Abstract
The translaminar pressure gradient (TLPG) refers to two forces at the lamina cribosa of the optic nerve: the anteriorly acting intracranial pressure (ICP), and posteriorly-acting intraocular pressure (IOP). It has been proposed that controlling the translaminar pressure gradient at regular intervals may preserve the optic nerve and slow the course of glaucoma. The precisional modulation of this TLPG is a recently introduced concept that may play a role in the treatment of ophthalmic diseases such as glaucoma. In this manuscript, we review the applications of pressurized goggles on ophthalmic diseases. We also elaborate upon current investigations in modulation of the TLPG including goggles and the multi-pressure dial goggle. We discuss future research directions for ophthalmic diseases including spaceflight associated neuro-ocular syndrome (SANS), a large physiological barrier to future long-duration spaceflight.
Keywords: Translaminar pressure gradient, glaucoma, spaceflight associated neuro-ocular syndrome, long-duration spaceflight
Introduction
The ophthalmic system is highly regulated by pressure both within the eye (i.e., intraocular pressure (IOP)) and in the brain (i.e., intracranial pressure (ICP)). Diseases such as glaucoma and idiopathic intracranial hypertension (IIH) represent disorders of the optic nerve that can lead to irreversible vision loss. The translaminar pressure gradient (TLPG) is the relationship between IOP and ICP. Modulation of the TLPG may mitigate ophthalmic IOP or ICP related diseases. In this paper, we describe the TLPG and its relationship to ophthalmic diseases; discuss the role of spontaneous venous pulsations (SVPs) as a potential non-invasive indicator of ICP; and review precisional modulation of the TLPG using pressurized goggles. Modulation of the TLPG has terrestrial (e.g., glaucoma or IIH) as well as extraterrestrial applications including spaceflight associated neuro-ocular syndrome (SANS).
Methods
Google Scholar, PubMed, Ovid MEDLINE searches from inception to October 30, 2022 were performed using the search terms: “Multi-Pressure Dial”, “Translaminar Pressure Gradient” and “Intraocular Pressure”. Selected articles were limited to English language only. Full references were manually searched to find other relevant studies.
Results
A total of 30 papers were reviewed and 16 papers were included in this review.
Discussion
Open angle glaucoma (OAG) is the most common optic nerve related cause of permanent visual loss in the world. OAG causes gradual degeneration of retinal ganglion cells (RGC) and up to 57.5 million people are estimated to be affected worldwide. 1 Risk factors for OAG include: increased IOP, age, myopia, gender, frailty, smoking, family history, systemic hypotension, systemic hypertension and use of topical or systemic steroids. 1 loss of RGC in OAG manifests clinically as loss of the retinal nerve fiber resulting in visual field reduction, while structural abnormalities in the optic nerve head (“cupping”) are a result of posterior bowing of the lamina cribrosa. 2
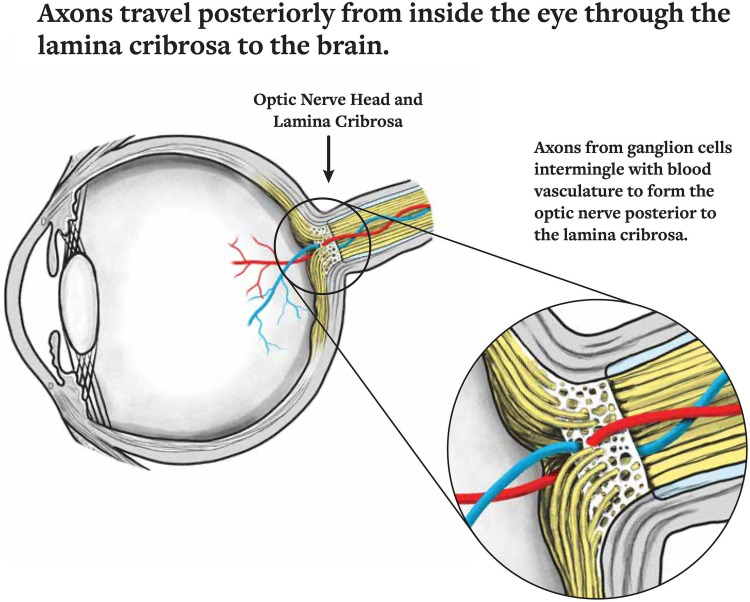
The lamina cribrosa is a connective tissue structure via which RGC axons transit the eye and is the main site for RGC axonal damage in OAG. (Figure 1) 3 Elevated IOP is the major risk factor for OAG but up to 25–50% of patients with OAG in the United States had IOP within the “normal” range (i.e., normal tension glaucoma). 4 Reducing IOP medically (e.g., eye drops), with lasers, or surgically are the only currently proven means of treating OAG and its subset normal tension glaucoma. 5 Additionally, despite reaching a target IOP certain patients may continue to show disease progression. 6
Figure 1.
The ocular axons travel from the eye through the lamina cribrosa backwards to the brain. This connection represents an important relationship between intraocular pressure and intracranial pressure. Reprinted from Berdahl JP, Ferguson TJ, Samuelson TW. Periodic normalization of the translaminar pressure gradient prevents glaucomatous damage. Med Hypotheses. Permissions obtained from Elsevier and Rightslink by Copyright Clearance Center, Inc.
In contrast to IOP, ICP is the pressure of the cerebrospinal fluid and central nervous system. Papilledema, transient visual obscuration, vision loss, double vision, headache, and pulsatile tinnitus are signs and symptoms of elevated ICP. 7 In addition, low ICP is characterized by orthostatic headache, vertigo, neck discomfort, vomiting, and horizontal diplopia.8,9 It is thought that IOP and ICP may be related in several ophthalmic diseases. In the following section, we discuss the relationship between these two pressures.
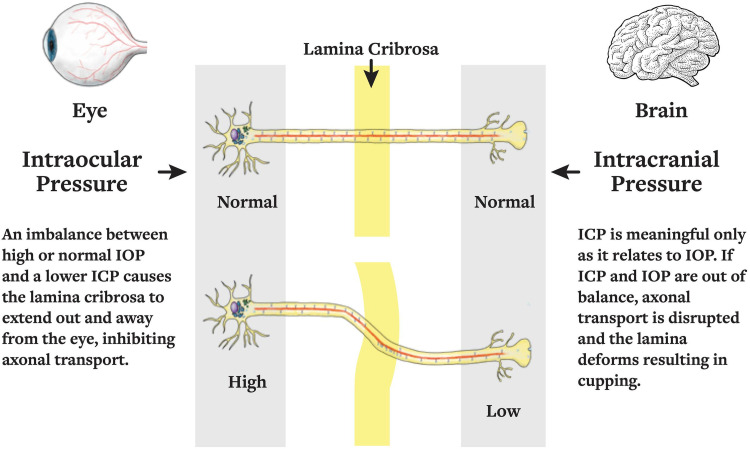
The lamina cribrosa is regulated by two distinct pressures: the anteriorly-acting ICP, and posteriorly-acting IOP. 10 The difference between these two pressures is the TLPG. The lamina cribrosa deforms anteriorly in papilledema and posteriorly in glaucomatous cupping due to mechanical pressure alterations. 10 Recent research suggests that an imbalance in IOP and ICP (i.e., the TLPG) may have a role in the development of OAG 10 and modulating this TLPG at regular intervals may preserve the optic nerve function and slow the course of POAG (Figure 2).
Figure 2.
The translaminar pressure gradient can be modulated with changes to intraocular pressure and intracranial pressure in disease. Thus, also representing that pressure modulation may help to reverse the translaminar pressure gradient back to equilibrium. Reprinted from Berdahl JP, Ferguson TJ, Samuelson TW. Periodic normalization of the translaminar pressure gradient prevents glaucomatous damage. Med Hypotheses. Permissions obtained from Elsevier and Rightslink by Copyright Clearance Center, Inc.
IOP and ICP- optic nerve head biomechanics
A study by Tong et al. 11 found that IOP and ICP have opposing effects on lamina cribrosa peak strain and lamina cribrosa depth. This study also found that the effects of ICP on the lamina cribrosa peak strain is three times lesser than the effect of IOP, while the effects of these forces of lamina cribrosa depth are equivalent. 11 Karimi et al conducted a study with three finite element models of the posterior eye, and found that IOP created a four time greater stress than cerebrospinal fluid pressure at the lamina cribrosa and peripapillary sclera. 12 A later study by Zhu et al. 13 imaged the eyes of adult monkeys with OCT under varying pressure conditions. This study found that both IOP and ICP had a significant effect on lamina cribrosa depth, aspect ratio and Bruch's membrane opening area. 13 This study also showed that by raising both ICP and IOP by the same amount, the effects do not cancel out, and that ICP may play a role in the sensitivity to IOP, which may affect glaucoma susceptibility. 13
Spontaneous venous pulsations
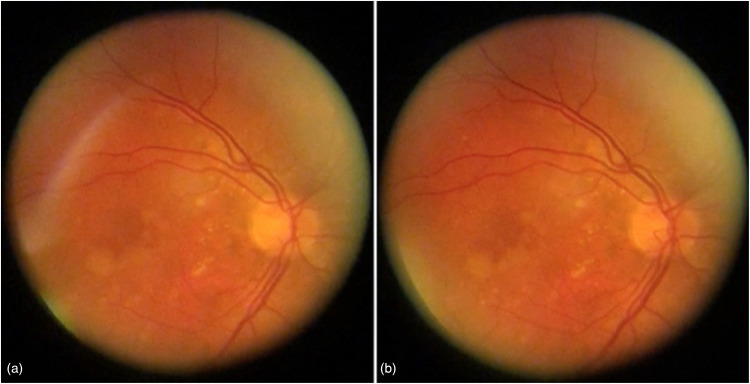
Spontaneous venous pulsation (SVP) is a pulse of the central retinal vein that occurs in both normal and pathological conditions and can be visualized using an ophthalmoscope. The presence of SVPs is decreased across glaucoma patients, but among normal individuals is around 87.6–98%.14–16 The cardiac cycle, which triggers the pulsation of cerebrospinal fluid (CSF) and in turn serves as a wave generator for SVP, is thought to be an important cause of a SVP. Transmural pressure, or the differential between IOP and retinal venous pressure, does, in fact, oscillate because of CSF pulsation.17,18 When the central retinal vein's volume notably changes, the SVP becomes prominent (Figure 3). The vessel must be compliant for this to happen and compliance diminishes at high volumes. 18 This physiological mechanism decreases compliance and prevents rupture. If the vessel is non-compliant then an SVP may not be observed but transmural pressure oscillations occurs at large retinal vein fill volumes.18,19 In contrast, the SVP is more noticeable when an oscillation occurs across a compliant vessel. While compliance is an important factor, change in compliance alone is not sufficient for the SVP to manifest. The SVP may not be seen even in a compliant vessel if the CSF pulse is not transferred to the central retinal vein.
Figure 3.
Spontaneous venous pulsations illustrated with still figures with (a) showcasing venous collapse of the retinal vein and (b) showcasing venous dilation of the retinal vein. Reprinted with permission from Laurent C, Hong SC, Cheyne KR, Ogbuehi KC. The Detection of Spontaneous Venous Pulsation with Smartphone Video Ophthalmoscopy. Clin Ophthalmol. under Creative Commons Attribution - Non Commercial (unported, v3.0) License.
The venous pulsation pressure (VPP) is the least IOP at which the central retinal vein is compliant enough to clearly pulse in response to CSF pulsations. 20 IOP raises the pressure difference between the blood in the retinal veins and the cavernous sinus, hence decreasing the volume of the retinal veins. As IOP falls, more blood flows into the retinal vein, increasing its volume and decreasing its compliance. In contrast, as IOP rises, less blood can fill the retinal vein, its volume drops, and its compliance rises.
SVP and VPP can be employed as an ophthalmic biomarker of pathological conditions, such as elevated ICP, 21 OAG, and possibly SANS. 22 Research indicates that patients with an elevated ICP are less likely to have SVPs. 21 The two alterations are believed to be driven by an increase in blood pressure within the retinal vein, which, in turn, causes the vessel's volume to grow beyond its compliance range. 19
Glaucoma patients were shown to be less likely to have SVP than non-glaucoma patients. 16 Furthermore, the VPP is greater in glaucoma patients. 23 These changes are believed to be a result of increased resistance at the central retinal vein at the lamina cribrosa, which increases retinal veinous volume upstream of the sclera, and attenuates the transmission of CSF pulsations causing non-compliance of the retinal venous system. 16 VPP levels are suggested to indicate disease progression. 24
Introduction to multi-pressure dial goggles
IOP exhibits a diurnal pattern of variation, with the greatest magnitude measured at night. Ocular perfusion diminishes as intraocular pressure rises. This is particularly crucial for ocular illnesses such as glaucoma. 25 IOP may be reduced non-pharmacologically and non-invasively with the use of negative pressure goggles (NPGs). For example, −10 mmHg administered by NPG across the orbit using a vacuum may reduce intraocular pressure by about 6 mmHg without impacting intracranial pressure.26,27 By exerting positive pressure, these eyewear may be able to increase IOP and normalize the TLPG in cases with increased ICP. The development of vacuum or pressure goggles for the orbital region is the subject of active study at present 28 and may have applications for OAG, 29 IIH, and SANS. The development of pressurized goggles and the most recent research are covered here.
Multi-pressure dial goggle for precisional TLPG modulation
The multi-pressure dial goggle is a device developed by Equinox (Equinox Ophthalmic, Inc., CA, USA) that comprises pressure-sensing goggles with a precisional modulating pump (Figure 4). Each eye can be connected to a separate pump which modulates the individual pressure of each eye. A handheld device can be used to control the pump and program individualized pressure values. An immediate lowering of IOP can then occur after the negative pressure microenvironment is established.
Figure 4.
Multi-pressure dial (MPD) goggles that allows for precisional application of negative pressure with an access port for pneumatonometry measurements during negative pressure application. Reprinted with permission from Ferguson TJ, Radcliffe NM, Van Tassel SH, Baartman BJ, Thompson VM, Lindstrom RL, Ibach MJ, Berdahl JP. Overnight Safety Evaluation of a Multi-Pressure Dial in Eyes with Glaucoma: Prospective, Open-Label, Randomized Study. Clin Ophthalmol. under Creative Commons Attribution – Non Commercial (unported, v3.0) License.
Specifications
The ophthalmic goggles include lenses with seals made of silicone, adjustable nasal bridge between the lenses, tubing to connect googles to the pump, a pump (programmed by investigators) and a head strap. Additionally to accommodate variations in facial anatomy, small, medium and large sized goggles are available. The mechanism of action of the goggles remains consistent at all goggle sizes. During studies, a modified version of the MPD system with a special pneumatonometry port was used, to allow IOP measurements to be performed while negative pressure is being applied (Figure 4).
Previous studies
Numerous studies have demonstrated the usefulness of NPGs as a feasible treatment option for ocular illnesses. Samuelson et al. 26 established that NPGs exhibited good safety parameters and the potential for well-tolerated, prolonged therapy administration in patients. In their investigation, 10 individuals with open-angle glaucoma had −10 mmHg applied to one eye and normal atmospheric pressure applied to the other eye for 8 h. At the completion of the trial, patient tolerance and interest in NPGs as prospective glaucoma treatments were reported. On a scale of 1–10 with 1 being the best, a positive interest response of 1.8 ± 0.5 was noted with a mean tolerability of 1.8 ± 0.4. 26
Ferguson et al. 30 examined the overnight safety of MPD in eyes with glaucoma over seven consecutive days with an randomized, open-label study. Prior to the testing period, the application of negative pressure (10 mmHg), caused IOP to 18.2 ± 3.8 mmHg to 14.0 ± 2.1 mmHg (p < 0.01). Following 7 days of the study, application of the negative pressure caused a reduction in IOP of 16.9 ± 4.3 mmHg to 13.5 ± 3.7 mmHg. This study showed that a statistically significant decrease in IOP (>20%) was seen, in addition to current therapy and that the MPD can be worn comfortably and safely overnight (Table 1).
Table 1.
Relevant literature relating to translaminar pressure gradients and spontaneous venous pulstations.
| The relationship between intraocular pressure (IOP) and intracranial pressure (ICP) | Ocular axons travel from the eye through the lamina cribrosa to the brain | https://pubmed.ncbi.nlm.nih.gov/33254565/ |
| Pressure changes cause lamina cribrosa distortion in papilledema and glaucomatous cupping. | The translaminar pressure gradient (difference between IOP and ICP) may have a role in the development of primary open-angle glaucoma (POAG) | https://pubmed.ncbi.nlm.nih.gov/33254565/ |
| Lamina cribrosa depth, aspect ratio, and Bruch's membrane opening area are all impacted by IOP and ICP. | Eyes of adult monkeys that were imaged revealed that both IOP and ICP had a significant impact on lamina cribrosa parameters | https://pubmed.ncbi.nlm.nih.gov/34736887/ |
| Using spontaneous venous pulsations (SVP) as an ophthalmic biomarker | SVPs are observed in both normal and pathological states, and their incidence decreases among individuals with glaucoma. | https://pubmed.ncbi.nlm.nih.gov/32099318/ |
| SVP and venous pulsation pressure (VPP) in glaucoma patients | Glaucoma patients are less likely to have SVPs and display higher VPP levels. | https://pubmed.ncbi.nlm.nih.gov/12881331/ |
| Negative pressure goggles (NPGs) for IOP reduction | NPGs can lower IOP without the need for pharmacologic or invasive procedures. | https://pubmed.ncbi.nlm.nih.gov/33061256/ |
| The application of multi-pressure dial (MPD) goggle for accurate modulation of the TLPG | The utilization of the MPD goggle helps with precise application of negative pressure, creating an immediate reduction of intraocular pressure. | https://pubmed.ncbi.nlm.nih.gov/33061256/ |
Ethier et al. 31 used a lumped parameter mathematical model to the effects of negative pressure goggles on IOP. The model used in this study considered episcleral venous pressure, aqueous humour dynamics and changes in the volume of ocular blood. The researchers found that clinical data is consistent with model-generated predictions if: 1) negative pressure goggles caused an increase in ocular globe volume and increased intraocular blood volume; 2) negative pressure causes a reduction in episcleral venous pressure, which alters aqueous humor dynamics and slows the speed of IOP adjustments. 31 It is important to note that the mechanism behind the increase of globe/blood volume as a rapid response to the application of negative pressure via goggles is not fully understood.
Shafer et al. 32 examined the effect of negative pressure in human cadaver eyes. The intra-goggle space, posterior segment and retrobulbar space were cannulated to provide measurements of retrobulbar pressure (RBP), IOP and intra-goggle pressure. This study found that negative pressure applied with the multi-pressure dial system reduced IOP without an effect on RBP.
In addition, it has been demonstrated that the goggles can deliver precise IOP measurements. Ferguson et al. 33 demonstrated that pneumotonometry may be performed using the excursion test method, which employs a Tono-Pen tip cover and NPGs to provide precise, reliable, and reproducible measurements of intraocular pressure (IOP). In this investigation, 480 paired IOP measurements were obtained with and without a Tono-Pen cover at four pressure levels of 7, 10, 20, and 30 mmHg. The results confirmed that pneumotonometry with a Tono-Pen cover in conjunction with NPG is an acceptable method for measuring IOP. 33
Lastly, a study by Swan et al. 34 demonstrated that among 65 participants randomized to receive either no negative pressure or negative pressure for 60 min at 25%, 50%, and 75% of baseline IOP, there was a clinically and statistically significant reduction in IOP at all negative pressure settings. This creates the potential for ocular illness applications in the real world.
Current IOP-controlling treatments include ocular drops, laser treatments, and surgical procedures.27,29 However, the IOP-lowering effects of these medications are frequently unpredictable and, in the case of eye drops, can be compromised by noncompliance. 29 With the use of NPG, a more targeted and precise method of decreasing IOP that is also accurate and well-tolerated by patients is available. 26
Future potential utilization of positive pressure goggles
SANS is found in astronauts after long-duration spaceflight (LDSF) and is characterized by globe flattening, optic disc edema, choroidal folds and a hyperopic refractive shift. 35 The pathophysiology of SANS is poorly known. Nonetheless, several ideas involving cephalad fluid changes, elevated intracranial pressure, ocular glymphatic system congestion, upward brain shift, and cerebral volume pulsatility have emerged.35–38 The findings of SANS were initially termed “Vision Impairment and Intracranial Pressure” (VIIP). Several findings have suggested that increased ICP may not be the sole reason for SANS; compared to terrestrial ICP elevations (e.g., IIH), astronauts do not display diplopia, severe headache, or pulsatile tinnitus. Post-flight lumbar puncture opening pressures have also been normal to only slightly elevated in SANS astronauts, and a much larger majority of SANS astronauts display asymmetric or unilateral optic disc edema as opposed to bilateral optic disc edema in IIH. While these differences occur, there is still a large focus on elevated ICP as a major role in SANS, as well as modulating the TLPG as a SANS countermeasure. Further research is required to fully understand the SANS pathophysiology, and our group has been developing a NASA-funded head mounted visual assessment system to finely assess the visual system during long duration spaceflight.39–41
As a mitigating method for SANS, swimming goggles have been studied. According to prior research, swimming goggles that exert direct pressure on the eye might artificially elevate IOP. 42 A terrestrial counterpart to SANS was created by placing swimming goggles with direct pressure on the eye on healthy subjects in a head-down tilt. During head-down tilt, swimming goggles were observed to raise IOP and the TLPG. The authors of the study propose that a moderate increase in IOP may aid in the safe mitigation of SANS; however, additional research is required to determine the safety of increasing IOP for extended periods. Positive pressure goggles may aid in increasing IOP more so than standard swimming goggles, hence giving an additional means of increasing the TLPG. 43
Conclusion
The TLPG is an emerging area of ophthalmic research on disorders of both IOP and ICP. Pressurized goggles may act as a potential treatment for terrestrial ocular disorders such as POAG or IIH by modulating this TLPG. By evaluating for SVPs during changes in pressure, these goggles may also permit indirect measurements of ICP. Using goggles to apply positive pressure as a therapeutic method for astronauts during extended spaceflight may be beneficial for the extraterrestrial condition, SANS. As an emerging countermeasure and therapeutic device for various ophthalmic diseases, further validation research is required for this modern technology for widespread clinical use.
Footnotes
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article. Dr. Berdahl is the founder and CEO of Equinox Ophthalmic, Inc.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: NASA Grant [NCC 9-58-SMST00012]: The Effects of Local Orbital Pressure Changes on Intraocular Pressure and Equinox Ophthalmic, Inc. (Newport Beach, CA).
ORCID iD: Ethan Waisberg https://orcid.org/0000-0001-8999-0212
References
- 1.Allison K, Patel D, Alabi O. Epidemiology of glaucoma: The past, present, and predictions for the future. Cureus 2020. doi: 10.7759/cureus.11686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Waisberg E, Micieli JA. Neuro-Ophthalmological optic nerve cupping: an overview. Eye Brain 2021; 13: 255–268. doi: 10.2147/EB.S272343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abe RY, Gracitelli CPB, Diniz-Filho A, et al. Lamina cribrosa in glaucoma: diagnosis and monitoring. Curr Ophthalmol Rep 2015; 3: 74–84. doi: 10.1007/s40135-015-0067-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schuster AK, Erb C, Hoffmann EM, et al. The diagnosis and treatment of glaucoma. Dtsch Arztebl Int 2020. doi: 10.3238/arztebl.2020.0225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Waisberg E, Ong J, Masalkhi M, et al. Anatomical considerations for reducing ocular emergencies during spaceflight. Ir J Med Sci 2023. doi: 10.1007/s11845-023-03407-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Waisberg E, Ong J, Masalkhi M, et al. Wearable, dropless reduction in intraocular pressure as an emerging therapy for glaucoma. Eye (Lond) 2023. doi: 10.1038/s41433-023-02448-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pinto VL, Tadi P, Adeyinka A. Increased intracranial pressure. In: StatPearls. Treasure Island, FL, USA: StatPearls Publishing, 2022. http://www.ncbi.nlm.nih.gov/books/NBK482119/ (accessed 16 October 2022). [PubMed] [Google Scholar]
- 8.Foltz EL, Blanks JP. Symptomatic low intracranial pressure in shunted hydrocephalus. J Neurosurg 1988; 68: 401–408. doi: 10.3171/jns.1988.68.3.0401 [DOI] [PubMed] [Google Scholar]
- 9.Lay CM. Low cerebrospinal fluid pressure headache. Curr Treat Options Neurol 2002; 4: 357–363. doi: 10.1007/s11940-002-0046-9 [DOI] [PubMed] [Google Scholar]
- 10.Berdahl JP, Ferguson TJ, Samuelson TW. Periodic normalization of the translaminar pressure gradient prevents glaucomatous damage. Med Hypotheses 2020; 144: 110258. doi: 10.1016/j.mehy.2020.110258 [DOI] [PubMed] [Google Scholar]
- 11.Tong J, Ghate D, Kedar Set al. et al. Relative contributions of intracranial pressure and intraocular pressure on Lamina cribrosa behavior. J Ophthalmol 2019; 2019: 1–8. doi: 10.1155/2019/3064949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karimi A, Razaghi R, Rahmati SM, et al. Relative contributions of intraocular and cerebrospinal fluid pressures to the biomechanics of the Lamina cribrosa and laminar neural tissues. Invest Ophthalmol Vis Sci 2022; 63: 14. doi: 10.1167/iovs.63.11.14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhu Z, Waxman S, Wang B, et al. Interplay between intraocular and intracranial pressure effects on the optic nerve head in vivo. Exp Eye Res 2021; 213: 108809. doi: 10.1016/j.exer.2021.108809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Levin BE. The clinical significance of spontaneous pulsations of the retinal vein. Arch Neurol 1978; 35: 37–40. doi: 10.1001/archneur.1978.00500250041009 [DOI] [PubMed] [Google Scholar]
- 15.Morgan WH, Hazelton ML, Azar SL, et al. Retinal venous pulsation in glaucoma and glaucoma suspects. Ophthalmology 2004; 111: 1489–1494. doi: 10.1016/j.ophtha.2003.12.053 [DOI] [PubMed] [Google Scholar]
- 16.Abegão Pinto L, Vandewalle E, De Clerck E, et al. Lack of spontaneous venous pulsation: possible risk indicator in normal tension glaucoma? Acta Ophthalmol (Copenh) 2013; 91: 514–520. doi: 10.1111/j.1755-3768.2012.02472.x [DOI] [PubMed] [Google Scholar]
- 17.Meyer-Schwickerath R, Kleinwächter T, Papenfuß HDet al. et al. Central retinal venous outflow pressure. Graefe’s Arch Clin Exp Ophthalmol 1995; 233: 783–788. doi: 10.1007/BF00184090 [DOI] [PubMed] [Google Scholar]
- 18.Moreno AH, Katz AI, Gold LDet al. et al. Mechanics of distension of dog veins and other very thin-walled tubular structures. Circ Res 1970; 27: 1069–1080. doi: 10.1161/01.res.27.6.1069 [DOI] [PubMed] [Google Scholar]
- 19.Morgan WH, DYi Y, Cooper RL, et al. Retinal artery and vein pressures in the dog and their relationship to aortic, intraocular, and cerebrospinal fluid pressures. Microvasc Res 1997; 53: 211–221. doi: 10.1006/mvre.1997.2010 [DOI] [PubMed] [Google Scholar]
- 20.Morgan WH, Hazelton ML, Yu DY. Retinal venous pulsation: expanding our understanding and use of this enigmatic phenomenon. Prog Retinal Eye Res 2016; 55: 82–107. doi: 10.1016/j.preteyeres.2016.06.003 [DOI] [PubMed] [Google Scholar]
- 21.Walsh TJ, Garden JW, Gallagher B. Obliteration of retinal venous pulsations. Am J Ophthalmol 1969; 67: 954–956. doi: 10.1016/0002-9394(69)90094-4 [DOI] [PubMed] [Google Scholar]
- 22.Mader TH, Gibson CR, Lee AG, et al. Unilateral loss of spontaneous venous pulsations in an astronaut. J Neuroophthalmol 2015; 35: 226–227. doi: 10.1097/WNO.0000000000000207 [DOI] [PubMed] [Google Scholar]
- 23.Jonas JB. Central retinal artery and vein collapse pressure in eyes with chronic open angle glaucoma. Br J Ophthalmol 2003; 87: 949–951. doi: 10.1136/bjo.87.8.949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Balaratnasingam C, Morgan WH, Hazelton ML, et al. Value of retinal vein pulsation characteristics in predicting increased optic disc excavation. Br J Ophthalmol 2007; 91: 441–444. doi: 10.1136/bjo.2006.105338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Konstas AG, Kahook MY, Araie M, et al. Diurnal and 24-h intraocular pressures in glaucoma: monitoring strategies and impact on prognosis and treatment. Adv Ther 2018; 35: 1775–1804. doi: 10.1007/s12325-018-0812-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Samuelson TW, Ferguson TJ, Radcliffe NM, et al. 8 h Safety evaluation of A multi-pressure dial in eyes with glaucoma: prospective, open-label, randomized study. OPTH 2019; 13: 1947–1953. doi: 10.2147/OPTH.S217736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chu N, Brambilla E, Yoo Pet al. et al. Evaluation of negative pressure transfer through tissue in a benchtop cornea and eyelid model. Ophthalmol Eye Dis 2020; 12: 251584142097140. doi: 10.1177/2515841420971406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Equinox Balance Goggles: The effects of local orbital pressure changes on intraocular pressure. National Space Biomedical Research Institute, http://nsbri.org/researches/equinox-balance-goggles-the-effects-of-local-orbital-pressure-changes-on-intraocular-pressure/ (accessed 16 October 2022).
- 29.Sheybani A, Scott R, Samuelson TW, et al. Open-Angle glaucoma: burden of illness, current therapies, and the management of nocturnal IOP variation. Ophthalmol Ther 2020; 9: 1–14. doi: 10.1007/s40123-019-00222-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ferguson TJ, Radcliffe NM, Van Tassel SH, et al. Overnight safety evaluation of a multi-pressure dial in eyes with glaucoma: prospective, open-label, randomized study. Clin Ophthalmol 2020; 14: 2739–2746. doi: 10.2147/OPTH.S256891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ethier CR, Yoo P, Berdahl JP. The effects of negative periocular pressure on intraocular pressure. Exp Eye Res 2020; 191: 107928. doi: 10.1016/j.exer.2020.107928 [DOI] [PubMed] [Google Scholar]
- 32.Shafer B, Ferguson TJ, Chu N, et al. The effect of periocular negative pressure application on intraocular and retrobulbar pressure in human cadaver eyes. Ophthalmol Ther 2022; 11: 365–376. doi: 10.1007/s40123-021-00442-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ferguson TJ, Knier CG, Chowdhury UR, et al. Intraocular pressure measurement with pneumatonometry and a tonometer tip cover. Ophthalmol Ther 2020; 9: 127–137. doi: 10.1007/s40123-020-00235-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Swan RJ, Ferguson TJ, Shah M, et al. Evaluation of the IOP-lowering effect of a multi-pressure dial at different negative pressure settings. Trans Vis Sci Tech 2020; 9: 19. doi: 10.1167/tvst.9.12.19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee AG, Mader TH, Gibson CR, et al. Spaceflight associated neuro-ocular syndrome (SANS) and the neuro-ophthalmologic effects of microgravity: a review and an update. npj Microgravity 2020; 6: 7. doi: 10.1038/s41526-020-0097-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wostyn P, Gibson CR, Mader TH. The odyssey of the ocular and cerebrospinal fluids during a mission to Mars: the “ocular glymphatic system” under pressure. Eye 2022; 36: 686–691. doi: 10.1038/s41433-021-01721-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Strangman GE, Zhang Q, Marshall-Goebel K, et al. Increased cerebral blood volume pulsatility during head-down tilt with elevated carbon dioxide: the SPACECOT study. J Appl Physiol 2017; 123: 62–70. doi: 10.1152/japplphysiol.00947.2016 [DOI] [PubMed] [Google Scholar]
- 38.Galdamez LA, Brunstetter TJ, Lee AGet al. et al. Origins of cerebral edema: implications for spaceflight-associated neuro-ocular syndrome. J Neuroophthalmol 2020; 40: 84–91. doi: 10.1097/WNO.0000000000000852 [DOI] [PubMed] [Google Scholar]
- 39.Ong J, Tavakkoli A, Zaman N, et al. Terrestrial health applications of visual assessment technology and machine learning in spaceflight associated neuro-ocular syndrome. npj Microgravity 2022; 8: 37. doi: 10.1038/s41526-022-00222-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Waisberg E, Ong J, Zaman N, et al. Head-Mounted dynamic visual acuity for G-transition effects during interplanetary spaceflight: technology development and results from an early validation study. aerosp med hum Perform 2022; 93: 800–805. doi: 10.3357/AMHP.6092.2022 [DOI] [PubMed] [Google Scholar]
- 41.Ong J, Zaman N, Kamran SA, et al. A multi-modal visual assessment system for monitoring Spaceflight Associated Neuro-ocular Syndrome (SANS) during long duration spaceflight. J Vis 2022; 22: 6. doi: 10.1167/jov.22.3.6 [DOI] [Google Scholar]
- 42.Morgan WH, Cunneen TS, Balaratnasingam Cet al. et al. Wearing swimming goggles can elevate intraocular pressure. Br J Ophthalmol 2008; 92: 1218–1221. doi: 10.1136/bjo.2007.136754 [DOI] [PubMed] [Google Scholar]
- 43.Scott JM, Tucker WJ, Martin D, et al. Association of exercise and swimming goggles with modulation of cerebro-ocular hemodynamics and pressures in a model of spaceflight-associated neuro-ocular syndrome. JAMA Ophthalmol 2019; 137: 652. doi: 10.1001/jamaophthalmol.2019.0459 [DOI] [PMC free article] [PubMed] [Google Scholar]