Abstract
Background
Betaine (trimethylglycine) lowers plasma homocysteine, a possible risk factor for cardiovascular disease. However, studies in renal patients and in obese individuals who are on a weight-loss diet suggest that betaine supplementation raises blood cholesterol; data in healthy individuals are lacking. Such an effect on cholesterol would counteract any favourable effect on homocysteine. We therefore investigated the effect of betaine, of its precursor choline in the form of phosphatidylcholine, and of the classical homocysteine-lowering vitamin folic acid on blood lipid concentrations in healthy humans.
Methods and Findings
We measured blood lipids in four placebo-controlled, randomised intervention studies that examined the effect of betaine (three studies, n = 151), folic acid (two studies, n = 75), and phosphatidylcholine (one study, n = 26) on plasma homocysteine concentrations. We combined blood lipid data from the individual studies and calculated a weighted mean change in blood lipid concentrations relative to placebo. Betaine supplementation (6 g/d) for 6 wk increased blood LDL cholesterol concentrations by 0.36 mmol/l (95% confidence interval: 0.25–0.46), and triacylglycerol concentrations by 0.14 mmol/l (0.04–0.23) relative to placebo. The ratio of total to HDL cholesterol increased by 0.23 (0.14–0.32). Concentrations of HDL cholesterol were not affected. Doses of betaine lower than 6 g/d also raised LDL cholesterol, but these changes were not statistically significant. Further, the effect of betaine on LDL cholesterol was already evident after 2 wk of intervention. Phosphatidylcholine supplementation (providing approximately 2.6 g/d of choline) for 2 wk increased triacylglycerol concentrations by 0.14 mmol/l (0.06–0.21), but did not affect cholesterol concentrations. Folic acid supplementation (0.8 mg/d) had no effect on lipid concentrations.
Conclusions
Betaine supplementation increased blood LDL cholesterol and triacylglycerol concentrations in healthy humans, which agrees with the limited previous data. The adverse effects on blood lipids may undo the potential benefits for cardiovascular health of betaine supplementation through homocysteine lowering. In our study phosphatidylcholine supplementation slightly increased triacylglycerol concentrations in healthy humans. Previous studies of phosphatidylcholine and blood lipids showed no clear effect. Thus the effect of phosphatidylcholine supplementation on blood lipids remains inconclusive, but is probably not large.
Folic acid supplementation does not seem to affect blood lipids and therefore remains the preferred treatment for lowering of blood homocysteine concentrations.
Lowering homocysteine might reduce the risk for heart disease. Betaine seems to have an adverse effect on blood lipids. This would make it less suitable than folic acid, which does not affect blood lipids.
Introduction
Cardiovascular disease (CVD) is a major cause of morbidity and mortality in Western societies. Diet may play a crucial role in the aetiology of CVD. However, clinical trials that test the effects of diet on CVD are complicated to conduct. Hence, risk factors that are predictive of CVD risk are frequently used to test the effects of diet on disease prevention. Examples of such risk factors are homocysteine concentrations and cholesterol concentrations. A limitation of studying effects of dietary changes on risk factors is that often a single factor is measured, whereas multiple factors might be affected by changes in diet. Whether homocysteine lowering indeed lowers risk of CVD is not yet established, but evidence for a causal relationship is accumulating [1–3]. The effects of increased blood low-density lipoprotein (LDL) cholesterol concentrations on CVD risk are well established.
Betaine is used as therapy to lower plasma homocysteine in hyperhomocysteinemic patients with genetic defects in their homocysteine metabolism who are unresponsive to pyridoxine, folic acid, and vitamin B12 [4–6]. It also lowers plasma homocysteine in healthy humans with homocysteine concentrations in the normal range [7–9]. Betaine occurs naturally in the diet, or it can be produced endogenously through oxidation of choline. Dietary intake of betaine is estimated at 0.5–2 g/d generally, and major food sources are wheat products (e.g., bread), beets, and spinach [10]. Recently we showed that supplementation with 1.5 g/d of betaine, which is in the range of daily intake, lowers fasting and post-methionine plasma homocysteine concentrations in healthy humans [8]. The homocysteine-lowering properties of betaine would predict a decrease in risk of CVD. However, betaine and its precursor choline are also involved in lipid metabolism. In animal studies betaine supplementation increases serum cholesterol concentrations relative to control [11,12]. Studies in humans have also found that betaine supplementation increases serum cholesterol in obese individuals who are on a weight-loss diet, and in patients with chronic renal failure [9,13]. However, in these studies betaine was not the only intervention, so the observed effects on lipid concentrations could have been due to factors other than betaine supplementation. Furthermore, in the study with obese individuals, weight changes might have contributed to the changes in serum lipids [9]. The effects of betaine on lipid metabolism in healthy humans are not well established.
Choline, the precursor of betaine, also occurs naturally in foods, mainly as phosphatidylcholine. Daily intake of choline is generally estimated to be 0.3–1 g [14]. Phosphatidylcholine is an essential component of very low density lipoproteins (VLDLs) and is therefore involved in lipid metabolism. Animal and human studies indicate that choline deficiency decreases serum cholesterol, which can be prevented by addition of choline [15–17]. Data on the effects of choline supplementation on blood lipids in humans are scarce.
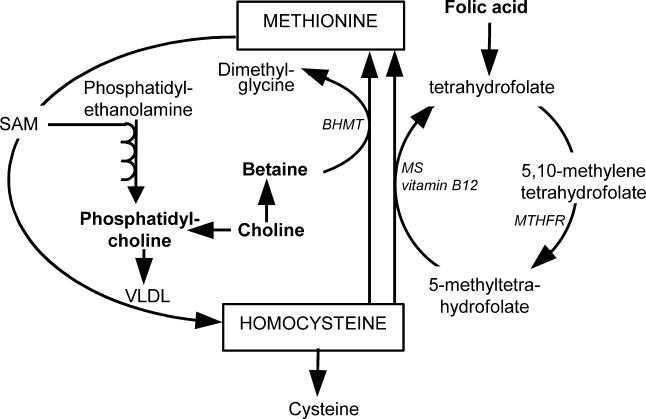
Increases in blood lipid concentrations due to betaine or choline supplementation might offset the potential beneficial effects of their homocysteine-lowering properties. Folic acid is the most commonly used homocysteine-lowering agent. The betaine remethylation and folic acid remethylation pathways are interrelated (Figure 1), so effects of folic acid supplementation on lipid metabolism cannot be excluded [18].
Figure 1. Role of Folic Acid, Choline, and Betaine in Homocysteine Metabolism and in Phosphatidylcholine Metabolism.
Phosphatidylcholine is necessary for synthesis of VLDL, which exports lipids from the liver.
In this study we investigated the effects of supplementation of three homocysteine-lowering nutrients, betaine, choline (as phosphatidylcholine), and folic acid, on blood lipid concentrations in healthy humans. For this, we combined data of four placebo-controlled studies in healthy humans, performed by our group. These are the only available randomized, controlled studies in healthy humans on this matter that we are aware of.
Methods
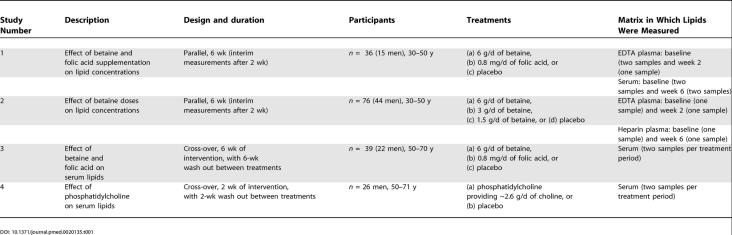
Data were derived from four placebo-controlled intervention studies (studies 1, 2, 3 and 4) into the effects of betaine, phosphatidylcholine, and folic acid on homocysteine concentrations (Table 1). All studies are reported in accordance with the CONSORT guidelines (Tables S1–S4).
Table 1. Overview of Study Designs into the Effects of Betaine, Folic Acid, and Phosphatidylcholine on Blood Lipid Concentrations.
Study 1
Study participants and design
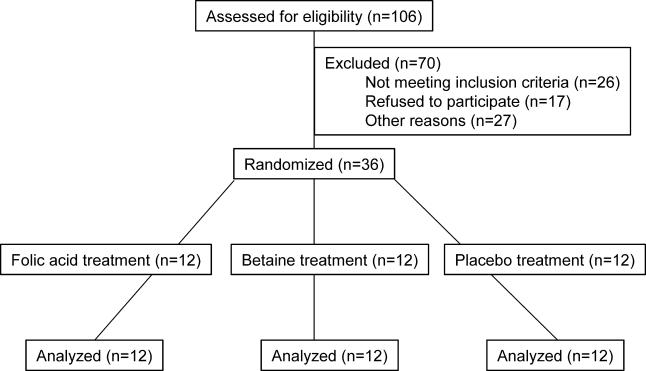
The primary endpoint of this study was plasma homocysteine concentrations, and these results have been published [7]. Blood lipid measurements were done post-hoc. Study 1 was conducted according to Good Clinical Practice guidelines at TNO Quality of Life (Zeist, the Netherlands). The local medical ethics committee approved the protocol, and all volunteers gave their written informed consent. Volunteers were recruited from April to May 2000. Eligible volunteers were healthy as assessed by routine medical screening and a general health questionnaire, had plasma total homocysteine concentrations below 25 μmol/l, had no history of cardiovascular disease, and had not used vitamin B supplements more than once a week in the 3 mo before entering the study. Out of the eligible participants, the 36 participants (15 males and 21 females, age 30–50 y) with the highest plasma total homocysteine concentrations (range 8.9 to 21.0 μmol/l) were included in this placebo-controlled, double-blind parallel study (Figure 2). Participants were stratified by gender and plasma homocysteine concentration, and then randomly assigned to one of three treatments for 6 wk: (a) 6 g/d of betaine (n = 12) (BUFA Pharmaceutical Products, Uitgeest, the Netherlands), (b) 0.8 mg/d of folic acid (n = 12), or (c) placebo (n = 12). One person at the local pharmacy assigned codes to the study treatments and provided the key in a sealed envelope to the principal investigator at TNO. The statistician then randomly allocated treatment codes to participant numbers. Randomization was done using a computerized procedure that produced combinations based on random seed numbers. The statistician supplied the medical investigator with sealed envelopes with the treatment allocation per participant. The participants and all others involved in this study were unaware of treatment allocation. The principal investigator performed unblinding of the treatment allocation after the study had ended, laboratory analyses were complete, and datasets were locked. The study supplements were dissolved in water and ingested on two daily occasions, one half of the daily dose after breakfast and the other half after the evening meal. Throughout the study, participants were asked to refrain from eating products based on animal liver and to consume no more than two eggs per week. (Eggs and liver are major sources of betaine and of choline, the dietary precursor for betaine.)
Figure 2. Flow Diagram of Participant Progress through Study 1.
Blood sampling and blood lipid analysis
Venous blood was taken from an anticubital vein in the forearm following an overnight fast at baseline and after 2 wk and 6 wk of intervention. Immediately after collection, blood was mixed well and put on ice. Within 30 min samples were centrifuged for 10 min at 2,000 g at 4 °C. Samples were stored below −20 °C. Samples were coded to hide the identity and treatment of participants. All samples obtained from one participant were analysed in the same run. We measured blood lipid concentrations in fasting EDTA plasma samples at baseline and after 2 wk of intervention, and in fasting serum samples collected at baseline and after 6 wk of intervention. Concentrations of total cholesterol, high-density lipoprotein (HDL) cholesterol, and triacylglycerol were measured with a Hitachi (Tokyo, Japan) 911 analyzer and enzymatic assays of Roche (Basel, Switzerland). LDL cholesterol concentrations were calculated with the formula of Friedewald et al. [19].
Study 2
Participants and design
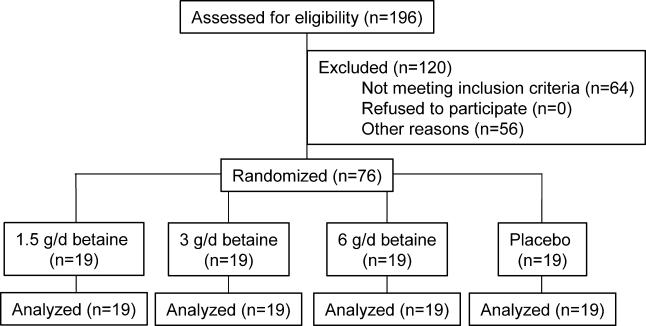
The primary endpoint of this study was plasma homocysteine concentrations, and these results have been published [8]. Blood lipid measurements were done post-hoc. Study 2 was conducted according to Good Clinical Practice guidelines at TNO Quality of Life (Zeist, the Netherlands). The local medical ethics committee approved the protocol, and all volunteers gave their written informed consent. Volunteers were recruited from September to October 2001. Eligible volunteers were healthy as assessed by a general health and lifestyle questionnaire, blood pressure measurement, and blood analyses of haematology, homocysteine, B vitamins, liver enzymes, creatinine, glucose, and lipids. Plasma total homocysteine concentrations were below 25 μmol/l. Volunteers had no history of CVD, and had not used vitamin B supplements more than once a week in the 3 mo before entering the study. Out of the eligible participants, the 76 participants (44 males and 32 females, age 30–50 y) with the highest plasma total homocysteine concentrations (range 8.4 to 22.2 μmol/l) were included in this placebo-controlled, double-blind parallel trial (Figure 3). Volunteers were stratified by gender, plasma homocysteine concentration, blood pressure, and smoking (yes or no), and then randomly assigned to one of four treatment groups for 6 wk: (a) 1.5 g/d of anhydrous betaine (BUFA) (n = 19), (b) 3 g/d of betaine (n = 19), (c) 6 g/d of betaine (n = 19), or (d) placebo (n = 19). One person at the local pharmacy assigned codes to the study treatments and provided the key in a sealed envelope to the principal investigator at TNO. The statistician then randomly allocated treatment codes to the participant numbers. Randomization was done using a computerized procedure that produced combinations based on random seed numbers. The statistician supplied the principal investigator with sealed envelopes with the treatment allocation per participant. The participants and all others involved in this study were unaware of treatment allocation. The statistician performed unblinding of the treatment allocation after the study had ended, laboratory analyses were complete, and datasets were locked. The study supplements were dissolved in water and ingested on two daily occasions, one half of the daily dose after breakfast and the other half after the evening meal. Throughout the study participants were asked not to consume liver products more than twice a week, and not to consume more than two eggs per week. Six grams per day of betaine is the lowest therapeutic dose used to lower plasma homocysteine in hyperhomocysteinemic patients with genetic defects in their homocysteine metabolism [20]. We chose to study doses of betaine lower than 6 g/d, e.g., 1.5 g/d and 3 g/d, because these are within the range of intake of betaine with foods. Intake of betaine with foods is generally estimated at 0.5–2 g/d.
Figure 3. Flow Diagram of Participant Progress through Study 2.
Blood sampling and blood lipid analysis
Venous blood was taken from the anticubital vein in the forearm following an overnight fast at baseline and after 2 wk and 6 wk of intervention. Samples were mixed and put on ice immediately after collection. Within 30 min samples were centrifuged for 10 min at 2,000 g at 4 °C. Samples were stored below −18 °C. Samples were coded to hide the identity and treatment of participants. All samples obtained from one participant were analyzed in the same run. We measured blood lipid concentrations in fasting EDTA plasma samples at baseline and after 2 wk of intervention, and in fasting heparin plasma samples collected at baseline and after 6 wk of intervention. Concentrations of total cholesterol, HDL cholesterol, and triacylglycerol were measured with a Hitachi 911 analyzer and enzymatic assays of Roche. LDL cholesterol concentrations were calculated with the formula of Friedewald et al. [19].
Study 3
Participants and design
The primary endpoints of this study were plasma homocysteine concentrations and vascular function. Blood lipid measurements were planned before the study took place, but power analysis was based on changes in the primary endpoint vascular function, and not on changes in blood lipids. The original study protocol of this study can be found in Protocol S1, and the trial was registered at clinicaltrials.gov under identifier NCT00102843. The study was conducted at the division of Human Nutrition, Wageningen University (Wageningen, the Netherlands). The local medical ethics committee approved the protocol, and all volunteers gave their written informed consent. Volunteers were recruited from June to September 2002. Eligible volunteers were healthy as assessed by routine medical screening and a general health questionnaire, had plasma total homocysteine concentrations below 26 μmol/l, had no history of CVD; and had not used vitamin B supplements more than once a week in the 3 mo before entering the study. Out of the eligible participants, the 40 participants (23 males and 17 females, age 50–70 y) with the highest plasma total homocysteine concentrations (range 10.2 to 21.7 μmol/l) were included in this placebo-controlled, double-blind cross-over trial (Figure 4). Participants were randomly assigned to one out of six treatment orders, and they received each of the following supplements for 6 wk, with a 6-wk wash out in between: (a) 6 g/d of betaine (BUFA), (b) 0.8 mg/d of folic acid, or (c) placebo. A person not further involved in the study assigned codes to the study treatments, and randomly allocated the selected participants to one out of six treatment orders, according to a computer-generated randomization list, and kept the key in a sealed envelope. The participants and all others involved in this study were unaware of treatment allocation. The principal investigator performed unblinding of the treatment allocation after the study had ended and laboratory analyses were complete. The study supplements were dissolved in water and ingested twice per day, one half of the daily dose after breakfast and the other half after the evening meal. During the study participants were not allowed to consume supplements containing B vitamins, antioxidant vitamins (A, beta-carotene, C, and E), or omega-3 fatty acids/fish oil supplements.
Figure 4. Flow Diagram of Participant Progress through Study 3.
Blood sampling and laboratory analyses
Venous blood was taken from the antecubital vein following an overnight fast on days 41 and 43 of each treatment period. Blood was collected in vacutainer tubes containing clot activator and a gel to separate serum and cells. About 30 min after collection, samples were centrifuged for 15 min at 2,000 g at 4 °C. All serum samples were stored below −70 °C. Samples were coded to hide the identity and treatment of participants. All samples obtained from one participant were analyzed in the same run. In serum samples we measured concentrations of total cholesterol, HDL cholesterol, and triacylglycerols on a Synchron LX 20 system (Beckman Coulter, Mijdrecht, the Netherlands). LDL cholesterol concentrations were calculated with the formula of Friedewald et al. [19].
Study 4
Participants and design
The primary endpoint of study 4 was plasma homocysteine concentrations. Blood lipid measurements were planned before the study took place, but power analysis was based on changes in the primary endpoint homocysteine concentrations, and not on changes in blood lipids. The original study protocol of this study can be found in Protocols S2–S4, and the trial was registered at clinicaltrials.gov under identifier NCT00102232. This study was conducted according to Good Clinical Practice guidelines at TNO Quality of Life (Zeist, the Netherlands). The local medical ethics committee approved the protocol, and all volunteers gave their written informed consent. Volunteers were recruited from March to May 2003. Eligible volunteers were healthy as assessed by physical examination, a general health and lifestyle questionnaire, blood pressure measurement, routine clinical laboratory tests, and blood analyses of homocysteine and B vitamins. Plasma homocysteine concentrations were below 26 μmol/l. Volunteers had no history of CVD, and had not used vitamin B supplements, lecithin, or supplements containing choline, choline derivatives, or betaine more than once a week during the 1 mo before screening. Out of the eligible men, the 26 men between 50–71 y of age with the highest plasma homocysteine concentrations (range 11.0 to 23.1 μmol/l) were included in this placebo-controlled, double-blind cross-over trial (Figure 5). Participants were stratified by plasma homocysteine concentrations at screening and by smoking habits and then randomly assigned to one of two treatment orders; they received each of the following supplements for 2 wk, with a 2-wk wash out in between: (a) 34.0 g of a soybean lecithin preparation, in which phosphatidylcholine is the only phospholipid (PhosChol Nutrasal, Oxford, Connecticut, United States) and (b) placebo oil, which consisted of 25.5 g of a mixture of edible oils that mimicked the fatty acid composition of the phosphatidylcholine supplement (provided by Unilever Research Laboratory, Vlaardingen, the Netherlands) (Table 2). A person not further involved in the study assigned codes to the study treatments and provided the key in a sealed envelope to the principal investigator at TNO. The statistician randomly allocated the selected participants to one of the two treatment orders. Randomization was done using a computerized procedure that produced combinations based on random seed numbers. The statistician supplied the principal investigator with sealed envelopes with the treatment allocation per participant. The participants and all others involved in this study were unaware of treatment allocation. The statistician performed unblinding of the treatment allocation after the study had ended, laboratory analyses were complete, and datasets were locked. Phosphatidylcholine and placebo supplements were matched for fat content and fatty acid composition (Table 2). The amount of choline in 34 g of the phosphatidylcholine supplement was 2.6 g, as measured by Koc et al. [21] and by TNO. The daily dose of 2.6 g of choline is well below the current tolerable upper intake level of 3.5 g of choline per day for adults [22]. Participants ingested half of the daily supplement dose two times per day (i.e., at breakfast and at dinner). The individual portions of half the daily dose of the supplements were mixed with 200 ml of custard. Participants returned 99.5% of the bowls empty, which indicated good compliance. From 2 wk before the start of the study until the end of the study participants were not allowed to consume food products rich in lecithin or betaine.
Figure 5. Flow Diagram of Participant Progress through Study 4.
Table 2. Composition of Treatment Supplements in Study 4.
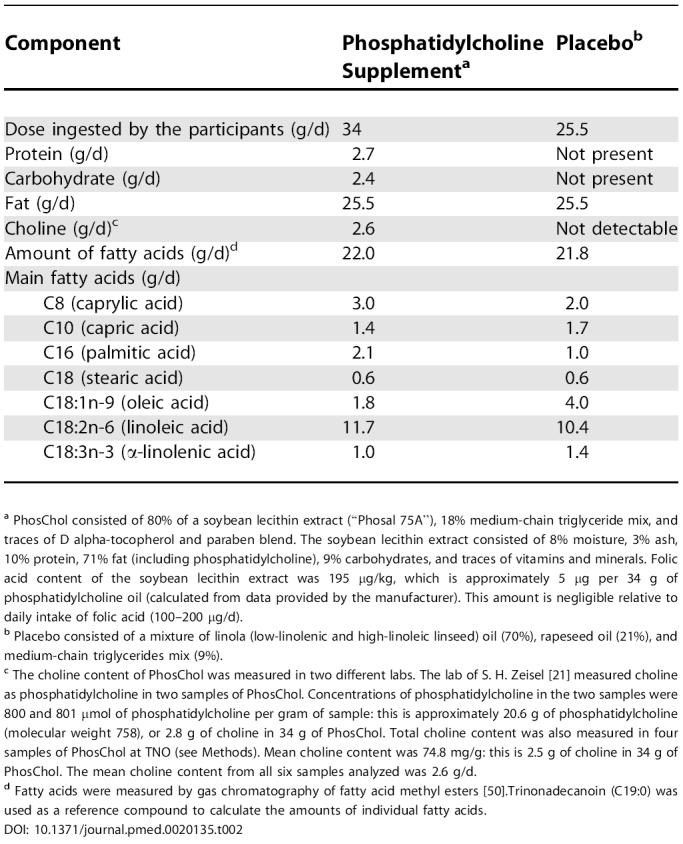
a PhosChol consisted of 80% of a soybean lecithin extract (“Phosal 75A”), 18% medium-chain triglyceride mix, and traces of D alpha-tocopherol and paraben blend. The soybean lecithin extract consisted of 8% moisture, 3% ash, 10% protein, 71% fat (including phosphatidylcholine), 9% carbohydrates, and traces of vitamins and minerals. Folic acid content of the soybean lecithin extract was 195 μg/kg, which is approximately 5 μg per 34 g of phosphatidylcholine oil (calculated from data provided by the manufacturer). This amount is negligible relative to daily intake of folic acid (100–200 μg/d).
b Placebo consisted of a mixture of linola (low-linolenic and high-linoleic linseed) oil (70%), rapeseed oil (21%), and medium-chain triglycerides mix (9%).
c The choline content of PhosChol was measured in two different labs. The lab of S. H. Zeisel [21] measured choline as phosphatidylcholine in two samples of PhosChol. Concentrations of phosphatidylcholine in the two samples were 800 and 801 μmol of phosphatidylcholine per gram of sample: this is approximately 20.6 g of phosphatidylcholine (molecular weight 758), or 2.8 g of choline in 34 g of PhosChol. Total choline content was also measured in four samples of PhosChol at TNO (see Methods). Mean choline content was 74.8 mg/g: this is 2.5 g of choline in 34 g of PhosChol. The mean choline content from all six samples analyzed was 2.6 g/d.
d Fatty acids were measured by gas chromatography of fatty acid methyl esters [50].Trinonadecanoin (C19:0) was used as a reference compound to calculate the amounts of individual fatty acids.
Blood sampling and laboratory analyses
Venous blood was taken from the antecubital vein following an overnight fast on days 13 and 15 of each treatment period. Blood was collected in vacutainer tubes containing clot activator and a gel to separate serum and cells. About 30 min after collection, samples were centrifuged for 15 min at 2,000 g at 4 °C. All serum samples were stored below −70 °C. Samples were coded to hide the identity and treatment of participants. All samples obtained from one participant were analyzed in the same run. In serum samples we measured concentrations of total cholesterol, HDL cholesterol, and triacylglycerols with a Hitachi 911 analyzer and enzymatic assays of Roche. LDL cholesterol concentrations were calculated with the formula of Friedewald et al. [19]. For the measurement of choline at TNO, choline was liberated from the sample by boiling the sample for 4 h with nitric acid (17 v/v%). After filtration of the sample, the pH of the extract was raised to 9.0 with sodium hydroxide solution. To an aliquot part of the extract a solution of ammonium reineckate in methanol was added, which formed the insoluble choline reineckate. The precipitate was filtered off and dissolved in acetone. The color of the acetone solution was determined with a spectrophotometer at a wavelength of 530 nm.
Statistics
The sample size of each study was based on power analysis for the primary outcome measure. To exclude effects of the matrix in which lipids were measured [23], all changes in lipid concentrations within persons or studies were based on measurements within the same matrix. So choice of matrix could not have been responsible for differences in results between the studies. First we calculated the mean change in lipid concentrations relative to placebo after supplementation with the treatments for each study. Means were compared with the General Linear Models procedure in SAS (ANOVA; SAS version 6.12, SAS Institute, Cary, North Carolina, United States). For the parallel studies 1 and 2, Dunnett's two-tailed t-test was used to test whether any of the treatments significantly differed from placebo. For the cross-over studies 3 and 4, a paired Student's t-test was used to test whether any of the treatments significantly differed from placebo. Then we combined the lipid data of the studies with betaine or folic acid supplementation. For this we calculated a combined effect of all four studies, which was a weighted mean and 95% confidence intervals of the change in lipid concentrations relative to placebo after betaine supplementation (studies 1, 2, and 3), and after folic acid supplementation (studies 1 and 3) according to the method used by Curtin et al. [24] (Tables 3 and 4). We tested whether the weighted mean changes differed from zero with a t-test. For all tests mean differences were considered statistically significant at p < 0.05.
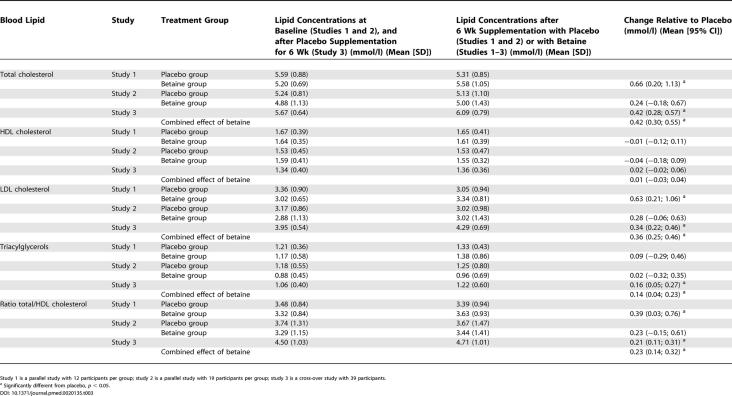
Table 3. Mean Changes in Blood Lipid Concentrations and the Ratio of Total to HDL Cholesterol in Fasting State in Studies 1, 2, and 3, and the Combined Effect of Supplementation with 6 g/d of Betaine.
Study 1 is a parallel study with 12 participants per group; study 2 is a parallel study with 19 participants per group; study 3 is a cross-over study with 39 participants.
a Significantly different from placebo, p < 0.05.
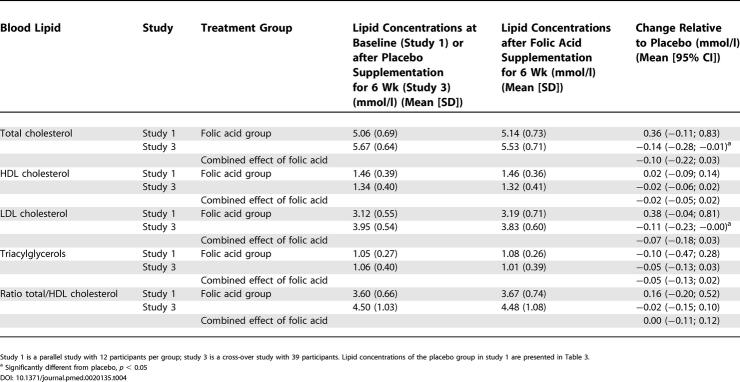
Table 4. Mean Changes in Blood Lipid Concentrations and the Ratio of Total to HDL Cholesterol in Fasting State in Studies 1 and 3, and the Combined Effect of Supplementation with 0.8 mg/d of Folic Acid.
Study 1 is a parallel study with 12 participants per group; study 3 is a cross-over study with 39 participants. Lipid concentrations of the placebo group in study 1 are presented in Table 3.
a Significantly different from placebo, p < 0.05
Results
All participants completed the study, except for one male participant who withdrew from study 3 due to emigration. No serious adverse events were reported in any of the studies. Non-serious adverse events are shown in Table S5.
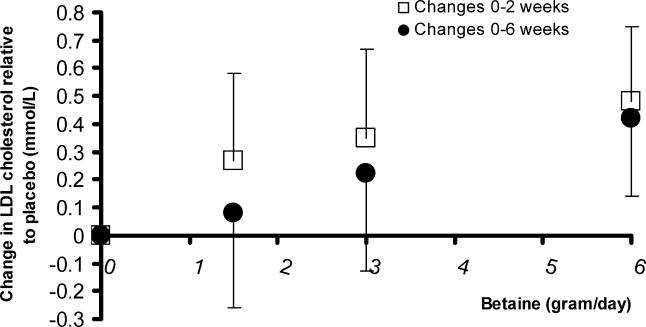
Supplementation of 6 g/d of betaine for 6 wk increased total cholesterol concentrations by 0.42 mmol/l (8%) relative to placebo (see Table 3). This increase was almost completely accounted for by an increase in LDL cholesterol concentrations of 0.36 mmol/l (11%). Betaine increased triacylglycerol concentrations by 0.14 mmol/l (13%). HDL cholesterol concentrations did not change, but the ratio of total to HDL cholesterol concentration increased by 0.23 (6%). The increases in LDL cholesterol concentrations in the groups that ingested 1.5 g/d of betaine and 3 g/d of betaine for 6 wk were higher than in the placebo group, but these changes did not reach statistical significance (Figure 6). The effects of betaine supplementation on LDL cholesterol concentrations were already evident after 2 wk of supplementation in studies 1 and 2.
Figure 6. Mean Change Relative to Placebo in LDL Cholesterol Concentrations.
Compared are the values after participants had ingested 1.5 g/d of betaine (study 2, n = 19), 3 g/d of betaine (study 2, n = 18), or 6 g/d of betaine (studies 1 and 2 combined, n = 31) after 2 wk (+95% confidence interval) and 6 wk (–95% confidence interval). In the group that ingested 3 g/d of betaine one participant missed the blood collection before treatment.
Supplementation of 0.8 mg/d of folic acid for 6 wk did not affect lipid concentrations in healthy volunteers (see Table 4).
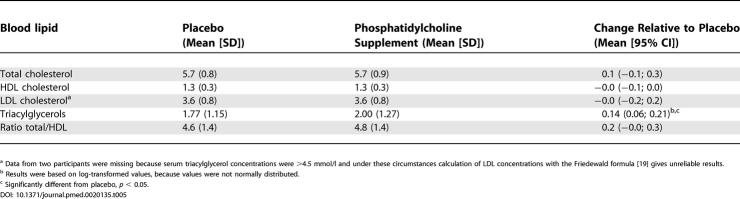
Supplementation with 2.6 g/d of choline (provided as phosphatidylcholine) for 2 wk increased serum triacylglycerols by 0.14 mmol/l (8%), but did not affect cholesterol concentrations (Table 5).
Table 5. Concentrations of Serum Lipids (mmol/l) and the Ratio of Total to HDL Cholesterol in Fasting State after 26 Healthy Men Had Ingested Phosphatidylcholine Providing ~2.6 g/d of Choline or Placebo for 2 Wk.
a Data from two participants were missing because serum triacylglycerol concentrations were >4.5 mmol/l and under these circumstances calculation of LDL concentrations with the Friedewald formula [19] gives unreliable results.
b Results were based on log-transformed values, because values were not normally distributed.
c Significantly different from placebo, p < 0.05.
Discussion
We found that supplementation of 6 g/d of betaine for 6 wk consistently increased total cholesterol concentrations, mainly LDL cholesterol, and triacylglycerol concentrations. Phosphatidylcholine supplementation for 2 wk increased only serum triacylglycerol concentrations. Folic acid supplementation did not affect lipid concentrations.
The three studies on betaine supplementation were not originally designed to investigate changes in blood lipids. Therefore, we did not perform power calculations for changes in blood lipids for each study beforehand. We combined the lipid data of the three studies to have a better estimate of the effects of betaine on blood lipids, and thereby increase the statistical power afterwards. Similarly we did not do power calculations for effects of folic acid on blood lipids. The increase in LDL cholesterol concentrations with betaine supplementation that we found in healthy humans is in accordance with results from previous studies in renal patients [13] and obese individuals who were on a weight-loss diet [9]. Doses of betaine lower than 6 g/d also raised LDL cholesterol, although this did not reach statistical significance (Figure 6). Our study was not designed to investigate the dose–response relationship between betaine doses and changes in LDL cholesterol. Establishing the dose–response relationship between betaine and LDL cholesterol might require more than three betaine dose groups. Furthermore, betaine supplementation affected blood lipids already after 2 wk of intervention (Figure 6). The increase in triacylglycerol concentrations with betaine supplementation is well in line with the results of Schwab et al. in obese individuals [9]. In the obese individuals betaine supplementation increased triacylglycerol concentrations by approximately 12%, although this increase did not reach statistical significance.
Our observations should be of specific concern for clinical practice as well as for the general population. Patients with inborn errors in the enzymes involved in homocysteine metabolism and who are not or only partially responsive to pyridoxine are generally prescribed betaine in doses of 6 g/d or higher in order to lower their plasma homocysteine [25]. Our data imply that betaine treatment in these patients may have adverse effects on lipid concentrations. No one has systematically reported changes in serum cholesterol concentrations in hyperhomocysteinemic patients who are on betaine treatment, although this may be warranted. Clearly in these patients, the benefit of the substantial homocysteine-lowering effect of betaine outweighs the effect of any modest lipid-related changes. Furthermore, our data imply that among the general population intake of extra betaine, e.g., from supplements or through increased intake of betaine-rich foods, may not bring the expected benefits for CVD prevention predicted from the homocysteine-lowering effect of betaine.
Phosphatidylcholine supplementation increased serum triacylglycerols, but did not affect serum cholesterol concentrations. We supplemented phosphatidylcholine for only 2 wk, and it is possible that this was too short to induce changes in serum cholesterol, although betaine supplementation did increase blood cholesterol concentrations within 2 wk. We carefully matched the fat content and fatty acid composition of the control and the phosphatidylcholine supplements. Previous studies that also attempted to control for the fatty acid component of lecithin (the trivial name for phosphatidylcholine) [26–30], or that tested choline bitartrate [31], suggest that there is little or no effect of the choline moiety of lecithin on blood lipids, provided that the fatty acids in lecithin are balanced in the placebo treatment. Only two of the studies that investigated phosphatidylcholine included a concurrent control group [27,30]. In the randomized cross-over study of Childs et al. [27] triacylglycerol concentrations in 12 normolipidemic individuals were 0.01 mmol/l lower after they had consumed lecithin for 3 wk than after they had consumed placebo. Oosthuizen et al. [30] found in a parallel study with seven hyperlipidemic men per group that the median triacylglycerol concentration decreased by 0.19 mmol/l in the lecithin group relative to the placebo group after 2 wk of intervention. After 4 wk of intervention this difference had disappeared. The increase in triacylglycerol concentrations with phosphatidylcholine treatment in our study could be a chance finding. However, our study was larger than previous studies, we used a cross-over design with a wash out period, and participants received supplements in random order. Moreover, the dose of phosphatidylcholine we used was higher than in the previous studies. Therefore, our study leaves open a real possibility that the choline moiety of phosphatidylcholine in high doses raises serum triacylglycerol concentrations.
We propose that the mechanism by which betaine and phosphatidylcholine supplementation increase blood lipid concentrations involves increased export of lipids from the liver into the circulation. Lipids are exported from the liver through VLDL particles, which consist of a triglyceride core packaged in an amphipatic shell of phospholipids, mostly phosphatidylcholine, and apolipoprotein B-100. More VLDL will increase transport of lipids from the liver into the blood, and concentrations of lipids in the liver decrease accordingly [11]. Betaine and phosphatidylcholine supplementation could increase VLDL synthesis via increased availability of phosphatidylcholine, an essential component of VLDL (see Figure 1) [32]. Furthermore, betaine might increase phosphatidylcholine synthesis owing to increased availability of S-adenosylmethionine via increased remethylation of homocysteine into methionine, because S-adenosylmethionine is the methyl donor for the formation of phosphatidylcholine out of phosphatidylethanolamine [33]. In addition, betaine supplementation could promote phosphatidylcholine synthesis through increasing the activity of the enzyme betaine–homocysteine methyltransferase [34]. Betaine–homocysteine methyltransferase catalyzes the transfer of a methyl group from betaine to homocysteine, but it also catalyzes the first step in the three-enzyme pathway that promotes methylation of phosphatidylethanolamine into phosphatidylcholine [35,36].
We speculate that betaine and phosphatidylcholine supplementation increase lipid concentrations in blood because of increased synthesis and availability of phosphatidylcholine, which promotes VLDL secretion from the liver into plasma, resulting in higher lipid concentrations in blood [32,37].
Our data indicate that folic acid supplementation does not affect serum lipid concentrations, which is in agreement with other studies [38–41].
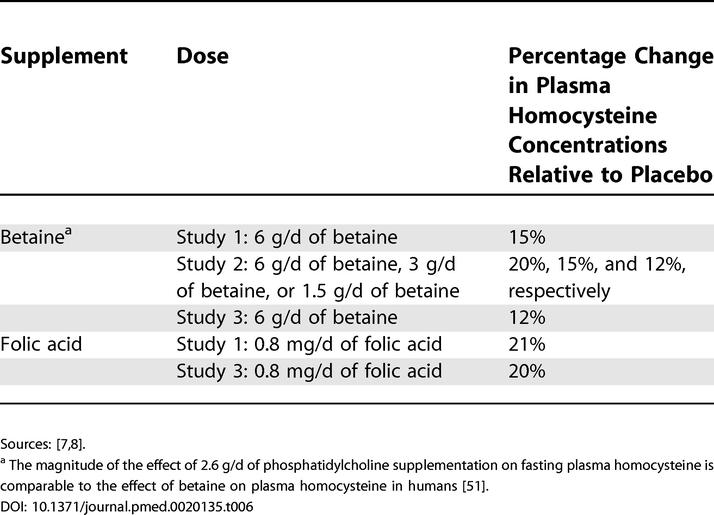
The consequences of betaine or phosphatidylcholine supplementation for risk of CVD are difficult to predict. In healthy participants, betaine supplementation lowers plasma homocysteine after methionine loading by 20%–40%. Betaine as well as phosphatidylcholine supplementation lower fasting plasma homocysteine by 12%–20% (Table 6) [7,8]. This is estimated to lower CVD risk by 10%–15% [1,42]. However, it is not yet firmly established that homocysteine lowering indeed lowers risk of CVD, although evidence for a causal relationship is accumulating [1–3,43]. We now find that betaine not only decreases homocysteine, but also increases LDL cholesterol by approximately 10% and the total/HDL cholesterol ratio by approximately 6%. This is estimated to increase CVD risk by approximately 10% [44–46]. Betaine (6 g/d) and phosphatidylcholine (providing approximately 2.6 g/d of choline) supplementation increase triacylglycerol concentrations by up to 13%, which is estimated to increase risk of CVD by approximately 6%, after adjustment for other risk factors [47–49]. This implies that betaine supplementation is expected to increase risk of CVD if homocysteine is not a cause of CVD, or does not affect risk if homocysteine is causally involved. Whether phosphatidylcholine affects CVD risk remains uncertain so far.
Table 6. Percentage Change in Fasting Concentrations of Plasma Homocysteine after Participants Had Ingested Betaine and Folic Acid for 6 Wk.
Observational studies could help in assessing the overall effect of phosphatidylcholine and betaine consumption on CVD risk. Recently, data on betaine and choline contents in foods became available [10], so it should now be feasible to assess the relationship between betaine and phosphatidylcholine intake and risk of CVD. In summary, the adverse effects of betaine on blood lipids make it less suitable as a homocysteine-lowering agent in healthy humans. Phosphatidylcholine may be more suitable because it only slightly increases serum triacylglycerol concentrations and does not affect LDL cholesterol. Folic acid has no adverse side effects on blood lipids, and therefore folic acid supplementation should remain the preferred homocysteine-lowering treatment in healthy humans.
Supporting Information
(95 KB DOC).
(840 KB PDF).
(585 KB PDF).
(470 KB PDF).
(47 KB DOC).
(47 KB DOC).
(47 KB DOC).
(38 KB DOC).
(25 KB DOC).
Trial Registration
Study 3 is registered at clinicaltrials.gov under identifier NCT00102843. Study 4 is registered at clinicaltrials.gov under identifier NCT00102232.
Patient Summary
Background
Homocysteine is an amino acid in the blood (amino acids are the building blocks of proteins). Too much homocysteine in the blood is related to a higher risk of stroke and heart disease. Mildly elevated levels (which are quite common) might promote atherosclerosis (furring up of the arteries), but this link has not been proven yet. Studies are currently under way to test whether reducing homocysteine levels in people who have mildly elevated levels could prevent heart disease or stroke. Again, the results are not yet known.
Why Was This Study Done?
In people who have dramatically elevated levels of homocysteine, different food supplements have been shown to bring down homocysteine concentrations and to reduce the risk of heart disease and strokes. These supplements include folic acid, vitamin B12, vitamin B6, betaine, and phosphatidylcholine. This study looked at the effects of betaine, folic acid, and phosphatidylcholine on blood lipids in healthy people. The researchers wanted to study blood lipids, especially “bad” cholesterol (LDL cholesterol), because they are known to influence the risk of heart disease.
What Did the Researchers Do?
They analyzed data from four different studies that looked at the ability of the three substances to lower homocysteine levels. They analyzed the blood samples (which had previously been used to measure homocysteine) for lipid levels.
What Did They Find?
They found that betaine increased the level of “bad” cholesterol. Folic acid did not affect lipid levels. The data for phosphatidylcholine were not conclusive.
What Does This Mean?
This suggests that the beneficial effects of betaine (which lowers homocysteine) might be undone at least in part by its negative effects on blood lipids. Based on these results, folic acid would be a better choice for people who want to lower their homocysteine levels, since folic acid doesn't cause a rise in “bad” cholesterol.
More Information Online
More information on the link between homocysteine and heart disease can be found at the following Web sites. Web site of the American Heart Association (search for “homocysteine” or “cholesterol”): http://www.americanheart.org/presenter.jhtml?identifier = 1200000
Web site of the Australian Heart Association (search for “homocysteine,” “cholesterol,” and “cardiovascular risk”): http://www.heartfoundation.com.au/
Web site of the British Heart Foundation (search for “homocysteine,” “cholesterol,” and “cardiovascular risk”): http://www.bhf.org.uk/
WebMD Web pages on homocysteine, cholesterol, and heart disease (or search http://webmd.com for “homocysteine” or “cholesterol”): http://my.webmd.com/content/pages/9/1675_57859; http://my.webmd.com/content/pages/9/1675_57815
Acknowledgments
We thank all volunteers for their participation, and our colleagues at TNO Quality of life and at the Division of Human Nutrition, Wageningen University, for their help. We thank J. Burema, MSc, for statistical advice, and Dr. H. J. Verkade, Academic Hospital Groningen, for advice on phosphatidylcholine metabolism.
Funding was provided by the Wageningen Centre for Food Sciences, an alliance of major Dutch food industries, Maastricht University, TNO Quality of Life, and Wageningen University and Research Centre, with financial support from the Dutch government. The funders approved the design of the studies, but had no role in data collection, analysis, and interpretation, in preparation of the manuscript, or the decision to publish
Abbreviations
- CVD
cardiovascular disease
- HDL
high-density lipoprotein
- LDL
low-density lipoprotein
- VLDL
very low density lipoprotein
Footnotes
Citation: Olthof MR, van Vliet T, Verhoef P, Zock PL, Katan MB (2005) Effect of homocysteine-lowering nutrients on blood lipids: Results from four randomised, placebo-controlled studies in healthy humans. PLoS Med 2(5): e135.
References
- Homocysteine Studies Collaboration. Homocysteine and risk of ischemic heart disease and stroke: A meta-analysis. JAMA. 2002;288:2015–2022. doi: 10.1001/jama.288.16.2015. [DOI] [PubMed] [Google Scholar]
- Schnyder G, Roffi M, Pin R, Flammer Y, Lange H, et al. Decreased rate of coronary restenosis after lowering of plasma homocysteine levels. N Engl J Med. 2001;345:1593–1600. doi: 10.1056/NEJMoa011364. [DOI] [PubMed] [Google Scholar]
- Schnyder G, Roffi M, Flammer Y, Pin R, Hess OM. Effect of homocysteine-lowering therapy with folic acid, vitamin B(12), and vitamin B(6) on clinical outcome after percutaneous coronary intervention: The Swiss Heart study: A randomized controlled trial. JAMA. 2002;288:973–979. doi: 10.1001/jama.288.8.973. [DOI] [PubMed] [Google Scholar]
- Wilcken DE, Wilcken B, Dudman NP, Tyrrell PA. Homocystinuria—The effects of betaine in the treatment of patients not responsive to pyridoxine. N Engl J Med. 1983;309:448–453. doi: 10.1056/NEJM198308253090802. [DOI] [PubMed] [Google Scholar]
- Smolin LA, Benevenga NJ, Berlow S. The use of betaine for the treatment of homocystinuria. J Pediatr. 1981;99:467–472. doi: 10.1016/s0022-3476(81)80352-6. [DOI] [PubMed] [Google Scholar]
- Bostom AG, Shemin D, Nadeau MR, Shih V, Stabler SP, et al. Short term betaine therapy fails to lower elevated fasting total plasma homocysteine concentrations in hemodialysis patients maintained on chronic folic acid supplementation. Atherosclerosis. 1995;113:129–132. doi: 10.1016/0021-9150(94)05466-v. [DOI] [PubMed] [Google Scholar]
- Steenge GR, Verhoef P, Katan MB. Betaine supplementation lowers plasma homocysteine in healthy men and women. J Nutr. 2003;133:1291–1295. doi: 10.1093/jn/133.5.1291. [DOI] [PubMed] [Google Scholar]
- Olthof MR, van Vliet T, Boelsma E, Verhoef P. Low dose betaine supplementation leads to immediate and long term lowering of plasma homocysteine in healthy men and women. J Nutr. 2003;133:4135–4138. doi: 10.1093/jn/133.12.4135. [DOI] [PubMed] [Google Scholar]
- Schwab U, Torronen A, Toppinen L, Alfthan G, Saarinen M, et al. Betaine supplementation decreases plasma homocysteine concentrations but does not affect body weight, body composition, or resting energy expenditure in human subjects. Am J Clin Nutr. 2002;76:961–967. doi: 10.1093/ajcn/76.5.961. [DOI] [PubMed] [Google Scholar]
- Zeisel SH, Mar MH, Howe JC, Holden JM. Concentrations of choline-containing compounds and betaine in common foods. J Nutr. 2003;133:1302–1307. doi: 10.1093/jn/133.5.1302. [DOI] [PubMed] [Google Scholar]
- Hayes KC, Pronczuk A, Cook MW, Robbins MC. Betaine in sub-acute and sub-chronic rat studies. Food Chem Toxicol. 2003;41:1685–1700. doi: 10.1016/s0278-6915(03)00196-0. [DOI] [PubMed] [Google Scholar]
- Matthews JO, Southern LL, Higbie AD, Persica MA, Bidner TD. Effects of betaine on growth, carcass characteristics, pork quality, and plasma metabolites of finishing pigs. J Anim Sci. 2001;79:722–728. doi: 10.2527/2001.793722x. [DOI] [PubMed] [Google Scholar]
- McGregor DO, Dellow WJ, Robson RA, Lever M, George PM, et al. Betaine supplementation decreases post-methionine hyperhomocysteinemia in chronic renal failure. Kidney Int. 2002;61:1040–1046. doi: 10.1046/j.1523-1755.2002.00199.x. [DOI] [PubMed] [Google Scholar]
- Zeisel SH. Dietary choline: Biochemistry, physiology, and pharmacology. Annu Rev Nutr. 1981;1:95–121. doi: 10.1146/annurev.nu.01.070181.000523. [DOI] [PubMed] [Google Scholar]
- Zeisel SH, Da Costa KA, Franklin PD, Alexander EA, Lamont JT, et al. Choline, an essential nutrient for humans. FASEB J. 1991;5:2093–2098. [PubMed] [Google Scholar]
- Olson RE, Vester JW, Gursey D, Davis N, Longman D. The effect of low-protein diets upon serum cholesterol in man. Am J Clin Nutr. 1958;6:310–324. doi: 10.1093/ajcn/6.3.310. [DOI] [PubMed] [Google Scholar]
- Olson RE, Jablonski JR, Taylor E. The effect of dietary protein, fat, and choline upon the serum lipids and lipoproteins of the rat. Am J Clin Nutr. 1958;6:111–118. doi: 10.1093/ajcn/6.2.111. [DOI] [PubMed] [Google Scholar]
- Niculescu MD, Zeisel SH. Diet, methyl donors and DNA methylation: Interactions between dietary folate, methionine and choline. J Nutr. 2002;132:2333S-2335S. doi: 10.1093/jn/132.8.2333S. [DOI] [PubMed] [Google Scholar]
- Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- Brenton DP, Cusworth DC, Dent CE, Jones EE. Homocystinuria. Clinical and dietary studies. Q J Med. 1966;35:325–346. [PubMed] [Google Scholar]
- Koc H, Mar MH, Ranasinghe A, Swenberg JA, Zeisel SH. Quantitation of choline and its metabolites in tissues and foods by liquid chromatography/electrospray ionization-isotope dilution mass spectrometry. Anal Chem. 2002;74:4734–4740. doi: 10.1021/ac025624x. [DOI] [PubMed] [Google Scholar]
- Institute of Medicine Food and Nutrition Board. Dietary references intakes for thiamin, riboflavin, niacin, vitamin B6, folate, vitamin B12, pantothenic acid, biotin, and choline. Washington (DC): National Academies Press. Available: http://www.nap.edu/books/0309065542/html/index.html . 1998 Accessed 4 April 2005. [PubMed] [Google Scholar]
- Beheshti I, Wessels LM, Eckfeldt JH. EDTA-plasma vs serum differences in cholesterol, high-density-lipoprotein cholesterol, and triglyceride as measured by several methods. Clin Chem. 1994;40:2088–2092. [PubMed] [Google Scholar]
- Curtin F, Altman DG, Elbourne D. Meta-analysis combining parallel and cross-over clinical trials. I: Continuous outcomes. Stat Med. 2002;21:2131–2144. doi: 10.1002/sim.1205. [DOI] [PubMed] [Google Scholar]
- Wilcken DE, Wilcken B. The natural history of vascular disease in homocystinuria and the effects of treatment. J Inherit Metab Dis. 1997;20:295–300. doi: 10.1023/a:1005373209964. [DOI] [PubMed] [Google Scholar]
- Greten H, Raetzer H, Stiehl A, Schettler G. The effect of polyunsaturated phosphatidylcholine on plasma lipids and fecal sterol excretion. Atherosclerosis. 1980;36:81–88. doi: 10.1016/0021-9150(80)90201-4. [DOI] [PubMed] [Google Scholar]
- Childs MT, Bowlin JA, Ogilvie JT, Hazzard WR, Albers JJ. The contrasting effects of a dietary soya lecithin product and corn oil on lipoprotein lipids in normolipidemic and familial hypercholesterolemic subjects. Atherosclerosis. 1981;38:217–228. doi: 10.1016/0021-9150(81)90119-2. [DOI] [PubMed] [Google Scholar]
- Prack M, Sanborn T, Waugh D, Simkin H, Bennett Clark S, et al. Perkins EG, Visek WJ. Dietary fats and health. Champaign (Illinois): American Oil Chemist's Society; 1983. Effects of polyunsaturated lecithin on plasma and lipoprotein cholesterol and fatty acids in normal men; pp. 689–697. [Google Scholar]
- 29. Kesaniemi YA, Grundy SM. Effects of dietary polyenylphosphatidylcholine on metabolism of cholesterol and triglycerides in hypertriglyceridemic patients. Am J Clin Nutr. 1986;43:98–107. doi: 10.1093/ajcn/43.1.98. [DOI] [PubMed] [Google Scholar]
- Oosthuizen W, Vorster HH, Vermaak WJ, Smuts CM, Jerling JC, et al. Lecithin has no effect on serum lipoprotein, plasma fibrinogen and macro molecular protein complex levels in hyperlipidaemic men in a double-blind controlled study. Eur J Clin Nutr. 1998;52:419–424. doi: 10.1038/sj.ejcn.1600580. [DOI] [PubMed] [Google Scholar]
- Dodson WL, Sachan DS. Choline supplementation reduces urinary carnitine excretion in humans. Am J Clin Nutr. 1996;63:904–910. doi: 10.1093/ajcn/63.6.904. [DOI] [PubMed] [Google Scholar]
- Yao ZM, Vance DE. The active synthesis of phosphatidylcholine is required for very low density lipoprotein secretion from rat hepatocytes. J Biol Chem. 1988;263:2998–3004. [PubMed] [Google Scholar]
- Ridgway ND, Vance DE. Kinetic mechanism of phosphatidylethanolamine N-methyltransferase. J Biol Chem. 1988;263:16864–16871. [PubMed] [Google Scholar]
- Emmert JL, Webel DM, Biehl RR, Griffiths MA, Garrow LS, et al. Hepatic and renal betaine-homocysteine methyltransferase activity in pigs as affected by dietary intakes of sulfur amino acids, choline, and betaine. J Anim Sci. 1998;76:606–610. doi: 10.2527/1998.762606x. [DOI] [PubMed] [Google Scholar]
- Shibata T, Akamine T, Nikki T, Yamashita H, Nobukuni K. Synthesis of betaine-homocysteine S-methyltransferase is continuously enhanced in fatty livers of thyroidectomized chickens. Poult Sci. 2003;82:207–213. doi: 10.1093/ps/82.2.207. [DOI] [PubMed] [Google Scholar]
- Sehayek E, Wang R, Ono JG, Zinchuk VS, Duncan EM, et al. Localization of the PE methylation pathway and SR-BI to the canalicular membrane: Evidence for apical PC biosynthesis that may promote biliary excretion of phospholipid and cholesterol. J Lipid Res. 2003;44:1605–1613. doi: 10.1194/jlr.M200488-JLR200. [DOI] [PubMed] [Google Scholar]
- Sowden MP, Collins HL, Smith HC, Garrow TA, Sparks JD, et al. Apolipoprotein B mRNA and lipoprotein secretion are increased in McArdle RH-7777 cells by expression of betaine-homocysteine S-methyltransferase. Biochem J. 1999;341:639–645. [PMC free article] [PubMed] [Google Scholar]
- Holven KB, Haugstad TS, Holm T, Aukrust P, Ose L, et al. Folic acid treatment reduces elevated plasma levels of asymmetric dimethylarginine in hyperhomocysteinaemic subjects. Br J Nutr. 2003;89:359–363. doi: 10.1079/BJN2002779. [DOI] [PubMed] [Google Scholar]
- Liem A, Reynierse-Buitenwerf GH, Zwinderman AH, Jukema JW, van Veldhuisen DJ. Secondary prevention with folic acid: Effects on clinical outcomes. J Am Coll Cardiol. 2003;41:2105–2113. doi: 10.1016/s0735-1097(03)00485-6. [DOI] [PubMed] [Google Scholar]
- Verhaar MC, Wever RM, Kastelein JJ, van Loon D, Milstien S, et al. Effects of oral folic acid supplementation on endothelial function in familial hypercholesterolemia. A randomized placebo-controlled trial. Circulation. 1999;100:335–338. doi: 10.1161/01.cir.100.4.335. [DOI] [PubMed] [Google Scholar]
- Toole JF, Malinow MR, Chambless LE, Spence JD, Pettigrew LC, et al. Lowering homocysteine in patients with ischemic stroke to prevent recurrent stroke, myocardial infarction, and death: The Vitamin Intervention for Stroke Prevention (VISP) randomized controlled trial. JAMA. 2004;291:565–575. doi: 10.1001/jama.291.5.565. [DOI] [PubMed] [Google Scholar]
- Wald DS, Law M, Morris JK. Homocysteine and cardiovascular disease: Evidence on causality from a meta-analysis. BMJ. 2002;325:1202–1206. doi: 10.1136/bmj.325.7374.1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klerk M, Verhoef P, Clarke R, Blom HJ, Kok FJ, et al. MTHFR 677C→T polymorphism and risk of coronary heart disease: A meta-analysis. JAMA. 2002;288:2023–2031. doi: 10.1001/jama.288.16.2023. [DOI] [PubMed] [Google Scholar]
- Natarajan S, Glick H, Criqui M, Horowitz D, Lipsitz SR, et al. Cholesterol measures to identify and treat individuals at risk for coronary heart disease. Am J Prev Med. 2003;25:50–57. doi: 10.1016/s0749-3797(03)00092-8. [DOI] [PubMed] [Google Scholar]
- Kinosian B, Glick H, Preiss L, Puder KL. Cholesterol and coronary heart disease: Predicting risks in men by changes in levels and ratios. J Investig Med. 1995;43:443–450. [PubMed] [Google Scholar]
- Stampfer MJ, Sacks FM, Salvini S, Willett WC, Hennekens CH. A prospective study of cholesterol, apolipoproteins, and the risk of myocardial infarction. N Engl J Med. 1991;325:373–381. doi: 10.1056/NEJM199108083250601. [DOI] [PubMed] [Google Scholar]
- Hokanson JE, Austin MA. Plasma triglyceride level is a risk factor for cardiovascular disease independent of high-density lipoprotein cholesterol level: A meta-analysis of population-based prospective studies. J Cardiovasc Risk. 1996;3:213–219. [PubMed] [Google Scholar]
- Jeppesen J, Hein HO, Suadicani P, Gyntelberg F. Triglyceride concentration and ischemic heart disease: An eight-year follow-up in the Copenhagen Male Study. Circulation. 1998;97:1029–1036. doi: 10.1161/01.cir.97.11.1029. [DOI] [PubMed] [Google Scholar]
- Stampfer MJ, Krauss RM, Ma J, Blanche PJ, Holl LG, et al. A prospective study of triglyceride level, low-density lipoprotein particle diameter, and risk of myocardial infarction. JAMA. 1996;276:882–888. [PubMed] [Google Scholar]
- Metcalfe LD, Schmitz AA, Pelka JR. Rapid preparation of fatty acid esters from lipids for gas chromatographic analysis. Anal Chem. 1966;38:514–515. [Google Scholar]
- Olthof MR, Brink EJ, Katan MB, Verhoef P. Choline supplemented as phosphatidylcholine decreases fasting and post-methionine plasma homocysteine concentrations in healthy men. Am J Clin Nutr. 2005 doi: 10.1093/ajcn.82.1.111. In press. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(95 KB DOC).
(840 KB PDF).
(585 KB PDF).
(470 KB PDF).
(47 KB DOC).
(47 KB DOC).
(47 KB DOC).
(38 KB DOC).
(25 KB DOC).