Abstract
Open reading frame 71 (ORF 71) of Kaposi's sarcoma-associated herpesvirus (KSHV) encodes a death effector domain-containing protein that is homologous to cellular FLIPs (FLICE-inhibitory proteins) and is proposed to inhibit Fas-mediated apoptosis. Transcripts bearing ORF 71 (v-FLIP) sequences are present in all latently infected cells. However, mapping studies reveal these to be bi- or tricistronic mRNAs with ORF 71 located 3′ to ORFs 72 (v-cyclin) and 73 (latency-associated nuclear antigen), raising the question of how efficient expression of v-FLIP is achieved. We explored this question by examining the expression of model bicistronic (v-cyclin/LUC) transcripts in which a luciferase (LUC) reporter replaced v-FLIP coding sequences. SLK spindle cells transfected with such constructs efficiently expressed luciferase from the 3′ position, and this expression was independent of the expression of the 5′ v-cyclin gene. Surprisingly, transcript mapping showed that in these cultures, efficient splicing occurred to remove v-cyclin sequences and generate monocistronic LUC transcripts. Similar splicing events produced monocistronic v-FLIP transcripts in KSHV-infected primary effusion lymphoma cells. However, these RNAs were of low abundance and were inducible by treatment with 12-O-tetradecanoylphorbol-13-acetate. Examination of the more abundant bicistronic latent RNAs revealed the presence of an efficient internal ribosome entry site (IRES) overlapping ORF 72 coding sequences. Thus, two potential mechanisms exist for v-FLIP expression, but the evidence suggests that IRES-mediated internal translational initiation on latent polycistronic mRNAs is the principal source of v-FLIP in latency.
Infection by Kaposi's sarcoma-associated herpesvirus (KSHV) (also called human herpesvirus 8) is strongly linked to development of Kaposi's sarcoma (4, 26). KSHV is a lymphotropic (γ-2) herpesvirus whose principal target is the B cell; rarely, infection of such cells leads to lymphoproliferative disease, including primary effusion lymphoma (PEL) and multicentric Castleman's disease (3, 21, 26). In all of these neoplasms, KSHV is principally found as a latent infection, though lytic reactivation is also found in a small subset of cells in these lesions (16, 17, 22). Since latent viral gene products are thought to be the prime effectors of cell proliferation in other gammaherpesvirus infections, much attention has been focused on the KSHV latency program. One important cluster of KSHV latency genes maps to open reading frames (ORFs) 73, 72, and 71, encoding the viral latency-associated nuclear antigen (LANA), v-cyclin, and v-FLIP, respectively. LANA plays a role in maintenance of the latent viral genome but also has been shown to block function of p53 and pRb (2, 6, 9, 14). v-cyclin is a viral homolog of cellular cyclin D and may play a role in cell cycle deregulation (13, 18), though cell immortalization has never been observed following KSHV infection of primary B cells. v-FLIP is a viral death effector domain-containing protein that is homologous to cellular FLICE (caspase-8)-inhibitory proteins (FLIPs). v-FLIPS of other herpesviruses are known to block Fas-mediated apoptosis (25) by preventing recruitment of caspase-8 to the death-inducing signaling complex, and a similar activity has been reported for KSHV v-FLIP (8). On this basis, v-FLIP is assumed to play an important role in extending the survival of latently infected cells.
Analysis of the fine structure of latent mRNAs from the ORF 71 to 73 coding regions has thus far revealed no monocistronic v-FLIP mRNA (7, 19, 20, 24). Rather, v-FLIP coding sequences are found as the 3′ ORF in bi- and tricistronic mRNAs bearing either ORF 72 alone or ORFs 73 and 72 upstream of the v-FLIP AUG (see Fig. 1A). This has raised the question of how v-FLIP expression is achieved in latency. Possibilities include (i) that v-FLIP is encoded from the bi- or tricistronic mRNAs via either leaky ribosomal scanning or direct, internal ribosome entry site (IRES)-mediated initiation or (ii) that v-FLIP is expressed from a previously overlooked monocistronic mRNA. Here we present data indicating that multiple mechanisms may operate in KSHV infection, but IRES-mediated internal initiation appears to be the predominant expression strategy in latency.
FIG. 1.
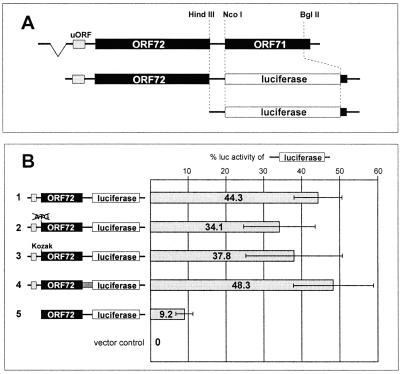
Luciferase expression from bicistronic ORF 72/luciferase reporter constructs. (A) Design of a bicistronic ORF 72/luciferase construct. A genomic fragment containing the second exon of the bicistronic ORF 72/71 mRNA was cloned in the vector pCDNA3, and ORF 71 was replaced by the coding region of the Photinus luciferase. From the resulting construct, pCMV 72/LUC, the region upstream of a HindIII site overlapping the ORF 72 stop codon was excised to generate a monocistronic luciferase construct (pCMV LUC) serving as a positive control. (B) Expression of luciferase from bicistronic reporter constructs in SLK cells. The bicistronic construct pCMV 72/LUC described above is shown in line 1. Additional reporters derived from this construct were modified as follows: the ORF 72 start codon was eliminated (line 2); the nucleotides surrounding the ORF 72 start codon were optimized for translational initiation (12) (line 3); the noncoding segment between ORF 72 and ORF 71 was replaced by an unrelated nucleotide sequence of similar size (line 4); and the region upstream of the ORF 72 start codon was deleted (line 5). The luciferase activity expressed by the monocistronic luciferase construct pCMV LUC described above was set to 100%. Relative luciferase activity of the bicistronic constructs is shown as a percentage of that activity. Each transfection was performed in triplicate per experiment, and the values shown are averaged from three independent experiments.
MATERIALS AND METHODS
Cell lines.
293 and SLK (10) cells were maintained in Dulbecco's modified Eagle medium H21 supplemented with 10% fetal calf serum (FCS). The KSHV-negative Burkitt's lymphoma cell line BJAB was grown in RPMI 1640 supplemented with 10% FCS. The KSHV-positive PEL cell lines BCBL-1 (17) and BC-3 (1) were cultured in RPMI 1640 supplemented with 10% FCS, 0.005 mM 2-mercaptoethanol, 1 mM sodium pyruvate, and 2 mM l-glutamine or RPMI 1640 supplemented with 20% FCS, respectively. For the induction of lytic viral replication, cells were treated with 12-O-tetradecanoylphorbol-13-acetate (TPA) at a final concentration of 20 ng/ml for 48 h.
Plasmids.
For the generation of bicistronic ORF 72/71 constructs, a genomic fragment containing the second exon of the bicistronic ORF 72/71 mRNA (nucleotide positions 123776 to 122037 of the KSHV genome; GenBank accession number U75698) which includes the 3′ nontranslated region (NTR) was PCR amplified with primers containing KpnI and EcoRI restriction sites and cloned in the vector pCDNA3 (Invitrogen) to produce construct pCMV 72/71. An alternative 5′ primer was used for the generation of construct pCMV 72/71ΔuORF (containing nucleotides 123583 to 122037 of the KSHV genome), which lacks most of the 5′ region of the bicistronic transcript (including the 5′ upstream open reading frame [uORF]).
For the generation of bicistronic ORF 72/luciferase (LUC) reporter constructs, a BamHI/EcoRI restriction fragment containing ORF 71 was removed from the construct pCMV 72/71 and cloned into the vector pRSETB (Invitrogen), ORF 71 was excised with the restriction enzymes NcoI and BglII and replaced by the coding region of the Photinus luciferase amplified from the vector pGL3-basic (Promega), and the cassette was reinserted into the parental construct pCMV 72/71 using the BamHI and EcoRI restriction sites to yield construct pCMV 72/LUC. Additional reporters were generated based on these constructs: (i) the region upstream of a HindIII site overlapping the ORF 72 stop codon was excised to generate a monocistronic luciferase construct (pCMV LUC) which served as a positive control; overlapping PCR with primers containing the desired mutations was used (ii) to eliminate the ORF 72 start codon (GCTCGCCACTCTATATGGCA → GCTCGCCACTCTATCGATCA; the ORF 72 start codon is shown in bold and mutated nucleotides are underlined) or (iii) to optimize the nucleotides surrounding the ORF 72 start codon with regard to the efficiency of translational initiation (GCTCGCCACTCTATATGGCA → GCTCGGCCGCCACCATGGCA) (12); (iv) the 82-bp noncoding segment between the HindIII and NcoI sites overlapping the ORF 72 stop codon or the ORF 71 start codon, respectively, was replaced by an unrelated nucleotide sequence of 83 bp consisting of a ScaI-NcoI polylinker fragment of the vector pGL3 basic (Invitrogen); (v) with the exception of the 16 nucleotides (nt) immediately upstream of the ORF 72 start codon, the complete 5′ region, including a small uORF, was removed.
Bicistronic Renilla/Photinus luciferase (RP) reporter constructs are based on the construct pRP (kindly provided by Anne E. Willis, University of Leicester, Leicester, United Kingdom [23]). The construct contains the coding region of the Photinus luciferase downstream of the gene for the Renilla luciferase. Fragments spanning 232, 474, 658, or 856 bp of the region upstream of the ORF 71 start codon were PCR amplified with primers containing EcoRI and NcoI restriction sites and inserted in the EcoRI and NcoI sites located in the intercistronic region of the construct pRP. To generate a negative control, an NcoI fragment containing 856 bp of the region upstream of ORF 71 was removed from the bicistronic pCMV 72/LUC construct optimized for translational initiation (see above) and inserted into the NcoI site of the construct pRP in an antisense orientation (−856as). Positive controls were obtained by cloning the IRES elements of the encephalomyocarditis virus (EMCV) or the poliovirus (nt 260 to 851 of the EMCV genome, GenBank accession number NC_001479, and nucleotides 66 to 755 of the poliovirus genome, GenBank accession number NC_002058). Finally, because the original vector pRP contains a chimeric intron immediately downstream of the SV40 promoter, the expression cassettes from all bicistronic constructs were excised with EcoRV and XbaI and inserted into the vector pCDNA3.1/Zeo(+) (Invitrogen) to yield constructs pCMV:RP, pCMV:RP −232, pCMV:RP −474, pCMV:RP −658, pCMV:RP −856, pCMV:RP −856as, pCMV:RP ECMV, and pCMV:RP Polio.
Transfections.
SLK cells were transfected with plasmid DNA using the Fugene 6 transfection reagent (Boehringer Mannheim) according to the manufacturer's recommendations and harvested after 36 h. For the introduction of in vitro-transcribed RNA into 293 cells by electroporation, cells were trypsinized, washed three times with phosphate-buffered saline and resuspended in phosphate-buffered saline at a concentration of 4 × 106 cells/ml. Aliquots (400 μl each) of the cell suspension (1.6 × 106 cells) were mixed with 10 μg of in vitro-transcribed RNA in cuvettes with a 0.2-cm electrode gap and immediately subjected to electroporation, using an Electro Cell Manipulator 600 (BTX Inc., San Diego, Calif.) with the following settings: 150 V, 500 μF, 24 Ω. After the pulse, cells were resuspended in complete medium and incubated at 37°C in a 5% CO2 incubator. Aliquots of the cells (4 × 105 cells) were harvested after 1.5, 2.5, 3.5, and 4.5 h and analyzed for luciferase expression.
RNA isolation and Northern blotting.
Total RNA was isolated using the RNAzol reagent (Tel-Test, Inc., Friendswood, Tex.) according to the supplier's instructions. For Northern blotting, 12 μg of RNA was separated on a 1% formaldehyde gel, transferred to nylon membranes, and analyzed by hybridization to radioactively labeled probes as described previously (11).
RT-PCR and Southern blotting.
Reverse transcription-PCR (RT-PCR) was performed using the Titan One-Tube RT-PCR System (Boehringer Mannheim) following the manufacturer's instructions. For the analysis of spliced RNA transcripts produced in transiently transfected SLK cells, total RNA was isolated from SLK cells transfected with pCMV 72/LUC and subjected to RT-PCR with primers specific for nt 123777 to 123762 and 122813 to 122790 of the KSHV genome, initiating 210 bp upstream and 4 bp downstream of the ORF 72 start and stop codons, respectively. RT-PCR products of 988 bp (amplified from the unspliced transcript) and 253 bp (amplified from the spliced transcript) were obtained and analyzed by sequencing.
For the detection of monocistronic ORF 71 transcripts in PEL cells, 1 μg of total RNA was subjected to cDNA synthesis with a 3′ primer specific for nt 122541 to 122566 of the KSHV genome (5′-CGCTAACAGGGGAAACGTTAACCTGC) for 35 min at 50°C, followed by 30 cycles of PCR amplification using the same 3′ primer and a 5′ primer specific for nt 127894 to 127871 of the KSHV genome (5′-GCGCCACGAAGCAGTCACGTCCC). Aliquots of the samples (15 μl of a 50-μl reaction mixture) were analyzed by Southern blotting as described previously (15) using a probe encompassing nucleotides 122645 to 122859 of the KSHV genome.
In vitro transcription and translation.
Transcription and translation of bicistronic ORF 71/72 constructs were carried out using the TNT T7 quick coupled transcription/translation system (Promega) following the manufacturer's instructions. Aliquots of the reaction mixtures (3 μl of a 50-μl reaction mixture) were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and subsequent autoradiography. For the transfection of bicistronic reporter transcripts into 293 cells, large-scale in vitro synthesis of capped RNAs was carried out using the mMESSAGE mMACHINE kit (Ambion, Austin, Tex.) according to the manufacturer's instructions. Briefly, 1 μg of linearized plasmid DNA was transcribed for 2 h at 37°C in a reaction mixture with a final 7-methyl-GTP:GTP ratio of 1.3:1. After addition of DNase 1, reaction mixtures were incubated for 15 min at 37°C to remove template DNA; the complete loss of template DNA was verified by gel electrophoresis. RNA was recovered by precipitation with 1/2 volumes of a 7.5 M lithium chloride–75 mM EDTA solution, washed twice with 70% ethanol, and resuspended in H2O.
Luciferase assays.
For quantification of luciferase expressed from bicistronic ORF 72/luciferase RNAs, SLK cells were cotransfected with the various bicistronic constructs and a β-galactosidase expression construct. Luciferase activity was determined using the luciferase assay system (Promega) according to the manufacturer's instructions in a TD-20/20 luminometer (Turner Designs). Luciferase activity was normalized for transfection efficiency, as determined by quantification of β-galactosidase activity with Promega's β-galactosidase enzyme assay system. The activities of Renilla and Photinus luciferase in SLK cells transfected with bicistronic RP constructs or 293 cells electroporated with bicistronic RP-RNA transcripts were quantitated simultaneously with Promega's dual-luciferase reporter assay system. The kit allows discrimination of the two enzymatic activities due to different cofactor requirements.
RESULTS AND DISCUSSION
Experimental strategy.
To explore the translational potential of the bicistronic ORF 72/71 mRNA, we constructed a model of this transcript in which the v-FLIP coding region was replaced by a luciferase reporter. Plasmid pCMV 72/LUC (Fig. 1A, line 2) employs a cytomegalovirus (CMV) promoter to drive expression of a transcript containing the authentic 5′ NTR, v-cyclin gene, intergenic region, and v-FLIP AUG of the bicistronic RNA's second exon; however, the v-FLIP start codon is fused to a LUC reporter to permit sensitive enzymatic detection of its translation product. If either leaky scanning or internal initiation is used, efficient luciferase expression should result. Scanning can be readily differentiated from internal ribosome entry in such a system, because in scanning models, altering the utilization of the 5′ AUG influences expression of the 3′ cistron; such maneuvers, however, do not affect IRES-mediated internal initiation at the 3′ cistron. Accordingly, in addition to the parental pCMV 72/LUC construct, we constructed derivatives in which the 5′ v-cyclin AUG was ablated (Fig. 1B, line 2) or in which its context was changed to conform to a consensus sequence optimized for translational initation (Fig. 1B, line 3) (12). In constructing these plasmids, we noted the existence of a previously unrecognized small ORF in the 5′ NTR of the ORF 72/71 mRNA (termed uORF) (Fig. 1A); since such small ORFs sometimes play a role in translational control, we also constructed a derivative in which it was deleted (Fig. 1B, line 5). Each construct was transfected into SLK cells, an immortalized but KSHV-negative spindle cell line derived from a classical Kaposi's sarcoma tumor (10). Thirty-six hours later, luciferase activity was measured in cell extracts and compared to that expressed by pCMV LUC, encoding a monocistronic RNA driven by the same promoter.
Expression of luciferase is efficient and is independent of v-cyclin translation.
As shown in Fig. 1B, substantial amounts of luciferase were expressed from all of the bicistronic constructs—levels were generally within 40% of that of the monocistronic control. Interestingly, ablation of the 5′ v-cyclin AUG did not enhance expression of the reporter, nor did optimization of that AUG impair reporter expression. These findings were consistent with an IRES-directed mechanism for v-FLIP expression. However, a paradoxical result with the remaining mutant led us to examine the situation more closely. Deletion of the uORF in the 5′-NTR appeared to reduce reporter expression to 10% of that of the monocistronic construct. This result does not accord well with either a scanning or an IRES model, unless the deletion incidentally affected the structure or accumulation of the mRNA.
mRNA structure analysis.
Accordingly, we examined the transcripts produced by all these constructs by Northern blotting (Fig. 2). Total RNA prepared from transfected SLK cells was analyzed with probes specific for either luciferase (Fig. 2A) or ORF 72/v-cyclin (Fig. 2B). Surprisingly, most constructs produced two sets of transcripts—an upper doublet that hybridized with both probes, consistent with the expected bicistronic RNA, and a lower doublet that annealed only to luciferase sequences. [The doublet character of each band is due to the alternative use of one of two poly(A) signals present in the construct.] Since there is no existing evidence for a promoter within ORF 72, we surmised that the lower species must have been derived by splicing out v-cyclin sequences from a bicistronic pre-mRNA. Indeed, inspection of the nucleotide sequence of the locus revealed a consensus splice donor and acceptor that could account for these findings. The candidate splice donor was located at nt 123595 between the 5′ uORF and the v-cyclin AUG, and the putative splice acceptor was positioned distally within ORF 72, 14 nt downstream of the last internal AUG (Fig. 3). Consistent with this, we noted that the deletion of the 5′ uORF (Fig. 1B, line 5) also encompassed the potential splice donor; this deletion ablated the production of the lower bands while preserving synthesis of the upper (unspliced) RNA species (Fig. 2, lane 5).
FIG. 2.
Analysis of luciferase-bearing transcripts in cells transfected with bicistronic reporters. SLK cells were transfected with the bicistronic constructs illustrated in Fig. 1 (lanes 1 to 5), the monocistronic luciferase control pCMV LUC (lane 6), or a vector control (rightmost lane). Total RNA was extracted 36 h posttransfection and analyzed by Northern blotting, using probes specific for luciferase (A) or the 5′-proximal 600 bp of ORF 72 (B). The expected transcript sizes were approximately 3.2 and 3.0 kb for the unspliced bicistronic transcripts in lanes 1 to 4 or lane 5, respectively, and 2.2 kb for the monocistronic luciferase construct in lane 6. The position of the spliced transcripts in lanes 1 to 4 is marked by an arrow in panel A. Bands appear as doublets as a result of usage of two different transcriptional termination sites. The constructs contain the KSHV polyadenylation signal downstream of ORF 71 in addition to the bovine growth hormone polyadenylation signal which is provided by the vector pCDNA3.
FIG. 3.
A 735-nt segment spanning most of the ORF 72 coding region is flanked by functional splice sites. The location of splice sites was determined by RT-PCR amplification and sequencing of splice products as described in Materials and Methods. The genomic sequences flanking the splice donor and acceptor sites are shown in the left and right text boxes, respectively. Within the text boxes, slashes indicate the exact position of the splice donor and acceptor sites, located at nt 123595 and 122859 of the KSHV genome, respectively. Conserved nucleotides (shown in bold) at both sites and a pyrimidine-rich tract (shown underlined) upstream of the acceptor site identify them as consensus splice sites. The start codon of ORF 72 is shown framed within the left text box. The structures of the bicistronic ORF 72/71 and the spliced monocistronic ORF 71 transcripts are illustrated in the lower part of the figure. The locations of the splice sites are indicated by lines emanating from the text boxes. Simple arrows indicate the start codon and the positions of additional AUG codons within the coding region of ORF 72. Arrows marked with asterisks indicate the Kozak start codons (12) which initiate the 5′ uORF and ORF 71. Note that the splice sites are situated to remove the ORF 72 start codon as well as all internal AUG codons, leaving a 66-bp fragment of ORF 72 devoid of potential translation initiation sites.
To verify that splicing was indeed occurring and to map the putative splice junctions, we used RT-PCR to determine the fine structure of the lower RNA band. Primers flanking ORF 72 were used to PCR amplify reverse transcripts of RNA from pCMV 72/LUC-transfected SLK cells (data not shown). Sequencing of the RT-PCR product established the splice junction shown in Fig. 3, exactly in accord with the predicted splice junctions.
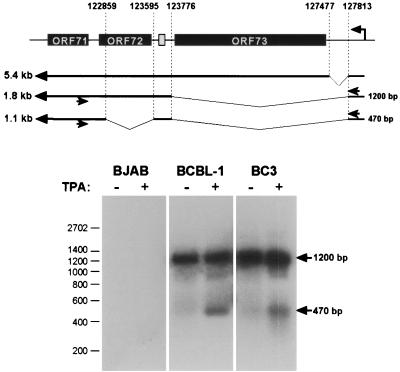
Does this splicing event occur in bona fide KSHV infection, or is it limited to cells transiently transfected with recombinant plasmids? To answer this question, we examined several PEL cell lines that harbor latent KSHV genomes, both before and after lytic induction with TPA. As previously reported (7, 19, 20, 24), neither BCBL-1 nor BC-3 cells harbored monocistronic v-FLIP mRNAs detectable by Northern blotting (data not shown). RT-PCR analysis was carried out to look for transcripts of lower abundance. Since the promoter for the ORF 73/72/71 transcription unit is 5′ to ORF 73, we positioned the 5′ primer in the NTR upstream of the LANA coding region, while the 3′ primer was located within v-FLIP coding sequences (Fig. 4, top). Utilization of the known splice sites of this transcription unit could generate a monocistronic v-FLIP RNA in two ways: (i) a single splice from the donor in the LANA 5′-NTR to the acceptor upstream of the v-FLIP AUG (generating a 290-nt PCR product) or (ii) a splice from the LANA 5′-NTR donor to the known acceptor 5′ to ORF 72, followed by removal of the cyclin AUGs by a second splicing event identical to that seen in our luciferase constructs (generating a 470-bp PCR product). In the latter case, the doubly spliced v-FLIP RNA would be derived from the same pre-RNA that generates singly spliced v-cyclin transcripts (Fig. 4, top). Figure 4 (bottom) shows that only doubly spliced monocistronic v-FLIP transcripts are detected by this analysis. Interestingly, these RNAs are barely detectable prior to TPA treatment but become somewhat more abundant following TPA exposure. Since 5′ primers located upstream of the LANA promoter did not detect these species, they do not emanate from read-through from upstream lytic promoters (not shown). The increase in their abundance may reflect stabilization of the RNA in the environment of lytic replication or use of a previously unrecognized cryptic lytic promoter in the region. The very low abundance of the RNA, however, precluded mapping of its start site.
FIG. 4.
A monocistronic ORF 71 transcript is generated in vivo in PEL cells. Top, location of hybridization sites of RT-PCR primers (indicated by arrows) specific for nt 122566 to 122541 (within ORF 71) and 127871 to 127849 (upstream of ORF 73) of the KSHV genome. The expected size of products amplified from the 1.8-kb bicistronic ORF 72/71 mRNA (1,200 bp) or a putative 1.1-kb monocistronic ORF 71 mRNA (470 bp) is indicated to the right of the transcripts. Bottom, RT-PCR from KSHV-positive and -negative cells using the primers described above. The KSHV-positive PEL cell lines BCBL-1 and BC-3 and the KSHV-negative Burkitt's lymphoma cell line BJAB were treated with TPA to induce lytic replication. Total RNA was isolated from uninduced or induced cells and subjected to RT-PCR as described in Materials and Methods. Aliquots of the samples were analyzed by Southern blotting using a probe encompassing nt 122645 to 122859 of the KSHV genome. The positions of amplification products derived from the bicistronic ORF 72/71 and the monocistronic ORF 71 transcripts are marked by arrows. Sequencing confirmed that the 470-bp product was amplified from a monocistronic RNA resulting from a double splice as illustrated. A product of 290 bp indicative of an ORF 71 transcript generated by a single splice removing nucleotides 122860 to 127812 (see the text) was not detected.
An IRES in the bicistronic ORF 72/71 mRNA.
The presence of a low-abundance monocistronic ORF 71 RNA as well as a high-abundance bicistronic ORF 72/71 RNA raises the question of which one contributes more to the translation of v-FLIP in KSHV infection. Our analysis of luciferase expression in reporter constructs (Fig. 1) was fully compatible with expression from the v-FLIP AUG via an IRES-mediated mechanism from the bicistronic ORF 72/71 RNA, but in transfected SLK cells the high abundance of the monocistronic transcript could have obscured a contribution from this mechanism. To search directly for an IRES within the 72/71 mRNA, we carried out additional experiments. First, we replaced the intergenic region in pCMV 72/LUC with a foreign sequence of comparable length. As shown in Fig. 1B (line 4), this substitution did not affect LUC expression, indicating that an IRES, if present, is likely to be upstream of these sequences.
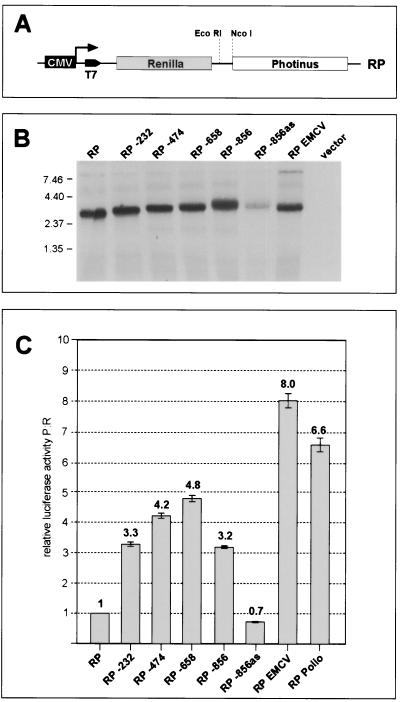
We then cloned fragments from the ORF 72 coding region into the intergenic region of a bicistronic transcription unit composed of two luciferase coding regions (Fig. 5A). The 5′ LUC gene is derived from Renilla, while the 3′ LUC is from Photinus. The two enzymes have different cofactor requirements and therefore can be readily differentiated from one another. All potential splice donor sites were removed from the 5′-NTR of this vector, to avoid problems with splicing to the acceptor site within ORF 72. Fragments of 232, 474, 658, or 856 bp from the region upstream of the ORF 71 AUG were inserted between the two LUC genes, and each construct was transfected into SLK cells. Analysis of the resulting RNAs by Northern blotting confirmed that only a single, bicistronic transcript was being produced in each case (Fig. 5B). We then measured the activities of the 5′ and 3′ luciferase proteins produced in such transfectants; the ratio of Photinus to Renilla activities (P:R) provides an index of the efficiency of internal initiation at the AUG codon of the 3′ reporter. The P:R ratio generated by the parental (IRES-negative) vector was set to 1.0. As shown in Fig. 5C, all four fragments of ORF 72 DNA cloned in the sense orientation allowed substantial expression of the downstream reporter, while a control fragment cloned in the antisense orientation did not. (Although the transcripts from this antisense control were expressed less efficiently [Fig. 5B], expressing the results as the P:R ratio normalizes for this effect and affirms the absence of IRES activity in this construct.) These findings indicate that an IRES element is indeed present within these ORF 72 sequences. The P:R activity ratios generated by these constructs were 30 to 50% of those produced by the strong IRES elements from EMCV and poliovirus (5), and a fragment as small as 232 nt was sufficient to allow efficient internal initiation.
FIG. 5.
An IRES is located upstream of ORF 71. (A) Basic bicistronic Renilla/Photinus (RP) luciferase reporter construct used to detect IRES activity. The construct pCDNA3.1 RP contains the coding region of the Photinus luciferase downstream of the gene for the Renilla luciferase. Expression is driven by the CMV promoter in transfection experiments; a T7 promoter is also available for in vitro transcription (see Fig. 6). Fragments of 232, 474, 658, or 856 bp (the largest fragment contains the complete ORF 72 coding region) upstream of the ORF 71 start codon were inserted in the EcoRI and NcoI sites located in the intercistronic region of the vectors. The authentic ORF 71 start codon was fused to the Photinus luciferase ORF. The 856-bp fragment was also inserted in an antisense orientation (pCMV:RP −856as). The IRES elements from the poliovirus and EMCV served as positive controls. (B) Analysis of transcripts expressed by bicistronic Renilla/Photinus luciferase constructs. Total RNA was isolated from SLK cells 36 h after transfection with the indicated constructs and analyzed by Northern blotting using a probe specific for the Photinus luciferase. Staining of the gel with ethidium bromide showed equal amounts of total RNA loaded in each lane (not shown). (C) IRES activity mediated by the region upstream of ORF 71. SLK cells transfected with the bicistronic constructs were harvested 36 h posttransfection, and Renilla and Photinus luciferase activities were quantitated. The relative luciferase activity (ratio of Photinus to Renilla luciferase activity [P:R]) was calculated and normalized against the activity of the construct pCDNA3.1 RP, whose P:R ratio was set to 1. Each transfection was performed in triplicate.
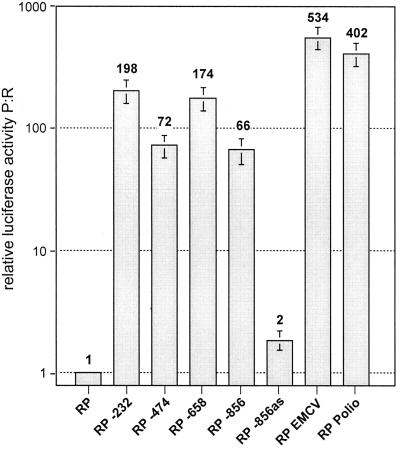
To further substantiate these findings, we transcribed these same constructs in vitro with T7 RNA polymerase and examined the in vivo translatability of the resulting RNAs (after degrading the DNA template with DNase) by transfecting them into SLK cells; 1.5 to 4.5 h later, cell extracts were assayed for both luciferase activities and the P:R activity ratio was calculated as before. The presence of KSHV sequences significantly increased the expression of the downstream Photinus luciferase relative to that of the upstream Renilla enzyme; in this assay, the P:R ratios mediated by the KSHV IRES were between 16 and 49% of those mediated by the poliovirus IRES (Fig. 6). Interestingly, the individual fragments did not all behave identically (relative to one another) in the two assays—for example, the 232-nt insert typically displayed more activity relative to the 474-nt insert in the RNA-based transfection (Fig. 6) than in the conventional DNA transfection experiment (Fig. 5C). While we do not know the reasons for this modest dichotomy, we speculate that the secondary structures adopted in vitro differ from those generated in vivo and may influence the accessibility of the ribosome to the IRES. But in any case, all fragments that were active in one assay were active in the second, and the parental vector and antisense control constructs were similarly negative in both assays.
FIG. 6.
Expression of Renilla/Photinus luciferase in SLK cells transfected with RNA transcribed in vitro from bicistronic RP constructs. RNA was transcribed in vitro as described in Materials and Methods and introduced into 293 cells by electroporation. Aliquots of the cells were harvested at 1.5, 2.5, 3.5, and 4.5 h after electroporation and analyzed for Renilla and Photinus luciferase activity. Shown are the mean values of normalized, relative luciferase activity (see the legend to Fig. 5C) over the total time period of 4.5 h.
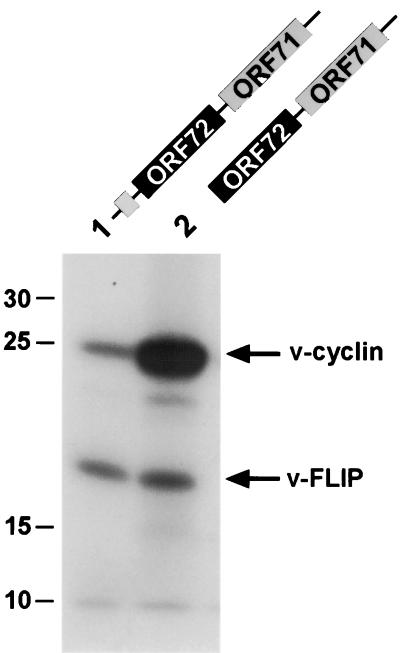
The experiments described above utilized reporter constructs in which ORF 71 was replaced by the luciferase coding region. To investigate v-FLIP expression from the intact bicistronic RNA, we subjected constructs encoding ORF 72/71 transcripts to in vitro transcription and translation. As shown in Fig. 7, lane 1, the v-FLIP protein was indeed translated from a bicistronic transcript. Furthermore, in accordance with the assumption that v-cyclin translation is initiated by ribosomal scanning, deletion of the 5′ uORF (Fig. 7, lane 2) led to an at least 10- to 20-fold increase in the levels of v-cyclin. In contrast, v-FLIP levels were virtually unaffected, as expected for internal initiation of v-FLIP translation mediated by an IRES present within the bicistronic transcript.
FIG. 7.
v-FLIP is translated from a bicistronic ORF 72/71 RNA in vitro. Constructs encoding either the complete second exon of the bicistronic ORF 72/71 RNA (pCMV 72/71; lane 1) or the second exon with a deletion of the 5′ uORF (pCMV 72/71ΔuORF; lane 2) were in vitro transcribed and translated. [35S]methionine-labeled translation products were subsequently analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and autoradiography. The positions of the v-cyclin and the v-FLIP proteins are indicated by arrows. The identity of the lower band (v-FLIP) was verified by in vitro transcription and translation of constructs in which ORF 71 was deleted or replaced by luciferase (data not shown).
These data demonstrate that cells infected with KSHV can employ two different strategies for v-FLIP expression. We believe that the use of the IRES within the bicistronic ORF 72/71 mRNA represents the dominant strategy for v-FLIP expression in latency. We note that the levels of luciferase produced by a model bicistronic construct (Fig. 1B, line 5) are ca. 10% of those generated from a monocistronic construct. Since the bicistronic ORF 72/71 RNA is at least 100- to 500-fold more abundant than the monocistronic ORF 71 RNA in latent PEL cells, translation of the latter RNA is predicted to make only a small contribution to the intracellular pool of v-FLIP in latency. (This assumes that the translational efficiencies of our reporters approximate those of the authentic messages—a reasonable assumption since they preserve the authentic v-FLIP AUG and IRES elements.) The function of the monocistronic transcript is unclear. It might be a device for assuring continued expression of v-FLIP in lytic infection, or it might be merely an incidental finding resulting from alternative processing of the bicistronic (or tricistronic) pre-mRNA. (If the latter, the alternatively processed RNAs must be stabilized by TPA treatment). The low efficiency of this processing in PEL cells in vivo is of interest, since the identical splice sites were efficiently utilized in the context of the ORF 72/LUC reporter in transiently transfected cells (Fig. 2). When this LUC gene was replaced by ORF 71 sequences, splicing of this transcript in transiently transfected SLK cells was suppressed (A. Grundhoff and D. Ganem, unpublished results), suggesting that cis-acting features of the ORF 72/71 RNA impair its efficient splicing. This is the presumed explanation for the low abundance of monocistronic v-FLIP RNA and almost certainly accounts for why it eluded earlier searches based largely on Northern blotting.
REFERENCES
- 1.Arvanitakis L, Mesri E A, Nador R G, Said J W, Asch A S, Knowles D M, Cesarman E. Establishment and characterization of a primary effusion (body cavity-based) lymphoma cell line (BC-3) harboring Kaposi's sarcoma-associated herpesvirus (KSHV/HHV-8) in the absence of Epstein-Barr virus. Blood. 1996;88:2648–2654. [PubMed] [Google Scholar]
- 2.Ballestas M E, Chatis P A, Kaye K M. Efficient persistence of extrachromosomal KSHV DNA mediated by latency-associated nuclear antigen. Science. 1999;284:641–644. doi: 10.1126/science.284.5414.641. [DOI] [PubMed] [Google Scholar]
- 3.Cesarman E, Chang Y, Moore P S, Said J W, Knowles D M. Kaposi's sarcoma-associated herpesvirus-like DNA sequences in AIDS-related body-cavity-based lymphomas. N Engl J Med. 1995;332:1186–1191. doi: 10.1056/NEJM199505043321802. [DOI] [PubMed] [Google Scholar]
- 4.Chang Y, Cesarman E, Pessin M S, Lee F, Culpepper J, Knowles D M, Moore P S. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi's sarcoma. Science. 1994;266:1865–1869. doi: 10.1126/science.7997879. [DOI] [PubMed] [Google Scholar]
- 5.Chappell S A, Edelman G M, Mauro V P. A 9-nt segment of a cellular mRNA can function as an internal ribosome entry site (IRES) and when present in linked multiple copies greatly enhances IRES activity. Proc Natl Acad Sci USA. 2000;97:1536–1541. doi: 10.1073/pnas.97.4.1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cotter M A, II, Robertson E S. The latency-associated nuclear antigen tethers the Kaposi's sarcoma-associated herpesvirus genome to host chromosomes in body cavity-based lymphoma cells. Virology. 1999;264:254–264. doi: 10.1006/viro.1999.9999. [DOI] [PubMed] [Google Scholar]
- 7.Dittmer D, Lagunoff M, Renne R, Staskus K, Haase A, Ganem D. A cluster of latently expressed genes in Kaposi's sarcoma-associated herpesvirus. J Virol. 1998;72:8309–8315. doi: 10.1128/jvi.72.10.8309-8315.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Djerbi M, Screpanti V, Catrina A I, Bogen B, Biberfeld P, Grandien A. The inhibitor of death receptor signaling, FLICE-inhibitory protein defines a new class of tumor progression factors. J Exp Med. 1999;190:1025–1032. doi: 10.1084/jem.190.7.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Friborg J, Jr, Kong W, Hottiger M O, Nabel G J. p53 inhibition by the LANA protein of KSHV protects against cell death. Nature. 1999;402:889–894. doi: 10.1038/47266. [DOI] [PubMed] [Google Scholar]
- 10.Herndier B G, Werner A, Arnstein P, Abbey N W, Demartis F, Cohen R L, Shuman M A, Levy J A. Characterization of a human Kaposi's sarcoma cell line that induces angiogenic tumors in animals. AIDS. 1994;8:575–581. doi: 10.1097/00002030-199405000-00002. [DOI] [PubMed] [Google Scholar]
- 11.Kirshner J R, Lukac D M, Chang J, Ganem D. Kaposi's sarcoma-associated herpesvirus open reading frame 57 encodes a posttranscriptional regulator with multiple distinct activities. J Virol. 2000;74:3586–3597. doi: 10.1128/jvi.74.8.3586-3597.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kozak M. Point mutations define a sequence flanking the AUG initiator codon that modulates translation by eukaryotic ribosomes. Cell. 1986;44:283–292. doi: 10.1016/0092-8674(86)90762-2. [DOI] [PubMed] [Google Scholar]
- 13.Li M, Lee H, Yoon D W, Albrecht J C, Fleckenstein B, Neipel F, Jung J U. Kaposi's sarcoma-associated herpesvirus encodes a functional cyclin. J Virol. 1997;71:1984–1991. doi: 10.1128/jvi.71.3.1984-1991.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Radkov S A, Kellam P, Boshoff C. The latent nuclear antigen of Kaposi sarcoma-associated herpesvirus targets the retinoblastoma-E2F pathway and with the oncogene hras transforms primary rat cells. Nat Med. 2000;6:1121–1127. doi: 10.1038/80459. [DOI] [PubMed] [Google Scholar]
- 15.Renne R, Blackbourn D, Whitby D, Levy J, Ganem D. Limited transmission of Kaposi's sarcoma-associated herpesvirus in cultured cells. J Virol. 1998;72:5182–5188. doi: 10.1128/jvi.72.6.5182-5188.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Renne R, Lagunoff M, Zhong W, Ganem D. The size and conformation of Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8) DNA in infected cells and virions. J Virol. 1996;70:8151–8154. doi: 10.1128/jvi.70.11.8151-8154.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Renne R, Zhong W, Herndier B, McGrath M, Abbey N, Kedes D, Ganem D. Lytic growth of Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8) in culture. Nat Med. 1996;2:342–346. doi: 10.1038/nm0396-342. [DOI] [PubMed] [Google Scholar]
- 18.Russo J J, Bohenzky R A, Chien M C, Chen J, Yan M, Maddalena D, Parry J P, Peruzzi D, Edelman I S, Chang Y, Moore P S. Nucleotide sequence of the Kaposi sarcoma-associated herpesvirus (HHV8) Proc Natl Acad Sci USA. 1996;93:14862–14867. doi: 10.1073/pnas.93.25.14862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sarid R, Flore O, Bohenzky R A, Chang Y, Moore P S. Transcription mapping of the Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8) genome in a body cavity-based lymphoma cell line (BC-1) J Virol. 1998;72:1005–1012. doi: 10.1128/jvi.72.2.1005-1012.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sarid R, Wiezorek J S, Moore P S, Chang Y. Characterization and cell cycle regulation of the major Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8) latent genes and their promoter. J Virol. 1999;73:1438–1446. doi: 10.1128/jvi.73.2.1438-1446.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Soulier J, Grollet L, Oksenhendler E, Cacoub P, Cazals-Hatem D, Babinet P, d'Agay M F, Clauvel J P, Raphael M, Degos L, et al. Kaposi's sarcoma-associated herpesvirus-like DNA sequences in multicentric Castleman's disease. Blood. 1995;86:1276–1280. [PubMed] [Google Scholar]
- 22.Staskus K A, Zhong W, Gebhard K, Herndier B, Wang H, Renne R, Beneke J, Pudney J, Anderson D J, Ganem D, Haase A T. Kaposi's sarcoma-associated herpesvirus gene expression in endothelial (spindle) tumor cells. J Virol. 1997;71:715–719. doi: 10.1128/jvi.71.1.715-719.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stoneley M, Paulin F E, Le Quesne J P, Chappell S A, Willis A E. C-Myc 5′ untranslated region contains an internal ribosome entry segment. Oncogene. 1998;16:423–428. doi: 10.1038/sj.onc.1201763. [DOI] [PubMed] [Google Scholar]
- 24.Talbot S J, Weiss R A, Kellam P, Boshoff C. Transcriptional analysis of human herpesvirus-8 open reading frames 71, 72, 73, K14, and 74 in a primary effusion lymphoma cell line. Virology. 1999;257:84–94. doi: 10.1006/viro.1999.9672. [DOI] [PubMed] [Google Scholar]
- 25.Thome M, Schneider P, Hofmann K, Fickenscher H, Meinl E, Neipel F, Mattmann C, Burns K, Bodmer J L, Schroter M, Scaffidi C, Krammer P H, Peter M E, Tschopp J. Viral FLICE-inhibitory proteins (FLIPs) prevent apoptosis induced by death receptors. Nature. 1997;386:517–521. doi: 10.1038/386517a0. [DOI] [PubMed] [Google Scholar]
- 26.Whitby D, Boshoff C. Kaposi's sarcoma herpesvirus as a new paradigm for virus-induced oncogenesis. Curr Opin Oncol. 1998;10:405–412. doi: 10.1097/00001622-199809000-00007. [DOI] [PubMed] [Google Scholar]