Abstract
ABSTRACT
Introduction
We undertook phenotypic characterization of early-onset and late-onset type 2 diabetes (T2D) in adult black African and white European populations with recently diagnosed T2D to explore ethnic differences in the manifestation of early-onset T2D.
Research design and methods
Using the Uganda Diabetes Phenotype study cohort of 500 adult Ugandans and the UK StartRight study cohort of 714 white Europeans with recently diagnosed islet autoantibody-negative T2D, we compared the phenotypic characteristics of participants with early-onset T2D (diagnosed at <40 years) and late-onset T2D (diagnosed at ≥40 years).
Results
One hundred and thirty-four adult Ugandans and 113 white Europeans had early-onset T2D. Compared with late-onset T2D, early-onset T2D in white Europeans was significantly associated with a female predominance (52.2% vs 39.1%, p=0.01), increased body mass index (mean (95% CI) 36.7 (35.2–38.1) kg/m2 vs 33.0 (32.4–33.6) kg/m2, p<0.001), waist circumference (112.4 (109.1–115.6) cm vs 108.8 (107.6–110.1) cm, p=0.06), and a higher frequency of obesity (82.3% vs 63.4%, p<0.001). No difference was seen with the post-meal C-peptide levels as a marker of beta-cell function (mean (95% CI) 2130.94 (1905.12–2356.76) pmol/L vs 2039.72 (1956.52–2122.92), p=0.62).
In contrast, early-onset T2D in Ugandans was associated with less adiposity (mean (95% CI) waist circumference 93.1 (89.9–96.3) cm vs 97.4 (95.9–98.8) cm, p=0.006) and a greater degree of beta-cell dysfunction (120 min post-glucose load C-peptide mean (95% CI) level 896.08 (780.91–1011.24) pmol/L vs 1310.10 (1179.24–1440.95) pmol/L, p<0.001), without female predominance (53.0% vs 57.9%, p=0.32) and differences in the body mass index (mean (95% CI) 27.3 (26.2–28.4) kg/m2 vs 27.9 (27.3–28.5) kg/m2, p=0.29).
Conclusions
These differences in the manifestation of early-onset T2D underscore the need for ethnic-specific and population-specific therapeutic and preventive approaches for the condition.
Keywords: Ethnic Differences; Adult; Diabetes Mellitus, Type 2
WHAT IS ALREADY KNOWN ON THIS TOPIC?
Globally, the burden of early-onset type 2 diabetes (T2D) is rapidly increasing.
Evidence from studies conducted in Asians and white populations of European ancestry has shown that early-onset T2D is characterized by a female preponderance, increased obesity, rapid decline in beta-cell function, and a high prevalence of diabetes-related complications.
WHAT THIS STUDY ADDS?
Striking differences in the manifestation of early-onset T2D between adult black Africans and white Europeans with recently diagnosed T2D exist.
Early-onset T2D in adult white populations of European ancestry was characterized by female predominance and increased adiposity. In contrast, less adiposity, lower pancreatic beta-cell function, and the absence of female predominance were observed in adult Ugandans with early-onset T2D.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY?
Because of these findings, the preventive and therapeutic strategies for early-onset T2D should be individualized to ethnicity and population.
Introduction
Globally, the burden of early-onset type 2 diabetes (T2D), defined as T2D diagnosed at <40 years, is rapidly increasing.1 2 Most of this evidence has been derived from Asian and white populations of European ancestry.3,8 Data from these populations, particularly in high-income countries, have shown that compared with late-onset T2D, early-onset T2D is characterized by a female preponderance, increased obesity, rapid decline in the beta-cell function (reflecting an aggressive phenotype), and a high prevalence of diabetes-related complications.79,13 The condition is thought to develop due to a close interplay between genetics and environmental factors, with adiposity and genetic susceptibility as key features driving the early development of this condition.9 10
In contrast, evidence on the clinical and genetic profile of early-onset T2D in adult black African populations is limited.14 15 We also lack rigorous studies comparing the phenotypic characteristics of early-onset T2D (where islet-cell autoimmunity has been excluded) in black adult Africans and white populations of European ancestry. To address this gap, we compared the demographic, clinical, anthropometric, and metabolic characteristics of adult Ugandans and white Europeans with early-onset T2D (diagnosed at <40 years) and late-onset T2D (diagnosed at ≥40 years) recruited in the Uganda Diabetes Phenotype (UDIP) and the United Kingdom StartRight studies, respectively, with an overarching aim of exploring whether differences in the manifestation of early-onset T2D exist between these two distinct populations.
Methods
The participants in this study were recruited from the UDIP and StartRight studies conducted in Uganda and the UK, respectively. The UDIP study aimed to undertake rigorous phenotypic characterization of 568 adult Ugandans (aged ≥18 years) with recently diagnosed diabetes (diabetes diagnosed in the preceding 3 months). The study participants were recruited from February 2019 to October 2020 from the adult diabetes outpatient clinics of seven public and faith-based private not-for-profit secondary hospitals in Central and Southwestern Uganda. All pregnant women with recently diagnosed diabetes were excluded from the study.
The StartRight study was a prospective multi-center study conducted across 55 sites in the UK that aimed to provide robust clinical evidence on accurate classification of diabetes at the time of diagnosis and early identification of patients who will rapidly require insulin treatment (https://www.clinicaltrials.gov/study/NCT02287506). The study recruited 1802 participants aged ≥18 years with a diagnosis of diabetes made within the previous 12 months. Participants with gestational and secondary diabetes were excluded from the study. The StartRight study was enriched for late-onset type 1 diabetes (T1D) by aiming for equal recruitment of those receiving and not receiving insulin therapy in those with diabetes onset after 50 years. For our analysis, we considered only individuals of white ancestry with a clinical diagnosis of T2D and confirmed negative islet autoantibody status.
Assessment of the phenotypic characteristics of interest
Regarding the UDIP study, information on the demographic (age, sex, and residence) and clinical (family history of diabetes and history of co-existing hypertension) characteristics of interest was collected from each study participant. Following this, resting blood pressure (systolic and diastolic) and anthropometric measurements (weight, height, waist circumference (WC), hip circumference (HC), BMI, waist: hip ratio (WHR)) were recorded.
A fasting venous blood sample was then drawn for the measurement of glycated hemoglobin (HbA1c), lipid profile, C-peptide, and three islet autoantibodies (glutamic acid decarboxylase-65 or GADA, tyrosine phosphatase or IA-2A, and zinc transporter 8 or ZnT8-A). All participants were subjected to a 75 g oral glucose tolerance test to measure the 120 min C-peptide concentrations. All the above tests were carried out at the Medical Research Council/Uganda Virus Research Institute and London School of Hygiene and Tropical Medicine Uganda Research Unit, Entebbe Uganda.
For the StartRight study, demographic and clinical information was collected at the recruitment visit with an assessment of height, weight, WC, and HC measurements to calculate the BMI and WHR. This was followed by the collection of a nonfasted (within 1–5 hours post-meal) blood sample for DNA extraction and measurement of C-peptide, glucose, HbA1c, and islet autoantibodies (GADA, IA-2A, and ZnT8A) concentrations. These tests were performed at the academic Blood Sciences Department at the Royal Devon and Exeter Hospital, Exeter, UK.
Assessment of T1D and T2D Genetic Risk Score
In addition to screening for the three islet autoantibodies, we calculated T1D and T2D Genetic Risk Scores (GRS). The T1D GRS was calculated based on the 67 single nucleotide polymorphisms (SNPs) reported to be associated with T1D, as described by Sharp et al.16 The T2D GRS was calculated using T2D-associated SNPs from a European population,17 which was shown to effectively predict T2D across racial/ethnic groups.18
Definition of the study outcomes
Early-onset and late-onset T2D were defined as T2D diagnosed at <40 years and ≥40 years, respectively, with confirmed islet autoantibody-negative status in both populations. Ugandan participants were considered to be islet autoantibody negative if the GADA, IA-2A, and ZnT8-A concentrations were ≤34 U/mL, ≤58 U/mL, and ≤67.7 U/mL, respectively, based on the 97.5th centile of 600 adult rural Ugandans without diabetes.
Islet autoantibody-negative status was considered in the white European participants if the GADA and IA-2A concentrations were <11 U/mL and <7.5 U/mL, respectively. Cut-offs of <65 U/mL and <10 U/mL were used for the ZnT8-A concentration in participants aged <30 years and ≥30 years, respectively.19 These cut-offs represented the ≥97.5th percentile of 1559 British participants without diabetes.
Obesity was defined based on the traditional WHO cut-off of ≥30 kg/m2.20
Statistical analysis
The demographic, clinical, anthropometric, and metabolic characteristics of the participants with early-onset and late-onset T2D were analyzed using Fisher’s exact test for categorical data and two-sided Wilcoxon rank-sum tests for continuous data, with the exception of T1D and T2D GRS, which were analyzed using two-sample t-tests. The categorical and continuous variables were expressed as proportions and mean with 95% CIs, respectively. All analyses were performed using STATA statistical software V.15 (StataCorp).
Results
Characteristics of the white European and Ugandan participants with islet autoantibody-negative T2D
The overall characteristics of the white European and Ugandan participants with islet autoantibody-negative T2D are summarized in table 1.
Table 1. Overall characteristics of the adult white Europeans and black Ugandans with recently diagnosed T2D.
| Characteristics | Adult white European participants with recently diagnosed T2D (n=714) | Adult black Ugandan participants with recently diagnosed T2D (n=500) | P value |
| Age at diagnosis (years) | 51.2 (50.2–52.2) | 48.4 (47.2–49.5) | <0.001 |
| Female, n (%) | 294 (41.2) | 283 (56.6) | <0.001 |
| Parental history of diabetes, n (%) | 318 (44.5) | 191 (38.2) | <0.001 |
| Co-existing hypertension, n (%) | 268 (37.5) | 171 (35.0) | 0.25 |
| Systolic blood pressure, mm Hg | 134.4 (133.1–135.7) | 126.8 (125.2–128.0) | <0.001 |
| Diastolic blood pressure, mm Hg | 81.0 (80.2–81.8) | 84.1 (83.1–85.0) | <0.001 |
| Body mass index, kg/m2 | 33.6 (33.0–34.2) | 27.7 (27.2–28.2) | <0.001 |
| Body mass index ≥30 kg/m2, n (%) | 474 (66.4) | 171 (34.2) | <0.001 |
| Waist circumference, cm | 109.4 (108.2–110.6) | 96.3 (94.9–97.6) | <0.001 |
| Hip circumference, cm | 113.6 (112.5–114.6) | 104.2 (102.3–106.1) | <0.001 |
| Waist: hip ratio | 0.96 (0.96–0.97) | 0.92 (0.91–0.93) | <0.001 |
| Total cholesterol, mmol/L | 5.20 (5.05–5.35) | 4.17 (4.05–4.29) | <0.001 |
| HDLC, mmol/L | 1.18 (1.13–1.24) | 0.95 (0.74–1.20) | <0.001 |
| LDLC, mmol/L | 2.95 (2.83–3.06) | 2.71 (2.61–2.81) | 0.001 |
| TGL, mmol/L | 3.40 (2.88–3.93) | 1.59 (1.50–1.67) | <0.001 |
| HbA1c, mmol/mol | 73.2 (71.2–75.2) | 88.2 (85.0–91.3) | <0.001 |
| HbA1c, % | 8.8 (8.7–9) | 10.2 (9.9–10.5) | <0.001 |
| Random/120 min post-OGTTC-peptide, pmol/L | 2054.16 (1975.73–2132.58) | 1197.79 (1096.34–1299.25) | <0.001 |
GRSGenetic Risk ScoreHbA1cglycated hemoglobinHDLChigh-density lipoprotein cholesterolLDLClow-density lipoprotein cholesterolOGTToral glucose tolerance testT1Dtype 1 diabetesT2Dtype 2 diabetes
Compared with the white European participants, adult Ugandans with islet autoantibody-negative T2D were younger (mean (95% CI) 48.4 (47.2–49.5) years vs 51.2 (50.2–52.2) years) with lower markers of adiposity (mean (95% CI) BMI of 27.7 (27.2–28.2) kg/m2 vs 33.6 (33.0–34.2) kg/m2 and BMI≥30 kg/m2, 34.2% vs 66.4%). Additionally, Ugandan participants had a higher HbA1c (mean (95% CI) HbA1c 88.2 (85.0–91.3) mmol/mol vs 73.2 (71.2–75.2) mmol/mol) and lower pancreatic beta-cell function (mean (95% CI) post-meal or 120 min post-glucose load C-peptide 1197.79 (1096.34–1299.25) pmol/L vs 2054.16 (1975.73–2132.58) pmol/L) at the time of recruitment.
In white Europeans, early-onset T2D is strongly associated with a female predominance and markers of obesity
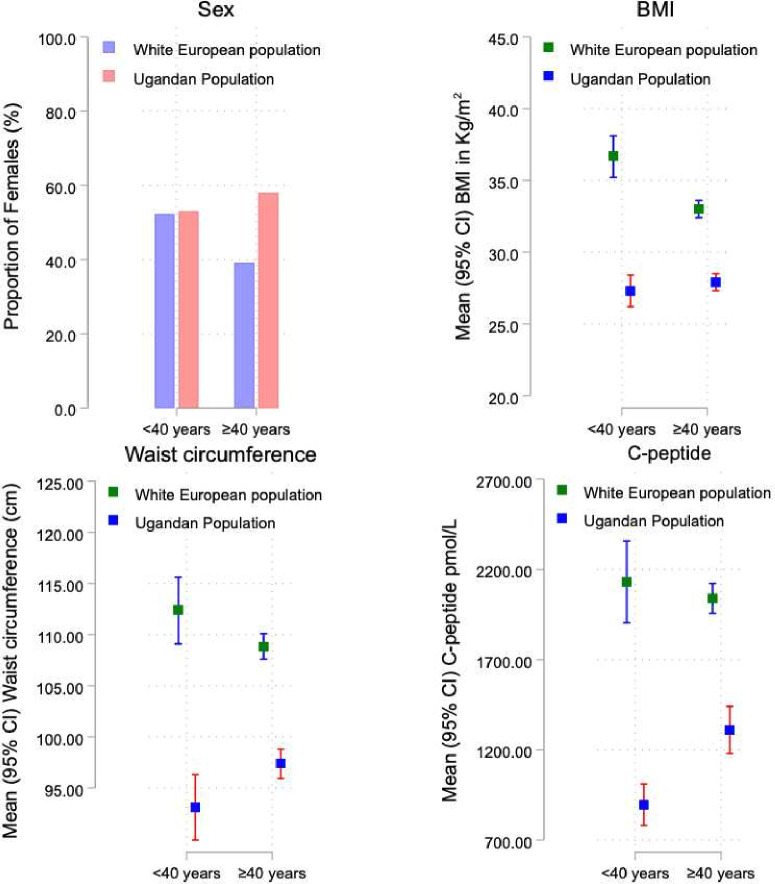
The characteristics of the adult white European and Ugandan participants with early-onset and late-onset T2D are shown in table 2, online supplemental table 1and figure 1.
Table 2. Characteristics of adult white European and Ugandan participants with early-onset and late-onset T2D.
| Characteristics | Adult white European population with recently diagnosed T2D(n=714) | Adult black Ugandan population with recently diagnosed T2D(n=500) | ||||
| <40 years(n=113, 15.8%) | ≥40 years(n=601, 84.2%) | P value | <40 years(n=134, 26.8%) | ≥40 years(n=366, 73.2%) | P value | |
| Age at diagnosis (years) | 32.7 (31.7–33.7) | 54.7 (53.8–55.5) | <0.001 | 32.9 (32.0–33.8) | 54.0 (53.0–55.0) | <0.001 |
| Female, n (%) | 59 (52.2) | 235 (39.1) | 0.01 | 71 (53.0) | 212 (57.9) | 0.32 |
| Parental history of T2D,n (%) | 56 (49.6) | 262 (43.6) | 0.26 | 54 (40.3) | 137 (37.4) | 0.68 |
| Co-existing hypertension, n (%) | 12 (10.6) | 256 (42.6) | <0.001 | 16 (11.9) | 155 (42.4) | <0.001 |
| Systolic blood pressure, mm Hg | 127.8 (125.3–130.3) | 135.6 (134.2–137.0) | <0.001 | 120 (117–122) | 129 (127–131) | <0.001 |
| Diastolic blood pressure, mm Hg | 80.2 (78.4–82.0) | 81.1 (80.3–82.0) | 0.27 | 81 (79–83) | 85 (84–86) | <0.001 |
| Body mass index, kg/m2 | 36.7 (35.2–38.1) | 33.0 (32.4–33.6) | <0.001 | 27.3 (26.2–28.4) | 27.9 (27.3–28.5) | 0.29 |
| Body mass index ≥30 kg/m2, n (%) | 93 (82.3) | 381 (63.4) | <0.001 | 43 (32.6) | 128 (35.2) | 0.48 |
| Waist circumference, cm | 112.4 (109.1–115.6) | 108.8 (107.6–110.1) | 0.06 | 93.1 (89.9–96.3) | 97.4 (95.9–98.8) | 0.006 |
| Hip circumference, cm | 119.4 (116.5–122.3) | 112.5 (111.4–113.6) | <0.001 | 104.2 (98.4–110.0) | 104.2 (102.7–105.7) | 0.99 |
| Waist: hip ratio | 0.94 (0.93–0.96) | 0.97 (0.96–0.98) | 0.003 | 0.89 (0.88–0.91) | 0.93 (0.92–0.94) | <0.001 |
| Total cholesterol, mmol/L | 5.65 (4.97–6.32) | 5.12 (5.00–5.25) | 0.17 | 4.03 (3.83–4.20) | 4.22 (4.08–4.36) | 0.17 |
| HDLC, mmol/L | 1.04 (0.96–1.13) | 1.21 (1.15–1.27) | 0.002 | 0.94 (0.70–1.15) | 0.96 (0.76–1.23) | 0.52 |
| LDLC, mmol/L | 3.11 (2.84–3.39) | 2.92 (2.80–3.05) | 0.22 | 2.56 (2.38–2.73) | 2.76 (2.64–2.88) | 0.07 |
| Triglycerides, mmol/L | 3.87 (1.68–6.06) | 3.33 (2.83–3.83) | 0.29 | 1.54 (1.39–1.70) | 1.60 (1.50–1.71) | 0.55 |
| HbA1c, mmol/mol | 77.6 (72.5–82.8) | 72.4 (70.2–74.5) | 0.049 | 96.3 (89.7–102.9) | 85.3 (81.7–88.8) | 0.004 |
| HbA1c, % | 9.3 (8.8–9.7) | 8.8 (8.6–9.0) | 0.049 | 10.9 (10.3–11.5) | 9.9 (9.6–10.3) | 0.004 |
| Random/120 min post-OGTT C-peptide, pmol/L | 2130.94 (1905.12–2356.76) | 2039.72 (1956.52–2122.92) | 0.62 | 896.08 (780.91–1011.24) | 1310.10 (1179.24–1440.95) | <0.001 |
| T1D GRS | 10.42 (9.93–10.92) | 10.07 (9.88–10.26) | 0.14 | 8.43 (8.03–8.81) | 8.12 (7.89–8.36) | 0.20 |
| T2D GRS | 11.25 (11.19–11.31) | 11.20 (11.17–11.22) | 0.08 | 11.28 (11.21–11.34) | 11.22 (11.18–11.25) | 0.09 |
GRSGenetic Risk ScoreHbA1cglycated hemoglobinHDLChigh-density lipoprotein cholesterolLDLClow-density lipoprotein cholesterolOGTToral glucose tolerance testT1Dtype 1 diabetesT2Dtype 2 diabetes
Figure 1. A comparison of the female sex distribution, body mass index, waist circumference, and post-glucose or meal C-peptide levels between the white European and Ugandan participants with early-onset and late-onset type 2 diabetes. BMI, body mass index.
Of the 500 Ugandan and 714 white European participants enrolled in the UDIP and UK StartRight studies with autoantibody-negative T2D, 134 (26.8%) and 113 (15.8%) had early-onset T2D, respectively.
Compared with late-onset T2D, early-onset T2D in the white European participants was significantly associated with a female predominance (52.2% vs 39.1%, p=0.01), increased BMI (mean (95% CI) 36.7 (35.2–38.1) kg/m2 vs 33.0 (32.4–33.6) kg/m2, p<0.001), WC (112.4 (109.1–115.6) cm vs 108.8 (107.6–110.1) cm, p=0.06), and a higher frequency of obesity (82.3% vs 63.4%, p<0.001).
Conversely, compared with those with late-onset T2D, Ugandan participants with early-onset T2D had lower markers of adiposity (mean (95% CI) WC 93.1 (89.9–96.3) cm vs 97.4 (95.9–98.8), p=0.006). No differences were noted in the proportion of females (53.0% vs 57.9%, p=0.32), frequency of obesity (32.6% vs 35.2%, p=0.48), and BMI levels (mean (95% CI) 27.3 (26.2–28.4) kg/m2 vs 27.9 (27.3–28.5) kg/m2) in Ugandan participants with early-onset and late-onset T2D.
Early-onset T2D in Ugandans is associated with marked hyperglycemia and pancreatic beta-cell failure
Ugandan participants with early-onset T2D had a greater degree of hyperglycemia (mean (95% CI) HbA1c: 96.3 (89.7–102.9) mmol/mol vs 85.3 (81.7–88.8) mmol/mol, p=0.004) and pancreatic beta-cell dysfunction (mean (95% CI) 120 min post-glucose load C-peptide level: 896.08 (780.91–1011.24) pmol/L vs 1310.10 (1179.24–1440.95) pmol/L, p<0.001) at the time of recruitment. In contrast, the post-meal C-peptide levels did not differ in the white European participants with early-onset and late-onset T2D (mean (95% CI): 2130.94 (1905.12–2356.76) pmol/L vs 2039.72 (1956.52–2122.92) pmol/L, p=0.62).
Genetic risk score of the adult Ugandan and white European participants with early-onset T2D
Ugandan participants with early-onset T2D had a higher T2D GRS when compared with those with late-onset T2D (11.28 (11.21–11.34) vs 11.22 (11.18–11.25), p=0.09).
Similarly, compared with those with late-onset T2D, white European participants with early-onset T2D also had a higher T2D GRS (11.25 (11.19–11.31) vs 11.20 (11.17–11.22), p=0.08), although the differences in both cohorts were not statistically significant.
Conclusions
To our knowledge, this is the first study to rigorously investigate the manifestation of early-onset and late-onset T2D in an adult black African population and to make comparisons with a white European population. We demonstrate striking differences in presentation between these two populations, where, in contrast to the white European cohort, early-onset T2D in adult Ugandans is likely to be seen more in patients without obesity (and other features of the metabolic syndrome) and is associated with marked pancreatic beta-cell dysfunction.
The association between obesity and early-onset T2D in white European populations has been well documented and suggests a common underlying mechanism, driven by insulin resistance as a primary defect and pancreatic beta-cell dysfunction occurring later.421,23 However, beta-cell failure appears to occur more rapidly in patients with early-onset T2D compared with those with late-onset T2D.9 11 In addition, early-onset T2D in white European participants was common among woman, which has also been widely reported in other studies of white populations.24 25
In contrast, the pathways that lead to the early onset of T2D in Africa, where it occurs in the absence of excessive adiposity, are unclear, but most evidence suggests that pancreatic beta-cell dysfunction is the primary defect. This may result from genetic predisposition as well as environmental exposures, such as early-life (in utero and/or early childhood) malnutrition and infections like tuberculosis, HIV, and malaria, which are prevalent in the region.26,29 This notion forms the basis of the developmental origins of health and disease, which explains that such early-life environmental exposures may induce changes, including epigenetic, that alter gene expression, cellular growth, composition, and physiology, increasing the future risk of developing cardiometabolic conditions like T2D.30 The high burden of infectious diseases, such as tuberculosis and HIV, may also act directly or indirectly in adulthood to increase the risk of developing early-onset T2D.26 27
The unique manifestation of early-onset T2D with a predominance of pancreatic beta-cell dysfunction, as seen in adult Ugandans, may also be due to genetic influences. Polymorphisms of genes that influence pancreatic beta-cell development, proliferation, neogenesis, apoptosis, and insulin secretion such as the transcription factor-7 like 2 genes (TCF7L2), Zinc Finger RANBP2-Type Containing 3 (ZRANB3), and the ATP-sensitive potassium channel Kir6.2 gene (KCNJ11), have been suggested as possible mechanisms for pancreatic beta-cell dysfunction in patients of African ancestry.31,33
In addition to genetics and early-life environmental factors, the differences seen in the phenotypic characteristics and manifestation of T2D in these two distinct populations may be due to the complex interplay of environmental factors including lifestyle, dietary habits, education, socioeconomic status, healthcare infrastructure, cultural practices, and public health policies. While the UK and other high-income countries generally have better resources aimed at diagnosing and managing diabetes early and optimally, Uganda and other African countries face unique challenges such as limited access to quality healthcare, which may impact early diagnosis and influence diabetes onset, presentation, progression, and treatment outcomes.34 The delay in diabetes diagnosis and the initiation of appropriate treatment (whether as a result of lack of education, access to healthcare, or any other social factor) may lead to a higher glycemic burden irrespective of underlying pancreatic beta-cell function, subsequently resulting in marked weight loss. This may explain the lower BMI and WC seen in the Ugandan participants with poor glycemic control.
Broadly, as observed in the comparison of the early-onset T2D cohorts, there were also striking differences in the overall population characteristics between the Ugandan and the UK populations. These differences have been generally reported elsewhere, emphasizing the racial differences in the manifestation of T2D. For example, compared with white populations living in high-income countries, T2D has been reported to occur at a lower mean age and BMI in native and migrant Africans.35 36 Additionally, the pathogenesis of T2D appears to be influenced more by pancreatic beta-cell dysfunction in most black African populations (as supported by the lower stimulated C-peptide concentrations in the Ugandan population) as opposed to insulin resistance.37 38
Strengths of the study include that this is the first study to undertake phenotypic characterization of early-onset and late-onset T2D (where islet-cell autoimmunity has been robustly screened for and excluded) in adult black African and white European patients with recently diagnosed diabetes to investigate if ethnic-related differences exist in the manifestation of early-onset T2D. Participants were phenotyped in detail, and GRS was available to investigate the potential contribution of antibody-negative T1D and classical T2D genetic susceptibility to early-onset antibody-negative T2D.
Limitations of this study include differences in the designs of the two studies, which may limit the interpretation of some findings. For example, the differences in the study design (including age-related recruitment in the StartRight study) suggest that these studies should not be used to directly compare the relative prevalence of early-onset T2D. In addition, the post-meal C-peptide measurement in the StartRight study may not be directly comparable to the 120 min post-glucose load C-peptide measurement used in the UDIP study, and differences in duration at recruitment may impact a direct comparison of glycemic control. Despite enrolling clinically stable participants (in the absence of an acute metabolic decompensation state), we acknowledge that the high glycemic levels at recruitment could also largely contribute to the lower 120 min post-glucose load C-peptide level (an effect of the glucotoxicity) in the Ugandan population. Ugandan participants were recruited only from seven secondary hospitals (in contrast to the combined primary and secondary healthcare recruitment in the StartRight study).
In conclusion, our study findings demonstrate that the phenotypic profile of early-onset T2D in adult Ugandans and white Europeans with recently diagnosed diabetes greatly differs. While obesity plays a central role in the pathogenesis of early-onset T2D in white Europeans, its effect on adult Ugandans is insignificant. Pancreatic beta-cell dysfunction appears to explain the early onset of T2D in this population. An in-depth understanding of the phenotype of early-onset T2D in black African and white European populations is important and has significant clinical and therapeutic implications. Because of these phenotypic differences, the therapeutic and preventive strategies for early-onset T2D should be tailored to ethnicity and population. Due to a lack of adequate clinical evidence, future research is needed to guide how to optimally manage and prevent early-onset T2D, especially in adult Ugandans.
supplementary material
Acknowledgements
The authors are grateful to all the study participants who consented to join the study, the UDIP and StartRight research teams, the staff of the Clinical Diagnostics Laboratory Services at the Medical Research Council/Uganda Virus Research Institute and London School of Hygiene and Tropical Medicine Uganda Research Unit, Entebbe, Uganda, and the academic Blood Sciences Department, Royal Devon and Exeter Hospital, UK, for conducting the laboratory tests. The authors also thank the ADDRESS-2 study team (Imperial College, London, UK) for support with participant recruitment in the StartRight study.
Footnotes
Funding: The UDIP study was supported by the UK Medical Research Council (MRC) and the UK Department for International Development (DFID) under the MRC/DFID Concordat agreement (Project Reference: MC_UP_1204/16), and the National Institute for Health Care Research (NIHR) (17/63/131). The StartRight study was funded by the National Institute for Health and Care Research (NIHR) (CS-2015-15-018) and Diabetes UK (17/0005624). Genetic analysis for the StartRight study was funded by the European Foundation for the Study of Diabetes (2016 Rising Star Fellowship). JCK is supported by the NIHR Exeter Biomedical Research Centre (NIHR Exeter BRC). ATH is supported by the NIHR Exeter Clinical Research Facility and an NIHR Senior Investigator award. AGJ was supported for this work by an NIHR Clinician Scientist award (CS-2015-15-018). MJN is an MRC Investigator. The study sponsor/funder was not involved in the design of the study; the collection, analysis, and interpretation of data; writing the report; and did not impose any restrictions regarding the publication of the report.
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient consent for publication: Not applicable.
Ethics approval: This study involves human participants and was approved by Uganda Virus Research Centre, Entebbe Uganda (GC/127/18/05/650), Uganda National Council of Science and Technology (HS 2431), and the South West–Cornwall and Plymouth NHS Research Ethics Committee (16/SW/0130). Participants gave informed consent to participate in the study before taking part.
Contributor Information
Davis Kibirige, Email: kibirigedavis@gmail.com.
Jean-Claude Katte, Email: jckatte@gmail.com.
Anita V Hill, Email: anita.hill@nhs.net.
Isaac Sekitoleko, Email: sekitolekoisaac7@gmail.com.
William Lumu, Email: dhabaguma@gmail.com.
Julieanne Knupp, Email: j.knupp@exeter.ac.uk.
Steven Squires, Email: s.squires@exeter.ac.uk.
Andrew T Hattersley, Email: A.T.Hattersley@exeter.ac.uk.
Liam Smeeth, Email: Liam.Smeeth@lshtm.ac.uk.
Angus G Jones, Email: Angus.Jones@exeter.ac.uk.
Moffat J Nyirenda, Email: Moffat.Nyirenda@mrcuganda.org.
Data availability statement
Data are available upon reasonable request.
References
- 1.Xie J, Wang M, Long Z, et al. Global burden of type 2 diabetes in adolescents and young adults, 1990-2019: systematic analysis of the Global Burden of Disease Study 2019. BMJ. 2022;379:e072385. doi: 10.1136/bmj-2022-072385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Perng W, Conway R, Mayer-Davis E, et al. Youth-Onset Type 2 Diabetes: The Epidemiology of an Awakening Epidemic. Diabetes Care. 2023;46:490–9. doi: 10.2337/dci22-0046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chowdhury TA, Lasker SS. Complications and cardiovascular risk factors in South Asians and Europeans with early-onset type 2 diabetes. QJM. 2002;95:241–6. doi: 10.1093/qjmed/95.4.241. [DOI] [PubMed] [Google Scholar]
- 4.Misra S, Holman N, Barron E, et al. Characteristics and care of young people with type 2 diabetes included in the national diabetes audit datasets for England. Diabet Med. 2023;40:e14940. doi: 10.1111/dme.14940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Prasad RB, Asplund O, Shukla SR, et al. Subgroups of patients with young-onset type 2 diabetes in India reveal insulin deficiency as a major driver. Diabetologia. 2022;65:65–78. doi: 10.1007/s00125-021-05543-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Siddiqui MK, Anjana RM, Dawed AY, et al. Young-onset diabetes in Asian Indians is associated with lower measured and genetically determined beta cell function. Diabetologia. 2022;65:973–83. doi: 10.1007/s00125-022-05671-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barker MM, Zaccardi F, Brady EM, et al. Age at diagnosis of type 2 diabetes and cardiovascular risk factor profile: A pooled analysis. World J Diabetes. 2022;13:260–71. doi: 10.4239/wjd.v13.i3.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Song SH, Hardisty CA. Early onset type 2 diabetes mellitus: a harbinger for complications in later years--clinical observation from a secondary care cohort. QJM. 2009;102:799–806. doi: 10.1093/qjmed/hcp121. [DOI] [PubMed] [Google Scholar]
- 9.Lascar N, Brown J, Pattison H, et al. Type 2 diabetes in adolescents and young adults. Lancet Diabetes Endocrinol. 2018;6:69–80. doi: 10.1016/S2213-8587(17)30186-9. [DOI] [PubMed] [Google Scholar]
- 10.Magliano DJ, Sacre JW, Harding JL, et al. Young-onset type 2 diabetes mellitus - implications for morbidity and mortality. Nat Rev Endocrinol. 2020;16:321–31. doi: 10.1038/s41574-020-0334-z. [DOI] [PubMed] [Google Scholar]
- 11.Misra S, Ke C, Srinivasan S, et al. Current insights and emerging trends in early-onset type 2 diabetes. Lancet Diabetes Endocrinol. 2023;11:768–82. doi: 10.1016/S2213-8587(23)00225-5. [DOI] [PubMed] [Google Scholar]
- 12.Wilmot E, Idris I. Early onset type 2 diabetes: risk factors, clinical impact and management. Ther Adv Chronic Dis. 2014;5:234–44. doi: 10.1177/2040622314548679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wilmot EG, Davies MJ, Yates T, et al. Type 2 diabetes in younger adults: the emerging UK epidemic. Postgrad Med J. 2010;86:711–8. doi: 10.1136/pgmj.2010.100917. [DOI] [PubMed] [Google Scholar]
- 14.Kiraka GN, Kunyiha N, Erasmus R, et al. Family history as a risk for early-onset type 2 diabetes in Kenyan patients. Afr J Diab Med. 2014;22:15–7. [Google Scholar]
- 15.Adadey SM, Mensah JA, Acquah KS, et al. Early-onset diabetes in Africa: A mini-review of the current genetic profile. Eur J Med Genet. 2023;66:104887. doi: 10.1016/j.ejmg.2023.104887. [DOI] [PubMed] [Google Scholar]
- 16.Sharp SA, Rich SS, Wood AR, et al. Development and Standardization of an Improved Type 1 Diabetes Genetic Risk Score for Use in Newborn Screening and Incident Diagnosis. Diabetes Care. 2019;42:200–7. doi: 10.2337/dc18-1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mahajan A, Taliun D, Thurner M, et al. Fine-mapping type 2 diabetes loci to single-variant resolution using high-density imputation and islet-specific epigenome maps. Nat Genet. 2018;50:1505–13. doi: 10.1038/s41588-018-0241-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oram RA, Sharp SA, Pihoker C, et al. Utility of Diabetes Type-Specific Genetic Risk Scores for the Classification of Diabetes Type Among Multiethnic Youth. Diabetes Care. 2022;45:1124–31. doi: 10.2337/dc20-2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grace SL, Cooper A, Jones AG, et al. Zinc transporter 8 autoantibody testing requires age-related cut-offs. BMJ Open Diabetes Res Care. 2021;9:e002296. doi: 10.1136/bmjdrc-2021-002296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.WHO Obesity: preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ Tech Rep Ser. 2000;894:1–253. [PubMed] [Google Scholar]
- 21.Benhalima K, Song SH, Wilmot EG, et al. Characteristics, complications and management of a large multiethnic cohort of younger adults with type 2 diabetes. Prim Care Diabetes. 2011;5:245–50. doi: 10.1016/j.pcd.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 22.Hillier TA, Pedula KL. Characteristics of an adult population with newly diagnosed type 2 diabetes: the relation of obesity and age of onset. Diabetes Care. 2001;24:1522–7. doi: 10.2337/diacare.24.9.1522. [DOI] [PubMed] [Google Scholar]
- 23.Hou C, Yang H, Qu Y, et al. Health consequences of early-onset compared with late-onset type 2 diabetes mellitus. Precis Clin Med. 2022;5:pbac015. doi: 10.1093/pcmedi/pbac015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Frayling TM, Wiltshire S, Hitman GA, et al. Young-onset type 2 diabetes families are the major contributors to genetic loci in the Diabetes UK Warren 2 genome scan and identify putative novel loci on chromosomes 8q21, 21q22, and 22q11. Diabetes. 2003;52:1857–63. doi: 10.2337/diabetes.52.7.1857. [DOI] [PubMed] [Google Scholar]
- 25.Zhou K, Donnelly LA, Morris AD, et al. Clinical and genetic determinants of progression of type 2 diabetes: a DIRECT study. Diabetes Care. 2014;37:718–24. doi: 10.2337/dc13-1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goedecke JH, Mendham AE. Pathophysiology of type 2 diabetes in sub-Saharan Africans. Diabetologia. 2022;65:1967–80. doi: 10.1007/s00125-022-05795-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kibirige D, Lumu W, Jones AG, et al. Understanding the manifestation of diabetes in sub Saharan Africa to inform therapeutic approaches and preventive strategies: a narrative review. Clin Diabetes Endocrinol. 2019;5:2. doi: 10.1186/s40842-019-0077-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Christensen DL, Hjort L, Mpagama SG, et al. Environmental exposures are important for type 2 diabetes pathophysiology in sub-Saharan African populations. Diabetologia. 2023;66:777–9. doi: 10.1007/s00125-022-05867-3. [DOI] [PubMed] [Google Scholar]
- 29.Ferdous F, Filteau S, Schwartz NB, et al. Association of postnatal severe acute malnutrition with pancreatic exocrine and endocrine function in children and adults: a systematic review. Br J Nutr. 2022;129:1–34. doi: 10.1017/S0007114522001404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hoffman DJ, Reynolds RM, Hardy DB. Developmental origins of health and disease: current knowledge and potential mechanisms. Nutr Rev. 2017;75:951–70. doi: 10.1093/nutrit/nux053. [DOI] [PubMed] [Google Scholar]
- 31.Adeyemo AA, Tekola-Ayele F, Doumatey AP, et al. Evaluation of Genome Wide Association Study Associated Type 2 Diabetes Susceptibility Loci in Sub Saharan Africans. Front Genet. 2015;6:335. doi: 10.3389/fgene.2015.00335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Adeyemo AA, Zaghloul NA, Chen G, et al. ZRANB3 is an African-specific type 2 diabetes locus associated with beta-cell mass and insulin response. Nat Commun. 2019;10:3195. doi: 10.1038/s41467-019-10967-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen J, Sun M, Adeyemo A, et al. Genome-wide association study of type 2 diabetes in Africa. Diabetologia. 2019;62:1204–11. doi: 10.1007/s00125-019-4880-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hill-Briggs F, Adler NE, Berkowitz SA, et al. Social Determinants of Health and Diabetes: A Scientific Review. Diabetes Care. 2020;44:258–79. doi: 10.2337/dci20-0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goff LM. Ethnicity and Type 2 diabetes in the UK. Diabet Med. 2019;36:927–38. doi: 10.1111/dme.13895. [DOI] [PubMed] [Google Scholar]
- 36.Goff LM, Ladwa M, Hakim O, et al. Ethnic distinctions in the pathophysiology of type 2 diabetes: a focus on black African-Caribbean populations. Proc Nutr Soc. 2020;79:184–93. doi: 10.1017/S0029665119001034. [DOI] [PubMed] [Google Scholar]
- 37.Ladwa M, Hakim O, Amiel SA, et al. A Systematic Review of Beta Cell Function in Adults of Black African Ethnicity. J Diabetes Res. 2019;2019:7891359. doi: 10.1155/2019/7891359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ishimwe MCS, Wentzel A, Shoup EM, et al. Beta-cell failure rather than insulin resistance is the major cause of abnormal glucose tolerance in Africans: insight from the Africans in America study. BMJ Open Diabetes Res Care. 2021;9:e002447. doi: 10.1136/bmjdrc-2021-002447. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available upon reasonable request.