Abstract
Pseudo-Meigs syndrome is a rare entity where pleural effusion and ascites disappear after resection of a benign or malignant pelvic tumour. We report a 48-year-old woman presented with shortness of breath and abdominal distention. She had a right-sided massive pleural effusion and ascites. Pleural and ascitic fluid analysis revealed exudative effusion in the absence of pyogenic foci, tuberculosis or malignant cells. Contrast-enhanced computed tomography of the abdomen showed bilateral ovarian malignancy with peritoneal deposits and ascites which was later confirmed as serous adenocarcinoma. Surgical resection of the tumour led to the resolution of the pleural effusion and ascites suggestive of Pseudo-Meigs syndrome. The presentation due to bilateral ovarian serous adenocarcinoma has not been reported in the literature.
Keywords: Pseudo-Meigs syndrome, Meigs syndrome, serous ovarian carcinoma
Introduction
Meigs and Cass reported a case series of patients associated with ascites, pleural effusion and ovarian fibroma in 1937 although it was first reported by Demons in 1887.1–3 The most commonly associated pathologies include fibromas, thecomas and granulosa cell tumours. These manifestations improve usually with tumour resection. Pseudo-Meigs syndrome (PMS) is a very rare condition characterized by the presence of a benign or malignant abdominal or pelvic tumour other than an ovarian fibroma accompanied by pleural effusion and ascites which disappear after tumour resection. We report this case due to the rarity of the occurrence and this is the first such report to be reported from Sri Lanka. Furthermore, there are no reported cases of bilateral ovarian serous adenocarcinoma giving rise to this syndrome.
Case
A previously well 48-year-old female, presented with progressively worsening shortness of breath and right-sided pleuritic-type chest pain accompanied by abdominal distension of 2 weeks duration. She did not have a fever, anorexia or cachexia suggestive of a chronic infection or malignancy. There were no other respiratory, gastrointestinal or genitourinary symptoms including vaginal bleeding or dyspareunia. The patient did not have any other skin rashes or joint pains suggestive of connective tissue disease or vasculitis. Furthermore, there was no history of tuberculosis or malignancy. She did not have a history of alcohol/narcotic use, herbal medicines or any significant occupational or environmental exposure to toxins or radiation.
She was a mesomorph with a body mass index of 21.9 kg/m2. The patient was not febrile, pale or icteric and there was no pedal oedema. The respiratory rate was 18/min and the pulse oximetry was 96% on air. There was a right-sided moderate pleural effusion with mediastinal shift and tracheal deviation. The abdominal examination revealed moderate ascites without hepatosplenomegaly. There were no stigmata of chronic liver cell disease. There was no ankle or sacral oedema. Her blood pressure was 130/80 mmHg and her pulse rate was 88/min. The rest of the cardiovascular and neurological examinations were normal.
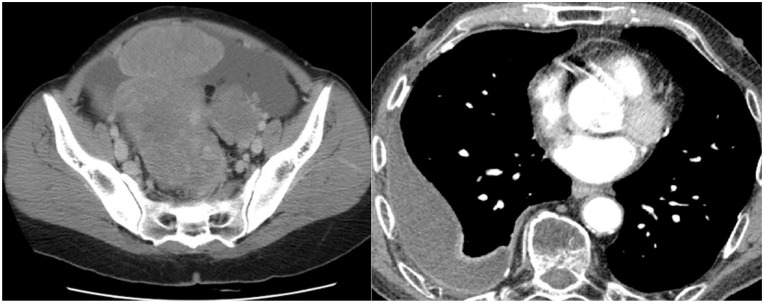
Chest radiography confirmed the presence of a right-sided massive pleural effusion for which a diagnostic and therapeutic aspiration was performed. The pleural fluid analysis revealed an exudative effusion with proteins 49.1 g/L, red cells 2–4/hpf, pus cells 1–3/hpf, pleural fluid lactate dehydrogenase (LDH) 206 u/L (serum LDH was 232.4 u/L). There were no malignant cells in cytology. The Gram stain was negative and acid/alcohol fast bacilli were not detected. Pleural fluid tuberculosis GeneXpert MTB-RIF test and pleural fluid adenosine deaminase levels were negative while three samples for sputum for acid-fast bacilli were unremarkable. The ascitic fluid analysis also revealed the presence of an exudate with a serum-ascites albumin gradient of 0.8 g/dl. There were 2–4 red cells/hpf and 2–4 pus cells in the absence of malignant cells or pyogenic organisms or acid-fast bacilli in cytology. The serum CA-125 level was significantly raised with a value of 1342 U/ml. A contrast-enhanced computed tomography demonstrated a right-sided pleural effusion in the background of bilateral ovarian carcinoma with peritoneal deposit and malignant ascites (Figure 1). There was no evidence of chronic liver disease, portal hypertension or the presence of lymphadenopathy. Her other investigations are summarized in Table 1.
Figure 1.
Contrast-enhanced computed tomography of pelvis and chest.
Table 1.
Summary of investigations.
| White blood cells | 8 × 109 cells/L Neutrophils 68%Lymphocytes 30 % |
4–11 × 109 |
|---|---|---|
| Haemoglobin | 12.5 g/dl | 13.5–16 |
| Platelets | 241 × 109/L | 150–400 × 109 |
| C-reactive protein | 6 mg/L | <6 |
| Erythrocyte sedimentation rate (ESR) | 80 mm in 1st h | <20 |
| Serum creatinine | 106 μmol/L | 65.4–119.3 |
| Serum sodium | 142 mmol/L | 135–145 |
| Serum potassium | 4.5 mmol/L | 3.5–5.5 |
| Serum calcium | 9.2 mg/dl | 8.6–10.3 |
| Aspartate transaminase (AST) | 38 U/L | 5–40 |
| Alanine transaminase (ALT) | 35 U/L | 5–55 |
| Alkaline phosphatase (ALP) | 87 U/L | 40–120 |
| Total bilirubin | 0.8 mg/dl | <1.2 |
| Albumin | 4.8 g/dl | 3.5–5.4 |
| Globulin | 2.1 g/dl | 2–3.5 |
| International normalised ratio (INR) | 1.0 | <1.1 |
The patient underwent a radical hysterectomy with bilateral salphingo-oophorectomy. Intraoperatively there was evidence of local spread of the tumour due to metastatic deposits into the fallopian tubes. Histologically it was composed of papillary structures, clusters and sheets of polygonal cells (Figure 2). The cells contain moderately pleomorphic vesicular nuclei and moderate eosinophilic cytoplasm. Further, it was a high-grade serous carcinoma of the ovary with capsular breach with vascular emboli. Bilateral Para-tubal tissue and bilateral parametrial deposits were noted. Tumour cells showed mutant-type positivity for P53 and focal nuclear positivity for Wilm tumour protein 1 (WT-1) and oestrogen receptor (ER). She underwent chemoradiation therapy. Resection of the tumour resulted in the resolution of the effusion and ascites. The pleural effusion and ascites had resolved completely at 1 month’s review. This phenomenon with the evidence of a malignant tumour of the ovaries that resolved following tumour resection was suggestive of PMS. The patient did not have any recurrence of ascites or pleural effusions and was clinically well at her outpatient visit in 6 months
Figure 2.
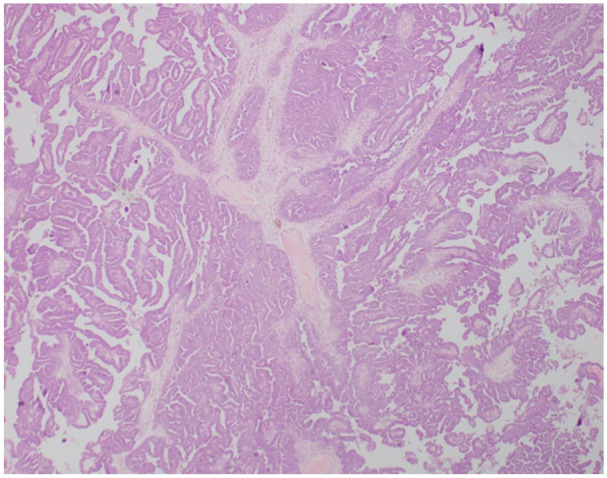
Histological appearance of the serous ovarian carcinoma showing papillary structures.
Discussion/conclusion
Meigs syndrome (MS) is diagnosed based on a triad of benign and solid ovarian fibroma, pleural effusion, and ascites which is supported by resolution following resection of the primary pathology.1,3,4 PMS or atypical MS is described when abdominal ascites and pleural effusion occur in association with a pelvic or abdominal tumour other than an ovarian fibroma. Usually, tumours such as uterine myoma, mature teratoma, struma ovarii and ovarian cancer with or without metastasis give rise to this entity. 5 Tjalma syndrome or Pseudo-PMS is seen in systemic lupus erythematosus patients which is characterized by the presence of pleural effusion and ascites with a high serum CA-125 level without any evidence of a tumour. 6
There have been some reports of PMS associated with malignancy of the uterus although benign uterine tumours such as leiomyomas are frequently associated with PMS.7,8 Only a few case reports of bilateral ovarian pathologies related to PMS exist in literature. Yin et al described a presentation of bilateral endometroid carcinoma while Naito et al reported metastatic breast cancer of both ovaries.9,10 This patient had the only presentation of a bilateral ovarian malignancy of serous adenocarcinoma type that the authors were able to find in the literature making this case report a unique one.
Several concepts were proposed for the underlying pathophysiology of ascites in these patients such as irritation of the visceral peritoneum overlying the tumour, tumour application of pressure on pelvic and abdominal lymphatics and venous circulation, and fluid leaking from fibroadenoma.11–15 Vascular endothelial growth factor is also postulated to be associated with ascites in MS. 16 Other agents include fibroblast growth factor and interleukin-6. 3 Studies have shown that the levels of these chemical agents reduce after resection of the primary tumour along with the resolution of serous fluid collection, thus supporting the existence of the clinical entity of MS/PMS.3,16 Pleural effusion in MS is believed to be due to the leakage of ascitic fluid into the pleural cavity via small pores in the diaphragm or diaphragmatic lymph channels.4,17,18 The possible explanation for the right-sided predilection of effusions to be present more in the right may be the presence of a greater number of large diaphragmatic lymphatic channels compared to the left. 19 However, there are reported cases on the left side and bilateral pleural effusions. 20
CA-125, a high molecular weight glycoprotein is expressed endometrium, Fallopian tubes and ovaries. 21 When there is vascular invasion, inflammation and tissue destruction even with malignancies, the levels are seen to rise. When ascites present with a high level of CA-125 they may be mistaken for malignancy that presents as peritoneal carcinomatosis. CA-125 levels are raised with peritoneal irritation as well as the large quantity of ascites. 22 Thus diagnostic difficulties are differentiated by histology as in our patient where solid features of malignancy were seen in ovarian histology but with normal cytology. 23
The strong grounds of this case report are based on the definitive histological diagnosis along with radiological evidence accompanied by the clinical presentation that resolved completely after resection of the primary pathology.
It is imperative that when a patient presents with right-sided pleural effusion and ascites, a pelvic tumour, in particular, arising from ovaries is evaluated. Such presentation leads to a diagnosis of PMS which is confirmed by the resolution of serous fluid by resection of the tumour.
Acknowledgments
We extend our thanks to the patient who provided us with the consent to publish this case report.
Footnotes
The author(s) declared no potential conflicts of interest concerning the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethics approval: Our institution does not require ethical approval for reporting individual cases or case series.
Informed consent: Written informed consent was obtained from the patient for their anonymized information to be published in this article.
ORCID iD: S. Silva  https://orcid.org/0000-0002-7348-4830
https://orcid.org/0000-0002-7348-4830
References
- 1. Meigs JV. Fibroma of the ovary with ascites and hydrothorax – Meigs’ syndrome. Am J Obstet Gynecol 1954; 67: 962–987. [DOI] [PubMed] [Google Scholar]
- 2. Brun J-L. Demons syndrome revisited: a review of the literature. Gynecol Oncol 2007; 105: 796–800. [DOI] [PubMed] [Google Scholar]
- 3. Iavarone I, Padovano M, Pasanisi F, et al. Meigs syndrome and elevated CA-125: case report and literature review of an unusual presentation mimicking ovarian cancer. Medicina (B Aires) 2023; 59: 1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. O’Flanagan SJ, Tighe BF, Egan TJ, et al. Meigs’ syndrome and pseudo-Meigs’ syndrome. J R Soc Med 1987; 80: 252–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Peparini N, Chirletti P. Ovarian malignancies with cytologically negative pleural and peritoneal effusions: demons’ or Meigs’ pseudo-syndromes? Int J Surg Pathol 2009; 17: 396–397. [DOI] [PubMed] [Google Scholar]
- 6. Gao F, Xu Y, Yang G. Pseudo-pseudo Meigs’ syndrome presenting with a combination of polyserositis, elevated serum CA 125 in systemic lupus erythematosus. Medicine 2019; 98: e15393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Huang SE, Huang SC, Lee WY, et al. Pseudo-Meigs syndrome caused by uterine smooth muscle tumor of uncertain malignant potential with low vascular endothelial growth factor expression. Int J Gynecol Cancer 2008; 18: 851–853. [DOI] [PubMed] [Google Scholar]
- 8. Marci R, Giugliano E, Carboni S, et al. Pseudo-Meigs’ syndrome caused by a uterine leiomyosarcoma: a new clinical condition. Gynecol Obstet Invest 2011; 72: 68–72. [DOI] [PubMed] [Google Scholar]
- 9. Yin H, Li XH, Xu HM, et al. Pseudo–Meigs’ syndrome secondary to bilateral ovarian endometrioid carcinomas. Int J Gynecol Obstetr 1999; 66: 293–295. [DOI] [PubMed] [Google Scholar]
- 10. Naito K, Oura S, Yasuoka H, et al. A case of pseudo-Meigs’ syndrome associated with ovarian metastases from breast cancer. J Breast Cancer 2012; 15: 474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Krenke R, Maskey-Warzechowska M, Korczynski P, et al. Pleural effusion in Meigs’ syndrome – Transudate or exudate? Medicine 2015; 94: e2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rubin IC, Novak J, Squire JJ. Ovarian fibromas and theca-cell tumors: report of 78 cases with special reference to production of ascites and hydrothorax (Meigs’ Syndrome). Am J Obstet Gynecol 1944; 48: 601–616. [Google Scholar]
- 13. Mitrou S, Manek S, Kehoe S. Cystic struma ovarii presenting as pseudo-Meigs’ syndrome with elevated CA125 levels. A case report and review of the literature. Int J Gynecol Cancer 2008; 18: 372–375. [DOI] [PubMed] [Google Scholar]
- 14. Chandanwale SS, Pal SS, Kumar HB, et al. Serous cystadenoma and fibrothecoma: a rare combination in collision tumor of ovary with pseudo-Meigs syndrome. J Pathol Transl Med 2015; 49: 163–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Weise M, Westphalen S, Fayyazi A, et al. Pseudo-Meigs syndrome: uterine leiomyoma with bladder attachment associated with ascitesand hydrothorax – a rare case of a rare syndrome. Oncol Res Treat 2002; 25: 443–446. [DOI] [PubMed] [Google Scholar]
- 16. Ishiko O, Yoshida H, Sumi T, et al. Vascular endothelial growth factor levels in pleural and peritoneal fluid in Meigs’ syndrome. Eur J Obstetr Gynecol Reprod Biol 2001; 98: 129–130. [DOI] [PubMed] [Google Scholar]
- 17. Riker D, Goba D. Ovarian mass, pleural effusion, and ascites. J Bronchology Interv Pulmonol 2013; 20: 48–51. [DOI] [PubMed] [Google Scholar]
- 18. Nagakura S, Shirai Y, Hatakeyama K. Pseudo-Meigs’ syndrome caused by secondary ovarian tumors from gastrointestinal cancer. Dig Surg 2000; 17: 418–419. [DOI] [PubMed] [Google Scholar]
- 19. Dalal N, Athwal PSS, Tharu B, et al. A rare case of pseudo-Meigs’ syndrome with ovarian metastasis presenting as Meigs’ syndrome. Cureus 2020; 12(10): e11022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Singh M, Singh S. An unusual case of sudden collapse in the immediate postoperative period in a young healthy female with myxofibroma of the maxilla. Case Rep Anesthesiol 2013; 2013: 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kabawat SE, Bast RC, Bhan AK, et al. Tissue distribution of a coelomic- epithelium-related antigen recognized by the monoclonal antibody OC125. Int J Gynecol Pathol 1983; 2: 275–285. [DOI] [PubMed] [Google Scholar]
- 22. Benjapibal M, Sangkarat S, Laiwejpithaya S, et al. Meigs’ syndrome with elevated serum CA125: case report and review of the literature. Case Rep Oncol 2009; 2: 61–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Qureshi FU, Alvi WA. A challenging case of pseudo Meigs syndrome: a case report. J Pak Med Assoc 2022; 72: 547–549. [DOI] [PubMed] [Google Scholar]