Key Points
Question
What is the association between electronic health record (EHR) nudges and health care quality and outcomes in primary care?
Findings
In this systematic review including 54 randomized clinical trials, EHR nudges were associated with improvements in some areas of health care quality, such as descriptive and patient-centeredness measures. However, the evidence on other health care quality areas and clinical outcomes was less consistent.
Meaning
These results suggest that EHR nudges may help improve clinicians’ documentation behaviors through higher completeness and accuracy of office notes.
This systematic review investigates whether there is an association between electronic health record (EHR) nudges and health care outcomes in primary care settings and describes implementation facilitators and barriers.
Abstract
Importance
Nudges have been increasingly studied as a tool for facilitating behavior change and may represent a novel way to modify the electronic health record (EHR) to encourage evidence-based care.
Objective
To evaluate the association between EHR nudges and health care outcomes in primary care settings and describe implementation facilitators and barriers.
Evidence Review
On June 9, 2023, an electronic search was performed in PubMed, Embase, PsycINFO, CINAHL, and Web of Science for all articles about clinician-facing EHR nudges. After reviewing titles, abstracts, and full texts, the present review was restricted to articles that used a randomized clinical trial (RCT) design, focused on primary care settings, and evaluated the association between EHR nudges and health care quality and patient outcome measures. Two reviewers abstracted the following elements: country, targeted clinician types, medical conditions studied, length of evaluation period, study design, sample size, intervention conditions, nudge mechanisms, implementation facilitators and barriers encountered, and major findings. The findings were qualitatively reported by type of health care quality and patient outcome and type of primary care condition targeted. The Risk of Bias 2.0 tool was adapted to evaluate the studies based on RCT design (cluster, parallel, crossover). Studies were scored from 0 to 5 points, with higher scores indicating lower risk of bias.
Findings
Fifty-four studies met the inclusion criteria. Overall, most studies (79.6%) were assessed to have a moderate risk of bias. Most or all descriptive (eg, documentation patterns) (30 of 38) or patient-centeredness measures (4 of 4) had positive associations with EHR nudges. As for other measures of health care quality and patient outcomes, few had positive associations between EHR nudges and patient safety (4 of 12), effectiveness (19 of 48), efficiency (0 of 4), patient-reported outcomes (0 of 3), patient adherence (1 of 2), or clinical outcome measures (1 of 7).
Conclusions and Relevance
This systematic review found low- and moderate-quality evidence that suggested that EHR nudges were associated with improved descriptive measures (eg, documentation patterns). Meanwhile, it was unclear whether EHR nudges were associated with improvements in other areas of health care quality, such as effectiveness and patient safety outcomes. Future research is needed using longer evaluation periods, a broader range of primary care conditions, and in deimplementation contexts.
Introduction
Primary care clinicians are pivotal in population health by lowering health care costs and reducing mortality globally.1,2,3 Despite its importance, primary care delivery varies substantially across settings. Across the many conditions treated in primary care, evidence-to-practice gaps exist.4,5,6 To address this, research has examined how to change clinician behavior to encourage the use of evidence-based care. Nudges, or interventions that subtly guide individuals toward specific behaviors while preserving freedom of choice, represent one approach of interest.7
Nudges have been increasingly studied for their potential use in facilitating behavior change among individuals.8,9,10,11 Nudges do not require extensive resources to implement and target automatic cognitive processing, which may be used in situations when time is limited (eg, primary care setting). Nudges may potentially affect primary care clinicians’ behaviors.10,12 For instance, studies have found that a variety of nudges (eg, public commitments, social norm feedback) can potentially improve antibiotic prescribing for viral infections.10 Consequently, these encouraging results have led some health systems to examine how to improve the reach and sustainability of nudge interventions.
Since adoption of electronic health record (EHR) systems is high,13 the EHR represents an additional medium to disseminate nudge interventions.14 The EHR also offers the opportunity to present the nudge to clinicians in their workflows and allows health systems to monitor the effectiveness of implemented nudges across different medical specialties and clinician types. Clinicians may also benefit from EHR nudges through the reduction of cognitive load, especially those that aim to provide reminders, offer preselected options at decision points for review, or provide additional contextual information (eg, relative costs of medications) at the point of decision-making. In turn, this may help, in part, facilitate desired clinician behaviors. In light of a growing number of randomized clinical trials (RCTs) evaluating EHR nudges in primary care, there is a growing need to summarize these findings and determine if the benefits of EHR nudges apply uniformly to the different types of conditions that primary care clinicians typically manage. To our knowledge, EHR nudges have only been reviewed in the inpatient setting,11 which may differ from those used in primary care settings due to differences in EHR workflows, patient complexity, and alert fatigue.
To address this research gap, we conducted a systematic review to summarize how clinician-facing EHR nudges are associated with health care quality (eg, documentation patterns, ordering behaviors) and patient outcomes in primary care settings. We also summarized implementation facilitators and barriers of EHR nudge interventions. Findings from this line of research may reveal future research areas and influence policies aimed at improving evidence-based care delivery in primary care.
Methods
We reported this systematic review according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) reporting guideline.15 This review was registered with PROSPERO (CRD42022309599).
Data Sources and Search
We considered clinician-facing EHR nudges to include subtle modifications integrated into an EHR’s choice architecture, specifically designed to influence medical decision-making without limiting clinicians’ autonomy. These nudges are intended to guide clinicians toward preferred actions or decisions (eg, generic formulations over brand-name drugs) while allowing them to disregard the suggestions if they are not suitable or practical for a given patient (eg, patient allergies, lack of insurance coverage). Clinician-facing EHR nudges could include order sets or default dosages that represent the health system’s preferred treatment pathways for a given clinical situation. They may include alerts and/or reminders designed to more easily complete a desired action (eg, adding immunization orders, adding diagnosis to EHR problem list) and redesigning checklists or dropdown menus in documentation templates to display the health system’s preferred options first. Accordingly, in consultation with a health sciences librarian, we developed a search strategy (eTable 1 in Supplement 1) that included search terms for clinicians, EHRs, and nudges.
On June 9, 2023, we ran the search strategy on MEDLINE, Embase, PsycINFO, CINAHL, and Web of Science to identify relevant peer-reviewed literature. Our inclusion criteria included articles written in English, conducted in primary care settings (ie, family medicine, general internal medicine, general pediatrics), used a RCT design, used nudges developed within the EHR environment, and evaluated how the nudge was associated with health care quality and patient outcome measures. We excluded articles that were nonempirical (ie, commentaries, other reviews) or did not explicitly discuss what clinicians could do if they disagreed with a recommendation. Lastly, we hand-searched the reference lists of included studies to identify other potentially relevant articles.
Study Selection
Two reviewers were randomly assigned to independently screen the titles and abstracts of included studies. For full-text reviews, 2 reviewers were also randomly assigned to independently assess each article for eligibility. In both review stages, conflicts were resolved by a third reviewer. All reviews were conducted using the web-based software, Covidence.
Data Extraction and Quality Assessment
For each included study, 2 reviewers abstracted the following elements: country, targeted clinician types, medical conditions studied, length of evaluation period, study design, sample size, intervention characteristics, nudge mechanisms, implementation facilitators and barriers encountered, and major findings. Since a nudging intervention could involve several mechanisms, we mapped identified nudges to the nudging ladder model used in a prior systematic review.16 We adapted the Risk of Bias 2.0 tools to evaluate the studies based on randomized clinical trial design (cluster, parallel, crossover). Our team scored studies from 0 to 5 points, where those that scored 0 to 1 point were considered high risk of bias, 2 to 3 points were considered moderate risk of bias, and 4 to 5 points were considered low risk of bias.
Statistical Analysis
We were unable to conduct a meta-analysis because outcome definitions across studies differed immensely, multiple studies had missing information on precision estimates, and there was high prevalence of studies of moderate or high risk of bias.17,18 Thus, we used the narrative review approach to report results by outcome type and clinical concept.17 For health care quality, measure types were derived from a quality framework and included patient safety, effectiveness, patient-centeredness, timeliness, efficiency, and descriptive.19 For patient outcomes, measure types were formed after identifying themes in the findings and included patient-reported outcomes, patient adherence, and clinical outcomes. For clinical concept, we adapted a previously published taxonomy of the most common primary care conditions to classify disease types.20 For each reported finding, we included point estimates and P values or 95% CIs. We considered a 2-sided threshold of P < .05 as statistically significant. We also examined potential differences in findings if studies with high risk of bias were excluded from the analysis.
Results
Study Characteristics
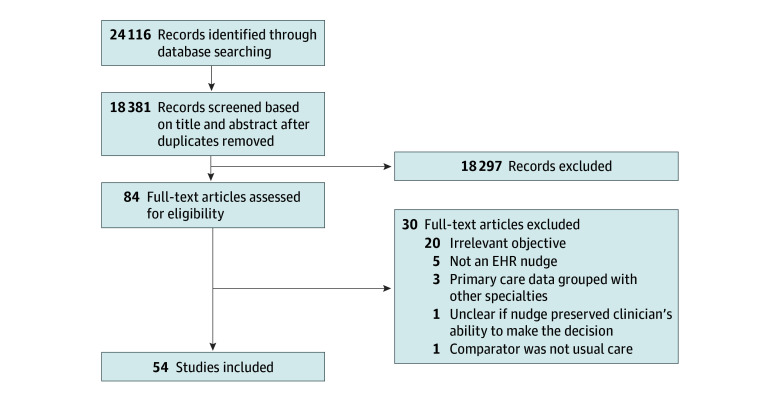
Overall, we found 18 381 articles and included 54 studies in this systematic review. The Figure illustrates the outcomes of our review process. Most studies were located in the US (79.6% [43 of 54]) and had a cluster RCT study design (63.0% [34 of 54]). Over time, there was a general trend in an increasing number of studies that use a RCT design to evaluate the EHR nudge. Approximately 68.5% of studies (37 of 54) implemented the EHR nudge alongside another intervention (eg, providing education, providing reference or support materials). Of the 34 studies that reported which EHR vendor their nudge was implemented in, 15 (44.1%) used Epic. Most studies evaluated process measures with few studies attempting to assess clinical outcome measures. Furthermore, when examining primary care conditions targeted, most measures pertained to chronic care management (eg, diabetes, hypertension) and preventative care (eg, breast cancer screening). Although physicians were the most studied clinician type, 15 studies also examined other types of clinicians, including nurse practitioners, physician assistants, registered nurses, and medical assistants. Most studies (79.6% [43 of 54]) used EHR nudges to facilitate the uptake of a targeted behavior while the remaining studies (20.4% [11 of 54]) used EHR nudges to facilitate a decrease in a targeted behavior. The number of studies that reported an association with improved outcomes was higher among studies facilitating an increase in a targeted behavior compared with those facilitating a decrease in a targeted behavior (54.7% vs. 9.1%). Lastly, only 7 studies formally assessed implementation factors. All of these studies identified implementation barriers, but none reported implementation facilitators (eTable 2 in Supplement 1). Table 1 displays a summary of the proportion of studies that showed positive, negative, and null associations of EHR nudges and each outcome type. Detailed results on how EHR nudges were associated with health care quality and outcome measures are shown in Table 2 and Table 3, respectively.
Figure. Study Flow Diagram.
EHR indicates electronic health record.
Table 1. Direction of Associations Between Electronic Health Record Nudges and Health Care Quality and Patient Outcomes.
| Outcome type | Total No. of measures | No. (%) | |||
|---|---|---|---|---|---|
| No. of measures with associations | No. of measures | ||||
| With improved outcomes | With worsened outcomes | With mixed associations | With null associations | ||
| Patient safety | 12 | 4 (33.3) | 0 | 2 (16.7) | 6 (50.0) |
| Effectiveness | 48 | 19 (39.6) | 0 | 13 (27.1) | 16 (25.0) |
| Patient-centeredness | 3 | 3 (100.0) | 0 | 0 | 0 |
| Timeliness | 0 | NA | NA | NA | NA |
| Efficiency | 4 | 0 | 0 | 0 | 4 (100.0) |
| Descriptive | 38 | 30 (78.9) | 0 | 1 (2.6) | 7 (18.4) |
| Patient-reported outcomes | 3 | 0 | 0 | 1 (33.3) | 2 (66.7) |
| Patient adherence | 2 | 1 (50.0) | 0 | 0 | 1 (50.0) |
| Clinical outcomes | 7 | 1 (14.3) | 0 | 0 | 6 (85.7) |
Abbreviation: NA, not applicable.
Table 2. Overall Findings of Association Between Electronic Health Record Nudges and Health Care Quality Measures.
| Measure type and concept | Citation(s) | Measures | Overall findingsa |
|---|---|---|---|
| Patient safety | |||
| Appropriate prescribing | Campbell et al,21 2021 | Anticholinergic discontinuation | 7.8% vs 8.2%; P = .65 |
| Appropriate prescribing | Kraemer et al,22 2022 | Opioid prescriptions at index visit | OR = 0.74 (95% CI, 0.46-1.18) |
| Appropriate prescribing | Kraemer et al,22 2022 | Continued use of opioid prescriptions | OR = 1.08 (95% CI, 0.94-1.24) |
| Appropriate prescribing | Kraemer et al,22 2022 | Concurrent opioid and benzodiazepine use | OR = 1.10 (95% CI, 0.94-1.29) |
| Appropriate prescribing | Tamblyn et al,23 2012 | Psychotropic prescription | Immediate-acting benzodiazepines: mean difference = −0.008 (95% CI, −0.05 to 0.03); long-acting benzodiazepines: mean difference = −0.006 (95% CI, −0.001 to −0.000); antidepressants: mean difference = −0.011 (95% CI, −0.03 to 0.01); immediate potency opiates: mean difference = 0.001 (95% CI, −0.00 to 0.01); low potency opiates: mean difference = −0.004 (95% CI, −0.01 to 0.00); anticonvulsants: mean difference = 0.006 (95% CI, −0.00 to 0.01); antipsychotics: mean difference = 0.005 (95% CI, −0.00 to 0.01) No.of psychotropic medications prescribed: mean difference = −0.02 (95% CI, −0.09 to 0.05) |
| Appropriate prescribing | Gill et al,24,2011; Abdel-Kader et al,25 2011 | Discontinued NSAIDs | Gill et al: OR = 1.18 (95% CI, 0.99-1.40); Abdel-Kader et al: OR = 1.43 (95% CI, 0.32-6.33) |
| Appropriate prescribing | Tamblyn et al,23 2012 | Drug-related injuries | Mean difference: −0.17 (95% CI, −0.32 to −0.02) |
| Appropriate prescribing | Flottorp et al,26 2002; Høye et al,27 2013; Meeker et al,28 2016; Gulliford et al,29 2019 | Ordering antibiotics for viral infections | Flottorp et al: −3.0% difference, P = .003 (pharyngitis); −0.4% difference, P = .64 (urinary tract infections); Høye et al: OR = 0.72 (95% CI, 0.60-0.86) (upper respiratory infections); Meeker et al: DiD: −5% (95% CI, −7.8 to 0.1) (suggested alternatives mechanism); DiD: −7% (95% CI, −9.1 to −2.9) (accountable justification mechanism); Gulliford et al: IRR = 0.88 (95% CI, 0.78-0.99 (upper respiratory infections) |
| Appropriate prescribing | Gill et al,24 2011 | Provided guideline-concordant care | OR = 1.19 (95% CI, 1.01-1.42) |
| Appropriate prescribing | Tamblyn et al,30 2003 | Inappropriate prescribing of new medications | RR = 0.82 (95% CI, 0.69-0.98) |
| Appropriate prescribing | Tamblyn et al,30 2003 | Inappropriate discontinuation of medications | RR = 1.06 (95% CI, 0.89-1.26) |
| Appropriate prescribing | Fortuna et al,31 2009 | Prescribing of heavily marketed hypnotics | RR = 0.74 (95% CI, 0.57-0.96) |
| Effectiveness | |||
| Diabetes | Sequist et al,32 2005 | Eye exam screening | HR = 1.38 (95% CI, 0.81-2.32) |
| Diabetes | Sequist et al,32 2005 | Receiving recommended diabetes care | OR = 1.30 (95% CI, 1.01-1.67) |
| Diabetes, hypertension | Sequist et al,32 2005; Abdel-Kader et al,25 2011; Sequist et al,33 2018; Tamblyn et al,34 2018 | Antihypertensive prescriptions | Sequist et al (2005): HR = 1.42 (95% CI, 0.94-2.14) (ACE inhibitors for patients with diabetes); HR = (95% CI, 0.72 - 1.63); (β-blockers for CAD); Abdel-Kader et al: OR = 0.84 (95% CI, 0.50-1.41) (ACE or ARB for CKD); Sequist et al (2018): 76% vs 79%, P = .17 (ACE or ARB for high-risk CKD); 64% vs 65%, P = .57 (ACE or ARB for low-risk CKD); Tamblyn et al: RR = 1.65 (95% CI, 1.17-2.33) (diuretics for newly diagnosed hypertension); RR = 0.61 (95% CI, 0.43-0.86) (other antihypertensives for newly diagnosed hypertension); RR = 1.10 (95% CI, 0.73-1.66) (prescribing 1 antihypertensive for newly diagnosed hypertension); RR = 0.91 (95% CI, 0.60-1.37) (prescribing 2 or more antihypertensives for newly diagnosed hypertension); RR = 1.09 (95% CI, 0.79-1.52) (diuretic for established hypertension); RR = 0.91 (95% CI, 0.66-1.27) (other antihypertensives for established hypertension); RR = 1.13 (95% CI, 0.90-1.42) (1 antihypertensive prescribed for established hypertension); RR = 0.88 (95% CI, 0.70-1.11) (2 or more antihypertensives prescribed for established hypertension) |
| Diabetes, other (CAD, hyperlipidemia) | Sequist et al,32 2005; Gill et al,35 2009; Adusumalli et al,36 2023 | Statin prescriptions | Sequist et al: HR = 1.10 (95% CI, 0.65-0.85 (only patients with diabetes); Sequist et al: HR = 1.51 (95% CI, 1.05-2.17) (only patients with CAD); Gill et al: OR = 0.05, P > .05 Adusumalli et al: 5.5% difference (95% CI, 3.2%-8.1%) |
| Immunizations | Frank et al,37 2004 | Tetanus immunizations | RR = 1.89 (95% CI, 1.59-2.25) |
| Immunizations | Frank et al,37 2004; Loo et al,38 2011 | Pneumococcal immunizations | Frank et al: RR = 1.70 (95% CI, 1.10-2.62); Loo et al: OR = 2.01 (95% CI, 1.30-3.11) |
| Immunizations | Frank et al,37 2004 | Measles, mumps, and rubella immunizations | RR = 1.25 (95% CI, 0.82-1.93) |
| Immunizations | Frank et al,37 2004; Fiks et al,39 2009; Loo et al,38 2011; Szilagyi et al,40 2015 | Influenza immunizations | Frank et al: RR = 0.96 (95% CI, 0.78-1.18); Fiks et al: OR = 1.22 (95% CI, 0.94-1.61); Loo et al: OR = 1.53 (95% CI, 1.23-1.91); Szilagyi et al: OR = 0.93 (95% CI, 0.69-1.25); OR = 0.89 (95% CI, 0.69-1.16) (reported data across 2 sites separately) |
| Immunizations | Fiks et al,41 2013; Szilagyi et al,40 2015 | HPV immunizations | Fiks et al: HR = 1.5 (95% CI, 1.2 - 2.0 (dose 1); HR = 1.0 (95% CI, 0.8-1.1) (dose 2); HR = 1.1 (95% CI, 0.9-1.3) (dose 3); Szilagyi et al: OR = 0.92 (95% CI, 0.60-1.40; OR = 0.96 (95% CI, 0.59-1.56) (dose 1 at 2 clinics); OR = 1.01 (95% CI, 0.57-1.77); OR = 1.06 (95% CI, 0.68-1.66) (dose 2 at 2 clinics); OR = 0.93 (95% CI, 0.69-1.25); OR = 1.13 (95% CI, 0.68-1.88) (dose 3 at 2 clinics) |
| Immunizations | Stockwell et al,42 2015; Szilagyi et al,40 2015; Stephens et al,43 2021 | Pediatric immunizations | Stockwell et al: RR = 0.90 (95% CI, 0.83-0.98) (up-to-date immunizations); Szilagyi et al: OR = 1.44 (95% CI, 0.82-2.56); OR = 1.16 (95% CI, 0.68-1.99) (Tdap from 2 clinics); OR = 1.15 (95% CI, 0.64-2.05); OR = 1.08 (95% CI, 0.82-1.41) (MCV4 at 3 clinics); Stephens et al: 3.7% difference (95% CI, 1.8%-5.6%) (among young children); 3.2% difference (95% CI, 0.6%-6.9%) (among adolescents); 0.8% difference, 95% CI, −0.3% to 1.8%) (under-immunizations) |
| Immunizations | Fiks et al,39 2009 | Having up-to-date influenza immunization | 3.4% difference (95% CI, −1.4 to 9.1) |
| Other (ADHD) | Co et al,44 2010 | Assessing ADHD care | OR = 2.2 (95% CI, 1.2-4.0) |
| Other (asthma) | Bell et al,45 2010 | Prescribing asthma controller | Urban practices: 6% difference, P = .006; suburban practices: 14% difference, P = .03 |
| Other (atrial fibrillation) | Karlsson et al,46 2018 | Anticoagulant prescription | 70.3% vs 70.0%, P = .01 |
| Other (atrial fibrillation) | McKie et al,47 2020 | Guideline-concordant care for atrial fibrillation | OR = 0.94 (95% CI, 0.15 - 5.94) |
| Other (CAD) | Sequist et al,32 2005; Sequist et al,48 2012 | Aspirin prescriptions | Sequist et al (2005): HR = 2.36 (95% CI, 1.37-4.07) (CAD); Sequist et al (2012): 20% vs 18%, P = .43 |
| Other (CAD) | Sequist et al,32 2005 | Receiving recommended coronary artery disease care | OR = 1.25 (95% CI, 1.01-1.55) |
| Other (chest pain) | Sequist et al,48 2012 | Echocardiograms | 51% vs 48%, P = .33 |
| Other (domestic violence screening) | Feder et al,49 2011 | Referral to domestic violence agency | IRR = 22.1 (95% CI, 11.5-42.4) |
| Other (GERD, drug side effects) | Player et al,50 2010; Gill et al,24 2011 | Prescribing gastroprotective medications | Player et al: OR = 1.11 (95% CI, 0.86-1.43) (newly diagnosed GERD); OR = 1.37 (95% CI, 1.12-1.68) (established patients with GERD); Gill et al: OR = 1.33 (95% CI, 1.01-1.74 (among patients receiving NSAIDs) |
| Other (heart failure) | McKie et al,47 2020 | Guideline-concordant care for heart failure | OR = 7.6 (95% CI, 1.2-47.5) |
| Other (hyperlipidemia) | McKie et al,47 2020 | Guideline-concordant care for hyperlipidemia | OR = 1.1 (95% CI, 0.6-1.8) |
| Other (kidney disease) | Abdel-Kader et al,25 2011; Sequist et al,33 2018 | Proteinuria assessment | Abdel-Kader et al: OR = 1.73 (95% CI, 0.77-3.87); Sequist et al: 71% vs 70%, P = .35 (patients with high risk of CKD); 45% vs 21%, P < .001 (patients with low risk of CKD) |
| Other (kidney disease) | Sequist et al,33 2018 | eGFR test | Patients with high risk of CKD: 89% vs 89%, P = .90; patients with low-risk of CKD: 82% vs 80%, P = .20 |
| Other (kidney disease) | Sequist et al,33 2018 | Hemoglobin test | Patients with high risk of CKD: 73% vs 73%, P = .63; patients with low risk of CKD: 61% vs 61%, P = .87 |
| Other (kidney disease) | Sequist et al,33 2018 | Phosphorous lab test | Patients with high risk of CKD: 49% vs 38%, P < .001; patients with low risk of CKD: 23% vs 13%, P < .001 |
| Other (kidney disease) | Sequist et al,33 2018 | 25-OH vitamin D lab | Patients with high risk of CKD: 53% vs 45%, P = .002; patients with low risk of CKD: 31% vs 24%, P = .004 |
| Other (kidney disease) | Sequist et al,33 2018 | Calcium lab | Patients with high risk of CKD: 75% vs 69%, P = .01; patients with low risk of CKD: 59% vs 54%, P = .11 |
| Other (kidney disease) | Sequist et al,33 2018 | Parathyroid hormone lab | Patients with high risk of CKD: 49% vs 39%, P < .001; patients with low risk of CKD: 24% vs 14%, P < .001 |
| Other (kidney disease) | Sequist et al,33 2018 | Annual nephrology visit | Patients with high risk of CKD: 45% vs 34%, P < .001 Patients with low risk of CKD: 17% vs 11%, P = .001 |
| Other (lab monitoring) | Palen et al,51 2006; Feldstein et al,52 2006 | Monitoring laboratory values | Palen et al: 56.6% vs 57.1%, P = .31; Feldstein et al: HR = 2.5 (95% CI, 1.8-3.5), P < .001 |
| Other (osteoporosis) | Loo et al,38 2011 | Bone density scan | OR = 1.43 (95% CI, 0.94-2.17) |
| Other (substance use) | Linder et al,66 2009 | Smoking cessation prescriptions | 2.0% vs 2.0%, P = .40 |
| Other (substance use) | Linder et al,66 2009 | Smoking counseling referrals | 4.5% vs 0.4%, P < .001 |
| Other (substance use) | Lee et al,54 2023 | Alcohol treatment initiation | 7.8% vs 6.2%, P = .04 |
| Other (substance use) | Lee et al,54 2023 | Positive alcohol screen | 18.0% vs 5.0%, P < .001 |
| Other (substance use) | Lee et al,54 2023 | Assessment DSM-5 Alcohol Symptom Checklist | 80.9% vs 4.1%, P < .001 |
| Other (weight issues) | Schriefer et al,55 2009 | Weight loss prescriptions | 0.5% vs 0.2%, P = .86 |
| Other (weight issues) | Schriefer et al,55 2009 | Weight loss program referrals | 1.1% vs 1.3%, P = 1.00 |
| Other (weight issues) | Schriefer et al,55 2009 | Bariatric surgery referrals | 0.8% vs 0.6%, P = 1.00 |
| Other (weight issues) | Schriefer et al,55 2009 | Combination therapy for patients with obesity | 11.9% vs 6.6%, P = .01 |
| Routine health maintenance (cancer screening) | Frank et al,37 2004 | Cervical smear test | RR = 1.09, 95% CI 0.91-1.29 |
| Routine health maintenance (cancer screening) | Hsu et al,56 2013 | HPV screening | 40.9% vs 1.1%, P < .001 |
| Routine health maintenance (hepatitis screening) | Hsu et al,56 2013; Chak et al,57 2018; Chak et al,58 2020 | Hepatitis B screening | Hsu et al: 34.1% vs 0.0%, P < .001; Chak et al (2018): OR = 3.13 (95% CI, 2.18-4.48); Chak et al (2020): OR = 3.23 (95% CI, 2.24-4.67) |
| Routine health maintenance (hepatitis screening) | Federman et al,59 2017 | Hepatitis C screening | OR = 8.99 (95% CI, 7.57-10.70) |
| Routine health maintenance (wellness screening) | Frank et al,37 2004; Sequist et al,32 2005; van Wyk et al,60 2008; Gill et al,35 2009; O’Connor et al,61 2011; Sequist et al,33 2018 | Lipids screening | Frank et al: RR = 0.89 (95% CI, 0.73-1.09); Sequist et al (2005): HR = 1.41 (95% CI, 1.15-1.72) (among patients with diabetes); HR = 0.99 (95% CI, 0.75-1.29) (among patients with CAD); van Wyk et al: RR = 1.76 (95% CI, 1.41-2.20) (dyslipidemia screening); RR = 1.40 (95% CI, 1.15-1.70) (dyslipidemia treatment); Gill et al: OR = 15.00, P < .05 (for patients of high risk); OR = 1.47, P > .05 (for patients of moderate risk); OR = 0.97, P > .05 (for patients of low risk); O’Connor et al: 3.3% difference, P = .14 (among patients with diabetes); Sequist et al (2018): 82% vs 83%, P = .24 (for patients of high risk of CKD); 72% vs 70%, P = .19 (for patients of low risk of CKD) |
| Routine health maintenance (wellness screening) | Frank et al,37 2004; Sequist et al,32 2005; O’Connor et al,61 2011; Zera et al,62 2015; Weiner et al,63 2020 | Diabetes screening | Frank et al: RR = 0.98 (95% CI, 0.65-1.48); Sequist et al: HR = 1.14 (95% CI, 0.89-1.46) (among patients with diabetes); O’Connor et al: 4.1% difference, P = .045 (among patients with diabetes); Zera et al: OR = 1.04 (95% CI, 0.79-1.38); Weiner et al: 0.72 vs 0.74, P = .07 (A1C tests); 1.55 vs 1.63, P = .49 (No.of glucose tests); 1.20 vs 1.22, P = .63 (No. of creatinine tests) |
| Routine health maintenance (wellness screening) | McDowell et al,64 1989; Frank et al,37 2004; O’Connor et al,61 2011; Kharbanda et al,65 2018 | Measuring blood pressure | McDowell et al: 30.7% vs 21.1%, P < .001; Frank et al: HR = 1.02 (95% CI, 0.90-1.16); O’Connor et al: 0.8% difference, P = .28 (patients with diabetes); Kharbanda et al: 14.3% vs 10.6%, P = .07 (within 30 d of index visit); 26.0% vs 23.4%, P = .46 (within 90 d of index visit) |
| Patient-centeredness | |||
| Other (weight issues) | Schriefer et al,55 2009 | Diet counseling for patients with obesity | 14.0% vs 7.3%, P = .002 |
| Other (weight issues) | Schriefer et al,55 2009 | Exercise counseling for patients with obesity | 12.1% vs 7.1%, P = .02 |
| Hypertension | Kressin et al,67 2016 | Blood pressure counseling | β = 1.10, P = .01 |
| Timeliness | |||
| NA | None | NA | NA |
| Efficiency | |||
| Upper respiratory infections, urinary tract infections | Flottorp et al,26 2002 | Ordering labs for viral infections | Urinary tract infections: −5.1% difference, P = .05; pharyngitis: −0.5% difference, P = .64 |
| Other (lab monitoring) | Lo et al,69 2009 | Appropriate ordering of labs | OR = 1.05 (95% CI, 0.75-1.46) |
| Other (chest pain) | Sequist et al,48 2012 | Cardiac stress testing | 10% vs 9%, P = .40 |
| Other (musculoskeletal pain) | Zafar et al,68 2019 | Lumbar spine MRI | Same-day orders: OR = 0.92 (95% CI, 0.68-1.25); orders within 30 d of visit: OR = 0.94 (95% CI, 0.70-1.25) |
| Descriptive | |||
| Routine health maintenance (wellness screening) | Frank et al,37 2004 | Documenting allergies | RR = 1.81 (95% CI, 1.63-2.02) |
| Routine health maintenance (wellness screening) | Frank et al,37 2004 | Documenting weight | RR = 1.28 (95% CI, 1.13-1.44) |
| Routine health maintenance (wellness screening) | Frank et al,37 2004 | Documenting smoking status | RR = 1.12 (95% CI, 0.90-1.39) |
| Other (weight issues) | Schriefer et al,55 2009; Tang et al,70 2012 | Diagnosis of obesity | Schriefer et al: 16.6% vs 10.7%, P = .02; Tang et al: OR = 4.1 (95% CI, 1.3-12.7) |
| Other (ADHD) | Co et al,44 2010 | Documenting ADHD symptoms | 100% vs 61.3%, P < .001 |
| Other (ADHD) | Co et al,44 2010 | Documenting ADHD treatment effectiveness | 96.6% vs 54.8%, P < .001 |
| Other (ADHD) | Co et al,44 2010 | Documenting ADHD adverse events | 96.6 vs 40.3%, P < .001 |
| Other (GERD) | Player et al,50 2010 | Diagnosis of GERD | OR = 1.33 (95% CI, 1.13-1.56) |
| Other (asthma) | Bell et al,45 2010 | Filing up-to-date asthma care plan | Urban practices: 1% difference, P > .05; suburban practices: 25% difference, P = .03 |
| Other (asthma) | Bell et al,45 2010 | Documenting spirometry | Urban practices: 3% difference, P = .04; suburban practices: 13% difference, P = .003 |
| Other (kidney disease) | Abdel-Kader et al,25 2011 | CKD documentation | OR = 1.23 (95% CI, 0.60-2.51) |
| Other (domestic violence screening) | Feder et al,49 2011 | Documenting domestic violence | IRR = 3.1 (95% CI, 2.2-4.3) |
| Other (weight issues) | Tang et al,70 2012 | Documenting results of weight counseling | OR = 2.1 (95% CI, 1.1-4.1) |
| Other (ADHD) | Wright et al,71 2012 | ADHD problem list | OR = 2.23 (P < .0001) |
| Other (asthma) | Wright et al,71 2012 | Asthma/COPD problem list | OR = 2.98 (P < .0001) |
| Other (cancer) | Wright et al,71 2012 | Breast cancer problem list | OR = 1.78 (P < .001) |
| Other (CAD) | Wright et al,71 2012 | CAD problem list | OR = 4.66 (P < .0001) |
| Other (coagulopathy) | Wright et al,71 2012 | Congenital coagulopathy problem list | OR = 2.06, P = .04, finding not significant due to a Bonferroni correction |
| Other (CHF) | Wright et al,71 2012 | CHF problem list | OR = 7.56, P < .0001 |
| Diabetes | Wright et al,71 2012 | Diabetes mellitus problem list | OR = 1.97, P < .0001 |
| Other (glaucoma) | Wright et al,71 2012 | Glaucoma problem list | OR = 3.78, P < .0001 |
| Hypertension | Wright et al,71 2012 | Hypertension problem list | OR = 4.12, P < .0001 |
| Other (thyroid issues) | Wright et al,71 2012 | Hyperthyroidism problem list | OR = 1.30, P = .29 |
| Other (thyroid issues) | Wright et al,71 2012 | Hypothyroidism problem list | OR = 3.99, P < .0001 |
| Other (autoimmune issues) | Wright et al,71 2012 | Myasthenia gravis problem list | OR = 2.10, P = .11 |
| Other (osteoporosis) | Wright et al,71 2012 | Osteoporosis/Osteopenia problem list | OR = 3.40, P < .0001 |
| Other (autoimmune issues) | Wright et al,71 2012 | Rheumatoid arthritis problem list | OR = 3.97, P < .0001 |
| Other (kidney issues) | Wright et al,71 2012 | Renal failure/insufficiency problem list | OR = 8.22, P < .0001 |
| Other (sickle cell disease) | Wright et al,71 2012 | Sickle cell disease problem list | OR = 1.66, P = .29 |
| Other (stroke) | Wright et al,71 2012 | Stroke problem list | OR = 2.35, P = .0002 |
| Other (social determinants of health screening) | Weiner et al,73 2022 | Contextual factors integrated into care plan | OR = 2.67 (95% CI, 1.32-5.41) |
| Other (gun storage screening) | Sigel et al,72 2023 | Documenting gun storage counseling | 51.2% vs 20.0%, P = .04 |
| Other (substance use) | Lee et al,54 2023 | Alcohol intervention documented | 57% vs 11%, P < .001 |
| Routine health maintenance (wellness screening) | Lee et al,54 2023 | Alcohol screening documented | 83.2% vs 20.8%, P < .001 |
| Other (substance use) | Lee et al,54 2023 | Alcohol diagnoses | 33.8% vs 28.8%, P = .003 |
| Other (designating a proxy) | Loo et al,38 2011 | Designation of health care proxy | OR = 1.55 (95% CI, 1.00-2.41) |
| Other (social determinants of health screening) | Weiner et al,63 2022 | Improving or resolving “red flags” | OR = 0.96 (95% CI, 0.57-1.63) |
| Other (social determinants of health screening) | Weiner et al,63 2022 | Probing “red flags” | OR = 2.12 (95% CI, 1.14-3.93) |
Abbreviations: ACE, angiotensin-converting enzyme inhibitors; ADHD, attention-deficit/hyperactivity disorder; ARB, angiotensin receptor blockers; CAD, coronary artery disease; CHF, coronary heart failure; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; DiD, difference-in-differences; eGFR, estimated glomerular filtration rate; EHR, electronic health records system; GERD, gastroesophageal reflux disease; HPV, human papillomavirus; HR, hazard ratio; IRR, incidence risk ratio; MRI, magnetic resonance imaging; NSAIDs, nonsteroidal anti-inflammatory drugs; OR, odds ratio.
All findings are reported with the EHR nudge first before the usual care. For studies reporting ratios, the comparator is usual care.
Table 3. Overall Findings of Association Between EHR Nudges and Patient Outcomes in Primary Care.
| Measure type and concept | Citation(s) | Measures | Overall findingsa |
|---|---|---|---|
| Patient-reported outcomes | |||
| Hypertension | Kressin et al,67 2016 | Antihypertensive adherence via self-reports | β = −0.34, P = .39 |
| Other (pain management) | Dhingra et al,74 2021 | Pain intensity | Worst pain intensity scores: β = −0.50 (95% CI, −0.91 to −0.08); average pain intensity scores: β = −0.12 (95% CI, −0.57 to 0.33) |
| Other (pain management) | Dhingra et al,74 2021 | Pain interference | β = −1.33 (95% CI, −3.05 to 0.39) |
| Patient adherence | |||
| Routine health maintenance (cancer screening) | Guiriguet et al,75 2016 | Colorectal cancer screening completed by patients | OR = 1.11 (95% CI, 1.02-1.22) |
| Hypertension | Tamblyn et al,34 2018 | Antihypertensive adherence via EHR data | Newly diagnosed uncomplicated hypertension: risk difference = 1.72 (95% CI, −6.91 to 10.36); established diagnoses: risk difference = 1.36 (95% CI, −1.55 to 4.28) |
| Clinical outcomes | |||
| Other (hyperlipidemia) | Gill et al,35 2009 | At-goal lipid values | High-risk patients: OR = 1.17, P > .05; moderate-risk patients: OR = 0.29, P > .05; low-risk patients: OR = 1.74, P > .05 |
| Hypertension | Abdel-Kader et al,25 2011 | Blood pressure <130/80 | OR = 2.07 (95% CI, 0.83-5.17) |
| Hypertension | Kressin et al,67 2016 | Diastolic blood pressure | β = −0.70, P = .06 |
| Hypertension | Kressin et al,67 2016 | Systolic blood pressure | β = −0.62, P = .05 |
| Other (atrial fibrillation) | Karlsson et al,46 2018 | Having stroke, transient ischemic attack, or systemic thromboembolism | 49 vs 47, P = .64 |
| Other (atrial fibrillation) | Karlsson et al,46 2018 | Having bleeding | 12 vs 16, P = .04 |
| Diabetes | Weiner et al,63 2020 | Hypoglycemia | 5% vs 5%, P = .99 |
Abbreviations: EHR, electronic health records system; OR, odds ratio.
All findings are reported with the EHR nudge first before the usual care. For studies reporting ratios, the comparator is usual care.
Study Quality Assessment Results
Overall, most studies (79.6%) were assessed to have a moderate risk of bias. For all cluster and parallel RCTs, there was limited measurement of clinicians’ adherence rate to the nudge intervention (ie, acknowledging the presence of the nudge by explicitly accepting or declining recommendations) in the final analysis. For crossover RCTs, there were concerns of short washout periods before administering the next phase. Across all RCTs, it was largely unclear whether outcome assessors were masked on study phases during the analysis and whether analytic methods used were predetermined prior to data collection. Some studies also experienced unexpected challenges in implementing or evaluating their EHR nudge as initially planned, such as hardware and software issues, concurrent quality improvement efforts, and changes in insurance coverage. Overall trends in study findings persisted even after removal of studies with high risk of bias (eTable 3 in Supplement 1).
Health Care Quality
Patient Safety
Eleven studies together assessed 12 unique patient safety measures with all targeting appropriate medication prescribing patterns.21,22,23,24,25,26,27,28,29,30,31 Medications examined included antibiotics, opiates, anticholinergics, hypnotics, benzodiazepines, and nonsteroidal anti-inflammatory drugs (NSAIDs). Overall, studies reported mixed associations. EHR nudges were associated with improvements for 4 of the 12 measures. Another 2 measures were associated with improvements but under specific contexts (ie, disease type, nudge characteristics). For example, EHR nudges were associated with improvements in antibiotic prescribing for pharyngitis (−3.0% difference; P = .003) but not for urinary tract infections (−0.4% difference; P = .64). Nudges that included accountable justification mechanisms were associated with more appropriate prescribing compared with nudges that suggested alternatives.
Effectiveness
Thirty-seven studies together assessed 48 unique effectiveness measures.24,25,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66 Clinical concepts included immunizations, wellness screening, cancer screening, and chronic care management. Overall, we observed mixed associations. EHR nudges were associated with improvements for 19 of the measures, such as increased rates of tetanus and pneumococcal immunizations; higher screening rates for hepatitis B and alcohol use; and providing guideline-concordant care for patients with heart failure or coronary artery disease. An additional 13 measures also were associated with improvements under specific contexts (eg, medical condition, patient risk level, time since diagnosis).
Patient-Centeredness
Two studies together assessed 3 unique patient-centeredness measures.55,67 Clinical concepts were limited to weight management and hypertension. Overall, EHR nudges were associated with improvements in counseling rates for exercise, diet, and blood pressure.
Timeliness
No study that we found assessed the association between EHR nudges and timeliness of care. Therefore, we were unable to assess for timeliness of care.
Efficiency
Four studies together assessed 4 unique efficiency measures in the context of reducing inappropriate or contraindicated laboratory tests and imaging.26,48,68,69 Overall, EHR nudges were not associated with improvements in any study.
Descriptive
Thirteen studies together assessed 38 unique descriptive measures.25,37,38,44,45,49,50,54,55,70,71,72,73 Overall, most studies found that EHR nudges were associated with improvements of various documentation and care planning patterns with fewer studies reporting null associations.
Patient Outcomes
Patient-Reported Outcomes
Two studies together assessed 3 unique patient-reported outcome measures. These included measures for pain symptoms and self-reported medication adherence.67,74 EHR nudges were associated with improvements in pain intensity and null associations with medication adherence and pain interference.
Patient Adherence
Two studies together assessed 2 unique patient adherence measures using objective data sources (eg, EHR data).34,75 One study found that EHR nudges were associated with improved rates of completed colorectal screening kits by patients, whereas the other study found null association for antihypertensive adherence based on EHR data.
Clinical Outcomes
Five studies together assessed 7 unique clinical outcomes, including blood pressure, blood glucose, and hyperlipidemia levels as well as risk levels for bleeding among patients with atrial fibrillation and stroke.25,35,46,63,67 Overall, EHR nudges had null associations for most measures. However, EHR nudges were associated with improvements in the risk of bleeding among patients with atrial fibrillation.
Implementation Facilitators and Barriers
Seven studies assessed clinicians’ viewpoints on implementation facilitators and barriers. Clinicians identified barriers, such as lack of knowledge or disagreement with guidelines and perceived errors in the programming of the nudge in the EHR (eTable 2 in Supplement 1).
Discussion
This systematic review aimed to summarize available RCTs evaluating how EHR nudges were associated with health care quality and outcomes in primary care settings. Overall, our findings suggest that EHR nudges may improve specific dimensions of health care quality (ie, descriptive, patient-centeredness). There were less consistent findings about how EHR nudges were associated with other health care quality measures (eg, effectiveness) and patient outcomes. To our knowledge, no study has attempted to assess how EHR nudges were associated with timeliness of care.
We found that EHR nudges may be associated with modest improvements in descriptive measures of health care quality. This suggests that EHR nudges may facilitate improvements in clinicians’ documentation patterns, such as completeness or accuracy. Interestingly, this pattern was less consistent for studies targeting clinicians’ ordering behaviors. This difference may be, in part, due to differences in nudge mechanisms and uptake of the nudge. For instance, templates were more common among studies that aimed to improve documentation patterns compared with ordering behaviors. Although most studies in our review did not measure clinician adherence to the nudge, other studies have found that primary care clinicians have increasingly adopted templates to support their documentation (29% in 2007-2008 vs more than 90% in 2018-2020).53,76 This suggests that higher clinician acceptance of a nudge or its fit in the workflow may be important factors that influence the effectiveness of the nudge. Other factors may include implementation contexts and approaches. Few studies in our review attempted to assess implementation facilitators and barriers of their nudges. Thus, further qualitative research on clinicians’ attitudes and perceptions on current EHR nudges are needed to inform efforts on how to optimize the delivery of EHR nudges in primary care contexts.
In comparison to descriptive and patient-centeredness measures, other types of health care quality measures (ie, patient safety, effectiveness, efficiency) showed relatively less consistent improvement. Although there were several studies that suggested associations between EHR nudges and improved ordering behaviors, there were also just as many studies reporting null associations. One reason for these different findings may be that the effect varies across clinical conditions. For instance, most studies examining EHR nudges related to substance use revealed a pattern of improved outcomes. Another reason for these differences in findings may stem from the presence of additional factors, such as patient preferences and insurance coverage, that may influence whether orders are placed. One dimension of health care quality that has been relatively understudied is timeliness in primary care, revealing an area in need of additional research.
Our review found that most of the studies assessing patient outcomes assessed them within a 1-year timeframe, suggesting that additional studies measuring sustainability of EHR nudges are needed. Consequently, our findings should be interpreted as short-term outcomes. Like other systematic reviews of nudge interventions in and outside of the EHR,10,11 a majority of the studies in our review only examined short-term outcomes. Thus, the long-term outcomes of EHR nudges remain unclear. Furthermore, most of the available studies examined metabolic diseases (hyperlipidemia, hypertension, diabetes). Meanwhile, to our knowledge, no RCT has been conducted that targets other diseases that are treated and managed in primary care, such as depression, anxiety, chronic obstructive pulmonary disease, and asthma. Consequently, additional research is needed on more diverse types of patient outcomes and with longer evaluation periods.
Our systematic review revealed that there were only 11 studies (20.4%) that used the EHR nudge to facilitate a decrease in a targeted behavior, such as reducing antibiotic prescribing for viral infections, imaging orders for low back pain, and reducing overprescribing of hypnotics and psychotropic drugs. Together, these accounted for 11 unique measures. Only 1 of the 11 measures (9.1%) reported that EHR nudges were associated with a decrease in the targeted behavior (a decrease in inappropriate prescribing across a variety of conditions). In contrast, EHR nudges were associated with improvements for 52 of the 95 measures (54.7%) where the EHR nudge was designed and implemented to facilitate an increase in a targeted behavior. Due to the low number of available studies involving EHR nudges to deimplement specific practices, it may be premature to conclude that EHR nudges may be better suited for increasing behavior frequency. Nonetheless, it remains unclear what the reasons are for this potential difference between nudges designed to decrease a behavior compared with those designed to increase a behavior. There exists an ongoing need for additional studies examining EHR nudges with the intent to decrease a behavior as well as qualitative research on how EHR nudges could help overcome inertia of reducing, restricting, removing, or replacing low-value clinical practices. These findings may provide additional insights as to whether EHR nudges should be designed differently to deimplement a particular practice or whether different concurrent interventions (eg, audit and feedback, insurance payment structures) need to be in place.
Limitations
This review has limitations. First, most studies targeted physicians or nurses. Furthermore, of the few studies that examined nonphysicians, combined samples of physicians and another clinician type were used. It remains unclear if different members of the care team respond differently to EHR nudges. Second, few studies were conducted in safety-net settings, such as federally qualified health centers, that may require different approaches to implementing EHR nudges due to lower available resources and less advanced EHR systems.77 Third, EHR nudges may be partly influenced by user interface design elements by EHR vendors. The most commonly used EHR was Epic, limiting generalizability to other EHR vendors. Fourth, most individual measures were assessed by only 1 study, precluding definitive conclusions on how EHR nudges are associated with performance in specific areas (eg, documenting allergies, blood pressure). Additionally, our quality appraisal revealed that none of the included studies controlled for adherence to evaluate EHR nudges. The observed outcomes of an intervention may be partly influenced by how often it is used, which makes the interpretation of findings challenging. For instance, it is unclear whether null results were due to low efficacy of the EHR nudge vs implementation issues of the nudge (eg, lack of awareness among clinicians of the nudge’s existence).
Based on these limitations, we discuss additional areas for future research. First, studies are needed that explicitly target other key members of the care team, such as social workers, pharmacists, and dietitians. Different care team members often have differing EHR workflows, which may influence how they respond to EHR nudges. Second, although outside of the scope of this review, patients can also be targeted by nudge interventions. Further studies are needed that assess how patients respond to nudges as well as studies that compare nudge effectiveness between patients and clinicians. Third, additional research is needed to assess potential facilitators and barriers that may be encountered when implementing EHR nudges in care settings. In a similar vein, there is a need for comparative evaluations of different EHR interfaces used to deliver the nudge to better understand what specific design elements facilitate the salience and effectiveness of the nudge. Additionally, because the number of studies that examined process measures outnumbered those that examined patient outcome measures, there persists a need for studies that assess how EHR nudges may influence downstream patient outcomes.
Conclusions
EHR-based nudge interventions are a promising strategy for improving evidence-based care delivery in primary care, a setting that is critical for supporting population health. Findings from this systematic review suggest that nudges may change specific dimensions of health care quality, such as descriptive measures (ie, documentation patterns). Their associations with other dimensions and clinical outcomes have been less consistent. To strengthen the evidence for nudge interventions, additional implementation studies are needed to understand the context of when nudge interventions work.
eTable 1. Search Strategy
eTable 2. Study Characteristics
eTable 3. Study Quality Assessment Results
Data Sharing Statement
References
- 1.Franks P, Fiscella K. Primary care physicians and specialists as personal physicians. health care expenditures and mortality experience. J Fam Pract. 1998;47(2):105-109. [PubMed] [Google Scholar]
- 2.Basu S, Berkowitz SA, Phillips RL, Bitton A, Landon BE, Phillips RS. Association of primary care physician supply with population mortality in the United States, 2005-2015. JAMA Intern Med. 2019;179(4):506-514. doi: 10.1001/jamainternmed.2018.7624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baker R, Freeman GK, Haggerty JL, Bankart MJ, Nockels KH. Primary medical care continuity and patient mortality: a systematic review. Br J Gen Pract. 2020;70(698):e600-e611. doi: 10.3399/bjgp20X712289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Akincigil A, Matthews EB. National rates and patterns of depression screening in primary care: results from 2012 and 2013. Psychiatr Serv. 2017;68(7):660-666. doi: 10.1176/appi.ps.201600096 [DOI] [PubMed] [Google Scholar]
- 5.Pedersen RA, Petursson H, Hetlevik I. Stroke follow-up in primary care: a prospective cohort study on guideline adherence. BMC Fam Pract. 2018;19(1):179. doi: 10.1186/s12875-018-0872-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Niven DJ, Mrklas KJ, Holodinsky JK, et al. Towards understanding the de-adoption of low-value clinical practices: a scoping review. BMC Med. 2015;13:255. doi: 10.1186/s12916-015-0488-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thaler RH, Sunstein CR. Nudge: Improving Decisions about Health, Wealth, and Happiness. Yale University Press; 2008. [Google Scholar]
- 8.Ledderer L, Kjær M, Madsen EK, Busch J, Fage-Butler A. Nudging in public health lifestyle interventions: a systematic literature review and metasynthesis. Health Educ Behav. 2020;47(5):749-764. doi: 10.1177/1090198120931788 [DOI] [PubMed] [Google Scholar]
- 9.Bucher T, Collins C, Rollo ME, et al. Nudging consumers towards healthier choices: a systematic review of positional influences on food choice. Br J Nutr. 2016;115(12):2252-2263. doi: 10.1017/S0007114516001653 [DOI] [PubMed] [Google Scholar]
- 10.Raban MZ, Gonzalez G, Nguyen AD, et al. Nudge interventions to reduce unnecessary antibiotic prescribing in primary care: a systematic review. BMJ Open. 2023;13(1):e062688. doi: 10.1136/bmjopen-2022-062688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Raban MZ, Gates PJ, Gamboa S, Gonzalez G, Westbrook JI. Effectiveness of non-interruptive nudge interventions in electronic health records to improve the delivery of care in hospitals: a systematic review. J Am Med Inform Assoc. 2023;30(7):1313-1322. doi: 10.1093/jamia/ocad083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Van Den Bulck S, Spitaels D, Vaes B, Goderis G, Hermens R, Vankrunkelsven P. The effect of electronic audits and feedback in primary care and factors that contribute to their effectiveness: a systematic review. Int J Qual Health Care. 2020;32(10):708-720. doi: 10.1093/intqhc/mzaa128 [DOI] [PubMed] [Google Scholar]
- 13.Henry J, Pylypchuk Y, Searcy T, Patel V. Adoption of electronic health record systems among U.S. non-federal acute care hospitals: 2008-2015. Accessed February 18, 2019. https://dashboard.healthit.gov/evaluations/data-briefs/non-federal-acute-care-hospital-ehr-adoption-2008-2015.php
- 14.Wolf A, Sant’Anna A, Vilhelmsson A. Using nudges to promote clinical decision making of healthcare professionals: a scoping review. Prev Med. 2022;164:107320. doi: 10.1016/j.ypmed.2022.107320 [DOI] [PubMed] [Google Scholar]
- 15.Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Last BS, Buttenheim AM, Timon CE, Mitra N, Beidas RS. Systematic review of clinician-directed nudges in healthcare contexts. BMJ Open. 2021;11(7):e048801. doi: 10.1136/bmjopen-2021-048801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shamseer L, Shea B, Hutton B, Moher D. Reporting and appraisal of systematic reviews. In: Egger M, Higgins JPT, Smith GD, eds. Systematic Reviews in Health Research: Meta-Analysis in Context. John Wiley & Sons; 2022:109-128. doi: 10.1002/9781119099369.ch7 [DOI] [Google Scholar]
- 18.McKenzie JE, Brennan SE. Synthesizing and presenting findings using other methods. Published 2023. Accessed May 10, 2024. https://training.cochrane.org/handbook/current/chapter-12#section-12-1
- 19.Agency for Healthcare Research and Quality . Examples of physician quality measures for consumers. Accessed December 20, 2023. https://www.ahrq.gov/talkingquality/measures/setting/physician/examples.html
- 20.Finley CR, Chan DS, Garrison S, et al. What are the most common conditions in primary care? systematic review. Can Fam Physician. 2018;64(11):832-840. [PMC free article] [PubMed] [Google Scholar]
- 21.Campbell NL, Holden RJ, Tang Q, et al. Multicomponent behavioral intervention to reduce exposure to anticholinergics in primary care older adults. J Am Geriatr Soc. 2021;69(6):1490-1499. doi: 10.1111/jgs.17121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kraemer KL, Althouse AD, Salay M, et al. Effect of different interventions to help primary care clinicians avoid unsafe opioid prescribing in opioid-naive patients with acute noncancer pain: a cluster randomized clinical trial. JAMA Health Forum. 2022;3(7):e222263. doi: 10.1001/jamahealthforum.2022.2263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tamblyn R, Eguale T, Buckeridge DL, et al. The effectiveness of a new generation of computerized drug alerts in reducing the risk of injury from drug side effects: a cluster randomized trial. J Am Med Inform Assoc. 2012;19(4):635-643. doi: 10.1136/amiajnl-2011-000609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gill JM, Mainous AG III, Koopman RJ, et al. Impact of EHR-based clinical decision support on adherence to guidelines for patients on NSAIDs: a randomized controlled trial. Ann Fam Med. 2011;9(1):22-30. doi: 10.1370/afm.1172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abdel-Kader K, Fischer GS, Li J, Moore CG, Hess R, Unruh ML. Automated clinical reminders for primary care providers in the care of CKD: a small cluster-randomized controlled trial. Am J Kidney Dis. 2011;58(6):894-902. doi: 10.1053/j.ajkd.2011.08.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Flottorp S, Oxman AD, Håvelsrud K, Treweek S, Herrin J. Cluster randomised controlled trial of tailored interventions to improve the management of urinary tract infections in women and sore throat. BMJ. 2002;325(7360):367. doi: 10.1136/bmj.325.7360.367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Høye S, Gjelstad S, Lindbæk M. Effects on antibiotic dispensing rates of interventions to promote delayed prescribing for respiratory tract infections in primary care. Br J Gen Pract. 2013;63(616):e777-e786. doi: 10.3399/bjgp13X674468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meeker D, Linder JA, Fox CR, et al. Effect of behavioral interventions on inappropriate antibiotic prescribing among primary care practices: a randomized clinical trial. JAMA. 2016;315(6):562-570. doi: 10.1001/jama.2016.0275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gulliford MC, Prevost AT, Charlton J, et al. Effectiveness and safety of electronically delivered prescribing feedback and decision support on antibiotic use for respiratory illness in primary care: REDUCE cluster randomised trial. BMJ. 2019;364:l236. doi: 10.1136/bmj.l236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tamblyn R, Huang A, Perreault R, et al. The medical office of the 21st century (MOXXI): effectiveness of computerized decision-making support in reducing inappropriate prescribing in primary care. CMAJ. 2003;169(6):549-556. [PMC free article] [PubMed] [Google Scholar]
- 31.Fortuna RJ, Zhang F, Ross-Degnan D, et al. Reducing the prescribing of heavily marketed medications: a randomized controlled trial. J Gen Intern Med. 2009;24(8):897-903. doi: 10.1007/s11606-009-1013-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sequist TD, Gandhi TK, Karson AS, et al. A randomized trial of electronic clinical reminders to improve quality of care for diabetes and coronary artery disease. J Am Med Inform Assoc. 2005;12(4):431-437. doi: 10.1197/jamia.M1788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sequist TD, Holliday AM, Orav EJ, Bates DW, Denker BM. Physician and patient tools to improve chronic kidney disease care. Am J Manag Care. 2018;24(4):e107-e114. [PubMed] [Google Scholar]
- 34.Tamblyn R, Winslade N, Qian CJ, Moraga T, Huang A. What is in your wallet? a cluster randomized trial of the effects of showing comparative patient out-of-pocket costs on primary care prescribing for uncomplicated hypertension. Implement Sci. 2018;13(1):7. doi: 10.1186/s13012-017-0701-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gill JM, Chen YX, Glutting JJ, Diamond JJ, Lieberman MI. Impact of decision support in electronic medical records on lipid management in primary care. Popul Health Manag. 2009;12(5):221-226. doi: 10.1089/pop.2009.0003 [DOI] [PubMed] [Google Scholar]
- 36.Adusumalli S, Westover JE, Jacoby DS, et al. Effect of passive choice and active choice interventions in the electronic health record to cardiologists on statin prescribing: a cluster randomized clinical trial. JAMA Cardiol. 2021;6(1):40-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Frank O, Litt J, Beilby J. Opportunistic electronic reminders. improving performance of preventive care in general practice. Aust Fam Physician. 2004;33(1-2):87-90. [PubMed] [Google Scholar]
- 38.Loo TS, Davis RB, Lipsitz LA, et al. Electronic medical record reminders and panel management to improve primary care of elderly patients. Arch Intern Med. 2011;171(17):1552-1558. doi: 10.1001/archinternmed.2011.394 [DOI] [PubMed] [Google Scholar]
- 39.Fiks AG, Hunter KF, Localio AR, et al. Impact of electronic health record-based alerts on influenza vaccination for children with asthma. Pediatrics. 2009;124(1):159-169. doi: 10.1542/peds.2008-2823 [DOI] [PubMed] [Google Scholar]
- 40.Szilagyi PG, Serwint JR, Humiston SG, et al. Effect of provider prompts on adolescent immunization rates: a randomized trial. Acad Pediatr. 2015;15(2):149-157. doi: 10.1016/j.acap.2014.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fiks AG, Grundmeier RW, Mayne S, et al. Effectiveness of decision support for families, clinicians, or both on HPV vaccine receipt. Pediatrics. 2013;131(6):1114-1124. doi: 10.1542/peds.2012-3122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stockwell MS, Catallozzi M, Camargo S, et al. Registry-linked electronic influenza vaccine provider reminders: a cluster-crossover trial. Pediatrics. 2015;135(1):e75-e82. doi: 10.1542/peds.2014-2616 [DOI] [PubMed] [Google Scholar]
- 43.Stephens AB, Wynn CS, Hofstetter AM, et al. Effect of electronic health record reminders for routine immunizations and immunizations needed for chronic medical conditions. Appl Clin Inform. 2021;12(5):1101-1109. doi: 10.1055/s-0041-1739516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Co JPT, Johnson SA, Poon EG, et al. Electronic health record decision support and quality of care for children with ADHD. Pediatrics. 2010;126(2):239-246. doi: 10.1542/peds.2009-0710 [DOI] [PubMed] [Google Scholar]
- 45.Bell LM, Grundmeier R, Localio R, et al. Electronic health record-based decision support to improve asthma care: a cluster-randomized trial. Pediatrics. 2010;125(4):e770-e777. doi: 10.1542/peds.2009-1385 [DOI] [PubMed] [Google Scholar]
- 46.Karlsson LO, Nilsson S, Bång M, Nilsson L, Charitakis E, Janzon M. A clinical decision support tool for improving adherence to guidelines on anticoagulant therapy in patients with atrial fibrillation at risk of stroke: A cluster-randomized trial in a Swedish primary care setting (the CDS-AF study). PLoS Med. 2018;15(3):e1002528. doi: 10.1371/journal.pmed.1002528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McKie PM, Kor DJ, Cook DA, et al. Computerized advisory decision support for cardiovascular diseases in primary care: a cluster randomized trial. Am J Med. 2020;133(6):750-756.e2. doi: 10.1016/j.amjmed.2019.10.039 [DOI] [PubMed] [Google Scholar]
- 48.Sequist TD, Morong SM, Marston A. Electronic risk alerts to improve primary care management of chest pain: a randomized, controlled trial. J Gen Intern Med. 2012;27:438-444. doi: 10.1007/s11606-011-1911-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Feder G, Davies RA, Baird K, et al. Identification and Referral to Improve Safety (IRIS) of women experiencing domestic violence with a primary care training and support programme: a cluster randomised controlled trial. Lancet. 2011;378(9805):1788-1795. doi: 10.1016/S0140-6736(11)61179-3 [DOI] [PubMed] [Google Scholar]
- 50.Player MS, Gill JM, Mainous AG III, et al. An electronic medical record-based intervention to improve quality of care for gastro-esophageal reflux disease (GERD) and atypical presentations of GERD. Qual Prim Care. 2010;18(4):223-229. [PubMed] [Google Scholar]
- 51.Palen TE, Raebel M, Lyons E, Magid DM. Evaluation of laboratory monitoring alerts within a computerized physician order entry system for medication orders. Am J Manag Care. 2006;12(7):389-395. [PubMed] [Google Scholar]
- 52.Feldstein A, Elmer PJ, Smith DH, et al. Electronic medical record reminder improves osteoporosis management after a fracture: a randomized, controlled trial. J Am Geriatr Soc. 2006;54(3):450-457. doi: 10.1111/j.1532-5415.2005.00618.x [DOI] [PubMed] [Google Scholar]
- 53.Linder JA, Schnipper JL, Middleton B. Method of electronic health record documentation and quality of primary care. J Am Med Inform Assoc. 2012;19(6):1019-1024. doi: 10.1136/amiajnl-2011-000788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lee AK, Bobb JF, Richards JE, et al. Integrating alcohol-related prevention and treatment into primary care: a cluster randomized implementation trial. JAMA Intern Med. 2023;183(4):319-328. doi: 10.1001/jamainternmed.2022.7083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schriefer SP, Landis SE, Turbow DJ, Patch SC. Effect of a computerized body mass index prompt on diagnosis and treatment of adult obesity. Fam Med. 2009;41(7):502-507. [PubMed] [Google Scholar]
- 56.Hsu L, Bowlus CL, Stewart SL, et al. Electronic messages increase hepatitis B screening in at-risk Asian American patients: a randomized, controlled trial. Dig Dis Sci. 2013;58(3):807-814. doi: 10.1007/s10620-012-2396-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chak E, Taefi A, Li CS, et al. Electronic medical alerts increase screening for chronic hepatitis B: a randomized, double-blind, controlled trial. Cancer Epidemiol Biomarkers Prev. 2018;27(11):1352-1357. doi: 10.1158/1055-9965.EPI-18-0448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chak E, Li CS, Chen MS Jr, MacDonald S, Bowlus C. Electronic health record alerts enhance mass screening for chronic hepatitis B. Sci Rep. 2020;10(1):19153. doi: 10.1038/s41598-020-75842-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Federman AD, Kil N, Kannry J, et al. An electronic health record-based intervention to promote hepatitis C virus testing among adults born between 1945 and 1965: a cluster-randomized trial. Med Care. 2017;55(6):590-597. doi: 10.1097/MLR.0000000000000715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.van Wyk JT, van Wijk MAM, Sturkenboom MCJM, Mosseveld M, Moorman PW, van der Lei J. Electronic alerts versus on-demand decision support to improve dyslipidemia treatment: a cluster randomized controlled trial. Circulation. 2008;117(3):371-378. doi: 10.1161/CIRCULATIONAHA.107.697201 [DOI] [PubMed] [Google Scholar]
- 61.O’Connor PJ, Sperl-Hillen JM, Rush WA, et al. Impact of electronic health record clinical decision support on diabetes care: a randomized trial. Ann Fam Med. 2011;9(1):12-21. doi: 10.1370/afm.1196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zera CA, Bates DW, Stuebe AM, Ecker JL, Seely EW. Diabetes screening reminder for women with prior gestational diabetes: a randomized controlled trial. Obstet Gynecol. 2015;126(1):109-114. doi: 10.1097/AOG.0000000000000883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Weiner M, Cummins J, Raji A, et al. A randomized study on the usefulness of an electronic outpatient hypoglycemia risk calculator for clinicians of patients with diabetes in a safety-net institution. Curr Med Res Opin. 2020;36(4):583-593. doi: 10.1080/03007995.2020.1717451 [DOI] [PubMed] [Google Scholar]
- 64.McDowell I, Newell C, Rosser W. A randomized trial of computerized reminders for blood pressure screening in primary care. Med Care. 1989;27(3):297-305. doi: 10.1097/00005650-198903000-00008 [DOI] [PubMed] [Google Scholar]
- 65.Kharbanda EO, Asche SE, Sinaiko A, et al. Evaluation of an electronic clinical decision support tool for incident elevated BP in adolescents. Acad Pediatr. 2018;18(1):43-50. doi: 10.1016/j.acap.2017.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Linder JA, Rigotti NA, Schneider LI, Kelley JHK, Brawarsky P, Haas JS. An electronic health record-based intervention to improve tobacco treatment in primary care: a cluster-randomized controlled trial. Arch Intern Med. 2009;169(8):781-787. doi: 10.1001/archinternmed.2009.53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kressin NR, Long JA, Glickman ME, et al. A brief, multifaceted, generic intervention to improve blood pressure control and reduce disparities had little effect. Ethn Dis. 2016;26(1):27-36. doi: 10.18865/ed.26.1.27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zafar HM, Ip IK, Mills AM, Raja AS, Langlotz CP, Khorasani R. Effect of clinical decision support-generated report cards versus real-time alerts on primary care provider guideline adherence for low back pain outpatient lumbar spine MRI orders. AJR Am J Roentgenol. 2019;212(2):386-394. doi: 10.2214/AJR.18.19780 [DOI] [PubMed] [Google Scholar]
- 69.Lo HG, Matheny ME, Seger DL, Bates DW, Gandhi TK. Impact of non-interruptive medication laboratory monitoring alerts in ambulatory care. J Am Med Inform Assoc. 2009;16(1):66-71. doi: 10.1197/jamia.M2687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tang JW, Kushner RF, Cameron KA, Hicks B, Cooper AJ, Baker DW. Electronic tools to assist with identification and counseling for overweight patients: a randomized controlled trial. J Gen Intern Med. 2012;27(8):933-939. doi: 10.1007/s11606-012-2022-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wright A, Pang J, Feblowitz JC, et al. Improving completeness of electronic problem lists through clinical decision support: a randomized, controlled trial. J Am Med Inform Assoc. 2012;19(4):555-561. doi: 10.1136/amiajnl-2011-000521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sigel EJ, Arredondo Mattson S, Runyan CW. Utilizing the electronic medical record to enhance health care provider delivery of messages about the safe storage of firearms. Clin Pediatr (Phila). 2023;62(8):894-900. doi: 10.1177/00099228221146998 [DOI] [PubMed] [Google Scholar]
- 73.Weiner SJ, Schwartz A, Weaver F, et al. Effect of electronic health record clinical decision support on contextualization of care: a randomized clinical trial. JAMA Netw Open. 2022;5(10):e2238231. doi: 10.1001/jamanetworkopen.2022.38231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dhingra L, Schiller R, Teets R, et al. Pain management in primary care: a randomized controlled trial of a computerized decision support tool. Am J Med. 2021;134(12):1546-1554. doi: 10.1016/j.amjmed.2021.07.014 [DOI] [PubMed] [Google Scholar]
- 75.Guiriguet C, Muñoz-Ortiz L, Burón A, et al. Alerts in electronic medical records to promote a colorectal cancer screening programme: a cluster randomised controlled trial in primary care. Br J Gen Pract. 2016;66(648):e483-e490. doi: 10.3399/bjgp16X685657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rule A, Hribar MR. Frequent but fragmented: use of note templates to document outpatient visits at an academic health center. J Am Med Inform Assoc. 2021;29(1):137-141. doi: 10.1093/jamia/ocab230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.van Zyl C, Badenhorst M, Hanekom S, Heine M. Unravelling ‘low-resource settings’: a systematic scoping review with qualitative content analysis. BMJ Glob Health. 2021;6(6):e005190. doi: 10.1136/bmjgh-2021-005190 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Search Strategy
eTable 2. Study Characteristics
eTable 3. Study Quality Assessment Results
Data Sharing Statement