Abstract
Abstract
Introduction
Undetected high-risk conditions in pregnancy are a leading cause of perinatal mortality in low-income and middle-income countries. A key contributor to adverse perinatal outcomes in these settings is limited access to high-quality screening and timely referral to care. Recently, a low-cost one-dimensional Doppler ultrasound (1-D DUS) device was developed that front-line workers in rural Guatemala used to collect quality maternal and fetal data. Further, we demonstrated with retrospective preliminary data that 1-D DUS signal could be processed using artificial intelligence and deep-learning algorithms to accurately estimate fetal gestational age, intrauterine growth and maternal blood pressure. This protocol describes a prospective observational pregnancy cohort study designed to prospectively evaluate these preliminary findings.
Methods and analysis
This is a prospective observational cohort study conducted in rural Guatemala. In this study, we will follow pregnant women (N =700) recruited prior to 18 6/7 weeks gestation until their delivery and early postpartum period. During pregnancy, trained nurses will collect data on prenatal risk factors and obstetrical care. Every 4 weeks, the research team will collect maternal weight, blood pressure and 1-D DUS recordings of fetal heart tones. Additionally, we will conduct three serial obstetric ultrasounds to evaluate for fetal growth restriction (FGR), and one postpartum visit to record maternal blood pressure and neonatal weight and length. We will compare the test characteristics (receiver operator curves) of 1-D DUS algorithms developed by deep-learning methods to two-dimensional fetal ultrasound survey and published clinical pre-eclampsia risk prediction algorithms for predicting FGR and pre-eclampsia, respectively.
Ethics and dissemination
Results of this study will be disseminated at scientific conferences and through peer-reviewed articles. Deidentified data sets will be made available through public repositories. The study has been approved by the institutional ethics committees of Maya Health Alliance and Emory University.
Keywords: Health informatics, PAEDIATRICS, OBSTETRICS, Fetal medicine
STRENGTHS AND LIMITATIONS OF THIS STUDY.
Active recruitment through public health centres will be used to recruit a diverse sample of pregnant women from the study town into the cohort.
Portable ultrasound devices will allow for the collection of two-dimensional ultrasound data even under challenging rural conditions.
Ultrasound data will be collected by nurses trained to international standards by a supervising physician.
Portable ultrasound records may be of lower resolution and quality than stand-alone devices.
Data from this rural Guatemalan population may not be generalisable to other low-resource settings globally.
Introduction
Around the world, almost 95% of all maternal deaths occur in low-income and middle-income countries. Factors that contribute to this mortality include social, economic, gender and ethnic inequities.1 2 In addition, neonatal mortality is associated with a lack of skilled care and treatment during and immediately after birth and first week of life. Globally, around 1 million newborns die annually within the first 24 hours of birth.3
These maternal and neonatal deaths are mostly preventable, assuming that adequate resources can be brought to bear to permit the timely detection of high-risk complications, such as maternal hypertensive disorders and fetal growth restriction (FGR). For example, in high-income settings, cardiotocography and two-dimensional (2D) ultrasound imaging are the standard of care for prenatal fetal monitoring and during labour. However, these technologies are high cost and highly dependent on the skill of trained operators.4 Lower-cost, accessible technologies are needed to improve monitoring during pregnancy and the perinatal period in low-resource settings.
Guatemala is a multicultural and multilingual country, which due to economic, social, cultural and language barriers, has some of the highest levels of perinatal morbidity and mortality in Latin America. This particularly affects Indigenous Maya women from rural areas of the country, who often have limited access to biomedical care and frequently prefer to deliver in the home attended by traditional midwives. These midwives are important front-line healthcare providers in their community, but they often lack the training and resources necessary for the timely detection of complications.5
Recently, we developed a smartphone-based technology to provide decision support to midwives in rural Guatemala for the identification of high-risk perinatal conditions. Coupled with a care navigation system for individuals requiring facility-based care, this system has significantly improved access to biomedical obstetrical care.5 6 This system includes fetal heart tone recordings obtained using a low-cost direct-to-consumer 1-D Doppler ultrasound (1-D DUS) device. Using signal processing algorithms and machine-learning approaches, our team has produced preliminary evidence that this low-cost 1-D DUS signal can be used to accurately predict hypertensive disorders of pregnancy and FGR.7 This approach has the potential to revolutionise pregnancy and perinatal care in low-resource settings; however, our findings so far are limited by the use of retrospective data analysis and limited longitudinal data points on maternal and fetal endpoints, especially early in pregnancy.
To address these limitations, we will conduct a prospective, well-powered observational pregnancy cohort study in Guatemala, as described in this protocol paper. In addition to providing detailed longitudinal data on maternal and fetal endpoints in this important setting, the overall goal of the cohort study is to validate the use of low-cost 1-D DUS signal, coupled with machine-learning algorithms, as a novel technology for early detection of maternal and fetal complications in low-resource settings. The Guatemala cohort study described here is part of a larger multisite collaboration dedicated to this overall goal.
Methods and analysis
Study setting and design
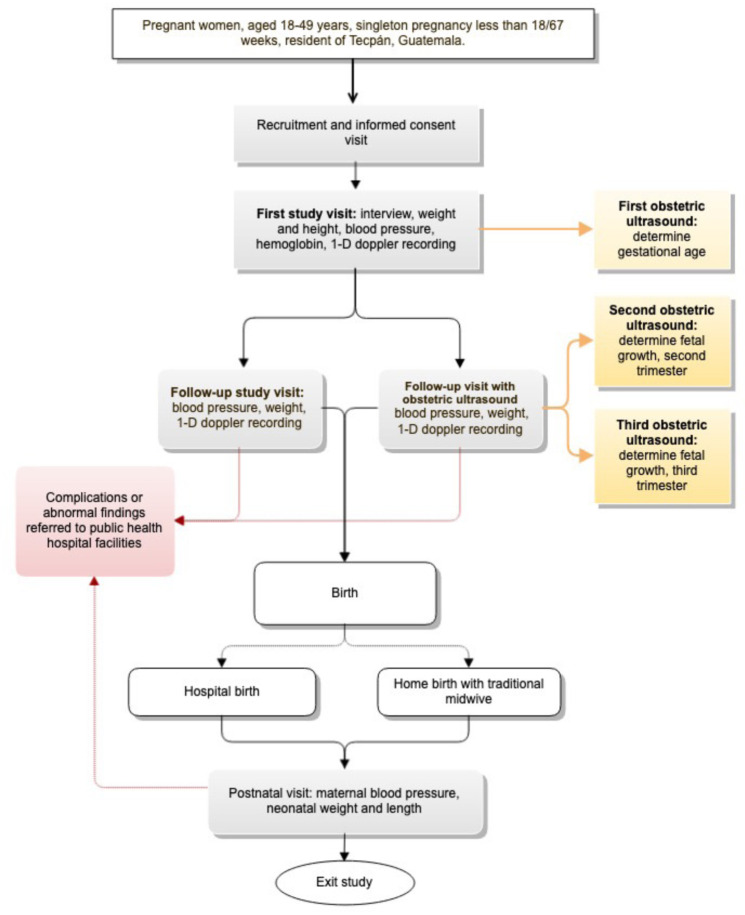
The Mobile Monitoring Doppler Ultrasound Study is the Guatemala arm of a prospective, multisite observational cohort study of pregnant women designed to validate the use of 1-D DUS signals to predict important maternal and fetal outcomes. In this study, we will collaborate with the local lead institution, Maya Health Alliance, to recruit pregnant women in the municipality of Tecpán, Chimaltenango, Guatemala. Maya Health Alliance has been the local partner for our team’s previous work developing a smartphone-based system for use with traditional midwives in this area.5 8 Tecpán is a highland (2200 m above sea level) agricultural community of majority Kaqchikel Maya ethnicity with an estimated population of 95 000. An overall study schematic is provided in figure 1.
Figure 1. Overall design and subject flow for a prospective, observational pregnancy cohort study in rural Guatemala.
Clinical endpoints
Gestational age will be determined by use of the four-component Hadlock equation on 2D fetal ultrasound performed between 15 and 18 6/7 weeks gestational age.9 10
FGR will be defined as:
Estimated fetal weight less than the third percentile for gestational age by prenatal ultrasound evaluation.
Estimated fetal weight between the 3rd and 10th percentile for gestational age with abnormal Doppler findings in the umbilical, uterine and middle cerebral arteries by prenatal ultrasound evaluation.11
Small for gestational age will be defined as a birth weight less than the 10th percentile using the WHO child growth reference standards.12
Gestational hypertension: New-onset hypertension will be defined as systolic blood pressure (SBP) >140 mm Hg or diastolic blood pressure (DBP) >90 mm Hg after 20 weeks of pregnancy when previous blood pressure was normal, in the absence of proteinuria and other signs or symptoms of pre-eclampsia.13
Pre-eclampsia will be defined according to international standards as either:
New-onset hypertension (SBP >140 mm Hg or DBP >90 mm Hg) and proteinuria on urine dipstick at least two occasions 4 hours apart.
New-onset hypertension with or without proteinuria but with at least one other significant symptom of end-organ dysfunction (eg, visual changes, altered mental status, severe headache, severe abdominal pain, pulmonary oedema, low platelets and elevated creatinine).13
Eligibility criteria
Study participants will be pregnant women recruited from the municipality of Tecpán. Maya Health Alliance clinics, as well as collaborating public health clinics, will be the primary source for recruitment. Additionally, the project will be promoted with fliers distributed through community centres.
Inclusion criteria are (1) pregnant women between 18 and 49 years of age and (2) committed to remaining engaged in prenatal care with an identified healthcare provider throughout the pregnancy.
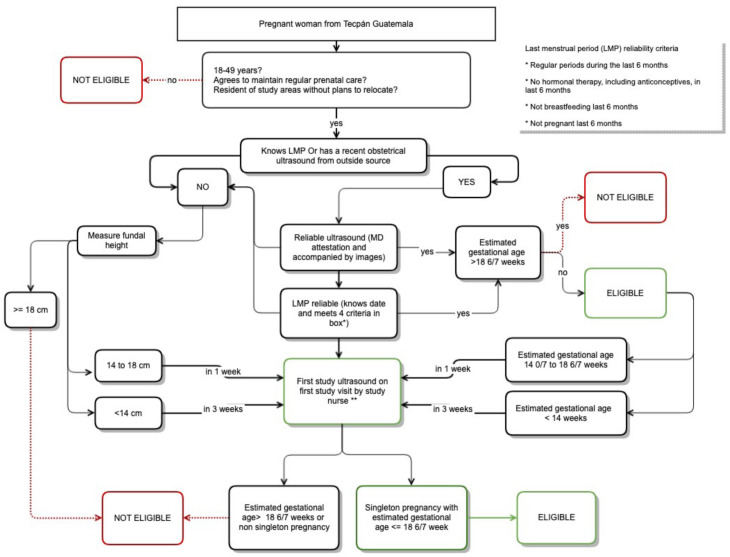
Exclusion criteria are (1) not willing or able to give informed consent; (2) primary residence or planned relocation outside the study area; (3) non-singleton pregnancy and (4) pregnancy >18 6/7 weeks by reliable last menstrual period (LMP) or ultrasound at the time of enrolment. Criteria for defining reliability of LMP are given in figure 2.
Figure 2. Overall recruitment and eligibility workflow for a prospective, observational pregnancy cohort study in rural Guatemala. *Box in upper right corner of figures gives study criteria for reliable LMP. **If body mass index is >40 ultrasound should be performed by study MD. LMP, last menstrual period.
Sample size
We will plan to enrol 700 pregnant subjects over a 5-year timeline using a rolling recruitment methodology. This sample size target is based on the overall project goal of comparing the predictive accuracy of our 1-D DUS machine-learning-derived algorithm against gold-standard diagnostic methods for pre-eclampsia and FGR.
For pre-eclampsia, we will use gold-standard oscillometric blood pressure device readings and the Bayesian pre-eclampsia risk prediction algorithm developed by the Fetal Medicine Foundation.14 This algorithm, not including optional biomarkers, has an area under the receiver operating characteristic curve (AUROC) for pre-eclampsia at >37 weeks of 0.74. Baseline prevalence of pre-eclampsia in Guatemala in our prior work is approximately 8%.5 Assuming an intertest correlation coefficient of 0.7, enrolling 560 subjects in Guatemala will provide 80% power at an alpha of 0.05 to detect an AUROC for the new 1-D DUS algorithm of within a 0.12 difference in AUROC as compared with the Fetal Medicine Foundation algorithm. Assuming a loss to follow-up rate of up to 20% requires at least 670 subjects.
For FGR, we will use as gold standard comprehensive 2D fetal ultrasound, which has an AUROC of 0.81.15 However, given the lack of any detailed fetal growth from any prior study in our population, we base our sample size calculation on the prevalence of small for gestational age based on a birth weight obtained by trained personnel in the first 48 hours of life, which is approximately 5%.16 Assuming an intertest correlation coefficient of 0.7, enrolling 380 subjects in Guatemala will provide 80% power at an alpha of 0.05 to detect an AUROC for the new 1-D DUS algorithm of 0.18 points. Given that the sample size estimates for pre-eclampsia detection above are larger, we will exceed enrolment targets for the FGR-based aims.
Recruitment and informed consent
Study staff will identify potential participants in usual care spaces run by Maya Health Alliance or collaborating public health centres, after receiving inquiries about the study from community-based promotional flyers. Subjects expressing interest will be rapidly screened for eligibility and then, if appropriate, study staff will administer informed consent. Research staff administering informed consent will be bilingual speakers of both Spanish and Kaqchikel Maya. They will be trained to administer informed consent and provide explanations of study details and procedures in these languages. A verbal informed consent procedure will be used, as is common in other minimal risk research studies conducted at Maya Health Alliance since literacy is low and obtaining signatures is not culturally common. The consent script will be read by study staff in the participant’s chosen language and a teach back methodology will be used to ensure comprehension.
Enrolment based on gestational age
Enrolment in this study early in pregnancy is important for obtaining accurate gestational age estimates. However, obtaining accurate gestational age estimates is challenging in rural Guatemala given lack of access to routine obstetric ultrasound, typically late presentation to prenatal care, and frequent short interpregnancy intervals and extended breast feeding making determination of LMP at times uncertain. To address these concerns, study nurses will use a detailed workflow outlined in figure 2, which helps guide the timing of clinical data collection, physical examination and point-of-care ultrasound to determine eligibility based on gestational age.
Collection of clinical data
Each participant will have a maximum of seven study visits, each lasting an estimated duration of 90 min. Study visits will include (1) initial visit to explain study and obtain informed consent; (2) first data collection visit to obtain sociodemographic and clinical history data, perform first dating ultrasound (between 15 and 18 6/7 weeks gestational age) and measure height, weight, blood pressure, capillary haemoglobin and 1-D fetal Doppler recording; (3) monthly follow-up study visits (up to a maximum of 4: 20 0/7–23 6/7 weeks; 24 0/7–27 6/7 weeks; 28 0/7–31 6/7 weeks; 32 0/7–37 6/7 weeks) to obtain blood pressure, weight and 1-D fetal Doppler recording, two sequential ultrasounds (second and third trimester, between 24–27 6/7 weeks and 32–36 6/7 weeks, respectively), and review any new antenatal events and (4) one final postnatal visit performed within 48 hours of birth to determine final pregnancy disposition, document any maternal or neonatal complications, and obtain maternal blood pressure and neonatal weight. For hospital births, neonatal weight and other immediate postnatal data will be obtained by review of hospital records. Table 1 details the study visit sequence and elements.
Table 1. Schedule of study visits and procedures.
| Data element | Visit 1 (15 0/7–18 6/7 weeks) | Visit 2 (15 0/7–18 6/7 weeks) | Visit 3 (20 0/7–23 6/7 weeks) | Visit 4 (24 0/7–27 6/7 weeks) | Visit 5 (28 0/7–31 6/7 weeks) | Visit 6 (32 0/7–36 6/7 weeks) | Visit 7 (<48 hours post partum) |
| Eligibility criteria | |||||||
| Informed consent | |||||||
| Social and clinical history | |||||||
| Interim history | |||||||
| 1-D DUS | |||||||
| Blood pressure | |||||||
| Maternal weight | |||||||
| Maternal height | |||||||
| Capillary haemoglobin | |||||||
| Dating ultrasound | |||||||
| 2°Trimester Fetal Survey Ultrasound | |||||||
| 3°Trimester Fetal Survey Ultrasound | |||||||
| Neonatal weight | |||||||
| Neonatal height |
1-D DUS1-D Doppler ultrasound
2D ultrasound data will be collected by trained study nurses with close study physician support using the Butterfly iQ+device (Butterfly Network, Burlington, Massachusetts, USA). Nurses will be trained using the recommendations of the International Society of Ultrasound in Obstetrics and Gynecology Education Committee for basic training in obstetric ultrasound.17
Height and weight data will be collected in triplicate on subjects wearing traditional clothing. Weights of representative sets of traditional clothing (typically woven cotton blouse, skirt and belt) will be recorded as we have previously described in detail in order to permit weight adjustments for worn clothing as needed for women.18 The Seca 213 portable stadiometer (Seca, Hamburg, Germany) and Tanita HD-395 portable digital scale (Tanita, Tokyo, Japan) will be used for adult measures. For infant measures, a Seca 310 hanging scale and portable length board locally constructed according to UNICEF specifications will be used.19 Seated arterial blood pressure will be obtained in duplicate at 1 min intervals after 5 min of rest in the right arm using an iHealth KN-550BT oscillometric automated blood pressure device (iHealth Labs, Sunnyvale, California, USA) and an appropriately sized cuff following instructions in the user manual (22–42 cm or 42–48 cm) supplied with the device.
Capillary haemoglobin will be obtained using the HemoCue Hb 201+Analyzer (Hemocue AB, Ängelholm, Sweden) following manufacturer’s instructions. Haemoglobin values will be adjusted for altitude.
1-D DUS data will be collected at each study visit as a 5 min recorded segment using a Google Pixel 6a smartphone (Alphabet, Mountain View, California, USA) running a bespoke data acquisition app developed by our team8 20 and the Contec Baby Sound-A Pocket Fetal Doppler (Contec Medical, Qinhuangdao Hebei, PRC).
Laboratory data are not routinely collected in antenatal care in Guatemala, but when elevated blood pressure or other concerns for hypertensive disorders of pregnancy are detected, referrals will be made to the local public hospital in Tecpán, where laboratory assessments for end-organ dysfunction (eg, proteinuria, serum creatinine and platelet count) are performed at the discretion of the on-call clinician. A study MD will review these data along with clinical notes to establish clinical endpoints.
Statistical methods
Clinical data will be pulled retrospectively from digital registries and associated with the 1-D DUS ultrasound and blood pressure recordings. We will calculate descriptive statistics (demographics and baseline clinical factors) for our study population using proportions for categorical variables and medians with iIQRs for continuous variables. We will present these data pooled and separately by final pre-eclampsia and FGR status. We will compare the test characteristics (receiver operator curves) of our 1-D Doppler algorithms with a comprehensive 2D fetal ultrasound survey (for FGR) and with the Fetal Medicine Foundations’ Bayesian pre-eclampsia risk prediction algorithm (for pre-eclampsia) using χ2 tests.14 15 Repeated bootstrap cross-validation will be applied to estimate the confidence intervals. Data will be analysed by using Stata V.18, SAS V.9.4 and R V.4.3.2 or a similar statistical analysis program.
Ethics and dissemination
Patient and public involvement
Patients or the public were not involved in the design, or conduct, or reporting, or dissemination plans of our research.
Ethics approval and consent to participate
The study has been approved by the institutional ethics committees of Maya Health Alliance (WK 2022 005) and Emory University (IRB Study # 00005252). All subjects will provide verbal informed consent, the requirement to document informed consent by signature has been waived by the Maya Health Alliance ethics committee.
Data management
All clinical data except for ultrasound images will be entered directly into Maya Health Alliance’s secure cloud-based electronic health record and subsequently extracted for analysis. Ultrasound data will be stored in Butterfly’s secure commercial cloud-based image storage solution.
Data monitoring, harm, auditing
Rates of subject recruitment, adherence to recruitment procedures and inclusion/exclusion criteria, and retention will be monitored closely by the study team. The study is of minimal risk to subjects. The anticipated risks include lost productivity or interference with home routines during study visits. There is also the risk of psychological distress if previously unknown maternal or fetal complications are discovered by the study team. Disclosure of unexpected or new complications will be closely overseen by a study physician. All subjects in the cohort study who require referral to public health facilities for the management of complications will receive obstetrical care navigation services from Maya Health Alliance care navigators to assist in accessing effective care in a timely fashion.6 A study physician will closely track the outcomes of these referrals to care and liaise with hospital medical staff as necessary. The study is not expected to have any study-related adverse events, but any perceived adverse events or complaints from participants will be reported to the institutional review boards.
Availability of data and materials
Deidentified data sets will be uploaded to the DASH repository (https://dash.nichd.nih.gov/) or PhysioNet.org, as appropriate, on completion of analysis and publication of primary manuscripts.
Acknowledgements
We thank the staff of Maya Health Alliance and collaborating traditional midwives from Tecpán, Guatemala.
Footnotes
Funding: This study was funded by a grant from the NIH/NICHD (5R01HD110480) to GDC after undergoing competitive peer review.
Prepublication history for this paper is available online. To view these files, please visit the journal online (https://doi.org/10.1136/bmjopen-2024-090503).
Patient consent for publication: Not applicable.
Provenance and peer review: Not commissioned; peer reviewed for ethical and funding approval prior to submission.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Contributor Information
Edlyn Ramos, Email: edlynramos@wuqukawoq.org.
Irma Piló Palax, Email: irmapilo@wuqukawoq.org.
Emily Serech Cuxil, Email: emilyserech@wuqukawoq.org.
Elsa Sebaquijay Iquic, Email: elsasebaquijay@wuqukawoq.org.
Ana Canú Ajqui, Email: anacanu@wuqukawoq.org.
Ann C Miller, Email: Ann_Miller@hms.harvard.edu.
Suchitra Chandrasekeran, Email: suchitra.chandrasekaran@emory.edu.
Rachel Hall-Clifford, Email: hall-clifford@emory.edu.
Reza Sameni, Email: rsameni@dbmi.emory.edu.
Nasim Katebi, Email: nasim.katebi@dbmi.emory.edu.
Gari D Clifford, Email: gari@dbmi.emory.edu.
Peter Rohloff, Email: prohloff@bwh.harvard.edu.
References
- 1.World Health Organization Fact sheets: maternal mortality. 2023. [08-Dec-2023]. https://www.who.int/news-room/fact-sheets/detail/maternal-mortality Available. Accessed.
- 2.World Health Organization Joint statement on reducing maternal morbidity and mortality. 2023. https://www.paho.org/en/documents/brochure-joint-statement-reducing-maternal-morbidity-and-mortality Available.
- 3.World health Organization Fact sheets: newborns: improving survival and well-being. 2020. https://www.who.int/news-room/fact-sheets/detail/newborns-reducing-mortality Available.
- 4.Valderrama CE, Ketabi N, Marzbanrad F, et al. A review of fetal cardiac monitoring, with a focus on low- and middle-income countries. Physiol Meas. 2020;41:11TR01. doi: 10.1088/1361-6579/abc4c7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martinez B, Ixen EC, Hall-Clifford R, et al. mHealth intervention to improve the continuum of maternal and perinatal care in rural Guatemala: a pragmatic, randomized controlled feasibility trial. Reprod Health. 2018;15:120. doi: 10.1186/s12978-018-0554-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Austad K, Juarez M, Shryer H, et al. Obstetric care navigation: results of a quality improvement project to provide accompaniment to women for facility-based maternity care in rural Guatemala. BMJ Qual Saf. 2020;29:169–78. doi: 10.1136/bmjqs-2019-009524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Katebi N, Sameni R, Rohloff P, et al. Hierarchical Attentive Network for Gestational Age Estimation in Low-Resource Settings. IEEE J Biomed Health Inform. 2023;27:2501–11. doi: 10.1109/JBHI.2023.3246931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stroux L, Martinez B, Coyote Ixen E, et al. An mHealth monitoring system for traditional birth attendant-led antenatal risk assessment in rural Guatemala. J Med Eng Technol. 2016;40:356–71. doi: 10.1080/03091902.2016.1223196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hadlock FP, Harrist RB, Sharman RS, et al. Estimation of fetal weight with the use of head, body, and femur measurements--a prospective study. Am J Obstet Gynecol. 1985;151:333–7. doi: 10.1016/0002-9378(85)90298-4. [DOI] [PubMed] [Google Scholar]
- 10.Committee Opinion No 700: Methods for Estimating the Due Date. Obstet Gynecol. 2017;129:e150–4. doi: 10.1097/AOG.0000000000002046. [DOI] [PubMed] [Google Scholar]
- 11.Melamed N, Baschat A, Yinon Y, et al. FIGO (international Federation of Gynecology and obstetrics) initiative on fetal growth: best practice advice for screening, diagnosis, and management of fetal growth restriction. Int J Gynaecol Obstet. 2021;152 Suppl 1:3–57. doi: 10.1002/ijgo.13522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Onis M, WHO MULTICENTRE GROWTH REFERENCE STUDY GROUP WHO Child Growth Standards based on length/height, weight and age. Acta Paediatr. 2006;95:76–85. doi: 10.1111/j.1651-2227.2006.tb02378.x. [DOI] [PubMed] [Google Scholar]
- 13.Brown MA, Magee LA, Kenny LC, et al. Hypertensive Disorders of Pregnancy: ISSHP Classification, Diagnosis, and Management Recommendations for International Practice. Hypertension. 2018;72:24–43. doi: 10.1161/HYPERTENSIONAHA.117.10803. [DOI] [PubMed] [Google Scholar]
- 14.Fetal Medicine Foundation Risk assessment: risk for preeclampsia. https://fetalmedicine.org/research/assess/preeclampsia/first-trimester n.d. Available.
- 15.Figueras F, Caradeux J, Crispi F, et al. Diagnosis and surveillance of late-onset fetal growth restriction. Am J Obstet Gynecol. 2018;218:S790–802. doi: 10.1016/j.ajog.2017.12.003. [DOI] [PubMed] [Google Scholar]
- 16.Garces A, McClure EM, Figueroa L, et al. A multi-faceted intervention including antenatal corticosteroids to reduce neonatal mortality associated with preterm birth: A case study from the Guatemalan Western Highlands. Reprod Health. 2016;13:63. doi: 10.1186/s12978-016-0178-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.ISUOG Education Committee recommendations for basic training in obstetric and gynecological ultrasound. Ultrasound Obstet Gynecol. 2014;43:113–6. doi: 10.1002/uog.13208. [DOI] [PubMed] [Google Scholar]
- 18.Chacón V, Liu Q, Park Y, et al. Diet quality, school attendance, and body weight status in adolescent girls in rural Guatemala. Ann N Y Acad Sci. 2021;1494:59–69. doi: 10.1111/nyas.14558. [DOI] [PubMed] [Google Scholar]
- 19.Contreras M, Palomino C. Elaboración y Mantenimiento de Infantómetros y Tallímetros de Madera. Lima, Peru: Instituto Nacional de Salud/UNICEF; 2007. [Google Scholar]
- 20.Martinez B, Hall-Clifford R, Coyote E, et al. Agile Development of a Smartphone App for Perinatal Monitoring in a Resource-Constrained Setting. J Health Inform Dev Ctries. 2017;11 [PMC free article] [PubMed] [Google Scholar]