Abstract
Background and aims
The single nucleotide polymorphisms (SNPs) in SLC30A8 gene have been recognized as contributing to type 2 diabetes (T2D) susceptibility and colorectal cancer. This study aims to predict the structural stability, and functional impacts on variations in non-synonymous SNPs (nsSNPs) in the human SLC30A8 gene using various computational techniques.
Materials and methods
Several in silico tools, including SIFT, Predict-SNP, SNPs&GO, MAPP, SNAP2, PhD-SNP, PANTHER, PolyPhen-1,PolyPhen-2, I-Mutant 2.0, and MUpro, have been used in our study.
Results
After data analysis, out of 336 missenses, the eight nsSNPs, namely R138Q, I141N, W136G, I349N, L303R, E140A, W306C, and L308Q, were discovered by ConSurf to be in highly conserved regions, which could affect the stability of their proteins. Project HOPE determines any significant molecular effects on the structure and function of eight mutated proteins and the three-dimensional (3D) structures of these proteins. The two pharmacologically significant compounds, Luzonoid B and Roseoside demonstrate strong binding affinity to the mutant proteins, and they are more efficient in inhibiting them than the typical SLC30A8 protein using Autodock Vina and Chimera. Increased binding affinity to mutant SLC30A8 proteins has been determined not to influence drug resistance. Ultimately, the Kaplan-Meier plotter study revealed that alterations in SLC30A8 gene expression notably affect the survival rates of patients with various cancer types.
Conclusion
Finally, the study found eight highly deleterious missense nsSNPs in the SLC30A8 gene that can be helpful for further proteomic and genomic studies for T2D and colorectal cancer diagnosis. These findings also pave the way for personalized treatments using biomarkers and more effective healthcare strategies.
Keywords: SLC30A8, T2D, Missense, Non-synonymous, Polymorphism, And colorectal cancer
1. Introduction
Diabetes is a metabolic disease affecting populations globally, arising from insulin production or utilization disruptions. Notably, type 2 diabetes (T2D) serves as a well-established risk factor for colorectal cancer, a highly prevalent form of malignancy worldwide. Based on the most recent cancer data, colorectal cancer is a significant contributor to cancer-related deaths, posing a substantial threat to global health, with 1.9 million new cases recorded in 2020 [1]. A gene associated with insulin signaling, specifically SLC30A8, could potentially influence the connection between diabetes and the risk of colorectal cancer. This connection may offer new perspectives on the biological mechanisms that link diabetes and colorectal cancer [2]. Several cancers closely relate to the solute carrier 30 (SLC30) family of genes during their development and progression [3]. The presence of a specific variant in the SLC30A8 gene was linked substantially to an increased risk of breast cancer in women [4]. The combined effects of uncontrolled blood sugar, hormone imbalances, and ongoing inflammation associated with T2D may linked to an increased risk of head and neck cancer development, metastasis, and severity [[5], [6], [7]]. Additionally, T2D is more prone to infection with Kaposi's sarcoma-associated herpesvirus (KSHV), the pathogen accountable for Kaposi's sarcoma [8].
SLC30A8, a gene responsible for encoding the zinc transporter 8 (ZnT8), is an influential variable in T2D risk [9]. ZnT8, which belongs to the ZnT family, is mostly found in pancreatic beta cells, and it is essential for transporting zinc from the cytoplasm to intracellular vesicles [10,11]. Residing inside insulin-storing granules, ZnT8 is essential for the synthesis, encapsulation, and discharge of insulin. Antibodies against ZnT8 are a potential breakthrough in diagnosing autoimmune diabetes [9].
Single nucleotide polymorphisms (SNPs) represent the predominant genetic variation within the DNA sequence. Their density varies across regions, yet they occur in approximately 100–300 bases of the human genome, constituting over 90 % of all genetic variation in humans [12]. SNPs are present in the human genome's coding and non-coding areas, potentially influencing transcription-factor binding, splicing, or gene expression. Some SNPs do not impact cell function, while others can increase susceptibility to illness or alter medication response [13,14]. Approximately 500,000 SNPs have been identified within the coding region of the human genome [15]. Among these, non-synonymous SNPs (nsSNPs), hold significance compared to other proteins as they can modify amino acid residues [16]. Although not all structural and functional modifications induced by nsSNPs have the potential to be harmful. While some nsSNPs have functional effects, others impact structural features [17]. Modifications in function can have a favorable or detrimental impact on the structure or functionality of proteins. Potentially harmful effects include changes in gene regulation [18,19], instability of protein structures, effects on protein charge, geometry, and hydrophobicity [20,21] and changes in protein structure [22]. Therefore, it can be assumed that nsSNPs are associated with several human illnesses. The significant impact of nsSNPs on human health and disease has made this research more effective in recent years.
Researchers have found hundreds of SNPs in the SLC30A8 gene's coding region because of advancements in genotyping methods. Nevertheless, more work needs to be done to determine which SNPs of the SLC30A8 gene are the most harmful and disease-prone. Several studies conducted according to these techniques, using NCBI dbSNP, UniProtKB, SIFT, Predict-SNP, SNAP2, PANTHER, SNPs&GO, PolyPhen, PhD-SNP, I-Mutant, ConSurf, GPS-MSP, NetPhos, Mutpred2, MUpro, Mutation 3D, Project Hope, EnrichR, STRING, Chimera, AutoDock Vina, SwissADME, pkCSM, Kaplan-Meier Plotter, etc., have been employed to forecast the functional consequences of the most harmful nsSNPs in SLC30A8 gene (Fig. 1(A-D)) [23,24] Additionally, we suggested novel treatment approaches that provide insightful information regarding their potential impact on the protein's functionality, stability, and structure. Our study also identified potentials biomarkers as drug targets for mutations linked to T2D and its progression to CRC. Therefore, it can be predicted how patients will respond to treatments, enabling more effective and developing personalized therapeutic strategies.
Fig. 1.
Flow diagram of overall in silico SNPs analysis on the SLC30A8 gene. A, B, C, and D periodically indicate the steps and their subsequent results.
2. Materials and Methods
2.1. Retrieval of SLC30A8 nsSNPs dataset
The dataset of the human SLC30A8 gene polymorphisms and protein sequence (Uniprot ID: Q8IWU4) was collected from NCBI dbSNP (https://www.ncbi.nlm.nih.gov/snp/) and UniProtKB (https://www.uniprot.org/) databases, respectively. The dataset comprised 82339 SNPs, including 336 nonsynonymous SNPs (nsSNPs) in the coding area and 945 in non-coding regions. Non-synonymous mutations are those that result in a change in the amino acid sequence of a protein and can have a significant impact on the structure and function of the protein. In our dataset preparation, we excluded only missense variant of nsSNPs because, its impact can vary from benign to highly detrimental, depending on the role of the substituted amino acid in the protein's function or structure compared to nonsense variant [25]. As a result, coding region of 336 nsSNPs were prioritized for further investigation due to their recognized ability to significantly influence protein structure and function, potentially responding to the progression of several disease states. Subsequently, all the 336 nsSNPs were isolated and retrieved for further investigation.
2.2. Evaluation of the deleterious nsSNPs
In our study, we excluded deleterious nsSNPs that increase the stability of the protein rather than over-stabilization effects. Because the role of SLC30A8 gene in insulin regulation and pancreatic beta-cell function, under-stabilization may be more immediately harmful. Over-stabilization, while potentially harmful in the long term, may not have as immediate an impact. Under-stabilization of the protein can quickly disrupt zinc homeostasis and insulin secretion, leading to metabolic disturbances and contributing to diabetes risk [26].
Besides, we utilized various bioinformatics tools, such as SIFT, Predict-SNP, MAPP, PANTHER, SNAP2, SNPs&GO, PhD-SNP, PolyPhen-1, and PolyPhen-2, to assess the functional consequences of the nsSNPs. The use of multiple methods ensured a rigorous and accurate screening process. Only modifications anticipated to exert harmful effects were chosen for additional scrutiny. SIFT (Sorting Intolerant From Tolerant) (https://sift.bii.a-star.edu.sg/) can forecast the functional outcomes of amino acid replacements by examining sequence homology and the physicochemical attributes of amino acids [27]. The PhD-SNP (https://snps.biofold.org/phd-snp/phd-snp.html) is the Predictor of Human Negative Single Nucleotide Polymorphisms. PhD-SNP uses support vector machines (SVMs) to identify a point mutation in a human that is associated with a genetic disorder or polymorphism [28]. MAPP, SNAP 2, PANTHER, and PolyPhen-1 tools were combined into a consensus classifier Predict-SNP (https://loschmidt.chemi.muni.cz/predictsnp1/), offering much better prediction performance while also providing data for each mutation, showing that consensus prediction is a reliable and accurate substitute for the predictions made by different tools [29]. SNPs&GO (https://snps-and-go.biocomp.unibo.it/snps-and-go/) approach predicts harmful single amino acid polymorphisms (SAPs). For each protein variant, the server's output gives the likelihood that it will be linked to a disease in humans. When the likelihood score is > 0.5, it suggests that the variations are disease-associated mutations, and when it is 0.5, it shows that the variations have no effect [30]. The software PolyPhen-2 (Polymorphism Phenotyping v2) (http://genetics.bwh.harvard.edu/pph2/) predicts the impact of amino acid alterations on the stability and functionality of human proteins, considering structural and evolutionary comparisons. Coding SNPs are mapped to gene transcripts, structural and functional information on proteins is collected, functional annotation of SNPs is completed, and conservation profiles are established. Subsequently, it computes the possibility of the missense mutation being harmful by integrating all these characteristics [31].
2.3. Analyzing protein stability
The I-Mutant 2.0 tool is built on a support vector machine (SVM) (https://folding.biofold.org/cgi-bin/i-mutant2.0.cgi). I-Mutant 2.0 is reasonably accurate, it is not infallible and can sometimes give erroneous predictions, especially for less common mutations or proteins not well represented in the training data. We employed the sequence-based edition of I-Mutant 2.0, which categorizes predictions into three distinct groups: substantial decrease (0.5 kcal/mol), notable increase (>0.5 kcal/mol), and neutral mutation (between −0.5 and 0.5 kcal/mol). The basis for the predicted free energy change (ΔΔG) by I-Mutant 2.0 is the variance between the unfolding Gibbs free energy change of the mutant and the native protein (in kcal/mol) [32,33].
Additionally, we utilized MUpro (http://mupro.proteomics.ics.uci.edu/), which has two machine-learning applications: neural networks and support vector machines. We used the program's sequence-based version and the default settings to run the SVM algorithm. The program's output indicates the energy change's sign (+ or -) [34]. With these methods, we could anticipate how nsSNPs could affect the stability of proteins and guide our subsequent investigation.
2.4. Evolutionary conservation analysis of the most damaging nsSNPs
A trade-off between the need to preserve the structural stability and functionalities of the macromolecule and the natural tendency of the molecular building blocks to mutate is indicated by the level of evolutionary conservation of nucleic acid in DNA/RNA or amino acid in proteins [35]. Compared to other popular approaches based on relative entropy and consensus techniques, ConSurf (https://consurf.tau.ac.il/) has a clear advantage. To estimate evolutionary rates, it considers the distinct dynamics of the sequences under study and the phylogenetic connections among the homologs using complex probabilistic evolutionary models [36]. The ConSurf server was utilized to evaluate the level of evolutionary conservation at each amino acid position in the SLC30A8 protein and identify the high-risk nsSNPs [23].
2.5. Prediction of post-translational modification (PTM) sites
Proteins are fine-tuned by over 400 complex chemical modifications. These act as molecular control panels, meticulously regulating diverse cellular functions by altering protein structure and behaviour [37]. We relied on NetPhos 3.1 (https://services.healthtech.dtu.dk/services/NetPhos-3.1/) [38] to pinpoint potential areas where phosphate molecules could latch onto our protein. This software uses several neural networks to anticipate possible phosphorylation sites on tyrosine, threonine, and serine residues. A score exceeding 0.5 on the software's scale indicated a high phosphorylation probability at that location. Additionally, we employed GPS-MSP 1.0 (http://msp.biocuckoo.org/) [39] to predict possible methylation sites on the protein chain.
2.6. Identification of structural and functional alternations
We used Mutpred2 (http://mutpred.mutdb.org/), which integrates genetic and molecular data using machine learning methods and employs the amino acid sequence to predict structural and functional alteration caused by amino acid changes. Additionally, the instrument provides information on the precise biological mechanisms behind the onset of diseases [23]. We provided amino acid substitution and protein FASTA sequences as input for the Mutpred2 study. The g-score, derived from the collective scores of all neural networks within MutPred2, indicates pathogenicity. A g-score surpassing 0.50 (g > 0.50) is considered indicative of pathogenicity [40].
2.7. Evaluation of cancer-associated nsSNPs
Mutation 3D (http://www.mutation3d.org/) is employed to detect a group of amino acid substitutions arising from somatic cancer mutations. It proves invaluable in investigating the spatial arrangement of amino acid alterations within protein models and structures. This software utilizes a 3D clustering method to detect possible cancer-causing changes in amino acids inside a protein. It requires a target protein and its mutations as input [41].
2.8. Estimating the effects of high-risk nsSNPs on the structure of protein
An automated approach for analyzing mutants called Project HOPE (https://www3.cmbi.umcn.nl/hope/input/) helps examine the structural, functional and metabolic consequences of point mutations within protein sequences. It investigates how changes in amino acid composition affect native structures and the variations in hydrophobicity, charge, and size between residues of the wild-type and mutant-types. Project HOPE combines structural data with knowledge from literature and databases to predict the functional impact of mutations. It often compares the mutation in question to similar known mutations. The accuracy can vary depending on the extent and relevance of available comparative data [42]. Several computational studies utilizing the Project HOPE server to identify significance mutations in specific proteins including human LYZ C gene [43], CDK4 gene [44], and human RASSF5 gene [24]. Protein Data Bank (https://www.rcsb.org/) provides the SLC30A8 protein sequence to Project HOPE for predicting the 3D structure of the mutated protein. It also provides a plethora of information on changing amino acids and estimates the composition and function of proteins after mutations [23,44].
2.9. Gene Ontology (GO) enrichment analysis
Genome-wide experiments generate massive datasets of genes, and enrichment analysis offers a powerful tool for extracting meaningful insights from these sets. Gene ontology analysis was performed using Enrichr (https://maayanlab.cloud/enrichr-kg) [45] to detect statistically noteworthy (P < 0.05) associations between the input gene set and curated databases of cellular components, molecular activities, and biological processes [46]. Employing SRplot (https://www.bioinformatics.com.cn/en), we constructed graphical summaries of the enriched analysis results [47].
2.10. Prediction of protein-protein interaction (PPI)
The online database STRING (Search Tool for Recurring Instances of Neighboring Genes) predicts the PPI network of the SLC30A8 protein (https://string-db.org/). It retrieves the genes that are tangentially (via other genes) linked to the query gene using an evolutionary method [48]. The STRING output furnishes details regarding the protein's expression, localization, transcription, experimental evidence, and graphical depictions of the interactions in which the queried protein participates [49].
2.11. Superimposition and molecular docking of raw and mutated proteins
Protein structure determination and modeling involve determining and positioning protein structures in 3D space, known as protein structure superimposition. The best superimposition reflects dynamic changes over time and aligning structures to reveal common structural or functional patterns [50]. We used Chimera 1.16 [51] for the superimpositioning of native SLC30A8 protein and its mutant variants.
Further, to assess the impact of deleterious point mutations on the binding affinity of SLC30A8 (UniProt accession no. Q8IWU4.2), we employed the Autodock Vina (version 1.2.1) [52] to perform molecular docking analysis. We generated a mutated form of the target protein using the crystal structure complex of SLC30A8 obtained from RCSB (https://www.rcsb.org/) [53] using phyr2 (http://www.sbg.bio.ic.ac.uk/phyre2/html/page.cgi?id=index) [54], and energy minimization employed by Swiss-PdbViewer (https://spdbv.unil.ch/) [55].
The mutated SLC30A8 protein containing eight nsSNPs is the docking procedure's target protein along with normal protein. Ligands like Luzonoid B and Roseoside [56] obtain from pubchem (https://pubchem.ncbi.nlm.nih.gov/) [57]. The PDB format of these input ligands was transformed into pdbqt format using Autodock Vina. We tailored the grid box size for maximum efficiency. The docking outcome and the binding interactions between the ligand and receptor proteins were observed through BIOVIA Discovery Studio (version 21.1.0) [58].
2.12. ADME properties and toxicity analysis
Compounds such as Luzonoid B and Roseoside [56] exhibit notable physicochemical properties, pharmacokinetics, and drug-likeness while demonstrating no significant adverse effects according to the ADMET assay. These compounds could be assessed as potential therapeutic agents targeting SLC30A8 in cancer and T2D [2]. Each compound was characterized in silico as a potential drug candidate to evaluate its pharmacokinetic properties, particularly absorption, distribution, metabolism, and excretion (ADME) [59]. Following the docking process, we assessed our chosen compounds' Pharmacokinetics and ADME characteristics using SwissADME (https://www.swissadme.ch/) [59] and the pkCSM (https://biosig.lab.uq.edu.au/pkcsm/) [60] online server.
2.13. Gene diseases Co-relation through survival analysis
The Kaplan-Meier technique (https://kmplot.com/analysis/) is a particularly effective way to evaluate changes associated with treatment interventions and forecast how they may affect cancer survival rates. This strategy makes use of overall survival (OS) and relapse-free data from the Cancer Genome Atlas (TCGA), European Genome Phenome Atlas (EGA), and Gene Expression Omnibus (GEO). It makes it possible to generate and assess cancer biomarkers through thorough meta-analyses. Once the survival job has been submitted, a Kaplan-Meier plot will demonstrate whether there is a substantial difference in the length of survival between the two patient groups [61].
3. Results
3.1. Retrieving missense variant of nsSNPs of SLC30A8 gene
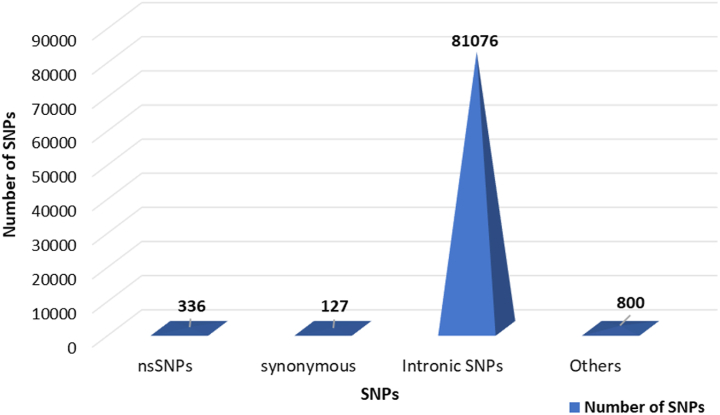
The NCBI dbSNP database was employed to get polymorphism data for the SLC30A8 gene. There were 82,339 SNPs, including 127 synonymous variants, 81,076 intron region variants, 336 missense variants of nsSNPs, and the remaining were different types (Fig. 2 and Supplementary Table S1). We excluded the other SNPs and only concentrated on 336 non-synonymous SNPs (missense variations) for our investigation. A missense variant is a single nucleotide change in the coding region for a different amino acid substitution, potentially affecting the protein's structure and function.
Fig. 2.
The clustered pyramid illustrates the quantity and arrangement of SNPs within the human SLC30A8 gene derived from the dbSNP database (nsSNPs: 336; synonymous SNPs: 127; intronic SNPs: 81076; others: 800).
3.2. Prediction and analysis of deleterious nsSNPs
We used nine different harmful SNP prediction algorithms (SIFT, Predict-SNP, MAPP, SNAP 2, SNPs&GO, PANTHER, PhD-SNP, PolyPhen-1, and PolyPhen-2) to identify deleterious nsSNPs capable of modifying the overall arrangement or function of the SLC30A8 protein. For analysis, a dataset of 336 polymorphic inputs was employed in total. All nine in silico tools predicted that 29 out of 336 nsSNPs were harmful (Table 1).
Table 1.
A compilation of nsSNPs predicted to be highly deleterious by nine computational methods.
| Rs no | A.A. change | SIFT | MAPP | PANTHER | SNP&GO | PHD-SNP | Predict-SNP | SNAP2 | PolyPhen1 | PolyPhen2 |
|---|---|---|---|---|---|---|---|---|---|---|
| Rs73317647 | R165C | DL | DL | DL | D | D | DL | E | DL | PD |
| Rs139489847 | G296R | DL | DL | DL | D | D | DL | E | DL | PD |
| Rs140404252 | L74R | DL | DL | DL | D | D | DL | E | DL | PD |
| Rs141730422 | H54R | DL | DL | DL | D | D | DL | E | DL | PD |
| Rs142407509 | W152R | DL | DL | DL | D | D | DL | E | DL | PD |
| Rs145677283 | R165H R165L |
DL | DL | DL | D | D | DL | E | DL | PD |
| Rs149524118 | A216T | DL | DL | DL | D | D | DL | E | DL | PD |
| Rs201697165 | S182F | DL | DL | DL | D | D | DL | E | DL | PD |
| Rs746249658 | R138L R138Q |
DL | DL | DL | D | D | DL | E | DL | PD |
| Rs751350884 | S182P | DL | DL | DL | D | D | DL | E | DL | PD |
| Rs762384069 | H301N | DL | DL | DL | D | D | DL | E | DL | PD |
| Rs763719329 | H304P H304L |
DL | DL | DL | D | D | DL | E | DL | PD |
| Rs765852756 | I141N | DL | DL | DL | D | D | DL | E | DL | PD |
| Rs771191320 | W136G | DL | DL | DL | D | D | DL | E | DL | PD |
| Rs771330275 | V219E | DL | DL | DL | D | D | DL | E | DL | PD |
| Rs776903420 | S230R | DL | DL | DL | D | D | DL | E | DL | PD |
| Rs780551100 | I349N | DL | DL | DL | D | D | DL | E | DL | PD |
| Rs866700996 | L303R | DL | DL | DL | D | D | DL | E | DL | PD |
| Rs1035358113 | R215G | DL | DL | DL | D | D | DL | E | DL | PD |
| Rs1255515664 | W306C | DL | DL | DL | D | D | DL | E | DL | PD |
| Rs1264823419 | H137P | DL | DL | DL | D | D | DL | E | DL | PD |
| Rs1271726667 | L111R | DL | DL | DL | D | D | DL | E | DL | PD |
| Rs1300885615 | E140A | DL | DL | DL | D | D | DL | E | DL | PD |
| Rs1305371427 | S234R | DL | DL | DL | D | D | DL | E | DL | PD |
| Rs1325443204 | L308Q | DL | DL | DL | D | D | DL | E | DL | PD |
| Rs1586608274 | Q227R | DL | DL | DL | D | D | DL | E | DL | PD |
| Rs1822266670 | D103V | DL | DL | DL | D | D | DL | E | DL | PD |
| Rs1822805541 | L222R | DL | DL | DL | D | D | DL | E | DL | PD |
| Rs2130999340 | L146R | DL | DL | DL | D | D | DL | E | DL | PD |
DL: deleterious; D: disease; E: effect; PD: probably damaging.
3.3. Analyzing the protein stability of 29 identified mutant variants
To evaluate protein stability, 29 nsSNPs previously identified as harmful were further analyzed using two additional tools: I-Mutant 2.0 and MUpro. I-Mutant showed that 25 nsSNPs decreased protein stability, while four nsSNPs were found to increase the stability, using the reliability index (RI) value and changes in free energy (ΔΔG). MUpro also predicted that 28 nsSNPs destabilized the protein, while one (R138L) nsSNP stabilized it. For further analysis, we only focused on missense variants of nsSNPs predicted to destabilize by all in silico tools (Table 2).
Table 2.
Changes in the stability of the SLC30A8 protein structure predicted by I-mutant 2.0 and MUpro.
| Rs no | Mutation | I-Mutant 2.0 | MUpro |
|---|---|---|---|
| Rs73317647 | R165C | D | D |
| Rs139489847 | G296R | D | D |
| Rs140404252 | L74R | D | D |
| Rs141730422 | H54R | D | D |
| Rs142407509 | W152R | D | D |
| Rs145677283 | R165H R165L |
D D |
D D |
| Rs149524118 | A216T | D | D |
| Rs201697165 | S182F | I | D |
| Rs746249658 | R138L R138Q |
D D |
I D |
| Rs751350884 | S182P | I | D |
| Rs762384069 | H301N | D | D |
| Rs763719329 | H304P H304L |
I I |
D D |
| Rs765852756 | I141N | D | D |
| Rs771191320 | W136G | D | D |
| Rs771330275 | V219E | D | D |
| Rs776903420 | S230R | D | D |
| Rs780551100 | I349N | D | D |
| Rs866700996 | L303R | D | D |
| Rs1035358113 | R215G | D | D |
| Rs1255515664 | W306C | D | D |
| Rs1264823419 | H137P | I | D |
| Rs1271726667 | L111R | D | D |
| Rs1300885615 | E140A | D | D |
| Rs1305371427 | S234R | D | D |
| Rs1325443204 | L308Q | D | D |
| Rs1586608274 | Q227R | D | D |
| Rs1822266670 | D103V | D | D |
| Rs1822805541 | L222R | D | D |
| Rs2130999340 | L146R | D | D |
Five SNPs including one isoform (H304P) of rs746249658 have been excluded for further analysis (Bold).
3.4. Evolutionary conservation analysis of high-risk nsSNPs
It is crucial for a protein's function and biology that its amino acids remain conserved throughout evolution. Mutations in these conserved regions often have detrimental effects and can disrupt the protein's normal function. It has been discovered that among 25 nsSNPs, 22 are located within highly conserved regions, with nine of them being buried and eight exposed (Fig. 3). The remaining of five nsSNPs were classified as buried or exposed based on the network algorithm employed by the Consurf online database. Three SNPs were excluded from this analysis as they fall within the variable region (Supplementary Table S2).
Fig. 3.
The ConSurf server conducted an analysis of evolutionary conservation for SLC30A8. Here, 'f' represents a predicted functional residue (highly conserved and exposed), while 's' denotes a predicted structural residue (highly conserved and buried).
3.5. Prediction of post-translation modification (PTM) site
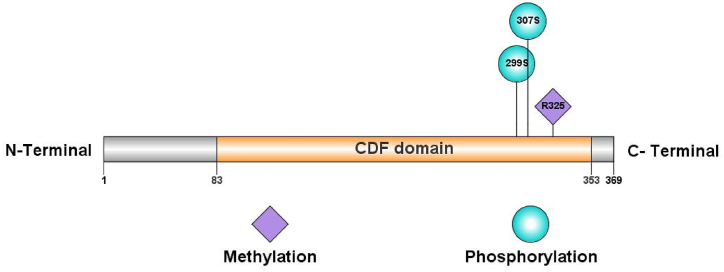
Using the GPS-MSP 1.0 tool, we identified R325 as a potential methylation site on the SLC30A8 protein. We turned to NetPhos 3.1 to predict phosphorylation sites (Fig. 4). It identified S307 in the native protein and S299 in the mutant as potential phosphorylation sites (Supplementary Table S3).
Fig. 4.
PTM sites (methylation and phosphorylation) predicted by GPS-MSP 1.0, and NetPhos 3.1 (using IBS software).
3.6. Evaluation of cancer-associated nsSNPs
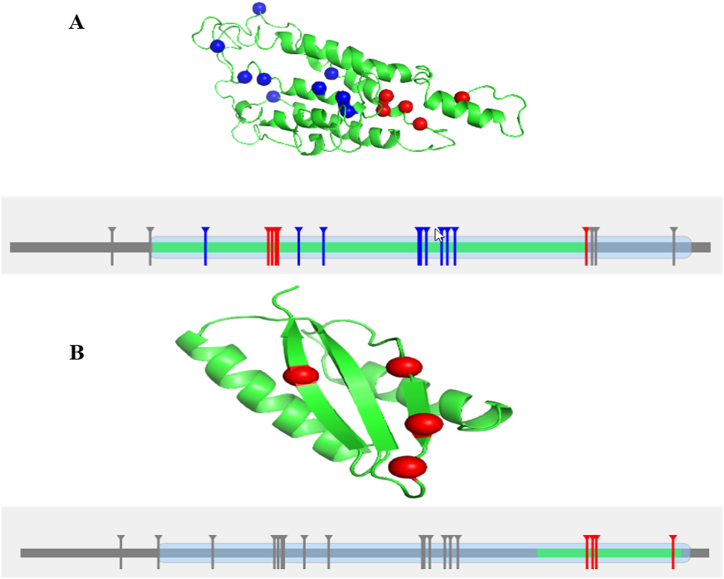
Mutation 3D server classified our reported nsSNPs into two groups. One group, comprising SNPs W152R, R215G, A216T, V219E, L222R, S230R, and S234R, is designated as covered mutations (represented in blue) in Fig. 5. The "covered mutation group” in the context of the Mutation 3D server typically refers to a set of mutations that are analyzed for their potential impact on protein structure and function. Mutation 3D server provided several default exclusion criteria for the selection of covered mutation group such as (i) mutations are typically annotated with their exact position in the protein sequence and structure, (ii) the specific substitution of amino acid such as missense at the given position, (iii) mutations in highly conserved regions indicating potential functional importance, (iv) finally, mutations that are predicted to affect the stability or folding of the protein structure.
Fig. 5.
The mutation 3D server identified certain nsSNPs as potential cancer-causing mutations (red mark). Red indicates clustered mutations, whereas blue signifies covered mutations. A represents cluster-1, where five nsSNPs (W136G, R138Q, E140A, I141N, and L303R) are present, and B represents cluster-2 where four nsSNPs are (L303R, W306C, L308Q, and I349N) present related with cancer (red mark).
Another group is the clustered mutations are categorized consisting of the eight nsSNPs (shown in red) and divided into two clusters. According to this research, our identified eight nsSNPs including W136G, R138Q, E140A, I141N, L303R, W306C, L308Q, and I349N are linked to cancer and being examined further (Fig. 5(A-B)).
3.7. Identification of functional and structural modifications
Eight nsSNPs were identified as potentially harmful and further analyzed using the MutPred2 tool. The results, including g-scores and p-values, are summarized in Table 3. Notably, all eight nsSNPs (W136G, R138Q, E140A, I141N, L303R, W306C, L308Q, and I349N) exhibited strong pathogenic potential, with g-scores exceeding 0.60 and p-values below 0.05.
Table 3.
MutPred2 predicts structural and functional modifications of SLC30A8 protein. The term "g-value” refers to the MutPred2 general score.
| Mutation | Actionable/confident hypothesis | g-value | p-value | Probability | Affected PROSITE and ELM Motifs |
|---|---|---|---|---|---|
| R138Q | Altered Transmembrane protein | 0.690 | 0.03 | 0.28 | None |
| Altered Ordered interface | 0.03 | 0.25 | |||
| I141N | Altered Transmembrane protein | 0.768 | 4.9e-03 | 0.34 | ELME000045 |
| Altered Ordered interface | 0.04 | 0.28 | |||
| Gain of Relative solvent accessibility | 0.02 | 0.28 | |||
| Gain of Allosteric site at R138 | 0.01 | 0.24 | |||
| W136G | Gain of Intrinsic disorder | 0.899 | 4.8e-03 | 0.44 | ELME000008, ELME000062, PS00008 |
| Altered Ordered interface | 2.8e-03 | 0.34 | |||
| Altered Transmembrane protein | 0.01 | 0.31 | |||
| Gain of Relative solvent accessibility | 0.01 | 0.29 | |||
| Gain of Catalytic site at R138 | 0.05 | 0.08 | |||
| I349N | Gain of Intrinsic disorder | 0.810 | 6.5e-03 | 0.41 | ELME000173 |
| Altered Stability | 5.5e-03 | 0.30 | |||
| L303R | Altered Metal binding | 0.888 | 4.5e-03 | 0.29 | ELME000051, ELME000063 |
| Altered Ordered interface | 0.04 | 0.24 | |||
| Loss of Catalytic site at H304 | 7.7e-03 | 0.23 | |||
| Gain of Allosteric site at W306 | 0.03 | 0.21 | |||
| Altered Stability | 0.04 | 0.11 | |||
| E140A | Altered Transmembrane protein | 0.823 | 3.8e-04 | 0.28 | None |
| Altered Ordered interface | 0.03 | 0.25 | |||
| Loss of Relative solvent accessibility | 0.04 | 0.24 | |||
| W306C | Altered Ordered interface | 0.839 | 3.3e-03 | 0.32 | None |
| Altered Metal binding | 4.5e-03 | 0.30 | |||
| Loss of Allosteric site at W306 | 0.02 | o.25 | |||
| Loss of Catalytic site at H304 | 0.01 | 0.21 | |||
| L308Q | Altered Metal binding | 0.707 | 8.3e-03 | 0.26 | ELME000202 |
| Altered Ordered interface | 0.04 | 0.24 | |||
| Loss of Catalytic site at H304 | 9.0e-03 | 0.22 | |||
| Gain of Allosteric site at W306 | 0.03 | 0.21 | |||
| Altered Stability | 0.04 | 0.11 |
3.8. Estimating the effects of high risk nsSNPs on the structure of protein
Project HOPE utilizes computational methods to analyze the structural alterations induced by amino acid changed in native protein. The study revealed that wild-type and mutant-type amino acids exhibit distinct physicochemical attributes, including size, charge, and hydrophobicity. All eight nsSNPs resulted in changes in amino acid size. Four mutant amino acids (I141N, L303R, L308Q, I349N) exhibited increased size compared to their wild-type counterparts, while the remaining four mutations displayed reduced size. Three nsSNPs (R138Q, E140A, L303R) were found to alter the amino acid charge. Additionally, E140A exhibited increased hydrophobicity in the mutant form. R138Q did not affect hydrophobicity, while all other six mutants showed reduced hydrophobicity compared to the wild type (Table 4).
Table 4.
Properties of wild-type and mutant-type amino acids regarding size, charge, and hydrophobicity analyzed by Project HOPE.
| AA change | Wild type AA |
Mutant type AA |
||||
|---|---|---|---|---|---|---|
| Size | Charge | Hydrophobicity | Size | Charge | Hydrophobicity | |
| R138Q | Larger | Positive | None | Smaller | Neutral | None |
| I141N | Smaller | _ | More hydrophobic | Larger | _ | Less hydrophobic |
| W136G | Larger | _ | More hydrophobic | Smaller | _ | Less hydrophobic |
| I349N | Smaller | _ | More hydrophobic | Larger | _ | Less hydrophobic |
| L303R | Smaller | Neutral | More hydrophobic | Larger | Positive | Less hydrophobic |
| E140A | Larger | Negative | Less hydrophobic | Smaller | Neutral | More hydrophobic |
| W306C | Larger | _ | More hydrophobic | Smaller | _ | Less hydrophobic |
| L308Q | Smaller | _ | More hydrophobic | Larger | _ | Less hydrophobic |
‘_’ indicates neutral effect.
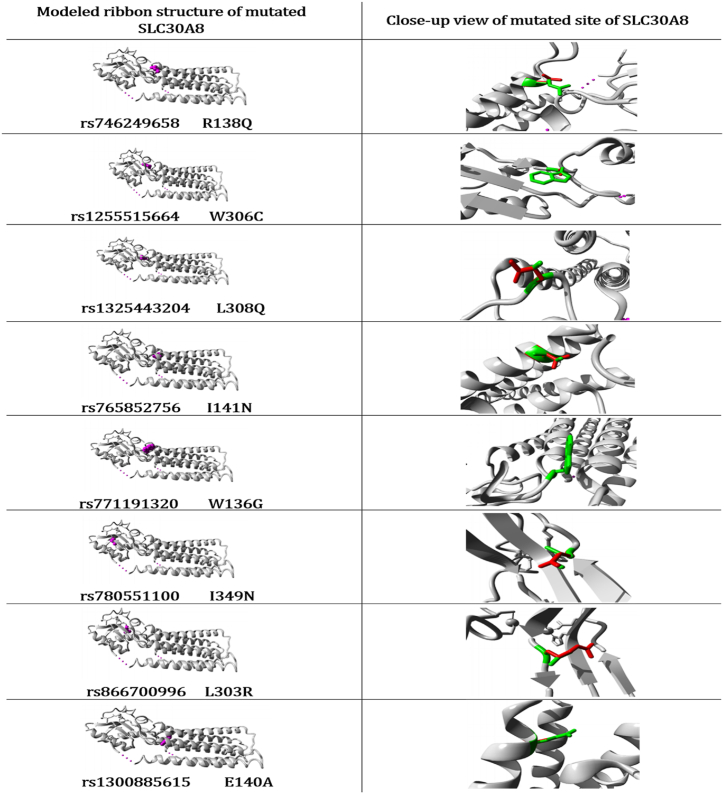
Project HOPE identified notable distinctions between wild-type and mutant-type amino acids regarding structural alterations, mutations within conserved domains, and properties of amino acids (Supplementary Table S4). The study employed computational methods to predict the physicochemical attributes, including structural features and conserved domains, which were then used to assess the possible molecular impact of high-risk nsSNPs on protein structure (Supplementary Table S5). Furthermore, Project HOPE generated 3D model structures for the eight mutated SLC30A8 proteins. Fig. 6 illustrates the ribbon presentation and resulting 3D models following the introduction of mutations. These pictures show how mutations in the SLC30A8 protein change its structure, which helps us understand valuable insights.
Fig. 6.
The 3D model structure of the SLC30A8 protein was predicted using Project HOPE. The location of the mutation is depicted in violet on the ribbon diagram. The native and mutated amino acids are represented by green and red colors, respectively.
3.9. Gene Ontology (GO) analysis
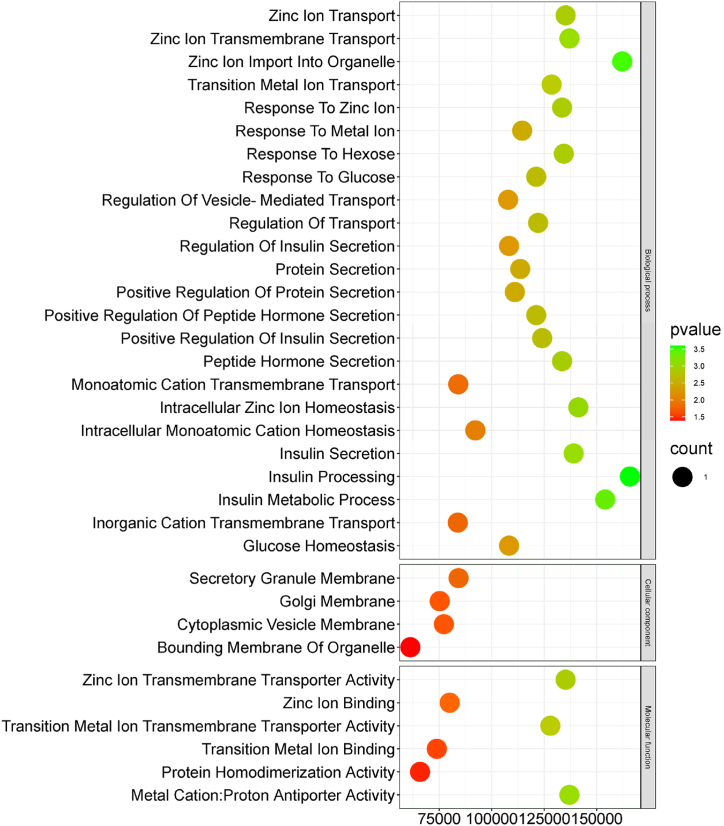
We assessed the biological properties of SLC30A8 by functionally annotating the primary targets on GO enrichment. The three components of the GO enrichment analysis are biological process (BP), cellular component (CC), and molecular function (MF), depicted by green, orange, and blue bubbles, respectively. About 34 GO words in all were produced, of which BP provided 24, CC provided 4, and MF provided 6. Node sizes indicated linked target genes, and green to red colors represented high to low p-values (Fig. 7).
Fig. 7.
Gene Ontology (GO) pathway study of SLC30A8 gene using Biological Process (BP), Cellular Component (CC), and Molecular Function (MF). The pathway term outcomes (based on P-value) were sorted using the combined score. The color gradient green to red color indicates the higher to lower p-value.
3.10. Prediction of protein-protein interaction (PPI)
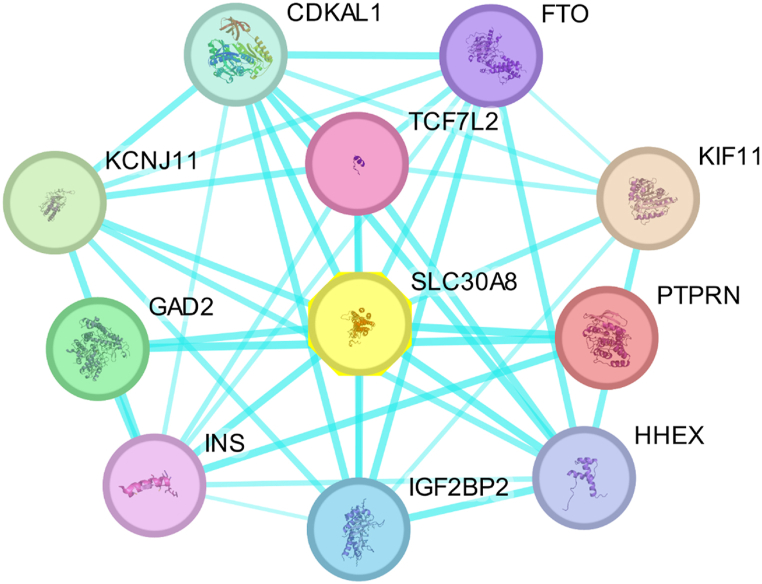
Changes in a protein's structure caused by mutations can affect its function. An analysis of the SLC30A8 protein's interaction network using the STRING server revealed interactions with PTPRN, HHEX, CDKAL1, INS, TCF7L2, GAD2, IGF2BP2, KCNJ11, FTO, and KIF11 (Fig. 8 and Supplementary Table S6). The SLC30A8 protein exhibits high connectivity within this network, interacting with ten other proteins to form a highly interconnected network with 39 edges and 11 nodes. The mean node degree stands at 7.09, while the PPI enrichment value is 6.27e-10. This network suggests these proteins have strong functional associations and potential regulatory relationships.
Fig. 8.
PPI network of SLC30A8 protein predicted by STRING server and Designed through Cytoscape software. The circular shape indicates the neighboring proteins and solid line indicates the interaction between those proteins.
3.11. Superimposition and molecular docking of raw and mutated proteins
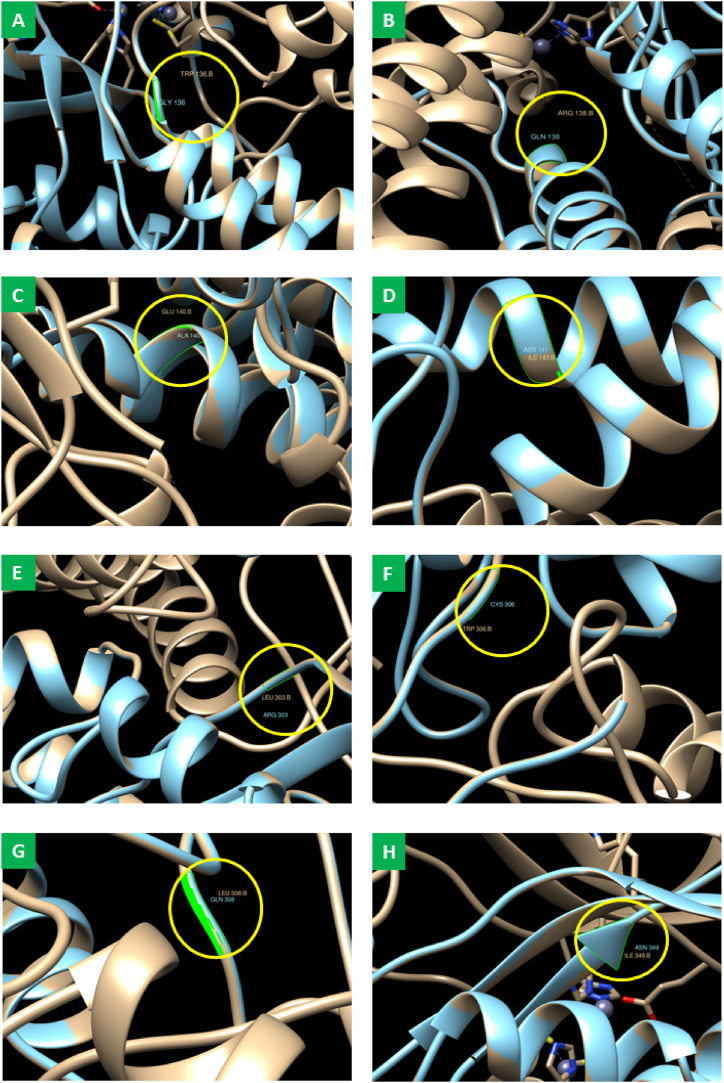
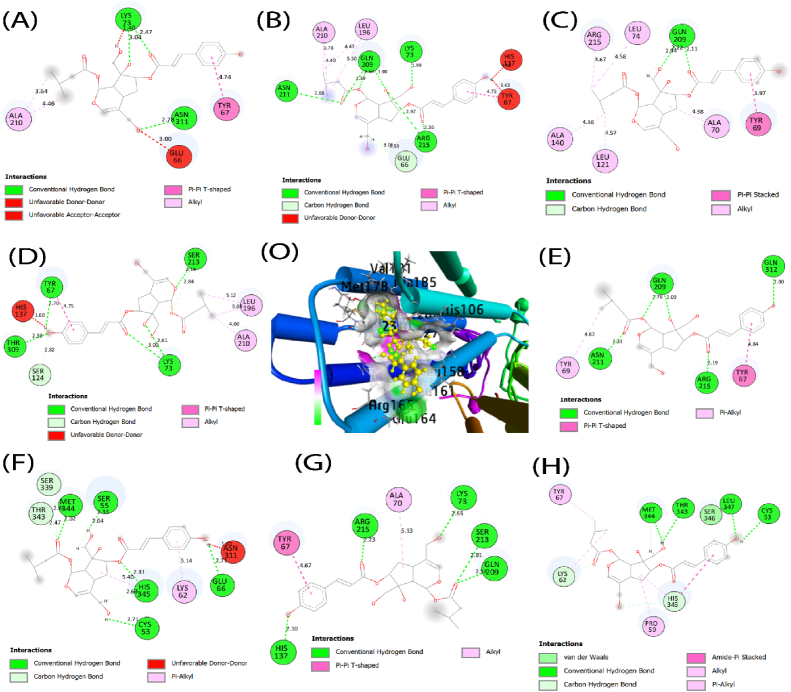
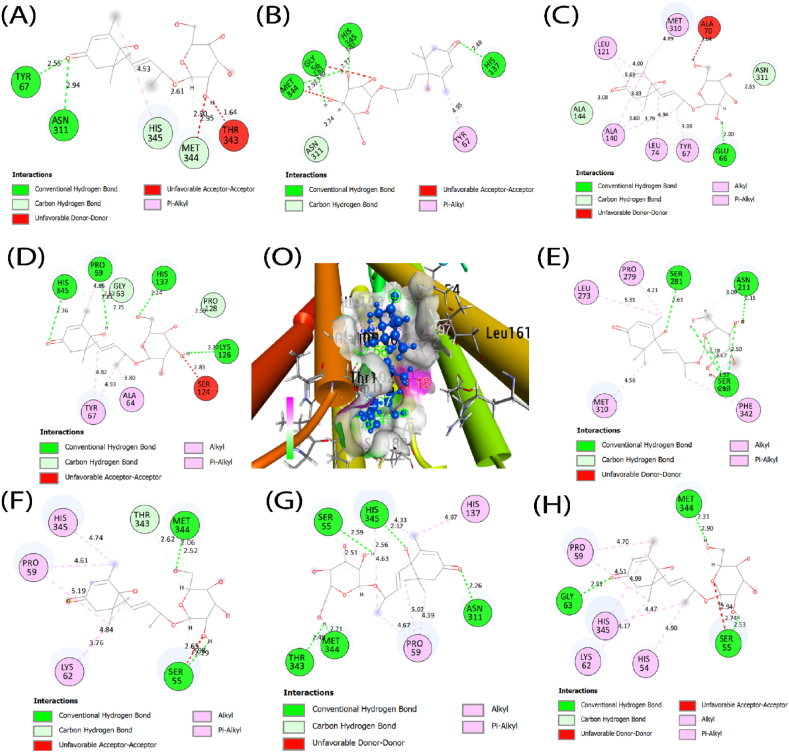
A molecular docking simulation predicted the binding affinities of eight mutated SLC30A8 for two target compounds. After matchmaking (superimposed) through chimera tools, the SLC30A8 protein and eight mutant proteins are structurally similar (Fig. 9(A-H)). There is no significant difference between the wild-type and mutant-type SLC30A8 protein structures after superposition. In the Fig. 9, we have only illustrated the substitution of amino acids. While validating our docking tools using RMSD analysis, we identified eight complexes with co-crystallized unique ligands to those complexes: luzonoid B and Roseoside, along with normal protein. The 6xpd exhibited a high binding affinity for luzonoid B (−6.9 kcal/mol) and Roseoside (−6.5 kcal/mol). Eight mutated proteins also show promising affinities with luzonoid B and Roseoside, as shown in Table 5. Among their hydrogen and hydrophobic bonds, the residues like Glu66, Tyr67, Asn311, Met344, and His345 share between mutated proteins (Fig. 10(A-H) and Fig. 11(A-H)).
Fig. 9.
Superimposition of Wild type of SLC30A8 protein with eight mutated proteins. Superimposed structure of wild-type SLC30A8 protein and mutant-type having mutations from (A) Tryptophan to Glycine at position 136, (B) Arginine to Glutamine at position 138, (C) Glutamic acid to Alanine at position 140, (D) Isoleucine to Asparagine at position 141, (E) Leucine to Arginine at position 303, (F) Tryptophan to Cysteine at position 306, (G) Leucine to Glutamine at position 308 and (H) Isoleucine to Asparagine at position 349. Ash color indicates wild-type protein, sky blue for mutant protein, and the yellow box for mutations between a wild-type protein and eight mutated proteins, along with their positions.
Table 5.
Interaction of ligands showing high docking scores for mutated SLC30A8 protein.
| Receptor type | Receptor | Binding Affinity of Luzonoid B | Binding Affinity of Roseoside | Interacting residues |
|||
|---|---|---|---|---|---|---|---|
| Hydrogen bond |
Hydrophobic bond |
||||||
| Luzonoid B | Roseoside | Luzonoid B | Roseoside | ||||
| Normal SLC30A8 protein | 6xpd | −6.9 | −6.5 | His106, Glu164, Arg165, Glu227, Ser228 | Thr102, Ser182 | Leu158, Leu161, Met178, Val181, Ala185 | Ala105, His106, Ile109, Val154, Leu158, Leu161 |
| Mutated SLC30A8 protein | W136G | −6.6 | −6.6 | Lys73, Asn311 | Tyr67, Asn311 | Glu66, Tyr67, Ala210 | Thr343, Met344, His345 |
| R138Q | −7.9 | −7.2 | Glu66, Lys73, Glu209, Asn211, Arg215 | Gly56, His137, Asn311, Met344, His345 | Tyr67, His137, Ala210, Leu196, Thr309 | Tyr67 | |
| E140A | −7.2 | −8.0 | Gln209 | Glu66, Asn311 | Tyr67, Ala70, Leu74, Leu121, Ala140, Arg215 | Tyr67, Ala70, Leu74, Leu121, Ala140, Met310 | |
| I141N | −7.4 | −6.5 | Tyr67, Lys73, Ser213, Thr309 | Pro59, Lys126, His137, His345 | His137, Leu196, Ala210 | Ala64, Tyr67, Ser124 | |
| L303R | −7.3 | −6.3 | Gln209, Asn211, Arg215, Gln312 | Asn211, Ser213, Ser281 | Tyr67, Tyr69, Thr309 | Leu273, Pro279, Met310, Phe342 | |
| W306C | −7.0 | −7.5 | Cys53, Ser55, Glu66, Ser339, Met344, His345 | Ser55, Met344 | Lys62, Asn311 | Gly56, Pro59, Lys62, His345 | |
| L308Q | −7.3 | −7.2 | Lys73, His137, Gln209, Ser213, Arg215 | Ser55, Asn311, Thr343, Met344, His345 | Tyr67, Ala70, Thr309 | Pro59, His137 | |
| I349N | −7.2 | −7.4 | Cys53, Thr343, Met344, His345, Leu347 | Ser55, Gly63, Met344 | Pro59, Lys62, Tyr67 | His54, Pro59, Lys62, His345 | |
Fig. 10.
Analysis of binding interactions of (O) raw and mutated SLC30A8 proteins using Autodock Vina. From the right side, (A) depicts the interaction between W136G and Luzonoid B, (B) R138Q and Luzonoid B, (C) E140A and Luzonoid B, (D) I141N and Luzonoid B, (E) L303R and Luzonoid B, (F) W306C and Luzonoid B, (G) L308Q and Luzonoid B, (H) I349N and Luzonoid B.
Fig. 11.
Analysis of binding interactions of (O) raw and mutated SLC30A8 proteins using Autodock Vina. From the right side, (A) depicts the interaction between W136G and Roseoside, (B) R138Q and Roseoside, (C) E140A and Roseoside, (D) I141N and Roseoside, (E) L303R and Roseoside, (F) W306C and Roseoside, (G) L308Q and Roseoside, (H) I349N and Roseoside.
3.12. ADME properties and toxicity analysis
The tools SwissADME and pkCSM analyze the molecular properties of selected compounds to ensure compliance with Lipinski's rule of five, a crucial factor in drug design. The chosen compounds obtained from PubChem were assessed for their drug-likeness and physiochemical characteristics. To determine the most promising candidates' drug-likeness, we evaluate selected compounds' ADME properties. This evaluation included Lipinski's rule of five compliance, bioactivity rating, and ADME parameters such as Total Solvent Accessible Surface Area (TPSA), Predicted Aqueous Solubility (PSA), Human Oral Absorption (HIA), Molecular Weight (MW), Blood-Brain Barrier (BBB) permeability, Hydrogen Bond Donor (HBD) count, Hydrogen Bond Acceptor (HBA) count, Central Nervous System (CNS) permeability, and Partition Coefficient (LogP). These factors are crucial for determining a compound's potential to effectively interact with its target site and be safely metabolized and eliminated from the body (Table 6). The toxicity evaluation of the selected compounds revealed encouraging results, suggesting their potential as drug candidates. The AMES test, Hepatotoxicity, Oral Rat Acute Toxicity (LD50), and Skin Sensitization tests all demonstrated low toxicity and minimal adverse effects, supporting the compounds' safety and suitability for further development (Table 6). These ligands' favorable physiochemical properties and ADME profiles suggest their potential as promising drug candidates for future development.
Table 6.
Analysis of reported compounds, considering their ADMET (Absorption, Distribution, Metabolism, Excretion, and Toxicity), physicochemical properties, lipophilicity, and drug-likeness.
| Properties | Model Name | luzonoid B | Roseoside |
|---|---|---|---|
| Absorption | Intestinal absorption (human) | 96.016 | 47.786 |
| Skin Permeability | −2.781 | −2.859 | |
| caco-2 | 0.176 | 0.379 | |
| Distribution | VDss (human) | −0.113 | −0.131 |
| Fraction unbound (human) | 0.228 | 0.601 | |
| BBB permeability | −1.227 | −1.067 | |
| CNS permeability | −3.526 | −3.632 | |
| Metabolism | CYP2D6 substrate | No | No |
| CYP3A4 substrate | Yes | No | |
| CYP2D6 inhibitor | No | No | |
| CYP3A4 inhibitor | No | No | |
| Excretion | Total Clearence | 0.873 | 1.389 |
| Toxicity | AMES toxicity | No | No |
| Oral Rat Acute Toxicity (LD5) | 2.808 | 2.2 | |
| Hepatotoxicity | No | No | |
| Skin Sensitization | No | No | |
| Physicochemical | Molecular weight | 462.49 g/mol | 386.44 g/mol |
| LogP | 1.4985 | −0.576 | |
| Num. H-bond acceptors | 9 | 8 | |
| Num. H-bond donors | 4 | 5 | |
| Molar Refractivity | 117.94 | 96.23 | |
| TSPA | 142.75 Å2 | 136.68 Å2 | |
| Lipophilicity | Consensus Log Po/w | 1.61 | 0.01 |
| Water Solubility | Log S (ESOL) | −2.91 | −1.24 |
| Solubility class | Soluble | Soluble | |
| Drug-likeness | Lipinski violation | Yes; 0 violation | Yes; 0 violation |
| Bioavailability Score | 0.55 | 0.55 | |
| Medicinal Chemistry | PAINs | 0 alert | 0 alert |
3.13. Gene diseases Co-relation through survival analysis
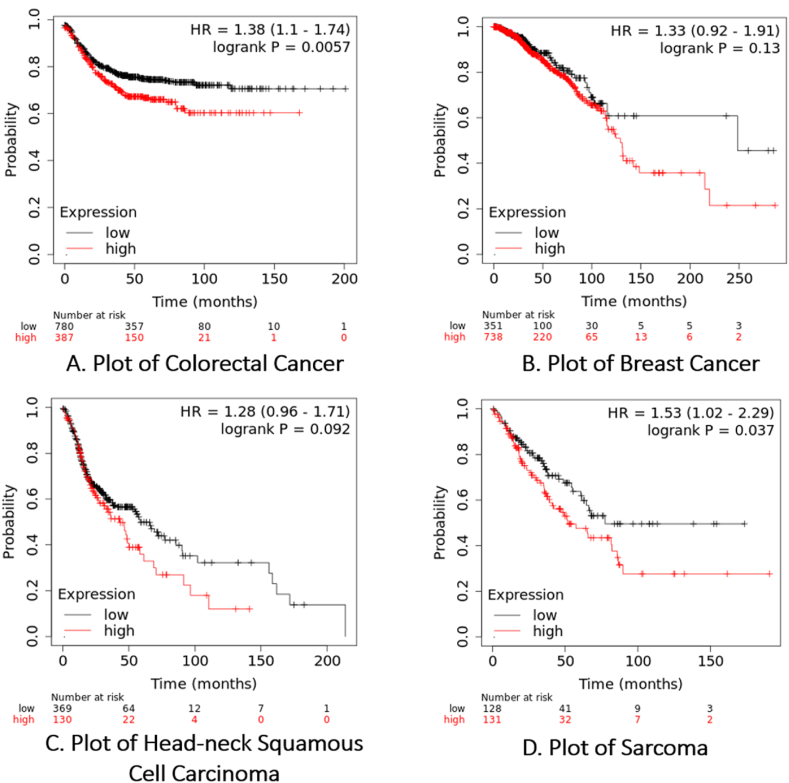
A research investigation was carried out to examine the correlation between the SLC30A8 gene and the survival rates of patients with breast cancer, colorectal cancer, head-neck squamous cell carcinoma, and sarcoma. The findings indicated that individuals exhibiting elevated levels of SLC30A8 expression faced reduced mortality risk across all four types of cancer. For colorectal and breast cancer, the HR ratio and P-value were (HR 1.38 [1.1–1.74], P = 0.0057) and (HR 1.33 [0.92–1.91], P = 0.13), respectively. Similarly, for head-neck squamous cell carcinoma and sarcoma, the HR ratio and P-value stood at (HR 1.28 [0.96–1.71], P = 0.092) and (HR 1.53 [1.02–2.29], P = 0.037), respectively (Fig. 12(A-D)). These findings imply that the SLC30A8 gene could be a prognostic indicator for these cancers. Furthermore, eight nsSNPs were detected within the SLC30A8 gene. These nsSNPs are anticipated to impair the function of the SLC30A8 protein, potentially fostering cancer progression.
Fig. 12.
SLC30A8 gene expression levels and survival rates in patients with various cancer types using microarray data by Kaplan–Meier Plotter.
3.14. Biological validation of reported SNP
According to our study, nsSNPs of the SLC30A8 gene may have a detrimental impact on several fatal diseases like T2D, Colorectal cancer, and so on. The SLC30A8 gene polymorphism rs13266634 raises the probability of developing T2D in Jordanian population (Mashal et al. [62]). A different study detected the SLC30A8 rs13266634 and rs16889462 genetic variations in Chinese individuals diagnosed with T2D, which were connected with susceptibility to the disease and the effectiveness of repaglinide as a treatment (Huang et al. [63]). Also, other research groups investigated and examined the correlation between the rs13266634 polymorphism and T2D in Iranian populations (Faghih et al. [64]) and the rs13266634 SLC30A8 gene variant in Han Chinese and minority ethnic groups in China (Wang et al. [65]).
The association between diabetes and colorectal cancer risk was modified by variants on chromosome 8q24.11 within the SLC30A8 gene, with rs3802177 being the genetic variant showing the most significant effect (Dimou et al. [2]). Four single-nucleotide polymorphisms (SNPs) in T2D were found to increase the risk of colorectal cancer: rs7578597 (THADA), rs864745 (JAZF1), rs5219 (KCNJ11), Rs7961581 (TSPAN8, LGR5) (Cheng et al. [66]). The patients who possessed the TCF7L2_rs7903146_T gene variant were more likely to develop colorectal cancer (Sainz et al. [67]).
Above all, the SLC30A8 gene with different rs IDs potentially impacts T2D and Colorectal cancer in humans. We evaluated eight rs IDs of nsSNPs of the SLC30A8 gene, possibly linked to several diseases, including T2D, colorectal cancer, etc.
4. Discussion
Single nucleotide polymorphisms (SNPs) can change the structure and function of proteins, thereby influencing human diseases, traits, drug responses, and therapeutic outcomes [68,69]). However, characterizing these SNPs is a laborious and expensive technique due to their complex relationship with specific phenotypes. In silico approaches, which utilize computer simulations to analyze biological data, can provide insights into genetic differences in disease susceptibility and their phenotypic impacts, hence lowering the number of candidates for molecular screening [70].
This research determines genetic variations within the SLC30A8 gene to assess their potential impact on gene function. Out of the 336 documented missense SNPs, eight high-risk SNPs (rs746249658, rs765852756, rs771191320, rs780551100, rs866700996, rs1300885615, rs1255515664, and rs1325443204) were determined to be harmful by nine distinct computational techniques (Table 1). Two computational approaches, MuPro and I-Mutant 2.0, were employed to assess the impact of 29 identified nsSNPs on protein stability. According to I-Mutant, the S182F, S182P, H304P, H304L, and H137P SNPs are anticipated to enhance protein stability. Meanwhile, MuPro suggested that the R138L SNP would also increase protein stability. Since mutations stabilizing the protein were not the focus of this study, they were excluded from this study. Only the 25 SNPs, which were consistently forecasted by both tools to instabilize the protein, were kept for further studies (Table 2).
Utilizing ConSurf, a study evaluated how the residues in the SLC30A8 protein have evolved according to their conservation. This analysis revealed that mutations are more likely to occur in conserved regions, where critical amino acid positions change less frequently due to their importance for protein function. An amino acid's evolutionary conservation indicates its mutability, with highly conserved and exposed residues most prone to harm (Ashkenazy et al. [36]). The Evolutionary Trace approach and its variations are the most well-known substitute for ConSurf for discovering functional areas [71,72] Among 25 nsSNPs, ConSurf revealed 22 nsSNPs with a conservation score of 8 or 9, indicating they were highly conserved (Supplementary Table S2, and Fig. 3). Afterward, the study of mutation 3D servers revealed the correlation of W136G, R138Q, E140A, I141N, L303R, W306C, L308Q, and I349N nsSNPs with cancer (Fig. 4). GPS-MSP 1.0 anticipated R325's methylation location. The NetPhos 3.1 program predicted the phosphorylation sites to be wild-type residues (S307) and mutant residues (S299) (Fig. 5(A-B)).
Protein stability alterations influence the conformational structure, dictating protein function [73]. In this research, the MutPred2 web server was employed to investigate potential molecular alterations in the structure or function of the SLC30A8 protein caused by mutations. According to their g and p scores, every detected deleterious SNP was categorized as "pathogenic” (Table 3). Among the eight analyzed nsSNPs, W136G possesses the highest probability score, indicating its significant potential to affect protein structure and function. Conversely, R138Q exhibits the lowest g-value, suggesting a comparatively less impact. This analysis reveals G47R as the most impactful mutation, with the remaining seven potentially influencing protein stability and possibly affecting structure and function. As demonstrated by previous studies, reduced protein stability can result in altered folding mechanisms, accelerated breakdown, and erroneous aggregation [74,75]).
Project HOPE provides new insights into the adverse effects of point mutations on protein structure. It demonstrates notable variances in physicochemical characteristics between wild and mutant amino acids, encompassing size, charge, and hydrophobicity. Notably, the mutations I141N, L303R, L308Q, and I349N result in increased amino acid size compared to the wild-type counterpart, whereas W136G, R138Q, E140A, and W306C mutations lead to a size decrease. Moreover, three mutations (R138Q, E140A, and L303R) exhibit altered amino acid charges in the mutant variant. Regarding hydrophobicity, W136G, I141N, L303R, W306C, L308Q, and I349N mutations render the protein less water-repellent than the wild-type residue, while E140A exhibits increased hydrophobicity, potentially impacting hydrophobic interactions. Notably, R138Q does not contribute to hydrophobic interactions (Table 4).
STRING analysis shows that the SLC30A8 protein plays crucial roles in several vital processes. It works with other proteins like PTPRN, INS, and TCF7L2 to maintain normal levels of necessary brain chemicals like norepinephrine, dopamine, and serotonin; boost critical metabolic processes in the liver, including glycolysis, the pentose phosphate cycle, and glycogen synthesis; and additionally, involves in the important Wnt signalling pathway (Fig. 8). ZnT8, a protein encoded by SLC30A8, may transport zinc ions from the cytoplasm to the granules that secrete insulin. While high zinc concentrations within these granules are well-documented, the precise physiological function of secreted zinc remains unclear. It validated three loci in a noncoding region close to CDKN2A and CDKN2B, in an intron of IGF2BP2, and in an intron of CDKAL1, which was shown to be related to T2D. Additionally, it replicated associations close to HHEX and in SLC30A8 [76,77].
We employed molecular docking to explore the possibility that luzonoid B and roseoside bind to a mutated SLC30A8 protein, which plays a role in both T2D and colorectal cancer [2,9] Based on their exceptional binding affinity to the eight mutated SLC30A8 proteins along with normal protein, luzonoid B and roseoside were identified as the most promising compounds with promising binding scores (Fig. 10, Fig. 11 and Table 5). This suggests that the two compounds exhibit the strongest attraction to the mutant proteins and are more effective at inhibiting them compared to the normal SLC30A8 protein. The increasing binding affinity towards mutant SLC30A8 proteins has been demonstrated not to contribute to drug resistance [78,79].
We evaluated the pharmacokinetics of the selected compounds to ensure they can be effectively absorbed, distributed, metabolized, and excreted, allowing them to reach their target sites and exert their desired effects [80]. Lower molecular weight (<500) enhances permeability, facilitating transport, diffusion, and absorption through the cell membrane [81]. Selected compounds displayed molecular weights under 500 g/mol, including Luzonoid B (462.49 g/mol) and Roseoside (386.44 g/mol). Higher positive LogP values suggest enhanced permeation of compounds across biological membranes, with a recommended threshold below 5 considered acceptable [82]. LogS indicates drug solubility, with lower values representing higher solubility [83]. These results demonstrate the compounds as promising candidates for further evaluation as drugs for T2D and colorectal cancer in living organisms (in vivo). Optimal membrane permeation for drugs requires a specific range of hydrogen bonds (H-donors: <5 H-acceptors: <10). Optimal oral bioavailability necessitates limiting the number of rotatable bonds in a drug molecule to a maximum of 10. ADMET prediction suggests that all two docked compounds possess properties within the allowed range for predicted drug development (Table 6).
The SLC30A8 gene dysregulation was found to have a prognostic significance and to impact the total survival rate for individuals who have suffering from breast cancer, colorectal cancer, sarcoma, and head-neck squamous cell carcinoma using the Kaplan-Meier plotter. All four malignancies combined posed a decreased death risk to those with greater expression of SLC30A8. These results suggest any dysregulation of SLC30A8 expression may significantly impact patient survival in these four cancers (Fig. 12). While the in-silico approach is powerful, the study would be strengthened by experimental validation including in vitro or in vivo of the predicted effects in near future. This approach allows researchers to efficiently predict the impact of genetic mutations on protein function and disease pathophysiology, thereby prioritizing variants for further experimental validation and saving time and resources in the research process [84]. Therefore, we will be emphasized the need for experimental validation to elucidate how SLC30A8 affects cancer progression and investigate its viability as a potential drug in the future of the predicted impacts of identified mutations.
5. Conclusion
Analyzing genetic variations (SNPs) in SLC30A8 suggests it might be an essential gene for impacting the onset of type 2 diabetes, a recognized risk factor for colorectal cancer. Eight potentially harmful nsSNPs of SLC30A8 were found in this study using various in silico methods. Finding these nsSNPs should facilitate quick and effective screening to identify diseases linked to SLC30A8 expression. After identifying nsSNPs of SLC30A8, we developed a novel drug against its harmful effects; this suggests that the two compounds exhibit the strongest attraction to the mutant proteins and are more effective at inhibiting them compared to the normal SLC30A8 protein. The high binding affinity towards mutant SLC30A8 proteins has been exhibited not to contribute to drug resistance, which might be detrimental to curing these fatal diseases. Moreover, it will greatly simplify the process of creating experiments for upcoming lab-based research.
Clinical significance
In our study, identification of specific genetic polymorphisms can serve as biomarkers for early detection of diseases progression, helping to tailor treatment plans based on individual risk profiles. In addition, studying such types of polymorphisms can provide insights into the molecular pathways as well as prognostic indicators allowing for more proactive management of T2D and CRC. In the area of pharmacogenomics, understanding how genetic variations affect drug metabolism can optimize drug selection and dosing, minimizing side effects and maximizing efficacy. Besides, advances in gene editing technologies, such as CRISPR, might allow for the correction of our identified genetic variants that contribute to T2D and CRC.
Limitations
In silico methods can predict SNP functional effects, although they are only sometimes accurate. Database accuracy and completeness are crucial to in silico SNP analysis. Reference data errors or gaps can affect SNP identification and interpretation. The SNP identification of SLC30A8 is just the beginning. Interpreting their functional significance or relationship with other genetic traits/diseases and environmental factors related to glucose homeostasis and insulin secretion is complicated and requires further experimental validation and in vitro or in vivo research.
Even though SLC30A8 has been linked to insulin secretion, diabetes risk, and many malignancies, our knowledge of its genetic diversity and the functional effects of its variants continues growing. This suggests that some crucial genetic variations and their impacts may be insufficiently identified using computer-based SNP analysis. Since SNPs in genes' coding regions are more likely to affect gene function, most in silico techniques concentrate on these regions. On the other hand, SNPs in non-coding areas should be more appropriately characterized for their potential importance. Finally, SNP impacts on SLC30A8 function typically necessitate experimental validation.
Data availability
The datasets supporting the conclusions of the study are included within the article and its additional file as a supplementary file. Correspondence and requests for materials should be addressed to Kawsar Ahmed and Md. Arju Hossain. All supporting data are available on a publicly available respiratory (Github) https://github.com/Md-Arju-Hossain/In-silico-Single-neucleotide-Polymorphisms.
Funding Statement
The Funding number is 4170008.
Ethical Statement
We declared that, our manuscript submitted to the Heliyon Journal has been done in accordance to Cell Press guidelines of publication ethics in a responsible way.
CRediT authorship contribution statement
Md. Moin Uddin: Writing – original draft, Visualization, Software, Resources, Methodology, Investigation, Formal analysis, Data curation. Md. Tanvir Hossain: Writing – original draft, Visualization, Software, Resources, Methodology, Investigation, Formal analysis, Data curation. Md. Arju Hossain: Writing – review & editing, Writing – original draft, Visualization, Validation, Software, Resources, Project administration, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. Asif Ahsan: Writing – original draft, Visualization, Resources, Methodology, Investigation, Formal analysis, Data curation. Kamrul Hasan Shamim: Writing – original draft, Visualization, Resources, Methodology, Investigation. Md. Arif Hossen: Writing – original draft, Visualization. Md. Shahinur Rahman: Writing – original draft, Visualization. Md Habibur Rahman: Writing – original draft, Validation, Supervision, Project administration, Methodology. Kawsar Ahmed: Writing – review & editing, Writing – original draft, Validation, Supervision, Project administration, Conceptualization. Francis M. Bui: Writing – review & editing, Validation, Project administration, Funding acquisition. Fahad Ahmed Al-Zahrani: Writing – review & editing, Funding acquisition.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors would like to thank the Deanship of Scientific Research at Umm Al-Qura University for supporting this work.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2024.e37280.
Contributor Information
Md. Moin Uddin, Email: moinbge12@gmail.com.
Md. Tanvir Hossain, Email: tanvirhossain69744@gmail.com.
Md. Arju Hossain, Email: arju.primer60@gmail.com.
Asif Ahsan, Email: asifahsanbge@gmail.com.
Kamrul Hasan Shamim, Email: khs.shamim1@gmail.com.
Md. Arif Hossen, Email: arifh6528@gmail.com.
Md. Shahinur Rahman, Email: dr.srahman90@yahoo.com.
Md Habibur Rahman, Email: habib@iu.ac.bd.
Kawsar Ahmed, Email: kawsar.ict@mbstu.ac.bd, k.ahmed@usask.ca.
Francis M. Bui, Email: francis.bui@usask.ca.
Fahad Ahmed Al-Zahrani, Email: fayzahrani@uqu.edu.sa.
Appendix A. Supplementary data
The following is the Supplementary data to this article.
References
- 1.Sung H., Ferlay J., Siegel R.L., et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Dimou N., Kim A.E., Flanagan O., et al. Probing the diabetes and colorectal cancer relationship using gene–environment interaction analyses. Br. J. Cancer. 2023:1–10. doi: 10.1038/s41416-023-02312-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guo Y., He Y. Comprehensive analysis of the expression of SLC30A family genes and prognosis in human gastric cancer. Sci. Rep. 2020;10 doi: 10.1038/s41598-020-75012-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nguyen L.T.D., Gunathilake M., Lee J., et al. Zinc intake, SLC30A8 rs3802177 polymorphism, and colorectal cancer risk in a Korean population: a case–control study. J. Cancer Res. Clin. Oncol. 2023;149:16429–16440. doi: 10.1007/s00432-023-05381-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ujpal M., Matos O., Bibok G., et al. Diabetes and oral tumors in Hungary: epidemiological correlations. Diabetes Care. 2004;27:770–774. doi: 10.2337/diacare.27.3.770. [DOI] [PubMed] [Google Scholar]
- 6.Wu C.H., Wu T.Y., Li C.C., et al. Impact of diabetes mellitus on the prognosis of patients with oral squamous cell carcinoma: a retrospective cohort study. Ann. Surg Oncol. 2010;17:2175–2183. doi: 10.1245/s10434-010-0996-1. [DOI] [PubMed] [Google Scholar]
- 7.Tseng C.H. Pioglitazone and oral cancer risk in patients with type 2 diabetes. Oral Oncol. 2014;50:98–103. doi: 10.1016/j.oraloncology.2013.10.015. [DOI] [PubMed] [Google Scholar]
- 8.Cui M., Fang Q., Zheng J., et al. Kaposi's sarcoma-associated herpesvirus seropositivity is associated with type 2 diabetes mellitus: a case–control study in Xinjiang, China. Int. J. Infect. Dis. 2019;80:73–79. doi: 10.1016/j.ijid.2019.01.003. [DOI] [PubMed] [Google Scholar]
- 9.Cheng L., Zhang D., Zhou L., et al. Association between SLC30A8 rs13266634 polymorphism and type 2 diabetes risk: a meta-analysis. Med Sci Monit Int Med J Exp Clin Res. 2015;21:2178. doi: 10.12659/MSM.894052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chimienti F., Devergnas S., Favier A., et al. Identification and cloning of a β-cell–specific zinc transporter, ZnT-8, localized into insulin secretory granules. Diabetes. 2004;53:2330–2337. doi: 10.2337/diabetes.53.9.2330. [DOI] [PubMed] [Google Scholar]
- 11.Chimienti F., Devergnas S., Pattou F., et al. In vivo expression and functional characterization of the zinc transporter ZnT8 in glucose-induced insulin secretion. J. Cell Sci. 2006;119:4199–4206. doi: 10.1242/jcs.03164. [DOI] [PubMed] [Google Scholar]
- 12.Lee J.E., Choi J.H., Lee J.H., et al. Gene SNPs and mutations in clinical genetic testing: haplotype-based testing and analysis. Mutat Res Mol Mech Mutagen. 2005;573:195–204. doi: 10.1016/j.mrfmmm.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 13.Prokunina L., Alarcón-Riquelme M.E. Regulatory SNPs in complex diseases: their identification and functional validation. Expert Rev Mol Med. 2004;6:1–15. doi: 10.1017/S1462399404007690. [DOI] [PubMed] [Google Scholar]
- 14.Stenson P.D., Mort M., Ball E.V., et al. The human gene mutation database: 2008 update. Genome Med. 2009;1:1–6. doi: 10.1186/gm13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Collins F.S., Brooks L.D., Chakravarti A. A DNA polymorphism discovery resource for research on human genetic variation. Genome Res. 1998;8:1229–1231. doi: 10.1101/gr.8.12.1229. [DOI] [PubMed] [Google Scholar]
- 16.Lander E.S. The new genomics: global views of biology. Science. 1996;80(274):536–539. doi: 10.1126/science.274.5287.536. [DOI] [PubMed] [Google Scholar]
- 17.Dobson R.J., Munroe P.B., Caulfield M.J., et al. Predicting deleterious nsSNPs: an analysis of sequence and structural attributes. BMC Bioinf. 2006;7:1–9. doi: 10.1186/1471-2105-7-217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barroso I., Gurnell M., Crowley V.E.F., et al. Dominant negative mutations in human PPARγ associated with severe insulin resistance, diabetes mellitus and hypertension. Nature. 1999;402:880–883. doi: 10.1038/47254. [DOI] [PubMed] [Google Scholar]
- 19.Petukh M., Kucukkal T.G., Alexov E. On human disease‐causing amino acid variants: statistical study of sequence and structural patterns. Hum. Mutat. 2015;36:524–534. doi: 10.1002/humu.22770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chasman D., Adams R.M. Predicting the functional consequences of non-synonymous single nucleotide polymorphisms: structure-based assessment of amino acid variation. J. Mol. Biol. 2001;307:683–706. doi: 10.1006/jmbi.2001.4510. [DOI] [PubMed] [Google Scholar]
- 21.Kucukkal T.G., Petukh M., Li L., et al. Structural and physico-chemical effects of disease and non-disease nsSNPs on proteins. Curr. Opin. Struct. Biol. 2015;32:18–24. doi: 10.1016/j.sbi.2015.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thomas R., McConnell R., Whittacker J., et al. Sandford, Identification of mutations in the repeated part of the autosomal dominant polycystic kidney disease type 1 gene, PKD1, by long-range PCR. Am. J. Hum. Genet. 1999;65:39–49. doi: 10.1086/302460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Das S.C., Rahman M.A., Das Gupta S. In-silico analysis unravels the structural and functional conseque’nces of non-synonymous SNPs in the human IL-10 gene. Egypt J Med Hum Genet. 2022;23:10. doi: 10.1186/s43042-022-00223-x. [DOI] [Google Scholar]
- 24.Hossain M.S., Roy A.S., Islam M.S. In silico analysis predicting effects of deleterious SNPs of human RASSF5 gene on its structure and functions. Sci. Rep. 2020;10 doi: 10.1038/s41598-020-71457-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Petrosino M., Novak L., Pasquo A., Chiaraluce R., Turina P., Capriotti E., et al. Analysis and interpretation of the impact of missense variants in cancer. Int. J. Mol. Sci. 2021;22(11):5416. doi: 10.3390/ijms22115416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zeng Q., Tan B., Han F., Huang X., Huang J., Wei Y., et al. Association of solute carrier family 30 A8 zinc transporter gene variations with gestational diabetes mellitus risk in a Chinese population. Front. Endocrinol. 2023;14 doi: 10.3389/fendo.2023.1159714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ng P.C., Henikoff S. Predicting the effects of amino acid substitutions on protein function. Annu. Rev. Genom. Hum. Genet. 2006;7:61–80. doi: 10.1146/annurev.genom.7.080505.115630. [DOI] [PubMed] [Google Scholar]
- 28.Venkata Subbiah H., Ramesh Babu P., Subbiah U. In silico analysis of non-synonymous single nucleotide polymorphisms of human DEFB1 gene. Egypt J Med Hum Genet. 2020;21:1–9. doi: 10.1186/s43042-020-00110-3. [DOI] [Google Scholar]
- 29.Bendl J., Stourac J., Salanda O., et al. PredictSNP: robust and accurate consensus classifier for prediction of disease-related mutations. PLoS Comput. Biol. 2014;10 doi: 10.1371/journal.pcbi.1003440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Capriotti E., Calabrese R., Fariselli P., et al. WS-SNPs&GO: a web server for predicting the deleterious effect of human protein variants using functional annotation. BMC Genom. 2013;14:1–7. doi: 10.1186/1471-2164-14-S3-S6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Adzhubei I., Jordan D.M., Sunyaev S.R. Predicting functional effect of human missense mutations using PolyPhen‐2. Curr Protoc Hum Genet. 2013;76:7–20. doi: 10.1002/0471142905.hg0720s76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Capriotti E., Fariselli P., Casadio R. I-Mutant2. 0: predicting stability changes upon mutation from the protein sequence or structure. Nucleic Acids Res. 2005;33:W306–W310. doi: 10.1093/nar/gki375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kamaraj B., Purohit R. In silico screening and molecular dynamics simulation of disease-associated nsSNP in TYRP1 gene and its structural consequences in OCA32013. BioMed Res. Int. 2013;2013 doi: 10.1155/2013/697051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Khan S., Vihinen M. Performance of protein stability predictors. Hum. Mutat. 2010;31:675–684. doi: 10.1002/humu.21242. [DOI] [PubMed] [Google Scholar]
- 35.Celniker G., Nimrod G., Ashkenazy H., et al. ConSurf: using evolutionary data to raise testable hypotheses about protein function. Isr. J. Chem. 2013;53:199–206. [Google Scholar]
- 36.Ashkenazy H., Abadi S., Martz E., et al. ConSurf 2016: an improved methodology to estimate and visualize evolutionary conservation in macromolecules. Nucleic Acids Res. 2016;44:W344–W350. doi: 10.1093/nar/gkw408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ramazi S., Zahiri J. Post-translational modifications in proteins: resources, tools and prediction methods. Database. 2021;2021 doi: 10.1093/database/baab012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Blom N., Gammeltoft S., Brunak S. Sequence and structure-based prediction of eukaryotic protein phosphorylation sites. J. Mol. Biol. 1999;294:1351–1362. doi: 10.1006/jmbi.1999.3310. [DOI] [PubMed] [Google Scholar]
- 39.Xue Y., Zhou F., Zhu M., et al. GPS: a comprehensive www server for phosphorylation sites prediction. Nucleic Acids Res. 2005;33:W184–W187. doi: 10.1093/nar/gki393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pejaver V., Urresti J., Lugo-Martinez J., et al. Inferring the molecular and phenotypic impact of amino acid variants with MutPred2. Nat. Commun. 2020;11:5918. doi: 10.1038/s41467-020-19669-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Meyer M.J., Lapcevic R., Romero A.E., et al. mutation3D: cancer gene prediction through atomic clustering of coding variants in the structural proteome. Hum. Mutat. 2016;37:447–456. doi: 10.1002/humu.22963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Venselaar H., Te Beek T.A.H., Kuipers R.K.P., Hekkelman M.L., Vriend G. Protein structure analysis of mutations causing inheritable diseases. An e-Science approach with life scientist friendly interfaces. BMC Bioinf. 2010;11:548. doi: 10.1186/1471-2105-11-548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Venkata Subbiah H., Ramesh Babu P., Subbiah U. Determination of deleterious single-nucleotide polymorphisms of human LYZ C gene: an in silico study. J. Genet. Eng. Biotechnol. 2022;20(1):92. doi: 10.1186/s43141-022-00383-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Islam R., Rahaman M., Hoque H., et al. Computational and structural based approach to identify malignant nonsynonymous single nucleotide polymorphisms associated with CDK4 gene. PLoS One. 2021;16 doi: 10.1371/journal.pone.0259691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen E.Y., Tan C.M., Kou Y., et al. Enrichr: interactive and collaborative HTML5 gene list enrichment analysis tool. BMC Bioinf. 2013;14:1–14. doi: 10.1186/1471-2105-14-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kuleshov M.V., Jones M.R., Rouillard A.D., et al. Enrichr: a comprehensive gene set enrichment analysis web server 2016 update. Nucleic Acids Res. 2016;44:W90–W97. doi: 10.1093/nar/gkw377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tang D., Chen M., Huang X., et al. SRplot: a free online platform for data visualization and graphing. PLoS One. 2023;18 doi: 10.1371/journal.pone.0294236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Snel B., Lehmann G., Bork P., et al. STRING: a web-server to retrieve and display the repeatedly occurring neighbourhood of a gene. Nucleic Acids Res. 2000;28:3442–3444. doi: 10.1093/nar/28.18.3442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Szklarczyk D., Franceschini A., Wyder S etal. STRING v10: protein–protein interaction networks, integrated over the tree of life. Nucleic Acids Res. 2015;43:D447–D452. doi: 10.1093/nar/gku1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wu D., Wu Z. Superimposition of protein structures with dynamically weighted RMSD. J. Mol. Model. 2010;16:211–222. doi: 10.1007/s00894-009-0538-6. [DOI] [PubMed] [Google Scholar]
- 51.Pettersen E.F., Goddard T.D., Huang C.C., et al. UCSF Chimera—a visualization system for exploratory research and analysis. J. Comput. Chem. 2004;25:1605–1612. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
- 52.Eberhardt J., Santos-Martins D., Tillack A.F., et al. AutoDock Vina 1.2. 0: new docking methods, expanded force field, and python bindings. J. Chem. Inf. Model. 2021;61:3891–3898. doi: 10.1021/acs.jcim.1c00203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rose P.W., Prlić A., Altunkaya A., et al. The RCSB protein data bank: integrative view of protein, gene and 3D structural information. Nucleic Acids Res. 2016 doi: 10.1093/nar/gkw1000. gkw1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kelley L.A., Mezulis S., Yates C.M., et al. The Phyre2 web portal for protein modeling, prediction and analysis. Nat. Protoc. 2015;10:845–858. doi: 10.1038/nprot.2015.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Guex N., Peitsch M.C. SWISS‐MODEL and the Swiss‐Pdb Viewer: an environment for comparative protein modeling. Electrophoresis. 1997;18(15):2714–2723. doi: 10.1002/elps.1150181505. [DOI] [PubMed] [Google Scholar]
- 56.Gupta M.K., Vadde R. Insights into the structure–function relationship of both wild and mutant zinc transporter ZnT8 in human: a computational structural biology approach. J. Biomol. Struct. Dyn. 2019 doi: 10.1080/07391102.2019.1567391. [DOI] [PubMed] [Google Scholar]
- 57.Kim S., Chen J., Cheng T., et al. PubChem 2023 update. Nucleic Acids Res. 2023;51(D1):D1373–D1380. doi: 10.1093/nar/gkac956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Biovia D.S. Dassault Systèmes; San Diego: 2021. Discovery Studio Visualizer V21. 1.0. 20298. [Google Scholar]
- 59.Pradeepkiran J.A., Kumar K.K., Kumar Y.N., et al. Modeling, molecular dynamics, and docking assessment of transcription factor rho: a potential drug target in Brucella melitensis 16M. Drug Des Devel Ther. 2015;2015:1897–1912. doi: 10.2147/DDDT.S77020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pires D.E.V., Blundell T.L., Ascher D.B. pkCSM: predicting small-molecule pharmacokinetic and toxicity properties using graph-based signatures. J. Med. Chem. 2015;58(9):4066–4072. doi: 10.1021/acs.jmedchem.5b00104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chen X., Miao Z., Divate M., et al. KM-express: an integrated online patient survival and gene expression analysis tool for the identification and functional characterization of prognostic markers in breast and prostate cancers. Database. 2018;2018:bay069. doi: 10.1093/database/bay069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mashal S., Khanfar M., Al-Khalayfa S., et al. SLC30A8 gene polymorphism rs13266634 associated with increased risk for developing type 2 diabetes mellitus in Jordanian population. Gene. 2021;768 doi: 10.1016/j.gene.2020.145279. [DOI] [PubMed] [Google Scholar]
- 63.Huang Q., Yin J.Y., Dai X.P., et al. Association analysis of SLC30A8 rs13266634 and rs16889462 polymorphisms with type 2 diabetes mellitus and repaglinide response in Chinese patients. Eur. J. Clin. Pharmacol. 2010;66:1207–1215. doi: 10.1007/s00228-010-0882-6. [DOI] [PubMed] [Google Scholar]
- 64.Faghih H., Khatami S.R., Azarpira N., Foroughmand A.M. SLC30A8 gene polymorphism (rs13266634 C/T) and type 2 diabetes mellitus in south Iranian population. Mol. Biol. Rep. 2014;41:2709–2715. doi: 10.1007/s11033-014-3158-x. [DOI] [PubMed] [Google Scholar]
- 65.Wang Y., Duan L., Yu S., et al. Association between" solute carrier family 30 member 8"(SLC30A8) gene polymorphism and susceptibility to type 2 diabetes mellitus in Chinese Han and minority populations: an updated meta-analysis. Asia Pac. J. Clin. Nutr. 2018;27(6):1374–1390. doi: 10.6133/apjcn.201811_27(6).0025. [DOI] [PubMed] [Google Scholar]
- 66.Cheng I., Caberto C.P., Lum-Jones A., et al. Type 2 diabetes risk variants and colorectal cancer risk: the Multiethnic Cohort and PAGE studies. Gut. 2011;60(12):1703–1711. doi: 10.1136/gut.2011.237727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sainz J., Rudolph A., Hoffmeister M., et al. Effect of type 2 diabetes predisposing genetic variants on colorectal cancer risk. J. Clin. Endocrinol. 2012;97(5):E845–E851. doi: 10.1210/jc.2011-2565. [DOI] [PubMed] [Google Scholar]
- 68.Miller M.P., Kumar S. Understanding human disease mutations through the use of interspecific genetic variation. Hum. Mol. Genet. 2001;10(21):2319–2328. doi: 10.1093/hmg/10.21.2319. [DOI] [PubMed] [Google Scholar]
- 69.Vignal A., Milan D., SanCristobal M., et al. A review on SNP and other types of molecular markers and their use in animal genetics. Genet. Sel. Evol. 2002;34(3):275–305. doi: 10.1186/1297-9686-34-3-275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dakal T.C., Kala D., Dhiman G., et al. Predicting the functional consequences of non-synonymous single nucleotide polymorphisms in IL8 gene. Sci. Rep. 2017;7(1):6525. doi: 10.1038/s41598-017-06575-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mihalek I., Reš I., Lichtarge O. A family of evolution–entropy hybrid methods for ranking protein residues by importance. J. Mol. Biol. 2004;336(5):1265–1282. doi: 10.1016/j.jmb.2003.12.078. [DOI] [PubMed] [Google Scholar]
- 72.Sankararaman S., Kolaczkowski B., Sjölander K. INTREPID: a web server for prediction of functionally important residues by evolutionary analysis. Nucleic Acids Res. 2009;37(suppl_2):W390–W395. doi: 10.1093/nar/gkp339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Deller M.C., Kong L., Rupp B. Protein stability: a crystallographer's perspective. Acta Crystallogr Sect F, Struct Biol Commun. 2016;72(Pt 2):72–95. doi: 10.1107/S2053230X15024619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Singh S.M., Kongari N., Cabello-Villegas J., et al. Missense mutations in dystrophin that trigger muscular dystrophy decrease protein stability and lead to cross-β aggregates. Proc Natl Acad Sci. 2010;107(34):15069–15074. doi: 10.1073/pnas.1008818107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Witham S., Takano K., Schwartz C., et al. A missense mutation in CLIC2 associated with intellectual disability is predicted by in silico modeling to affect protein stability and dynamics. Proteins. 2011;79(8):2444–2454. doi: 10.1002/prot.23065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Saxena R., Voight B.F., Lyssenko V., et al. Genome-wide association analysis identifies loci for type 2 diabetes and triglyceride levels. Science. 2007;316(80):1331–1336. doi: 10.1126/science.1142358. 5829. [DOI] [PubMed] [Google Scholar]
- 77.Scott L.J., Mohlke K.L., Bonnycastle L.L., et al. A genome-wide association study of type 2 diabetes in Finns detects multiple susceptibility variants. Science (80-) 2007;316(5829):1341–1345. doi: 10.1126/science.1142382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Flannick J., Thorleifsson G., Beer N.L., et al. Loss-of-function mutations in SLC30A8 protect against type 2 diabetes. Nat. Genet. 2014;46(4):357–363. doi: 10.1038/ng.2915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sui L., Du Q., Romer A., et al. ZnT8 loss of function mutation increases resistance of human embryonic stem cell-derived beta cells to apoptosis in low zinc condition. Cells. 2023;12(6):903. doi: 10.3390/cells12060903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yang M., Liu X., Hou T., et al. Synthesis and luminescent properties of GdNbO4: Bi3+ phosphors via high temperature high pressure. J. Alloys Compd. 2017;723:1–8. doi: 10.1016/j.jallcom.2017.06.204. [DOI] [Google Scholar]
- 81.Walters W.P. Going further than Lipinski's rule in drug design. Expert Opin Drug Discov. 2012;7(2):99–107. doi: 10.1517/17460441.2012.648612. [DOI] [PubMed] [Google Scholar]
- 82.Al-Shabib N.A., Khan J.M., Malik A., et al. Molecular insight into binding behavior of polyphenol (rutin) with beta lactoglobulin: spectroscopic, molecular docking and MD simulation studies. J. Mol. Liq. 2018;269:511–520. doi: 10.1016/j.molliq.2018.07.122. [DOI] [Google Scholar]
- 83.Daina A., Michielin O., Zoete V. SwissADME: a free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017;7(1) doi: 10.1038/srep42717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hoda A., Lika M., Kolaneci V. Identification of deleterious nsSNPs in human HGF gene: in silico approach. J. Biomol. Struct. Dyn. 2023;41(21):11889–11903. doi: 10.1080/07391102.2022.2164060. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets supporting the conclusions of the study are included within the article and its additional file as a supplementary file. Correspondence and requests for materials should be addressed to Kawsar Ahmed and Md. Arju Hossain. All supporting data are available on a publicly available respiratory (Github) https://github.com/Md-Arju-Hossain/In-silico-Single-neucleotide-Polymorphisms.