Abstract
Adaptive behaviour requires the ability to focus on a task and protect it from distraction (cognitive stability) and to rapidly switch tasks when circumstances change (cognitive flexibility). Burgeoning research literatures have aimed to understand how people achieve task focus and task switch readiness. In this Perspective, I integrate these literatures to derive a cognitive architecture and functional rules underlying the regulation of cognitive stability and flexibility. I propose that task focus and task switch readiness are supported by independent mechanisms. However, I also suggest that the strategic regulation of both mechanisms is governed by shared learning principles: an incremental, online learner that nudges control up or down based on the recent history of task demands (a recency heuristic) and episodic reinstatement when the current context matches a past experience (a recognition heuristic). Finally, I discuss algorithmic and neural implementations of these processes, as well as clinical implications.
Introduction
Imagine you were reading this article in a busy café. You would probably notice that the degree of attentional focus required for reading waxes and wanes in unison with the noise from other patrons around you. However, even at a time of great concentration, a buzz from your cell phone would cause you to swiftly shift tasks from reading to answering the call. This example illustrates two fundamental capacities of human cognition: cognitive stability, reflected in the ability to focus on a task at hand while ignoring task-irrelevant stimuli, and cognitive flexibility, reflected in the ability to switch focus to another task when the goals or circumstances of an individual change.
Cognitive stability and flexibility are components of cognitive control, which is the ability to use internal goals to drive behaviour1. Stability and flexibility are mediated by working memory, a mental workspace that supports the temporary maintenance and use of internal information2,3. This information includes procedural representations4, such as the current goal of an individual and the associated rules for attending and responding to stimuli, known as a task set5,6 (Fig. 1). In the café example, the goal of reading this paper and the corresponding attentional strategy (focus on reading, ignore auditory chatter) would be maintained and protected in working memory, thereby granting stable task performance. This protective function of working memory is complemented by a gating mechanism that allows for selective updating7: the current task set can be switched out in response to cues indicating that a different goal deserves higher priority, enabling flexibility (Fig. 1). In this example, the buzzing phone would trigger the gate to open, which would facilitate the replacement of a paper-reading task set with a phone-answering task set, characterized by a different goal and attentional strategy (focus on voice on the other end of the line, ignore written words of the paper).
Figure 1. Working memory in the use and updating of task sets.
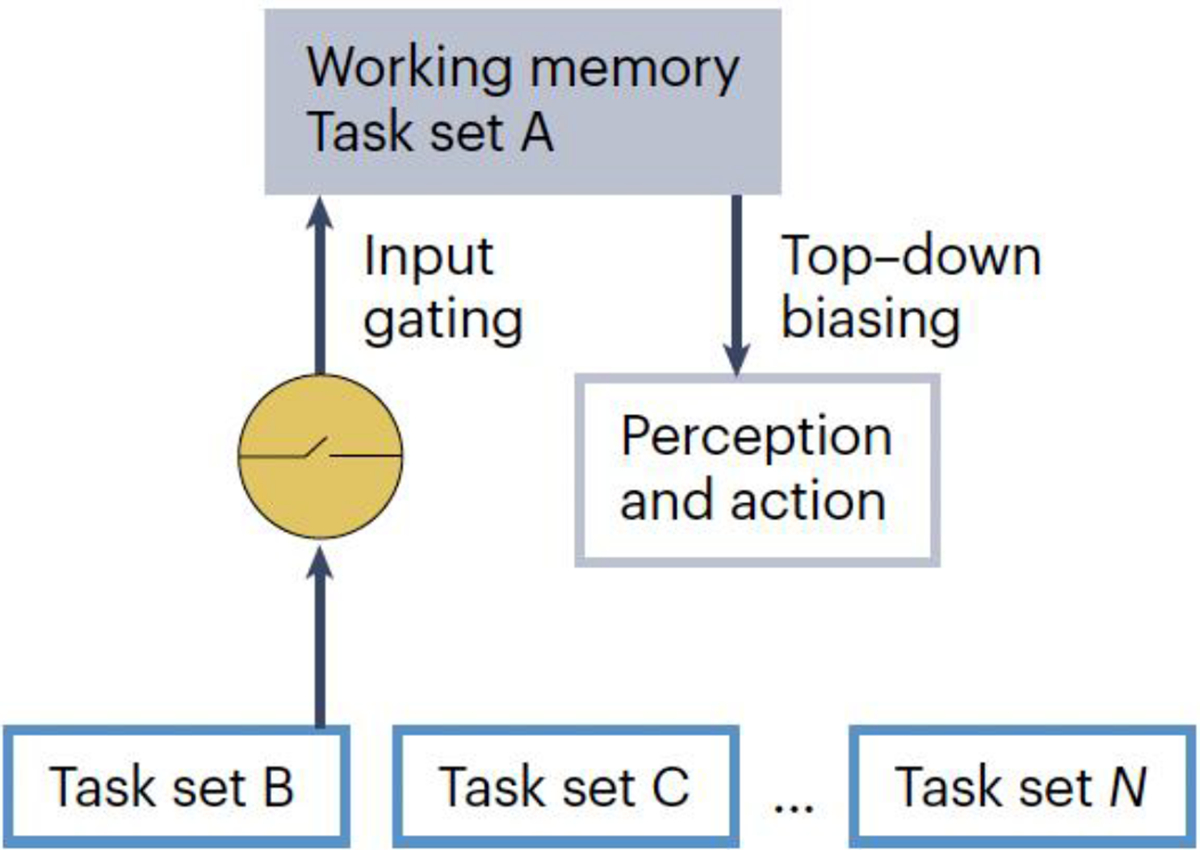
A task set maintained in working memory (task set A) biases perception and action in line with the stimulus-response mapping rules of the task set. The task set can be switched out (here, with task set B) when the gate to working memory is opened.
Critically, the degrees of cognitive stability and flexibility are not fixed. There is also no single optimal set point; in a dynamic environment, levels of task focus and switch readiness need to adapt to changing demands8,9. In the café example, stability and therefore task focus can be increased when the environment becomes noisier. Similarly, flexibility and therefore the readiness to switch tasks would be enhanced if, for instance, you expected to receive an important phone call. Such context-sensitive regulation of cognitive stability and flexibility enables people to pursue their goals in the face of distraction without getting stuck in a suboptimal task set. Conversely, dysregulation of stability and/or flexibility can result in inappropriate fixedness or distraction and is seen in numerous clinical conditions10, including attention deficit hyperactivity disorder11,12, autism spectrum disorders12–14, schizophrenia15 and Parkinson’s disease16.
Two distinct research literatures have sought to determine how cognitive stability and flexibility are regulated to match situational demands. The conflict-control literature is concerned with cognitive stability, asking how people manage to focus on information that is relevant to their task while ignoring irrelevant, distracting information17. By contrast, the task switching literature is concerned with cognitive flexibility, asking how people manage to switch their focus from one task to another when cued to do so18. These research traditions can be seen as complementary, in that conflict-control studies are concerned with regulating attentional focus within a task, whereas task switching studies ask how focus is shifted between tasks. Moreover, it is commonly assumed that cognitive stability and flexibility are interdependent, trading off against each other8,9: being more stable, or more focused on a current task, implies being less flexible, or less ready to switch to another task. Despite these strong conceptual connections, an overarching framework of control over task focus and task switching has been lacking in the literature.
In this Perspective, I review these two traditionally disconnected research literatures to provide an integrative answer to how stability and flexibility are regulated to produce adaptive behaviour. The existing findings regarding task switching and task focus are often interpreted as indicating that stability and flexibility are opposite ends of a single dimension of cognitive control8,19. However, based on mounting empirical evidence documenting that task focus and task switch readiness can be adapted independently of each other, I argue that cognitive stability and flexibility map onto two independent dimensions of control. After explaining this theoretical position, I explore the underlying learning principles that guide stability and flexibility. Despite reflecting independent control dimensions, I conclude that stability and flexibility are regulated according to the same learning principles, which can be thought of as recency and recognition heuristics. In closing, I address clinical implications and open questions regarding the interplay between different sources of information in guiding cognitive control.
Adaptation of stability and flexibility
At the turn of the twenty-first century, the conflict-monitoring model pushed the question of how attentional task focus is dynamically controlled into the limelight of cognitive psychology and neuroscience17. This model tried to account for a range of behavioural phenomena by suggesting that task focus is subject to strategic adaptation, being ramped up in contexts with more distraction and relaxed in contexts with less distraction. Subsequent research has shown that task switching processes are similarly subject to strategic adaptation20,21. Here I introduce canonical protocols and foundational findings supporting adaptive control from these two literatures. This research enterprise has included robust debates about potential confounds (Box 1); in this Perspective, I focus on effects that have been documented using best-practice designs.
Box 1. Measuring adaptive control.
One challenge of measuring putative effects of adaptive control is that the learning of stimulus-response contingencies can mimic these effects127,128. For instance, in an LWPC Stroop protocol, blocks with a high proportion of incongruent trials typically contain many more instances of specific incongruent stimuli (such as ‘BLUE’ in red) than the blocks with low proportion of incongruent blocks, and the reverse is true of congruent stimuli26. Thus, the faster responses to incongruent stimuli in blocks with a high rate of incongruent trials could simply reflect frequency-based stimulus-response learning for these contextually probable stimuli rather than adjustments in control127,128. Similarly, in an ISPC Stroop protocol, if a specific colour word was paired more frequently with a particular incongruent ink colour, the faster responses to that item could simply reflect the learning of a contingency between the colour word and a specific action rather than the reactive recruitment of task focus33,127,128. Thus, it is essential to devise experiments that tap adaptive control in a manner that accounts for stimulus-response learning confounds129.
One way to minimize these confounds is to not use recurring stimuli at all. For instance, one study asked participants to name line drawings of common objects that were overlaid with congruent or incongruent distractor words (such as a table with the word ‘TABLE’ or ‘CHAIR’). Because it is possible to create hundreds of unique stimuli of this kind, this paradigm allows for assessing an LWPC effect free of any stimulus-response learning80. Similarly, LWPS effects can be obtained on task switch protocols that employ trial-unique stimuli, with tasks such as classifying common objects by their size (‘is it smaller or larger than a soccer ball?’) or origin (‘is it natural or man-made?’)91. Another common approach is to devise tasks that distinguish between ‘inducer’ and ‘diagnostic’ items, in which the former create the biased statistical distribution of control demands, and the latter are unbiased probes that measure the effects of control129. For example, in a Stroop LWPC protocol, a subset of colour word combinations can be presented frequently as incongruent stimuli to create a block with high control demand, and the effect of that manipulation is assessed in a different subset of colour word combinations that are presented equally often as congruent and incongruent stimuli28,81–88. An equivalent approach can be used in LWPS protocols44–46.
Similarly, for measuring ISPC effects, specific colours can be assigned to be mostly congruent or incongruent by pairing them with a subset of congruent or incongruent colour words on inducer trials, and item-specific control effects can then be gauged on diagnostic trials in which distractor words are not congruency biased36 (see also refs.34,130). For assessing ISPS effects, each stimulus can be presented equally often under each task rule (and their respective response requirements) despite being biased in terms of switch likelihood47,49. For instance, in the parity and magnitude paradigm (Fig. 2c), one could present the digit 3 on task switch trials 80% of the time, but half of those switch trials could involve the magnitude task and the other half the parity task.
Finally, the Stroop and parity and magnitude paradigms (Fig. 2a,c) are only representative examples of probes of task focus and flexibility. These specific protocols are not without their critics (for an alternative take on the Stroop task, see ref.131), but the literature reviewed and conclusions drawn in this Perspective encompass a much wider array of conceptually similar tasks.
A classic probe of task focus, or cognitive stability, is the Stroop task22 (Fig. 2a). In this task, participants are presented with colour words that are printed in various ink colours and asked to name the ink colours rather than reading the colour words. Naming the ink colours constitutes a task set, in this case one that is distinct from the more-common task set of reading when visual words are presented. The efficacy of imposing the colour-naming task set is usually measured by comparing response times and error rates between trials in which the word meaning coincides with the ink colour (congruent condition, such as ‘GREEN’ in green ink) and trials where they conflict (incongruent condition, such as ‘GREEN’ in blue ink). Almost invariably, participants display a substantial congruency or conflict effect, with slower and more error-prone responses to incongruent stimuli as they struggle to overcome the highly practiced behaviour of reading the words23,24. The size of this effect is considered a measure of cognitive stability17,25, with a smaller congruency effect indexing a more stable implementation of the colour-naming task set, or greater task focus.
Figure 2. Classic tasks and results indexing adjustments in cognitive stability and flexibility.
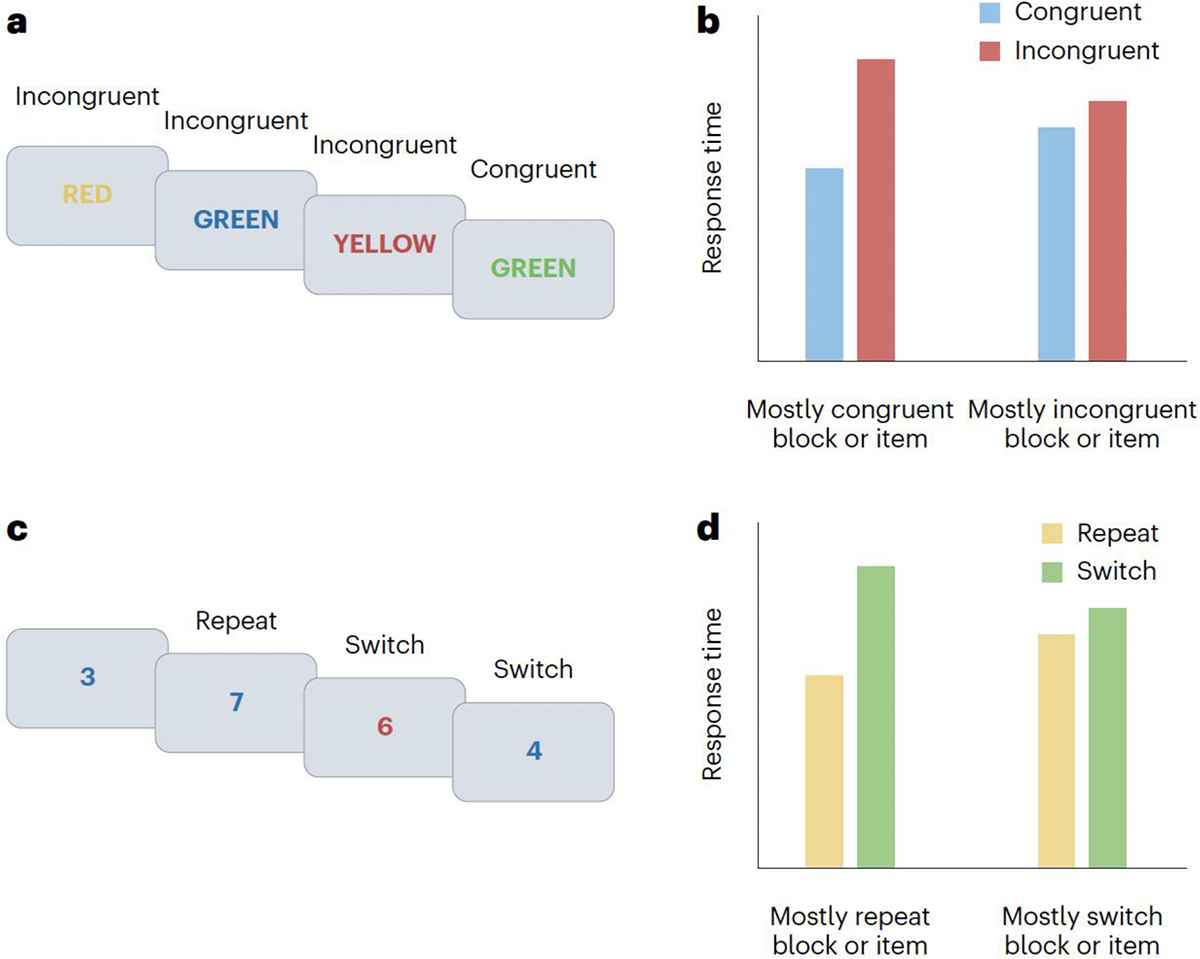
a, In a typical Stroop task, participants are required to name the ink colours while ignoring the words. b, A typical response time pattern is that the size of the conflict effect (the difference between incongruent and congruent trials) is reduced for blocks and items or stimuli that are associated with frequent conflict. The list-wide proportion congruent effect and the item-specific proportion congruent effect can be seen by comparing the difference of differences between the two bars on the left and the two bars on the right sides of the graph. c, In a typical cued task switching protocol, the colour of each digit cues the participants whether to perform a parity or magnitude task. d, A typical response time pattern in cued task switching, in which the performance cost of switching tasks (the difference between switch and repeat trials) is reduced for blocks and items that are associated with frequent switching. The list-wide proportion switch effect and the item-specific proportion switch effect can be seen in the larger difference between the left set of bars than between the right set of bars.
Crucially, the size of the congruency effect and, therefore, the efficiency of the ability of an individual to maintain the colour-naming task set can vary substantially depending on context. First, the size of the congruency effect scales with the proportion of congruent trials encountered over time26,27. This phenomenon is dubbed the list-wide proportion congruent (LWPC) effect28,29: the mean congruency effect is larger in mostly congruent blocks of trials in which incongruent trials are rare than in mostly incongruent blocks when incongruent trials are common (Fig. 2b). This data pattern suggests that people adapt their task focus to changing demands, enhancing stability during times in which interference from distractors (here, incongruent colour words) is more probable. This phenomenon can also be observed from one trial to the next, as the mean congruency effect tends to be smaller following an incongruent trial than a congruent one27, known as the congruency sequence effect30,31.
A second way that task focus can be adjusted based on context is in relation to cues that are predictive of distraction. Studies have found that control over task focus is adapted in response to specific stimuli or stimulus components that are predictive of congruent or incongruent distractors32. For instance, when a specific colour word33,34 or ink colour35,36 in the Stroop task is frequently part of incongruent stimuli, the mean congruency effect is smaller for stimuli involving these features than for stimuli with features that are predictive of congruent stimuli. This finding is referred to as the item-specific proportion congruent (ISPC) effect29,32: there is a larger difference between congruent and incongruent response time for mostly congruent items than for mostly incongruent items (Fig. 2b). In sum, this literature has documented that people adapt their level of task focus to match changing levels of distraction over time, as reflected in the LWPC effect, and to match levels of distraction associated with specific stimuli, as reflected in the ISPC effect.
A parallel literature in the domain of task switching has documented corresponding adaptation effects for cognitive flexibility20,21. A classic task switching protocol involves presenting participants with a single digit number (1–9, excluding 5) and cuing them on each trial to categorize the number either by magnitude (lesser or greater than 5) or by parity (even or odd)6,37 (Fig. 2c). The canonical finding is a switch cost: slower and more error-prone responses on trials in which the task set has to be changed (such as a magnitude trial after a parity trial) than on trials in which the task stays the same (a magnitude trial after a magnitude trial)5,6,18,38. This switch cost has been variously attributed to interference from the task set of the previous trial5, the configuration of the newly relevant set6 and the undoing of stimulus–task bindings39 (reviewed in ref.40). Regardless of the exact source or mixture of sources, switch costs provide an index of cognitive flexibility, with a smaller switch cost reflecting greater flexibility or ‘switch readiness’20,21. Similar to the congruency effect, the size of the switch cost is also strongly dependent on context.
First, temporal contexts (blocks of trials) in which switching is required more frequently are associated with smaller mean switch costs than contexts in which switching is required less frequently41–46. This finding is referred to as the list-wide proportion switch (LWPS) effect44: there is a larger switch cost for mostly repeat blocks, in which switch trials are rare, than for mostly switch blocks, in which switching is required more often (Fig. 2d). Thus, switch likelihood manipulated at the block level appears to modulate the ease with which tasks can be switched, relative to repeated. Second, if specific stimuli are predictive of switch demands, switch costs are also modulated in an item-specific manner, with lower switch costs for switch-predictive stimuli and higher switch costs for repeat-predictive stimuli. This effect is known as the item-specific proportion switch (ISPS) effect46,47: mostly repeat items are associated with a larger switch cost than mostly switch items (Fig. 2d). For example, if the digits 3 and 6 were shown primarily on task switch trials and the digits 4 and 7 primarily on task repeat trials, the mean switch cost for the former digits would be smaller than for the latter. Finally, list-wide and item-based switch frequency also modulate the willingness of people to switch tasks when task selection is voluntary48,49.
These literatures suggest that people learn about variations in control demands over time and in association with specific stimuli or cues, which leads to adaptation of stability-supporting and flexibility-supporting control processes. In the next sections, I first discuss the relationship between stability and flexibility, followed by a detailed treatment of the nature of the learning processes that drive their regulation. Although in principle the question of the structure of stability and flexibility is independent of how they are regulated, I discuss them together to emphasize that distinct control mechanisms can be guided by shared learning processes.
Independent mechanisms of stability and flexibility
Task focus and task switching are located at different levels of the functional hierarchy of working memory (Fig. 1). Task focus is a property of the task set that is currently active in working memory and can be conceptualized as the strength with which that task set is being Implemented17,25. By contrast, task switching processes are responsible for gating task sets into working memory and, hence, operate on a given task set rather than within it. Despite this fundamental distinction, stability and flexibility have traditionally been viewed as two opposing poles of a single continuum of functional states8,9,20,44,50 (Fig. 3a). After explaining this account, I will review how newer evidence strongly indicates that stability and flexibility are regulated independently51,52. This independence account is in line with the inherent flexibility afforded by a working memory model with separate components serving maintenance (task focus) versus updating (task switching) functions7 (Fig. 1). Furthermore, the independence of stability and flexibility suggests a two-dimensional state space (Fig. 3b) that enables the possibility that people can be both stable and flexible at the same time (for a more elaborate treatment of this idea, see ref.53).
Figure 3. One-dimensional and two-dimensional conceptions of cognitive stability and flexibility.
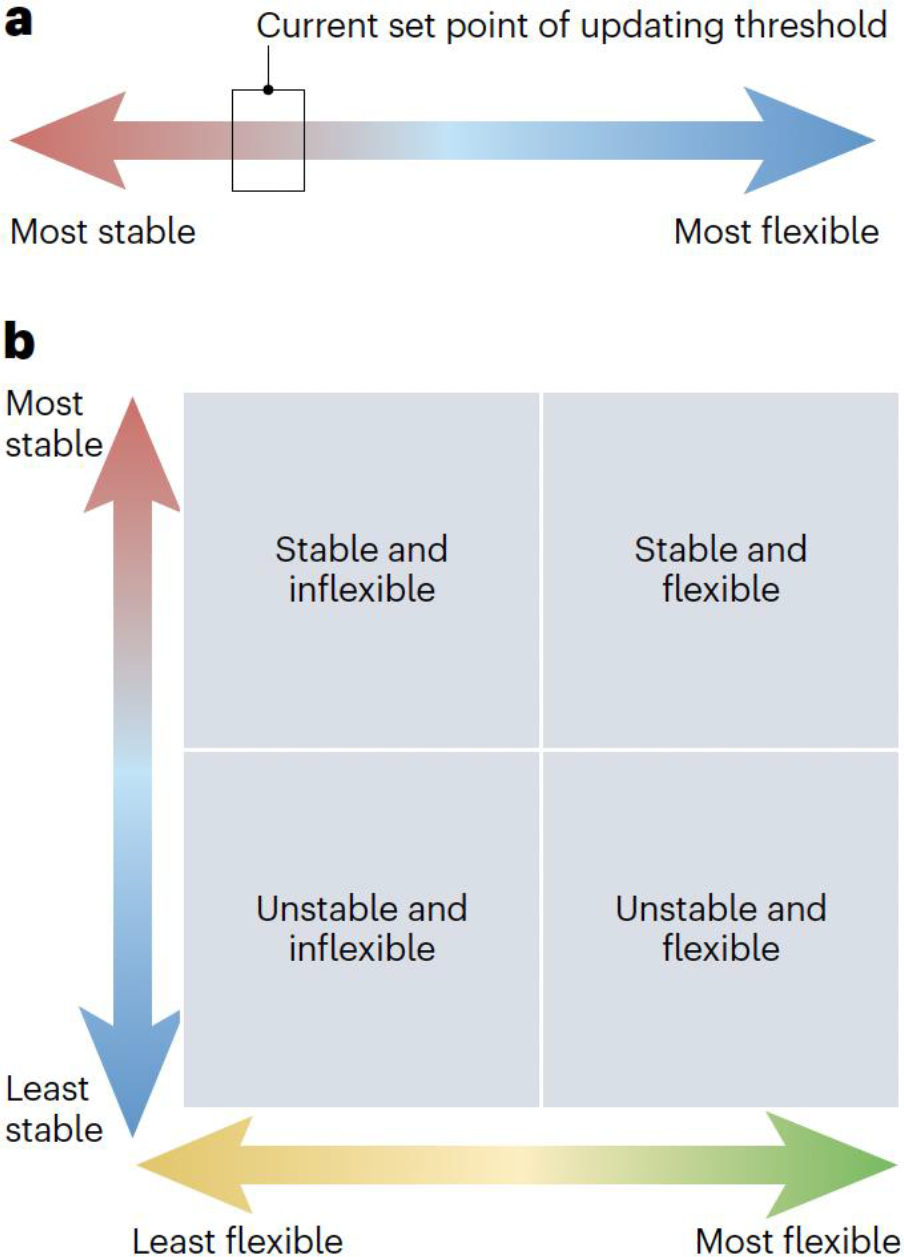
a, The traditional assumption that stability and flexibility are opposing poles of a one dimensional continuum that trades off stability against flexibility. The present set point on the continuum is conceptualized as an ‘updating threshold’ meta-control parameter. b, A two-dimensional model in which stability and flexibility are independent dimensions. This view accommodates states in which stability and flexibility appear to trade off (upper left and lower right quadrants) and states in which both stability and flexibility are low or high (lower left and upper right quadrants, respectively).
A one-dimensional conception of stability and flexibility reflects the basic intuition that being more stable implies being less flexible, and vice versa. Moreover, even within a working memory model that situates task focus and task switching in different components, it is quite plausible that the two could be reciprocal or functionally yoked54. Specifically, a closed-gate state could prevent task switching and also confer protection upon the task set currently held in working memory, lending low flexibility and high stability7,55. This notion is captured in the influential proposal that the degree of stability (and, ergo, flexibility) is governed by a single meta-control parameter, a working memory ‘updating threshold’8,19 (Fig. 3a). When the updating threshold is low, the current task set is poorly shielded (low stability) and it is easy to switch to a different task (high flexibility). By contrast, when the threshold is high, the current task set is well protected (high stability) but at the cost of making switching more difficult (low flexibility). This conception leads to an obligatory trade-off between stability and flexibility55, and it renders any putative metric of stability (such as the congruency effect) an inverse measure of flexibility and any putative metric of flexibility (such as the switch cost) an inverse measure of stability20.
Empirical support for a trade-off between stability and flexibility derives from several findings in the task switching literature. First, it is often observed that the degree to which a currently irrelevant task interferes with the currently relevant one is greater on switch than on repeat trials6,56,57. This cross-task interference is measured in switch protocols with bivalent stimuli — stimuli that both tasks can be performed on — and overlapping responses, a scenario that produces task-congruent and task-incongruent stimuli6,37. For example, in the magnitude and parity task described in Fig. 2c, if the responses ‘less than 5’ and ‘odd’ were both mapped onto one response button and ‘greater than 5’ and ‘even’ onto another button, the digits 2, 4, 7 and 9 would require different (incongruent) responses between the two task sets, whereas 1, 3, 6 and 8 would require the same (congruent) response. The relative slowdown and greater error rate for incongruent than for congruent stimuli is the cross-task congruency effect and can be used as a (inverse) measure of cognitive stability20. The fact that this effect tends to be enhanced during switch trials is in line with the idea that a low updating threshold required for task switching also results in poor protection against interference from the alternate task set.
Second, the switch cost also tends to be enhanced on trials that follow an incongruent trial, which could be attributable to the stability-enhancing effect of conflict17, making it harder to be flexible on the subsequent trial58,59. To wit, encountering an incongruent stimulus under the magnitude task set would nudge up focus on that task, which in turn would make it more difficult to switch to the parity task on the next trial — reflecting a stability–flexibility trade-off. Third, affect and reward manipulations have often been found to elicit opposing effects on measures of stability and flexibility60,61, which would be expected if stability and flexibility were inversely related to each other. For instance, presentation of task-irrelevant pleasant (compared to neutral or negative) images can result in poorer proactive task cue maintenance (low stability) but better reactive target detection (high flexibility)62. By contrast, reward prospect can enhance cue maintenance but impair adaptation to changing task and reward contingencies63,64. Finally, one study showed that rewarding cued switch trials can induce a greater voluntary switch rate on free-choice trials, but at the cost of larger cross-task congruency effects65.
Despite this putative support, the trade-off account can be criticized on both conceptual and empirical grounds51,53. Conceptually, the theoretical premise of a single stability–flexibility continuum renders the hypothesis unfalsifiable: if one assumes that any metric of stability is also an inverse metric of flexibility, and vice versa20, it is impossible to test for their potential independence, as the two constructs cannot be manipulated or measured independently53. Although it is intuitive to consider stability and flexibility as intrinsically yoked in the abstract, once these constructs are operationalized as task focus and task switch readiness, the issue of their relationship reduces to the empirical question of whether a greater task focus necessarily results in less efficient switching, and whether greater task switch readiness necessarily comes at the cost of less task focus.
On the empirical side, many findings in the literature speak against an obligatory inverse relationship between stability and flexibility. One such finding was noted in several of the foundational studies of task switching: whereas a longer time interval between a task cue and the subsequent target stimulus reliably reduces switch cost, it does not tend to alter cross-task congruency effects5,6,38, violating the trade-off assumption. Another line of evidence comes from studies of cognitive training. On the one hand, extensive training on the Stroop task led to reduced congruency effects but had no reciprocal impact on measures of task set shifting or working memory updating66. On the other hand, extensive training on cued task switching led to reduced switch costs but had no reciprocal effect on Stroop congruency effects67. Thus, increasing stability through training does not seem to reduce flexibility, and increasing flexibility through training does not seem to reduce stability; both findings run counter to the trade-off account. Individual difference data also speak against the idea of a single factor mediating task focus and task switching68,69. For instance, a large-scale 2022 study had participants perform both LWPC and LWPS protocols to probe the cognitive structure of learned adaptation in task focus and switch readiness52. Structural equation modelling revealed that learned adjustments of stability and flexibility relied on distinct latent factors52.
Finally, particularly compelling evidence against a single stability– flexibility dimension comes from studies in which incentives for stability and flexibility were manipulated independently. In one such study, the proportion of forced versus free task selection trials in a task switching protocol was varied between groups of participants, and prospective reward was manipulated at the trial-to-trial level within groups70. A low rate of forced choices was found to increase stability (eliciting fewer voluntary switches) at the block level. However, a trial-by-trial change in reward prospect induced greater switch rates, reflecting concurrent local flexibility70. Under a strict trade-off assumption, it should not be possible for participants to be both stable and flexible at the same time. The most direct demonstration of independent adaptations of stability and flexibility comes from a study in which the rate of task switches and cross-task congruency was varied orthogonally within the same group of participants51 (Fig. 4a). If task focus and switching were inversely yoked, conditions with a high switch rate should result in smaller switch costs (the LWPS effect) but also larger congruency effects. Conversely, conditions with a high rate of incongruent trials should result in smaller congruency effects (the LWPC effect) but also greater switch costs. The results did not support these predictions: Switch rate modulated switch costs but not congruency effects, and congruency rate modulated congruency effects but not switch costs51 (Fig. 4b). Thus, the findings support independence between the regulation of task focus and task switching processes.
Figure 4. Independent effects of control over stability and flexibility.
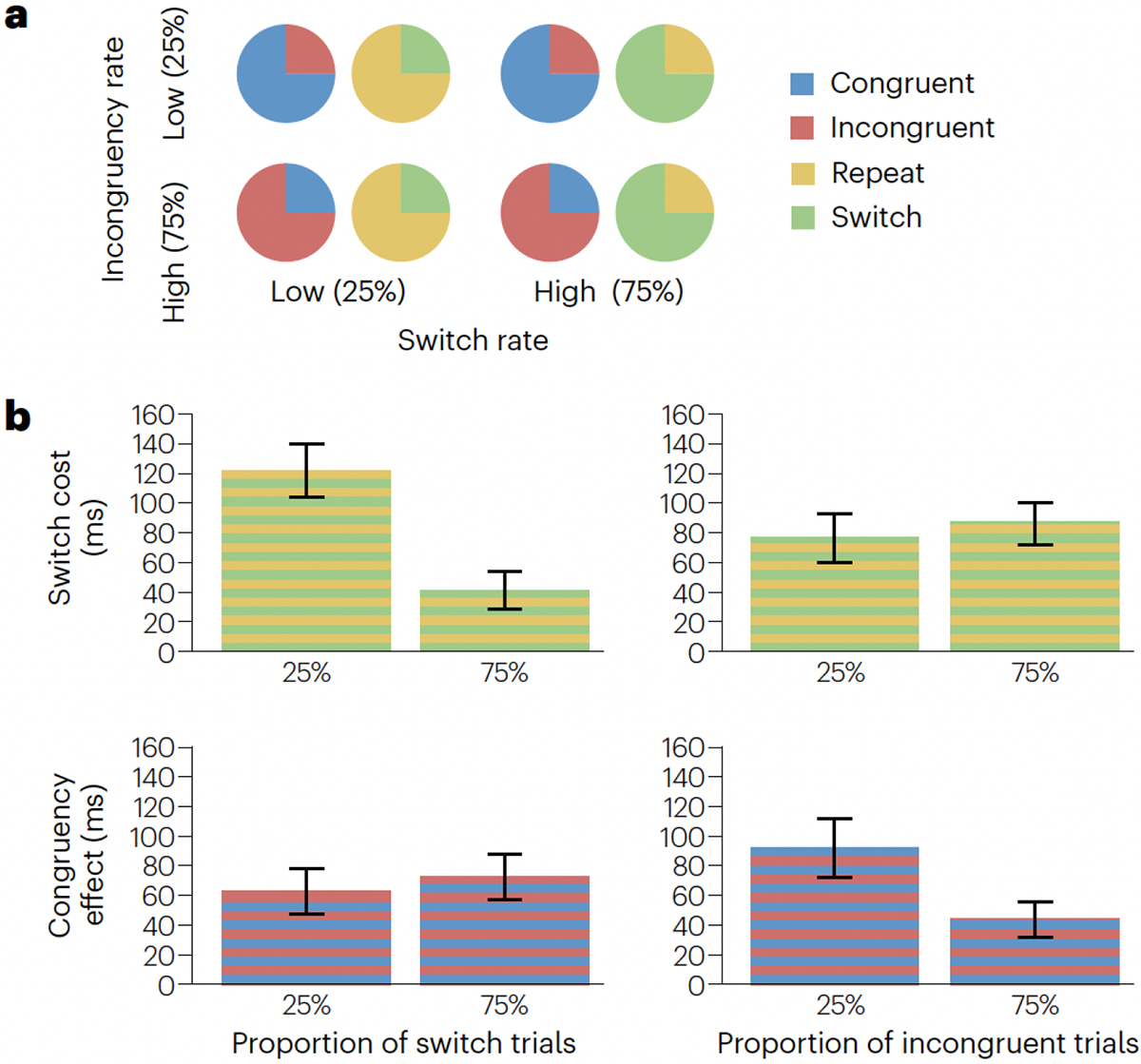
a, The relationship between the regulation of stability and flexibility can be investigated by independently and simultaneously varying demands on task focus, via the rate of incongruent trials, and on switch readiness, via the rate of switch trials. b, The rate of incongruent trials modulates the congruency effect but not switch costs, and the rate of switch trials modulates the switch cost but not congruency effects, suggesting independent control of cognitive stability and flexibility. Data from ref.51.
Together, the empirical data are incompatible with a trade-off or one-dimensional account of stability and flexibility, which cannot account for situations in which the two modes are not inversely yoked. By contrast, the two-dimensional or independent-mechanisms view can accommodate situations in which this inverse relationship is present and situations in which stability and flexibility are both low or both high51,53 (Fig. 3b). The working memory model sketched out in Fig. 1 can easily span this state space by assuming independent regulation of the strength of task set implementation in working memory versus the ease of input gating. Furthermore, a bulk of neuroscientific evidence maps working memory maintenance of an ongoing task set and the switching between task sets onto distinct (but interacting) brain structures (Box 2). A related proposal to account for the possibility of concurrent stable and flexible processing modes suggests that stability and flexibility can be modulated by two distinct means, either by varying a working memory updating threshold or by varying how many task sets are being held in working memory20. In this scheme, one of the two mechanisms could promote stability (such as a high updating threshold) while the other one could promote flexibility (holding more than one task set in working memory), therefore producing a state in which stability and flexibility are both high70.
Box 2. Neural mechanisms of task focus and switching.
The stable maintenance of working memory content has long been associated with the lateral prefrontal cortex (LPFC)132,133. Subsequent work expanded this association to the representation of task rules134–137 and their use to impose attentional biases on stimulus processing and response selection1,138–141. Building on the assumption that stability is subserved by the LPFC, conflict-monitoring theory proposed that the degree of stability (and top–down influence) of task goals held in LPFC is modulated as a function of conflict detected by the dorsal anterior cingulate cortex (dACC)17. Although there has been much debate about the specifics of dACC function, there is broad agreement that it has a key role in evaluating performance and initiating behavioural adjustments71,142–144. In line with the basic idea of a conflict-control loop for adapting task focus that involves these structures, many studies have documented increased dACC activity in response to conflict145–147 and greater LPFC involvement when conflict is successfully adapted to146–149. Neuroimaging studies that applied computational modelling of incremental learning to estimate the updating of conflict predictions from trial to trial have additionally implicated the dorsal striatum in linking contextual cues to adjustments in task focus119,150,151.
Studies of task switching and working memory updating have also implicated frontal152–154 and striatal sites155–159. These co-activations can be accounted for by the influential proposal that working memory input gating is mediated by interactions between the basal ganglia and the LPFC, in which the former functions as the gate that, if opened, allows representations in LPFC to be updated7. Neural mechanisms of adjusting flexibility to varying demands have been much less studied than adjustments in stability, but imaging studies that have manipulated switch rates across blocks of trials have documented context-sensitive activation changes in frontoparietal cortex160,161. These findings perhaps suggest that frontal cortex regulates flexibility by modulating the gating threshold in the basal ganglia162. (For a dynamic brain network perspective on cognitive flexibility, see ref.10).
The interplay between the basal ganglia and LPFC in regulating task sets raises the question of the interdependence between the stability of task sets maintained in the LPFC and the flexibility granted by the basal ganglia gating function. However, although stability-supporting and flexibility-supporting functions of the LPFC and basal ganglia can display a seesaw-like relationship suggestive of functional yoking157, an obligatory reciprocity of function has been considered not probable54. Moreover, genetic and pharmacological studies selectively targeting lPFC versus striatal function have demonstrated double dissociations of stability and flexibility163,164. For instance, selective pharmacological disruption of LPFC function impairs stability but leaves flexibility unaffected, whereas the opposite is true when disrupting striatal function164. Finally, the basal ganglia LPFC gating model itself envisages the updating of working memory to be selective, by assuming multiple cortico-striatal loops with independent basal ganglia gates7. This assumption means that some working memory content can be stably maintained even while other items are being updated7, making it possible to have stability and flexibility at the same time.
Nevertheless, one might wonder why behaviour often seems to resemble a stability–flexibility trade-off. A probable reason is cognitive effort or the cost of control71. Both task focus and task switching are experienced as effortful and preferably avoided72–74. Thus, if a task context primarily incentivizes stability, switch readiness tends to be low — not because it is inversely yoked to task focus but because high switch readiness is not required and would involve unnecessary effort. The obverse argument applies to situations that primarily incentivize flexibility and are therefore associated with a low need for costly stability51,53. Having argued that cognitive stability and flexibility should be considered distinct dimensions of cognitive control, I next turn to the question of how they are adapted to suit changing contexts.
Shared principles of adaptive control
The fact that people adjust their levels of task focus and switch readiness to meet changing demands over time and different contexts raises the key question: what kind of learning processes guide these adaptations? Although stability and flexibility are regulated independently, the underlying learning principles that attune task focus and switch readiness to situational demands seem to be shared and have two main forms. The first learning principle is incremental, online adjustment of control, which continually nudges stability and flexibility settings up or down based on recent demands (exemplified by LWPC and LWPS effects) (Fig. 5a,b). This principle represents a proactive, anticipatory form of control75, driven by the implicit assumption that the near future tends to resemble the recent past76. The second learning principle is episodic binding of specific stimuli and contexts with the control processes that were active during their encoding and are subsequently reinstated when those stimuli or contexts are re-encountered (exemplified by ISPC and ISPS effects) (Fig. 5c,d). By contrast to the first principle, this one represents a reactive, stimulus-driven application of control75. In this section, I unpack these learning mechanisms in cognitive terms, and in the ‘Heuristics and algorithms’ section, I review possible implementations in algorithmic terms.
Figure 5. Incremental and episodic guidance of stability and flexibility.
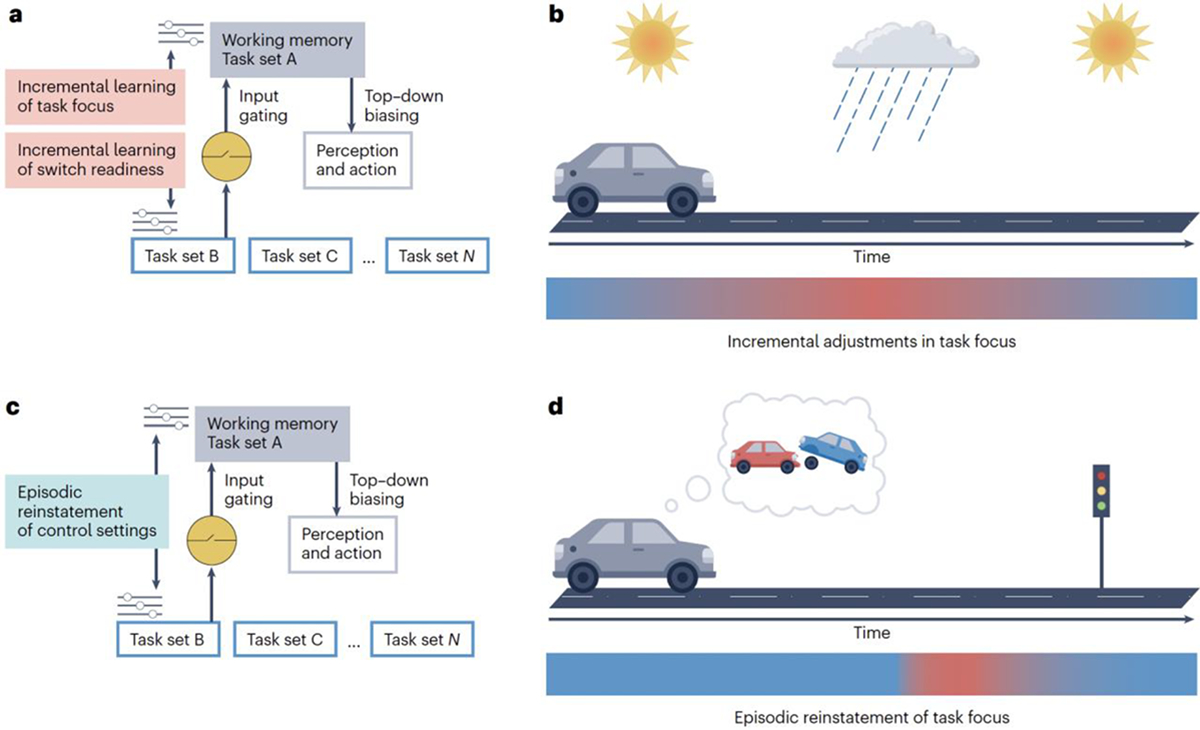
a, Incremental learning mechanisms nudge the strength of the currently active task set, and the ease of access to alternative task sets, up or down in line with the recent history of task demands. b, The incremental learner tracks ongoing changes in task difficulty and gradually nudges task focus up or down in line with changing demands (incremental learning of task focus). While driving, task focus would be nudged up gradually if it begins to rain or traffic density increases (indicated by the red colour in the colour gradient). c, An episodic memory mechanism reinstates task focus and switch readiness settings from prior experiences if they closely resemble the present situation. d, The episodic learner detects matches between perception and memory and, when recognizing a familiar situation, reinstates matching memories, including task focus settings associated with the prior experience (episodic reinstatement of task focus). While driving, an intersection where one previously encountered a crash would lead to retrieval of a high task focus (indicated by the sharp transition to the red colour in the colour gradient).
Incremental, trial-by-trial adjustments in control lie at the heart of the influential conflict-monitoring model17. The basic idea is that the cognitive apparatus monitors for the occurrence of conflict — the co-activation of mutually incompatible responses — as a proxy for whether the current level of task focus is appropriate for performing the task well. Task focus is then nudged up or down from trial to trial based on whether the level of conflict experienced on the current trial is greater or less than a running average17,77. Accordingly, if a participant performing the Stroop task encountered several incongruent, conflict-inducing stimuli in a row, their level of task focus would steadily increase. In an everyday scenario, this adjustment in control would be akin to a driver increasing their focus on the road in response to poorer visibility with the onset of a rainstorm (Fig. 5b). Conversely, several congruent trials in a row in the Stroop task would result in a gradual relaxation of task focus. Incremental online learning based on conflict detection can, therefore, account for adjustments in task focus to varying demands over time, both at the trial-by-trial level (the congruency sequence effect) and the block level (the LWPC effect)17,77 (Fig. 2b).
This type of incremental control mechanism can also learn about alternative trial characteristics or performance metrics other than response conflict. Thus, although it has received much less attention in the literature, the same logic of trial-by-trial adaptation can also be applied to task switching processes, either by treating switching as a form of conflict59 or by making task sets the target of the learning process78,79. Accordingly, an incremental learning mechanism could also detect task switches and nudge switch readiness up or down based on deviations from a recent running average of switch demands (Fig. 5a).
Importantly, the incremental learning mechanism envisaged here (and in the conflict-monitoring model) operates at the level of task sets or goal representations and is not concerned with learning about specific stimulus features. Accordingly, control is adapted by modulating the strength of the task set rather than the representations of particular stimuli17. Owing to their distinct functional roles with respect to task sets, incremental adjustments to the strength of task sets have different consequences for the regulation of stability versus flexibility.
For stability, operating within a task set, learned adjustments in task focus are generalizable or transferrable across stimuli. For instance, in the Stroop task, an increase in the strength of the goal representation because of conflict will lead to greater focus on colour processing, which will reduce the impact of distractors regardless of whether the forthcoming stimulus is ‘BLUE’ printed in red or ‘RED’ printed in yellow, or some other stimulus that has never been encountered previously. The fact that incremental learning of control demand occurs at the level of task goals is crucial because it permits the conflict-monitoring and related models to account for commonly observed adjustments in task focus that are unrelated to the specifics of the stimuli in question17,77, such as LWPC effects obtained in the absence of reoccurring stimuli80 or in relation to stimuli that are themselves not predictive of control demands28,81–88 (Box 1).
However, for the regulation of flexibility, the fact that the incremental learning mechanism operates on task goals means that learned switch readiness is tied to specific task sets and does not tend to transfer to other task sets44,89,90 even in the same temporal context44,91 (but see ref.79 for an empirical demonstration that transfer can occur under some circumstances). For example, consider an LWPS study design involving three tasks. Like in the traditional design, two of the tasks are ‘switch frequency-biased’ in that they are most frequently presented on task switch trials in some blocks (creating blocks with a high switch rate) and on task repetition trials in other blocks (creating blocks with a low switch rate). Critically, the third task is ‘unbiased’, presented equally often on task switch and task repetition trials in all blocks. This set-up allows researchers to probe whether the LWPS effect would occur only for tasks whose switch rate is varied across blocks or would also transfer to the third task that is intermixed in the same blocks but itself is not associated with a biased switch rate. It turns out that only the two switch frequency-biased tasks display a LWPS effect44,91. However, in spite of a lack of transfer to the unbiased task set, for the two switch frequency-biased task sets, the LWPS effect generalizes over all task stimuli, meaning that the reduced switch cost can be observed even for task stimuli that themselves are not associated with frequent switching44,91. This observation fits with the assumption that incremental learning operates at the level of task sets and with a variety of related findings suggesting that task sets form the units or boundaries of cognitive control strategies76,91–94. Together, these findings support the idea of incremental control that modulates the strength of the current task set for regulating stability and modulates the accessibility of specific alternative task sets for regulating flexibility (rather than making all other task sets more accessible) (Fig. 5a).
However, a task-set-based incremental learning mechanism cannot produce item-specific effects of control for two reasons. First, this learning mechanism has no means of linking control settings with specific stimuli95,96. Second, ISPC and ISPS effects are not rooted in block-level biases of the proportion of incongruent or switch trials, with the consequence that participants cannot anticipate whether the next trial will be an incongruent or switch trial36,47. Instead, these item-level effects suggest that re-encountering a demand-predicting stimulus leads to a swift reinstatement of the appropriate control setting gleaned from prior encounters35, a reactive and episodic mode of control75,76 (Fig. 5c). Thus, to account for ISPC and ISPS phenomena, control settings have to become associated with the particular features of demand-predicting stimuli or contexts: episodic or event-based information76,97,98. In everyday life, this form of control learning is evident when one approaches a particular intersection where they experienced a car crash in the past, which now triggers the retrieval of a high focus of attention toward traffic (Fig. 5d).
One way this episodic form of control could be accomplished in the case of task focus is if conflict were to enhance the binding between the task goal and the specific relevant feature value of a conflict-inducing stimulus (such as the colour red)95,96. A broader version of this proposal accommodates stimulus-specific and context-specific control effects by extending the theory of event coding99 to include cognitive control states76,97,100,101. Event coding theory states that memory binds together features of ongoing experience into ‘event files’ and subsequent encounters with some or all of those features lead to the retrieval of similar event files, which can serve as a shortcut for perceptual inference and response selection99,100. Accordingly, the binding of object features with actions performed in response to those features facilitates repeat performance but impairs performance when a different action is required in the presence of those stimulus features102,103. By adding the assumption that internal states such as the current task set104 and level of focus76,105 of an individual are also part of this episodic feature integration process, one can naturally account for item-specific and other context-specific control effects. Re-encountering a particular stimulus would retrieve not only prior associated motor responses but also the associated cognitive settings, such as the level of task focus or switch readiness76,97,101,105 (Fig. 5c,d). However, episodic reinstatement cannot account for adaptation effects in the absence of item-level control-demand associations, as observed in many LWPC28,80–88 and LWPS protocols44–46,91.
In summary, to explain the full range of how cognitive stability and flexibility settings are adapted to meet varying demands, one has to posit incremental learning mechanisms that continually adjust the strength of and access to goal representations (Fig. 5a,b) and event-based learning mechanisms that bind together episodic details with internal control states (Fig. 5c,d). These learning mechanisms would operate simultaneously in influencing context-appropriate levels of cognitive stability and flexibility on a moment-by-moment basis. This proposal, in addition to reconciling many findings across the conflict-control and task switching literatures, also connects the cognitive control literature with developments in the study of value-based decision-making, in which it has been shown that choices can be guided by a combination of incremental online learning and the recall of specific prior experiences106.
Heuristics and algorithms
At the cognitive level, the learning principles discussed in the previous sections can be neatly summarized in terms of two fundamental heuristics guiding control: the incremental learner is an expression of a recency heuristic, in which stability and flexibility are adjusted to match recent demands (Fig. 5a,b) and the episodic learner is an expression of a recognition heuristic, in which control settings are driven by a match between the current situation and a similar past experience (Fig. 5c,d). One unifying property of the two is that they reflect ‘sticky’ control states107. Stability and flexibility settings stick around over time108 and stick to other event features during memory encoding and retrieval109,110. At the algorithmic level, computational models have been built to account for the regulation of cognitive stability and, to a lesser extent, flexibility. An initial algorithmic implementation of incremental control was provided by the conflict-monitoring model17 that, within a neural network model framework, adopts a common reinforcement learning algorithm (temporal difference learning)111 to guide task focus. The strength with which the current task set biases task-relevant over task-irrelevant processing pathways on trial N is a function of the strength of the task set on trial N − 1, updated by the product of the trial N − 1 control prediction error and a learning rate. The prediction error refers to the difference between control demand on trial N – 1 and a running average of demand on the preceding trials. The learning rate determines how many of those preceding trials are averaged over: the higher the rate, the shorter the trial history on which the estimate is based, and the greater an influence the trial N − 1 prediction error will have on the updated strength of the task set for trial N. In the original model, learning rates were fixed for a given individual, but this is suboptimal because the model had to employ vastly different rates to separately account for task focus adjustments at the trial-by-trial level versus the block level17.
To overcome this limitation, the updated ‘flexible control model’ incorporated a self-adjusting learning rate that is driven by the volatility, or rate of change, of control demand77. This extension embraces the insight that learning rates should be higher in a volatile than in a stable environment because in a volatile environment, a surprising observation has a higher tendency to indicate a true change in the circumstances of an individual112. Thus, a Stroop task sequence of many incongruent trials in a row would not only result in increasing control (as in the original conflict-monitoring model) but also produce a steadily decreasing learning rate because the level of control demand is stable, which would attenuate the prediction error-driven updating77. This more flexible incremental learning mechanism can therefore account for both trial-by-trial and block-level effects of conflict on task focus adjustments simultaneously77. As noted in the ‘Shared principles of adaptive control’ section, the same logic of incremental updating of demand expectations instantiated in these models can also be applied to the challenge of task set selection78,79. However, neither of these models can account for item-based control learning because they operate at the level of goal representations only.
One way to produce item-level effects in variants of the conflict-monitoring model is to enable conflict to modulate the connections between goal representations and item-level or feature representations (such as colours and words in the Stroop task)95,96. For instance, using activation-dependent (Hebbian) learning to strengthen connections between active goal representations and stimulus features in response to increased conflict can account for both the ISPC and the congruency sequence effect96. However, this type of model has trouble accounting for adaptation effects in cases in which list-wide effects transfer to unbiased stimuli28,81–88, and when stimulus sets are large113 or trial unique80,91. Accordingly, there remains a need for a hybrid model that combines incremental adjustments at the level of goal representations with item-level, episodic learning. Moreover, none of the models discussed in the previous sections has attempted to combine the regulation of stability with concurrent adjustments in flexibility (but see ref.59 for a model that incorporates both conflict and task switching components).
I suggest that a comprehensive model of learned adjustments in task focus and switch readiness requires two independent incremental stability and flexibility learning mechanisms, complemented by an episodic reinstatement mechanism (Fig. 5a,c). Inspiration for implementing the latter can be found in the decision-making literature, in which models relying on ‘episodic sampling’ have been successful in describing reward-guided choices114,115. In these models, choices are based on stochastic sampling of prior decisions116, which can be weighted not only by recency (mimicking incremental learning) but also by contextual feature matching with more remote episodes117. Thus, future work could use these models to explain at an algorithmic level how re-encountering a control-demanding situation reinstates the associated control settings. A plausible neural mechanism for this can be found in the phenomenon of hippocampal pattern completion118. In summary, promising models have been developed to account for the learned adaptation of cognitive stability over time and in relation to specific items, but an algorithmic integration of incremental and episodic learning mechanisms, and of concurrent adjustments in stability and flexibility, remains a challenging research target for the future.
Conclusion
In this Perspective, I have synthesized the past two decades’ worth of research on how people regulate cognitive stability and flexibility, deriving a cognitive architecture in which task focus and switch readiness reflect two independent mechanisms whose control is guided by the same two general learning principles. The first learning principle reflects an incremental, online learning process that adapts stability and flexibility settings to match recent demands. This process operates by modulating the strength and/or accessibility of task goal representations, seems to be well-captured by reinforcement learning algorithms and can be thought of as regulating cognitive control settings via a recency heuristic. The second learning principle reflects the encoding and similarity-based retrieval of episodic event files, which enable the reinstatement of previously used control setting when specific items or contexts are re-encountered. This process could be captured by extending episodic memory sampling models to incorporate cognitive control settings and can be thought of as matching control states to current demands via a recognition heuristic. I hope that the integrative perspective laid out here stimulates further progress in understanding how people achieve context-sensitive control over task focus and task switching, as well as lends some future insight into clinical conditions (Box 3). I close by highlighting a few important remaining challenges.
Box 3. Clinical implications.
Cognitive stability and/or flexibility are impaired in normal ageing165,166 and many psychiatric and neurological conditions10, with particularly well-documented deficits in attention deficit hyperactivity disorder (ADHD)11,12, autism spectrum disorder12–14, schizophrenia15 and Parkinson’s disease16. Thus, these constructs are targets in the search for transdiagnostic biomarkers of potential failure modes in neural processing167. However, there is little agreement on optimal assessment and treatment of putative deficits in task focus and switch readiness. The current Perspective can provide some conceptual clarification to aid in fine tuning expectations for behaviour related to cognitive stability and flexibility and the assessment of deficits.
First, an appreciation that stability and flexibility are independent of each other immediately demystifies the co-occurrence of deficits in both task focus and switching, as is common in conditions such as ADHD168 and schizophrenia15, and which is currently considered ‘paradoxical’10. According to the two-dimensional account advocated here (Fig. 3b), there is no reason to assume that a condition that is characterized by low flexibility would necessarily be associated with high stability. The independence account also predicts that therapeutic interventions that aim to, for instance, improve cognitive stability should not necessarily alter cognitive flexibility or vice versa.
A second lesson from the studies reviewed here is that any comprehensive assessment of cognitive stability and flexibility should tap into the dynamic learning processes that are involved in matching task focus and switch readiness to changing contexts. For example, it is clear that individuals with ADHD do not have a generic inability to focus attention. Rather, they can become extremely engrossed in some activities, a state known as hyperfocus169. Instead, the ability of these individuals to properly align attentional focus with contextual demands seems to be impaired. The same deficit in learning to adapt control probably applies to other conditions in which individuals are thought to have deficits in cognitive stability or flexibility — it might not be an inability to focus or switch per se, but rather an impairment in context-sensitive regulation of these abilities. However, there is a relative dearth of clinical assessments involving dynamic, context-dependent changes in demands on stability and flexibility. An emphasis on adaptive learning has quite naturally emerged in assessing clinical deficits in reward processing170,171 and adopting a similar approach to characterizing potential impairments in cognitive control should prove very useful172.
Finally, the distinction drawn in the current Perspective between incremental and episodic learning contributions to regulating stability and flexibility is unexplored in characterizing cognitive control deficits in clinical populations. However, given the well-documented distinction between the neural systems that underpin reinforcement learning versus episodic memory173,174, it stands to reason that differential deficits in these two ways of guiding control could be observed across individuals with different clinical profiles.
One key issue to resolve is the degree to which the binding phenomena investigated in the event coding literature correspond to episodic memories100. The former effects are usually assessed by averaging over many trial pairs with a prime–probe structure, in which stimulus and response features either repeat or change across successive trials102,103. By contrast, episodic memories are typically assessed in the form of recall or recognition memory tests following a single exposure and much longer encoding–retrieval delays. Given that most ISPC and ISPS studies also use recurring stimuli, one might wonder whether item-based control learning effects are truly episodic or whether they could instead reflect incremental learning of item-level associations119. Some recent studies have provided critical proof-of-principle support for episodic effects by documenting that item-specific task focus and switch readiness associations can be formed based on a single experience (one-shot learning)109,110 and that the effects of such associations can endure for at least several minutes120. However, the exact relationship between feature integration phenomena in event coding studies, item-level control, and associative episodic memories remains to be fully worked out.
Another major challenge is to determine the specifics of the interplay between incremental and episodic contributions to control. Assuming parallel learning processes, the respective influences of incremental and episodic learning on guiding control settings would need to be reconciled in some way, but the details of this process are currently unknown. One possibility is a competitive relationship, in which some evaluative metric determines which learning mechanism dominates at a given point in time121. A plausible possibility could be some form of recognition memory threshold: if the current situation does not evoke a sufficiently close match, episodic reinstatement does not occur, but above the threshold, the best-matching episodic event file would be reinstated and determine control settings. In line with this idea, the decision-making literature has produced some clear examples of episodic reinstatement overriding choices based on incremental learning106,117. Alternatively, there could be a more symbiotic or cooperative relationship between these learning mechanisms, in line with proposals that reinforcement learning might draw on episodic memories to produce maximally adaptive behaviour122. However, testing and modelling both these ideas in the domain of cognitive stability and flexibility remain to be done.
Finally, I have focused on online incremental adaptation and episodic reinstatement of control because these two learning schemes appear both necessary and sufficient for explaining a large body of work on trial-by-trial, list-wide, and item-based adjustments in task focus and switch readiness. However, learning-guided control can take additional forms. For instance, control adjustments can be based on other contextual cues to control demand, such as stimulus location123, or stimulus–stimulus associations124. Zooming out further, in everyday scenarios, context-appropriate task goals and the means to achieve them often have to be derived from inferences based on rich representations of world knowledge in long-term memory, such as schemas or cognitive maps125. For example, a person might use their knowledge of typical airport layouts to inform temporary task sets that enable them to achieve goals such as finding their gate or a café in an unfamiliar airport126. The current perspective assumes that, as more abstract knowledge is converted into actionable task sets126, the latter become subject to the regulatory mechanisms described in this paper, but the intricacies of the interplay between inference-based versus incremental learning and episodic influences on control settings are yet to be explored.
Acknowledgements
The author thanks J. Jiang, Y.-C. Chiu, C. Bejjani, P. Whitehead, A. Siqi-Liu and R. Geddert for collaborations and discussions leading to this Perspective. This work was supported by grant R01MH116967 by the National Institutes of Health.
Footnotes
Competing interests
The author declares no competing interests.
Additional information
Peer review information Nature Reviews Psychology thanks Maria Augustinova, David Badre and Gesine Dreisbach for their contribution to the peer review of this work.
References
- 1.Miller EK & Cohen JD An integrative theory of prefrontal cortex function. Annu. Rev. Neurosci 24, 167–202 (2001). [DOI] [PubMed] [Google Scholar]
- 2.Baddeley AD & Hitch G in The Psychology of Learning and Motivation: Advances in Research and Theory Vol. 8 (ed Bower GH) pp. 47–89 (Academic, 1974). [Google Scholar]
- 3.Cowan N Evolving conceptions of memory storage, selective attention, and their mutual constraints within the human information-processing system. Psychol. Bull 104, 163–191 (1988). [DOI] [PubMed] [Google Scholar]
- 4.Oberauer K in The Psychology of Learning and Motivation Vol. 51 (ed Ross BH) pp. 45–100 (Elsevier Academic, 2009). [Google Scholar]
- 5.Allport A, Styles EA & Hsieh S in Attention and Performance Vol. XV (eds Moscovitch M & Umilta C) pp. 421–452 (MIT Press, 1994). [Google Scholar]
- 6.Rogers RD & Monsell S Costs of a predictable switch between simple cognitive tasks. J. Exp. Psychol. Gen 124, 207–231 (1995). [Google Scholar]
- 7.Frank MJ, Loughry B & O’Reilly RC Interactions between frontal cortex and basal ganglia in working memory: a computational model. Cogn. Affect. Behav. Neurosci 1, 137–160 (2001). [DOI] [PubMed] [Google Scholar]
- 8.Goschke T in Voluntary Action: Brains, Minds, and Sociality (eds Prinz W et al. ) pp. 49–85 (Oxford University, 2003). [Google Scholar]
- 9.Hommel B in Advances in Motivation Science Vol. 2 (ed Elliot AJ) pp. 33–67 (Elsevier, 2015). [Google Scholar]
- 10.Uddin LQ Cognitive and behavioural flexibility: neural mechanisms and clinical considerations. Nat. Rev. Neurosci 22, 167–179 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cepeda NJ, Cepeda ML & Kramer AF Task switching and attention deficit hyperactivity disorder. J. Abnorm. Child Psychol 28, 213–226 (2000). [DOI] [PubMed] [Google Scholar]
- 12.Craig F et al. A review of executive function deficits in autism spectrum disorder and attention-deficit/hyperactivity disorder. Neuropsychiatr. Dis. Treat 12, 1191–1202 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.D’Cruz AM et al. Reduced behavioral flexibility in autism spectrum disorders. Neuropsychology 27, 152–160 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Uddin LQ Brain mechanisms supporting flexible cognition and behavior in adolescents with autism spectrum disorder. Biol. Psychiatry 89, 172–183 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nieuwenstein MR, Aleman A & de Haan EH Relationship between symptom dimensions and neurocognitive functioning in schizophrenia: a meta-analysis of WCST and CPT studies. Wisconsin Card Sorting Test. Continuous performance test. J. Psychiatr. Res 35, 119–125 (2001). [DOI] [PubMed] [Google Scholar]
- 16.Robbins TW & Cools R Cognitive deficits in Parkinson’s disease: a cognitive neuroscience perspective. Mov. Disord 29, 597–607 (2014). [DOI] [PubMed] [Google Scholar]
- 17.Botvinick MM, Braver TS, Barch DM, Carter CS & Cohen JD Conflict monitoring and cognitive control. Psychol. Rev 108, 624–652 (2001). [DOI] [PubMed] [Google Scholar]
- 18.Monsell S Task switching. Trends Cogn. Sci 7, 134–140 (2003). [DOI] [PubMed] [Google Scholar]
- 19.Goschke T in Action Science: Foundations of an Ermerging Discipline (eds Beisert A et al. ) pp. 409–434 (MIT Press, 2013). [Google Scholar]
- 20.Dreisbach G & Frober K On how to be flexible (or not): modulation of the stability-flexibility balance. Curr. Dir. Psychol. Sci 28, 3–9 (2018). [Google Scholar]
- 21.Braem S & Egner T Getting a grip on cognitive flexibility. Curr. Dir. Psychol. Sci 27, 470–476 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stroop JR Studies of interference in serial verbal reactions. J. Exp. Psychol 18, 643–662 (1935). [Google Scholar]
- 23.MacLeod CM Half a century of research on the Stroop effect: an integrative review. Psychol. Bull 109, 163–203 (1991). [DOI] [PubMed] [Google Scholar]
- 24.Parris BA, Hasshim N, Wadsley M, Augustinova M & Ferrand L The loci of Stroop effects: a critical review of methods and evidence for levels of processing contributing to color-word Stroop effects and the implications for the loci of attentional selection. Psychol. Res 86, 1029–1053 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cohen JD, Dunbar K & McClelland JL On the control of automatic processes: a parallel distributed processing account of the Stroop effect. Psychol. Rev 97, 332–361 (1990). [DOI] [PubMed] [Google Scholar]
- 26.Logan GD & Zbrodoff NJ When it helps to be misled: facilitative effects of increasing the frequency of conflicting stimuli in a Stroop-like task. Mem. Cogn 7, 166–174 (1979). [Google Scholar]
- 27.Gratton G, Coles MG & Donchin E Optimizing the use of information: strategic control of activation of responses. J. Exp. Psychol. Gen 121, 480–506 (1992). [DOI] [PubMed] [Google Scholar]
- 28.Bugg JM & Chanani S List-wide control is not entirely elusive: evidence from picture-word Stroop. Psychon. Bull. Rev 18, 930–936 (2011). [DOI] [PubMed] [Google Scholar]
- 29.Bugg JM & Crump MJ In support of a distinction between voluntary and stimulus-driven control: a review of the literature on proportion congruent effects. Front. Psychol 3, 367 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Egner T Congruency sequence effects and cognitive control. Cogn. Affect. Behav. Neurosci 7, 380–390 (2007). [DOI] [PubMed] [Google Scholar]
- 31.Egner T The Wiley Handbook of Cognitive Control (ed Egner T) pp. 64–78 (Wiley-Blackwell, 2017). [Google Scholar]
- 32.Bugg JM Dissociating levels of cognitive control: the case of Stroop interference. Curr. Dir. Psychol. Sci 21, 302–309 (2012). [Google Scholar]
- 33.Jacoby LL, Lindsay DS & Hessels S Item-specific control of automatic processes: Stroop process dissociations. Psychon. Bull. Rev 10, 638–644 (2003). [DOI] [PubMed] [Google Scholar]
- 34.Spinelli G & Lupker SJ Item-specific control of attention in the Stroop task: contingency learning is not the whole story in the item-specific proportion-congruent effect. Mem. Cogn 48, 426–435 (2020). [DOI] [PubMed] [Google Scholar]
- 35.Bugg JM, Jacoby LL & Chanani S Why it is too early to lose control in accounts of item-specific proportion congruency effects. J. Exp. Psychol. Hum. Percept. Perform 37, 844–859 (2011). [DOI] [PubMed] [Google Scholar]
- 36.Bugg JM & Hutchison KA Converging evidence for control of color-word Stroop interference at the item level. J. Exp. Psychol. Hum. Percept. Perform 39, 433–449 (2013). [DOI] [PubMed] [Google Scholar]
- 37.Sudevan P & Taylor DA The cuing and priming of cognitive operations. J. Exp. Psychol. Hum. Percept. Perform 13, 89–103 (1987). [DOI] [PubMed] [Google Scholar]
- 38.Meiran N Reconfiguration of processing mode prior to task performance. J. Exp. Psychol. Learn. Mem. Cogn 22, 1423–1442 (1996). [Google Scholar]
- 39.Waszak F, Hommel B & Allport A Task-switching and long-term priming: role of episodic stimulus-task bindings in task-shift costs. Cogn. Psychol 46, 361–413 (2003). [DOI] [PubMed] [Google Scholar]
- 40.Vandierendonck A, Liefooghe B & Verbruggen F Task switching: interplay of reconfiguration and interference control. Psychol. Bull 136, 601–626 (2010). [DOI] [PubMed] [Google Scholar]
- 41.Dreisbach G & Haider H Preparatory adjustment of cognitive control in the task switching paradigm. Psychon. Bull. Rev 13, 334–338 (2006). [DOI] [PubMed] [Google Scholar]
- 42.Monsell S & Mizon GA Can the task-cuing paradigm measure an endogenous task-set reconfiguration process? J. Exp. Psychol. Hum. Percept. Perform 32, 493–516 (2006). [DOI] [PubMed] [Google Scholar]
- 43.Schneider DW & Logan GD Priming cue encoding by manipulating transition frequency in explicitly cued task switching. Psychon. Bull. Rev 13, 145–151 (2006). [DOI] [PubMed] [Google Scholar]
- 44.Siqi-Liu A & Egner T Contextual adaptation of cognitive flexibility is driven by task- and item-level learning. Cogn. Affect. Behav. Neurosci 20, 757–782 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bejjani C, Siqi-Liu A & Egner T Minimal impact of consolidation on learned switch-readiness. J. Exp. Psychol. Learn. Mem. Cogn 47, 1622–1637 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kang MS & Chiu YC Proactive and reactive metacontrol in task switching. Mem. Cogn 49, 1617–1632 (2021). [DOI] [PubMed] [Google Scholar]
- 47.Chiu YC & Egner T Cueing cognitive flexibility: item-specific learning of switch readiness. J. Exp. Psychol. Hum. Percept. Perform 43, 1950–1960 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Frober K & Dreisbach G Keep flexible — keep switching! The influence of forced task switching on voluntary task switching. Cognition 162, 48–53 (2017). [DOI] [PubMed] [Google Scholar]
- 49.Chiu YC, Frober K & Egner T Item-specific priming of voluntary task switches. J. Exp. Psychol. Hum. Percept. Perform 46, 434–441 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Musslick S & Cohen JD Rationalizing constraints on the capacity for cognitive control. Trends Cogn. Sci 25, 757–775 (2021). [DOI] [PubMed] [Google Scholar]
- 51.Geddert R & Egner T No need to choose: independent regulation of cognitive stability and flexibility challenges the stability-flexibility tradeoff. J. Exp. Psychol. Gen 151, 3009–3027 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bejjani C, Hoyle RH & Egner T Distinct but correlated latent factors support the regulation of learned conflict-control and task-switching. Cogn. Psychol 135, 101474 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nack C & Chiu YC A dual-dimension framework of cognitive flexibility and stability. Preprint at PsyArXiv 10.31234/osf.io/knmr7 (2022). [DOI] [Google Scholar]
- 54.Cools R & D’Esposito M Inverted-U-shaped dopamine actions on human working memory and cognitive control. Biol. Psychiatry 69, e113–e125 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dreisbach G Mechanisms of cognitive control: the functional role of task rules. Curr. Dir. Psychol. Sci 21, 227–231 (2012). [Google Scholar]
- 56.Meiran N in Control of Cognitive Processes: Attention and Performance Vol. XVIII (eds Driver J & Monsell S) Ch. 16, pp. 377–399 (MIT Press, 2000). [Google Scholar]
- 57.Kiesel A et al. Control and interference in task switching — a review. Psychol. Bull 136, 849–874 (2010). [DOI] [PubMed] [Google Scholar]
- 58.Goschke T in Control of Cognitive Processes: Attention and Performance XVIII (eds Monsell S & Driver J) pp. 331–355 (MIT Press, 2000). [Google Scholar]
- 59.Brown JW, Reynolds JR & Braver TS A computational model of fractionated conflict-control mechanisms in task-switching. Cogn. Psychol 55, 37–85 (2007). [DOI] [PubMed] [Google Scholar]
- 60.Dreisbach G & Goschke T How positive affect modulates cognitive control: reduced perseveration at the cost of increased distractibility. J. Exp. Psychol. Learn. Mem. Cogn 30, 343–353 (2004). [DOI] [PubMed] [Google Scholar]
- 61.Goschke T & Bolte A Emotional modulation of control dilemmas: the role of positive affect, reward, and dopamine in cognitive stability and flexibility. Neuropsychologia 62, 403–423 (2014). [DOI] [PubMed] [Google Scholar]
- 62.Dreisbach G How positive affect modulates cognitive control: the costs and benefits of reduced maintenance capability. Brain Cogn 60, 11–19 (2006). [DOI] [PubMed] [Google Scholar]
- 63.Hefer C & Dreisbach G How performance-contingent reward prospect modulates cognitive control: increased cue maintenance at the cost of decreased flexibility. J. Exp. Psychol. Learn. Mem. Cogn 43, 1643–1658 (2017). [DOI] [PubMed] [Google Scholar]
- 64.Chiew KS & Braver TS Dissociable influences of reward motivation and positive emotion on cognitive control. Cogn. Affect. Behav. Neurosci 14, 509–529 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Braem S Conditioning task switching behavior. Cognition 166, 272–276 (2017). [DOI] [PubMed] [Google Scholar]
- 66.Talanow T & Ettinger U Effects of task repetition but no transfer of inhibitory control training in healthy adults. Acta Psychol 187, 37–53 (2018). [DOI] [PubMed] [Google Scholar]
- 67.Zhao X, Wang H & Maes JHR Training and transfer effects of extensive task-switching training in students. Psychol. Res 84, 389–403 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Miyake A et al. The unity and diversity of executive functions and their contributions to complex “frontal lobe” tasks: a latent variable analysis. Cogn. Psychol 41, 49–100 (2000). [DOI] [PubMed] [Google Scholar]
- 69.Miyake A & Friedman NP The nature and organization of individual differences in executive functions: four general conclusions. Curr. Dir. Psychol. Sci 21, 8–14 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Frober K, Raith L & Dreisbach G The dynamic balance between cognitive flexibility and stability: the influence of local changes in reward expectation and global task context on voluntary switch rate. Psychol. Res 82, 65–77 (2018). [DOI] [PubMed] [Google Scholar]
- 71.Shenhav A, Botvinick MM & Cohen JD The expected value of control: an integrative theory of anterior cingulate cortex function. Neuron 79, 217–240 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kool W & Botvinick M A labor/leisure tradeoff in cognitive control. J. Exp. Psychol. Gen 143, 131–141 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kool W, McGuire JT, Rosen ZB & Botvinick MM Decision making and the avoidance of cognitive demand. J. Exp. Psychol. Gen 139, 665–682 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Westbrook A, Kester D & Braver TS What is the subjective cost of cognitive effort? Load, trait, and aging effects revealed by economic preference. PLoS ONE 8, e68210 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Braver TS The variable nature of cognitive control: a dual mechanisms framework. Trends Cogn. Sci 16, 106–113 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Egner T Creatures of habit (and control): a multi-level learning perspective on the modulation of congruency effects. Front. Psychol 5, 1247 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jiang J, Heller K & Egner T Bayesian modeling of flexible cognitive control. Neurosci. Biobehav. Rev 46, 30–43 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jiang J, Wagner AD & Egner T Integrated externally and internally generated task predictions jointly guide cognitive control in prefrontal cortex. eLife 7, e39497 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wen T, Geddert RM, Madlon-Kay S & Egner T Transfer of learned cognitive flexibility to novel stimuli and task sets. Psychol. Sci 34, 435–454 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Spinelli G, Perry JR & Lupker SJ Adaptation to conflict frequency without contingency and temporal learning: evidence from the picture-word interference task. J. Exp. Psychol. Hum. Percept. Perform 45, 995–1014 (2019). [DOI] [PubMed] [Google Scholar]
- 81.Bugg JM Conflict-triggered top-down control: default mode, last resort, or no such thing. J. Exp. Psychol. Learn. Mem. Cogn 40, 567–587 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hutchison KA The interactive effects of listwide control, item-based control, and working memory capacity on Stroop performance. J. Exp. Psychol. Learn. Mem. Cogn 37, 851–860 (2011). [DOI] [PubMed] [Google Scholar]
- 83.Gonthier C, Braver TS & Bugg JM Dissociating proactive and reactive control in the Stroop task. Mem. Cogn 44, 778–788 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Spinelli G & Lupker SJ Proactive control in the Stroop task: a conflict-frequency manipulation free of item-specific, contingency-learning, and color-word correlation confounds. J. Exp. Psychol. Learn. Mem. Cogn 47, 1550–1562 (2021). [DOI] [PubMed] [Google Scholar]
- 85.Spinelli G & Lupker SJ Robust evidence for proactive conflict adaptation in the proportion-congruent paradigm. J. Exp. Psychol. Learn. Mem. Cogn 49, 675–700 (2022). [DOI] [PubMed] [Google Scholar]
- 86.Bugg JM & Gonthier C List-level control in the flanker task. Q. J. Exp. Psychol 73, 1444–1459 (2020). [DOI] [PubMed] [Google Scholar]
- 87.Bejjani C, Tan S & Egner T Performance feedback promotes proactive but not reactive adaptation of conflict-control. J. Exp. Psychol. Hum. Percept. Perform 46, 369–387 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bejjani C & Egner T Evaluating the learning of stimulus-control associations through incidental memory of reinforcement events. J. Exp. Psychol. Learn. Mem. Cogn 47, 1599–1621 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sabah K, Dolk T, Meiran N & Dreisbach G When less is more: costs and benefits of varied vs. fixed content and structure in short-term task switching training. Psychol. Res 83, 1531–1542 (2019). [DOI] [PubMed] [Google Scholar]
- 90.Sabah K, Dolk T, Meiran N & Dreisbach G Enhancing task-demands disrupts learning but enhances transfer gains in short-term task-switching training. Psychol. Res 85, 1473–1487 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Siqi-Liu A & Egner T Task sets define boundaries of learned cognitive flexibility in list-wide proportion switch manipulations. J. Exp. Psychol. Hum. Percept. Perform 49, 1111–1122 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hazeltine E, Lightman E, Schwarb H & Schumacher EH The boundaries of sequential modulations: evidence for set-level control. J. Exp. Psychol. Hum. Percept. Perform 37, 1898–1914 (2011). [DOI] [PubMed] [Google Scholar]
- 93.Grant LD, Cookson SL & Weissman DH Task sets serve as boundaries for the congruency sequence effect. J. Exp. Psychol. Hum. Percept. Perform 46, 798–812 (2020). [DOI] [PubMed] [Google Scholar]
- 94.Schumacher EH & Hazeltine E Hierarchical task representation: task files and response selection. Curr. Dir. Psychol. Sci 25, 449–454 (2016). [Google Scholar]
- 95.Blais C, Robidoux S, Risko EF & Besner D Item-specific adaptation and the conflict-monitoring hypothesis: a computational model. Psychol. Rev 114, 1076–1086 (2007). [DOI] [PubMed] [Google Scholar]
- 96.Verguts T & Notebaert W Hebbian learning of cognitive control: dealing with specific and nonspecific adaptation. Psychol. Rev 115, 518–525 (2008). [DOI] [PubMed] [Google Scholar]
- 97.Abrahamse E, Braem S, Notebaert W & Verguts T Grounding cognitive control in associative learning. Psychol. Bull 142, 693–728 (2016). [DOI] [PubMed] [Google Scholar]
- 98.Verguts T & Notebaert W Adaptation by binding: a learning account of cognitive control. Trends Cogn. Sci 13, 252–257 (2009). [DOI] [PubMed] [Google Scholar]
- 99.Hommel B, Musseler J, Aschersleben G & Prinz W The Theory of Event Coding (TEC): a framework for perception and action planning. Behav. Brain Sci 24, 849–878 (2001). [DOI] [PubMed] [Google Scholar]
- 100.Frings C et al. Binding and retrieval in action control (BRAC). Trends Cogn. Sci 24, 375–387 (2020). [DOI] [PubMed] [Google Scholar]
- 101.Dignath D, Johannsen L, Hommel B & Kiesel A Reconciling cognitive-control and episodic-retrieval accounts of sequential conflict modulation: binding of control-states into event-files. J. Exp. Psychol. Hum. Percept. Perform 45, 1265–1270 (2019). [DOI] [PubMed] [Google Scholar]
- 102.Hommel B Event files: evidence for automatic integration of stimulus-response episodes. Vis. Cogn 5, 183–216 (1998). [Google Scholar]
- 103.Hommel B Event files: feature binding in and across perception and action. Trends Cogn. Sci 8, 494–500 (2004). [DOI] [PubMed] [Google Scholar]
- 104.Kikumoto A & Mayr U Conjunctive representations that integrate stimuli, responses, and rules are critical for action selection. Proc. Natl Acad. Sci. USA 117, 10603–10608 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Spape MM & Hommel B He said, she said: episodic retrieval induces conflict adaptation in an auditory Stroop task. Psychon. Bull. Rev 15, 1117–1121 (2008). [DOI] [PubMed] [Google Scholar]
- 106.Duncan KD & Shohamy D Memory states influence value-based decisions. J. Exp. Psychol. Gen 145, 1420–1426 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Mayr U & Bryck RL Sticky rules: integration between abstract rules and specific actions. J. Exp. Psychol. Learn. Mem. Cogn 31, 337–350 (2005). [DOI] [PubMed] [Google Scholar]
- 108.Schiltenwolf M, Kiesel A & Dignath D No temporal decay of cognitive control in the congruency sequence effect. J. Exp. Psychol. Learn. Mem. Cogn 49, 1247–1263 (2022). [DOI] [PubMed] [Google Scholar]
- 109.Brosowsky NP & Crump MJC Memory-guided selective attention: single experiences with conflict have long-lasting effects on cognitive control. J. Exp. Psychol. Gen 147, 1134–1153 (2018). [DOI] [PubMed] [Google Scholar]
- 110.Whitehead PS, Pfeuffer CU & Egner T Memories of control: one-shot episodic learning of item-specific stimulus-control associations. Cognition 199, 104220 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Sutton R & Barto A Reinforcement Learning (MIT Press, 1998). [Google Scholar]
- 112.Behrens TE, Woolrich MW, Walton ME & Rushworth MF Learning the value of information in an uncertain world. Nat. Neurosci 10, 1214–1221 (2007). [DOI] [PubMed] [Google Scholar]
- 113.Blais C & Verguts T Increasing set size breaks down sequential congruency: evidence for an associative locus of cognitive control. Acta Psychol 141, 133–139 (2012). [DOI] [PubMed] [Google Scholar]
- 114.Plonsky O, Teodorescu K & Erev I Reliance on small samples, the wavy recency effect, and similarity-based learning. Psychol. Rev 122, 621–647 (2015). [DOI] [PubMed] [Google Scholar]
- 115.Bornstein AM, Khaw MW, Shohamy D & Daw ND Reminders of past choices bias decisions for reward in humans. Nat. Commun 8, 15958 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wang S, Feng SF & Bornstein AM Mixing memory and desire: how memory reactivation supports deliberative decision-making. Wiley Interdiscip. Rev. Cogn. Sci 13, e1581 (2021). [DOI] [PubMed] [Google Scholar]
- 117.Bornstein AM & Norman KA Reinstated episodic context guides sampling-based decisions for reward. Nat. Neurosci 20, 997–1003 (2017). [DOI] [PubMed] [Google Scholar]
- 118.Horner AJ, Bisby JA, Bush D, Lin WJ & Burgess N Evidence for holistic episodic recollection via hippocampal pattern completion. Nat. Commun 6, 7462 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Chiu YC, Jiang J & Egner T The caudate nucleus mediates learning of stimulus-control state associations. J. Neurosci 37, 1028–1038 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Whitehead PS, Pfeuffer CU & Egner T Assessing the durability of one-shot stimulus-control bindings. J. Cogn 5, 26 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Nicholas J, Daw ND & Shohamy D Uncertainty alters the balance between incremental learning and episodic memory. eLife 10.7554/eLife.81679 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Gershman SJ & Daw ND Reinforcement learning and episodic memory in humans and animals: an integrative framework. Annu. Rev. Psychol 68, 101–128 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Crump MJ & Milliken B The flexibility of context-specific control: evidence for context-driven generalization of item-specific control settings. Q. J. Exp. Psychol 62, 1523–1532 (2009). [DOI] [PubMed] [Google Scholar]
- 124.Bejjani C, Zhang Z & Egner T Control by association: transfer of implicitly primed attentional states across linked stimuli. Psychon. Bull. Rev 25, 617–626 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Behrens TEJ et al. What is a cognitive map? Organizing knowledge for flexible behavior. Neuron 100, 490–509 (2018). [DOI] [PubMed] [Google Scholar]
- 126.Vaidya AR & Badre D Abstract task representations for inference and control. Trends Cogn. Sci 26, 484–498 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Schmidt JR Questioning conflict adaptation: proportion congruent and Gratton effects reconsidered. Psychon. Bull. Rev 20, 615–630 (2013). [DOI] [PubMed] [Google Scholar]
- 128.Schmidt JR Evidence against conflict monitoring and adaptation: an updated review. Psychon. Bull. Rev 26, 753–771 (2019). [DOI] [PubMed] [Google Scholar]
- 129.Braem S et al. Measuring adaptive control in conflict tasks. Trends Cogn. Sci 23, 769–783 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Spinelli G, Morton JB & Lupker SJ Both task-irrelevant and task-relevant information trigger reactive conflict adaptation in the item-specific proportion-congruent paradigm. Psychon. Bull. Rev 29, 2133–2145 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Algom D, Fitousi D & Chajut E Can the Stroop effect serve as the gold standard of conflict monitoring and control? A conceptual critique. Mem. Cogn 50, 883–897 (2022). [DOI] [PubMed] [Google Scholar]
- 132.Goldman-Rakic PS in Handbook of Physiology, Section1: The Nervous System Vol. 5 (eds Plum F & Mountcastle VB) pp. 373–417 (American Physiological Society, 1987). [Google Scholar]
- 133.Fuster JM & Alexander GE Neuron activity related to short-term memory. Science 173, 652–654 (1971). [DOI] [PubMed] [Google Scholar]
- 134.Freedman DJ, Riesenhuber M, Poggio T & Miller EK Categorical representation of visual stimuli in the primate prefrontal cortex. Science 291, 312–316 (2001). [DOI] [PubMed] [Google Scholar]
- 135.Stokes MG et al. Dynamic coding for cognitive control in prefrontal cortex. Neuron 78, 364–375 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Woolgar A, Hampshire A, Thompson R & Duncan J Adaptive coding of task-relevant information in human frontoparietal cortex. J. Neurosci 31, 14592–14599 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Waskom ML, Kumaran D, Gordon AM, Rissman J & Wagner AD Frontoparietal representations of task context support the flexible control of goal-directed cognition. J. Neurosci 34, 10743–10755 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Miller EK The prefrontal cortex and cognitive control. Nat. Rev. Neurosci 1, 59–65 (2000). [DOI] [PubMed] [Google Scholar]
- 139.Duncan J An adaptive coding model of neural function in prefrontal cortex. Nat. Rev. Neurosci 2, 820–829 (2001). [DOI] [PubMed] [Google Scholar]
- 140.Desimone R & Duncan J Neural mechanisms of selective visual attention. Annu. Rev. Neurosci 18, 193–222 (1995). [DOI] [PubMed] [Google Scholar]
- 141.Gazzaley A & Nobre AC Top-down modulation: bridging selective attention and working memory. Trends Cogn. Sci 16, 129–135 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kolling N, Behrens T, Wittmann MK & Rushworth M Multiple signals in anterior cingulate cortex. Curr. Opin. Neurobiol 37, 36–43 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Alexander WH & Brown JW Medial prefrontal cortex as an action-outomce predictor. Nat. Neurosci 14, 1338–1344 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Mansouri FA, Egner T & Buckley MJ Monitoring demands for executive control: shared functions between human and nonhuman primates. Trends Neurosci 40, 15–27 (2017). [DOI] [PubMed] [Google Scholar]
- 145.Botvinick MM, Nystrom LE, Fissell K, Carter CS & Cohen JD Conflict monitoring versus selection-for-action in anterior cingulate cortex. Nature 402, 179–181 (1999). [DOI] [PubMed] [Google Scholar]
- 146.MacDonald AW 3rd, Cohen JD, Stenger VA & Carter CS Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science 288, 1835–1838 (2000). [DOI] [PubMed] [Google Scholar]
- 147.Kerns JG et al. Anterior cingulate conflict monitoring and adjustments in control. Science 303, 1023–1026 (2004). [DOI] [PubMed] [Google Scholar]
- 148.Egner T & Hirsch J Cognitive control mechanisms resolve conflict through cortical amplification of task-relevant information. Nat. Neurosci 8, 1784–1790 (2005). [DOI] [PubMed] [Google Scholar]
- 149.Muhle-Karbe PS, Jiang J & Egner T Causal evidence for learning-dependent frontal lobe contributions to cognitive control. J. Neurosci 38, 962–973 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Jiang J, Beck J, Heller K & Egner T An insula-frontostriatal network mediates flexible cognitive control by adaptively predicting changing control demands. Nat. Commun 6, 8165 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Chiu YC & Egner T Cortical and subcortical contributions to context-control learning. Neurosci. Biobehav. Rev 99, 33–41 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Owen AM, McMillan KM, Laird AR & Bullmore E N-back working memory paradigm: a meta-analysis of normative functional neuroimaging studies. Hum. Brain Mapp 25, 46–59 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Kim C, Cilles SE, Johnson NF & Gold BT Domain general and domain preferential brain regions associated with different types of task switching: a meta-analysis. Hum. Brain Mapp 33, 130–142 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Worringer B et al. Common and distinct neural correlates of dual-tasking and task switching: a meta-analytic review and a neuro-cognitive processing model of human multitasking. Brain Struct. Funct 224, 1845–1869 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Nir-Cohen G, Kessler Y & Egner T Neural substrates of working memory updating. J. Cogn. Neurosci 32, 2285–2302 (2020). [DOI] [PubMed] [Google Scholar]
- 156.Murty VP et al. Selective updating of working memory content modulates meso-cortico-striatal activity. Neuroimage 57, 1264–1272 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Cools R, Sheridan M, Jacobs E & D’Esposito M Impulsive personality predicts dopamine-dependent changes in frontostriatal activity during component processes of working memory. J. Neurosci 27, 5506–5514 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Chatham CH & Badre D Multiple gates on working memory. Curr. Opin. Behav. Sci 1, 23–31 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Leber AB, Turk-Browne NB & Chun MM Neural predictors of moment-to-moment fluctuations in cognitive flexibility. Proc. Natl Acad. Sci. USA 105, 13592–13597 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.De Baene W & Brass M Switch probability context (in)sensitivity within the cognitive control network. Neuroimage 77, 207–214 (2013). [DOI] [PubMed] [Google Scholar]
- 161.Sayali C & Badre D Neural systems underlying the learning of cognitive effort costs. Cogn. Affect. Behav. Neurosci 21, 698–716 (2021). [DOI] [PubMed] [Google Scholar]
- 162.Cools R Chemistry of the adaptive mind: lessons from dopamine. Neuron 104, 113–131 (2019). [DOI] [PubMed] [Google Scholar]
- 163.den Ouden HE et al. Dissociable effects of dopamine and serotonin on reversal learning. Neuron 80, 1090–1100 (2013). [DOI] [PubMed] [Google Scholar]
- 164.Furman DJ et al. Effects of dopaminergic drugs on cognitive control processes vary by genotype. J. Cogn. Neurosci 32, 804–821 (2020). [DOI] [PubMed] [Google Scholar]
- 165.Salthouse TA, Babcock RL & Shaw RJ Effects of adult age on structural and operational capacities in working memory. Psychol. Aging 6, 118–127 (1991). [DOI] [PubMed] [Google Scholar]
- 166.Cepeda NJ, Kramer AF & Gonzalez de Sather JC Changes in executive control across the life span: examination of task-switching performance. Dev. Psychol 37, 715–730 (2001). [PubMed] [Google Scholar]
- 167.Cuthbert BN & Insel TR Toward new approaches to psychotic disorders: the NIMH research domain criteria project. Schizophr. Bull 36, 1061–1062 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Barkley RA Behavioral inhibition, sustained attention, and executive functions: constructing a unifying theory of ADHD. Psychol. Bull 121, 65–94 (1997). [DOI] [PubMed] [Google Scholar]
- 169.Hupfeld KE, Abagis TR & Shah P Living “in the zone”: hyperfocus in adult ADHD. Atten. Defic. Hyperact. Disord 11, 191–208 (2019). [DOI] [PubMed] [Google Scholar]
- 170.Maia TV & Frank MJ From reinforcement learning models to psychiatric and neurological disorders. Nat. Neurosci 14, 154–162 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Kishida KT, King-Casas B & Montague PR Neuroeconomic approaches to mental disorders. Neuron 67, 543–554 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Gillan CM, Kosinski M, Whelan R, Phelps EA & Daw ND Characterizing a psychiatric symptom dimension related to deficits in goal-directed control. eLife 5, e11305 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Atallah HE, Frank MJ & O’Reilly RC Hippocampus, cortex, and basal ganglia: insights from computational models of complementary learning systems. Neurobiol. Learn. Mem 82, 253–267 (2004). [DOI] [PubMed] [Google Scholar]
- 174.Squire LR Memory systems of the brain: a brief history and current perspective. Neurobiol. Learn. Mem 82, 171–177 (2004). [DOI] [PubMed] [Google Scholar]


