Abstract
Introduction:
Various treatment protocols have been recommended since the beginning of the COVID-19 pandemic and have gradually evolved. This study aimed to assess the effectiveness and safety of incentive spirometer exercise (ISE) in outcomes of hospitalized patients with moderate-to-severe COVID-19 pneumonia.
Methods:
A 3-month single-blind, two parallel-armed randomized controlled trial was conducted at Imam Hossein Hospital, Tehran, Iran. Participants aged >18 years with documented COVID-19 pneumonia were randomly allocated to 2 groups of IS (ISE in addition to the usual treatment) and control (usual care alone). The IS group was also asked to perform ISE after discharge for three months. The primary outcomes were peripheral O2 saturation (SpO2), VBG parameters (pCO2, PH, HCO3), dyspnea level measured by Modified Borg Scale (MBS), length of hospital stay (LOS), and respiratory rate (RR). Secondary outcomes included mortality rate, intubation rate (IR), and ICU admission rate.
Results:
A total of 160 eligible patients were randomly assigned to either the IS (n = 80) or control (n=80) groups. Although there were no significant differences in primary and secondary outcomes between the groups post-intervention, adjusted analysis showed that participants allocated to the IS group had significantly higher SpO2 levels and lower RR, MBS levels, and LOS. Also, the adjusted model analysis showed a marginal statistically significant difference between groups in secondary outcomes, such as IR, the 1-month mortality rate, and the 3-month mortality rate.
Conclusion:
It seems that adding the ISE to usual care in the early treatment setting of COVID-19 patients resulted in a relatively significant increase in SpO2 levels, improved respiratory status, and marginally decreased LOS. Additionally, ISE minimally reduced ICU admissions and intubation rates, with no significant impact on in-hospital or long-term mortality in patients with COVID-19 pneumonia.
1. Introduction:
The World Health Organization (WHO) declared COVID-19 a global pandemic on March 11, 2020, leading to a high hospitalization rate and overwhelmed intensive care units (ICU). Many countries have faced a lot of challenges in their healthcare systems and economics due to COVID-19. (1) (2) (3)
COVID-19 manifests as a mild disease (primarily affecting the respiratory system, which is usually presented by cough, dyspnea, and fever and Spo2>94%), a moderate to severe infection requiring oxygen (Spo2<94% on room air + lung infiltration), or a critical illness requiring ventilation and life support (e.g., in acute respiratory distress syndrome or septic shock). (4)(5) Subsequently, increased capillary permeability, inflammatory response, diffusion abnormalities, and pulmonary edema, with hypoxic vasoconstriction lead to ventilation/perfusion mismatch (V/Q mismatch) and severe hypoxemia. Moreover, COVID-19 affects other organs such as the heart, liver, and kidneys to various degrees. (6) (7) (8)
Pulmonary rehabilitation (PR) is a conservative treatment with several programs, such as exercise, health education, and breathing techniques. PR was deemed effective and safe for improving overall respiratory function, lung volume, airway secretions, V/Q mismatch, and quality of life, along with reducing the related complications and minimizing disability. (9) (10) (11) Based on the evidence, a three-day PR program, with an emphasis on inspiratory hold exercise, airway clearance techniques, and mild aerobic exercise is safe and effective on functional capacity, level of dyspnea, and gas exchange in severe COVID-19 pneumonia. (12) Moreover, respiratory function is negatively associated with mortality, the need for ventilator support, and length of stay among patients with pulmonary diseases. (11) (13)
Incentive spirometer exercise (ISE) is a type of deep breathing technique widely used for lung expansion and the prevention of pulmonary complications among hospitalized and non-hospitalized patients with various diseases. ISE is used for contactless therapy among patients requiring oxygen (via a nasal cannula), or even patients who don’t require oxygen supplementation. Further, ISE encourages deep and controlled inspiratory and expiratory breathing by having the patient take long and deep breaths with subsequent pauses and emphasizes lung expansion, increasing tidal volume, and keeping the smaller airways open. (9) (14) (15) (16) As there is a knowledge gap about applying ISE in COVID-19 pneumonia management, the present study aimed to prospectively evaluate the efficacy and safety of ISE among patients with moderate to severe COVID-19 pneumonia during the acute phase.
2.Methods:
2.1 Study design and setting
This single-blind two-arm parallel randomized controlled trial (RCT) compared the efficacy and safety of ISE during five days added to the normal care with patients who received the normal care alone among hospitalized patients with moderate-to-severe COVID-19 pneumonia. The participants were enrolled from Imam Hossein Hospital (IHH), Tehran, Iran from August 19th to October 21st, 2021. IHH was designated as a referral COVID-19 hospital during the third COVID-19 spread peak in Tehran.
The study was approved by the Research Ethics Committee of Shahid Beheshti University of Medical Sciences (IR.SBMU.RETECH.REC.1400.519) and registered in the Iranian Registry of Clinical Trials (IRCT Identifier: IRCT20201012049010N2). A written informed consent form was obtained from all patients after giving a wide explanation of the study procedure.
2.2 Participants
Participants meeting the following criteria were deemed eligible for enrollment in the study: age equal to 18 years or older, patients with COVID-19 pneumonia (pulmonary CT involvement), positive RT-PCR test, and hospitalization in the last 6 hours. The following criteria rendered individuals ineligible for study participation: any case of a central nervous system disorder, need to be admitted to the ICU or intubated at enrollment, history of lung disease, SpO2 94% or above (room air), pregnant woman, history of congestive heart failure, requiring mechanical ventilation at enrollment, evidence of multi-organ failure at enrollment, or a recent diagnosis of acute coronary syndrome. Individuals meeting any of the following criteria were excluded from the study: inability to continue the study procedure for any reason, ICU admission during the study period before the fifth day, and dissatisfaction to continue the study for any reason.
2.3 Intervention
Eligible individuals were assigned (1:1) to the IS (received ISE added to usual care) or the control group (received usual care only) by simple randomization.
The randomization was done using a computer-generated allocation order. A non-involved researcher in the sampling process used a sealed opaque envelope with random numbers (group I or group II) inside them, which were opened at the time of randomization. There was no way to see the allocation type in the envelopes before opening. Opening the envelopes was done by another researcher who was responsible for the patients’ intervention before applying the allocated interventions. The assessor researchers and statistical analyzers were blinded to the randomization process and interventions used for the recruited participants. They had no access to the patient’s medical records.
Both groups received the same routine care in terms of medical treatment and other procedures based on WHO COVID-19 management guidelines. (17) The IS group was asked to do ISE along with routine care. A pulmonary physical therapist explained the instructions of ISE to the patients in the experimental group and continued training until they were assured that they were using ISE correctly.
The participants were trained to inhale for 3-5 seconds deeply and slowly, hold it for about one second, and then exhale slowly into the spirometer as their lips made a tight seal around the mouthpiece in the sitting or semi-fowler positions. Further, they were asked to cough, have a short period of rest, and repeat the entire cycle five times in three sets every day during the hospitalization period. They were also asked to continue ISE after discharge for three months. The participants received oxygen through a nasal cannula during the ISE, if needed. The interventionist researcher visited patients twice daily until discharge or dropout to ensure they were using IS correctly. The patients had a direct connection to the interventionist researcher and called him if they developed symptoms of chest pain, excessive fatigue, hemoptysis, or worsening dyspnea.
2.4 Data gathering
In order to confirm patients’ compliance with the intervention program, an ISE team member personally contacted the participants in the experimental group twice daily at the end of each shift to evaluate the ISE duration and possible side effects. The researcher contacted patients in the control group daily to ensure ISE had not been started by the medical service and followed up with the patients weekly by phone calls until the end of the third month after discharge. The team members collected estimates about patients’ compliance based on their statements at the time of the record. The patient was dropped out of the study when he/she reported discontinuing ISE for any reason in the intervention group.
Data from participants’ medical records and demographic characteristics were collected immediately after enrollment. Patients were clinically evaluated at baseline and daily (until the fifth day), including venus blood gas (VBG) analysis, quantitative level of blood C-reactive protein (CRP), D-dimer, peripheral O2 saturation (SpO2), blood pressure (BP), respiratory rate (RR), pulse rate (PR), and dyspnea level. Data were collected daily for data monitoring and participants’ safety to find ISE-related possible adverse events; however, they had no effect on the statistical analysis procedure. If the research team found any possible adverse events in one participant, the patient was excluded from the study, and a second evaluation was not applied if a patient died, got intubated, or was admitted to the ICU before the fifth day.
For VBG analysis, blood samples were taken from the upper extremity peripheral veins after breathing free air for two minutes and were immediately analyzed. The severity of dyspnea was measured based on the Modified 0-10 Borg Dyspnea Scale (MBS). The patients were asked to report the severity of dyspnea after the five-minute sitting (resting) and also after the 50-meter walking with a finger pulse oximeter (activity) as a self-assessment, on a scale of zero indicating no breathlessness at all and 10 indicating the highest perceivable level of dyspnea. (18) BP, PR, and RR were recorded after two minutes of sitting and SpO2 was recorded in the mode of O2 therapy after breathing free-air for five minutes, using an index finger pulse oximeter.
An expert radiologist determined the CT severity score (CT-score) for each lobe based on the involvement percentage (0: no involvement, to 5: >75% involvement). The total CT-score was the sum of each lobar score, ranging from 0-25. (19)
One-month and three-month mortality rates were assessed by following up to three months using a telephone-based assessment. Moderate to severe COVID-19 infection was defined as SpO2 ≥94% or <94% on room air + lung infiltration.
2.5 Outcome
Given that the present study aimed to evaluate the efficacy of ISE on the level of blood O2 saturation, the main primary outcome was SpO2 levels, along with MBS levels, RR, VBG parameters (pCO2, PH, HCO3), and length of hospital stay (LOS). In addition, the overall mortality rate, intubation rate, and ICU admission rate were considered as the secondary outcome measurements.
All outcomes were assessed before and after the day fifth except the mortality rate, which was collected during the one and three-month period.
2.6 Statistical analysis
The sample size was calculated based on a previous pilot study using Bargahi, et al. (20) for achieving 80% power and 5% α error, a sample size of 70 patients per group was calculated to detect a significant mean difference (MD) of 4% in SpO2 outcome measurements between the groups. The final sample size was considered 80 participants in each group for a more accurate assessment and possible drop-out during the study.
STATA version 13 (Stata, College. Statin, Texas, USA) was employed for statistical analysis. The normality of continuous variables was initially checked using the P-P plot, Q-Q plot, and Shapiro-Wilk test. To ensure a statistically balanced random distribution between groups at the baseline, Fisher’s exact test was used for gender, smoking status, ventilation type, and comorbidities, and independent sample T-test was applied for age, body mass index (BMI), and the levels of CRP and D-dimer.
The analysis of variance (ANOVA) was used as a crude analysis to determine the statistical differences between post-intervention outcome measurements and continuous variables. Further, ANCOVA was performed considering CRP, D-dimer, and CT-score as co-variances (one factor, three covariates). These factors were determined due to the following reasons: although there was no statistical difference between groups among all baseline outcome measures, the MD related to most of them, especially SpO2 as primary outcome measurement, was more than 0.15 × standard deviation (SD) among all participants. Considering the combination of quantitative CRP (qCRP) and D-dimer, CT-score was reported as an important predictive value for disease progression and mortality rate among hospitalized patients with COVID-19 pneumonia. (21) MD with a 95% confidence interval (CI), standardized mean difference (SMD) with 95% CI, and adjusted R2 were reported as effect sizes for each type of analysis. Cohen’s d analysis was used to report SMD and various intervals were considered in the interpretation of Cohen’s, including less than 0.02 (very small), 0.2-0.49 (small), 0.5-0.79 (moderate), 0.8-1.19 (large), and more than 1.2 (very large). Further, adjusted R2 was reported to compare the effects of considering co-founders/co-variables in statistical analyses. The variation of adjusted R2>15% between ANOVA and ANCOVA analysis models was considered important. To analyze the categorical data, the binary logistic regression was applied in the models for the crude and adjusted to baseline measurements, including qCRP, D-dimer, and CT-score. In addition, odds ratio (OR) was reported as the effect size, and intention-to-treat (ITT) analysis was utilized for categorical data analysis.
3. Result:
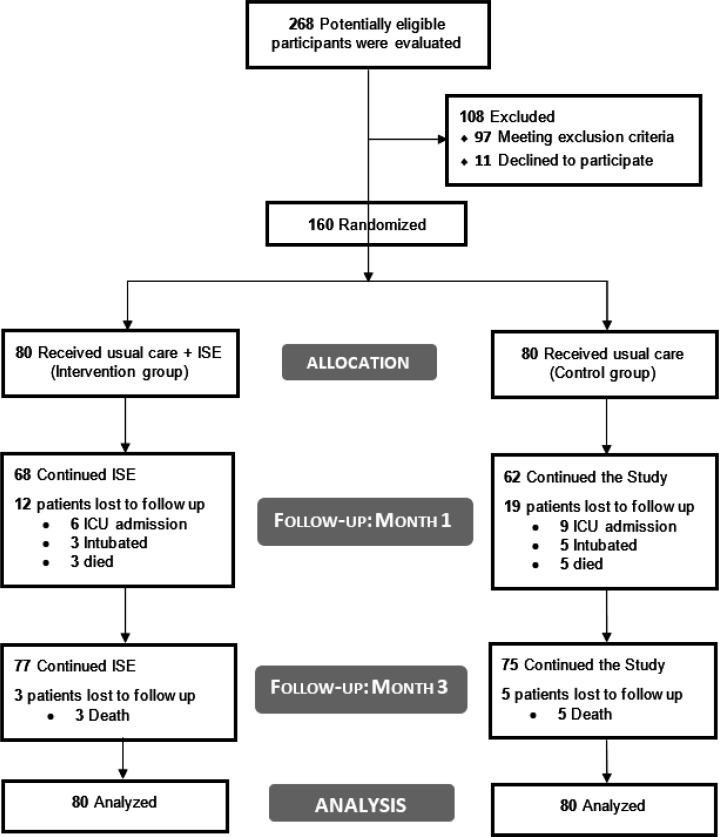
A total of 268 patients were screened for eligibility, of whom 160 met the inclusion criteria and were randomly assigned to IS (n = 80) or control (n=80) groups (Figure 1). At the time of the study, none of the Iranians had been vaccinated against Covid-19. No patient was admitted to the ICU, intubated, or died before the fifth day and all patients participated in the second assessment time. The groups were statistically similar regarding the baseline characteristics (Table 1).
Figure 1.
Study diagram.
Table 1.
Comparing the baseline characteristics of studied patients between groups underwent the incentive spirometer (IS) and control group
| Variables | Control group | IS group | P-value |
|---|---|---|---|
| Age, years | |||
| Mean ± SD | 49.66 ± 12.15 | 47.77 ± 9.83 | 0.28 |
| Sex | |||
| Male | 38 (47.5) | 42 (52.5) | 0.53 |
| Female | 42 (52.5) | 38 (47.5) | |
| BMI (kg/m2) | |||
| Mean ± SD | 23.39 ± 3.86 | 24.06 ± 4.53 | 0.32 |
| SpO 2 (%) | |||
| Free air | 87.80 ± 4.7 | 87.80 ± 5.05 | 0.99 |
| With O2 therapy | 95.20 ± 3.67 | 95.94 ± 3.41 | 0.19 |
| VBG parameters | |||
| pCO2 (mmHg) | 44.80 ± 6.71 | 43.92 ± 6.79 | 0.41 |
| HCO3 (mEq/L) | 28.22 ±3.7 | 27.89 ± 3.68 | 0.56 |
| pH | 7.41 ± .04 | 7.42 ± .04 | 0.18 |
| Modified Borg Scale | |||
| At rest | 2.23 ± 2.23 | 2.41 ± 2.09 | 0.32 |
| After activity | 4.41 ± 3.27 | 4.9 ± 3.43 | 0.32 |
| RR (n/minute) | |||
| Mean ± SD | 24.55 ± 7.54 | 25.83 ± 6.58 | 0.25 |
| Length of stay (day) | |||
| Mean ± SD | 7.01 ± 4.84 | 6.5 ± 5.34 | 0.53 |
| CRP (mg/L) | |||
| Mean ± SD | 47.76 ± 25.35 | 55.77 ± 39.04 | 0.13 |
| D-dimer (ng/mL) | |||
| Mean ± SD | 581.63 ± 607.19 | 669.30±683.78 | 0.89 |
| Smoking | |||
| Yes | 16 (20) | 15 (18.75) | 0.84 |
| Comorbidities | |||
| Diabetes mellitus | 15 (18.75) | 13 (16.5) | 0.78 |
| Heart disease | 6 (7.5) | 7 (8.75) | |
| Hypertension | 13 (16.5) | 16 (20) | |
| Kidney disease | 1 (1.25) | 2 (2.5) | |
| Other | 6 (7.5) | 8 (10) | |
| Oxygenation method | |||
| Cannula | 6 (7.5) | 5 (6.25) | 0.86 |
| Mask | 74 (92.5) | 75 (93.75) |
Data are presented as mean ± standard deviation or frequency (%). BMI: body mass index; CRP: C-reactive protein; IS: Incentive Spirometer; pCO2: Mixed venous CO2 pressure; SpO2: Oxygen saturation; VBG: venous blood gas analyses; RR: respiratory rate; M: Mean; SD: standard deviation; n: Number.
After five days, three out of 80 (3.7%) and five out of 80 (6.3%) were intubated in IS and control groups, respectively. Six participants in the IS group (7.5%) and 11 of the patients in the control group (13.8%) experienced ICU admission after the fifth day. Among 160 patients, three in the IS group and five in the control group died in the hospital. Although no more patients in the IS group died in months one and three, two patients in the control group died in this period (Figure 1).
The results of the post-intervention between groups analysis in the ANOVA versus the ANCOVA models indicated that adjusting baseline measurement of each variable, D-dimer, CRP, and CT-score changed the amount of adjusted R2 to more than 15% among all outcome measurements except for pH. The results of ANCOVA model demonstrated that the participants in the IS group had statistically greater SpO2 after five-minute free air breathing [MD: 0.57, 95%CI: -1.29, 2.44; p <0.001] and SpO2 in the mode O2 therapy [MD: 0.29, 95%CI: -1.23, 1.8; p = <0.001] and lower RR [MD: -0.55, 95%CI: -2.35, 1.26; p = 0.07], MBS at rest [MD: -0.45, 95%CI: -1.24, 0.34; p = <0.001], MBS after activity [MD: -0.07, 95%CI: -1.06, 0.92; p = <0.001], and LOS [MD: -1, 95%CI: -2.5, 0.57; p = 0.001]. No significant difference was observed between the groups in terms of VBG parameters (pCO2, PH, and HCO3) outcomes in both crude and adjusted models (Table 2).
Table 2.
Comparing different clinical and laboratory outcomes between two groups
| Outcomes | Groups | F (P value) * | SMD [95 % CI] | Adjusted R 2 | |
|---|---|---|---|---|---|
| IS (n =80) | Control (n =80) | ||||
| SpO2 (Free air) (%) | 90.11 ± 5.9 | 90.10 ± 6.9 | 0.0 (0.99) a | 0.00 [-.31, .31] | 0.006 |
| 8.07 (0.00) b | 0.10 [-.21, .41] | 0.152 | |||
| SpO2 (With O2 therapy) (%) | 95.83 ± 5.22 | 95.89 ± 5.09 | 0.01 (0.94) a | -0.01 [-.32, .3] | -0.006 |
| 6.73 (0.00) b | 0.06 [-.25, .37] | 0.126 | |||
| pCO2 (mmHg) | 49.79 ± 7.71 | 50.1 ± 9.87 | 0.05 (0.83) a | -0.03 [-.35, .28] | -0.006 |
| 1.53 (0.2) b | -0.04 [-.35, .27] | 0.013 | |||
| pH | 7.40 ± 0.04 | 7.40 ± .05 | 0.37 (0.05) a | -0.1 [-.41, .22] | -0.004 |
| 0.76 (0.55) b | -0.09 [-.4, .22] | -0.006 | |||
| HCO3 (mEq/L) | 33.92 ± 3.69 | 30.71 ± 4.44 | 0.57 (0.45) a | -0.12 [-.43, .19] | -0.003 |
| 0.40 (0.80) b | -0.13 [.18, -.45] | -0.026 | |||
| RR (number/minute) | 23.45 ± 5.92 | 23.73 ± 5.73 | 0.09 (0.77) a | -0.05 [-.36, .26] | -0.006 |
| 2.18 (0.07) b | -0.09 [-.41, .21] | 0.029 | |||
| Modified Borg Scale at rest | 1.46 ± 2.42 | 1.75 ± 2.89 | 0.47 (0.49) a | -0.11 [-.42, .2] | -0.003 |
| 6.14 (0.00) b | -0.18 [-.49, .13] | 0.114 | |||
| Modified Borg Scale after activity | 3.24 ± 3.11 | 3.1 ± 3.4 | 0.08 (0.77) a | 0.05 [-.26, .36] | -0.006 |
| 3.91 (0.00) b | -0.02 [-.33, .29] | 0.068 | |||
| Length of stay (day) | 6.5± 5.34 | 7.01 ± 4.84 | 0.40 (0.53) a | -0.1 [-.21, .41] | -0.004 |
| 4.88 (0.001) b | -0.2 [-.51, .11] | 0.089 | |||
Data are expressed as mean ± standard deviation. IS: Incentive Spirometer; RR: respiratory rate; pCO2: Mixed venous CO2 pressure; SpO2: Oxygen saturation; CI: Confidence interval; SMD: Standardized mean difference (based on Cohen’s d test), ANOVA: Analysis of variance, ANCOVA: Analysis of covariance.
*Analyzed using ANOVA/ANCOVA tests. a Crude analysis; b Adjusted to baseline measurement of the D-dimer, CRP, and CT-score (as covariances).
The results of Cohen’s d analysis presented very small differences, i.e. < 0.19, related to all outcome measurements except LOS which was small [SMD in the adjusted model: -0.2, 95%CI: -0.51, .11]. Table 2 shows more details of the statistical analysis of continuous data.
Although there was no statistically significant difference in Intubation rate (p = 0.47), ICU admission rate (p = 0.20), one and three-month mortality rates in crude analysis (p = 0.58), the adjusted model analysis showed a marginal statistically significant difference between the groups in secondary outcomes, including intubation rate [OR: 0.42, 95%CI: 0.08, 2.21, p = 0.06], one-month mortality rate [OR: 0.47, 95%CI: 0.09, 2.46, p = 0.05], and three-month mortality rate [OR: 0.47, 95%CI: 0.09, 2.46, p = 0.05] (Table 3).
Table 3.
Comparing the mortality rate, ICU admission, and intubation rate of participants allocated to groups
| Variables | Yes | No | OR [95% CI] | P-Value* |
|---|---|---|---|---|
| Intubation rate | ||||
| IS group | 3 (3.7) | 77 (96.3) | 0.58a [.13, 2.53] | 0.47a |
| Control group | 5 (6.3) | 75 (93.7) | 0.42b [0.08, 2.21] | 0.06b |
| ICU admission rate | ||||
| IS group | 6 (7.5) | 74 (92.5) | 0.51a [0.18, 1.45] | 0.20a |
| Control group | 11 (13.8) | 69 (86.2) | 0.45b [0.15, 1.34] | 0.48b |
| Hospital mortality | ||||
| IS group | 3 (3.7) | 77 (96.3) | 1a [0.19, 5.1] | 1.00a |
| Control group | 3 (3.7) | 77 (96.3) | 0.82b [0.13, 5.25] | 0.06b |
| One-month mortality | ||||
| IS group | 3 (3.7) | 77 (96.3) | 0.58a [0.13, 2.53] | 0.47a |
| Control group | 5 (6.3) | 75 (93.7) | 0.47b [0.09, 2.46] | 0.05b |
| Three-month mortality | ||||
| IS group | 3 (3.7) | 77 (96.3) | 0.58a [0.13, 2.53] | 0.47a |
| Control group | 5 (6.3) | 75 (93.7) | 0.47b [0.09, 2.46] | 0.05b |
Data are presented as number (%). ICU: Intensive care unit; IS: Incentive spirometry. CI: Confidence interval; OR: odds ratio. a Crude analysis; b Adjusted to baseline measurement of the D-dimer, CRP, and Ct-score (as covariances); * Using logistic regression analysis.
4.Discussion:
The present study aimed to evaluate the efficacy and safety of ISE as an additional treatment for hospitalized patients with moderate to severe COVID-19 pneumonia in the acute setting.
The result of ANOVA indicated no significant difference by adding ISE to the routine care in the IS group on the fifth day. Although by considering covariance, including baseline qCRP, D-dimer, and Ct-score as the most important factors for predicting the disease severity, the ANCOVA model showed a very slight improvement in SpO2 levels, MBS scores, and RR among the patients who received ISE. In addition, the result revealed that patients in the IS group stayed in the hospital for a shorter period compared to the patients in the control group (about half a day less), supporting some suggestions about the application of ISE provided by the guideline and expert advice mildly. (22) (23) (24) (25)
The patients in the IS group had a slightly lower rate of intubation, death, and ICU admission within three months compared to the patients in the control group. Although the score needed to treat (NNT) levels for the secondary outcomes was not clinically significant (>10), the logistic test adjusted to the baseline covariates in one and three-month mortality, intubation, and ICU admission rates outcomes verified the advantages of ISE application. This study confirms the recommendations from experts and guidelines for starting PR like ISE early in the disease. (12) (22) (23) (25) Although the results of ANCOVA and binary logistic regression analysis presented significant or marginal differences in most outcomes, the results of the efficacy of ISE application should be generalized with caution since very slight or slight effect sizes were reached.
Currently, there is a controversy regarding the use of various PR strategies during the acute phase of COVID-19. A number of researchers and specialists have prohibited the use of ISE due to conceivable side effects and worsening of the disease course. (2) (24) (25) (26) (27) On the other hand, some have proposed ISE as a safe procedure in the early stage of COVID-19. (25) (28) (29) In the present study, a few patients who performed ISE reported mild dizziness, fatigue, and minor worsening in breathing within the to-begin-with were inquired to proceed with the intervention with long pauses between the cycles to more compliance. Eventually, no serious complications were observed among the patients who performed ISE which forced them to terminate the intervention during the whole period of time. Furthermore, no statistical analysis revealed any results about the participants’ worsening during the study. Nevertheless, the results support the ISE as a safe procedure to start in the early setting of the disease. Consequently, it is suggested that patients take sufficient O2 support and be under accurate monitoring by an experienced pulmonary physical therapist during exercises.
4.1 Limitations:
The results of the present study should be considered in light of some limitations. The trial was run in a single center only and given the nature of the ISE, it was essentially outlandish to blind the patients and the health staff. Due to the synchronization of the study, the third peak of COVID-19 in Tehran, and the overwhelming of accessible centers, it was impossible to perform more detailed clinical and para-clinical tests such as spirometry and imaging, and the long-term mortality rate was satisfactory. Also, we avoided reporting the intention to treat. Eventually, further studies should explore the financial esteem of utilizing the integrated treatment method compared to the routine treatment alone.
5. Conclusion:
Among the patients with moderate to severe COVID-19 pneumonia, the use of ISE integrated into routine care compared with routine care alone in the early setting seems led to a relatively statistically significant increase in SpO2 level, an improvement in respiratory status, and a reduction of the length of stay over five days. Considering that ISE as a safe, contactless, feasible, low-price, potentially add-on intervention might minimally reduce the ICU admission and Intubation rate, it seems to have no side effect on the in-hospital and long-term mortality among patients with COVID-19 pneumonia. Further trials should be considered to run in more centers with more long-term evaluation and wider clinical outcomes.
6. Declarations:
6.1 Funding
This study was funded by the Shahid Beheshti University of Medical Sciences. The funder of the study had no role in study design, data collection, analysis, interpretation, or writing the manuscript. The first author and corresponding author had full access to all the data and are responsible for publishing the results.
6.2 Acknowledgments
The researchers sincerely thank all physicians, physical therapists and nurses of COVID-19 wards of IHH. The authors would like to appreciate the methodologist’s support and constructive comments (s) research development office, IHH, Tehran, Iran.
6.3 Author Contributions
Conceptualization and Methodology: Mohammad Bargahi, Mostafa Alavi-Moghaddam, Mohammad Javaherian.
Investigators: Mehdi Karimi, Zahra Azizan, Fateme Jafarzadeh, Ayda Allahyari, Seyed Hamidreza Mirbehbahani, Hussein Soleimantabar.
Statistical analysis: Mohammad Javaherian, Mohammad Bargahi.
Project administration: Mohammad Bargahi.
Supervision: Mostafa Alavi-Moghaddam, Mohammad Bargahi.
Visualization: Mohammad Javaherian, Mohammad Bargahi.
Writing – original draft and review: Mohammad Bargahi, Mohammad Javaherian, Mostafa Alavi-Moghaddam.
6.4 Conflicts of interest
The authors declare no conflicts of interest.
6.5 Using artificial intelligence chatbots
NO.
6.6 Data availability
Upon official request to the corresponding author, data are available to release.
References
- 1.Jamil S, Mark N, Carlos G, Cruz CSD, Gross JE, Pasnick S. Diagnosis and management of COVID-19 disease. American journal of respiratory and critical care medicine. 2020;201(10):P19–P20. doi: 10.1164/rccm.2020C1. [DOI] [PubMed] [Google Scholar]
- 2.Thomas P, Baldwin C, Bissett B, Boden I, Gosselink R, Granger CL, et al. Physiotherapy management for COVID-19 in the acute hospital setting: clinical practice recommendations. Journal of physiotherapy. 2020;66(2):73–82. doi: 10.1016/j.jphys.2020.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nasserie T, Hittle M, Goodman SN. Assessment of the frequency and variety of persistent symptoms among patients with COVID-19: a systematic review. JAMA network open. 2021;4(5):e2111417–e. doi: 10.1001/jamanetworkopen.2021.11417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Organization WH. Living guidance for clinical management of COVID-19: living guidance, 23 November 2021. World Health Organization; 2021. [Google Scholar]
- 5.He Z-f, Zhong N-s, Guan W-j. The benefits of pulmonary rehabilitation in patients with COVID-19. Eur Respiratory Soc. 2021 doi: 10.1183/23120541.00212-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Makic MBF. Prone position of patients with COVID-19 and acute respiratory distress syndrome. Journal of PeriAnesthesia Nursing. 2020;35(4):437–8. doi: 10.1016/j.jopan.2020.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Groff D, Sun A, Ssentongo AE, Ba DM, Parsons N, Poudel GR, et al. Short-term and long-term rates of postacute sequelae of SARS-CoV-2 infection: a systematic review. JAMA network open. 2021;4(10):e2128568–e. doi: 10.1001/jamanetworkopen.2021.28568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang L, Yang T. Pulmonary Rehabilitation for Patients with Coronavirus Disease 2019 (COVID-19) Chronic Diseases and Translational Medicine. 2020 doi: 10.1016/j.cdtm.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alahmri F, Muaither S, Alsharhan H, Alotaibi S, Alotaibi H, Alsadi S. The effect of pulmonary rehabilitation on covid-19 patients. Phys Med Rehabil Res. 2020;5:1–4. [Google Scholar]
- 10.Fila E, Rocco I, Rocco G, Zuccon U, Ruberti E. Recommendations for the respiratory rehabilitation of hospitalized and discharged COVID-19 patients: A sistematic review. G Ital Med Lav Ergon. 2021;43:56–65. [Google Scholar]
- 11.Kader M, Hossain MA, Reddy V, Perera NKP, Rashid M. Effects of short-term breathing exercises on respiratory recovery in patients with COVID-19: a quasi-experimental study. BMC Sports Science, Medicine and Rehabilitation. 2022;14(1) doi: 10.1186/s13102-022-00451-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Javaherian M, Shadmehr A, Keshtkar A, Beigmohammadi MT, Dabbaghipour N, Syed A, et al. Safety and efficacy of pulmonary physiotherapy in hospitalized patients with severe COVID-19 pneumonia (PPTCOVID study): A prospective, randomised, single-blind, controlled trial. Plos one. 2023;18(1):e0268428. doi: 10.1371/journal.pone.0268428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhu Y, Wang Z, Zhou Y, Onoda K, Maruyama H, Hu C, et al. Summary of respiratory rehabilitation and physical therapy guidelines for patients with COVID-19 based on recommendations of World Confederation for Physical Therapy and National Association of Physical Therapy. Journal of physical therapy science. 2020;32(8):545–9. doi: 10.1589/jpts.32.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jiandani MP, Agarwal B, Baxi G, Kale S, Pol T, Bhise A, et al. Evidence-based national consensus: recommendations for physiotherapy management in COVID-19 in acute care Indian setup. Indian Journal of Critical Care Medicine: Peer-reviewed, Official Publication of Indian Society of Critical Care Medicine. 2020;24(10) doi: 10.5005/jp-journals-10071-23564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Battaglini D, Caiffa S, Gasti G, Ciaravolo E, Robba C, Herrmann J, et al. An experimental pre-post study on the efficacy of respiratory physiotherapy in severe critically III COVID-19 patients. Journal of clinical medicine. 2021;10(10):2139. doi: 10.3390/jcm10102139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Restrepo RD, Wettstein R, Wittnebel L, Tracy M. Incentive spirometry: 2011. Respiratory care. 2011;56(10):1600–4. doi: 10.4187/respcare.01471. [DOI] [PubMed] [Google Scholar]
- 17.COVID W. Clinical management: living guidance. OMS/2019-nCoV/Clinical. 2021:659–63. [Google Scholar]
- 18.Kendrick KR, Baxi SC, Smith RM. Usefulness of the modified 0-10 Borg scale in assessing the degree of dyspnea in patients with COPD and asthma. Journal of Emergency Nursing. 2000;26(3):216–22. doi: 10.1016/s0099-1767(00)90093-x. [DOI] [PubMed] [Google Scholar]
- 19.Pan F, Ye T, Sun P, Gui S, Liang B, Li L, et al. Time course of lung changes at chest CT during recovery from coronavirus disease 2019 (COVID-19) Radiology. 2020;295(3):715–21. doi: 10.1148/radiol.2020200370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bargahi M, Rastgoo N, Aryanejad F, Esmaielzade S, Nemati R, Ghaebi M, et al. Effect of Balloon-Blowing on Dyspnea and Oxygenation in Hospitalized COVID-19 Patients: A Pilot Study. Acta Medica Iranica. . 2022:338–44. [Google Scholar]
- 21.Zhu J, Chen C, Shi R, Li B. Correlations of CT scan with high-sensitivity C-reactive protein and D-dimer in patients with coronavirus disease 2019. Pakistan journal of medical sciences. 2020;36(6):1397. doi: 10.12669/pjms.36.6.2961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lan C-C, Hsieh P-C, Yang M-C, Su W-L, Wu C-W, Huang H-Y, et al. Early pulmonary rehabilitation of COVID-19 patients in an isolation ward and intensive care unit. Tzu Chi Medical Journal. 2023;35(2):137–42. doi: 10.4103/tcmj.tcmj_136_22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ambrose AF, Kurra A, Tsirakidis L, Hunt KC, Ayers E, Gitkind A, et al. Rehabilitation and in-hospital mortality in COVID-19 patients. The Journals of Gerontology: Series A. 2022;77(4):e148–e54. doi: 10.1093/gerona/glab321. [DOI] [PubMed] [Google Scholar]
- 24.Verma CV, Arora RD, Mistry HM, Kubal SV, Kolwankar NS, Patil PC, et al. Changes in mode of oxygen delivery and physiological parameters with physiotherapy in covid-19 patients: a retrospective study. Indian Journal of Critical Care Medicine: Peer-reviewed, Official Publication of Indian Society of Critical Care Medicine. 2021;25(3):317. doi: 10.5005/jp-journals-10071-23763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mistry HM, Natesan A, Verma CV. Early Intervention of Physiotherapy Helps to Reduce Hospital Stay and Improve Functional Capacity of Patients with Severe COVID–ARDS. Indian Journal of Medical Sciences. 2020;73(1):75. [Google Scholar]
- 26.Lazzeri M, Lanza A, Bellini R, Bellofiore A, Cecchetto S, Colombo A, et al. Respiratory physiotherapy in patients with COVID-19 infection in acute setting: a Position Paper of the Italian Association of Respiratory Physiotherapists (ARIR) Monaldi archives for chest disease. 2020;90:1. doi: 10.4081/monaldi.2020.1285. [DOI] [PubMed] [Google Scholar]
- 27.Righetti RF, Onoue MA, Politi FVA, Teixeira DT, Souza PNd, Kondo CS, et al. Physiotherapy care of patients with coronavirus disease 2019 (COVID-19)-a Brazilian experience. Clinics. 2020;75:e2017. doi: 10.6061/clinics/2020/e2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Seyller H, Gottlieb M, Colla J. A breath of fresh air: The role of incentive spirometry in the treatment of COVID-19. The American Journal of Emergency Medicine. 2021;48:369. doi: 10.1016/j.ajem.2021.01.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sheth AR, Faraji M, Boparai S, Dhaliwal L, Kulkarni S. Judicious use of incentive spirometry in resource limited times of COVID-19 pandemic. The American Journal of Emergency Medicine. 2021;47:290. doi: 10.1016/j.ajem.2021.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Upon official request to the corresponding author, data are available to release.