Abstract
Abstract
Objective
Evidence of geographical variation in total hip replacement (THR) and deviations from treatment guidelines persists. In this exploratory study, we aim to gain an in-depth understanding of patients’ healthcare trajectories by identifying and visualising medication use patterns in coxarthrosis patients before surgery. We examine their association with patient characteristics and THR, and compare them with recommendations on mild analgesics, opioid prescription and exhaustion of conservative therapy.
Methods
In this exploratory study, we apply State Sequence Analysis (SSA) on German health insurance data (2012–2015). We analyse a cohort of coxarthrosis patients, half of whom underwent THR after a 1 year observation period and half of whom did not undergo surgery until at least 1 year after the observation period. Hierarchical states are defined based on prescriptions. We construct sequences, calculate sequence similarity using optimal matching and identify medication use patterns via clustering. Patterns are visualised, descriptive statistics are presented and logistic regression is employed to investigate the association of medication patterns with subsequent THR.
Results
Seven distinct medication use patterns are identified, correlating strongly with patient characteristics and subsequent THR. Two patterns leading to THR demonstrate exhaustion of pharmacological therapy. Opioid use is concentrated in two small patterns with low odds for THR. The most frequent pattern lacks significant pharmacological therapy.
Conclusions
This SSA uncovers heterogeneity in medication use patterns before surgery in coxarthrosis patients. Cautious opioid handling and adherence to a stepped prescription approach are observed, but many patients display low medication therapy usage and lack evidence of exhausting conservative options before surgery.
Keywords: chronic disease, observational study, orthopaedic & trauma surgery, health services administration & management, hip
STRENGTHS AND LIMITATIONS OF THIS STUDY.
This study employs innovative sequence clustering methods to identify, visualise and investigate medication patterns among patients with coxarthrosis.
Correlation analyses with patient characteristics and logistic regression modelling, with the occurrence of total hip replacement as the outcome, are used to characterise and comprehend the identified patterns.
The analysis is based on a large dataset from German health insurance records spanning multiple years.
This study examines prescription medications only.
Introduction
Numerous evidence-based guidelines exist for the treatment of coxarthrosis, that aims to reduce pain, slow joint degeneration and maintain or restore joint functionality and mobility.1,8 Despite their availability, significant regional variations in total hip replacement (THR) rates have been observed in Germany9 and internationally,10,12 which cannot be fully accounted for by morbidity differences in the population. Studies have identified and discussed various non-morbidity-related factors, including supply structures, regional differences in medical practice paradigms, physician preferences, as well as social and economic factors.9 11 12 These factors can influence treatment decisions for coxarthrosis patients and may lead to deviations from guidelines.
Many studies have investigated healthcare utilisation among coxarthrosis patients, focusing on the appropriate use of conservative therapy options, potential opioid misuse, and the appropriateness and timeliness of THR indications. Several German and international studies have reported potential underutilisation of conservative therapy options, especially physiotherapy and medications from step 1 of the WHO analgesic ladder (including non-opioid analgesics, non-steroidal anti-inflammatory drugs (NSAID) and cyclo-oxygenase-2 inhibitors, hereinafter referred to as ‘mild analgesics’).13,18 Some studies have criticised deviations from the stepped prescription approach,15 16 although positive evidence for its application exists.19
Another concern pertains to the potential misuse or overuse of opioids (steps 2 and 3 of the WHO analgesic ladder13), particularly in light of the US opioid epidemic.20,22 While the extent of opioid use in Germany is not considered epidemic, researchers have noted the rising number of opioid prescriptions overall and among osteoarthritis patients.14 19 23 Furthermore, the observed geographical variation in THR rates9,12 raises questions about the appropriateness and timeliness of the decision for THR surgery. Although assessing the appropriateness of indications for THR at the population level remains challenging, one study compared healthcare utilisation of patients prior to surgery with the recommendation to exhaust conservative therapy options before undergoing surgery, suggesting that many patients do not receive conservative treatments before undergoing THR surgery.24
The majority of these studies and treatment guidelines possess a predominantly normative character. They focus on how patients’ care should be provided and how their care pathways should be designed. However, in recent years, there has been a growing interest in empirical, exploratory patient pathway analyses, that focus on how patients actually receive care and navigate the healthcare system.25 These analyses aim to offer a comprehensive view of the care situation for the patient population under investigation and allow for the identification of characteristic care patterns.26,28 By comparing these observed, real-world patterns with ideal, normative pathways or guideline-based treatment recommendations, gaps in care and deviations from desired care pathways can be identified.26 29 30 Analysing the correlation between identified patterns and patient characteristics, supply-side factors or health outcomes can enhance our understanding of healthcare in the studied patient population, explain unexpected patterns or deviations from guidelines, and pinpoint areas for further research.2627 29,31
Various methods, often drawn from the field of data mining, are employed for this purpose.25 In recent years, a combination of sequencing and clustering methods called ‘State Sequence Analysis’ (SSA), originally from the social sciences,32 33 has been successfully applied to healthcare data or health insurance claims data to identify healthcare utilisation patterns of patients.26 27 29 30 SSA enables the identification, investigation and holistic visualisation of characteristic healthcare patterns.26 27 29 30
This study aims to offer insights into the care of coxarthrosis patients who have not yet undergone THR surgery. Our objective is to identify, visualise and investigate empirical pain medication use patterns in these patients, while also examining whether specific medication patterns lead to THR. We will interpret our findings on care patterns in relation to patient characteristics and compare them to guideline recommendations. Key aspects of interest include appropriate prescribing of mild analgesics, the stepped prescription approach, cautious handling of opioid prescriptions and the exhaustion of pharmacological treatment options prior to surgery. To achieve these goals, we will employ innovative sequencing and clustering techniques known as SSA on comprehensive health insurance claims data, use descriptive statistics and apply logistic regression.
Methods
Data, sample and observation period
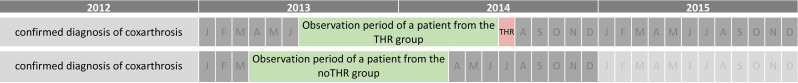
Our analysis used comprehensive data from two German statutory health insurers, the Allgemeine Ortskrankenkasse Bayern, which operates in Bavaria, and the Siemens Betriebskrankenkasse, a nationwide operating insurance. The choice of either of these health insurances, beyond geographical limitation, is a free choice of the insured individuals. There are no differences in the services and reimbursements provided by these insurers with respect to the care events analysed in this study. The data, spanning 2012–2015, includes reimbursable claims entailing prescription, diagnoses and demographics for individuals aged 18+ diagnosed with coxarthrosis in 2012. The prescription dataset includes all prescribed and dispensed medications, their quantities, Anatomical Therapeutic Chemical (ATC) classifications and daily defined doses. Only patients with complete demographic information were used for the analysis. We formed an analytical sample comprising two groups, excluding patients with femur fractures, femoral osteonecrosis or complications from orthopaedic devices. The first group consisted of patients with confirmed coxarthrosis who underwent THR surgery between 2013 and 2015 but not in 2012 (THR group). The second group included an equal number of randomly selected coxarthrosis patients without THR surgery between 2012 and 2015 (noTHR group). A coxarthrosis diagnosis was considered confirmed if diagnosed twice in different quarters within the outpatient sector or once in the inpatient sector in 2012. For ICD and procedure codes, see online supplemental material. The observation period (see figure 1) for the THR group was set to 12 months before the month of THR surgery. For the noTHR group, the 12-month observation period start was randomly chosen between 1 January 2012 and 31 December 2013, ensuring that patients did not undergo THR surgery for at least 1 year postobservation.
Figure 1. Visualization of the timeline. An exemplary observation period of a patient from the THR and noTHR group is shown. THR indicates total hip replacement; J to D, months.
Definition of medication use sequences
We aimed to identify patterns of pain medication use. An individual patient’s medication use sequence is constructed by consecutive states, with each state defined by the prescription events a patient encounters within a specified timeframe. Since medication prescriptions are accurately recorded in German health insurance claims, we used the small time unit of months. Consequently, the 12-month observation period corresponds to sequences of 12 successive states in SSA terminology.
Since the pain management approach for osteoarthritis can also be aligned with the established stage framework of the WHO analgesic ladder,3 13 we have structured the pharmacological interventions according to the classification outlined in this scheme. We identified prescribed medications based on ATC codes (refer to online supplemental material for ATC codes used) and calculated the days covered by the prescription based on the daily defined dose. A patient was considered a user of the respective medication in a given month if the prescription covered ten or more days. Medication therapies were grouped into three states: ‘N’ for no prescription, ‘M’ for mild analgesics (step 1 of the WHO ladder), and ‘O’ for opioids (steps 2 or 3 of the WHO ladder). It is important to note that small doses of mild analgesics are available over the counter in Germany, which could lead to underestimating their use. States were designed hierarchically, classifying patients based on the highest stage medication if they used medications from multiple stages.
Cluster analysis
To determine medication use patterns, we clustered the defined sequences into groups of similar sequences. This necessitates defining when two sequences are similar or, conversely, dissimilar. We used optimal matching to calculate sequence dissimilarity32 34 35 employing a feature dataset that reflects the hierarchical nature of the defined states.35 36 We used partitioning around medoids clustering to group sequences into clusters.37 To identify the optimal number of clusters, we evaluated quality measures and cluster cut-off criteria for 2–15 clusters, including the Average Silhouette Width (ASW) which reflects intercluster heterogeneity and intracluster homogeneity.38 39 To choose between solutions with comparable levels of cluster quality, we assessed each cluster solution based on its content validity, cluster size and whether an increased cluster solution contributed an additional cluster with theoretical significance. Based on these factors, we selected the 7-cluster solution. For more information on the cluster analysis, refer to online supplemental material.
In sensitivity analyses, we redraw randomly the noTHR group, conducted analyses with a bootstrapped THR group, and compared the 7-cluster solution to solutions of different orders with comparable quality criteria.
Clusters were visualised using frequency and distribution plots. We calculated summary statistics and performed χ2 tests to examine clusters and their unadjusted correlation with patient characteristics.
Investigating the relationship between medication use patterns and THR
To investigate the correlation between identified medication use patterns and the likelihood of receiving THR, we employed logistic regression with cluster membership as a categorical predictor variable. Additional independent variables included sociodemographic patient characteristics (age, gender, residential area), Elixhauser comorbidity score for general comorbidity adjustment40 41 and separate adjustment for opioid dependence. We used a hierarchical, categorical variable for pain based on ICD codes, following the approach of Freytag et al42 with minor modifications (see online supplemental material) to operationalise pain based on ICD diagnoses. We adjusted for the presence of diagnoses in pain categories above the osteoarthritis pain category (cancer, back pain, disc prolapse). Finally, we included a binary variable for physical therapy use. We used directed acyclic graphs to inform the theoretical model for logistic regression,43 with source code and visualisation available in online supplemental material.
Software used
Analyses were performed using R44 and Stata.45 Packages employed include the TraMineR,33 35 Weighted Cluster38 and co-morbidity package.46
Results
We identified 9975 patients with THR, resulting in a total study population of 19 950 individuals. Patient characteristics for the entire sample, as well as for the THR and noTHR groups, are presented in table 1. The majority of the study population is female, resides in rural regions, and is over 70 years old. By design, 50% of the study population undergoes THR, while less than half of the patients receive physical therapy during the observation period. Patients of the THR group tend to be younger and have fewer comorbidities, reside more frequently in cities and receive physical therapy to a greater extent.
Table 1. Unadjusted summary statistics for the entire sample and for THR and noTHR group.
| Total | THR group | noTHR group | P value | |
| Patients (n, %) | 19 950 (100.00) | 9975 (50.00) | 9975 (50.00) | |
| Patients (%) aged | ||||
| Below 60 years | 20.83 | 22.88 | 18.79 | |
| 60–69 years | 25.81 | 27.45 | 24.18 | |
| 70–79 years | 36.57 | 37.59 | 35.54 | |
| 80 years and older | 16.79 | 12.08 | 21.49 | <0.0001 |
| Mean age (95% CI) | 68.84 (68.69, 69.00) | 67.73 (67.52, 67.94) | 69.96 (69.73, 70.18) | <0.0001 |
| Women (%) | 58.03 | 58.36 | 57.69 | 0.344 |
| Patients living in (%) | ||||
| Urban region | 27.37 | 30.36 | 24.38 | |
| Region with moderate urbanisation | 22.10 | 22.29 | 21.90 | |
| Rural region | 50.54 | 47.36 | 53.71 | <0.0001 |
| Mean Elixhauser comorbidity score (95% CI) | 1.07 (1.02, 1.13) | 0.90 (0.83, 0.97) | 1.24 (1.16, 1.33) | <0.0001 |
| Patients in the following pain category (%) | ||||
| Cancer | 3.79 | 3.49 | 4.10 | 0.024 |
| Back pain | 33.00 | 32.87 | 33.12 | 0.707 |
| Disc prolapse | 13.02 | 13.24 | 12.80 | 0.355 |
| Patients with vertigo (%) | 14.48 | 12.33 | 16.63 | <0.0001 |
| Patients with opioid dependence (%) | 0.14 | 0.15 | 0.13 | 0.705 |
| Patients receiving physical therapy (%) | 45.97 | 52.73 | 39.22 | <0.0001 |
| Patients undergoing total hip replacement (%) | 50.00 | 100.00 | 0.00 |
P value comparing patients from THR group and noTHR group using appropriate correlation tests (Pearson’s χ2, Mann-Whitney, t-test).
CIconfidence intervalTHRtotal hip replacement
Cluster identification and visualisation
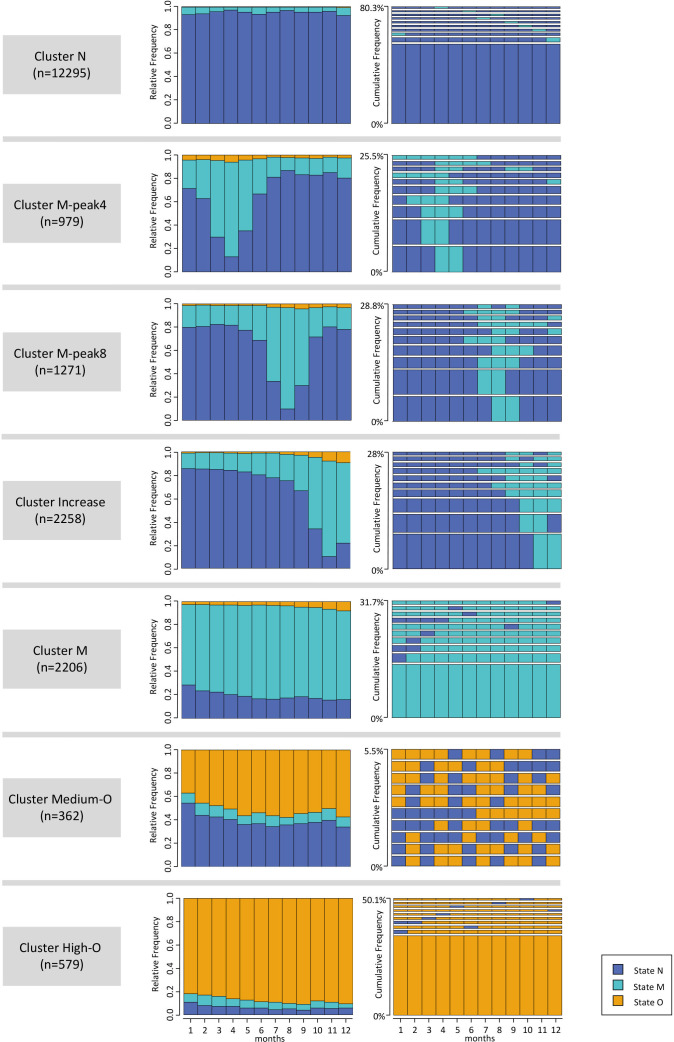
We identified seven clusters (ASW=0.52), ranging in size from 362 to 12 295 patients. Figure 2 displays the 10 most frequent sequences and the monthly distribution of states for each cluster.
Figure 2. Distribution and frequency plots for each of the seven identified clusters. The distribution plots show the distribution of states in each month of the observation period. The frequency plots show the 10 most frequent sequences in each group. The y-axis shows cumulative frequency; thus, the height of the sequences is relative to their occurrence. State N denotes the state with no prescription, state M with mild analgesic prescription, state O with an opioid prescription.
The visualisation of clusters in figure 2, shows, that each cluster is dominated by one or two states, with clusters differing in their dominant state(s) and observed dynamics over time. To simplify the presentation and discussion of results, clusters are henceforth referred to as ‘cluster N’ (state N dominant), ‘cluster M-peak4’ (rising and falling levels of M, peak in month 4 of the observation period), ‘cluster M-peak8’ (rising and falling levels of M, peak in month 8), ‘cluster Increase’ (escalating medication levels towards the end of the observation period), ‘cluster M’ (consistently high levels of M), ‘cluster Medium-O’ (continuous levels of around 50% of state O), and ‘cluster High-O’ (consistently high levels of O).
Medication use levels and cluster dynamics
The most dominant state overall is the prescription-free state N. Cluster N, the largest cluster, characterised by the prevalence of state N throughout the observation period, includes patients without significant pharmacologic therapy and accounts for 61.6% of the study population. State N is also the dominant state in clusters M-peak4, M-peak8 and Increase (combined 22.6% of the study population) for significantly more than half of the observation time.
State M is the most frequently observed medication state, particularly present in clusters M, M-peak4, M-peak8 and Increase (collectively 33.7% of the study population). While state M levels vary in clusters M-peak4, M-peak8 and Increase (rates between 11.0% and 87.1%), patients’ medication sequences in cluster M are characterised by continuous medication (rates between 69.3% and 80.7%).
Opioid use is primarily concentrated in clusters Medium-O and High-O which together constitute 4.7% of the study population. Cluster High-O exhibits consistently high opioid use (rates between 82.0% and 90.9%), while cluster Medium-O displays monthly medication levels around 50% (rates between 36.74% and 57.5%). The frequency plot of cluster Medium-O reveals that most patients alternate between states with and without prescriptions. Overall, medication levels increase toward the end of the observation period. This increase is most noticeable in cluster Increase, while clusters M, High-O and Medium-O also exhibit modest increases.
Regarding cluster dynamics, clusters N, High-O and M can be classified as stable throughout the observation time, while clusters M-peak4, M-peak-8 and Increase are considered dynamic. Cluster Medium-O occupies a unique position: few patients share the same sequences, but the alternating pattern between state N and state O and the state distribution remain consistent over time.
Patient characteristics by sequence clusters
The patient populations within individual clusters exhibit distinct characteristics (refer to table 2).
Table 2. Unadjusted summary statistics by sequence clusters.
| Cluster | N | M-peak4 | M-peak8 | Increase | M | Medium-O | High-O | P value |
| Patients (n, %) | 12 295 (61.63) | 979 (4.91) | 1271 (6.37) | 2258 (11.32) | 2206 (11.06) | 362 (1.81) | 579 (2.90) | |
| Patients (%) aged | ||||||||
| Below 60 years | 20.94 | 21.35 | 21.40 | 20.50 | 21.85 | 10.50 | 20.21 | |
| 60–69 years | 25.70 | 24.51 | 27.22 | 28.12 | 26.97 | 15.19 | 20.55 | |
| 70–79 years | 37.00 | 37.69 | 37.14 | 36.45 | 33.86 | 39.23 | 33.33 | |
| 80 years and older | 16.36 | 16.45 | 14.24 | 14.92 | 17.32 | 35.08 | 25.91 | 0.0001 |
| Mean age | 68.71 | 68.75 | 68.45 | 68.60 | 68.66 | 74.59 | 70.71 | |
| (95% CI) | (68.51, 68.91) | (68.04, 69.47) | (67.85, 69.05) | (68.15, 69.04) | (68.20, 69.13) | (73.48, 75.70) | (69.72, 71.70) | 0.0001 |
| Women (%) | 55.53 | 61.70 | 62.08 | 60.01 | 61.56 | 74.59 | 64.42 | <0.0001 |
| Patients living in (%) | ||||||||
| Urban region | 27.54 | 25.03 | 27.85 | 30.29 | 25.02 | 27.90 | 23.83 | |
| Region with moderate urbanisation | 22.03 | 23.29 | 21.24 | 21.70 | 22.48 | 21.82 | 23.49 | |
| Rural region | 50.43 | 51.69 | 50.90 | 48.01 | 52.49 | 50.28 | 52.68 | 0.02 |
| Mean Elixhauser comorbidity score | 0.92 | 1.27 | 0.98 | 1.14 | 1.17 | 2.94 | 2.36 | |
| (95% CI) | (0.86, 0.99) | (1.02, 1.53) | (0.78, 1.18) | (0.97, 1.31) | (0.99, 1.34) | (2.25, 3.63) | (1.87, 2.86) | 0.0001 |
| Patients in the following pain category (%) | ||||||||
| Cancer | 3.53 | 4.19 | 3.15 | 4.07 | 3.72 | 6.63 | 7.60 | <0.0001 |
| Back pain | 29.08 | 38.00 | 38.47 | 35.25 | 38.98 | 49.45 | 53.71 | <0.0001 |
| Disc prolapse | 12.22 | 17.16 | 14.71 | 15.63 | 13.24 | 8.84 | 11.05 | <0.0001 |
| Patients with vertigo (%) | 14.37 | 14.40 | 13.77 | 14.57 | 14.87 | 21.27 | 12.44 | 0.012 |
| Patients with opioid dependence (%) | 0.04 | 0.20 | 0.16 | 0.09 | 0.09 | 0.55 | 2.25 | <0.0001 |
| Patients receiving physical therapy (%) | 40.79 | 56.18 | 56.57 | 54.56 | 53.81 | 53.31 | 47.67 | <0.0001 |
| Patients undergoing THR (%) | 41.18 | 49.13 | 62.31 | 74.62 | 68.77 | 42.54 | 48.88 | <0.001 |
P value comparing patients from THR group and noTHR group using appropriate correlation tests (Pearson’s χ2, Kruskal-Wallis, analysis of variance).
THRtotal hip replacement
Cluster N includes the smallest percentage of women and is marked by relatively low or the lowest comorbidity levels (based on Elixhauser scores, opioid dependence and pain-related diagnoses) and healthcare utilisation concerning physical therapy (40.79%) and THR (41.18%).
The relatively young patients in the Increase cluster predominantly reside in urban areas and exhibit significantly higher rates of physical therapy (54.56%) and the highest THR rate (74.62%) compared with the other clusters.
Cluster M patients closely resemble those in the Increase cluster in terms of age, gender, comorbidity and physical therapy rates (53.81%). However, they are more likely to live in less urbanised areas. This cluster has the second-highest THR rate (68.77%).
Patients in clusters M-peak4 and M-peak8 share similar sociodemographic traits with those in cluster M. These two clusters exhibit the highest physical therapy rates, 56.15% and 56.75%, respectively. M-peak4 has a comparatively low THR rate of 49.13%, while M-peak8 has a higher THR rate of 62.31%.
Clusters Medium-O and High-O, with the oldest patients and highest women percentages, show significant differences from other clusters. These clusters exhibit the highest expression for all comorbidity variables and comparatively low THR and physical therapy rates.
Correlation with THR
Using logistic regression, we investigated whether the identified patterns exhibited increased or decreased odds for subsequent THR. The regression analysis (see table 3) revealed that, after adjusting for patient characteristics and morbidity, cluster membership is significantly associated with THR. Cluster Increase demonstrates the strongest association with THR, having fourfold odds compared with cluster N. Cluster M and M-peak8 follow with ORs above 3 and 2, respectively, while High-O and M-peak4 have ORs above 1. Cluster Medium-O displays only a slight, non-significant difference in odds compared with cluster N.
Table 3. Results of logistic regression for outcome total hip replacement surgery.
| Independent variables | OR | 95% CI | P value |
| Age, reference: below 60 years | |||
| 60–69 years | 0.927 | 0.850, 1.010 | 0.084 |
| 70–79 years | 0.909 | 0.838, 0.987 | 0.023 |
| 80 years and older | 0.486 | 0.439, 0.538 | <0.0001 |
| Gender, reference: male | |||
| Female | 1.020 | 0.960, 1.084 | 0.521 |
| Residence, reference: urban region | |||
| Region with moderate urbanisation | 0.797 | 0.733, 0.866 | <0.0001 |
| Rural region | 0.690 | 0.643, 0.740 | <0.0001 |
| Physical therapy, reference: none | |||
| Receiving physical therapy | 1.571 | 1.479, 1.669 | <0.0001 |
| Elixhauser comorbidity score | 0.982 | 0.975, 0.990 | <0.0001 |
| Pain category, reference: osteoarthritis | |||
| Cancer | 0.835 | 0.712, 0.979 | 0.026 |
| Back pain | 0.855 | 0.798, 0.916 | <0.0001 |
| Disc prolapse | 0.816 | 0.744, 0.894 | <0.0001 |
| Opioid dependence, reference: none | |||
| Opioid dependence | 1.029 | 0.471, 2.247 | 0.943 |
| Cluster membership, reference: cluster N | |||
| Cluster Increase | 4.160 | 3.751, 4.613 | <0.0001 |
| Cluster M-peak8 | 2.278 | 2.017, 2.572 | <0.0001 |
| Cluster M-peak4 | 1.352 | 1.183, 1.544 | <0.0001 |
| Cluster Medium-O | 1.209 | 0.972, 1.503 | 0.088 |
| Cluster M | 3.204 | 2.901, 3.539 | <0.0001 |
| Cluster High-O | 1.540 | 1.295, 1.831 | <0.0001 |
| Constant | 0.938 | 0.854, 1.029 | 0.175 |
Area under ROCreceiver operating characteristic curve: 67.7%. OR indicates Odds Ratio, Confidence Interval.
CIconfidence intervalORodds ratio
Older and more comorbid patients, as well as those in pain categories above the osteoarthritis pain category, exhibit significantly decreased odds for THR. Patients living in less urban regions also show decreased odds. Patients receiving physical therapy demonstrate increased odds for THR.
Discussion
In our study, we used SSA on German health insurance data to identify medication patterns for coxarthrosis patients and their correlation with patient characteristics and THR surgery. Our findings revealed seven distinct patterns, with the patient population showing significant differences in demographic, regional and health or healthcare use-related characteristics. Logistic regression showed a strong correlation between cluster membership and THR surgery. Below, we discuss our findings focusing on the recommended use of mild analgesics, a stepped prescription approach, cautious opioid use and the exhaustion of conservative therapy prior surgery.
Four clusters are characterised by the presence of state M: clusters Increase, M, M-peak4 and M-peak8. The medication pattern in all four clusters can be interpreted as a reflection of pain symptomatology.
The significant rise in mild analgesics in cluster Increase can be attributed to rapid disease progression and increasing symptom severity. In contrast, cluster M includes patients with continuous high-level analgesic use, indicating long-lasting and persistent pain. Both clusters do not show any particularly high comorbidity expression, suggesting osteoarthritis drives the medication patterns. This is supported by the high THR rates observed. The increase in opioid use in the final months can be reliably attributed to the goal of bridging the time to surgery, as recommended in guidelines.1,3 Cluster Increase and cluster M exhibit significant differences in age distribution and residential area. Since urbanity levels influence local healthcare infrastructure,47 an association between urban patients and high THR rates can be expected, as observed in cluster Increase. Surprisingly, even cluster M, with its more rural population, shows elevated medication use and THR rates.
Logistic regression confirms that these clusters represent two distinct medication use patterns, both leading to THR surgery. Both patterns reflect the exhaustion of conservative medication therapy prior to surgery, as recommended. An intriguing question for future research is whether long-term outcomes after surgery differ between patients with a dynamic movement to surgery, as seen in cluster Increase, and those with prolonged consistent pain management before surgery, as seen in cluster M.
The peaks of state M in M-peak4 and M-peak8 clusters can be attributed to acute pain episodes, typical for the intermittent course of coxarthrosis.48 The subsequent decrease in medication use may result from symptom improvement, which could occur spontaneously or as a result of changes in lifestyle or physical therapy use. Indeed, the two clusters show the highest rates of physical therapy. Alternatively, the decrease could stem from concerns over long-term analgesic use and associated side effects, leading to reduced medication use despite persisting pain. THR rates and ORs for THR surgery are significantly lower in these two clusters than in the Increase and M clusters, suggesting slower disease progression and less frequent decisions to undergo surgery. However, ORs for THR surgery compared with cluster N are significantly increased in both clusters. Given an appropriate indication for THR surgery, we cannot infer that the low medication rates in the final months of the observation period suggest an equally low level of pain symptomatology in these patients.
The two opioid clusters, Medium-O and High-O, show high long-term opioid use, exceeding recommendations,2 5 but represent a small fraction of the study population. These patterns may be attributed, at least partially, to other conditions, as indicated by pain and comorbidity variables and an older age demographic. Previous studies link comorbidity and age to opioid use, possibly due to increased NSAID contraindications.16 19 49 The clusters show low THR rates, potentially because coxarthrosis pain is deprioritised amidst multiple pain conditions and multifactorial immobility. However, given the evidence of higher osteoarthritis burden in patients with higher opioid use,14 underuse of physical therapy and THR surgery is possible. Future research should investigate whether the reduced utilisation of surgery and physical therapy benefits these older and more comorbid patients or represents treatment underuse.
Overall, in terms of opioid prescription, we found a generally low use of opioids. In clusters Increase, M, M-peak4 and M-peak8 short-term increases in use toward THR surgery or at time of the peaks are visible. Clusters with high opioid use are small and patient’s characteristics suggest the presence of other factors causing opioid use. These findings suggest a cautious prescribing of opioids and general adherence to the stepped prescription approach in these clusters, which is consistent with other German studies.14 19
It is important to note that the medication use patterns discussed affect less than half of patients, as 61.6% of patients belong to cluster N, which is characterised by the absence of any significant pharmacological therapy. Also in other clusters, clusters M-peak4, M-peak8 and Increase, the prescription-free state N is dominant for large parts of the observation time. While a high prescription rate of analgesics is not desirable per se, this high proportion of patients without significant pain medication raises concerns about whether all patients receive adequate access to and counselling about conservative treatment options. It is unlikely that this proportion of patients without medication reflects an equally high proportion of patients without pain, considering that all patients had a confirmed diagnosis of coxarthrosis prior to the observation period and many patients underwent hip surgery at the end of the observation period (with the lowest rate being as high as 41.18% in cluster N). Thus, we find no evidence of exhaustion of conservative medical treatment options prior THR in a significant proportion of patients. Additionally, the low use of physical therapy, especially in cluster N, suggests the underuse of physical therapy options. Previous national and international studies have raised similar concerns about the use of conservative therapy options.16 24 50 51
The logistic regression revealed further that the likelihood for THR surgery decreases with age and comorbidities, consistent with studies showing a clear correlation between these factors and THR surgery rates.10 16 The increase in complication rates, longer length of stay and higher readmission rates in patients with more comorbidities52 might discourage physicians from performing surgery in elderly patients, or induce concerns about surgery in elderly patients.53 In line with previous studies, the logistic regression results show the rural-urban divide9 and the correlation of THR rates with comorbidities.16
Strengths and limitations
The quality criteria for the presented 7-cluster solution indicate a reasonable data structure. Compared with previous studies applying SSA to insurance data, the quality achieved is very good.26 27 29 30 54 Sensitivity analyses confirm the cluster solution’s stability. We used a theory-driven approach to obtain meaningful measures of state dissimilarity and sequence dissimilarity by using a set of state features reflecting the hierarchical nature of prescription states. In doing so, we respond to criticisms levelled at purely data-driven methods.35
Our analysis, based on a large dataset from two German health insurance companies, benefits from low selection bias and comprehensiveness. The sizeable population allowed us to establish an expansive study cohort, extend our observation period to a full year and exclusively include patients in the noTHR group who had not undergone surgery for at least 1 year after the observation period.
Health insurance claims also have some limitations, many arising from the fact that they depict only prescribed medications, not actual use. First, WHO Level I analgesics are available in small doses over the counter. Thus, utilisation rates of mild analgesics are likely to be underestimated. However, since health insurers only cover the cost if a prescription is presented, there is an incentive, especially for frequent users, to obtain a prescription. Also, the low physical therapy rates not subject to this limitation confirm the prevailing pattern of low utilisation.
Second, we assumed purchased medications were used immediately after purchase. However, patients may use leftover medications from previous prescriptions, resulting in an underestimation of medication use rates. Conversely, patients can purchase prescribed medications but use less than the entire doses, which could lead to overestimated rates. However, we can reasonably assume that all mentioned scenarios occur on a much smaller scale than the macrolevel picture enabled by our analysis.
Third, we can only see prescriptions redeemed at the pharmacy. Thus, we cannot determine whether underserved patients lack prescriptions or simply have not redeemed them.
Another challenge with claims data is the operationalisation of pain. We addressed this problem by using hierarchical pain categories defined by Freytag et al for this purpose.42
Finally, health insurance claims data lack clinical and lifestyle information. As a result, imaging findings on joint wear progression, body mass index and physical activity are not available. This also means that non-billable components of arthritis-relevant conservative therapy measures, such as physician-recommended weight reduction or exercise, could not be included in our analysis.
Conclusion
In conclusion, using innovative methods like SSA on health insurance claims data enables us to identify, visualise and analyse medication use patterns, providing a comprehensive understanding of care patterns in coxarthrosis patients. Our analysis reveals the heterogeneity of medication use patterns and their strong correlation with sociodemographic and health-related characteristics.
Our findings show cautious opioid prescribing and a gradual prescription strategy in most clusters, in line with guidelines. However, the significant proportion of patients in cluster N, lacking substantial pharmacological therapy, suggests potential underuse of conservative pain management. This is supported by overall low physiotherapy rates. Thus, many patients do not seem to exhaust conservative therapy options before surgery.
Future research should investigate factors determining medication use, reasons for low conservative therapy utilisation and the impact of medication use patterns and surgery timing on long-term outcomes. Our findings underscore the ongoing need for health policy efforts to encourage patients and providers to exhaust conservative therapy options prior surgery.
supplementary material
Acknowledgements
The authors thank the AOK Bayern and the SBK health insurance companies for providing data for this study.
Footnotes
Funding: This study was conducted as part of the MobilE-PRO project, funded by the German Federal Ministry of Education and Research (Bundesministerium für Bildung und Forschung) under grant number 01GY1603A.
Prepublication history and additional supplemental material for this paper are available online. To view these files, please visit the journal online (https://doi.org/10.1136/bmjopen-2023-080348).
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient consent for publication: Not applicable.
Ethics approval: Not applicable.
Data availability free text: The datasets generated during and/or analysed during the current study are not publicly available due to German Federal data protection laws. Methodological details are provided in online supplemental material.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Contributor Information
Anna Novelli, Email: anna.novelli@tum.de.
Julia Frank-Tewaag, Email: julia.frank-tewaag@outlook.de.
Sebastian Franke, Email: sebastian.franke@iqtig.org.
Martin Weigl, Email: Martin.Weigl@med.uni-muenchen.de.
Leonie Sundmacher, Email: leonie.sundmacher@tum.de.
Data availability statement
No data are available.
References
- 1.Deutsche Gesellschaft für Orthopädie und Orthopädische Chirurgie (DGOOC) S2k-Leitlinie Koxarthrose, AWMF-register nummer: 033-001. 2019. https://register.awmf.org/assets/guidelines/033-001l_S2k_Koxarthrose_2019-07_1_01.pdf Available.
- 2.Deutsche Gesellschaft für Orthopädie und Orthopädische Chirurgie (DGOOC) S3-Leitlinie Koxarthrose. 2009. [Google Scholar]
- 3.Arzneimittelkommission der deutschen Ärzteschaft . Empfehlungen Zur Therapie von Degenerativen Gelenkerkrankungen. Vol. 35. Arzneiverordnung in der Praxis; 2008. p. 1. [Google Scholar]
- 4.Fernandes L, Hagen KB, Bijlsma JWJ, et al. EULAR recommendations for the non-pharmacological core management of hip and knee osteoarthritis. Ann Rheum Dis. 2013;72:1125–35. doi: 10.1136/annrheumdis-2012-202745. [DOI] [PubMed] [Google Scholar]
- 5.Häuser W. Aktualisierung der S3 Leitlinie Langzeitanwendungen von Opioiden bei chronischen nicht-tumorbedingten Schmerzen. Der Schmerz. 2020;34:204–44. doi: 10.1007/s00482-020-00472-y. [DOI] [PubMed] [Google Scholar]
- 6.Deutsche Gesellschaft für Unfallchirurgie S1-Leitlinie Endoprothese bei Koxarthrose. 2008.
- 7.Deutsche Gesellschaft für Orthopädie und Orthopädische Chirurgie (DGOOC) Evidenz- und konsensbasierte Indikationskriterien zur Hüfttotalendoprothese bei Koxarthrose (EKIT-Hüfte), Version 1.0. 2021. https://www.awmf.org/leitlinien/detail/ll/187-001.html Available.
- 8.Nelson AE, Allen KD, Golightly YM, et al. A systematic review of recommendations and guidelines for the management of osteoarthritis: The chronic osteoarthritis management initiative of the U.S. bone and joint initiative. Semin Arthritis Rheum. 2014;43:701–12. doi: 10.1016/j.semarthrit.2013.11.012. [DOI] [PubMed] [Google Scholar]
- 9.Schäfer T, Pritzkuleit R, Jeszenszky C, et al. Trends and geographical variation of primary hip and knee joint replacement in Germany. Osteoarthr Cartil. 2013;21:279–88. doi: 10.1016/j.joca.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 10.Judge A, Welton NJ, Sandhu J, et al. Geographical variation in the provision of elective primary hip and knee replacement: the role of socio-demographic, hospital and distance variables. J Public Health (Bangkok) 2009;31:413–22. doi: 10.1093/pubmed/fdp061. [DOI] [PubMed] [Google Scholar]
- 11.Fisher ES, Bell JE, Tomek IE, et al. Trends and regional variation in dip, knee, and shoulder replacement: A dartmouth atlas surgery report. The Dartmouth Inst for Health Policy and Clinical Practice. 2010 [PubMed] [Google Scholar]
- 12.Weeks WB, Jardin M, Dufour JC, et al. Geographic variation in admissions for knee replacement, hip replacement, and hip fracture in France: evidence of supplier-induced demand in for-profit and not-for-profit hospitals. Med Care. 2014;52:909–17. doi: 10.1097/MLR.0000000000000211. [DOI] [PubMed] [Google Scholar]
- 13.World Health Organization Cancer pain relief. 1986. https://apps.who.int/iris/bitstream/handle/10665/43944/9241561009_eng.pdf?sequence=1&isAllowed=y Available.
- 14.Callhoff J, Albrecht K, Redeker I, et al. Disease burden of patients with osteoarthritis: Results of a cross‐sectional survey linked to claims data. Arthritis Care Res (Hoboken) 2020;72:193–200. doi: 10.1002/acr.24058. [DOI] [PubMed] [Google Scholar]
- 15.Koopman C, Vaartjes I, Heintjes EM, et al. Persisting gender differences and attenuating age differences in cardiovascular drug use for prevention and treatment of coronary heart disease, 1998-2010. Eur Heart J. 2013;34:3198–205. doi: 10.1093/eurheartj/eht368. [DOI] [PubMed] [Google Scholar]
- 16.Postler A, Ramos AL, Goronzy J, et al. Prevalence and treatment of hip and knee osteoarthritis in people aged 60 years or older in Germany: an analysis based on health insurance claims data. Clin Interv Aging. 2018;13:2339–49. doi: 10.2147/CIA.S174741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Knoop J, van Tunen J, van der Esch M, et al. Analgesic use in patients with knee and/or hip osteoarthritis referred to an outpatient center: a cross-sectional study within the Amsterdam Osteoarthritis Cohort. Rheumatol Int. 2017;37:1747–55. doi: 10.1007/s00296-017-3785-3. [DOI] [PubMed] [Google Scholar]
- 18.Weigl M, Pietzner J, Kisch R, et al. Effects of a medical second opinion programme on patients’ decision for or against knee arthroplasty and their satisfaction with the programme. BMC Musculoskelet Disord . 2021;22:595. doi: 10.1186/s12891-021-04465-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hradetzky E, Ohlmeier C, Brinkmann C, et al. Epidemiology and routine care treatment of patients with hip or knee osteoarthritis and chronic lower back pain: real-world evidence from Germany. J Public Health (Berl) 2022;30:2855–67. doi: 10.1007/s10389-022-01700-8. [DOI] [Google Scholar]
- 20.Deveza LA, Hunter DJ, Van Spil WE. Too much opioid, too much harm. Osteoarthr Cartil. 2018;26:293–5. doi: 10.1016/j.joca.2017.12.003. [DOI] [PubMed] [Google Scholar]
- 21.DeMik DE, Bedard NA, Dowdle SB, et al. Are we still prescribing opioids for osteoarthritis? J Arthroplasty. 2017;32:3578–82. doi: 10.1016/j.arth.2017.07.030. [DOI] [PubMed] [Google Scholar]
- 22.Allen KD, Golightly YM, White DK. Gaps in appropriate use of treatment strategies in osteoarthritis. Best Pract Res Clin Rheumatol. 2017;31:746–59. doi: 10.1016/j.berh.2018.05.003. [DOI] [PubMed] [Google Scholar]
- 23.Werber A, Marschall U, L’hoest H, et al. Opioid therapy in the treatment of chronic pain conditions in Germany. Pain Physician. 2015;18:E323–31. [PubMed] [Google Scholar]
- 24.Lange T, Luque Ramos A, Albrecht K, et al. Verordnungshäufigkeit physikalischer Therapien und Analgetika vor dem Einsatz einer Hüft- bzw. Kniegelenks-Endoprothese: Eine versorgungsepidemiologische Analyse basierend auf GKV-Routinedaten aus Deutschland [Prescription frequency of physical therapy and analgesics before total hip and knee arthroplasy: An epidemiological analysis of routine health care data from Germany] Orthop. 2018;47:1018–26. doi: 10.1007/s00132-018-3629-1. [DOI] [PubMed] [Google Scholar]
- 25.Flothow A, Novelli A, Sundmacher L. Analytical methods for identifying sequences of utilization in health data: a scoping review. BMC Med Res Methodol . 2023;23 doi: 10.1186/s12874-023-02019-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Novelli A, Frank-Tewaag J, Bleek J, et al. Identifying and investigating ambulatory care sequences before invasive coronary angiography. Med Care. 2022;60:602–9. doi: 10.1097/MLR.0000000000001738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roux J, Grimaud O, Leray E. Use of state sequence analysis for care pathway analysis: The example of multiple sclerosis. Stat Methods Med Res. 2019;28:1651–63. doi: 10.1177/0962280218772068. [DOI] [PubMed] [Google Scholar]
- 28.Hanson CL, Osberg M, Brown J, et al. Conducting patient-pathway analysis to inform programming of tuberculosis services: methods. J Infect Dis. 2017;216:S679–85. doi: 10.1093/infdis/jix387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vogt V, Scholz SM, Sundmacher L. Applying sequence clustering techniques to explore practice-based ambulatory care pathways in insurance claims data. Eur J Public Health. 2018;28:214–9. doi: 10.1093/eurpub/ckx169. [DOI] [PubMed] [Google Scholar]
- 30.Le Meur N, Vigneau C, Lefort M, et al. Categorical state sequence analysis and regression tree to identify determinants of care trajectory in chronic disease: Example of end-stage renal disease. Stat Methods Med Res. 2019;28:1731–40. doi: 10.1177/0962280218774811. [DOI] [PubMed] [Google Scholar]
- 31.Lakshmanan GT, Rozsnyai S, Wang F. In: Business process management, lecture notes in computer science. Daniel F, Wang J, Weber B, editors. Berlin/Heidelberg Springer; 2013. Investigating clinical care pathways correlated with outcomes; pp. 323–38. [Google Scholar]
- 32.Abbott A, Forrest J. Optimal matching methods for historical sequences. J Interdiscip Hist. 1986;16:471. doi: 10.2307/204500. [DOI] [Google Scholar]
- 33.Gabadinho A, Ritschard G, Müller NS. Analyzing and visualizing state sequences in R with TraMineR. J Stat Soft. 40 doi: 10.18637/jss.v040.i04. n.d. [DOI] [Google Scholar]
- 34.Abbott A, Tsay A. Sequence analysis and optimal matching methods in sociology. Sociol Methods Res. 2000;29:3–33. doi: 10.1177/0049124100029001001. [DOI] [Google Scholar]
- 35.Studer M, Ritschard G. What matters in differences between life trajectories: A comparative review of sequence dissimilarity measures. J R Stat Soc Ser A Stat Soc. 2016;179:481–511. doi: 10.1111/rssa.12125. [DOI] [Google Scholar]
- 36.Gower JC. A general coefficient of similarity and some of its properties. Biometrics. 1971;27:857. doi: 10.2307/2528823. [DOI] [Google Scholar]
- 37.Kaufman L, Rousseeuw PJ. Finding groups in data. John Wiley Sons Inc; 1990. Partitioning around medoids (program pam) pp. 68–125. [Google Scholar]
- 38.Studer M. Weighted cluster library manual: A practical guide to creating typologies of trajectories in the social sciences with R. LIVES WP. 2013;2013:1–32. doi: 10.12682/lives.2296-1658.2013.24. [DOI] [Google Scholar]
- 39.Rousseeuw PJ. Silhouettes: A graphical aid to the interpretation and validation of cluster analysis. J Comput Appl Math. 1987;20:53–65. doi: 10.1016/0377-0427(87)90125-7. [DOI] [Google Scholar]
- 40.Elixhauser A, Steiner C, Harris DR, et al. Comorbidity measures for use with administrative data. Med Care. 1998;36:8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 41.van Walraven C, Austin PC, Jennings A, et al. A modification of the Elixhauser comorbidity measures into a point system for hospital death using administrative data. Med Care. 2009;47:626–33. doi: 10.1097/MLR.0b013e31819432e5. [DOI] [PubMed] [Google Scholar]
- 42.Freytag A, Schiffhorst G, Thoma R. Identifikation und Gruppierung von Schmerzpatienten anhand von Routinedaten einer Krankenkasse [Identification and grouping of pain patients according to claims data] Schmerz. 2010;24:12–22. doi: 10.1007/s00482-009-0861-y. [DOI] [PubMed] [Google Scholar]
- 43.Textor J, van der Zander B, Gilthorpe MS, et al. Robust causal inference using directed acyclic graphs: the R package “dagitty.”. Int J Epidemiol. 2016;45:1887–94. doi: 10.1093/ije/dyw341. [DOI] [PubMed] [Google Scholar]
- 44.The R Foundation R: A language and environment for statistical computing. R foundation for statistical computing, Vienna, Austria. 2014. http://www.R-project.org Available.
- 45.StataCorp . Stata statistical software: Release 14. StataCorp LP, College Station, TX; 2015. [Google Scholar]
- 46.Gasparini A. comorbidity: An R package for computing comorbidity scores. JOSS . 2018;3:648. doi: 10.21105/joss.00648. [DOI] [Google Scholar]
- 47.Vogt V. The contribution of locational factors to regional variations in office-based physicians in Germany. Health Policy. 2016;120:198–204. doi: 10.1016/j.healthpol.2016.01.006. [DOI] [PubMed] [Google Scholar]
- 48.Hackenbroch MH. Coxarthrose [Coxarthrosis] Orthop. 1998;27:659–67. doi: 10.1007/s001320050283. [DOI] [PubMed] [Google Scholar]
- 49.Rosner B, Neicun J, Yang JC, et al. Opioid prescription patterns in Germany and the global opioid epidemic: Systematic review of available evidence. PLoS One. 2019;14:e0221153. doi: 10.1371/journal.pone.0221153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shrier I, Feldman DE, Gaudet MC, et al. Conservative non-pharmacological treatment options are not frequently used in the management of hip osteoarthritis. J Sci Med Sport. 2006;9:81–6. doi: 10.1016/j.jsams.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 51.Sussmann KE, Jacobs H, Hoffmann F. Physical therapy use and associated factors in adults with and without osteoarthritis-an analysis of the population-based German halth update study. Healthcare (Basel) -> Healthc (Basel) 2021;9:1544. doi: 10.3390/healthcare9111544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schwartz FH, Lange J. Factors that affect outcome following total joint arthroplasty: a review of the recent literature. Curr Rev Musculoskelet Med. 2017;10:346–55. doi: 10.1007/s12178-017-9421-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hudak PL, Armstrong K, Braddock C, et al. Older patients’ unexpressed concerns about orthopaedic surgery. J Bone Joint Surg Am. 2008;90:1427–35. doi: 10.2106/JBJS.G.01077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Le Meur N, Gao F, Bayat S. Mining care trajectories using health administrative information systems: the use of state sequence analysis to assess disparities in prenatal care consumption. BMC Health Serv Res. 2015;15:200. doi: 10.1186/s12913-015-0857-5. [DOI] [PMC free article] [PubMed] [Google Scholar]