Abstract
Agroindustry factory's such as the table olive industry etc. that gain importance in the economies of Mediterranean countries. Conventional treatment methods are not effective for treating table olive processing wastewater due to its unique composition. Ultrasound/Ultraviolet light (US/UV) oxidation was used to treat wastewater of table olive industry to improve hydroxyl radicals and enhance organic compound removals. A statistical experimental design was used on table olive processing wastewater to examine the effects of the UV/US oxidation process. The highest removal efficiency for chemical oxygen demand, total organic carbon, color, suspended solids and phenol were obtained as 64 %, 52 % 60 %, 87.5 % and 22.3 %, respectively. These results were obtained under optimal conditions of 20 min reaction time for ultrasound process, intensity of 50 W/cm2, and 20 min reaction time for ultraviolet process in the US/UV process. The study also showed that the ultrasound/ultraviolet oxidation process resulted in small reaction time and low chemical requirements. Sludge production and operational cost decreased at best experimental conditions due to small reaction time and low chemical requirement.
Keywords: Box-Behnken design, Table olive industry, UV oxidation, US oxidation
1. Introduction
Agroindustry factory's such as the table olive industry etc. that gain importance in the economies of Mediterranean countries. Production of table olive has also increased in last decades and has gained importance, especially in Mediterranean countries [1]. Three types of table olives production are used in table olive industry, and these are green, black, and black through oxidation. The primary commercial varieties of table olives include Spanish-style green olives, Californian-style black ripe olives, and naturally black olives in brine. The most common method for producing table olives is “Spanish-style” processing (∼50 % of total production) [2]. The table olive industry has grown significantly, with global production exceeding 2900 tons in 2017/18, up from under 2500 tons in 2011/12 [3]. The characteristics and volume of table olive processing wastewater (TOPW) vary based on the olive variety and the processing methods employed. TOPW contains a range of pollutants including phenols, refractory organics, dissolved inorganic solids depending on the type of olive and the production methods used. Refractory organic compounds such as phenols are toxic effect on olives, resulted in low olive production or nutritional loss in olive [4]. California-style black ripe olive production has the highest pollution potential, generating up to 6 L of wastewater per kilogram of olives produced. Spanish-style green olive processing produces wastewater, which is alkaline wastewater that originates from embittering and washing processes and acidic wastewater that comes from fermentation brine processes. Nevertheless, table olive washing wastewaters are generally discharged into the surface water or discharging area without treatment, causing surface water, aquifers, and water pollution and affecting discharged areas such as turbidity, high color and bad smells of waters. In addition, soil and aquifer contamination with toxic compounds is a significant risk after uncontrolled discharge or insufficient treatment.
Conventional treatment methods are used to treat table olive processing wastewater (TOPW). However, some of these methods result in low efficiency, and some methods are expensive despite insufficient treatment. Therefore, TOPW cannot be treated easily by conventional methods due to the composition of the wastewater. Seasonal variability in wastewater composition and high organic loads, phenols, and salts in the wastewater are the most important issues for evaluating the preference of treatment methods to achieve sufficient treatment efficiency.
Advanced oxidation processes (AOPs) are effective to mineralize or degrade refractory organic compounds in wastewater because of the presence of unselective hydroxyl radicals. Several studies have used advanced oxidation processes to increase hydroxyl radical production. A new approach to advanced oxidation processes is to combine the different AOPs to improve hydroxyl radicals in the process and enhance the large-scale compound treatment.
The ultraviolet process (UV) is useful and simple oxidation process to produce hydroxyl radical (•OH, E0 = 2.8 V). Reactions in UV process is given below as Equation (1) shows that water molecules converted to hydroxyl radicals via ultraviolet light.
| (Eq.1) |
The ultraviolet process (UV) has been used as an advanced oxidation process in conjunction with other AOPs such as H2O2 [5,6], Fenton process [[7], [8], [9]], ZnO [10], TiO2 [11,12], ozone [13] and ultrasound [[14], [15], [16], [17]], in order to improve or accelerate hydroxyl radical formation.
The use of ultrasounds is assumed to be the next type of AOPs. In the ultrasound (US) process, cavitation is a notable phenomenon where gaseous or vapor bubbles form, grow, and then collapse violently in a liquid [18]. This implosive collapse generates intense “hot spots,” which lead to the dissociation of water molecules and the formation of reactive oxygen species (ROS). The chemical effect of ultrasound in the solution can be presented by Equations (Eq.2), (Eq.3), (Eq.4), (Eq.5).
| (Eq.2) |
| (Eq.3) |
| (Eq.4) |
| (Eq.5) |
where (((represents the ultrasound waves.
The chemical effect of ultrasound is occurred by the formation of highly reactive unselective radical species such as •OH and •H in the liquid, which can directly oxidize refractory organic compounds in wastewater [19].
Although usage ultrasound alone can effectively treat refractory organics compounds in wastewater, high energy consumption is the main disadvantages and limitation factor of ultrasound process. The use of ultrasound in the combined treatment process is more effective than in a single treatment. Thus, nowadays, ultrasound process combined with other advanced oxidation process to enhance hydroxyl radical formation, to minimize energy consumption and to achieve a high treatment efficiency. In addition, the combination of ultrasound and UV processes can be suitable treatment methods for the removal of various pollutants from wastewater. The addition of ultrasonic irradiation to the UV process can accelerate hydroxyl radical formation to overcome the restrictions of reactions in the UV process. The ultrasound can eliminate the negative effect of UV oxidation in wastewater contains high turbidity and suspended solids, which could effective in US/UV process better experimental results than that of US oxidation or UV oxidation alone. In addition, ultrasonic sound minimizes the amount of chemicals addition in UV process because of the acceleration of hydroxyl radical formation. Thus, the combination of ultrasound and ultraviolet light processes has been executed to increase the yields of oxidation processes. Therefore, the use of ultrasonic irradiation with AOPs has gained attention for treating wastewater.
The US/UV oxidations are alternative processes to eliminate drawbacks of only usage of UV or US oxidation process. This study examines the table olive processing wastewater treatment using ultrasound alone or in combination with UV light. In the US/UV oxidation process, optimum reaction conditions were examined by the Box-Behnken statically design to treat table olive processing wastewater. In addition, the synergistic effect of sonolysis and photolysis on TOPW treatment was evaluated by means of the UV/US oxidation processes and to evaluate the economic and commercial feasibility of hybrid oxidation process. The US/UV process needs lower chemical addition than the other oxidation process. Ultrasound-ultraviolet combined treatment processes can be an effective oxidation method for wastewater treatment, particularly for TOPW, when applied under optimal reaction conditions.
2. Materials and methods
Table 1 presents the characteristics of the table olive processing wastewater used in the study. Due to its high pollutant levels, characterizing this wastewater is challenging, complicating its treatment. Table olive processing wastewater is rich in organic contaminants and phenolic compounds, with a notably high suspended solid content. The wastewater also exhibits a dark brown color. The methods used for the parameters listed in Table 1 [20].
Table 1.
Table olive processing wastewater characterization.
| Parameter | Unit | Average Concentration |
Method |
|---|---|---|---|
| pH | – | 5.50 ± 0.1 | SM 4500-H+ B |
| Chemical oxygen demand (COD) | mg/L | 4000 ± 10 | SM 5220 B |
| Total organic carbon (TOC) | mg/L | 1000 ± 10 | SM 5310 B |
| Suspended Solids (SS) | mg/L | 2800 ± 10 | SM 2540 D |
| Phenol | mg/L | 4.6 ± 0.1 | SM 5530 D |
| Conductivity | mS/cm | 22.6 ± 0.1 | SM 2510 B |
| Color | ABS | 3.175 ± 0.1 | SM 2120 C |
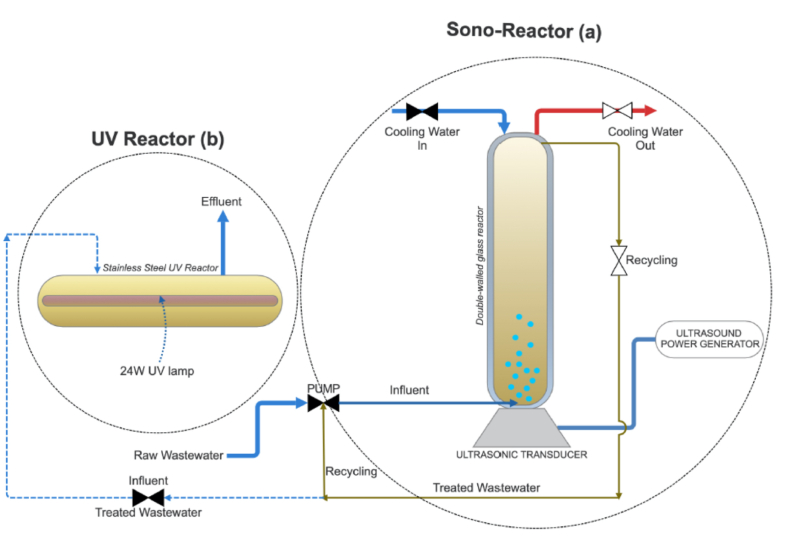
In ultrasound (US) experiments, a Meinhardt UST02 model glass reactor, a Meinhardt E/805/T model ultrasonic transducer, and a generator were used, as shown in Fig. 1-a. In addition, the pump is used in the system to provide the return wastewater cycle. The glass reactor, with a volume of 1500 mL and a diameter of 500 mm, is double-walled. The glass reactor is fixed to the ultrasonic transducer with clamps. The ultrasound frequency of the power supply can be adjusted to 850 kHz and above. The power output in the reactor is 120 W. The power supply intensity can be adjusted in 1-2-3-4 (25-50-75-100 W/cm2) steps. The ultrasound power generator used is also presented in Fig. 1-a.
Fig. 1.
Reactors used in the US/UV process: a. US reactor; b. UV reactor.
In the experimental studies, a Purfect-6 model stainless steel UV reactor was employed as the second reactor. The dimensions of the reactor are 515 × 125 × 185 mm, and its total power is 24 W. The wastewater that comes from the US reactor is filled into the UV reactor from the upper part of the reactor with the help of a pump. The UV reactor is shown in Fig. 1-b. In the US/UV process, wastewater was first filled in the US reactor, and the US reactor was started with different intensities (50-75-100 W/cm2) and reaction times (5–30 min). After the US process, the treated wastewater was discharged to the UV reactor by pump, and the UV reactor was used between 0- and 30-min. reaction times. The raw pH value used in the experiments was not changed to decrease chemical additions.
In the US/UV process, the effects of the US reaction time, the intense reaction time, and the UV reaction time were investigated by Box-Behnken statistical design. The Box-Behnken design (BBD), a variation of the central composite experimental design, is an independent, rotatable quadratic design that does not incorporate embedded factorial or fractional factorial designs [21]. This statistical method is notable for requiring fewer experimental runs, needing only 15 runs for a 3-factor experiment. Additionally, BBD allows for the calculation of response functions at intermediate levels that may not have been directly studied in the experiments [22,23]. Comparative analysis with other response surface designs, including central composite, Doehlert matrix, Taguchi method, the process optimization should be used in a three-step approach comprising of parameters design and three-level full factorial designs, reveals that both BBD and Doehlert matrix are slightly more efficient than central composite design and significantly more efficient than three-level factorial designs [24,25]. The Box-Behnken design as an effective statically design optimizes three-variable response functions, estimating the responses of fitted models [[26], [27], [28], [29], [30]]. The optimization process entails studying the responses of statistically designed combinations, predicting coefficients by fitting experimental data to response functions, estimating the responses of fitted models, and reaching model adequacy through analysis of variance tests.
In this study, Design Expert 10.0 version was applied to evaluate and optimize the experimental results. The US reaction time, intensity, and UV reaction times were determined as independent variables in the Box-Behnken experimental design. Among the variables, the US reaction time is shown as “X1”, the intensity is “X2”, the UV reaction time is abbreviated as “X3”. According to previous experiments, US reaction time, UV reaction time, and US intensity values are determined, and independent variable ranges of the US/UV process are predetermined by means of Box-Behnken design and are presented in Table 2. The low, center, and high levels of each variable, represented as −1, 0, and +1 respectively, using a statistical approach, are shown in Table 2.
Table 2.
Independent variables levels for the US/UV process in the Box-Behnken design.
| Level | US Reaction Time (min) |
Intense (W/cm2) |
UV Reaction Time (min) |
|---|---|---|---|
| X1 | X2 | X3 | |
| −1 | 5 | 50 | 0 |
| 0 | 17.5 | 75 | 15 |
| +1 | 30 | 100 | 30 |
Removal efficiency of COD (Y1), removal efficiency of TOC (Y2), removal efficiency of color (Y3), removal efficiency of suspended solids (Y4), and removal efficiency of phenol removal efficiency (Y5) were determined as dependent variables in the design.
The experimental conditions of the Box-Behnken experiment design were presented in Table 3. The independent variables effect on the US/UV oxidation process evaluated with these experimental conditions.
Table 3.
Experimental conditions for the US/UV process.
| Run | US reaction time (min), X1 | Intense (W/cm2), X2 | UV reaction time (min), X3 |
|---|---|---|---|
| 1 | +1 (30) | +1 (100) | 0 (15) |
| 2 | +1 (30) | −1 (50) | 0 (15) |
| 3 | −1 (5) | +1 (100) | 0 (15) |
| 4 | −1 (5) | −1 (50) | 0 (15) |
| 5 | +1 (30) | 0 (75) | +1 (30) |
| 6 | −1 (5) | 0 (75) | −1 (0) |
| 7 | +1 (30) | 0 (75) | −1 (0) |
| 8 | −1 (5) | 0 (75) | +1 (30) |
| 9 | 0 (17.5) | +1 (100) | +1 (30) |
| 10 | 0 (17.5) | +1 (100) | −1 (0) |
| 11 | 0 (17.5) | −1 (50) | +1 (30) |
| 12 | 0 (17.5) | −1 (50) | −1 (0) |
| 13 | 0 (17.5) | 0 (75) | 0 (15) |
| 14 | 0 (17.5) | 0 (75) | 0 (15) |
| 15 | 0 (17.5) | 0 (75) | 0 (15) |
3. Results and discussion
This study compares US and UV/US processes for table olive processing wastewater. To achieve this purpose, the US and UV/US processes were investigated and compared using the following parameters: UV reaction time, US reaction time, and intensity of the ultrasound process. The Box-Behnken design approach is a successful and useful way to optimize the response functions, predicting the response of the fitted model by the analysis of variance (ANOVA) tests [31,32]. The Box-Behnken experimental design was applied to TOPW to investigate the independent variables effect on removal efficiencies in the US/UV oxidation process. The reaction conditions for each experiment and the removal efficiencies are given in Table 4. The observed and predicted removal efficiencies as dependent variables are also presented in Table 5.
Table 4.
Reaction conditions for each experiment and removal efficiencies of independent variables from the Box-Behnken Experimental Design.
| Run | X1 |
X2 |
X3 |
Y1 |
Y2 |
Y3 |
Y4 |
Y5 |
|---|---|---|---|---|---|---|---|---|
| US Reac. Time (min) | Intense (W/cm2) | UV Reac. Time (min) | COD (%) | TOC (%) | Color (%) | SS (%) | Phenol (%) | |
| 1 | 5 | 50 | 15 | 57 | 43.9 | 56.8 | 92.8 | 23 |
| 2 | 30 | 50 | 15 | 60 | 46 | 55.2 | 35 | 15 |
| 3 | 5 | 100 | 15 | 50 | 34.4 | 52 | 25 | 25 |
| 4 | 30 | 100 | 15 | 46 | 29 | 54.4 | 21.4 | 30.7 |
| 5 | 5 | 75 | 0 | 50 | 30.7 | 47.4 | 42.9 | 31.6 |
| 6 | 30 | 75 | 0 | 44 | 29.5 | 45 | 14.3 | 30 |
| 7 | 5 | 75 | 30 | 48 | 45.6 | 45 | 85.7 | 33 |
| 8 | 30 | 75 | 30 | 58 | 44 | 56.8 | 45 | 33.9 |
| 9 | 17.5 | 50 | 0 | 58 | 46.8 | 53.6 | 78.6 | 27.6 |
| 10 | 17.5 | 100 | 0 | 44 | 30 | 50 | 50 | 32.2 |
| 11 | 17.5 | 50 | 30 | 60 | 51 | 60.1 | 85.7 | 29 |
| 12 | 17.5 | 100 | 30 | 58 | 46.9 | 56.8 | 64.3 | 34.2 |
| 13 | 17.5 | 75 | 15 | 60 | 45.5 | 54.5 | 85.7 | 28.7 |
| 14 | 17.5 | 75 | 15 | 60 | 45.2 | 55.7 | 85.7 | 29.3 |
| 15 | 17.5 | 75 | 15 | 60 | 45.1 | 55 | 85.7 | 29.6 |
Table 5.
Observed and predicted removal efficiencies as dependent variables in the Box-Behnken design.
| Run | Observed removal efficiencies (%) |
Predicted removal efficiencies (%) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| COD | TOC | Color | SS | Phenol | COD | TOC | Color | SS | Phenol | |
| 1 | 50 | 34.4 | 52 | 25 | 25 | 50 | 35 | 50.8 | 29.9 | 23.8 |
| 2 | 60 | 45.5 | 54.6 | 85.7 | 28.7 | 60 | 45.3 | 55.1 | 85.7 | 29.2 |
| 3 | 46 | 29 | 54.4 | 21.4 | 30.7 | 47.3 | 29.8 | 55.3 | 24.4 | 29.9 |
| 4 | 50 | 30.7 | 47.4 | 42.9 | 31.6 | 50.1 | 31.8 | 47.9 | 48.4 | 32 |
| 5 | 60 | 45.1 | 55 | 85.7 | 29.6 | 60 | 45.3 | 55.1 | 85.7 | 29.2 |
| 6 | 44 | 29.5 | 45 | 14.3 | 30 | 42.9 | 30.5 | 43.4 | 21.8 | 30 |
| 7 | 44 | 30 | 50 | 50 | 32.2 | 43.9 | 28.3 | 50.6 | 39.6 | 32.9 |
| 8 | 48 | 45.6 | 45 | 85.7 | 33 | 49.1 | 44.6 | 46.6 | 78.2 | 33 |
| 9 | 60 | 51 | 60.1 | 85.7 | 29 | 60.1 | 52.7 | 59.5 | 96.1 | 28.2 |
| 10 | 60 | 45.2 | 55.7 | 85.7 | 29.3 | 60 | 45.3 | 55.1 | 85.7 | 29.2 |
| 11 | 58 | 46.8 | 53.6 | 78.6 | 27.6 | 59.1 | 46.5 | 53.9 | 76 | 26.3 |
| 12 | 60 | 46 | 55.2 | 35 | 15 | 60 | 45.4 | 56.5 | 30.1 | 16.2 |
| 13 | 58 | 46.9 | 56.8 | 64.3 | 34.2 | 56.9 | 47.2 | 56.5 | 66.9 | 35.4 |
| 14 | 57 | 43.9 | 56.8 | 92.8 | 23 | 55.7 | 43.2 | 55.9 | 89.9 | 23.8 |
| 15 | 58 | 44 | 56.8 | 45 | 33.9 | 57.9 | 42.9 | 56.2 | 39.5 | 33.5 |
3.1. Regression model
RSM provides an empirical relationship between the dependent and independent variables. The relationship between the response function, dependent variable (Y), and independent variables (X) can be approximated using a quadratic (second-order) polynomial equation as shown below:
| Eq. (6) |
This method was chosen due to relatively fewer combinations of variables. A total of 15 experiments were planned to determine all the coefficients of the second-order polynomial regression model. The coefficients of the response functions for various dependent variables were obtained by correlating the experimental data with the relevant functions through a Stat-Ease regression program [26]. Equations with the determined coefficients for different response functions are presented as eqs Eq. (7), Eq. (8), Eq. (9), Eq. (10), Eq. (11).
-
•
The response function for removal percentage of COD (Y1) is expressed as follows:
| Eq. (7) |
-
•
The response function for removal percentage of TOC (Y2) is expressed as follows:
| Eq. (8) |
-
•
The response function for removal percentage of color (Y3) is expressed as follows:
| Eq. (9) |
-
•
The response function for removal percentage of suspended solid (Y4) is expressed as follows:
| Eq. (10) |
-
•
The response function for removal percentage of phenol (Y5) is expressed as follows:
| Eq. (11) |
The model's predictability is confirmed at a 95 % confidence level according to ANOVA. The coefficient of determination (R2) is 0.99, indicating a strong fit. Additionally, the F value exceeds 3.70, highlighting the treatment's high significance. P values greater than 0.05 for any factor in the ANOVA underscore the significant impact of the independent variables on the dependent variables.
3.2. Chemical oxygen demand removal
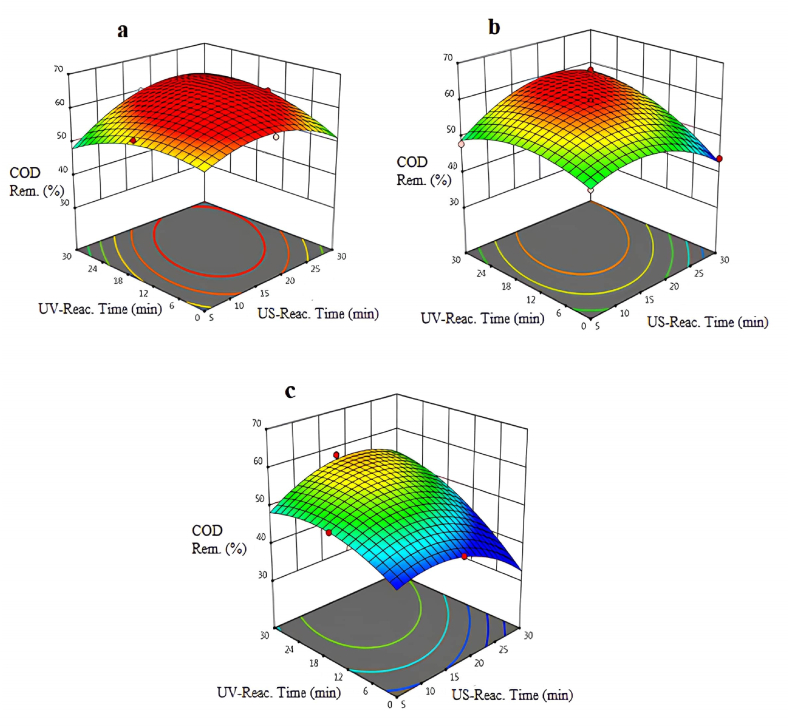
The removal efficiencies of chemical oxygen demand obtained with the US/UV process using different intensities of US are shown in Fig. 2(a–c). In the US/UV combined oxidation process as AOPs, the removal efficiency of chemical oxygen demand was achieved as 60 % with the intensity of US, which was the minimum value accepted as 50 W/cm2 when experiments were performed with the US reaction time (15 min) and the UV reaction time (20 min). After evaluating of the experimental results at minimum, average, and maximum intensity values, the optimum removal efficiency of COD was observed at the minimum intensity. Therefore, minimum intensity has a positive contribution to chemical oxygen demand removal percentage.
Fig. 2.
Variation of COD removal percentage at pH = 5.5 and different intensities of US: a-intense:50 W/cm2: b-intense:75W/cm2: c-intense:100 W/cm2.
The COD removal efficiency was observed as 43 % when the US process was used alone and the US process was carried out in 5 min. Briefly, it was interpreted that only 43 % of COD removal was observed with the US process alone with minimum intensity. When the US/UV reactors were operated in combination, the COD removal efficiency accelerated to 65 % at minimum intensity (50 W/cm2). In addition, the maximum removal efficiency of COD was obtained at a reaction time of 20 min for UV and US. When the advanced oxidation processes were used as a single, the COD removal efficiencies decreased. UV light accelerates hydroxyl radical production in the US/UV process. Some untreated organic compounds or intermediate products can be treated with the addition of UV light after the US process. For that reason, combined advanced oxidation processes are useful to treat some special wastewaters. This hybrid process aims to treat TOPW efficiently, not produce a high amount of sludge, and minimize chemical usage and addition.
Beltran-Heradia et al. [33] observed that using ozone alone in the treatment of TOPW observes removal efficiency of COD changing from 42 % to 55 %. The utilization of ozone as a standalone treatment for TOPW yields a removal percentage of COD changing from 42 % to 55 % [34]. Nevertheless, ozone process using alone was not useful method for removing organic compounds, TOPW was treated using the ozone/H2O2/UV process, producing nearly 80 % COD removal efficiency [35]. However, ozone oxidation or hybrid ozone oxidation process needs high chemical usages such as hydrogen peroxide (H2O2). So, operating costs and sludge production of these processes could be increased.
In contrast, our study demonstrates a significant COD removal efficiency of 65 % through the implementation of the US/UV process alone, entirely devoid of any chemical additives. This underscores the effectiveness of our approach in treating TOPW while minimizing operational complexities and environmental impact associated with chemical usage and sludge generation.
According to ANOVA test, it can be said that the intensity of the ultrasound process is an important parameter for the maximum removal efficiency of COD. The model F-value of 37.72 shows that the model is significant. In this case X2, X3, X1X2, X1X3, X2X3, X12, X32 are important model terms.
3.3. Total organic carbon removal
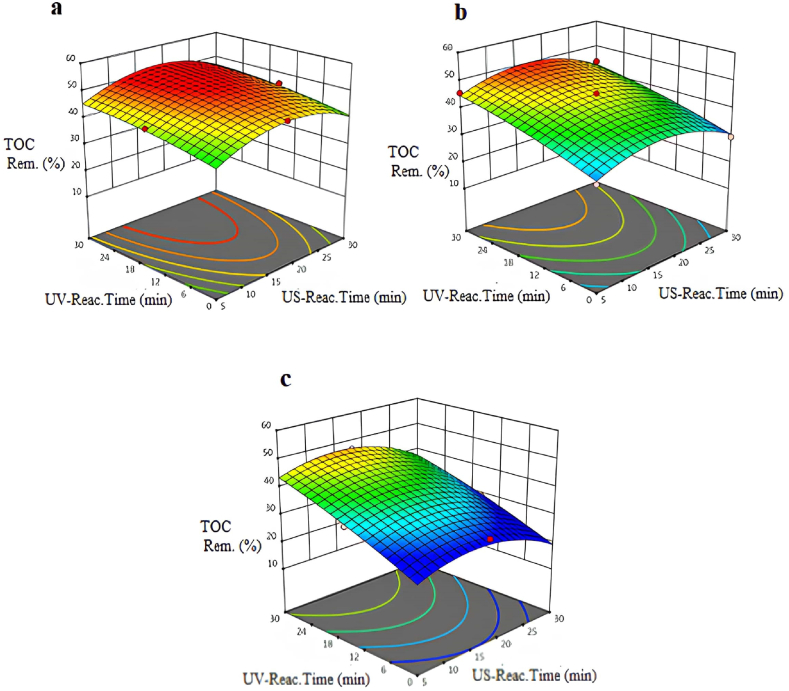
The removal efficiencies of total organic carbon obtained with the US/UV process using different intensities of US are shown in Fig. 3(a–c). In the US/UV process, as an advanced oxidation process, the intensity was adjusted to different values via the experimental design, and the US reactor was started. The removal efficiency of total organic carbon (TOC) was observed to be nearly 55 % when the intensity was adjusted to the minimum value at the US reaction time (17 min) and the UV reaction time (18 min). According to an experimental studies executed by Beltran et al. [34], it was found that TOPW could be effectively treated with ozone. Initially, a nearly 7 % removal efficiency of total carbon (TC) was observed after using 10 g of ozone at a rate of 7 × 10−4 mol/min for 5-h reaction time. Subsequently, when H2O2 was added, the removal efficiency of TC only raised to 30 %, using nearly 340 mg/L of H2O2 and 4.3 g/L of ozone dose. In contrast, we achieved a 55 % removal efficiency of TOC using the US/UV process in our own study, US/UV process was executed with short reaction times, unaltered pH values, and without the addition of any chemical substances. These reaction conditions minimize the cost of operation.
Fig. 3.
Variation of TOC removal percentages at pH = 5.5 and different intensities of US: a-intense:50 W/cm2: b-intense:75W/cm2: c-intense:100 W/cm2.
It was determined that the increase in intensity did not have a positive effect on removal efficiencies of TOC. While an increase in the UV reaction time has a negative impact on TOC removal efficiency, an increase in the US reaction time has a positive effect on removal efficiency of TOC. Also, high removal efficiencies of TOC were achieved when the intensity of US was arranged to the minimum value. The intense and US reaction time contribute positively to the TOC removal efficiency to treat table olive wastewater. The model F-value of 36.16 indicates that the model is significant. In this case, X2, X3, X2X3, X12 are important model terms. Combining the US and UV reactors resulted in a TOC removal efficiency of 55 % at the lowest intensity (50 W/cm2). In addition, the maximum TOC removal efficiency obtained after a 20-min reaction time is the same as the COD removal efficiency in the US/UV process.
3.4. Color removal
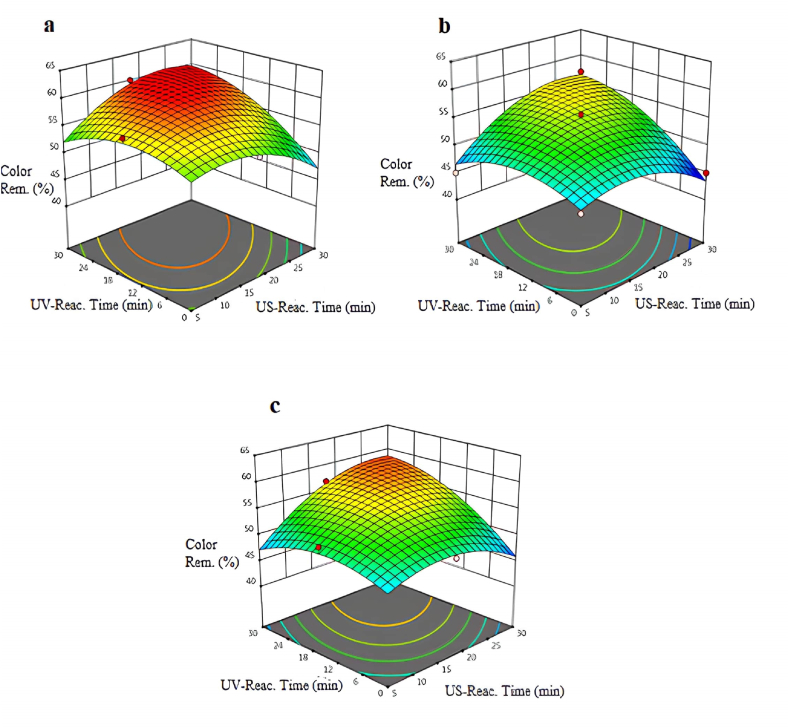
The color removal efficiencies obtained with the US/UV process using different intensities of US are shown in Fig. 4(a–c). In the US/UV advanced oxidation process, the removal efficiency of color was observed as 61.5 % when the intensity was adjusted to a minimum value (50 W/m2) at the US time (15 min) and the UV time (8 min). However, when the US reaction time was increased to 30 min, the color removal efficiency decreased to 45 %. In addition, the removal efficiency of color decreased at high US intensity. Therefore, the increase in intensity had negatively affect the color removal efficiency. In addition, the use of only the ultrasound process did not positively affect the color removal efficiency. This situation can be explained by bubbles in the US reactor at high intensity. Turbidity, caused by bubbles in the US process, has negatively affect color removal efficiencies.
Fig. 4.
Variation of color removal percentages at pH = 5.5 and different intensities of US: a-intense:50 W/cm2: b-intense:75W/cm2: c-intense:100 W/cm2.
According to a study studied by Chatzisymeon et al. [35], it was observed that TOPW could be effectively treated using photocatalytic oxidation. 80 % removal efficiency of color was achieved with the UV process after using 1.5 g/L TiO2 at a reaction time of 180 min. Subsequently, upon the addition of hydrogen peroxide as an oxidant to the UV/TiO2 process, the removal efficiency of color raised to 90 %, utilizing nearly 0.1 g/L of H2O2 and 1 g/L of TiO2 at a 180 min reaction time. In contrast, a 61.5 % color removal efficiency was achieved with the US/UV process in our own study, US/UV oxidation process was conducted with reaction time (23 min) and without the addition of any chemical substances. Reaction time involves both efficiency and expenses. Extending the processes may improve pollution removal but raise energy and maintenance expenses [36].
According to the experimental results, UV reaction time is a very important parameter for eliminating the color from wastewater. The UV reaction time has a positive effect on the color removal efficiency at the minimum, average, and maximum intensities. According to the ANOVA test, the model F-value of 12.64 indicates that the model is significant. In this case, X2, X3, X1X3, X12, X22, X32 are important model terms.
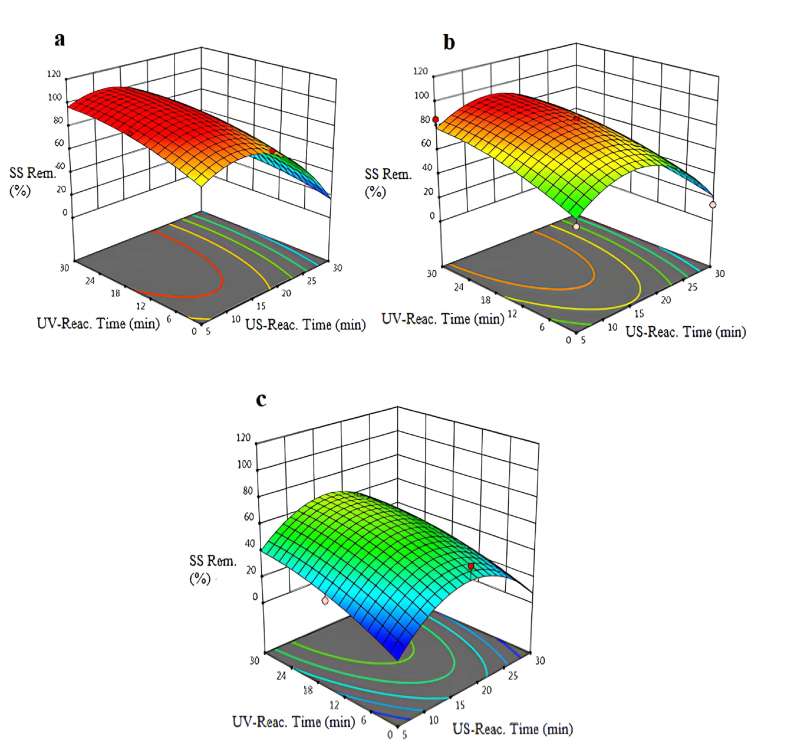
3.5. Suspended solid removal
The suspended solid (SS) removal efficiencies obtained with the US/UV process using different intensities of US are shown in Fig. 5(a–c). In the US/UV combined oxidation process, when experiments were performed with the intense minimum value, the suspended solids removal efficiency was observed as 98 % with the US time (10 min) and the UV time (20 min). The suspended solids removal efficiency was observed to be 78 % when the US process was used alone and the reaction time was adjusted to 10 min. When the US reaction time was increased to 25 min, the removal efficiency of suspended solids again decreased to 42 %. It was determined that the increase in the US reaction time at all intense values affected the SS removal efficiency negatively, similar to the color removal efficiency. Because bubbles resulted in turbidity during the oxidation process, low SS removal efficiencies were obtained at especially high intense values. When the UV reactor was operated as a single process, the SS removal efficiency was observed to be 65 % at an 18 min reaction time.
Fig. 5.
Variation of suspended solids removal percentages at pH = 5.5 and different intensities of US: a-intense:50 W/cm2: b-intense:75W/cm2: c-intense:100 W/cm2.
In brief, the SS removal efficiency should be adjusted to minimum intense values to obtain high suspended solids removals at low reaction times. Because the increase in the intensity value decreased the SS removal efficiency. US reaction time at all intense values had a negative effect on removal efficiency of SS. According to ANOVA test, the model F-value of 12.18 indicates that the model is significant. In this case X1, X2, X3, X1X2, X12 are important model terms.
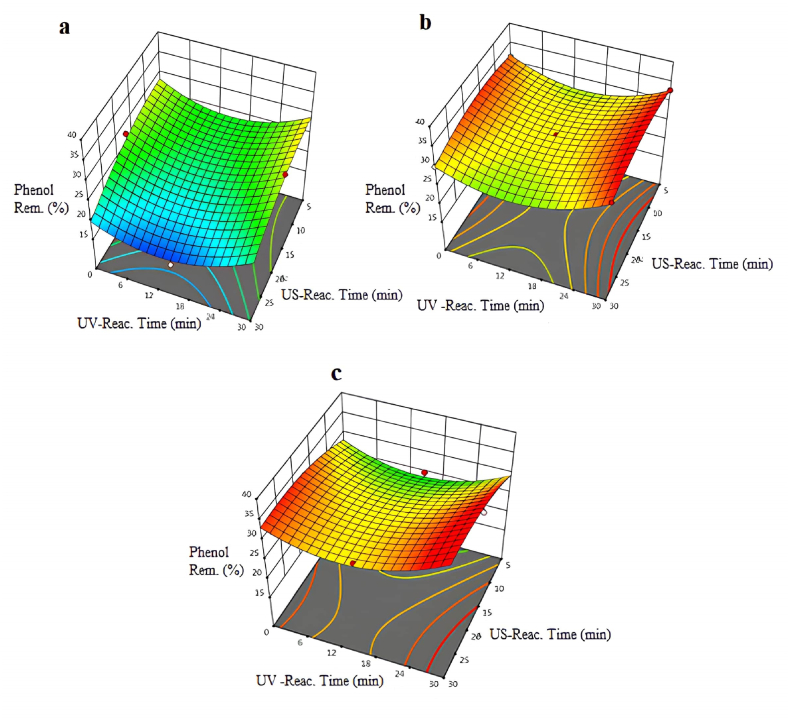
3.6. Phenol removal
The removal efficiencies of phenol obtained with the US/UV process using different intensities of US are shown in Fig. 6(a–c). According to the results, the incensement in the US reaction time had a positive effect on the removal efficiency of phenol at medium and low intensity of US in the US/UV combined process. When experiments were carried out at maximum intense values, the removal efficiency of phenol was observed to be 30 % at maximum reaction times. It was determined that the removal efficiency of phenol in the experiments decreased where the US reaction time was high at maximum intensity.
Fig. 6.
Variation of phenol removal percentages at pH = 5.5 and different intensities of US: a-intense:50 W/cm2: b-intense:75W/cm2: c-intense:100 W/cm2.
In a study executed by Rivas et al. [37], TOPW was treated using the wet air oxidation process (WAO). Results indicated that chemical oxygen demand (COD) reduction ranged from 30 % to 60 % after 6 h of treatment under specific reaction conditions (443–483 K and 3.0–7.0 MPa of total pressure using air). Furthermore, WAO was noted to effectively decrease phenolic content in the wastewater. In another investigation by Sounni et al. [38], electrocoagulation was employed to degrade olive oil mill wastewater (OOMW), resulting in the phenol removals as 80 % percentage within 50 min. Conversely, the usage of US/UV oxidation process at ambient conditions and short reaction times produced nearly 30 % removal efficiency of phenol in own study. This underscores the comparative effectiveness of different treatment methods and highlights the potential of combined approaches for short reaction times.
As a result, the efficiency of phenol removal was very low at minimum US or UV reaction times when the intensity was set to the minimum value. It was observed that the intensity of ultrasound was an important parameter for phenol removal efficiency. A model F-value of 20.20 indicates that the model is significant. In this case, X2, X1X2, X12, X22, X32 are important model terms.
When experimental results were evaluated with all parameters/independent variables with a statistical program, maximum COD, TOC, color, SS, and phenol removal efficiencies were achieved as 64 %, 52 %, 60 %, 87.5 %, and 22.3 %, respectively, under the same conditions in the US/UV oxidation process. The optimum reaction conditions were US reaction time (20 min), intensity (50 W/cm2), and UV reaction time (20 min) in the US/UV oxidation process for maximum removal efficiency. The present investigation introduces a modified ultrasound process in conjunction with ultraviolet light as an alternative method for TOPW. This oxidation process as an AOPs, specifically utilizing ultrasound and ultraviolet processes, is advocated for its capacity to eliminate or reduce sludge without the need for chemical additives. Furthermore, the ultrasound process, either stand-alone or combined with UV, emerges as a recommended treatment step for table olive wastewaters prior to aerobic biological oxidation. This is particularly crucial as it ensures the removal of toxic organic compounds, such as polyphenols, from the wastewater, enhancing the subsequent efficacy of the biological treatment unit.
Beltran et al. [33] found that 2.9 g of ozone, along with 240 mg of hydrogen peroxide, were required for an 80 % COD removal efficiency. However, in the absence of hydrogen peroxide, only 48 % COD removal efficiency was observed. In our study, the US/UV treatment process observed as a 64 % COD removal efficiency without the addition of any chemicals, thereby obviating the formation of sludge.
Another noteworthy aspect is the comparison with a study employing photocatalyst oxidation for table olive production wastewater treatment by Chatzisymeon, 2008. The latter involved varying concentrations of chemicals, including anatase TiO2 and H2O2. While this method exhibited some success in terms of COD reduction and color removal, the efficiency was highly dependent on the specific concentrations used. Depending on the reaction conditions applied, nearly complete color removal efficiency (>90 %) could be observed, while TOC removal efficiencies never exceeded 50 %. In this study, after adding a high amount of different oxidants and catalysts, COD removal efficiency and TOC removal efficiency did not reach 50 % removal efficiency. For that reason, the US/UV process is a suitable oxidation method to treat TOPW without any chemical addition. In addition, this process needs short reaction times. In this process, 62 % removal efficiency of COD was observed with not addition of any chemical.
According to experimental results, the US/UV oxidation process has significant potential for TOPW treatment. Although the disadvantage of AOPs is declared as energy consumption, at the optimized UV/US oxidation process, the low reaction time obtained from experiments will offer energy savings. UV/US oxidation process provides higher removal efficiencies at short reaction time, could be resulted in lower chemical or energy expenses. The advantage of the US/UV oxidation process is the no need addition of chemical that resulted in low sludge formation and low operational costs. The US/UV oxidation process can be executed to real wastewater. When US/UV oxidation process as a pretreatment process applied to TOPW before biological treatment methods, organic load, refractory organics compounds could be reduced and negative effects of toxic and recalcitrant compounds on biological process could be eliminated.
Operation costs that are the disadvantages for the large-scale of US/UV oxidation processes, should be studied and optimized in future works. In addition, intermediate products which may be more toxic than main product after oxidation process can be occurred, so this phenomenon should be evaluated more detailed in future works.
4. Conclusion
The US/UV oxidation process is an AOPs applied to TOPW. In the US/UV process, evaluations were made based on COD, phenol, TOC, color, and SS parameters. The reaction conditions and dependent variables affecting system efficiency varied for each parameter. The maximum removal efficiencies achieved with each parameter are given in the results section. When experimental results were evaluated with all parameters and independent variables using a statistical program, maximum COD, TOC, color, SS, and phenol removal efficiencies were achieved as 64 %, 52 %, 60 %, 87.5 %, and 22.3 %, respectively. The optimum reaction conditions were US reaction time (20 min), intensity (50 W/cm2), and UV reaction time (20 min) for maximum removal efficiencies. Based on these findings, the ultrasound/ultraviolet process, as an advanced oxidation method, emerges as a viable solution for treating table olive processing wastewater. This approach offers reduced reaction times, eliminated chemical expenses and minimized operational costs.
While US/UV process as a single process may not entirely treat the table olive processing wastewater (TOPW), integrating a biological or conventional treatment step post or pre-treatment unit enables safe discharge into the receiving environment. Moreover, for facilities situated within organized industrial zones, implementing the US/UV process allows for direct discharge into the zone's treatment facility without compromising operational efficiency or effectiveness.
Data availability statement
The data associated with this study has not been deposited into a publicly available repository because all experimental data have been included within the article itself.
Ethics and consent declarations
This study did not involve any human or animal subjects, and therefore, ethical approval and informed consent were not required.
CRediT authorship contribution statement
Ebru Çokay: Writing – review & editing, Writing – original draft, Validation, Software, Methodology, Formal analysis. Serkan Eker: Writing – review & editing, Validation, Software. Ecem Taşkın: Formal analysis.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
I would like to thank Prof. Dr. Nuri Azbar, a faculty member of Ege University Bioengineering Department, for his support.
References
- 1.Aldana J.C., Acero J.L., Alvarez P.M. Membrane filtration, activated sludge and solar photocatalytic technologies for the effective treatment of table olive processing wastewater. J. Environ. Chem. Eng. 2021;9 doi: 10.1016/j.jece.2021.105743. [DOI] [Google Scholar]
- 2.Delgado A., Chammem N., Issaoui M., Ammar E. Bioactive phytochemicals from olive (olea europaea) processing byproducts. Reference Series in Phytochemistry. 2022;10–1:1–31. doi: 10.1007/978-3-030-63961-7_10-1. [DOI] [Google Scholar]
- 3.Unal B.H., Canbolat C.B., Dizge N., Keskinler B. Treatability studies on optimizing coagulant type and dosage in combined coagulation/membrane processes for table olive processing wastewater. J. Water Proc. Eng. 2018;26:301–307. doi: 10.1016/j.jwpe.2018.10.023. [DOI] [Google Scholar]
- 4.Fernandez A.G., Adams M.R., Fernandez-Diez M.J. Springer Science & Business Media; Newyork: 1997. Table Olives: Production and Processing. [Google Scholar]
- 5.Asadollahfardi G., Hessami A., Gholizade A., Rezaei R. Removal of Reactive Blue 19 dye from synthetic wastewater using UV/H2O2 and UV/Cl advanced oxidation processes. Remed. J. 2023;33(2):167–176. doi: 10.1002/rem.21744. [DOI] [Google Scholar]
- 6.Shokri A. Investigation of UV/H2O2 process for removal of ortho-toluidine from industrial wastewater by response surface methodology based on the central composite design. Desalination Water Treat. 2017;58:258–266. doi: 10.5004/dwt.2017.0292. [DOI] [Google Scholar]
- 7.Kim J.K., Martinez F., Metcalfe I.S. The beneficial role of use of ultrasound in heterogeneous Fenton-like system over supported copper catalysts for degradation of p-chlorophenol. Catal. Today. 2007;124:224–231. doi: 10.1016/j.cattod.2007.03.040. [DOI] [Google Scholar]
- 8.Bagal M.V., Gogate P.R. Wastewater treatment using hybrid treatment schemes based on cavitation and Fenton chemistry: a review. Ultrason. Sonochem. 2014;21:1–14. doi: 10.1016/j.ultsonch.2013.07.009. [DOI] [PubMed] [Google Scholar]
- 9.Gholizade A., Asadollahfardi G., Rezaei R. Reactive Blue 19 dye removal by UV-LED/chlorine advanced oxidation process. Environ. Sci. Pollut. Res. 2023;30:1719. doi: 10.1007/s11356-022-22545-4. [DOI] [PubMed] [Google Scholar]
- 10.Shokri A., Mahanpoor K. Using UV/ZnO process for degradation of Acid Red 283 in synthetic wastewater. Bulg. Chem. Commun. 2018;50(1):27–32. [Google Scholar]
- 11.Zarei M., Khataee A.R., Ordikhani-Seyedlar R., Fathinia M. Photoelectro-Fenton combined with photocatalytic process for degradation of an azo dye using supported TiO2 nanoparticles and carbon nanotube cathode: neural network. Electrochim. Acta. 2010;55:7259–7265. doi: 10.1016/j.electacta.2010.07.050. [DOI] [Google Scholar]
- 12.Shokri A., Mahanpoor K. Removal of ortho-toluidine from industrial wastewater by UV/TiO2 process. Journal of Chemical Health Risks. 2016;6(3):213–223. [Google Scholar]
- 13.Abu Amr S.S., Aziz H.A. New treatment of stabilized leachate by ozone/Fenton in the advanced oxidation process. Waste Manag. 2012;32:1693–1698. doi: 10.1016/j.wasman.2012.04.009. [DOI] [PubMed] [Google Scholar]
- 14.Basturk E., Karatas M. Advanced oxidation of Reactive Blue 181 solution: a comparison. Ultrason. Sonochem. 2014;21:1881–1885. doi: 10.1016/j.ultsonch.2014.03.026. [DOI] [PubMed] [Google Scholar]
- 15.Gogate P.R., Pandit A.B. A review of imperative technologies for wastewater treatment II: hybrid methods. Adv. Environ. Res. 2004;8:553–597. doi: 10.1016/S1093-0191(03)00031-5. [DOI] [Google Scholar]
- 16.Zhong X., Royer S., Zhang H. Mesoporous silica irondoped as stable and efficient heterogeneous catalyst for the degradation of C.I. Acid Orange 7 using sono-photo-Fenton process. Separ. Purif. Technol. 2011;80:163–171. doi: 10.1016/j.seppur.2011.04.024. [DOI] [Google Scholar]
- 17.Poblete R., Cortes E., Salihoglu G., Salihoglu N.K. Ultrasound and heterogeneous photocatalysis for the treatment of vinasse from pisco production. Ultrason. Sonochem. 2020;61 doi: 10.1016/j.ultsonch.2019.104825. [DOI] [PubMed] [Google Scholar]
- 18.Bilińska L., Gmurek M. Novel trends in AOPs for textile wastewater treatment. Enhanced dye by-products removal by catalytic and synergistic actions. Water Resour. Ind. 2021;26 doi: 10.1016/j.wri.2021.100160. [DOI] [Google Scholar]
- 19.Cardenas Sierra R.S., Zuniga-Benitez H., Penuela G.A. Elimination of cephalexin and doxycycline under low frequency ultrasound. Ultrason. Sonochem. 2021;79 doi: 10.1016/j.ultsonch.2021.105777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.APHA (American Public Health Association) nineteenth ed. APHA; Washington, DC: 1992. Standard Methods for the Examination of Water and Wastewater. [Google Scholar]
- 21.Ragonese R., Macka M., Hughes J., Petocz P. The use of the Box- Behnken experimental design in the optimisation and robustness testing of a capillary electrophoresis method for the analysis of ethambutol hydrochloride in a pharmaceutical formulation. J. Pharm. Biomed. Anal. 2002;27:995–1007. doi: 10.1016/s0731-7085(01)00659-8. [DOI] [PubMed] [Google Scholar]
- 22.Sastry S.V., Khan M.A. Aqueous based polymeric dispersion: plackett- Burman design for screening of formulation variables of atenolol gastrointestinal therapeutic system. Pharm. Acta Helv. 1998;73:105–112. doi: 10.1016/s0031-6865(97)00052-6. [DOI] [PubMed] [Google Scholar]
- 23.Hamed E., Sakr A. Application of multiple response optimization technique to extended release formulations design. J. Contr. Release. 2001;73:329–338. doi: 10.1016/s0168-3659(01)00356-x. [DOI] [PubMed] [Google Scholar]
- 24.Ferreira S.L.C., Bruns R.E., Ferreira H.S., Matos G.D., David J.M., Brandão G.C., da Silva E.G.P., Portugal L.A., dos Reis P.S., Souza A.S., dos Santos W.N.L. Box-Behnken design: an alternative for the optimization of analytical methods. Anal. Chim. Acta. 2007;597(2):179–186. doi: 10.1016/j.aca.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 25.Shokri A. The treatment of spent caustic in the wastewater of olefin units by ozonation followed by electrocoagulation process. Desalination and Water. 2018;111:173–182. doi: 10.5004/dwt.2018.22248. [DOI] [Google Scholar]
- 26.Charles R.H., Kennneth V.T. Oxford: University Press; 1999. Fundamental Concepts in the Design of Experiments. [Google Scholar]
- 27.Abbasi A.G.F., Ahmad M., Wasim M. Optimization of concrete mix proportioning using reduced factorial experimental technique. American Concrete Institute, Journal of. 1987;9:55–63. doi: 10.14359/15162. [DOI] [Google Scholar]
- 28.Shokri A., Karimi S. Treatment of aqueous solution containing acid red 14 using an electro peroxone process and a box-behnken experimental design. Archives of Hygiene Sciences. 2020;9(1):48–57. doi: 10.29252/ArchHygSci.9.1.48. [DOI] [Google Scholar]
- 29.Shokri A., Nasernejad B. Treatment of spent caustic wastewater by electro-Fenton process: kinetics and cost analysis. Process Saf. Environ. Protect. 2023;172:836–845. doi: 10.1016/j.psep.2023.02.077. [DOI] [Google Scholar]
- 30.Shokri A. Degradation of 4-Chloro phenol in aqueous media thru UV/Persulfate method by Artificial Neural Network and full factorial design method. Int. J. Environ. Anal. Chem. 2022;102(17):5077–5091. doi: 10.1080/03067319.2020.1791328. [DOI] [Google Scholar]
- 31.Çokay E. Hydrogen gas production from food wastes by electrohydrolysis using a statical design approach. Int. J. Hydrogen Energy. 2018;43:10555–10561. doi: 10.1016/j.ijhydene.2018.01.079. [DOI] [Google Scholar]
- 32.Catalkaya E.C., Kargi F. Degradation and mineralization of simazine in aqueous solution by ozone/hydrogen peroxide advanced oxidation. J. Environ. Eng. 2009;135:1357–1364. doi: 10.1061/(ASCE)EE.1943-7870.0000112. [DOI] [Google Scholar]
- 33.Beltran-Heredia Torregrosa J., Dominguez J., Garcia J. Ozonation of black-table-olive industrial wastewaters: effect of an aerobic biological pretreatment. J. Chem. Technol. Biotechnol. 2000;75(7):561–568. doi: 10.1002/1097-4660(200007)75:7%3C561::AID-JCTB254%3E3.0.CO;2-B. [DOI] [Google Scholar]
- 34.Beltrań J.F., Garcia-Araya F.J., Frades J., Alvarez P., Gimeno O. Effects of single and combined ozonation with hydrogen peroxide or UV radiation on the chemical degradation and biodegradability of debittering table olive industrial wastewaters. Water Res. 1999;33:723–732. doi: 10.1016/S0043-1354(98)00239-5. [DOI] [Google Scholar]
- 35.Chatzisymeon E., Stypas E., Bousios S., Xekoukoulotakis N.P., Mantzavinos D. Photocatalytic treatment of black table olive processing wastewater. J. Hazard Mater. 2008;154:1090–1097. doi: 10.1016/j.jhazmat.2007.11.014. [DOI] [PubMed] [Google Scholar]
- 36.Besharatian S., Asadollahfardi G., Moussavi G. Feasibility study of metformin removal from synthetic wastewater using doped-TiO2 as catalyst and UVA-LED. Desalination Water Treat. 2020;175:68–78. doi: 10.5004/dwt.2020.24883. [DOI] [Google Scholar]
- 37.Rivas F.J., Beltran F.J., Gimeno O., Alvarez P. Chemical biological treatment of table olive manufacturing wastewater. J. Environ. Eng. 2001;127:611–619. doi: 10.1061/(ASCE)0733-9372(2001)127:7(611. [DOI] [Google Scholar]
- 38.Sounni F., Aissam H., Ghomari O., Merzouki M., Benlemlih M. Electrocoagulation of olive mill wastewaters to enhance biogas production. Biotechnol. Lett. 2018;40:297–301. doi: 10.1007/s10529-017-2464-5. 10.1007/s10529-017-2464-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data associated with this study has not been deposited into a publicly available repository because all experimental data have been included within the article itself.