Abstract
Background
Tick-borne pathogens are understudied among domestic animals in sub-Saharan Africa but represent significant threats to the health of domestic animals and humans. Specifically, additional data are needed on tick-borne pathogens in Chad, Africa. Surveillance was conducted among domestic dogs in Chad for selected tick-borne pathogens to measure (1) the prevalence of antibodies against Anaplasma spp., Borrelia burgdorferi, and Ehrlichia spp.; (2) the prevalence of infections caused by Hepatozoon spp., Ehrlichia canis, Anaplasma platys, and Babesia spp.; and (3) associations of pathogens with demographic, spatial, and temporal factors. Blood samples were collected from domestic dogs at three time points (May 2019, November 2019, June 2020) across 23 villages in southern Chad.
Results
Of the 428 dogs tested with the IDEXX SNAP 4Dx test in May 2019, 86% (n = 370, 95% CI = 83–90%) were positive for antibodies to Ehrlichia spp., 21% (n = 88, 95% CI = 17–25%) were positive for antibodies to Anaplasma spp., and 0.7% (n = 3, 95% CI = 0.1–2%) were positive for antibodies to Borrelia burgdorferi. Four different pathogens were detected via PCR. Hepatozoon spp. were most commonly detected (67.2–93.4%, depending on the time point of sampling), followed by E. canis (7.0-27.8%), A. platys (10.1–22.0%), and Babesia vogeli (0.4–1.9%). Dogs were coinfected with up to three pathogens at a single time point, and coinfections were most common in May 2019 compared to November 2019 and May 2020.
Conclusions
Overall, this study provides new data about the epidemiology of tick-borne pathogens in domestic dogs in Chad, with potential implications for dog and human health.
Keywords: Africa, Canine health, Tick-borne diseases, Zoonoses
Background
Vector-borne diseases, especially those caused by tick-borne pathogens, are a significant health concern for humans and domestic animals in sub-Saharan Africa and should be studied in the context of One Health [1]. Although tick-borne pathogens are widespread in Africa, there are still considerable knowledge gaps regarding pathogen prevalence, vectors, geographic distribution, and host ranges. Furthermore, changes in climate and habitat alter the distributions of ticks and their pathogens, resulting in the introduction of ticks and tick-borne pathogens into novel areas with naïve host populations [2]. Populations of domestic dogs can harbor a high prevalence and diversity of pathogens that can cause morbidity and mortality, and because some of these tick-borne pathogens are zoonotic, dogs may serve as reservoirs or sentinels for these zoonotic pathogens [3, 4]. Additionally, dog population dynamics, such as birth and mortality rates, as well as human-driven movement, pose unique challenges when attempting to control pathogens such as rabies and canine distemper viruses via vaccination campaigns [5].
One of the most common ticks found on dogs in Africa is the brown dog tick (Rhipicephalus sanguineus sensu lato [s.l.], in particular R. linnaei), which can transmit numerous important pathogens [3, 6, 7]. For example, Ehrlichia canis is the causative agent of canine monocytotropic ehrlichiosis, which may be subclinical or cause multisystemic effects, including fever, anorexia, hemorrhagic tendencies (dermal petechiae and/or ecchymoses), and ophthalmological lesions [8]. Hepatozoon canis causes canine hepatozoonosis, which can be asymptomatic or associated with extreme lethargy, cachexia, and anemia [9]. Babesia vogeli is one of the causative agents of babesiosis in dogs but typically results in only moderate disease or nonclinical infection [10]. Finally, Anaplasma platys causes infectious cyclic thrombocytopenia (ICT) in dogs but rarely in humans. Rhipicephalus sanguineus s.l. is the suspected primary vector of A. platys [7].
Currently, there are few data on tick-borne pathogens in domestic dogs in Chad. These mixed-breed dogs live outdoors in rural villages and are primarily free-roaming and free-foraging, but some are intermittently fed by humans. Few dogs receive regular veterinary or preventative care. Dogs are commonly used to protect livestock and help with hunting. Due to their free-roaming nature, they are exposed to a wide variety of wildlife, livestock, habitats, vectors (i.e. ticks), and pathogens, and their regular interactions with humans can result in both direct and indirect pathogen transmission. Previous studies by this group on domestic Chadian dogs revealed high rates of tick infestation [11], a lack of heartworm (Dirofilaria immitis) infections, and infections with a Brugia sp. that was previously unreported in dogs in Central Africa [12]. To further evaluate the health of these dogs, a survey was conducted of selected tick-borne pathogens of known significance to dog health in Africa. Specifically, this study aimed to conduct surveillance for tick-borne pathogens among domestic dogs in Chad and determine (1) the prevalence of antibodies against Anaplasma spp., Borrelia burgdorferi, and Ehrlichia spp.; (2) the prevalence of Hepatozoon spp., E. canis, A. platys, and Babesia spp.; and (3) associations between these pathogens and demographic, spatial, and temporal factors.
Results
A total of 428 dogs were sampled in May 2019. Of these initial 428 dogs, 314 were again sampled in November 2019, and 257 of these second round 314 were again sampled in June 2020.
Serologic testing with the IDEXX SNAP 4Dx test
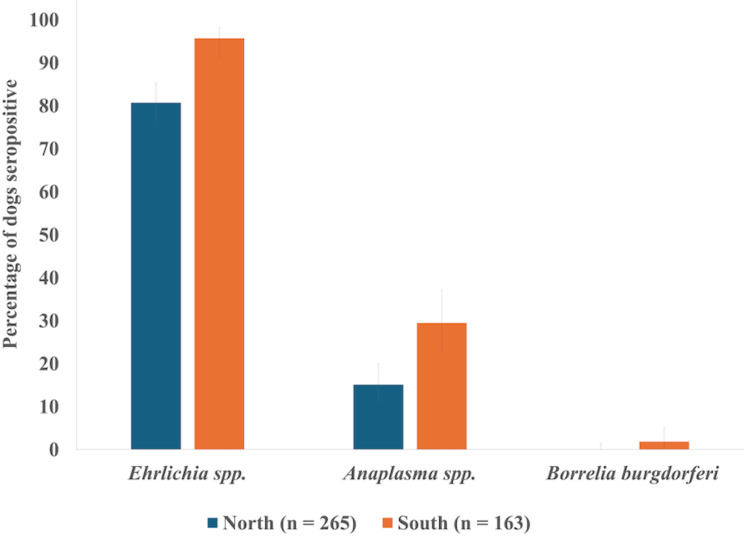
Of the 428 dogs that were tested with the SNAP 4Dx test in May 2019, 86% (n = 370, 95% CI = 83–90%) were positive for antibodies to Ehrlichia spp., 21% (n = 88, 95% CI = 17–25%) were positive for antibodies to Anaplasma spp., and 0.7% (n = 3, 95% CI = 0.1–2%) were positive for antibodies to B. burgdorferi (Table 1; Fig. 1). The results of D. immitis antigen testing and filarial worm molecular testing have been previously reported [12]. Of these 428 dogs, 0.7% (n = 3, 95% CI = 0.1–2%) were positive for all four tests; 6.3% (n = 27, 95% CI = 4–9%) had positive results for Ehrlichia spp., Anaplasma spp., and D. immitis; 19% (n = 80, 95% CI = 15–23%) had positive results for Ehrlichia and D. immitis; and 13% (n = 57, 95% CI = 10–17%) had positive results for Ehrlichia spp. and Anaplasma spp.
Table 1.
Number of dogs seropositive for each of the three pathogen groups based on the IDEXX snap 4DX test
| Pathogen | Total n = 428 |
Region | Sex1 | Age in years2 | |||||
|---|---|---|---|---|---|---|---|---|---|
| North n = 265 |
South n = 163 |
Male n = 263 |
Female n = 164 |
1-1.5 n = 94 |
2-2.5 n = 151 |
3-3.5 n = 113 |
4–5 n = 70 |
||
| Anaplasma spp. | 88 (20.5)3 | 40 (15.1) | 48 (29.4) | 52 (19.8) | 36 (22.0) | 23 (24.5) | 31 (20.5) | 24 (20.5) | 10 (14.3) |
| Borrelia burgdorferi | 3 (0.7) | 0 | 3 (1.8) | 1 (0.4) | 2 (1.2) | 0 | 2 (1.3) | 1 (0.9) | 0 |
| Ehrlichia spp. | 370 (86.4) | 214 (80.8) | 156 (95.7) | 224 (85.2) | 146 (89.0) | 75 (79.8) | 132 (87.4) | 100 (88.5) | 63 (90) |
1Sex was unknown for one dog
2Dogs were aged to the nearest 0.5 years
3Percentages are provided in parentheses
Fig. 1.
Percent of dogs from Chad, Africa (2019–2020), in the northern and southern regions of the study area seropositive for Ehrlichia spp., Anaplasma spp., and Borrelia burgdorferi according to the 4Dx SNAP test. Error bars indicate 95% confidence intervals
Generalized linear regression models revealed that geographic region of origin within the study area was a significant predictor of dogs being seropositive for Ehrlichia spp., with dogs in southern regions being more likely to test positive than those in northern regions (OR = 6.2, 95% CI = 2.6–14.8, p < 0.0001; Table 1; Fig. 1). Region of origin within the study area was also a significant predictor of Anaplasma spp. seropositivity, with dogs in southern regions more likely to have a positive result than dogs in the northern regions (OR = 2.4, 95% CI = 1.5–3.8, p = 0.0004; Table 1; Fig. 1). Neither sex nor age was a significant predictor of dogs testing positive for antibodies against these pathogens (Table 1). Three dogs that were seropositive for B. burgdorferi were from the southern region (two from Dankolo and one from Kaimamba) and included two females and one male (Table 1).
Molecular testing with PCR
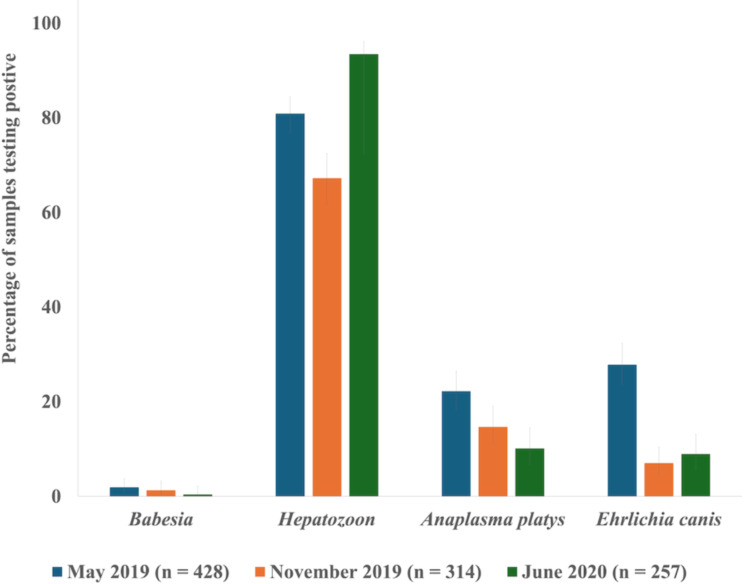
Based on PCR testing, four different pathogens were detected: Hepatozoon spp., B. vogeli, E. canis, and A. platys (Table 2; Fig. 2). The most commonly detected pathogen was Hepatozoon spp. Due to the large number of positive samples, only 83 of the 797 Hepatozoon-positive samples were randomly selected and sequenced; all were 98–100% similar to numerous H. canis sequences in GenBank. Similar prevalence levels of E. canis and A. platys were detected (Fig. 2). A random subset of the E. canis- and A. platys-positive samples (24 out of 164 and 33 out of 166, respectively) was sequenced and confirmed to be the expected pathogens. Thirteen samples across the entire study period were positive for Babesia spp., and sequence analysis of all amplicons confirmed infection with B. vogeli.
Table 2.
Number of dogs whose blood samples were PCR-positive for each of the four pathogens at each of the three time points
| May 2019 | Region | Sex1 | Age in years2 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Pathogen | Total n = 428 |
North n = 265 |
South n = 163 |
Male n = 263 |
Female n = 164 |
1-1.5 n = 94 |
2-2.5 n = 151 |
3-3.5 n = 113 |
4–5 n = 70 |
| Anaplasma platys | 94 (22.0)3 | 54 (20.4) | 40 (24.5) | 55 (20.9) | 39 (23.7) | 26 (27.6) | 42 (27.8) | 19 (16.8) | 7 (10.0) |
| Babesia vogeli | 8 (1.9) | 0 | 8 (4.9) | 4 (1.5) | 4 (2.4) | 2 (2.1) | 5 (3.3) | 0 | 1 (1.4) |
| Ehrlichia canis | 119 (27.8) | 56 (21.1) | 63 (38.6) | 84 (31.9) | 35 (21.3) | 25 (26.6) | 43 (28.5) | 33 (29.2) | 18 (25.7) |
| Hepatozoon canis | 346 (80.8) | 194 (73.2) | 152 (93.2) | 214 (81.37) | 131 (79.9) | 75 (79.8) | 123 (81.5) | 94 (83.2) | 54 (77.1) |
| November 2019 | Region | Sex 1 | Age in years 2 | ||||||
| Pathogen |
Total n = 314 |
North n = 185 |
South n = 125 |
Male n = 191 |
Female n = 119 |
1-1.5 n = 66 |
2-2.5 n = 106 |
3-3.5 n = 85 |
4–5 n = 54 |
| Anaplasma platys | 46 (14.6) | 38 (20.5) | 8 (6.4) | 28 (14.7) | 18 (15.1) | 20 (30.3) | 14 (13.2) | 7 (8.2) | 5 (9.3) |
| Babesia vogeli | 4 (1.3) | 0 | 4 (3.2) | 1 (0.5) | 3 (2.5) | 0 | 2 (1.9) | 2 (2.4) | 0 |
| Ehrlichia canis | 22 (7.0) | 17 (9.2) | 5 (4) | 14 (7.3) | 8 (6.7) | 3 (4.5) | 8 (7.5) | 7 (8.2) | 4 (7.4) |
| Hepatozoon canis | 211 (67.2) | 161 (87.0) | 50 (40) | 137 (71.7) | 73 (61.3) | 50 (75.8) | 67 (63.2) | 54 (63.5) | 40 (74.1) |
| June 2020 | Region | Sex | Age in years 2 | ||||||
| Pathogen |
Total n = 257 |
North n = 159 |
South n = 98 |
Male n = 157 |
Female n = 97 |
1-1.5 n = 51 |
2-2.5 n = 84 |
3-3.5 n = 77 |
4–5 n = 42 |
| Anaplasma platys | 26 (10.1) | 19 (11.9) | 7 (7.1) | 16 (10.2) | 10 (10.3) | 8 (15.7) | 7 (8.3) | 8 (10.4) | 3 (7.1) |
| Babesia vogeli | 1 (0.4) | 0 | 1 (1.0) | 0 | 1 (1.0) | 0 | 0 | 1 (1.3) | 0 |
| Ehrlichia canis | 23 (9.0) | 13 (8.2) | 10 (10.2) | 16 (10.2) | 6 (6.2) | 4 (7.8) | 8 (9.5) | 9 (11.7) | 1 (2.4) |
| Hepatozoon canis | 240 (93.4) | 147 (92.5) | 93 (94.9) | 147 (93.6) | 91 (93.8) | 45 (88.2) | 82 (97.6) | 70 (90.9) | 41 (97.6) |
1Sex was unknown for one dog sampled in May and November 2019
2Dogs were aged to the nearest 0.5 years
3Percentages are provided in parentheses
Fig. 2.
Percent of blood samples from domestic dogs in Chad, Africa, testing positive according to PCR for each of the four pathogens across the three time points in 2019 and 2020. The error bars show 95% confidence intervals
Dog sex, geographic region, sampling time, and season were significant predictors of the presence of Hepatozoon spp. The best mixed-effects generalized linear regression model included the interaction between region and sampling time (Table 3). According to this model, dogs in the northern region were more likely to test positive for Hepatozoon spp. in November 2019 and June 2020 than in May 2019. Dogs in the southern region were more likely to test positive in May 2019 and June 2020 than in November 2019 (Table 4).
Table 3.
AICc table of generalized linear models predicting the detection of Hepatozoon spp. in Chadian dogs in 2019 and 2020 based on region of origin (northern vs. southern), time point of testing, season of testing, and dog age and sex
| Model | K1 | AICc2 | ΔAICc3 | ωi4 |
|---|---|---|---|---|
| Region * time point | 7 | 833.5 | 0.00 | 0.504 |
| Sex + region * time point | 9 | 833.5 | 0.02 | 0.496 |
| Sex + region + time point | 7 | 940.7 | 107.23 | 0.000 |
| Region + time point | 5 | 941.7 | 108.22 | 0.000 |
| Sex + time point | 6 | 943.3 | 109.82 | 0.000 |
| Time point | 4 | 945.4 | 111.97 | 0.000 |
| Sex + region + season | 6 | 961.8 | 128.33 | 0.000 |
| Region + season | 4 | 961.8 | 128.36 | 0.000 |
| Sex + season | 5 | 964.2 | 130.73 | 0.000 |
| Season | 3 | 965.4 | 131.99 | 0.000 |
| Sex + region | 5 | 1001.8 | 168.33 | 0.000 |
| Region | 3 | 1002.3 | 168.89 | 0.000 |
| Sex | 4 | 1004.8 | 171.32 | 0.000 |
| Null | 2 | 1006.7 | 173.22 | 0.000 |
1K = number of parameters
2AICc = second-order Akaike information criterion
3ΔAICc = difference in AICc between ranked models
4ωi = Akaike weight
Table 4.
Odds ratios with 95% confidence intervals for the detection of Hepatozoon spp. based on the top-ranked linear regression model incorporating the interaction of region and time point of sampling. Only significant pairwise comparisons are shown
| Level | vs. | Odds Ratio | 95% CI lower limit | 95% CI upper limit | P-value |
|---|---|---|---|---|---|
| North May 2019 | South November 2019 | 5.39 | 2.34 | 12.41 | < 0.0001 |
| South May 2019 | North May 2019 | 6.11 | 2.10 | 17.77 | < 0.0001 |
| South May 2019 | South November 2019 | 32.91 | 9.62 | 112.57 | < 0.0001 |
| North November 2019 | North May 2019 | 2.60 | 1.19 | 5.65 | 0.0063 |
| North November 2019 | South November 2019 | 13.98 | 5.07 | 38.61 | < 0.0001 |
| North June 2020 | North May 2019 | 4.95 | 1.84 | 13.30 | 0.0001 |
| South June 2020 | North May 2019 | 7.47 | 1.80 | 30.98 | 0.0008 |
| North June 2020 | South November 2019 | 26.65 | 7.93 | 89.55 | < 0.0001 |
| South June 2020 | South November 2019 | 40.32 | 8.59 | 188.46 | < 0.0001 |
The dog age and time point of sampling were found to be significant predictors of A. platys detection, and the best model included the additive effects of these two factors (Table 5). According to this model, younger dogs were more likely to test positive, and dogs sampled in May 2019 were more likely to test positive for A. platys than were those sampled in November 2019 (OR = 1.65, 95% CI = 1.02–2.70, p = 0.0395) and June 2020 (OR = 2.54, 95% CI = 1.42–4.55, p = 0.0005).
Table 5.
AICc table of generalized linear models predicting the detection of Anaplasma platys in Chadian dogs in 2019 and 2020 based on region of origin (northern vs. southern), time point of testing, season of testing, and dog age and sex
| Model | K1 | AICc2 | ΔAICc3 | ωi4 |
|---|---|---|---|---|
| Age + time point | 5 | 863.2 | 0.00 | 0.999 |
| Age | 3 | 876.3 | 13.10 | 0.001 |
| Time point | 4 | 884.8 | 21.63 | 0.000 |
| Null | 2 | 899.3 | 36.09 | 0.000 |
1K = number of parameters
2AICc = second-order Akaike information criterion
3ΔAICc = difference in AICc between ranked models
4ωi = Akaike weight
The significant predictors of E. canis infection were dog sex, region, time point of sampling, and season. The top model included the additive effects of dog sex and the interaction between region and time point of sampling (Table 6). In general, based on this model, there were greater odds of E. canis being detected in both regions in May 2019. There were no significant pairwise comparisons according to sex (Table 7). Dog sex, age, geographic region, timing of sampling, and season were not significant predictors of Babesia spp. infection.
Table 6.
AICc table of generalized linear models predicting the detection of Ehrlichia canis in Chadian dogs in 2019 and 2020 based on region of origin (northern vs. southern), time point of testing, season of testing, dog age, and dog sex
| Model | K1 | AICc2 | ΔAICc3 | ωi4 |
|---|---|---|---|---|
| Sex + region * time point | 9 | 804.5 | 0.00 | 0.788 |
| Region * time point | 7 | 807.2 | 2.73 | 0.201 |
| Sex + region + time point | 7 | 813.6 | 9.13 | 0.008 |
| Region + time point | 5 | 816.4 | 11.97 | 0.002 |
| Sex + time point | 6 | 819.9 | 15.40 | 0.000 |
| Time point | 4 | 821.5 | 17.00 | 0.000 |
| Sex + region + season | 6 | 853.3 | 48.88 | 0.000 |
| Region + season | 4 | 856.1 | 51.66 | 0.000 |
| Sex + season | 5 | 859.5 | 55.02 | 0.000 |
| Season | 3 | 860.8 | 56.31 | 0.000 |
| Sex + region | 5 | 887.7 | 83.24 | 0.000 |
| Region | 3 | 890.6 | 86.11 | 0.000 |
| Sex | 4 | 892.9 | 88.44 | 0.000 |
| Null | 2 | 894.3 | 89.81 | 0.000 |
1K = number of parameters
2AICc = second-order Akaike information criterion
3ΔAICc = difference in AICc between ranked models
4ωi = Akaike weight
Table 7.
Odds ratios with 95% confidence intervals for the detection of Ehrlichia canis based on the top-ranked linear regression model incorporating the effects of dog sex and the interaction between region and time point of sampling. Only significant pairwise comparisons are shown
| Level | Vs | Odds Ratio | 95% CI lower limit | 95% CI upper limit | P value |
|---|---|---|---|---|---|
| South May 2019 | North May 2019 | 2.99 | 1.35 | 6.59 | 0.0011 |
| North May 2020 | North November 2019 | 3.02 | 1.22 | 7.48 | 0.0067 |
| North May 2021 | South November 2019 | 7.13 | 1.70 | 29.90 | 0.0013 |
| North May 2022 | North June 2020 | 3.49 | 1.29 | 9.46 | 0.0047 |
| South May 2019 | North November 2019 | 9.03 | 3.16 | 25.82 | < 0.0001 |
| South May 2020 | South November 2019 | 21.29 | 4.90 | 92.54 | < 0.0001 |
| South May 2021 | North June 2020 | 10.43 | 3.37 | 32.26 | < 0.0001 |
| South May 2022 | South June 2020 | 7.58 | 2.27 | 25.35 | < 0.0001 |
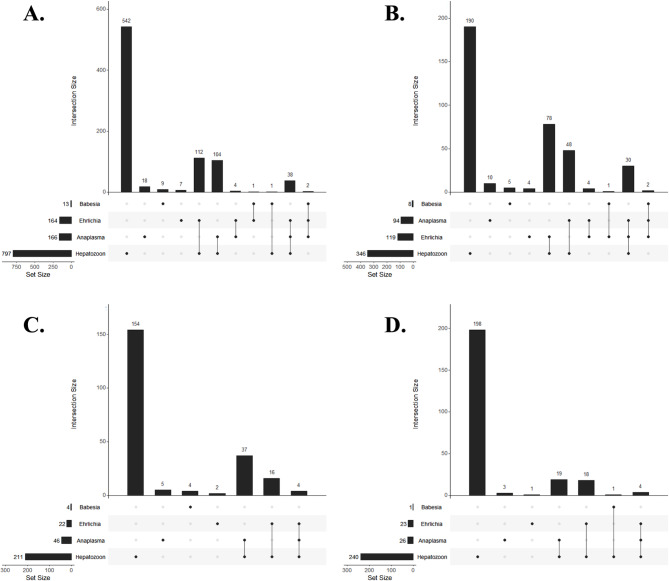
PCR revealed that 26% of the 999 samples tested (n = 262, 95% CI = 24–29%) had two or three pathogens detected (Fig. 3). The most common coinfection overall was Hepatozoon spp. with E. canis (Fig. 3A), and compared with those in November 2019 and May 2020, coinfections were most common, with the highest number of pathogen combinations occurring in May 2019 (Fig. 3B-D). Out of all the samples, 4% (n = 40, 95% CI = 3–5%) were positive for three pathogens; of these 40 samples, 38 were positive for A. platys, E. canis, and Hepatozoon spp., and two were positive for A. platys, E. canis, and B. vogeli. The latter combination was found only in May 2019. Out of all the samples, 22% (n = 222, 95% CI = 20–25%) were positive for two pathogens; of these 222 samples 112 were positive for E. canis and Hepatozoon; 104 were positive for A. platys and Hepatozoon spp.; four were positive for A. platys and E. canis; one was positive for B. vogeli and E. canis; and one was positive for B. vogeli and Hepatozoon (Fig. 3A). Considering longitudinal trends, no dogs were positive for B. vogeli at multiple time points; however, 33% of the 428 dogs sampled (n = 142, 95% CI = 29–38%) were positive for Hepatozoon twice, and 30% (n = 128, 95% CI = 26–34%) were positive at all three time points. Fewer dogs were positive for A. platys twice (n = 20, 5%, 95% CI = 3–7%) or at all three time points (n = 2, 0.5%, 95% CI = 0.06–1.7%), while 5% of dogs were positive for E. canis twice (n = 20, 95% CI = 3–7%) and 0.2% (n = 1, 95% CI = 0.1–1.3%) were positive at all three time points. Multiple positive detections over time may represent persistent infection or re-infection.
Fig. 3.
Frequency of blood samples from domestic dogs in Chad, Africa, testing positive for each of four pathogens, as determined via PCR, individually and in combination, in 2019 and 2020. (A) Data combined across all three time points; (B) May 2019; (C) November 2019; (D) June 2020
Discussion
Tick-borne pathogens represent an important One Health issue, as many can cause disease in domestic and agricultural animals, wildlife, and humans. The present study found evidence of exposure to and/or infection with numerous tick-borne pathogens in dogs from Chad. Of these pathogens A. platys, B. burgdorferi, and Ehrlichia spp., are, or have the potential to be, zoonotic, and many of the tick species found on Chadian dogs also infest humans [13–16]. Additional studies are needed in Chad to monitor the prevalence and transmission of these pathogens, specifically, to understand the risks they pose to the health of domestic animals and humans.
Similar to other studies of African domestic dogs, Hepatozoon spp., specifically H. canis, was the most common pathogen detected, with a 40–94% prevalence depending on the region and time point of sampling (e.g [3, 17–19]). A multi-country study revealed a commensurately high prevalence of H. canis (average of 59%; Tanzania: 67–77%; Kenya: 54–85%; Uganda: 86–98%; Nigeria: 26–56%; Ghana: 46–68%; Namibia: 9–29%) [3]. Other studies also found a high prevalence in Sudan (42%), Ghana (40%), and Nigeria (41%) [17–19]. This characteristically high prevalence with wide distribution has been attributed to a large number of known vectors, including Rhipicephalus spp., and the potential for vertical transmission to puppies [20–22].
The detection of antibodies against Ehrlichia spp. and the molecular detection of E. canis were not surprising, as this pathogen has been reported in dogs from Chad and other African countries. Moreover, most dogs in this study were infested with R. sanguineus s.l [11], the primary vector of E. canis [3, 16–19, 23–29]. The 86% seroprevalence of Ehrlichia spp. in Chadian dogs in this study was considerably greater than that in a previous study in Chad (5/18 clinically normal military dogs, 28%) and against comparable studies in Ghana (21–35%), Sierra Leone (40%), and Nigeria (32–54%); however, the data in this study were consistent with those of a study from Senegal (89%) [3, 18, 24–26]. Generally, the prevalence of E. canis antibodies in southern and eastern African countries was lower (e.g., Tanzania: 29–32%; Kenya: 15–22%; Uganda 4–10%; Namibia: 25–40%) [16]; however, variation does exist, and higher prevalence rates have been reported (e.g., 73% in Zimbabwe, 96% in Sudan, and 87% among sick dogs in Namibia) [26–28]. The PCR prevalence of E. canis in Chadian dogs ranged from 4 to 39%, depending on the region and time point of sampling, similar to the findings of studies in numerous other sub-Saharan African countries, including neighboring Nigeria [19, 29]. While 86.4% of dogs were seropositive for Ehrlichia spp. in May 2019, only 27.8% were confirmed to be actively infected with E. canis at that time. A similar trend was noted in dogs from Zimbabwe, as well as several other southern and sub-Saharan African countries [3, 28]. This can be explained by dogs having been infected previously and cleared the infection but still having antibodies present in the blood. Alternatively, these animals may have been infected by an Ehrlichia spp. other than E. canis, e.g., with E. ewingii, E. ruminantium, E. chaffeensis, and potentially new species of Ehrlichia also reported in West Africa [1]. Variation in prevalence similar to that documented in this study has been observed among other western and sub-Saharan African countries, including 20% of dogs from Ghana, 7.3% of dogs from the Ivory Coast, 12.7% of dogs from Nigeria, and 6.4% of dogs from Algeria [18, 19, 30, 31].
Canine cyclic thrombocytopenia, caused by A. platys, is a significant disease of dogs in many regions of the world, and similar to E. canis, R. sanguineus s.l. is a suspected vector [1, 6, 7]. This pathogen is also a rare zoonosis [13]. This study’s finding of 21% seroprevalence for Anaplasma spp. among dogs in Chad is comparable to that in other countries in southern and sub-Saharan Africa, e.g., Ghana: 0–30%; Sierra Leone: 19%; Kenya: 8–10%; Nigeria: 4–20%; Tanzania: 20–21%; Uganda: 4–24%; and Namibia: 8–23% [3, 18, 25]. In Zimbabwe, 10% of 225 samples were seropositive [28]. Interestingly, in May 2019, only 20.5% of the dogs were seropositive for Anaplasma spp., while 22% of the dogs were PCR positive for A. platys. This difference is likely due to recently infected animals not having yet mounted an antibody response, as the response is first detectable 16 days after infection [32]. In the current study, the prevalence of A. platys via PCR varied from 6 to 24%, depending on the region and time point of sampling, which is similar to the findings in nearby countries in sub-Saharan Africa (Kenya (10–23%), Ghana: 10%; Ivory Coast: 1.5% and 0–30%; Gabon: 1.2%; and Nigeria: 6.6%), as well as northern Africa (Algeria: 5.4%) [7, 18, 19, 30, 31].
A small number of the dogs sampled in this study (n = 13) were positive for B. vogeli, one of three closely related canine species including B. canis, B. rossi, and B. vogeli that are distinguished by biological characteristics and molecular methods [10, 33]. For example, B. rossi is transmitted by Haemaphysalis spp., and infection is typically fatal, while B. vogeli is transmitted by R. sanguineus s.l. and is considered the least pathogenic [10]. The prevalence of Babesia spp. in dogs in sub-Saharan Africa varies considerably from 0 to 12% depending on country and rural vs. urban area [3]. In countries neighboring Chad, 9% of dogs from Sudan were positive for Babesia spp. (five with B. rossi and two with B. vogeli) [17], and both B. rossi and B. vogeli have been detected in dogs in Nigeria, but B. rossi was more common [19, 34, 35]. The lack of B. rossi in the dogs in this study may be due to the low number (n = 14) of dogs infested with Haemaphysalis leachii [16]. Additional studies to determine the distribution and factors related to the presence and intensity of H. leachii are needed to better understand the risk of severe babesiosis to the health of dogs in Chad.
The high number of dogs that were positive for antibodies against both Anaplasma spp. and Ehrlichia spp. is not surprising, given that both pathogens are transmitted by R. sanguineus s.l [6, 25]. Among 53 dogs from Sierra Leone tested with the SNAP 4Dx test, 9.4% were positive for both Ehrlichia spp. and Anaplasma spp. antibodies, and 5.7% were positive for Ehrlichia spp. antibodies, Anaplasma spp. antibodies, and D. immitis [25]. Furthermore, antibodies against these pathogens have been shown to persist for months to years [36, 37]. Coinfections with two or three pathogens were detected by PCR in 24.2% of the samples in the current study, with the most common combination being Hepatozoon spp. and Ehrlichia canis, and 4.0% of the samples had three pathogens detected. This finding is similar to that of a multinational study of African dogs, in which 30.9% of the dogs were coinfected with at least two pathogens, the most common combination (10.1%) being H. canis and E. canis, and 5.1% of the dogs had three or four pathogens in their blood [3]. Coinfections are not surprising given that these pathogens share at least one tick vector group, Rhipicephalus sanguineus s.l., and these ticks were commonly detected on dogs in this study.
There were several significant spatiotemporal and demographic factors associated with the detection of exposure or infection with multiple pathogens included in this study. Dogs in the southern region were more likely to be seropositive for Ehrlichia spp. and Anaplasma spp. and to be infected with Hepatozoon spp. and E. canis than dogs in the northern region were. This may be explained by climate variation within Chad, with differences between regions that can impact tick populations: the northern study areas are more arid, and the southern region of Chad receives more rainfall [38]. For all three pathogens (Hepatozoon, A. platys and E. canis), the time point was a significant predictor of detection. In the northern region, Hepatozoon spp. were more likely to be detected later in the study (November 2019 and June 2020 > May 2019), whereas in the southern region, Hepatozoon spp. were more likely to be detected earlier in the study (May 2019 and June 2020 > November 2019). Overall, A. platys and E. canis were more likely to be detected earlier in the present study (May 2019 vs. November 2019 and June 2020). Ehrlichia canis infection typically occurs during the dry-hot season when the tick Rhipicephalus sanguineus s.l. is active [8]. It is also possible that the removal of ticks from the study dogs at the three time points may have reduced the pathogen prevalence at later time points, as ticks were no longer present to transmit the pathogens of interest; however, that only represented a few days throughout the year.
Dog age was a significant predictor for the detection of A. platys by PCR. The finding that younger dogs were more likely to be infected with A. platys agrees with the findings of previous work in Kenya and Ivory Coast that showed a 19.8% prevalence in dogs younger than one year, compared to 6.7% in adult dogs [7]. This supports the finding that dogs were more likely to be infected in May 2019 than at the two later time points of the study, as dogs were youngest at the first time point of the study. Furthermore, while infections with A. platys persist for months, dogs may clear infections after 100–150 days [32]. This is consistent with the finding that dogs were more often positive for A. platys at two consecutive time points (14 dogs) than at the first and third time points (six dogs) or at all three time points (two dogs).
The detection of antibodies against B. burgdorferi was unexpected based on the historical range of this pathogen in North America and Eurasia and its predominant association with Ixodes spp. ticks [15]. However, there are sporadic reports of this pathogen outside the expected range. In Africa, 1.4% of dogs in rural Kenya were seropositive for Borrelia spp [3], and a single dog in Egypt and an associated R. sanguineus s.l. tick tested positive via PCR [39]. Another study in Egypt detected B. burgdorferi via PCR in 23% of dogs (n = 26), 16% of cattle (n = 25), 58% of dog-associated R. sanguineus s.l. (n = 12), and 21% of bovine-associated Hyalomma anatolicum excavatum (n = 14) [40]. Although no Ixodes were found on any of the dogs in this study, the three B. burgdorferi- positive dogs were infested with R. sanguineus s.l. In addition to the true exposure of Chadian dogs to B. burgdorferi, there are other possible explanations for these findings. It is possible that these results represent cross-reaction with other Borrelia spp. or false positives. In Africa, relapsing fever group (RFG) Borrelia spp., such as B. recurrentis in countries east of Chad and B. crocidurae in countries north of Chad, have been reported, but rarely do RFG Borrelia cross-react with C6-based serologic tests [41, 42]. However, only a limited number of Borrelia species have been evaluated, so it is possible that some RFG Borrelia may cross-react. Additionally, a novel lineage of Borrelia, distinct from both the relapsing fever and Lyme disease groups, has been reported in Amblyomma spp. from Ethiopia and the Ivory Coast, including A. variegatum, a tick species found on dogs in Chad [16, 43, 44]. The cross-reactivity of this group with B. burgdorferi C6 assays is not known.
Aspects of this study limit the conclusions that can be drawn from the data. Importantly, ticks were opportunistically collected from dogs enrolled in an experimental therapeutic trial for the treatment and prevention of Guinea worms (Dracunculus medinensis) [13]. Therefore, sample size calculations and counts of total tick burden per dog were not performed, which limits the interpretability of the results. Additionally, there were only three time points of sample collection, and the SNAP 4DX tests were performed only at the first time point. More robust conclusions about prevalence trends could be drawn from data collected over many years, with multiple years of sampling during each season. Another limitation was that outwardly sick dogs were excluded based on the primary study criteria; therefore, analyzing data for associations between pathogen detection and clinical illness was not possible. Moreover, the number of dogs sampled decreased over time as dogs either died or moved with their owners away from the village where they were originally sampled. In addition, the ticks found on each dog were removed for subsequent testing at each time point, potentially reducing pathogen transmission and impacting prevalence estimates at later time points. Finally, animals testing positive for a pathogen multiple times over the course of the study may represent persistent infection or re-infection with that pathogen. This cannot be differentiated by the methods of this study, so these repeat positives were included in the statistical analysis to represent the probability of a given dog testing positive at each time point. However, a positive test for a pathogen at an earlier time point may influence the status of that dog at later points.
Conclusions
In summary, this study found that many domestic dogs in Chad had evidence of exposure to and/or infection with multiple pathogens, including E. canis, A. platys, B. burgdorferi, B. vogeli, and Hepatozoon spp. (some confirmed to be H. canis). Given the high prevalence of several pathogens in dogs (H. canis, E. canis, and A. platys), veterinarians in Chad should consider tick-borne diseases in dogs that present with appropriate clinical signs or abnormalities. Dog owners should also be encouraged to use appropriate preventatives to limit exposure to ticks and other vectors. Given that some of these pathogens are known, or are suspected to be, zoonoses, this study presents a One Health approach to understanding pathogen dynamics in Chad and indicates that additional work is needed to understand the risks these pathogens pose to domestic animals, wildlife, and humans.
Materials and methods
Sample collection
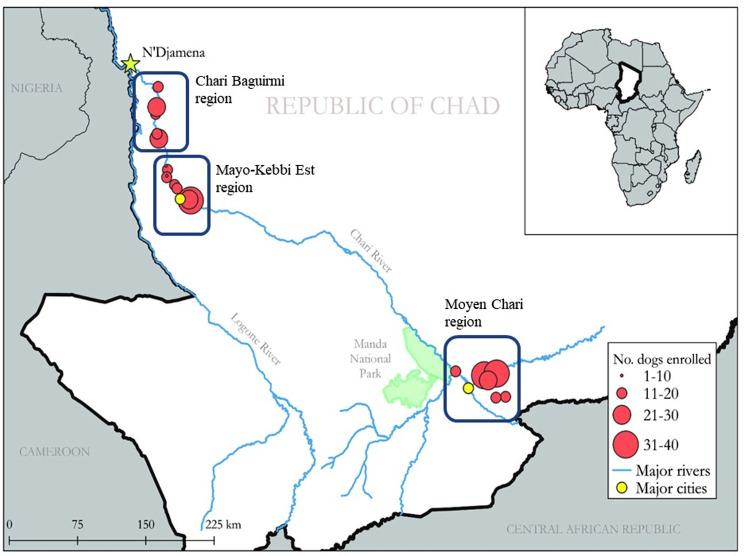
Blood samples were serially collected from the same individual domestic dogs in Chad, Africa, at three time points: May 2019, November 2019, and June 2020. In Chad, May and June are during the wet season, and November is the dry season. As part of a concurrent study [45], dogs were sampled in 23 villages from three regions (Moyen-Chari, Chari Baguirmi, and Mayo-Kebbi Est) (Fig. 4) based on the following criteria: owner approval for sample collection; dog age between one and five years; dogs lacking overt signs of significant illness; and dog demeanor allowing approach and restraint for sampling. The sex, age (estimated to the nearest 0.5 years), and village of origin of each dog were recorded.
Fig. 4.
Map of the study area in Chad, Africa, showing the regions from which dogs were sampled. This map was previously published by Cleveland et al. (2022) under the terms “creative common attribution” (CC-BY) license (https://creativecommons.org/licenses/by/4.0/) and has not been modified from its original form
Venipuncture sites were aseptically prepared with 70–90% EtOH, and a blood sample (~ 0.7 mL) was collected from the cephalic vein. Blood was placed in 3 mL EDTA vacutainer tubes (Becton, Dickinson and Company, Franklin Lakes, New Jersey, USA) and stored in a field cooler with an ice pack. Upon return to the field laboratory, ~ 125 µL of blood was transferred to Whatman™ FTA™ cards (Cytiva, Marlborough, Massachusetts, USA) for pathogen screening. In May 2019, whole blood was tested for antibodies against Anaplasma spp., Ehrlichia spp., and Borrelia burgdorferi, as well as Dirofilaria immitis antigens, using an IDEXX SNAP 4Dx test (IDEXX Laboratories, Portland, Maine, USA) per the manufacturer’s instructions. All animal procedures were reviewed and approved by the University of Georgia’s Institutional Animal Care and Use Committee (A2019 04–005), the Chad Ministry of Health, and the Institut de Recherche en Elevage pour le D´eveloppement (IRED), which is the research institution in charge of animal research in Chad and authorized by the Ministry of Livestock and Animal Production.
DNA extraction and molecular assays
DNA was extracted from the FTA cards according to the manufacturer’s protocol using a commercially available DNA extraction kit (QIAamp DNA Investigator Kit, Qiagen, Valencia, CA, USA) [46]. DNA was screened for four pathogens using the PCR protocols in Table 8. Two different PCR protocols were used to detect Babesia spp. and Hepatozoon spp. based on different gene targets: 18 S PCR provided sequences for species-level identification, while ITS PCR produced amplicons of different sizes for the two genera, allowing genus-level differentiation based on the band location on the gel rather than requiring sequencing for every amplicon. For all the assays, the amplicons were purified from a 0.8% agarose gel stained with Gel Red (Biotium, Inc., Hayward, California, USA) using a commercial gel purification kit (Qiagen). Bidirectional Sanger sequencing was conducted by Genewiz (South Plainfield, New Jersey, USA), and the sequences were edited and assembled using Geneious 10.2.6 (Biomatters Limited, Auckland, New Zealand). The consensus sequences were subsequently used as queries for BLASTN searches against the National Center for Biotechnology Information (NCBI) GenBank nucleotide sequence database.
Table 8.
PCR protocols used to screen blood samples from domestic dogs in Chad, Africa
| Pathogen | Gene Target (size in bp) | Primers | Primer Sequence (5’➔ 3’) | Reference |
|---|---|---|---|---|
|
Babesia spp. and Hepatozoon spp. |
18 S rRNA (500 for Babesia, 600 for Hepatozoon) | Primary: 5.1 / B |
CCTGGTTGATCCTGCCAGTAGT TGATCCTTCTGCAGGTTCACCTAC |
[47, 48] |
| Secondary: Babesia F / Babesia R |
GTGAAACTGCGAATGGCTCA CCATGCTGAAGTATTCAAGAC |
|||
| Internal Transcribed Spacer rRNA (ITS) (300 for Babesia, < 200 for Hepatozoon) | Primary: 15 C / 13B |
CGATCGAGTGATCCGGTGAATTA GCTGCGTCCTTCATCGTTGTG |
[50] | |
| Secondary: 15D / 13 C |
AAGGAAGGAGAAGTCGTAACAAGG TTGTGTGAGCCAAGACATCCA |
[51] | ||
| Ehrlichia canis | 16 S rRNA (389) | Primary: ECC / ECB | AGAACGAACGCTGGCGGCAAGCC CGTATTACCGCGGCTGCTGGCA | [52] |
| Secondary: ECA / HE3 |
CAATTATTTATAGCCTCTGGCTATAGG TATAGGTACCGTCATTATCTTCCCTAT |
[52, 53] | ||
| Anaplasma platys | 16 S rRNA (400) | Primary: ECC / ECB |
AGAACGAACGCTGGCGGCAAGCC CGTATTACCGCGGCTGCTGGCA |
[52] |
| Secondary: PLA2 / GA1UR |
TTTGTCGTAGCTTGCTATG- GAGTTTGCCGGGACTTCTTCT |
[54, 55] |
Statistical analyses
The prevalence of each pathogen, with corresponding 95% confidence intervals (CIs), was calculated for dogs with positive results for each pathogen or group of pathogens on the SNAP 4Dx test and for dogs with positive PCR results for each pathogen at each time point. Generalized linear regression models (function glm in the R package stats [56]) were used to predict SNAP-positivity based on dog age (continuous variable) and sex, as well as geographic region of origin within the study area (south [Moyen-Chari region] or north [Chari Baguirmi and Mayo-Kebbi Est regions]). A series of mixed-effects generalized linear regression models were created using the function glmer in the R package lme4 [57] to predict the PCR results for each pathogen based on the fixed effects of dog age, sex, geographic region of origin, time point of sampling, season (May and June during the wet season and November during the dry season), and the random effect of dog ID to account for repeated sampling of the same dogs. Predictors with p > 0.2 in univariable models were included in a set of multivariable models examining the additive and interactive effects of significant predictors. Models were evaluated using an information theoretic approach [58]. Statistical analyses were performed in RStudio version 2022.07.0 [59], with statistical significance assessed at α = 0.05.
Acknowledgements
The authors thank the team members of Afrique One ASPIRE (http://afriqueoneaspire.org/), Institut de Recherche en Elevage pour le Developpement (IRED) for their outstanding contributions in facilitating this work in the field, as well as the technical staff of The Carter Center and the Programme National d’Eradication du Ver de Guinee, Ministry of Health, N’Djamena, Chad for their support. In particular, Hubert Zirimwabagabo, Mario Romero, and Karmen Unterwegner at The Carter Center are acknowledged for supporting and facilitating this research. Finally, thanks to IDEXX Laboratories, Inc. for providing the SNAP tests used in this study.
Author contributions
EH analyzed the data and was a major contributor to the writing of the manuscript. KG and RG conducted the laboratory analyses. JB, MS, and CC conducted field work, and CC oversaw the project, was a supervisor, and was a major contributor to the writing of the manuscript. PTO and BNRN provided approval, oversight and field assistance. MJY oversaw the project, was a supervisor and was a major contributor to the writing of the manuscript. All the authors read and approved the final manuscript.
Funding
This work was supported by The Carter Center as part of concurrent guinea worm research in Chadian dogs. A full listing of Carter Center supporters is available at http://www.cartercenter.org/donate/corporate-government-foundation-partners/index.html. Additional support was provided by the wildlife management agencies of the Southeastern Cooperative Wildlife Disease Study member states through the Federal Aid to Wildlife Restoration Act (50 Stat. 917).
Data availability
All the data generated or analyzed in this study are included in the article, and any additional inquiries can be directed to the corresponding author.
Declarations
Ethics approval and consent to participate
All procedures involving dogs were reviewed and approved by the University of Georgia’s IACUC committee (A2019 04–005). Details on dog enrollment, consent, and sampling are provided in Cleveland et al. [13]; however, briefly, dogs were selected for study inclusion based on assessments of affect, age, physical condition, and owner consent. Assessments were conducted by project veterinarians and took place on site upon arrival in each study village. Passing assessments were required to secure qualification. Owner consent was required for each dog enrolled in the study. The owners were provided an explanation of the study, highlighting aspects of participation in both written and oral forms. Once informed and questions answered, owners signed the study consent form.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Ellen Haynes, Email: Ellen.haynes@uga.edu.
Michael J. Yabsley, Email: myabsley@uga.edu
Christopher A. Cleveland, Email: ccleve@uga.edu
References
- 1.Diarra AZ, Kelly P, Davoust B, Parola P. Tick-borne diseases of humans and animals in West Africa. Pathogens. 2023;12(11):1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Leger E, Vourch G, Vial L, Chevillon C, McCoy KD. Changing distributions of ticks: causes and consequences. Exp Appl Acarol. 2013;59(1–2):219–44. [DOI] [PubMed] [Google Scholar]
- 3.Heylen D, Day M, Schunack B, Fourie J, Labuschange M, Johnson S, et al. A community approach of pathogens and their arthropod vectors (ticks and fleas) in dogs of African Sub-sahara. Parasit Vectors. 2021;14(1):576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu Y, Nordone SK, Yabsley MJ, Lund RB, McMahan CS, Gettings JR. Quantifying the relationship between human Lyme disease and Borrelia burgdorferi exposure in domestic dogs. Geospat Health. 2019;14(1):111–20. [DOI] [PubMed] [Google Scholar]
- 5.Taylor LH, Wallace RM, Balaram D, Lindenmayer JM, Eckery DC, Mutonono-Watkiss B, et al. The role of dog population management in rabies elimination: a review of current approaches and future opportunities. Front Vet Sci. 2017;4:109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dantas-Torres F. The brown dog tick, Rhipicephalus sanguineus (Latreille, 1806) (Acari: Ixodidae): from taxonomy to control. Vet Parasitol. 2008;152(3–4):173–85. [DOI] [PubMed] [Google Scholar]
- 7.Matei IA, D’Amico G, Yao PK, Ionica AM, Kanyari PW, Daskalaki AA, et al. Molecular detection of Anaplasma platys infection in free-roaming dogs and ticks from Kenya and Ivory Coast. Parasites Vectors. 2016;9:157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harrus S, Waner T. Diagnosis of canine monocytotropic ehrlichiosis (Ehrlichia canis): an overview. Vet J. 2011;187(3):292–6. [DOI] [PubMed] [Google Scholar]
- 9.Baneth G, Mathew JS, Shkap V, Macintire DK, Barta JR, Ewing SA. Canine hepatozoonosis: two disease syndromes caused by separate Hepatozoon spp. Trends Parasitol. 2003;19(1):27–31. [DOI] [PubMed] [Google Scholar]
- 10.Zahler M, Schein E, Rinder H, Gothe R. Characteristic genotypes discriminate between Babesia canis isolates of differing vector specificity and pathogenicity to dogs. Parasitol Res. 1998;84:844–548. [DOI] [PubMed] [Google Scholar]
- 11.Cleveland CA, Friedman M, Thompson AT, Haynes E, Coker SM, Bryan JA et al. One health in practice: multi-season survey of ixodid tick species collected from domestic dogs in Chad. bioRxiv. 2024:2024.06.05.597634.
- 12.Haynes E, Cleveland CA, Garrett KB, Grunert RKA, Bryan IIJA, Sidouin M, et al. Characterization of the genetics and epidemiology of Brugia sp. in domestic dogs in Chad, Africa. Vet Parasitol Reg Stud Rep. 2022;35:100784. [DOI] [PubMed] [Google Scholar]
- 13.Arraga-Alvarado CM, Qurollo BA, Parra OC, Berrueta MA, Hegarty BC, Breitschwerdt EB. Case report: molecular evidence of Anaplasma platys infection in two women from Venezuela. Am J Trop Med Hyg. 2014;91(6):1161–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Perez M, Bodor M, Zhang C, Xiong Q, Rikihisa Y. Human infection with Ehrlichia canis accompanied by clinical signs in Venezuela. Ann N Y Acad Sci. 2006;1078:110–7. [DOI] [PubMed] [Google Scholar]
- 15.Hussain S, Hussain A, Aziz U, Song B, Zeb J, George D, et al. The role of ticks in the emergence of Borrelia burgdorferi as a zoonotic pathogen and its vector control: a global systemic review. Microorganisms. 2021;9(12):2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cleveland CA, Friedman M, Haynes E, Coker SM, Bryan II JA, Sidouin M et al. Multi-season survey of ixodid tick species collected on domestic dogs in Chad, Africa. In preparation.
- 17.Oyamada M, Davoust B, Boni M, Dereure J, Bucheton B, Hammad A, et al. Detection of Babesia canis rossi, B. canis vogeli, and Hepatozoon canis in dogs in a village of eastern Sudan by using a screening PCR and sequencing methodologies. Clin Diagn Lab Immunol. 2005;12(11):1343–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Clarke LL, Ballweber LR, Allen K, Little SE, Lappin MR. Prevalence of select vector-borne disease agents in owned dogs of Ghana. J S Afr Vet Assoc. 2014;85(1):996. [DOI] [PubMed] [Google Scholar]
- 19.Kamani J, Baneth G, Mumcuoglu KY, Waziri NE, Eyal O, Guthmann Y, et al. Molecular detection and characterization of tick-borne pathogens in dogs and ticks from Nigeria. PLoS Negl Trop Dis. 2013;7(3):e2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baneth G, Samish M, Shkap V. Life cycle of Hepatozoon canis (Apicomplexa: Adeleorina: Hepatozoidae) in the tick Rhipicephalus sanguineus and domestic dog (Canis familiaris). J Parasitol. 2007;93(2):283–99. [DOI] [PubMed] [Google Scholar]
- 21.Giannelli A, Lia RP, Annoscia G, Buonavoglia C, Lorusso E, Dantas-Torres F, et al. Rhipicephalus turanicus, a new vector of Hepatozoon canis. Parasitology. 2017;144(6):730–7. [DOI] [PubMed] [Google Scholar]
- 22.Schafer I, Muller E, Nijhof AM, Aupperle-Lellbach H, Loesenbeck G, Cramer S, et al. First evidence of vertical Hepatozoon canis transmission in dogs in Europe. Parasit Vectors. 2022;15(1):296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Groves MG, Dennis GL, Amyx HL, Huxsoll DL. Transmission of Ehrlichia canis to dogs by ticks (Rhipicephalus sanguineus). Am J Vet Res. 1975;36:937–40. [PubMed] [Google Scholar]
- 24.Brouqui P, Davoust B, Haddad S, Vidor E, Raoult D. Serological evaluation of Ehrlichia canis infections in military dogs in Africa and Reunion Island. Vet Microbiol. 1991;26:103–5. [DOI] [PubMed] [Google Scholar]
- 25.Dobinson R, Wright I. Exotic bloodborne parasites and dogs imported from Africa. Vet Rec. 2022;191(2):67–9. [DOI] [PubMed] [Google Scholar]
- 26.Davoust B, Parzy D, Demoncheaux JP, Tine R, Diarra M, Marie JL, et al. Usefulness of a rapid immuno-migration test for the detection of canine monocytic ehrlichiosis in Africa. Comp Immunol Microbiol Infect Dis. 2014;37(1):31–7. [DOI] [PubMed] [Google Scholar]
- 27.Manyarara R, Tubbesing U, Soni M, Noden BH. Serodetection of Ehrlichia canis amongst dogs in central Namibia. J S Afr Vet Assoc. 2015;86(1):e1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kennedy MA, Thompson RE, McRee Bakker A, Fung C, Dawson J, Parry R, et al. Detection and analysis of tick-borne infections in communal dogs of northwest Zimbabwe. J S Afr Vet Assoc. 2021;92(0):e1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kamani J, Lee CC, Haruna AM, Chung PJ, Weka PR, Chung YT. First detection and molecular characterization of Ehrlichia canis from dogs in Nigeria. Res Vet Sci. 2013;94(1):27–32. [DOI] [PubMed] [Google Scholar]
- 30.Marie JL, Shaw SE, Langton DA, Bourry O, Gomez J, Davoust B. Sub-clinical infection of dogs from the Ivory Coast and Gabon with Ehrlichia, Anaplasma, Mycoplasma and Rickettsia species. Clin Microbiol Infect. 2009;15(Suppl 2):284–5. [DOI] [PubMed] [Google Scholar]
- 31.Dahmani M, Loudahi A, Mediannikov O, Fenollar F, Raoult D, Davoust B. Molecular detection of Anaplasma platys and Ehrlichia canis in dogs from Kabylie, Algeria. Ticks Tick Borne Dis. 2015;6(2):198–203. [DOI] [PubMed] [Google Scholar]
- 32.Gaunt S, Beall M, Stillman B, Lorentzen L, Diniz P, Chandrashekar R, et al. Experimental infection and co-infection of dogs with Anaplasma platys and Ehrlichia canis: hematologic, serologic and molecular findings. Parasit Vectors. 2010;3(1):33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Uilenberg G, Franssen FF, Perie NM, Spanjer AA. Three groups of Babesia canis distinguished and a proposal for nomenclature. Vet Q. 1989;11(1):33–40. [DOI] [PubMed] [Google Scholar]
- 34.Hirata H, Omobowale T, Adebayo O, Asanuma N, Haraguchi A, Murakami Y, et al. Identification and phylogenetic analysis of Babesia parasites in domestic dogs in Nigeria. J Vet Med Sci. 2022;84(3):338–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sasaki M, Omobowale O, Tozuka M, Ohta K, Matsuu A, Nottidge HO, et al. Molecular survey of Babesia canis in dogs in Nigeria. J Vet Med Sci. 2007;69(11):1191–3. [DOI] [PubMed] [Google Scholar]
- 36.Egenvall A, Lilliehook I, Bjoersdorff A, Engvall EO, Karlstam E, Artursson K, et al. Detection of granulocytic Ehrlichia species DNA by PCR in persistently infected dogs. Vet Rec. 2000;146(7):186–90. [DOI] [PubMed] [Google Scholar]
- 37.Schafer I, Kohn B, Silaghi C, Fischer S, Marsboom C, Hendrickx G et al. Molecular and serological detection of Anaplasma phagocytophilum in dogs from Germany (2008–2020). Anim (Basel). 2023;13(4). [DOI] [PMC free article] [PubMed]
- 38.Maharana P, Abdel-Lathif AY, Pattnayak KC. Observed climate variability over Chad using multiple observational and reanalysis datasets. Global Planet Change. 2018;162:252–65. [Google Scholar]
- 39.Elhelw R, Elhariri M, Hamza D, Abuowarda M, Ismael E, Farag H. Evidence of the presence of Borrelia burgdorferi in dogs and associated ticks in Egypt. BMC Vet Res. 2021;17(1):49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Elhelw RA, El-Enbaawy MI, Samir A. Lyme borreliosis: a neglected zoonosis in Egypt. Acta Trop. 2014;140:188–92. [DOI] [PubMed] [Google Scholar]
- 41.Elbir H, Raoult D, Drancourt M. Relapsing fever borreliae in Africa. Am J Trop Med Hyg. 2013;89(2):288–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gettings JR, Lopez JE, Krishnavajhala A, Armstrong BA, Thompson AT, Yabsley MJ. Antibodies to Borrelia turicatae in experimentally infected dogs cross-react with Borrelia burgdorferi serologic asays. J Clin Microbiol. 2019;57(9):e00628–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kumsa B, Socolovschi C, Raoult D, Parola P. New Borrelia species detected in ixodid ticks in Oromia, Ethiopia. Ticks Tick Borne Dis. 2015;6(3):401–7. [DOI] [PubMed] [Google Scholar]
- 44.Ehounoud CB, Yao KP, Dahmani M, Achi YL, Amanzougaghene N, Kacou N, Douba A, et al. Multiple pathogens including potential new species in tick vectors in Cote d’Ivoire. PLoS Negl Trop Dis. 2016;10(1):e0004367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cleveland CA, Garrett KB, Box EK, Thompson AT, Haynes EK, Elder DL, et al. Investigating flubendazole as an anthelmintic treatment for Guinea worm (Dracunculus medinensis): clinical trials in laboratory-reared ferrets and domestic dogs in Chad. Am J Trop Med Hyg. 2022;106(5):1456–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Healthcare GE. Reliable extraction of DNA from Whatman FTA cards application note 28-9822-22 AA. 2010.
- 47.Medlin L, Elwood HJ, Stickel S, Sogin ML. The characterization of enzymatically amplified eukaryotic 16S-like rRNA-coding regions. Gene. 1988;71(2):491–9. [DOI] [PubMed] [Google Scholar]
- 48.Yabsley MJ, Work TM, Rameyer RA. Molecular phylogeny of Babesia poelea from brown boobies (Sula leucogaster) from Johnston Atoll, central Pacific. J Parasitol. 2006;92(2):423–5. [DOI] [PubMed] [Google Scholar]
- 49.Inokuma H, Yoshizaki Y, Shimada Y, Sakata Y, Okuda M, Onishi T. Epidemiological survey of Babesia species in Japan performed with specimens from ticks collected from dogs and detection of new Babesia DNA closely related to Babesia odocoilei and babesia divergens DNA. J Clin Microbiol. 2003;41(8):3494–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bostrom B, Wolf C, Greene C, Peterson DS. Sequence conservation in the rRNA first internal transcribed spacer region of Babesia Gibsoni genotype Asia isolates. Vet Parasitol. 2008;152(1–2):152–7. [DOI] [PubMed] [Google Scholar]
- 51.Shock BC, Moncayo A, Cohen S, Mitchell EA, Williamson PC, Lopez G, et al. Diversity of piroplasms detected in blood-fed and questing ticks from several states in the United States. Ticks Tick Borne Dis. 2014;5(4):373–80. [DOI] [PubMed] [Google Scholar]
- 52.Dawson JE, Biggie KL, Warner CK, Cookson K, Jenkins S, Levine JF, et al. Polymerase chain reaction evidence of Ehrlichia chaffeensis, an etiologic agent of human ehrlichiosis, in dogs from southeast Virginia. Am J Vet Res. 1996;57(8):1175–9. [PubMed] [Google Scholar]
- 53.Anderson BE, Sumner JW, Dawson JE, Tzianabos T, Greene CR, Olson JG, et al. Detection of the etiologic agent of human ehrlichiosis by polymerase chain reaction. J Clin Microbiol. 1992;30(4):775–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kordick SK, Breitschwerdt EB, Hegarty BC, Southwick KL, Colitz CM, Hancock SI, et al. Coinfection with multiple tick-borne pathogens in a Walker Hound kennel in North Carolina. J Clin Microbiol. 1999;37(8):2631–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Little SE, Stallknecht DE, Lockhart JM, Dawson JE, Davidson WR. Natural coinfection of a white-tailed deer (Odocoileus virginianus) population with three Ehrlichia Spp. J Parasitol. 1998;84(5):897–901. [PubMed] [Google Scholar]
- 56.Venables WN, Ripley BD. Modern Applied Statistics with S, Fourth Edition. New York: Springer; 2002. 495 p.
- 57.Bates D, Mächler M, Bolker B, Walker S. Fitting linear mixed-effects models using lme4. J Stat Soft. 2015;67(1):1–48. [Google Scholar]
- 58.Blankenship EE, Perkins MW, Johnson RJ. The information-theoretic approach to model selection: description and case study. Conference on Applied Statistics in Agriculture. 2002.
- 59.Team RC. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2020. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All the data generated or analyzed in this study are included in the article, and any additional inquiries can be directed to the corresponding author.