Abstract
The escalating demand for green hydrogen (H2) as a sustainable energy carrier has attracted intensive research into efficient water electrolysis methods. Promising candidates have emerged as binder-less metal electrodes, which enhance electrochemical performance and durability by reducing electron hindrance and avoiding binder degradation. Despite their potential, a comprehensive understanding of various binder-less fabrication techniques remains limited in the existing literature. As the main objective, this review paper aims to bridge this gap by providing an in-depth analysis of state-of-the-art fabrication methods for binder-less metal electrodes utilized in electrochemical water splitting. Recognizing the critical need for sustainable hydrogen production, the advantages of binder-less electrodes over conventional binder-based counterparts are elucidated, with emphasis placed on their role in promoting cost-effectiveness, improved stability, and enhanced catalytic activity. Techniques such as Hydrothermal/Solvothermal, Electrodeposition, Chemical/Vapor Deposition, and Laser-based fabrication are systematically examined, with their respective advantages, drawbacks, and comparison being highlighted. Drawing upon relevant examples from literature, insights on other aspects and recent trends are also provided, such as the performance of binder-less metal electrodes at industrial-scale current densities (0.1–1 A/cm2) or their potential as photoactive catalysts. Additionally, future directions in the field of binder-less electrode fabrication and the exploration of innovative techniques are also discussed, ensuring that the trajectory of research aligns with the evolving demands of sustainable energy production. The "what's next" section highlights areas of further investigation and potential avenues for technological advancement.
Keywords: Water electrolysis, Electrocatalysis, Electrode, Fabrication, Binderless
Highlights
-
•
Rising green hydrogen demand pushes innovation in electrocatalyst and electrode design.
-
•
Binder-less electrodes improve performance, yet fabrication methods need more study.
-
•
Methods include hydrothermal, electrodeposition, CVD, electrophoresis, and laser tech.
-
•
Future work: optimizing parameters for sustainable, industrial-scale hydrogen output.
List of abbreviations & nomenclature
- OER
Oxygen Evolution Reaction
- HER
Hydrogen Evolution Reaction
- PVDF
Polyvinylidene Fluoride
- DMF
Dimethylformamide
- CBD
Chemical Bath Deposition
- CVD
Chemical Vapor Deposition
- PVD
Physical Vapor Deposition
- EPD
Electrophoretic Deposition
- η10
Overpotential at 10 mA/cm2 of current density (η stands for overpotential and 10 denotes current density value)
- LDH
Layered Double Hydroxide
- PLD
Pulsed Laser Deposition
- ORR
Oxygen Reduction Reaction
- LSV
Linear Sweep Voltammetry
- CV
Cyclic Voltammetry
- ALD
Atomic Layer Deposition
- TOF
Turnover Frequency
- UOR
Urea Oxidation Reaction
- ECSA
Electrochemical active surface area
- FE
Faradaic Efficiency
- TM
Transition Metal
- AACVD/AA-CVD
aerosol-assisted chemical vapor deposition
- GO
zraphene Oxide
- PLAL
Pulsed Laser Ablation in Liquid
- ULPING
Ultra-Short Laser Pulse for In-situ Nanostructure Generation
- AI/ML
Artificial Intelligence/Machine Learning
1. Introduction
The pressing need to reduce carbon emissions and transition towards renewable energy sources has propelled green hydrogen to the forefront of global energy discussions [[1], [2], [3]]. As nations and industries seek cleaner alternatives to fossil fuels, the allure of hydrogen as a clean and versatile energy carrier has garnered significant attention. Green hydrogen, produced through the electrolysis of water using renewable energy sources such as solar and wind power, offers a promising pathway towards a carbon-neutral future [[4], [5], [6], [7]]. Unlike traditional hydrogen production methods that rely on fossil fuels, green hydrogen production harnesses the power of renewable resources to split water molecules into hydrogen and oxygen. This process, known as electrolysis, holds immense potential for storing renewable energy in the form of hydrogen, thereby enabling grid balancing, energy storage, and fuel production for various applications. At the heart of green hydrogen production lies the electrolysis of water, a simple yet transformative process that involves the decomposition of water molecules into hydrogen and oxygen gases. Despite the potential of water electrolysis for green hydrogen production, the process is inherently slow and inefficient without the presence of electrocatalysts. Electrocatalysts play a crucial role in lowering the activation energy barrier for the water splitting reaction, thereby enhancing reaction kinetics and overall efficiency. By facilitating the transfer of electrons and ions at the electrode-electrolyte interface, electrocatalysts enable the conversion of electrical energy into chemical energy with high efficiency. Most importantly, by the use of electrocatalysts, the practical potential required for the gas evolution reactions to occur experimentally (i.e. overpotential) is reduced and hence the process becomes practically possible [8,9]. Metal-based materials, including noble metals such as platinum (Pt), iridium (Ir), and ruthenium (Ru), as well as transition metals such as nickel (Ni), cobalt (Co), and iron (Fe), have been extensively studied as electrocatalysts for water electrolysis. Each metal exhibits unique catalytic properties and reactivity towards the oxygen evolution reaction (OER) and hydrogen evolution reaction (HER), the two half-reactions involved in water splitting. While noble metals offer excellent catalytic activity, their high cost and limited availability hinder widespread adoption. Hence, even there have been various alternatives, the use of transition metals, still proves to be cost-effective and practicable for electrochemical storage, conversion or catalytic applications [10]. In principle, the key challenges include lower electrochemical or catalytic activity and stability. The use of transition metals has been quite prominent in the case of other applications also, such as electrochemical energy storage devices including batteries, supercapacitors and pseudocapacitors but for the purpose of electrocatalysis, still more progress is necessary to match the activity of noble metals [11,12]. For this various innovative strategies and hybridization routes are being explored [[13], [14], [15], [16]].
Apart from the materials used as catalyst, the fabrication of electrodes is also a crucial factor that determines the working of the electrolyzer as well as its scalability and practicality. In conventional electrode fabrication methods, binders are commonly used to immobilize the active catalyst particles onto the electrode substrate and improve electrode stability. Binders, typically organic polymers such as polyvinylidene fluoride (PVDF) or Nafion, serve as a matrix to hold the catalyst particles together and adhere them to the electrode surface. While binders enhance mechanical strength and electrode integrity, they also introduce several challenges that can impede electrochemical performance. Despite their utility, binders pose several drawbacks that limit their effectiveness in electrode fabrication. One major issue is the insulating nature of certain binders, which can hinder electron transfer and mass transport within the electrode. Additionally, binders may undergo degradation under harsh electrolysis conditions, leading to the release of toxic byproducts or contamination of the electrolyte. Furthermore, binder-induced agglomeration of catalyst particles can reduce the effective surface area and active sites available for catalysis, thereby diminishing overall electrode performance. To overcome the limitations associated with binder-based electrode fabrication, researchers have turned to binderless fabrication methods as a promising alternative. Binderless electrodes eliminate the need for organic binders, relying instead on intrinsic interactions between the catalyst particles and the electrode substrate to achieve adhesion and cohesion. By eliminating the insulating barrier imposed by binders, binderless electrodes offer improved electron transfer kinetics, enhanced mass transport, and higher catalytic activity. As shown in Fig. 1, the number of steps involved in binderless electrode fabrication is much lesser than those in traditional electrode fabrication, making it suitable for scalable industrial innovations. [17]
Fig. 1.
– Steps involved in traditional electrode fabrication compared with binder-less electrode fabrication.
Binderless electrode fabrication encompasses a diverse array of techniques that leverage physical, chemical, and mechanical interactions to assemble catalyst particles into robust electrode structures. Hydrothermal synthesis, for example, involves the controlled growth of catalyst particles on conductive substrates under high-pressure, high-temperature conditions, resulting in intimate contact between the catalyst and the electrode surface. Chemical bath deposition utilizes chemical reactions to deposit thin films of catalyst materials directly onto electrode substrates, while electrodeposition enables the electrodeposition of metal ions onto conductive supports to form catalytic layers. Chemical vapor deposition involves the deposition of thin films of catalyst materials from vapor-phase precursors onto electrode substrates, offering precise control over film thickness and composition. Laser fabrication techniques utilize laser-induced processes such as laser ablation or laser sintering to pattern and shape catalyst materials directly on electrode surfaces, enabling precise control over electrode morphology and structure.
Despite the growing interest in binderless electrode fabrication for electrochemical water splitting, a comprehensive review of the existing literature and research developments is currently lacking. There are several reviews focusing on binderless fabrication of battery electrodes and energy storage device electrodes [[18], [19], [20], [21]]. A review article may offer researchers, engineers, and policymakers working in the field of electrochemical energy conversion and storage invaluable direction and guidance by compiling the most recent findings and insights from a wide range of studies. By elucidating the underlying principles, challenges, and future prospects of binderless electrode fabrication, this review seeks to accelerate the development and deployment of efficient and sustainable water electrolysis technologies for green hydrogen production.
2. Overview on water splitting and use of binders
Electrochemical water splitting is a pivotal process in the production of green hydrogen, a clean and renewable energy carrier. This method involves the use of an electrolyzer, which comprises various materials and components to facilitate the splitting of water molecules into hydrogen (H2) and oxygen (O2) gases [4,22]. The electrolyzer typically consists of an electrolyte solution, electrodes, and an external power source [23,24]. At the heart of electrochemical water splitting are two half-reactions: the hydrogen evolution reaction (HER) and the oxygen evolution reaction (OER). And as shown in Fig. 2(a), these reactions occur at the cathode and anode, respectively, and proceed as follows:
| (1) |
| (2) |
| (3) |
Fig. 2.
– a) Schematic showing Oxygen Evolution Reaction (OER) and Hydrogen Evolution Reaction (HER) in electrocatalytic water splitting Figure reproduced with permission from Ref. [24]. b) Conventionally coated electrode compared with c) binder-less/self-supporting electrode Figure reproduced with permission from Ref. [25].
Various components are involved in the electrolytic splitting of water. The electrolyte serves as a medium for ion transport between the electrodes and facilitates the flow of current during electrolysis. Common electrolytes include aqueous solutions of acids (e.g., sulfuric acid), bases (e.g., potassium hydroxide), or salts (e.g., potassium phosphate). The electrodes play a crucial role in catalyzing the HER and OER. Typically, the cathode is composed of a catalyst material that promotes the reduction of water to hydrogen, while the anode comprises a catalyst material that promotes the oxidation of water to oxygen.
In conventional electrode fabrication methods, binders are commonly employed to immobilize the active catalyst particles onto the electrode substrate. Binders, typically organic polymers such as polyvinylidene fluoride (PVDF) or Nafion, serve as a matrix to hold the catalyst particles together and adhere them to the electrode surface. While binders offer certain advantages, they also present several disadvantages and issues that can impact electrode performance and stability. However, there are various disadvantages and issues with the use of binders. Certain binders possess insulating properties, which can hinder electron transfer and ion transport within the electrode. This insulating barrier impedes the efficient utilization of catalyst sites and limits the overall electrochemical activity of the electrode. Binders undergo degradation under harsh electrolysis conditions, such as high temperatures, acidic or alkaline environments, and prolonged operation. This degradation can lead to the release of toxic byproducts or contamination of the electrolyte, compromising the integrity of the electrode and the purity of the hydrogen produced.
Several investigations have demonstrated the substantial impact of polymer binders on the overall efficacy of various electrochemical devices, particularly concerning mechanical attributes and interactions with electrolytes and active materials [[26], [27], [28], [29], [30], [31]]. While Poly(vinylidene fluoride) (PVDF) remains a conventional choice for organic electrodes in lithium-ion batteries [18,32], its lifespan is constrained, as evidenced by escalating contact resistance over extended operational periods and the identification of degradation byproducts within the devices. As compared in Fig. 2(b) and (c), coating the mixture of catalyst and conductive agent using a binder on the substrate is often uniform leading to blockage of electron pathways. On the other hand, in the case of binder-less or self-supported catalysts, there is a seamless contact providing an optimum channel for electron transfer [25]. In aqueous systems, the adoption of solely hydrophobic binders is discouraged, prompting the utilization of alternatives such as Nafion™-based binders. Nafion, renowned for its ion and water conductive membrane properties, is not engineered to endure the rigorous reductive and oxidative conditions prevalent in contemporary electrodes. Moreover, in the presence of binders can promote the agglomeration of catalyst particles, leading to the formation of large clusters or aggregates on the electrode surface. This phenomenon reduces the effective surface area available for catalysis and diminishes the catalytic activity of the electrode. Binders may exhibit limited durability and stability over time particularly in dynamic electrochemical environments. The mechanical stresses and chemical reactions occurring during electrolysis can cause binders to degrade or delaminate from the electrode substrate, resulting in loss of adhesion and structural integrity. Binders can create barriers to mass transport within the electrode, restricting the diffusion of reactants and products to and from the active catalytic sites. This limitation hampers the efficiency of the water splitting process and may lead to uneven electrode performance across the electrode surface.
Addressing these challenges through the development of binderless electrode fabrication methods is essential for advancing the scalability and sustainability of green hydrogen production technologies. Various such methods including Hydrothermal/Solvothermal, Chemical Bath Deposition (CBD), Electrodeposition, and Chemical/Physical Vapor Deposition (CVD/PVD) have been compared in Table 1. The methods being diverse in nature, the key advantages and disadvantages are provided along with parameters that govern the fabrication process. Hydrothermal/Solvothermal methods offer good control over morphology and particle size but are energy-intensive, with key parameters including temperature, pressure, and solvent type. CBD is simple and low-cost, ideal for conformal coating, but can produce films with impurities, with solution chemistry and deposition time being critical parameters. Electrodeposition provides precise control over film thickness and is scalable, though it can result in non-uniform deposition, with potential and current density being essential factors. CVD/PVD methods are excellent for producing high-purity, uniform films and complex material structures but involve high equipment costs and can be slow, with precursor chemistry, pressure, and temperature as key parameters. The following sections cover these methods in detail, along with examples of experimental works that suitably demonstrate these.
Table 1.
– Comparison of various binder-less electrode fabrication methods.
| Methods | Advantages | Disadvantages | Key Parameters |
|---|---|---|---|
| Hydrothermal/Solvothermal | Good control over morphology and particle size of active material | Can be energy-intensive (high temperature and pressure) |
|
| Chemical Bath Deposition (CBD) | Simple, low-cost setup, good for conformal coating on complex substrates | Can produce films with impurities, film thickness control can be challenging |
|
| Electrodeposition | Precise control over film thickness, can be scalable for larger substrates, room temperature operation is possible | Potential for non-uniform deposition, substrate conductivity is essential, solution composition is critical |
|
| Chemical/Physical Vapor Deposition (CVD/PVD) | Excellent for producing high-purity, uniform films, can create complex material structures | High equipment cost, can be slow for thick films, CVD often requires high temperatures |
|
| Laser Fabrication (e.g., Laser Ablation or Pulsed Laser Deposition) | Highly precise control over film properties, can fabricate complex, multi-material structures, versatile for different materials | High initial equipment cost, required knowledge and optimization of parameters |
|
3. Binder-less fabrication methods for non-noble metal electrodes
The utilization of non-noble metal electrocatalysts for water splitting presents several advantages over their noble metal counterparts, including cost-effectiveness, abundance, and tunable catalytic activity. Non-noble metals such as Iron (Fe), Cobalt (Co), Manganese (Mn), Copper (Cu) and Nickel (Ni) offer promising alternatives for catalyzing the hydrogen evolution reaction (HER) and oxygen evolution reaction (OER) in electrochemical water splitting systems. Non-noble metals are significantly more abundant and less expensive than noble metals such as platinum (Pt) and iridium (Ir). Non-noble metals such as copper, nickel, iron, cobalt etc. Are abundantly available in the Earth's crust, ensuring a stable and sustainable supply for large-scale hydrogen production [[33], [34], [35], [36], [37], [38]]. In the case of binder-less fabrication of non-noble metal electrodes, the choice of catalyst or catalyst's composition, is also crucial for enhancing electrical conductivity and electrochemical performance. It helps improve the structural integrity of the electrodes by promoting better adhesion of metal particles. Additionally, catalysts contribute to cost efficiency by boosting the performance of non-noble metals and increasing their durability. Thus, an effective catalyst is key to optimizing the performance and stability of binder-less electrodes. Hence various works have focused on developing efficient catalysts, followed by their binder-less deposition on a metal substrate and electrochemical characterization.
Among non-noble metals, copper and nickel have emerged as two of the most promising candidates for catalyzing water splitting reactions due to their favorable catalytic properties, abundance, and tunability [[39], [40], [41], [42], [43]]. Copper exhibits excellent catalytic activity for both the HER and OER, making it a versatile candidate for overall water splitting. Its rich redox chemistry and tunable surface properties enable precise control over catalytic activity and selectivity. Copper-based electrocatalysts have been shown to achieve competitive performance with noble metal catalysts while offering significant cost savings and scalability. Nickel is another attractive option for electrocatalytic water splitting, particularly for the HER. Nickel-based catalysts exhibit high catalytic activity, robustness, and corrosion resistance under acidic conditions, making them well-suited for practical applications. Moreover, nickel is abundant and cost-effective, further enhancing its appeal for large-scale hydrogen production. In addition to their utility as electrocatalysts, copper and nickel are commonly chosen as substrates for binder-less electrode fabrication due to their inherent conductivity, stability, and compatibility with fabrication processes. Copper and nickel possess excellent electrical conductivity, ensuring efficient electron transfer within the electrode material. Copper and nickel exhibit robust chemical stability under harsh electrochemical conditions, including acidic or alkaline environments and elevated temperatures. Copper and nickel substrates are well-suited for a variety of fabrication techniques, including deposition, etching, and surface modification. These metals can be easily processed and patterned to create tailored electrode architectures with precise control over morphology and surface properties. With an objective of providing comparison of various binder-less electrode fabrication methods, this section focuses on electrocatalysts based on Cu or Ni or those which utilize Cu or Ni metals as substrates.
Hydrothermal and solvothermal fabrication methods are versatile techniques used in the synthesis of binder-less electrodes for electrochemical applications. These methods involve the controlled growth of electrode materials under high-pressure, high-temperature conditions in aqueous or organic solvent environments, respectively. Hydrothermal synthesis utilizes water as the solvent and operates at elevated temperatures and pressures to promote the formation of crystalline electrode materials. This method enables precise control over particle size, morphology, and crystallinity, leading to tailored electrode architectures with enhanced catalytic activity and stability. Solvothermal synthesis, on the other hand, employs organic solvents such as ethanol or dimethylformamide (DMF) to facilitate the synthesis of binder-less electrodes. Solvothermal conditions allow for the dissolution of precursor materials and the formation of homogeneous electrode coatings, resulting in electrodes with improved adhesion, conductivity, and electrochemical performance [[44], [45], [46]].
For the case of copper, its electrocatalytic ability in hydrothermal conditions arises from its versatile oxidation states, encompassing Cu⁰, Cu⁺, Cu2⁺, and Cu³⁺, along with its semiconducting properties. A notable method for crafting binder-less self-supported Cu electrocatalysts involves depositing copper oxide onto readily available substrates like copper foam or foil. In a study by Ma et al., CuₓO (comprising CuO and Cu₂O) nanowires were synthesized on three-dimensional copper foam using a two-step process: alkali corrosion-oxidation followed by controlled annealing under different atmospheres [47]. The initial growth of nanowires of Cu(OH)₂ was brought about by dipping of Cu foam substrate in a Alkali-Persulfate (NaOH + (NH₄)₂S₂O₈) solution. Subsequent annealing under air and N₂ atmospheres produced CuₓO NWs/CF and CuₓO NWs/CF-N₂, respectively. A comparison was made with untreated Cu foam, annealed solely in air, yielding CuₓO/CF without nanostructure formation. Annealing significantly influenced the material's morphology, evident from SEM images revealing bent nanowires with pinhole formation post-calcination, enhancing specific surface area and catalytic activity. Evaluation of water splitting performance in 1 M KOH revealed CuₓO NW/CF as the most active catalyst for both HER and OER, attributed to its dense porous nanowire array morphology providing ample catalytic sites. The similar Tafel slopes between CuₓO NWs/CF-N₂ and CuₓO/CF for HER suggested equal active sites, influenced by specific surface area. For OER, similar overpotentials and Tafel slopes were observed among CuₓO NWs/CF-N₂, CuₓO/CF, and Cu(OH)₂/CF, with lower specific surface areas hindering catalytic performance. Nyquist plots indicated minimal interfacial reaction resistance for all samples, with CuₓO NWs/CF exhibiting the lowest, suggesting CuO as the active OER site. This study showcases the feasibility of binder-less Cu nanostructure fabrication through alkali-dipped corrosion, although achieving desired activity may necessitate more intricate fabrication methods. Consequently, further exploration into nuanced fabrication techniques could enhance both the structural integrity and electrocatalytic performance of these materials, offering promising prospects for applications in diverse electrochemical processes.
However, copper foam has found versatile applications beyond serving only as a substrate for growth of copper nanostructures, extending to the deposition of various metallic compounds or materials. In a pioneering study by Shi et al., a film of Nickel (II) Selenide was grown onto a Cu foam using electrodeposition, acting a dual-function catalyst for both hydrogen evolution reaction (HER) and oxygen evolution reaction (OER) in alkaline media [48]. This 3D electrode exhibited remarkable activity, achieving a HER overpotential of ∼100 mV (at 10 mA/cm2 current density) and OER overpotential of ∼350 mV (at 50 mA/cm2 current density). Moreover, the bifunctionality was examined in a two-electrode alkaline water electrolyzer cell and the said catalyst delivered a current density of 10 mA/cm2 at a potential of 1.65 V. The electrodeposition-mediated growth was carried out at pH 3.5 in an electrolytic bath comprising of a mixture of Ni (II) Acetate as Ni source and SeO2 as Se source. For the deposition, the Cu Foam substrate was potentially biased at −0.5 V vs. SCE for 1 h. The purity and uniformity of the deposition of the said Ni3Se2 catalyst was confirmed by XPS and SEM analysis. And hence, the practicability of electrodeposition in depositing catalyst materials with precision, contributing to the low overpotential, favorable Tafel slopes, and commendable stability over long hours (as like 10+ hours in this case) for both HER and OER was exemplified in this work.
Another study by Ma et al. (2018) focuses on growth of forest-like nanostructures of NiCoP-Cu3P hybrid were grown on a copper foam substrate through a combination of chemical oxidation and a solvothermal process, the electrocatalytic HER performance is depicted in Fig. 3(a) [49]. Initially, Cu(OH)2 nanowires (NWs) were synthesized by oxidizing Cu-foam, followed by the solvothermal reaction to obtain NiCo layered double hydroxide (LDH) nanosheets, which were subsequently phosphidated to yield NiCoP@Cu3P/CF. The optimization of Ni/Co content was done as shown in Fig. 3(b). The solvothermal process involved using nitrate salts of Cobalt and Nickel, NH4F, and urea as chemical precursors. The resulting 3D micro-hierarchical structure provided a large surface area with enhanced exposure of active sites and efficient electron transport, facilitated by the unique morphology. The catalyst was also found to be very stable and durable as found from comparison before and after 1000 cycles, as shown in Fig. 3(c). Additionally, Cu3P contributed to improved catalytic performance through synergistic effects and excellent conductivity. EDS mapping confirmed the even distribution of Cu throughout the nanosheet, indicating its role in facilitating nucleation and growth during the formation of NiCo LDH around the nanowires through hydrothermal treatment. From the SEM images, the growth of Cu(OH)2 nanowires (length <10 μm, diameter in the range of 100–200 nm) having highly useful active surface area was validated, as seen in Fig. 3(d) that was suitable for facilitating the growth of the nucleation and growth of foreign materials. Further process enabled growth of nanosheets, followed by slight change in morphology after phosphidization, as shown in Fig. 3(e). This synthesis strategy underscores the potential of copper foam as a versatile substrate for the growth of complex nanostructures with tailored properties for diverse catalytic applications.
Fig. 3.
– a) Electrocatalytic HER performance of Ni-Co Phosphide compared with other catalyst through LSV b) Optimization of Ni-Co atomic ratio c) Catalytic stability before and after 1000 cycles d) SEM images of Cu(OH)2 NWs e) SEM images of NiCoP@Cu3P/CF Figures reproduced with permission from Ref. [49]. f) HER Tafel slopes of electrodeposited Ni-Fe-Co ternary electrocatalyst g) OER Tafel slopes of electrodeposited Ni-Fe-Co ternary electrocatalyst Figures reproduced with permission from Ref. [50].
Nickel foam stands out as a notable substrate for binder-free water-splitting electrodes, offering numerous advantages that bolster catalyst performance and durability. Its highly porous structure and large surface area facilitate efficient electrolyte access to active sites, promoting rapid mass transport of reactants and products to enhance reaction kinetics. Additionally, the porous nature of nickel foam ensures effective gas diffusion throughout the electrode, minimizing mass transport limitations and ensuring uniform gas distribution, crucial for achieving high catalytic activity. Furthermore, nickel foam exhibits excellent electrical conductivity, enabling efficient electron transfer between the catalyst and the electrode substrate. It also demonstrates chemical stability under the harsh conditions of electrochemical water splitting by resisting corrosion and degradation over prolonged operating periods. Also, Nickel/Nickel oxide is known for its unique properties such as the formation of heterogeneous interfaces, nanoscale structures, and variable oxidation states of Ni2⁺ and Ni³⁺ during electrochemical processes and hence is widely useful as an efficient and practical water splitting catalyst.
In a recent study by Bibi et al., a binder-less hydrothermal synthesis approach was employed to fabricate Cu-Co (binary) and Cu-Co-Ni (ternary) composites on a nickel foam substrate for targeted Oxygen Evolution Reaction (OER) [51]. The ternary catalyst (CCoN8) demonstrated an outstanding electrocatalytic performance, achieving an OER overpotential of ∼400 mV at 50 mA/cm2 current density and a favorable Tafel slope of 85 mV dec−1. Also, it was observed that the said catalyst was able to withstand prolonged chronoamperometric analysis for over 30 h, confirming its stability and durability for water splitting. Moreover, with the addition of p-type NiO, the electrocatalysts' efficiency for water oxidation was further increased. The improved electrocatalytic characteristics were due to the complementary actions of Cu, Co, and Ni metal ions. Similarly, hydro/solvothermal fabrication methods have also been been applied to Glassy Carbon Electrode (GCE) [52]. In one of the studies, CuO was deposited onto GCE, along with Ni, Co, and Zn as dopants, resulting in notable electrocatalytic performance, particularly in the case of Co-doped CuO at GCE, which exhibited a low Tafel slope of approximately 70 mV dec−1 and a current density of 4.4 mA/g at 2.1 V (vs RHE). The addition of guest ions onto the CuO lattice was found to enhance the overall charge transportation mechanism of the modified electrode, as evidenced by a decrease in charge transfer resistance in electrochemical impedance spectroscopy (EIS) curves. Moreover, the Co-doped catalyst demonstrated good stability over 24 h of continuous electrolysis.
Another notable application of the hydrothermal method was reported by Xu et al., wherein a Ni-Mo alloy was deposited onto a nickel foam substrate in a multistage sheet/particle-type array structure at different temperatures [53]. This was achieved through a combination of hydrothermal methods and hydrogen reduction at varying temperatures, as shown in Fig. 4. For varying temperatures used for reduction in H2 environment, the color changes with increasing temperature assumed to be due to decrease in catalyst loading. Espescially at 750 °C, the color as the Ni foam surface blank resembles Ni foam itself. The resulting Ni-Mo@NF catalyst synthesized at 550 °C exhibited a highly hydrophilic-aerophobic surface, facilitating the deactivation of bubbles from the electrode surface and thereby improving catalytic efficiency. A remarkably low HER overpotential of ∼50 mV (at 10 mA/cm2 current density) and of ∼110 mV (at 40 mA/cm2 current density) was observed, along with favorable Tafel slope values. This comprehensive study highlights the versatility and efficacy of hydrothermal synthesis methods for fabricating advanced catalysts on nickel foam substrates, paving the way for enhanced performance in various electrochemical applications.
Fig. 4.
– a) Schematic showing fabrication of NiMoO4 catalyst, supported on NF- Nickel Foam, in a two-step process involving hydrothermal and H2 reduction, with inset showing the variation in appearance with changing temperature in H2 reduction Adapted and redrawn from Ref. [53].
Chemical bath deposition (CBD), often used along with hydrothermal and other chemical-based methods, is a widely-used technique for synthesizing thin films of metal oxides or sulfides on various substrates, making it an attractive method for binder-less electrode fabrication in electrochemical applications. In CBD, the substrate is immersed or dipped in a chemical bath containing precursor solutions, which react to deposit thin layers of the desired material onto the substrate surface. The deposition process is governed by chemical reactions occurring in the solution, leading to the formation of uniform and adherent electrode coatings. CBD offers several advantages for binder-less electrode fabrication, including simplicity, scalability, and versatility. Deposition parameters such as precursor concentration, bath temperature, and deposition time are crucial in controlling the composition, thickness, and morphology of the deposited films for optimizing the electrochemical performance. In a study by Lv et al. (2022), a multi-step process was employed to fabricate Copper Oxide (CuO) – Cu3P hybrid nanowires with a core-shell structure, involving chemical bath treatment followed by oxidation and phosphidization. The synthesis process of the said hybrid catalyst is depicted in Fig. 5 [54]. The resulting hybrid nanowires exhibited distinct functionalities, with hydrogen evolution occurring at the Cu3P active center and oxygen evolution at the CuO center. The core-shell structure also enhanced the hydrophilic properties of the nanowires, leading to low η10 overpotentials of approximately 145 mV and 265 mV for HER and OER, respectively. Additionally, the hybrid nanowires demonstrated remarkable electrochemical surface area (ECSA), low Tafel slopes, and excellent stability over 14 h in both independent catalytic tests and bifunctional electrolysis evaluations. First, the growth of Cu(OH)2 nanowires was initiated by dipping Cu foam in a bath of alkali-persulfate solution. Subsequent furnace oxidation converted nanowires to CuO, then Cu3P was formed by subjecting the catalyst to a NaH2PO2 environment. Cu3P, as like other metal phosphides, demonstrated good charge transfer, enhancing adsorption of eOH and hydrogen bonding. The CuO@Cu3P/CF surface showed superhydrophilicity, aiding H2 bubble separation and electrolyte rewetting. The rough, stacked nanosheet structure facilitates electrolyte penetration. OER activity stems from Cu (III) formed during cathodic oxidation of Cu (II).
Fig. 5.
– Schematic showing fabrication of CuO at Cu3P through three different steps Figures reproduced with permission from Ref. [54].
Chemical vapor deposition (CVD) and physical vapor deposition (PVD) stand as two highly sophisticated methodologies extensively employed in the fabrication of binder-free electrodes, particularly in the realm of hydrogen evolution reaction (HER) and oxygen evolution reaction (OER) catalysts. CVD, a process known for its precision, operates by initiating chemical reactions that facilitate both polymerization and coating phases simultaneously, resulting in the formation of copolymers comprising various monomers tailored for diverse applications. This intricate technique affords meticulous control over the thickness and composition of the resulting thin film, making it invaluable in industries requiring exacting specifications. In contrast, PVD harnesses physical reactions to transform liquid source materials into a gaseous state, which are then deposited as thin layers on substrate surfaces upon solidification. Utilizing techniques such as sputtering or evaporation, solid source materials undergo physical vaporization, with the resultant atoms or molecules being meticulously deposited onto the substrate. This process ensures not only exceptional adhesion to the substrate due to the energetic nature of the deposition but also guarantees uniform coating distribution and high material purity, prerequisites in industries demanding impeccable quality standards. Moreover, the absence of binder materials in these techniques eliminates potential interference with catalytic activity, thereby bolstering electrochemical performance and ensuring the long-term stability of the catalyst. The synergy between precise control, meticulous deposition, and material purity underscores the indispensability of CVD and PVD in cutting-edge technological advancements, where even the slightest deviation from perfection could have profound consequences [[55], [56], [57]].
Utilizing Nickel foam as a substrate, recent research has employed aerosol-assisted chemical vapor deposition (referred to as AA-CVD) to deposit a bimetallic Ruthenium-Cobalt alloy (Ru-Co), as shown in Fig. 6 (a) [58]. The resulting Ru-Co@NF electrocatalyst demonstrates outstanding performance in the hydrogen evolution reaction (HER) within a 1M KOH electrolyte, showcasing low overpotentials of 17 mV (η10) and 100 mV (η100), which are among the lowest reported values in the literature. Additionally, the catalyst exhibits a minimal Tafel slope (∼40 mV/dec) and maintains stability for over 24 h, surpassing the performance of the benchmark Pt/C catalyst. DFT-based analysis further validates these findings, revealing a lower Gibbs free energy value for the RuCo alloy compared to metallic Ru and Co, favoring the HER process. The AA-CVD process, utilizing a homogeneous mixture of Cobalt (II) and Ruthenium (II) acetylacetonate salt solutions (in a 1.25:1 mass ratio in methanol solvent), deposited onto the Nickel substrate with inert Nitrogen as the aerosol carrier, provides a fabrication strategy that is both simple and scalable. This approach eliminates the need for reducing agents and hydrothermal treatments, streamlining the production process. The three-dimensional porous network of NF facilitates charge transfer and electrolyte infiltration during HER, enhancing catalytic activity. In contrast, the bimetallic RuCo alloy deposited under AACVD conditions exhibits spherical particles that neatly decorate the NF skeleton. This phenomenon may arise from various factors, including synergistic effects, favorable hydrogen adsorption intermediates, charge redistribution, and electronic effects. The Ru sites in the alloy catalyst facilitate the adsorption of hydrogen intermediates (H*) during the HER process, while the Co sites promote the dissociation of water into OH* and H*. Concurrently, the Ru sites promote the adsorption of H* and subsequent combination to form H2, accelerating the overall HER process and enhancing catalytic performance. Moreover, the differing electronegativity of Ru and Co in the alloy structure induces charge redistribution and electronic effects, modifying the electronic structure and further enhancing catalytic activity, particularly at Ru sites. Additionally, the alloy structure exposes a variety of active surface sites, including uncoordinated atoms, step sites, and defects, which serve as highly active sites for the HER. These sites possess lower hydrogen adsorption energies, facilitating the HER process and contributing to high current densities at low overpotentials. In terms of acidic water electrolysis for hydrogen production, direct use of substrates such as Nickel foam (NF) is hindered by corrosion in the acidic environment. One approach to address this issue involves coating or alloying non-metallic elements such as Carbon. For instance, utilizing CVD with methanol and water as material precursors, researchers have coated Graphene Oxide (GO) onto NF to obtain an optimum electrocatalyst with anti-corrosive properties [59]. This approach demonstrates the applicability of CVD for depositing carbon-based materials on metallic substrates such as Nickel or Copper, providing promising avenues for advancing electrocatalysis research. This robust deposition method not only ensures precise control over the catalyst composition and morphology but also enables scalability for industrial applications, paving the way for efficient and sustainable energy conversion technologies.
Fig. 6.
– The use of aerosol-assisted Chemical Vapor Deposition (CVD) for a) deposition of VOx b) Ru-Co hybrid on Ni Foam Figures reproduced with permission from Refs. [58,60].
In another study by Ehsan et al., aerosol-assisted CVD has been applied to deposit nanoscale vanadium oxide (VOx) thin films onto porous nickel foam substrates for efficient Oxygen Evolution Reactions (OER) [60]. The process of deposition of VOx on Ni Foam, as depicted in Fig. 6 (b), involved varying deposition times to optimize catalytic performance. Specifically, the VOx sample having an optimized deposition time of half hour exhibited a remarkable OER performance and a high industrial-level current density of 1 A/cm2 was achieved at 1.7 V vs RHE. Also, a small and favorable Tafel slope of ∼70 mV/dec was observed with high conductivity, and long-term durability over 48 h of continuous exposure. Such a catalytic activity outperforms several conventional OER catalysts under similar conditions, highlighting the efficacy of the aerosol-assisted CVD method for practical or industrial applications. Moreover, the AACVD process offers the advantage of tuning surface morphology and thickness by adjusting deposition parameters, enhancing reproducibility and versatility compared to other synthetic approaches.
In a recent investigation by Babar et al., bimetallic nickel vanadium oxide (NiVOx) thin-film electrocatalysts were synthesized on 3D Nickel Foam substrates using aerosol-assisted chemical vapor deposition (AACVD) [61]. The study varied deposition durations (60–180 min), observing morphological shifts from micro-nano to nanoscale catalytic structures. Optimized deposition for 180 min yielded a catalyst with low OER onset overpotential and high current density of 1.2 A/cm2 at 1.65 V. Additionally, it exhibited a low Tafel slope of 60 mV/dec and maintained stability over 24 h at 0.5 A/cm2. This catalyst outperformed Ru-based counterparts under similar alkaline conditions, attributed to finely modulated local coordination and electronic structure of the Ni/V compound within the nanotextured catalyst. AACVD offers a straightforward route to efficient anodic oxygen evolution and chemical energy conversion catalysts.
Additionally, Ehsan & Khan also explored the impact of varying CVD deposition times on catalytic activity, focusing on NiMoO4 2D flower-like nanosheets [62]. Their investigation revealed that a deposition time of 1 h yielded superior results compared to 2 h, showcasing low overpotential, Tafel slope, and good OER stability. These findings underscore the importance of meticulous control over deposition parameters in tailoring catalyst properties and performance for diverse electrocatalytic applications. Such studies highlight the versatility and efficacy of AACVD as a versatile and scalable technique for synthesizing advanced catalyst materials with tailored properties, paving the way for advancements in energy conversion and storage technologies.
Sun et al. tackle the challenge of developing cost-effective and high-performance electrocatalysts for water splitting by introducing a high-throughput method for fabricating bi-metallic Ni–Mo alloy catalysts tailored for oxygen/hydrogen evolution reactions (OER/HER) [63]. Using a simple co-sputtering technique, they synthesize a range of Ni–Mo alloy catalysts with adjustable compositions. These catalysts benefit from the synergistic effects between Ni and Mo, resulting in enhanced intrinsic electrocatalytic activity for both HER and OER. Remarkably, the Ni90Mo10 catalyst exhibits outstanding HER performance with a low overpotential of approximately 60 mV, while the Ni40Mo60 catalyst shows an overpotential of about 300 mV. Moreover, the assembled Ni40Mo60//Ni90Mo10 electrolyzer demonstrates remarkable efficiency, achieving 10 mA/cm2 for overall water splitting at a cell voltage of only 1.57 V. This study highlights the potential of co-sputtering for producing tunable Ni–Mo alloy electrocatalysts, offering promising prospects for advancing electrochemical hydrogen production technologies and addressing the need for efficient and cost-effective catalysts in sustainable energy systems.
Electrodeposition emerges as a widely adopted technique for fabricating binder-less electrocatalysts tailored to facilitate Hydrogen Evolution Reaction (HER) and Oxygen Evolution Reaction (OER). This method entails the precise reduction of metal ions from an electrolytic bath onto a conductive substrate, affording meticulous control over the resulting thin film's thickness and composition. By obviating the need for binders, electrodeposition fosters direct contact between the catalyst and the electrode surface, thereby enhancing electron transfer kinetics and catalytic activity. Moreover, its scalability, versatility in catalyst material and composition, as well as its simplicity and cost-effectiveness, render it an ideal method for mass-producing catalyst electrodes for diverse applications in renewable energy conversion and storage systems. The selection of substrates such as Cu, Ni, Stainless Steel, or Carbon foams/foils further underscores the adaptability of this approach, facilitating the deposition of various catalyst materials to meet specific performance criteria and application requirements [[64], [65], [66], [67]].
Barati et al. present a notable study showcasing the fabrication of a ternary Ni-Fe-Co hybrid catalyst for both Hydrogen Evolution Reaction (HER) and Oxygen Evolution Reaction (OER) on copper foam via a straightforward electrodeposition approach [50]. The process involved treating copper foam with HCl before immersing it in a solution containing chloride salts of Ni, Co, and Fe in specific molar ratios, with Ni having the highest concentration. Utilizing Ni as the anode, a two-step electrodeposition technique was employed: an initial 10-min deposition at a current density of 20 mA/cm2 followed by a subsequent 1-min deposition at a higher current density of 50 mA/cm2, which was 2.5 times the original. This methodology facilitated the creation of a catalyst with a high active surface area, benefiting from the synergistic effects of the mixed transition metal (TM) elements and nanostructuring, which aided in the rapid removal of bubbles from the electrode surface, thus enhancing electrocatalytic activity. Also, highly favorable Tafel slopes were observed, both in the case of HER and OER, as shown in Fig. 3(f) and (g). Furthermore, the versatility of this catalyst was demonstrated through bifunctional performance validation in an alkaline electrolyzer setup, where a remarkably low voltage of 1.6 V sufficed to achieve a current density of 10 mA/cm2. The rapidity of the electrodeposition process not only underscores its efficiency but also positions it as an ideal method for scalable high-throughput production of binder-less electrodes.
Similarly, Zhou et al. showcased a rapid synthesis of metallic foams on copper substrates in under a minute using the electrodeposition technique [68]. This process, as depicted in Fig. 7 enabled the creation of unique three-dimensional channels conducive to rapid electrolyte diffusion and gas release, coupled with robust integrated structures facilitating enhanced conductivity and charge transport. The hydrogen bubbles generated during the electrodeposition process served as templates, facilitating the formation of the foam's surface in a manner akin to natural foaming processes. Such methodologies not only demonstrate the versatility and efficacy of electrodeposition in catalyst fabrication but also highlight its potential for advancing electrochemical applications. The Nickel-Cobalt (NiCo) and Nickel-Iron (NiFe) foam catalysts demonstrated remarkable performance with very low overpotentials for the Hydrogen Evolution Reaction (HER) and Oxygen Evolution Reaction (OER) in an alkaline 1 M KOH electrolyte. Subsequent utilization of NiCo as the anode and NiFe as the cathode in a lab-scale electrolyzer exhibited superior performance compared to a standard Pt/C//RuO2 electrolyzer, as evidenced by lower η10 and η100 overpotentials, alongside excellent stability over prolonged durations. Moreover, the Faradaic Efficiency (FE) for both Hydrogen (H2) and Oxygen (O2) production reached optimal levels, attaining 97 % and 91 % respectively.
Fig. 7.
– Schematic showing fast electrodeposition (<1 min) of Ni-Co-Fe electrocatalysts on Copper substrates using different salts Figures reproduced with permission from Ref. [68].
The exceptional HER capability of NiCo can be ascribed to its three-dimensional foam structure, which provides abundant open channels facilitating intermediate species adsorption, electrolyte diffusion, and efficient release of gaseous bubbles. Additionally, the synergistic effects of both nickel and cobalt contribute significantly to hydrogen production. Conversely, the highly porous nature of NiFe foam enhances oxygen evolution by promoting reactant adsorption, electrolyte diffusion, and gas release. Moreover, NiFe foam can readily produce highly active Ni/FeOOH species at a minimal overpotential, resulting in enhanced intrinsic OER activity. In the electrodeposition-based fabrication process, Cu foil substrates were subjected to 12 V for 50 s in a bath containing a mixture of either NiCl2⋅6H2O and CoSO4⋅7H2O (for Ni-Co foam) or NiCl2⋅6H2O and FeSO4⋅7H2O (for Ni-Fe foam). This method facilitates the controlled deposition of desired metal ions onto the conductive substrate, enabling the precise engineering of catalyst morphology and composition, thus optimizing its electrochemical performance. Overall, these findings underscore the potential of electrodeposition as a versatile and efficient technique for fabricating high-performance catalysts for various electrochemical applications.
Wei et al. conducted a study wherein they utilized a bubble-template electrodeposition technique, followed by phosphidization, to synthesize Ni-Cu Phosphide nano-foam as a versatile water splitting electrocatalyst [69]. This catalyst, characterized by a hierarchical pore structure and a substantial electrochemical active surface area (ECSA), exhibited remarkably low HER & OER overpotentials (at 50 mA/cm2 current densities), along with low Tafel slope values. The NiCuP catalyst exhibited exceptional water splitting performance, achieving nearly 100 % Faradaic efficiency as both the anode and cathode. It outperformed commercial Pt/C and IrO2 catalyst pairs. The catalyst's success can be attributed to factors such as the conductivity of NiCu substrates, a large electrochemically active surface area with varied pore sizes, and the presence of active Ni/Cu oxides/hydroxides and Ni/Cu phosphides in the nano-foam structure. This emphasizes the significance of optimizing catalyst morphology and composition for improved electrocatalytic performance, positioning Ni-Cu Phosphide nano-foam as a promising option for efficient water splitting in renewable energy applications. Li et al. reported the successful growth of grass-like Ni/Cu nanosheet arrays on copper foam using a straightforward galvanostatic electrodeposition technique [70]. These nanosheet arrays exhibited exceptional electrocatalytic performance for the hydrogen evolution reaction (HER). In a 1 M KOH solution, they demonstrated a remarkably low overpotential of ∼40 mV to achieve a current density of −10 mA/cm2. The catalyst demonstrated prolonged durability, retaining its performance over 50 h of continuous catalysis at 30 mA/cm2 without a notable increase in overpotential. Its exceptional electrocatalytic activity is credited to its unique grass-like structure, which enhances exposure of active sites, facilitates electrolyte penetration, and promotes gas diffusion. Unlike other complex catalysts, the Ni/Cu binary catalyst is simple to synthesize, cost-effective, and highly efficient for HER and hence offers promising prospects for practical water splitting.
Apart from the methods mentioned in Table 1, few other binder-less fabrication methods, have also been used for catalytic electrode fabrication. Some of them have achieved adequate attention while few still require additional research. One of them is electroless plating or electroless deposition, a novel approach for fabricating efficient catalytic electrodes by depositing conductive catalysts onto readily available insulating substrates such as paper, textiles, and sponge, without the need of applying electric field (as in its counterpart ‘electroplating’). For this, a chemical bath is prepared containing the salt of the desired metal which is to be plated, such as NiCl2 for plating Nickel. Simultaneously, the bath also contains a complexing agent to stabilize the metal ions and a reducing agent to facilitate the reduction of metallic ions to their metallic (zero oxidation) state. The process is autocatalytic and doesn't require any highly specific conditions such as high temperature or vacuum. Most importantly, it can also act on non-conductive substrates such as paper, fabrics, non-metallic elements (unlike in electrodeposition where there is the need of a conductive substrate) and hence has been widely used in various electrochemical applications including making electrodes for water splitting and energy storage [71,72]. Such a method also results in desired morphologies such as nanowires as depicted in Fig. 8 (a). These electrodes exhibit remarkable activity for overall water splitting. For instance, the Ni–P–B/paper electrode showcases outstanding electrocatalytic performance with highly favorable overpotentials both for hydrogen evolution (80 mV at 50 mA/cm2) and oxygen evolution reactions (260 mV at 50 mA/cm2) [73]. Even when operated at very high current density of 1 A/cm2 it remains stable for over 240 h in KOH (1 M) solution. Also, as shown in Fig. 8 (b), the superior bifunctional ability is also validated by low operating voltage (1.6 V for 50 mA/cm2) required in a two-electrode electrolyzer setup, quite comparable to Pt–C/Ni and IrO2/Ni electrodes. Additionally, a functional Ni–P–B/paper ring electrode with in situ separation capability allows simultaneous generation, separation, and collection of hydrogen and oxygen. The insulating substrate undergoes activation by alternate immersion in NiSO4 and NaBH4 solutions, followed by electroless plating of nickel boride nanoparticles. Submerging pre-treated filter paper in an EP bath creates the Ni–P–B/paper electrode, enabling scalability and flexibility. The paper electrode's insulating substrate boasts a significantly larger BET surface area than Ni foil, enhancing catalytic activity. It exhibits stable performance over 5000 cycles and 240 h at high current densities in 1 M KOH, surpassing IrO2/paper and IrO2/Ni foil electrodes in OER with lower overpotentials and a Tafel slope of ∼70 mV/dec, highlighting its stability and efficiency for water electrolysis. As depicted in Fig. 8 (c), the comparison of the said Ni-P-B/paper catalyst with other plausible water splitting catalysts shows that it stands superior in terms of HER/OER overpotential as well as electrode mass. Hence, this preparation process, depicted in detail in Fig. 8 (d), helps fabricate practical catalytic electrodes that are efficient, cost-effective, lightweight, flexible, derived from earth-abundant materials, and recyclable. In another recent work, an electroless deposition of binder-free NiP on a flexible cotton-polyester fabric is demonstrated for not only OER and HER functionalities, but additionally for Urea Oxidation Reaction (UOR) also. Along with low overpotentials, industrial-scale applicability was shown using an electrolyzer based on NiP-fabric electrodes that reported hydrogen generation using water and urea electrolysis with cell voltage of 1.883 V and 1.611 V, respectively to generate the current density of 100 mA/cm2. For this work, NiNO3 and NaH2PO2 were used as the metal precursor and chemical reducing agent, respectively, for the deposition of NiP on cotton fabric having porous three-dimensional structure along with hydroxyl & epoxy groups for ensuring good deposition [74].
Fig. 8.
– a) Photograph of Ni-P-B paper electrode with FESEM image as inset b) Overall two-electrode water splitting of paper electrodes compared via LSV c) Different paper electrodes compared on the basis of their HER/OER overpotential (shown in Y and X axis) and weight (shown by radius of the marker circle) Figures reproduced with permission from Ref. [73]. d) Re-drawn schematic showing fabrication of Ni–P–B/paper electrode using electroless plating. Adapted and redrawn from Ref. [73]. e) Schematic showing fabrication of high-surface area and high-TOF NiO film using Atomic Layer Deposition f) OER performance comparison for differently treated NiO (Fe - trace, Fe - satd, or Fe -two steps) g) OER performance comparison for differently soaked NiO Figures reproduced with permission from Ref. [75].
Atomic Layer Deposition (ALD) offers precise control over film thickness and uniformity, making it ideal for fabricating binderless electrodes. By depositing thin layers of materials one atomic layer at a time, ALD enables the creation of nanostructured electrodes with increased surface area and tailored properties. This method allows for the incorporation of nanostructuring into water splitting devices, enhancing light absorption, charge transport, and stability. Conditioning ALD catalysts is crucial to achieve desired surface area and activity. Optimization of variables in the process renders ALD a versatile method applicable to various material systems. Ongoing advancements in ALD technology hold promise for further improving the efficiency and efficacy of nanostructured water splitting devices, fostering their widespread adoption in diverse applications.
In a different approach, atomic layer deposition (ALD) is employed to grow NiO thin films as electrocatalysts for the OER, with enhanced activity achieved by incorporating Fe from the electrolyte [75]. Although direct deposition of ternary oxides like Ni–Fe can be challenging via ALD, the technique offers potential for improving catalyst performance through precise control over film composition and thickness. This strategy underscores the versatility of ALD in tailoring catalyst properties for efficient electrochemical applications. NiO deposition via atomic layer deposition (ALD) is followed by in-situ Fe addition through electrolyte, as depicted in Fig. 8 (e). The electrocatalytic activity of the films for OER is examined in two different variants of 0.1 M KOH electrolytes, one which has Fe in trace amounts and other has saturated Fe content. With Fe conditioning, it was seen that Fe-saturated electrolyte boosts geometric activity but reduces electrochemically accessible redox sites. This was first accomplished by preconditioning a thin, high surface area 3-nm ALD NiO catalyst in 0.1 M KOH with trace Fe. Subsequent immersion in Fe(NO3)3-saturated solution enhanced OER activity, as depicted in Fig. 8 (f). Also, NiO/two-step sample showing superior performance, reflecting Fe's beneficial role. Despite lower TOF, NiO/two-step sample displayed higher geometric activity than solely Fe-conditioned sample, attributed to increased surface area. Conversely, NiO/Fe-saturated sample performed similarly to NiO/Fe-saturated in 0.1 M KOH, indicating Fe concentration's insignificance post-incorporation. This confirms genuine catalyst transformation due to observed activity increase. The application of potential during cycling proves advantageous for enhancing the activity of ALD NiO treated with Fe(NO3)3 electrolyte. Comparing NiO films soaked in 0.1 M KOH or 0.1 M KOH with Fe(NO3)3 for 24 h, subsequent cyclic voltammetry (CV) shows similar activities to NiO/Fe trace samples, as shown in Fig. 8 (g). Cycling enhances activity due to increased hydration, but geometric activity remains lower than NiO/Fe satd. Optimization of pre-conditioning parameters may improve thin film activity and catalyst customization. Control of Fe concentration and exposure duration is crucial for NiO surface restructuring.
Another method for fabricating binder-free electrodes involves the technique of electrospinning, exemplified by the incorporation of Co nanoparticles onto porous carbon nanofibers for applications such as the oxygen evolution reaction (OER) and oxygen reduction reaction (ORR), often with intrinsic dopants like nitrogen or fluorine [76]. While electrospinning is extensively utilized in the production of electrodes for batteries, supercapacitors, and energy conversion systems, its application in HER/OER contexts remains relatively limited [[77], [78], [79], [80]]. In one of the works by Favors et al. a scalable binder-less electrode fabrication of carbon-coated Si nanofibers is depicted for the use of Lithium-ion batteries. The electrospinning and subsequent reduction process, as depicted in Fig. 9(a), were useful in fabrication of Si-based nanofibrous papers, followed by its etched and carbon-coated variants, see Fig. 9(b)–(d). As a result of self-standing binder-less electrode, a great enhancement in capacity and coulombic efficiency is seen, when used with Li-ion batteries. Also, the increase in % weight of the actual active material was found crucial. However, the appropriate use of electrospinning for binder-less fabrication in the case of water splitting is still to be explored in depth. Similarly, electrophoretic deposition (EPD) is also an effective and convenient method to directly deposit catalyst materials onto a conductive substrate, without the use of binders. It involves applying direct current to a suspension of charged particles. These particles are typically polymers with ionizable groups and the charge on the particles is determined by the specific ionizable group present. The charged particles suspended in the liquid are driven towards an electrode with opposite charge under an applied electric field. Hence, factors such as operating voltage, medium viscosity, coating time and temperature etc. can affect the process. A few works have demonstrated the successful use of EPD for fabricating binder-free electrodes for electrocatalytic water splitting. A recent work by Pataniya & Sumesh reports the deposition of nanosheets of MoS2, exfoliated in the liquid phase, onto conductive Cu foil (in a relatively larger area, 1 cm × 2 cm) by using EPD [81]. The coating being photosensitive, the electrode shows a ∼36 % reduction in HER overpotential upon shining light (i.e. from −110 mV in dark to −70 mV under illumination), due to photogenerated electrons. The charge transfer resistance also drops to half (4.2 Ω cm2 to 2.1 Ω cm2) in the presence of light and the electrode shows a stability up to 40 h. Another similar work by the same group used EPD for making photosensitive binder-free electrodes of 2D-MoSe2–MoOx [82]. Along with an appreciable overpotential and low charge-transfer resistance, the photoelectrode is seen to have optimized light-harvesting abilities as seen from the photoresponsivity. The overall performance was found to be better than bulk as well as nanostructured MoSe2, highlighting the novelty both with the material as well as the EPD process. Another noteworthy and highly-practicable fabrication method which has gained good attention in the recent past but still required more exploration is laser-based fabrication. Laser ablation involves the use of high-energy laser pulses to remove material from a target, generating nanoparticles or nanostructures with tailored properties. This method can be employed to create nanostructured surfaces on electrodes, increasing their active surface area and enhancing catalytic activity. Laser ablation allows for the precise control of nanostructure size, shape, and distribution, leading to optimized electrode performance. Pulsed Laser Deposition (PLD) is a versatile technique that utilizes laser pulses to ablate material from a target and deposit it onto a substrate, forming a thin film. In the context of binder-less electrode fabrication, PLD can be used to directly deposit catalyst materials onto conductive substrates without the need for binders or additives. This results in electrodes with improved electrical conductivity and reduced interfacial resistance, leading to enhanced charge transfer and catalytic efficiency. One of the recent works by Naik et al. reports the synthesis of layered double hydroxide (LDH nanosheets of Co-Fe hybrid using pulsed laser ablation in aqueous medium, and their subsequent electrocatalytic performance analysis. The optimized Co0.5Fe0.5 – LDH catalyst demonstrated ultralow η10 overpotentials (HER ∼ 270 mV, OER ∼ 365 mV) with a bifunctional cell voltage ∼ 1.9 V (for 10 mA/cm2). The LSV curves for HER performance analysis and Tafel slopes of the said catalysts are depicted in Fig. 9(e) and (f) respectively. In another work, strawberry-shaped clusters of Cobalt Oxide (Co3O4) was fabricated using laser on using Ag as a substrate. The said catalyst demonstrated remarkably low overpotentials for both HER (η10 ∼ 50 mV) & OER (η10 ∼ 200 mV), with a bifunctional electrode potential of ∼1.5 V only. Such a performance that even outnumbers Pt/C + RuO2 can be attributed to dual functionality of Ag NPs and Co3O4 clusters. Ag NPs prepared during laser ablation possesses good H2 absorption capability. Similarly, the O2 vacancies developed during the laser processing are also able to absorb hydroxyl and O2 intermediated appropriately. Moreover, the role of addition of Cobalt Oxide clusters is validated from the fact that Co3O4 enhances water dissociation, augments hydrogen adsorption on Ag via strain induction and low coordination, and improves OER performance through electron transfer to Ag. The similar use of laser ablation for electrode fabrication has been reported in some other cases also [[83], [84], [85]]. However, it is mostly Pulsed Laser Ablation in Liquid (PLAL) that is used for nanostructure generation. Use of pulsed laser in ambient air, as well as the study of laser processing parameters is lacking. In contrast, the use of laser for nanostructure generation for making self-supported, binder-free electrodes for batteries, supercapacitors & energy storage has been widely studied and proved to be effective & hence taking clue from such works is necessary. The works by our group also outline the conception and use of a laser-based technique named Ultra-Short Laser Pulse for In-situ Nanostructure Generation (ULPING) for fabricating nanostructured NiO, TiO2 and CuO electrodes for use as high capacity and high-performance binder-free electrodes in supercapacitors and pseduocapacitor [[86], [87], [88]]. A schematic in Fig. 9(g) shows the laser equipment for generation of laser pulses, operated in ambient air. The manner in which the laser pulses help create high surface area metal oxide nanostructures (NiO/CuO, in this case) is depicted in Fig. 9(f). Such a technique that allows rapid, high-throughput electrode fabrication along with accurate control over nanostructure size and morphology is dependent on the right use of parameters such as laser power intensity, frequency, scan speed or pulse duration. Hence, the use of advancing technologies such as AI/ML proves to be an effective option for reaching an optimization of parameters that gives an enhanced electrochemical activity [[89], [90], [91]]. Looking at the several advantages that laser-based fabrication offers, it can be an effective way for creating cost-effective industrial-scale electrodes for practical water electrolysis with a good electrocatalytic performance. Table 2 outlines and compares different electrocatalysts made through binder-less fabrication, some of which were not covered in this section but stand as appropriate examples. Apart from the comparative numerical data, the graphical plots comparing the HER/OER performance can be found in Fig. 6, Fig. 8 or 9 (a few) or by referring to the original works.
Fig. 9.
a) Schematic showing the binder-less electrode fabrication using electrospinning. Digital images of b) as-spun SiO2 NF, c) etched SiNF paper, d) C-coated SiNF Figures reproduced with permission from Ref. [92]. e) Linear sweep voltammograms comparing HER performance of Co-Fe-LDH f) Tafel slopes of the said catalyst Figures reproduced with permission from Ref. [93] g) Schematic showing the laser equipment build that is used for pulse generation h) Nanostructured oxide formation using laser irradiation Figures reproduced with permission from Ref. [88].
Table 2.
– Few water splitting electrocatalysts prepared using binder-less fabrication techniques.
| Material | Binder-less Fabrication Method | Electrolyte | Overpotential (unit - mV, at 10 mA/cm2 or unless mentioned otherwise) | Tafel Slope (unit – mV/dec) | Reference |
|---|---|---|---|---|---|
| CuO-Cu2O Nanowires//Cu Foam | Chemical oxidation of a Cu foam with NaOH and (NH4)2S2O, followed by annealing | 1 M KOH | HER -135 OER – 315 |
HER - 135 OER – 63 |
[47] |
| Ni3Se3//Cu Foam | Electrodepostion from a bath of Ni(OCOCH3)2. 4H2O, SeO2, LiCl | 1 M KOH | HER – 100 OER – 340 (at 50 mA/cm2) |
HER – 98 OER – 60 |
[48] |
| NiCoP@Cu3P//Cu Foam | Direct oxidation of Cu Foam | 1 M KOH | HER – 54 OER – 309 |
HER – 72 OER - 42 |
[49] |
| Ni-Fe-Co nanocones | Electrodeposition in a bath mixture of Ni, Fe and Co chlorides at a varying current density in 2-step process | 1 M KOH | HER – 91 OER – 316 |
HER -86 OER - 43 |
[50] |
| NiOx - Fe | Atomic Layer Deposition, followed by electrodeposition | 1 M KOH | OER: −480 mV (Saturated Fe electrolyte) −500 mV (Trace Fe electrolyte) |
OER – 30 | [75] |
| NiCo and NiFe on Cu Foams | Electrodeposition, in a bath of Ni, Co, Fe salts | 1 M KOH | NiCo (HER) −86 mV NiFe (OER) −206 mV |
HER - 62.1 mv/dec 43.5 mv/dec |
[68] |
| RuCo Alloy on Ni Foam | Chemical Vapor Deposition (Aerosol-Assisted) | 1 M KOH | HER – 17 mV (for 10 mA/cm2) 100 mV (for 100 mA/cm2) |
HER – 40 mV/dec | [58] |
| Graphene Oxide @ Ni Foam | Chemical Vapor Deposition | 1 M H2SO4 | HER – 83.2 mV | HER – 55.7 mV/dec | [59] |
| VOx-30 @ Ni Foam | Chemical Vapor Deposition (Aerosol-Assisted) | 1 M KOH | OER – 290 mV (for 10 mA/cm2) −470 (for 1000 mA/cm2) |
OER – 68 mV/dec | [60] |
| Co-Fe-LDH Nanosheets (Co0.5Fe0.5 – LDH optimized) | Pulsed Laser Ablation (aqueous medium) | 1 M KOH | OER – 270 mV | OER – 115.7 mV | [93] |
| Various Co-based electrocatalysts | Pulses Laser Ablation in Liquid | 1 M KOH | OER - 230 mV (for Co3(PO4)2) HER – 361 mV (for Co9S8) |
OER – 48.5 mV (HER - 95.8 mV | [85] |
One of the other important things to consider is the electrochemical parameters used for benchmarking the electrocatalysts at the lab scale, espescially the operating current density used for evaluating the overpotential and the stability. Conventionally, the catalytic performance of HER/OER catalysts, espescially the overpotential, has been measured at 10 mA/cm2, which is only suitable for lab-scale evaluation. The industrial-level electrolyzers need to run at very high current densities, around 1 A/cm2 or more, to ensure the production of H2 and O2 gases at an appreciable volume. Hence, there has been growing attention in designing and evaluating binder-less electrodes for catalytic performance at high current densities. Along with advancements in materials chemistry, eliminating the use of binders is also necessary for ensuring the efficacy of electrodes at high current densities. As an example, one of the recent works by Trivedi et al. demonstrated the use of one-step hydrothermal synthesis for the growth of Cr-Cu2S nanostructures for HER/OER in different electrolytes including alkaline and sea water (KOH or H2SO4 along with NaCl) at high current densities [94]. Using an optimized chemical composition of Cr-Cu2S (8 mM of Cr precursor in synthesis mix), an overpotential of ∼400 mV and ∼350 mV was observed for HER and OER respectively, measured at 0.1 A/cm2 current density in alkaline electrolyte. Encouragingly, oxygen generation at current density 0.5 A/cm2 was also achieved at overpotential 480 mV for 8 mM Cr-Cu2S@CF electrode, showing the capability for industrial scale oxygen generation. In addition to increasing HER activity because of increased electron density at Cu-sites, alloying Cr in Cu2S networks improves oxygen desorption at the anode by lowering the energy of adsorption of *OH intermediates. The alkaline electrolyzer, using Cr-Cu2S@CF electrodes as both anode and cathode, achieves a high current density of 0.6 A/cm2 at 30 °C, and in a two-electrode configuration, reaches 0.8 A/cm2 at 2.25 V and 80 °C, demonstrating efficient mass and charge transport for self-supported Cr-Cu2S@CF electro-catalysts. In a simulated sea water electrolyte also (0.5 M H2SO4 + NaCl), the optimized electrode demonstrated a stable HER for 10+ hours at a current density of 50 mA/cm2. The electrochemical activity is found to be much superior than other similar works based on Cr [95] and is novel in terms of industrial-scale applicability and this can be attributed to the optimized composition as well as the elimination of the use of binders. Some other works also report the use of electroplating and hydrothermal synthesis for binder-less fabrication of other metallic electrodes (based out of Fe, Ni, Mo etc) that can perform well at high current densities [[96], [97], [98]]. However, such studies are still scarce and more efforts in this direction is expected to bridging the gap between lab research and industrial applicability.
4. Conclusion and future scope
In the pursuit of sustainable energy solutions, green hydrogen has emerged as a promising avenue for decarbonizing various sectors and mitigating the impacts of climate change. Electrochemical water splitting, driven by renewable energy sources, offers a viable pathway for producing green hydrogen at scale. However, the efficiency and scalability of water electrolysis are contingent upon the development of robust electrocatalysts and electrode materials. In this review, we have explored the fabrication of binder-less metal electrodes for electrochemical water splitting, highlighting the drawbacks of binder-based electrode fabrication and the potential of binder-less approaches to address these challenges. The use of binders in electrode fabrication poses significant limitations, including decreased electrochemical activity, hindered mass transport, and potential contamination of electrolytes. These drawbacks underscore the need for alternative fabrication methods that eliminate the reliance on binders. Binder-less electrode fabrication techniques offer several advantages, including enhanced catalytic activity, improved electron transfer kinetics, and reduced manufacturing costs. Through methods such as hydrothermal synthesis, chemical bath deposition, electrodeposition, chemical vapor deposition, and laser fabrication, researchers have demonstrated the feasibility of fabricating binder-less electrodes with superior performance and stability. Table 1 gives an apt comparison of various binder-less fabrication methods and Table 2 outlines various electrocatalyst reports fabricated by binder-free method.
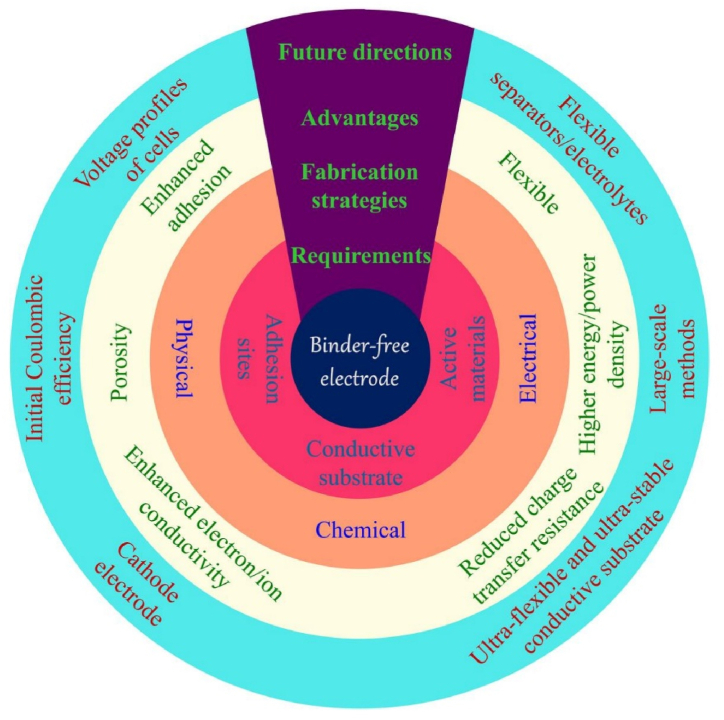
Moving forward, the future scope of binderless electrode fabrication for electrochemical water splitting is promising and multifaceted, as depicted in Fig. 10. One key area of focus is the exploration of novel catalyst materials and synthesis strategies to further enhance the catalytic activity and selectivity of binder-less electrodes. By leveraging advanced characterization techniques and computational modeling, researchers can gain deeper insights into the structure-function relationships of binder-less catalysts and tailor their properties for specific applications.
Fig. 10.
– Schematic summarizing various aspects of binder-less electrode fabrication such as advantages, applications and future directions Figures reproduced with permission [18].
Moreover, the scalability and commercial viability of binderless electrode fabrication methods are critical considerations for widespread adoption in industrial-scale water electrolysis systems. Efforts to optimize fabrication processes, scale up production, and reduce material costs will be essential for realizing the full potential of binder-less electrodes in large-scale hydrogen production facilities. In addition to material and process optimization, the integration of binder-less electrodes into electrolyzer designs and system architectures will play a pivotal role in advancing electrochemical water splitting technologies. By optimizing electrode configurations, reactor geometries, and operating conditions, researchers can maximize the efficiency, durability, and overall performance of binder-less electrode systems. Furthermore, the synergy between binder-less electrode fabrication and emerging technologies such as photoelectrochemical water splitting and electrolysis coupled with renewable energy sources holds immense promise for achieving sustainable and carbon-neutral hydrogen production. By combining electrochemical and photovoltaic processes, researchers can harness the complementary strengths of both approaches to overcome the limitations of individual systems and maximize overall energy conversion efficiency.
In conclusion, the fabrication of binder-less metal electrodes represents a transformative approach to electrochemical water splitting, offering a path towards efficient, scalable, and sustainable hydrogen production. By addressing the drawbacks of binder-based electrode fabrication and harnessing the potential of binder-less approaches, researchers can accelerate the transition towards a clean energy future powered by green hydrogen. Through continued innovation, collaboration, and investment, binder-less electrode fabrication has the potential to revolutionize the field of electrocatalysis and drive the widespread adoption of green hydrogen as a clean and renewable energy source.
5. What's next?
Splitting of water using electricity is one of the most sustainable and desirable methods for producing green Hydrogen as an energy carrier. To minimize the energy required to split a molecule of water, the need of efficient and cost-effective catalysts is paramount. Not just this, the method in which catalyst materials are bonded onto the electrode surface decides its performance in the long run. By eliminating the use of binders which are non-conductive in nature, and by making binder-less or self-supported electrodes, the flow of electrons is made easier and hence the electrocatalytic ability is found to be much better. The fabrication of binder-less electrodes is made possible by using various methods such as hydrothermal method, electrodeposition, chemical/physical vapor deposition, chemical bath deposition, laser fabrication etc. Each of the method has its pros and cons and hence based on the kind of material, various binder-free fabrication techniques can be employed. Various parameters such as morphology control, speed of fabrication, operation conditions (temperature & pressure), practicality & scalability etc. Also help determine which fabrication technique to be used. Also, it is most important that by controlling the fabrication parameters, the nanostructure morphology and electrochemical performance can also be accurately determined. Hence, keeping the needs of the developing industrial-level electrolyzers in mind, fast, cost-effective and highly controllable fabrication techniques such as electrodeposition, chemical vapor deposition and laser fabrication seem to emerge as the “next” common choices of the times ahead. Out of these, laser-based fabrication, though used for making battery and supercapacitor electrodes, still requires a lot of attention in the case of water splitting. By optimizing various factors such power of laser, frequency or pulse duration, laser ablation in air can be utilized as effective method for making catalytic nanostructures with desired electrochemical parameters such as overpotential, Tafel slope, stability etc. Moreover, with increasing employment of such techniques, the use of Artificial Intelligence or Machine Learning for establishing correlations and predicting optimum parameters for electrode fabrication is very much essential. Transitioning beyond laboratory-scale investigations, there should be a growing focus on deploying binder-less electrodes in pilot-scale or industrial-level configurations to know about their real-time dynamics, operational challenges across multiple levels, and the necessary adaptations for sustained long-term applications.
Funding sources
This work was supported by the NSERC and MITACS.
Data availability statement
The data that support the findings of this study are included within the article and are referenced within the article.
CRediT authorship contribution statement
Sandra Susan Koshy: Writing – review & editing, Writing – original draft, Methodology, Investigation. Jyotisman Rath: Writing – review & editing, Writing – original draft, Methodology, Investigation, Formal analysis. Amirkianoosh Kiani: Writing – review & editing, Writing – original draft, Validation, Supervision, Resources, Project administration, Investigation, Funding acquisition, Conceptualization.
Declaration of competing interest
The authors declare no competing interest, financial or otherwise.
Acknowledgment
The authors thanks and acknowledge the partial support provided by NSERC.
References
- 1.Hassan Q., Algburi S., Sameen A.Z., Salman H.M., Jaszczur M. Green hydrogen: a pathway to a sustainable energy future. Int. J. Hydrogen Energy. 2024;50:310–333. doi: 10.1016/j.ijhydene.2023.08.321. [DOI] [Google Scholar]
- 2.Squadrito G., Maggio G., Nicita A. The green hydrogen revolution. Renew. Energy. 2023;216 doi: 10.1016/j.renene.2023.119041. [DOI] [Google Scholar]
- 3.Yang S., Yang D., Shi W., Deng C., Chen C., Feng S. Global evaluation of carbon neutrality and peak carbon dioxide emissions: current challenges and future outlook. Environ. Sci. Pollut. Control Ser. 2022 doi: 10.1007/s11356-022-19764-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shiva Kumar S., Lim H. An overview of water electrolysis technologies for green hydrogen production. Energy Rep. 2022;8:13793–13813. doi: 10.1016/j.egyr.2022.10.127. [DOI] [Google Scholar]
- 5.Oliveira A.M., Beswick R.R., Yan Y. A green hydrogen economy for a renewable energy society. Curr Opin Chem Eng. 2021;33 doi: 10.1016/j.coche.2021.100701. [DOI] [Google Scholar]
- 6.Zainal B.S., Ker P.J., Mohamed H., Ong H.C., Fattah I.M.R., Rahman S.M.A., Nghiem L.D., Mahlia T.M.I. Recent advancement and assessment of green hydrogen production technologies. Renew. Sustain. Energy Rev. 2024;189 doi: 10.1016/j.rser.2023.113941. [DOI] [Google Scholar]
- 7.Yu M., Wang K., Vredenburg H. Insights into low-carbon hydrogen production methods: green, blue and aqua hydrogen. Int. J. Hydrogen Energy. 2021;46:21261–21273. doi: 10.1016/j.ijhydene.2021.04.016. [DOI] [Google Scholar]
- 8.Eftekhari A. Electrocatalysts for hydrogen evolution reaction. Int. J. Hydrogen Energy. 2017;42:11053–11077. doi: 10.1016/j.ijhydene.2017.02.125. [DOI] [Google Scholar]
- 9.Anwar S., Khan F., Zhang Y., Djire A. Recent development in electrocatalysts for hydrogen production through water electrolysis. Int. J. Hydrogen Energy. 2021;46:32284–32317. doi: 10.1016/j.ijhydene.2021.06.191. [DOI] [Google Scholar]
- 10.Rath J., Ramasubramanian B., Ramakrishna S., Chellappan V. Advancements in metalloid anodes (Si/Ge/B) for air batteries. Memories - Materials, Devices, Circuits and Systems. 2024;7 doi: 10.1016/j.memori.2023.100097. [DOI] [Google Scholar]
- 11.Yuan S., Duan X., Liu J., Ye Y., Lv F., Liu T., Wang Q., Zhang X. Recent progress on transition metal oxides as advanced materials for energy conversion and storage. Energy Storage Mater. 2021;42:317–369. doi: 10.1016/j.ensm.2021.07.007. [DOI] [Google Scholar]
- 12.Khot M., Kiani A. A review on the advances in electrochemical capacitive charge storage in transition metal oxide electrodes for pseudocapacitors. Int. J. Energy Res. 2022;46:21757–21796. doi: 10.1002/er.8763. [DOI] [Google Scholar]
- 13.Ali A., Long F., Shen P.K. Innovative strategies for overall water splitting using nanostructured transition metal electrocatalysts. Electrochem. Energy Rev. 2022;5:1. doi: 10.1007/s41918-022-00136-8. [DOI] [Google Scholar]
- 14.Muzammil A., Haider R., Wei W., Wan Y., Ishaq M., Zahid M., Yaseen W., Yuan X. Emerging transition metal and carbon nanomaterial hybrids as electrocatalysts for water splitting: a brief review. Mater. Horiz. 2023;10:2764–2799. doi: 10.1039/D3MH00335C. [DOI] [PubMed] [Google Scholar]
- 15.Li S., Li E., An X., Hao X., Jiang Z., Guan G. Transition metal-based catalysts for electrochemical water splitting at high current density: current status and perspectives. Nanoscale. 2021;13:12788–12817. doi: 10.1039/D1NR02592A. [DOI] [PubMed] [Google Scholar]
- 16.Mushtaq M., Sathyakam P.U., Vijayaraghavan R. Performance, comprehension and applications of hematite-based photoanodes in PEC water splitting. Materials. 2024;3 doi: 10.1016/j.nxmate.2024.100159. [DOI] [Google Scholar]
- 17.Reynolds C.D., Slater P.R., Hare S.D., Simmons M.J.H., Kendrick E. A review of metrology in lithium-ion electrode coating processes. Mater. Des. 2021;209 doi: 10.1016/j.matdes.2021.109971. [DOI] [Google Scholar]
- 18.Kang Y., Deng C., Chen Y., Liu X., Liang Z., Li T., Hu Q., Zhao Y. Binder-free electrodes and their application for Li-ion batteries. Nanoscale Res. Lett. 2020;15:112. doi: 10.1186/s11671-020-03325-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shen K., Zhai S., Wang S., Ru Q., Hou X., San Hui K., Nam Hui K., Chen F. Recent progress in binder‐free electrodes synthesis for electrochemical energy storage application. Batter Supercaps. 2021;4:860–880. doi: 10.1002/batt.202000271. [DOI] [Google Scholar]
- 20.How Y.Y., Numan A., Mustafa M.N., Walvekar R., Khalid M., Mubarak N.M. A review on the binder-free electrode fabrication for electrochemical energy storage devices. J. Energy Storage. 2022;51 doi: 10.1016/j.est.2022.104324. [DOI] [Google Scholar]
- 21.Gerard O., Numan A., Krishnan S., Khalid M., Subramaniam R., Kasi R. A review on the recent advances in binder-free electrodes for electrochemical energy storage application. J. Energy Storage. 2022;50 doi: 10.1016/j.est.2022.104283. [DOI] [Google Scholar]
- 22.Chatenet M., Pollet B.G., Dekel D.R., Dionigi F., Deseure J., Millet P., Braatz R.D., Bazant M.Z., Eikerling M., Staffell I., Balcombe P., Shao-Horn Y., Schäfer H. Water electrolysis: from textbook knowledge to the latest scientific strategies and industrial developments. Chem. Soc. Rev. 2022;51:4583–4762. doi: 10.1039/D0CS01079K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hisatomi T., Domen K. Overall water splitting: what's next? Next Energy. 2023;1 doi: 10.1016/j.nxener.2023.100006. [DOI] [Google Scholar]
- 24.Chen X., Yang J., Cao Y., Kong L., Huang J. Design principles for tungsten oxide electrocatalysts for water splitting. Chemelectrochem. 2021;8:4427–4440. doi: 10.1002/celc.202101094. [DOI] [Google Scholar]
- 25.Sun H., Yan Z., Liu F., Xu W., Cheng F., Chen J. Self‐supported transition‐metal‐based electrocatalysts for hydrogen and oxygen evolution. Adv. Mater. 2020;32 doi: 10.1002/adma.201806326. [DOI] [PubMed] [Google Scholar]
- 26.Cho M.K., Park H.-Y., Lee H.J., Kim H.-J., Lim A., Henkensmeier D., Yoo S.J., Kim J.Y., Lee S.Y., Park H.S., Jang J.H. Alkaline anion exchange membrane water electrolysis: effects of electrolyte feed method and electrode binder content. J. Power Sources. 2018;382:22–29. doi: 10.1016/j.jpowsour.2018.02.025. [DOI] [Google Scholar]
- 27.Cho M.K., Park H.-Y., Choe S., Yoo S.J., Kim J.Y., Kim H.-J., Henkensmeier D., Lee S.Y., Sung Y.-E., Park H.S., Jang J.H. Factors in electrode fabrication for performance enhancement of anion exchange membrane water electrolysis. J. Power Sources. 2017;347:283–290. doi: 10.1016/j.jpowsour.2017.02.058. [DOI] [Google Scholar]
- 28.Guy D., Lestriez B., Bouchet R., Guyomard D. Critical role of polymeric binders on the electronic transport properties of composites electrode. J. Electrochem. Soc. 2006;153 doi: 10.1149/1.2168049. [DOI] [Google Scholar]
- 29.Entwistle J., Ge R., Pardikar K., Smith R., Cumming D. Carbon binder domain networks and electrical conductivity in lithium-ion battery electrodes: a critical review. Renew. Sustain. Energy Rev. 2022;166 doi: 10.1016/j.rser.2022.112624. [DOI] [Google Scholar]
- 30.Wu M., Xiao X., Vukmirovic N., Xun S., Das P.K., Song X., Olalde-Velasco P., Wang D., Weber A.Z., Wang L.-W., Battaglia V.S., Yang W., Liu G. Toward an ideal polymer binder design for high-capacity battery anodes. J. Am. Chem. Soc. 2013;135:12048–12056. doi: 10.1021/ja4054465. [DOI] [PubMed] [Google Scholar]
- 31.Zhu Z., Tang S., Yuan J., Qin X., Deng Y., Qu R., Haarberg G.M. Effects of various binders on supercapacitor performances. Int. J. Electrochem. Sci. 2016;11:8270–8279. doi: 10.20964/2016.10.04. [DOI] [Google Scholar]
- 32.Zhong X., Han J., Chen L., Liu W., Jiao F., Zhu H., Qin W. Binding mechanisms of PVDF in lithium ion batteries. Appl. Surf. Sci. 2021;553 doi: 10.1016/j.apsusc.2021.149564. [DOI] [Google Scholar]
- 33.Yu F., Yu L., Mishra I.K., Yu Y., Ren Z.F., Zhou H.Q. Recent developments in earth-abundant and non-noble electrocatalysts for water electrolysis. Materials Today Physics. 2018;7:121–138. doi: 10.1016/j.mtphys.2018.11.007. [DOI] [Google Scholar]
- 34.Yu J., Dai Y., He Q., Zhao D., Shao Z., Ni M. A mini-review of noble-metal-free electrocatalysts for overall water splitting in non-alkaline electrolytes. Materials Reports: Energy. 2021;1 doi: 10.1016/j.matre.2021.100024. [DOI] [Google Scholar]
- 35.Wu H., Feng C., Zhang L., Zhang J., Wilkinson D.P. Non-noble metal electrocatalysts for the hydrogen evolution reaction in water electrolysis. Electrochem. Energy Rev. 2021;4:473–507. doi: 10.1007/s41918-020-00086-z. [DOI] [Google Scholar]
- 36.Li Y., Sun Y., Qin Y., Zhang W., Wang L., Luo M., Yang H., Guo S. Recent advances on water‐splitting electrocatalysis mediated by noble‐metal‐based nanostructured materials. Adv. Energy Mater. 2020;10 doi: 10.1002/aenm.201903120. [DOI] [Google Scholar]
- 37.Manojkumar K., Kandeeban R., Brindha R., Sangeetha V., Saminathan K. Non-precious metal-based integrated electrodes for overall alkaline water splitting. J. Indian Chem. Soc. 2022;99 doi: 10.1016/j.jics.2022.100775. [DOI] [Google Scholar]
- 38.Mondal P., Satra J., Srivastava D.N., Bhadu G.R., Adhikary B. Pd δ+ -mediated surface engineering of AgMnO 4 nanorods as advanced bifunctional electrocatalysts for highly efficient water electrolysis. ACS Catal. 2021;11:3687–3703. doi: 10.1021/acscatal.0c05638. [DOI] [Google Scholar]
- 39.Bulakhe S., Shinde N., Kim J., Mane R.S., Deokate R. Recent advances in non‐precious Ni‐based promising catalysts for water splitting application. Int. J. Energy Res. 2022;46:17829–17847. doi: 10.1002/er.8458. [DOI] [Google Scholar]
- 40.Li Y., Bao X., Chen D., Wang Z., Dewangan N., Li M., Xu Z., Wang J., Kawi S., Zhong Q. A minireview on nickel‐based heterogeneous electrocatalysts for water splitting. ChemCatChem. 2019;11:5913–5928. doi: 10.1002/cctc.201901682. [DOI] [Google Scholar]
- 41.Sabir A.S., Pervaiz E., Khosa R., Sohail U. An inclusive review and perspective on Cu-based materials for electrochemical water splitting. RSC Adv. 2023;13:4963–4993. doi: 10.1039/D2RA07901A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kannimuthu K., Sangeetha K., Sam Sankar S., Karmakar A., Madhu R., Kundu S. Investigation on nanostructured Cu-based electrocatalysts for improvising water splitting: a review. Inorg. Chem. Front. 2021;8:234–272. doi: 10.1039/D0QI01060J. [DOI] [Google Scholar]
- 43.Rajput A., Kundu A., Chakraborty B. Recent progress on copper‐based electrode materials for overall water‐splitting. Chemelectrochem. 2021;8:1698–1722. doi: 10.1002/celc.202100307. [DOI] [Google Scholar]
- 44.Devaraju M.K., Honma I. Hydrothermal and solvothermal process towards development of LiMPO 4 (M = Fe, Mn) nanomaterials for lithium‐ion batteries. Adv. Energy Mater. 2012;2:284–297. doi: 10.1002/aenm.201100642. [DOI] [Google Scholar]
- 45.Ndlwana L., Raleie N., Dimpe K.M., Ogutu H.F., Oseghe E.O., Motsa M.M., Msagati T.A.M., Mamba B.B. Sustainable hydrothermal and solvothermal synthesis of advanced carbon materials in multidimensional applications: a review. Materials. 2021;14:5094. doi: 10.3390/ma14175094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Feng S.-H., Li G.-H. Modern Inorganic Synthetic Chemistry. Elsevier; 2017. Hydrothermal and solvothermal syntheses; pp. 73–104. [DOI] [Google Scholar]
- 47.Ma X.-X., Chen L., Zhang Z., Tang J.-L. Electrochemical performance evaluation of CuO@Cu2O nanowires array on Cu foam as bifunctional electrocatalyst for efficient water splitting. Chin. J. Anal. Chem. 2020;48:e20001–e20012. doi: 10.1016/S1872-2040(19)61211-9. [DOI] [Google Scholar]
- 48.Shi J., Hu J., Luo Y., Sun X., Asiri A.M. Ni 3 Se 2 film as a non-precious metal bifunctional electrocatalyst for efficient water splitting. Catal. Sci. Technol. 2015;5:4954–4958. doi: 10.1039/C5CY01121C. [DOI] [Google Scholar]
- 49.Ma X., Chang Y., Zhang Z., Tang J. Forest-like NiCoP@Cu 3 P supported on copper foam as a bifunctional catalyst for efficient water splitting. J Mater Chem A Mater. 2018;6:2100–2106. doi: 10.1039/C7TA09619D. [DOI] [Google Scholar]
- 50.Barati Darband Gh, Aliofkhazraei M., Rouhaghdam A.S. Facile electrodeposition of ternary Ni-Fe-Co alloy nanostructure as a binder free, cost-effective and durable electrocatalyst for high-performance overall water splitting. J. Colloid Interface Sci. 2019;547:407–420. doi: 10.1016/j.jcis.2019.03.098. [DOI] [PubMed] [Google Scholar]
- 51.Bibi H., Mansoor M.A., Asghar M.A., Ahmad Z., Numan A., Haider A. Facile hydrothermal synthesis of highly durable binary and ternary cobalt nickel copper oxides for high-performance oxygen evolution reaction. Int. J. Hydrogen Energy. 2024 doi: 10.1016/j.ijhydene.2024.02.321. [DOI] [Google Scholar]
- 52.Rani B.J., Ravi G., Yuvakkumar R., Hasan Z.M., Ravichandran S., Hong S.I. Binder free, robust and scalable CuO@GCE modified electrodes for efficient electrochemical water oxidation. Mater. Chem. Phys. 2020;239 doi: 10.1016/j.matchemphys.2019.122321. [DOI] [Google Scholar]
- 53.Xu S., He Y., Yan S., He T., Li H., Guo X., Fan Y. Superhydrophilic and superaerophobic Ni–Mo alloy grown on 3D Ni foam with heterogeneous structure as self-standing electrocatalysts toward efficient alkaline HER. Solid State Sci. 2023;136 doi: 10.1016/j.solidstatesciences.2022.107089. [DOI] [Google Scholar]
- 54.Lv S., Li J., Zhang B., Shi Y., Liu X., Wang T. In-situ growth of hierarchical CuO@Cu3P heterostructures with transferable active centers on copper foam substrates as bifunctional electrocatalysts for overall water splitting in alkaline media. Int. J. Hydrogen Energy. 2022;47:9593–9605. doi: 10.1016/j.ijhydene.2022.01.037. [DOI] [Google Scholar]
- 55.Shishkovsky I.V., Lebedev P.N. Nanocoatings and Ultra-thin Films. Elsevier; 2011. Chemical and physical vapor deposition methods for nanocoatings; pp. 57–77. [DOI] [Google Scholar]
- 56.Manawi Y., Ihsanullah, Samara A., Al-Ansari T., Atieh M. A review of carbon nanomaterials' synthesis via the chemical vapor deposition (CVD) method. Materials. 2018;11:822. doi: 10.3390/ma11050822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sun L., Yuan G., Gao L., Yang J., Chhowalla M., Gharahcheshmeh M.H., Gleason K.K., Choi Y.S., Hong B.H., Liu Z. Chemical vapour deposition. Nature Reviews Methods Primers. 2021;1:5. doi: 10.1038/s43586-020-00005-y. [DOI] [Google Scholar]
- 58.Ehsan M.A., Khan A., Ali M., Gondal M.A., Hakeem A.S. Facile deposition of a spherical ruthenium–cobalt alloy on nickel foam as a high-performance electrocatalyst for alkaline hydrogen production. ACS Appl. Energy Mater. 2024 doi: 10.1021/acsaem.4c00320. [DOI] [Google Scholar]
- 59.Sarawutanukul S., Phattharasupakun N., Sawangphruk M. 3D CVD graphene oxide-coated Ni foam as carbo- and electro-catalyst towards hydrogen evolution reaction in acidic solution: in situ electrochemical gas chromatography. Carbon N Y. 2019;151:109–119. doi: 10.1016/j.carbon.2019.05.058. [DOI] [Google Scholar]
- 60.Ehsan M.A., Khan A., Al-Ahmed A., Hakeem A.S., Afzaal M., Pandey S., Mahar N. Aerosol-assisted chemical vapour deposited vanadium oxide thin films on nickel foam with auspicious electrochemical water oxidation properties. Int. J. Hydrogen Energy. 2024;52:718–727. doi: 10.1016/j.ijhydene.2023.05.364. [DOI] [Google Scholar]
- 61.Babar N.-U.-A., Hakeem A.S., Ehsan M.A. Direct fabrication of nanoscale NiVO x electrocatalysts over nickel foam for a high-performance oxygen evolution reaction. ACS Appl. Energy Mater. 2022;5:4318–4328. doi: 10.1021/acsaem.1c03940. [DOI] [Google Scholar]
- 62.Ehsan M.A., Khan A. Aerosol-assisted chemical vapor deposition growth of NiMoO 4 nanoflowers on nickel foam as effective electrocatalysts toward water oxidation. ACS Omega. 2021;6:31339–31347. doi: 10.1021/acsomega.1c05209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sun J., Yu B., Tan F., Yang W., Cheng G., Zhang Z. High throughput preparation of Ni–Mo alloy thin films as efficient bifunctional electrocatalysts for water splitting. Int. J. Hydrogen Energy. 2022;47:15764–15774. doi: 10.1016/j.ijhydene.2022.03.065. [DOI] [Google Scholar]
- 64.Hu X., Tian X., Lin Y.-W., Wang Z. Nickel foam and stainless steel mesh as electrocatalysts for hydrogen evolution reaction, oxygen evolution reaction and overall water splitting in alkaline media. RSC Adv. 2019;9:31563–31571. doi: 10.1039/C9RA07258F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Raza A., Deen K.M., Asselin E., Haider W. A review on the electrocatalytic dissociation of water over stainless steel: hydrogen and oxygen evolution reactions. Renew. Sustain. Energy Rev. 2022;161 doi: 10.1016/j.rser.2022.112323. [DOI] [Google Scholar]
- 66.Shang K., Gao J., Yin X., Ding Y., Wen Z. An overview of flexible electrode materials/substrates for flexible electrochemical energy storage/conversion devices. Eur. J. Inorg. Chem. 2021;2021:606–619. doi: 10.1002/ejic.202001024. [DOI] [Google Scholar]
- 67.Tiurin O., Ein‐Eli Y. A critical review: the impact of the battery electrode material substrate on the composition and properties of atomic layer deposition (ALD) coatings. Adv Mater Interfaces. 2019;6 doi: 10.1002/admi.201901455. [DOI] [Google Scholar]
- 68.Zhou J., Yu L., Zhou Q., Huang C., Zhang Y., Yu B., Yu Y. Ultrafast fabrication of porous transition metal foams for efficient electrocatalytic water splitting. Appl. Catal., B. 2021;288 doi: 10.1016/j.apcatb.2021.120002. [DOI] [Google Scholar]
- 69.Wei L., Goh K., Birer Ö., Karahan H.E., Chang J., Zhai S., Chen X., Chen Y. A hierarchically porous nickel–copper phosphide nano-foam for efficient electrochemical splitting of water. Nanoscale. 2017;9:4401–4408. doi: 10.1039/C6NR09864A. [DOI] [PubMed] [Google Scholar]
- 70.Li S., Li M., Ni Y. Grass-like Ni/Cu nanosheet arrays grown on copper foam as efficient and non-precious catalyst for hydrogen evolution reaction. Appl. Catal., B. 2020;268 doi: 10.1016/j.apcatb.2019.118392. [DOI] [Google Scholar]
- 71.Muench F. Electroless plating of metal nanomaterials. Chemelectrochem. 2021;8:2993–3012. doi: 10.1002/celc.202100285. [DOI] [Google Scholar]
- 72.Shimizu M., Tsushima Y., Arai S. Electrochemical Na-Insertion/Extraction property of Ni-coated black phosphorus prepared by an electroless deposition method. ACS Omega. 2017;2:4306–4315. doi: 10.1021/acsomega.7b00950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hao W., Wu R., Huang H., Ou X., Wang L., Sun D., Ma X., Guo Y. Fabrication of practical catalytic electrodes using insulating and eco-friendly substrates for overall water splitting. Energy Environ. Sci. 2020;13:102–110. doi: 10.1039/C9EE00839J. [DOI] [Google Scholar]
- 74.Sharma P.J., Modi K.H., Sahatiya P., Sumesh C.K., Pataniya P.M. Electroless deposited NiP-fabric electrodes for efficient water and urea electrolysis for hydrogen production at industrial scale. Appl. Surf. Sci. 2024;644 doi: 10.1016/j.apsusc.2023.158766. [DOI] [Google Scholar]
- 75.Nardi K.L., Yang N., Dickens C.F., Strickler A.L., Bent S.F. Creating highly active atomic layer deposited NiO electrocatalysts for the oxygen evolution reaction. Adv. Energy Mater. 2015;5 doi: 10.1002/aenm.201500412. [DOI] [Google Scholar]
- 76.Singhal R., Kalra V. Cobalt nanoparticle‐embedded porous carbon nanofibers with inherent N‐ and F‐doping as binder‐free bifunctional catalysts for oxygen reduction and evolution reactions. ChemPhysChem. 2017;18:223–229. doi: 10.1002/cphc.201600771. [DOI] [PubMed] [Google Scholar]
- 77.Hsiao M., Lo C. Hierarchical nanostructured electrospun carbon fiber/<scp> NiCo 2 O 4 composites </scp> as <scp>binder‐free</scp> anodes for <scp>lithium‐ion</scp> batteries. Int. J. Energy Res. 2020;44:8606–8621. doi: 10.1002/er.5549. [DOI] [Google Scholar]
- 78.Joshi B., Samuel E., Kim Y., Yarin A.L., Swihart M.T., Yoon S.S. Review of recent progress in electrospinning-derived freestanding and binder-free electrodes for supercapacitors. Coord. Chem. Rev. 2022;460 doi: 10.1016/j.ccr.2022.214466. [DOI] [Google Scholar]
- 79.Joshi B., Samuel E., Kim Y., Yarin A.L., Swihart M.T., Yoon S.S. Progress and potential of electrospinning-derived substrate-free and binder-free lithium-ion battery electrodes. Chem. Eng. J. 2022;430 doi: 10.1016/j.cej.2021.132876. [DOI] [Google Scholar]
- 80.Kundu M., Liu L. Binder-free electrodes consisting of porous NiO nanofibers directly electrospun on nickel foam for high-rate supercapacitors. Mater. Lett. 2015;144:114–118. doi: 10.1016/j.matlet.2015.01.032. [DOI] [Google Scholar]
- 81.Pataniya P.M., Sumesh C.K. MoS2 nanosheets on Cu-foil for rapid electrocatalytic hydrogen evolution reaction. J. Electroanal. Chem. 2022;912 doi: 10.1016/j.jelechem.2022.116270. [DOI] [Google Scholar]
- 82.Pataniya P.M., Patel V., Sumesh C.K. Electrophoretic deposition of MoSe 2 –MoO x nanosheets for enhanced electrocatalytic hydrogen evolution reaction. ACS Appl. Energy Mater. 2021;4:7891–7899. doi: 10.1021/acsaem.1c01239. [DOI] [Google Scholar]
- 83.Shukla A., Singh S.C., Saraj C.S., Verma G., Guo C. Ni-based overall water splitting electrocatalysts prepared via laser-ablation-in-liquids combined with electrophoretic deposition. Mater. Today Chem. 2022;23 doi: 10.1016/j.mtchem.2021.100691. [DOI] [Google Scholar]
- 84.Wang H.-B., Wang J.-Q., Mintcheva N., Wang M., Li S., Mao J., Liu H., Dong C.-K., Kulinich S.A., Du X.-W. Laser synthesis of iridium nanospheres for overall water splitting. Materials. 2019;12:3028. doi: 10.3390/ma12183028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Begildayeva T., Theerthagiri J., Lee S.J., Yu Y., Choi M.Y. Unraveling the synergy of anion modulation on Co electrocatalysts by pulsed laser for water splitting: intermediate capturing by in situ/operando Raman studies. Small. 2022;18 doi: 10.1002/smll.202204309. [DOI] [PubMed] [Google Scholar]
- 86.Khot M., Kiani A. Synthesis of self-grown nanostructured NiO via pulse ionization for binderless psuedocapacitor electrode. J. Energy Storage. 2022;55 doi: 10.1016/j.est.2022.105779. [DOI] [Google Scholar]
- 87.Gholami A., Kiani A. Laser-induced nanofibrous titania film electrode: a new approach for energy storage materials. J. Energy Storage. 2020;31 doi: 10.1016/j.est.2020.101654. [DOI] [Google Scholar]
- 88.Khot M., Shaik R.S., Touseef W., Kiani A. Binder-free NiO/CuO hybrid structure via ULPING (Ultra-short laser pulse for in-situ nanostructure generation) technique for supercapacitor electrode. Sci. Rep. 2023;13:6975. doi: 10.1038/s41598-023-34274-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Khosravinia K., Kiani A. Unlocking pseudocapacitors prolonged electrode fabrication via ultra-short laser pulses and machine learning. iScience. 2023;26 doi: 10.1016/j.isci.2023.106438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Khosravinia K., Kiani A. Protocol for fabricating pseudocapacitor electrodes using ultra-short laser pulses for in situ nanostructure generation. STAR Protoc. 2023;4 doi: 10.1016/j.xpro.2023.102469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Khosravinia K., Kiani A. Optimizing the ultrashort laser pulses for in situ nanostructure generation technique for high-performance supercapacitor electrodes using artificial neural networks and simulated annealing algorithms. ACS Omega. 2023;8:17220–17233. doi: 10.1021/acsomega.3c01676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Favors Z., Bay H.H., Mutlu Z., Ahmed K., Ionescu R., Ye R., Ozkan M., Ozkan C.S. Towards scalable binderless electrodes: carbon coated silicon nanofiber paper via Mg reduction of electrospun SiO2 nanofibers. Sci. Rep. 2015;5:8246. doi: 10.1038/srep08246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Shankar Naik S., Theerthagiri J., Nogueira F.S., Lee S.J., Min A., Kim G.-A., Maia G., Pinto L.M.C., Choi M.Y. Dual-cation-coordinated CoFe-layered double-hydroxide nanosheets using the pulsed laser ablation technique for efficient electrochemical water splitting: mechanistic screening by in situ/operando Raman and density functional theory calculations. ACS Catal. 2023;13:1477–1491. doi: 10.1021/acscatal.2c05017. [DOI] [Google Scholar]
- 94.Trivedi N., Joshi K.K., Siraj S., Sahatiya P., Patel V., Sumesh C.K., Pataniya P.M. Self-supported Cr–Cu2S nanoflakes for hydrogen production from seawater. Int. J. Hydrogen Energy. 2024;49:1113–1122. doi: 10.1016/j.ijhydene.2023.11.036. [DOI] [Google Scholar]
- 95.Yang D., Cao L., Huang J., Liu Q., Li G., He D., Wang J., Feng L. Vanadium-doped hierarchical Cu2S nanowall arrays assembled by nanowires on copper foam as an efficient electrocatalyst for hydrogen evolution reaction. Scr Mater. 2021;196 doi: 10.1016/j.scriptamat.2021.113756. [DOI] [Google Scholar]
- 96.Mandavkar R., Habib M.A., Lin S., Kulkarni R., Burse S., Jeong J.-H., Lee J. Electron enriched ternary NiMoB electrocatalyst for improved overall water splitting: better performance as compared to the Pt/C || RuO2 at high current density. Appl. Mater. Today. 2022;29 doi: 10.1016/j.apmt.2022.101579. [DOI] [Google Scholar]
- 97.Shah A.M., Modi K.H., Pataniya P.M., Joseph K.S., Dabhi S., Bhadu G.R., Sumesh C.K. Self-supported Mn-Ni 3 Se 2 electrocatalysts for water and urea electrolysis for energy-saving hydrogen production. ACS Appl. Mater. Interfaces. 2024;16:11440–11452. doi: 10.1021/acsami.3c16244. [DOI] [PubMed] [Google Scholar]
- 98.Thakkar H.K., Modi K.H., Joshi K.K., Bhadu G., Siraj S., Sahatiya P., Pataniya P.M., Sumesh C.K. Vertically oriented FeNiO nanosheet array for urea and water electrolysis at industrial-scale current density. ACS Sustain Chem Eng. 2024;12:8340–8352. doi: 10.1021/acssuschemeng.4c00591. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are included within the article and are referenced within the article.