Abstract
Background
Total body irradiation (TBI)-based allogeneic hematopoietic stem cell transplantation (allo-HSCT) is a curative treatment for selected patients with acute myeloid leukemia (AML). Yet, secondary malignancies contribute to long-term morbidity and mortality with TBI potentially influencing these risks.
Methods
This retrospective study analyzed the cumulative incidences of secondary solid malignancies and precancerous lesions of 89 consecutive AML patients after TBI-based conditioning before 1st allo-HSCT between 2000 and 2016. TBI was performed with an average dose rate of 4 cGy/min and a twice-daily fractionation. Cause-specific hazard models analyzed risk factors for secondary malignancies/precancerous lesions and the competing risks of dying before developing secondary malignancies/precancerous lesions.
Results
The median patient age at TBI was 42.5 years (interquartile range, 32.5–51.2), while the median follow-up was 15.2 years (interquartile range, 13.0-18.2). Most patients received a myeloablative conditioning (MAC) containing 8 Gy (n = 47) and 12 Gy TBI (n = 11). Reduced-intensity regimens (RIC, 4 Gy TBI) were applied in 31 patients. Of note, patients receiving RIC were older than patients receiving MAC. The most common cancer types were non-squamous cell carcinomas (n = 14) after exclusion of a patient diagnosed with sarcoma within less than a year after TBI. The cumulative incidences of secondary malignancies and precancerous lesions were 8% (95%CI, 4–16), 14% (95%CI, 7–23), and 17% (95%CI, 9–27) at 10, 15 and 20 years, while the cumulative incidences of premature deaths were 59% (95%CI, 48–69), 59% (95%CI, 48–69), and 64% (95%CI, 49–76). In multivariate analyses, higher patient age at TBI was associated with lower rates of secondary malignancies/precancerous lesions, while higher patient age translated into a trend towards premature deaths (before patients could develop malignancies). Higher TBI doses, mainly applied in younger patients, translated into lower rates of secondary malignancies/precancerous lesions while lacking associations with mortality. Chronic GVHD requiring systemic immunosuppression was associated with premature deaths.
Conclusions
Although this study indicates an inverse relationship between TBI doses applied and treatment-related malignancies, confounding by competing risks is present. The age dependency may be explained by the fact that older patients had a lower life expectancy independent of malignancies, illustrating the pitfalls of competing risks.
Trial registration
The study was retrospectively registered.
Keywords: Total body irradiation, Low-dose radiotherapy, Secondary solid malignancies, Allogeneic hematopoietic stem cell transplantation, Carcinogenesis, Acute myeloid leukemia
Background
Allogeneic hematopoietic stem cell transplantation (allo-HSCT) applying TBI and non-TBI-based conditioning regimens is a curative treatment option for selected patients with acute myeloid leukemia (AML). Yet, secondary malignancies contribute to long-term morbidity and mortality with conditioning regimens potentially modulating these risks [1]. Myeloablative conditioning (MAC) containing higher doses of TBI (8–12 Gy) is usually applied in fit and young patients (< 50 years of age) [2, 3]. Reduced-intensity conditioning (RIC) combining lower doses of chemotherapy and/or TBI (doses ranging from ≥ 4 Gy to < 8 Gy) is used particularly in patients ineligible for myeloablative conditioning to reduce transplant-related complications while maintaining efficacy [2, 4]. Both recipients of TBI and non-TBI-based conditioning regimens are at risk of developing secondary malignancies after allo-HSCT [5, 6]. Literature indicates that TBI is associated with basal cell carcinomas, and breast, thyroid, and brain cancers occurring with a long delay after allo-HSCT [7–9]. In contrast, chronic graft-versus-host disease (cGVHD)-related squamous cell carcinomas (SCCs) of the skin and oropharynx seem to occur after shorter latency [5]. Cumulative incidences of secondary solid malignancies after allo-HSCT as reported in the literature vary depending on statistical methodologies, patient population, conditioning regimens, and cGVHD and are as high as 13.5% at 15 years [10] and 22% at 30 years [11]. Yet, data are sparse regarding incidences of secondary solid malignancies after allo-HSCT applying modern and uniform TBI techniques. In this retrospective study, we, therefore, estimated the cumulative incidences of secondary solid malignancies and precancerous lesions in the presence of the competing risks of premature mortality after applying a standardized fractionated TBI technique for AML patients and 1st allo-HSCT. Cause-specific hazard models analyzed associations between transplant characteristics and the rates of secondary solid malignancies/precancerous lesions including the competing risks of premature death before developing malignancies.
Methods
Data collection
We retrospectively analyzed the cumulative incidences of secondary solid malignancies and precancerous lesions in adult patients with primary or secondary AML receiving TBI as part of the conditioning regimen before 1st allo-HSCT at the Departments of Radiation Oncology and Hematology of the University Hospital Regensburg between 01/2000 and 10/2016. Patients conditioned with TBI after 10/2016 were not analyzed to ensure sufficient follow-up. As patients conditioned with non-TBI-based regimens were older, this study didn´t analyze non-TBI-based regimens to prevent an age bias [12]. Secondary solid malignancies were subdivided into squamous cell carcinomas (SCCs) and non-squamous cell carcinomas (non-SCCs). Clinical data were extracted from the medical charts of the Departments of Radiation Oncology and Hematology of the University Hospital Regensburg. Transplantation variables included patient age at the time of TBI, conditioning regimens (RIC, 4 Gy TBI; MAC, 8 Gy and 12 Gy TBI), sex, diagnosis, Karnofsky performance score (KPS), hematopoietic cell transplantation-comorbidity index (HCT-CI) [13], 2017 European LeukemiaNet (ELN) genetic risk stratification [14], disease status before TBI, stem cell source, recipient and donor characteristics and GVHD prophylaxis. Graft-versus-host disease prophylaxis and conditioning regimens were dependent on the patient´s age, comorbidities and disease risk. The use of antithymocyte globulin (ATG) as part of GVHD prophylaxis was standard in unrelated donor transplantation and at the discretion of the physicians in sibling donor transplantation. Variables related to outcome were the cumulative incidences of secondary solid malignancies and precancerous lesions, relapse, non-relapse mortality (NRM), cGVHD (requiring systemic immunosuppression) and grade II-IV aGVHD. All patients received screening examinations for cutaneous malignancies before TBI. The screening of secondary malignancies after allo-HSCT included annual physical examinations, which encompassed examinations of the thyroid glands, skin, oropharynx and oral cavity. Patients suffering from cGVHD and those at high risk for developing cancers of the oropharynx and oral cavity were examined every 6 months. Gynecological and urological screenings for secondary solid malignancies were performed annually. Data closing was in October 2023. The local Ethics Board of the University of Regensburg approved this study (approval number, 20-1810-101).
TBI
Details of our TBI technique were previously published [15, 16]. We used Siemens Primus linear accelerators (Siemens Medical Systems, Inc., Concord, CA) and linear accelerators of type Elekta Synergy ™ with an Agility ™ head (Elekta Ltd, Crawley, UK) during the study period. All patients received rotational arcs with 6 megavoltage (MV) photon beams. We used a twice-daily fractionation and a minimum of 6 h between fractions. The average dose rate to the body was 4 cGy/min. Two individual lung shields of MCP96 with calculated thickness were designed if doses exceeded 8 Gy to reduce the total dose to the center of the lung to 7 Gy. Areas of the chest wall shielded by the lung blocks were irradiated once a day with electron beams to achieve full doses to the thoracic walls [15].
Definitions and statistical endpoints
Competing risks are common in survival data describing an event (e.g. premature deaths in patients before they could develop secondary malignancies) that prevents the event of interest, e.g. development of secondary malignancies [17, 18]. We used, therefore, the cumulative incidence function (CIF) to describe the cumulative incidences of secondary solid malignancies and precancerous lesions accounting for the competing risks of premature deaths among patients who did not develop secondary solid malignancies/precancerous lesions. Cause-specific hazard models analyzed the impact of pre-transplantation variables on the rates of secondary malignancies/precancerous lesions and the competing risks of premature deaths. Risk factors evaluated were patient age at TBI, TBI dose, ATG as part of GVHD prophylaxis, and cGVHD requiring immunosuppressive therapy. Age at TBI and TBI doses were analyzed as continuous variables. The impact of cGVHD changing value throughout the observation period on the rates of secondary solid malignancies/precancerous lesions was analyzed by applying a counting process format [19], while patients entered the risk set at the age of TBI. Secondary solid malignancies included solid cancers of any site and histology after TBI while excluding post-transplant lymphoproliferative disorders. The study recorded the times to the first secondary malignancy for patients developing ≥ 2 secondary malignancies. Acute GVHD and cGVHD were defined according to described standard criteria [20, 21]. Acute GVHD was classified as clinically significant at grade II-IV aGVHD. For the cumulative incidences of cGVHD requiring systemic immunosuppressive therapy, relapse or death without prior cGVHD was counted as a competing event. We captured non-relapse mortality (NRM) and relapse of AML. NRM was defined as deaths from any cause in the absence of prior relapse of the initial AML, with relapse considered a competing event. Relapse was defined as manifest hematologic relapse requiring treatment.
Statistical analysis
This study presents continuous variables as median and interquartile range (IQR) and categorical variables as absolute and relative frequencies. We used the cumulative incidence function (CIF) to estimate cumulative incidences of secondary malignancies and precancerous lesions, relapse, NRM, and cGVHD in the presence of competing risks [18]. CIF were compared using Gray´s test. The effects of transplantation variables on the rates of secondary malignancies/precancerous lesions and the competing risks of premature deaths were estimated with cause-specific hazard (CSH) analyses treating all other events as censored. The proportional hazards assumption of the CSH model was tested using Schoenfeld residuals. Hazard Ratio (HR) and 95% confidence intervals (95% CI) were presented as effect estimates. Median follow-up time was estimated using the reverse Kaplan-Meier method. All P-values were two-sided, and P-values < 0.05 were considered significant. Statistical analysis was performed using R, version 4.3.2 (R Core Team. R: A language for statistical computing. 2014. The R Foundation for Statistical Computing, Vienna, Austria) and SPSS 26.0 (SPSS Inc., Chicago, IL, USA).
Results
Patient and transplantation characteristics
Eighty-nine patients received TBI-based conditioning before 1st allo-HSCT. Table 1 summarizes transplant characteristics. Median patient age at TBI was 42.5 years (IQR, 32.5–51.2). The median follow-up time was 15.2 years (IQR, 13.0-18.2).
Table 1.
Patients characteristics (n = 89)
| Characteristics | value |
|---|---|
| Follow-up, years, median (IQR) | 15.2 (13.0-18.2) |
| Patient age, median (IQR) | 42.5 (32.5–51.2) |
| Sex, n (%) | |
|
male female |
56 (62.9%) 33 (37.1%) |
| Diagnosis, n (%) | |
|
de novo acute myeloid leukemia secondary acute myeloid leukemia |
68 (76.4%) 21 (23.6%) |
| Karnofsky performance score | |
|
< 80 ≥ 80 |
8 (9.0%) 81 (91.0%) |
| Hematopoietic cell transplantation-comorbidity index (HCT-CI), n (%) | |
|
0 1–2 ≥ 3 |
40 (44.9%) 34 (38.2%) 15 (16.9%) |
| 2017 ELN genetic risk stratification, n (%) | |
|
favorable intermediate adverse |
13 (14.6%) 36 (40.4%) 40 (44.9%) |
| Disease status at allo-HSCT, n (%) | |
|
First complete remission, CR1 CR2, first partial remission, PR1 > CR2, refractory, active disease |
31 (34.8%) 32 (36.0%) 26 (29.2%) |
| Donor type, n (%) | |
|
matched sibling donor matched unrelated donor mismatched unrelated donor |
32 (36.0%) 50 (56.2%) 7 (7.9%) |
| Stem cell source, n (%) | |
|
peripheral blood bone marrow |
82 (92.1%) 7 (7.9%) |
| Conditioning regimens, n (%) | |
|
reduced intensity (4 Gy TBI) myeloablative intensity (8 Gy TBI, 12 Gy TBI) |
31 (34.8%) 58 (65.2%) |
| Donor age, years, median (IQR) | 38.0 (30.5–44.0) |
| Female donors to male recipients, n (%) | |
|
yes no |
14 (15.7%) 75 (84.3%) |
| Graftversushost disease prophylaxis, n (%) | |
|
Cyclosporine, MTX Cyclosporine, MMF Post-transplant Cyclophosphamide, Tacrolimus, MMF |
57 (64.0%) 30 (33.7%) 2 (2.2%) |
| Antithymocyte globulin (ATG) *, n (%) | |
|
yes no |
61 (68.5%) 28 (31.5%) |
CMV, cytomegalovirus; ELN, European LeukemiaNet; * ATG was part of graft-versus-host disease prophylaxis; MTX, Methotrexate; MMF, Mycophenolate Mofetil
Most patients (n = 47) received a myeloablative conditioning (MAC) regimen containing 8 Gy TBI. Reduced-intensity conditioning (RIC) regimens comprising 4 Gy TBI were applied in 31 patients, while eleven patients received MAC with 12 Gy TBI (Table 2). The median age of patients receiving RIC (4 Gy) and MAC (8–12 Gy) was 50.7 years (IQR, 41.0–59.0) and 38.9 years (IQR, 29.7–45.1). This difference was statistically significant (P < 0.001). ELN genetic risk classification was similar between RIC and MAC groups (P = 0.443), while the distribution of disease status at allo-HSCT was different (P < 0.001). Fifteen patients receiving RIC were in > 2nd complete remission (including refractory AML), while 11 patients receiving MAC were in > 2nd complete remission (including refractory AML). Most patients receiving MAC were in first complete remission (n = 28), while only 3 patients receiving RIC were in first complete remission at allo-HSCT. PR1 and CR2 were present in 13 patients receiving RIC and 19 patients receiving MAC.
Table 2.
Conditioning regimens before allo-HSCT (n = 89)
| Regimens | n (%) |
|---|---|
|
TBI 8 Gy, Cyclophosphamide, Fludarabine (myeloablative conditioning) 8 Gy TBI (four 2 Gy doses on two consecutive days, d -8, d -7), Cyclophosphamide 2 × 60 mg/kg (d -4, d -3), Fludarabine 3 × 30 mg/m² (d -6, d -5, d -4) |
42 (47.2%) |
|
FLAMSA-RIC, TBI 4 Gy, Cyclophosphamide (reduced-intensity conditioning) FLAMSA regimen (d -12 to d -9), Fludarabine 4 × 30 mg/m², HD-Ara-C 4 × 2000 mg/m², Amsacrine 4 × 100 mg/m². Reduced intensity conditioning regimen after 3 days of rest: 4 Gy TBI on d -5 (two 2 Gy doses), Cyclophosphamide (2 × 40 mg/kg for MRD or 2 × 60 mg/kg for MUD, MMRD or MMUD) on d -4 to d -3, Antithymocyte globulin (ATG) 10 mg/kg for MRD or 20 mg/kg for MUD, MMRD, MMUD from d -4 to d -2, prophylactic donor lymphocyte infusions at day + 120 or 30 days after discontinuation of immunosuppression, 1–5 × 106 CD3+cells/kg |
31 (34.8%) |
|
TBI 12 Gy, Cyclophosphamide (myeloablative conditioning) 12 Gy TBI (six 2 Gy doses, on three consecutive days, d -7 to d -5), Cyclophosphamide 2 × 60 mg/kg on 2 consecutive days (d -4, d -3) |
11 (12.4%) |
|
TBI 8 Gy, Fludarabine (myeloablative conditioning) 8 Gy TBI (four 2 Gy doses on 2 consecutive days, d -5 and d -4), Fludarabine 4 × 30 mg/m² (d -5 to d -2) |
5 (5.6%) |
MRD, matched related donor; MUD, matched unrelated donor; MMRD, mismatched related donor; MMUD, mismatched unrelated donor
Chronic graft-versus-host disease
Two-year and 5-year cumulative incidences of cGVHD requiring systemic immunosuppression were 33% (95%CI, 23–42) and 36% (95%CI, 26–46). Twenty-nine patients had a history of grade II-IV aGVHD without cGVHD (requiring systemic immunosuppression), while 26 patients had neither aGVHD nor cGVHD. Eighteen patients had a history of aGVHD and cGVHD, while 16 patients had a history of cGVHD without prior aGVHD. Severe cGVHD was the most frequent maximum grade of cGVHD (n = 17) while twelve patients had moderate cGVHD, and five patients had mild cGVHD. Most patients had three or more cGVHD organ sites (median 3, IQR, 2–4). The most common sites of cGVHD in patients suffering from cGVHD were the skin (n = 24), oral mucosa (n = 20), eyes (n = 14), and liver (n = 11). The cumulative incidences of cGVHD (requiring systemic immunosuppression) in patients receiving RIC and MAC were similar over the entire follow-up period (P = 0.91). Two-year and 5-year cumulative incidences of cGVHD were 35% (95%CI, 19–53) and 39% (95%CI, 21–56) in patients receiving RIC, while patients receiving MAC showed 2-year and 5-year cumulative incidences of cGVHD of 31% (95%CI, 20–43) and 34% (95%CI, 22–47), respectively.
Secondary malignancies
Table 3 shows details of all secondary solid malignancies and precancerous lesions in 89 patients. Patient no. 290 was diagnosed with a pleomorphic undifferentiated sarcoma which appeared within less than a year after TBI. It was assumed that the sarcoma was present at the time of allo-HSCT. Therefore, this patient was excluded from further analyses resulting in 88 patients.
Table 3.
Secondary solid malignancies and precancerous lesions after total body irradiation-based conditioning (n = 89)
| No. | Secondary malignancies (SMs), precancerous lesions | TBI dose | Years from TBI to SMs, † death due to SMs |
Age at TBI | Sex | Chronic GVHD requiring systemic immunosuppression, organs of involvement |
Smoker |
|---|---|---|---|---|---|---|---|
| Non-squamous cell carcinomas (non-SCCs) in 8 patients | |||||||
| 419 | Mucoepidermoid cancer, lower lip, T1 G1 R0 | TBI 4 Gy | 11.69 | 26.2 | male | yes, skin. liver, joints | no |
| 266 | Medullary thyroid cancer, pT1a pN0 L0 V0 R0 | TBI 4 Gy | 5.33 | 40.2 | male | yes, skin, oral mucosa, eyes | no |
| 266 | Cutaneous basal cell carcinoma, face | TBI 4 Gy | 5.33 | 40.2 | male | yes, skin, oral mucosa, eyes | no |
| 19 | Mucoepidermoid carcinoma, major salivary gland, pT2 pN1 cM0 | TBI 8 Gy | 9.63 | 22.8 | male | yes, oral mucosa, eyes, gastrointestinal, liver | no |
| 240 | Cutaneous basal cell carcinoma, neck | TBI 8 Gy | 12.73 | 45.8 | male | no | yes |
| 240 | Cutaneous basal cell carcinoma, face | TBI 8 Gy | 12.73 | 45.8 | male | no | yes |
| 290 | Pleomorphic undifferentiated sarcoma, pT3 pN1 cM0 R1 * | TBI 8 Gy | 0.88, † death after 0.74 years | 51.6 | female | yes, gastrointestinal | no |
| 117 | Papillary thyroid cancer, pT1a L0 V0 R0 | TBI 4 Gy | 6.02 | 19.4 | male | yes, skin, eyes | no |
| 96 | Prostate cancer, adenocarcinoma | TBI 4 Gy | 12.45, † death after 3.55 years | 60.2 | male | no | no |
| 96 | Cutaneous basal cell carcinoma, face | TBI 4 Gy | 16.0 | 60.2 | male | no | no |
| 307 | Cutaneous basal cell carcinoma, face | TBI 8 Gy | 14.5 | 38.6 | female | yes, skin | no |
| 307 | Cutaneous basal cell carcinoma, face | TBI 8 Gy | 14.5 | 38.6 | female | yes, skin | no |
| 307 | Cutaneous basal cell carcinoma, face | TBI 8 Gy | 15.2 | 38.6 | female | yes, skin | no |
| 307 | Cutaneous basal cell carcinoma, face | TBI 8 Gy | 16.7 | 38.6 | female | yes, skin | no |
| 307 | Cutaneous eccine carcinoma, face | TBI 8 Gy | 18.2 | 38.6 | female | yes, skin | no |
| Squamous cell carcinomas (SCCs) in 3 patients | |||||||
| 428 | Cutaneous squamous cell carcinoma, face | TBI 8 Gy | 3.20 | 52.7 | male | yes, skin | no |
| 428 | Cutaneous squamous cell carcinoma, face | TBI 8 Gy | 3.50 | 52.7 | male | yes, skin | no |
| 432 | Cutaneous squamous cell carcinoma, face | TBI 8 Gy | 16.98 | 22.9 | male | no | yes |
| 9 | Squamous cell carcinoma, lip, pT1 cN0 cM0 | TBI 8 Gy | 9.40 | 20.3 | male | yes, skin, oral mucosa, CNS, gastrointestinal | no |
| Precancerous lesions, carcinomas in situ in 3 patients | |||||||
| 33 | Severe intraepithelial neoplasia of the vagina (VIN3) | TBI 8 Gy | 7.41 | 25.1 | female | yes, skin, oral mucosa, liver, CNS, eyes, vaginal | no |
| 307 | Cutaneous carcinoma in situ, ear | TBI 8 Gy | 18.22 | 38.6 | female | yes, skin | no |
| 412 | Cutaneous carcinoma in situ, skin | TBI 4 Gy | 3.79 | 48.6 | male | yes, skin, oral mucosa, lung, eyes | no |
* It was assumed that the pleomorphic undifferentiated sarcoma which appeared within less than a year after TBI was present at the time of allo-HSCT. Therefore, this patient was excluded from further analyses
The cumulative incidences of secondary solid malignancies and precancerous lesions were 8% (95%CI, 4–16), 14% (95%CI, 7–23) and 17% (95%CI, 9–27) at 10, 15 and 20 years, while the cumulative incidences of premature deaths were 59% (95%CI, 48–69), 59% (95%CI, 48–69), and 64% (95%CI, 49–76), respectively. Seven patients developed at least one non-SCC. The most common non-SCCs were cutaneous basal cell carcinomas of the face, while two male patients developed thyroid cancers. The mean time from allo-HSCT to the development of the first non-SCC was 10 years (95%CI, 7–14). Four secondary SCCs occurred in 3 patients. Cutaneous SCCs were the most frequent SCCs. The mean time from allo-HSCT to the first SCC was 10 years (95%CI, -7-27).
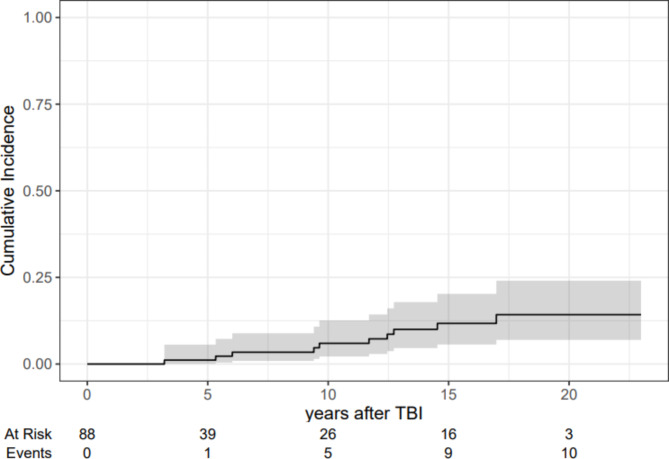
Figure 1 shows the estimates of the cumulative incidences of secondary solid malignancies with 95% confidence intervals treating premature deaths among patients who did not develop secondary solid malignancies as competing risks. The cumulative incidences of secondary solid malignancies were 6% (95%CI, 2–13), 12% (95%CI, 6–20), and 14% (95%CI, 7–24) at 10, 15 and 20 years.
Fig. 1.
Estimates of the cumulative incidences of secondary solid malignancies with 95% confidence intervals (n = 88)
The cumulative incidences of invasive non-SCCs were 4% (95%CI, 1–9), 10% (95%CI, 4–18), and 9% (95%CI, 4–18) at 10, 15 and 20 years. The cumulative incidences of invasive SCCs were 2% (95%CI, 1–8), 2% (95%CI, 1–8), and 5% (95%CI, 1–14) at 10, 15 and 20 years, respectively.
The cumulative incidences of relapse at 5, 10 and 15 years were 43% (95%CI, 33–53), 44% (95%CI, 34–54) and 44% (95%CI, 34–54), while the cumulative incidences of non-relapse mortality (NRM) were 16% (95%CI, 9–24), 18% (95%CI, 11–27) and 18% (95%CI, 11–27), respectively.
Hazard models for secondary solid malignancies and precancerous lesions and the competing risks of premature deaths
Table 4 shows the cause-specific hazards (HR CS) for secondary solid malignancies and precancerous lesions and the competing risks of premature deaths. In the multivariate regression models, older age at TBI translated into lower rates of secondary malignancies/precancerous lesions (HR cs 0.95, 95% CI, 0.90-1.00; P = 0.043). The analysis of competing events revealed that older patients showed a trend towards premature deaths (deaths before they could develop secondary malignancies/precancerous lesions, HR cs 1.02, 95%CI, 1.00-1.05; P = 0.069). Higher TBI doses (mainly applied in younger patients) translated into reduced rates of secondary malignancies/precancerous lesions (HR cs 0.76, 95% CI, 0.59–0.98; P = 0.035) while lacking associations with premature deaths. Chronic GVHD translated into increased rates of deaths before patients could develop secondary malignancies/precancerous lesions (HR cs 8.04, 95%CI, 3.97–16.3; P < 0.001).
Table 4.
Cause-specific hazards (HR CS) for secondary malignancies/precancerous lesions and the competing risks of premature deaths (n = 88)
| Cause-specific hazard model for secondary malignancies * |
Cause-specific hazard model for premature deaths before the development of secondary malignancies * |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariate analysis | Multivariate analysis | Univariate analysis | Multivariate analysis | |||||||||
| HRCS | 95%CI | Pvalue | HRCS | 95%CI | Pvalue | HRCS | 95%CI | Pvalue | HRCS | 95%CI | Pvalue | |
| Patient age † | 0.96 | 0.91–1.02 | 0.2 | 0.95 | 0.90-1.00 | 0.043 | 1.03 | 1.00-1.05 | 0.028 | 1.02 | 1.00-1.05 | 0.069 |
| TBI dose ◊ | 0.82 | 0.65–1.03 | 0.091 | 0.76 | 0.59–0.98 | 0.035 | 0.92 | 0.83–1.02 | 0.11 | 1.00 | 0.89–1.12 | > 0.9 |
| Chronic GVHD # | 1.86 | 0.50–6.91 | 0.4 | 1.31 | 0.31–5.59 | 0.7 | 7.85 | 3.91–15.8 | < 0.001 | 8.04 | 3.97–16.3 | < 0.001 |
| ATG ‡ | 0.89 | 0.27–2.96 | 0.8 | 0.67 | 0.19–2.37 | 0.5 | 1.00 | 0.57–1.77 | > 0.9 | 1.34 | 0.74–2.42 | 0.3 |
* secondary solid malignancies of any histology including precancerous lesions; † patients entered the risk set at the age of TBI; ◊ TBI dose was analyzed metrically; # Chronic graft-versus-host disease (GVHD) requiring systemic immunosuppression was analyzed as time-dependent variable; ‡ Antithymocyte globulin (ATG) was part of GVHD prophylaxis
Discussion
The present study analyzed the cumulative incidences of secondary solid malignancies and precancerous lesions in AML patients conditioned with TBI before 1st allo-HSCT over 16 years. The cumulative incidences of secondary solid malignancies of any histology including precancerous lesions were 14% and 17% at 15 and 20 years, respectively. We acknowledge a selection bias as RIC containing 4 Gy TBI was applied in older patients with advanced disease status, while younger patients received higher doses of TBI. Multivariate analyses revealed that higher patient age at TBI translated into lower rates of secondary malignancies. The age dependency may be explained by the confounding of competing risks. Older patients mainly receiving lower doses of TBI died more frequently before they could develop treatment-related secondary malignancies. Nevertheless, our results indicate that higher doses of TBI, mainly applied in young patients, were associated with lower rates of secondary malignancies while lacking associations with other causes of mortality. The results partly support radiobiological assumptions and theoretical predictions of carcinogenesis after ionizing radiotherapy suggesting, that the incidence of radiation-induced malignancies increases with increasing radiation dose until a peak is reached and then decreases rapidly [22–24]. Low-dose radiotherapy is not immediately lethal but induces sub-lethal DNA damages and mutations, increasing the risks of carcinogenesis over time. Contrarily, high doses of radiotherapy inducing direct cell death and apoptosis may prevent mutations, and thus carcinogenesis as cell kill becomes the predominant effect. However, the exact dose peak at which cell killing outweighs cell mutation is difficult to predict as molecular, cellular, and tissue-specific factors are integral [22, 23]. Boice et al. [24] analyzed the relationship between radiation doses and leukemia risk after radiotherapy for cancer of the cervix, supporting the radiobiological considerations of carcinogenesis after therapy showing an increased risk for radiation-induced leukemia up to doses of about 4 Gy and a decreased risk at higher doses. Regardless of these radiobiological considerations, we acknowledge the bias of competing risks and the small number of secondary malignancies as confounders in the present analysis.
A reason for making a comparison between studies focusing on the second cancer risk after TBI difficult is the fact that the literature on TBI shows variability in planning and treatment with TBI [25]. Although dose rates of 7.5 cGy/min or less and a twice-daily fractionation are recommended, dose rates and photon energy vary from 2.25 to 37.5 cGy/min and 6 to 25 MV, influencing the risks of secondary solid malignancies after TBI [25]. Furthermore, some studies provide no information about dose rates and the application of lung shielding, which contributes to different toxicity and organ damage after treatment.
Several studies examined the influence of pre-transplantation variables on the risks of second cancers after TBI- and non-TBI-based conditioning [11, 26, 27]. In summary, carcinogenesis after allo-HSCT remains multifactorial, while the number of pretransplant chemotherapy cycles [27], age at exposure, GVHD [12] and its treatment seem to be relevant. Environmental and genetic factors with variabilities in individual susceptibility to DNA-damaging therapies additionally influence the risks of secondary malignancies after allo-HSCT [22, 23]. Scott et al. [28] indicated that clinically photodamaged skin and a history of cutaneous SCC are important risk factors for non-melanoma skin cancer after allo-HSCT, factors not analyzed in the present study. However, the results of the present study are not entirely consistent with other recent studies, which excluded non-melanoma skin cancers or comprised different primary diagnoses. Leisenring et al. [7] analyzed skin and mucosal SCCs and BCCs after TBI- and non-TBI-based conditioning concluding that TBI increases the risks of BCCs but not of SCCs. Modern fractionation with fractionated doses < 13 Gy did not affect the hazards of BCCs in contrast to TBI applied as a single dose and fractionated doses ≥ 13 Gy. Both are no longer recommended as standard [7]. Chronic GVHD and its immunosuppressive therapy seem to increase the risks of SCCs of the skin and mucosa [5, 29], while associations of cGVHD with non-SCCs are less pronounced. The present analysis lacks an association of cGVHD with secondary malignancies (of any histology), which is most likely due to the small patient group. Yet, cGVHD, which represents a main cause of long-term morbidity and mortality, was associated with premature deaths before patients could develop secondary malignancies. Life-long surveillance for secondary malignancies is mandatory for all transplant survivors. Examinations include examinations of the thyroid glands, skin, genitals, and oropharynx [30]. With the help of yearly follow-up examinations, two thyroid cancers were diagnosed in 2 men suffering from cGVHD, which is a known additional risk factor for secondary thyroid cancers [8]. In summary, cutaneous non-melanocytic cancers (BCCs, SCCs) were the most frequent cancer types after TBI, which is in line with recent data demonstrating non-melanocytic skin cancers as the most common cancer types in Germany [31]. The retrospective design and the small number of patients conditioned with TBI limit this study. The study was not powered to analyze risk factors for secondary malignancies of specific anatomical sites and histological subtypes. Moreover, the small number of secondary malignancies apart from non-melanoma skin cancer reduced the generalizability of the results. In addition, the selection bias to treat young patients with TBI and older patients with non-TBI-based regimens, prohibited comparisons of TBI and non-TBI patients regarding secondary malignancies. Nevertheless, the primary strength of the present study is the consistent delivery of modern TBI over 16 years.
Conclusions
This study indicates a potential inverse relationship between the risk for secondary malignancies and TBI doses applied and is consistent with radiobiological considerations, which assume a decrease in secondary malignancies at high doses. Yet, confounding by competing risks remains a limitation. The use of lower TBI doses in older patients interferes with the age-related increased mortality not caused by secondary malignancies. Therefore, older patients appeared to have lower risks for secondary malignancies than younger patients. This study illustrates the pitfalls of not reporting and considering competing risks in survival data which is relevant in evaluation of newer technologies, such as total marrow irradiation (TMI) and volumetric modulated arc therapy (VMAT) and associated risk assessment for carcinogenesis.
Acknowledgements
Not applicable.
Abbreviations
- TBI
Total body irradiation
- allo-HSCT
Allogeneic hematopoietic stem cell transplantation
- AML
Acute myeloid leukemia
- SSM
Secondary solid malignancy
- IQR
Interquartile range
- NRM
Non-relapse mortality
- cGVHD
Chronic graft-versus-host disease
- MAC
Myeloablative conditioning
- RIC
Reduced-intensity conditioning
- SCCs
Squamous cell carcinomas
- KPS
Karnofsky performance score
- HCT-CI
Hematopoietic cell transplantation-comorbidity index
- ELN
European LeukemiaNet
- ATG
Antithymocyte globulin
- MV
Megavoltage
- CIF
Cumulative incidence function
- CSH
Cause-specific hazard
- HR
Hazard ratio
- CI
Confidence interval
- CR1
First complete remission
- PR1
First partial remission
- CR2
Second complete remission
- FLAMSA
Fludarabine, HD-Ara-C, Amsacrine
- MRD
Matched related donor
- MUD
Matched unrelated donor
- MMRD
Mismatched related donor
- MMUD
Mismatched unrelated donor
- VIN
Intraepithelial neoplasia of the vagina
- CNS
Central nervous system
- TMI
Total marrow irradiation
- VMAT
Volumetric modulated arc therapy
Author contributions
Material preparation, data collection and analysis were performed by GI. The manuscript was written by GI. WD provided patient samples and contributed to the conception and interpretation of the work. KO contributed to the interpretation of the work including the radiobiological considerations. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Funding
GI and KO received no funding. WD received research support from Novartis and honoraria from Novartis, Sanofi, Incyte, Behring, Neovii, Takeda and Mallinckrodt.
Open Access funding enabled and organized by Projekt DEAL.
Data availability
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
Declarations
Ethics approval and consent to participate
The study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of University Regensburg (number, 20-1810-101, date, February 10, 2021). Informed consent was obtained from all individual participants included in the study.
Consent for publication
Not applicable.
Competing interests
Wolff Daniel received research support from Novartis and honoraria from Novartis, Sanofi, Incyte, Behring, Neovii, Takeda and Mallinckrodt.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kolb HJ, Socié G, Duell T, Van Lint MT, Tichelli A, Apperley JF, et al. Malignant neoplasms in long-term survivors of bone marrow transplantation. Late effects Working Party of the European Cooperative Group for Blood and Marrow Transplantation and the European late Effect Project Group. Ann Intern Med. 1999;131(10):738–44. [DOI] [PubMed] [Google Scholar]
- 2.Bacigalupo A, Ballen K, Rizzo D, Giralt S, Lazarus H, Ho V, et al. Defining the intensity of conditioning regimens: working definitions. Biol Blood Marrow Transpl. 2009;15(12):1628–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scott BL, Pasquini MC, Logan BR, Wu J, Devine SM, Porter DL, et al. Myeloablative Versus reduced-intensity hematopoietic cell transplantation for Acute myeloid leukemia and myelodysplastic syndromes. J Clin Oncol off J Am Soc Clin Oncol. 2017;35(11):1154–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sengsayadeth S, Savani BN, Blaise D, Malard F, Nagler A, Mohty M. Reduced intensity conditioning allogeneic hematopoietic cell transplantation for adult acute myeloid leukemia in complete remission - a review from the Acute Leukemia Working Party of the EBMT. Haematologica. 2015;100(7):859–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rizzo JD, Curtis RE, Socié G, Sobocinski KA, Gilbert E, Landgren O, et al. Solid cancers after allogeneic hematopoietic cell transplantation. Blood. 2009;113(5):1175–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Majhail NS, Brazauskas R, Rizzo JD, Sobecks RM, Wang Z, Horowitz MM, et al. Secondary solid cancers after allogeneic hematopoietic cell transplantation using busulfan-cyclophosphamide conditioning. Blood. 2011;117(1):316–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leisenring W, Friedman DL, Flowers MED, Schwartz JL, Deeg HJ. Nonmelanoma skin and mucosal cancers after hematopoietic cell transplantation. J Clin Oncol. 2006;24(7):1119–26. [DOI] [PubMed] [Google Scholar]
- 8.Cohen A, Rovelli A, Merlo DF, Van Lint MT, Lanino E, Bresters D, et al. Risk for secondary thyroid carcinoma after hematopoietic stem-cell transplantation: an EBMT late effects working party study. J Clin Oncol. 2007;25(17):2449–54. [DOI] [PubMed] [Google Scholar]
- 9.Friedman DL, Rovo A, Leisenring W, Locasciulli A, Flowers MED, Tichelli A, et al. Increased risk of breast cancer among survivors of allogeneic hematopoietic cell transplantation: a report from the FHCRC and the EBMT-Late Effect Working Party. Blood. 2008;111(2):939–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martelin E, Volin L, Itälä-Remes M, Niittyvuopio R, Lindström V, Heiskanen J, et al. Incidence and risk factors of secondary cancers after allogeneic stem cell transplantation: analysis of a single centre cohort with a long follow-up. Bone Marrow Transpl. 2019;54(2):334–7. [DOI] [PubMed] [Google Scholar]
- 11.Baker KS, Leisenring WM, Goodman PJ, Ermoian RP, Flowers ME, Schoch G, et al. Total body irradiation dose and risk of subsequent neoplasms following allogeneic hematopoietic cell transplantation. Blood. 2019;133(26):2790–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Isabella G, Katharina A, Matthias E, Oliver K, Daniel W. Secondary solid malignancies and precancerous lesions after allogeneic hematopoietic stem cell transplantation using non-total body irradiation-based conditioning in acute myeloid leukemia. J Cancer Res Clin Oncol. 2024;150(3):152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sorror ML, Maris MB, Storb R, Baron F, Sandmaier BM, Maloney DG, et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood. 2005;106(8):2912–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Döhner H, Estey E, Grimwade D, Amadori S, Appelbaum FR, Büchner T, et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood. 2017;129(4):424–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gruber I, Koelbl O, Treutwein M, Zeman F, Herr W, Holler E, et al. Analysis of long-term mortality after total body irradiation-based and melphalan-based chemotherapy conditioning for acute myeloid leukemia. Ann Hematol. 2023;102(8):2199–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Härtl PM, Treutwein M, Hautmann MG, März M, Pohl F, Kölbl O, et al. Total body irradiation-an attachment free sweeping beam technique. Radiat Oncol. 2016;11:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lau B, Cole SR, Gange SJ. Competing risk regression models for epidemiologic data. Am J Epidemiol. 2009;170(2):244–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Austin PC, Lee DS, Fine JP. Introduction to the Analysis of Survival Data in the Presence of competing risks. Circulation. 2016;133(6):601–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Austin PC, Latouche A, Fine JP. A review of the use of time-varying covariates in the Fine-Gray subdistribution hazard competing risk regression model. Stat Med. 2020;39(2):103–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Filipovich AH, Weisdorf D, Pavletic S, Socie G, Wingard JR, Lee SJ, et al. National Institutes of Health Consensus Development Project on Criteria for clinical trials in chronic graft-versus-host disease: I. diagnosis and staging Working Group Report. Biol Blood Marrow Transpl. 2005;11(12):945–56. [DOI] [PubMed] [Google Scholar]
- 21.Jagasia MH, Greinix HT, Arora M, Williams KM, Wolff D, Cowen EW, et al. National Institutes of Health Consensus Development Project on Criteria for clinical trials in chronic graft-versus-host disease: I. The 2014 diagnosis and staging Working Group Report. Biol Blood Marrow Transpl. 2015;21(3):389–e4011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lindsay KA, Wheldon EG, Deehan C, Wheldon TE. Radiation carcinogenesis modelling for risk of treatment-related second tumours following radiotherapy. Br J Radiol. 2001;74(882):529–36. [DOI] [PubMed] [Google Scholar]
- 23.Epstein R, Hanham I, Dale R. Radiotherapy-induced second cancers: are we doing enough to protect young patients? Eur J Cancer. 1997;33(4):526–30. [DOI] [PubMed] [Google Scholar]
- 24.Boice JDJ, Blettner M, Kleinerman RA, Stovall M, Moloney WC, Engholm G, et al. Radiation dose and leukemia risk in patients treated for cancer of the cervix. J Natl Cancer Inst. 1987;79(6):1295–311. [PubMed] [Google Scholar]
- 25.Giebel S, Miszczyk L, Slosarek K, Moukhtari L, Ciceri F, Esteve J, et al. Extreme heterogeneity of myeloablative total body irradiation techniques in clinical practice: a survey of the acute leukemia working party of the European group for blood and marrow transplantation. Cancer. 2014;120(17):2760–5. [DOI] [PubMed] [Google Scholar]
- 26.Sieker K, Fleischmann M, Trommel M, Ramm U, Licher J, Bug G, et al. Twenty years of experience of a tertiary cancer center in total body irradiation with focus on oncological outcome and secondary malignancies. Strahlentherapie Und Onkol. 2022;198(6):547–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nunez L, Abedin T, Naqvi S, Shen H, Chaudhry A, Bellerby S, et al. Cumulative incidence of subsequent malignancy after allo-HCT conditioned with or without low-dose total body irradiation. Blood Adv. 2022;6(3):767–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Scott JF, Brough KR, Grigoryan KV, Muzic JG, Kim GY, Conic RRZ, et al. Risk factors for Keratinocyte Carcinoma in recipients of allogeneic hematopoietic cell transplants. JAMA Dermatology. 2020;156(6):631–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Curtis RE, Metayer C, Rizzo JD, Socié G, Sobocinski KA, Flowers MED, et al. Impact of chronic GVHD therapy on the development of squamous-cell cancers after hematopoietic stem-cell transplantation: an international case-control study. Blood. 2005;105(10):3802–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Socié G, Rizzo JD. Second solid tumors: screening and management guidelines in long-term survivors after allogeneic stem cell transplantation. Semin Hematol. 2012;49(1):4–9. [DOI] [PubMed] [Google Scholar]
- 31.Nanz L, Keim U, Katalinic A, Meyer T, Garbe C, Leiter U. Epidemiology of keratinocyte skin Cancer with a focus on cutaneous squamous cell carcinoma. Cancers (Basel). 2024;16(3):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.