Abstract
Background:
There are major challenges in determining the etiology of vascular cognitive impairment (VCI) clinically, especially in the presence of mixed pathologies, such as vascular and amyloid. Most recently, two criteria (American Heart Association/American Stroke Association (AHA/ASA) and Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-V)) have been proposed for the clinical diagnosis of VCI but have not as yet been validated using neuroimaging.
Aims:
This study aims to determine whether the AHA/ASA and DSM-V criteria for VCI can distinguish between cases with predominantly vascular pathology and cases with mixed pathology.
Methods:
A total of 186 subjects were recruited from a cross-sectional memory clinic–based study at the National University Hospital, Singapore. All subjects underwent clinical and neuropsychological assessment, magnetic resonance imaging (MRI) and carbon 11-labeled Pittsburgh Compound B ([11C] PiB) positron emission tomography (PET) scans. Diagnosis of the etiological subtypes of VCI (probable vascular mild cognitive impairment (VaMCI), possible VaMCI, non-VaMCI, probable vascular dementia (VaD), possible VaD, non-VaD) were performed following AHA/ASA and DSM-V criteria. Brain amyloid burden was determined for each subject with standardized uptake value ratio (SUVR) values ⩾1.5 classified as amyloid positive.
Results:
Using κ statistics, both criteria had excellent agreement for probable VaMCI, probable VaD, and possible VaD (κ = 1.00), and good for possible VaMCI (κ = 0.71). Using the AHA/ASA criteria, the amyloid positivity of probable VaMCI (3.8%) and probable VaD (15%) was significantly lower compared to possible VaMCI (26.7%), non-VaMCI (33.3%), possible VaD (73.3%), and non-VaD (76.2%) (p < 0.001). Similarly, using the DSM-V criteria, the amyloid positivity of probable VaMCI (3.8%) and probable VaD (15%) was significantly lower compared to possible VaMCI (26.3%), non-VaMCI (32.1%), possible VaD (73.3%), and non-VaD (76.2%) (p < 0.001). In both criteria, there was good agreement in differentiating individuals with non-VaD and possible VaD, with significantly higher (p < 0.001) global [11C]-PiB SUVR, from individuals with probable VaMCI and probable VaD, who had predominant vascular pathology.
Conclusion:
The AHA/ASA and DSM-V criteria for VCI can identify VCI cases with little to no concomitant amyloid pathology, hence supporting the utility of AHA/ASA and DSM-V criteria in diagnosing patients with predominant vascular pathology.
Data access statement:
Data supporting this study are available from the Memory Aging and Cognition Center, National University of Singapore. Access to the data is subject to approval and a data sharing agreement due to University policy.
Keywords: Vascular cognitive impairment, [11C] PiB-PET, AHA/ASA criteria, DSM-V criteria, vascular mild cognitive impairment, vascular dementia
Introduction
Vascular cognitive impairment (VCI) refers to a spectrum of cognitive dysfunction associated with cerebrovascular disease (CeVD), ranging from vascular mild cognitive impairment (VaMCI) to more severe cognitive impairment accompanied by functional loss in cases with vascular dementia (VaD). The underlying pathology also ranges from CeVD alone or combined with other pathologies, most commonly Alzheimer’s disease with concomitant CeVD (AD + CeVD).1,2 One of the major challenges in VCI diagnosis is differentiating cases with predominantly vascular pathologies from those with additional AD pathology as indicated by the presence of significant brain amyloid beta (Aβ) burden. Distinguishing between single and mixed pathologies is clinically relevant as the prognosis and management may differ.1,3
Positron emission tomography (PET) allows researchers to visualize significant Aβ burden in vivo. Carbon 11-labeled Pittsburgh Compound B [ 11 C]-PIB is one of the most studied and used tracer for PET.4,5 It is selective for fibrillar Aβ with high affinity to single sites with minimal binding to the cerebellum. 6 It is binding to Aβ has been validated by comparing with post-mortem assays and showed excellent agreement.4,7,8
In vivo visualization of Aβ deposition using PET has been explored in VCI.3,9,10 However, these studied only a limited range of VCI. They either investigated only subjects with dementia but excluded milder forms of cognitive impairment3,9 or only examined a subtype of VCI presenting with white matter hyperintensities (WMHs) on magnetic resonance imaging (MRI) but excluded other markers of CeVD.3,10
Several efforts have been made to re-evaluate and standardize the diagnostic criteria for VCI.1,11–14 Recently, two diagnostic criteria, the American Psychiatric Association’s Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-V) 15 and the American Heart Association/American Stroke Association (AHA/ASA) 16 (see Supplemental Tables S1 and S2) criteria were proposed to further expand the definition of VCI by including classifications that cover both dementia and mild cognitive impairment (MCI), including other markers of CeVD, and introducing two levels of certainty, probable and possible. To date, there are no studies assessing the utility of these criteria in distinguishing cases with predominantly vascular pathology from cases with co-existing AD (Aβ) pathology.
Aims and hypothesis
Our aim therefore is to investigate, using carbon [ 11 C]-PiB amyloid PET studies, whether the AHA/ASA and DSM-V criteria for VCI can distinguish between cases with predominantly vascular pathology and cases with mixed vascular and Aβ pathology. We hypothesize that using the AHA/ASA and DSM-V criteria, probable VaMCI and VaD will have a lower frequency of Aβ positivity compared to possible VaMCI, possible VaD, and non-VCI cases.
Materials and methods
Subjects
From April 2016 to September 2018, 186 subjects were recruited from a cross-sectional memory clinic–based study at the National University Hospital, Singapore. All subjects underwent physical, clinical and neuropsychological assessments, 3T MRI, and [ 11 C]-PiB-PET scans at the Clinical Imaging Research Centre (CIRC), National University of Singapore (NUS). Ethical approval for this study was obtained from the National Health Group Domain-Specific Review Board (NHG DSRB Ref: 2015/00406). Written informed consent was obtained from all subjects or their legal representatives prior to their participation in the study.
Subjects with major neuropsychiatric conditions, chronic alcoholism, Parkinson’s disease, and Lewy body dementia were excluded. Subjects with severe aphasia that precluded them from performing for completing formal neuropsychological testing were also excluded.
Demographics and risk factors
A demographic questionnaire was administered to obtain information on age, years of education, sex, ethnicity, and smoking history. A history of hypertension, diabetes, and hyperlipidemia was obtained during clinical examination, and verified by medical records. The following risk factors were defined as follows: hypertension—systolic blood pressure >140 mm Hg and/or diastolic blood pressure >90 mm Hg or use of antihypertensive medication; diabetes mellitus and hyperlipidemia—self-report of having the condition and/or use of medications.
Magnetic resonance imaging
All participants underwent scanning on a 3T Siemens Magnetom Trio Tim (Siemens Healthineers, Erlangen, Germany) with a 32-channel head coil, at the Clinical Imaging Research Center of the NUS. T1-weighted images, Fluid Attenuated Inversion Recovery (FLAIR), T2-weighted images and Susceptibility Weighted Images were utilized for assessing cortical infarcts, lacunes, cerebral microbleeds, and WMHs. For each subject, MRI markers of CeVD were graded blinded to the patient characteristics and using the standards for reporting vascular changes on neuroimaging (STRIVE) Criteria.17–19
PiB-PET acquisition
PET-MR imaging was conducted on an mMR synchronous PET/MR scanner (Siemens Healthineers). 18 To obtain Aβ-standard uptake value ratio (SUVR) values, a 30-min brain PET scan was performed on all individuals 40 min after injection of 370 (±10%) MBq of [ 11 C]-PiB. Each list-mode datum was rebinned before tomographic reconstruction into a single static frame during which motion correction was applied using an in-house developed rebinner. 19 Corrected scans were then reconstructed into a 344 × 344 × 127 voxel volume with a voxel size of 2.09 × 2.09 × 2.03 mm3 using 3D ordinary Poisson ordered-subsets expectation maximization with all corrections applied including resolution modeling and using three iterations and 21 subsets. 20 SUVR images and global SUVR values were generated using cerebellar gray matter as the reference region, then computed and analyzed using an automated pipeline. SUVR values ⩾1.5 were classified as amyloid positive while SUVR values below 1.5 were classified as amyloid negative. 21
Diagnosis according to clinical criteria for VCI
For each subject, diagnoses were made according to the AHA/ASA and DSM-V criteria by a clinical neurology fellow (S.G.V.) together with an experienced neurologist (C.L.-H.C.), blinded to the amyloid PET data. Both criteria have been previously described in other studies and reviews.1,15,16 Briefly, the AHA/ASA criteria uses a definition of VCI based on a construct that first identifies presence of cognitive impairment and then evaluates the presence of vascular biomarkers and the relationship with cognitive decline. 16 The DSM-V criteria for vascular neurocognitive disorders also first establish the presence of cognitive impairment and then proceed to excludes cases with cognitive deficits due to other mental disorders, before finally evaluating the presence of vascular biomarkers and relationship with cognitive decline. 15 For this study, both VaMCI and mild neurocognitive disorder were grouped under the term VaMCI, and VaD and major neurocognitive disorder were grouped under VaD. Cases not diagnosed as either probable or possible VaMCI or VaD were classified under the terms non-VaMCI or non-VaD, respectively.
Statistical analysis
Statistical analysis was performed using standard statistical software (Statistical Package for Social Sciences, SPSS V25, SPSS Inc, Chicago, IL, USA). The kappa statistic was used to measure agreement among the diagnostic groups of both criteria. Aβ positivity was entered as a binary variable and analyzed using χ2. Continuous variables were presented as means with standard deviations (SD) or medians with interquartile ranges (IQRs) when applicable. In cases of skewed distribution of continuous variables, differences between groups were determined using the Kruskal–Wallis test. Significance level was set at p < 0.05. Significance values have been adjusted by the Bonferroni correction for multiple tests.
Results
A total of 186 subjects were recruited; Table 1 shows the number of participants diagnosed with VaMCI and VaD using the AHA/ASA and DSM-V criteria for VCI. For both criteria, the concordances were good to excellent. The agreement between the criteria was assessed using κ statistics, with similar results: excellent for the AHA/ASA and DSM-V criteria for probable VaMCI, probable VaD, and possible VaD (κ = 1.00), and good for possible VaMCI (κ = 0.71). Eleven subjects met the criteria for AHA/ASA possible VaMCI, but they were classified as non-VaMCI by DSM-V due to having a gradually progressive cognitive decline suggestive of AD and absence of any stroke history.
Table 1.
Number of participants diagnosed with VaMCI and VaD using the AHA/ASA and DSM-V criteria.
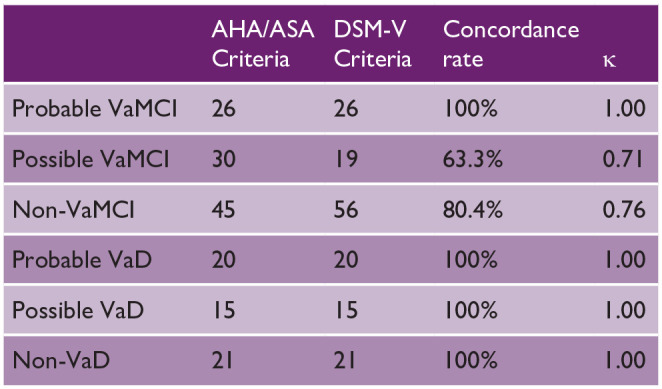
|
AHA/ASA: American Heart Association/American Stroke Association; DSM-V: Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition.
Using the AHA/ASA criteria (Figure 1), there was a significantly lower frequency of amyloid positivity in probable VaMCI (3.8%) compared to possible VaMCI (26.7%, p < 0.05), non-VaMCI (33.3%, p < 0.01), possible VaD (73.3%, p < 0.001), and non-VaD (76.2%, p < 0.001). There was also a significantly lower frequency of amyloid positivity in probable VaD (15%) compared to possible VaD (73.3%, p < 0.001) and non-VaD (76.2%, p < 0.001). In addition, subjects with probable VaMCI, possible VaMCI, and non-VaMCI have significantly lower frequency of amyloid positivity compared to subjects with possible VaD and non-VaD (p < 0.001).
Figure 1.
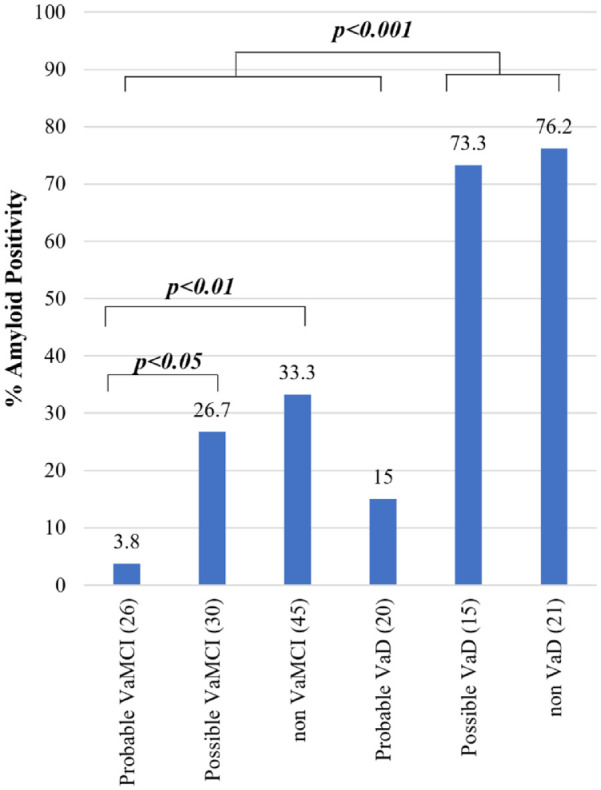
Amyloid positivity among diagnoses using the AHA/ASA criteria for VCI.
Using the DSM-V criteria (Figure 2), there was a significantly lower frequency of amyloid positivity in probable VaMCI (3.8%) compared to possible VaMCI (26.3%, p < 0.05), non-VaMCI (32.1%, p < 0.01), possible VaD (73.3%, p < 0.001), and non-VaD (76.2%, p < 0.001). Likewise, there was a significantly lower frequency of amyloid positivity in probable VaD (15%) compared to possible VaD (73.3%, p < 0.001) and non-VaD (76.2%, p < 0.001). Subjects with probable VaMCI, possible VaMCI, and non-VaMCI have significantly lower frequency of amyloid positivity compared to subjects with possible VaD and non-VaD (p < 0.001).
Figure 2.
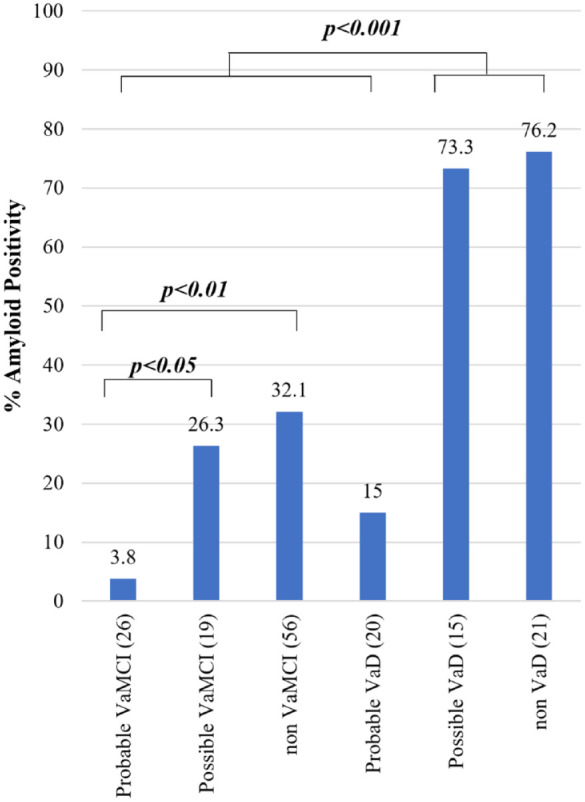
Amyloid positivity among diagnoses using the DSM-V criteria for VCI.
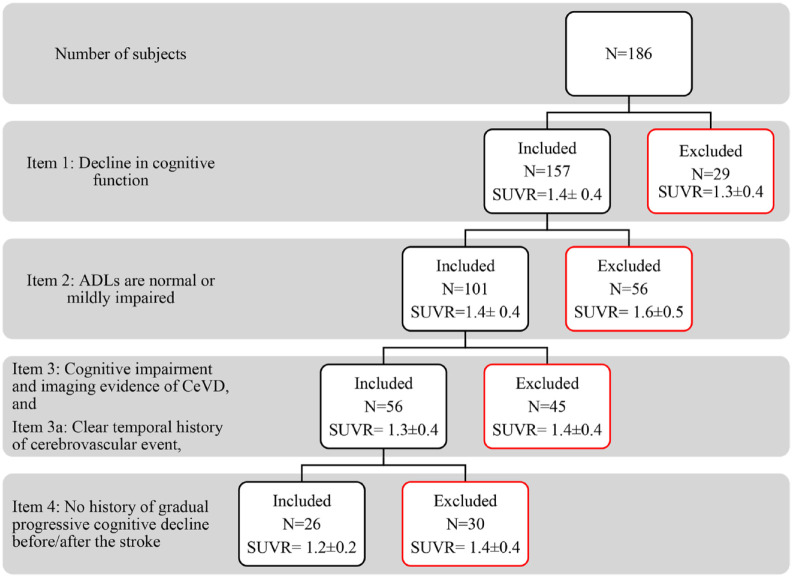
In the AHA/ASA criteria for VaMCI (Figure 3), the diagnosis of MCI was established by items (1) “Decline in cognitive function” and (2) “ADLs are normal or mildly impaired.” The 56 subjects with dementia excluded by item (2) had a high mean SUVR (1.6 ± 0.5). Subsequently, criteria such as item (3) “cognitive impairment and imaging evidence of CeVD,” sub-item (a) “clear temporal history of cerebrovascular event,” and item (4) “no history of gradual progressive cognitive decline before/after the stroke” further identified a group of MCI subjects with a lower mean SUVR (1.2 ± 0.2). This suggests that while item (3) and sub-item (a) identified those with MCI most likely due to CeVD, item (4) was an important criterion to exclude subjects with possible mixed pathologies causing cognitive decline.
Figure 3.
AHA/ASA criteria for probable VaMCI with each item and its corresponding SUVR values.
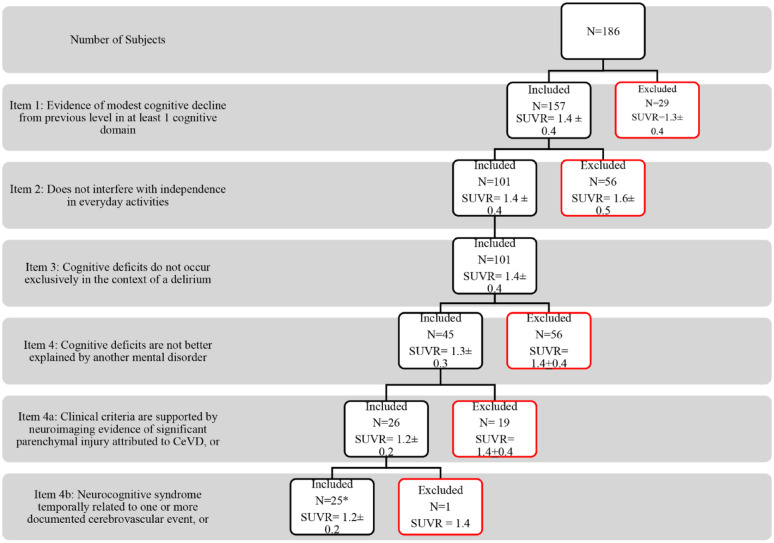
Similarly, in the DSM-V criteria for VaMCI (Figure 4), items (1) “Evidence of modest cognitive decline from previous level in at least 1 cognitive domain” and (2) “Does not interfere with independence in everyday activities” established the clinical diagnosis of MCI. Item (2) excluded subjects with a high mean SUVR (1.6 ± 0.5). Item (4) “cognitive deficits are not better explained by another mental disorder” excluded 56 subjects who presented with gradually progressive cognitive decline suggestive of Alzheimer’s disease and had a high mean SUVR (1.4 ± 0.4). A total of 45 subjects were eligible to proceed for evaluation of the MRI. Sub-item 4(a) “clinical criteria are supported by neuroimaging evidence of significant parenchymal injury attributed to CeVD” was able to identify 26 subjects with a low mean SUVR (1.2 ± 0.2). Sub-item 4(b) “neurocognitive syndrome temporally related to one or more documented cerebrovascular event” was able to identify 25 subjects with a low mean SUVR (1.2 ± 0.2). All 25 subjects that fulfilled sub-item 4(b) also fulfilled sub-item 4(a). The 19 subjects that were excluded by sub-item (a) only had mild WMH on MRI and a higher mean SUVR (1.4 ± 0.4). The only subject (SUVR = 1.4) that did not fulfill sub-item 4(b) but fulfilled sub-item 4(a) was classified under probable VaMCI.
Figure 4.
DSM-V criteria for probable VaMCI with each item and its corresponding SUVR values.
*All 25 subjects that fulfilled sub-item (b) also fulfilled sub-item (a).
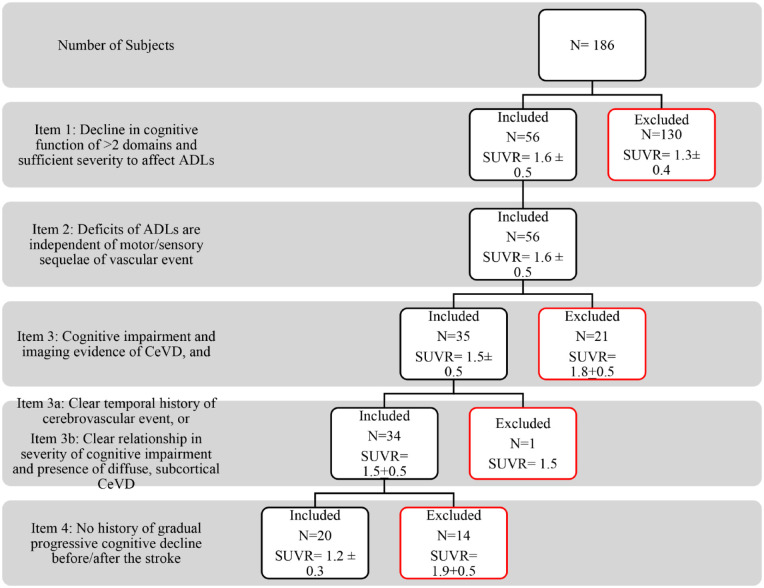
In the AHA/ASA criteria for VaD (Figure 5), the clinical diagnosis of dementia was established by items (1) “Decline in cognitive function of >2 domains and sufficient severity to affect ADLs” and (2) “Deficits of ADLs are independent of motor/sensory sequelae of vascular event.” Item (3) “cognitive impairment and imaging evidence of CeVD” was able to exclude 21 subjects with no CeVD on MRI and with a high mean SUVR (1.8 ± 0.5), suggestive of Alzheimer’s disease. The mean SUVR of all the 34 subjects who fulfilled sub-items (a) or (b) was 1.5 ± 0.5. Of these 34 subjects, 14 were excluded by item (4) “no history of gradually progressive cognitive decline before/after the stroke” and had a high mean SUVR (1.9 + 0.5). The 20 subjects who fulfilled the criteria for probable VaD had a low mean SUVR (1.2 + 0.3).
Figure 5.
AHA/ASA criteria for probable VaD with each item and its corresponding SUVR values.
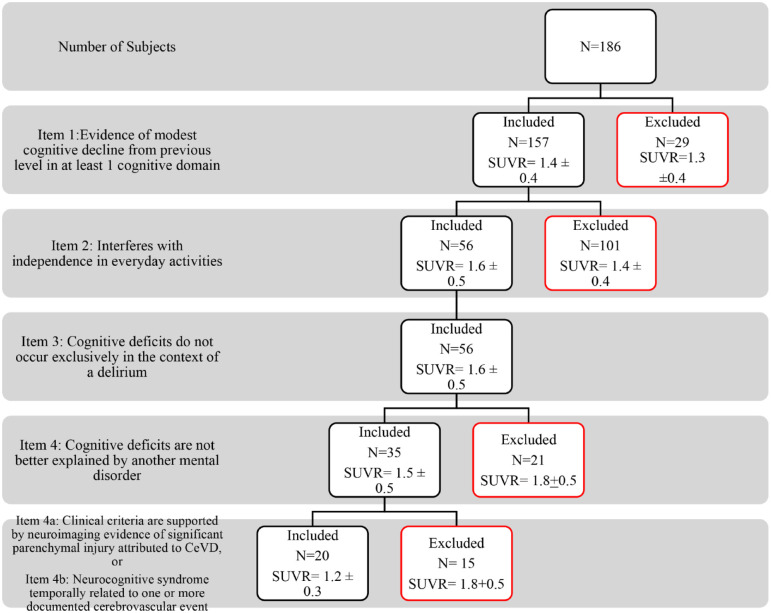
In the DSM-V criteria for VaD (Figure 6), item (1) established the presence of cognitive impairment while item (2) established the clinical diagnosis of dementia. Item (4) “cognitive deficits are not better explained by another mental disorder” excluded 21 subjects with a clinical history consistent with Alzheimer’s disease and had a high mean SUVR (1.8 ± 0.5). A total of 35 subjects were eligible to proceed for the evaluation of the neuroimaging. Sub-items (a) “clinical criteria are supported by neuroimaging evidence of significant parenchymal injury attributed to CeVD” and (b) “neurocognitive syndrome temporally related to one or more documented cerebrovascular event” were able to identify 20 subjects with probable VaD and a low mean SUVR (1.2 + 0.3). Fifteen subjects who were excluded by both sub-items (a) and (b) were diagnosed as possible VaD had a high mean SUVR (1.8 ± 0.5).
Figure 6.
DSM-V criteria for probable VaD with each item and its corresponding SUVR values.
For both criteria (Table 2), the individuals with non-VaD and possible VaD had significantly higher global [ 11 C]-PiB SUVR compared to individuals with prob VaMCI and prob VaD (p < 0.001) (see Supplemental Tables S3 to S6 for detailed analyses).
Table 2.
Subject’s SUVR according to criteria classification.
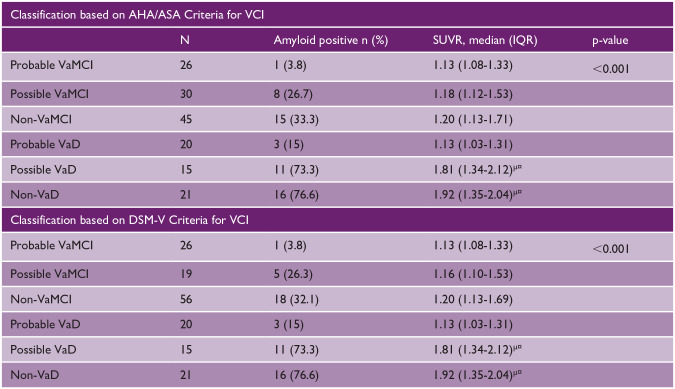
|
AHA/ASA: American Heart Association/American Stroke Association; VCI: vascular cognitive impairment; SUVR: standardized uptake value ratio; IQR: interquartile range; DSM: Diagnostic and Statistical Manual of Mental Disorders.
Significance values have been adjusted by the Bonferroni correction for multiple tests.
Versus subjects with Prob VaMCI.
Versus subjects with Prob VaD.
Discussion
This study is the first study to demonstrate that the widely used AHA/ASA and DSM-V criteria for VCI can identify subjects with a predominant vascular pathology and little to no co-existing significant amyloid pathology. The major findings of this study are as follows: first, both criteria show a substantial level of agreement, particularly for the diagnosis of probable VCI. Second, for the AHA/ASA criteria, a diagnosis of probable VaMCI and VaD having the lowest amyloid positivity was identified after establishing a clear relationship with a vascular injury through neuroimaging confirmation of having significant CeVD and/or a clear history of cognitive decline after a vascular event. Furthermore, the exclusion of cases with concomitant cognitive decline before and/or after a stroke identified cases with the lowest positivity. Finally, for the DSM-V criteria, a diagnosis of probable VaMCI and VaD having the lowest amyloid positivity was identified after establishing the presence of neuroimaging evidence of CeVD and/or having a clear temporal relationship with a vascular event.
The diagnosis of VCI involves two steps—establishing the presence of a cognitive disorder and determining that this is a direct result of cerebrovascular pathology.16,22,23 Both criteria follow the same step-wise approach leading to excellent agreement. The difference between the criteria appeared when diagnosing possible VaMCI and was likely due to the different stages in the criteria at which a history of a gradually progressive cognitive decline suggesting a neurodegenerative disorder was evaluated. In the DSM-V, this was assessed prior to neuroimaging evaluation, whereas for the AHA/ASA, it was assessed after neuroimaging evaluation. This led to more cases being considered as vascular in the AHA/ASA criteria than in DSM-V. Notably, all the 11 cases that were classified as non-VaMCI in DSM-V but classified as possible VaMCI in AHA/ASA only had extensive WMH on MRI, which may be due to neurodegeneration rather than direct vascular injury.24,25 Another reason for the excellent agreement between both criteria is in the determination of a probable level of certainty where emphasis on the role of neuroimaging in assessing vascular lesions is highlighted.1,2,16,23 Neuroimaging remains to be a crucial component of evaluating patients with cognitive decline.26,27 Objective evidence of significant vascular pathology in the brain is typically obtained through computed tomography (CT) or structural MRI, with the latter being more sensitive. 28 Therefore, the applicability of these criteria is seen in clinical and research settings where neuroimaging is readily available. In the present cohort, MRI was performed in all subjects which allowed sufficient evaluation for evidence of CeVD. This is also the reason why the sub-item “no neuroimaging available” was not applicable in this study. It is possible that the frequencies of possible VaMCI and VaD will be increased in situations where MRI is not readily available.
Although structural neuroimaging is helpful in evaluating CeVD, it cannot determine the presence of Aβ, which can co-occur with CeVD and contribute to cognitive impairment. Previous studies have successfully used PiB-PET to determine the amyloid positivity in VCI which showed that amyloid positivity was approximately 30% for VaD and 33% for VaMCI. However, the conclusions were limited because these studies only looked at a subset of degree of cognitive impairment (MCI or dementia only) or a subtype of vascular disease.3,9,10 In addition, these studies used different criteria in defining VaMCI and VaD, and there was a variety of PET amyloid ligands used. In this study, we used PiB-PET to determine whether the AHA/ASA and DSM-V criteria were able to distinguish which diagnostic groups had the least frequency of amyloid PET positive cases. We have shown that probable VaMCI (3.8%) and probable VaD (15%) had the lowest amyloid positivity compared to other diagnostic groups. Moreover, we have identified that possible VaD (1.92 (1.35-2.04)) and non-VaD (1.81 (1.34-2.12)) had high global [ 11 C]-PiB SUVR. Examining both criteria suggests that the diagnostic features of the diagnosis of probable VCI represent the typical presentations of post-stroke dementia, multi-infarct dementia, or strategic infarct dementia. These descriptions are consistent with the report that VCI is most commonly due to direct ischemic tissue injury, such as multiple or strategic macroscopic infarcts.1,2,16 In addition, the number, location, and size of the infarct further increase the likelihood that it will be associated with cognitive impairment or dementia.29,30 Notably, all subjects in our cohort diagnosed as probable VaMCI or probable VaD had lacunes, cortical infarcts, or a combination of both. For cases diagnosed as possible VCI, the certainty of having a predominant vascular syndrome is diminished suggesting the presence of another disease process causing cognitive deficits. This is supported by the current findings of significantly more amyloid positivity in possible VaMCI and possible VaD compared to probable VaMCI and probable VaD for both criteria. As amyloid positivity for possible VaMCI and possible VaD range from 26.3% up to 75.0%, it suggests that cognitive decline may also be due to other pathologies such as α-synucleinopathy, tubulin-associated unit (tau) pathology, or transactivation-responsive DNA-binding protein (TDP) 43 pathology, aside from amyloid.31,32 These findings highlight the need for an accurate biomarker–based dementia diagnosis using PET, cerebrospinal fluid (CSF), and blood biomarkers. 33 This will help the patients and their caregivers understand the prognosis and most importantly, in the advent of antiamyloid therapy, their eligibility for disease-modifying therapy.34,35
Strengths of the study include the use of a cohort that consisted of subjects with varying degrees of cognitive impairment allowing the comparison of amyloid positivity across the spectrum of VCI. Second, this study demonstrated that the AHA/ASA and DSM-V criteria, using an in vivo assessment of amyloid using PiB-PET, can identify cases with mixed pathologies. Finally, this study used a novel Aβ PET quantification method that does not require parcellation of subject’s MRI, thus allowing evaluation of SUVR for subjects with larger strokes, where conventional parcellation methods often fail. 21 We also acknowledge the limitations of the study. First, no neuropathological examinations were performed; hence, we were unable to measure other pathologies such as neurofibrillary tangles or Lewy bodies. Second, although PIB-PET has shown good correlation with neuropathological investigations, [ 11 C]-PiB only binds with the larger fibrillar and protofibril forms of Aβ, but has weak binding to the soluble forms which may be more potent in causing cognitive decline.8,36,37 This limits the ability of [ 11 C]-PiB to totally “rule out” amyloid pathology. Third, the study is limited by its cross-sectional design. The sample size in each diagnostic group was not equally distributed and small in some groups. Finally, the results were obtained from an Asian memory clinic cohort, limiting its generalizability to the general population. Therefore, replication in community and memory clinic–based studies is needed.
The AHA/ASA and DSM-V criteria for VCI may be used in the clinical and research setting to identify patients with a predominant vascular pathology and no co-existing significant amyloid pathology. Future studies include using other emerging modalities to determine the presence of other pathologies to further validate these criteria.
Supplemental Material
Supplemental material, sj-docx-1-wso-10.1177_17474930241252556 for The AHA/ASA and DSM-V diagnostic criteria for vascular cognitive impairment identify cases with predominant vascular pathology by Melmar C Folloso, Steven G Villaraza, Lo Yi-Wen, Khong Pek-Lan, Tomotaka Tanaka, Saima Hilal, Narayanaswamy Venketasubramanian and Christopher Li-Hsian Chen in International Journal of Stroke
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Yong Loo Lin School of Medicine Aspiration Fund (NUHSRO/2014/102AF-WORLD CLASS/01) and the National Medical Research Council of Singapore (NMRC/CG/M009/2017_NUH/NUHS and NMRC/CIRG/1485/2018).
ORCID iDs: Melmar C Folloso  https://orcid.org/0000-0002-3710-5215
https://orcid.org/0000-0002-3710-5215
Saima Hilal  https://orcid.org/0000-0001-5434-5635
https://orcid.org/0000-0001-5434-5635
Data availability statement: The anonymized data are available from the corresponding author upon reasonable request.
Supplemental material: Supplemental material for this article is available online.
References
- 1. Van der Flier W, Skoog I, Schneider JA, et al. Vascular cognitive impairment. Nat Rev Dis Primers 2018; 4: 18003. [DOI] [PubMed] [Google Scholar]
- 2. O’Brien JT, Erkinjuntti T, Reisberg B, et al. Vascular cognitive impairment. Lancet Neurol 2003; 2: 89–98. [DOI] [PubMed] [Google Scholar]
- 3. Lee JH, Kim SH, Kim GH, et al. Identification of pure subcortical vascular dementia using 11C-Pittsburgh compound B. Neurology 2011; 77: 18–25. [DOI] [PubMed] [Google Scholar]
- 4. Vlassenko AG, Benzinger TL, Morris JC. PET amyloid-beta imaging in preclinical Alzheimer’s disease. Biochim Biophys Acta 2012; 1822: 370–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Klunk WE, Engler H, Nordberg A, et al. Imaging brain amyloid in Alzheimer’s disease with Pittsburgh Compound-B. Ann Neurol 2004; 55: 306–319. [DOI] [PubMed] [Google Scholar]
- 6. Lopresti BJ, Klunk WE, Mathis CA, et al. Simplified quantification of Pittsburgh Compound B amyloid imaging PET studies: a comparative analysis. J Nucl Med 2005; 46: 1959–1972. [PubMed] [Google Scholar]
- 7. Bacskai BJ, Frosch MP, Freeman SH, et al. Molecular imaging with Pittsburgh Compound B confirmed at autopsy: a case report. Arch Neurol 2007; 64: 431–434. [DOI] [PubMed] [Google Scholar]
- 8. Ikonomovic MD, Klunk WE, Abrahamson EE, et al. Post-mortem correlates of in vivo PiB-PET amyloid imaging in a typical case of Alzheimer’s disease. Brain 2008; 131: 1630–1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ossenkoppele R, Jansen WJ, Rabinovici GD, et al. Prevalence of amyloid PET positivity in dementia syndromes: a meta-analysis. JAMA 2015; 313: 1939–1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lee MJ, Seo SW, Na DL, et al. Synergistic effects of ischemia and β-amyloid burden on cognitive decline in patients with subcortical vascular mild cognitive impairment. JAMA Psychiatry 2014; 71: 412–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hachinski V, Iadecola C, Petersen RC, et al. National Institute of Neurological Disorders and Stroke-Canadian Stroke Network vascular cognitive impairment harmonization standards. Stroke 2006; 37: 2220–2241. [DOI] [PubMed] [Google Scholar]
- 12. Skrobot OA, Black SE, Chen C, et al. Progress toward standardized diagnosis of vascular cognitive impairment: guidelines from the Vascular Impairment of Cognition Classification Consensus Study. Alzheimers Dement 2018; 14: 280–292. [DOI] [PubMed] [Google Scholar]
- 13. Skrobot OA, Attems J, Esiri M, et al. Vascular cognitive impairment neuropathology guidelines (VCING): the contribution of cerebrovascular pathology to cognitive impairment. Brain 2016; 139: 2957–2969. [DOI] [PubMed] [Google Scholar]
- 14. Skrobot OA, O’Brien J, Black S, et al. The vascular impairment of cognition classification consensus study. Alzheimers Dement 2017; 13: 624–633. [DOI] [PubMed] [Google Scholar]
- 15. American Psychiatric Association (APA). DSM-V: Dijagnostički i Statistički Priručnik Za Duševne Poremećaje [Diagnostic and statistical manual of mental disorders]. 5th ed. Washington, DC: APA, 2013. [Google Scholar]
- 16. Gorelick PB, Scuteri A, Black SE, et al. Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2011; 42: 2672–2713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wardlaw JM, Smith EE, Biessels GJ, et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol 2013; 12: 822–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Delso G, Fürst S, Jakoby B, et al. Performance measurements of the Siemens mMR integrated whole-body PET/MR scanner. J Nucl Med 2011; 52: 1914–1922. [DOI] [PubMed] [Google Scholar]
- 19. Reilhac A, Merida I, Irace Z, et al. Development of a dedicated rebinner with rigid motion correction for the mMR PET/MR scanner, and validation in a large cohort of 11C-PIB scans. J Nucl Med 2018; 59: 1761–1767. [DOI] [PubMed] [Google Scholar]
- 20. Panin VY, Kehren F, Michel C, Casey M. Fully 3-D PET reconstruction with system matrix derived from point source measurements. IEEE Trans Med Imaging 2006; 25: 907–921. [DOI] [PubMed] [Google Scholar]
- 21. Tanaka T, Stephenson MC, Nai YH, et al. Improved quantification of amyloid burden and associated biomarker cut-off points: results from the first amyloid Singaporean cohort with overlapping cerebrovascular disease. Eur J Nucl Med Mol Imaging 2020; 47: 319–331. [DOI] [PubMed] [Google Scholar]
- 22. Sachdev PS, Lipnicki DM, Crawford JD, Brodaty H. The Vascular Behavioral and Cognitive Disorders criteria for vascular cognitive disorders: a validation study. Eur J Neurol 2019; 26: 1161–1167. [DOI] [PubMed] [Google Scholar]
- 23. Román GC, Tatemichi TK, Erkinjuntti T, et al. Vascular dementia: diagnostic criteria for research studies. Report of the NINDS-AIREN International Workshop. Neurology 1993; 43: 250–260. [DOI] [PubMed] [Google Scholar]
- 24. McAleese KE, Walker L, Graham S, et al. Parietal white matter lesions in Alzheimer’s disease are associated with cortical neurodegenerative pathology, but not with small vessel disease. Acta Neuropathol 2017; 134: 459–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Scott JA, Braskie MN, Tosun D, et al. Cerebral amyloid is associated with greater white-matter hyperintensity accrual in cognitively normal older adults. Neurobiol Aging 2016; 48: 48–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mahalingam S, Chen MK. Neuroimaging in dementias. Semin Neurol 2019; 39: 188–199. [DOI] [PubMed] [Google Scholar]
- 27. Román G, Pascual B. Contribution of neuroimaging to the diagnosis of Alzheimer’s disease and vascular dementia. Arch Med Res 2012; 43: 671–676. [DOI] [PubMed] [Google Scholar]
- 28. Brainin M, Tuomilehto J, Heiss WD, et al. Post-stroke cognitive decline: an update and perspectives for clinical research. Eur J Neurol 2015; 22: 229–238, e13–e16. [DOI] [PubMed] [Google Scholar]
- 29. Schneider JA, Wilson RS, Cochran EJ, et al. Relation of cerebral infarctions to dementia and cognitive function in older persons. Neurology 2003; 60: 1082–1088. [DOI] [PubMed] [Google Scholar]
- 30. Xu X, Hilal S, Collinson SL, et al. Association of magnetic resonance imaging markers of cerebrovascular disease burden and cognition. Stroke 2015; 46: 2808–2814. [DOI] [PubMed] [Google Scholar]
- 31. Lin WL, Castanedes-Casey M, Dickson DW. Transactivation response DNA-binding protein 43 microvasculopathy in frontotemporal degeneration and familial Lewy body disease. J Neuropathol Exp Neurol 2009; 68: 1167–1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bejanin A, Schonhaut DR, La Joie R, et al. Tau pathology and neurodegeneration contribute to cognitive impairment in Alzheimer’s disease. Brain 2017; 140: 3286–3300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chong JR, Ashton NJ, Karikari TK, et al. Blood-based high sensitivity measurements of beta-amyloid and phosphorylated tau as biomarkers of Alzheimer’s disease: a focused review on recent advances. J Neurol Neurosurg Psychiatry 2021; 92: 1231–1241. [DOI] [PubMed] [Google Scholar]
- 34. The Lancet Neurology. Dementia diagnosis in the anti-amyloid era. Lancet Neurol 2024; 23: 219. [DOI] [PubMed] [Google Scholar]
- 35. Laforce R, Rabinovici GD. Amyloid imaging in the differential diagnosis of dementia: review and potential clinical applications. Alzheimers Res Ther 2011; 3: 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Cohen AD, Klunk WE. Early detection of Alzheimer’s disease using PiB and FDG PET. Neurobiol Dis 2014; 72PA: 117–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ferreira ST, Lourenco MV, Oliveira MM, De Felice FG. Soluble amyloid-β oligomers as synaptotoxins leading to cognitive impairment in Alzheimer’s disease. Front Cell Neurosci 2015; 9: 191. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-wso-10.1177_17474930241252556 for The AHA/ASA and DSM-V diagnostic criteria for vascular cognitive impairment identify cases with predominant vascular pathology by Melmar C Folloso, Steven G Villaraza, Lo Yi-Wen, Khong Pek-Lan, Tomotaka Tanaka, Saima Hilal, Narayanaswamy Venketasubramanian and Christopher Li-Hsian Chen in International Journal of Stroke