ABSTRACT
The Arctic Monitoring Assessment Program (AMAP) is tasked with monitoring and assessing the status of environmental contaminants in the Arctic, documenting levels and trends, and producing science-based assessments. The objectives of this paper are to present the current levels of persistent organic pollutants (POPs) across the Arctic, and to identify trends and knowledge gaps as detailed in the most recent AMAP Human Health Assessment Report. Many Arctic populations continue to have elevated levels of these contaminants, and the highest levels of POPs were observed in populations from Greenland, Faroe Islands, and Nunavik (Canada), as well as populations in the coastal Chukotka district (Russia) for legacy POPs only. Concentrations of most POPs are declining in Arctic populations in regions where time trends data exist, although the declines are not consistent across all regions. The exceptions are per- and polyfluoroalkyl substances, with concentrations of some long-chain PFAS such as perfluorononanoic acid increasing in populations in Nunavik, Greenland and Sweden. This paper provides a more extensive summary of levels of contaminants in adults, pregnant women, and children across the Arctic than previous AMAP human health assessments, particularly for levels of long-chain PFAS, which are currently under consideration for inclusion in the Stockholm Convention.
KEYWORDS: Arctic, biomonitoring, contaminants, POPs, PFAS
Introduction
The harvesting of traditional foods (also commonly known as country foods or subsistence foods, which may include berries, plants, birds, terrestrial mammals, fish and marine mammals) have a significant benefit on the wellbeing of northerners, including social, cultural, economic and spiritual wellbeing for many Arctic Indigenous populations [1,2]. However, some traditional foods may also contain elevated levels of contaminants. Due to atmospheric and oceanic transport of contaminants to the Arctic, the Arctic has become a “sink” for many persistent chemicals that bioaccumulate and biomagnify through Arctic food chains. Several species of wildlife, especially some marine mammals at the top of the Arctic food web, can have elevated levels of particular contaminants including persistent organic pollutants (POPs) [3]. As a result, human consumption of some parts of such marine mammals and other traditional foods, can lead to elevated exposures to these contaminants. Human biomonitoring studies conducted over the last few decades have provided important data on current levels of these contaminants across the Arctic as well as temporal and spatial trends. This article presents human biomonitoring data from all eight circumpolar Arctic countries with the aim to provide an update on the presence of contaminants in human populations living in the Arctic, including the current state of exposure, spatial differences, and temporal trends. The article focuses on contaminants that continue to be a priority in Arctic regions, principally POPs including organochlorine compounds, brominated flame retardants and per- and polyfluoroalkyl substances (PFAS). Contaminants such as metals and trace elements are presented in another article of this special issue [4].
Materials and methods
Biomonitoring data are presented for pregnant women, adult men and women, women of childbearing age, children and youth. These data have been collected as part of regional cross-sectional studies or ongoing cohort studies. Contaminant concentrations are reported in blood, either in blood plasma, serum or whole blood.
The mere participation in an external QA/QC program has been shown to result in improved performance over time [5]. Data used in this assessment are primarily from laboratories that are active participants in different Quality Assurance (QA)/Quality Control (QC) programs, particularly the AMAP Ring test [6]. Initiated in 2001, the AMAP Ring test was designed to ensure comparability of Arctic POPs data provided from participating laboratories. Further details on the AMAP Ring and other QA/QC methodology are described in greater detail elsewhere [6,7]. Only in a few cases, data included here have come from laboratories which have used rigorous laboratory analysis procedures but have not participated in external QA/QC programs.
The data for POPs presented or discussed in this article, including polybrominated diphenyl ethers (PBDEs), polychlorinated biphenyls (PCBs) and organochlorines such as chlordanes, trans-nonachlor, oxychlordane, p,p’-dichlorodiphenyldichloroethylene (p,p’-DDE), p,p’-dichlorodiphenyltrichloroethane (p,p’-DDT), β-hexachlorocyclohexane (β-HCH), hexachlorobenzene, mirex, toxaphene no. 26, and toxaphene no. 50, are reported on lipid weight basis (µg/kg plasma or serum lipids), unless otherwise stated, due to their accumulation in lipids. This also enables accurate maternal blood monitoring during pregnancy due to changing lipid levels during pregnancy and lipid levels adjusting in response to consumption of meals. For other POPs such as PFAS, which do not accumulate in lipid tissue but in plasma proteins, data are instead reported on a wet weight basis in plasma or serum (µg/L or µg/kg plasma or serum). Although the same list of congeners was not analysed in all studies, POP congener concentrations measured in serum or plasma can be compared between studies. Any comparisons made between populations or contaminants are descriptive comparisons only, unless specified in text as statistically significant comparisons and provided with original reference citations that detail the statistical methods used.
Here, we present highlights of the biomonitoring data from the Arctic. Readers should refer to Chapter 3 of the 2021 AMAP Human Health Assessment Report [6] for a complete and extensive presentation of all available biomonitoring data.
Results
Alaska
Sivuqaq (St. Lawrence Island) is the largest island in the Bering Sea and home to approximately 1600 Yupik residents between the two villages of Gambell and Savoonga. Organochlorine compounds were measured among local residents in 2001 [8], while PBDEs and PFAS were measured in 2013–2014 [9]. PBDEs and PFAS data are shown in Table 1. Concentrations of organochlorine compounds were generally higher in men than women, and after controlling for age and sex, concentrations were highest for those residents with activities near the Northeast Cape formerly-used defence site, compared to residents from Gambell [8]. Serum concentrations of long-chain PFAS were higher than short-chain PFAS, and the highest concentrations were observed for perfluorooctane sulphonic acid (PFOS) and perfluorononanoic acid (PFNA). PFAS and PBDEs were also measured in household dust, and while there was a lack of statistically significant correlations between serum and household dust concentrations for PFAS, there was a weakly significant correlation between serum and household dust concentrations for PBDE47 in women [9]. Caution should be used when comparing levels of POPs in women to those among pregnant women, however levels of PBDEs and PFOS previously observed in pregnant Yupik women from the Yukon-Kuskokwim River Delta region of Alaska between 2009 and 2012 [7], were lower than among Yupik women of Sivuqaq. In contrast, levels of perfluorooctanoic acid (PFOA) were lower among women from Sivuqaq.
Table 1.
Blood concentrations of PBDEs and PFAS in yupik adults. Data presented as geometric means (range); PBDEs presented in serum (µg/kg lipids) and PFASs in serum (µg/L serum). Source [9].
| Men |
Women |
|||
| Year(s) | 2013–2014 | 2013–2014 | ||
| Mean age (range) | 29 (19–45) | 28 (18–45) | ||
| Sample size |
n = 38 |
n = 47 |
||
| Concentrations |
% <LOD1 |
Concentrations |
% <LOD1 |
|
| PBDE47 | 9.96 (<LOD–80) | 2.6 | 10.4 (0.85–554) | 0 |
| PBDE153 | 10.7 (3.7–27.0) | 0 | 7.28 (0.9–54.9) | 0 |
| PBDE209 | 3.86 (<LOD–46.6) | 2.6 | 3.28 (0.1–38.6) | 0 |
| PFOA | 1.45 (0.51–2.9) | 0 | 0.85 (<LOD–1.44) | 15 |
| PFNA | 2.75 (0.73–10.8) | 0 | 2.07 (<LOD–12.1) | 2 |
| PFUnDA | 1.04 (<LOD–3.74) | 32 | 0.88 (<LOD–1.73) | 26 |
| PFOS | 6.96 (3.07–16) | 0 | 3.29 (<LOD–9.69) | 2 |
1LOD: Limit of detection.
Canada
POPs data are presented here for several different regions of the Canadian Arctic. Blood concentrations of POPs in adult Gwich’in men and women from Old Crow (Yukon) recruited in 2019 [10] are presented in Table 2. The predominant organochlorine compounds in plasma were p,p’-DDE, HCB, and PCBs. Among PBDEs, the most commonly detected congeners were PBDE153 and PBDE47. One-way ANOVA and Mann-Whitney U tests were performed to determine if there were significant differences in levels of POPs between men and women, and the majority of POPs showed similar levels, with the exception of trans-nonachlor, HCB and toxaphene Parlar 50, which were higher in men (p < 0.05) [10]. The PFAS congeners with the highest geometric mean concentrations measured in serum were PFOS, PFNA and PFOA among both men and women. Significant differences across age and sex were also noted for PFAS, with men having higher levels than women for PFHxS, PFOS and PFOA (p < 0.05). When the data were stratified by age (i.e. those under 40 years in age and those over 40 years in age), PFAS levels were higher among the older participants [11].
Table 2.
Blood concentrations of POPs in adults in Old Crow, Yukon, Canada. Data presented as geometric means (10th − 95th percentile) in blood plasma (µg/kg lipids) for organochlorines and PBDEs, and blood serum (µg/l) for PFAS. Source [10,12].
| Men |
Women |
|||
| Year(s) | 2019 | 2019 | ||
| Mean age (range) | 43 (21–75) | 39 (20–72) | ||
| Sample size |
n = 26 |
n = 28 |
||
| Concentrations |
% <LOD |
Concentrations |
% <LOD |
|
| trans-Nonachlor | 5.6 (<1.42–120) | 8 | 2.7 (<1.43–14) | 32 |
| Hexachlorobenzene | 18 (7.0–76) | 0 | 12 (4.0–47) | 0 |
| p,p’-DDE | 46 (19–290) | 0 | 38 (13–220) | 0 |
| PCB138 | 3.8 (<1.42–38) | 15 | 2.7 (<1.43–17) | 29 |
| PCB153 | 7.8 (2.6–66) | 3.8 | 5.2 (<1.4b–32) | 14 |
| PCB180 | 5.2 (<1.42–30) | 12 | 3.7 (<1.43–24) | 29 |
| PBDE47 | 5.4 (<4.32–56) | 38 | <4.23 (<4.23–25) | 61 |
| PBDE153 | 6.1 (<4.32–35) | 31 | 4.2 (<4.23–32) | 50 |
| PFOA | 1.1 (0.65–1.7) | 0 | 0.76 (0.41–1.7) | 0 |
| PFNA | 1.2 (0.46–3.9) | 0 | 0.77 (0.37–2.0) | 0 |
| PFDA | 0.20 (<0.09–0.57) | 13 | 0.16 (<0.09–0.35) | 13 |
| PFHxS | 0.56 (0.28–1.2) | 0 | 0.26 (0.10–1.4) | 0 |
| PFOS | 1.4 (0.60–3.9) | 7.7 | 0.78 (<0.40–2.4) | 14 |
1.LOD: Limit of detection; values <LOD were replaced by LOD/2 for the purposes of calculating means.
2.based on the geometric mean (7.0 g/L) of blood lipids observed for male participants.
3.based on the geometric mean (7.1 g/L) of blood lipids observed for female participants.
In the Mackenzie Valley of the Northwest Territories, adults from nine First Nations communities from the Dehcho and Sahtú regions were recruited between 2016 and 2018 [11] and concentrations of POPs were measured in blood (Tables 3 and 4). Mann-Whitney U tests showed that concentrations of serum organochlorine compounds were similar in adult men and women and were generally low with many of the contaminants at concentrations below the limit of detection for a large proportion of participants. The main POPs detected were PCB153, PCB180 and p,p’-DDE. Brominated flame retardants were also measured in serum; however, other than PBDE47, relatively few participants had detectable levels of PBDEs. Several PFAS were measured in plasma, and the highest concentrations were observed for PFOS and PFNA in men and women. PFAS concentrations between sexes and age groups were compared using Mann–Whitney U tests and men had higher levels than women for PFHxS, PFOS and PFOA (p < 0.05). When stratified according to age, PFAS concentrations generally increased with age [11].
Table 3.
Blood concentrations of POPs in adults across multiple first nations communities in the Northwest Territories, Canada. Data presented as geometric means (10th−95th percentile) in blood serum (µg/kg lipids). Source [13].
| Men |
Women |
|||
| Year(s) | 2016–2018 | 2016–2018 | ||
| Mean age (range) |
47.6 (18–88) |
45.6 (18–80) |
||
| Sample size |
n = 124 |
n = 122 |
||
| Concentrations |
%<LOD1 |
Concentrations |
% <LOD1 |
|
| trans-Nonachlor | 8 (<1.62–101) |
10.5 | 6 (<1.63–71) |
16.4 |
| Hexachlorobenzene | 12 (6–48) |
4 | 12 (6–48) |
0.8 |
| p,p’-DDE | 54 (22–214) |
0 | 59 (17–346) |
0 |
| PCB138 | 7 (1–49) |
8.9 | 6 (<1.63–61) |
18.9 |
| PCB153 | 18 (4–165) |
2.4 | 14 (1–187) |
5.7 |
| PCB180 | 14 (<1.62–134) |
9.7 | 9 (<1.63–98) |
15.6 |
| PBDE47 | 5 (<4.92–23) |
45.2 | 6 (<4.83–102) |
42.6 |
| PBDE153 | nc4 (<4.92–20) |
59.7 | nc4 (<4.83–20) |
71.3 |
1.LOD: Limit of detection; values <LOD were replaced by LOD/2 for the purposes of calculating means.
2.based on the geometric mean (6.1 g/L) of blood lipids observed for male participants.
3.based on the geometric mean (6.3 g/L) of blood lipids observed for female participants.
4.nc: not calculated due to high number of non-detects.
Table 4.
Concentration of blood plasma PFASs in adults across multiple first nations communities in the Northwest Territories, Canada. Data presented as geometric means (10th−95th percentile) in plasma (µg/l). Source [13].
| Men |
Women |
|||
| Year(s) | 2019 | 2019 | ||
| Mean age (range) |
48 (18–79) |
45 (21–71) |
||
| Sample size |
n = 57 |
n = 55 |
||
| Concentrations |
% <LOD1 |
Concentrations |
% <LOD1 |
|
| PFOA | 1.1 (0.61–3.1) |
0 | 0.72 (0.41–2.8) |
0 |
| PFNA | 1.5 (0.50–11) |
0 | 1.3 (0.46–6.4) |
0 |
| PFDA | 0.22 (<0.09–1.4) |
15 | 0.20 (<0.09–0.90) |
11 |
| PFHxS | 0.58 (0.28–1.5) |
0 | 0.23 (0.10–0.55) |
1.8 |
| PFOS | 2.5 (1.0–12) |
0 | 1.6 (0.74–4.9) |
1.8 |
1LOD: Limit of detection.
In Nunavik (northern region of Quebec), organochlorine compounds and PBDEs were measured in plasma of Inuit adults (18 years and above) and youth (16–17 years of age) in a representative subsample of 500 participants of the Qanuilirpitaa? Nunavik Inuit Health Survey in 2017 Survey results presented in Table 5 are weighted by survey weights. Concentrations of POPs were very similar between men and women, and the POPs with the highest concentrations were p,p’-DDE, PCB153, and trans-nonachlor. Concentrations of POPs also increased with age among participants, as concentrations of organochlorine compounds in older men (50+ years of age) were approximately two- to six-fold higher than in younger men (18–49 years of age). This trend was more pronounced among women, where geometric mean concentrations in older women (50+ years of age), were approximately three- to nine-fold higher than in younger women (18–49 years of age). Few participants had detectable levels of PBDEs. Compared to the results of the Qanuippitaa? Nunavik Inuit Health Survey in 2004 [14] which were described in the previous AMAP assessment [7], plasma levels of POPs in Inuit men and women in 2017 declined by a large margin. In the 2017 survey report, authors noted that the majority of organochlorine compounds declined by approximately 50%, with the p,p’-DDE and PCB congeners showing some of the largest declines [15]. The declines for geometric mean concentrations in men and women were similar, with declines of up to 76% and 77% (p,p’-DDE) for men and women, respectively [15].
Table 5.
Blood concentrations of POPs in inuit adult men and women from Nunavik, Canada, in a representative sub-sample of the qanuilirpitaa? Nunavik inuit health survey in 2017. Data are weighted by survey weights and presented as geometric means (10th−95th percentile) in blood plasma (µg/kg plasma lipids). Source [15].
| Men |
Women |
|||
| Year | 2017 | 2017 | ||
| Mean age (range) |
38.9 (18–86) |
38.4 (18–81) |
||
| Concentrations |
% <LOD |
Concentrations |
% <LOD |
|
| trans-Nonachlor | 59 (12-540) |
0.4 | 63 (14-540) |
0.5 |
| p,p’-DDE | 190 (54-1040) |
0 | 200 (48-1080) |
11.3 |
| HCB | 32 (10-170) |
0 | 38 (11-180) |
0.4 |
| PCB138 | 29 (6.4-200) |
1.2 | 29 (6.0-240) |
0.2 |
| PCB153 | 70 (16-660) |
0 | 62 (12-570) |
0 |
| PCB180 | 40 (6.9-470) |
0.2 | 28 (4.9-350) |
2.0 |
| PBDE47 | nc (<LOD-12) |
71.7 | nc (<LOD-12) |
76.4 |
| PBDE153 | nc (<LOD-18) |
48.6 | nc (<LOD-11) |
70.5 |
1LOD: Limit of detection.
nc:geometric mean not calculated due to number of samples
Thirty pooled plasma samples were also established (grouped according to age, sex, and region of residence) from the Qanuilirpitaa? 2017 survey for measurement of PFAS, and results are weighted by survey weights. The predominant PFAS measured were PFOS, followed by PFNA and then PFOA (Table 6). The only exception was among the youngest age groups (16–19 years of age), where concentrations of PFNA were greater than for both PFOS and PFOA (for both males and females). For short-chain PFAS, while some samples had detectable concentrations of PFBA, none of the pooled samples had detectable concentrations of PFHxA and PFBS. Concentrations of PFAS were generally similar between men and women, with the exception of higher concentrations of PFDA and lower concentrations of PFHxS in women compared to men. Concentrations of PFOS, PFDA, PFUnDA and PFHxS appeared to increase steadily with age in all five age groups, whereas PFNA levels followed a u-shape with age.
Table 6.
Concentrations of PFASs in pooled blood samples of adult inuit from Nunavik. A total of n = 30 pooled samples were established, divided by sex, five age groups, and three regions of Nunavik; data presented here with regions combined. Data presented as means (min – max) in blood plasma for PFAS (µg/l). Source [16].
| Men |
Women |
|||||||||||
| Year |
2017 |
2017 |
||||||||||
| Age |
16–86 (all) |
16–19 |
20–29 |
30–39 |
40–59 |
60+ |
16–81 (all) |
16–19 |
20–29 |
30–39 |
40–59 |
60+ |
| Sample size | n = 15 | n = 3 | n = 3 | n = 3 | n = 3 | n = 3 | n = 15 | n = 3 | n = 3 | n = 3 | n = 3 | n = 3 |
| PFOA | 1.4 (1.1–2.7) |
1.2 (1.1–1.2) |
1.3 (1.2–1.3) |
1.4 (1.2–1.4) |
1.5 (1.4–1.6) | 2.2 (2–2.7) |
0.97 (0.57–2.5) |
0.82 (0.78–0.9) |
0.7 (0.57–0.8) |
0.74 (0.65–0.84) |
1.3 (1.2–1.4) |
2 (1.7–2.5) |
| PFNA | 4.7 (2.2–9.9) |
5.3 (5.2–5.4) |
3.9 (3.8–4.1) |
4.3 (2.2–5.2) |
4.6 (3.6–5.2) |
7.1 (5.5–9.9) |
4.5 (2.7–14) |
3.9 (3.3–4.2) |
3.1 (2.7–3.3) |
3.6 (3.1–4.3) |
5.9 (4.9–6.8) |
10 (5.9–14) |
| PFDA | 0.84 (0.4–2.3) |
0.55 (0.4–0.67) |
0.68 (0.66–0.78) |
0.9 (0.62–1.1) | 0.96 (0.84–1.1) | 1.6 (1.2–2.3) |
0.95 (0.44–3) |
0.57 (0.44–0.7) |
0.72 (0.61–0.82) |
0.84 (0.6–1.1) |
1.3 (0.95–1.5) |
2.1 (1.4–3) |
| PFUnDA | 0.85 (0.52–2) |
0.61 (0.52–0.7) |
0.68 (0.62–0.91) | 0.87 (0.73–1) |
0.96 (0.86–1.1) |
1.6 (1.2–2) |
1 (0.51–2.8) |
0.64 (0.51–0.75) |
0.81 (0.66–0.96) |
0.91 (0.65–1.1) |
1.2 (0.94–1.6) |
2.1 (1.5–2.8) |
| PFHxS | 0.97 (0.52–2.8) | 0.57 (0.52–0.63) | 0.76 (0.72–0.9) | 0.98 (0.86–1) |
1.2 (1.1–1.4) |
2 (1.6–2.8) |
0.62 (0.34–2.2) |
0.36 (0.34–0.37) |
0.42 (0.38–0.44) |
0.47 (0.4–0.56) |
0.92 (0.71–1.4) |
1.9 (1.7–2.2) |
| PFOS | 7.2 (3.4–17) |
4.3 (3.4–5.3) |
5.7 (4.9–6.6) |
7.7 (5.7–9.5) |
8.7 (7.6–10) |
14 (11–17) |
6.3 (2.6–24) |
3.6 (2.6–4.4) |
4.6 (3.5–5.3) |
5.3 (3.6–6.3) |
8.8 (5.6–11) |
17 (12–24) |
Over the past 30 years, substantial data have been collected allowing assessment of time trends for POPs in Inuit pregnant women with data for most POPs going back to 1992 (Table 7), while data for PFOS and PFOA extend back to 2004 and 2007 respectively, and other long-chain PFAS back to 2012 (Table 8). Levels of organochlorine compounds and PBDEs in blood of pregnant women in Nunavik are declining, with current levels of POPs in 2017 between 70% and 87% lower than levels observed in 1992. Statistically significant declining trends over time were observed for all POPs listed in Table 7, except for PBDE47. While data are limited to more recent years for PFAS, PFOS was the most predominant in 2017 followed by PFNA. It is important to note that PFBA, PFHxA and PFBS were measured in 2012 and 2017, but are excluded from Table 8 because they were not detected in one or both years. Statistically significant declines were also observed for PFOS, PFOA and PFHxS. In contrast to these PFAS, increasing concentrations were observed for long-chain PFCAs such as PFNA, PFDA, and PFUnDA (2012 to 2017), with respective increases of 19%, 13% and 21%, although only the increase in PFNA was statistically significant [17].
Table 7.
Blood concentrations of POPs in pregnant Inuit women from Nunavik, Canada. Organochlorine and PBDE data presented as geometric means (range) in µg/kg serum lipids. Results presented only for contaminants with 60% and more of data detected. Source [7,16].
| Year |
1992 |
1996 |
1997 |
1998 |
1999 |
2000 |
2001 |
2004 |
2007 |
2012 |
2013 |
2017 |
|
| Mean age (range) |
24 (18–35) |
24 (16–33) |
24 (15–40) |
24 (14–37) |
25 (17–35) |
26 (16–39) |
27 (17–39) |
26 (19–35) |
23 (17–37) |
24 (18–39) |
24 (18–41) |
24 (15–38) |
p-value1 |
| Sample size | n = 11 | n = 25 | n = 53 | n = 46 | n = 26 | n = 36 | n = 20 | n = 22 | n = 39 | n = 112 | n = 95 | n = 97 | |
| trans-Nonachlor | 110 (49–320) |
65 (15–250) |
75 (14–330) |
53 (12–580) |
59 (21–170) |
61 (13–300) |
49 (11–200) |
65 (19–200) |
47 (2.5–250) |
37 (<LOD2–220) |
42 (2.0–220) |
34 (2.8–230) |
<0.0001 |
| p,p’-DDE | 660 (290–1570) |
290 (71–1020) |
370 (59–1440) |
260 (67–2270) |
280 (140–900) |
280 (64–1330) |
210 (54–1690) |
230 (63–720) |
160 (30–720) |
120 (11–520) |
130 (22–480) |
100 (<17–490) |
<0.0001 |
| HCB | 97 (47–220) |
41 (15–120) |
51 (9.2–190) |
35 (6.7–350) |
36 (14–100) |
37 (12–110) |
32 (11–140) |
34 (8.9–92) |
24 (5.1–83) |
18 (<LOD2–110) |
20 (2.0–92) |
19 (<LOD2–110) |
<0.0001 |
| PCB138 | 110 (45–220) |
57 (10–210) |
69 (12–320) |
45 (13–390) |
60 (17–220) |
56 (9.7–300) |
49 (11–170) |
38 (12–120) |
23 (3.0–91) |
17 (<LOD2–77) |
19 (2.0–120) |
14 (<1.3–91) |
<0.0001 |
| PCB153 | 170 (71–290) |
100 (19–410) |
130 (23–610) |
80 (27–710) |
110 (29–470) |
98 (15–500) |
76 (16–420) |
73 (22–240) |
43 (4.5–220) |
39 (2.4–230) |
40 (3.5–320) |
30 (3.1–190) |
<0.0001 |
| PCB180 | 90 (34–150) |
43 (7.6–190) |
51 (11–220) |
34 (12–280) |
51 (13–380) |
42 (5.0–260) |
40 (7.5–240) |
30 (8.1–120) |
18 (2.0–95) |
17 (<LOD2–160) |
16 (1.7–200) |
13 (1.8–100) |
<0.0001 |
| PBDE47 | na3 | na3 | na3 | na3 | na3 | na3 | na3 | 7.0 (<LOD2–33) |
6.2 (<LOD2–49) |
nc4 (<LOD2–29)5 |
nc4 (<LOD2–210) |
nc4 (<LOD2–24) |
0.6106 |
| PBDE153 | na3 | na3 | na3 | na3 | na3 | na3 | na3 | 2.0 (<LOD2–12) |
2.6 (<LOD2–23) |
2.9 (<LOD2–15 5 |
nc4 (<LOD2–13) |
nc4 (<LOD2–19) |
0.0392 |
1.p-value based on orthogonal polynomial contrast for linear trend, using regression adjusted for age, smoking status (smoker vs. non-smoker) and multiparous woman (yes vs. no).
2.LOD: Limit of detection; for statistical purposes, values <LOD were replaced by LOD/2.
3.na: not available.
4.nc: geometric mean not calculated due to less than 60% of samples >LOD.
5.n = 95.
Table 8.
Blood concentrations of PFASs in pregnant Inuit women from Nunavik, Canada. The data are presented as geometric means (range) in µg/L serum. Results presented only for contaminants with 60% and more of data detected. Source [7,16].
| Year | 2004 | 2007 | 2012 | 2017 | |
|---|---|---|---|---|---|
| Mean age (range) |
27 (19–37) |
23 (17–37) |
24 (18–39) |
24 (15–37) |
p-value1 |
| Sample size |
n = 25 |
n = 40 |
n = 111 |
n = 91 |
|
| PFOA | na2 | 0.86 (0.40–1.9) |
0.69 (0.20–2.4) |
0.55 (0.19–1.4) |
<0.0001 |
| PFNA | na2 | na2 | 2.1 (0.80–12) |
2.5 (0.75–10) |
0.0358 |
| PFDA | na2 | na2 | 0.49 (<LOD3–4.0)4 |
0.52 (0.10–3.1)5 |
0.6396 |
| PFUnDA | na2 | na2 | 0.53 (<LOD3–4.5)6 |
0.60 (0.090–3.8) |
0.3788 |
| PFHxS | na2 | 0.44 (<LOD3–7.0) |
0.35 (<LOD3–1.5)7 |
0.26 (0.060–1.2) |
<0.0001 |
| PFOS | 9.8 (3.1–20) |
5.4 (1.5–15) |
3.9 (0.70–23) |
3.3 (0.70–19) |
<0.0001 |
1.p-value based on orthogonal polynomial contrast for linear trend, using regression adjusted for age, smoking status (smoker vs. non-smoker) and multiparous woman (yes vs. no).
2.na: not available.
3.LOD: Limit of detection; for statistical purposes, values <LOD were replaced by LOD/2.
4.n = 106.
5.n = 90.
6.n = 110.
7.n = 107.
Among pregnant women in Nunavik, strong positive associations were found between several PFAS congeners (PFHxS, PFOS, PFNA, PFDA, PFUnDA) and the omega-3/omega-6 polyunsaturated fatty acid ratio, indicating a positive association with consumption of marine country foods [17]. While shorter-chain PFAS such as PFOA are often associated with exposure though consumer goods [18], the bioaccumulation potential of PFAS congeners is higher for PFOS and among the C9–C14 congeners (e.g. PFNA, PFDA, PFUnDA, and above) compared to many of the shorter congeners [19,20]. The higher ratios (long versus short-chain PFAS) noted in pregnant women from Nunavik may therefore indicate an increasing exposure to these compounds occurring through their bioaccumulation in marine country foods [17]). Moreover, currently used fluorotelomer alcohols are known to be capable of transport to the Arctic and degrade into a number of PFAS (i.e. PFOA, PFNA, PFDA, PFUnDA), but compared to PFOA, long-chain PFAS (C9-C14) have greater bioaccumulation potential which could lead to higher accumulation in Arctic wildlife, and ultimately circumpolar populations which rely on these species for subsistence as observed in this Nunavik study [17].
Concentrations of POPs vary across populations in the Canadian Arctic. Levels of POPs in the Yukon were relatively low compared to other Arctic regions (particularly Nunavik) and were comparable to populations in Southern Canada (non-Arctic). One exception was HCB for which results were higher in Yukon than levels reported in the nationally representative Canadian Health Measures Survey (CHMS) Cycle 1, 2007–2009 [21] and in the First Nations Biomonitoring Initiative (FNBI) in 2011 [22].
In contrast concentrations of many POPs were much higher among Inuit in Nunavik than the Canadian general population. In 2017, several organochlorines remained 7 to 10 fold higher among Nunavik Inuit aged 16 years old and over than the latest general Canadian measurements in CHMS Cycle 1 (2007–2008). Additionally, mean PFOS was 1.5 higher, and long-chain PFAAs (i.e. PFNA, PFDA and PFUnDA) were 4 to 7 fold higher in Nunavik youth and adults in 2017 compared to CHMS Cycle 5 in 2016–2017 [23]. With the exception of PFOA, PFAS exposure levels were far higher in Nunavik than elsewhere in the Arctic. Similarly, concentrations of PFOS, PFNA and PFDA in Inuit pregnant women from Nunavik were 1.8-, 6.3-, and 3.3-fold higher, respectively, than childbearing age women (18–40 years of age) from the general Canadian population (CHMS Cycle 5, 2016–2017), while levels of PFOA and PFHxS were lower [17]. As a whole, the sum of PFAS in Nunavik pregnant women was twice that of women in CHMS Cycle 5.
Greenland
Greenland has historically had some of the highest concentrations of contaminants in the Arctic, although several studies have noted that concentrations vary significantly by region. The ACCEPT mother-childbirth cohort (2010–2015) involved the recruitment of pregnant Inuit women from a total of 19 communities divided into 5 distinct regions. Significant regional differences (p < 0.0001) were observed for all organochlorine compounds, and higher levels were observed in eastern and northern Greenland (Table 9). The levels of POPs in pregnant Inuit women from the ACCEPT cohort were lower than levels previously reported [24], when comparing to previous levels in Disko Bay and Nuuk (Figure 1).
Table 9.
Blood concentrations of Organochlorines and PFAS in Greenlandic inuit pregnant women in ACCEPT cohort 2010–2015. Data presented as geometric means (range) in blood plasma (µg/kg lipid) for organochlorines, and in serum (µg/l) for PFAS. Source [25].
| % <LOQ |
North |
Disko Bay |
West |
South |
East |
p-value1 |
All |
|
| Mean age | 28.4 | 27.1 | 27.3 | 28.6 | 27.4 | 27.5 | ||
| (Range) | (20–36) | (18–41) | (18–42) | (20–41) | (19–42) | (18–42) | ||
| Sample size |
n = 33 |
n = 117 |
n = 280 |
n = 42 |
n = 19 |
|
n = 491 |
|
| trans-Nonachlor | 1.9 | 75.2 (7.60–320) |
50.3 (7.60–220) |
32.9 (1.00–580) |
38.9 (3.20–110) |
264 (47.0–1600) |
<0.0001 | 42.3 (1.00–1600) |
| Hexachloro-benzene | 0.3 | 35.9 (5.80–130) |
31.5 (9.60–100) |
23.1 (2.50–170) |
22.9 (8.90–56.0) |
68.4 (25.0–240) |
<0.0001 | 26.7 (2.50–240) |
| p,p’ DDE | 0.7 | 208 (18.0–990) |
135 (22.0–540) |
104 (5.00–2500) |
127 (26.0–430) |
1037 (110–8800) |
<0.0001 | 129 (5.00–8800) |
| PCB138 | 0 | 38.9 (4.80–180) |
27.6 (4.30–110) |
23 (2.40–410) |
28.6 (6.20–82.0) |
200 (22.0–1300) |
<0.0001 | 27.6 (2.4–1300) |
| PCB153 | 0 | 82.5 (8.90–950) |
57.3 (8.40–210) |
47.8 (5.10–910) |
60.3 (12.0–180) |
414.5 (43.0–2700) |
<0.0001 | 57.4 (5.10–2700.0) |
| PCB180 | 0 | 39.5 (6.60–810) |
25.3 (3.90–110) |
24.1 (3.80–370) |
30.5 (7.30–82.0) |
186 (23.0–1100) |
<0.0001 | 27.8 (3.80–1100) |
| n = 32 | n = 122 | n = 283 | n = 43 | n = 19 | n = 499 | |||
| PFOA | 0.2 | 0.97 (0.23–2.27) |
1.1 (0.24–7.26) |
1.04 (0.10–6.33) |
0.91 (0.30–2.42) |
1.12 (0.33–2.31) |
0.3 | 1.04 (0.10–7.26) |
| PFNA | 0 | 1.42 (0.34–7.34) |
1.3 (0.39–7.87) |
1.1 (0.21–7.71) |
0.94 (0.43–3.35) |
2.52 (0.75–5.93) |
<0.0001 | 1.19 (0.21–7.87) |
| PFDA | 0.1 | 0.98 (0.22–3.30) |
0.88 (0.21–3.92) |
0.67 (0.12–7.84) |
0.55 (0.19–1.62) |
1.51 (0.43–4.35) |
<0.0001 | 0.74 (0.12–7.84) |
| PFUnDA | 0.7 | 1.91 (0.28–12.1) |
1.77 (0.21–16.3) |
1.25 (0.08–14.9) |
1.01 (0.16–5.36) |
3.4 (0.64–18.2) |
<0.0001 | 1.42 (0.08–18.2) |
| PFHxS | 0.2 | 0.67 (0.10–4.48) |
0.49 (0.13–2.52) |
0.49 (0.04–2.57) |
0.42 (0.17–1.37) |
1.49 (0.23–4.34) |
<0.0001 | 0.52 (0.04–4.48) |
| PFOS | 0 | 12.2 (2.04–50.7) |
10.4 (2.35–43.6) |
8.17 (1.45–61.3) |
7.12 (3.20–18.0) |
18.3 (5.35–42.5) |
<0.0001 | 9.06 (1.45–61.3) |
LOQ: limit of quantification.
1difference among regions was tested by one-way ANOVA analysis.
Figure 1.
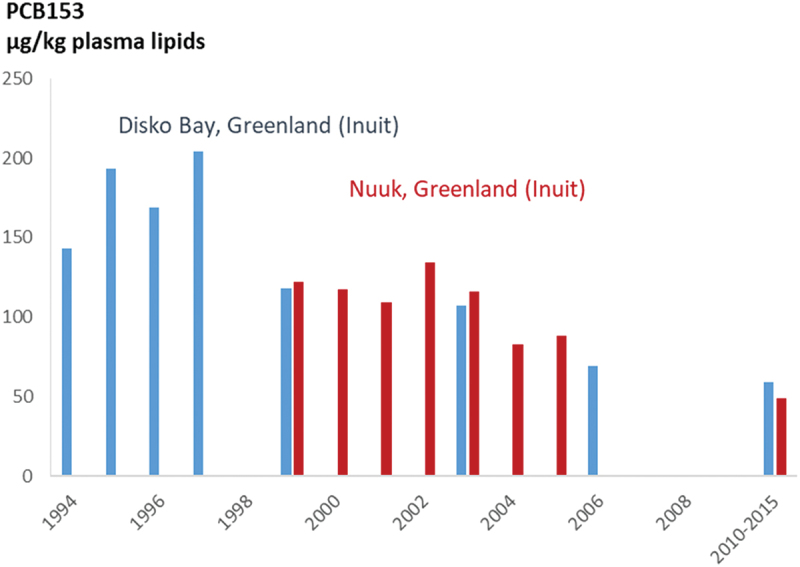
Trends in PCB153 in pregnant women from Disko Bay and Nuuk, Greenland. PCB153 presented in plasma (µg/kg lipid). Blue (Disko Bay), red (Nuuk). Source: [6].
A total of 16 PFASs were measured in the ACCEPT cohort, but only PFOS, PFHxS, PFHpS, PFOA, PFNA, PFDA and PFUnDA were detected in majority of samples (74.7%) [25]. Similar to the pattern of lipophilic POPs, significant regional differences in PFAS congeners (except for PFOA, p = 0.3) were observed with the highest levels seen in eastern Greenland (Table 9).
In an earlier cohort, the INUENDO birth cohort (2002–2004) [26–28], in Greenlanders the concentrations of PFOS were over ten-fold higher than for other PFASs detected for pregnant women and their male partners [6]. Geometric mean concentrations of PFASs in men were much higher than for pregnant women, often twice as high or more (for PFOS, PFOA, PFNA, PFDA)). When comparing levels of PFAS in pregnant women from the ACCEPT cohort to the earlier INUENDO birth cohort, levels of PFOS, PFOA, and PFHxS in pregnant women appear to have declined, while levels of other PFAS such as PFNA, PFDA and PFUnDA have increased (Table S2).
In addition to adults, PCBs and PFAS were measured in children (OCEANS study) and area of residence was significantly associated with contaminant levels [29]. Concentrations of PFASs were detected in almost all children, except for PFHxA (94% < LOD), PFUnDA (1% < LOD), and PFHpA which was below the limit of detection for 23% and 21% of boys and girls, respectively. The predominant PFAS measured in serum were PFOS, PFOA and PFNA, which were much higher than all other PFAS measured.
Statistically significant regional differences (p < 0.001) were found for PCBs and most PFAS in children (Table 10). The lowest geometric mean concentrations of PCBs were observed in Nuuk, while the highest were observed in Tasiilaq (eastern Greenland). While statistically significant differences were observed for all PFASs measured, except PFHxA, regional trends were more varied. The lowest mean concentrations of PFOA and PFDA were observed in western Greenland (Sisimiut and Maniitsoq), while PFOS was lowest in Nuuk. Similar to PCBs, most PFASs were observed at higher concentrations in eastern Greenland. While increased consumption of traditional Greenlandic food was associated with higher levels of all contaminants, levels of PFOA were not as strongly associated with food consumption, which may suggest other sources of exposure [29].
Table 10.
Regional comparisons of blood concentrations of contaminants in Greenlandic children between 2012 and 2015. Data presented as geometric means (range); PCBs in lipid adjusted serum (µg/kg plasma lipids), PFAS in serum (µg/l). Source [29,30].
| Region |
Greenland (all) |
Nuuk |
Disko Bay1 |
West2 |
East3 |
|
| Mean age (range) |
9.8 (7.1–12.1) |
9.3 (7.3-11.0) |
9.5 (7.1–11.5) |
10.4 (8.1–11.6) |
10.5 (9.1–12.1) |
p-value4 |
| Sample size |
n = 338 |
n = 84 |
n = 130 |
n = 100 |
n = 24 |
|
| PCB138 | 43 (<3 –1137) |
24 (<3 –230) |
45 (<3 –1137) |
45 (<3 –235) |
223 (<3 –997) |
<0.001 |
| PCB153 | 66 (<3 –1531) |
39 (<3 –363) |
66 (<3 –504) |
68 (<3 –454) |
351 (<3 –1532) |
<0.001 |
| PCB180 | 29 (<3 –834) |
16 (<3 –198) |
31 (<3 –413) |
29 (<3 –342) |
165 (<3 –834) |
<0.001 |
| ƩPCBs5 | 283 (<18 –6600) |
162 (<18–1463) |
292 (18–2677) |
290 (49–2062) |
1484 (289–6600) |
<0.001 |
| PFOA | 2.33 (0.824–6.33) |
2.18 (1.05–4.35) |
2.70 (1.23–6.33) |
2.01 (0.824–5.25) |
2.37 (1.60–3.70) |
<0.001 |
| PFNA | 1.48 (0.434–8.04) |
1.23 (0.434–2.93) |
1.59 (0.613–5.10) |
1.37 (0.459–2.93) |
2.60 (0.865–8.04) |
<0.001 |
| PFDA | 0.440 (0.078–3.64) |
0.436 (0.106–1.07) |
0.565 (0.083–1.63) |
0.258 (0.078–2.41) |
1.07 (0.339–3.64) |
<0.001 |
| PFUnDA | 0.413 (<0.03–5.99) |
0.244 (<0.03–1.22) |
0.532 (0.068–2.74) |
0.357 (<0.03–2.92) |
1.20 (0.234–5.99) |
<0.001 |
| PFHxS | 0.731 (0.234–5.83) |
0.636 (0.234–1.43) |
0.699 (0.260–1.80) |
0.676 (0.291–1.70) |
2.11 (0.675–5.83) |
<0.001 |
| PFOS | 8.89 (2.05–53.1) |
6.97 (2.05–18.3) |
9.84 (3.23–32.1) |
8.08 (2.90–25.1) |
18.0 (5.11–53.1) |
<0.001 |
1.Qeqertarsuaq, Aasiaat, and Ilulissat.
2.Sisimiut and Maniitsoq.
3.Tasiilaq.
4.differences between areas were tested using Kruskal Wallis test.
5.ƩPCB = 2×(PCB138+PCB153+PCB180).
Iceland
Levels of POPs in maternal plasma have been monitored at approximately five-year intervals since 1995. Iceland has a socially and culturally homogenous population, and results from maternal sampling in 1999 (Reykavik) and 2004 (across all Iceland) have indicated that observed exposures and contaminant concentrations are similar [31]. The time series show a clear downward trend in levels of most POPs, especially from 2004 to 2015 (Table 11). Levels observed in 2015 are roughly 20–30% of those recorded in 1995. PBDEs were analysed in 2009 and 2015 and levels appear similar at these two time points. While PBDE47 continues to be the predominant congener, while concentrations of PBDEs are low and close to the limit of detection.
Table 11.
Trends of POPs in pregnant Icelandic women in their third trimester. Data presented as geometric means (range) for POPs (µg/kg plasma lipid) and for PFOS and PFOA (µg/L plasma). Lipid normalisation of data in 1999 and 2004 based on average lipid concentrations from 1995. Source [7,32].
| Reykjavik |
All Iceland |
Reykjavik |
|||
| Year(s) | 1995 | 1999 | 2004 | 2009 | 2015 |
| Mean age (range) |
30 (18–41) |
28.7 (20–42) |
30.3 (20–40) |
30.4 (21–43) |
31.6 (22–43) |
| Sample size; mean parity |
n = 40; para1 = 1.9 |
n = 39; para1 = 1.9 |
n = 40; para1 = 1.8 |
n = 33; para1 = 1.7 |
n = 50; para1 = 2.0 |
| trans-Nonachlor | 12 (3.8–50) |
15 (6.4–47) |
7.1 (1.3–29) |
6.7 (3.6–15.5) |
4.6 (1.5–12) |
| p,p’-DDE | 113 (42–514) |
100 (33–306) |
54 (19–226) |
36 (12.1–139) |
21 (6.0–67) |
| HCB | 41 (17–147) |
49 (23–96) |
27 (13–51) |
20 (12–35) |
11 (5.3–17) |
| β-HCH | 32 (11–142) |
24 (10–71) |
9.0 (2.5–20) |
7.1 (3.0–28) |
3.3 (0.81–9.9) |
| PCB138 | 46 (18–99) |
40 (17–90) |
23 (11–57) |
15 (6.0–60) |
8.7 (3.4–20) |
| PCB153 | 68 (26–158) |
60 (24–143) |
40 (19–98) |
34 (18–108) |
16 (6.1–37) |
| PCB180 | 34 (14–106) |
35 (14–98) |
22 (6.4–60) |
16 (6.1–79) |
8.0 (2.6–17) |
| ƩPCBs2 | 297 (115–132) |
266 (114–662) |
172 (78.7–429) |
114 (53.4–273) |
65.5 (26.3–141) |
| PBDE47 | na3 | na3 | na3 | 1.7 (<1.3–21) |
1.9 (0.59–11) |
| PBDE153 | na3 | na3 | na3 | <1.3 (<1.3–3.9) |
na3 |
| PFOA | na3 | na3 | na3 | 4.8 (1.4–40)4 |
na3 |
| PFOS | na3 | na3 | na3 | 6.2 (4.2–13)4 |
na3 |
1.para = parity of mothers.
2.ƩPCB = 2×(PCB138+PCB153+PCB180).
3.na: not analysed.
4.n = 10.
Faroe Islands
Several cohorts have been initiated in the Faroe Islands since the late 1980s due to concerns associated with high exposure from pilot whale consumption. Pregnant women and children have been followed-up over multiple time points and concentrations of POPs for Cohorts 1, 3 and 5 are presented here.
Children from the first Faroe Islands birth cohort were followed up to adulthood and POPs were measured at 22 and 28 years of age in 2008–2009 and 2013-2016, respectively. Concentrations of most POPs did not appear to change between 2008 and 2016, however concentrations were much lower than when measured at 7 and 14 years of age during childhood, and lower than concentrations observed in cord blood (Table 12).
Table 12.
Time series of blood POPs concentrations from the Faroe Islands cohort 1. All participants are Faroese children born in 1986–1987. Data presented as geometric means (range), POPs in µg/kg plasma lipid, PFASs in µg/L plasma. Source [7,33].
| Year(s) | 1986–1987 | 1993–1994 | 2000–2001 | 2008–2009 | 2013–2016 |
|---|---|---|---|---|---|
| Mean age | Cord blood | 6.9 | 13.8 | 22.1 | 28 |
| Sample size | n = 1022 | n = 922 | n = 792 | n = 849 | n = 703 |
| p,p’-DDE | 270 (4.2–4487) |
na2 | 468 (25.4–8050) |
122 (5.4–3257) |
189 (6.5–4501) |
| HCB | 45.9 (3.4–1469) |
na2 | 94.3 (22.1–858) |
18.9 (3.1–164) |
16.3 (0.4–243) |
| PCB138 | 83 (0.3–1068) |
na2 | na2 | 64.6 (0.1–790) |
65.7 (1.5–798) |
| PCB153 | 130 (0.3–1127) |
na2 | na2 | 93 (8.2–1006) |
101 (4.8–1227) |
| PCB180 | 72 (0.3–889) |
na2 | na2 | 60.8 (3.4–673) |
74.4 (8.3–792) |
| ƩPCBs1 | 604 (17–5606) |
1525 (210–7040) |
708 (4.2–4941) |
443 (36–4940) |
489 (35.4–5479) |
| PFOA | na2 | 5.4 (1.3–17.3) |
na2 | na2 | 1.226 (0.108–13.2) |
| PFNA | na2 | na2 | na2 | na2 | 0.941 (0.157–4.17) |
| PFDA | na2 | na2 | na2 | na2 | 0.328 (0.034–2.61) |
| PFUnDA | na2 | na2 | na2 | na2 | 0.375 (0.015–3.10) |
| PFOS | na2 | 31.1 (7.2–96.9) |
na2 | na2 | 6.27 (0.554–28.7) |
1.Sum of PCBs = 2×(PCB138+PCB153+PCB180).
2.na: not available.
In the third birth cohort in the Faroe Islands (Table 13), concentrations of PFAS in mothers, and in children across multiple time points have been measured. Breastfeeding has shown to be an important exposure pathway for PFAS among infants, as the duration of exclusive breastfeeding was associated with increases in most PFAS measured (except PFHxS) by up to 30% per month, with lower increases observed with partial breastfeeding [34]. After breastfeeding ended, serum concentrations of PFAS declined. Serum concentrations of PFAS were similar among children at ages 5 and 7.5 years, however a large decline in levels of PFOS and PFOA was observed in children at age 13 years. PFAS including PFHxS, PFNA, and PFDA showed no clear upward or downward trend among children between the ages of 5 and 13 years.
Table 13.
Time series of blood PFAS concentrations in faroese women and their children from Faroe Islands cohort 3 (1998–2000). Data presented as geometric means (range). PFASs in serum (µg/l). Source [33].
| |
Mothers |
Children |
||
| Year(s) | 1998–2000 | 2002–2005 | 2005–2007 | 2011–2012 |
| Mean age (range) |
30 (16–43) |
5.0 (4.8–5.2) |
7.5 (7.0–7.9) |
13.2 (12.6–14.3) |
| Sample size |
n = 618 |
n = 545 |
n = 500 |
n = 526 |
| PFOA | 3.2 (0.8–8.4) |
4.1 (0.8–15.4) |
4.5 (1.7–19.2) |
2.0 (0.6–6.1) |
| PFNA | 0.6 (0.1–2.5) |
0.6 (0.02–19.5) |
0.5 (0.5–9.5) |
0.7 (0.2–2.1) |
| PFDA | 0.3 (0.03–1.2) |
0.3 (0.05–1.2) |
0.4 (0.07–2.2) |
0.3 (0.09–1.2) |
| PFHxS | 4.4 (0.6–26.5) |
0.6 (0.02–19.5) |
0.5 (0.1–8.9) |
0.4 (0.07–4.1) |
| PFOS | 27.4 (9.4–68.8) |
16.7 (3.3–48.2) |
15.3 (5.6–35.5) |
6.6 (1.0–16.6) |
In the fifth birth cohort, concentrations of PCBs, p,p’-DDE, and HCB have declined in Faroese children from 18 months of age in 2009–2011 to 9 years of age in 2016–2018 (Table 14). A decline in PFAS was also observed for children between 5 and 9 years of age, and PFOS continues to be the highest of the PFAS.
Table 14.
Time series of blood organochlorines and PFAS in faroese women and their children from Faroe Islands cohort 5 (2007–2009). Data presented as geometric means (range) in blood serum (µg/kg lipid) for organochlorines, and in whole blood (µg/L blood) for PFAS and Hg. Source [7,33].
| Mothers |
Children |
|||
| Year(s) | 2007–2009 | 2009–2011 | 2012–2014 | 2016–2018 |
| Mean age (range) |
30.7 (17.2–49.4) |
1.5 (1.4–1.7) |
5 | 9 |
| Sample size |
n = 500 |
n = 363 |
n = 347 |
n = 381 |
| p,p-DDE | 131 (6.0–1517) |
180 (15–4414) |
185.2 (13–2575) |
90.8 (1.5–1051.5) |
| HCB | 17.3 (3.0–116) |
26.5 (15–144) |
23.9 (7–85) |
11.6 (1.5–68.5) |
| PCB138 | 53.7 (3.0–383) |
80.1 (15–796) |
60.2 (2–526) |
33.3 (1.5–636.5) |
| PCB153 | 91.2 (1.0–694) |
105 (15–1214) |
84.8 (2–739) |
54.9 (1.5–1033.6) |
| PCB180 | 60.1 (3.0–496) |
61 (3.0–872) |
49.9 (2–724) |
30.1 (1.5–943.9) |
| ƩPCBsa | 420 (16–2965) |
500 (70–5760) |
397.8 (10–3470) |
249.4 (9–5228) |
| PFOA | Na | 2.9 (0.5–22.5) |
2.22 (0.682–13.34) |
1.44 (0.672–2.983) |
| PFNA | Na | na | 1.12 (0.124–5.745) |
0.65 (0.147–3.404) |
| PFDA | Na | na | 0.33 (0.015–1.715) |
0.24 (0.045–0.828) |
| PFUnDA | Na | na | 0.17 (0.015–1.773) |
0.17 (0.015–0.93) |
| PFHxS | Na | na | 0.34 (0.077–3.252) |
0.27 (0.1–1.751) |
| PFOS | Na | 6.5 (1.4–28.3) |
4.68 (1.066–16.275) |
3.27 (0.652–11.654) |
In an effort to better understand changes in exposure sources to PFAS in Faroese communities, child data from between 1993 and 2012 (Faroe Islands Cohorts 1, 3, and 5) were analysed for 19 PFAS [35]. Analysis of time trend data revealed that exposure in Faroese children peaked in 2000 at just under 50 µg/L, and that PFAS (total sum) have decreased by 14.4% per year since 2000. The majority of this decrease is attributed to a rapid decrease in levels of PFOA and PFOS, which aligns temporally with the voluntary withdrawal of PFOS from markets in 2000. In addition, principle component analysis allowed the differentiation of seafood from other possible sources of PFAS. Pilot whale consumption was an ongoing source of exposure but was not the driving factor behind the observed changes in PFAS levels. Modelling work, however, indicated that consumption of pilot whale was still a significant source of PFNA and other new generation PFAS. The marine environment may take longer to reflect changes in emissions, and the rapid decreases observed in levels of PFAS were not wholly explained by seafood consumption. Use of and exposure to PFAS from consumer products, may have a greater role in explaining the observed rapid declines in PFAS levels in the Faroese population.
Norway
As previously reported by Nøst et al., repeated measurements in older men from Tromsø across five time points between 1979 and 2007 revealed that earlier-born cohorts had higher concentrations of POPs than later-born cohorts [36]. To build on this work and to determine the relevance of age-period-cohort effects by including a younger age group and keeping age constant among the participants, a new study was designed for individual measurements in repeated cross-sectional samples of 30 year-olds in the same Tromsø population surveys as in the previous longitudinal study [37]. This design enabled evaluation of changing levels of POPs in 30-year-old adults (representing reproductive age) from population surveys (n = 45 per survey) over a 22-year period (1986, 1994, 2001, 2007) as shown in Table 15. Concentrations of a sum of 14 POPs (eight PCB congeners, HCB, p,p’-DDE, p,p’-DDT, β-HCH, oxychlordane, trans-nonachlor) decreased among 30 year-olds between 1986 and 2007, with median decreases (relative to 1986) in 1994, 2001 and 2007 of 71%, 81%, and 86% among women, respectively, and 65%, 77% and 87% among men, respectively [37]. Decreasing concentrations over time were observed for all POPs measured, with the strongest declines observed for HCB and β-HCH. Median concentrations of POPs were higher among men in 1986, 1994 and 2001, although no differences between men and women were observed in 2007, except for β-HCH which was slightly higher in women. Median concentrations of PCB153 were lower in 30 year-olds, compared to older men at the same time points (1986, 1994, 2001, 2007) that were part of the previous longitudinal study. In addition, the observed relative decreases in 30 year-olds over time were greater than the decreases in older men. The magnitude of decreases in 30 year-olds were similar to those found in an earlier study including repeated cross-sectional samples among Norwegian men between 40 and 50 years old [38] The declines in the periods studied since the 1970s in Norway are considerable and convincingly follow national and international action to regulate the production and use of these compounds [39]. By comparing the biomonitoring results using two different study designs [36,39], the authors could observe that regardless of age group, declining trends were evident for most POPs and that time trends differed between age groups and were stronger in the younger age group.
Table 15.
Concentrations of POPs in 30 year-olds from the tromsø study. Data presented as medians (range), POPs in serum (µg/kg lipids). Source [37].
| Men | Women | Men | Women | Men | Women | Men | Women | |
|---|---|---|---|---|---|---|---|---|
| Year | 1986 | 1994 | 2001 | 2007 | ||||
| Age | 30 | 30 | 30 | 30 | ||||
| Sample size |
n = 14 |
n = 31 |
n = 17 |
n = 28 |
n = 21 |
n = 24 |
n = 20 |
n = 25 |
| trans-nonachlor | 38 (11–62) |
15 (5–45) |
12 (4–66) |
7 (<LOD–15) |
12 (5–23) |
7 (<LOD–20) |
6 (<LOD–14) |
4 (<LOD–12) |
| HCB | 78 (50–152) |
61 (28–140) |
16 (9–33) |
11 (4–23) |
19 (8–46) |
16 (9–41) |
11 (5–18) |
12 (5–23) |
| p,p’-DDE | 438 (106–718) |
533 (28–2340) |
110 (57–366) |
114 (31–295) |
73 (32–1650) |
67 (19–1180) |
39 (16–79) |
46 (12–328) |
| β-HCH | 20 (14–53) |
25 (7–58) |
4 (<LOD–8) |
4 (<LOD–14) |
4 (<LOD–32) |
5 (<LOD–12) |
nc (<LOD–5) |
3 (<LOD–18) |
| PCB138 | 121 (68–200) |
101 (9–291) |
52 (30–209) |
40 (11–68) |
35 (20–102) |
21 (10–116) |
18 (8–35) |
18 (5–88) |
| PCB153 | 218 (119–338) |
165 (30–425) |
99 (61–348) |
65 (23–120) |
60 (38–171) |
43 (19–204) |
35 (19–66) |
30 (10–183) |
| PCB180 | 164 (95–226) |
94 (49–235) |
71 (46–226) |
46 (21–89) |
39 (24–98) |
25 (14–128) |
24 (13–50) |
19 (8–134) |
LOD: limit of detection.
nc:not calculated.
As part of a sub-project of the Tromsø study, the Fit Future 1 project was developed to measure serum concentrations of PFAS (Table 16) in youth and to investigate associations with dietary and lifestyle variables [37]. The most abundant PFAS measured were PFOS, followed by PFOA, PFHxS, PFNA and PFDA, and it was noted by authors that concentrations of PFAS including PFOS and PFOA were inversely associated with age [40]. Girls had statistically significantly higher PFHpA, PFOA, PFNA, PFDA and PFUnDA concentrations than boys, while boys had significantly higher ΣPFHxS and ΣPFOS concentrations than girls [37]. ΣPFOS, linear PFOS, PFNA, PFDA and PFUnDA were positively associated with reindeer consumption, while PFNA was also positively associated with “junk food” consumption. Fatty fish (salmon, trout, mackerel, herring) consumption was positively associated with the concentrations of ΣPFHxS, linear PFHxS, ΣPFHpS, PFHpA, PFOA, ΣPFOS, linear PFOS, PFNA and PFDA. Only PFUnDA concentrations were positively associated with the consumption of both fatty and lean fish (cod, haddock, saithe).
Table 16.
PFASs in in adolescents from the Tromsø Fit study, Norway. Data collected from 2010 to 2011 and presented as geometric means (range) in blood serum (µg/L serum). Source [40].
| Girls |
Boys |
|||
| Year(s) | 2010–2011 | 2010–2011 | ||
| Mean age (range) |
16.5 (15–19) |
16.3 (15–19) |
||
| Sample size |
n = 445 |
n = 495 |
||
| µg/L serum |
% <LOD1 |
µg/L serum |
% <LOD1 |
|
| PFOA | 2.14 (<0.3–13.97) |
0 | 1.86 (0.51–5.44) |
0 |
| PFNA | 0.61 (0.15–5.35) |
0 | 0.48 (0.12–1.91) |
0 |
| PFDA | 0.27 (0.05–1.89) |
0 | 0.19 (<0.03–0.801) |
0 |
| PFUnDA | 0.17 (<0.03–0.85) |
3 | 0.14 (<0.03–0.64) |
6 |
| PFHxS | 0.8 (0.19–84.72) |
0 | 0.95 (0.18–44.18) |
0 |
| PFOS | 5.71 (1.28–99.2) |
0 | 6.52 (1.33–19.44) |
0 |
1LOD: limit of detection.
In an exposure assessment of adult men and women from Oslo, Norway, between the ages of 20 and 66 and recruited in 2013–2014, concentrations of PFAS (including linear and branched) were measured in multiple different blood matrices to better assess the presence and distribution of PFAS in whole blood, plasma and serum [41]. Strong positive correlations were noted among all matrices for perfluoroalkyl sulphonates (PFSAs) and perfluoroalkyl carboxylates (PFCAs). PFHpS, PFOS, PFOA, PFHxPA and PFNA were detected in all three matrices, while PFHxA was only detected in whole blood samples (below limits of detection in serum and plasma samples) [41]. The authors suggest that whole blood is the most suitable matrix for measuring PFHxA and that studies investigating PFAS in serum or plasma may overlook the presence of this compound in humans [41]. In this sample of Norwegian men and women, the predominant PFAS measured in whole blood were in decreasing order: PFOS, PFOA, PFHxA, PFHxS, PFNA, PFDA, PFUnDA. Whole blood concentrations of PFHxS, PFHpS, and PFOS were significantly lower in women than men. In addition, concentrations of several PFAS were significantly higher in older adults compared to other age groups (<36, 36–45, >45 years old).
Between 2003 and 2009, plasma concentrations of PFAS were measured in women from the Norwegian Mother and Child Cohort study (MoBa) across two consecutive pregnancies to investigate determinants of change across pregnancies [42]. Concentrations of 10 PFAS were measured approximately 18 months apart (median time) and maternal concentrations in the second pregnancy were statistically significantly lower for all PFAS measured (Wilcoxon signed-rank test < 0.05), compared to the first pregnancy. For almost all the women, concentrations of PFOS, PFOA and PFHxS were lower in the second pregnancy, however for 30 women concentrations of PFNA, PFUnDA and PFDA actually increased in the second pregnancy. Time between pregnancies proved an important factor for concentrations of some PFAS, particularly PFNA and PFDA where there was a statistically significant 31% and 75% increase for every additional year between pregnancies. Concentrations of PFAS during the first pregnancy were an important determinant of concentrations in the second pregnancy, and the degree of correlation between the repeated PFAS measurements closely matched the half-lives of the measured compounds as described in Papadopoulou et al. [42]. Breastfeeding (negatively associated with PFAS concentration in the second pregnancy), and seafood consumption (particularly shellfish, positively associated) were also important determinants for some PFAS [42].
Sweden
In a case-control cohort within the Västerbotten Intervention Programme (VIP), plasma concentrations of PCBs, DDE, HCB, and PFAS were measured in adults in the county of Västerbotten in northern Sweden [43–46]. Adults participated in the study twice, ten years apart, with the baseline visit occurring between 1990 and 2003 and the follow-up visit between 2000 and 2013. There was a decrease in chlorinated POPs over the 10-year period with relative changes of −27% (sum of dioxin-like PCBs), −25% (sum of non-dioxin like PCBs), −41% (HCB) and −39% (DDE). PFAS were also measured for 187 of the case-control pairs [45] and concentrations of PFAS that were above the limit of quantification are presented in Table 17. PFOA and PFOS decreased by 15% (interquartile range −33% to 11%) and 29% (IQR −42% to −8%), respectively, from baseline to follow-up. For PFNA and PFHxS there was instead an increase of 53% (IQR 23% to 94%) and 13% (IQR −15% to 37%), respectively.
Table 17.
Concentrations of PFAS levels in Västerbotten intervention programme participants (Sweden) who are free of diabetes at baseline and follow-up. Data presented as median concentrations (interquartile range) in blood plasma (µg/l). Source [45].
| Baseline 1990–2003 |
Follow-up 2000–2013 |
|
| Sample size |
n = 187 |
n = 187 |
| PFOA | 2.9 (2.2–4.2) |
2.7 (1.9–3.6) |
| PFNA | 0.53 (0.42–0.74) |
0.83 (0.64–1.1) |
| PFDA | 0.23 (0.08–0.31) |
0.33 (0.25–0.45) |
| PFUnDA | 0.19 (0.08–0.28) |
0.22 (0.08–0.37) |
| PFHxS | 1.0 (0.74–1.4) |
1.2 (0.82–1.5) |
| PFOS | 20 (15–26) |
15 (9.7–21) |
The cross-sectional national dietary survey Riksmaten Adolescents 2016–2017 was undertaken by the Swedish Food Agency during the 2016–2017 school year [47] and included students in grades 5, 8 and 11 (mean ages 12, 15 and 18 years). Concentrations of POPs among the sum of adolescents are presented in Table 18, however it was noted that concentrations of several POPs including HCB, p,p’-DDE, PCB138, PCB153, PCB180, PFNA, PFHxS and PFOS were significantly higher in boys than girls (as shown in the Swedish Food Agency survey report), which suggests possible sex-related differences in dietary habit or elimination [48]. Consumption of fish for example, an important contributor to PCB and PFAS exposure, was higher in male than female participants [49]. Further breakdown of study data by age/grade are presented in greater detail in the original survey report, however key findings included that the concentrations of PCBs and chlorinated pesticides tended to increase with increasing age/grade, significantly for PCB153, PCB170, PCB180 and p,p’-DDE [45]. In contrast, the concentrations of PFOA, PFHxS and PFOS tended to be higher in individuals in grade 5, compared to those adolescents in grades 8 and 11. An age-associated decrease in the levels of PFAS might be explained by differences in dietary habit between age groups or by growth dilution from grade 5 to grade 11 [48]. The brominated flame-retardants PBDE-47, −99 and −153 were below the quantification limit in almost all individuals [48].
Table 18.
Blood serum concentrations of POPs in Swedish adolescents participating in the national representative study riksmaten adolescents 2016–2017. POPs data presented as arithmetic means (5th−95th percentile); chlorinated and brominated POPs presented per volume in blood serum (ng/L wet weight); PFASs data presented in blood serum (µg/kg wet weight). Source [48].
| Year(s) | 2016–2017 | |
| Mean age | 14.7 (10-21) | |
| Sample size |
n = 1096 |
|
| ng/L |
% <LOQ1 |
|
| HCB | 52 (22–75) |
0.0 |
| trans-Nonachlor | 52 (<LOQ1–10) |
76.9 |
| p,p’-DDE | 191 (<LOQ1–575) |
7.1 |
| PCB138 | 32 (6–76) |
0.8 |
| PCB153 | 53 (13–129) |
0.1 |
| PCB180 | 32 (5–87) |
3.8 |
| PBDE47 | <LOQ1 | 98.0 |
| PBDE153 | <LOQ1 | 99.5 |
| µg/kg |
% <LOQ1 |
|
| PFOA | 1.4 (0.6–2.5) |
0.2 |
| PFNA3 | 0.4 (<LOQ1–0.9) |
7.2 |
| PFDA3 | 0.2 (<LOQ–0.4) |
37.5 |
| PFHxS3 | 1.8 (0.2–2.7) |
7.8 |
| PFOS3 | 4.6 (1.2–9.7) |
0.0 |
1.LOQ: limit of quantification; concentrations below the LOQ were replaced by LOQ/√2 for calculating means.
2.Interpret data with caution due to the high % of samples below the LOQ.
3.n = 1098.
Levels of p,p’-DDE, PCB153, PFOS and PFHxS per region and adjusted for grade and sex are shown in Figure 2. The different letters in the figures indicate significant differences between regions (p < 0.05) according to Tukey’s multiple comparison test. Umeå is the region that represents the northern part of Sweden and had significantly lower levels of p,p’-DDE, PFOS and PFHxS than some of the other regions.
Figure 2.
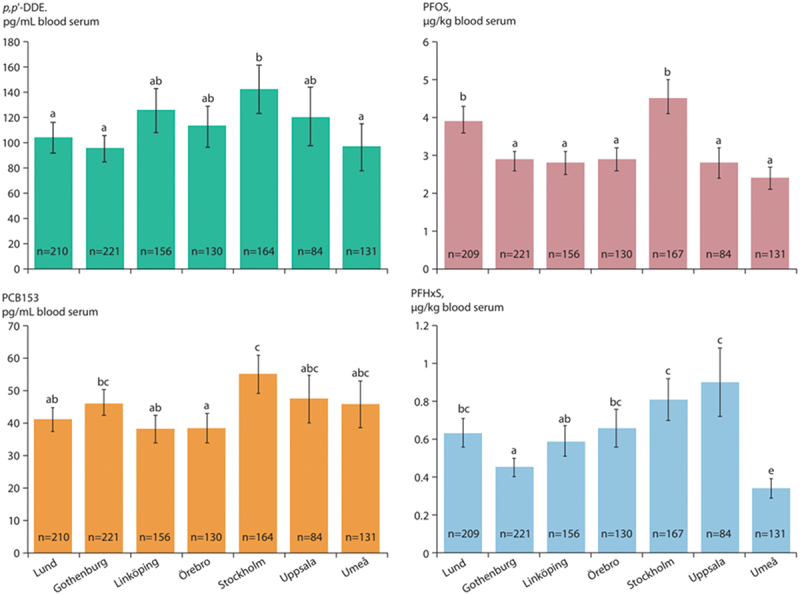
Concentrations of p,p’-dde, PCB153, PFOS and PFHxS in blood serum in Swedish adolescents per region adjusted for grade and gender, and interactions between all factors (back-transformed least squares means with 95% confidence intervals from the analysis of log values). Different letters indicate significant differences between regions (p < 0.05) according to Tukey’s multiple comparison test. The number of observations per region is shown at the base of each bar. Source [6].
A time series of POPs concentrations in breastmilk samples from 1996 to 2016 in Swedish first-time mothers in Uppsala (Figure 3) shows a decline for many POPs, such as PCBs, p,p’-DDE and HCB. Trends for PBDEs are also downward, with PBDE153 showing a slower decline than PBDE47 after levels peaked around 2006. Trends for PFAS in serum samples are less consistent (Figure 4), with PFOS and PFOA showing a declining trend and levels of PFNA, PFDA and PFUnDA increasing.
Figure 3.
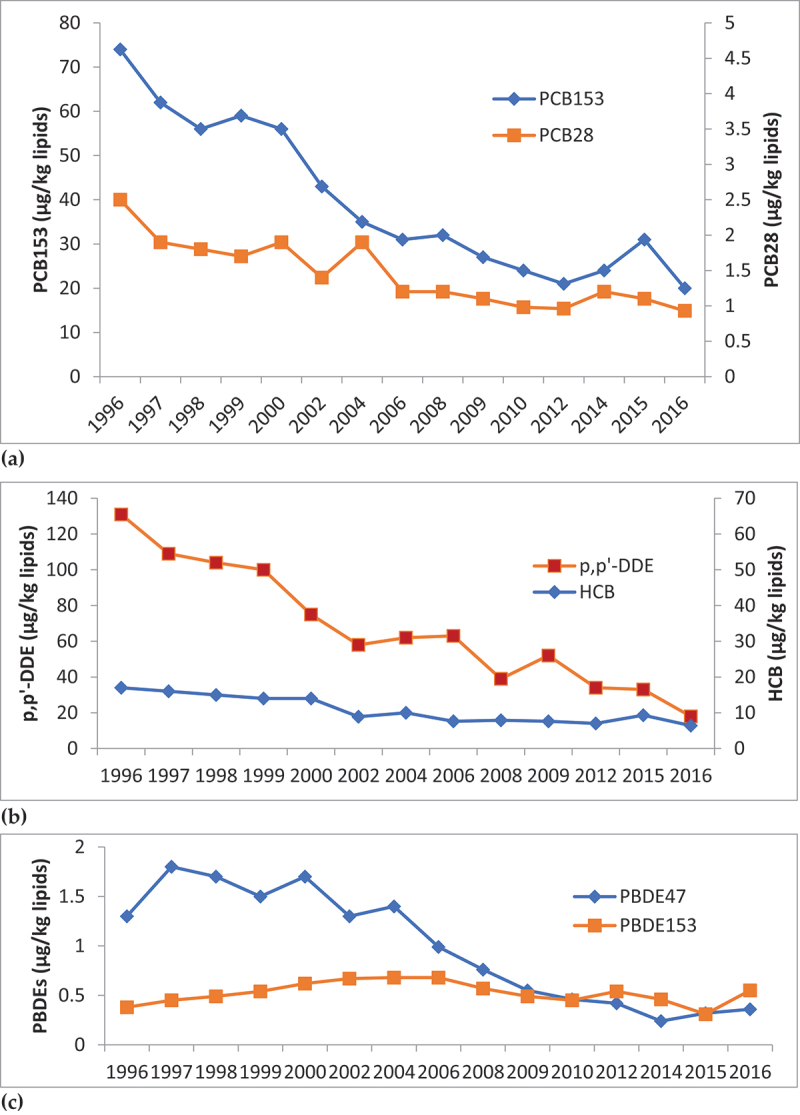
Trends in (a) PCBs, (b) p,p’-DDE & HCB, and (c) PBDEs concentrations in breast milk samples from Swedish first-time mothers (µg/kg lipid weight). Samples collected 3 weeks after delivery. Data presented as median concentrations. Source [50].
Figure 4.
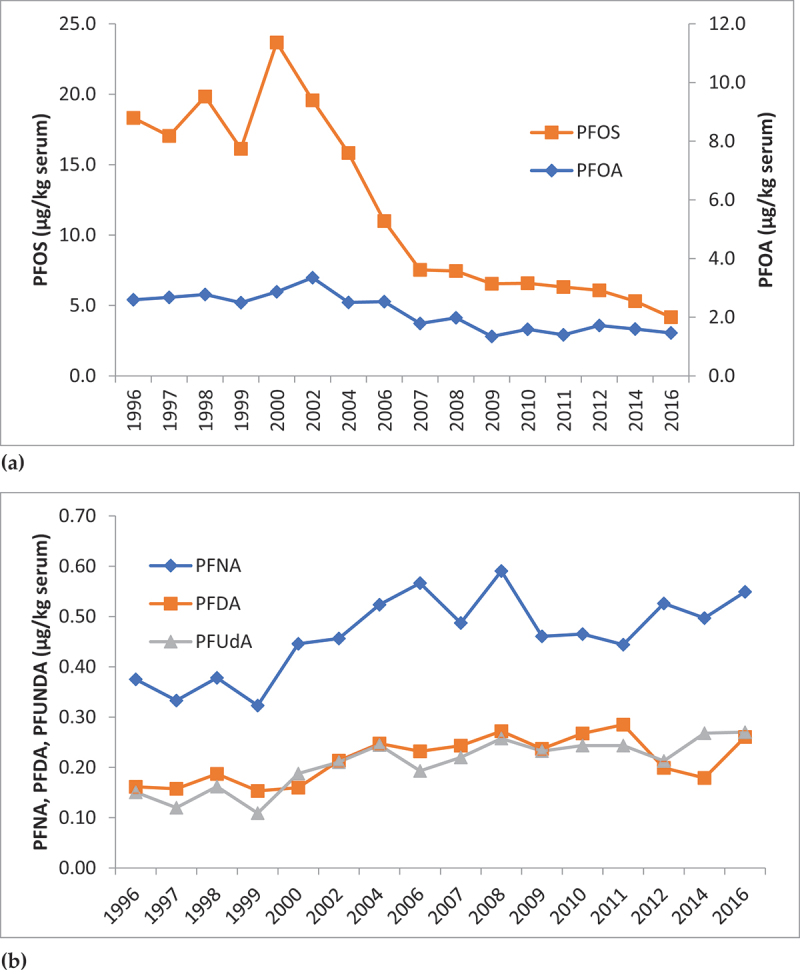
Trends in PFAS concentrations in serum samples from Swedish first-time mothers (µg/kg serum); (a) PFOS and PFOA, and (b) PFNA, PFDA, PFUdA. Samples collected 3 weeks after delivery. Data presented as median concentrations. Source [51].
Finland
In eastern Finland, a group of 54 children born in 2004–2005 to mothers recruited in the birth cohort study (LUKAS2), were followed-up at ages 1, 6, and 10.5 years between 2005/2006 and 2014/2015. Between 2005 and 2015, serum concentrations of PFAS decreased significantly (except PFHxS which declined but was not statistically significant, p = 0.16) as presented in Table 19 [52]. Comparing time trends between boys and girls, no differences were observed except for PFNA (where statistical declines were not observed in boys between 6 and 10.5 years of age). Concentrations between boys and girls were not significantly different with the exception of PFOA which was found to be significantly higher in boys at 10.5 years of age (p < 0.01). The predominant PFAS measured were PFOA followed closely by PFOS, which together accounted for 73–80% of median concentrations. Significant increases in estimated body burdens of PFNA were observed between children at 1 and 6 years of age and between 1 and 10.5 years of age. Significant increases in PFHxS were also seen between children at 1 and 6 years of age, however significant increases between 1 and 10.5 years of age was only observed for boys [52].
Table 19.
Median concentrations of Finnish PFASs in children, boys and girls, at 1, 6, and 10.5 years of age. Data presented as medians (range) concentrations presented in µg/L serum. Source [52].
| All |
Boys |
Girls |
All |
Boys |
Girls |
All |
Boys |
Girls |
|
| 2005/2006 | 2010/2011 | 2014/2015 | |||||||
| Mean Age (range) |
1 (0.97–1.06) |
6 (5.71–6.32) |
10.5 (9.90–10.95) |
||||||
| Sample size |
n = 54 |
n = 26 |
n = 28 |
n = 54 |
n = 26 |
n = 28 |
n = 54 |
n = 26 |
n = 28 |
| PFOA | 6.6 | 5.6 (1.4–15) |
7.1 (2.0–13) |
2.7 | 2.8 (1.4–5.7) |
2.7 (1.9–3.4) |
1.5 | 1.6 (0.80–2.5) |
1.4 (0.96–2.1) |
| PFNA | 0.8 | 0.69 (0.22–2.1) |
0.84 (0.29–1.8) |
0.54 | 0.5 (0.25–1.2) |
0.56 (<LOQ–0.90) |
0.36 | 0.37 (0.23–1.2) |
0.35 (<LOQ–0.67) |
| PFHxS | 0.47 | 0.42 (<LOQ–1.5) |
0.49 (<LOQ–1.2) |
0.42 | 0.4 (<LOQ–0.94) |
0.43 (<LOQ–0.84) |
0.21 | 0.23 (<LOQ–0.56) |
0.2 (<LOQ–0.37) |
| PFOS | 5.5 | 4.6 (2.0–40) |
6.3 (1.7–16) |
2.1 | 2.1 (0.98–4.5) |
2.3 (1.3–3.3) |
1.5 | 1.5 (0.63–3.4) |
1.6 (0.62–3.4) |
LOQ: limit of quantification.
Russia
Biomonitoring conducted among residents in the Pechenga district of Murmansk Oblast (under the KolArctic project) provided information on a broad suite of POPs in adult men and women, and pregnant women [53] as presented in Table 20. Residents were recruited between 2013 and 2014 and came from settlements located near a copper-nickel ore smelter (in Nickel) and concentrating plants and roasting shop (in Zapolyarny) and are therefore not reflective of contaminant exposures among Indigenous people (Sami) living in the Lovozersky district of Murmansk Oblast.
Table 20.
POPs in the population of the Pechenga district of Murmansk oblast, Russia. Data presented as geometric means (range) in blood serum (µg/kg lipids). Source [53].
| Men |
Women |
Pregnant women |
||||
| |
2013 |
2013 |
2013–2014 |
|||
| Mean age (range) |
39.9 (27–54) |
45.2 (26–65) |
29.2 (20–42) |
|||
| Sample size | n = 18 | n = 32 | n = 50 | |||
| µg/kg lipids |
% <LOD1 |
µg/kg lipids |
% <LOD1 |
µg/kg lipids |
% <LOD1 |
|
| HCB | 42.4 (10.9–189) |
6 | 32.3 (12.8–74.4) |
0 | 18.2 (5.3–251.6) |
0 |
| β-HCH | 49.1 (27.9–111.9) |
22 | 54.5 (17.6–157.1) |
9 | 8.5 (0.8–145.8) |
0 |
| 4,4’ DDE | 169.8 (51.6–940) |
0 | 139.3 (39.4–537.7) |
0 | 101.9 (16–1220.8) |
0 |
| 4,4’ DDT | 41.8 (11.6–131.9) |
6 | 17.7 (6.5–123.9) |
19 | 11.4 (1.3–376.4) |
0 |
| PCB118 | 48.3 (9.9–133.9) |
11 | 34.5 (12.1–94.2) |
0 | 26.1 (9.4–119.3) |
0 |
| PCB138 | 45.5 (14.8–106.4) |
11 | 27.2 (6.4–74.9) |
6 | 9.2 (1–48.2) |
0 |
| PCB153 | 48.5 (21.7–141.3) |
11 | 26.8 (8.5–61) |
3 | 12.2 (1.3–56.7) |
0 |
| PCB180 | 27.4 (14.5–106.2) |
33 | 17.3 (8.4–56.5) |
44 | 15.7 (5–46.8) |
702 |
| ƩPCBs3 | 242.8 (102–707.8) |
33 | 142.6 (46.6–384.8) |
44 | 74.2 (14.6–303.4) |
702 |
| ƩPCBs154 | 284.4 (125–753) |
0 | 200.2 (69.7–456) |
0 | 105.5 (40.7–368.8) |
0 |
1.1LOD: limit of detection.
2.Interpret data with caution due to the high % of samples below the LOD.
3.ƩPCBs = 2×(PCB138+PCB153+PCB180).
4.ƩPCBs15 = sum of fifteen PCB congeners: PCB28, PCB31, PCB52, PCB99, PCB101, PCB105, PCB118, PCB128, PCB138, PCB153, PCB156, PCB170, PCB180, PCB183, PCB187.
Many of the POPs measured were below the limit of detection for a majority of the samples, however the most predominant organochlorine compounds were 4,4’-DDE, HCB and PCBs 118, 138 and 153. Differences were noted between men and women, with concentrations of POPs generally higher in men than women (except for β-HCH). Concentrations of POPs were lowest in pregnant women, although it should be noted that the pregnant women sampled were younger (average age 29.2 years) than non-pregnant women (average age 45.2 years). Sources of exposure to POPs from local foods were also investigated [54]. While β-HCH was highly detected in residents, HCH was not detected in local foods which would suggest that these exposures may be due to different exposure source. While not investigated in this study, additional potential sources of exposure including household sources of POPs have been studied in other regions of the Russian Arctic, as described below.
In the Chukotka Autonomous Okrug (far northeast of the Russian Federation), previous studies have identified high levels of organochlorine compounds in women from coastal eastern Chukotka [55,56]. Further investigation of serum levels of POPs in pregnant women living in the coastal and inland areas of Chukotka, was conducted in 2014–2015 (Table 21) and statistically significant regional differences were observed for concentrations of several POPs [57]. Coastal communities generally had higher levels of POPs than inland communities, and pregnant women from coastal eastern Chukotka communities had the highest concentrations of POPs, with the strongest differences observed for PCBs (3.3- to 4.2-fold higher than the geometric mean for the whole study population). This is thought to reflect dietary factors, as mothers from these coastal communities have a diet that includes whale, walrus, and seal blubber. Linear multivariate analysis showed residence to be an important factor in explaining POPs exposure, particularly for PCBs, β-HCH and mirex.
Table 21.
POPs in pregnant women from the Chukotka Autonomous Okrug (both inland and coastal settlements), Russia. Data presented as geometric means (range) in blood serum (µg/kg lipids). Source [57].
| Year(s) |
2014–2015 |
|
| Mean age (range) |
27.8 (15–44) |
|
| Sample size |
n = 246 |
|
| µg/kg lipids | %<LOD | |
| HCB | 35 (<LOD–850) |
1 |
| β-HCH | 35 (<LOD–660) |
3 |
| 4,4’ DDE | 120 (<LOD–1100) |
1 |
| PCB118 | 10 (<LOD–1100) |
2 |
| PCB138 | 17 (1.3–440) |
0 |
| PCB153 | 31 (3.9–880) |
0 |
| PCB180 | 5.4 (<LOD–230) |
22 |
LOD: limit of detection.
When comparing concentrations of POPs in pregnant women from the Pechenga district of Murmansk Oblast with the Chukotka Okrug, concentrations appear higher in Chukotka for many POPs including HCB (18 vs 35 µg/kg lipids, respectively), β-HCH (8.5 vs 35 µg/kg lipids), DDE (102 vs 120 µg/kg lipids), and PCB153 (12.2 vs 31 µg/kg lipids). These results support previous studies that found high levels of POPs in Chukotka [55,56].
Studies of POPs in local food sources found low levels among fish, land and marine mammal meat, relative to marine mammal blubber [58]. Estimated daily intakes of POPs show that over 90% of dietary exposure to POPs among local people due to the consumption of marine mammal blubber. Several marine mammal fatty tissues were studied, and grey whale blubber and mantak (a Yupik name for the layer of whale skin with a thin layer of adjacent blubber) had the highest concentrations of HCHs, chlordanes, HCB, and the second highest levels of DDTs and PCBs (seal blubber had higher concentrations of DDT and PCB). Compared to previous study of local foods 15 years earlier, levels of most POPs appear to be decreasing, with the exception of HCB which is rising in marine mammals.
This study also found additional sources of in-home food contamination, as relatively high levels of HCHs, DDTs and PCBs were found in home-brewed alcohol (produced in plastic barrels), although these levels were lower than had been observed 15 years previously. The impact of this potential exposure source and its relative proportion of the total POPs intake is unclear and needs further study [58].
Discussion
POPs continue to be detected in populations across the circumpolar Arctic, although concentrations vary widely by region and many POPs are decreasing over time. These spatial and temporal trends are evident in Figure 5, which show mean concentrations of some predominant organochlorine compounds and PBDE, among women of childbearing age and pregnant women from across the circumpolar Arctic over multiple time periods. While not illustrated in Figure 5, multiple cohort studies in the Faroe Islands also show levels of POPs are decreasing, probably due to decreasing consumption of pilot whale meat, as the concentrations of POPs in participants in the Faroe Islands Cohort 5 (2007–09) are much lower than those observed in the first Faroe Island cohort (1986–87). As maternal levels of POPs decrease, so too does maternal transfer of POPs to infants.
Figure 5.
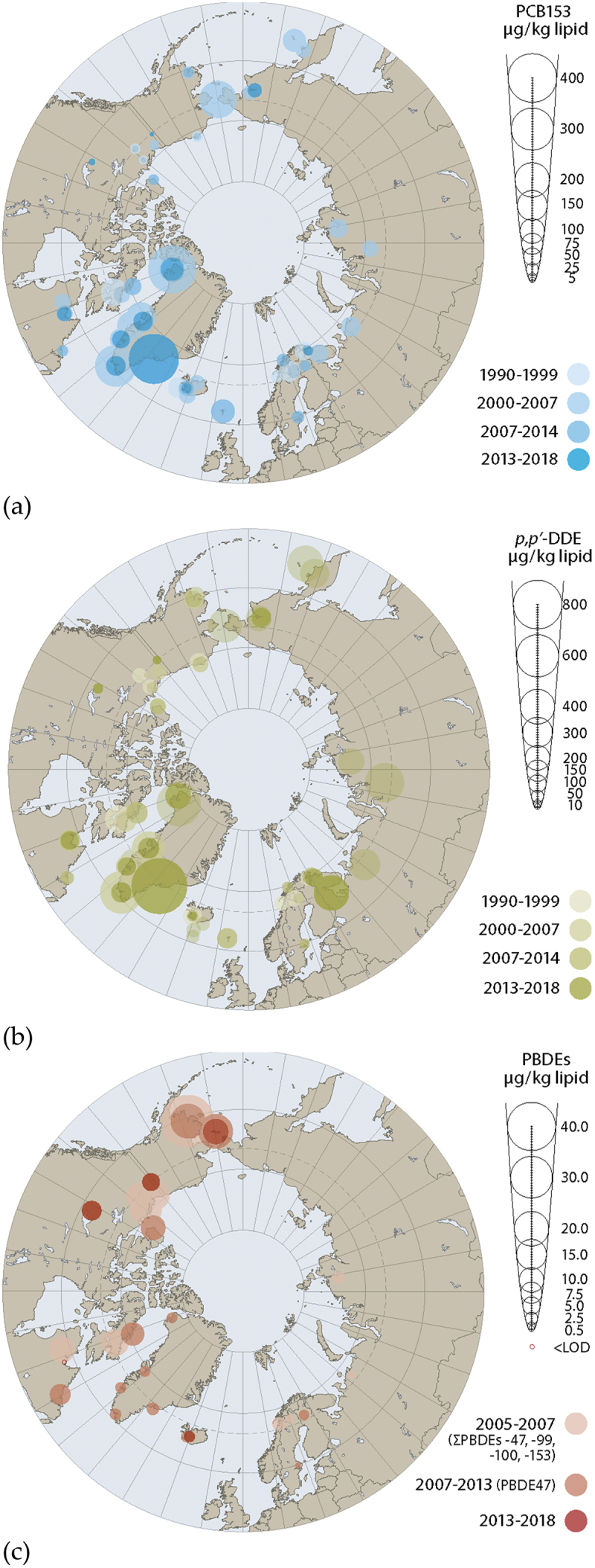
Circumpolar concentrations of (a) PCB153, (b) p,p’-DDE, and (c) PBDEs, presented in μg/kg lipid. Data from women of childbearing age (Yukon, DehCho/Sahtu region, inuvialuit settlement region, Nunavut and nunatsiavut [Canada]), maternal blood (Alaska, Faroe Islands, Sweden, and coastal Chukotka [Russia]), blood of pregnant women (Nunavik [Canada], Greenland, Iceland, Norway, Pechenga district of murmansk oblast, and Chukotka [Russia]), and from breast milk (Sweden, Finland). Source [6].
Although time trends vary by contaminant and region concentrations of POPs such as PCBs and p,p’-DDE have generally continued to decline in many of the Arctic regions. However, it should be noted that only a subset of studies described herein have sufficient time series data available. In Nunavik, extensive time trend series for pregnant women (starting in the early 1990s) show continued declines of many POPs, while the limited time points available for PBDEs and PFOS show a decline since 2004, although this is less pronounced between 2012 and 2016/2017, and an increasing trend for PFNA since 2012 (as seen in Table 8). In Greenlandic pregnant women, the most recent data from the ACCEPT study show that levels of POPs such as PCB153 and p,p’-DDE have continued to decline across Greenland, with the exception of eastern Greenland. Time trends of POPs in Disko Bay extend back to the mid-1990s, and late-1990s for Nuuk. Levels of POPs declined substantially over this period in both areas, as shown in Figure 1. Levels of PFOS in Greenlandic women have also been recorded across several studies since the late 1990s and appear to have declined between 1997 and 2015 [25,59,60]. It should be noted however that the age of participants from these time points varies widely with the most recent time points representing much younger populations (median age of 53 years in 1997 and 27 years in 2015). Levels of POPs in Iceland and Scandinavian countries continue to decline based on available time points. In the Faroe Islands, time series data show POPs levels are continuing to decline among children, including PFAS in Cohort 3 children (between 2002 and 2012) and organochlorine compounds in Cohort 5 children (between 2009 and 2018). The exception is children from Cohort 1, who are now 28 years old. Previous levels reported in this cohort showed declines in organochlorine compounds concentrations between children at 7, 14 and 22 years of age, however the most recent levels among cohort participants (age 28 years) appear to have plateaued and even slightly increased for some contaminants (p,p’-DDE, PCBs). Levels of PFOS and PFOA appear to have decreased between 1993–1994 and 2013–2016.
In addition to temporal trends, several spatial trends are evident. The highest levels of many POPs continue to be in Greenland (particularly eastern Greenland), followed by the Faroe Islands, Nunavik, and Pechenga district of Murmansk Oblast (Russia), while the coastal eastern Chukotsky district of the Chukotka Autonomous Okrug had some of the highest levels of several POPs including HCB and β-HCH. The lowest levels were observed in Iceland and the Dehcho and Sahtú regions of the NWT in Canada. In the Canadian Arctic, levels of POPs range widely among pregnant women and women of childbearing age from different regions. Women of childbearing age in First Nations communities from the NWT appear to have the lowest concentrations in the Canadian Arctic, and when compared to 2007–2008 Inuit Health Survey data for the Inuvialuit Settlement region, Nunavut and Nunatsiavut [7], levels were roughly 4- to 5-fold lower than levels in the Nunatsiavut region. A different trend has been observed for PBDEs and older PFAS. The highest levels of PBDEs are found in Alaska (Sivuqaq and the Kuskokwim region) and are roughly an order of magnitude higher than levels in some Arctic European countries, while they are almost no longer detected in Nunavik. Similar to many organochlorine compounds, levels of PFOS were highest in Greenland, particularly northern and eastern Greenland, while PFNA were the highest in Nunavik followed by Alaska. Conversely, levels of PFOA were fairly similar, across the Arctic. In Alaska, levels of PFAS among Yupik women from Sivuqaq appear similar to levels in mothers from the Kuskokwim region of Alaska.
In addition to differences in concentrations of contaminants between regions, there were also sex based differences observed in several regions across Arctic regions where data was available for comparison. In Alaska, concentrations of PFAS were generally higher in men compared to women on St. Lawrence Island [9]. In the Yukon, Canada, while many POPs were statistically similar between men and women (exceptions included trans-nonachlor, HCB and toxaphene Parlar 50, which were higher in men), concentrations of PFAS were generally higher in men, with men having higher concentrations of PFHxS, PFOS and PFOA [10–12]. In the Northwest Territories, Canada, concentrations of POPs were generally similar between men and women although men had higher levels of PFHxS, PFOS and PFOA [11,12]. In Nunavik, Canada, concentrations of many POPs were similar between men and women (except for HCB, toxaphenes, mirex, PFOA and PFHxS), although when comparing between different age groups, more differences were observed as POPs were generally higher among younger men compared to younger women, and higher among older women compared to older men, but these differences were not always statistically significant [15]. Among Greenlandic women and their male partners (INUENDO study) concentrations of PFAS were generally higher in men (Table S2). In Tromso, Norway, median concentrations of most POPs were generally higher in adult men compared to women in 1986, 1994, and 2001, however no differences were observed in 2007 except for β -HCH (higher in women) [37]. Among adolescents in Tromso, Norway, differences varied by contaminant, with girls having higher concentrations of PFHpA, PFOA, PFNA, PFDA and PFUnDA, while boys had significantly higher concentrations of PFHxS and PFOS [40]. Among men and women from Oslo, Norway, concentrations of several PFAS including PFHxS, PFHpS and PFOS were higher in men [41]. Among Swedish adolescents, levels many POPs were significantly higher in boys compared to girls [48]. Concentrations of PFAS among Finnish boys and girls were generally not significantly different [52]. In Russia, among men and women from the Pechenga district of Murmansk Oblast, concentrations of POPs were generally higher in men with the exception of β-HCH [53]. As summarised here, in many regions of the Arctic there are sex-based differences in concentrations of POPs, and while men generally have higher concentrations, this is not consistent across all regions and for all POPs (with women having higher concentrations of some contaminants in certain regions). Further researcher is needed to understand these observed differences and potential sex-based differences in dietary exposure.
A growing number of studies suggest country foods, especially seafood and marine mammals are the key sources of PFOS and long-chain PFAS exposure [9,17,35]. Compared to past AMAP human health assessments, there are now substantial data available for numerous PFAS, not just PFOS and PFOA. Several of these substances have recently been added to the Annexes to the Stockholm Convention that list prohibited or restricted POPs (e.g. PFOA and its salts) or are under review for their inclusion in the Annexes (e.g. long-chain PFAS C9 to C21). The human levels reported here represent some of the first measurements of these substances in the Arctic, and so will establish a baseline for future measurements across the Arctic. Data from several Arctic cohorts and cross-sectional studies have measured PFAS among the adult population (men and women, including pregnant women) as well as children and youth, and comparisons between these populations in Figures 6 and 7 respectively, are shown for the following PFAS: PFHxS, PFOS, PFOA, PFNA, and PFDA. In most Arctic regions the most predominant PFAS were PFOS followed by PFOA, with a few exceptions including adult men and women from Alaska, the Canadian Arctic (including the Yukon, the NWT, and Nunavik), and pregnant women from Nunavik and Greenland where levels of PFNA were higher than PFOA (in Nunavik especially), although not as high as PFOS. The highest levels of PFOS were observed in pregnant women from Greenland (Figure 6).
Figure 6.
Blood concentrations (plasma and serum) of PFAS across Arctic countries. Data presented as geometric/arithmetic* means in adults and pregnant women. Source [9–11,16,25,33,41].
Figure 7.

Blood concentrations (plasma, serum and whole blood) of PFAS across Arctic countries. Data presented as geometric/arithmetic means* or medians** in children and youth. Source [11,16,30,33,40,48,52,].
Similar to adults, the most predominant PFAS in children were PFOS and PFOA, with a few exceptions including: adolescents in Nunavik (ages 16–19 years) where levels of PFNA were substantially higher than PFOA and PFOS; adolescents in Sweden with higher levels of PFHxS than PFOA which were also the highest levels of PFHxS seen in children/adolescents across the Arctic; and children in Finland who had similar or higher levels of PFOA than PFOS (Figure 7). In general, the highest levels of PFNA in children are found in Nunavik and Greenland. It is interesting to note the changes over time in PFAS levels measured in several of the Faroese cohorts at different time points. For example, levels of PFOS and PFOA were much higher in 13-year old Faroese children from 2011–2012 than in 9-year old Faroese children in 2016–2018. In contrast to PFOS and PFOA, levels of other PFAS such as PFNA and PFDA did not substantially change.
Despite being banned in many countries worldwide, levels of POPs are still elevated in some Arctic human populations, such as in Greenland, the Faroe Islands, Nunavik (Canada), the Pechenga district of Murmansk Oblast (Russia), and the Chukotka Autonomous Okrug (Russia).
While there are still some regions of the Arctic with limited biomonitoring data, levels of most POPs are declining in Arctic regions where time trend data exist, although these declines are neither uniform nor consistent across all regions. Large declines are observed when comparing current levels with levels first measured in the 1990s; however, the declines observed in recent years have been much smaller for many contaminants in some regions.
While biomonitoring and exposure information for PBDEs and PFAS may be more limited than for other POPs and metals, new Arctic data have established baseline levels, and in some regions limited temporal trend data. PBDEs have been measured in some Arctic regions, although levels appear very low in most regions with some congeners barely above detection limits (with the exception of Alaska). Baseline levels of PFAS are now available for several Arctic regions (Alaska, Yukon, Nunavik, Greenland) and time trend data for several other regions (Nunavik, Faroe Islands, Sweden, Finland). Temporal patterns of PFAS are not consistent and vary by region, however available trend data suggest that levels of PFOS and PFOA are declining in Arctic populations, including in Nunavik, Greenland, the Faroe Islands, Sweden and Finland, while levels of long-chain PFAS are rising in Nunavik, Greenland, and Sweden.
There is a need for biomonitoring data to support chemicals management under the Stockholm Convention and subsequent effectiveness evaluations. This is particularly true for long-chain PFAS which are under review for inclusion in the Stockholm Convention, as data are limited for PFAS with a chain length of C12 to C21. Future biomonitoring is needed to establish baseline levels of POPs of emerging Arctic concern, monitoring contaminants in identified vulnerable populations (e.g. children, women of childbearing age, pregnant women), and to establish and monitor temporal changes of levels of these contaminants.
While there have been several regional studies that have generated valuable information on contaminant levels in children and adults, there is still a need for maternal blood biomonitoring studies. Many of the earliest Arctic biomonitoring studies prioritised the sampling of pregnant women and this has provided AMAP with valuable time trend information. It is important to continue this work, either through maternal blood monitoring programs or with new birth cohorts to follow existing trends and improve our understanding of the impact of Arctic contaminants on human health. Continued monitoring for establishing new temporal trend data will become ever more important as chemicals are phased-out and replacement chemicals are developed. Indeed, strong efforts will be needed to identify contaminants of emerging concern in biomonitoring studies, as the numbers of new chemicals in commerce is expected to double by 2030 [61]. Predictive models have attempted to identify persistent contaminants of potential concern in the Arctic and several of these chemical substances should be considered for human biomonitoring, some of which have already been detected in Arctic biota [62]. Biomonitoring among Arctic populations coupled with dietary intake data and wildlife monitoring are essential to inform Arctic health authorities and support the health and wellbeing of Arctic populations.
Supplementary Material
Acknowledgments
This article is based on Chapter 3 “Human biomonitoring and exposure” of the AMAP Assessment 2021: Human Health in the Arctic, which was completed prior to February 2022.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Author contributions
Conceptualization, B. A., E.B-J., A.D., K.O.; writing – original draft preparation, B. A., E.B-J., A.D., K.O., K.A., M.A., S.B., É.C-B., M.D., P.D., J.G-B., B.L., M.L., K.N., S.p-M., M.P., M.R., A.R., A.T., G.T., P.W., T.N., M.W.; writing – review and editing, P.A., S.L., M.L., A.A. All authors have read and agreed to the published version of the manuscript.
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/22423982.2024.2392405
References
- [1].Donaldson SG, Van Oostdam J, Tikhonov C, et al. Environmental contaminants and human health in the Canadian Arctic. Sci Total Environ. 2010;408(22):5165–5234.2. doi: 10.1016/j.scitotenv.2010.04.059 [DOI] [PubMed] [Google Scholar]
- [2].Vaktskjold A, Deutch B, Skinner K, et al. Food, diet, nutrition and contaminants. In: AMAP assessment 2009: human health in the Arctic. Oslo, Norway; 2009. p. 21–27. Arctic Monitoring and Assessment Programme (AMAP). [Google Scholar]
- [3].AMAP . AMAP assessment 2015: temporal trends in persistent organic pollutants in the Arctic. In: Arctic monitoring and assessment programme (AMAP). Oslo, Norway; 2016. p. vi+71. [Google Scholar]
- [4].Adlard B, Bonefeld-Jorgensen EC, Dudarev AA, et al. Levels and trends of metals in human populations living in the Arctic. Int J Circumpolar Health. 2024;83:2386140. doi: 10.1080/22423982.2024.2386140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Olafsdottir K, Sandandger T, Weber J-P.. Analytical quality assurance and quality control. In: AMAP assessment 2009: human health in the Arctic. Oslo, Norway; 2009. p. 49–60. Arctic Monitoring and Assessment Programme (AMAP). [Google Scholar]
- [6].Adlard B, Bonefeld-Jørgensen EC, Dudarev AA, et al. Human biomonitoring and exposure. In: AMAP assessment 2021: human health in the Arctic. Norway: Arctic Monitoring and Assessment Programme (AMAP). Tromsø; 2021. p. 47–114. [Google Scholar]
- [7].AMAP . AMAP assessment 2015: human health in the Arctic. In: Arctic monitoring and assessment programme (AMAP). Oslo, Norway; 2015. p. vii + 165. [Google Scholar]
- [8].Byrne S, Miller P, Waghiyi V, et al. Persistent organochlorine pesticide exposure related to a formerly used defense site on st. Lawrence Island, Alaska: data from sentinel fish and human sera. J Toxicol Environ Health A. 2015;78(15):976–992. doi: 10.1080/15287394.2015.1037412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Byrne S, Seguinot-Medina S, Miller P, et al. Exposure to polybrominated diphenyl ethers and perfluoroalkyl substances in a remote population of Alaska Natives. Environ Pollut. 2017;231:387–395. doi: 10.1016/j.envpol.2017.08.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Drysdale M, Ratelle M, Skinner K, et al. Human biomonitoring results of contaminant and nutrient biomarkers in Old Crow, Yukon, Canada. Sci Total Environ. 2021;760:143339. doi: 10.1016/j.scitotenv.2020.143339 [DOI] [PubMed] [Google Scholar]
- [11].Garcia-Barrios J, Drysdale M, Ratelle M, et al. Biomarkers of poly- and per-fluoroalkyl substances (PFAS) in sub-Arctic and Arctic communities in Canada. Int J Hyg Environ Health. 2021;235:113754. doi: 10.1016/j.ijheh.2021.113754 [DOI] [PubMed] [Google Scholar]
- [12].Drysdale S, Garcia-Barrios J, Ratelle M, et al. Personal communication. In: School of public health and health systems, faculty of applied health. Waterloo, Canada: University of Waterloo; 2020. [Google Scholar]
- [13].Laird B, Ratelle M. Personal communication. In: School of public health and health systems, faculty of applied health. Waterloo, Canada: University of Waterloo; 2019. [Google Scholar]
- [14].Dewailly É, Dallaire R, Pereg D, et al. Qanuippitaa? How are we? Exposure to environmental contaminants in Nunavik: persistent organic pollutants and new contaminants of concern. Nunavik Reg Board Health Soc Serv, Institut National de santé Publique. 2007. https://www.inspq.qc.ca/sites/default/files/publications/711_esi_exposure_env_cont.pdf [Google Scholar]
- [15].Aker A, Lemire M, Ayotte P. Environmental contaminants: persistent organic pollutants and contaminants of emerging arctic concern. Nunavik inuit health survey 2017 Qanuilirpitaa? How are we now? Quebec: Nunavik Regional Board of Health and Social Services (NRBHSS) & Institut national de santé publique du Québec (INSPQ). 2021. https://nrbhss.ca/sites/default/files/health_surveys/Environmental_Contaminants_POPs_fullreport_en.pdf [Google Scholar]
- [16].Lemire M, Blanchette C. Personal Communication. Axe Santé des populations et pratiques optimales en santé. In: Centre de Recherche du CHU de Québec -. Université Laval, Québec, Canada; 2020. [Google Scholar]
- [17].Caron-Beaudoin É, Ayotte P, Blanchette C, et al. Perfluoroalkyl substances in pregnant women from Nunavik, Quebec: trends in exposure and associations with country foods consumption. Environ Int. 2020;145:106169. doi: 10.1016/j.envint.2020.106169 [DOI] [PubMed] [Google Scholar]
- [18].Xie S, Lu Y, Wang T, et al. Estimation of PFOS emission from domestic sources in the eastern coastal region of China. Environ Int. 2013;59:336–343. doi: 10.1016/j.envint.2013.06.015 [DOI] [PubMed] [Google Scholar]
- [19].Haukås M, Berger U, Hop H, et al. Bioaccumulation of per- and polyfluorinated alkyl substances (PFAS) in selected species from the Barents Sea food web. Environ Pollut. 2007;148(1):360–371. doi: 10.1016/j.envpol.2006.09.021 [DOI] [PubMed] [Google Scholar]
- [20].Xu J, Guo C-S, Zhang Y, et al. Bioaccumulation and trophic transfer of perfluorinated compounds in a eu-trophic freshwater food web. Environ Pollut. 2014;184:254–261. doi: 10.1016/j.envpol.2013.09.011 [DOI] [PubMed] [Google Scholar]
- [21].Health Canada . Report on human biomonitoring of environmental chemicals in Canada. In: Results of the Canadian health measures survey cycle 1 (2007–2009). Ottawa (ON): Health Canada; 2010. p. 292. [Google Scholar]
- [22].Assembly of First Nations . 2013. First nations biomonitoring initiative: national results. 2011. Available from: www.afn.ca/uploads/files/afn_fnbi_en_-_2013-06-26.pdf
- [23].Health Canada . Fifth report on human biomonitoring of environmental chemicals in Canada (2016–2017). In: Results of the Canadian health measures survey cycle 5. Ottawa (ON): Health Canada; 2019. [Google Scholar]
- [24].Bjerregaard P, Pedersen HS, Nielsen NO, et al. Population surveys in Greenland 1993-2009: temporal trend of PCBs and pesticides in the general Inuit population by age and urbanisation. Sci Total Environ. 2013;454-455:283–288. doi: 10.1016/j.scitotenv.2013.03.031 [DOI] [PubMed] [Google Scholar]
- [25].Hjermitslev MH, Long M, Wielsøe M, et al. Persistent organic pollutants in Greenlandic pregnant women and indices of foetal growth: the ACCEPT study. Sci Total Environ. 2020;698:134118. doi: 10.1016/j.scitotenv.2019.134118 [DOI] [PubMed] [Google Scholar]
- [26].Lindh CH, Rylander L, Toft G, et al. Blood serum concentrations of perfluorinated compounds in men from Greenlandic inuit and European populations. Chemosphere. 2012;88(11):1269–1275. doi: 10.1016/j.chemosphere.2012.03.049 [DOI] [PubMed] [Google Scholar]
- [27].Lenters V, Portengen L, Smit LA, et al. Phthalates, perfluoroalkyl acids, metals and organochlorines and reproductive function: a multipollutant assessment in Greenlandic, Polish and Ukrainian men. Occup Environ Med. 2015;72(6):385–393. doi: 10.1136/oemed-2014-102264 [DOI] [PubMed] [Google Scholar]
- [28].Lenters V, Portengen L, Rignell-Hydbom A, et al. Prenatal phthalate, perfluoroalkyl acid, and organochlorine exposures and term birth weight in three birth cohorts: multi-pollutant models based on elastic net regression. Environ Health Perspect. 2016;124(3):365–372. doi: 10.1289/ehp.1408933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Timmermann CAG, Pedersen HS, Budtz-Jorgensen E, et al. Environmental chemical exposures among Greenlandic children in relation to diet and residence. Int J Circumpolar Health. 2019;78(1):1642090. doi: 10.1080/22423982.2019.1642090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].. Timmermann CAG. 2019. Personal communication, department of public health, university of southern Denmark, Odense, denmark. [Google Scholar]
- [31].AMAP . AMAP assessment 2009: human health in the Arctic. In: Arctic monitoring and assessment programme (AMAP). Oslo, Norway; 2009. p. xiv+254. [Google Scholar]
- [32].Olafsdottir K. Personal communication, department of pharmacology and toxicology. Reykjavik, Iceland: University of Iceland; 2019. [Google Scholar]
- [33].Petersen MS, Skytthe A, Erikstrup C. The healthy donor effect impacts self-reported physical and mental health - results from the Danish blood donor study (DBDS). Transfus Med. 2019;29 Suppl 1:65–69. doi: 10.1111/tme.12478 [DOI] [PubMed] [Google Scholar]
- [34].Mogensen UB, Grandjean P, Nielsen F, et al. Breastfeeding as an exposure pathway for perfluorinated alkylates. Environ Sciamp; Technol. 2015;49(17):10466–10473. doi: 10.1021/acs.est.5b02237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Dassuncao C, Hu XC, Nielsen F, et al. Shifting global exposures to poly- and perfluoroalkyl substances (PFAS) evident in longitudinal birth cohorts from a seafood-consuming population. Environ Sciamp; Technol. 2018;52(6):3738–3747. doi: 10.1021/acs.est.7b06044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Nøst TH, Breivik K, Fuskevag O-M, et al. Persistent organic pollutants in Norwegian men from 1979 to 2007: intraindividual changes, age–period–cohort effects, and Model predictions. Environ Health Perspect. 2013;121(11–12):1292–1298. doi: 10.1289/ehp.1206317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Nøst TH, Berg V, Hanssen L, et al. Time trends of persistent organic pollutants in 30 year olds sampled in 1986, 1994, 2001 and 2007 in Northern Norway: measurements, mechanistic modeling and a comparison of study designs. Environ Res. 2019;172:684–692. doi: 10.1016/j.envres.2019.02.047 [DOI] [PubMed] [Google Scholar]
- [38].Thomsen C, Liane VH, Becher G. Automated solid-phase extraction for the determination of polybrominated diphenyl ethers and polychlorinated biphenyls in serum—application on archived Norwegian samples from 1977 to 2003. J Chromatogr. 2007;846(1–2):252–263. doi: 10.1016/j.jchromb.2006.09.011 [DOI] [PubMed] [Google Scholar]
- [39].Nøst TH, Sandanger TM, Nieboer E, et al. The impacts of emission trends of POPs on human concentration dynamics: lessons learned from a longitudinal study in Norway (1979–2007). Int J Hyg Environ Health. 2017;220(4):776–781. doi: 10.1016/j.ijheh.2017.01.015 [DOI] [PubMed] [Google Scholar]
- [40].Averina M, Brox J, Huber S, et al. Perfluoroalkyl substances in adolescents in northern Norway: lifestyle and dietary predictors. The Tromsø study, fit futures 1. Environ Int. 2018;114:123–130. doi: 10.1016/j.envint.2018.02.031 [DOI] [PubMed] [Google Scholar]
- [41].Poothong S, Thomsen C, Padilla-Sanchez JA, et al. Distribution of novel and well-known poly- and perfluoroalkyl substances (PFAS) in human serum, plasma, and whole blood. Environ Sciamp; Technol. 2017;51(22):13388–13396. doi: 10.1021/acs.est.7b03299 [DOI] [PubMed] [Google Scholar]
- [42].Papadopoulou E, Haug LS, Sabaredzovic A, et al. Reliability of perfluoroalkyl substances in plasma of 100 women in two consecutive pregnancies. Environ Res. 2015;140:421–429. doi: 10.1016/j.envres.2015.04.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Donat-Vargas C, Akesson A, Tornevi A, et al. Persistent organochlorine pollutants in plasma, blood pressure, and hypertension in a longitudinal study. Hypertension. 2018;71(6):1258–1268. doi: 10.1161/HYPERTENSIONAHA.117.10691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Donat-Vargas C, Bergdahl I, Tornevi A, et al. Perfluoroalkyl substances and risk of type II diabetes: a prospective nested case-control study. Environ Int. 2019;123:390–398. doi: 10.1016/j.envint.2018.12.026 [DOI] [PubMed] [Google Scholar]
- [45].Donat-Vargas C, Bergdahl I, Tornevi A, et al. Associations between repeated measure of plasma perfluoroalkyl substances and cardiometabolic risk factors. Environ In-Ternational. 2019;124:58–65. doi: 10.1016/j.envint.2019.01.007 [DOI] [PubMed] [Google Scholar]
- [46].Tornevi A, Sommar J, Rantakokko P, et al. Chlorinated persistent organic pollutants and type 2 diabetes – a population-based study with pre- and post- diagnostic plasma samples. Environ Res. 2019;174:35–45. doi: 10.1016/j.envres.2019.04.017 [DOI] [PubMed] [Google Scholar]
- [47].Moraeus L, Lemming EW, Koivisto Hursti U-K, et al. Riksmaten adolescents 2016-17: a national dietary survey in Sweden - design, methods, and participation. Food & Nutr Res. 2018;62:1381. doi: 10.29219/fnr.v62.1381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Livsmedelsverket and Naturvårdsverket . Contaminants in blood and urine from adolescents in Sweden. Results from the national dietary survey riksmaten adolescents 2016–17. Livsmedelsverket and Naturvårdsverket; 2020. p. 102. [Google Scholar]
- [49].Warensjö Lemming E, Moraeus L, Petrelius Sipinen J, et al. Riksmaten ungdom 2016-17. In: Livsmedelskonsumtion bland ungdomar i Sverige. Livsmedelsverket; 2018. p. 118. [Google Scholar]
- [50].Gyllenhammar I, Glynn A, Fridén U, et al. Levels of persistent halogenated organic pollutants (POP) in mother’s milk from first-time mothers in Uppsala, Sweden: results from year 2015 and 2016, and temporal trends for the time period 1996-2016. Swed Environ Protect Agency (EPA). 2017;17. [Google Scholar]
- [51].Glynn A, Benskin J, Gyllenhammar I, et al. Temporal trends of perfluoroalkyl substances (PFAS) in individual serum samples from first-time mothers in Uppsala 1996-2016. Swed Environ Pro-Tection Agency (EPA). 2017;12. [Google Scholar]
- [52].Koponen J, Winkens K, Airaksinen R, et al. Longitudinal trends of per- and polyfluoroalkyl substances in children’s serum. Environ Int. 2018;121:591–599. doi: 10.1016/j.envint.2018.09.006 [DOI] [PubMed] [Google Scholar]
- [53].Dudarev AA, Dushkina EV, Sladkova YN, et al. Levels of exposure to persistent organic pollutants (POPs) of the population of Pechenga district of Murmansk oblast. Toxicologichesky vestnik (Russ Fed). 2016;3:2–9. [Article in Russian]. [Google Scholar]
- [54].Dudarev AA, Dushkina EV, Sladkova YN, et al. Persistent organic pollutants (POPs) in local food in the Pechenga district of the Murmansk region. Toxicologichesky Vestnik (Russ Fed). 2015;4(133):18–25. [Article in Russian. [Google Scholar]
- [55].Sandanger TM, Brustad M, Odland JØ, et al. Human plasma levels of POPs, and diet among native people from Uelen, Chukotka. J Environ Monit. 2003;5(4):689–696. doi: 10.1039/b302025h [DOI] [PubMed] [Google Scholar]
- [56].Anda EE, Nieboer E, Dudarev AA, et al. Intra- and intercompartmental associations between levels of organochlorines in maternal plasma, cord plasma and breast milk, and lead and cadmium in whole blood, for indigenous peoples of Chukotka, Russia. J Environ Monit. 2007;9(8):884–893. doi: 10.1039/b706717h [DOI] [PubMed] [Google Scholar]
- [57].Bravo N, Grimalt JO, Chashchin M, et al. Drivers of maternal accumulation of organo-halogen pollutants in Arctic areas (Chukotka, Russia) and 4,4’-DDT effects on the newborns. Environ Int. 2019;124:541–552. doi: 10.1016/j.envint.2019.01.049 [DOI] [PubMed] [Google Scholar]
- [58].Dudarev AA, Chupakhin VS, Vlasov SV, et al. Traditional diet and environmental contaminants in coastal chukotka II: legacy POPs. Int J Environ Res Public Health. 2019;16(5):695. doi: 10.3390/ijerph16050695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Long M, Bossi R, Bonefeld-Jørgensen EC. Level and temporal trend of perfluoroalkyl acids in Greenlandic Inuit. Int J Circumpolar Health. 2012;71(1):17998. doi: 10.3402/ijch.v71i0.17998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Wielsøe M, Kern P, Bonefeld-Jørgensen EC. Serum levels of environmental pollutants is a risk factor for breast cancer in inuit: a case control study. Environ Health. 2017;16(1):56. doi: 10.1186/s12940-017-0269-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].UNEP . Global chemicals outlook. 2020. [cited 2020 Oct]. Available from: www.unenvironment.org/explore-topics/chemicals-waste/what-we-do/policy-and-governance/global-chemicals-outlook
- [62].Gibson JC. Emerging persistent chemicals in human biomonitoring for populations in the Arctic: a Canadian per-spective. Sci Of The Total Environ. 2020;708:134538. doi: 10.1016/j.scitotenv.2019.134538 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


