Abstract
Introduction
The main risk factors of necrotising enterocolitis (NEC) are prematurity and low birth weight. The aim of our study was to identify risk factors for NEC in patients with duct-dependent congenital heart disease (CHD).
Study design
Newborns with duct-dependent CHD and NEC were matched 1:1 to those without NEC. Matched criteria were gestational age, birth weight, antenatal versus postnatal diagnosis and type of CHD.
Results
Twenty-three infants were included in each group. In the NEC group, mortality, length of intensive care unit stay and length of hospital stay were significantly higher (p=0.035; p<0.0001; p<0.0001). Lower diastolic blood pressure (DBP), negative flow balance, peritoneal dialysis and epinephrine-infusion were significantly associated with NEC (respectively, p=0.008, p=0.002, p=0.007, p=0.017). In multivariate analysis, DBP≤30 mm Hg remained the only independent risk factor of NEC (OR=8.70; 95% CI (1.46 to 53.50), p=0.019).
Conclusion
A DBP lower than 30 mm Hg was in our matched population of newborns with duct-dependent CHD, independently associated with NEC.
Keywords: Neonatology, Cardiology
WHAT IS ALREADY KNOWN ON THIS TOPIC.
WHAT THIS STUDY ADDS
In a matched duct-dependent congenital heart disease population, we identified some specific conditions associated with NEC. In congenital heart disease patient, even Bell’s stage I NEC seems to have a poor prognosis.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
Monitoring and management of clinical factors (inotropic support, control of the fluid balance and peritoneal dialysis) may be an interesting tool to reduce risk of NEC. Diastolic hypotension is an important risk factor for NEC and must be controlled.
Introduction
Congenital heart disease (CHD) is the most frequent cause of death in infants.1 2 Prostaglandins E1 infusion is needed in duct-dependent CHD before surgical or transcatheter management, to maintain adequate pulmonary or systemic circulation. Hospitalisation in intensive care units (ICU) is usually required for those patients.
Necrotising enterocolitis (NEC) is a well-known complication in newborns and is associated with significant morbidity and mortality.3 The main risk factors for NEC are prematurity4 and low birth weight.5 Children with CHD, especially those with duct-dependent circulation, carry an increased risk of NEC46,10 with an incidence and mortality up to 3.7 and 24.4%, respectively.611,14 Previous studies investigating NEC in neonates with heart disease are mainly cross-sectional observational retrospective studies or retrospective cohort studies comparing NEC in newborns with heart disease and NEC in newborns without heart disease.7 8 The objective of our study was to identify specific risk factors for NEC in a matched duct-dependent CHD population.
Methods
A retrospective, single-centre, case–control study was performed. All neonates (less than 28 days) with duct-dependent CHD, treated between 2014 and 2019, were considered for inclusion. Patients were identified through the institutional informatics system based on diagnostic codes. A total of 187 newborns responded to those initial criteria. Analysis of the medical charts allowed to identify 23 (12%) CHD patients who developed a stage I–III necrotising enterocolitis according to Bell’s criteria15 modified by Walsh and Kliegman.16 All patient with NEC suspicion were included in NEC group.
A propensity-score methodology was used to identify within the group without NEC, comparable patients (control group) to those with NEC. A 1/1 matching was performed according to gestational age, birth weight, antenatal diagnosis and type of heart disease. Indeed, patients were grouped into three categories: (1) duct-dependent pulmonary circulation (eg, pulmonary atresia and tetralogy of Fallot), (2) duct-dependent systemic circulation (eg, hypoplastic left heart syndrome, coarctation of aorta and interruption of the aortic arch) and (3) duct-dependent without an adequate mixing of blood between the two circulations, named parallel circulation (eg, transposition of the great arteries).17 Patients were matched using the nearest neighbour method without replacement and with a calliper of width equal to 0.25. Other collected general data were as follows: the presence or absence of antenatal diagnosis, age at surgery or cardiac catheterisation, length of stay in intensive care, total length of hospitalisation and mortality.
During ICU period, invasive blood pressure was routinely monitored and recorded. Input and output balance were hourly recorded and daily calculated (using an intake–output chart). The given medications were traced. For 74% of our patients, somatic (visceral) near infrared spectroscopy (NIRS) (NIRS, INVOS, Covidien, Ireland) sensor was positioned in the lumbar region for continuous measurement. Data were recorded on dedicated monitoring sheets and archived in the patient file. Following ICU data were extracted: minimum diastolic blood pressure (DBP min; average of 3 lowest consecutive values), lowest visceral NIRS value (average of the five lowest consecutive values), input–output fluid balance (mL/kg/day), length of prostaglandin E1 infusion (days), minimum and maximum doses of prostaglandin E1, maximum dose of inotropic support, need for peritoneal dialysis (PD) and number of days and feeding volumes (mL/kg/day). All these data were collected for the period before development of NEC for the NEC group and with an equivalent duration for each control in the no-NEC group. Following surgical data were extracted: duration of extracorporeal circulation, duration of aortic clamping and minimum temperature during surgery. Following biological data were extracted: the presence of acute renal failure (ARF) (defined as serum creatinine level greater than 1.5 mg/dL (133 micromol/L) or an increase of at least 0.2–0.3 mg/dL (17–27 micromol/L) per day from a previous value), minimum (min) and maximum (max) pH, minimum and maximum haematocrit, maximum lactate (arterial or venous, data from capillary gas measurements were excluded). Finally, the number of transfusions and number of days spent on antibiotic and antifungal treatment were also extracted.
In the NEC-group, we additionally collected age at NEC-diagnosis, weight at NEC-diagnosis Bell stage according to the modified criteria of Walsh and Kliegman and treatment of NEC.
The extracted brain natriuretic peptide values were not usable due to a local change from BNP to NT-proBNP measurement during the period study.
Ethical statement
La Timone Children’s Hospital Clinical Investigation Committee and the Ethics Committee approved the study protocol (local reference number: 2019‐92). The study was performed in accordance with the relevant guidelines and regulations and with the Declaration of HelsinkiDeclaration of Helsinki.
Statistical analysis
Quantitative variables are expressed as means±SD for normal distribution variables and as medians and IQR for non-normal distribution variables. Qualitative variables are expressed as number and percentage. For normally distributed variables, comparison of qualitative data between groups was performed by χ2 test (or Fisher’s exact test in case of sizes<5) and comparison of continuous data was performed by using Student’s t test. For non-normally distributed variables, comparison of qualitative data between groups was performed by non-parametric χ2 test (or Fisher’s exact test). The comparison of continuous data was made using a Mann-Whitney test. The accuracy of the DBP min values for predicting the occurrence of NEC was assessed by calculating the areas under the curve using the receiver operator characteristics (ROC) curves and the best cut-off value was defined as the one with the greatest sensitivity and specificity. In order to assess the association between the various parameters found to be significant during the univariate analysis, multivariate analysis was performed using binary logistic regression, all p-values were two-sided and significance was pronounced for a p-value at 5% (p<0.05). Statistical analysis was performed using the SPSS software, V.17.0 (SPSS, Chicago, IL, USA).
Results
Initial data
A total of 187 newborns responded to the initial criteria. We identified 23 (12%) CHD patients who developed (or suspected of developing) a NEC (NEC group). Using the propensity score, we selected 23 comparable patients out of the 164 newborns without NEC (no-NEC group). The distribution of the matching criteria in the initial population and the comparison between the final two groups is given in table 1.
Table 1. Demographic characteristics before and after matching.
| Before matching | After matching | |||||
| NEC groupn=23 | No NEC groupn=164 | P value | NEC groupn=23 | No NEC groupn=23 | P value | |
| Term (weeks) | 38.1±1.9 | 38.7±1.6 | 0.113 | 38.2±2.0 | 38.1±2.4 | 0.947 |
| Moderate to late preterm, n (%) | 3 (13) | 26 (15.8) | 0.173 | 3 (13) | 5 (21.7) | 0.699 |
| Weight (kg) | 3.05±0.76 | 3.16±0.51 | 0.176 | 3.05±0.75 | 3±0.62 | 0.813 |
| Type of CHD, n (%) | 0.226 | 1 | ||||
| Duct-dependent pulmonary circulation | 7 (30.4) | 27 (16.5) | 7 (30.4) | 7 (30.4) | ||
| Duct-dependent systemic circulation | 10 (43.5) | 75 (45.7) | 10 (43.5) | 10 (43.5) | ||
| Parallel circulation | 6 (26.1) | 62 (37.8) | 6 (26.1) | 6 (26.1) | ||
| Prenatal diagnosis, n (%) | 16 (69.5) | 116 (70.7) | 0.209 | 16 (69.5) | 16 (69.5) | 1 |
CHD, congenital heart diseaseNECnecrotising enterocolitis
Table 2 details demographic data of patients in NEC group. Seventeen (73.9%) were male. NEC occurred postoperatively in 12 patients (52.2%). The median age at the NEC diagnosis was 14 days35,24 and the mean weight was 3.2±0.79 kg. Only 21.7% of the patients in the NEC group required surgical NEC treatment. Subgroup analysis in NEC group comparing stage I to other stages of NEC showed no statistical difference in length of ICU stay, length of hospital stay, age at surgery or death. The analysis is detailed in table 3.
Table 2. NEC group characteristics.
| Characteristics | NEC groupn=23 |
| Male gender, n (%) | 17 (73.9) |
| Postoperative NEC, n (%) | 12 (52.2) |
| NEC stage, n (%) | |
| Ia | 4 (17.4) |
| Ib | 4 (17.4) |
| IIa | 3 (13) |
| IIb | 7 (30.4) |
| IIIa | 0 |
| IIIb | 5 (21.7) |
| NEC requiring surgery, n (%) | 5 (21.7) |
| Age at diagnosis (day), median (IQR) | 14 (6–26) |
| Weight at diagnosis (kg), mean (SD) | 3.2±0.79 |
NEC, necrotising enterocolitis
Table 3. Subgroup analysis comparing NEC stage I to other stages of NEC.
| Variables | NEC stage In=8 | NEC other stagesn=15 | P value |
| Length of ICU stay (days), median (IQR) | 17 (9.5–33.3) | 31 (19–44) | 0.451 |
| Length of hospital stay (days), median (IQR) | 48.5 (20.7–86.0) | 69 (40–143) | 0.097 |
| Age at surgery (days), median (IQR) | 13.5 (7.2–27.2) | 18 (7–26.7) | 0.977 |
| Death, n (%) | 4 (50) | 4 (26.7) | 0.210 |
ICU, intensive care unitNECnecrotising enterocolitis
The comparison between the two groups (NEC group vs no-NEC group) is detailed in table 4. Because of occurrence of NEC, cardiac surgery or catheterisation was generally delayed (16.5 days (7–26.5) in the NEC group vs 7.5 days (6.75–13.25) in the no-NEC group, p=0.018). The length of stay in intensive care and hospitalisation were significantly longer in the NEC group with a median length of stay in intensive care unit of 28 days35 14,40 versus 9 days5,17 (p<0.0001) and a median total hospital stay of 59 days (39–103) versus 23 days (20–45) (p<0.001). Eight newborns (34.8%) died in the NEC group versus 2 (8.7%) in no-NEC group (p=0.035).
Table 4. Univariate analysis of factors associated with NEC occurrence.
| Variables | NEC group(n=23) | No NEC group(n=23) | P value |
| Prostaglandin (days), median (IQR) | 6.8 (3.83–11.00) | 6.21 (4.00–8.00) | 0.39 |
| Maximal dose of prostaglandin (µg/kg/min), median (IQR) | 0.05 (0.038–0.05) | 0.047 (0.029–0.06) | 0.08 |
| Minimal dose of prostaglandin (µg/kg/min), median (IQR) | 0.025 (0.013–0.042) | 0.016 (0.006–0.034) | 0.36 |
| Epinephrine, n (%) | 11 (47.8) | 19 (82.6) | 0.017 |
| Maximal dose of epinephrine (µg/kg/min), median (IQR) | 0 (0–0.06) | 0.04 (0.02–0.14) | 0.057 |
| Norepinephrine, n (%) | 9 (39.1) | 11 (47.8) | 0.55 |
| Maximal dose of norepinephrine (µg/kg/min), median (IQR) | 0 (0–0.09) | 0 (0–0.07) | 0.32 |
| ECMO, n (%) | 3 (13.0) | 4 (17.4) | 0.5 |
| Antibiotics, n (%) | 15 (65.2) | 18 (78.3) | 0.326 |
| Antifungal, n (%) | 3 (13.0) | 2 (8.1) | 0.636 |
| Transfusion, n (%) | 17 (73.9) | 14 (60.9) | 0.345 |
| Peritoneal dialysis, n (%) | 4 (17.4) | 13 (56.5) | 0.007 |
| Breastfeeding, n (%) | 15 (68.2) | 10 (43.5) | 0.095 |
| Feeding volume (mL/kg/day), mean (SD) | 47.6 (±39.3) | 61.2 (±24.6) | 0.368 |
| Fasting (days), median (IQR) | 3 (0–6) | 2 (1–4) | 0.31 |
| Clinical data | |||
| Minimal DBP, (mm Hg), mean (SD) | 26.1 (±3.9) | 30.3 (±5.8) | 0.008 |
| Minimal NIRSv, (%), median (IQR) | 37.9 (25.8–44.5) | 44.6 (33.6–53.9) | 0.083 |
| Input–output fluid balance (mL/kg/day), median (IQR) | −19.6 (−31.9–−2.18) | −49.4 (−60.2–−29.38) | 0.002 |
| Biological data | |||
| Acute renal failure | 16 (69.5) | 15 (65.2) | 0.518 |
| Lactates (mmol/L), median (IQR) | 5.5 (4–10.7) | 5.6 (4.3–10.8) | 0.771 |
| Haematocrit (%), mean (SD) | 33.3±7.3 | 30.2±5.6 | 0.531 |
| pH min, mean (SD) | 7.14±0.14 | 7.2±0.14 | 0.098 |
| pH max, mean (SD) | 7.52±0.09 | 7.59±0.08 | 0.083 |
| Peroperative data | |||
| Age at surgery, (days), median (IQR) | 16.5 (7–26.5) | 7.5 (6.75–13.25) | 0.018 |
| Length of CPB (min), median (IQR) | 144.5 (0–206.25) | 79 (0–166.25) | 0.2 |
| Length of clamping (min), median (IQR) | 50 (0–109.25) | 28 (6–95) | 0.39 |
| Temperature min (°C), mean (SD) | 28.8 (±7.4) | 31.2 (±5.3) | 0.25 |
| Length of stay | |||
| ICU (days), median (IQR) | 28 (14–42) | 9 (6–18) | 0.0001 |
| Hospital (days), median (IQR) | 59 (39–103) | 23 (20–45) | 0.001 |
| Death, n (%) | 8 (34.8) | 2 (8.7) | 0.035 |
CPB, cardiopulmonary bypass; DBP, diastolic blood pressure; ECMO, extracorporeal membrane oxygenation; ICU, intensive care unitMax, maximal; Min, minimal; NECnecrotising enterocolitisNIRSv, visceral near infrared spectroscopy
Risk factor analysis
Nutrition-wise, 25 (54.3%) patients was exclusively breastfed. There was no statistical difference, between the NEC and no-NEC group, with regard to breastfeeding, feeding volume or fasting duration before the occurrence of NEC (table 4). Following the NEC (for the NEC group), nutritional management was conducted as recommended for NEC.
There was a clear tendency towards lower visceral NIRS values in the NEC group (37.9 (25.8–44.5) vs 44.6 (33.6–53.9), p=0.083), however not reaching statistical significance. DBP, inotropic support and fluid balance were significantly different between the two groups, with a larger use of epinephrine and PD, a more negative input–output fluid balance and a higher mean DBP in the no-NEC group (table 3). The lowest values of DBP were recorded 48–72 hours before the suspicion of NEC. Subgroup analysis in NEC group found no difference between preoperative (n=11) and postoperative NEC (n=12) concerning DBP (24.8±3.6 vs 26.8±4.1 mm Hg respectively, p=0.432). The ROC curve of the DPB min, using a threshold at 30 mm Hg, is shown in figure 1. A DBP min≤30 mm Hg appeared as a relevant prognostic value for predicting the development of an NEC (area under the curve=0.71). Other known risk factors for NEC did not significantly differ between our two matched populations (table 4). After multivariate analysis (table 5), a DBP min≤30 mm Hg was significantly associated with the NEC and NEC suspicion (OR: 8.7; 95% CI: 1.4 to 53.5).
Figure 1. ROC curve for prediction of NEC based on minimum diastolic arterial pressure. AUC, area under the curve; NEC, necrotising enterocolitis; ROC, receiver operator characteristics.
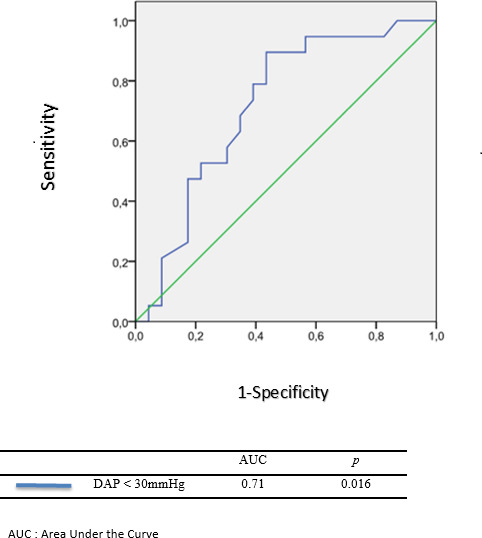
Table 5. Multivariate analysis of risk factors of NEC.
| Risk factors | OR (95% CI) | P value |
| Inotropic support | 1.25 (0.13 to 12) | 0.84 |
| Input–output fluid balance | 2.3 (0.15 to 33.5) | 0.53 |
| Peritoneal dialysis | 0.27 (0.04 to 1.77) | 0.17 |
| DBP<30 mm Hg | 8.7 (1.4 to 53.5) | 0.019 |
DBP, diastolic blood pressureNECnecrotising enterocolitis
Discussion
The strength of our case–control study lies in the careful selection and matching on type of duct-dependent heart disease but also on gestational age and birth weight, which are two important risk factors for NEC.4 5 We also matched patients according to prenatal versus postnatal diagnosis to avoid bias linked to the delay in treatment.
Characteristics of necrotising enterocolitis
In our study, NEC occurred equally in the cardiac preoperative and postoperative period as has been shown in previous studies.10 18 Preoperative NEC may be favoured by the drop in mesenteric perfusion pressure, either by ductal steal in CHDs with right-way obstruction, or by insufficient systemic flow in CHDs with left-sided obstruction.19 20 Postoperative NEC may be favoured by stress and inflammation associated with surgery and bypass, which undoubtedly increase the fragility of the intestinal tract.21 The median age at the time of diagnosis of NEC was 14 days,35,24 which is comparable with the results of two previous cohort studies on NEC in CHD.5 22
NEC in CHD patients is a serious and life-threatening complication associated with a 34.8% mortality in our study. Kessler et al found a similar mortality rate of 35% in a similar cohort.22 Becker et al described a higher mortality (48%), but their cohort included preterm babies.5 After excluding premature babies, their mortality dropped to 14%.5 Mortality in newborns with NEC appears to be higher in CHD than in the general population, as shown in a recent meta-analysis (mortality, respectively 38% vs 27%).10
NEC is associated with an increased length of stay in intensive care and in hospital, and in consequence cost related to medical care.6 14 Our findings corroborate these data.
Risk factors of NEC
Our matched comparison showed that a DBP≤30 mm Hg was significantly associated with an increased risk of NEC. A significant association between arterial hypotension and the occurrence of NEC in full-term newborns has been previously shown.3 8 In the study by McElhinney et al episodes of low cardiac output and shocks were significantly associated with the occurrence of NEC.4 Carlo et al found a persistent reversal of diastolic flow in the abdominal aorta of neonates with CHD and NEC.20 In the study of Van Der Heide, comparing 16 CHD infants with NEC (Bell stages II and III) and selected 16 controls, difference in DBP did not reach statistical significance, but demonstrated the same direction towards (relative) hypoxia/ischaemia in NEC infants.23 These data support the hypothesis according to which a drop in mesenteric perfusion pressure resulting from aortic diastolic steal or global hypotension leads to intestinal ischaemia and subsequent necrosis in newborns with duct-dependent CHD.
Our study did not show significant difference in visceral NIRS value between the two groups. This may be explained by a lack of power since 26% of the data on visceral NIRS were missing. Indeed, the NIRS was only recently introduced systematically in the preoperative period. NIRS-monitoring must be encouraged as it is interesting in the management of these patients. Stapleton et al support the hypothesis that an altered mesenteric oxygen supply is an important factor in the development of NEC in CHD and could justify systematic NIRS monitoring in the preoperative period.24 Other parameters related to NIRS such as NIRS variability and cerebral to splanchnic ratio are interesting, and probably require prospective studies.25
In our study, significantly more children who did not develop a NEC received an epinephrine infusion. Epinephrine may be a protective factor in NEC, limiting episodes of hypotension and low cardiac output. This association did however not persist in our multivariate analysis, probably due to the confounding effect of lower DBP.
The maximum dose and the number of days spent on prostaglandin E1 were not associated with the occurrence of NEC. This is consistent with the study by Carlo et al,20 who did not find association between NEC in newborns with CHD and the use of prostaglandin E1. They did however noticed an increased risk of NEC with high doses of prostaglandin E1 (greater than 0.05 µg/kg/min), but this association disappeared in their multivariate analysis. Low doses (<0.05 µg/kg/min) were mostly used in our unit. These data differ from those reported by Leung et al and Becker et al, who found an association between prostaglandin E1 infusion and the occurrence of NEC.3 5 Those discordances may be explained by the fact that the persistence of the ductus arteriosus is the risk factor for NEC rather than the prostaglandin E1 infusion itself.
Interestingly, our study shows that patients without NEC had a significantly more negative fluid balance than patients in the NEC group. Several studies have shown that after paediatric cardiac surgery, an increase in fluid balance was associated with more complications including an increased risk of ARF, ventilator-acquired pneumonia, increased use of inotropes and vasopressors, increased duration of mechanical ventilation, length of hospital stay and death.26,31 Oedema increases intra-abdominal pressure and decreases renal perfusion.32 It can be hypothesised that the same is true for mesenteric perfusion. Another hypothesis is that interstitial oedema in the intestinal wall alters the epithelial barrier promoting bacterial translocation.33 Finally, oedema impairs oxygen tissue diffusion and is responsible for cellular hypoxia, particularly if oxygen transport is already impaired.34
In our study, PD was associated with a reduced risk of NEC. PD can have a dual effect in preventing NEC. It is both a safe and effective method of removing fluids after paediatric cardiopulmonary surgery35 36 and results in faster and greater fluid balance negativation.37,39 The early onset of PD has also been associated with a lower level of postoperative proinflammatory cytokines, which could be beneficial.39,41
Limits of our study
An obvious limitation of our study is its retrospective nature causing data loss. Conducting a prospective study is however difficult, due to the low incidence of NEC.
Another limitation is the inclusion of stage I. This decision was taken because there was no difference between stage I and other stages (concerning length of ICU stay, length of hospital stay and age at surgery or death). We considered Bell stage I as major event impacting management and prognosis of those patients. Therefore, our study concern NEC suspicion in duct-dependent CHD. The inclusion of preoperative and postoperative NEC in the same group, complicates the interpretation of the results and made it impossible to analyse the data on feeding volumes and the number of fasting days.
Although the number of cases included in our study is higher than in previously cited studies,4 18 21 it remains a small sample. The design of our study, comparing matched populations, increases however the power of our data.
Conclusion
NEC is a serious complication in newborns with CHD, responsible for increased mortality and increased length of hospital stay, including intensive care. Diastolic hypotension is an important risk factor for NEC and must be controlled. Management of inotropic support and a better control of the fluid balance, including PD seems to be important tools to reduce the risk of NEC.
Footnotes
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Patient consent for publication: Not applicable.
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting or dissemination plans of this research.
Contributor Information
Fedoua El Louali, Email: fedoua.el-louali@ap-hm.fr.
Camille Prom, Email: camille.prom@ap-hm.fr.
Belghiti Alaoui Myriem, Email: myriem.belghiti-alaoui@ap-hm.fr.
Celia Gran, Email: celia.gran@ap-hm.fr.
Virginie Fouilloux, Email: virginie.fouilloux@ap-hm.fr.
Marien Lenoir, Email: marien.lenoir@ap-hm.fr.
Isabelle Ligi, Email: isabelle.ligi@ap-hm.fr.
Caroline Ovaert, Email: Caroline.OVAERT@ap-hm.fr.
Fabrice Michel, Email: fabrice.michel@ap-hm.fr.
Data availability statement
Data are available upon reasonable request.
References
- 1.Jortveit J, Øyen N, Leirgul E, et al. Trends in Mortality of Congenital Heart Defects. Congenit Heart Dis. 2016;11:160–8. doi: 10.1111/chd.12307. [DOI] [PubMed] [Google Scholar]
- 2.Wren C, Irving CA, Griffiths JA, et al. Mortality in infants with cardiovascular malformations. Eur J Pediatr . 2012;171:281–7. doi: 10.1007/s00431-011-1525-3. [DOI] [PubMed] [Google Scholar]
- 3.Leung MP, Chau KT, Hui PW, et al. Necrotizing enterocolitis in neonates with symptomatic congenital heart disease. J Pediatr . 1988;113:1044–6. doi: 10.1016/s0022-3476(88)80580-8. [DOI] [PubMed] [Google Scholar]
- 4.McElhinney DB, Hedrick HL, Bush DM, et al. Necrotizing enterocolitis in neonates with congenital heart disease: risk factors and outcomes. Pediatrics. 2000;106:1080–7. doi: 10.1542/peds.106.5.1080. [DOI] [PubMed] [Google Scholar]
- 5.Becker KC, Hornik CP, Cotten CM, et al. Necrotizing enterocolitis in infants with ductal-dependent congenital heart disease. Am J Perinatol . 2015;32:633–8. doi: 10.1055/s-0034-1390349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mukherjee D, Zhang Y, Chang DC, et al. Outcomes analysis of necrotizing enterocolitis within 11 958 neonates undergoing cardiac surgical procedures. Arch Surg . 2010;145:389–92. doi: 10.1001/archsurg.2010.39. [DOI] [PubMed] [Google Scholar]
- 7.Motta C, Scott W, Mahony L, et al. The association of congenital heart disease with necrotizing enterocolitis in preterm infants: a birth cohort study. J Perinatol. 2015;35:949–53. doi: 10.1038/jp.2015.96. [DOI] [PubMed] [Google Scholar]
- 8.Lambert DK, Christensen RD, Henry E, et al. Necrotizing enterocolitis in term neonates: data from a multihospital health-care system. J Perinatol . 2007;27:437–43. doi: 10.1038/sj.jp.7211738. [DOI] [PubMed] [Google Scholar]
- 9.Bolisetty S, Lui K, Oei J, et al. A regional study of underlying congenital diseases in term neonates with necrotizing enterocolitis. Acta Paediatr . 2000;89:1226–30. doi: 10.1080/080352500750027619. [DOI] [PubMed] [Google Scholar]
- 10.Siano E, Lauriti G, Ceccanti S, et al. Cardiogenic Necrotizing Enterocolitis: A Clinically Distinct Entity from Classical Necrotizing Enterocolitis. Eur J Pediatr Surg . 2019;29:014–22. doi: 10.1055/s-0038-1668144. [DOI] [PubMed] [Google Scholar]
- 11.Iannucci GJ, Oster ME, Mahle WT. Necrotising enterocolitis in infants with congenital heart disease: the role of enteral feeds. Cardiol Young . 2013;23:553–9. doi: 10.1017/S1047951112001370. [DOI] [PubMed] [Google Scholar]
- 12.Natarajan G, Anne SR, Aggarwal S. Outcomes of congenital heart disease in late preterm infants: double jeopardy? Acta Paediatr. 2011;100:1104–7. doi: 10.1111/j.1651-2227.2011.02245.x. [DOI] [PubMed] [Google Scholar]
- 13.Cognata A, Kataria-Hale J, Griffiths P, et al. Human Milk Use in the Preoperative Period Is Associated with a Lower Risk for Necrotizing Enterocolitis in Neonates with Complex Congenital Heart Disease. J Pediatr. 2019;215:11–6. doi: 10.1016/j.jpeds.2019.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Spinner JA, Morris SA, Nandi D, et al. Necrotizing Enterocolitis and Associated Mortality in Neonates With Congenital Heart Disease: A Multi-Institutional Study. Pediatr Crit Care Med . 2020;21:228–34. doi: 10.1097/PCC.0000000000002133. [DOI] [PubMed] [Google Scholar]
- 15.Bell MJ, Ternberg JL, Feigin RD, et al. Neonatal necrotizing enterocolitis. Therapeutic decisions based upon clinical staging. Ann Surg . 1978;187:1–7. doi: 10.1097/00000658-197801000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kliegman RM, Walsh MC. Neonatal necrotizing enterocolitis: Pathogenesis, classification, and spectrum of illness. Curr Probl Pediatr. 1987;17:219–88. doi: 10.1016/0045-9380(87)90031-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Akkinapally S, Hundalani SG, Kulkarni M, et al. Prostaglandin E1 for maintaining ductal patency in neonates with ductal-dependent cardiac lesions. Cochrane Database Syst Rev. 2018;2:CD011417. doi: 10.1002/14651858.CD011417.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pickard SS, Feinstein JA, Popat RA, et al. Short- and long-term outcomes of necrotizing enterocolitis in infants with congenital heart disease. Pediatrics . 2009;123:e901–6. doi: 10.1542/peds.2008-3216. [DOI] [PubMed] [Google Scholar]
- 19.Wong SN, Lo RN, Hui PW. Abnormal renal and splanchnic arterial Doppler pattern in premature babies with symptomatic patent ductus arteriosus. J Ultrasound Med . 1990;9:125–30. doi: 10.7863/jum.1990.9.3.125. [DOI] [PubMed] [Google Scholar]
- 20.Carlo WF, Kimball TR, Michelfelder EC, et al. Persistent diastolic flow reversal in abdominal aortic Doppler-flow profiles is associated with an increased risk of necrotizing enterocolitis in term infants with congenital heart disease. Pediatrics . 2007;119:330–5. doi: 10.1542/peds.2006-2640. [DOI] [PubMed] [Google Scholar]
- 21.Kargl S, Maier R, Gitter R, et al. Necrotizing enterocolitis after open cardiac surgery for congenital heart defects--a serious threat. Klin Padiatr . 2013;225:24–8. doi: 10.1055/s-0032-1331724. [DOI] [PubMed] [Google Scholar]
- 22.Kessler U, Schulte F, Cholewa D, et al. Outcome in neonates with necrotizing enterocolitis and patent ductus arteriosus. World J Pediatr . 2016;12:55–9. doi: 10.1007/s12519-015-0059-6. [DOI] [PubMed] [Google Scholar]
- 23.van der Heide M, Mebius MJ, Bos AF, et al. Hypoxic/ischemic hits predispose to necrotizing enterocolitis in (near) term infants with congenital heart disease: a case control study. BMC Pediatr. 2020;20:553. doi: 10.1186/s12887-020-02446-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stapleton GE, Eble BK, Dickerson HA, et al. Mesenteric oxygen desaturation in an infant with congenital heart disease and necrotizing enterocolitis. Tex HeartInst J. 2007;34:442–4. [PMC free article] [PubMed] [Google Scholar]
- 25.Howarth C, Banerjee J, Leung T, et al. Could Near Infrared Spectroscopy (NIRS) be the new weapon in our fight against Necrotising Enterocolitis? Front Pediatr. 2022;10:1024566. doi: 10.3389/fped.2022.1024566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hassinger AB, Wald EL, Goodman DM. Early postoperative fluid overload precedes acute kidney injury and is associated with higher morbidity in pediatric cardiac surgery patients. Pediatr Crit Care Med . 2014;15:131–8. doi: 10.1097/PCC.0000000000000043. [DOI] [PubMed] [Google Scholar]
- 27.Hazle MA, Gajarski RJ, Yu S, et al. Fluid overload in infants following congenital heart surgery. Pediatr Crit Care Med . 2013;14:44–9. doi: 10.1097/PCC.0b013e3182712799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Seguin J, Albright B, Vertullo L, et al. Extent, risk factors, and outcome of fluid overload after pediatric heart surgery*. Crit Care Med . 2014;42:2591–9. doi: 10.1097/CCM.0000000000000517. [DOI] [PubMed] [Google Scholar]
- 29.Lex DJ, Tóth R, Czobor NR, et al. Fluid Overload Is Associated With Higher Mortality and Morbidity in Pediatric Patients Undergoing Cardiac Surgery. Pediatr Crit Care Med . 2016;17:307–14. doi: 10.1097/PCC.0000000000000659. [DOI] [PubMed] [Google Scholar]
- 30.Bhaskar P, Dhar AV, Thompson M, et al. Early fluid accumulation in children with shock and ICU mortality: a matched case-control study. Intensive Care Med . 2015;41:1445–53. doi: 10.1007/s00134-015-3851-9. [DOI] [PubMed] [Google Scholar]
- 31.Piggott KD, Soni M, Decampli WM, et al. Acute Kidney Injury and Fluid Overload in Neonates Following Surgery for Congenital Heart Disease. World J Pediatr Congenit Heart Surg . 2015;6:401–6. doi: 10.1177/2150135115586814. [DOI] [PubMed] [Google Scholar]
- 32.Wauters J, Claus P, Brosens N, et al. Pathophysiology of renal hemodynamics and renal cortical microcirculation in a porcine model of elevated intra-abdominal pressure. J Trauma . 2009;66:713–9. doi: 10.1097/TA.0b013e31817c5594. [DOI] [PubMed] [Google Scholar]
- 33.Mehta RL, Bouchard J, Soroko SB, et al. Sepsis as a cause and consequence of acute kidney injury: Program to Improve Care in Acute Renal Disease. Intensive Care Med . 2011;37:241–8. doi: 10.1007/s00134-010-2089-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Leach RM, Treacher DF. Oxygen transport-2. Tissue hypoxia. BMJ. 1998;317:1370–3. doi: 10.1136/bmj.317.7169.1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pedersen KR, Hjortdal VE, Christensen S, et al. Clinical outcome in children with acute renal failure treated with peritoneal dialysis after surgery for congenital heart disease. Kidney Int. 2008;73:S81–6. doi: 10.1038/sj.ki.5002607. [DOI] [PubMed] [Google Scholar]
- 36.Sorof JM, Stromberg D, Brewer ED, et al. Early initiation of peritoneal dialysis after surgical repair of congenital heart disease. Pediatr Nephrol. 1999;13:641–5. doi: 10.1007/s004670050672. [DOI] [PubMed] [Google Scholar]
- 37.Kwiatkowski DM, Menon S, Krawczeski CD, et al. Improved outcomes with peritoneal dialysis catheter placement after cardiopulmonary bypass in infants. J Thorac Cardiovasc Surg. 2015;149:230–6. doi: 10.1016/j.jtcvs.2013.11.040. [DOI] [PubMed] [Google Scholar]
- 38.Saini A, Delius RE, Seshadri S, et al. Passive peritoneal drainage improves fluid balance after surgery for congenital heart disease. Eur J Cardiothorac Surg. 2012;41:256–60. doi: 10.1016/j.ejcts.2011.05.037. [DOI] [PubMed] [Google Scholar]
- 39.Sasser WC, Dabal RJ, Askenazi DJ, et al. Prophylactic Peritoneal Dialysis Following Cardiopulmonary Bypass in Children Is Associated with Decreased Inflammation and Improved Clinical Outcomes. Congenit Heart Dis . 2014;9:106–15. doi: 10.1111/chd.12072. [DOI] [PubMed] [Google Scholar]
- 40.Dittrich S, Aktuerk D, Seitz S, et al. Effects of ultrafiltration and peritoneal dialysis on proinflammatory cytokines during cardiopulmonary bypass surgery in newborns and infants. Eur J Cardiothorac Surg. 2004;25:935–40. doi: 10.1016/j.ejcts.2004.02.008. [DOI] [PubMed] [Google Scholar]
- 41.Barhight MF, Soranno D, Faubel S, et al. Fluid Management With Peritoneal Dialysis After Pediatric Cardiac Surgery. World J Pediatr Congenit Heart Surg. 2018;9:696–704. doi: 10.1177/2150135118800699. [DOI] [PubMed] [Google Scholar]


