Abstract
BALB/c mice are resistant to measles virus (MV)-induced encephalitis due to their strong MV-specific CD4+ T-cell response. Resistance is broken by neutralization of gamma interferon with monoclonal antibodies, indicating an important role for this pleiotropic cytokine. Here, we demonstrate that mouse gamma interferon has no direct antiviral effect in vitro and in vivo. The breakdown of resistance is due neither to a switch in the T-helper response nor to an impaired migration of CD4+ T cells. Neutralization of gamma interferon interferes with the major histocompatibility complex class II-dependent antigen presentation and subsequent proliferation of CD4+ T cells in vitro and in vivo. In consequence, the reduction in numbers of CD4+ T cells below a protective threshold leads to susceptibility to MV-induced encephalitis.
Gamma interferon (IFN-γ) is a pleiotropic cytokine with multiple functions in the defense against pathogens (1). IFN-γ is produced by activated natural killer (NK) cells and activated CD4 as well as CD8 T cells of the type 1 subset (T-helper 1 [TH1] [20] and Tc1 [25], respectively). It induces antiviral activity by stimulating the transcription of the double-stranded RNA-activated protein kinase, the 2′-5′-oligoadenylate synthetase, and double-stranded RNA-specific adenosine deaminase (1). IFN-γ is also an important regulator of the development of TH1 or TH2 responses by suppressing the secretion of interleukin-4 (IL-4) and stimulating in an autocrine loop the secretion of IFN-γ and the development of a TH1 response (20). Furthermore, it stimulates antigen processing and presentation of both major histocompatibility complex class I (MHC-I)- and MHC-II-restricted antigens. The stimulation of antigen processing and presentation via MHC-I is due to simultaneous induction of a variety of different molecules (MHC-I, transporter of antigen presentation [TAP], LMP, etc.), whereas all key genes for MHC-II antigen presentation are regulated by the single IFN-γ-inducible transcription factor CIITA (class II transactivator) (17). In addition, IFN-γ stimulates the expression of adhesion molecules, thereby probably influencing the migration of lymphocytes (21). Studies of mice with a disruption of the IFN-γ gene have shown that IFN-γ is crucial in mounting an immune response against some intracellular bacteria and parasites (3, 14, 26). For mice unable to respond to IFN-γ due to a deletion of the IFN-γ receptor gene, increased susceptibility to vaccinia virus and lymphocytic choriomeningitis virus was observed (14). In contrast, deletion of the IFN-γ gene had no influence on the immune response against influenza A virus (9) and Sendai virus (19).
The protective function of IFN-γ has also been investigated in the mouse model of measles virus (MV)-induced encephalitis (MVE) (7). After infection with a neurotropic rodent-adapted MV (strain CAM/RBH), susceptible C3H mice succumb to encephalitis after 5 to 9 days (22), and this correlates with the development of a TH2-like response (no IL-4, but IL-6 and IL-10) (7). In contrast, BALB/c mice are resistant to MVE and develop CD4 T cells of the TH1 type secreting IL-2 and IFN-γ, which alone are sufficient to protect against MVE (7, 8). After neutralization of IFN-γ by injection of neutralizing monoclonal antibodies (MAbs), resistance is abolished and BALB/c mice are highly susceptible to encephalitis (7). This is correlated with the development of a typical TH2 response with CD4 T cells secreting IL-4, IL-6, and IL-10 (7). As the susceptible C3H mice also develop a TH2-like response, it was assumed that the TH2 phenotype correlates with the breakdown of resistance.
Here, we investigated whether the breakdown of resistance after neutralization of IFN-γ is due to a lack of direct antiviral activity of IFN-γ, a switch in the TH response from the type 1 to the type 2 phenotype, an impairment of migration of T cells, or MHC-II-dependent antigen presentation.
MATERIALS AND METHODS
Mice.
BALB/c and C3H mice were bought from Harlan Winkelmann (Borchem, Germany), and B6.129S7-Ifngtm1Ts (IFN-γ−/−) and C57BL/6J (IFN-γ+/+) mice were bought from the Jackson Laboratory (Bar Harbor, Maine). Mice were specific pathogen free (specification according to the company). Every 3 to 4 months, animals were checked for pathogens by serological examination. Animals were kept in a barrier system with light negative pressure (100 mPa) and a 12-h day (artificial light) and were fed and watered ad libitum. The room temperature (21 ± 2°C) and the humidity (50% ± 5%) were regulated by air conditioning. Mice were used between the ages of 6 and 18 weeks.
Cells and antibodies.
Vero cells (African green monkey kidney cells) were grown in minimal essential medium with 5% fetal calf serum (FCS), and the human fibroblast cell line HEp-2 was grown in minimal essential medium plus 10% FCS. Hybridoma cells secreting anti-IL-4 antibody (clone 11B11), anti-IFN-γ (R4-6A2; American Type Culture Collection), anti-IL-10 (clone JES2A5; kindly provided by Anne O'Garra, Department of Immunobiology, DNAX Research Institute of Molecular and Cellular Biology, Palo Alto, Calif.), or anti-CD4 (YTS 191; European Collection of Cell Cultures, Porton Down, United Kingdom) were grown in RPMI/10. MAbs were purified from tissue culture supernatants on protein G-Sepharose columns (Pharmacia, Hamburg, Germany) according to standard protocols (7).
Virus and bacteria.
The rodent-adapted neurotropic MV strain CAM/RBH was grown and used as described previously (22). MV (Edmonston strain) was grown in Vero cells, and for the stimulation of CD4+ T cells, it was purified and UV inactivated as described previously (23). Both viruses are closely related and belong to clade A (24). Recently, differences in neuropathogenicity were shown to be due to single amino acid differences in the MV hemagglutinin (6). Escherichia coli K-12 (strain TG1) was grown in Luria-Bertani (LB) broth medium overnight. In plateau phase, bacteria were centrifuged, resuspended in phosphate-buffered saline (PBS), and heat inactivated at 70°C for 1 h. Inactivated bacteria were controlled for growth on microbiological plates.
Infection and treatment of mice.
Mice were infected intracerebrally (i.c.) with 104 50% tissue culture infective doses (TCID50) of MV (strain CAM/RBH) in a 20-μl volume or intraperitoneally (i.p.) with MV (Edmonston strain). For switching the TH response, 200 μg of anti-IL-4 antibody (clone 11B11) and 200 μg of anti-IL-10 (clone JES2A5) or 200 μg of heat-inactivated E. coli (as a source of lipopolysaccharide [LPS]) was injected i.p.
To test the effect of IFN-γ in vivo, BALB/c mice were infected at the age of 5 weeks. For neutralization of IFN-γ, mice were treated daily with 2.5 × 103 neutralizing units of the MAb R4-6A2. The neutralization titer of anti-murine IFN-γ antibody R4-6A2 was assessed by a plaque reduction assay as described previously (10). Briefly, equal volumes of recombinant IFN-γ (5 U/ml) were incubated with serially diluted R4-6A2 (for 24 h at 37°C) before L929 cells (1.8 × 105/ml) and vesicular stomatitis virus (VSV) (multiplicity of infection, 0.1) were added. After 24 h, cultures were examined for cytopathic effect. The neutralization titer was defined as the reciprocal of the highest antibody dilution that reduced the protective effect of recombinant IFN-γ by more than 50%.
T-cell cultures, cytokine secretion, and ex vivo proliferation.
For the growth of CD4+ T cells, 3 × 107 to 4 × 107 spleen cells from primed animals were cultured in an upright 50-ml flask in 15 ml of RPMI/10 with 60 μg of UV-inactivated MV for 3 days. Blast cells were obtained by a Percoll gradient (1,077 g/ml) and cultured in RPMI/10 and 2% T-cell supernatant containing IL-2 at a density of 6 × 105/ml. For restimulation, 3 × 106 to 4 × 106 T cells were mixed with 3 × 107 to 4 × 107 irradiated spleen cells and 60 μg of UV-inactivated MV.
Supernatants of CD4+ T-cell cultures were harvested after 24 to 72 h after the second in vitro stimulation with UV-inactivated MV. MAb pairs for a sandwich enzyme-linked immunosorbent assay (ELISA) for the detection of IFN-γ, IL-4, IL-5, and IL-10 as well as the respective recombinant cytokines as standards were obtained from PharMingen (Hamburg, Germany). Cytokine ELISA was performed according to the manufacturer's recommendations.
For proliferation assays investigating the influence of IFN-γ on antigen presentation, IFN-γ+/+ and IFN-γ−/− spleen cells were inactivated with mitomycin C. Cells were seeded at a density of 2.5 × 105 cells/well in a 96-well plate, and effector cells were added at a density of 5 × 105 cells/well. Concentrations of recombinant mouse IFN-γ were 2.5 pg/ml, and those of IFN-γ-neutralizing antibody R4-6A2 were 12.5 μg/ml.
For direct ex vivo proliferation assays, single-cell suspensions of spleen cells were plated in triplicate at 5 × 105 cells/well into 96-well flat-bottomed plates in RPMI 1640–1% mouse serum with or without 2.5 μg of gradient-purified UV-inactivated MV/ml. After 2 days, cultures were labeled with [3H]thymidine, and after 16 to 20 h, they were harvested as described previously (22). The stimulation index (SI) was calculated as the ratio of counts of MV-stimulated cells per minute to medium controls. For antigen-independent stimulation, cells were stimulated with 2.5 μg of concanavalin A (Sigma)/ml.
Determination of IgG isotypes by ELISA.
For determination of immunoglobulin G (IgG) isotype, 10 μg of gradient-purified, UV-inactivated MV/ml was coated with 200 mM NaCO3 buffer (pH 9.6) at 4°C overnight, blocked with PBS–10% FCS–0.05% Tween 20, and incubated with MV-specific mouse serum at 4°C for 1 h. After washing, the plate was incubated for 45 min at room temperature with alkaline phosphatase-coupled goat anti-mouse antibodies, specific for the different IgG subtypes (Southern Biotechnology, Birmingham, Ala.), and was subsequently developed with 0.5 mg of p-nitrophenylphosphate (Sigma)/ml in diethanolamine buffer (pH 9.8).
Adoptive transfer of MV-specific CD4+ T cells.
For adoptive transfer, virus-specific T cells were purified using nylon wool columns as described previously (15). Briefly, spleen cell suspensions were loaded onto nylon wool columns at a density of 5 × 107 cells/ml in Hanks balanced salt solution containing 5% FCS and incubated for 45 min at 37°C. The columns were washed with 2 column volumes of warm (37°C) Hanks balanced salt solution–5% FCS, and the cells in the effluent were pelleted at 300 × g for 15 min at 4°C. The efficiency of purification was determined by flow cytometry. The preparations always contained less than 4% Ig-bearing cells. One spleen equivalent of the pelleted cells was resuspended in 100 μl of PBS–0.1% FCS and injected into the tail vein of the recipient animal. Immediately after transfer, the animals were infected i.c. and depleted of CD8+ T cells by injection of 0.25 mg of MAb. Supernatants from hybridoma YTS 169 (specific for mouse CD8) were purified using a Sepharose G column (Pharmacia) and dialyzed against PBS. The amount of antibody was determined with the Bio-Rad protein assay, and the effectiveness of the in vivo depletion of the CD8+ T-cell subsets was confirmed by flow cytometry with MAb MCA 609 G (Serotech). Depletion was repeated every fourth day. This scheme of depletion was monitored by flow cytometry. Within the limit of detection (2%), no CD8+ T cells were found on days 1 and 7 after a single injection of MAb. In previous experiments, injection of an isotype-matched MAb of irrelevant specificity did not influence the CD4+ and CD8+ T-cell subsets.
Antiviral effect of IFN-γ in vitro.
The antiviral effect of IFN-γ was assessed as described previously (16). Cells were incubated for 24 h with or without IFN-γ and then infected with a multiplicity of infection of 0.1 (VSV) or 1 (MV) for another 24 h. For VSV infection and MV infection of human cells, virus inhibition was directly assessed by scoring cytopathic effects in a 96-well plate. MV-infected mouse L929 and 3T3 cells were harvested from six-well plates and freeze-thawed three times, and viral titers were determined by a plaque assay. Human IFN-γ was purchased from R & D Systems. Mouse IFN-γ contained in T-cell supernatants was measured by ELISA and tested for biological activity against VSV, and the specific activity was calculated as units per milligram = 1/50% effective dose (ED50) in micrograms per milliliter.
RESULTS
No antiviral effect of mouse IFN-γ.
IFN-γ has been shown to exert (at least in vitro) a direct antiviral effect on some viruses like VSV, encephalomyocarditis virus, or Semliki Forest virus (16). Neutralization of IFN-γ might simply neutralize its antiviral action. Therefore, the antiviral activity of mouse IFN-γ on MV was tested in vitro and in vivo. MV replicates relatively poorly in rodent cells, but the mouse fibroblast cell lines L929 (derived from C3H mice) and 3T3 (derived from Swiss mice) have been found to support MV replication quite well (5). The antiviral activity of mouse IFN-γ against VSV (as a control) and MV was tested on these cell lines. Whereas the ED50 (protection of 50% of cells) for VSV was determined as 2 to 4 μg of mouse IFN-γ/ml, the ED50 for MV was higher than 400 μg/ml. To investigate whether this effect was specific for mouse IFN-γ, we tested the protective capacity of human IFN-γ against MV on human HEp-2 cells. Human IFN-γ was effective against MV at an ED50 of 1 to 5 μg/ml. It therefore seems that, in contrast to human IFN-γ, mouse IFN-γ is extremely inefficient in inhibiting MV replication in vitro.
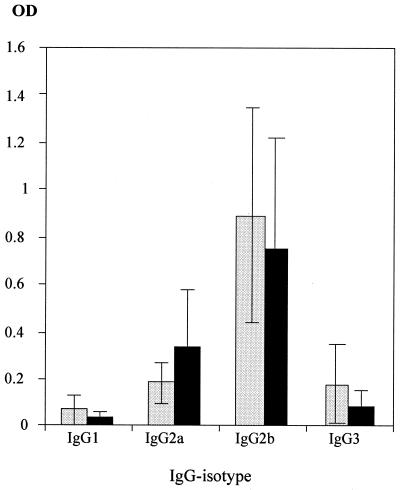
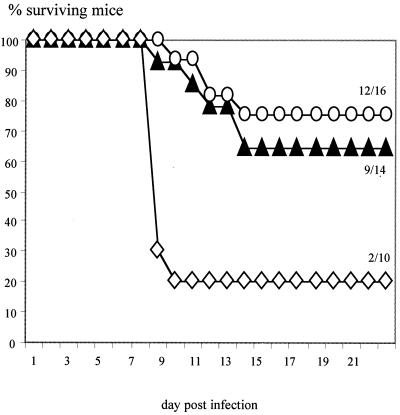
To test the action of mouse IFN-γ in vivo, C57BL/6 mice with a disruption of the IFN-γ gene (IFN-γ−/−) (4) and wild-type C57BL/6 mice (IFN-γ+/+) were used. After immunization with MV, both IFN-γ+/+ and IFN-γ−/− mice develop MV-specific CD4 T cells which proliferate well after in vitro stimulation (Table 1). CD4 T cells from both strains stimulate mainly the production of IgG2b (Fig. 1) and do not secrete IL-4 or IL-5 (Table 1). Due to the disruption of the IFN-γ gene, IFN-γ−/− CD4 T cells do not secrete IFN-γ, in contrast to IFN-γ+/+ T cells. Wild-type C57BL/6 mice (IFN-γ+/+) are highly susceptible to MVE after depletion of CD8 T cells (27). The transfer of MV-specific CD4 T cells (IFN-γ+/+) protected IFN-γ+/+ mice depleted of CD8 T cells against disease (Fig. 2). After transfer of MV-specific CD4 T cells (IFN-γ−/−), the same level of protection was observed. These data demonstrate that mouse IFN-γ has no direct antiviral effect against MV in vitro and in vivo.
TABLE 1.
Proliferation and cytokine production of MV-specific CD4+ T cellsa
| CD4+ T-cell type | MV-specific T-cell proliferation (SI ± SD) | Amt of cytokine produced (± SD)
|
||
|---|---|---|---|---|
| IFN-γ (ng/ml) | IL-4 (ng/ml) | IL-5 (pg/ml) | ||
| IFN-γ−/− | 8.6 ± 1.5 | ND | ND | ND |
| IFN-γ+/+ | 7.8 ± 1.2 | 18 ± 4.36 | ND | ND |
Proliferation and cytokine production of MV-specific CD4+ T cells are identical. C57BL/6 mice (IFN-γ+/+ and IFN-γ−/−) were immunized with 3 × 106 PFU of MV (Edmonston strain). One week later, the generation of MV-specific CD4+ T cells was measured in a proliferation assay. To determine the cytokine profile of MV-specific CD4+ T cells, spleen cells were stimulated with UV-inactivated MV. After 72 h, the amounts of cytokines in the supernatant (IFN-γ, IL-4, and IL-5) were measured. SI, stimulation index; ND, not detected.
FIG. 1.
Antibody isotypes from IFN-γ+/+ and IFN-γ−/− C57BL/6 mice are identical. C57BL/6 mice (IFN-γ+/+ [black bars] and IFN-γ−/− [light gray bars]) were immunized with 3 × 106 PFU of MV (Edmonston strain). Isotypes of MV-specific antibodies in the sera of immunized mice were determined by an MV-specific ELISA (error bars indicate standard deviations). OD, optical density.
FIG. 2.
Protection by MV-specific CD4 T cells in vivo is independent of IFN-γ secretion. MV-specific CD4 T cells (IFN-γ+/+ or IFN-γ−/−) were transferred into C57BL/6 mice (IFN-γ+/+) which had been depleted of CD8 T cells by MAb. Weight loss and clinical signs were monitored over time, and moribund animals were sacrificed. Numbers signify ratios of surviving animals to total animals. Symbols: solid triangles, IFN-γ−/− T cells; open circles, IFN-γ+/+ T cells; open diamonds, no T cells.
Switch from TH2 to TH1 in the C3H mouse does not protect against i.c. infection with MV.
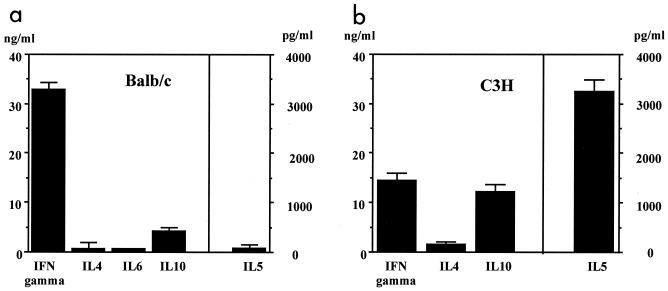
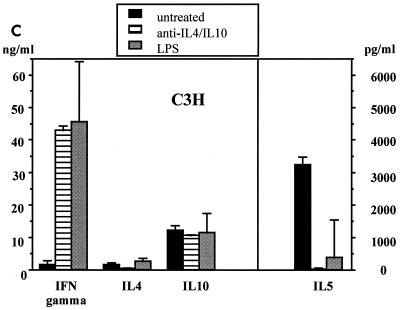
As no direct antiviral activity by IFN-γ was observed, the importance of its regulatory functions was analyzed. First, it was confirmed that BALB/c mice develop a typical TH1 response after i.p. immunization with MV (Fig. 3a). In contrast, C3H mice generated TH2-like T cells which secreted IL-5 and IL-10, but little IL-4 (Fig. 3b). To investigate whether the TH2-like response is the cause of susceptibility, we tried to protect C3H mice by switching the MV-specific T-cell response to a TH1 response. Two methods were used to induce a TH1 response: the injection of MAbs against TH2 cytokines (anti-IL-4 and anti-IL-10) and injection of LPS (which induces IL-12 secretion, stimulating IFN-γ production and a TH1 response) (12, 13). Treatment of mice infected i.p. with MV (strain Edmonston) led to the development of an MV-specific TH1 response (Fig. 3c). To test the protective capacity of a switch to a TH1 response, 5-week-old C3H mice (10 per group) were infected i.c. with 5 × 104 TCID50 of neurotropic MV strain CAM/RBH and treated with either anti-IL-4 and anti-IL-10 MAbs (200 μg each) or heat-inactivated E. coli LPS (to induce IL-12; 200 μg). There was no difference in mortality between treated and untreated animals: 9 of 10 untreated mice, 10 of 10 MAb-treated mice, and 9 of 10 LPS-treated mice died. To exclude the possibility that treatment would be more effective in animals with a more mature immune system, 8-week-old C3H mice were used. Again, no difference in morbidity or mortality was observed between treated and untreated controls (data not shown).
FIG. 3.
Switch of a TH2 to a TH1 response in susceptible C3H mice after i.p. infection. Animals from the resistant (BALB/c) (a) and the susceptible (C3H) (b) mouse strains were infected i.p. with 107 PFU of MV (strain Edmonston). Other groups of C3H mice were infected i.p. with 107 PFU of MV (strain Edmonston) and coinjected with MAbs against IL-4 and IL-10 or heat-inactivated E. coli (as a source of LPS). Supernatants from MV-specific CD4+ T cells (derived from spleen cells) were assayed 48 to 72 h after stimulation for the presence of IFN-γ, IL-4, IL-5, and IL-10 by ELISA (c).
Differential generation of TH responses after i.c. and i.p. infection.
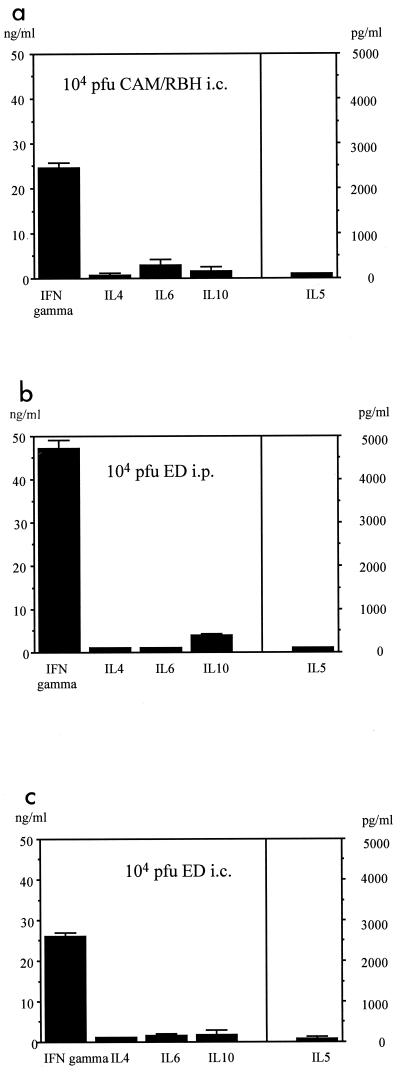
A possible explanation for the failure of the TH1 switch to protect against disease is a differential induction of the TH response after i.p. and i.c. infection. Therefore, C3H mice who had survived MVE were tested and found to have generated (without treatment) MV-specific T cells which secreted IFN-γ but not IL-4 or IL-5 (Fig. 4a). In addition, 2 weeks after i.c. infection the isotype profile of MV-specific antibodies in C3H mice was identical to that of BALB/c mice, with a preference for IgG2a (indicating a TH1 response) (data not shown). In the leishmania system, the difference in the development of a TH1 or TH2 response has been shown to be due to the number of pathogens (amount of antigen) inoculated (2). Therefore, the differential TH responses after i.p. immunization and i.c. infection could be due to differences in virus strain (Edmonston or CAM/RBH) or virus dose (107 PFU i.p. and 104 PFU i.c.). To test which one was responsible for this effect, the TH response was tested after i.p. and i.c. injection of Edmonston virus. i.p. infection with a low dose of virus (104 PFU) led to a TH1 response (Fig. 4b), whereas the high dose (107 PFU) resulted in a TH2 response (Fig. 3b). i.c. infection with the Edmonston strain resulted (as did i.c. infection with the CAM/RBH strain) in a TH1 response (Fig. 4c). In summary, these data demonstrate that the development of a TH1 response after i.c. infection with MV is not correlated with resistance and susceptibility.
FIG. 4.
Influence of viral titer and route of infection on development of a TH1 or TH2 response. C3H mice were infected i.c. with 104 PFU of MV (strain CAM/RBH), and the MV-specific TH response of surviving animals was tested (a). Other animals were infected i.p. (b) or i.c. (c) with 104 PFU of MV (strain Edmonston [ED]). i.c. infected animals did not display clinical signs. Supernatants from MV-specific CD4+ T cells (derived from spleen cells) were assayed 48 to 72 h after stimulation for the presence of IFN-γ, IL-4, IL-5, and IL-10 by ELISA (error bars indicate standard deviations).
Lack of IFN-γ has no effect on migration of CD4 T cells.
To investigate the influence of IFN-γ on migration, MV-specific CD4 T cells from both IFN-γ+/+ and IFN-γ−/− mice were transferred into IFN-γ−/− mice. No significant difference in protection was noted between the two groups (6 out of 15 IFN-γ+/+ animals survived and 4 out of 6 IFN-γ−/− mice survived). In addition, MV-specific CD4 T cells from IFN-γ+/+ mice were labeled with the fluorescent dye CSFE (5-carboxyfluorescein diacetate succinimidyl ester) and transferred into both IFN-γ+/+ and IFN-γ−/− mice. After transfer, the same number of lymphocytes was recovered from brain tissue of the two groups by Percoll gradient on days 5 and 6 after infection (data not shown). In addition, CD4 T cells were counted in coronal cryostat sections of the brain and no difference in the number of cells was found (per coronary section, 27 ± 5.2 CD4+ T cells in IFN-γ+/+ mice and 33.1 ± 9.7 CD4 T cells in IFN-γ−/− mice). In aggregate, these data indicate that migration of CD4 T cells was not impaired by the lack of IFN-γ.
IFN-γ stimulates antigen presentation and T-cell proliferation in vitro and in vivo.
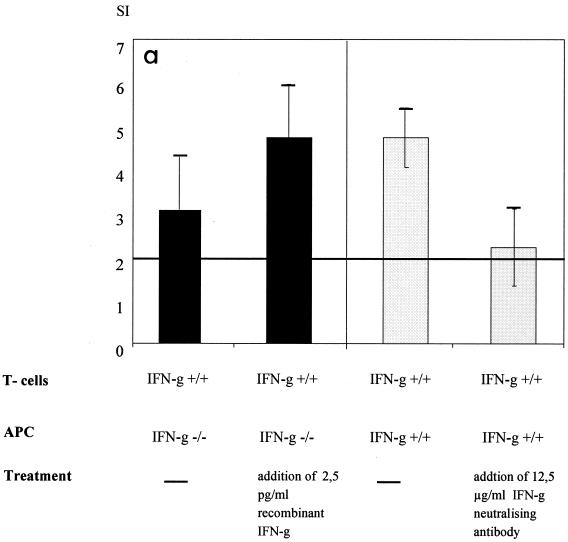
To investigate the influence of IFN-γ on MHC-II-dependent antigen presentation, IFN-γ−/− and IFN-γ+/+ antigen-presenting cells (APC) were used to stimulate MV-specific CD4 T cells (IFN-γ+/+) in vitro. APC (IFN-γ−/−) unable to secrete IFN-γ did not stimulate MV-specific proliferation as well as did normal APC (IFN-γ+/+) (Fig. 5a). The addition of recombinant IFN-γ significantly restored the stimulatory capacity of IFN-γ−/− APC. In a reverse experiment, the neutralization of IFN-γ significantly reduced the stimulatory capacity of IFN-γ+/+ APC.
FIG. 5.
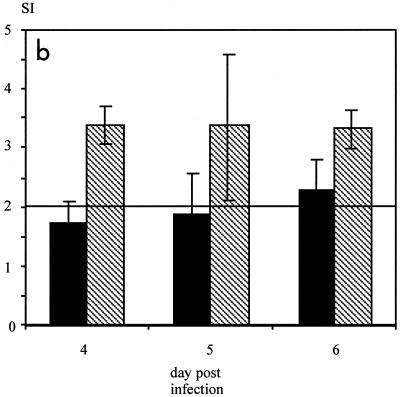
Neutralization of IFN-γ inhibits antigen processing and T-cell proliferation in vitro and in vivo. (a) MV-specific CD4 T cells were stimulated with UV-inactivated MV and IFN-γ+/+ or IFN-γ−/− APC. In addition, either recombinant IFN-γ (2.5 pg/ml) or IFN-γ-neutralizing antibodies (12.5 μg/ml) were added. The differences in the SI with and without the addition of recombinant IFN-γ were significant (P < 0.05, Kruskal-Wallis test), as were the differences with and without neutralizing antibody (P < 0.05, t test) (error bars indicate standard deviations). The lack of IFN-γ did not reduce constitutive MHC-II expression on APC, whereas the addition of IFN-γ increased it (data not shown). Concanavalin A-dependent proliferation was not influenced by the lack or addition of IFN-γ (data not shown). In addition, concanavalin A-dependent proliferation of spleen cells from naive animals was not influenced by IFN-γ concentrations ranging from 40 ng to 0.04 pg/ml (data not shown). (b) BALB/c mice were infected i.c. with 5 × 104 TCID50 of MV strain CAM/RBH and injected daily with IFN-γ-neutralizing antibodies (black bars) or left untreated (striped bars). On days 4, 5, and 6, MV-specific T-cell proliferation was measured from spleen cells (error bars indicate standard deviations). The levels of proliferation of spleen cells from these animals after concanavalin A stimulation were comparable (data not shown).
To evaluate whether neutralization of IFN-γ also reduced proliferation of MV-specific T cells in vivo, i.c. infected BALB/c mice were treated with IFN-γ-neutralizing antibodies and T-cell proliferation was tested. MV-specific proliferation of CD4 T cells from spleen cells of treated mice was reduced on days 4 to 6 after infection in comparison to that for nontreated controls (Fig. 5b).
DISCUSSION
MV-specific CD4+ T cells are the important T-cell subset for protection against MVE in the mouse model. Depletion of this subset in resistant mice leads to the breakdown of resistance (8), whereas immunization with a CD4+ T-cell epitope protects susceptible mice, as does the transfer of MV-specific CD4+ T cells (27). In contrast, primary CD8+ T cells alone are never able to protect against MVE (27), and resistance is not affected by depletion of CD8+ T cells (8). After neutralization of IFN-γ, breakdown of resistance was observed with resistant mice (7). To analyze the underlying mechanism, we first investigated the direct antiviral effect of IFN-γ against MV in vitro and in vivo. Interestingly enough, human but not mouse IFN-γ protected fibroblast cells against MV infection. Also in vivo, the protective capacity of transferred MV-specific CD4+ T cells was independent of their ability to secrete IFN-γ. This is in contrast to findings by Maloy et al. (18), who demonstrated that clearance of vaccinia virus is due to the secretion of IFN-γ by T cells.
A causal correlation between TH2 response and susceptibility was assumed (7) based on the finding that neutralization of IFN-γ led to a TH2 response in BALB/c mice. In addition, susceptible C3H mice generated a TH2-like response after i.p. inoculation of a high virus dose. However, as shown in this study the development of a TH2 response is due to the high titer of virus inoculated. After i.c. infection with a low titer of the neuropathogenic MV, a TH1 response (with relatively low levels of IFN-γ secretion) is generated. Even inducing a stronger TH1 response by neutralizing IL-4 and IL-10 or induction of IL-12 does not protect against disease.
Other regulatory mechanisms of IFN-γ in T-cell function include the influence on the expression of adhesion molecules, thereby putatively affecting migration. Similar to infection with lymphocytic choriomeningitis virus (21), no influence of IFN-γ on T-cell migration was observed in MVE. After in vivo neutralization of IFN-γ in mice infected with murine cytomegalovirus, a decreased generation of peptide epitopes for CD8+ T cells was observed (11). Because CD8+ T cells are important in determining the outcome of murine cytomegalovirus infection, the inhibition of MHC-I presentation is fundamental. In MVE, neutralization of IFN-γ in vitro and in vivo clearly inhibited antigen presentation toward CD4+ T cells. In contrast to MHC-I-dependent antigen presentation, presentation via MHC-II molecules is exclusively regulated by CIITA, a transcriptional coactivator, which is required for constitutive and IFN-γ-induced expression of the MHC-II antigen presentation pathway (1). The lack of IFN-γ did not, however, decrease the constitutive expression of MHC-II molecules (data not shown). Also, IFN-γ did not act on T-cell proliferation directly (e.g., as a growth factor) as shown by the independence of concanavalin A-stimulated proliferation of IFN-γ.
It has been shown previously that the activation of MV-specific CD4+ T cells in spleens of resistant mice correlates with low virus titers in brain tissue, resulting in lack of clinical signs and protection (27). In contrast, a lack of MV-specific CD4+ T-cell proliferation in spleens of susceptible mice correlates with high virus titers in brain tissue and death (27). Therefore, the neutralization of IFN-γ inhibits MHC-II-dependent antigen presentation and subsequent proliferation of CD4+ T cells, resulting in breakdown of resistance.
In mice with a deletion in the IFN-γ gene, the primary immune response toward measles is also affected by the lack of IFN-γ because all IFN-γ−/− mice die after MV infection with faster kinetics than those of IFN-γ+/+ control mice (data not shown). However, in the secondary immune response T-cell proliferation is comparable to that for IFN-γ+/+ mice (Table 1) and the transfer of MV-specific CD4+ T cells (IFN-γ−/−) into IFN-γ−/− mice protects against encephalitis. This demonstrates that the influence of IFN-γ on antigen presentation is overcome during the secondary immune response in these animals. It seems to be obvious that the constant lack of IFN-γ in these animals has led to so far unknown adaptations in antigen processing. In contrast, in animals with an intact genome the neutralization of IFN-γ inhibits antigen processing and T-cell proliferation.
REFERENCES
- 1.Boehm U, Klamp T, Groot M, Howard J C. Cellular responses to interferon-gamma. Annu Rev Immunol. 1997;15:749–796. doi: 10.1146/annurev.immunol.15.1.749. [DOI] [PubMed] [Google Scholar]
- 2.Bretscher P A, Wei G, Menon J N, Bielefeldt O H. Establishment of stable cell-mediated immunity that makes “susceptible” mice resistant to Leishmania major. Science. 1992;257:539–542. doi: 10.1126/science.1636090. [DOI] [PubMed] [Google Scholar]
- 3.Cooper A M, Dalton D K, Stewart T A, Griffin J P, Russell D G, Orme I M. Disseminated tuberculosis in interferon γ gene-disrupted mice. J Exp Med. 1993;178:2243–2247. doi: 10.1084/jem.178.6.2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dalton D K, Pitts-Meek S, Keshav S, Figari I S, Bradley A, Stewart T A. Multiple defects of immune cell function in mice with disrupted interferon-gamma. Science. 1993;259:1739–1742. doi: 10.1126/science.8456300. [DOI] [PubMed] [Google Scholar]
- 5.Dunster L M, Schneider-Schaulies J, Dehoff M H, Holers V M, Schwartz-Albiez R, ter Meulen V. Moesin, and not the murine functional homologue (Crry/p65) of human membrane cofactor protein (CD46) is involved in the entry of measles virus (strain Edmonston) into susceptible murine cell lines. J Gen Virol. 1995;76:2085–2089. doi: 10.1099/0022-1317-76-8-2085. [DOI] [PubMed] [Google Scholar]
- 6.Duprex W P, Duffy I, McQuaid S, Hamill L, Cosby S L, Billeter M A, Schneider-Schaulies J, ter Meulen V, Rima B K. The H gene of rodent brain-adapted measles virus confers neurovirulence to the Edmonston vaccine strain. J Virol. 1999;73:6916–6922. doi: 10.1128/jvi.73.8.6916-6922.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Finke D, Brinckmann U G, ter Meulen V, Liebert U G. Gamma interferon is a major mediator of the antiviral defense in experimental measles virus-induced encephalitis. J Virol. 1995;69:5469–5474. doi: 10.1128/jvi.69.9.5469-5474.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Finke D, Liebert U G. CD4+ T cells are essential in overcoming experimental murine measles encephalitis. Immunology. 1994;83:184–189. [PMC free article] [PubMed] [Google Scholar]
- 9.Graham B M, Dalton D K, Giltinan D, Braciale V L, Stewart T A, Braciale T J. Response to influenza infection in mice with a targeted disruption in the interferon gamma gene. J Exp Med. 1993;178:1725–1732. doi: 10.1084/jem.178.5.1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Havell E A, Spitalny G L. Production and characterization of anti-murine interferon-gamma sera. J Interferon Res. 1983;3:191–198. doi: 10.1089/jir.1983.3.191. [DOI] [PubMed] [Google Scholar]
- 11.Hengel H, Lucin P, Jonjic S, Ruppert T, Koszinowski U H. Restoration of cytomegalovirus antigen presentation by gamma interferon combats viral escape. J Virol. 1994;68:289–297. doi: 10.1128/jvi.68.1.289-297.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hsieh C S, Macatonia S E, O'Garra A, Murphy K M. Pathogen-induced Th1 phenotype development in CD4+ alpha beta-TCR transgenic T cells is macrophage dependent. Int Immunol. 1993;5:371–382. doi: 10.1093/intimm/5.4.371. [DOI] [PubMed] [Google Scholar]
- 13.Hsieh C S, Macatonia S E, Tripps C S, Wolf S F, O'Garra A, Murphy K M. Development of TH1 CD4+ T cells through IL-12 produced by Listeria-induced macrophages. Science. 1993;260:496–497. doi: 10.1126/science.8097338. [DOI] [PubMed] [Google Scholar]
- 14.Huang S, Hendriks W, Althage A, Hemmi S, Bluethmann H, Kamijo R, Vilcek J, Zinkernagel R M, Aguet M. Immune response in mice that lack the interferon-γ receptor. Science. 1993;259:1742–1745. doi: 10.1126/science.8456301. [DOI] [PubMed] [Google Scholar]
- 15.Julius H M, Simpson E, Herzenberg L A. A rapid method for the isolation of functional thymus-derived murine lymphocytes. Eur J Immunol. 1973;3:645–649. doi: 10.1002/eji.1830031011. [DOI] [PubMed] [Google Scholar]
- 16.Lewis J A. Antiviral activity of cytokines. In: Balkwill F R, editor. Cytokines. Oxford, United Kingdom: Oxford University Press; 1991. pp. 109–120. [Google Scholar]
- 17.Mach B, Steimle V, Martinez-Soria E, Reith W. Regulation of MHC class II genes: lessons from a disease. Annu Rev Immunol. 1996;14:301–331. doi: 10.1146/annurev.immunol.14.1.301. [DOI] [PubMed] [Google Scholar]
- 18.Maloy K, Burkhart C, Freer G, Rulicke T, Pircher H, Kono D H, Theofilopoulos A, Ludewig B, Hoffmann-Rohrer U, Zinkernagel R, Hengartner H. Qualitative and quantitative requirements for CD4+ T cell-mediated antiviral protection. J Immunol. 1999;162:2867–2874. [PubMed] [Google Scholar]
- 19.Mo X Y, Tripp R A, Sangster M Y, Doherty P C. The cytotoxic T-lymphocyte response to Sendai virus is unimpaired in the absence of gamma interferon. J Virol. 1997;71:1906–1910. doi: 10.1128/jvi.71.3.1906-1910.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mosmann T R, Coffman R L. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–174. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- 21.Nansen A, Christensen J, Ropke C, Marker O, Scheynius A, Thomsen A. Role of interferon-gamma in the pathogenesis of LCMV-induced meningitis: unimpaired leucocyte recruitment, but deficient macrophage activation in interferon-gamma knock-out mice. J Neuroimmunol. 1998;86:202–212. doi: 10.1016/s0165-5728(98)00055-1. [DOI] [PubMed] [Google Scholar]
- 22.Niewiesk S, Brinckmann U, Bankamp B, Sirak S, Liebert U G, ter Meulen V. Susceptibility to measles virus-induced encephalitis in mice correlates with impaired antigen presentation to cytotoxic T lymphocytes. J Virol. 1993;67:75–81. doi: 10.1128/jvi.67.1.75-81.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reich A, Erlwein O, Niewiesk S, ter Meulen V, Liebert U G. CD4+ T cells control measles virus infection of the central nervous system. Immunology. 1992;76:185–191. [PMC free article] [PubMed] [Google Scholar]
- 24.Rima B, Earle J, Baczko K, ter Meulen V, Liebert U, Carstens C, Carabana J, Caballero M, Celma M, Fernandez-Munoz R. Sequence divergence of measles virus haemagglutinin during natural evolution and adaptation to cell culture. J Gen Virol. 1997;78:97–106. doi: 10.1099/0022-1317-78-1-97. [DOI] [PubMed] [Google Scholar]
- 25.Sad S, Marcotte R, Mosmann T R. Cytokine-induced differentiation of precursor mouse CD8+ T cells into cytotoxic CD8+ T cells secreting Th1 or Th2 cytokines. Immunity. 1995;2:271–279. doi: 10.1016/1074-7613(95)90051-9. [DOI] [PubMed] [Google Scholar]
- 26.Wang Z E, Reiner S L, Zheng S, Dalton D K, Locksley R M. CD4+ effector cells default to the Th2 pathway in interferon-γ-deficient mice infected with Leishmania major. J Exp Med. 1994;179:1367–1371. doi: 10.1084/jem.179.4.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weidinger G, Czub S, Neumeister C, Harriott P, ter Meulen V, Niewiesk S. Role of CD4+ and CD8+ T cells in the prevention of measles virus induced encephalitis in mice. J Gen Virol. 2000;81:2707–2713. doi: 10.1099/0022-1317-81-11-2707. [DOI] [PubMed] [Google Scholar]