Abstract
The size and shape of the cells in the basal cell layer of the oral epithelium in 100 specimens from oral mucosa were studied by using an interactive image analysis system (IBAS-1). Four groups of white lesions (traumatic keratosis, lichen planus, leucoplakia, and a "risk group") in addition to two control groups (normal mucosa and squamous cell carcinoma) were studied retrospectively. The results showed a progressive increase in the dimensions (area, perimeter, and maximum diameter) of the nuclei from normal mucosa through traumatic keratosis, lichen planus, leucoplakia and the "risk group" to carcinoma, with considerable differences. The nucleus in squamous cell carcinoma was twice as large as in normal mucosa. A substantial increase in the dimensions of both the cell and the nucleus was found in the "risk group." The nucleo:cytoplasmic ratio, contrary to what might have been anticipated in risk lesions, did not show considerable differences between the diagnostic groups. Furthermore, it was slightly decreased in the risk group compared with the normal mucosa. The shape factors (form PE and contour index) seemed to be less helpful in the identification of the "risk group." The size of the basal cell and its nucleus can be of diagnostic value for lesions with a high risk of malignant transformation.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boysen M., Reith A. A morphometric model for light microscopic analysis of metaplastic, dysplastic, and carcinomatous alterations of the nasal mucosa in nickel workers. Pathol Res Pract. 1980;166(2-3):362–371. doi: 10.1016/S0344-0338(80)80140-3. [DOI] [PubMed] [Google Scholar]
- Boysen M., Reith A. Stereological analysis of nasal mucosa. III. Stepwise alterations in cellular and subcellular components of pseudostratified, metaplastic and dysplastic epithelium in nickel workers. Virchows Arch B Cell Pathol Incl Mol Pathol. 1982;40(3):311–325. [PubMed] [Google Scholar]
- Böhm N., Sandritter W. DNA in human tumors: a cytophotometric study. Curr Top Pathol. 1975;60:151–219. doi: 10.1007/978-3-642-66215-7_5. [DOI] [PubMed] [Google Scholar]
- CHASE H. B., MONTAGNA W., MALONE J. D. Changes in the skin in relation to the hair growth cycle. Anat Rec. 1953 May;116(1):75–81. doi: 10.1002/ar.1091160107. [DOI] [PubMed] [Google Scholar]
- Craig G. T., Franklin C. D. The effect of turpentine on hamster cheek pouch mucosa: a model of epithelial hyperplasia and hyperkeratosis. J Oral Pathol. 1977 Sep;6(5):268–277. doi: 10.1111/j.1600-0714.1977.tb01649.x. [DOI] [PubMed] [Google Scholar]
- Eveson J. W., MacDonald D. G. Hamster tongue carcinogenesis II. Quantitative morphologic aspects of preneoplastic epithelium. J Oral Pathol. 1981 Oct;10(5):332–341. doi: 10.1111/j.1600-0714.1981.tb01285.x. [DOI] [PubMed] [Google Scholar]
- Eveson J. W., MacDonald D. G. Quantitative histological changes during early experimental carcinogenesis in the hamster cheek pouch. Br J Dermatol. 1978 Jun;98(6):639–644. doi: 10.1111/j.1365-2133.1978.tb03582.x. [DOI] [PubMed] [Google Scholar]
- FORAKER A. G., REAGAN J. W. Nuclear size and nuclear: cytoplasmic ratio in the delineation of atypical hyperplasia of the uterine cervix. Cancer. 1956 May-Jun;9(3):470–479. doi: 10.1002/1097-0142(195605/06)9:3<470::aid-cncr2820090307>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
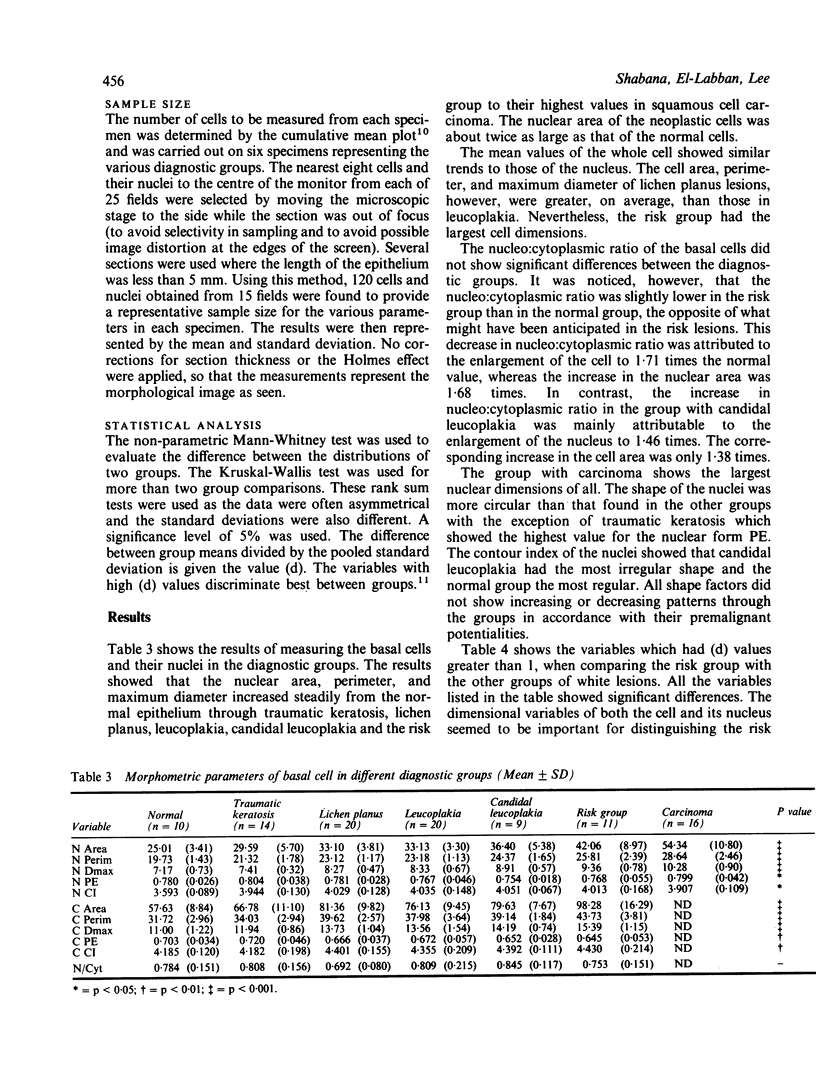
- Franklin C. D., Smith C. J. Stereological analysis of histological parameters in experimental premalignant hamster cheek pouch epithelium. J Pathol. 1980 Mar;130(3):201–215. doi: 10.1002/path.1711300309. [DOI] [PubMed] [Google Scholar]
- Jagoe R., Sowter C., Slavin G. Shape and texture analysis of liver cell nuclei in hepatomas by computer aided microscopy. J Clin Pathol. 1984 Jul;37(7):755–762. doi: 10.1136/jcp.37.7.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer I. R., Lucas R. B., el-Labban N., Lister L. A computer-aided study on the tissue changes in oral keratoses and lichen planus, and an analysis of case groupings by subjective and objective criteria. Br J Cancer. 1970 Sep;24(3):407–426. doi: 10.1038/bjc.1970.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackenzie I. C., Miles A. E. The effect of chronic frictional stimulation on hamster cheek pouch epithelium. Arch Oral Biol. 1973 Nov;18(11):1341–1349. doi: 10.1016/0003-9969(73)90107-6. [DOI] [PubMed] [Google Scholar]
- Murti P. R., Daftary D. K., Bhonsle R. B., Gupta P. C., Mehta F. S., Pindborg J. J. Malignant potential of oral lichen planus: observations in 722 patients from India. J Oral Pathol. 1986 Feb;15(2):71–77. doi: 10.1111/j.1600-0714.1986.tb00580.x. [DOI] [PubMed] [Google Scholar]
- PINKUS H. Examination of the epidermis by the strip method. II. Biometric data on regeneration of the human epidermis. J Invest Dermatol. 1952 Dec;19(6):431–447. doi: 10.1038/jid.1952.119. [DOI] [PubMed] [Google Scholar]
- Pindborg J. J., Reibel J., Holmstrup P. Subjectivity in evaluating oral epithelial dysplasia, carcinoma in situ and initial carcinoma. J Oral Pathol. 1985 Oct;14(9):698–708. doi: 10.1111/j.1600-0714.1985.tb00549.x. [DOI] [PubMed] [Google Scholar]
- Potten C. S., Allen T. D. The fine structure and cell kinetics of mouse epidermis after wounding. J Cell Sci. 1975 Mar;17(3):413–447. doi: 10.1242/jcs.17.3.413. [DOI] [PubMed] [Google Scholar]
- Schrek R. Ultrastructure of blood lymphocytes from chronic lymphocytic and lymphosarcoma cell leukemia. J Natl Cancer Inst. 1972 Jan;48(1):51–64. [PubMed] [Google Scholar]
- Silverman S., Jr, Gorsky M., Lozada-Nur F. A prospective follow-up study of 570 patients with oral lichen planus: persistence, remission, and malignant association. Oral Surg Oral Med Oral Pathol. 1985 Jul;60(1):30–34. doi: 10.1016/0030-4220(85)90210-5. [DOI] [PubMed] [Google Scholar]
- Tarin D. Further electron microscopic studies on the mechanism of carcinogenesis: the specificity of the changes in carcinogen-treated mouse skin. Int J Cancer. 1968 Nov 15;3(6):734–742. doi: 10.1002/ijc.2910030606. [DOI] [PubMed] [Google Scholar]
- Valeri V., Cruz A. R., Brandão H. J., Lison L. Relationship between cell nuclear volume and deoxyribonucleic acid of cells of normal epithelium, of carcinoma in situ and of invasive carcinoma of the uterine cervix. Acta Cytol. 1967 Nov-Dec;11(6):488–496. [PubMed] [Google Scholar]


