Abstract
Familial nonautoimmune hyperthyroidism (NAH) is a rare type of autosomal dominant hyperthyroidism caused by constitutively active pathogenic variants of the thyrotropin receptor (TSHR) gene. Although affected family members present with varied levels of hyperthyroid features, even when the same pathogenic variant is present, total thyroidectomy followed by radioiodine therapy is recommended for long-term management. Herein, we present the case of an 18-year-old proband and her family members with NAH (TSHR-I640V), who presented with diverse thyroid dysfunctions: fluctuations between euthyroid and subclinical hyperthyroidism, mild hyperthyroidism, and overt hyperthyroidism. Almost all affected adult family members, except the proband, showed no progression of hyperthyroidism or thyroid enlargement. A family history of thyrotropin receptor antibodies (TRAb)-negative hyperthyroidism is important for the identification of NAH in adults before TSHR genetic testing can be performed. Ablative therapy is not necessary when familial NAH presents with late-onset mild hyperthyroidism without coexisting diseases.
Keywords: familial nonautoimmune hyperthyroidism, TSH receptor gene pathogenic variant, subclinical hyperthyroidism, no increased vascularity in the thyroid, normal thyroid size
Introduction
Nonautoimmune hyperthyroidism (NAH) is a rare disease caused by constitutively active pathogenic variants of the thyrotropin receptor (TSHR) gene. The prevalence of germline pathogenic TSHR gene variants is < 5% among patients with subclinical or overt hyperthyroidism with diffuse goiter and negative for thyrotropin receptor antibodies (TRAb) (1). Compared with sporadic NAH, familial NAH shows varied levels of thyroid hormones and goiter size in addition to a wide range of hyperthyroid onset ages, even when the same pathogenic variant is present. All affected members of a family with TSHR-E575K presented with subclinical hyperthyroidism and no increase in thyroid size, even without any treatment for > 10 years (2). Meanwhile, according to the guidelines of the European Thyroid Association (3), total thyroidectomy followed by radioiodine therapy are strongly recommended as management strategies for familial NAH to prevent relapse of hyperthyroidism and the development of goiter enlargement.
In this report, we describe and explore the best treatment plan for an 18-year-old proband and her family members with NAH (TSHR-I640V) who presented with diverse levels of hyperthyroidism.
Case Presentation
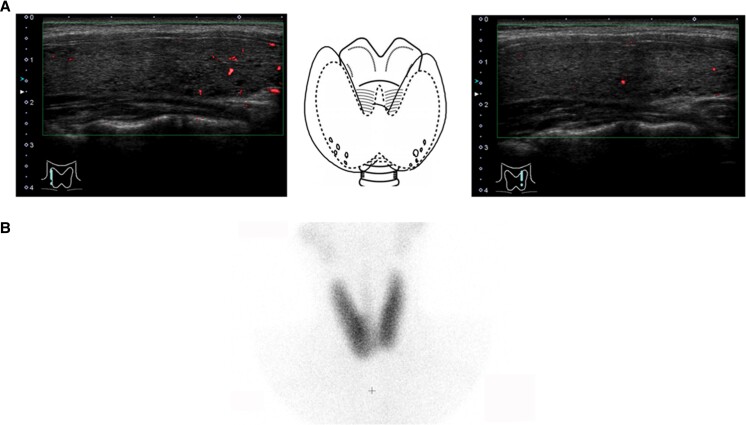
The proband was an 18-year-old Japanese female individual who presented with several thyrotoxic symptoms, including general fatigue and palpitations. Her thyroid function tests showed that serum thyrotropin (TSH) was < 0.01 mIU/L (reference range, 0.5-5.0) and free thyroxine (FT4) was 3.26 ng/dL (41.9 pmol/L) (reference range, 0.9-1.7 ng/dL [11.5-21.8 pmol/L]). Tests for serum anti-thyroglobulin, anti-thyroid peroxidase, and TRAb were negative. Technetium scintigraphy showed diffuse uptake of 4.47% (reference range, 0.5%-4.0%) in the thyroid. She was started on thiamazole and inorganic iodine 1 year before visiting our hospital. At the initial examination at our hospital, her thyroid function tests indicated that she was still hyperthyroid and negative for TRAb and thyroid stimulating antibodies (TSAb) (III-2 in Table 1). Ultrasonography of the thyroid showed mild goiter (28.2 mL) with no increased thyroid blood flow, and technetium scintigraphy showed diffuse uptake of 2.0% in the thyroid (Fig. 1). We performed thyroid function tests and ultrasonography on all available family members who had not been treated for any thyroid disease (Table 1). Consequently, 3 additional members presented with mild or overt hyperthyroidism but without TRAb or TSAb (Table 1).
Table 1.
Relationship between thyroid function tests and genetic abnormality in the family members
| Member | Age | FT4 | FT3 | TSH | TRAb | TSAb | Thyroid volume | TPOAb/TgAb | Thyroid blood flow | Uptake | TSHR-I640V | Treatment |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| I-1 | 76 years | 1.24 ng/dL [15.9 pmol/L] |
2.63 pg/mL [4.03 pmol/L] |
1.29 μIU/mL [1.29 mIU/L] |
<0.08 IU/L | NT | 15.2 mL | Neg/neg | 2.4% | NT | Neg | None |
| I-2 | 72 years | 1.22 ng/dL [15.7 pmol/L] |
3.22 pg/mL [4.94 pmol/L] |
0.95 μIU/mL [0.95 mIU/L] |
<0.08 IU/L | NT | 7.7 mL | Neg/neg | 1.5% | NT | Pos | None |
| II-1 | 50 years | 1.55 ng/dL [19.9 pmol/L] |
4.53 pg/mL
[6.95 pmol/L] |
0.015 μIU/mL
[0.015 mIU/L] |
1.35 IU/L | 101% | 16.6 mL | Neg/neg | 0.5% | NT | Pos | None |
| II-2 | 47 years | 1.05 ng/dL [13.5 pmol/L] |
3.55 pg/mL [5.45 pmol/L] |
1.46 μIU/mL [1.46 mIU/L] |
<0.80 IU/L | NT | 13.7 mL | Neg/neg | 2.7% | NT | Neg | None |
| II-3 | 44 years |
2.66 ng/dL
[34.2 pmol/L] |
4.55 pg/mL
[6.98 pmol/L] |
<0.005 μIU/mL
[<0.005 mIU/L] |
<0.80 IU/L | 111% | 21.4 mL | Neg/neg | 0.9% | 5.8%a | Pos | MMI |
| III-1 | 21 years | 1.70 ng/dL [21.8 pmol/L] |
4.76 pg/mL
[7.31 pmol/L] |
0.007 μIU/mL
[0.007 mIU/L] |
<0.80 IU/L | 100% | 15.2 mL | Neg/neg | 0.5% | NT | Pos | None |
| III-2 | 18 years |
2.93 ng/dL
[37.7 pmol/L] |
8.78 pg/mL
[13.4 pmol/L] |
<0.005 μIU/mL
[<0.005 mIU/L] |
0.99 IU/L | 99% | 28.2 mL | Neg/neg | 0.5% | 2.0%b | Pos | MMI |
| III-3 | 8 years | 1.45 ng/dL [18.6 pmol/L] |
4.91 pg/mL
[7.54 pmol/L] |
0.93 μIU/mL [0.93 mIU/L] |
<0.80 IU/L | NT | 5.4 mL | Neg/neg | 1.1% | NT | Neg | None |
Reference intervals: FT4, 0.90-1.70 ng/dL [11.5-21.8 pmol/L]; FT3, 2.30-4.00 pg/mL [3.53-6.14 pmol/L]; TSH, 0.50-5.00 μIU/mL [0.50-5.00 mIU/L]; TRAb, <2.00 IU/L; TSAb, <120%. The discriminative findings are bolded.
Abbreviations: MMI, thiamazole; Neg, negative; NT, not tested; Pos, positive; TgAb, anti-thyroglobulin antibodies; TPOAb, anti-thyroid peroxidase antibodies.
a Radioiodine uptake in 3 hours, reference interval: 5.6%-15.8%.
b 99mTc-pertechnetate uptake, reference interval: 0.5%-4.0%.
Figure 1.
Ultrasonography (A) and technetium scintigraphy (B) in the thyroid of the proband.
Diagnostic Assessment
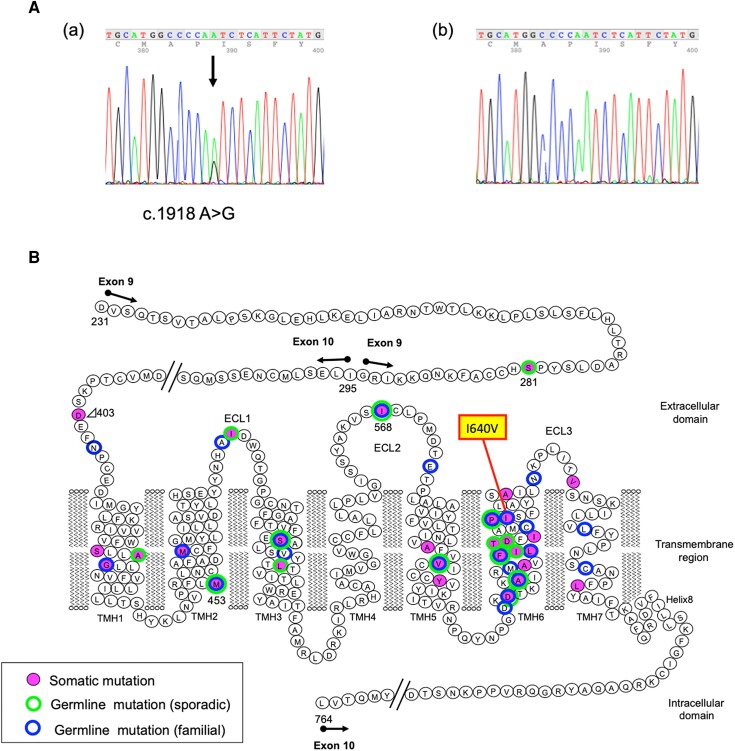
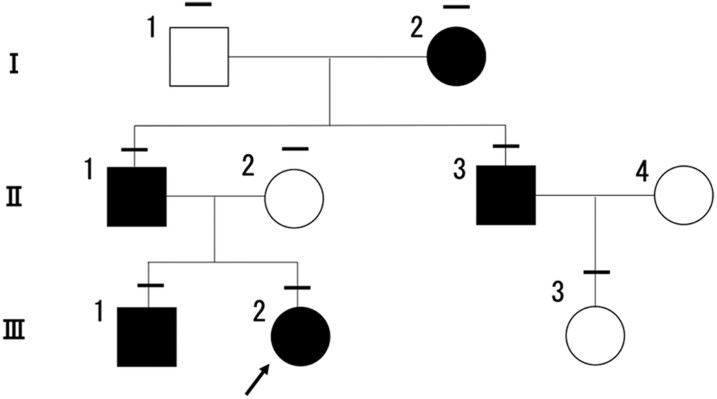
To further investigate familial NAH, sequence analysis of the genomic DNA of the proband and other family members was performed. Consequently, a heterozygous TSHR pathogenic variant (c.1918 A>G, p. I640V) was detected in the proband (III-2), her grandmother (I-2), father (II-1), brother (III-1), and uncle (II-3), as shown in Figs. 2 and 3.
Figure 2.
Sequencing analysis results of the TSHR gene in genomic DNA. (A) Heterozygous adenine to guanine transition at position 1918 (arrow) in the proband (a) and none in her mother (b). (B) The locations of TSHR pathogenic variants.
Figure 3.
Pedigree of the investigated family. The bars above each symbol indicate individuals who underwent a sequencing analysis for the TSHR gene. Individuals with the germline pathogenic variant, TSHR-I640V, are indicated by filled symbols. The open symbols represent unaffected family members without the germline pathogenic TSHR variant. The arrow shows the proband.
Treatment
Among the 4 patients with hyperthyroidism, 2 were prescribed medical therapy with thiamazole. The daily thiamazole dosage of the proband (III-2) was reduced from 15 mg to 7.5 mg over a 3-year period while maintaining normal thyroid function. Another family member with overt hyperthyroidism (II-3) started on 10 mg of thiamazole daily and is currently receiving 2.5 mg. Other family members with mild hyperthyroidism (II-1 and III-1) were not treated.
Outcome and Follow-Up
All members harboring the TSHR-I640V pathogenic variant were older than 18 years and were negative for anti-thyroglobulin antibodies, anti-thyroid peroxidase antibodies, and TRAb upon initial examination (Table 1). No complications, including cardiac disease or osteoporosis, were observed in the affected members. The oldest woman (I-2), aged 72 years, presented with normal thyroid function at our hospital. We inquired about the previous medical records of her periodic physical examinations and found fluctuations between euthyroidism and subclinical hyperthyroidism, with mild TSH suppression without any treatment (Table 2).
Table 2.
Fluctuation between euthyroid and subclinical hyperthyroidism in an older affected member (I-2)
| Age | 67 years | 68 years | 69 years | 70 years | 71 years | 72 years |
|---|---|---|---|---|---|---|
| TSH |
0.22 μIU/mL
[0.22 mIU/L] |
0.90 μIU/mL [0.90 mIU/L] |
1.12 μIU/mL [1.12 mIU/L] |
0.80 μIU/mL [0.80 mIU/L] |
0.53 μIU/mL
[0.53 mIU/L] |
0.67 μIU/mL [0.67 mIU/L] |
| FT4 | 1.34 ng/dL [17.2 pmol/L] |
1.20 ng/dL [15.4 pmol/L] |
1.21 ng/dL [15.5 pmol/L] |
1.31 ng/dL [16.8 pmol/L] |
1.23 ng/dL [15.8 pmol/L] |
1.24 ng/dL [15.9 pmol/L] |
Reference intervals: TSH, 0.54-4.54 μIU/mL [0.54-4.54 mIU/L]; FT4, 0.97-1.72 ng/dL [12.4-22.1 pmol/L]. The discriminative findings are bolded.
Initial ultrasound examination showed that 2 family members (II-3 and III-2) with overt hyperthyroidism presented with mild goiter; however, the other affected family members (II-1 and III-1) with mild hyperthyroidism and euthyroidism (I-2) presented with a normal thyroid size (Table 1). In addition, none of the affected family members showed nodules or increased vascular flow in the thyroid gland (Table 1). Sequential ultrasound examination showed that the thyroid size of II-3 had not changed (21.4 to 24.9 mL), but that of the proband (III-2) increased from 28.2 to 47.9 mL after thiamazole treatment for 3 years.
Discussion
Herein, we describe a family presenting with NAH harboring a germline pathogenic variant, TSHR-I640V. The I640, which is located at transmembrane helix 6 in TSHR, was previously shown to have a spatial and functional relationship with I568 in extracellular loop 2 by in vitro experiments (Fig. 2B) (4). The TSHR-I640V pathogenic variant, which has a reduced side chain length, showed 2.4-fold cAMP accumulation compared to the wild-type TSHR, suggesting constitutive activity (4). Another pathogenic variant with a different amino acid substitution, TSHR-I640K, was previously detected in functional thyroid nodules and showed constitutive activity in vitro (5). Very recently, 2 families with NAH with TSHR-I640V have been reported and all affected members were initially diagnosed with subclinical hyperthyroidism (6). In this study, we present another family with NAH caused by TSHR-I640V, who presented with variable levels of hyperthyroidism, consistent with genetic test results (Tables 1 and 2).
TRAb-positivity is critical for the diagnosis of Graves hyperthyroidism; however, approximately 30% of patients with continuous subclinical or overt hyperthyroidism with diffuse goiter and negative TRAb have latent Graves disease, including those with early stage with mild hyperthyroidism or near remission (1). Vascular flow in the thyroid gland can be very useful in the differential diagnosis between Graves disease and painless thyroiditis, with a cutoff of 4% (7). However, some patients with Graves disease present with no increase in vascular flow (< 4%) when levels of hyperthyroidism are mild. In the current study, none of the affected members showed increased vascular flow in the thyroid (Table 1), which suggests that the relatively mild hyperthyroidism was caused by TSHR-I640V.
In familial NAH, both onset of thyrotoxicosis and thyroid size are highly variable (8). This family included 5 affected members ranging in age from 18 to 72 years, and the thyroid size of the proband increased even with normalization of thyroid function maintained by thiamazole; the thyroid sizes of other members were normal without treatment or stable with thiamazole treatment (Table 1). This partly depended on the intensity of the activating pathogenic variant allele. Other genetic, epigenetic, and environmental factors such as insulin-like growth factor 1 and iodine supply are likely modifying factors (8).
The proband with the youngest onset age in this family qualified for ablative therapy, according to the guidelines of the European Thyroid Association (3). No patients with NAH relapse after total thyroidectomy or radioiodine therapy alone have been reported at our institute (9-11). Previously, fractionated radioiodine therapy led to the remission of hyperthyroidism without relapse and reduced goiter volume in the absence of TRAb, which is advantageous in preventing hyperthyroid exacerbation after radioiodine therapy (9, 11). Therefore, we believe that total thyroidectomy followed by radioiodine therapy should not be the only option considered for the proband and that the remaining affected adult family members do not immediately require ablative therapy.
In conclusion, a family history of TRAb-negative hyperthyroidism is essential for identifying NAH in adults before TSHR genetic testing can be performed. When there is late-onset mild hyperthyroidism and no coexisting diseases in familial NAH, treatment should be selected according to the clinical course, and complete ablative therapy is not necessary.
Learning Points
A family history of TSH receptor antibody–negative hyperthyroidism should raise the possibility of familial NAH.
No increased blood flow in the thyroid on ultrasound examination may be a distinctive finding of familial NAH.
Complete ablative therapy is not necessary when patients with familial NAH present with late-onset mild hyperthyroidism without coexisting diseases.
Acknowledgments
We are grateful to the staff of Kuma Hospital for their valuable contributions.
Abbreviations
- FT4
free thyroxine
- NAH
nonautoimmune hyperthyroidism
- TSH
thyrotropin (thyroid stimulating hormone)
- TSHR
thyrotropin receptor
- TRAb
thyrotropin receptor antibody
- TSAb
thyroid stimulating antibody
Contributor Information
Sawako Takahashi, Center for Excellence in Thyroid Care, Kuma Hospital, Kobe 650-0011, Japan.
Eijun Nishihara, Center for Excellence in Thyroid Care, Kuma Hospital, Kobe 650-0011, Japan.
Shuji Fukata, Center for Excellence in Thyroid Care, Kuma Hospital, Kobe 650-0011, Japan.
Akira Miyauchi, Center for Excellence in Thyroid Care, Kuma Hospital, Kobe 650-0011, Japan.
Takashi Akamizu, Center for Excellence in Thyroid Care, Kuma Hospital, Kobe 650-0011, Japan.
Contributors
S.T. and E.N. extracted the data and wrote the manuscript. S.T., E.N., and S.F. contributed to the patient's care and acquisition of data. A.M. and T.A. reviewed the manuscript critically. All the authors discussed the results and approved the final version of the manuscript.
Funding
No public or commercial funding.
Disclosures
The authors have nothing to disclose. Takashi Akamizu is an Editorial Board Member for JCEM Case Reports and played no role in the Journal’s evaluation of the manuscript.
Informed Patient Consent for Publication
Signed informed consent obtained directly from the patient.
Data Availability Statement
Original data generated and analyzed during this study are included in this published article.
References
- 1. Nishihara E, Fukata S, Hishinuma A, Amino N, Miyauchi A. Prevalence of thyrotropin receptor germline mutations and clinical courses in 89 hyperthyroid patients with diffuse goiter and negative anti-thyrotropin receptor antibodies. Thyroid. 2014;24(5):789‐795. [DOI] [PubMed] [Google Scholar]
- 2. Nishihara E, Chen CR, Higashiyama T, et al. Subclinical nonautoimmune hyperthyroidism in a family segregates with a thyrotropin receptor mutation with weakly increased constitutive activity. Thyroid. 2010;20(11):1307‐1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Paschke R, Niedziela M, Vaidya B, Persani L, Rapoport B, Leclere J. 2012 European thyroid association guidelines for the management of familial and persistent sporadic non-autoimmune hyperthyroidism caused by thyroid-stimulating hormone receptor germline mutations. Eur Thyroid J. 2012;1(3):142‐147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kleinau G, Claus M, Jaeschke H, et al. Contacts between extracellular loop two and transmembrane helix six determine basal activity of the thyroid-stimulating hormone receptor. J Biol Chem. 2007;282(1):518‐525. [DOI] [PubMed] [Google Scholar]
- 5. Gozu HI, Bircan R, Krohn K, et al. Similar prevalence of somatic TSH receptor and Gsalpha mutations in toxic thyroid nodules in geographical regions with different iodine supply in Turkey. Eur J Endocrinol. 2006;155(4):535‐545. [DOI] [PubMed] [Google Scholar]
- 6. Makkonen K, Jännäri M, Crisóstomo L, et al. Mechanisms of thyrotropin receptor-mediated phenotype variability deciphered by gene mutations and M453T-knockin model. JCI Insight. 2024;9(4):e167092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ota H, Amino N, Morita S, et al. Quantitative measurement of thyroid blood flow for differentiation of painless thyroiditis from Graves’ disease. Clin Endocrinol (Oxf). 2007;67(1):41‐45. [DOI] [PubMed] [Google Scholar]
- 8. Hebrant A, van Staveren WC, Maenhaut C, Dumont JE, Leclere J. Genetic hyperthyroidism: hyperthyroidism due to activating TSHR mutations. Eur J Endocrinol. 2011;164(1):1‐9. [DOI] [PubMed] [Google Scholar]
- 9. Nishihara E, Nagayama Y, Amino N, et al. A novel thyrotropin receptor germline mutation (Asp617Tyr) causing hereditary hyperthyroidism. Endocr J. 2007;54(6):927‐934. [DOI] [PubMed] [Google Scholar]
- 10. Nishihara E, Tsugawa M, Ozaki Y, et al. Long-term follow-up of a patient with sporadic non-autoimmune hyperthyroidism due to a thyrotropin receptor mutation (D619G). AACE Clin Case Rep. 2018;4(1):e84‐e89. [Google Scholar]
- 11. Nishihara E, Fukata S, Miyauchi A, Akamizu T. Long-term disproportional TSH hyposecretion in a patient with nonautoimmune hyperthyroidism after radioiodine therapy. JCEM Case Rep. 2023;1(2):luad026. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Original data generated and analyzed during this study are included in this published article.