Abstract
Background
Mpox is a severe viral zoonosis that has emerged as a public health concern due to its potential for human-to-human transmission and severe illness. Understanding its clinical manifestations is crucial for effective management and control. Several systematic reviews have assessed various manifestations of Mpox. This umbrella review synthesizes evidence on Mpox’s manifestations across different organ systems.
Method
We conducted an umbrella review, adhering to Joanna Briggs Institute (JBI) methodology and Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines, focusing on systematic reviews of Mpox manifestations. We performed a literature search up to 25th September 2023, in databases like PubMed, Embase, and Web of Science. We included systematic reviews of observational studies, case reports, case series, or RCTs reporting any manifestations of Mpox in humans, focusing on a global scope. AMSTAR 2 was used to evaluate the quality of systematic reviews, and data has been synthesized in narrative and tabular manners.
Results
A total of 25 systematic reviews were included, uncovering diverse manifestations of Mpox, such as cutaneous, cardiovascular, oral, ophthalmic, gastrointestinal, respiratory, and pregnancy-related. Cutaneous manifestations (up to 100%) were the most prevalent, featuring lesions and rashes. Constitutional symptoms of viral illness were reported in ~ 60% to > 85% of the cases. Significant respiratory symptoms were present in ~ 50% of cases overall. Headaches were the leading neurological symptom present in > 30%. Symptoms of gastrointestinal involvement ranged from 39% (oral lesions) with decreasing frequency to low diarrhea at ~ 5%, with proctitis percentages ranging from high teens to mid-twenties. Ophthalmic manifestations (6% but with wide variations among studies). Many primary studies included in the systematic reviews consisted of case reports and case series. A wide range of manifestations across different organ systems was observed. Negative outcomes for pregnancies were reported, but evidence is limited. Adverse cardiovascular and neurological outcomes were identified, though only a few studies provided insights into these findings.
Conclusion
Mpox exhibits diverse manifestations, impacting multiple organ systems, with substantial variations. The findings highlight the importance of ongoing, nuanced, and region-specific research and management strategies for Mpox. The reliance on case reports and series underscores the need for more high-quality, long-term studies to deepen our understanding and management of this significant public health concern.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12879-024-09884-y.
Keywords: Mpox, Umbrella review, Monkeypox, Infection
Introduction
Mpox is a rare but potentially severe viral zoonosis, likened to human smallpox due to similarities in clinical presentation and pustular rash manifestation [1]. It was first discovered in 1958 when outbreaks of a pox-like disease occurred in monkeys kept for research, lending the disease its name. It is caused by the Mpox virus, a member of the Orthopoxvirus genus, which includes variola virus, the cause of smallpox. The virus predominantly circulates in remote parts of Central and West Africa, near tropical rainforests. Human monkeypox (HMPX) was first discovered in 1970 in the Democratic Republic of the Congo (DRC) [2].
The prevalence of Mpox has been relatively low; however, it has substantial public health significance due to its potential for human-to-human transmission and its capacity to cause severe illness, including fatalities [3, 4]. The disease typically presents with fever, headache, muscle aches, and a characteristic progressive pox-like rash. The manifestations of Mpox vary widely, affecting different organ systems and causing a broad spectrum of clinical signs and symptoms, which are often correlated with the extent and severity of the disease [3]. Given the significance of Mpox as a public health concern, especially in a world that has eradicated its relative, smallpox, it is essential to have a robust understanding of its clinical manifestations. Understanding the myriad of clinical presentations is crucial to identify, diagnose, and manage cases effectively and to implement appropriate control measures to prevent further spread.
Several systematic reviews have been published on various manifestations of Mpox. These cover epidemiology, symptoms, clinical presentations, mortality, and effects on different organ systems [5–8]. The current understanding of Mpox is predominantly centered around its cutaneous manifestations, but there are significant gaps in knowledge regarding its effects on other organ systems. For instance, the extent and nature of Mpox’s involvement in the respiratory, gastrointestinal, and neurological systems remain largely elusive. Moreover, there’s a limited understanding of the factors contributing to the variability in the disease’s severity and progression. The reasons why some individuals experience only mild symptoms while others suffer from severe, life-threatening complications are not yet fully understood, necessitating further research in this area. Another area that requires more attention is the long-term effects of Mpox. The potential chronic conditions or sequelae that may follow recovery from Mpox are not well-documented, which is a crucial gap, especially for guiding long-term patient care and rehabilitation strategies. Additionally, the impact of Mpox on special populations, such as immunocompromised individuals, children, pregnant women, and the elderly, is not adequately understood. These groups may have different disease experiences and outcomes, highlighting the need for more detailed investigation. These gaps in knowledge underscore the need for comprehensive research to fully understand the multifaceted nature of Mpox and its impact on diverse patient populations.
Umbrella reviews are an innovative methodological approach to synthesize evidence from multiple systematic reviews into one overarching review, providing high-level, comprehensive insights [9]. The primary objective of this umbrella review is to synthesize evidence from multiple systematic reviews on Mpox, focusing on its various clinical manifestations across different organ systems. By consolidating data from diverse studies, this review aims to provide a holistic view of Mpox’s clinical characteristics and the variability in its presentations. This comprehensive synthesis will help in enhancing our understanding of the disease, guiding healthcare professionals in its management, and informing public health strategies to curb its spread.
Methods
This umbrella review has been conducted, adhering to the established standards and protocols outlined in the Joanna Briggs Institute (JBI) methodology and the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (Table S1) [10–12]. An umbrella review, also known as a review of reviews, is a distinct form of evidence synthesis that compiles data from multiple systematic reviews related to a specific topic or question, in this case, the clinical manifestations of Mpox. This methodology is especially valuable when addressing complex, multifaceted health issues that have been the subject of numerous reviews, allowing for a broader and more comprehensive understanding of the available evidence. We employed rigorous criteria for the selection of systematic reviews to ensure relevance and quality. Each selected review was required to meet specific standards regarding methodology, scope, and the robustness of findings. Our synthesis process involved extracting key data points from each review, analyzing patterns and discrepancies, and summarizing the evidence to provide an overarching insight into the clinical spectrum of Mpox. This structured approach enables us to present a consolidated view of the disease’s impact on different organ systems, capturing variability in clinical presentations and outcomes across different populations and study settings. The review has been registered in PROSPERO: CRD42023468364.
Inclusion criteria
We included studies involving the general population, without restricting to specific subgroups, to provide a comprehensive overview of Mpox’s impact across diverse demographics. The exposure of interest was clearly defined as cases of Mpox, excluding other infections to eliminate confounding influences. The outcomes of our review were categorized into three main groups: Major Health Outcomes and Associated Risk Factors (such as atherosclerosis and high blood pressure); Specific Organ System Effects (including respiratory diseases and neurological effects); and Broader Health Implications (like effects on pregnancy and developmental outcomes). These outcomes were chosen to explore both the direct effects of the virus and its broader health implications. Regarding study designs, our review included systematic reviews of randomized controlled trials (RCTs), observational studies, longitudinal studies, and other empirical study types up to case reports, providing a rich variety of data sources. We excluded policy discussions, opinion pieces, reviews that do not include original data, and animal studies to maintain a focus on empirical human data. Only articles available in English are included. The geographic scope of our review was global, considering studies from any region to ensure a wide epidemiological perspective. The detailed criteria for inclusion in our review can be found in Table S2.
Literature search
We performed comprehensive literature search in databases such as PubMed, Embase, Web of Science, and the Cochrane Database of Systematic Reviews from inception upto 25th September 2023. We employed search terms including “mpox,” “monkeypox,” and “mpxv,” coupled with “meta-analysis” or “systematic review” to curate relevant systematic reviews. To refine the search results, an English language filter was applied. Detailed search strategy is furnished in Table S3.
Screening
The process of screening and selecting systematic reviews was undertaken by two independent reviewers (SG and PS). They utilized the Nested Knowledge software for the removal of duplicates and screening purposes [13, 14]. The preliminary screening involved reviewing the titles and abstracts of each article, followed by a thorough reading of the full texts. In instances where discrepancies between the reviewers arose, a third, senior reviewer was consulted to reconcile differences and reach a resolution.
Data extraction
Data extraction was conducted by two reviewers, utilizing a pre-tested data extraction form. The data collated from the included systematic reviews comprised the name of the authors, year of publication, databases searched and date, the number and nature of the included studies, the tools used for assessing risk of bias, geographical location/region of the studies, the range of years of the studies, the results of the quality assessment, and the pertinent outcomes.
Quality assessment
To assess the methodological quality of included systematic reviews in our study on Mpox, we employed the AMSTAR 2 tool, recognized for its comprehensive and rigorous approach tailored specifically to systematic reviews [15, 16]. This tool is adept at evaluating critical domains crucial for the validity of a review’s conclusions, such as literature search adequacy, risk of bias, and handling of study heterogeneity. This approach aligns with our objective to ensure the reliability and accuracy of our synthesis, given the diverse clinical presentations of Mpox. The quality assessment was collaboratively performed by two reviewers, with studies graded as high, moderate, low, or critically low based on AMSTAR 2 criteria. Reviews were categorized as high quality if they addressed all critical domains with no more than one non-critical weakness, including a comprehensive literature search, explicit statement of research questions, thorough risk of bias assessment, and robust discussion on study heterogeneity and publication bias. Moderate quality reviews met most critical criteria but had several minor methodological weaknesses in non-critical areas, such as less detailed search strategies or incomplete bias discussions. Low quality reviews had multiple flaws across both critical and non-critical domains, including incomplete literature searches and insufficient bias consideration, affecting the reliability of their conclusions. Finally, reviews were deemed critically low quality if they failed to meet multiple critical domains, displaying major methodological shortcomings that likely significantly impaired the validity of their findings, such as lacking transparent reporting and inadequate risk of bias assessments. Any discrepancies in the quality assessment were resolved by a third reviewer, ensuring a thorough and unbiased evaluation.
Data synthesis
Data synthesis and summarising is performed through a coherent narrative synthesis and are also structured in tabular form to facilitate comprehension and interpretation. We have categorized the manifestations of Mpox by the affected organ systems: Oral, Ophthalmic, Cardiovascular, Neurological, cutaneous, gastrointestinal, respiratory, and general manifestations. Each of these categories is appended with a succinct summary to offer clear insights into the varied manifestations within each organ system. A detailed table outlines the specifics of each systematic review involved in our analysis, presenting various pertinent aspects and main information of each review. Different manifestations are comprehensively summarized, and tables are integrated to heighten clarity and alignment with the available systematic reviews. Priority is assigned to the findings of the highest-rated systematic reviews by AMSTAR-2.
Results
Literature search outcome
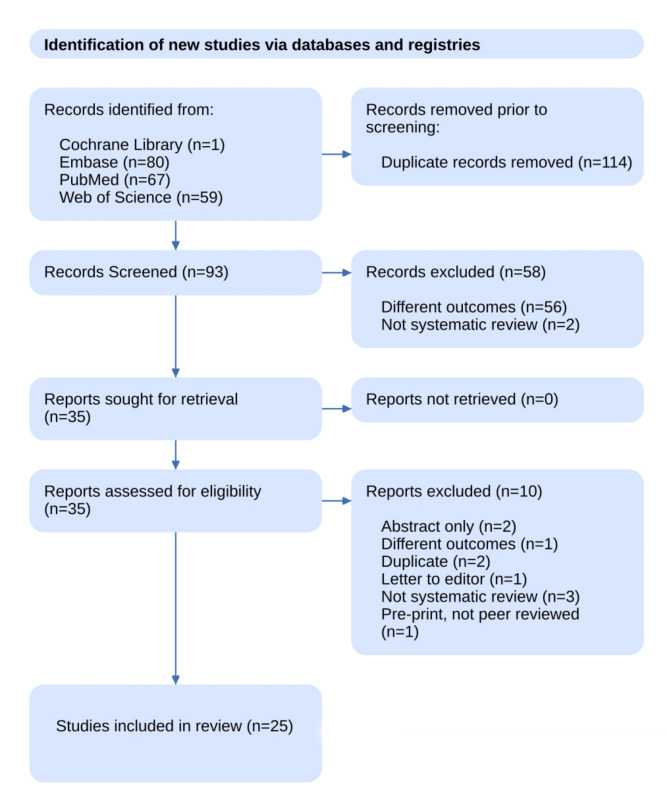
A total of 207 records were identified from various databases, of which 114 were duplicates. Consequently, 93 records underwent primary screening, with 35 of these progressing to full-text screening. Ten records were subsequently excluded for various reasons. Ultimately, 25 systematic reviews were deemed eligible and included in the synthesis. The screening process and selection of articles are depicted in Fig. 1.
Fig. 1.
PRISMA flow chart depicting screening and study selection process
Characteristics of included systematic reviews
The important characteristics of the included systematic reviews are given in Table 1. The range of included studies by reviews is diverse, encompassing cross-sectional studies, case reports, case controls, case series, and cohorts. Most of the systematic reviews were published in recent years; however, they include primary studies dating from previous decades up to the present. These reviews incorporate studies from various regions, providing a comprehensive overview of global-level data. The Newcastle–Ottawa Scale (NOS), the Joanna Briggs Institute (JBI) checklist, the National Heart, Lung, and Blood Institute (NHLBI) quality assessment tools, and the National Institutes of Health (NIH) quality assessment tools are the predominant methodologies employed for quality assessment in these reviews. The clinical manifestations discussed within the systematic reviews are varied, including cutaneous, cardiovascular, neurological, respiratory, gastrointestinal, oral, and ophthalmic manifestations, among others. The overall quality of the primary studies varied, ranging from very poor to high quality. Out of the 25 systematic reviews, 16 performed meta-analysis. Most of the reviews were categorized as critically low quality according to AMSTAR 2 criteria. Many of them did not register the protocol, and some reviews did not perform or consider accounting for the risk of bias in primary studies Table S4.
Table 1.
Summary of included systematic reviews
| Author | Publication Year | Databases searched and date | Type of studies | Number of studies | Year of included studies | Location | Meta-analysis performed | Quality assessment tool used | Overall quality of studies | Outcomes of concern |
|---|---|---|---|---|---|---|---|---|---|---|
| Abdelaal [7] | 2023 | PubMed, Scopus, Web of Science, EMBASE, and Google Scholar (On August 7, 2022) | Case series, Surveillance-based study, cross-sectional, Prospective observational, Chart reviews, Retrospective observational study | 11 | During 2022, Before 2022 | UK, Spain, DRC, US, Zaire, Nigeria, England, 16 countries, Switzerland | Yes | NIH | Average-fair | Ophthalmic manifestations |
| Badenoch[29] | 2022 | MEDLINE, EMBASE, PsycINFO and AMED (31 may 2022) | Cohort, cross-sectional, case series, case reports | 19 | 1987–2022 | Nigeria, USA, UK, DRC | Yes | NOS and JBI | NOS = Studies lost scores due to comparability and lack of follow-up | Neurological manifestations |
| Benites-Zapata [21] | 2022 | PubMed, Scopus, Embase, Ovid-Medline and Web of Science (June 7 2022) | Cross-sectional, cohort, case series, case reports | 19 | 1980–2022 | Sudan, DRC, Central and west Africa, UK, Nigeria, Portugal, Italy, Sierra Leone, Spain, Australia, Israel | Yes | NOS | Low risk of bias | Cutaneous, Oral, ophthalmic and general clinical manifestations |
| D’Antonio[33] | 2023 | Medline, Embase, and Cochrane (June 25, 2022) | Case report and case series | 4 | 1983–2020 | Nigeria, DRC, and Zaire | Yes | Standardized tool adapted from Murad et al. | Three studies = low risk, one study = high risk | Pregnancy related manifestations |
| Deb [26] | 2023 | PubMed, Embase, and Scopus (October 2022) | Case report, case series, cohort studies | 130 | NA | USA, India, South Africa, Morocco, Italy, Brazil, France, Romania, Argentina, Turkey, UK, Chile, Iran, Spain, KSA, Canada, Istanbul, Indiana, Sweden, Multiple countries, Peru, Belgium, Germany, Egypt, Zaire | No | NOS and JBI | NA | Neurological manifestations |
| Gandhi P [4] | 2023 | PubMed, Scopus, Web of Science, EMBASE, and Cochrane, medRxiv, arXiv, bioRxiv, BioRN, ChiRxiv, ChiRN and SSRN (15 November 2022) | Cross-sectional, retrospective, Prospective observational studies | 19 | 1988–2022 | Nigeria, DRC, Portugal, Sudan, USA | Yes | NHLBI | 13/19 studies found to be fair or good quality | Oral manifestations |
| Gandhi [30] | 2023 | PubMed, Scopus, Web of Science, EMBASE, ProQuest, EBSCOHost, Cochrane, medRxiv, arXiv, bioRxiv, BioRN, ChiRxiv, ChiRN, and SSRN (12 December 2022) | Case series, cross sectional, retrospective and prospective studies | 12 | 1987–2022 | Spain, USA, UK, Nigeria, DRC, Australia, Israel, Portugal, Europe | Yes | NHLBI | Fair to good | Opthalmic manifestations |
| Hatami [20] | 2023 | PubMed/Medline, Embase, and Scopus (from 1 January 2012 up to 15 February 2023) | Cross-sectional studies, Case series, Letters, Brief reports, Rapid communications, Shortcommunications, Correspondences | 67 | 2014–2023 | Spain, USA, UK, Africa, Central African Republic, DRC, Nigeria | Yes | JBI | Good | Cutaneous, Oral manifestations |
| Jaiswal [24] | 2023 | PubMed, Embase, and Scopus (January 5th, 2023) | Case reports, Case series | 6 | 2022 | USA, France, Canada, Portugal | No | NA | NA | Cardiovascular manifestations |
| Jaiswal [17] | 2022 | Databases = NA (December 2019 to 14th June 2022) | Case report, Case series, Observational studies | 10 | 2019–2022 | UK, Nigeria, DRC, Singapore, USA, Australia, Sierra Leone | No | NA | NA | Clinical and general manifestations |
| Khan [25] | 2023 | PubMed, Scopus, Cochrane library, and Google scholar (April 13, 2023) | NA | 22 | 1980–2023 | NA | No | NA | NA | Neurological and general clinical manifestations |
| Li [22] | 2023 | Medline, Embase, Web of Science, Cochrane Central, and Google Scholar (10 January 2023) | NA | 73 | NA | NA | Yes | JBI | Good | Cutaneous, Oral, respiratory, gastrointestinal and general clinical manifestations |
| Liu [18] | 2023 | PubMed, EMBASE, and Web of Science (From 1 January and 11 November 2022) | Cross sectional, case series | 77 | NA | Europe, America, Asia, Africa | Yes | JBI | Moderate Quality | Cutaneous, Neurological, Oral and general clinical manifestations |
| Morris [34] | 2023 | NA | NA | NA | 2007–2011 | DRC | No | NA | NA | Pregnancy related manifestations |
| Okoli [27] | 2023 | MEDLINE, Embase, CENTRAL, CINAHL and Scopus (August 30, 2022) | Cross-sectional, Case reports | 79 | 1972–2022 | USA, Italy, DRC, UK, Germany, Spain, Portugal, France, Nigeria, Netherlands, Australia, Belgium, Canada, Central African Republic, Czech Republic, Greece, India, Israel, Republic of Korea, Romania, Singapore, Switzerland | No | NOS | Very poor quality | Cutaneous, Neurological, ophthalmic and general clinical manifestations |
| Pourriyahi [8] | 2023 | PubMed, Scopus, Web of science, and Embase (from January 1, 2022 up until August 11, 2022) | Case reports, Case series, Observational studies | 46 | 2013–2022 | USA, Canada, UK, Spain, Portugal, Germany, France, Italy, Romania, Czech Republic, Nigeria, Central African Republic, Kora, Australia, Poland, Netherlands | No | NA | NA | Cutaneous, Oral, gasrtointestinal and general clinical manifestations |
| Rogers [50] | 2021 | MEDLINE, EMBASE, PsycINFO and AMED (9 September 2020) | Ecological, cohort, case-control, cross-sectional, search engine results | 57 | 1992–2020 | Spain, Japan, Uganda, Hong Kong, Nigeria, USA, UK, Sierra Leone, Australia, India, Iran, Korea, Bangladesh, Denmark, Taiwan China, Worldwide, France, Liberia, Italy, Guinea, Greece | Yes | NOS | Low quality | Suicide |
| Rojas-Carabali [32] | 2023 | PubMed, Embase, VHL (Virtual Health Library), and MedxRiv (February 28, 2023) | Case report, case series, cross-sectional, cohort, case-control | 60 | 1980–2023 | USA, Brazil, Italy, Spain, UK, Switzerland, Colombia, Spain, Australia, DRC, Portugal, Nigeria, Mexico | Yes | Tool by CLARITY group, ROBINS-I | NA | Ophthalmic manifestations |
| Sayad [23] | 2023 | PubMed, Cochrane, Medline, Scopus, and Web of Science (December 01, 2022) | Case report, case series | 9 | 2022–2023 | Puerto Rico, Canada, France, USA, Portugal | No | NIH tool for case reports and case series | Fair to good |
Cutaneous, Cardiovascular manifestations |
| Shah [28] | 2023 | PubMed, Cochrane Library, Scopus, Embase, Web of Science, Google Scholar, and OpenGrey (September 2022) | Case series, Case reports, Observational studies | 38 | 1980–2022 | USA, UK, Spain, France, DRC, Europe | Yes | JBI | High quality | Neurological, oral, respiratory and general clinical manifestations |
| Sharma [6] | 2023 | PubMed, Google Scholar, and Science Direct (from January to September 2022) | Case series, case reports, and original articles | 26 | NA | USA, Brazil, Columbia, Singapore, India, Thailand, New Zealand, Spain, France, Germany, Israel | No | NA | NA | Cutaneous, ophthalmic, respiratory, and general clinical manifestations |
| Shin [51] | 2023 | PubMed/MEDLINE, Embase, and Google Scholar (up to March 7, 2023) | Cross sectional, case series, case report | 99 | 2022–2023 | UK, Canada, USA, Germany, Israel, Netherlands, India, Spain, Czech Republic | Yes | NOS, JBI | NA | General clinical manifestations |
| Simadibrata [31] | 2023 | MEDLINE, EMBASE, and SCOPUS (October 21,2022) | Case- series, cohort, cross sectional, | 31 | 1987–2022 | Spain, USA, Congo, France, Nigeria, Israel, Sudan, UK | Yes | NIH | 14 studies were rated good, 10 studies were rated fair, 7 rated poor | Oral and gastrointestinal manifestations |
| Yon [19] | 2023 | PubMed/ MEDLINE, Embase, CINAHL, Google Scholar, Cochrane Database (16 September 2022) | Cohort, Cross-sectional, case series report | 27 | 2005–2022 | Congo, France, Germany, UK, USA, Italy, USA, Nigeria, Portugal, Spain, Israel, Multiple countries | Yes | JBI and NOS | Poor quality for NOS and Good for JBI | Cutaneous, Neurological, ophthalmic, respiratory and general clinical manifestations |
Manifestation of Mpox in humans
These findings categorized based on different organ systems, are summarized in Table 2.
Table 2.
Summary of manifestations of Mpox in human organ systems
| Author | Publication Year | Databases searched and date | Type of studies | Number of studies | Year of included studies | Location | Meta-analysis performed | Quality assessment tool used | Overall quality of studies | Outcomes of concern |
|---|---|---|---|---|---|---|---|---|---|---|
| Abdelaal [7] | 2023 | PubMed, Scopus, Web of Science, EMBASE, and Google Scholar (On August 7, 2022) | Case series, Surveillance-based study, cross-sectional, Prospective observational, Chart reviews, Retrospective observational study | 11 | During 2022, Before 2022 | UK, Spain, DRC, US, Zaire, Nigeria, England, 16 countries, Switzerland | Yes | NIH | Average-fair | Ophthalmic manifestations |
| Badenoch[29] | 2022 | MEDLINE, EMBASE, PsycINFO and AMED (31 may 2022) | Cohort, cross-sectional, case series, case reports | 19 | 1987–2022 | Nigeria, USA, UK, DRC | Yes | NOS and JBI | NOS = Studies lost scores due to comparability and lack of follow-up | Neurological manifestations |
| Benites-Zapata [21] | 2022 | PubMed, Scopus, Embase, Ovid-Medline and Web of Science (June 7 2022) | Cross-sectional, cohort, case series, case reports | 19 | 1980–2022 | Sudan, DRC, Central and west Africa, UK, Nigeria, Portugal, Italy, Sierra Leone, Spain, Australia, Israel | Yes | NOS | Low risk of bias | Cutaneous, Oral, ophthalmic and general clinical manifestations |
| D’Antonio[33] | 2023 | Medline, Embase, and Cochrane (June 25, 2022) | Case report and case series | 4 | 1983–2020 | Nigeria, DRC, and Zaire | Yes | Standardized tool adapted from Murad et al. | Three studies = low risk, one study = high risk | Pregnancy related manifestations |
| Deb [26] | 2023 | PubMed, Embase, and Scopus (October 2022) | Case report, case series, cohort studies | 130 | NA | USA, India, South Africa, Morocco, Italy, Brazil, France, Romania, Argentina, Turkey, UK, Chile, Iran, Spain, KSA, Canada, Istanbul, Indiana, Sweden, Multiple countries, Peru, Belgium, Germany, Egypt, Zaire | No | NOS and JBI | NA | Neurological manifestations |
| Gandhi P [5] | 2023 | PubMed, Scopus, Web of Science, EMBASE, and Cochrane, medRxiv, arXiv, bioRxiv, BioRN, ChiRxiv, ChiRN and SSRN (15 November 2022) | Cross-sectional, retrospective, Prospective observational studies | 19 | 1988–2022 | Nigeria, DRC, Portugal, Sudan, USA | Yes | NHLBI | 13/19 studies found to be fair or good quality | Oral manifestations |
| Gandhi [30] | 2023 | PubMed, Scopus, Web of Science, EMBASE, ProQuest, EBSCOHost, Cochrane, medRxiv, arXiv, bioRxiv, BioRN, ChiRxiv, ChiRN, and SSRN (12 December 2022) | Case series, cross sectional, retrospective and prospective studies | 12 | 1987–2022 | Spain, USA, UK, Nigeria, DRC, Australia, Israel, Portugal, Europe | Yes | NHLBI | Fair to good | Opthalmic manifestations |
| Hatami [20] | 2023 | PubMed/Medline, Embase, and Scopus (from 1 January 2012 up to 15 February 2023) | Cross-sectional studies, Case series, Letters, Brief reports, Rapid communications, Shortcommunications, Correspondences | 67 | 2014–2023 | Spain, USA, UK, Africa, Central African Republic, DRC, Nigeria | Yes | JBI | Good | Cutaneous, Oral manifestations |
| Jaiswal [24] | 2023 | PubMed, Embase, and Scopus (January 5th, 2023) | Case reports, Case series | 6 | 2022 | USA, France, Canada, Portugal | No | NA | NA | Cardiovascular manifestations |
| Jaiswal [17] | 2022 | Databases = NA (December 2019 to 14th June 2022) | Case report, Case series, Observational studies | 10 | 2019–2022 | UK, Nigeria, DRC, Singapore, USA, Australia, Sierra Leone | No | NA | NA | Clinical and general manifestations |
| Khan [25] | 2023 | PubMed, Scopus, Cochrane library, and Google scholar (April 13, 2023) | NA | 22 | 1980–2023 | NA | No | NA | NA | Neurological and general clinical manifestations |
| Li [22] | 2023 | Medline, Embase, Web of Science, Cochrane Central, and Google Scholar (10 January 2023) | NA | 73 | NA | NA | Yes | JBI | Good | Cutaneous, Oral, respiratory, gastrointestinal and general clinical manifestations |
| Liu [18] | 2023 | PubMed, EMBASE, and Web of Science (From 1 January and 11 November 2022) | Cross sectional, case series | 77 | NA | Europe, America, Asia, Africa | Yes | JBI | Moderate Quality | Cutaneous, Neurological, Oral and general clinical manifestations |
| Morris [34] | 2023 | NA | NA | NA | 2007–2011 | DRC | No | NA | NA | Pregnancy related manifestations |
| Okoli [27] | 2023 | MEDLINE, Embase, CENTRAL, CINAHL and Scopus (August 30, 2022) | Cross-sectional, Case reports | 79 | 1972–2022 | USA, Italy, DRC, UK, Germany, Spain, Portugal, France, Nigeria, Netherlands, Australia, Belgium, Canada, Central African Republic, Czech Republic, Greece, India, Israel, Republic of Korea, Romania, Singapore, Switzerland | No | NOS | Very poor quality | Cutaneous, Neurological, ophthalmic and general clinical manifestations |
| Pourriyahi [8] | 2023 | PubMed, Scopus, Web of science, and Embase (from January 1, 2022 up until August 11, 2022) | Case reports, Case series, Observational studies | 46 | 2013–2022 | USA, Canada, UK, Spain, Portugal, Germany, France, Italy, Romania, Czech Republic, Nigeria, Central African Republic, Kora, Australia, Poland, Netherlands | No | NA | NA | Cutaneous, Oral, gasrtointestinal and general clinical manifestations |
| Rogers [50] | 2021 | MEDLINE, EMBASE, PsycINFO and AMED (9 September 2020) | Ecological, cohort, case-control, cross-sectional, search engine results | 57 | 1992–2020 | Spain, Japan, Uganda, Hong Kong, Nigeria, USA, UK, Sierra Leone, Australia, India, Iran, Korea, Bangladesh, Denmark, Taiwan China, Worldwide, France, Liberia, Italy, Guinea, Greece | Yes | NOS | Low quality | Suicide |
| Rojas-Carabali [32] | 2023 | PubMed, Embase, VHL (Virtual Health Library), and MedxRiv (February 28, 2023) | Case report, case series, cross-sectional, cohort, case-control | 60 | 1980–2023 | USA, Brazil, Italy, Spain, UK, Switzerland, Colombia, Spain, Australia, DRC, Portugal, Nigeria, Mexico | Yes | Tool by CLARITY group, ROBINS-I | NA | Ophthalmic manifestations |
| Sayad [23] | 2023 | PubMed, Cochrane, Medline, Scopus, and Web of Science (December 01, 2022) | Case report, case series | 9 | 2022–2023 | Puerto Rico, Canada, France, USA, Portugal | No | NIH tool for case reports and case series | Fair to good |
Cutaneous, Cardiovascular manifestations |
| Shah [28] | 2023 | PubMed, Cochrane Library, Scopus, Embase, Web of Science, Google Scholar, and OpenGrey (September 2022) | Case series, Case reports, Observational studies | 38 | 1980–2022 | USA, UK, Spain, France, DRC, Europe | Yes | JBI | High quality | Neurological, oral, respiratory and general clinical manifestations |
| Sharma [6] | 2023 | PubMed, Google Scholar, and Science Direct (from January to September 2022) | Case series, case reports, and original articles | 26 | NA | USA, Brazil, Columbia, Singapore, India, Thailand, New Zealand, Spain, France, Germany, Israel | No | NA | NA | Cutaneous, ophthalmic, respiratory, and general clinical manifestations |
| Shin [51] | 2023 | PubMed/MEDLINE, Embase, and Google Scholar (up to March 7, 2023) | Cross sectional, case series, case report | 99 | 2022–2023 | UK, Canada, USA, Germany, Israel, Netherlands, India, Spain, Czech Republic | Yes | NOS, JBI | NA | General clinical manifestations |
| Simadibrata [31] | 2023 | MEDLINE, EMBASE, and SCOPUS (October 21,2022) | Case- series, cohort, cross sectional, | 31 | 1987–2022 | Spain, USA, Congo, France, Nigeria, Israel, Sudan, UK | Yes | NIH | 14 studies were rated good, 10 studies were rated fair, 7 rated poor | Oral and gastrointestinal manifestations |
| Yon [19] | 2023 | PubMed/ MEDLINE, Embase, CINAHL, Google Scholar, Cochrane Database (16 September 2022) | Cohort, Cross-sectional, case series report | 27 | 2005–2022 | Congo, France, Germany, UK, USA, Italy, USA, Nigeria, Portugal, Spain, Israel, Multiple countries | Yes | JBI and NOS | Poor quality for NOS and Good for JBI | Cutaneous, Neurological, ophthalmic, respiratory and general clinical manifestations |
Cutaneous manifestations
Skin lesions and rashes are a universal manifestation in Mpox, with a rash being observed in 100% of patients in one study [17], and skin lesions having a prevalence of 95.2% (CI: 93.3 to 96.9%) [18]. Another study revealed a notable prevalence of 85.7% (95% CI : 68.3 to 94.30) for rashes [19]. The disease is marked by a diversity in lesion types. Pustular lesions were observed in 46% of cases, papular and ulcerating lesions in 33%, vesicular lesions in 27%, and macular lesions in 13% [8]. The lesions have a typical progression, starting as vesicular eruptions, evolving into pustules, with some ulcerating, forming scabs, and subsequently resolving. Exanthem and maculopapular pinkish exanthem were also identified as types of lesions present in Mpox [6]. The majority of patients before 2022 experienced moderate skin rash severity (53.6%, 95% CI: 46.6 to 60.5), whereas, post-2022, most patients predominantly experienced mild skin rash severity (84.9%, 95% CI: 71.9 to 92.5) [20]. Complications such as ocular lesions, secondary bacterial skin infections, hemorrhagic pustules, and ulcerated or necrotic lesions were also reported [21].
The anatomical distribution of lesions before 2022 was extensive. Facial lesions were exceedingly prevalent, found in 98.0% (95% CI: 97.1 to 98.6) of patients [20]. Trunk lesions were reported in 93.8-95.2% of patients, and limb lesions were observed in 91.0-94.9% of cases [20]. Genital lesions and oral lesions were also significant, observed in 53.5%(95% CI: 36.8 to 69.5) to 53.58% and 37.47% of cases, respectively [20]. In the 2022 outbreak and subsequent ones, there was a shift in the prevalence of lesion locations. The genital area emerged as a common site with a prevalence of 50.83% (95% CI: 40.12 to 61.50) [22]. Perianal lesions were noted in 35.44% (95% CI: 28.04 to 43.19), limb lesions in 30.82% (95% CI: 14.24–50.37%); facial lesions in 27.67% (95% CI: 22.78 to 32.82), trunk lesions in 26.74% (95% CI: 18.16 to 36.24), and oral lesions in 9.86% (95% CI: 2.13 to 21.59) of the patients [22]. The prevalence of lesions in the anal/perianal area, a previously unreported location, was marked at 39.8% (95% CI: 30.4 to 49.9) [20]. Lesions on the limbs were most common on arms, forearms, and legs [8]. Anogenital lesions, including vesicular, macular, pustular, or ulcerated lesions on the penile shaft, were often accompanied by painful inguinal lymphadenopathies or pubic erythema and pruritus [8]. Numerous rectal lesions with few perianal and cutaneous lesions were also reported [8]. The location of the rash was notably related to the site of sexual contact, including the genital, anal, or oral regions [23]. A significant variance in rash distribution was noted between African and European studies. Rash frequency was significantly higher in African studies (100%) compared to European studies (22%, 95% CI: 14 to 32). Additionally, the distribution of the rash in the pelvic area and groins was significantly higher in European studies (75%, 95% CI: 65 to 84) compared to African studies (30%, 95% CI: 28 to 33%) [21].
Cardiovascular manifestations
Cardiovascular complications in Mpox virus cases are notable for a variety of symptoms and diagnostic findings, primarily involving myocarditis. The complications are often associated with distinctive symptoms like chest pain sometimes radiating to the left arm and dyspnea, were identified in examined cases [23, 24]. Electrocardiographic evaluations across studies have disclosed various abnormalities, including sinus tachycardia and widespread ST-segment elevation, reflecting the diversified cardiac involvements in Mpox [23, 24]. Specifically, ST-Elevation was identified in 44% of patients, with sinus tachycardia and normal sinus rhythm recorded in 22% and 33% of patients, respectively [24]. This finding was based on the findings of 6 studies which include only total of 9 patients. CMR imaging elucidated areas of increased signal intensity and findings consistent with necrosis and myocardial edema [23]. Late gadolinium enhancement and edema were prevalent in 40% of the patients analyzed through CMR [24]. Echocardiographic assessments depicted normal wall motion in 67% and reduced ejection fraction in 43% of the patients [24]. The elevated levels of high-sensitivity troponins, ranging from 0.165 to 21.20 ng/ml, were a consistent finding [23]. Other cardiac biomarkers like Creatine Kinase (291–740 U/L) and N-terminal prohormone B-type natriuretic peptide (155–1258 pg/ml) were also found to be elevated, indicating extensive cardiac implications [23].
Neurological manifestations
The neurological manifestations of Mpox are extensive, with headache being the leading symptom, reported in 18 out of 22 studies, affecting 47.84% of participants [25]. The pooled prevalence of headaches was reported at 31% [26], and varied findings have been reported regarding its prevalence in different outbreaks, with 48% in previous outbreaks compared to 36% in 2022 outbreaks [27]. Myalgia and fatigue are also prevalent, reported in 27.5% and 17.73% of participants, respectively [25]. Myalgia was reported with a prevalence of 36.0% and 30.8% [18, 19]. Fatigue, asthenia, or malaise were particularly prevalent, affecting 38.7% of patients [18]. There is variation in the prevalence of neurological symptoms between different studies and outbreaks. Headache was more prevalent in non-endemic regions (36%, 95% CI: 24 to 47) compared to endemic regions (24%, 95% CI: 1 to 57)) [28]. It was also more prevalent in prior outbreaks (37%, 95% CI: 11 to 66) compared to the 2022 outbreak (23%, 95% CI: 14 to 34) [28].
Seizure, confusion, and encephalitis were reported with prevalences of 2.7% (95% CI: 0.7 to 10.2%), 2.4% (95% CI: 1.1 to 5.2), and 2.0% (95% CI: 0.5 to 8.2) respectively [29]. Seizures were particularly noted in three patients, with one case involving a 43-year-old man with HIV-1 infection who died following repeated seizures [25]. Encephalitis details include cases involving a female neonate and a 6-year-old girl [25]. Photophobia was reported in 39 patients (4.43%), and visual deficits were reported in five patients (0.57%) [25]. Other reported manifestations included dizziness, coma, encephalomyelitis, and transverse myelitis [25].
Several pediatric cases have been reported, with symptoms like headache and malaise being common. A 2-year-old female developed headache and malaise [26]. Another 6-year-old female presented with malaise, anorexia, and headache, and a provisional diagnosis of encephalitis was made [26]. Brain MRI in this case showed diffuse cortical, thalamic, and brainstem edema, meningeal enhancement, and signal abnormalities [26].
Oral manifestations
The prevalence of oropharyngeal rash was 52.4% pre-2022 outbreak, reducing to 18.3% (95% CI: 13.2 to 24.9) in post-2022 outbreaks [20]. Variations in prevalence are also seen based on geographic locations, with a higher prevalence of 62.67% (95% CI: 52.75 to 72.12) in endemic countries compared to 15.62% (95% CI: 10.24 to 21.69) in non-endemic ones [30]. A meta-analysis investigated the prevalence of oral manifestations of human Mpox infection in 4042 individuals, out of which 1433 exhibited oral manifestations [30]. The overall pooled prevalence of oral manifestations was 36.75% (95% CI: 23.77 to 50.65), with sore throat being the predominant oral manifestation at 39.96% (95% CI: 21.42 to 59.91), followed by mouth sore at 24.80% (95% CI: 8.14 to 46.32), tonsillitis at 18.24% (95% CI: 0.34 to 52.54), and mouth rash at 17.99% (95% CI: 15.66 to 20.43) [30].
Oral ulcers are a common manifestation, recorded in 96% of the patients in one study [17], and another study observed a pooled prevalence of 7% (95% CI: 1 to 19), with a higher occurrence in prior outbreaks (13%, 95% CI: 2.0 to 30) compared to the 2022 outbreak (2%, 95% CI: 0.0 to 5.0) [28]. Tonsillar signs and oral exanthem are reported with a pooled prevalence of 3% (95% CI: 0.00 to 9.0), with variations in prevalence between prior outbreaks and the 2022 outbreak [28]. The prevalence of oral ulcers has been reported to be 33% (95% CI: 31 to 35), while dysphagia has been observed in 57% (95% CI: 54 to 59) of cases, and odynophagia in 31% (95% CI: 27 to 35) of cases [31]. There have been highlighted instances showcasing significant oral, perioral, enoral, and tonsillar lesions. These instances include cases of isolated enoral peritonsillar abscesses with unilateral cervical lymphadenopathy (LAP) without the presence of cutaneous lesions, and cases displaying oropharyngeal erythema, tonsillar enlargement, and associated bilateral cervical LAP [8]. The prevalence of oral lesions was reported to be 37.47% for outbreaks occurring prior to 2022 and 9.86% for outbreaks post-2022 for Mpox [22].
Specific studies have reported a prevalence of 62.67% (95% CI: 52.75 to 72.12) in Africa, 27.48% (95% CI: 7.19 to 52.99) in North America, and 15.07% (95% CI: 7.02 to 24.95) in Europe [30]. A prospective observational study from the DRC reported a 77.78% prevalence of sore throat, while another study observed a 70% prevalence of oral lesions in patients with Mpox from the DRC [30].
Ophthalmic manifestations
The prevalence of conjunctivitis is notably high, recorded at 96% in one study [17], and 12% in another, with a remarkable difference in prevalence noted between outbreaks before 2022 (17%) and the current multicountry outbreak (1%). Another study reported the prevalence of conjunctivitis at 7.1% [19], and the weighted prevalence is reported as 6%, with reports of up to 60% prevalence in certain latitudes [32]. Geographic variations are also observed, with no patients from non-African countries reporting vision loss [5]. There are notable differences in the rate of ophthalmic manifestations between previous MPX outbreaks and the current outbreak, with a higher prevalence of conjunctivitis, eye lesions involving the cornea and/or conjunctiva, and involvement of the eyelids reported in previous outbreaks [7]. Various studies have documented a range of ophthalmic manifestations such as photophobia, eye pain, eye lesions involving the cornea and conjunctiva, and lesions involving the eyelids, including vesicles, pustules, or pseudo-pustules [5, 7]. Specific complications associated with these manifestations include keratitis, corneal ulceration, unilateral blindness, and impaired vision [7]. The involvement of the eyelids can present single or multiple umbilicated papules in 3% of patients and can lead to eyelid deformation, with severe cases of skin necrosis reported [32]. A pooled prevalence of 13.89% (95% CI: 6.92 to 22.67) for conjunctival lesions and a prevalence of 14.37% (95% CI: 6.91 to 23.71) for eye rash is reported [5]. Redness, pain, and discharge in the eye are observed in 9.26% of the cases, with vision loss reported in 7.69% and vision changes in 2.31%. Cases of severe conjunctivitis with erythematous sclera, corneal edema, opacity, and keratitis with confluent corneal lesions spreading into the sclera have been reported [32]. Additionally, uveitis, described in four case reports, had mild inflammation accompanied by corneal compromise, with keratic precipitates and superior limbitis observed [32].
Respiratory manifestations of Mpox
Respiratory manifestations are a significant aspect of Mpox, with cough being a common symptom, reported with an overall prevalence of 48.55% (95% CI: 34.67% to 62.54) [22]. Another study indicated a similar prevalence for cough, noting it in 53% of cases in previous outbreaks compared to 17% in 2022 outbreaks [19]. In contrast, the prevalence of cough in nonendemic countries during the 2022 outbreak was significantly lower, recorded at 10.17% (95% CI: 1.68 to 23.50) [22]. Upper respiratory symptoms are also predominant, with an overall prevalence of 97% [17]. One review also categorized respiratory symptoms as rare clinical features, with a prevalence of 19.5% [19]. The significant variations in the prevalence of different respiratory symptoms, especially between different outbreaks, highlight the dynamic nature of Mpox and its respiratory manifestations. Beyond cough, other respiratory symptoms like nasal congestion and shortness of breath have a pooled prevalence of 1%, with similar prevalence in both prior and the 2022 outbreaks and in both endemic and non-endemic regions [28]. A remarkable difference in the prevalence of sore throat is also noted, being 71% in previous outbreaks versus 28% in 2022 outbreaks [19]. A rare case of nasal necrosis has been reported in a 40-year-old HIV-positive man [19].
Gastrointestinal manifestations
Abdominal pain is a recognized gastrointestinal symptom of Mpox, noted to have an overall prevalence of 9% (95%: 8 to 10) [31]. There is documented variance in the prevalence of abdominal pain, with African studies reporting higher incidences compared to non-African studies [31]. Anorexia, characterized by a decreased appetite, has also been reported, with a prevalence of 47% (95% CI: 41 to 53), underscoring the substantial impact of Mpox on the gastrointestinal system and dietary patterns [31]. Nausea and vomiting are common, with prevalences of 10% and 12% respectively [22, 31]. Diarrhea, on the other hand, is less common, with a 5% (95% CI: 4 to 6) prevalence [31].
The prevalence of proctitis is documented at 11% (95% CI: 12 to 13), and issues related to rectal/anal pain and rectal bleeding are also notable, with prevalences of 25% (95% CI: 24 to 27) and 12% (95% CI: 11 to 13) respectively [31]. In the 2022 outbreak in nonendemic countries, a significant prevalence of 16.82% for rectal pain was reported [22]. Dysphagia has a varied prevalence, with reports as high as 70.08% (95% CI: 19.55 to 100) [22] and as low as 2% [8]. There is also a reported general prevalence of 23% for either pharyngitis or dysphagia.
Pregnancy related manifestations
Only two systematic reviews reported details on pregnancy related manifestations mainly conducted in DRC [33, 34]. Mpox infection during pregnancy has been associated with a high incidence of spontaneous early miscarriages and second-trimester losses [34]. Another review revealed that 39% of the cases experienced miscarriages and a substantial 77% (95% CI: 26.0 to 100) incidence of overall fetal and perinatal loss [33]. Furthermore, the incidence of fetal loss was found to be 67% following first-trimester infection and increased to 82% with second-trimester infection. Vertical transmission of Mpox is a significant concern, with an estimated incidence of 62% (95 CI: 9 to 99) [33]. It is further reported that only 23% (95% CI: 0 to 74) of fetuses surviving to birth [33]. Although no Neonatal Death (NND) was reported within 30 days from birth, there has been a reported case of NND at 6.5 weeks of age due to malnutrition and a neonatal rash. The occurrence of preterm births before 37 weeks of gestation has been seen as another complication with an incidence of 8.0% (95% CI: 0 to 92) [33].
Other general and constitutional symptoms
Fever is widely identified as a prominent manifestation of Mpox, with studies documenting its prevalence at 81.34% (95% CI: 60.36 to 96.21) and 58.4% (95% CI: 54.9 to 61.8) [18, 22]. Lymphadenopathy, notably impacting the cervical and inguinal regions, is a recurring feature, observed in 53.0% (95% CI: 48.7 to 57.3) to 85% of cases [17, 18]. The reports significantly contribute to the understanding of its prevalence in Mpox. Fatigue is another common symptom, with its prevalence ranging from 46.18% in nonendemic regions during the 2022 outbreak to 75% [21]. The incidence of chills is reported at 23.8% and 67.91% (95% CI: 47.05% to 85.77) [18, 22], and other systemic manifestations like myalgia and sweating are reported in approximately 43% (17.47 to 70.58) of cases [22]. A specific emphasis is placed on the occurrence of scrotal or penile edema, with a reported prevalence of 10.7% (95% CI: 6.3 to 17.7) [19]. Instances of axillary, cervical, and inguinal lymphadenopathy have been recorded in various outbreaks [27].
Discussion
This study constitutes the inaugural umbrella review conducted on Mpox, aiming to aggregate and comprehensively summarize its various manifestations to date. Our objective was to provide a bird’s eye view of the extensive range of MPox’s effects on human organ systems, offering a broader understanding of its diverse manifestations. It became evident that Mpox impacts the human body as a whole, with its manifestations prevailing across different organ systems, the most common of which was cutaneous. We were able to identify a sufficient number of systematic reviews evaluating the clinical manifestations of Mpox, allowing for a more inclusive analysis of its effects. However, concerns arose regarding the types of primary studies included. Many consisted of case series and case reports, contributing to a substantial portion of the systematic reviews. The prevalence of such studies highlights a significant gap in the availability of long-term studies for numerous outcomes. For instance, there is a pronounced need for more expansive research into the cardiovascular manifestations of Mpox. Similarly, the complications of Mpox infection during pregnancy are another area where information is notably limited.
Mpox exhibits a multifaceted and evolving nature, characterized by varied manifestations impacting multiple organ systems. The prevalence and types of manifestations, particularly cutaneous ones, indicate significant variability and evolution, hinting at possible mutations or adaptations of the virus over time, as evidenced by changes observed in post-2022 outbreaks. The near-universal presence of diverse skin lesions is pivotal for diagnosis, reflecting the virus’s primary interaction with the host [35]. The geographical variations observed in the prevalence of symptoms between African and non-African regions suggest the influence of environmental and genetic factors on disease presentation, necessitating region-specific approaches to management and treatment, though the portal of entry and the mode of exposure likely also play a role in diverse manifestations. Moreover, the systemic impact of Mpox is evident through the prevalence of severe cardiovascular and neurological complications, emphasizing the need for comprehensive multisystem monitoring and holistic management to detect and address potential long-term health implications. Particularly vulnerable populations, such as pediatric patients and pregnant women, experience heightened risks and severe consequences, underscoring the importance of specialized care and stringent prenatal monitoring to mitigate adverse outcomes related to vertical transmission, like spontaneous miscarriages and neonatal complications. The variability in respiratory and gastrointestinal symptoms between different outbreaks highlights the dynamic nature of the disease and the necessity for ongoing research and surveillance to develop targeted interventions and treatments. In essence, the complex interplay of manifestations in Mpox requires a nuanced, multidisciplinary approach, informed by continuous research and a deep understanding of the disease’s evolving nature and geographical variations, to effectively manage and mitigate its impacts.
While our umbrella review has provided valuable insights into the diverse clinical manifestations of Mpox, it is important to note the inclusion of studies categorized as low or critically low quality, according to AMSTAR 2 criteria, presents certain challenges. The presence of these studies inherently affects the reliability and generalizability of our findings. Low-quality studies often suffer from methodological flaws such as inadequate control for confounding factors, poor handling of data, and insufficient reporting of outcomes, which can introduce bias into the synthesis process. These methodological shortcomings might lead to overstated or inaccurate representations of Mpox’s clinical manifestations, impacting our ability to draw definitive conclusions. Moreover, the generalizability of the results is compromised as studies with such deficiencies do not adequately represent the broader patient population or the full spectrum of clinical presentations.
The clinical manifestations of Mpox can vary based on gender and different transmission pathways. In terms of gender, the recent outbreak of Mpox has shown unique clinical signs, especially among men who have sex with men (MSM) [36]. In this group, rashes have been observed primarily around the genital or anal area, with subsequent spread throughout the body. Severe cases in this population can lead to complications such as hemorrhagic disease, necrotic disease, and inflammation of vital organs. Additionally, the majority of reported cases among homosexual or bisexual males have shown a high co-infection rate with human immunodeficiency virus (HIV), with a significant percentage of lesions occurring in the anal and genital regions. Regarding transmission pathways, Mpox can be transmitted through various routes, including animal-to-human, person-to-person, and fomites [37–39]. Human-to-human transmission is believed to occur through direct contact with respiratory droplets from infected individuals, and vertical transmission can occur from infected mothers to their newborns [40]. The recent outbreak of Mpox has seen a shift in transmission patterns, with most cases not associated with contact with infected animals or travel but linked to sexual contact between individuals. This outbreak has highlighted the importance of understanding the diverse transmission pathways of Mpox, especially in the context of the 2022 epidemic that mainly affected MSM. Clade I and Clade II of the Mpox virus exhibit distinct characteristics that significantly impact their transmission pathways and the severity of the disease [41]. Clade I is primarily associated with transmission through direct contact with infected wild animals, close contact with infected individuals, and contact with contaminated materials. Clade II shares similar transmission routes, including direct contact with infected animals and individuals, as well as through contaminated materials. These differing pathways are crucial for understanding the spread of Mpox and have implications for the severity of outbreaks, necessitating targeted public health interventions and control measures to manage transmission effectively. Additionally, the Mpox virus shows specific tropism for tissues such as the skin, mucous membranes, and lymph nodes, yet the precise virus ligands responsible for this tissue specificity remain unidentified [42]. This lack of identification shows the complexity of the interactions between the Mpox virus and host cells, influencing the virus’s ability to infect particular tissues and propagate within the host. Understanding these mechanisms is vital for elucidating the pathogenesis of Mpox and developing targeted therapeutic and preventive strategies, enhancing our ability to effectively combat this virus and minimize its public health impact.
The findings from this study on Mpox have significant public health and clinical implications, underscoring the need for an adaptable, informed response to this evolving disease. Several viral infectious diseases are emerging [43–45]. The wide range of manifestations across multiple organ systems, particularly the variability in cutaneous symptoms and the emergence of severe cardiovascular and neurological complications, call for heightened surveillance and a multidisciplinary approach to patient care. Public health strategies must consider the geographical and temporal variations in symptom prevalence, tailoring interventions to specific regional needs and potential virus adaptations [46]. Clinically, this necessitates vigilance in diagnosis and treatment, especially for vulnerable groups like pregnant women and pediatric patients, to mitigate the risks of severe outcomes and vertical transmission. The diverse presentations highlight the importance of ongoing research and capacity building in healthcare systems, ensuring they are equipped to handle the multifaceted challenges posed by this virus. The effectiveness of vaccines in preventing Mpox infection underscores the necessity for strategic vaccination programs, particularly targeting high-risk groups to prevent outbreaks and control the disease’s spread [47]. Alongside vaccination, the importance of contact tracing and monitoring, including healthcare personnel, is crucial in preventing further transmission and containing outbreaks. To effectively manage Mpox, healthcare systems must be prepared with robust surveillance and detection mechanisms that promptly identify cases and track outbreaks. Infection control measures in healthcare facilities, including the use of personal protective equipment (PPE), are vital to prevent transmission to healthcare workers and other patients [48]. Additionally, the global nature of Mpox outbreaks demands international collaboration and coordination to share data, best practices, and resources, ensuring a unified response. Public education and awareness campaigns play a critical role in promoting prevention measures, reducing stigma, and encouraging individuals to seek medical attention if symptoms arise, further bolstering the public health response to Mpox [49]. In essence, these findings call for a concerted effort in public health planning, clinical management, and research to effectively address the complexities of Mpox and safeguard community health.
Our study has several limitations. We restricted our inclusion to articles published solely in English, potentially overlooking relevant studies in other languages. There is also a high possibility of overlap in primary studies included in multiple systematic reviews within our umbrella review, which could potentially skew the findings. We could not perform a meta-analysis due to the high variability of study designs and variations in PICO (Population, Intervention, Comparison, Outcome). Moreover, many of our findings predominantly rely on case reports and case series, which could introduce a degree of bias into these results. More high-quality observational studies are required to comprehensively understand the impact of Mpox in humans.
Conclusion
This umbrella review provides an extensive synthesis of the clinical manifestations of Mpox, underscoring the disease’s complex and dynamic nature. Our findings reveal the prevalence of severe complications across various organ systems, including cutaneous, cardiovascular, neurological, and respiratory systems, highlighting the urgent need for detailed and region-specific management strategies. The study significantly contributes to the existing knowledge by delineating the broad spectrum of Mpox effects, which is critical for developing targeted interventions and enhancing diagnostic accuracy. However, our conclusions are tempered by the predominance of case reports and series in the included systematic reviews, which limits the robustness and generalizability of our results. This limitation underlines an urgent need for more comprehensive, high-quality observational studies that can provide deeper insights into the long-term effects of Mpox and its varying clinical presentations across different populations. Future research should focus on longitudinal studies to better understand the disease’s progression, treatment outcomes, and potential chronic conditions following recovery. By addressing these gaps, we can better tailor our clinical and public health strategies to effectively combat the evolving threat of Mpox and safeguard vulnerable communities.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors acknowledge Nested-Knowledge, MN, USA for providing access for the software.
Author contributions
Author contributions: - Author Contributions: Conceptualization and Methodology - PS, MNK, AFH-M, MFA-S, NAAK; Data Curation - SG, QSZ, MG, MA, TS; Formal Analysis - PS, MNK, MK, AAR, CFP; Writing - Original Draft Preparation - HAR, HAS, RS, NAAK, MFA-S; Writing - Review & Editing - MG, MA, TS, AAR, MK; Final Approval of the Version to be Published - All authors.
Funding
This study received no funding.
Data availability
Availability of data and materials: Data pertaining to this study are available with the authors. and can be obtained by contacting the corresponding author.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Prakasini Satapathy and Mahalaqua Nazli Khatib contributed equally to this work.
Contributor Information
Quazi Syed Zahiruddin, Email: zahirquazi@gmail.com.
Ranjit Sah, Email: ranjitsah@iom.edu.np.
References
- 1.Alakunle EF, Okeke MI. Monkeypox virus: a neglected zoonotic pathogen spreads globally. Nat Rev Microbiol. 2022;20(9):507–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bunge EM, Hoet B, Chen L, Lienert F, Weidenthaler H, Baer LR, et al. The changing epidemiology of human monkeypox—A potential threat? A systematic review. PLoS Negl Trop Dis. 2022;16(2):e0010141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Di Gennaro F, Veronese N, Marotta C, Shin JI, Koyanagi A, Silenzi A, et al. Human monkeypox: a comprehensive narrative review and analysis of the public health implications. Microorganisms. 2022;10(8):1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Saloni S, Srilatha M, Biju S, Arabindo T, Azra k, Amit Kumar G et al. Global resurgence of monkeypox (mpox) virus: a review of current outbreaks and public health strategies. Evid. 2024;2(3).
- 5.Gandhi AP, Gupta PC, Padhi BK, Sandeep M, Suvvari TK, Shamim MA, et al. Ophthalmic manifestations of the monkeypox virus: a systematic review and meta-analysis. Pathogens. 2023;12(3):452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sharma A, Prasad H, Kaeley N, Bondalapati A, Edara L, Kumar YA. Monkeypox epidemiology, clinical presentation, and transmission: a systematic review. Int J Emerg Med. 2023;16(1):20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abdelaal A, Reda A, Hassan AR, Mashaal A, Serhan HA, Katamesh BE, et al. Monkeypox-Associated manifestations and complications Involving the Eye: a systematic review and Meta-analysis of previous and current outbreaks. Asia-Pacific J Ophthalmol. 2023;12(3):326–37. [DOI] [PubMed] [Google Scholar]
- 8.Pourriyahi H, Aryanian Z, Afshar ZM, Goodarzi A. A systematic review and clinical atlas on mucocutaneous presentations of the current monkeypox outbreak: with a comprehensive approach to all dermatologic and nondermatologic aspects of the new and previous monkeypox outbreaks. J Med Virol. 2023;95(2):e28230. [DOI] [PubMed] [Google Scholar]
- 9.Belbasis L, Bellou V, Ioannidis JP. Conducting umbrella reviews. BMJ Med. 2022;1(1). [DOI] [PMC free article] [PubMed]
- 10.Aromataris E, Fernandez R, Godfrey C, Holly C, Khalil H, Tungpunkom P. Chapter 10: Umbrella Reviews. JBI Manual for Evidence Synthesis; 2020. [cited 2021 May].
- 11.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Int J Surg. 2021;88:105906. [DOI] [PubMed] [Google Scholar]
- 12.Goel S, Shabil M, Kaur J, Chauhan A, Rinkoo AV. Safety, efficacy and health impact of electronic nicotine delivery systems (ENDS): an umbrella review protocol. BMJ open. 2024;14(1):e080274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Swarup SS, Padhi BK, Satapathy P, Shabil M, Bushi G, Gandhi AP et al. Cardiovascular consequences of financial stress: a systematic review and meta-analysis. Curr Probl Cardiol. 2023:102153. [DOI] [PubMed]
- 14.Shabil M, Bushi G, Yadav A, Ahmed M, Kishore J, Lekamwasam S, et al. Effect of metformin on cardiovascular outcomes: a systematic review and meta-analysis of observational studies and RCTs. Evid. 2023;1(1):23–34. [Google Scholar]
- 15.Shea BJ, Reeves BC, Wells G, Thuku M, Hamel C, Moran J et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. 2017;358. [DOI] [PMC free article] [PubMed]
- 16.Alrahbeni T, Mahal A, Alkhouri A, Alotaibi HF, Rajagopal V, Behera A et al. Surgical interventions for intractable migraine: a systematic review and meta-analysis. Int J Surg. 2024:101097. [DOI] [PMC free article] [PubMed]
- 17.Jaiswal V, Nain P, Mukherjee D, Joshi A, Savaliya M, Ishak A, et al. Symptomatology, prognosis, and clinical findings of Monkeypox infected patients during COVID-19 era: a systematic‐review. Immun Inflamm Dis. 2022;10(11):e722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu Q, Fu L, Wang B, Sun Y, Wu X, Peng X, et al. Clinical characteristics of human Mpox (monkeypox) in 2022: a systematic review and meta-analysis. Pathogens. 2023;12(1):146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yon H, Shin H, Shin JI, Shin JU, Shin YH, Lee J et al. Clinical manifestations of human mpox infection: a systematic review and meta-analysis. Rev Med Virol. 2023:e2446. [DOI] [PubMed]
- 20.Hatami H, Jamshidi P, Arbabi M, Safavi-Naini SAA, Farokh P, Izadi-Jorshari G, et al. Demographic, epidemiologic, and clinical characteristics of human monkeypox disease pre-and post-2022 outbreaks: a systematic review and meta-analysis. Biomedicines. 2023;11(3):957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Benites-Zapata VA, Ulloque-Badaracco JR, Alarcon-Braga EA, Hernandez-Bustamante EA, Mosquera-Rojas MD, Bonilla-Aldana DK, et al. Clinical features, hospitalisation and deaths associated with monkeypox: a systematic review and meta-analysis. Ann Clin Microbiol Antimicrob. 2022;21(1):36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li P, Li J, Ayada I, Avan A, Zheng Q, Peppelenbosch MP et al. Clinical features, antiviral treatment, and patient outcomes: a systematic review and comparative analysis of the previous and the 2022 Mpox outbreaks. J Infect Dis. 2023:jiad034. [DOI] [PMC free article] [PubMed]
- 23.Sayad R, Siddiq A, Hashim A, Elsaeidy AS. Can the current monkeypox affect the heart? A systematic review of case series and case report. BMC Cardiovasc Disord. 2023;23(1):328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jaiswal V, Sultana Q, Lahori S, Mukherjee D, Agrawal V, Doshi N et al. Monkeypox-Induced myocarditis: a systematic review. Curr Probl Cardiol. 2023:101611. [DOI] [PMC free article] [PubMed]
- 25.Khan SA, Parajuli SB, Rauniyar VK. Neurological manifestations of an emerging zoonosis—human monkeypox virus: a systematic review. Medicine. 2023;102(35):e34664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Deb N, Roy P, Biswakarma A, Mary T, Mahajan S, Khan J, et al. Neurological manifestations of Coronavirus Disease 2019 and Monkeypox in Pediatric patients and their management: a state-of-the-art systematic review. Pediatr Neurol. 2023;146:65–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Okoli GN, Van Caeseele P, Askin N, Abou-Setta AM. Comparative evaluation of the clinical presentation and epidemiology of the 2022 and previous Mpox outbreaks: a rapid review and meta-analysis. Infect Dis. 2023:1–19. [DOI] [PubMed]
- 28.Shah J, Saak TM, Desai AN, Gudis DA, Cheema HA, Abuelazm M et al. Otolaryngologic manifestations among MPOX patients: a systematic review and meta-analysis. Am J Otolaryngol. 2023:103991. [DOI] [PubMed]
- 29.Badenoch JB, Conti I, Rengasamy ER, Watson CJ, Butler M, Hussain Z et al. Neurological and psychiatric presentations associated with human monkeypox virus infection: a systematic review and meta-analysis. EClinicalMedicine. 2022. [DOI] [PMC free article] [PubMed]
- 30.Gandhi A, Patro SK, Sandeep M, Satapathy P, Shamim MA, Kumar V et al. Oral manifestation of the monkeypox virus: a systematic review and meta-analysis. Eclinicalmedicine. 2023;56. [DOI] [PMC free article] [PubMed]
- 31.Simadibrata DM, Lesmana E, Pratama MIA, Annisa NG, Thenedi K, Simadibrata M. Gastrointestinal symptoms of Monkeypox infection: a systematic review and meta-analysis. J Med Virol. 2023. [DOI] [PubMed]
- 32.Rojas-Carabali W, Cifuentes-González C, Agrawal R, de-la-Torre A. Spectrum of ophthalmic manifestations in monkeypox virus infection worldwide: systematic review and meta-analysis. Heliyon. 2023. [DOI] [PMC free article] [PubMed]
- 33.D’Antonio F, Pagani G, Buca D, Khalil A. Monkeypox infection in pregnancy: a systematic review and metaanalysis. Am J Obstet Gynecol MFM. 2023;5(1):100747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morris S, Joshi P, Soni P, Jakhmola V, Kalita K, Nainwal N et al. A systematic review on human monkeypox virus disease and infection in pregnancy. J Pure Appl Microbiol. 2023;17(2).
- 35.Shabil M, Khatib MN, Gaidhane S, Zahiruddin QS, Rustagi S, Satapathy P et al. Emerging Threats in Public Health: H5N1 Transmission from Dairy Cattle to Humans. New Microbes and New Infections. 2024:101429. [DOI] [PMC free article] [PubMed]
- 36.Lu J, Xing H, Wang C, Tang M, Wu C, Ye F, et al. Mpox (formerly monkeypox): pathogenesis, prevention, and treatment. Signal Transduct Target Therapy. 2023;8(1):458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pinto P, Costa MA, Gonçalves MF, Rodrigues AG, Lisboa C. Mpox person-to-person transmission—where have we got so far? A systematic review. Viruses. 2023;15(5):1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shabil M, Murti K, Kumar VU, Kumar R, Kumar N, Dhingra S, et al. Older PLHIV are at Higher Cardiovascular Risk with Poor Quality of Life. Curr HIV Res. 2023;21(6):354–60. [DOI] [PubMed] [Google Scholar]
- 39.Shabil M, Kumar VU, Dhingra S, Ravichandiran V, Parihar VK, Kumar N, et al. Current scenario and strategies to Tackle Cardiovascular Disease Risk in HIV Geriatrics. Curr Pharmacol Rep. 2023;9(6):523–39. [Google Scholar]
- 40.Schwartz DA, Ha S, Dashraath P, Baud D, Pittman PR, Adams Waldorf KM. Mpox Virus in pregnancy, the Placenta, and Newborn: an emerging Poxvirus with similarities to Smallpox and other Orthopoxvirus agents causing maternal and fetal disease. Arch Pathol Lab Med. 2023;147(7):746–57. [DOI] [PubMed] [Google Scholar]
- 41.Masirika LM, Kumar A, Dutt M, Ostadgavahi AT, Hewins B, Nadine MB, et al. Complete genome sequencing, annotation, and mutational profiling of the Novel Clade I Human Mpox Virus, Kamituga Strain. J Infect Developing Ctries. 2024;18(04):600–8. [DOI] [PubMed] [Google Scholar]
- 42.Ritter JM, Martines RB, Bhatnagar J, Rao AK, Villalba JA, Silva-Flannery L et al. Pathology and Monkeypox virus localization in tissues from immunocompromised patients with severe or fatal mpox. J Infect Dis. 2024:jiad574. [DOI] [PubMed]
- 43.Shabil M, Khatib MN, Gaidhane S, Zahiruddin QS, Rustagi S, Satapathy P et al. Emerging threats in public health: H5N1 transmission from dairy cattle to humans. New Microbes and New Infections. 2024;60. [DOI] [PMC free article] [PubMed]
- 44.Shabil M, Yadav A, Shamim MA, Ahmed M, Satapathy P, Zaidan AA, et al. Prevalence of hepatitis B and C infections among HIV-positive men who have sex with men: a systematic review and meta‐analysis. Health Sci Rep. 2024;7(6):e2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bushi G, Vajjala SM, Irfan FB, Aggarwal D, Jamil S, Sagar D et al. One health implications of bovine leukemia virus seroprevalence: a global systematic review and meta-analysis. Evid. 2024;2(1).
- 46.Branda F, Romano C, Ciccozzi M, Giovanetti M, Scarpa F, Ciccozzi A, et al. Mpox: an overview of Pathogenesis, diagnosis, and Public Health Implications. J Clin Med. 2024;13(8):2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cohn H, Bloom N, Cai GY, Clark JJ, Tarke A, Bermúdez-González MC, et al. Mpox vaccine and infection-driven human immune signatures: an immunological analysis of an observational study. Lancet Infect Dis. 2023;23(11):1302–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Decousser J-W, Romano-Bertrand S, Glele LA, Baron R, Carre Y, Cassier P, et al. Healthcare worker protection against mpox contamination: position paper of the French Society for Hospital Hygiene. J Hosp Infect. 2023;140:156–64. [DOI] [PubMed] [Google Scholar]
- 49.Norberg AN, Norberg PRBM, Manhães FC, Souza DGd, Queiroz MMC, Neto CHG, et al. Public Health Strategies against Social Stigma in the Mpox outbreak: a systematic review. J Adv Med Med Res. 2024;36(2):33–47. [Google Scholar]
- 50.Rogers J, Chesney E, Oliver D, Begum N, Saini A, Wang S, et al. Suicide, self-harm and thoughts of suicide or self-harm in infectious disease epidemics: a systematic review and meta-analysis. Epidemiol Psychiatric Sci. 2021;30:e32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shin H, Shin H, Rahmati M, Koyanagi A, Jacob L, Smith L et al. Comparison of clinical manifestations in mpox patients living with HIV versus without HIV: a systematic review and meta-analysis. J Med Virol. 2023. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Availability of data and materials: Data pertaining to this study are available with the authors. and can be obtained by contacting the corresponding author.