Highlights
-
•
Freshwater biodiversity responses to multiple stressors were studied.
-
•
Hydrology and water quality primarily drove all individual biodiversity indices.
-
•
Fish and phytoplankton showed greater responses to studied stressors.
-
•
Natural, land use, and hydrological factors affected integrated indices indirectly.
-
•
Individual and integrated indices are needed for conservation and management.
Keywords: Hydrological modification, α and β diversity, Land use, Water quality, Dams
Abstract
Freshwater biodiversity is increasingly threatened by dams and many other anthropogenic stressors, yet our understanding of the complex responses of different biotas and their multiple facets remains limited. Here, we present a multi-faceted and integrated-indices approach to assess the differential responses of freshwater biodiversity to multiple stressors in the Yangtze River, the third longest and most dam-densely river in the world. By combining individual biodiversity indices of phytoplankton, zooplankton, periphyton, macroinvertebrates, and fish with a novel integrated aquatic biodiversity index (IABI), we disentangled the effects of hydrology, water quality, land use, and natural factors on both α and β diversity facets in taxonomic, functional, and phylogenetic dimensions. Our results revealed that phytoplankton and fish species and functional richness increased longitudinally, while fish taxonomic and phylogenetic β diversity increased but phytoplankton and macroinvertebrate β diversity remained unchanged. Hydrology and water quality emerged as the key drivers of all individual biodiversity indices, followed by land use and natural factors, with fish and phytoplankton showed the strongest responses. Importantly, we found that natural, land use, and hydrological factors indirectly affected biodiversity by altering water quality, which in turn directly influenced taxonomic and phylogenetic IABIs. Our findings highlight the complex interplay of multiple stressors in shaping freshwater biodiversity and underscore the importance of considering both individual and integrated indices for effective conservation and management. We propose that our multi-faceted and integrated-indices approach can be applied to other large, dam-modified river basins globally.
Graphical abstract
1. Introduction
Earth's biodiversity in the Anthropocene is undergoing severe changes, at least when compared to those recorded before (Pimm et al., 2014; Ceballos et al., 2020). The fluctuating presence, frequency, and intensity of various stressors across different dimensions lead to a wide spectrum of responses, from individuals to ecosystems (Jackson et al., 2021). The decline in freshwater biodiversity, for instance, is significantly correlated with the dramatic increase in dams and many other human activities (Chen et al., 2020). However, studies on patterns of aquatic organisms based on large-scale multi-biota groups under multiple stressors are relatively uncommon, even in global biodiversity hotspots and key ecosystems (Cincotta et al., 2000). These studies are urgently needed to accurately identify those species groups at high risk and to determine priority conservation actions (Di Marco et al., 2018).
Accurate mapping and analysis of biodiversity variation under anthropogenic stressors is a prerequisite to halt global biodiversity decline (Kraft et al., 2008). Analysis of species diversity patterns and responses to stressors can predict changes in ecosystem dynamics (Wilson et al., 2004). Such assessment based on mapping, analysis and prediction allows for the identification of single species or biotic communities currently at high risk in the ecosystem and provide the basis for forecasting the potential impacts of influential environmental stressors on organisms (Urban et al., 2016). However, multi-trophic biodiversity (i.e., plant, animal, or microbial) and their multiple dimensions (i.e., taxonomic, functional, and phylogenetic diversity) may differ in their responses to environmental stressors (Heino, 2015). Such varied responses make it difficult to estimate the overall biodiversity response to multiple stressors, which is the main challenge in the research on biodiversity change (Su et al., 2021). It is therefore necessary to develop a research framework that includes both individual and integrated biological indices. Individual indices can reveal the sensitivity and adaptation of a specific taxa to environmental changes while the integrated index can inform the overall biological response to stressors.
In addition, simultaneously considering taxonomic diversity (TD), functional diversity (FD), and phylogenetic diversity (PD) could provide a more comprehensive perspective when studying biodiversity variations (Meynard et al., 2011; Teichert et al., 2018). Moreover, aquatic organisms at different trophic levels could respond differently to the same set of anthropogenic stressors (Qu et al., 2023), which also showed advantages over individual indices (Allan et al., 2014; Moi et al., 2022). Therefore, it is necessary to integrate different levels and dimensions of diversity across multiple trophic levels to reflect the effects of dams and many other stressors on freshwater biodiversity accurately.
In this study, we used comprehensive datasets of five biological groups in the Yangtze River basin (YRB) to detect basin-wide freshwater biodiversity patterns and test how multiple natural factors and anthropogenic stressors affect individual biodiversity indices and the integrated aquatic biodiversity index (IABI). Our research questions include: (1) what are the overall biodiversity patterns across multiple biological groups in multiple facets in the YRB? (2) How do dams and many other stressors affect individual and integrated biodiversity indices? Our results highlight multi-trophic biodiversity patterns and anthropogenic stressors affecting freshwater biodiversity, which have implications on management, conservation and restoration priorities in dam-modified river basins.
2. Results
2.1. Patterns in α diversity
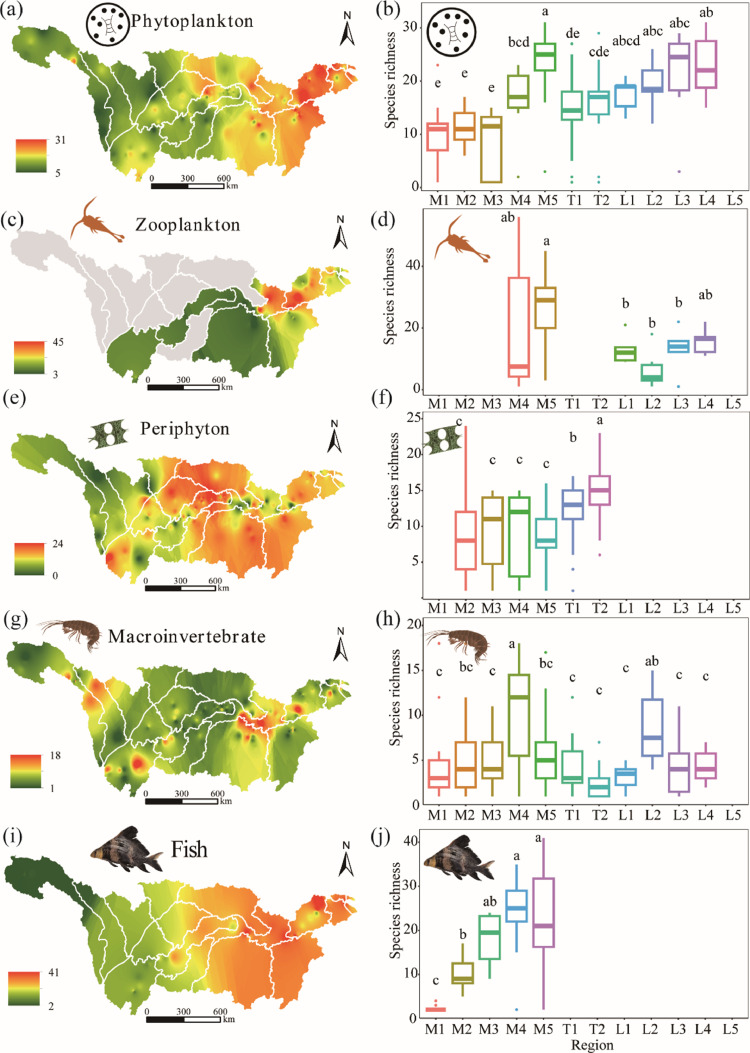
Phytoplankton and fish species richness increased from the higher elevation plateau region HYZ to lower elevation floodplain region LYZ. Periphyton species richness varied consistently in the mainstem but was significantly higher in MTYZ and TTGD. Macroinvertebrate species richness was significantly higher in MYZ than in other mainstem reaches and tributaries (Fig. 1). Similar patterns were observed for Shannon diversity (Fig. S1). However, fish evenness showed a decreasing trend from HYZ to LYZ (Fig. S2).
Fig. 1.
Maps of species richness of five biological groups in the Yangtze River basin. Note: Different lowercase letters indicate significant differences among different parts of the YRB. M1=HYZ, M2=JSR, M3= UYZ, M4=MYZ, M5=LYZ; T1=MTYZ, T2= TTGD; L1=DCL, L2=DTL, L3=PYL, L4=CHL, L5=THL.
Phytoplankton and fish functional richness increased from the higher elevation plateau region HYZ to lower elevation floodplain region LYZ. Periphyton functional richness did not vary appreciably in the mainstem but increased slightly in the direction of MTYZ and TTGD (Fig. S3). Phytoplankton and fish functional evenness decreased from HYZ to LYZ (Fig. S4). Functional divergence of phytoplankton increased, while functional divergence of fish decreased from HYZ to LYZ (Fig. S5).
Fish average taxonomic distinctness (Delta+) increased from the higher elevation plateau region HYZ to lower elevation floodplain region MYZ, while other biological groups showed a consistent pattern among locations (Fig. S6).Variation in fish taxonomic distinctness (Lambda+) increased from HYZ to MYZ, while other biological groups did not show considerable variation among locations (Fig. S7).
2.2. Patterns in β diversity
Total taxonomic β diversity of phytoplankton and its components were relatively consistent among locations, and it was mainly contributed by turnover (Fig. S8a-c). Total taxonomic β diversity of zooplankton decreased from MYZ to LYZ (Fig. S9). Total taxonomic β diversity of periphyton and its components showed significant decreases from the mainstem JSR to tributaries (Fig. S10). Total taxonomic β diversity of macroinvertebrates and its components were consistent among locations, and it was mainly contributed by turnover (Fig. S11). Total taxonomic β diversity of fish and its turnover increased from HYZ to LYZ (Fig. S12a,b).
Total functional β diversity of phytoplankton and its components decreased from HYZ to LYZ, and it was mainly contributed by nestedness (Fig. S8d-f). Zooplankton functional total β diversity decreased from the MYZ to LYZ (Fig. S9). Periphyton functional total β diversity and its components had significant decreases from the mainstem JSR to the tributaries (Fig. S10). Total functional β diversity of macroinvertebrates and its components were consistent among locations, and it was mainly contributed by turnover (Fig. S11). Total functional β diversity of fish and its major contributing component, turnover, decreased from HYZ to LYZ (Fig. S12d, e).
Total phylogenetic β diversity of phytoplankton and its components were relatively consistent among locations, and total β diversity was mainly contributed by turnover (Fig. S8g-i). Total phylogenetic β diversity of zooplankton decreased from MYZ to LYZ (Fig. S9). Total phylogenetic β diversity of periphyton and its components exhibited significant decreases from the mainstem JSR to the tributaries (Fig. S10). Total phylogenetic β diversity of macroinvertebrates and its components were consistent among locations, and total β diversity was mainly contributed by turnover (Fig. S11). Total phylogenetic β diversity of fish and its major contributing component, turnover, increased from HYZ to LYZ (Fig. S12g, h).
2.3. Driving factors
2.3.1. Individual biodiversity index responses
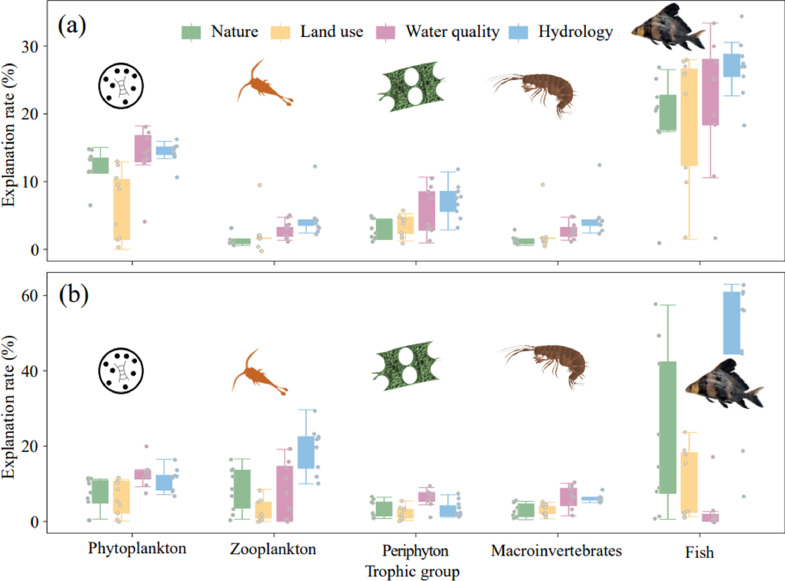
For α diversity, phytoplankton diversity was mainly driven by hydrology (14.25 %), water quality (13.88 %), and natural factors (12.32 %), followed by land use (6.68 %) (Fig. 2a). Zooplankton diversity was mainly driven by water quality and hydrology (Fig. 2a). Periphyton diversity was mainly driven by hydrology, followed by water quality, land use, and natural factors (Fig. 2a). Macroinvertebrate diversity was mainly driven by hydrology, followed by water quality, land use, and natural factors (Fig. 2a). Fish diversity was mainly driven by hydrology and water quality, followed by land use and natural factors (Fig. 2a).
Fig. 2.
Individual freshwater biodiversity (a. α level, b. β level) responses to multiple stressors in the Yangtze River basi.
For β diversity, phytoplankton assemblage variation was mainly driven by water quality (12.47 %) and hydrology (11.33 %), followed by natural factors (7.12 %), and land use(6.00 %) (Fig. 2b). Zooplankton assemblage variation was mainly driven by hydrology, followed by natural factors, water quality, and land use (Fig. 2b). Periphyton assemblage variation was mainly driven by water quality, followed by natural factors, hydrology, and land use (Fig. 2b). Macroinvertebrate assemblage variation was mainly driven by water quality and hydrology, followed by land use and natural factors (Fig. 2b). Fish assemblage variation was mainly driven by hydrology and natural factors, followed by land use and water quality (Fig. 2b).
2.3.2. Integrated biodiversity index (IABI) responses
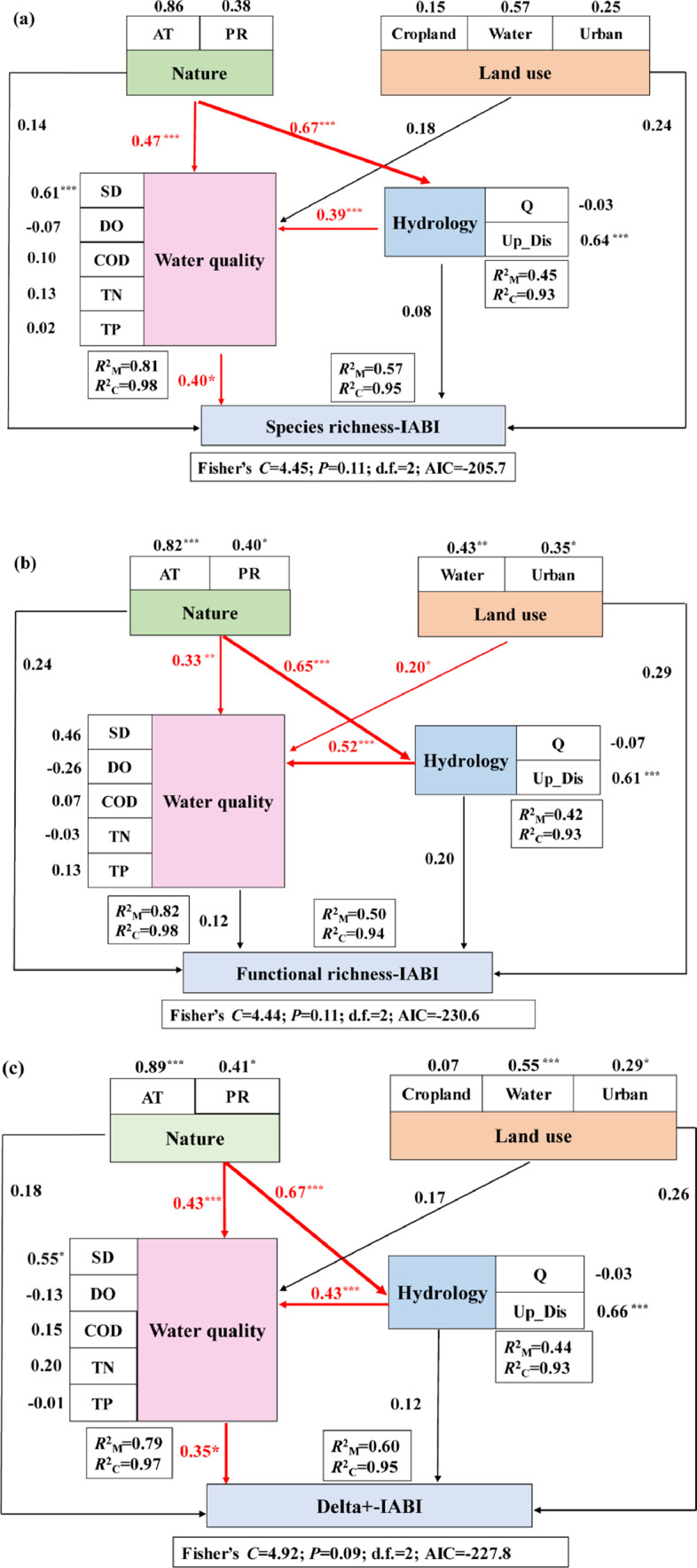
Water quality had the highest contribution to species richness-IABI (coefficient = 0.40), followed by land use (0.24), nature (0.14), and hydrology (0.08) (Fig. 3a). Natural factors had significant direct effects on both water quality and hydrology. Hydrology had a significant direct effect on water quality (Fig. 3a).
Fig. 3.
Integrated freshwater biodiversity (a. taxonomic diversity based IABI, b. functional diversity based IABI, c. phylogenetic diversity based IABI) responses to multiple anthropogenic stressors (Land use, Water quality, and Hydrology) and natural factors (Nature) in the Yangtze River basin. Note: IABI refers to Integrated Aquatic Biodiversity Index. The numbers adjacent to the arrows are path coefficients, which indicate the direct standardized effect size of the relationship. Significant paths and their corresponding coefficients were highlighted in red. The sign of * indicates the degree of significance where * refers to p < 0.05, ** refers to p < 0.01, and *** refers to p < 0.001, respectively.
Functional richness-IABI was most strongly contributed by land use (coefficient = 0.29) and natural factors (0.24), followed by hydrology (0.20) and water quality (0.12) (Fig. 3b). Natural factors had significant direct effects on water quality and hydrology. Hydrology and land use had significant direct effects on water quality (Fig. 3b).
IABI based on combined taxonomic distinctness (delta+) was mostly strongly related to water quality (coefficient = 0.35) and land use (0.26), followed by natural factors (0.18) and hydrology (0.12) (Fig. 3c). Natural factors had significant direct effects on water quality and hydrology. Hydrology had a significant direct effect on water quality (Fig. 3c).
3. Discussion
At the alpha level, phytoplankton and fish species and functional richness increased from HYZ to LYZ. Such pattern in the relationship between river biodiversity and longitudinal gradient has been detected elsewhere (Green et al., 2022), which is consistent with previously reported fish patterns in the YRB (Kang et al., 2018). Spatial differences in ecological community structure at the watershed scale are mostly shaped by a combination of abiotic factors, biotic interactions, and dispersal processes (Heino et al., 2015). For instance, habitat topography and channel geomorphology determine the distribution pattern of species at the watershed scale (Birk et al., 2020). Different habitat characteristics dictate that distributions of aquatic organisms match to a considerable degree with the natural features and geography in the YRB (Kang et al., 2018). In the upper reaches of the Yangtze River, alpine environments determine which species are filtered by the natural environmental factors, resulting in lower species diversity at high elevations. As elevation decreases in the middle and lower parts of the Yangtze River, warmer climate favors an increase in species diversity (Peters et al., 2016). Under the dominant influence of environmental filters, FD increased significantly as suitable functional traits facilitated species to cope with specific local environmental conditions (Liu et al., 2023). For periphyton, tributaries have less water volume and flow than the mainstem, which facilitates survival and reproduction of many taxa. This has been detected in tributaries entering the Three Gorges Reservoir (TGR) (Li et al., 2021). Fish usually have strong migratory and dispersal capabilities, being able to migrate and reproduce freely within a river basin, provided there are no barriers for their movements along the watercourses. The middle and lower reaches of the Yangtze River possess a high degree of riverine connectivity that allow for relatively unrestricted migration and dispersal of fish (Xiong et al., 2023). These migratory and dispersal possibilities in turn facilitate gene flow and exchange of individuals between fish assemblages in different regions, which may lead to increased phylogenetic diversity in fish communities. Other aquatic taxa showed a more uniform variation across sites, possibly due to their relatively lower migratory and dispersal capacities (Qu et al., 2023). The importance of both phytoplankton and fish in response to stressors could be partially related to our sampling focus (i.e., large river, main stem and major tributaries), which is consistent with previous studies (Gao et al., 2023; Xiong et al., 2023).
The main novelty of this study is that it revealed that the multidimensional diversity of aquatic taxa comprising multiple trophic levels does not always vary consistently across sites and regions, increasing understanding of spatial β-diversity patterns of aquatic communities and different organism groups at the river basin scale. Total taxonomic and functional β-diversity of phytoplankton and macroinvertebrates were not significantly different between the regions of YRB, which may indicate that environmental stress in the YRB has homogenized phytoplankton and macroinvertebrates to a certain extent. Previous studies have demonstrated that eutrophication could promote taxonomic homogenization of diatom and macroinvertebrate assemblages (Zhang et al., 2019; Zorzal-Almeida et al., 2021). In general, biotic homogenization is a worldwide phenomenon resulting from various anthropogenic factors, yet the degree of biotic change depends on many factors (Rolls et al., 2023).
Total taxonomic and phylogenetic beta diversity of fish and their turnover components increased from HYZ to LYZ, suggesting that turnover, or the presence or absence of species contributed more than nestedness to beta diversity at the watershed scale (Araujo et al., 2019). Metacommunity theory suggests that ecological community structure is determined not only by local abiotic environmental conditions, but also by biotic interactions and dispersal-related effects (Leibold et al., 2004), which results in substitution of species between sites and may be associated with both natural and anthropogenic factors (Heino, 2013). Aquatic organisms in the upper reaches of YRB are strongly isolated by mountain ranges, elevation, and human-made engineering structures (e.g., dams), which contributes to dispersal limitation among aquatic species. In turn, fish total functional β-diversity showed a decreasing trend from HYZ to LYZ and was mainly contributed by nestedness. This is consistent with previous studies, as the diversity of fish traits suitable to unique environments in the Qinghai-Tibet Plateau Region is different from those in other parts of YRB (Kang et al., 2018). A possible explanation for the dominance of nestedness in functional β-diversity is selective extinction or environmental selection in general; whereby some species traits are more common while others are rare due to environmental filtering (Heino and Tolonen, 2017). The relatively high geographical isolation of the headwater reaches of the YRB, coupled with the unidirectional flow of water and limited dispersal of organisms, may also have resulted in aquatic communities that are more strongly reflecting high nestedness (Heino et al., 2015). Variation in environmental heterogeneity among sites within the watershed may also contribute to high nestedness and low turnover in the functional beta diversity index (Liu et al., 2023). However, distinguishing whether environmental heterogeneity results in high nestedness or high turnover of biotic communities would require simultaneous analysis of nestedness and turnover of environmental conditions among sites (Heino and Tolonen, 2017).
Diversity indices of individual taxonomic groups responded similarly to environmental stressors at α and β diversity levels. Hydrology and water quality were key stressors driving all individual biodiversity indices examined, followed by land use and natural factors. With rapidly developing landscape change under the Anthropocene (Ellis, 2021), anthropogenic pressures may drive changes in diversity more than other factors (Soliveres et al., 2016). In general, local and regional factors affect biotic communities directly via changes in habitat conditions (e.g., changes in water quality and flow) or indirectly through biotic (e.g., invasion, predation) factors (Qu et al., 2023). Variations in water quality across landscapes may have promoted opportunities for environmental filtering to form novel assemblages that are largely a product of environmental alteration (Chen et al., 2020). Water quality (e.g., measured as TN or TP) is not only directly involved in aquatic organismal and ecosystem processes, such as development and reproduction of algae, but can also ‘reshape’ habitat complexity and heterogeneity (e.g., SD, NTU), which in turn affects aquatic community structures (Coffey et al., 2019).
Hydrological factors (Up_Dis, WL, and Do_Dis) had influential effects on multiple aquatic biodiversity indices in the YRB. Dams affect the integrity of freshwater ecosystems through inundation of terrestrial habitats, unexpected hydrological events, and increased fragmentation (Barbarossa et al., 2020). With the continuous development of water conservancy and engineering projects, the YRB has formed the world's top ten dams (Lehner et al., 2011). Natural factors, such as air temperature, elevation, and precipitation, could be expected to be major factors shaping diversity patterns of riverine aquatic organisms (Eros et al., 2020). With global climate change, local extinctions and distributional changes of aquatic species are expected in the future with decreasing number and area of suitable habitats (Barbarossa et al., 2021). Although land use explained less change in aquatic biodiversity than other predictors, these factors can influence indirectly the structure of aquatic communities via effects on water quality, habitat complexity and environmental heterogeneity (Yang et al., 2024). We found that fish and phytoplankton were more sensitive to stressors than the other taxa we examined in the YRB. This finding is consistent with previous studies that both fish and phytoplankton were good indicators of the ecological health of the Yangtze River (Gao et al., 2023; Xiong et al., 2023).
SEM results suggested that natural factors, land use, and hydrological factors affected biodiversity indirectly by altering water quality, which in turn had significant direct effects on taxonomic and phylogenetic IABIs. This finding further indicates that water quality is the most influential factor that directly contributes to variation in aquatic biodiversity in the YRB, adding to the role of geographic characteristics detected in previous studies (Chen et al., 2020). This finding suggests that we need to pay special attention on water quality improvements in future conservation and management actions in the YRB. Effects and interactions of multiple stressors on water quality and subsequently on aquatic biodiversity further proved previous findings that multiple anthropogenic stressors influence water quality in the Yangtze River (Xiong et al., 2022). As an integrated diversity index, the IABI may blur the response of a particular aquatic organism to environmental stress, but it generates a general picture of the overall biological response to multiple stressors. IABI may also have advantages over individual taxa-based indices in assessment and management of river ecosystem health.
Although we have invested significant resources and efforts in the YRB, limitations still exist in the current study. For instance, our sampling locations were primarily concentrated on the main stem and major tributaries of the Yangtze River, which lacks data on smaller tributaries where human activities are usually more intensive. Additionally, due to the relatively short duration of basin-scale aquatic organism monitoring in China, longer term and more continuous biological monitoring is required. Furthermore, relationships between human stressors and nature factors and their effects on both individual and IABI indices should be more thoroughly investigated as more data become available in near future.
4. Conclusions
We have reached the following conclusions.
First, at the alpha level, phytoplankton and fish species and functional richness exhibited a longitudinal increase from the headwaters to the lower reaches of the Yangtze River, while their functional evenness decreased. Fish phylogenetic diversity also increased downstream, while other biological groups showed consistent patterns among locations.
Second, at the beta level, fish taxonomic and phylogenetic diversity increased, while their functional diversity decreased from the headwaters to the lower parts of the Yangtze River.
Third, hydrology and water quality were the primary drivers of all individual biodiversity indices, followed by land use and natural factors. Among the biological groups examined, fish and phytoplankton exhibited greater responses to these stressors compared to other groups.
Fourth, natural factors, land use, and hydrological factors indirectly affected biodiversity by altering water quality, which in turn had significant direct impacts on taxonomic and phylogenetic IABIs.
Finally, based on our findings, we recommend that fish and phytoplankton be used as priority indicators to detect multiple stressors in the Yangtze River Basin. We urge policy makers and environmental regulators to consider both individual and integrated indices when developing freshwater biodiversity conservation and management plans.
5. Materials and methods
5.1. Study area and biological data collection
The Yangtze River is the third longest river in the world and the first longest in Asia, and its special geomorphology with higher west and lower east contains rich water energy resources (Chen et al., 2016). The Yangtze River Basin (YRB) is not only a global biodiversity hotspot with over 400 species of fish, but it also supports >40 % of China's population and economy. Spanning three of China's major urban centers (i.e., Shanghai, Wuhan, and Chengdu-Chongqing), it drains 19 of China's provinces, municipalities, and autonomous regions, which makes it one of the largest and most densely populated river basins in the world. The YRB is primarily influenced by the East Asian subtropical monsoon climate with a mean annual temperature of 15 °C and precipitation of 1127 mm/year. The geographical characteristics of the YRB make the river ecosystem subject to different natural conditions (e.g., elevation, riparian landscapes), which leads to natural spatial variation in distributions of aquatic organisms from west to east, with many endemic species present in the upper YRB (Liu et al., 2021). Over the past several decades, however, aquatic habitats and biodiversity of the Yangtze River basin have been heavily affected by multiple anthropogenic stressors, including dams, urbanization, industry, mining, navigation, overfishing, shoreline modification, agriculture, and others (Chen et al., 2017).
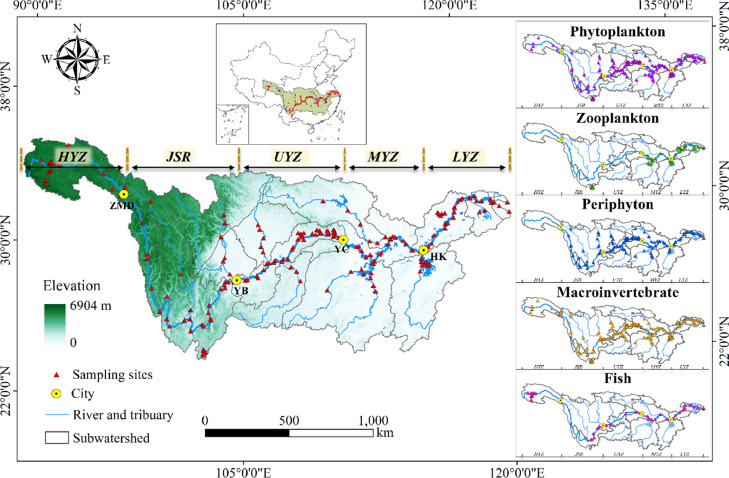
In the present study, we examined freshwater biodiversity in the Yangtze River mainstem, major tributaries, and key large lakes. We divided the Yangtze River mainstem into five reaches: the headwaters of the Yangtze River (HYZ), the Jinsha River (JSR), the upper reaches of the Yangtze River (UYZ), the middle reaches of the Yangtze River (MYZ), and the lower reaches of the Yangtze River (LYZ) (Chen et al., 2016). Major tributaries studied include major tributaries of the Yangtze River (MTYZ) and tributaries of the Three Gorges Reservoir (TTGD). Key lakes included were Dianchi Lake (DCL), Dongting Lake (DTL), Poyang Lake (PYL), Chaohu Lake (CHL), and Taihu Lake (THL) (Fig. 4; Tab. S1).
Fig. 4.
Study area and sampling sites in the Yangtze River basin, China. Note: The Yangtze River mainstem is divided into five reaches: the headwaters of the Yangtze River (HYZ), the Jinsha River (JSR), the upper reaches of the Yangtze River (UYZ), the middle reaches of the Yangtze River (MYZ), and the lower reaches of the Yangtze River (LYZ).
There were 162, 47, 119, 157 and 46 sites sampled for phytoplankton, zooplankton, periphyton, macroinvertebrates, and fish, respectively (Fig. 4). The zooplankton data were only available for the Yangtze River mainstem sites and key lakes. The fish data were only available in the Yangtze River mainstem (Fig. 4). Field surveys were conducted between August and November in 2020. Field collection and laboratory identification methods for each taxon followed standard protocols in China and were consistent across all geographic areas (Zhang et al., 2018; Divya et al., 2021; Liu et al., 2022; Xiong et al., 2022). More detailed field sampling and laboratory processing of samples were presented in the N materials.
5.2. Quantification of stressors
We collected 48 environmental factors on a number of human-induced stressors and other factors, and grouped them into four categories, i.e., land use, water quality, natural factors (nature), and hydrology (Tab.S2). Land use within a 5 km buffer zone surrounding each sampling site was collected to represent the land use stressor (Xiong et al., 2021). The land use types including included cropland, urban, forest, shrub, grassland, water, snow/ice, barren, and wetland (Yang and Huang, 2021). Water quality samples were collected at the same sites where the above-mentioned biological samples were taken. Standard protocols were followed in the field and laboratory for analyzing water quality samples (GB3838-2002). A total of 25 water quality parameters, including conductivity, pH, dissolved oxygen, turbidity, total nitrogen, total phosphorus, ammonia, chemical oxygen demand, lead, zinc, selenium, arsenic, cadmium, and others, were analyzed (Tab. S2). Natural variables (Tab. S2) including elevation, air and water temperature (°C), and precipitation (mm) were obtained from the National Earth System Science Data Center of China (http://www.geodata.cn).
Hydrology factors were water level, flow, and eight dam-related factors associated with each sampling site (Tab. S2). The eight dam-related factors included water storage level of the closest upstream hydropower plant, installed capacity of upstream hydropower plants, distance to the closest upstream hydropower plant, generation capacity of the closest upstream hydropower plant, water storage level of the closest downstream hydropower plant, installed capacity of downstream hydropower plants, distance to the closest downstream hydropower plant, and generation capacity of the closest downstream hydropower plant. These data were collected from China Institute of Water Resource and Hydropower Research, Google satellite maps, and other sources (http://www.hydropower.org.cn).
5.3. Biodiversity indices
For α diversity, we calculated taxonomic, functional, and phylogenetic diversity indices. The “Vegan” package of R software was used to calculate species richness (Richness), Shannon-Weiner index (Shannon), and Pielou evenness index (Pielou) as indices of taxonomic diversity. To calculate functional diversity, we focused on traits known to be associated with spatial distribution patterns and individual adaptations of freshwater organisms, which may also be considered proxies of ecosystem processes (Moi et al., 2022). These traits fall into four broad categories: (1) body form (e.g., body length, body shape), (2) resource acquisition (e.g., habitat use, trophic roles), (3) mobility (e.g., vertical position, flow preferences), and (4) life strategy (e.g., reproductive strategy, life history strategy) (Tab. S3). The FD package of R software was used to calculate functional richness index (FRic), functional divergence index (FDiv), and functional evenness index (FEve) as indices of functional diversity (Villéger et al., 2013). The statistical software PRIMER was used to calculate average taxonomic distinctness (Delta+) and variation in taxonomic distinctness (Lambda+) as indices of phylogenetic diversity (Heino et al., 2007).
For β diversity, overall taxonomic species beta diversity (TDsor) is decomposed into turnover (TDsim) and nestedness (TDsne) components (Baselga, 2010). The functional dissimilarity matrix was developed, and then the function "functional.beta.pair" from the R package “betapart” was used to calculate the three functional Sorensen dissimilarity matrices and to characterize the total functional beta diversity, functional turnover components, and functional nested components as FDsor, FDsim, and FDsne, respectively (Baselga and Orme, 2012). The taxonomic level (e.g., kingdom, phylum, order, order, family, genus, etc.) of different groups of organisms was arranged in a phylogenetic matrix, and then we used the R package function "phylo.beta.pair" to calculate three phylogenetic Sorensen dissimilarity matrices and characterize the total phylogenetic beta diversity, phylogenetic turnover components and phylogenetic nested components as PDsor, PDsim, and PDsne, respectively (Baselga et al., 2018).
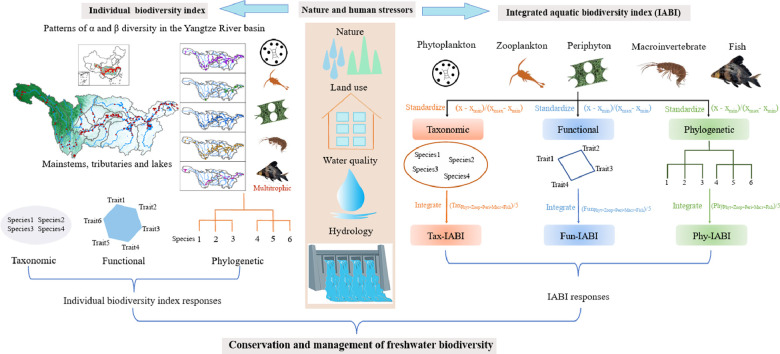
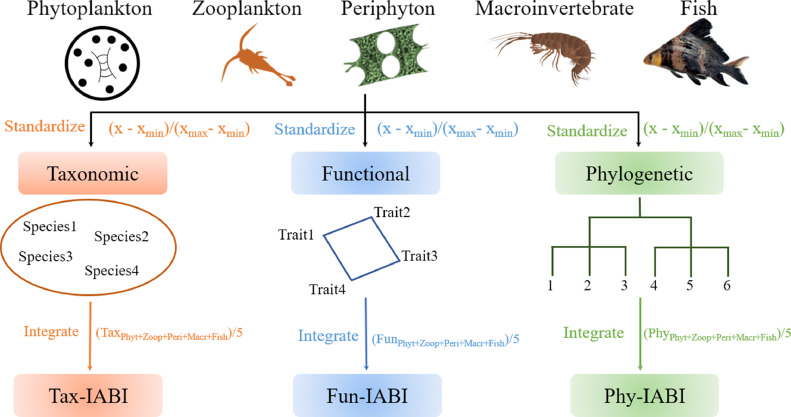
Furthermore, we also developed an integrated biodiversity index, the Integrated Aquatic Biodiversity Index (IABI) to comprehensively represent the diversity of five biological groups in combination. The IABI index was devised by considering the individual taxonomic, functional, and phylogenetic diversity indices from all the five aquatic biological groups (i.e., phytoplankton (Phyt), zooplankton (Zoop), periphyton (Peri), macroinvertebrate (Macr), and fish). The diversity index values of taxonomic (Tax), functional (Fun), and phylogenetic (Phy) α diversity from each biological group were standardized, scaled to the maximum observed value, and then these standardized diversity index values were averaged for each site. This process ensures that the diversity of each biological group contributes equally to the integrated diversity index (Allan et al., 2014; Moi et al., 2022). The formula to calculate the IABI is as follows:
Where D represents the diversity index. S represents standardization. Diversity indices species richness, FRic and Delta+ were used to calculate Tax-IABI, Fun-IABI, and Phy-IABI, respectively. More details on development and calculation of the IABIs were presented in Fig. 5.
Fig. 5.
A framework to show the calculation of the integrated aquatic biodiversity index (IABI). The flow of analysis was as follows: 1) for each biological group, individual taxonomic, functional or phylogenetic index values were calculated separately, 2) the individual indices were standardized, and 3) standardized indices of all biological groups were combined into taxonomic, functional or phylogenetic IABIs.
5.4. Data analysis
Analysis of variance (ANOVA), Permutational Multivariate ANOVA (PERMANOVA), and multiple comparisons (LSD) tests were used to examine differences in taxonomic, phylogenetic and functional diversity indices at both the α and β levels in each of the five biological groups among different aquatic ecosystem types in the YRB. Data were log(x + 1) transformed prior to analysis to improve chi-square and normality. For indices that did not meet normality, we used the non-parametric Kruskal-Wallis test.
The random forest decomposition analysis was used to screen the top 10 stressor factors that influenced α diversity indices. Subsequently, we conducted Redundancy analysis (RDA) and Variance Partitioning Analysis (VPA) to assess the impact of various stressor factors on the α diversity indices. To analyze β diversity indices, dissimilarity matrices (pertaining to total beta diversity) and their turnover and nested components were extracted as response variables, and significant stressor factors were screened using the distance-based RDA forward selection. The VPA was used to quantify contributions of different stressor factors in influencing β diversity indices. These analyses were performed using the "vegan", "ape", and "betapart" packages in R.
By utilizing the IABI index as the dependent variable and considering human stressor and natural factors as independent variables, we employed random forests to identify the top 10 factors that exerted the greatest influence on the IABI index. Then PiecewiseSEM were used to analyze direct and indirect influencing pathways from stressors to IABI indices (Tian et al., 2021). All model construction and statistical analyses in this study were done in R software, PRIMER6.0, and Excel 2021.
CRediT authorship contribution statement
Zhongyang Li: Writing – review & editing, Writing – original draft, Methodology, Investigation, Formal analysis, Data curation. Huiyu Xie: Writing – review & editing, Writing – original draft, Methodology, Investigation, Formal analysis, Data curation. Zhiqi Peng: Writing – review & editing, Writing – original draft, Methodology, Investigation, Formal analysis, Data curation. Jani Heino: Writing – review & editing, Methodology, Formal analysis. Yu Ma: Writing – review & editing, Investigation, Formal analysis, Data curation. Fangyuan Xiong: Writing – review & editing, Investigation, Data curation. Wenqi Gao: Writing – review & editing, Investigation, Data curation. Wei Xin: Writing – review & editing, Investigation, Data curation. Chiping Kong: Writing – review & editing, Investigation, Data curation. Lekang Li: Writing – review & editing, Investigation, Data curation. Lei Fang: Writing – review & editing, Investigation, Data curation. Haihua Wang: Writing – review & editing, Investigation, Data curation. Guangpeng Feng: Writing – review & editing, Investigation, Data curation. Beixin Wang: Writing – review & editing, Writing – original draft, Methodology, Investigation, Formal analysis, Conceptualization. Xiaowei Jin: Writing – review & editing, Writing – original draft, Methodology, Investigation, Formal analysis, Conceptualization. Yushun Chen: Writing – review & editing, Writing – original draft, Validation, Supervision, Methodology, Investigation, Funding acquisition, Formal analysis, Data curation, Conceptualization.
Declaration of competing interest
The authors declare that there is no competing conflict of interest.
Acknowledgements
ZL, FX, WG, WX, and YC were mainly supported by National Key R&D Program of China (2023YFC3209002), National Natural Science Foundation of China (32303011), and Jiangsu Province (HSXT30322). HW and GF were supported by the Innovative and Entrepreneurial Talents Project of Jiangxi Province (JXSQ2020102109). HX and XJ were supported by National Natural Science Foundation of China (42322710), National Key R&D Program of China (2021YFC3200105), and the Joint Research Project for the Yangtze River Conservation (Phase II).
Footnotes
This work is a joint research effort mainly contributed by teams of BW, XJ, and YC while collaborating with others in the Yangtze River Basin.
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.wroa.2024.100251.
Contributor Information
Beixin Wang, Email: wangbeixin@njau.edu.cn.
Xiaowei Jin, Email: jinxw@cnemc.cn.
Yushun Chen, Email: yushunchen@ihb.ac.cn.
Appendix. Supplementary materials
Data availability
The data that support the findings are available from the authors upon reasonable request.
References
- Allan E., Bossdorf O., Dormann C.F., Prati D., Gossner M.M., Tscharntke T., Blüthgen N., Bellach M., Birkhofer K., Boch S. Interannual variation in land-use intensity enhances grassland multidiversity. Proc. Natl. Acad. Sci. U.S.A. 2014;111:308–313. doi: 10.1073/pnas.1312213111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araujo F.G., Costa de Azevedo M.C., Gomes-Goncalves R.d.S.L., Penha Guedes A.P. Taxonomic and functional β-diversity patterns reveal random assembly rules in nearshore fish assemblages. Mar. Ecol. Prog. Ser. 2019;627:109–123. [Google Scholar]
- Barbarossa V., Bosmans J., Wanders N., King H., Bierkens M.F.P., Huijbregts M.A.J., Schipper A.M. Threats of global warming to the world’s freshwater fishes. Nat. Commun. 2021;12:1701. doi: 10.1038/s41467-021-21655-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbarossa V., Schmitt R.J., Huijbregts M.A., Zarfl C., King H., Schipper A.M. Impacts of current and future large dams on the geographic range connectivity of freshwater fish worldwide. Proc. Natl. Acad. Sci. U.S.A. 2020;117:3648–3655. doi: 10.1073/pnas.1912776117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baselga A. Partitioning the turnover and nestedness components of beta diversity. Global Ecol. Biogeogr. 2010;19:134–143. [Google Scholar]
- Baselga A., Orme C.D.L. Betapart: an R package for the study of beta diversity. Methods Ecol. Evol. 2012;3:808–812. [Google Scholar]
- Baselga, A., D. Orme, S. Villéger, J. De Bortoli, F. Leprieur, and M. Logez. 2018. Betapart: partitioning beta diversity into turnover and nestedness components. R package version.
- Birk S., Chapman D., Carvalho L., Spears B.M., Andersen H.E., Argillier C., Auer S., Baattrup-Pedersen A., Banin L., Beklioglu M., Bondar-Kunze E., Borja A., Branco P., Bucak T., Buijse A.D., Cardoso A.C., Couture R.-M., Cremona F., de Zwart D., Feld C.K., Ferreira M.T., Feuchtmayr H., Gessner M.O., Gieswein A., Globevnik L., Graeber D., Graf W., Gutierrez-Canovas C., Hanganu J., Iskin U., Jarvinen M., Jeppesen E., Kotamaki N., Kuijper M., Lemm J.U., Lu S., Solheim A.L., Mischke U., Moe S.J., Noges P., Noges T., Ormerod S.J., Panagopoulos Y., Phillips G., Posthuma L., Pouso S., Prudhomme C., Rankinen K., Rasmussen J.J., Richardson J., Sagouis A., Santos J.M., Schaefer R.B., Schinegger R., Schmutz S., Schneider S.C., Schuelting L., Segurado P., Stefanidis K., Sures B., Thackeray S.J., Turunen J., Uyarra M.C., Venohr M., von der Ohe P.C., Willby N., Hering D. Impacts of multiple stressors on freshwater biota across spatial scales and ecosystems. Nat. Ecol. Evol. 2020;4:1060–1068. doi: 10.1038/s41559-020-1216-4. [DOI] [PubMed] [Google Scholar]
- Ceballos G., Ehrlich P.R., Raven P.H. Vertebrates on the brink as indicators of biological annihilation and the sixth mass extinction. Proc. Natl. Acad. Sci. U.S.A. 2020;117:13596–13602. doi: 10.1073/pnas.1922686117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Zhang S., Huang D., Li B.-L., Liu J., Liu W., Ma J., Wang F., Wang Y., Wu S. The development of China’s Yangtze River Economic Belt: how to make it in a green way. Sci. Bull. 2017;62:648–651. doi: 10.1016/j.scib.2017.04.009. [DOI] [PubMed] [Google Scholar]
- Chen Y., Chapman D.C., Jackson J., Chen D., Eggleton M. American Fisheries Society; 2016. Fishery Resources, Environment, and Conservation in the Mississippi and Yangtze (Changjiang) River Basins. [Google Scholar]
- Chen Y., Qu X., Xiong F., Lu Y., Wang L., Hughes R.M. Challenges to saving China’s freshwater biodiversity: fishery exploitation and landscape pressures. Ambio. 2020;49:926–938. doi: 10.1007/s13280-019-01246-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cincotta R.P., Wisnewski J., Engelman R. Human population in the biodiversity hotspots. Nature. 2000;404:990–992. doi: 10.1038/35010105. [DOI] [PubMed] [Google Scholar]
- Coffey R., Paul M.J., Stamp J., Hamilton A., Johnson T. A review of water quality responses to air temperature and precipitation changes 2: nutrients, algal blooms, sediment, pathogens. JAWRA J. Am. Water Resour. Assoc. 2019;55:844–868. doi: 10.1111/1752-1688.12711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Marco M., Venter O., Possingham H.P., Watson J.E. Changes in human footprint drive changes in species extinction risk. Nat. Commun. 2018;9:4621. doi: 10.1038/s41467-018-07049-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Divya K.R., Zhao S., Chen Y., Cheng F., Zhang L., Qin J., Arunjith T.S., Schmidt V.B., Xie S. A comparison of zooplankton assemblages in Nansi Lake and Hongze Lake, potential influences of the East Route of the South-to-North Water Transfer Project, China. J. Oceanol. Limnol. 2021;39:623–636. [Google Scholar]
- Ellis E.C. Land use and ecological change: a 12,000-year history. Annu. Rev. Environ. Resour. 2021;46:1–33. [Google Scholar]
- Eros T., Czegledi I., Toth R., Schmer D. Multiple stressor effects on alpha, beta and zeta diversity of riverine fish. Sci. Total Environ. 2020;748 doi: 10.1016/j.scitotenv.2020.141407. [DOI] [PubMed] [Google Scholar]
- Gao W., Xiong F., Lu Y., Qu X., Xin W., Chen Y. Development of a phytoplankton-based index of biotic integrity for ecological health assessment in the Yangtze River. Ecol. Process. 2023;12:41. [Google Scholar]
- GB3838- China Environmental Science Press; Beijing: 2002. Editorial Board of State Environmental Protection Administration of China. [Google Scholar]
- Green M.D., Anderson K.E., Herbst D.B., Spasojevic M.J. Rethinking biodiversity patterns and processes in stream ecosystems. Ecol. Monogr. 2022;92:e1520. [Google Scholar]
- Heino J. The importance of metacommunity ecology for environmental assessment research in the freshwater realm. Biol. Rev. 2013;88:166–178. doi: 10.1111/j.1469-185X.2012.00244.x. [DOI] [PubMed] [Google Scholar]
- Heino J. CSIRO Publishing and CRC Press; Melbourne and London: 2015. Approaches, Potential and Pitfalls of Applying Bioindicators in Freshwater ecosystems. Surrogates and Indicators of Biodiversity and Environmental Change; pp. 91–100. [Google Scholar]
- Heino J., Melo A.S., Siqueira T., Soininen J., Valanko S., Bini L.M. Metacommunity organisation, spatial extent and dispersal in aquatic systems: patterns, processes and prospects. Freshw. Biol. 2015;60:845–869. [Google Scholar]
- Heino J., Mykrä H., Kotanen J., Muotka T. Ecological filters and variability in stream macroinvertebrate communities: do taxonomic and functional structure follow the same path? Ecography. 2007;30:217–230. [Google Scholar]
- Heino J., Tolonen K.T. Ecological drivers of multiple facets of beta diversity in a lentic macroinvertebrate metacommunity. Limnol. Oceanogr. 2017;62:2431–2444. [Google Scholar]
- Jackson M.C., Pawar S., Woodward G. The temporal dynamics of multiple stressor effects: from individuals to ecosystems. Trends Ecol. Evol. (Amst.) 2021;36:402–410. doi: 10.1016/j.tree.2021.01.005. [DOI] [PubMed] [Google Scholar]
- Kang B., Huang X., Yan Y., Yan Y., Lin H. Continental-scale analysis of taxonomic and functional fish diversity in the Yangtze river. Global Ecol. Conserv. 2018;15:e00442. [Google Scholar]
- Kraft N.J., Valencia R., Ackerly D.D. Functional traits and niche-based tree community assembly in an Amazonian forest. Science. 2008;322:580–582. doi: 10.1126/science.1160662. [DOI] [PubMed] [Google Scholar]
- Lehner B., Liermann C.R., Revenga C., Vörösmarty C., Fekete B., Crouzet P., Döll P., Endejan M., Frenken K., Magome J. High-resolution mapping of the world’s reservoirs and dams for sustainable river-flow management. Front. Ecol. Environ. 2011;9:494–502. [Google Scholar]
- Leibold M.A., Holyoak M., Mouquet N., Amarasekare P., Chase J.M., Hoopes M.F., Holt R.D., Shurin J.B., Law R., Tilman D. The metacommunity concept: a framework for multi-scale community ecology. Ecol. Lett. 2004;7:601–613. [Google Scholar]
- Li P., Yao Y., Lian J., Ma C. Effect of thermal stratified flow on algal blooms in a tributary bay of the Three Gorges reservoir. J. Hydrol. 2021;601 [Google Scholar]
- Liu H., Guo C., Qu X., Xiong F., Paukert C.P., Chen Y., Su W. Fish diversity, endemism, threats, and conservation in the Jinsha river basin (upper yangtze river), China. North Am. J. Fish. Manage. 2021;41:967–984. [Google Scholar]
- Liu H., Qu X., Xia W., Chen Y. Taxonomic, functional, and phylogenetic diversity patterns reveal different processes shaping river fish assemblages in the Eastern Huai River Basin, China. Water Biol. Secur. 2023;2 [Google Scholar]
- Liu S., Garcia-Palacios P., Tedersoo L., Guirado E., van der Heijden M.G., Wagg C., Chen D., Wang Q., Wang J., Singh B.K. Phylotype diversity within soil fungal functional groups drives ecosystem stability. Nat. Ecol. Evol. 2022;6:900–909. doi: 10.1038/s41559-022-01756-5. [DOI] [PubMed] [Google Scholar]
- Meynard C.N., Devictor V., Mouillot D., Thuiller W., Jiguet F., Mouquet N. Beyond taxonomic diversity patterns: how do α, β and γ components of bird functional and phylogenetic diversity respond to environmental gradients across France? Global Ecol. Biogeogr. 2011;20:893–903. [Google Scholar]
- Moi D.A., Lansac-Tôha F.M., Romero G.Q., Sobral-Souza T., Cardinale B.J., Kratina P., Perkins D.M., Teixeira de Mello F., Jeppesen E., Heino J. Human pressure drives biodiversity–multifunctionality relationships in large Neotropical wetlands. Nat. Ecol. Evol. 2022;6:1279–1289. doi: 10.1038/s41559-022-01827-7. [DOI] [PubMed] [Google Scholar]
- Peters M.K., Hemp A., Appelhans T., Behler C., Classen A., Detsch F., Ensslin A., Ferger S.W., Frederiksen S.B., Gebert F. Predictors of elevational biodiversity gradients change from single taxa to the multi-taxa community level. Nat. Commun. 2016;7:13736. doi: 10.1038/ncomms13736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pimm S.L., Jenkins C.N., Abell R., Brooks T.M., Gittleman J.L., Joppa L.N., Raven P.H., Roberts C.M., Sexton J.O. The biodiversity of species and their rates of extinction, distribution, and protection. Science. 2014;344 doi: 10.1126/science.1246752. [DOI] [PubMed] [Google Scholar]
- Qu X., Olden J.D., Xia W., Liu H., Xie Z., Hughes R.M., Chen Y. Hydrology and water quality shape macroinvertebrate patterns and facilitate non-native species dispersals in an inter-basin water transfer system. J. Environ. Manage. 2023;329 doi: 10.1016/j.jenvman.2022.117111. [DOI] [PubMed] [Google Scholar]
- Rolls R.J., Deane D.C., Johnson S.E., Heino J., Anderson M.J., Ellingsen K.E. Biotic homogenisation and differentiation as directional change in beta diversity: synthesising driver–response relationships to develop conceptual models across ecosystems. Biol. Rev. 2023;98:1388–1423. doi: 10.1111/brv.12958. [DOI] [PubMed] [Google Scholar]
- Soliveres S., Van Der Plas F., Manning P., Prati D., Gossner M.M., Renner S.C., Alt F., Arndt H., Baumgartner V., Binkenstein J. Biodiversity at multiple trophic levels is needed for ecosystem multifunctionality. Nature. 2016;536:456–459. doi: 10.1038/nature19092. [DOI] [PubMed] [Google Scholar]
- Su G., Logez M., Xu J., Tao S., Villéger S., Brosse S. Human impacts on global freshwater fish biodiversity. Science. 2021;371:835–838. doi: 10.1126/science.abd3369. [DOI] [PubMed] [Google Scholar]
- Teichert N., Lepage M., Lobry J. Beyond classic ecological assessment: the use of functional indices to indicate fish assemblages sensitivity to human disturbance in estuaries. Sci. Total Environ. 2018;639:465–475. doi: 10.1016/j.scitotenv.2018.05.179. [DOI] [PubMed] [Google Scholar]
- Tian Peng, Liu S., Zhao X., Sun Z., Yao X., Niu S., Crowther T.W., Wang Q. Past climate conditions predict the influence of nitrogen enrichment on the temperature sensitivity of soil respiration. Commun. Earth Environ. 2021;2:251. [Google Scholar]
- Urban M.C., Bocedi G., Hendry A.P., Mihoub J.B., Pe’er G., Singer A., Bridle J., Crozier L., De Meester L., Godsoe W. Improving the forecast for biodiversity under climate change. Science. 2016;353:aad8466. doi: 10.1126/science.aad8466. [DOI] [PubMed] [Google Scholar]
- Villéger S., Grenouillet G., Brosse S. Decomposing functional β-diversity reveals that low functional β-diversity is driven by low functional turnover in E uropean fish assemblages. Global Ecol. Biogeogr. 2013;22:671–681. [Google Scholar]
- Wilson R.J., Thomas C.D., Fox R., Roy D.B., Kunin W.E. Spatial patterns in species distributions reveal biodiversity change. Nature. 2004;432:393–396. doi: 10.1038/nature03031. [DOI] [PubMed] [Google Scholar]
- Xiong F., Chen Y., Zhang S., Xu Y., Lu Y., Qu X., Gao W., Wu X., Xin W., Gang D.D. Land use, hydrology, and climate influence water quality of China’s largest river. J. Environ. Manage. 2022;318 doi: 10.1016/j.jenvman.2022.115581. [DOI] [PubMed] [Google Scholar]
- Xiong F., Infante D.M., Olden J.D., Gao W., Wang L., Chen Y. River–lake connectivity, wetland, and human stress factors shape fish diversity (alpha and beta) patterns in the middle and lower Yangtze River, China. Landsc Ecol. 2023:1–16. [Google Scholar]
- Xiong F., Olden J.D., Lu Y., Liu H., Qu X., Xia W., Guo C., Wu X., Infante D.M., Wang L., Chen Y. Riparian land use and in-channel stressors drive fish community structure in the Yangtze River. Landsc Ecol. 2021;36:3079–3095. [Google Scholar]
- Yang B., Qu X., Liu H., Yang M., Xin W., Wang W., Chen Y. Urbanization reduces fish taxonomic and functional diversity while increases phylogenetic diversity in subtropical rivers. Sci. Total Environ. 2024;908 [Google Scholar]
- Yang J., Huang X. The 30 m annual land cover dataset and its dynamics in China from 1990 to 2019. Earth Syst. Sci. Data. 2021;13:3907–3925. [Google Scholar]
- Zhang Y., Cheng L., Li K., Zhang L., Cai Y., Wang X., Heino J. Nutrient enrichment homogenizes taxonomic and functional diversity of benthic macroinvertebrate assemblages in shallow lakes. Limnol. Oceanogr. 2019;64:1047–1058. [Google Scholar]
- Zhang Y., Wang R., Qu X., Xia W., Xin W., Guo C., Chen Y. Effects of aquaculture on phytoplankton communities of lakes in the middle reach of the Yangtze River Basin. Acta Hydrobiol. Sin. 2018;42:1135–1143. [Google Scholar]
- Zorzal-Almeida S., Bartozek E.C.R., Bicudo D.C. Homogenization of diatom assemblages is driven by eutrophication in tropical reservoirs. Environ. Pollut. 2021;288 doi: 10.1016/j.envpol.2021.117778. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings are available from the authors upon reasonable request.