB-1.
2.3. Characterisation
The original PET and the whitened PET were chosen for characterisation. The whitened PET was processed in a medium composed of a water-D5 mixture (at a ratio of 1:99) with an OB-1 weight concentration of 0.0125 % o.w.f and whitened at 130 °C for 60 min, after which the fabric was thoroughly washed with ethanol and soaped to complete the treatment protocol. The whiteness of PET fabric was detected at random 10 positions using a spectrophotometer (Hangzhou Chnspec Technology Co., Ltd.) with the built-in software of WI (CIE) method at 10° field of view and a D65 light source. A mean of 10 records was used to characterise the performance of whiteness. In transmission mode, the PET fabric cut into powder form with scissors was prepared with KBr for FTIR analysis with an FT-IR spectrometer (FTIR, Nicolet iS5, Thermo Fisher Scientific, USA). Also, the powder form of PET fibre was applied to thermogravimetric analysis (TGA) using TGA/DSC1 thermogravimetric analyser (Mettler-Toledo, LLC, Shanghai, China) in a temperature range of 50–800 °C at a heating rate of 10 °C/min under nitrogen atmosphere. The powder form of PET fibre was analysed by a Rigaku Ultima III X-ray diffractometer with CuKα radiation (λ = 1.54056 Å) (Tokyo, Japan). The XRD data was collected in a range of 5–80° of 2θ with 0.02° step size. The morphology of PET fabric coated with gold was observed at 10 kV accelerated voltage with a Philips SEM 515 field-emission scanning electron microscope (Germany). The fastness to washing and crocking (dry and wet) of whitened PET fabric were treated as per the ISO 105-C06: 2010 (Test number: C2S) and ISO 105-X12:2001 standard methods, respectively. The fastness to crocking was observed in a Colour Assessment Cabinet (CAC-5000, Foshan Huibao Dyeing and Finishing Machinery Factory, China).
3. Results and discussion
3.1. Influence of water: D5 ratio on the whiteness of PET fabric
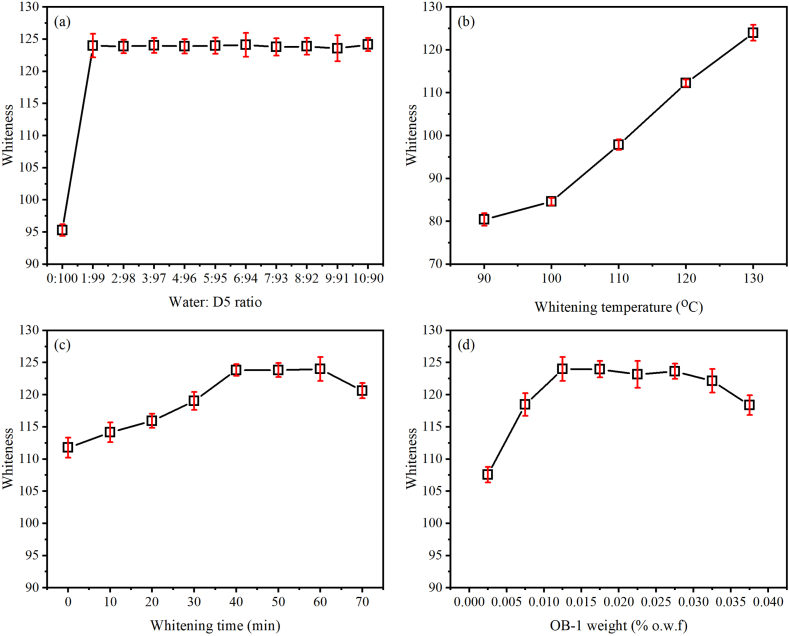
The PET fabric was whitened in a medium composed of a water-D5 mixture (at varied ratios from 0:100 to 10:90) with 0.0125 % o.w.f of OB-1 weight at 130 °C for 60 min of the whitening period, followed by the ethanol washing and soaping, and the whiteness values of whitened PET fabrics are shown in Fig. 3a. Interestingly, the whiteness jumped to 123.99 from 95.30 (pure D5 medium) when 1 % of water was added to the D5 medium (1:99). Subsequently, increasing water content in the D5 medium from the ratio of 2:98 to 10:90, the whiteness levelly maintained. This indicates that the small addition of water has a significant role in whitening the PET fabric using OB-1. Generally, OB-1 can be treated as a disperse dye due to its insolubility in water [30], but it has a better solubility in D5. Thus, the whitening performance of PET fabric was achieved in pure D5 (0:100) with 95.30 of whiteness because of the solubility of OB-1 in D5 and the accessibility of D5 into the interior of PET fibre [11]. In contrast, it is impossible to whiten PET fibre with raw OB-1 powder in water [9]. Besides, the addition of water into D5 possibly decreased the solubility of OB-1, resulting in an increase of OB-1 molecules diffusion into the PET fibre [13]. Meanwhile, the addition of water contributed to the swelling of PET fibre [13], and it was accompanied by the generation of larger free volumes in the PET fibre, which was beneficial for the OB-1 uptake [31].
Fig. 3.
Influence of (a) water:D5 ratio, (b) whitening temperature, (c) whitening time, and (d) OB-1 weight on whiteness of PET fabric whitened by OB-1.
3.2. Influence of whitening temperature on the whiteness of PET fabric
The PET fabric was whitened in a mixture of water and D5 media (1:99) with 0.0125 % o.w.f of OB-1 weight at various whitening temperatures from 90 to 130 °C for 60 min of whitening period, followed by the ethanol washing and soaping, and the whiteness values of whitened PET fabrics are shown in Fig. 3b. The whiteness of the control PET fabric is 78.1, and it increased after the whitening treatment, showing an increased tendency with the promotion of whitening temperature. Especially, when whitening at 100 °C or higher, the whiteness linearly increased, and achieved 123.99 whiteness at 130 °C.
The PET fibre was swelled and softened well at high temperatures [32] in a D5 medium, which produced more accessibility of the free volume in the noncrystalline regions [33]. Meanwhile, a small amount of raw OB-1 agent was dissolved in the D5 medium with a single particle form, which was adsorbed on the surface of PET fibre, migrated in the interior of PET fibre, and absorbed in the fibre. Whitening at 90 °C, the PET fibre did not swell well, causing a low whiteness of 80.44 because of a slight difficulty on diffusion of OB-1 into the interior of fibre. Thus, the whitening temperature was sensitive on the whitening performance.
It should be pointed out that the whiteness of whitened PET fabric at 140 °C or higher could be promoted according to the increased tendency. However, on a laboratory and industrial scale, the dyeing machine was designed for 130 °C as the maximum application temperature. Thus, a whitening temperature higher than 130 °C was not considered in the present work, and the whitening temperature at 130 °C was fixed for other factors’ investigations.
3.3. Influence of whitening time on the whiteness of PET fabric
The PET fabric was whitened in a mixture of water and D5 media (1:99) with 0.0125 % o.w.f of OB-1 weight at 130 °C for 0–70 min of whitening period, followed by the ethanol washing and soaping, and the whiteness values of whitened PET fabrics are shown in Fig. 3c. The whiteness of PET fabric gradually increased with the extension of whitening time and arrived at equilibrium after 40 min whitening, with whiteness of 111.77 increased to 123.82 for the whitening time from 0 min to 40 min. Next, the whiteness was stabilised in the whitening period from 40 to 60 min but slightly decreased to 120.63 for 70 min of the whitening period. This indicates that the adsorption equilibrium arrived at 40 min in the whitening period. Compared with the disperse dyeing of PET fabric in a D5 medium, this adsorption process is faster to be at equilibrium due to its low weight of OB-1 usage [34]. The decrease of whiteness for 70 min of the whitening period was possibly caused by the aggregation of OB-1 molecules on the surface of PET fibre because of excessive adsorption, which probably resulted in a quenching of fluorescence [35].
3.4. Influence of OB-1 weight on the whiteness of PET fabric
The PET fabric was whitened in a mixture of water and D5 media (1:99) with OB-1 weight varied from 0.0025 to 0.0375 % o.w.f at 130 °C for 60 min of the whitening period, followed by the ethanol washing and soaping, and the whiteness values of whitened PET fabrics are shown in Fig. 3d. The whiteness of PET increased from 107.58 with 0.0025 % o.w.f to a maximum value of 123.99 with 0.0125 % o.w.f and gradually decreased to 118.37 with 0.0375 % o.w.f. The excessive OB-1 molecules in the substance easily generated a high interaction between the molecules and formed a multilayer, causing a fluorescent extinction or self-quenching [36], i.e., reduction of whitening performance. Therefore, it concludes that the OB-1 weight usage should be no more than 0.0325 % o.w.f for whitening the PET.
3.5. Taguchi analysis
An orthogonal experimental scheme using an L16 (4^4) array was carried out to obtain the experimental data for Taguchi analysis to elucidate the influence order of the factors on the whitening performance and their interactions. The L16 (4^4) array of the orthogonal experimental scheme and their whiteness and S/N ratio values are presented in Table 1, and the signals of A, B, C, and D stand for whitening temperature (oC), OB-1 weight (%, o.w.f), water:D5 ratio, and whitening time (min), respectively. The S/N was evaluated employing the "larger is better" criterion [37], yielding an optimal whiteness level of 123.7. In comparison, a prior study [9] examined the use of OB-1 nanoparticles for whitening PET fabric, achieving a maximum whiteness of only 94.1. This previous work involved a preparation process for OB-1 nanoparticles that incorporated a significant amount of dispersant through a milling process. Consequently, the current whitening technology demonstrates enhanced cleaner and a marked improvement in whitening efficacy.
Table 1.
L16 (4^4) orthogonal array-based experimental data with their S/N ratios.
| Experiment | A | B | C | D | Whiteness | S/N ratio (dB) |
|---|---|---|---|---|---|---|
| 1 | 100 | 0.0125 | 0:100 | 40 | 78.94 | 37.8663 |
| 2 | 100 | 0.0175 | 1:99 | 50 | 89.32 | 39.0110 |
| 3 | 100 | 0.0225 | 2:98 | 60 | 89.84 | 39.0883 |
| 4 | 100 | 0.0275 | 3:97 | 70 | 89.38 | 39.0935 |
| 5 | 110 | 0.0125 | 1:99 | 60 | 103.07 | 40.3313 |
| 6 | 110 | 0.0175 | 0:100 | 70 | 84.73 | 38.5797 |
| 7 | 110 | 0.0225 | 3:97 | 40 | 103.37 | 40.2800 |
| 8 | 110 | 0.0275 | 2:98 | 50 | 102.36 | 40.1229 |
| 9 | 120 | 0.0125 | 2:98 | 70 | 114.10 | 41.1370 |
| 10 | 120 | 0.0175 | 3:97 | 60 | 118.41 | 41.3881 |
| 11 | 120 | 0.0225 | 0:100 | 50 | 97.01 | 39.8050 |
| 12 | 120 | 0.0275 | 1:99 | 40 | 119.28 | 41.5503 |
| 13 | 130 | 0.0125 | 3:97 | 50 | 123.72 | 41.8888 |
| 14 | 130 | 0.0175 | 2:98 | 40 | 123.15 | 41.8773 |
| 15 | 130 | 0.0225 | 1:99 | 70 | 123.16 | 41.7297 |
| 16 | 130 | 0.0275 | 0:100 | 60 | 108.23 | 40.6790 |
The ANOVA (analysis of variance) of the orthogonal array experimental data is listed in Table 2. The p-values of whitening temperature (A) and water: D5 ratio (C) are lower than 0.05, hinting that both factors in the experimental conditions are significant [33]. i.e., both factors substantially influenced the whitening performance. Furthermore, the P values (contribution percentage) claim that the whitening temperature factor (74.2 %) primarily influenced the whitening performance and the water: D5 factor (24.5 %) was the second one. Both factors of whitening time (D) and OB-1 weight (B) had an ignorable influence on the whiteness change of the PET fabric during whitening treatment.
Table 2.
ANOVA for S/N ratios of whitenessa.
| Source | DF | SS | MS | F | p-value | Remarks | P (%) |
|---|---|---|---|---|---|---|---|
| A | 3 | 2428.48 | 809.494 | 641.06 | 0.000 | Significant | 74.2 |
| B | 3 | 11.13 | 3.71 | 2.94 | 0.200 | Insignificant | 0.3 |
| C | 3 | 803.5 | 267.834 | 212.11 | 0.001 | Significant | 24.5 |
| D | 3 | 27.79 | 9.264 | 7.34 | 0.068 | Insignificant | 0.8 |
| Residual Error | 3 | 3.79 | 1.263 | ||||
| Total | 15 | 3274.69 |
DF: degree of freedom, SS: sum of square, MS: Mean square, F: Fisher's test, p-value: statistical significance; P (%): percentage of contribution.
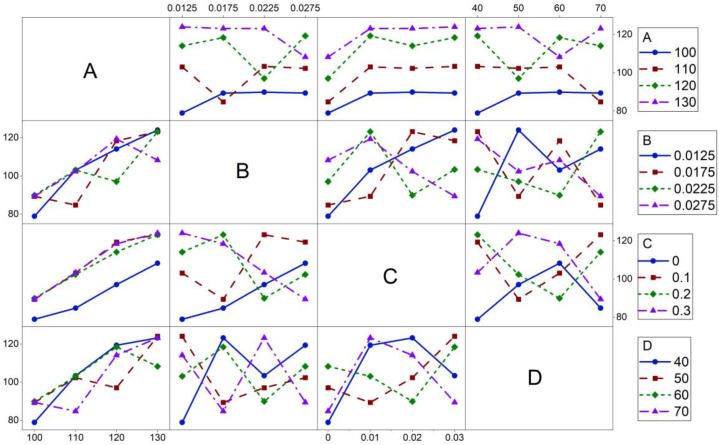
The interactions of these investigated factors (in the whitening conditions) during the whitening treatment of PET fabric are shown in Fig. 4. The interaction between the A and the C was not obvious, based on the evaluation of parallel curves [38] in the related figures. Their substantial contributions to whitening performance may cause this. In other words, a small change in each factor distinguished the whiteness, which distinction was extensive and resulted in an unobservable interaction. The interactions between B and C, B and D, and C and D were intense, based on observing disordered cross curves. Although some interactions occurred in relation to A, the cross curves are not tangled. Thus, it concludes that the factor of whitening temperature (A) had a substantial contribution (74.2 %) for the whitening performance tends to have low interaction with the other factors.
Fig. 4.
Full interaction plot for S/N ratios in whiteness.
3.6. FT-IR
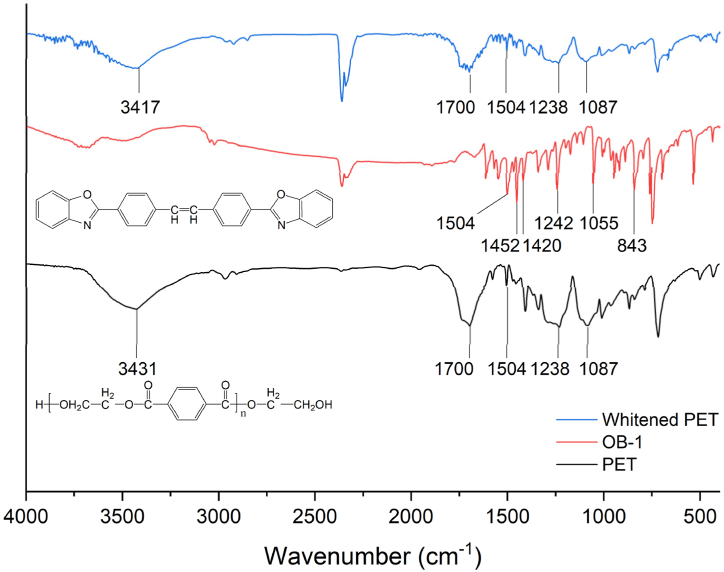
In the FT-IR spectrum of PET in Fig. 5, a distinct absorption band associated with the ester linkage was observed at 1700 cm−1 (stretching vibration of C=O), while absorption bands at 1087 cm−1 and 1238 cm−1 corresponded to the stretching vibration of the C-O-C bond. The presence of free –OH groups in polymer chains was indicated by a weak broad absorption band at 3437 cm−1 [39]. The absorption band observed at 1504 cm−1 was attributed to the symmetric structure of the benzene ring [40]. The FT-IR spectrum of OB-1 exhibited prominent absorption bands at 1504 cm−1 and 1452 cm−1, originating from the bending vibrations of the C=C bonds within the phenyl ring. Furthermore, an absorption band at 843 cm−1 was assigned to the vibration of C-H bonds in the phenyl ring. A previous report [41] mentioned a bending vibration bond of -C=N at the 1420 cm−1 absorption band and stretching vibration bonds of -C-O at the 1242 cm−1 and 1055 cm−1 absorption bands. The FT-IR spectrum of whitened PET exhibited similar characteristics to that of PET, indicating the inability to distinguish the low concentration of OB-1 within the PET matrix.
Fig. 5.
FT-IR spectra of PET, OB-1, and whitened PET.
3.7. SEM
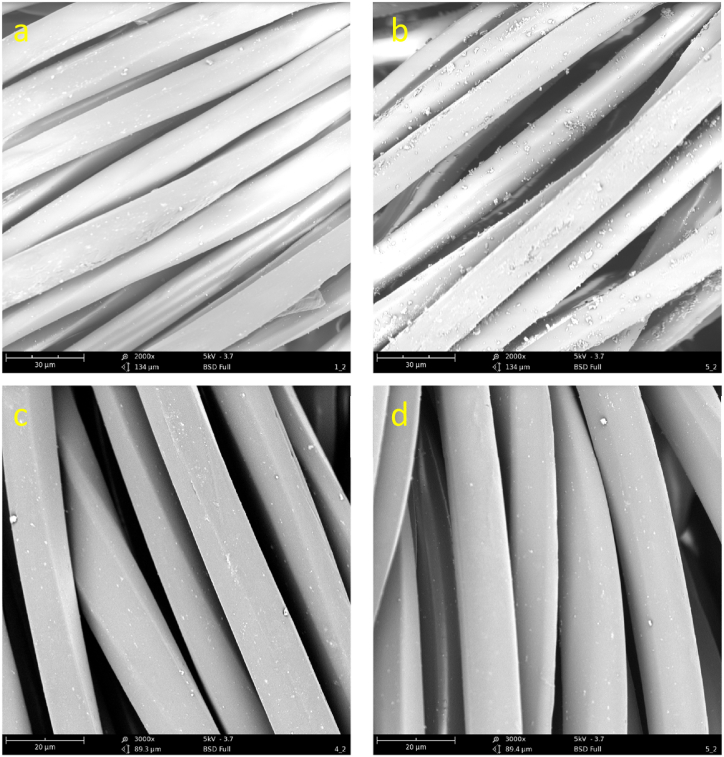
The longitudinal surface topographies of PET and whitened PET with 2000 magnification times are shown in Fig. 6. The round and smooth PET fibre with few particles stained on the surface was observed in Fig. 6a. However, after whitening treatment, more particles were stained on the surface, which was possibly caused by the aggregation of OB-1 molecules. Meanwhile, the surface of the whitened PET fibre did not exhibit any erosion, which means that the mixture medium of water and D5 did not damage the PET fibre [42].
Fig. 6.
Longitudinal topography images (2000 × ) of (a) PET and (b) whitened PET, and (3000 × ) of (c) PET and (d) whitened PET.
3.8. XRD
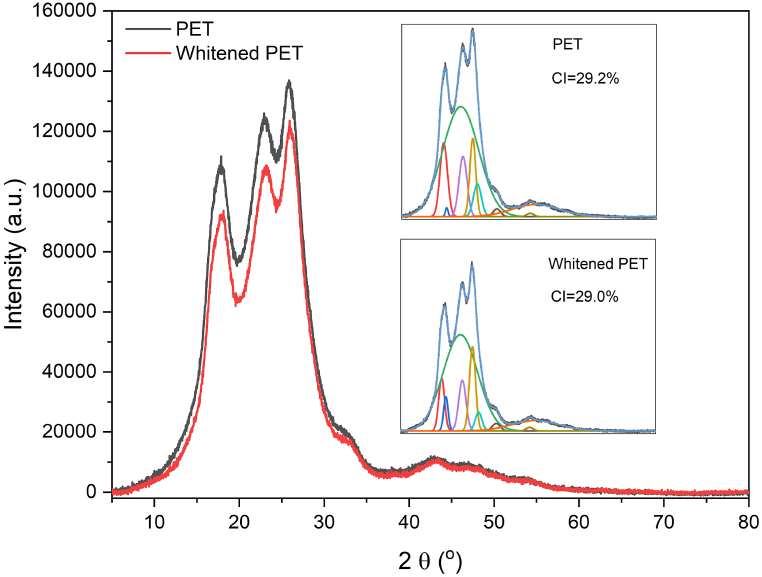
The XRD curves and deconvolution peaks of PET and whitened PET fibres were analysed using Fityk software (version 1.3.1), as shown in Fig. 7. The diffraction peaks and shapes of both fibres were found to be similar. The main crystallinity peaks of both samples were around 17°, 23°, and 26° of 2θ values. The crystallinity indices (CIs) of PET and whitened PET, as identified by the deconvolution peaks in their separated curves, were 29.2 % and 29.0 %, respectively, which CIs are consistent with previous studies [43]. Therefore, it can be concluded that the differences in the CIs between the two fibres are negligible, suggesting that the whitening process did not significantly affect the structure of the PET fibres, which is consistent with the report in the literature [42].
Fig. 7.
XRD curves of the PET and whitened PET.
3.9. TGA
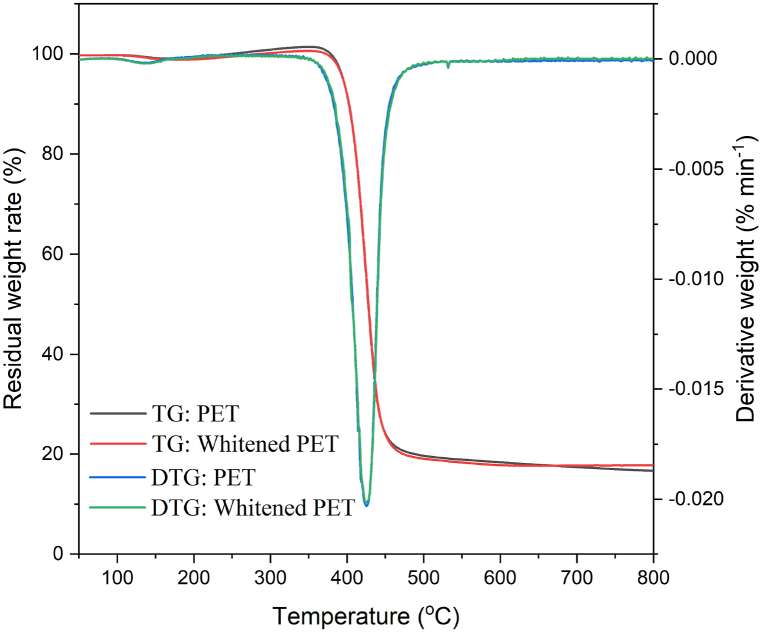
The thermal stability of both PET and whitened PET fibres is depicted in Fig. 8. The SEM and XRD analyses indicated that the whitening process did not cause any damage to the PET fibre, and the FT-IR analysis showed no generation of distinct chemical groups. This suggests that the primary matrix of PET remained unchanged, resulting in similar thermal behaviour before and after treatment. Consequently, the TGA curves of both fibres exhibit significant overlap. The initial peaks in the DTG curves can be attributed to the evaporation of negligible moisture in both fibres. Subsequently, a major decomposition process occurred between 355 and 484 °C with a maximum decomposition temperature of 426 °C, leading to a weight loss of approximately 80 % of the sample weight for both fibres. At 800 °C, the residual chars decreased gradually to 16.6 % and 17.7 % for PET and whitened PET, respectively. The analysis revealed a significant proportion of volatile matter, approximately 83 %, present in both PET fibres, which can be attributed to their high organic content [44].
Fig. 8.
TGA and DTG analyses of the PET and whitened PET.
3.10. Fastnesses to washing and crocking
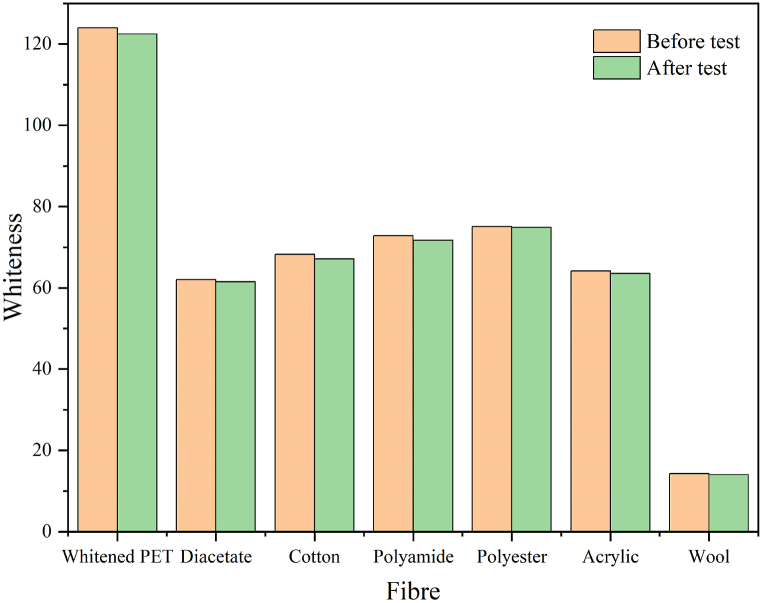
The whitening performance fastnesses of whitened PET to washing and crocking were shown in Fig. 9, Fig. 10, respectively. It was hard to evaluate the fading scale of whitened PET and staining scales on the multi-fibres’ fabric (cotton, polyamide, polyester, acrylic, and wool fibres) using the normal observation method in D65 light. Thus, the whiteness values of the whitened PET and the multi-fibres fabric were measured to express the fastness to washing. Obviously, the fastness to washing was strong due to the whiteness values before and after the test was closed, i.e., the whiteness changes of fading and staining rates were ignorable.
Fig. 9.
Whiteness of whitened PET and the multi-fibres’ fabric before and after the test of fastness to washing.
Fig. 10.
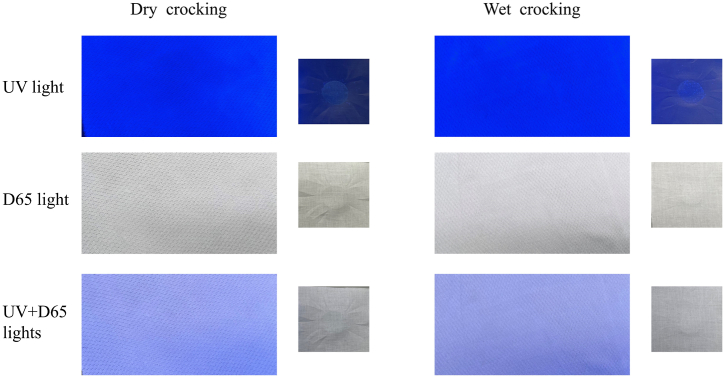
Images of whitened PET and the tested cotton fabric after the test of fastness to crocking (dry and wet).
In the aspect of fastness to crocking, the whitened PET and the tested cotton fabric (dry and wet crockings) after the test were observed in UV light, D65 light, and a combination of UV and D65 lights. The staining of the fluorescent phenomenon on the tested cotton fabrics with dry and wet conditions was unobservable in D65 light. While somewhat fluorescent staining was observed on the tested cotton fabrics in UV light, it changed to pale in combination with UV and D65 light. These observations suggested that the whitened PET has a high fastness for crocking.
3.11. Tensile strength
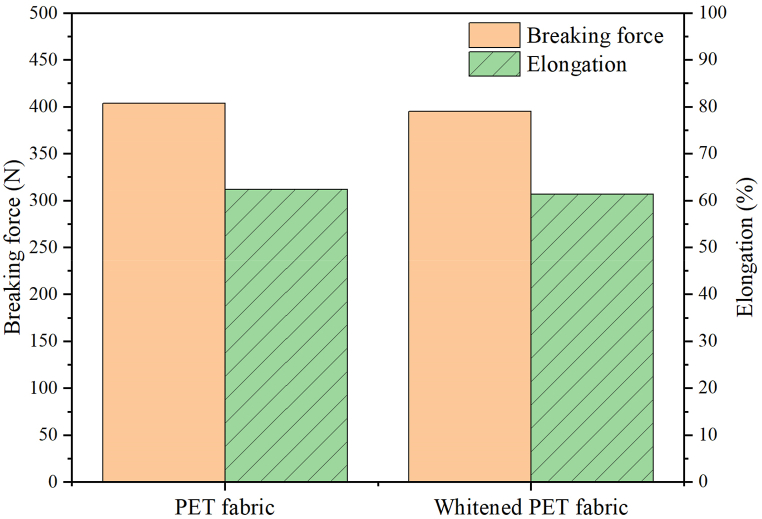
The tensile strength of PET fabric and whitened PET fabric is shown in Fig. 11. Obviously, after the whitening treatment, the breaking forces of both fabrics are close, around 400 N. Meanwhile, the elongation distinction of both fabrics is negligible. The results state that the tensile strength of the PET fabric was not damaged during the whitening treatment, which supports the results of the XRD analysis of both fabrics.
Fig. 11.
Tensile strength of PET fabric and whitened PET fabric.
4. Conclusions
The research introduced a novel technique for eco-friendly whitening of polyester fabric using OB-1 in a mixture of water and D5 medium, focusing on addressing environmental concerns in the textile sector. The PET fabric was exposed to a solution of water and D5 media (1:99) with an OB-1 weight concentration of 0.0125 % o.w.f at 130 °C for 60 min, resulting in the greatest whiteness level of 123.99. The application of the Taguchi methodology subsequently enabled the significant influence of temperature and water: D5 ratio on the whitening process parameters on the whiteness of polyester fabric (P < 0.05), with contribution percentages of 74.2 % and 25.2 %. Moreover, adding water to D5 may have reduced the solubility of OB-1, causing more OB-1 molecules to diffuse into the PET fibre. This process also led to the creation of bigger free spaces inside the PET fibre, which facilitated the absorption of OB-1. The analytical analysis shows PET's morphological structure was unchanged after using OB-1 whitening in water and D5 medium. The color fastness to washing and crocking qualities showed promising results, indicating a potential route for developing a sustainable textile whitening process using a combination of water and D5.
Ethics declarations
The ethics are not applicable.
Data availability statement
The data underlying this article will be shared upon reasonable request to the corresponding author.
CRediT authorship contribution statement
Yingjie Cai: Writing – original draft, Methodology, Investigation, Conceptualization. Jianhua Xiong: Methodology, Data curation. Le Li: Methodology, Data curation. Md Reazuddin Repon: Writing – original draft, Formal analysis. Md Nahid Pervez: Writing – review & editing, Writing – original draft, Investigation, Formal analysis. Ai Chen: Writing – review & editing, Funding acquisition. Xiaohua Zhao: Writing – review & editing, Funding acquisition. Shuang Han: Writing – review & editing, Supervision, Funding acquisition. Xiaorong Xiong: Writing – review & editing, Supervision, Funding acquisition, Conceptualization. Vincenzo Naddeo: Writing – review & editing, Supervision. Lina Lin: Writing – review & editing, Formal analysis, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was financially supported by industry funding from Hubei Hongxin Chemical Co., Ltd.
Contributor Information
Shuang Han, Email: S_Han_WTU@163.com.
Vincenzo Naddeo, Email: vnaddeo@unisa.it.
Lina Lin, Email: linalin@wtu.edu.cn.
References
- 1.Chen S., et al. Insight into multifunctional polyester fabrics finished by one-step eco-friendly strategy. Chem. Eng. J. 2019;358:634–642. [Google Scholar]
- 2.Karmakar S.R., editor. Textile Science and Technology. Elsevier; 1999. Bleaching of textiles; pp. 160–216. [Google Scholar]
- 3.Um S.-I. The synthesis and properties of benzoxazole fluorescent brighteners for application to polyester fibers. Dyes Pigments. 2007;75(1):185–188. [Google Scholar]
- 4.Al-Sayed W., Abdelrahman S.H. In: Green Chemistry for Sustainable Textiles. Ibrahim N., Hussain C.M., editors. Woodhead Publishing; 2021. Sustainable chemistry in textile processes (pretreatment, coloration and chemical finishing) pp. 93–111. [Google Scholar]
- 5.Kabir S.F., et al. Optimization of parameters of cotton fabric whiteness. Eur. Sci. J. 2014;10(36) [Google Scholar]
- 6.Salas H., et al. Respirometric study of optical brighteners in textile wastewater. Materials. 2019;12(5):785. doi: 10.3390/ma12050785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saad F., et al. Polyester fabric with fluorescent properties using microwave technology for anti-counterfeiting applications. J. Fluoresc. 2022;32(1):327–345. doi: 10.1007/s10895-021-02845-7. [DOI] [PubMed] [Google Scholar]
- 8.Shariful Islam S.M., Alam M., Akter S. Identifying the values of whiteness index, strength and weight of cotton spandex woven fabric in peroxide bleaching of different concentration. Fibers and Textiles. 2019;26(4):96–109. [Google Scholar]
- 9.Cai Y., et al. The optimization of whiteness of polyester fabric treated with nanoparticles of 2,2′-(vinylenedi-p-phenylene)bis-benzoxazole (OB-1) by the Taguchi method. Colloids Surf. A Physicochem. Eng. Asp. 2023;676 [Google Scholar]
- 10.Cai Y., et al. Decamethylcyclopentasiloxane-based sustainable and recyclable polyester fabric whitening using OB-1 fluorescent brightener. Arab. J. Chem. 2024;17(5) [Google Scholar]
- 11.An Y., et al. High-efficiency dispersant-free polyester dyeing using D5 non-aqueous medium. Dyes Pigments. 2021;190 [Google Scholar]
- 12.Zhang H., et al. Distribution mechanism of disperse dyes with similar substituents in a waterless dyeing system based on molecular dynamic simulation. Dyes Pigments. 2022;202 [Google Scholar]
- 13.Wang J., et al. Mechanism of accelerant on disperse dyeing for PET fiber in the silicone solvent dyeing system. Polymers. 2019;11 doi: 10.3390/polym11030520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ding L., et al. Correlation between dye structure with dyeing properties in anhydrous dyeing systems: insights from Crystallographic, DFT, kinetic, and thermodynamic analyses. Dyes Pigments. 2024;223 [Google Scholar]
- 15.Saleem M.A., et al. Sustainable dyeing of nylon fabric with acid dyes in decamethylcyclopentasiloxane (D5) solvent for improving dye uptake and reducing raw material consumption. J. Clean. Prod. 2021;279 [Google Scholar]
- 16.Saleem M.A., et al. Sustainable dyeing of nylon with disperse dyes in Decamethylcyclopentasiloxane waterless dyeing system. J. Clean. Prod. 2020;276 [Google Scholar]
- 17.Saleem M.A., Pei L., Wang J. Single-step single bath dyeing and finishing of nylon with disperse dye and chitosan by using decamethylcyclopentasiloxane solvent as dyeing media. Fibers Polym. 2022;23(6):1631–1640. [Google Scholar]
- 18.Hossain M.Y., et al. Effluent-free deep dyeing of cotton fabric with cacao husk extracts using the Taguchi optimization method. Cellulose. 2021;28(1):517–532. [Google Scholar]
- 19.Hossain M.Y., et al. Adsorption, kinetics, and thermodynamic studies of cacao husk extracts in waterless sustainable dyeing of cotton fabric. Cellulose. 2021;28(4):2521–2536. [Google Scholar]
- 20.Hossain M.Y., et al. Green and sustainable method to improve fixation of a natural functional dye onto cotton fabric using cationic dye-fixing agent/D5 microemulsion. J. Nat. Fibers. 2022;19(15):11283–11298. [Google Scholar]
- 21.Cai Y., et al. Green penetration dyeing of wool yarn with natural dye mixtures in D5 medium. J. Mater. Res. Technol. 2023;25:6524–6541. [Google Scholar]
- 22.Luo Y., et al. Environment-friendly high-efficiency continuous pad dyeing of non-shrinkable wool fabric by the silicon fixation method without auxiliary chemicals. ACS Sustain. Chem. Eng. 2022;10(11):3557–3566. [Google Scholar]
- 23.Feng L., et al. Sustainable dyeing of wool yarn with Monascus pigments in non-aqueous medium. J. Text. Inst. 2023;114(10):1550–1556. [Google Scholar]
- 24.Lin L., et al. Sustainable traditional grass cloth fiber dyeing using the Taguchi L16 (4^4) orthogonal design. Sci. Rep. 2022;12(1) doi: 10.1038/s41598-022-18213-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pervez M.N., et al. Multi-response optimization of resin finishing by using a taguchi-based grey relational analysis. Materials. 2018;11(3):426. doi: 10.3390/ma11030426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morshed M.N., et al. Statistical modeling and optimization of heterogeneous Fenton-like removal of organic pollutant using fibrous catalysts: a full factorial design. Sci. Rep. 2020;10(1) doi: 10.1038/s41598-020-72401-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cai Y., et al. Improved reactive dye fixation on ramie fiber in liquid ammonia and optimization of fixation parameters using the Taguchi approach. Dyes Pigments. 2020;183 [Google Scholar]
- 28.Cai Y., et al. Improving the dyeability of ramie fibre by sequential alkaline and alcohol pretreatments. Ind. Crop. Prod. 2024;212 [Google Scholar]
- 29.Pervez M.N., et al. Optimization and prediction of the cotton fabric dyeing process using Taguchi design-integrated machine learning approach. Sci. Rep. 2023;13(1) doi: 10.1038/s41598-023-39528-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ketema A., Worku A. Review on intermolecular forces between dyes used for polyester dyeing and polyester fiber. J. Chem. 2020;2020 [Google Scholar]
- 31.Lee K.W., Chung Y.S., Kim J.P. Characteristics of ultrasonic dyeing on poly(ethylene terephthalate) Textil. Res. J. 2003;73(9):751–755. [Google Scholar]
- 32.Zheng W., et al. Molecular-level swelling behaviors of poly (ethylene terephthalate) glycolysis using ionic liquids as catalyst. Chem. Eng. Sci. 2023;267 [Google Scholar]
- 33.Burkinshaw S.M., The roles of elevated temperature and carriers in the dyeing of polyester fibres using disperse dyes: Part 3 model of dye adsorption based on dye solubility, Color. Technol. 140 (4) 513–555.
- 34.Li S.Z., et al. Study on the dyeing of PET fiber with disperse dyes in D5 media. Adv. Mater. Res. 2011;331:334–337. [Google Scholar]
- 35.Liu H., et al. Influence of fluorescent dyes for dyeing of regenerated cellulose fabric. Textil. Res. J. 2020;90(11–12):1385–1395. [Google Scholar]
- 36.Zhao Q., et al. Coloring properties of novel 1,4-distyrylbenzene and 4,4′-distyrylbiphenyl fluorescent brighteners and their arrangement in cotton and polyester fiber. Cellulose. 2014;21(4):2937–2950. [Google Scholar]
- 37.Behmanesh E., Pannek J. Taguchi analysis for improving optimization of integrated forward/reverse logistics. Journal of the Operations Research Society of China. 2023;11(3):529–552. [Google Scholar]
- 38.Shafiq F., et al. Structural relationships and optimization of resin-finishing parameters using the Taguchi approach. Cellulose. 2018;25(10):6175–6190. [Google Scholar]
- 39.Natarajan S., Moses J.J. Surface modification of polyester fabric using polyvinyl alcohol in alkaline medium. Indian J. Fibre Text. Res. 2012;37(3):287–291. [Google Scholar]
- 40.Xiao X., et al. Surface modification of polyester nonwoven fabrics by Al2O3 sol–gel coating. J. Coating Technol. Res. 2009;6(4):537–541. [Google Scholar]
- 41.Pan Q., et al. Direct identification and quantitation of fluorescent whitening agent in wheat flour based on multi-molecular infrared (MM-IR) spectroscopy and stereomicroscopy. Spectrochim. Acta Mol. Biomol. Spectrosc. 2021;250 doi: 10.1016/j.saa.2020.119353. [DOI] [PubMed] [Google Scholar]
- 42.Li H., et al. Extraction of cyclic oligomer and their influence on polyester dyeing in a silicone waterless dyeing system. Polymers. 2021;13(21):3687. doi: 10.3390/polym13213687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Murthy N.S., Zero K., Minor H. Resolution enhancement of polymer X-ray diffraction scans using maximum entropy methods: poly(ethylene terephthalate) Macromolecules. 1994;27(6):1484–1488. [Google Scholar]
- 44.Das P., Tiwari P. Thermal degradation study of waste polyethylene terephthalate (PET) under inert and oxidative environments. Thermochim. Acta. 2019;679 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data underlying this article will be shared upon reasonable request to the corresponding author.