Abstract
Pulmonary mucociliary clearance (MCC) is an important defence mechanism of the respiratory system and clears pathogens and foreign particles from the airways. Understanding the effect of disease states, drugs, toxins and airway manipulations on MCC could be beneficial in preventing early pulmonary disease and developing new pulmonary therapeutics. This review summarises the current methods and future efforts to detect pulmonary MCC in vivo.
Shareable abstract
Scintigraphy has long been the mainstay for detecting pulmonary mucociliary clearance (MCC). However, newer methods of measuring MCC exist and should be explored in clinical settings to improve disease management and therapeutic development. https://bit.ly/4cGOP8x
Introduction
Mucociliary clearance (MCC) is the primary defence mechanism of the lungs. When functioning properly, inhaled particles and microbes are trapped by the mucous layer and transported proximally by the coordinated beating of cilia. This coordinated process facilitates the removal of pathogens and foreign particles and limits accumulation of mucus in the airways. By doing so, MCC helps to prevent infection, inflammatory reactions and airway obstruction within the alveoli and smaller airways. Impairment of MCC occurs in many respiratory diseases such as cystic fibrosis (CF), primary ciliary dyskinesia (PCD), asthma and COPD [1]. In these conditions, impaired MCC is one of the first functional changes in the respiratory tract and contributes to disease progression, repeated infection and architectural changes such as bronchiectasis and mucous plugging [2, 3]. Imaging methods such as computed tomography (CT) are utilised for detecting structural changes but often fail to detect more subtle alterations, particularly in early pulmonary disease. Thus, measurement of MCC in the clinical setting could be highly valuable for early prognostication of airway disease.
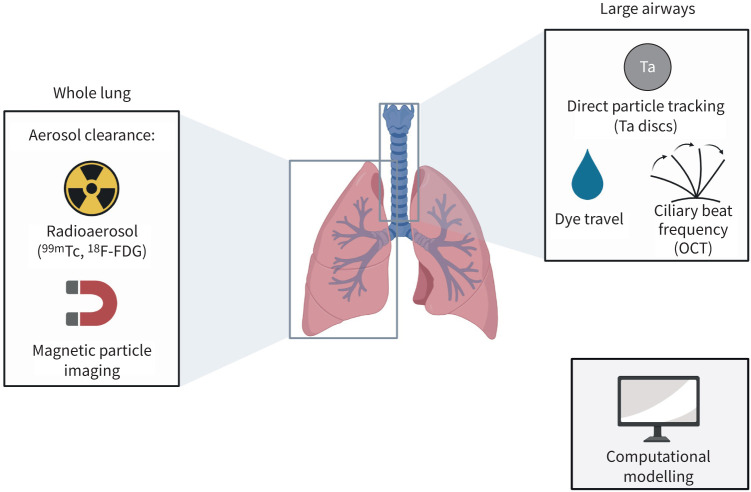
With the increase in global pulmonary disease and growing concerns about air quality and the damaging effects of inhaled ozone on ciliated cells, early assessment of pulmonary disease via MCC detection could be a critical tool to drive earlier medical management and reduce healthcare burden [4–6]. Despite an increasing utilisation of inhaled pharmaceuticals, their effect on MCC is rarely studied [7]. Currently, there are barriers to MCC measurement preventing wider use in both the clinical and research settings [4–6]. This review will focus on how MCC can be utilised to detect early changes in pulmonary function, the main methods of detecting MCC in vivo and the current barriers to measuring MCC in clinical trials and clinical settings (figure 1).
FIGURE 1.
Summary of methods to detect pulmonary mucociliary clearance (MCC). 99mTc: technetium-99m; 18F-FDG: fluorine-18-fluorodeoxyglucose; Ta: tantalum; OCT: optical coherence tomography. Figure created using BioRender.com.
Measurement of physiological MCC
Pulmonary MCC can be detected by the change of inhaled particles over time by directly measuring particle velocity or by measuring the frequency at which cilia move, known as the ciliary beat frequency (CBF). In physiological conditions, individual cilia beat at a frequency of 10–20 Hz, which results in a clearance velocity or mucous transport velocity (MTV) of roughly 5.5 mm·min−1 in the trachea [8]. Global MCC, or the change in MCC throughout both lungs, is reported as the percent clearance of the inhaled substance over time. The rates of MCC can vary based on the specific measurement technique utilised but normal rates of global pulmonary MCC have been reported as 13.3% radioaerosol clearance over 60 min and 49.8% clearance of inhaled particles over 24 h [9].
MCC detection: what is it good for?
MCC has been measured in most pulmonary diseases to better characterise functional changes [2]. Patients with mild stable asthma were shown to have markedly reduced MCC rates [10] and regional defects in MCC have been identified in CF [11]. MCC rates have been shown to be significantly reduced in patients with chronic bronchitis as well as patients with PCD, a historically difficult disease to diagnose even with advancements in DNA sequencing [12, 13]. More recently, studies have related MCC to disease severity and exposure history. For instance, MCC rates have been shown to correlate with years of smoking [12].
Measuring MCC has also been used to assess therapeutic efficacy in pulmonary diseases. While hypertonic saline has shown mixed improvement on MCC rates in chronic bronchitis, it has shown persistent improvement of MCC rates in CF patients [14–16]. Outside of hypertonic saline, mannitol has been shown to significantly improve whole-lung MCC in asthma and bronchiectasis [17–19]. More recently, the CF drugs elexacaftor/tezacaftor/ivacaftor (E/T/I) were shown to improve MCC after 1 month of therapy [20]. Another study found that CF patients on E/T/I had no change in MCC following cessation of hypertonic saline and dornase alpha, demonstrating the utility of measuring MCC in guiding disease management [21].
In addition to disease states, detection of MCC has also been used to measure the effects of drugs, surgical procedures and airway manipulation to reduce the risk of infection and to maintain the architecture of healthy airways. In one study, general anaesthesia with midazolam, fentanyl, pancuronium and nitrous oxide showed no change in bronchial MTV [22]. However, another anaesthetic, high-dose lidocaine significantly reduced MTV [23]. In terms of airway manipulation, endotracheal intubation during surgery is a risk factor for pneumonia [24]. Bronchial MTV was shown to be significantly decreased in endotracheal intubations compared to less-invasive laryngeal mask airways [25]. Studies have also demonstrated changes in MCC in various surgical groups. Infants undergoing surgery for congenital heart defects showed consistently low MCC rates in the immediate post-operative period with a correlation between pre-operative MCC and post-operative respiratory support [26]. Understanding the effects of airway procedures on MCC could improve patient outcomes and reduce post-operative complication rates. Additionally, with the increased utilisation of stem cell therapies and higher transplant volumes, measuring MCC could be utilised in understanding early functional changes in the airways from transplantation.
Despite the clinical benefits of detecting early changes in pulmonary function via MCC, there remain multiple hurdles preventing more routine, widespread use. Measurement of MCC in the clinical setting has largely been neglected, with most imaging methods to detect MCC reserved for research purposes. MCC measurement is also not routinely done in clinical trials. In a systematic review of 17 trials of hypertonic saline for the treatment of CF, only five reported measuring MCC [27]. Many questions need to be addressed to increase the utilisation of in vivo MCC detection in the clinic and in clinical trials. Where should we focus our standardisation efforts? With standardisation, can the utilisation of MCC detection in clinical trials be improved upon? If we are gathering useful information from MCC measurements, how do we increase utilisation in clinical trials and potentially translate these methods to the clinic for routine use?
Radioaerosols
Scintigraphy
The most common method to detect MCC in vivo is two-dimensional pulmonary scintigraphy, by which inhaled radioisotopes are noninvasively detected in the lungs and measured over time to determine clearance. Scintigraphy was established as a method to detect MCC in the 1950s and continues to be the mainstay as it is relatively simple to analyse and has been the most standardised method of measuring MCC [28]. From 2019 to 2023, only 14 clinical trials measured pulmonary MCC in vivo, but 86% utilised gamma scintigraphy (Cochrane database, figure 2). To noninvasively measure MCC with scintigraphy, a patient inhales technetium-99 m (99mTc)-labelled particles (e.g. 99mTc-labeled colloidal albumin or sulphur) that are small enough to deposit throughout the lungs and large enough to be cleared via MCC and not absorbed. The inhaled particles emit gamma radiation which is then detected via a gamma camera typically every 10 min and up to 24 h (due to 99mTc's half-life of 6.04 h), producing dynamic changes in radioaerosol clearance over time. Regional changes in MCC can then be quantified by establishing an outline of the lung borders before inhalation of the 99mTc-labelled particles using either a co-registered CT scan or inhalation of cobalt-57 (57Co) or xenon-133 (133Xe) [29].
FIGURE 2.
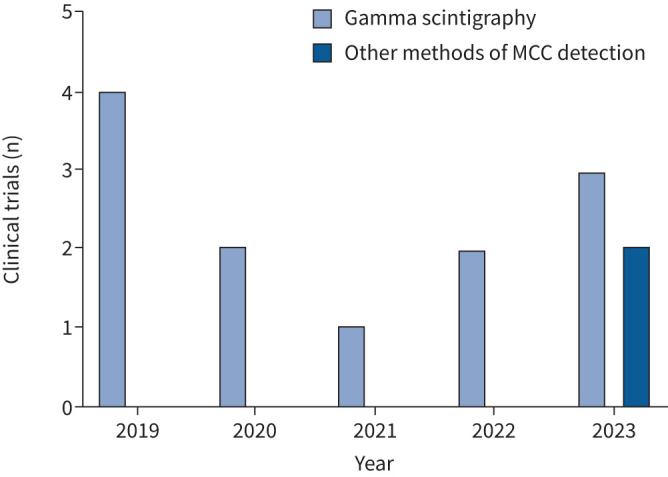
Clinical trials measuring mucociliary clearance (MCC) from 2019 to 2023. Data acquired from the Cochrane library of clinical trials.
Recent clinical trials have utilised gamma scintigraphy to identify clearance techniques and therapies for CF. In a recent trial, there were no measurable clearance differences in whole-body vibration, high-frequency chest-wall oscillatory vests and oscillatory positive pressure ventilation when compared to baseline pulmonary clearance [30].
Scintigraphy continues to be the mainstay of measuring pulmonary MCC because it is easy to quantify, exposes patients to relatively low doses of radiation and has been standardised the most out of other MCC detection methods [28].
In a meeting of pulmonary researchers in 2009, a list of objectives was discussed for increasing the utilisation of scintigraphy as a method for assessing drug efficacy in clinical trials [31]. The first objective was to improve standardisation between studies and institutions. The largest discrepancies between MCC studies are the radioaerosol particle size, inhalation techniques and accuracy of transmission scans [31]. Now, more than a decade later, studies using scintigraphy to measure MCC continue to possess variability in experimental set-up. However, unlike older studies, recent studies document all details of the transmission scan, inhalation techniques with the number of coughs per patient and specific information on nebulisers used for radioaerosol administration [26, 30, 32]. Further efforts to standardise MCC scintigraphy have been notable in the CF community and consortiums have been formed in the United States [21].
Despite the continued use of scintigraphy and standardisation efforts, intersubjective and interinstitutional variability remains high. Inhalation methods are often different between studies, which limits the ability to compare scintigraphy results between institutions [29]. Therefore, scintigraphy is best used in crossover studies, where patients can receive multiple therapies after a baseline measurement of MCC as a control. However, patient cooperation can be challenging due to the specific inhalation techniques to ensure proper radioaerosol deposition, which largely limits scintigraphy use to older children and adults, although MCC scintigraphy has been measured in young children and infants [26]. Measurement of coughing and/or throat clearing is also crucial as these actions can result in falsely elevated rates of MCC due to particles being expelled by cough clearance in addition to MCC, although measuring cough clearance alone can be useful for assessing functional airway clearance [31]. Another limitation of scintigraphy is a limited assessment of the trachea and left lung due to radioaerosol accumulation in the oesophagus and stomach. Many groups therefore isolate the right lung when measuring MCC with scintigraphy [29].
Single photon computed emission tomography
A large barrier to the broader utilisation of two-dimensional scintigraphy imaging is the poor visualisation of regional changes in MCC [29]. To obtain better regional MCC information, single photon computed emission tomography (SPECT) provides three-dimensional images of radioaerosol distribution that can be co-registered with CT images to quantify more specific regional changes in MCC. The main benefit of hybrid SPECT/CT imaging is the ability to measure MCC in heterogenic lung disorders such as CF and COPD, where regional changes are more important to quantify as opposed to global MCC in diseases such as asthma.
In the past 5 years, there have been no clinical trials utilising SPECT/CT imaging to quantify pulmonary MCC and this is likely due to the time-consuming nature of capturing and quantifying data in three dimensions compared to two-dimensional scintigraphy (Cochrane database). However, pulmonary SPECT imaging in general is becoming more commonplace in hospitals and clinical trials. With new advances in data analysis software, the stitching of circumferential two-dimensional radioaerosol transmission images is more efficiently performed in the clinic [33]. A barrier to use of SPECT is the acquisition time, which can take upwards of 20 min depending on the camera used, limiting the interpretation of MCC over shorter time periods [34]. Another barrier to the wider implementation of hybrid SPECT/CT imaging for MCC measurement is the higher levels of ionising radiation compared to scintigraphy used without CT, which may limit the number of SPECT/CT scans that can be performed on one patient [33, 35].
Summary of radioaerosols in detecting pulmonary MCC
Overall, radioaerosol clearance scans for measurement of MCC are incredibly useful in small cohort crossover studies comparing various pulmonary therapies. While lung clearance may vary greatly between patients, with low radiation exposure techniques such as scintigraphy, multiple scans can be performed and patients can be used as their controls. However, when visualisation of regional changes is required, scintigraphy fails to give sufficient data while hybrid SPECT/CT imaging exposes patients to higher levels of radiation. If radiation exposure can be minimised in SPECT/CT imaging with the utilisation of newer ultrafast CT scans, hybrid SPECT/CT imaging may eventually prove to be the more useful MCC imaging technique [33]. Until that time, scintigraphy is the mainstay of detection of MCC.
Positron emission tomography
Instead of utilising gamma-emitting radioisotopes, positron emission tomography (PET) scans rely on positron-emitting radioisotopes, which undergo a process of annihilation that produces two high-energy photons that subsequently travel in opposite directions from the body and are detected and allow for reconstruction of these events into PET images [36]. Studies that use PET imaging to detect in vivo MCC have subjects inhale fluorine-18-fluorodeoxyglucose (18F-FDG). To measure MCC, 18F-FDG is inhaled by the patient, and serial scans are performed (typically at 60 and 120 min) where the residual 18F-FDG activity is measured and compared to the initial activity to obtain the MCC. High-resolution CT is performed to give anatomical detail/localisation of 18F-FDG uptake on PET images.
Because the origin of emission can be more accurately detected with PET imaging, it is several magnitudes more sensitive than SPECT with greater spatial resolution [37]. However, despite improved resolution, to date, PET imaging has not been routinely used to measure MCC. One study from 1990 utilised PET to measure the MCC of five healthy adults and reported a mean 18F-FDG retention after 60 min of 24% [32]. This was significantly lower than the average retention of 99mTc-conjugate scintigraphy scans of 86% and is likely due to increased epithelial absorption of 18F-FDG [9]. Due to the absorbance and cellular uptake of 18F-FDG, more recent studies have utilised PET to measure drug absorption and clearance rather than measuring MCC [36, 38]. 18F-FDG also has a much shorter half-life than 99mTc, 110 min versus 6 h, making long-term MCC measurements impractical due to prolonged decay [36, 37]. Finally, PET scanner technology and PET radioisotope production remain more expensive than SPECT, thus making PET imaging less practical in the near future for MCC imaging studies [39].
Direct particle velocity measurement
MCC measurement via radioaerosol clearance measures global or regional changes in gamma emission. With advancements in imaging resolution and molecular engineering, some research labs are detecting MCC in larger airways by measuring the transit time of individual particles. To directly measure particle velocity, groups have used various methods.
Tantalum discs
The most common method to measure single particle clearance and velocity of clearance is with tantalum discs. Tantalum is an extremely dense metal and tantalum microdiscs (0.35–1.0 mm in diameter) can be inhaled or directly placed into larger airways to measure direct particle velocity over time via continuous X-ray (fluoroscopy) or CT. Direct particle velocity measurement is effective for the measurement of MCC of the larger airways such as the trachea and bronchi, which are harder areas to isolate with scintigraphy. Additionally, tantalum discs remain inert in physiological conditions making them relatively safe compared to directly inhaling radioactive particles [40]. In the last 20 years, only a handful of papers have been published describing MCC measurement with tantalum discs and studies are limited to large animal models of lung disease with few trials in humans [41–43]. While the experimental setup for direct particle velocity measurement is less complicated than scintigraphy, the radiation exposure is greater, with one research study conducting CT scans every 2 min for 1 h [41]. Additionally, the long-term consequences of insufflating metal discs into the lungs are not entirely understood, limiting clinical trials in humans.
Magnetic particle imaging
While tantalum discs need to be visualised via serial X-rays or CT, one group has recently published a method of direct MCC measurement with magnetic resonance imaging (MRI) using magnetic beads [44]. The benefits of this method are the lack of radiation exposure and the increased resolution that MRI has over gamma scintigraphy and CT. This technique is limited by expensive and highly technical experimental setup and complex image analysis but could be a valuable method to measure MCC in the future [45].
Dye
Methylene blue dye has been used extensively in measuring nasal MCC but studies on measuring pulmonary MCC are more limited. The method is simple; by placing a drop of coloured dye at a specific location and measuring the travel distance after 2–10 min, a rough estimate of MCC can be obtained [41]. Using dye to measure MCC is low-cost and typically only requires a bronchoscope to visualise the dye over time. While simple to measure, methylene blue dye spreads rapidly and visual measurement of the dye lacks sensitivity to detect subtle changes in MCC [46]. Additionally, to measure MCC below the vocal cords, patients would need to be anesthetised, limiting its use to operative settings [47].
Optical coherence tomography
Perhaps the most sensitive and direct measurement of MCC is the direct visualisation and calculation of the CBF. Optical coherence tomography (OCT) is a noninvasive interference imaging technique that produces extremely high-resolution cross-sectional images at the micron scale [48]. OCT has been used to capture the CBF of ex vivo tissue, but more recently, the technique has been utilised to measure the CBF of nasal mucosa in vivo [49]. In 2016, the first in vivo imaging of vocal folds using OCT was published [50]. More recently, one group has published efforts to eventually translate OCT to the clinical setting to measure tracheal CBF in a rabbit model in real-time, obtaining resolution on the scale of picometres [51]. When applied to larger airways, OCT will enable real-time measurement of CBF that does not involve any radiation [52]. While the in vivo technique is still in the early stages of development, the ability to directly measure MCC on the level of ciliary beating will be a valuable asset for the development of pulmonary diagnostic tests and validation of MCC-enhancing therapeutics.
Computational modelling
All current methods of measuring MCC involve some degree of invasive procedure from inhalation of radiopaque particles to direct visualisation of MCC with OCT or bronchoscopy. However, the ability to computationally model MCC and the effects of various drugs, toxins and environments is increasingly relevant to better understanding early pulmonary disease progression and limiting drug and environmental effects on the airways. Understanding how particle size and density determine pulmonary clearance will be valuable for future drug development and improving methods of drug delivery. Computational models of pulmonary MCC have expanded immensely in the last decade. As with any computational model design, the complexity of the modelling has built upon itself. A good model for pulmonary MCC must consider pulmonary architecture, particle dimensions, individual and grouped ciliary movement, mucous layers, and effects of the microenvironment such as temperature, humidity and airflow rates [53].
In the 2010s, most computational models were focused on individual ciliary movement [54] or MCC at small regions such as single airway bifurcations [55]. Recent models consider MCC through multiple airway bifurcations, the presence of more than one viscous layer (the thick mucus layer and thinner periciliary layer) and the regional differences in mucous thickness and constitution [56, 57]. While full three-dimensional modelling of MCC throughout multiple branch generations has not yet been achieved, these simpler models can help us better understand regional MCC and the effects of particle size on lung deposition and clearance.
Conclusions
MCC is an essential regulator of pulmonary homeostasis. Detection of MCC has been used to better describe disease states and examine the effects of drugs and therapeutics in the airways. With the increased pulmonary disease burden forecasted in the next decades, early detection of disease via MCC measurement could be a useful technique for the pulmonary radiologic field. While MCC detection via radioaerosols has been the mainstay of the field, this review has covered newer methods that, with standardisation efforts, could give greater-resolution images with lower radiation exposure. A summary of methods of in vivo MCC detection is listed in table 1. With an increased understanding of physiological MCC, computational models will be vital in the quick analysis of pulmonary therapeutics. Overall, the future of MCC imaging shows much promise.
TABLE 1.
Summary of in vivo mucociliary clearance (MCC) methods
| Method | Location | Radiation exposure | Spatial resolution | Measurement time | Advantages | Disadvantages |
|---|---|---|---|---|---|---|
| Scintigraphy | Lung: global | Low | 8–10 mm [55] | 1–24 h | Established method | Poor spatial resolution; 2D; radiation exposure |
| SPECT/CT | Lung: global and regional | Moderate | ∼5–8 mm3 [56] | 1–24 h | Regional MCC | Capture time; radiation exposure |
| PET/CT | Lung: global, regional and large airways | High | ∼3–5 mm3 [36] | 30 min–1.5 h | Sensitive, good spatial resolution of entire airway | Radiation exposure; costly radiochemistry and equipment |
| Tantalum discs | Large airways | Low–high | <1–3 mm [57] | Minutes–1 h | Large airway MCC; high temporal resolution | Radiation exposure |
| MPI | Lung: global and regional | None | 1 mm [45] | Minutes–days | High spatial resolution; lack of radiation | Not studied clinically |
| Dye | Large airways, larynx | None | Dependent on scope | Minutes | Fast; inexpensive | Nonspecific; requires scope and sedation |
| OCT | Large airways | None | 20–25 µm [52] | Real-time | CBF measurement | Not studied clinically |
2D: two dimensional; CBF: ciliary beat frequency; CT: computed tomography; MPI: magnetic particle imaging; OCT: optical coherence tomography; PET: positron emission tomography; SPECT: single photon emission computed tomography.
Questions for future research
What is the feasibility of detecting pulmonary MCC in the clinic?
What is the current interinstitutional variability in pulmonary MCC measured via scintigraphy?
Can computational models be utilised for pulmonary drug deposition and retention studies?
Footnotes
Provenance: Submitted article, peer reviewed.
Conflict of interest: All authors have nothing to disclose.
References
- 1.Whitsett JA. Airway epithelial differentiation and mucociliary clearance. Ann Am Thorac Soc 2018; 15: S143–S148. doi: 10.1513/AnnalsATS.201802-128AW [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Munkholm M, Mortensen J. Mucociliary clearance: pathophysiological aspects. Clin Physiol Funct Imaging 2014; 34: 171–177. doi: 10.1111/cpf.12085 [DOI] [PubMed] [Google Scholar]
- 3.Chalmers JD, Chotirmall SH. Bronchiectasis: new therapies and new perspectives. Lancet Respir Med 2018; 6: 715–726. doi: 10.1016/S2213-2600(18)30053-5 [DOI] [PubMed] [Google Scholar]
- 4.Boers E, Barrett M, Su JG, et al. Global burden of chronic obstructive pulmonary disease through 2050. JAMA Netw Open 2023; 6: e2346598. doi: 10.1001/jamanetworkopen.2023.46598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tran HM, Tsai FJ, Lee YL, et al. The impact of air pollution on respiratory diseases in an era of climate change: a review of the current evidence. Sci Total Environ 2023; 898: 166340 doi: 10.1016/j.scitotenv.2023.166340 [DOI] [PubMed] [Google Scholar]
- 6.Krishna MT, Springall D, Meng QH, et al. Effects of ozone on epithelium and sensory nerves in the bronchial mucosa of healthy humans. Am J Respir Crit Care Med 1997; 156: 943–950. doi: 10.1164/ajrccm.156.3.9612088 [DOI] [PubMed] [Google Scholar]
- 7.Hickey AJ. Emerging trends in inhaled drug delivery. Adv Drug Deliv Rev 2020; 157: 63–70. doi: 10.1016/j.addr.2020.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bustamante-Marin XM, Ostrowski LE. Cilia and mucociliary clearance. Cold Spring Harb Perspect Biol 2017; 9: a028241. doi: 10.1101/cshperspect.a028241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bennett WD, Wu J, Fuller F, et al. Duration of action of hypertonic saline on mucociliary clearance in the normal lung. J Appl Physiol (1985) 2015; 118: 1483–1490. doi: 10.1152/japplphysiol.00404.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Corcoran TE, Huber AS, Hill SL, et al. Mucociliary clearance differs in mild asthma by levels of type 2 inflammation. Chest 2021; 160: 1604–1613. doi: 10.1016/j.chest.2021.05.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Robinson M, Eberl S, Tomlinson C, et al. Regional mucociliary clearance in patients with cystic fibrosis. J Aerosol Med 2000; 13: 73–86. doi: 10.1089/089426800418604 [DOI] [PubMed] [Google Scholar]
- 12.Xavier RF, Ramos D, Ito JT, et al. Effects of cigarette smoking intensity on the mucociliary clearance of active smokers. Respiration 2014; 86: 479–485. doi: 10.1159/000348398 [DOI] [PubMed] [Google Scholar]
- 13.Walker WT, Young A, Bennett M, et al. Pulmonary radioaerosol mucociliary clearance in primary ciliary dyskinesia. Eur Respir J 2014; 44: 533–535. doi: 10.1183/09031936.00011814 [DOI] [PubMed] [Google Scholar]
- 14.Corcoran TE, Godovchik JE, Donn KH, et al. Overnight delivery of hypertonic saline by nasal cannula aerosol for cystic fibrosis. Pediatr Pulmonol 2017; 52: 1142–1149. doi: 10.1002/ppul.23749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bennett WD, Henderson AG, Ceppe A, et al. Effect of hypertonic saline on mucociliary clearance and clinical outcomes in chronic bronchitis. ERJ Open Res 2020; 6: 00269-2020. doi: 10.1183/23120541.00269-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Trimble AT, Whitney Brown A, Laube BL, et al. Hypertonic saline has a prolonged effect on mucociliary clearance in adults with cystic fibrosis. J Cyst Fibros 2018; 17: 650–656. doi: 10.1016/j.jcf.2018.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Daviskas E, Anderson SD, Eberl S, et al. Beneficial effect of inhaled mannitol and cough in asthmatics with mucociliary dysfunction. Respir Med 2010; 104: 1645–1653. doi: 10.1016/j.rmed.2010.05.019 [DOI] [PubMed] [Google Scholar]
- 18.Daviskas E, Anderson SD, Eberl S, et al. Effect of increasing doses of mannitol on mucus clearance in patients with bronchiectasis. Eur Respir J 2008; 31: 765–772. doi: 10.1183/09031936.00119707 [DOI] [PubMed] [Google Scholar]
- 19.Daviskas E, Anderson SD, Eberl S, et al. Inhalation of dry powder mannitol improves clearance of mucus in patients with bronchiectasis. Am J Respir Crit Care Med 1999; 159: 1843–1848. doi: 10.1164/ajrccm.159.6.9809074 [DOI] [PubMed] [Google Scholar]
- 20.Konrad F, Schreiber T, Grunert A, et al. Measurement of mucociliary transport velocity in ventilated patients; short-term effect of general anesthesia on mucociliary transport. Chest 1992; 102: 1377–1383. doi: 10.1378/chest.102.5.1377 [DOI] [PubMed] [Google Scholar]
- 21.Gosselink R, Gayan-Ramirez G, Houtmeyers E, et al. High-dose lidocaine reduces airway mucus transport velocity in intubated anesthetized dogs. Respir Med 2006; 100: 258–263. doi: 10.1016/j.rmed.2005.04.028 [DOI] [PubMed] [Google Scholar]
- 22.Zolfaghari PS, Wyncoll DLA. The tracheal tube: gateway to ventilator-associated pneumonia. Crit Care 2011; 15: 310. doi: 10.1186/cc10352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Keller C, Brimacombe J. Bronchial mucus transport velocity in paralyzed anesthetized patients: a comparison of the laryngeal mask airway and cuffed tracheal tube. Anesth Analg 1998; 86: 1280–1282. doi: 10.1097/00000539-199806000-00028 [DOI] [PubMed] [Google Scholar]
- 24.Adams PS, Corcoran TE, Lin JH, et al. Mucociliary clearance scans show infants undergoing congenital cardiac surgery have poor airway clearance function. Front Cardiovasc Med 2021; 8: 652158. doi: 10.3389/fcvm.2021.652158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wark P, McDonald VM, Smith S. Nebulised hypertonic saline for cystic fibrosis. Cochrane Database Syst Rev 2023; 6: CD001506. 10.1002/14651858.CD001506.pub5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bennett WD. Design of in vivo deposition and clearance experiments. J Aerosol Med Pulm Drug Deliv 2022; 35: 286–290. doi: 10.1089/jamp.2022.29069.wdb [DOI] [PubMed] [Google Scholar]
- 27.Newman S, Bennett WD, Biddiscombe M, et al. Standardization of techniques for using planar (2D) imaging for aerosol deposition assessment of orally inhaled products. J Aerosol Med Pulm Drug Deliv 2012; 25: Suppl. 1, S10–S28. doi: 10.1089/jamp.2012.1Su4 [DOI] [PubMed] [Google Scholar]
- 28.Trimble A, Zeman K, Wu J, et al. Effect of airway clearance therapies on mucociliary clearance in adults with cystic fibrosis: a randomized controlled trial. PLoS One 2022; 17: e0268622. doi: 10.1371/journal.pone.0268622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Scheuch G, Bennett W, Borgström L, et al. Deposition, imaging, and clearance: what remains to be done? J Aerosol Med Pulm Drug Deliv 2010; 23: Suppl. 2, S39–S57. doi: 10.1089/jamp.2010.0839 [DOI] [PubMed] [Google Scholar]
- 30.Bennett WD, Burbank A, Almond M, et al. Acute and durable effect of inhaled hypertonic saline on mucociliary clearance in adult asthma. ERJ Open Res 2021; 7: 00062-2021. doi: 10.1183/23120541.00062-2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fleming JS. The use of single photon emission computed tomography in aerosol medicine. J Aerosol Med Pulm Drug Deliv 2023; 36: 44–53. doi: 10.1089/jamp.2023.29077.jsf [DOI] [PubMed] [Google Scholar]
- 32.Corcoran TE. Imaging in aerosol medicine. Respir Care 2015; 60: 850–857. doi: 10.4187/respcare.03537 [DOI] [PubMed] [Google Scholar]
- 33.Venegas JG. Measuring anatomical distributions of ventilation and aerosol deposition with PET-CT. J Aerosol Med Pulm Drug Deliv 2023; 36: 210–227. doi: 10.1089/jamp.2023.29086.jgv [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hatazawa J, Yanai M, Itoh M, et al. Tracheobronchial mucociliary clearance and alveolar epithelial permeability measured by PET with 18FDG powder. J Comput Assist Tomogr 1990; 14: 175–181. doi: 10.1097/00004728-199003000-00003 [DOI] [PubMed] [Google Scholar]
- 35.Shingaki T, Katayama Y, Nakaoka T, et al. Visualization of drug translocation in the nasal cavity and pharmacokinetic analysis on nasal drug absorption using positron emission tomography in the rat. Eur J Pharm Biopharm 2016; 99: 45–53. doi: 10.1016/j.ejpb.2015.11.014 [DOI] [PubMed] [Google Scholar]
- 36.Lu FM, Yuan Z. PET/SPECT molecular imaging in clinical neuroscience: recent advances in the investigation of CNS diseases. Quant Imaging Med Surg 2015; 5: 433–447. doi: 10.3978/j.issn.2223-4292.2015.03.16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Black J. Review paper: biological performance of tantalum. Clin Mater 1994; 16: 167–173. doi: 10.1016/0267-6605(94)90113-9 [DOI] [PubMed] [Google Scholar]
- 38.Trawöger R, Kolobow T, Patroniti N, et al. Intratracheal pulmonary ventilation keeps tracheal tubes clean without impairing mucociliary transport. Scand J Clin Lab Invest 2002; 62: 351–356. doi: 10.1080/00365510260296500 [DOI] [PubMed] [Google Scholar]
- 39.Ash JJ, Hilkin BM, Gansemer ND, et al. Tromethamine improves mucociliary clearance in cystic fibrosis pigs. Physiol Rep 2022; 10: e15340. doi: 10.14814/phy2.15340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bassi GL, Zanella A, Cressoni M, et al. Following tracheal intubation, mucus flow is reversed in the semirecumbent position: possible role in the pathogenesis of ventilator-associated pneumonia. Crit Care Med 2008; 36: 518–525. doi: 10.1097/01.CCM.0000299741.32078.E9 [DOI] [PubMed] [Google Scholar]
- 41.Tay ZW, Chandrasekharan P, Zhou XY, et al. In vivo tracking and quantification of inhaled aerosol using magnetic particle imaging towards inhaled therapeutic monitoring. Theranostics 2018; 8: 3676–3687. doi: 10.7150/thno.26608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ledowski T, Manopas A, Lauer S. Bronchial mucus transport velocity in patients receiving desflurane and fentanyl vs. sevoflurane and fentanyl. Eur J Anaesthesiol 2008; 25: 752–755. doi: 10.1017/S0265021508004304 [DOI] [PubMed] [Google Scholar]
- 43.O'Neil LM, Jefferson ND. Direct visualization of laryngeal mucociliary clearance in adults. Ann Otol Rhinol Laryngol 2019; 128: 1048–1053. doi: 10.1177/0003489419859376 [DOI] [PubMed] [Google Scholar]
- 44.Podoleanu AG. Optical coherence tomography. J Microsc 2012; 247: 209–219. doi: 10.1111/j.1365-2818.2012.03619.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wu XLC, Zhang XY, Steinberg XG, et al. A review of magnetic particle imaging and perspectives on neuroimaging. Am J Neuroradiol 2019; 40: 206–212. doi: 10.3174/ajnr.A5896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cho DY, Rivers NJ, Lim D, et al. Glutathione and bicarbonate nanoparticles improve mucociliary transport in cystic fibrosis epithelia. Int Forum Allergy Rhinol 2023; 14: 1026–1035. doi: 10.1002/alr.23301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Coughlan CA, Chou LD, Jing JC, et al. In vivo cross-sectional imaging of the phonating larynx using long-range Doppler optical coherence tomography. Sci Rep 2016; 6: 22792. doi: 10.1038/srep22792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.He Y, Jing JC, Qu Y, et al. Spatial mapping of tracheal ciliary beat frequency using real time phase-resolved Doppler spectrally encoded interferometric microscopy. ACS Photonics 2020; 7: 128–134. doi: 10.1021/acsphotonics.9b01235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xu L, Jiang Y. Mathematical modeling of mucociliary clearance: a mini-review. Cells 2019; 8: 736. doi: 10.3390/cells8070736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sears PR, Thompson K, Knowles MR, et al. Human airway ciliary dynamics. Am J Physiol Lung Cell Mol Physiol 2013; 304: L170–L183. doi: 10.1152/ajplung.00105.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Farkas A, Szöke I. Simulation of bronchial mucociliary clearance of insoluble particles by computational fluid and particle dynamics methods. Inhal Toxicol 2013; 25: 593–605. doi: 10.3109/08958378.2013.815666 [DOI] [PubMed] [Google Scholar]
- 52.Popescu DP, Choo-Smith LP, Flueraru C, et al. Optical coherence tomography: fundamental principles, instrumental designs and biomedical applications. Biophys Rev 2011; 3: 155. doi: 10.1007/s12551-011-0054-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Farkas Á. Simulation of the effect of mucociliary clearance on the bronchial distribution of inhaled radon progenies and related cellular damage using a new deposition and clearance model for the lung. Radiat Environ Biophys 2020; 59: 651–661. doi: 10.1007/s00411-020-00868-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bartlett BA, Feng Y, Fromen CA, et al. Computational fluid dynamics modeling of aerosol particle transport through lung airway mucosa. Comput Chem Eng 2023; 179: 108458. doi: 10.1016/j.compchemeng.2023.108458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cecchin D, Poggiali D, Riccardi L, et al. Analytical and experimental FWHM of a gamma camera: theoretical and practical issues. PeerJ 2015; 3: e722. doi: 10.7717/peerj.722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ljungberg M, Pretorius PH. SPECT/CT: an update on technological developments and clinical applications. Br J Radiol 2018; 91: 20160402. doi: 10.1259/bjr.20160402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yucel-Finn A, Mckiddie F, Prescott S, et al. Farr's Physics for Medical Imaging, 3rd edn. Amsterdam, Elsevier, 2023. [Google Scholar]