Abstract
Context
Cognitive deficits are neuropsychiatric syndromes associated with systemic lupus erythematosus. In our context, there are no data on the frequency of cognitive deficit as a manifestation of neuropsychiatric SLE or the associated conditions.
Objective
To define determinants of cognitive deficit in a cohort of Colombian patients with SLE attending a third-level hospital.
Methods and Patients
This descriptive cross-sectional study included patients with SLE, explored the presence of cognitive impairment through screening testing using the Montreal Cognitive Assessment (MoCA test), and diagnostic confirmation with a specific neuropsychological test battery recommended by the American College of Rheumatology. Quality of life was assessed using the LupusCol questionnaire and depression using the Beck Depression Inventory.
Results
Most patients were women, with a median age of 37 years (IQR, 28.0 - 46.7). Most patients had a level of higher education or technical education. Fifty-nine (62.9%) patients presented with a normal MoCA test result ≥26 points, and 35 (37.1%) patients with a score <26 points that were considered abnormal. The comprehensive neuropsychological test battery was applied to 31 patients (33.0%) with an abnormal MoCA test. Forty-one patients (48.8%) had some degree of depression. The median loss of quality of life was 21.03% (IQR 10.2 - 40.3). 19 patients (20%) presented some degree of cognitive deficit, 15 (15.95% of the total sample) had cognitive impairment, and 4 (4.25%) had cognitive decline. In a logistic regression analysis using data from patients undergoing specific tests, variables related to cognitive deterioration were found to be associated with a lower quality of life, showing an adjusted odds ratio of 1.05 (CI 1.01-0.09). No association was demonstrated with SLEDAI, prednisolone use, cyclophosphamide use, and the presence of depression.
Conclusion
In this study, it was found in 16% of patients evaluated with the complete neuropsychological test battery and in 37% with the MoCA screening test. Our results suggest that it is crucial to implement strategies to assess cognitive deficit, depression, and quality of life in the consultation of patients with SLE and to raise awareness among health providers who care for patients with lupus about their presence and impact.
Keywords: Systemic lupus erythematosus, cognitive deficit, depression, quality of life
Introduction
Cognitive deficit (CD) is a neuropsychiatric syndrome associated with systemic lupus erythematosus (SLE). 1 It is an insidious manifestation, generally subclinical, and its diagnosis is difficult because of the variability of the tests and the lack of standardization. 2 Some comorbidities can generate or increase CD levels and have a confounding effect. In addition, medications such as corticosteroids and immunomodulators, which are frequently used in these patients, are associated with the presence of cognitive deficit. For these reasons, studies to estimate the prevalence of CD present heterogeneity and variable results between 5% and 80%. 3 The relative risk of cognitive impairment in SLE was higher than that in rheumatoid arthritis (1.80) and healthy individuals (2.80). 4 In many cases, it is difficult to establish whether the presence of CD is primary, i.e., associated with SLE, or if it is secondary to medications or comorbidities. 5 Cognitive deficits in SLE can affect the quality of life and social role 6 and lead to a progression to dementia. 7 In our setting, the frequency of CD associated with lupus, the associated factors, and determining conditions are unknown. Therefore, it is important to know the characteristics of CD associated with lupus in our country in addition to other countries. 8 In addition, it will enable the development of rehabilitation programs in the future and include neuropsychological evaluation as part of the comprehensive care of these patients.
Our study defined the determinants of CD in a cohort of Colombian patients with SLE who attended a third-level hospital.
Materials and methods
This study was approved by the Ethics Committee of the Universidad Nacional de Colombia and the Hospital Universitario Nacional de Colombia, with the ID: CEI-2020-11-06,), and it was conducted under the International Guidelines for Biomedical Research in Humans, the Declaration of Helsinki, amendment 2008, the Colombian Law 29 of 1990, and Resolution 008,430 of 1993. All participants provided informed consent upon entering the study.
This cross-sectional observational study that consecutively included 94 patients diagnosed with SLE who met the criteria of the European League Against Rheumatism/American College of Rheumatology 2019. 9
They were treated at the rheumatology service of the Hospital Universitario Nacional de Colombia from January 2016 to April 2021. The exclusion criteria were a personal history of neurological disease (neurological pathology recorded in the medical record or clinical history before the study evaluation), some neurological alteration detected during the evaluation of the study, and patients with acute neuropsychiatric activity or an episode of neurolupus in the last 6 months (evaluated with the BILAG questionnaire). 10 Incomplete basic primary education, inability to read and write, and history of a diagnosis of depression before the diagnosis of SLE. Patients older than 64 years of age or hospitalized.
The collection of sociodemographic, clinical, and laboratory data in routine clinical practice during the last 6 months was conducted. Information on the Systemic Lupus Erythematous Disease Activity Index-2000 (SLEDAI-2K) was collected. 11
The patients were contacted by phone and scheduled for the first face-to-face or virtual session to apply the Montreal Cognitive Assessment (MoCA) cognitive deficit screening test, which has demonstrated adequate psychometric properties and diagnostic utility for CI in SLE.8,12
The Beck Depression Inventory (BDI-II) measurement test was used to search for depression. 13 In addition, LupusCol is an instrument for the evaluation of health-related quality of life in Colombian adult patients with SLE. LupusCol is composed of 44 questions and seven domains and significant values for validity, reliability, and sensitivity to change in the studied population. Pearson’s correlation for the criteria validity is −0,48; a Cronbach’s α coefficient for internal consistency was 0, 96, intraclass correlation coefficient for personal test-retest-telephone was 0.96, and intraclass correlation coefficient personal test-retest-personal was 0.96. For interrater concordance, Pearson’s correlation was 0.8, intraclass correlation coefficient was 0.77, and Lin’s coefficient was 0.86. Sensitivity to change was demonstrated through analysis of variance, obtaining significant indicators about the scale, demonstrating the instrument’s ability to detect changes in health-related quality of life.14,15
Subsequently, patients with a score below 26 points on the MoCA test were given a specific battery test for CD that included: verbal and auditory memory, attention, constructive praxis, verbal fluency, and executive functions using the following tests:
(1) Learning and memory test with free encoding 16 ;
(2) Copy and evocation of the Rey–Osterrieth complex Figure 17 :
(3) Trail making test, forms A and B 18 ;
(4) Phonemics (FAS) and semantic verbal fluency (animal naming) 19 ;
(5) Stroop test 20 ;
(6) WAIS-III Digit Symbol Substitution Test (DSST) 21 and
(7) Symbol Digit Modalities Test (SDMT). 22
These specific tests were applied within a maximum of 60 days after applying the MoCA test.
This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE). 23
Statistical analysis
Normative data were used to qualify neuropsychological tests based on studies conducted in the Colombian context. For the verbal memory test, the means and standard deviations proposed by Alarcón were used as a reference 16.
The Stroop test was evaluated using the normative data proposed by Barreto. 20 The SDMT 24 and the TMT 25 with the normative data proposed by Chavarro et al. 24 and the Rey–Osterrieth complex figure, the semantic fluency, the phonological fluency, and the DSST with the normative data proposed by Torres. 26
Patients with scores lower than 26 points on the MoCA test and a score below the average range in any cognitive domain of the battery of neuropsychological tests were included in the CD group. Patients who obtained an initial MoCA test score of fewer than 26 points, in addition to a score of less than 2.0 standard deviations below the mean in any cognitive domain, were considered to have cognitive impairment; scores between −1.5 and −1.9 standard deviations below the mean were interpreted as cognitive decline.
Patients who were identified as not having cognitive deficit were patients with a MoCA result equal to or greater than 26 points (who did not require the battery of specific tests), and those patients with MoCA less than 26 in addition to normal specific tests.
These criteria are those proposed by the ACR. 27
A descriptive analysis of the sociodemographic, clinical, laboratory, and imaging variables of the population was performed. The normality of the variables was examined using the Shapiro– Wilk test. Continuous data are presented as mean with standard deviation (SD) or median with interquartile range (IQR), as appropriate, and categorical variables are presented with frequencies and percentages. The frequency of cognitive impairment was calculated in each test and was defined based on the approach described by Crawford 1998, which allows for comparing the performance of a case with that of a group of paired controls. The use of this method had better psychometric properties than a Z-score when the normative sample was less than 50, and the performance was equal when the sample was greater than 50.28,29
According to the case, association analysis of the variables was performed using Fisher’s exact test and the U-Mann-Whitney U test. A logistic regression model analysis was performed to evaluate the association between cognitive impairment and age, adjusting for dyslipidemia, depression, quality of life, SLEDAI-2K, and prednisone use. The statistical significance threshold was α = 0.05 using the two-tailed tests. Confounding and interaction variables were controlled.
Results
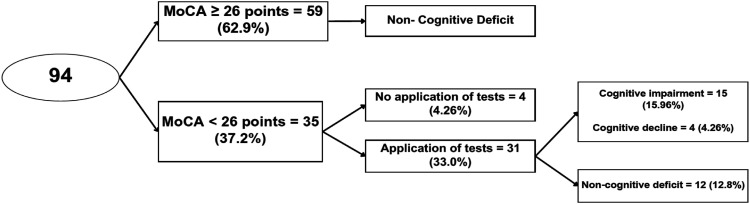
One hundred one patients were consecutively invited to participate; 94 patients agreed and underwent MoCA screening. Four patients with MoCA 26 did not complete the specific tests, three of them had problems due to the COVID-19 pandemic, and the other had problems traveling. The other seven patients who refused to undergo all tests were mainly due to issues with the COVID-19 pandemic, time constraints, or competing priorities. The rejection rate was 10.9% (Figure 1). Most of the 94 patients were women (n = 90; 95.7%) with a median age of 37 years (IQR, 28.0 - 46.7). The age of the patients ranged from 18 years to 59 years: 32 patients (34.0%) in the 18–29-year-old group; 22 patients (23.5%) in the 30–39-year-old group; 23 patients (24.5%) in the 40–49-year-old group; and 17 patients (18.0%) in the 50–59-year-old group. The median duration of the disease was 9 years (IQR 4-16). The majority of patients had a level of higher education or technical education. The demographic and clinical characteristics, comorbidities, medications, and classification of patients according to CD status are shown in Table 1. When comparing clinical and laboratory characteristics between groups (univariate analysis) according to CD status, there were no differences (Table 2). In addition, when classifying the patients as never/ever used prednisolone and comparing the two groups, no differences were found (Table 3). Antinuclear antibodies were positive in all patients, and positive anti-DNA antibodies and consumed complement were present in approximately a quarter of the patients (Table 1). The median loss of quality of life was 21.03% (IQR 10.2 - 40.3). Domains of the quality of life scale with the highest affectation related to the presence of CD were: fatigue, physical dominance, and the feeling of being a burden to others. Specific tests were performed on 31 (33.0%) patients with an abnormal MoCA test; the remaining four patients did not undergo specific tests. Forty-one patients (48.8%) had some degree of depression (Figure 1). 15 patients (15.95% of the total sample) had cognitive impairment, and 4 (4.25%) had cognitive decline. Therefore, 19 patients (20%) presented with some degree of CD.
Figure 1.
Flowchart of patients (n = 94) according to the presence of cognitive deficit: 101 patients were invited to participate, 94 performed the MoCA test. Specific tests were performed on 31 (33.0%) patients with an abnormal MoCA test, the remaining four patients did not attend the specific tests (rejection rate was 10.9%). Due to problems caused by the COVID-19 pandemic, problems traveling to the study center, or competing priorities. 15 patients (15.95% of the total sample) had cognitive impairment, and 4 (4.25%) had cognitive decline. Therefore, 19 patients (20%) presented some degree of CD.
Table 1.
Characteristics of the population.
| Variable | Total: 94 n (%) |
|---|---|
| Demographics | |
| Age (median, IQR) | 37 years (28.0 - 46.7) |
| Women | 90 (95.7%) |
| Education level | |
| Primary | 3 (3.2%) |
| Incomplete high school | 3 (3.2%) |
| Graduated from high school | 15 (16.0%) |
| Technician/technologist | 22 (23.4%) |
| Professional | 41 (43.6%) |
| Postgraduate education | 10 (10.6%) |
| Clinic | |
| Age of disease onset, median (IQR) | 25 (18.25-31.5) |
| Duration of disease, median (IQR) | 9 (4.0-16.0) |
| Renal impairment | 53 (56.4%) |
| Hematologic alteration | 49 (52.1%) |
| Mucocutaneous involvement | 87 (92.6%) |
| Drugs | |
| Prednisolone | |
| 1 to 10 mg daily | 53 (56.4%) |
| 11-30 mg day | 7 (7.4%) |
| More than 31 mg a day | 2 (2.1%) |
| Not use | 32 (34.0%) |
| Cyclophosphamide | 3 (3.2%) |
| Comorbidities | |
| Diabetes mellitus | 0 (0%) |
| Dyslipidemia | 21 (19.8%) |
| Essential hypertension | 2 (2.1%) |
| Score of LupusCol (N = 82), median (IQR) | 21.3 (10.7- 40.3) |
| Depression (beck equal to or greater than 13 points) | 41.0 (48.8%) |
| SLEDAI-2K (79), median (RIQ) | 6 (2-8) |
| Laboratory | |
| Positive antinuclear antibodies | 94 (100%) |
| C3 consumed (N = 79) | 28 (35.4%) |
| C4 consumed (N = 79) | 24 (30.4%) |
| Positive Anti-DNA (N = 79) | 26 (32.9%) |
Table 2.
Univariate analysis of patients according to cognitive deficit status and clinical data.
| Variable | Patients with cognitive deficit | Patients without cognitive deficit | Wald test | p-value |
|---|---|---|---|---|
| Women | 23 | 71 | 1.05 | 0.57 |
| Level of education | ||||
| Complete high school | 6 | 12 | 1.39 | 0.16 |
| Technical | 4 | 18 | 0.47 | 0.63 |
| Professional | 4 | 31 | −0.11 | 0.91 |
| Postgraduate | 2 | 14 | 0.75 | 1.00 |
| Type occupation | ||||
| Student | 0 | 4 | 1.15 | 0.12 |
| Unemployed | 0 | 6 | 0.47 | 0.63 |
| Employee | 13 | 50 | 0.72 | 0.06 |
| Retiree | 0 | 5 | 0.30 | 0.48 |
| Independent | 0 | 6 | 0.19 | 0.32 |
| Renal | 53 | 41 | 1.17 | 0.24 |
| Hematological | 49 | 45 | 1.07 | 0.28 |
| Mucocutaneous | 7 | 87 | −0.36 | 0.71 |
| Anti-DNA | 26 | 68 | −0.79 | 0.42 |
| C3 | 28 | 66 | −1.27 | 0.20 |
| C4 | 24 | 70 | −1.32 | 0.18 |
| Cyclophosphamide | 3 | 91 | 1.73 | 1.08 |
Table 3.
Univariate analysis of patients according to never/ever used prednisolone.
| Variable | Never used | Ever used | Wald test | p-value |
|---|---|---|---|---|
| Women | 31 | 59 | −0.39 | 0.69 |
| Level of education | ||||
| Complete high school | 7 | 11 | −0.46 | 0.62 |
| Technical | 8 | 14 | −0.33 | 0.74 |
| Professional | 12 | 23 | −0.21 | 0.83 |
| Postgraduate | 5 | 11 | −0.19 | 0.87 |
| Type occupation | ||||
| Student | 2 | 2 | −0.70 | 0.48 |
| Unemployed | 1 | 5 | 0.59 | 0.55 |
| Employee | 22 | 41 | −0.30 | 0.76 |
| Retiree | 2 | 3 | −0.39 | 0.69 |
| Independent | 2 | 4 | −0.14 | 0.88 |
| Renal | 32 | 62 | 0.89 | 0.37 |
| Hematological | 32 | 62 | 0.30 | 0.76 |
| Mucocutaneous | 32 | 62 | −0.83 | 0.40 |
| Anti-DNA | 32 | 62 | −0.85 | 0.39 |
| C3 | 32 | 62 | −0.66 | 0.51 |
| C4 | 32 | 62 | −1.04 | 0.29 |
| Hypertension | 32 | 62 | 1.05 | 0.54 |
The results of the specific neuropsychological tests showed that the patients exhibited alteration in verbal memory, attention, and executive functions (Table 4).
Table 4.
Results of specific neuropsychological tests (n = 31).
| Cognitively impaired (11/36.6%) | Without cognitive impairment (19/63.3%) | |||||
|---|---|---|---|---|---|---|
| Absent | Present | Absent | Present | p | Odds Ratio | |
| Verbal Memory | 6 | 9 | 13 | 2 | 0.02 | 0.1 |
| (20%) | (30%) | (86.66%) | (13.33%) | [0.01-0.62] | ||
| Visual Memory | 13 | 2 | 15 | 0 | 0.48 | 0.17 |
| (43.33%) | (6.66%) | (100%) | (0%) | [0.00-3.96] | ||
| Attention | 7 | 8 | 14 | 1 | 0.01 | 0.06 |
| (23.33%) | (26.66%) | (93.33%) | (6.66%) | [0.00 - 0.604] | ||
| Verbal Fluency | 14 | 1 | 15 | 0 | 1.00 | 0.31 |
| (46.66%) | (3.33%) | (100%) | (0%) | [0.01-8.28] | ||
| Praxias and | 15 | 0 | 15 | 0 | 1.00 | N/A |
| Visuo-constructive ability | (50%) | (0%) | (100%) | (0%) | ||
| 5 | 10 | 14 | 1 | <0.00 | 0.03 | |
| Executive functions | (16.66%) | (33.33%) | (93.33%) | (6.66%) | [0.00-3.5] | |
The variables of interest related to CD were included in the logistic regression model with the data obtained from the patients who attended the specific tests, and it was observed that cognitive deterioration was associated with a low quality of life (adjusted OR of 1.05; CI 1.01-0.09). No association of cognitive impairment with SLEDAI-2K score, prednisolone use at different doses, cyclophosphamide use, or the presence of depression was demonstrated (Table 5).
Table 5.
Logistic regression model analysis.
| Variable | Crude odd ratio (COR) (95%CI) | Adjusted odds ratio (AOR) (95%CI) | p (Wald test) | p (LR test) |
|---|---|---|---|---|
| Age (years) | 0.99 (0.63-1.56) | 1.77 (0.87- 3.57) | 0.11 | 0.99 |
| Depression | 1.00 (0.95-1.06) | 1.00 (0.93-1.06) | 0.96 | 0.75 |
| LupusCol | 1.03 (1.00-1.06) | 1.05 (1.01-1.09) | 1.05 | 0.02 |
| SLEDAI | 0.95 (0.2-2.58) | 0.96 (0.83-1.09) | 0.57 | 0.47 |
| Dyslipidemia | 1.31 (0.41-4.20) | 2.36 (0.51-10.8) | 0.26 | 0.64 |
| Prednisone dosage | 1.12 (0.70-1.45) | 1.19 (0.75-1.87) | 0.44 | 0.94 |
Discussion
In this study, we explored the frequency and type of cognitive impairment in a cohort of Colombian patients with SLE and the associated factors. These data can take health measures to mitigate this problem in the future. For this reason, the research is considered relevant because knowing the frequency and type of cognitive alterations could help to make visible the need to include neuropsychological evaluation as part of the comprehensive care of our patients and to develop rehabilitation programs in our country. In this sample of 94 patients, a frequency of CD of approximately 16% was found, which coincides with some reports in the literature. 4 The variability of the frequency of CD in the literature has been found to be high, partly because different diagnostic tools have been used. Some studies have included the application of a specific neuropsychological battery, whereas others have included screening tests such as the Mini-mental State Examination or the MoCA test. 30 In our study, there was an approximate 3:1 relationship between abnormal MoCA test and diagnosis of CD by neuropsychological specific tests, evidencing that the application of the MoCA test as a screening method is not sufficient to identify this entity and that it is necessary to perform specific neuropsychological test batteries. For this reason, we decided to administer MoCA as a screening test and, subsequently, the battery of specific tests to seek greater specificity in identifying patients with CI. We found that the specific domains with the highest affectation were verbal memory and executive functions, which were similar to those reported in previous studies. 31
MoCA is considered a valid screening tool for detecting CD in SLE, both for epidemiological studies and routine clinical care, where its routine use has been recommended. The MoCA cut-off established for our study was based on the cut-off reported as useful for detecting patients with cognitive impairment in SLE.32–34 Using a cut-off point of 26, the sensitivity of MoCA was 83% and the specificity 73% compared with Automated Neuropsychological Assessment Metrics (ANAM). 12 The area under the curve (0.76–0.78) of the MoCA cut-off of <27 had the highest diagnostic accuracy in diagnosing cognitive impairment when compared to a battery of neuropsychological tests recommended by ACR. 31
It is not common for treating physicians to actively search for cognitive impairment in patients with SLE. In a study conducted by Pamfil et al. in 2 European centers, only 27% of patients with SLE and suspicion of possible CD were referred for evaluation by neuropsychology and application of the battery of tests. 35
However, an increasing number of studies have included this specific diagnostic tool for the search for CD. 36 For this reason, this study is useful in our country to consider CD in patients who attend lupus care clinics. 37
Concerning other research in Latin America, the frequencies reported are variable but generally higher than those reported in our study. 16 A multicenter study conducted in Argentina by D'Amico et al. reported a CD frequency of 65% using the battery of neuropsychological tests proposed by the ACR. In addition, a depression frequency of 48% was found, similar in our study.
Similar to this study, Calderón et al. found that 16% of their patients had CD. They did not find an association between the presence of CD and depressive symptoms. In patients with CD, special impairment was found in sustained attention tasks and visuospatial working memory. 38 In addition, in this study, an association was found between the presence of CD and a negative perception of the quality of life in patients. 39 This association was confirmed in a systematic literature review developed by Mendelsonh et al. 40
In our study, the lowest scores reported by patients in the quality of life questionnaire were in the domains of fatigue, physical domain, and burden for others, showing a more significant compromise in these areas. The study by Calderón et al. reported the association between the affectation of quality of life and the presence of CD, mainly due to executive function, 38 findings that were similar to our study.
Conditions associated with SLE that can generate CD deficit have been described in the literature, such as the use of corticosteroids, 6 immunosuppressants such as cyclophosphamide, 3 the presence of depression, 41 and comorbidities such as arterial hypertension and diabetes mellitus. 42 We did not find a relationship between these conditions and the presence of CD. Although we did not find an association between depression and CD, we found a high frequency of depressive symptoms (49.8%), consistent with the prevalence reported in the literature between 17 % and 75%. 43 For the Beck Depression Inventory, a 13-point cut-off point was established, with a sensitivity of 95.2% and a specificity of 85.1% in patients with SLE. 44
In our study, no relationship was found between CD and the presence of lupus activity (measured using SLEDAI 2K), probably in part because patients with acute neuropsychiatric activity or with an episode of neurolupus in the last 6 months were excluded, and the literature has reported that these patients have a higher frequency of CD.45,46
Antiphospholipid antibodies have been reported to be related to cognitive impairment.5,47,48 However, we could not include them because only 14 patients provided the results, so it was impossible to establish associations.
Our study excluded patients over 64 years of age to avoid confounding factors such as the presence of comorbidities as much as possible cardiovascular disorders and mainly due to the possible coexistence with dementia syndromes, entities that can generate cognitive alterations. In the D'Amico et al. 37 study, ethnicity was included as a variable, and a higher frequency of CD was found in white patients. Studies reported in the GLADEL cohort have shown that ethnicity can influence different alterations associated with organ disease. 49 In our study, we did not consider this variable, given that in the region of the country where the study was conducted, most patients were mixed-race, and only this type of patient was included.
Among the strengths of the present study, it should be mentioned that a battery of neuropsychological tests was used that covered all cognitive domains and complied with the ACR recommendations for neuropsychological assessment in patients with SLE. 50 In addition, we attempted to analyze the most significant number of variables that could be involved with the development of CD in the evaluated population. To measure the quality of life, we used a tool specific to patients with SLE and validated in Colombia, 15 giving greater validity to our results.
Our study has some limitations. It was impossible to obtain the information of four patients (about 5%), so the results are based on the patients in whom all the tests could be performed, and probably the frequency of cognitive decline could have been higher. It was impossible to obtain this information from the results of the antiphospholipid antibodies. The type of design of this study does not allow the establishment of definitive conclusions; therefore, the characteristics of this cross-sectional study have less statistical power because patient collection was difficult due to the nature of the study and the coincidence with the onset of the SARS-COV-2 pandemic. No MRI was reported. MRI may be relevant, since p because with SLE are more likely to develop ischemic stroke and vascular dementia, even at younger ages. In addition, it is advisable that every patient with dementia undergo brain imaging. However, not all patients had MRI result information, and our objective was to describe the cognitive deficit of these patients regardless of their specific causes.
Conclusion
Cognitive deficits in patients with SLE are frequently subclinical and poorly evaluated. The prevalence of CD in the Colombian population with SLE is not known, and in our study, CD was found in 16% of the population with specific tests and 37% with the MoCA screening test. The domains of the quality of life scale with the highest affectation related to the presence of CD were fatigue, physical dominance, and the feeling of being a burden to others. In the specific tests, verbal memory and executive function alterations were observed. Cognitive deficits in patients with SLE were associated with low scores on a specific quality of life scale. No association was found between CD and depression. However, we found that approximately half of the patients had some degree of depression. It is important to implement strategies to assess CD, depression, and quality of life in the follow-up of patients with SLE and to sensitize health personnel about their presence and impact. We consider this research relevant because knowing the frequency and type of cognitive alterations could help develop rehabilitation programs and include neuropsychological evaluation as part of the comprehensive care of our patients.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Dirección de Investigación, Universidad Nacional de Colombia (Hermes/51292).
ORCID iD
Carlos M Rodriguez-Plata https://orcid.org/0000-0003-4134-1891
References
- 1.Loukkola J, Laine M, Ainiala H, et al. Cognitive impairment in systemic lupus erythematosus and neuropsychiatric systemic lupus erythematosus: a population-based neuropsychological study. J Clin Exp Neuropsychol 2003; 25(1): 145–151. [DOI] [PubMed] [Google Scholar]
- 2.Reeves DL, Winter KP, Bleiberg J, et al. ANAM® Genogram: historical perspectives, description, and current endeavors. Arch Clin Neuropsychol 2007; 22(SUPPL. 1): 15–37. [DOI] [PubMed] [Google Scholar]
- 3.Kello N, Anderson E, Diamond B. Cognitive dysfunction in systemic lupus erythematosus: a case for initiating trials. Arthritis Rheumatol 2019; 71(9): 1413–1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rayes HA, Tani C, Kwan A, et al. What is the prevalence of cognitive impairment in lupus and which instruments are used to measure it? A systematic review and meta-analysis. Semin Arthritis Rheum 2018; 48(2): 240–255. [DOI] [PubMed] [Google Scholar]
- 5.Appenzeller S, Lapa AT, De Carvalho JF, et al. Cognitive dysfunction and antiphospholipid antibodies. Curr Rheumatol Rep 2012; 14(1): 95–98. [DOI] [PubMed] [Google Scholar]
- 6.Montero-López E, Santos-Ruiz A, Navarrete-Navarrete N, et al. The effects of corticosteroids on cognitive flexibility and decision-making in women with lupus. Lupus 2016; 25(13): 1470–1478. [DOI] [PubMed] [Google Scholar]
- 7.Zhao Z, Rocha NP, Salem H, et al. The association between systemic lupus erythematosus and dementia: a meta-analysis. Dement e Neuropsychol 2018; 12(2): 143–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kanapathy A, Nik Jaafar NR, Shaharir SS, et al. Prevalence of cognitive impairment using the Montreal Cognitive Assessment questionnaire among patients with systemic lupus erythematosus: a cross-sectional study at two tertiary centres in Malaysia. Lupus 2019; 28(7): 854–861. [DOI] [PubMed] [Google Scholar]
- 9.Shantanam S. European League against rheumatism/American College of rheumatology classification criteria for systemic lupus erythematosus. Physiol Behav. 2018;176(1):139–148. [Google Scholar]
- 10.Isenberg DA, Rahman A, Allen E, et al. Development and initial validation of an updated version of the British Isles Lupus Assessment Group’s disease activity index for patients with systemic lupus erythematosus.;Rheumatology 2005;44(7):902–906. [DOI] [PubMed] [Google Scholar]
- 11.Touma Z, Urowitz MB, Gladman DD. Systemic lupus erythematosus disease activity index 2000. J Rheumatol 2002; 29: 288–291. [PubMed] [Google Scholar]
- 12.Adhikari T, Piatti A, Luggen M. Cognitive dysfunction in SLE: development of a screening tool. Lupus 2011; 20(11): 1142–1146. [DOI] [PubMed] [Google Scholar]
- 13.Beck AT, Ward CH, Mendelson M, et al. An Inventory for Measuring Depression the difficulties inherent in obtaining, 1960, pp. 561–571. [DOI] [PubMed] [Google Scholar]
- 14.McElhone K, Abbott J, Shelmerdine J, et al. Development and validation of a disease-specific health-related quality of life measure, the LupusQoL, for adults with systemic lupus erythematosus. Arthritis Care Res 2007; 57(6): 972–979. [DOI] [PubMed] [Google Scholar]
- 15.Quintana LG, Muñetón López G, Coral-Alvarado P, et al. Design and validation of LupusCol, an instrument for the evaluation of health-related quality of life in colombian adult patients with systemic lupus erythematosus. Rheumatology 2014; 54(1): 104–112. [DOI] [PubMed] [Google Scholar]
- 16.Alarcón AN, Ayala OD, García JR, et al. Validation of the brief international cognitive assessment for multiple Sclerosis (BICAMS) in a Colombian population. Mult Scler Relat Disord 2020; 42(March): 102072. [DOI] [PubMed] [Google Scholar]
- 17.Brown RR, Partington JE. The intelligence of the narcotic drug addict. J Gen Psychol 1942; 26(1): 175–179. [Google Scholar]
- 18.Bowie CR, Harvey PD. Administration and interpretation of the trail making test. Nat Protoc 2006; 1(5): 2277–2281. [DOI] [PubMed] [Google Scholar]
- 19.Range K, Moser YA. Validity of brief screening tools for cognitive impairment in rheumatoid arthritis and systemic lupus erythematosus. Bone 2012; 23(1): 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barreto LCR, Pulido NDC, Roa CAP. Psychometric properties of the Stroop color-word test in non-pathological Colombian population. Univ Psychol 2016; 15(2): 255–272. [Google Scholar]
- 21.Silva MA. Development of the WAIS-III: a brief overview, history, and description. Grad J Couns Psychol 2008; 1(1): 117–135. [Google Scholar]
- 22.Sheridan LK, Fitzgerald HE, Adams KM, et al. Normative Symbol Digit Modalities test performance in a community-based sample. Arch Clin Neuropsychol 2006; 21(1): 23–28. [DOI] [PubMed] [Google Scholar]
- 23.Vandenbroucke JP, Von Elm E, Altman DG, et al. Strengthening the reporting of observational studies in Epidemiology (STROBE): explanation and elaboration. Epidemiology 2007; 18(6): 805–835. [DOI] [PubMed] [Google Scholar]
- 24.Chavarro L. Neuropsychological characterization of executive functioning in adults healthy young people residing in the city of Bogotá. Universidad Nacional de Colombia, 2020. [Google Scholar]
- 25.Espitia A. Funciones ejecutivas en el envejecimiento normal: Datos normativos con la batería Neuronorma. Colombia. In: Universidad Nacional de Colombia Sede Bogotá Facultad de Ciencias Humanas Departamento de Psi. 2017. [Google Scholar]
- 26.Torres VL, Vila-Castelar C, Bocanegra Y, et al. Normative data stratified by age and education for a Spanish neuropsychological test battery: results from the Colombian Alzheimer’s prevention initiative registry. Appl Neuropsychol 2021; 28(2): 230–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Esdaile JM, Alarcón GS, Crofford L, et al. Proposed response criteria for neurocognitive impairment in systemic lupus erythematosus clinical trials. Lupus 2007; 16(6): 418–425. [DOI] [PubMed] [Google Scholar]
- 28.Crawford JR, Howell DC. Comparing an individual’s test score against norms derived from small samples. Clin Neuropsychol 1998; 12(4): 482–486. [Google Scholar]
- 29.Crawford JR, Garthwaite P. Single-case research in neuropsychology: a comparison of five forms of t-test for comparing a case to controls. Cortex 2012; 48(8): 1009–1016. [DOI] [PubMed] [Google Scholar]
- 30.Meara A, Davidson N, Steigelman H, et al. Screening for cognitive impairment in SLE using the self-administered gerocognitive exam. Lupus 2018; 27(8): 1363–1367. [DOI] [PubMed] [Google Scholar]
- 31.Raghunath S, Glikmann-Johnston Y, Morand E, et al. Evaluation of the Montreal Cognitive Assessment as a screening tool for cognitive dysfunction in SLE. Lupus Sci Med 2021; 8(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pedraza OL, Salazar AM, Sierra FA, et al. Reliability, criterion and discriminant validity of the Montreal cognitive assessment test (MoCA) in a group of adults from bogotá. Acta Méd Colomb 2017; 41(4): 221–228. [Google Scholar]
- 33.Gõmez F, Zunzunegui MV, Lord C, et al. Applicability of the MoCA-S test in populations with little education in Colombia. Int J Geriatr Psychiatr 2013; 28(8): 813–820. [DOI] [PubMed] [Google Scholar]
- 34.Pedraza L, Sánchez E, Plata SJ, et al. MoCA and MMSE scores in patients with mild cognitive impairment and dementia in a memory clinic in Bogotá. Acta Neurol Colomb 2014; 30(1): 22–31. [Google Scholar]
- 35.Pamfil C, Fanouriakis A, Damian L, et al. EULAR recommendations for neuropsychiatric systemic lupus erythematosus vs usual care: results from two European centres. Rheumatology 2015; 54(7): 1270–1278. [DOI] [PubMed] [Google Scholar]
- 36.Yuen K, Green R, Bingham K, et al. Metrics and definitions used in the assessment of cognitive impairment in systemic lupus erythematosus: a systematic review. Semin Arthritis Rheum 2021; 51(4): 819–830. [DOI] [PubMed] [Google Scholar]
- 37.D’Amico MA. Multicenter study of cognitive impairment in systemic lupus erythematosus: ecles. Rev Argent Reumatol 2015; 26(2): 28–32. [Google Scholar]
- 38.Calderón J, Flores P, Aguirre JM, et al. Impact of cognitive impairment, depression, disease activity, and disease damage on quality of life in women with systemic lupus erythematosus. Scand J Rheumatol 2017; 46(4): 273–280. [DOI] [PubMed] [Google Scholar]
- 39.Jolly M, Katz P. Predictors of stress in patients with Lupus. Front Med 2022; 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mendelsohn M, Khoja L, Alfred S, et al. Cognitive impairment in systemic lupus erythematosus is negatively related to social role participation and quality of life: a systematic review. Lupus 2021; 30(10): 1617–1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Petri M, Naqibuddin M, Carson KA, et al. Depression and cognitive impairment in newly diagnosed systemic lupus erythematosus. J Rheumatol 2010; 37(10): 2032–2038. [DOI] [PubMed] [Google Scholar]
- 42.Range K, M D, Moser YA. White matter abnormalities and working memory impairment in systemic lupus erythematosus. Bone 2012; 23(1): 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Palagini L, Mosca M, Tani C, et al. Depression and systemic lupus erythematosus: a systematic review. Lupus 2013; 22(5): 409–416. [DOI] [PubMed] [Google Scholar]
- 44.Macêdo EA, Appenzeller S, Costallat LTL. Depression in systemic lupus erythematosus: gender differences in the performance of the Beck depression inventory (BDI), center for epidemiologic studies depression scale (CES-D), and hospital anxiety and depression scale (HADS). Lupus 2018; 27(2): 179–189. [DOI] [PubMed] [Google Scholar]
- 45.Dorman G, Micelli M, Cosentino V, et al. Disfunción cognitiva en lupus eritematoso sistémico y su asociación con actividad y daño. Méd 2017; 77(1669–9106): 257–260. [PubMed] [Google Scholar]
- 46.Zabala A, Salgueiro M, Sáez-Atxukarro O, et al. Cognitive impairment in patients with neuropsychiatric and non- neuropsychiatric systemic lupus erythematosus: a systematic review and meta-analysis. J Int Neuropsychol Soc 2018; 24(6): 629–639. [DOI] [PubMed] [Google Scholar]
- 47.Yelnik CM, Kozora E, Appenzeller S. Cognitive disorders and antiphospholipid antibodies. Autoimmun Rev 2016; 15(12): 1193–1198. [DOI] [PubMed] [Google Scholar]
- 48.Pasoto SG, Chakkour HP, Natalino RR, et al. Lupus anticoagulant: a marker for stroke and venous thrombosis in primary Sjögren’s syndrome. Clin Rheumatol 2012; 31(9): 1331–1338. [DOI] [PubMed] [Google Scholar]
- 49.Pons-Estel BA, Catoggio LJ, Cardiel MH, et al. The GLADEL multinational Latin American prospective inception cohort of 1,214 patients with systemic lupus erythematosus: ethnic and disease heterogeneity among “hispanics.”. Medicine (Baltimore), 2004; 83: 1–17. [DOI] [PubMed] [Google Scholar]
- 50.Ad ACR, Committee HOC, Neuropsychiatric ON, et al. ARC Ad Hoc committee on neuropsychiatric lupus nomenclature. The American College of Rheumatology nomenclature and case definitions for neuropsychiatric lupus. Arthritis Rheum 1999; 42(4): 599–608. [DOI] [PubMed] [Google Scholar]