Abstract
Background
Campylobacteriosis in kidney transplant recipients (KTRs) is the most common identified bacterial cause of diarrhea. Risk factors in KTRs are unknown.
Methods
A 10-year multicentric, retrospective 1:1 case-control study was performed in France between 2010 and 2020. The main aim was to identify factors associated with Campylobacter-related infection in KTRs. The KTRs with a functional graft and campylobacteriosis (positive stool culture and/or blood culture and/or positive nucleic amplification test) and their controls matched on transplantation date within the same center were included.
Results
We identified 326 patients with campylobacteriosis. The estimated incidence rate of campylobacteriosis was 2.3/1000 patient-years. The infection occurred at a median of 2.4 years posttransplantation. The independent risk factors for campylobacteriosis were use of corticosteroids as maintenance regimen (75.8% vs 66%; P < .001), acute rejection (8.9% vs 4%; P = .048), low lymphocyte count (0.96 vs 1.4 giga/liter (G/L); P < .001), and low basal estimated glomerular filtration rate (eGFR) (44.2 vs 57.5 mL/minute/1.73 m2; P < .001). A fluoroquinolone was initiated in 64 (21.4%) patients, with 51.1% of antimicrobial resistance, whereas almost all strains were erythromycin sensitive.
Conclusions
Campylobacteriosis has a higher incidence in the 2 first years of transplantation. The factors independently associated with campylobacteriosis are corticosteroids as maintenance immunosuppressive regimen, low lymphocyte counts, low eGFR, and a history of acute rejection. Due to high antimicrobial resistance with fluoroquinolone, the first line of treatment should be azithromycin.
Keywords: Campylobacter spp, Campylobacter-related infection, diarrhea, immunodepression, kidney transplant recipients
Campylobacter-related infections are frequent among kidney recipients and occur preferentially shortly after transplantation. The independent risk factors for campylobacteriosis are corticosteroids as maintenance immunosuppressive regimen, low basal estimated glomerular filtration rate, history of acute rejection, and low absolute lymphocyte count.
Campylobacter is the most common identified bacterial cause in food-borne gastroenteritis worldwide. In low-income countries, the infection mainly occurs in children and young adults. In France, campylobacteriosis often affects the elderly. The infection mainly occurs after consumption of undercooked poultry. In high-income countries it is usually a mild infection; however, in 1% of cases, bacteremia and secondary localization can occur, with significant mortality (3%–28%) [1–3]. The species mainly encountered in human disease are Campylobacter jejuni, Campylobacter coli, and Campylobacter fetus. The main reservoir of this zoonosis is the digestive tract of wild or domestic birds, especially poultry [4].
In kidney transplant recipients (KTRs), few epidemiological data are available. In various studies, the frequency of Campylobacter spp varies between 5% and 28% of infectious diarrheas [5–8]. Campylobacter spp are even responsible for 29% of severe diarrhea when molecular diagnosis is available, representing the most frequently isolated bacterial microorganism [7]. When van den Bogaart et al studied bacterial food-borne infections in solid organ transplant recipients, 88% of the identified microorganisms were Campylobacter spp [9]. Thus, Campylobacter spp are a leading cause of bacterial diarrhea in transplant recipients.
Immunosuppressive treatment could increase susceptibility to Campylobacter infections and eventually bacteremia, but little is known about Campylobacter diarrhea and bacteremia in KTRs [1, 2, 10, 11]. The risk factors of Campylobacter diarrhea and invasive infections in KTRs have not been studied and the outcomes are poorly known.
The main aim of our study was to identify factors associated with Campylobacter spp diarrhea and bacteremia in KTRs. The secondary objectives were to analyze the impact of Campylobacter spp infection on KTRs, focusing on early hospitalization and acute kidney injury (AKI) rates. Finally, we focused on the epidemiology of Campylobacter spp in this population together with the antibiotic susceptibility profile and the efficiency of prescribed antibiotic therapy.
PATIENTS AND METHODS
Study Design and Patients
The incidence was calculated as the number of infections per patient-year among KTRs with a functional graft, in 9 French university hospitals (Bordeaux, Toulouse, Lyon, Reims, Strasbourg, Paris Necker, Besançon, Caen, Tours).
Then, a case-control study was conducted in those 9 centers. Patients >18 years old with a functional kidney transplant and who suffered from a Campylobacter spp infection between 1 January 2010 and 31 December 2020 were included.
Cases were defined by a Campylobacter sp–positive stool culture and/or nucleic acid amplification test (NAAT) and/or a positive blood culture [12, 13]. For detailed microbiological methods, see the Supplementary Methods.
The cases were identified either through a systematic search in bacteriology laboratories in 5 centers (Tours, Toulouse, Paris Necker, Besançon, and Reims) or a search of the term “Campylobacter” in the medical records of KTRs in the last 3 centers (Bordeaux, Lyon, and Caen), confirmed by positive microbiological assay. In Strasbourg, both methods were used.
Each case was matched with 1 control patient from the same center. Control patients had no Campylobacter spp infection during the study period. Cases were matched by transplantation date (±3 months) and had a functional graft at the time of diagnosis of the matched case.
For cases, diagnosis date (day 0) was the date of the first positive microbiological sample for Campylobacter spp. If not available, it was the first date on which the diagnosis was written in the medical record. For control patients, reference day 0 was determined from the interval between transplantation and diagnosis of the matched case.
Case and control patients were followed from the reference day up to 1 year.
Data Collection
Data on demographic, medical history, kidney transplantation, induction and maintenance immunosuppressive treatment at time of infection, biological features, and antibiotic treatment were retrospectively extracted from medical records through a standardized database. When available, microbiological data were also extracted, especially the identification of Campylobacter spp and susceptibility to ampicillin, amoxicillin-clavulanate, erythromycin, tetracycline, gentamicin, and ciprofloxacin. Details of the methods of antimicrobial resistance testing are shown in the Supplementary Methods.
Immunosuppressive Regimen
Immunosuppressive regimens were similar in the 9 centers. For detailed immunosuppressive regimens, see the Supplementary Methods.
Definitions
Acute kidney injury and its severity (stage 1–3) was defined according to the Kidney Disease—Improving Global Outcomes (KDIGO) universal definition [14]. Allograft rejection was biopsy-proven. The day 0 of rejection was the date on which the graft biopsy was performed. Allograft failure at 1 year was defined as requirement for permanent dialysis, and death at 1 year was defined as death from all causes 1 year after Campylobacter spp infection. Estimated glomerular filtration rate (eGFR) was calculated with the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) formula and expressed in mL/minute/1.73 m2. Basal eGFR was collected 1 to 6 months before the campylobacteriosis.
Antibiotic treatment was considered as appropriate if the strain was at least susceptible to 1 of the drugs prescribed. First-line antibiotic therapy was either empirical, when based on clinical data without microbiological results, or adapted to bacterial identification and/or susceptibility results.
Nonsevere Campylobacter spp infections were defined as cases that were not hospitalized, had no AKI, no bacteremia, and neither death nor allograft failure at 1 year. “Severe at infection” patients were defined as cases that were hospitalized, had an AKI or had bacteremia.
Outcomes
The primary objective of this study was to assess factors associated with Campylobacter spp infections. The secondary endpoints were to assess the impact of Campylobacter spp infection in KTRs: hospitalization, hospitalization in intensive care unit (ICU), AKI, allograft failure, and death at 1 year after infection. A focus was also made on the epidemiology of Campylobacter spp in this population together with the antibiotic susceptibility and the effectiveness of received antimicrobial therapy.
Statistical Analyses
Descriptive statistics are expressed as percentages for categorical variables and as the mean with standard deviation (SD) and median with interquartile range (IQR) for continuous variables. Mann-Whitney test for continuous variables and χ2 test for qualitative variables were used when appropriate. Alternatively, Fisher exact test was used for low numbers of patients. A P value <.05 was considered statistically significant.
Univariate logistic regression analysis of Campylobacter infection risk factors was first performed, then covariables with a P value <.20 were included in a model. Then, covariables were selected by iterative backward elimination, keeping only the covariables significantly associated with the outcome (P < .05). Results were expressed as odds ratios (ORs) with 95% confidence intervals (CIs).
GraphPad Prism software (version 9.0.1) and RStudio statistical software (version 2022.12.0) were used.
RESULTS
Epidemiological and Clinical Characteristics of Patients
The global incidence rate of Campylobacter-related infection was 2.3 per 1000 patient-years
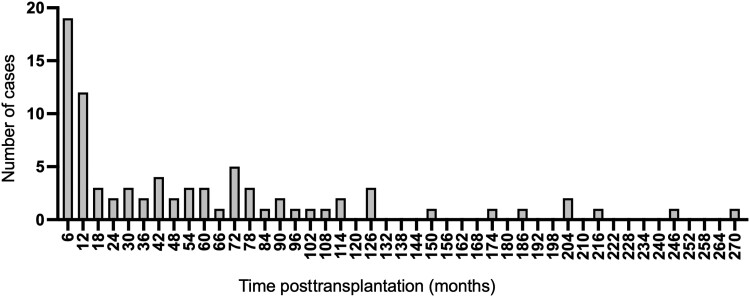
After transplantation, the highest incidence was within the first year after transplantation (Figure 1). Campylobacteriosis occurred at a median of 2.4 years posttransplantation (IQR, 0.58–6.4).
Figure 1.
Development of Campylobacter infection cases in transplant recipients after transplantation, in 6-month time periods.
During the study period, 326 patients with at least 1 episode of Campylobacter spp infection were identified and matched with 326 controls. Baseline characteristics of the study population are described in Table 1.
Table 1.
Characteristics of the Study Population
| Characteristics | Cases (n = 326) |
Controls (n = 326) | OR (95% CI) | P Value |
|---|---|---|---|---|
| Age at infection, y, median (IQR) | 58 (47–67) | 55 (45–65) | 1.01 (.99–1.02) | .071 |
| Male sex | 191 (58.6) | 205 (62.9) | 1.26 (.91–1.73) | .153 |
| Medical history | ||||
| Cardiovascular disease | 210 (64.4) | 217 (66.6) | 0.93 (.67–1.29) | .677 |
| Cancer | 68 (20.9) | 46 (14.1) | 1.62 (1.08–2.45) | .021 |
| Diabetes | 84 (25.8) | 87 (26.7) | 0.95 (.67–1.35) | .791 |
| Transplantation rank | 1.24 (.89–1.74) | .201 | ||
| 1 | 263 (80.7) | 276 (84.7) | … | |
| >1 | 61 (18.7) | 50 (15.3) | … | |
| Previous kidney replacement therapy | 0.82 (.60–1.13) | .232 | ||
| Preemptive | 39 (12) | 47 (14.4) | … | |
| Hemodialysis | 236 (72.4) | 236 (72.4) | … | |
| Peritoneal dialysis | 38 (11.7) | 33 (10.1) | … | |
| CMV status | ||||
| D−/R− for CMV | 67 (20.6) | 81 (24.8) | … | |
| D+/R− for CMV | 71 (21.8) | 55 (16.9) | … | |
| D−/R+ for CMV | 68 (20.9) | 91 (27.9) | … | |
| D+/R+ for CMV | 97 (29.8) | 84 (25.8) | … | |
| Anti-HLA antibodies | 113 (34.7) | 111 (34) | 1.09 (.79–1.51) | .602 |
| DSA | 41 (12.6) | 27 (8.3) | 1.69 (1.01–2.84) | .044 |
| Initial nephropathy | 0.99 (.92–1.07) | .931 | ||
| Vascular | 18 (5.5) | 16 (4.9) | … | |
| Tubulo-interstitial | 17 (5.2) | 17 (5.2) | … | |
| Glomerular | 74 (22.7) | 98 (30.1) | … | |
| Diabetes | 19 (5.8) | 16 (4.9) | … | |
| Malformative | 29 (8.9) | 27 (8.3) | … | |
| PKD | 77 (23.6) | 69 (21.2) | … | |
| Undetermined | 69 (21.2) | 71 (21.8) | … | |
| Other | 22 (6.7) | 11 (3.4) | … | |
| Induction treatment | ||||
| rATG | 130 (39.8) | 125 (38.2) | 1.07 (.77–1.49) | .672 |
| Basiliximab | 155 (47.5) | 156 (47.9) | … | |
| Maintenance IS at infection | ||||
| CNI (tacrolimus or cyclosporin A) | 279 (85.3) | 283 (78.6) | 0.80 (.49–1.28) | .351 |
| Tacrolimus | 200 (61.34) | 208 (64.4) | … | |
| Cyclosporin A | 79 (24.2) | 76 (23.3) | … | |
| mTORi | 47 (14.4) | 32 (9.8) | 1.54 (.96–2.50) | .075 |
| Belatacept | 11 (3.4) | 13 (4) | 0.83 (.36–.89) | .665 |
| Azathioprine | 7 (2.1) | 12 (3.7) | 0.58 (.21–1.45) | .247 |
| MMF | 282 (86.5) | 274 (84) | 1.23 (.73–2.10) | .437 |
| Corticosteroids | 247 (75.8) | 215 (66) | 14.71 (6.39–42.60) | <.001 |
| Rejection 1 y before infection | ||||
| Acute rejection | 29 (8.9) | 13 (4) | 2.27 (1.26–4.24) | .005 |
| TCMR | 17 (5.2) | 10 (3) | 1.81 (.83–4.16) | .137 |
| ABMR | 13 (4) | 3 (0.9) | 4.65 (1.48–20.44) | .007 |
| Chronic rejection | 6 (1.8) | 4 (1.2) | 1.57 (.44–6.19) | .484 |
| Treatment of acute rejection | ||||
| Corticosteroids | 22 (6.7) | 10 (3) | 2.36 (1.13–5.29) | .022 |
| Plasma exchange | 8 (2.5) | 4 (1.2) | 2.09 (.65–7.88) | .22 |
| Rituximab | 6 (1.8) | 2 (0.6) | 3.13 (.71–21.45) | .135 |
| IVIG | 8 (2.6) | 5 (1.5) | 1.65 (.55–5.54) | .380 |
| Lymphocyte count, giga/liter (G/L), mean (SD) | 0.96 (0.8) | 1.4 (0.84) | 0.42 (.32–.54) | <.001 |
| IgG, g/L, mean (SD) | 8.6 (3.7) | 9.0 (2.9) | 0.97 (.90–1.04) | .417 |
| Basal eGFR, mL/min/1.73 m2, median (IQR) | 44.2 (30.5–59.3) | 57.5 (39.4–79) | 0.97 (.96–.98) | <.001 |
Data are presented as No. (%) unless otherwise indicated.
Abbreviations: ABMR, antibody-mediated rejection; CI, confidence interval; CMV, cytomegalovirus; CNI, calcineurin inhibitor; D–, donor negative; D+, donor positive; DSA, donor-specific antibody; eGFR, glomerular filtration rate; HLA, human leukocyte antigen; IgG, immunoglobulin G; IQR, interquartile range; IS, immunosuppressive; IVIG, intravenous immunoglobulin; MMF, mycophenolic acid; mTORi, mammalian target of rapamycin inhibitor; OR, odds ratio; PKD, polycystic kidney disease; rATG, rabbit anti-thymoglobulin; R–, recipient negative; R+, recipient positive; SD, standard deviation; TCMR, T-cell–mediated rejection.
Cases were comparable in terms of age, sex ratio, transplantation rank, and initial kidney disease. The covariables with P < .05 were included in the analysis of Campylobacter-related infection risk factors.
Lower basal eGFR, maintenance immunosuppressive regimen with corticosteroids, lower basal lymphocyte count, and acute rejection were independently associated with a higher risk of Campylobacter-related infection after multivariate analysis (Table 2).
Table 2.
Multivariable Analyses for Risk Factors of Campylobacter-Related Infection in Kidney Transplant Recipients
| Variable | aOR | (95% CI) | P Value |
|---|---|---|---|
| Corticosteroids | 10.22 | (4.20–30.84) | <.001 |
| Acute rejection | 2.20 | (1.03–4.95) | .048 |
| Lymphocyte count, giga/liter (G/L) | 2.10 | (1.53–2.95) | <.001 |
| Basal eGFR | 1.03 | (1.02–1.04) | <.001 |
Covariables with a P value <.20 were included in a multivariable logistic regression analysis.
Abbreviations: aOR, adjusted odds ratio; CI, confidence interval; eGFR, estimated glomerular filtration rate.
The mean corticosteroid dose was 7.3 mg/day (SD, 4.6) in cases and 6.4 mg/day (SD, 6.5) in controls. Cases were treated more frequently with an immunosuppressive regimen comprising 3 molecules always including a calcineurin inhibitor (CNI) + antiproliferative or 2 antiproliferative agents (either mammalian target of rapamycin inhibitor [mTORi] or mycophenolic acid) and corticosteroids (n = 209 [64%]) compared with controls (n = 180 [55%]) (P = .02).
Severe Campylobacter-Related Infection
Among cases, we analyzed factors associated with severe Campylobacter-related infection.
Hospitalization and Acute Kidney Injury
Hospitalization occurred in 210 patients (64.4%); 7 were admitted in ICU (2.1%) and 141 KTRs had an AKI at the time of infection (43.3%). AKI was mainly stage 1 (118 [36.2%]) and, less frequently, stage 2 (15 [4.6%]) and stage 3 (8 [2.5%]).
Use of corticosteroids as maintenance therapy and lower basal eGFR were factors associated with hospitalization and AKI.
Bacteremia
Bacteremia occurred in 16 patients (4.9% of cases). Factors associated with bacteremia were age (median, 68.5 years [IQR, 65–71] vs 56 [IQR, 42–64]; P = .001), the absence of CNI (62.5% vs 89.2%; P = .007), use of mTORi (37.5 vs 9.7%; P = .007), corticosteroid prescription as maintenance immunosuppressive therapy (93.75 vs 62.4%; P = .01), and lower basal eGFR (median, 42 mL/minute/1.73 m2 [IQR, 26–48] vs 52 [IQR, 40–68]; P = .02). These results are summarized in Supplementary Table 1.
As expected, the proportion of C fetus infection was higher in patients with positive blood cultures (37.5% vs 2.5%). The most frequent pathogen was C jejuni (10 [62.5%]); the others were C fetus (6 [37.5%]). Treatment duration was between 5 and 28 days (mean, 17 days).
Outcome of Campylobacter on Patient and Graft Outcomes
One-year graft loss occurred in 18 (5.5%) cases and 1-year death in 14 (4.3%) cases. In the controls, graft loss occurred in 4 (1.2%) and death in 5 (1.5%). For a detailed description and comparison with nonsevere patients, see Supplementary Table 2.
Microbiological Results
Of the 326 cases, 297 (91.1%) had a positive stool culture and 19 (5.8%) had a positive Campylobacter spp stool NAAT. Only 16 patients had a positive blood culture (4.9%) (Supplementary Table 3). Apart from 1 patient with a Campylobacter spp endocarditis with a positive cardiac valve polymerase chain reaction (PCR), there were no other secondary localizations.
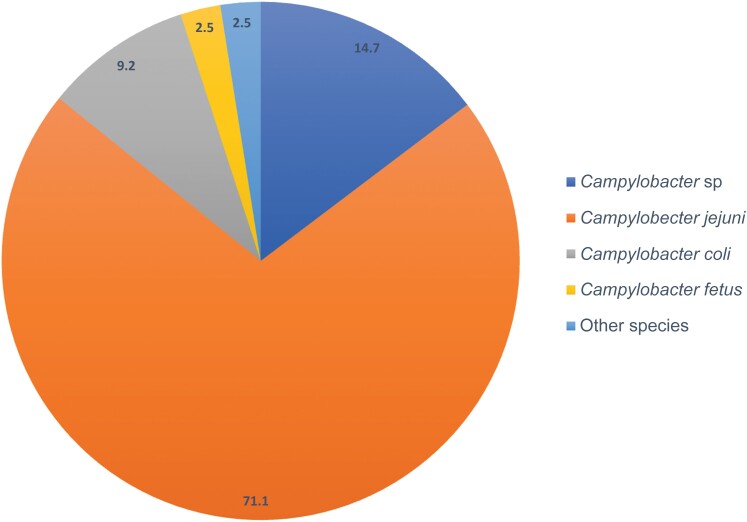
Campylobacter jejuni was the most frequently identified species (71.1%), followed by C coli and C fetus (respectively, 9.2% and 2.5%). Campylobacter spp were the cases for which we did not have the microbiological identification on the species level (PCR) or missing data from the microbiological results. The other species involved were Campylobacter lari, Campylobacter showae, Campylobacter hyointestinalis, and Aliarcobacter butzleri (Figure 2).
Figure 2.
Campylobacter species involved in Campylobacter-related infection. Campylobacter sp: cases with campylobacteriosis without species-level identification (nucleic acid amplification test or missing microbiological data). “Other” includes Campylobacter lari, Campylobacter showae, Campylobacter hyointestinalis, and Aliarcobacter butzleri.
Antimicrobial resistance data were available for 197 patients (60.4%). Some antimicrobial data was not available because they were realized in outpatient settings or were too old to be collected. No resistance to carbapenem was described in the 51 cases tested. For C jejuni, resistance rates were 47.3% for ampicillin, 61.2% for ciprofloxacin, and 0.6% for erythromycin (Table 3).
Table 3.
Antimicrobial Resistance According to Species
| Antimicrobial Susceptibilitya | Total (n = 197) |
Campylobacter jejuni
(n = 165) |
Campylobacter coli
(n = 25) |
Campylobacter fetus
(n = 7) |
|---|---|---|---|---|
| Ampicillin | 86 (43.7) | 78 (47.3) | 8 (32) | 0 |
| Amoxicillin-clavulanate | 2 (1) | 1 (0.6) | 1 (4) | 0 |
| Ciprofloxacin | 121 (61.4) | 101 (61.2) | 17 (68) | 3 (42.8) |
| Erythromycin | 2 (1) | 1 (0.6) | 1 (4) | 0 |
| Tetracycline | 107 (54.3) | 84 (50.9) | 23 (92) | 0 |
| Gentamicin | 1 (0.5) | 1 (0.6) | 0 | 0 |
Data are presented as No. (%).
aSusceptibility testing was interpreted according to the Antibiogram Committee of the French Society of Microbiology and European Committee on Antimicrobial Susceptibility Testing recommendations.
An epidemiologic risk factor was described in only 18 cases: 8 patients had traveled in foreign countries, 4 had eaten chicken, and 6 had sick people in their entourage.
A coinfection occurring simultaneously, at time of campylobacteriosis, was documented in 82 cases (25.2%), of which 15 had at least 2 other infections. Cytomegalovirus (CMV) infection was diagnosed in 25 cases (15 with a DNAemia and 10 with CMV disease), 20 patients had a urinary tract infection, and 6 patients had a bacteremia (4 with enterobacteria, 1 Staphylococcus aureus, 1 Staphylococcus epidermidis).
Coinfection in stools were diagnosed in 19 patients (5.3%): norovirus infection (n = 9), Clostridium difficile (n = 3), rotavirus (n = 2), sapovirus (n = 1), Yersinia spp (n = 1), and adenoviruses (n = 3).
Treatment
The received treatments are detailed in Table 4. The most prescribed therapy were macrolides (40.1%), for the shortest duration (mean, 5.4 days) with no documented acquired resistance. The second antibiotic most frequently prescribed was fluoroquinolones (21.4%) for a mean duration of 7.7 days, with 51.1% of acquired resistance. Third-generation cephalosporins (3GC) were inappropriately prescribed in 12% of cases. Among the treated patients, 25 received dual therapy (mostly 3GC + metronidazole).
Table 4.
First-line Antimicrobial Therapy Received, Resistance, and Mean Duration
| Treatment | Cases (N = 299) |
Resistance (n = 189) |
Duration, Mean (SD) |
|---|---|---|---|
| Amoxicillin | 16 (5.4) | 0/10 (0) | 9.5 (4) |
| Amoxicillin-clavulanate | 22 (7.4) | 0/17 (0) | 9.2 (6.1) |
| Fluoroquinolone | 64 (21.4) | 24/47 (51.1) | 7.7 (4.3) |
| Macrolide | 120 (40.1) | 0/79 (0) | 5.4 (2.5) |
| 3GCa | 36 (12) | 36/36 (100) | 9.1 (4.7) |
| Otherb | 8 (2.7) | NA | NA |
| No antimicrobial treatment | 33 (11) | NA | NA |
Data are presented as No. (%) unless otherwise indicated.
Abbreviations: 3GC, third-generation cephalosporin; NA, not applicable; SD, standard deviation.
a3GC was ceftriaxone. 3GCs are not tested in Campylobacter panels; it is a natural resistance.
bOther included vancomycin, clindamycin, piperacillin-tazobactam, metronidazole, imipenem, doxycycline.
Sixteen (17.2%) patients with a nonsevere infection did not receive any antimicrobial treatment. All experienced spontaneous clinical remission without microbiological relapse. There was no significant difference between these patients and the nonsevere cases who were treated but a higher basal eGFR (Supplementary Table 4). A resistance to the first-line prescribed antimicrobial treatment was identified in 24 of 47 (51.1%) cases treated with fluoroquinolones and, as expected, in 36 of 36 (100%) cases treated with 3GC. Among patients with a resistance to fluoroquinolones as first-line treatment (n = 24), 2 experienced a relapse of infection (8.4%). For patients treated with fluoroquinolones as first line but susceptible (n = 23), 1 patient needed antibiotic modification to macrolides because of clinical state worsening (4.3%). For patients with macrolide in first-line treatment, 0 experienced bad evolution or relapse (no statistical analysis performed). Moreover, among the severe patients, we compared 101 with an effective first line of antibiotics versus 59 with an ineffective first line, and observed 1 relapse among 101 (1%) and 4 among 59 patients (6.8%) (P = .062).
DISCUSSION
In this study conducted in 9 French centers on 326 KTRs with Campylobacter-related infection, the incidence rate was 2.3 per 1000 patient-years. In Swiss solid organ transplant recipients, the incidence rate of Campylobacter spp infection was 6.6 per 1000 patients per year [9]. Even underestimated, compared with the general population of matched age in France (mean age of 55 years in our study), the incidence (2.3/1000 patient-years) is 15-fold higher (0.15/1000 patient-years) [15].
Risk factors associated with campylobacteriosis were identified, notably corticosteroids as maintenance immunosuppressive regimen, a low basal eGFR, a history of acute rejection, and a low lymphocyte count. Lymphopenia is a known risk factor for opportunistic infections (fungal, parasitic, viral, and bacterial) [16]. The distribution from transplantation date and corticosteroids as maintenance immunosuppressive drug suggests also that the burden of immunosuppression is a risk factor for Campylobacter-related infection in this population. However, like urinary tract infections, Campylobacter infection can occur regardless of the posttransplant period [17].
Severe Campylobacter-related symptoms at day 0 of infection occurred mainly among elderly patients with comorbidities and an immunosuppressive regimen containing maintenance corticosteroids and no CNI. Even with low doses of corticosteroids (5 mg), the risk of Campylobacter infection remained higher. Corticosteroids’ immunosuppressive effect is linked to the inhibition of cytokine production and lymphocyte migration, which is the first step of the Campylobacter immune response [18–20], so corticosteroids in that way may promote the dissemination of the infection. However, since corticosteroids were almost always a third immunosuppressive drug in addition to CNI and antiproliferatives, it was not possible to independently analyze its own effect compared to the effect of a global higher immunosuppression burden (having 2 vs 3 immunosuppressive molecules). Death and graft loss occurred in patients with many comorbidities and since the median time to occurrence was 4 months, those outcomes are more related to the patients' comorbidities rather than the Campylobacter infection itself. We do not have the precise cause of death in our database but in term of timeline, we can consider that death within 30 days could be more directly linked to the event “campylobacteriosis,” and only 2 patients died within the month after the diagnosis of campylobacteriosis.
When comparing our microbiological results with the French epidemiological report from 2021, the distribution of species and the antimicrobial resistance were similar to those in the general population [15].
Macrolides were mainly initiated as first-line treatment, but 3GCs with natural resistance or fluoroquinolones with two-thirds of acquired resistance were also significantly used, according to French and international guidelines for the treatment of infectious diarrhea [21, 22]. However, Campylobacter has been assessed as the most common identified bacterial cause of severe infectious diarrhea [5–7], so regarding our results in this representative KTR population in France, this suggests that first-line empirical antimicrobial therapy for severe diarrhea should include a macrolide (azithromycin).
An obstacle to the use of macrolides in KTRs is their interaction with immunosuppressive treatments. Macrolides inhibit CYP450 3A4, thus inhibiting the elimination of CNI. However, azithromycin is a weak CYP450 3A4 inhibitor and is less likely to lead to acute kidney injury under treatment, compared to clarithromycin and erythromycin [23–25], and it is also prescribed for a short time. Consequently, azithromycin can safely be prescribed with monitoring of graft function and CNI levels.
Fluoroquinolones are on the “watch” list of the AWaRe (access, watch, and reserve) classification from the World Health Organization [26] and must be spared. In our study, avoiding fluoroquinolones in first-line treatment would have allowed fluoroquinolone sparing in 64 patients (ie, 21.4% of the patients in the cohort).
Other than basal eGFR, characteristics of patients in the nonsevere Campylobacter-related infection group who were treated were similar to those of the nonsevere patients who did not receive any antibiotic treatment. For future practice, it could be argued to wait for microbiological identification before introducing antimicrobial treatment in those patients.
Our study has limitations, the first of which is being retrospective, which has led notably to a nonsystematic inclusion of patients with Campylobacter-related infection, mainly inpatients. Indeed, the incidence found is probably underestimated because not all mild or self-limiting diarrhea benefited from a stool culture or a consultation at a tertiary care center. Exposure factors were researched but often missing from medical records. In this study, the suspected origin of Campylobacter spp infection was identified for only 18 patients, mainly international travel, which is a known risk for campylobacteriosis [27]. Minimization of immunosuppressive treatment was not analyzable. Moreover, antibiotic exposure before campylobacteriosis has not been recorded, although it is a known risk factor [28]. Consequently, a prospective multicentric cohort could be interesting to confirm our results. However, to our knowledge this study is the first to assess risk factors and severity of campylobacteriosis in KTRs, and it is the largest cohort of KTRs with Campylobacter-related infection.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Contributor Information
Feline Bos, Infectious and Tropical Diseases Department, Centre Hospitalier Universitaire de Bordeaux, Bordeaux, France; Department of Nephrology, Transplantation, Dialysis and Apheresis, Centre Hospitalier Universitaire de Bordeaux, Bordeaux, France.
Romain Gueneau, Department of Infectious Diseases and Tropical Medicine, Assistance Publique-Hôpitaux de Paris, Necker-Enfants Malades University Hospital, Paris, France.
Thomas Crepin, Department of Nephrology, Dialysis, and Renal Transplantation, Centre Hospitalier Universitaire Besançon, Besançon, France.
Claire Tinévez, Infectious and Tropical Diseases Department, Centre Hospitalier Universitaire de Bordeaux, Bordeaux, France.
Benjamin Taton, Department of Nephrology, Transplantation, Dialysis and Apheresis, Centre Hospitalier Universitaire de Bordeaux, Bordeaux, France.
Lionel Couzi, Department of Nephrology, Transplantation, Dialysis and Apheresis, Centre Hospitalier Universitaire de Bordeaux, Bordeaux, France.
Karine Moreau, Department of Nephrology, Transplantation, Dialysis and Apheresis, Centre Hospitalier Universitaire de Bordeaux, Bordeaux, France.
Betoul Schvartz, Nephrology and Transplant Department, Centre Hospitalier Universitaire de Reims, Reims, France.
Peggy Perrin, Nephrology-Transplantation Department, Centre Hospitalier Universitaire de Strasbourg, Strasbourg, France.
Philippe Gatault, Nephrology-Transplantation Department, Centre Hospitalier Universitaire de Tours, Tours, France.
Anne Scemla, Department of Infectious Diseases and Tropical Medicine, Assistance Publique-Hôpitaux de Paris, Necker-Enfants Malades University Hospital, Paris, France.
Valérie Chatelet-Pouliquen, Nephrology-Transplantation Department, Centre Hospitalier Universitaire de Caen, Caen, France.
Charlène Levi, Department of Transplantation, Centre Hospitalier Universitaire de Lyon-Est, Hospices Civils de Lyon, Edouard Herriot Hospital, Nephrology and Clinical Immunology, Lyon, France.
Nassim Kamar, Department of Nephrology and Organ Transplantation, Toulouse Rangueil University Hospital, Institut national de la santé et de la recherche médicale, Unité mixte de recherche 1291, Toulouse Institute for Infectious and Inflammatory Diseases, University Paul Sabatier, Toulouse, France.
Fanny Lanternier, Department of Infectious Diseases and Tropical Medicine, Assistance Publique-Hôpitaux de Paris, Necker-Enfants Malades University Hospital, Paris, France.
Philippe Lanotte, Unité de Bactériologie - Hôpital Bretonneau, Service de Bactériologie - Virologie - Hygiène, CHU de Tours, 2 boulevard Tonnellé, 37044 Tours cedex, France.
Didier Neau, Infectious and Tropical Diseases Department, Centre Hospitalier Universitaire de Bordeaux, Bordeaux, France.
Pierre Merville, Department of Nephrology, Transplantation, Dialysis and Apheresis, Centre Hospitalier Universitaire de Bordeaux, Bordeaux, France.
Philippe Lehours, Bacteriology Department, Centre Hospitalier Universitaire de Bordeaux, National Reference Center for Campylobacter and Helicobacter, Bordeaux, France; Institut national de la santé et de la recherche médicale, Université de Bordeaux, Unité mixte de recherche 1312, Bordeaux Institute of Oncology, Bordeaux, France.
Mathilde Puges, Infectious and Tropical Diseases Department, Centre Hospitalier Universitaire de Bordeaux, Bordeaux, France.
Hannah Kaminski, Department of Nephrology, Transplantation, Dialysis and Apheresis, Centre Hospitalier Universitaire de Bordeaux, Bordeaux, France.
Notes
Author contributions. F. B. and H. K. participated in the writing of the manuscript. F. B., H. K., P. L., M. P., C. T., B. T., L. C., and K. M. participated in research design and the data analysis. R, G., T. C., B. S., P. P., P. G., A. S., V. C.-P., C. L., N. K., and F. L. helped with the data collection in the various centers. D. N. and P. M. did proofreading.
Acknowledgments. We thank the participating centers and the Immunodepression and Infection study group (G2I) from the Société de pathologie infectieuse de langue française.
Patient consent. In accordance with French legislation, our study was classified as “MR–004” for which patients who were still alive did not object to analysis of their data for research issues. Then, the data were pseudonymized. We declared our study to the French National Institute of Health Data and the French Data Protection Authority (CNIL n° 2216503).
Financial support. This work was supported by G2I (Groupe Infection et Immunodépression).
References
- 1. Tinévez C, Velardo F, Ranc AG, et al. Retrospective multicentric study on Campylobacter spp. bacteremia in France: the Campylobacteremia Study. Clin Infect Dis 2022; 75:702–9. [DOI] [PubMed] [Google Scholar]
- 2. Feodoroff B, Lauhio A, Ellström P, Rautelin H. A nationwide study of Campylobacter jejuni and Campylobacter coli bacteremia in Finland over a 10-year period, 1998–2007, with special reference to clinical characteristics and antimicrobial susceptibility. Clin Infect Dis 2011; 53:e99–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gazaigne L, Legrand P, Renaud B, et al. Campylobacter fetus bloodstream infection: risk factors and clinical features. Eur J Clin Microbiol Infect Dis 2008; 27:185–9. [DOI] [PubMed] [Google Scholar]
- 4. Chlebicz A, Śliżewska K. Campylobacteriosis, salmonellosis, yersiniosis, and listeriosis as zoonotic foodborne diseases: a review. Int J Environ Res Public Health 2018; 15:863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Maes B, Hadaya K, de Moor B, et al. Severe diarrhea in renal transplant patients: results of the DIDACT study. Am J Transplant 2006; 6:1466–72. [DOI] [PubMed] [Google Scholar]
- 6. Aulagnon F, Scemla A, DeWolf S, Legendre C, Zuber J. Diarrhea after kidney transplantation: a new look at a frequent symptom. Transplantation 2014; 98:806–16. [DOI] [PubMed] [Google Scholar]
- 7. Coste JF, Vuiblet V, Moustapha B, et al. Microbiological diagnosis of severe diarrhea in kidney transplant recipients by use of multiplex PCR assays. J Clin Microbiol 2013; 51:1841–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Echenique IA, Penugonda S, Stosor V, Ison MG, Angarone MP. Diagnostic yields in solid organ transplant recipients admitted with diarrhea. Clin Infect Dis 2015; 60:729–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. van den Bogaart L, Lang BM, Neofytos D, et al. Epidemiology and outcomes of medically attended and microbiologically confirmed bacterial foodborne infections in solid organ transplant recipients. Am J Transplant 2022; 22:199–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pacanowski J, Lalande V, Lacombe K, et al. Campylobacter bacteremia: clinical features and factors associated with fatal outcome. Clin Infect Dis 2008; 47:790–6. [DOI] [PubMed] [Google Scholar]
- 11. Barton Behravesh C, Jones TF, Vugia DJ, et al. Deaths associated with bacterial pathogens transmitted commonly through food: foodborne diseases active surveillance network (FoodNet), 1996–2005. J Infect Dis 2011; 204:263–7. [DOI] [PubMed] [Google Scholar]
- 12. Bessède E, Delcamp A, Sifré E, Buissonnière A, Mégraud F. New methods for detection of campylobacters in stool samples in comparison to culture. J Clin Microbiol 2011; 49:941–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Comité de l’Antibiograme de la Société Française de Microbiologie . Société Française de Microbiologie. 2023. Available at: https://www.sfm-microbiologie.org/wp-content/uploads/2023/06/CASFM2023_V1.0.pdf. Accessed 15 November 2023.
- 14. Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract 2012; 120:c179–84. [DOI] [PubMed] [Google Scholar]
- 15. Santé Publique France . Bilan de la surveillance des infections à Campylobacter en France en 2021. 2022. Available at: https://www.santepubliquefrance.fr/maladies-et-traumatismes/maladies-infectieuses-d-origine-alimentaire/campylobacter/documents/bulletin-national/bilan-de-la-surveillance-des-infections-a-campylobacter-en-france-en-2021. Accessed 15 January 2023.
- 16. Attias P, Melica G, Boutboul D, et al. Epidemiology, risk factors, and outcomes of opportunistic infections after kidney allograft transplantation in the era of modern immunosuppression: a monocentric cohort study. J Clin Med 2019; 8:594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fishman JA. Infection in organ transplantation. Am J Transplant 2017; 17:856–79. [DOI] [PubMed] [Google Scholar]
- 18. de Zoete MR, Keestra AM, Roszczenko P, van Putten JPM. Activation of human and chicken Toll-like receptors by Campylobacter spp. Infect Immun 2010; 78:1229–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hu L, Hickey TE. Campylobacter jejuni induces secretion of proinflammatory chemokines from human intestinal epithelial cells. Infect Immun 2005; 73:4437–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Callahan SM, Dolislager CG, Johnson JG. The host cellular immune response to infection by Campylobacter spp. and its role in disease. Infect Immun 2021; 89:e0011621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Iovine NM. Resistance mechanisms in Campylobacter jejuni. Virulence 2013; 4:230–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lachance N, Gaudreau C, Lamothe F, Larivière LA. Role of the beta-lactamase of Campylobacter jejuni in resistance to beta-lactam agents. Antimicrob Agents Chemother 1991; 35:813–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jeong R, Quinn RR, Lentine KL, et al. Outcomes following macrolide use in kidney transplant recipients. Can J Kidney Health Dis 2019; 6:2054358119830706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Paterson DL, Singh N. Interactions between tacrolimus and antimicrobial agents. Clin Infect Dis 1997; 25:1430–40. [DOI] [PubMed] [Google Scholar]
- 25. Fleet JL, Shariff SZ, Bailey DG, et al. Comparing two types of macrolide antibiotics for the purpose of assessing population-based drug interactions. BMJ Open 2013; 3:e002857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. World Health Organization . AWaRe classification. 2021. Available at: https://www.who.int/publications-detail-redirect/2021-aware-classification. Accessed 17 April 2023.
- 27. Kaakoush NO, Castaño-Rodríguez N, Mitchell HM, Man SM. Global epidemiology of Campylobacter infection. Clin Microbiol Rev 2015; 28:687–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Koningstein M, Simonsen J, Helms M, Hald T, Mølbak K. Antimicrobial use: a risk factor or a protective factor for acquiring campylobacteriosis? Clin Infect Dis 2011; 53:644–50. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.