Abstract
The elasmobranch population is declining in the Bay of Bengal of Bangladesh due to large-mesh gill net fishing, locally known as the Lakkha net, which primarily targets Indian threadfin (Leptomelanosoma indicum). This study was the first attempt to identify megafaunal bycatch in Lakkha fishing and assess its vulnerability using Productivity Susceptibility Analysis. A total of 40 elasmobranch bycatch species were identified, with sharks comprising 13 species from three families, while 27 rays belonged to six families, with the majority belonging to the Myliobatiformes order (60 %). Productivity and susceptibility scores were assigned to all identified species, with values ranging from 1.27 to 2.73 and 1.50 to 2.63, respectively. The target Lakkha fish exhibited the highest susceptibility score, followed by several pelagic sharks and eagle rays. Vulnerability assessment revealed that 31.7 % (n = 13) of species were highly vulnerable, while 43.9 % (n = 18) were classified as moderate, and 24.4 % (n = 10) were considered to have low vulnerability. All the high-risk megafauna species (n = 13) are classified as threatened by the global IUCN Red List. Sensitivity analysis highlighted susceptibility as a major contributor to species’ vulnerability. Alterations in susceptibility scores led to significant changes in the vulnerability status of many species. The overall data quality assessment indicated moderate data quality across species, with variability observed between productivity (76 % of species received a poor data quality score) and susceptibility attributes. However, vulnerability of these species can be reduced through adequate gear modification, shorter net deployment periods, adoption of safe discharge techniques, identification of critical habitats, and establishment of marine protected areas within this region. This study provides valuable insights into the species composition and vulnerability of elasmobranchs in the Lakkha gill net fishery, emphasizing the need for conservation measures to mitigate bycatch impacts on threatened species.
Keywords: Risk assessment, Productivity Susceptibility Analysis, Large meshed gill net, Megafauna, Bay of Bengal, Bangladesh
Highlights
-
•
Ecological risk assessment conducted on Elasmobranch bycatch in Bay of Bengal of Bangladesh
-
•
40 species of elasmobranch bycatch identified in Lakkha net (large mesh gill net)fishery
-
•
Vulnerability assessment shows that 31.7 % of species are highly vulnerable
-
•
76 % of bycatch stocks exibit poor data quality for productivity attributes
-
•
Immediate conservation measures required to reduce the impact of Lakkha net fishing
1. Introduction
Elasmobranchs (Sharks, rays, and skates) are opportunistic predators that play a significant role in marine ecosystems. They constitute one of the oldest and most ecologically diverse vertebrate lineages, occupying the upper tier of the aquatic food web [1,2]. In the northeastern part of the Indian Ocean lies the Bay of Bengal, a vibrant ecosystem that shelters a fascinating array of elasmobranch species, with a total of 111 species taxonomically validated from the Bay of Bengal of Bangladesh [3]. However, the precipitous decline of elasmobranch populations has raised the utmost concern among researchers and conservationists around the world [4]. Their population is significantly influenced by human-induced environmental changes. For instance, excessive fishing has led to adverse ecological impacts on elasmobranchs [5,6]. Generally, elasmobranchs and other marine mammals (e.g., Cetaceans, Sea turtles, Seabirds, etc.) are result of incidental bycatch in commercial or recreational fishing activities [[7], [8], [9], [10]].
Similarly, in Bangladesh, sharks and rays are not specifically targeted species; rather, they are incidental bycatch of artisanal and commercial fishing of Hilsa (Tenualosa ilisha) and other species. However, a specialized gillnet called the “Shark net” (mesh: 400–520 mm) is also used for shark fishing in the Bay of Bengal region of Bangladesh [11]. While it holds economic value in the local market, the international fin trade has become a more dependable source for commercial fishing. Moreover, the absence of statistical data regarding potential stocks, home range, distribution range, and natural habitat has contributed to the overexploitation of many elasmobranch species in this region. Consequently, the inaccuracies in stock assessment have resulted in management decisions that are often unrealistic or inadequate [12]. Therefore, ecological risk assessments, gear specificity, and taxonomic identification are prerequisites for shielding and implementing effective management actions.
For effective fisheries management, ecological risk assessment is a fundamental precondition, which focuses on sustainability and establishes harmony between ecology and human welfare. Productivity Susceptibility Analysis (PSA), a semi-quantitative method of ecological risk assessment, has become a reliable method to deal with complex bycatch assemblage in fishery due to its adaptability and fewer data requirements. This method evaluates the relative vulnerability when data on fishing activity or biological characteristics are insufficient [13,14]. This method also incorporates stakeholders' and fishers' knowledge as well as economic aspects of the fishery or stocks into the assessment and decision-making processes. Thus, PSA has been utilized for a number of years to evaluate data-limited fisheries resources in order to advocate the degree of threats as well as aid in sustainable management. This method has been used for many fisheries risk analyses with some regional adjustment in attribute's scoring criteria, for instance, otter trawl fishery in southeastern Australia [15,16], small-scale artisanal fisheries in Baja California, Mexico [17], tuna purse-seine and longline fisheries in the Atlantic [18], Pacific [5] and Indian Ocean [18,19] to estimate potential relative risk caused by anthropogenic stressors [[20], [21], [22], [23]]. Bangladesh contributes significantly to the elasmobranch bycatch each year due to its choice of fishing practices and unregulated management regime. Multiple fishing gear (e.g., gill net, set-bag net, trammel net, longline) operates in this region, predominantly unselective or less selective in nature, generating a significant number of bycatches in Bangladesh. Since limited information is available about the gear-specific species composition, it may put marine baycatch species at greater risk of overfishing. In addition, the majority of fisheries regulations primarily prioritize the protection of the Hilsa population, resulting in other species receiving less protection [24]. Moreover, in Bangladesh, no former risk assessment was carried out addressing the impact of unregulated fishing on the globally threatened elasmobranch species. Therefore, for the first time, our research was designed to identify the Lakkha net-specific species composition of elasmobranchs in the Bay of Bengal of Bangladesh. The second aim was to assess the gear-specific relative risk of the identified species using PSA. Finally, identify the data gaps in the life history attributes of sharks and rays, and recommend some guidelines to improve the vulnerability of the elasmobranchs for their long-term sustainability.
2. Materials and methods
2.1. Study site and gear selection
Bangladesh, a sovereign coastal state, is situated in the northern littoral region of the Bay of Bengal, a shallow embayment of the Indian Ocean. The Bay of Bengal is the sole adjacent marine ecosystem that supports the Bangladesh's marine fishery, where the country has obtained legal rights to a total of 118,813 km2 to this region [25]. Bangladesh has several fish landing sites across the 19 coastal districts adjacent to the Bay of Bengal. In this study, we examined various governmental reports, and literature to identify the potential zones associated with landing and trading of elasmobranch species. Two districts of Bangladesh, Chattogram and Cox's Bazar, were selected as the sites for data collection, as they are the major contributors to elasmobranch [11] production in Bangladesh as shown in Fig. 1. We have selected a total of seven sampling points, two of which were located along the bank of the Karnafuli river in Chattogram district. The first station, S1, is New Fishery Ghat (Lat: 22.32863; Long: 91.84800), and the second station, S2, is the Old Fish Market (Lat: 22.32931; Long: 91.84373). The other five station–S3 (BFDC Fishery Ghat; Lat: 21.45213, Long: 91.96813), S4 (Najeertek; Lat: 21.46507, Long: 91.95038), S5 (Chowfaldandi; Lat: 21.51352, Long: 92.01188), S6 (Nuniachara; Lat: 21.47814, Long: 91.97005), and S7 (Moheskhali; Lat: 21.51832, Long: 91.97073)–were situated along the coasts in Cox's Bazar district.
Fig. 1.
Maps showing the survey points of this study. The map on the left displays the coastal zones of Bangladesh,
while the map on the right illustrates the seven sampling points in the two coastal districts, Chattogram and Cox's Bazar, of Bangladesh. (S1– New Fishery Ghat, S2–Old Fish Market, S3–BFDC Fishery Ghat, S4–Najeertek, S5–Chowfaldandi, S6–Nuniachara, S7–Moheskhali).
In the Bay of Bengal region, local fishers operate a considerable variety of fishing gear to target multiple species, especially Hilsa, Lakkha (Common Name: Indian threadfin; Leptomelanosoma indicum), Pomfret (e.g., Pampus argenteus, Pampus chinensis), etc. [26]. Among them, Large Mesh Drift Gill Net (LDGN) stand out as one of the predominant types of net in the area. This group consists of a wide variety of nets that differ in terms of mesh size, depth, length, and the specific species they target. The Lakkha net, named after its primary target fish, Lakkha, is a commonly used LDGN. Although its main purpose is to catch Lakkha, it has been observed to incidentally capture various elasmobranch species. The Lakkha net, made of 60-inch cotton twine, is 5000 m long and 12 m wide. Its mesh size ranges from 300 to 400 mm, and it is mainly deployed in the open sea for around 5–6 h before being hauled back [26]. However, limited scientific research has been conducted on the impacts of the Lakkha fishery despite its significant contribution to the megafauna bycatch in the region. Therefore, we conducted an ecological risk assessment of the Lakkha fishing in this study. The field study was conducted for two years, from February 2021 to January 2023.
2.2. Identification of elasmobranch bycatch from Lakkha net
In Bangladesh, data on gear-specific species composition and their abundance in a particular habitat are often limited. This situation is exacerbated for elasmobranch species, as many local fishing communities are unaware of the morphological differences among the species. To overcome the challenges of species identification, we employed a series of techniques. First, we compiled a list of elasmobranch species with regional importance by analyzing the data from previous catch analysis, market assessment, and commercial importance as documented in various literature [3,11,[27], [28], [29], [30]]. We then created a fish photo album containing all listed elasmobranch species, along with their local names, common names, scientific names, and identifying characteristics, accompanied by photographs. Subsequently, we conducted face-to-face interviews with local Lakkha fishers to identify elasmobranch species captured in that region throughout the year, utilizing the lists and photographs from the album. The number of Lakkha fishers interviewed (210 in total; 30 from each sampling point) was determined based on the principle outlined by Ref. [31]. We also asked the fishers to report any additional species they might come across during fishing trips or had previously landed but were not included in the current photo album. Once we collected the preliminary data from the locals, we summarized the identified species and formed a secondary sorted list for further validation. Then, we conducted field surveys and closely observed the landed catch to understand the composition of the elasmobranch bycatch associated with the Lakkha fishing net. We scientifically identified species by following the established taxonomic keys published in the literature [[32], [33], [34], [35], [36]]. We continued our surveys for six to seven consecutive days per month at each station over the course of two years. Subsequently, species that were not observed in the landed catch during the survey period were excluded from the study. Moreover, none of the fishers reported catching any species that were not listed in the photo album. Finally, all scientific names of bycatch species were validated using FishBase [37], and Eschmeyer's Catalog of Fishes [38].
2.3. Focus group discussion
The focus group discussion (FGD) is an interactive and efficient method for obtaining information on a specific topic. FGDs are commonly used to gather qualitative and quantitative information in field-based research [39,40]. We formed focus groups with 10 participants each, representing one-third of the interviewed fishers at each sampling station [24]. We utilized purposive sampling, also known as selective or judgmental sampling, to select experienced Lakkha fishers with a minimum of ten years of fishing experience. This approach aimed to ensure that the groups could provide more insightful information [41]. We asked them about seasonal availability, gear-specific catch trend, abundance, size of the catch, and previous historic catches of elasmobranch in that area. Additionally, we collected information regarding the fishing area, depth of gear operation, gear selectivity, fishing persistency, gear availability, and mesh composition, as these factors resemble the vertical and horizontal overlap between fish and gear. We also obtained information on market demand (local and international), market structure, and the degree of enforcement of laws on capture and trades of elasmobranch from focus group discussion. This information was used to assign susceptibility scores, that includes survival after capture and release, aggregation and migratory behaviors, vertical overlap of species, management strategy, and market demand. It also supported few arguments within the discussion section. The focus group discussions lasted for three to four hours at each sampling station.
2.4. Selection of productivity and susceptibility attributes and data sources
In this study, eleven productivity (P) attributes (i.e., species biological characteristics which determine the intrinsic rate of growth) (Table 1) and eight susceptibility (S) attributes (i.e., species mortality due to fishing activity imposed by different types of fishing gear) (Table 2) were considered for analyzing the relative vulnerability of the species caught in the Lakkha net fishery. This method is widely preferred to estimate potential threats to a species when empirical data is limited [16]. The attributes of both productivity and susceptibility were selected based on the availability of information and their representativeness in the risk analysis [42]. The definitions of these attributes are presented in the supplementary section (see Table A.1 and A.2).
Table 1.
Scoring thresholds for selected attributes to estimate the productivity of species.
| Productivity Attributes | High Productivity (Low risk, score = 3) | Moderate Productivity (Moderate risk, score = 2) | Low Productivity (High risk, score = 1) |
|---|---|---|---|
| Maximum size (Lmax, cm) | <97 | 97–222 | >222 |
| Maximum age (tmax, year) | <15 | 15–25 | >25 |
| von Bertalanffy growth coefficient (K, yr−1) | >0.20 | 0.13–0.20 | <0.13 |
| Estimated natural mortality (M, yr−1) | >0.47 | 0.30–0.47 | <0.30 |
| Measured fecundity (MF) | >5 | 3–5 | <3 |
| Breeding strategy (BS) | Egg layer | Live birth unattended | Live birth and care |
| Size at first maturity (Lmat, cm) | <55 | 55–123 | >123 |
| Mean trophic level (MTL) | <3.6 | 3.6–3.9 | >3.9 |
| Age at first maturity (tmat, year) | <4.67 | 4.67–6.5 | >6.5 |
| Lmat/Lmax | <0.53 | 0.53–0.68 | >0.68 |
| tmat/tmax | <0.25 | 0.25–0.34 | >0.34 |
Table 2.
Scoring thresholds for selected attributes to estimate susceptibility of species.
| Susceptibility Attributes | High Susceptibility High risk (3) |
Moderate Susceptibility Moderate risk (2) |
Low Susceptibility Low risk (1) |
|---|---|---|---|
| Availability (distribution; Av) | Mostly in the country/fishery | Limited range in the region | Throughout the region/global |
| Encounterability (vertical overlap of species and fishing gear; E) | High vertical overlap with fishing gear (>50 % of species depth range) | Moderate vertical overlap with fishing gear (25–50 % of species depth range) | Low vertical overlap with fishing gear (<25 % of species depth range) |
| Schooling/Aggregation and other behavioral responses (SABR) | Species or stocks show large aggregation and/or affecting catchability of the gear | Normally found in loose aggregation and/or has distinct pairing between male and female | Solitary species and/or does not affect catchability of the gear |
| Management Strategy (MSt) | Catch and trades are not regulated by laws/act/rules | Catch and trades are regulated by laws/act/rules and compliance of regulation moderate | Catch and trades are regulated by laws/act/rules and compliance of regulation is widespread |
| Survival after capture and release (SCR) | Often retained or has value as a by-product species, or majority dead when released | Likely to be released alive | Evidence of post-capture release and survival |
| Market Demand (MD) | High | Medium | Low |
| Gear Selectivity (GS) | Species is a by-product, has similar physical and life history traits to targeted species, or has traits that attract it to the fishing gear | Species has some similarities to targeted species, but there is evidence it sometimes escapes | Species is not physically similar to targeted species (e.g. much larger or much smaller, different foraging ecology, habitat use) or there is evidence species can escape the gear |
| Price category (TK)/kg (PC); 1 TK = 0.0091USD | >250 | 150–250 | <150 |
From the selected life history attributes (P), namely maximum size, maximum age, von Bertalanffy growth coefficient, estimated natural mortality, measured fecundity, breeding strategy, mean trophic level, and age at maturity were chosen based on [42]. These attributes are widely recognized by researchers for their strong correlation with life history characteristics [43]. Additionally, we included other traits such as size at first maturity [16], maturity size ratio, and age ratio [44], which have been found influential in determining species productivity.
Species-specific biological data for each species were compiled using a hierarchical method. First, life-history data were gathered from relevant published articles, books, reports, and online libraries (e.g., FishBase, IUCN; Table A.4). Priority was given to species-specific information based on its regional availability. When specific information was lacking, life-history data were collected from various sources, including neighboring countries (i.e., India, Myanmar, etc.), the broader Indian subcontinent, or international committee operating in the Indian Ocean (i.e., IOTTC), or globally when necessary. Close relatives within the same genus were also considered as proxies, following the protocols described in Ref. [16], to overcome limitations. In addition, we employed an empirical approach to estimate the productivity values, as there is a strong relationship among the life history traits, as proposed by Ref. [45]. For instance, empirical equation such as tmax = 3/k (where k is the von Bertalanffy growth parameter and tmax is maximum age), Lmat = L∞10 (0.8979–0.0782T) (where L∞ is the asymptotic maximum length, T is the water temperature and Lmat is length at maturity), tmat = -Loge(1 - Lmat/L∞)/k (tmat is age at maturity) and M = 0.985 L∞ −0.279k0.6543T0.4634 (M is natural mortality at a temperature of 28 °C) were used to calculate the necessary values [24,46,47]. However, the estimated data we used for scoring did not violate the further calling for data collection as in data quality scoring, we used to score 4 (i.e., very limited data) for data derived from empirical equations.
Each of the productivity attributes was ranked on an ordinal scale of 1–3, where “1” symbolized low productivity, “2” represented moderate productivity, and “3” indicated high productivity. The scoring thresholds were determined by dividing the attribute values into 1/3 and 2/3 quantiles [48,49], ensuring equal probabilities for each range (see Table 1, Table A.4). The scoring thresholds for “breeding strategy” were retained from Ref. [50], where species with egg-laying capabilities were assumed to be at low risk (value 1) because of their high productivity nature. Conversely, live bearer was given a low productivity score (value 3). Finally, the average productivity score was calculated for each species.
Susceptibility is an indicator of the impact that fishing activities have on species. Eight susceptibility attributes were selected based on available data. Each scoring criterion for susceptibility attributes, such as “availability” [51]; “encounterability’’ and “gear selectivity’’ [50]; “survival after capture and release”, and “schooling/aggregation and other behavioral responses” [52], was drawn from different published articles due to its applicability with elasmobranch species. Attributes and scoring criteria for “Market Demand”, and “Price Category’’ were retained from Ref. [24]. However, in our study, we developed specific criteria for the “Management Strategy” that align with regional practices aimed at protecting a particular species. Species whose catch and trades are protected by law and act were assumed to be less susceptible to fishing. Conversely, species with limited catch regulation were assumed to be highly susceptible to fishing. Like productivity, susceptibility attributes were also scored on a scale of 1–3, with 1 representing low, 2 indicating moderate, and 3 indicating high susceptibility (see Table 2; Table A.5). The cutoff values for the “price category” were determined using the quantile method, providing equal probabilities to each range. Finally, average susceptibility was calculated for each species, and all the selected attributes were given the equal weighting of 2 as proposed by Ref. [42].
2.5. Vulnerability analysis
Vulnerability is a measure that indicates species' regeneration capacity when exposed to higher fishing mortality. Vulnerability for all species was quantified by incorporating the weighted average values of both productivity and susceptibility on a two-dimensional x-y scatter plot using the equation: [42]
In the scatter plot, the X-axis was assigned the weighted average productivity score (P-value) from High (3) to Low (1) value, and the Y-axis was marked with a weighted average susceptibility score (S-value) from Low (1) to High (3) value. The vulnerability score was classified into three categories (where low <1.32, moderate 1.32 ≤ V < 1.70, and V ≥ 1.70) following the quantile approach [53].
2.6. Sensitivity analysis of productivity and susceptibility attributes
After vulnerability analysis through PSA, a sensitivity analysis was conducted on the productivity and susceptibility attributes for all 41 identified species. This was done to determine the level of contribution of each attribute to the vulnerability score of the species. This highlights the influential potentiality of attributes in a scoring system. Each attribute was removed in turn, and Root Mean Squared Error (RMSE) was calculated for all 19 attributes [54].
Where, j is different attribute, i denotes species, represent vulnerability after removal of attribute j, is the relative error between vulnerability scores [47]. A higher RMSE value indicates greater impacts of attributes on the vulnerability score of species, while a lower value indicates less influence on species vulnerability. Furthermore, we determined vulnerability changes in each species by altering average susceptibility by ± 5 %, ±10 %, ±15 % and ±20 %. Then, for each of these eight scenarios, we compared changes in the species' vulnerability category.
2.7. Data quality analysis
Data quality analysis is a key factor in scoring attributes for comprehensive PSA analysis. This technique assists in identifying species with limited data (uncertainty estimation) and suggests strategies to enhance data collection for those species. Therefore, each PSA attribute was assigned a data quality score (DQ) using an ordinal scale of 5 tiers (1–5), ranging from best data (1) to no data (5). Table 3 provides a comprehensive overview of data quality. The overall data quality scores were determined by calculating weighted means of individual productivity and susceptibility scores. The data was further classified into high (DQ < 2.0), moderate (2.0 ≤ DQ < 3.0), and low quality (DQ ≥ 3.0) followed by Patrick et al. (2010) and Ormseth and Spencer (2011) [42,43].
Table 3.
Data quality tiers used in Productivity Susceptibility Analysis (PSA) [42].
| DQ Scores | DQ | Description | Example |
|---|---|---|---|
| 1 | Best | Information is based on collected data for the stock and area of interest that is established and substantial | Data-rich stock assessment; published literature for which multiple methods are used, etc. |
| 2 | Adequate | Information is based on limited coverage and corroboration, or for some other reason is deemed not as reliable as tier-1 data | Limited temporal or spatial data, relatively old information, etc. |
| 3 | Limited | Estimates with high variation and limited confidence, and which may be based on studies of similar taxa or life history strategies | Similar genus or family, etc. |
| 4 | Very limited | Information based on expert opinion or general literature reviews from a wide range of species, or from outside of the region, or data derived by equations using the correlated life history parameter | General data not referenced |
| 5 | No data | No information |
2.8. Species vulnerability comparison with other assessments
Since PSA has credibility concerns among stakeholders due to its semi-quantitative nature [16], we conducted a vulnerability comparison with other assessments to gain a deeper understanding of species' relative risk. According to Osio et al. (2015) [55], credibility concerns regarding species' relative risk can be resolved through comparative analysis for fish species. Therefore, we compared our findings with the IUCN Red List (IUCN, 2024) status of species. The IUCN Red List determines the threat status of species by assessing the relative potential ecological risk of extinction, considering both qualitative and quantitative information. In this study, we considered global assessment categories, since national or regional threat assessments were unavailable for the majority of elasmobranch bycatch species. The study compared the findings of PSA risk status with the IUCN Red List Categories (Critically Endangered (CR), Endangered (EN), Vulnerable (VU), Near Threatened (NT), Least Concern (LC), Data Deficient (DD), and Not Evaluated (NE)) to examine the potential relationship [16,24,50,55,56]. This implies that species identified as high risk by PSA were anticipated to be categorized as threatened by the IUCN Red List.
3. Results
3.1. Species composition
The Lakkha gill net fishery is a significant source of elasmobranch bycatch in the Bay of Bengal region of Bangladesh. A total of 40 elasmobranch species were identified to interact with the Lakkha fishing net throughout the region. The identified species showed a diverse range of groups, with representation from four different orders (Carcharhiniformes, Myliobatiformes, Orectolobiformes, and Rhinopristiformes) and nine families. Sharks bycatch comprised 13 species belonging to three different families (Carcharhinidae, Sphyrnidae, and Hemiscyllidae) whereas, rays were from six families (Dasyatidae, Glaucostegidae, Gymnuridae, Mobulidae, Rhinidae, and Rhinopteridae) comprising 27 species (Fig. 2; Table A.3). The majority of the bycatch species identified belonged to the myliobatiformes order (60 %, n = 24), followed by carcharhiniformes order (30 %, n = 12), rhinopristiformes order (8 %, n = 3), and finally the orectolobiformes order (2 %, n = 1) (Fig. 2). Most interacted shark species observed were the Scalloped hammerhead shark (Sphyrna lewini), Spottail shark (Carcharhinus sorrah), Spadenose shark (Scoliodon laticaudus), Tiger shark (Galeocerdo cuvier), Pigeye shark (Carcharhinus amboinensis) and ray species including Bleeker's whipray (Pateobatis bleekeri), Long-tailed butterflyray (Gymnura poecilura), Honeycomb whipray (Himantura undulata), and Coach whipray (Himantura uarnak) (see Table A.3).
Fig. 2.
Composition of identified elasmobranch species by order and family.
The diagram displays four orders (A = Rhinopristiformes, n = 3; B= Carcharhiniformes, n = 12; C= Orectolobiformes, n = 1; D = Myliobatiformes, n = 24). Each order is classified into respective families [e.g., Rhinoprsitiformes (n = 3) = Rhinidae (n = 1) + Glaucostegidae (n = 2); Carcharhiniformes = Carcharhinidae (n = 10) + Sphyrnidae (n = 2)].
3.2. Species vulnerability
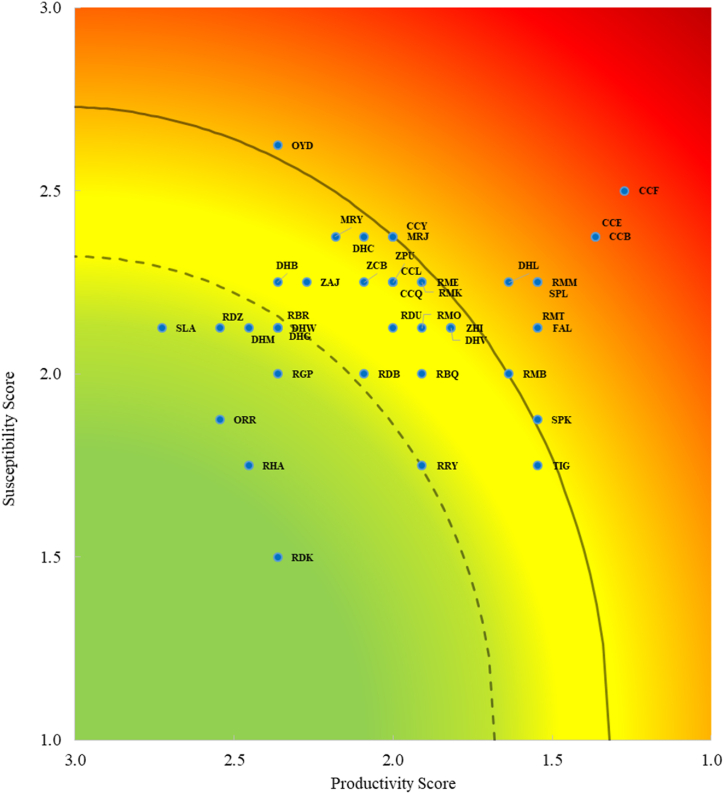
The target and the 40 identified bycatch species were assigned productivity and susceptibility scores, as presented in Table 4. The productivity and susceptibility scores ranged from 1.27 to 2.73 and 1.50 to 2.63, respectively (Table 4). The highest susceptibility value was received by the target Lakkha fish (Leptomelanosoma indicum; S = 2.63) followed by several pelagic sharks and eagle rays. Conversely, the Pigeye shark (Carcharhinus amboinensis), a large pelagic shark, received the lowest productivity score of 1.27 (Table 4).
Table 4.
PSA results of elasmobranch bycatch species of the Lakkha net fishery including the target Lakkha fish in the Bay of Bengal of Bangladesh.
| Scientific Name | Common Name | FAO code | P | S | V | VC | DQSP | DQCP | DQSS | DQCS | IUCN |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Leptomelanosoma indicum | Indian threadfin | OYD | 2.36 | 2.63 | 1.75 | H | 3.45 | L | 2.38 | M | NE |
| Carcharhinus amblyrhynchoides | Graceful shark | CCY | 2.00 | 2.38 | 1.70 | H | 2.91 | M | 2.25 | M | VU |
| Carcharhinus amboinensis | Pigeye shark | CCF | 1.27 | 2.50 | 2.29 | H | 3.18 | L | 2.25 | M | VU |
| Carcharhinus brevipinna | Spinner shark | CCB | 1.36 | 2.38 | 2.14 | H | 3.18 | L | 2.25 | M | VU |
| Carcharhinus falciformis | Silky shark | FAL | 1.55 | 2.13 | 1.84 | H | 3.36 | L | 2.00 | M | VU |
| Carcharhinus leucas | Bull shark | CCE | 1.36 | 2.38 | 2.14 | H | 2.82 | M | 2.25 | M | VU |
| Carcharhinus limbatus | Blacktip shark | CCL | 2.00 | 2.25 | 1.60 | M | 3.09 | L | 2.25 | M | VU |
| Carcharhinus sorrah | Spot-tail shark | CCQ | 2.00 | 2.25 | 1.60 | M | 2.82 | M | 2.00 | M | NT |
| Galeocerdo cuvier | Tiger shark | TIG | 1.55 | 1.75 | 1.64 | M | 2.91 | M | 2.13 | M | NT |
| Rhizoprionodon acutus | Milk shark | RHA | 2.45 | 1.75 | 0.93 | L | 2.73 | M | 2.00 | M | VU |
| Scoliodon laticaudus | Spadnose shark | SLA | 2.73 | 2.13 | 1.16 | L | 2.82 | M | 2.13 | M | NT |
| Sphyrna lewini | Scalloped hammerhead shark | SPL | 1.55 | 2.25 | 1.92 | H | 2.73 | M | 2.00 | M | CR |
| Sphyrna mokarran | Great hammerhead shark | SPK | 1.55 | 1.88 | 1.71 | H | 3.09 | L | 2.25 | M | CR |
| Chiloscyllium griseum | Grey bamboo shark | ORR | 2.55 | 1.88 | 0.99 | L | 3.27 | L | 2.38 | M | VU |
| Brevitrygon imbricata | Bengal whipray | DHM | 2.45 | 2.13 | 1.25 | L | 3.45 | L | 2.00 | M | VU |
| Brevitrygon walga | Scaly whipray | DHW | 2.36 | 2.13 | 1.29 | L | 3.00 | L | 2.25 | M | NT |
| Hemitrygon bennettii | Bennett's stingray | RDB | 2.09 | 2.00 | 1.35 | M | 3.36 | L | 2.25 | M | VU |
| Hemitrygon fluviorum | Estuary stingray | ZHI | 1.82 | 2.13 | 1.63 | M | 3.36 | L | 2.25 | M | NT |
| Himantura uarnak | Coach whipray | DHV | 1.82 | 2.13 | 1.63 | M | 2.91 | M | 2.00 | M | EN |
| Himantura undulata | Honeycomb whipray | DHL | 1.64 | 2.25 | 1.85 | H | 2.82 | M | 2.13 | M | EN |
| Hypanus guttatus | Longnose stingray | RDU | 2.00 | 2.13 | 1.51 | M | 3.27 | L | 2.25 | M | NT |
| Maculabatis bineeshi | Short-tailed whipray | ZAJ | 2.27 | 2.25 | 1.45 | M | 3.36 | L | 2.25 | M | CR |
| Maculabatis gerrardi | White-spotted whipray | DHG | 2.36 | 2.13 | 1.29 | L | 3.36 | L | 2.25 | M | EN |
| Maculabatis pastinacoides | Round whipray | ZCB | 2.09 | 2.25 | 1.55 | M | 3.09 | L | 2.25 | M | EN |
| Neotrygon kuhlii | Blue-spotted stingray | RDK | 2.36 | 1.50 | 0.81 | L | 2.91 | M | 2.13 | M | DD |
| Pateobatis bleekeri | Bleeker's whipray | DHB | 2.36 | 2.25 | 1.40 | M | 3.09 | L | 2.25 | M | EN |
| Pateobatis uarnacoides | Whitenose whipray | ZPU | 2.00 | 2.25 | 1.60 | M | 3.09 | L | 2.25 | M | EN |
| Telatrygon zugei | Pale-edged stingray | RDZ | 2.55 | 2.13 | 1.21 | L | 3.09 | L | 2.00 | M | VU |
| Urogymnus polylepis | Giant freshwater whipray | DHC | 2.09 | 2.38 | 1.65 | M | 3.00 | L | 2.25 | M | EN |
| Gymnura poecilura | Long-tailed butterflyray | RGP | 2.36 | 2.00 | 1.19 | L | 3.27 | L | 2.25 | M | VU |
| Mobula birostris | Giant mantaray | RMB | 1.64 | 2.00 | 1.70 | H | 3.00 | L | 2.25 | M | EN |
| Mobula eregoodoo | Longhorned pygmy devilray | RME | 1.91 | 2.25 | 1.66 | M | 3.27 | L | 2.13 | M | EN |
| Mobula kuhlii | Shortfin devilray | RMK | 1.91 | 2.25 | 1.66 | M | 3.45 | L | 2.25 | M | EN |
| Mobula mobular | Spinetail devilray | RMM | 1.55 | 2.25 | 1.92 | H | 3.18 | L | 2.13 | M | EN |
| Mobula tarapacana | Sicklefin devilray | RMT | 1.55 | 2.13 | 1.84 | H | 3.36 | L | 2.13 | M | EN |
| Mobula thurstoni | Bentfin devilray | RMO | 1.91 | 2.13 | 1.57 | M | 3.27 | L | 2.25 | M | EN |
| Rhinoptera javanica | Javanese cownoseray | MRJ | 2.00 | 2.38 | 1.70 | H | 2.73 | M | 2.13 | M | EN |
| Rhinoptera jayakari | Oman cownoseray | MRY | 2.18 | 2.38 | 1.60 | M | 3.45 | L | 2.25 | M | EN |
| Glaucostegus granulatus | Sharpnose guitarfish | RBR | 2.36 | 2.13 | 1.29 | L | 3.00 | L | 2.25 | M | CR |
| Glaucostegus typus | Giant shovelnoseray | RBQ | 1.91 | 2.00 | 1.48 | M | 3.09 | L | 2.38 | M | CR |
| Rhina ancylostomus | Bowmouth guiterfish | RRY | 1.91 | 1.75 | 1.32 | M | 3.36 | L | 2.38 | M | CR |
P = weighted average productivity scores; S = weighted average susceptibility scores; V = vulnerability score; VC = vulnerability categories (low (L): V < 1.32, moderate (M): 1.32 ≤ V < 1.70, and high (H): V ≥ 1.70); DQSP = weighted average data quality scores of productivity; DQCP = data quality categories for productivity attributes (high (H): DQ < 2, moderate (M): 2 ≤ DQ < 3, and low (L): DQ ≥ 3); DQSS = weighted average data quality scores of susceptibility; DQCS = data quality categories for susceptibility attributes; IUCN is the Red List indicating towards extinction risk of species.
The X–Y scatter plot (Fig. 3) displays the results of the PSA analysis, showing three tiers that visualize the relative risk experienced by bycatch species from the target fishery. Vulnerability scores ranged between 0.81 and 2.29, with the Blue-spotted stingray (Neotrygon kuhlii) receiving the lowest score (V = 0.81), while the Pigeye shark (Carcharhinus amboinensis) received the highest score (V = 2.29). Among the evaluated species, 31.7 % (n = 13) were identified as highly vulnerable, followed by 43.9 % (n = 18) as moderately vulnerable, and 24.4 % (n = 10) as low vulnerable species. Out of the 41 evaluated species, alongside the targeted Indian threadfin fish (Leptomelanosoma indicum), seven species of sharks—Graceful shark (Carcharhinus amblyrhynchoides), Pigeye shark (Carcharhinus amboinensis), Spinner shark (Carcharhinus brevipinna), Silky shark (Carcharhinus falciformes), Bull shark (Carcharhinus leucas), Scalloped hammerhead shark (Sphyrna lewini), Great hammerhead shark (Sphyrna mokarran), and five species of rays—Honeycomb whipray (Himantura undulate), Giant devilray (Mobula mobular), Sicklefin devilray (Mobula tarapacana), Javanese cownoseray (Rhinoptera javanica) and Gaint mantaray (Mobula birostris)—obtained higher cut-off score (Table 4; Fig. 3). Eight species at high risk exceeded the vulnerability score of the targeted Lakkha fish. Among the moderately vulnerable species, scores of Mobula eregoodoo (V = 1.66), Mobula kuhlii (V = 1.66), Urogymnus polylepis (V = 1.65), and Galeocerdo cuvier (V = 1.64) were close to the cut-off value of high risk, indicating a probability of high risk in the near future due to continuous fishing activity. Furthermore, three small-sized sharks and seven smaller Dasyatidae rays were ranked in the lower-risk category according to the PSA.
Fig. 3.
Two-dimensional PSA plot for target species (Lakkha, OYD) and elasmobranch bycatch species.
The contour line shows the vulnerability values (V) of 1.32 and 1.70, where vulnerability categories are defined as low (V < 1.32), moderate (1.32 ≤ V < 1.70), and high (V ≥ 1.70). The scientific and common names associated with species identification codes (FAO codes) are provided in Table 4.
3.3. Vulnerability comparison with IUCN red list category
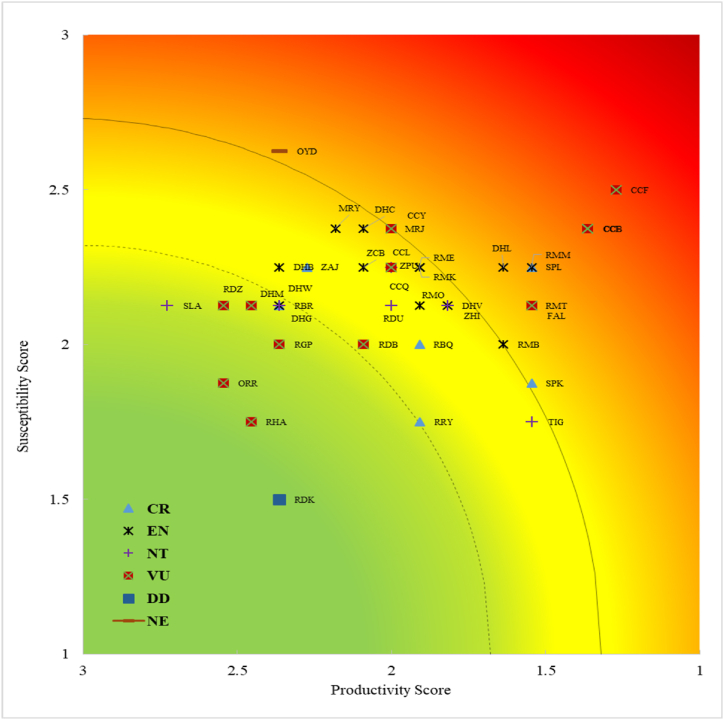
According to IUCN Red List categories, 37.5 % (n = 15) of the 40 elasmobranch bycatch species were categorized as “Endangered,” with 30 % (n = 12) labeled as “Vulnerable”, 15 % (n = 6) as “Critically Endangered” and the remainder falling into the “Near threatened” category (Fig. 4a). Additionally, species within the families Rhinidae, Glaucostegidae, and Sphyrnidae were marked as Critically Endangered, while those in the Mobulidae and Rhinopteridae families were classified as Endangered by the IUCN (Fig. 4b). On the other hand, the majority of the identified sharks were found to be threatened on a global scale. Fig. 5 shows the comparative analysis of PSAs and the global IUCN Red List status of all identified species.
Fig. 4.
Distribution of elasmobranch species across global IUCN Red List categories: (a) percentage (%) distribution and (b) distribution by families of species
(DD = Data Deficient, NT = Near Threatened, VU = Vulnerable, EN = Endangered, and CR = Critically Endangered). (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
Fig. 5.
Comparative analysis of global IUCN Red List status with species vulnerability to Lakkha fishery.
Different marker represents different IUCN Red List categories (DD = Data Deficient, NT = Near Threatened, VU = Vulnerable, EN = Endangered, and CR = Critically Endangered). (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
The five requiem sharks—Carcharhinus amboinensis (V = 2.29), Carcharhinus brevipinna (V = 2.14), Carcharhinus leucas (V = 2.14), Carcharhinus falciformis (V = 1.84) and Carcharhinus amblyrhynchoides (V = 1.70)—identified as high-risk species by PSA, were classified as threatened (Vulnerable) on the global IUCN Red List. The hammerhead sharks—Sphyrna lewini and Sphyrna mokarran—were also in the threatened (Critically Endangered) category by IUCN and received high risk in our analysis. However, species from rhinopristiformes order (Glaucostegus granulatus (V = 1.29), Glaucostegus typus (V = 1.48), and Rhina ancylostomus (V = 1.32) were classified as Critically Endangered (CR) on the global IUCN Red List, whereas our study evaluated them as low to moderately vulnerable species based on PSA (Fig. 5).
Furthermore, 15 of the ray species we identified were assessed to be “Endangered” by IUCN. Among them, five species (Himantura undulata, Mobula mobular, Mobula tarapacana, Mobula birostris, and Rhinoptera javanica) were ranked as high-risk, nine as moderate, and only one, Maculabatis gerrardi (V = 1.29), as low-risk species in our analysis (Fig. 5). Moderately vulnerable species such as Milk shark, Pale-edged stingray, and Longnose stingray were classified as “Near Threatened” on the IUCN Red List.
3.4. Sensitivity analysis of productivity and susceptibility attributes
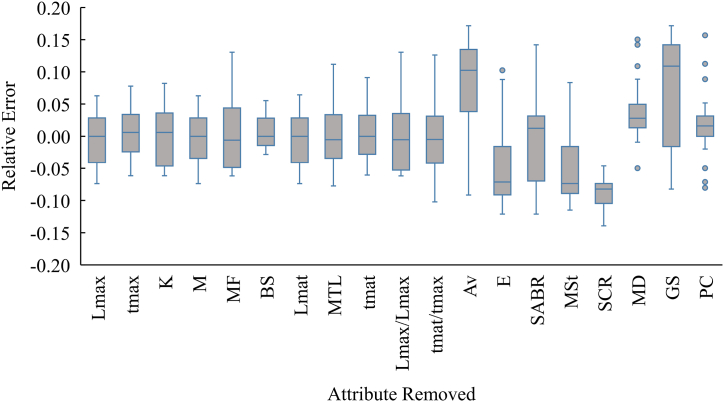
After excluding each attribute in turn, we observed minimal changes in the RMSE value for productivity attributes. The top two attributes were measured fecundity (MF; RMSE = 0.06) and mean trophic level (MTL; RMSE = 0.05) (Table 5). On the other hand, most susceptibility attributes scored significantly higher than the productivity attributes. Our result showed that gear selectivity (GS), availability (Av), and species survival after capture and release (SCR) were the top three attributes that had a greater impact on species vulnerability, with RMSE values of 0.11, 0.11, and 0.09, respectively (Table 5). This indicates a notable shift in the risk status of the species, with a relative average error increase of 10.8 % and 10.5 % for 41 species when “gear selectivity (GS)” and “availability (Av)” were excluded, respectively (Fig. 6). Additionally, the number of highly vulnerable species was increased almost twice (by 52 %) after excluding “availability” from the calculated vulnerability. These outcomes suggest that susceptibility was the primary driver and had a stronger influence on species vulnerability than productivity attributes.
Table 5.
Root Mean Squared Error (RMSE) between vulnerability scores when removing 19 attributes sequentially from the PSA of Lakkha gill net fishery of Bangladesh.
| Attribute removed | Lmat | tmax | k | M | MF | BS | Lmat | tmat | MTL | Lmat/Lmax | tmat/tmax |
|---|---|---|---|---|---|---|---|---|---|---|---|
| RMSE | 0.04 | 0.04 | 0.04 | 0.04 | 0.06 | 0.02 | 0.04 | 0.04 | 0.05 | 0.05 | 0.05 |
| Attribute removed | Av | E | SABR | MSt | SCR | MD | GS | PC | |||
| RMSE | 0.11 | 0.08 | 0.06 | 0.07 | 0.09 | 0.07 | 0.11 | 0.05 |
Fig. 6.
Relative error of vulnerability after excluding 11 productivity and 8 susceptibility attributes sequentially from PSA.
Table 6 shows that the majority of the identified elasmobranch species experienced a significant change in their vulnerability status when the susceptibility score was altered. Throughout the eight scenarios, a consistent pattern has emerged: the number of high-risk species increased when the susceptibility was increased, whereas many species shifted from higher-risk to lower-risk categories when the susceptibility score was decreased. According to the analysis, the vulnerability status of 7, 13, 16, and 17 species changed from moderate to high-risk categories as the susceptibility increased to 5 %, 10 %, 15 %, and 20 %, respectively. Several low-risk species also changed their status to moderate when their susceptibility increased. Conversely, as susceptibility scores decreased from 5 % to 20 %, many species shifted their risk categories toward lower vulnerability. Moreover, with a 20 % reduction in susceptibility, over half (n = 22) of the identified species changed their status to the lower-risk category, with only three species remaining in the high-risk group (C. amboinensis, C. brevipinna, and C. leucas). However, as susceptibility values rose (20 %), about 73 % (n = 29) of the total species received a high-risk status.
Table 6.
Alterations in the vulnerability scores of 40 elasmobranch species captured by the Lakkha gill net in the Bay of Bengal, Bangladesh occurred when susceptibility scores changed by ±5 %, ±10 %, ±15 %, and ±20 %. The spectrum of different colors; green, yellow, and red represent low, moderate, and high vulnerability, respectively.
3.5. Data quality assessment
Data quality score of productivity varied between 2.73 and 3.45, with 76 % of the species acquiring a low data quality category (Table 4). Only ten elasmobranch species obtained moderate data quality scores in productivity. However, scores related to data quality of the susceptibility attribute did not exceed 3.0 and all the species were categorized as moderate data quality (Table 4). Overall data quality score ranged from 2.36 to 2.91, indicating that most of the species ranked in the moderate category. The target species received the highest data quality score (2.91), followed by Brown mouth guitar fish (2.87) and Oman cownoseray (2.85).
4. Discussion
4.1. Species composition
In Bangladesh, 161 elasmobranchs (95 rays, 66 sharks) species were recorded, but only 111 species were further validated taxonomically and confirmed to be the most common species found in the Bay of Bengal [3]. We identified 40 bycatch species of sharks and rays that have the potential to engage with the Lakkha fishing net. The extent of species interaction with the fishing gear depends on various factors such as their depth preference, habitat, abundance of species, depth of fishing operation, gear efficiency, fishing hours, etc. [57]. In particular, more than half of the species that interacted with fishing gear were large pelagic sharks and rays, as Lakkha net (are mostly deployed at certain depths in the water column, drifting in the ocean, targeting schools of Indian threadfin salmon. Besides, the species composition supported the existence of diversification of elasmobranch species since multiple families were identified [3].
4.2. Species vulnerability
In this study, we employed the PSA method to identify the relative impact of Lakkha fishing on the data-limited elasmobranch species in the Bay of Bengal, Bangladesh. We chose this approach because it has demonstrated its efficacy in assessing risks in data-limited scenarios, especially when comprehensive biological or catch data are unavailable or traditional stock assessments are not feasible [49], a situation common in elasmobranch fisheries worldwide. It has also increased confidence among researchers and stakeholders in assessing potential risk to target or bycatch species affected by fishing practices. Furthermore, it facilitates the formulation of guideline, the establishment adequate management strategies, and implementation of proper surveillance to mitigate anthropogenic impacts on vulnerable species within a relatively short timeframe [16].
Results of the study confirmed that the target Lakkha fish was one of the highly vulnerable species, with a susceptibility score of 2.63 compared to other species. This finding aligns with those reported by Faruque and Matsuda (2021a) and Ormseth and Spencer (2011) [24,43] for their target stocks of Hilsa fish and Alaskan ground fish, respectively. The high susceptibility of the Indian threadfin is likely due to the significant modification of the fishing gear used to catch them. This might justify the high vulnerability score obtained by the target species, even though they possess a high productive score.
Furthermore, certain elasmobranch species showed substantial cumulative risk from the fishing gear. The current assessment classified seven sharks and five rays as highly vulnerable species to Lakkha fishery. This high risk might result from their intrinsic biology (poor productivity), influenced collectively by several factors, including poor fecundity, low birth rate, long life span, slow to moderate growth rate, large size, long interval between breeding period, and late maturity [[58], [59], [60]]. Additionally, the high-risk elasmobranch species also received a high susceptibility score ranging from 2.25 to 2.50 suggesting a high likelihood of overlap with the gear and habitat, along with their aggregation behavior, morphological characteristics, feeding and breeding dynamics. This implies that these species might not sustain the existing fishing pressure.
In our study, the comparison showed a notable alignment between PSA and the IUCN Red List, with all high-risk bycatch species listed in the threatened categories of global IUCN Red List. This finding is consistent with the equivalences established by Ref. [61], suggesting that high vulnerability in PSA should correspond with the threatened category (VU, EN, and CR) of the IUCN Red List. This implies that species with high vulnerability are at risk of facing extinction or experiencing a massive decline in population within the study region. This underscores the urgent need for immediate conservation efforts for elasmobranch from the Lakkha fishery. Species such as Sphyrna mokkarran and Carcharchinus brevipinna are highly migratory sharks that exhibit oceanodromus migration, making it more challenging to estimate their current biomass and population risk, thereby increasing the challenge for implementing adequate conservation measures. Furthermore, the Devil ray (Mobula mobular and Mobula tarapacana) and Cownose ray (Rhinoptera javanica), already categorized as Endangered (EN), face substantial market demand that raises their chance for future extinction. Moreover, Spot-tail shark (Carcharhinus sorrah), Tiger shark (Galeocerdo cuvier), Longnose stingray (Hypanus guttatus), and Estuary stingray (Hemitrygon fluviorum), classified as nearly threatened by the IUCN Red List on a global scale, scored moderate risk in the present study, which also aligns with the findings of [61]. Species from the Mobulidae and Rhinopteridae families, classified as moderately vulnerable by PSA, have risk scores nearing the threshold of high-risk status. This suggests a heightened risk of extinction with increased fishing pressure, aligning with the Endangered status designated by the IUCN.
Our results revealed some inconsistencies between the global IUCN Red List status and the low–risk species evaluated by the PSA. For instance, the majority of low-risk species were classified as threatened by the global IUCN Red List. This inconsistency may result from various factors, such as their limited capture frequency, body shape, and size of species [57], which suggest their minimal encounter with the studied gear due to its larger mesh size, gear dynamics, and position in the water column. However, elasmobranchs are highly susceptible to multiple gear types, and evidence suggests that these species are more susceptible to long line, set bag net, and bottom set gill net rather than large meshed gill net [49,50,[61], [62]].
The susceptibility of elasmobranch species to multiple fishing gear makes them more fragile in the ecosystem. This susceptibility is certainly influenced by the structural and anatomical features of species, their vertical distribution in the water, aggregative nature, depth allocation of fishing gear, fishers’ willingness to retain catch, and socioeconomic importance [[63], [64], [65]]. Besides, researchers need to consider other anthropogenic pressures such as habitat loss, oil spills, and climate change while evaluating the risk of these species [66,67].
The target, Indian threadfin fish, obtained a moderate overall data quality score despite being the target species due to the absence of thorough research. Most of the bycatch species received poor ratings in data quality analysis for productivity, indicating a scarcity of biological knowledge relevant to individual species from Bangladesh [24]. Species that are quite conspicuous and accessible, such as Whiprays (Dasyatidae family) and Spinetail devil rays (Myliobatidae), are also rarely investigated. In addition, we provided species with less market value and demand poor data quality ratings similar to tropical marine fisheries [24,47].
4.3. Sensitivity analysis
In PSA, each productivity and susceptibility trait does not have the same impact when determining a species' vulnerability [22]. Our sensitivity results for attributes showed higher RMSE value for susceptibility attribute rather than productivity, suggesting that susceptibility traits were the primary drivers [22,47,52,68], acknowledging the fact that susceptibility traits contributed more towards species' vulnerable condition. Among all 19 attributes, availability (Av- 0.11), gear selectivity (GS- 0.11), and survival after catch and release (SCR- 0.09) received comparatively higher scores. Removal or minimizing of availability (species horizontal overlap with fishing gear) might create a significant difference in their vulnerability [68]. Similarly, morphological features of species influence the entanglement with the fishing gear (which is likely to increase gear selectivity) [24]. Along with this, the mesh size of the Lakkha net and gear position might enhance the susceptibility of the elasmobranch. These could be the probable reason for the high susceptibility score for shark and shark-like species.
The alteration of susceptibility values caused many species to change their risk status (Table 6), indicating possible improvement in species' ecological status by regulated and controlled fishing practices [69]. Another important outcome is more species shifted to high risk when susceptibility was altered positively, rather than changing to low risk while susceptibility decreased. This demonstrates that existing fishing pressure has the potential to damage more species unless appropriate management measures are undertaken. The analysis also highlighted the probable areas, which would guide the authorities to establish and reform management measures and policies to mitigate species vulnerability to Lakkha fishery.
4.4. Reducing elasmobranch mortality in Lakkha fishing net
Recognizing and understanding the dynamics of Lakkha fishing gear is the primary step in developing interventions and conservation measures for the bycatch species [70]. Gill net industry in Bangladesh is highly diversified and largely unmonitored, similar to other developing nations [71]. Therefore, authorities should develop an inventory and appropriate protocol to monitor the catch and fishing vessels operating in the Bay of Bengal. Bycatch mortality can be reduced by limiting encounterability and decrease post-capture mortality [72,73]. Encounterability can be addressed by establishing closures or marine protected areas , modifying gears, and changing gear deployment techniques. Shortening the period of gear deployment in the feeding area would lessen the fishing mortality of bycatch species [74]. Safe discharge technique should be approached with caution, as most entangled elasmobranch species die before the Lakkha net is hauled [7]. Hence, low-cost strategic adjustments to the deployment technique of the gear and limiting its operation for a certain period of time could be the most effective methods to lessen the impact of overfishing on the vulnerable bycatch [75,76]. However, the socioeconomic consequences of limiting the operation of Lakkha net should be accessed. Mandates and criminal charges should be established to control illegal skin and fin trade activities. Moreover, adequate action plans, proper tactics, compliance with national and international rules and regulations, formation of an enforcement body, and comprehensive research are necessary to safeguard the globally threatened elasmobranch species.
4.5. Future research needs
Our study utilized morphological identification keys, a common approach in species identification in fisheries science, to identify elasmobranch bycatch. However, this technique can be ambiguous and challenging when identifying species with similar morphometrics. A molecular approach in species identification could be ideal in this regard, providing more authentic identification of elasmobranch species in the Bay of Bengal.
While calculating productivity scores we used maximum sizes documented in literature, instead of landed sizes in the study area. Furthermore, many identified species encounter other gear types, such as hooks and lines, set-bag nets, seine nets, and gill nets (varying mesh size), collectively increasing their risk from fishing practices. In addition, varying sizes of the species at different life stages, and their differing susceptibility to multiple gear types were not considered when assessing the risk of elasmobranchs. Therefore, a comprehensive study on multi-gear risk assessment of each identified species should be undertaken to understand their collective risks with greater certainty. In addition, research on species biology and population structure should be conducted to generate robust data and revise the vulnerability precisely, aiding in the establishment of adequate management measures to safeguard these megafauna species in the Bay of Bengal, Bangladesh.
5. Conclusion
Our study sheds light on the significant impact of the Lakkha gill net fishery on elasmobranch species in the Bay of Bengal, Bangladesh. We identified a diverse array of elasmobranch species interacting with the Lakkha net, with sharks and rays from various families being the most affected. Through Productivity Susceptibility Analysis, we assessed the vulnerability of these species, revealing high susceptibility scores for many, including those already classified as Endangered or Critically Endangered by the IUCN Red List. Our findings underscore the necessity of immediate action to mitigate the threat posed by unregulated fishing to elasmobranch populations, highlighting the importance of data-driven management strategies for their long-term sustainability. Additionally, the assessment of data quality emphasizes the need for further research and data collection to enhance our understanding of these vulnerable species and inform effective conservation efforts.
Data availability
Data will be made available on request.
CRediT authorship contribution statement
Rupesh Das: Writing – original draft, Methodology, Funding acquisition, Formal analysis, Data curation, Conceptualization. Md. Hasan Faruque: Writing – review & editing, Supervision, Methodology, Funding acquisition, Formal analysis, Data curation, Conceptualization. Sadman Sakib: Writing – original draft, Formal analysis, Data curation. Md. Taslim Ahmad: Writing – original draft, Methodology, Formal analysis, Data curation. Rubaia Nishat Seba: Writing – original draft, Methodology, Data curation. Md. Al Zahid: Writing – review & editing, Methodology, Conceptualization. Most. Nilufa Yeasmin: Writing – review & editing, Methodology, Conceptualization. Md. Mazharul Islam: Writing – review & editing, Methodology, Data curation.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The work was supported by grants from Centennial Research, University of Dhaka (Grant No: Memo No-Reg./Admin-3/70926) and the National Science and Technology (NST) Fellowship (Grant No: 39.00.0000.012.02.007.22.159) from the Ministry of Science and Technology, Bangladesh. We would like to express our heartfelt gratitude to the authorities for providing the research funding. We also extend our sincere thanks to the Department of Fisheries, Ministry of Fisheries and Livestock, Bangladesh, for their invaluable support in data collection. We are grateful to the local fishery authorities in the surveyed areas for their significant support during our surveys. Additionally, we are deeply thankful to all the Lakkha fishers who participated in personal interviews and focus group discussions (FGDs), providing us with valuable insights into the Lakkha fishery and its bycatch. We express our appreciation to Md. Shamim for his unwavering assistance during the survey.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2024.e37331.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Kriwet J., Witzmann F., Klug S., Heidtke U.H.J. First direct evidence of a vertebrate three-level trophic chain in the fossil record. Proc. R. Soc. B Biol. Sci. 2008;275:181–186. doi: 10.1098/rspb.2007.1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Compagno L.J.V. Alternative life-history styles of cartilaginous fishes in time and space. Environ. Biol. Fishes. 1990;28:33–75. doi: 10.1007/BF00751027. [DOI] [Google Scholar]
- 3.Haque A.B., Cavanagh R.D., Seddon N. Evaluating artisanal fishing of globally threatened sharks and rays in the Bay of Bengal, Bangladesh. 2021. [DOI] [PMC free article] [PubMed]
- 4.Jorgensen S.J., Micheli F., White T.D., Van Houtan K.S., Alfaro-Shigueto J., Andrzejaczek S., Arnoldi N.S., Baum J.K., Block B., Britten G.L., Butner C., Cardeñosa D., Chapple T.K., Clarke S., Cortés E., Dulvy N.K., Fowler S., Gallagher A.J., Shea B.D., Gilman E., Godley B.J., Graham R.T., Hammerschlag N., Harry A.V., Heithaus M.R., Hutchinson M., Huveneers C., Lowe C.G., Lucifora L.O., MacKeracher T., Martins A.P.B., Mull C., Mangel J.C., McCauley D.J., McClenachan L., Natanson L.J., Pauly D., Pazmiño D.A., Simpfendorfer C.A., Pazmiño D.A., Pistevos J.C.A., Queiroz N., Roff G., Shea B.D., Ferretti F., Sims D.W., Ward-Paige C., Worm B. Emergent research and priorities for shark and ray conservation. Endanger. Species Res. 2022;47:171–203. doi: 10.3354/ESR01169. [DOI] [Google Scholar]
- 5.Griffiths S.P., Kesner-Reyes K., Garilao C., Duffy L.M., Román M.H. Ecological assessment of the sustainable impacts of fisheries (EasI-FiSh): a flexible vulnerability assessment approach to quantify the cumulative impacts of fishing in data-limited settings. Mar. Ecol. Prog. Ser. 2019;625:89–113. doi: 10.3354/meps13032. [DOI] [Google Scholar]
- 6.Myers R.A., Baum J.K., Shepherd T.D., Powers S.P., Peterson C.H. Cascading effects of the loss of apex predatory sharks from a coastal ocean. Science. 2007;315:1846–1850. doi: 10.1126/science.1138657. [DOI] [PubMed] [Google Scholar]
- 7.Brownell R., Jr., Reeves R., Read A., Smith B., Thomas P., Ralls K., Amano M., Berggren P., Chit A., Collins T., Currey R., Dolar M., Genov T., Hobbs R., Kreb D., Marsh H., Zhigang M., Perrin W., Phay S., Rojas-Bracho L., Ryan G., Shelden K., Slooten E., Taylor B., Vidal O., Ding W., Whitty T., Wang J. Bycatch in gillnet fisheries threatens critically endangered small cetaceans and other aquatic megafauna. Endanger. Species Res. 2019;40:285–296. doi: 10.3354/esr00994. [DOI] [Google Scholar]
- 8.Costello M.J., Coll M., Danovaro R., Halpin P., Ojaveer H., Miloslavich P. A census of marine biodiversity knowledge, resources, and future challenges. PLoS One. 2010;5 doi: 10.1371/journal.pone.0012110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lewison R.L., Crowder L.B., Read A.J., Freeman S.A. Understanding impacts of fisheries bycatch on marine megafauna. Trends Ecol. Evol. 2004;19:598–604. doi: 10.1016/j.tree.2004.09.004. [DOI] [Google Scholar]
- 10.Ripple W.J., Wolf C., Newsome T.M., Betts M.G., Ceballos G., Courchamp F., Hayward M.W., Van Valkenburgh B., Wallach A.D., Worm B. Are we eating the world's megafauna to extinction? Conserv. Lett. 2019:1–10. doi: 10.1111/conl.12627. [DOI] [Google Scholar]
- 11.Hoq M.E., Yousuf Haroon A.K., Hussain M.G. 2011. Shark Fisheries in the Bay of Bengal, Bangladesh: Status and Potentialities.papers2://publication/uuid/FF094496-F73B-4361-B939-CEE5DE5FAC8A [Google Scholar]
- 12.Salas S., Chuenpagdee R., Seijo J.C., Charles A. Challenges in the assessment and management of small-scale fisheries in Latin America and the Caribbean. Fish. Res. 2007;87:5–16. doi: 10.1016/j.fishres.2007.06.015. [DOI] [Google Scholar]
- 13.Stobutzki I.C., Miller M.J., Jones P., Salini J.P. Bycatch diversity and variation in a tropical Australian penaeid fishery. Fish. Res. 2001;53:283–301. [Google Scholar]
- 14.Milton D.A. Assessing the susceptibility to fishing of populations of rare trawl bycatch: sea snakes caught by Australia's northern prawn fishery. Biol. Conserv. 2001;101:281–290. doi: 10.1016/S0006-3207(00)00232-9. [DOI] [Google Scholar]
- 15.Hobday A., Fuller M., Griffiths S., Kenyon R., Bulman C., Dowdney J., Williams A., Sporcic M. Ecological risk assessment for the effects of fishing: southern bluefin tuna purse seine sub-fishery. 2007. http://ecite.utas.edu.au/92589/
- 16.Hobday A.J., Smith A.D.M., Stobutzki I.C., Bulman C., Daley R., Dambacher J.M., Deng R.A., Dowdney J., Fuller M., Furlani D., Griffiths S.P., Johnson D., Kenyon R., Knuckey I.A., Ling S.D., Pitcher R., Sainsbury K.J., Sporcic M., Smith T., Turnbull C., Walker T.I., Wayte S.E., Webb H., Williams A., Wise B.S., Zhou S. Ecological risk assessment for the effects of fishing. Fish. Res. 2011;108:372–384. doi: 10.1016/j.fishres.2011.01.013. [DOI] [Google Scholar]
- 17.Micheli F., De Leo G., Butner C., Martone R.G., Shester G. A risk-based framework for assessing the cumulative impact of multiple fisheries. Biol. Conserv. 2014;176:224–235. doi: 10.1016/j.biocon.2014.05.031. [DOI] [Google Scholar]
- 18.Lucena Frédou F., Frédou T., Gaertner D., Kell L., Potier M., Bach P., Travassos P., Hazin F., Ménard F. Life history traits and fishery patterns of teleosts caught by the tuna longline fishery in the South Atlantic and Indian Oceans. Fish. Res. 2016;179:308–321. doi: 10.1016/j.fishres.2016.03.013. [DOI] [Google Scholar]
- 19.Maura H., Santiago J., Coelho R., Zudaire I., Neves C., Rosa D., Zudaire I., Semba Y., Geng P., Arrizabalga H., Bach P., Baez J.C., Ramos M.L., Zhu J.F., Ruiz J. Updated Ecological Risk Assessment (ERA) for shark species caught in fisheries managed by the Indian Ocean Tuna Commission (IOTC) Indian Ocean Tuna Comm. Mahé, Seychelles. 2018:1–22. [Google Scholar]
- 20.Brown S.L., Reid D., Rogan E. Spatial and temporal assessment of potential risk to cetaceans from static fishing gears. Mar. Policy. 2015;51:267–280. doi: 10.1016/j.marpol.2014.09.009. [DOI] [Google Scholar]
- 21.Clarke T.M., Espinoza M., Romero Chaves R., Wehrtmann I.S. Assessing the vulnerability of demersal elasmobranchs to a data-poor shrimp trawl fishery in Costa Rica, Eastern Tropical Pacific. Biol. Conserv. 2018;217:321–328. doi: 10.1016/j.biocon.2017.11.015. [DOI] [Google Scholar]
- 22.Hordyk A.R., Carruthers T.R. A quantitative evaluation of a qualitative risk assessment framework: examining the assumptions and predictions of the Productivity Susceptibility Analysis (PSA) PLoS One. 2018;13 doi: 10.1371/JOURNAL.PONE.0198298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Patrick W., Spencer P., Ormseth O., Cope J., Field J., Kobayashi D., Gedamke T., Coretes E., Bigelow K., Overholtz W., Link J., Lawson P. NOAA Tech. Memo; 2009. Use of Productivity and Susceptibility Indices to Determine the Vulnerability of a Stock: with Example Applications to Six US Fisheries; p. 90. [Google Scholar]
- 24.Faruque H., Matsuda H. Assessing the vulnerability of bycatch species from Hilsa gillnet fishing using productivity susceptibility analysis: insights from Bangladesh. Fish. Res. 2021;234 doi: 10.1016/j.fishres.2020.105808. [DOI] [Google Scholar]
- 25.Alam M.W., Xiangmin X., Ahamed R., Mozumder M.M.H., Schneider P. Ocean governance in Bangladesh: necessities to implement structure, policy guidelines, and actions for ocean and coastal management. Reg. Stud. Mar. Sci. 2021;45 doi: 10.1016/j.rsma.2021.101822. [DOI] [Google Scholar]
- 26.Rashed M., Hossain M.A., Rahman M.M. A case study of the gears and craft used for artisanal fishing in Chittagong Patharghata Fishery Ghat, Bangladesh and socio-economic condition of the fishermen. Asian J. Med. Biol. Res. 2017;2:712–726. doi: 10.3329/ajmbr.v2i4.31019. [DOI] [Google Scholar]
- 27.Roy B.J., Singha M.G., Kumar Rhaman Nripendra, Ali A.S.M.H. Status and recorded of sharks and rays in the Bay of bengal of Bangladesh region. Brazilian J. Biol. Sci. 2015;2:343–367. http://revista.rebibio.net [Google Scholar]
- 28.Jit R.B., Hasan Ali S., Kumar Singha N., Gaziur Rahman M. Sharks and rays fisheries of the Bay of Bengal at the landing centers of chittagong and Cox’S bazar, Bangladesh, Bangladesh J. Zool. 2013;41:49–60. [Google Scholar]
- 29.Jit R.B., Alam F., Rhaman M.G., Singha N.K., Akhtar A. Landing trends, species composition and percentage composition of sharks and rays in chittagong and Cox's bazar, Bangladesh. Glob. J. Sci. Front. Res. D Agric. Vet. 2014;14 [Google Scholar]
- 30.Badhon M.K., Uddin M.K., Nitu F.K., Siddique E.M.K. Identifying priorities for shark conservation in the Bay of Bengal, Bangladesh. Front. Mar. Sci. 2019;6:1–6. doi: 10.3389/fmars.2019.00294. [DOI] [Google Scholar]
- 31.Dworkin S.L. Sample size policy for qualitative studies using in-depth interviews, Arch. Sex. Beyond Behav. 2012;41:1319–1320. doi: 10.1007/S10508-012-0016-6. [DOI] [PubMed] [Google Scholar]
- 32.Grace M.A. Field guide to the requiem sharks (elasmobranchiomorphi: Carcharhinidae) of the western North Atlantic, NOAA tech. Reports. 2001;153:1–32. [Google Scholar]
- 33.Haroon Y., Kibria G. ResearchGate Online Publication; 2021. Shark Fisheries (Taxonomy , Biology , Ecology) of Bangladesh and Pollution Impacts. [Google Scholar]
- 34.shahadat Hossain M., Chowdhury S.R., Sharifuzzaman S., Islam M.M., Hasan J., Haque M.A., zulfikar Ali M., Hoq M.E., Mahmud Y. 2020. Marine Fishes of Bangladesh, Bangladesh Fisheries Research Institute (BFRI) Mymensingh-2201, Bangladesh. [Google Scholar]
- 35.Rahman A.K.A., Kabir S.M.H., Ahmad M., Ahmed A.T.A., Ahmed Z.U., Begum Z.N.T., Hassan M.A., Khondker M. 2009. Encyclopedia of Flora and Fauna of Bangladesh Vol. 24 Marine Fishes. [Google Scholar]
- 36.Wildlife Conservation Society Identification training on shark and rays: species visual ID and design monitoring. 2019. https://www.coraltriangleinitiative.org/sites/default/files/resources/IdentificationtrainingonSharksandRaysModule.pdf
- 37.Froese R., Pauly D. Publ.; 2024. FishBase., World Wide Web Electron.www.fishbase.org,version(02/2024 [Google Scholar]
- 38.Fricke R., Eschmeyer W.N., Van Der Laan R. ESCHMEYER’S catalog of fishes: genera, species, references. 2024. http://researcharchive.calacademy.org/research/ichthyology/catalog/fishcatmain.asp
- 39.Calder B.J. Focus groups groups and the nature of qualitative marketing research. J. Mark. Res. 1977;14:353–364. doi: 10.1177/002224377701400311. [DOI] [Google Scholar]
- 40.Nyumba O.T., Wilson K., Derrick C.J., Mukherjee N. The use of focus group discussion methodology: insights from two decades of application in conservation. Methods Ecol. Evol. 2018;9:20–32. doi: 10.1111/2041-210X.12860. [DOI] [Google Scholar]
- 41.Creswell J.W., Clark V.L.P. 2017. Designing and Conducting Mixed Methods Research. [Google Scholar]
- 42.Patrick W.S., Spencer P., Link J., Cope J., Field J., Kobayashi D., Lawson P., Gedamke T., Cortés E., Ormseth O., Bigelow K., Overholtz W. Using productivity and susceptibility indices to assess the vulnerability of United States fish stocks to overfishing. Fish. Bull. 2010;108:305–322. http://hdl.handle.net/1834/25396 [Google Scholar]
- 43.Ormseth O.A., Spencer P.D. An assessment of vulnerability in Alaska groundfish. Fish. Res. 2011;112:127–133. doi: 10.1016/j.fishres.2011.02.010. [DOI] [Google Scholar]
- 44.Manjaji-Matsumoto B., Last P. Two new whiprays, Maculabatis arabica sp. nov. and M. bineeshi sp. nov.(Myliobatiformes: Dasyatidae), from the northern Indian Ocean. Zootaxa. 2016;4144:335–353. doi: 10.11646/zootaxa.4144.3.3. [DOI] [PubMed] [Google Scholar]
- 45.Froese R., Binohlan C. Empirical relationships to estimate asymptotic length, length at first maturity and length at maximum yield per recruit in fishes, with a simple method to evaluate length frequency data. J. Fish. Biol. 2000;56:758–773. doi: 10.1111/J.1095-8649.2000.TB00870.X. [DOI] [Google Scholar]
- 46.Faruque H., Matsuda H. Conservative scoring approach in productivity susceptibility analysis leads to an overestimation of vulnerability: a study from the Hilsa gillnet bycatch stocks of Bangladesh. Fishes. 2021;6:33. doi: 10.3390/FISHES6030033/S1. [DOI] [Google Scholar]
- 47.Lin C.-Y., Wang S., Chiang W., Griffiths S., Yeh H. Ecological risk assessment of species impacted by fisheries in waters off eastern Taiwan. Fish. Manag. Ecol. 2020;27:345–356. doi: 10.1111/fme.12417. [DOI] [Google Scholar]
- 48.Duffy L.M., Lennert-Cody C.E., Olson R.J., Minte-Vera C.V., Griffiths S.P. Assessing vulnerability of bycatch species in the tuna purse-seine fisheries of the eastern Pacific Ocean. Fish. Res. 2019;219 doi: 10.1016/j.fishres.2019.105316. [DOI] [Google Scholar]
- 49.Lucena-Frédou F., Kell L., Frédou T., Gaertner D., Potier M., Bach P., Travassos P., Hazin F., Ménard F. Vulnerability of teleosts caught by the pelagic tuna longline fleets in South Atlantic and Western Indian Oceans. Deep. Res. Part II Top. Stud. Oceanogr. 2017;140:230–241. doi: 10.1016/j.dsr2.2016.10.008. [DOI] [Google Scholar]
- 50.Roberson L., Wilcox C., Boussarie G., Dugan E., Garilao C., Gonzalez K., Green M., Kark S., Kaschner K., Klein C.J., Rousseau Y., Vallentyne D., Watson J.E.M., Kiszka J.J. Spatially explicit risk assessment of marine megafauna vulnerability to Indian Ocean tuna fisheries. Fish Fish. 2022;23:1180–1201. doi: 10.1111/faf.12676. [DOI] [Google Scholar]
- 51.Jutagate T., Sawusdee A. Catch composition and risk assessment of two fishing gears used in small-scale fisheries of Bandon Bay, the Gulf of Thailand. PeerJ. 2022;10 doi: 10.7717/peerj.13878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lin Q., Chen Y., Zhu J. A comparative analysis of the ecological impacts of Chinese tuna longline fishery on the Eastern Pacific Ocean. Ecol. Indic. 2022;143 doi: 10.1016/j.ecolind.2022.109284. [DOI] [Google Scholar]
- 53.Lira A.S., Le Loc’h F., Andrade H.A., Lucena-Frédou F. Vulnerability of marine resources affected by a small-scale tropical shrimp fishery in Northeast Brazil. ICES J. Mar. Sci. 2022;79:633–647. doi: 10.1093/icesjms/fsac004. [DOI] [Google Scholar]
- 54.Chai T., Draxler R.R. Root mean square error (RMSE) or mean absolute error (MAE)? -Arguments against avoiding RMSE in the literature. Geosci. Model Dev. (GMD) 2014;7:1247–1250. doi: 10.5194/gmd-7-1247-2014. [DOI] [Google Scholar]
- 55.Osio G.C., Orio A., Millar C.P. Assessing the vulnerability of Mediterranean demersal stocks and predicting exploitation status of un-assessed stocks. Fish. Res. 2015;171:110–121. doi: 10.1016/j.fishres.2015.02.005. [DOI] [Google Scholar]
- 56.Fujita R., Thornhill D.J., Karr K., Cooper C.H., Dee L.E. Assessing and managing data-limited ornamental fisheries in coral reefs. Fish Fish. 2014;15:661–675. doi: 10.1111/faf.12040. [DOI] [Google Scholar]
- 57.Rincón-Sandoval L.A., Velázquez-Abunader I., Brulé T. Factors affecting catchability in longline fishing of red grouper in the southeastern gulf of Mexico. Trans. Am. Fish. Soc. 2019;148:857–868. doi: 10.1002/tafs.10178. [DOI] [Google Scholar]
- 58.Da Silva V.E.L., Teixeira E.C., Fabré N.N., da Silva Batista V. Reproductive biology of the longnose stingray Hypanus guttatus (Bloch & Schneider, 1801) from the northeastern coast of Brazil, Cah. Biol. Mar. 2018;59:467–472. doi: 10.21411/CBM.A.C4BC192C. [DOI] [Google Scholar]
- 59.Pinheiro P., Broadhurst M.K., V Hazin F.H., Bezerra T., Hamilton S. Reproduction in Bagre marinus (ariidae) off pernambuco, northeastern Brazil. J. Appl. Ichthyol. 2006;22:189–192. doi: 10.1111/j.1439-0426.2006.00704.x. [DOI] [Google Scholar]
- 60.Simpfendorfer C.A., Heupel M.R., White W.T., Dulvy N.K. The importance of research and public opinion to conservation management of sharks and rays: a synthesis. Mar. Freshw. Res. 2011;62:518–527. doi: 10.1071/MF11086. [DOI] [Google Scholar]
- 61.De Freitas A.J.R., Passarone R., Lira A.S., Pelage L., Lucena-Frédou F. Vulnerability assessment of species caught by the shrimp trawl fishery in northeastern Brazil. Reg. Stud. Mar. Sci. 2023;61 doi: 10.1016/j.rsma.2023.102949. [DOI] [Google Scholar]
- 62.Temple A.J., Wambiji N., Poonian C.N.S., Jiddawi N., Stead S.M., Kiszka J.J., Berggren P. Marine megafauna catch in southwestern Indian Ocean small-scale fisheries from landings data. Biol. Conserv. 2019;230:113–121. doi: 10.1016/j.biocon.2018.12.024. [DOI] [Google Scholar]
- 63.Skomal G.B. Evaluating the physiological and physical consequences of capture on post‐release survivorship in large pelagic fishes. Fsheries Manag. 2007;14:81–89. doi: 10.1111/j.1365-2400.2007.00528.x. [DOI] [Google Scholar]
- 64.Stein A.B., Friedland K.D., Sutherland M. Atlantic sturgeon marine bycatch and mortality on the continental shelf of the northeast United States. North Am. J. Fish. Manag. 2004;24:171–183. doi: 10.1577/M02-123. [DOI] [Google Scholar]
- 65.Raby G.D., Colotelo A.H., Blouin-Demers G., Cooke S.J. Freshwater commercial bycatch: an understated conservation problem. Bioscience. 2011;61:271–280. doi: 10.1525/bio.2011.61.4.7. [DOI] [Google Scholar]
- 66.Sherman C.S., Simpfendorfer C.A., Pacoureau N., Matsushiba J.H., Yan H.F., Walls R.H.L., Rigby C.L., VanderWright W.J., Jabado R.W., Pollom R.A., Carlson J.K., Charvet P., Bin Ali A., Fahmi, Cheok J., Derrick D.H., Herman K.B., Finucci B., Eddy T.D., Palomares M.L.D., Avalos-Castillo C.G., Kinattumkara B., Blanco-Parra M. del P., Dharmadi, Espinoza M., Fernando D., Haque A.B., Mejía-Falla P.A., Navia A.F., Pérez-Jiménez J.C., Utzurrum J., Yuneni R.R., Dulvy N.K. Half a century of rising extinction risk of coral reef sharks and rays. Nat. Commun. 2023;14:1–11. doi: 10.1038/s41467-022-35091-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chin A., Kyne P.M., Walker T.I., McAuley R.B. An integrated risk assessment for climate change: analysing the vulnerability of sharks and rays on Australia's Great Barrier Reef. Glob. Chang. Biol. 2010;16:1936–1953. doi: 10.1111/j.1365-2486.2009.02128.x. [DOI] [Google Scholar]
- 68.Georgeson L., Rigby C.L., Emery T.J., Fuller M., Hartog J., Williams A.J., Hobday A.J., Duffy C.A.J., Simpfendorfer C.A., Okuda T., Stobutzki I.C., Nicol S.J. Ecological risks of demersal fishing on deepwater chondrichthyan populations in the Southern Indian and South Pacific Oceans. ICES J. Mar. Sci. 2020;77:1711–1727. doi: 10.1093/icesjms/fsaa019. [DOI] [Google Scholar]
- 69.Pacoureaua N., Carlson J.K., Kindsvatera H.K., Rigby C.L., Winker H., Simpfendorferd C.A., Charvetf P., Pollom R.A., Barretoi R., Sherman C.S., Talwar B.S., Skerritt D.J., Sumaila R.U., Matsushibab J.H., VanderWright H.F., Yan Wade J., Dulvy N.K. Conservation successes and challenges for wide-ranging sharks and rays. Ecol. Environ. Sci. 2023;120 doi: 10.1073/pnas.2216891120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Teh L.S., Teh L.C., Hines E., Junchompoo C., Lewison R.L. Contextualising the coupled socio-ecological conditions of marine megafauna bycatch. Ocean Coast Manag. 2015;116:449–465. doi: 10.1016/j.ocecoaman.2015.08.019. [DOI] [Google Scholar]
- 71.Davies T.K., Mees C.C., Milner-Gulland E.J. The past, present and future use of drifting fish aggregating devices (FADs) in the Indian Ocean. Mar. Policy. 2014;45:163–170. doi: 10.1016/j.marpol.2013.12.014. [DOI] [Google Scholar]
- 72.Carruthers E.H., Schneider D.C., Neilson J.D. Estimating the odds of survival and identifying mitigation opportunities for common bycatch in pelagic longline fisheries. Biol. Conserv. 2009;142:2620–2630. doi: 10.1177/002224377701400311. [DOI] [Google Scholar]
- 73.Senko J., White E., Heppell S. Comparing bycatch mitigation strategies for vulnerable marine megafauna. Anim. Conserv. 2013;17:5–18. doi: 10.1111/acv.12051. [DOI] [Google Scholar]
- 74.Zollett E.A., Swimmer Y. Safe handling practices to increase post-capture survival of cetaceans, sea turtles, seabirds, sharks, and billfish in tuna fisheries. Endanger. Species Res. 2019;38:115–125. doi: 10.3354/esr00940. [DOI] [Google Scholar]
- 75.Hamilton S., Baker G.B. Technical mitigation to reduce marine mammal bycatch and entanglement in commercial fishing gear: lessons learnt and future directions. Rev. Fish Biol. Fish. 2019;29:223–247. doi: 10.1007/S11160-019-09550-6. [DOI] [Google Scholar]
- 76.Kiszka J.J., Moazzam M., Boussarie G., Shahid U., Khan B., Nawaz R. Setting the net lower: a potential low‐cost mitigation method to reduce cetacean bycatch in drift gillnet fisheries. Mar. Freshw. Ecosyst. 2021;31:3111–3119. doi: 10.1002/aqc.3706. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.