Abstract
Pathogenic African swine fever virus (ASFV) isolates primarily target cells of the mononuclear-phagocytic system in infected swine and replicate efficiently in primary macrophage cell cultures in vitro. ASFVs can, however, be adapted to grow in monkey cell lines. Characterization of two cell culture-adapted viruses, MS16 and BA71V, revealed that neither virus replicated in macrophage cell cultures. Cell viability experiments and ultrastructural analysis showed that infection with these viruses resulted in early macrophage cell death, which occurred prior to viral progeny production. Genomic cosmid clones from pathogenic ASFV isolate E70 were used in marker rescue experiments to identify sequences capable of restoring MS16 and BA71V growth in macrophage cell cultures. A cosmid clone representing a 38-kbp region at the left terminus of the genome completely restored the growth of both viruses. In subsequent fine-mapping experiments, an 11-kbp subclone from this region was sufficient for complete rescue of BA71V growth. Sequence analysis indicated that both MS16 and BA71V had significant deletions in the region containing members of multigene family 360 (MGF 360) and MGF530. Deletion of this same region from highly pathogenic ASFV isolate Pr4 significantly reduced viral growth in macrophage cell cultures. These findings indicate that ASFV MGF360 and MGF530 genes perform an essential macrophage host range function(s) that involves promotion of infected-cell survival.
African swine fever virus (ASFV) is a large, enveloped, double-stranded DNA virus; it is the sole member of the newly named Asfarviridae family (L. K. Dixon et al., personal communication). Although the icosahedral morphology of the ASFV virion resembles that of iridoviruses, both the genomic organization, which includes terminal cross-links and inverted terminal repeats, and its cytoplasmic replication strategy suggest some relationship with the Poxviridae family (19, 28, 36).
ASFV is the only known DNA arbovirus (8, 10). ASFV infects both warthogs (Phacochoerus aethiopicus) and bushpigs (Potamochoerus spp.), as well as ticks of the genus Ornithodoros, in sub-Saharan Africa (30, 31, 41, 45). In the warthog host, ASFV infection is subclinical, characterized by low viremia titers (32, 42).
In domestic pigs, ASF occurs in several disease forms, ranging from highly lethal to subclinical infections, depending on contributing viral and host factors (9, 24, 32). ASFV infects cells of the mononuclear-phagocytic system, including fixed tissue macrophages, and specific lineages of reticular cells in the spleen, lymph nodes, lungs, kidneys, and liver (9, 21, 22, 24, 25). This ability to replicate and induce marked cytopathology in these cell types in vivo appears to be critical for ASFV virulence. Viral and host factors responsible for the differing outcomes of infection with highly virulent strains and strains of lesser virulence are largely unknown.
Variation in genome size and restriction fragment patterns is observed among different ASFV isolates. Like poxviruses, the diversity within the ASFV genome is localized primarily to terminal genomic regions (6, 7, 12, 37, 44). With poxviruses, genes contained within the terminal variable regions are often nonessential in vitro, instead performing functions related to viral host range (17, 23, 35). ASFV terminal variable regions comprise the left 35-kbp and the right 15-kbp ends of the genome and contain at least five multigene families (MGFs): MGF100, MGF110, MGF300, MGF360, and MGF530 (4, 5, 11, 18, 43, 47). Variations within these regions, including gene deletion events, are observed during ASFV adaptation to monkey cell lines (6, 38). Given the similarities to poxviruses, it is likely that ASFV variable-region genes are associated with important host range functions in the pig or tick host.
Previously, we have identified two ASFV right variable region genes, NL-S and UK, with functions involving virulence and host range in the pathogenic European isolate E70 (48, 49). While these genes are important for ASFV virulence, they alone are not sufficient, indicating that other viral determinants must play significant roles in determining host range and viral virulence (3, 48, 49).
Here, we describe an additional and novel macrophage host range determinant(s) in the left variable region of the ASFV genome. Our data indicate that MGF360 and MGF530 genes in this region perform an essential macrophage host range function that involves promotion of infected-cell survival.
MATERIALS AND METHODS
Cell cultures and viruses.
Vero cells were propagated in Dulbecco's minimal essential medium supplemented with 10% fetal bovine serum. Primary porcine macrophage cell cultures were prepared from heparinized swine blood as previously described (16, 48). Porcine alveolar macrophages were obtained at necropsy by bronchoalveolar lavage from uninfected pigs. These cells were purified and cultivated as described above for primary macrophages.
The pathogenic European ASFV isolate E70, MS16 (E70 passaged 16 times in MS monkey cells [38]), and BA71V (Vero cell culture-adapted ASFV strain BA71) were provided by J. M. Escribano (Instituto Nacional Investigaciones Agrarias, Madrid, Spain). Pathogenic ASFV strain Pretoriuskop/96/4 (Pr4) was isolated from Ornithodoros porcinus porcinus ticks collected from the Republic of South Africa in 1996 (20). A cell culture-adapted variant, Pr4V, was prepared by repeated passaging of Pr4 on Vero cell cultures (L. Zsak, unpublished data).
Cell viability assay.
Porcine primary macrophage cell cultures (2 × 106 cells per well in a six-well plate) were infected with ASFVs (multiplicity of infection [MOI] = 5). Trypan blue dye exclusion viability assays were performed as previously described (27).
Ultrastructural analysis of ASFV-infected macrophages.
Macrophage cell cultures were either mock infected or infected (MOI = 10) with ASFV strain E70, MS16, or BA71V and harvested at 8, 12, 16, and 24 h postinfection (hpi) by gentle removal of the adherent cells with prewarmed phosphate-buffered saline containing 2 mM EDTA. Electron microscopy was performed as previously described (27).
DNA manipulation, cloning, and sequencing.
Viral DNAs were isolated from purified virions using proteinase K and sodium dodecyl sulfate lysis, followed by phenol extraction and ethanol precipitation (44). Southern blot, dot blot, radiolabeling, and hybridization analyses were performed by using standard methods (34). Plasmid DNA was prepared and manipulated essentially as described by Sambrook et al. (34).
Pathogenic European ASFV isolate E70 was passaged three times in swine, and viral DNA was purified from viremic pig blood (44, 49). A cosmid library was constructed from E70 genomic DNA as previously described (49). Cosmid clone G7, representing the 38-kbp left terminus of the genome, was identified and sequenced in its entirety with an Applied Biosystems PRISM 377 automated DNA sequencer (Perkin-Elmer, Foster City, Calif.). Applied Biosystems sequence software (version 3.3) was used for lane tracking and trace extraction. Chromatogram traces were base called with Phred (15); sequences were assembled with Phrap (14) and analyzed by the FASTA method (29), as well as other phylogenetic programs (39, 40). Using a similar approach, cosmid clone M25, from the left 35-kbp genomic region of cell culture-adapted ASFV strain MS16, was identified and sequenced (Lu et al., unpublished data). A 10.3-kbp fragment of cosmid clone G7 was subcloned by digestion with restriction enzymes EcoRI and Pmel and inserted into EcoRI/SmaI-digested plasmid BlueScript II KS (Stratagene, La Jolla, Calif.) to yield pBS-EP (EP).
Marker rescue of MS16 and BA71V growth in macrophage cell cultures.
Primary porcine macrophage cell cultures were infected with either virus strain MS16 or BA71V (MOI = 10) and transfected with DNA clone G7 or EP, respectively, as previously described (49). Cell cultures were harvested 24 h later and sonicated, and serial 10-fold dilutions of the lysates were plated on swine macrophages in 24-well plates and incubated for 5 to 7 days at 37°C. The infected-cell cultures were passaged three additional times in macrophage cell cultures, and putative rescued recombinant viruses were purified by endpoint dilution. Virus stocks of recombinant virus strains MS16-C2 and BA71V-E5 were made in macrophage cell cultures, and viral DNAs were analyzed and characterized by Southern blot hybridization to verify the genomic structure of the recombinants.
Construction of recombinant BA71V viruses containing genomic regions of E70.
To facilitate mutant construction, a β-glucaronidase (GUS)-expressing variant of the BA71V virus, BA71VG, was constructed by introducing the p72GUS reporter gene cassette into a noncoding, intergenic region located between open reading frames (ORFs) A224L and A104R of the BA71V genome at nucleotide position 29900 (46). BA71VG exhibited unaltered BA71V growth characteristics on Vero cell cultures (data not shown).
To insert genomic regions from the pathogenic E70 virus into the cell culture-adapted BA71VG viral genome (see Fig. 8A), an engineered recombination transfer vector was constructed by PCR amplification using BA71V genomic DNA as a template. Genomic regions flanking the deleted region between nucleotide positions 17469 and 17496 in the BA71V genome (46) were amplified using primer sets each of which introduced a BamHI restriction site adjacent to the insertion region and a BglII site (right flanking fragment) at the opposite end. The primer sets were as follows (boldface sequences are restriction cleavage sites for BamHI, GGATCC, and BglII, AGATCT): left flank forward primer, 5′-AAGAGGACGTGCCGTTAAAGTATT-3′; left flank reverse primer, 5′-GGATCCACCTTCACGAGCTGTACG-3′; right flank forward primer, 5′-GGATCCGGCCAACGTTTGTAAAGA-3′; right flank reverse primer, 5′-AGATCTCTTTACGGCTTGGGTCAGGAC-3′. The PCR products were sequentially cloned into the TA cloning vector pCR2.1 (Invitrogen, San Diego, Calif.) to give p71V2. E70 genomic regions were amplified by PCR using cosmid G7 DNA as a template. Primer sets each of which introduced a BamHI restriction site at both ends of the fragments were as follows: fragment A forward primer, 5′-GCAAGGAGAGGATCCTAACTTCTT-3′; reverse primer, 5′-ATATGAGGATCCTCCTTTCCTATG-3′; fragment B forward primer, 5′-ACGCTCAGGATCCTACTAATATCA-3′; reverse primer, 5′-AAACGGATCCCCCTACTTCATTAA-3′. The amplified fragments were digested with BamHI and inserted into BamHI-digested p71V2 to yield the p71V2-A and p71V2-B transfer vectors. Primary porcine macrophage cell cultures were infected with BA71VG (MOI = 10) and transfected with the p71V2-A or p71V2-B transfer vector as previously described (49). The resulting recombinant viruses, BA71VG-A and BA71VG-B, were purified by plaque assay on macrophage cell cultures and characterized by Southern blot analysis as previously described (49).
FIG. 8.
Construction and characterization of recombinants BA71VG-A and BA71VG-B. (A) Diagram of the genomic structure of pathogenic ASFV isolate E70; cell culture-adapted, GUS-containing BA71VG; and recombinant viruses BA71VG-A and BA71VG-B. (B) Southern blot hybridization of BA71VG (lane 1), E70 (lane 2), BA71VG-A (lane 3), and BA71VG-B (lane 4). Viral DNAs were digested with BamHI, electrophoresed, blotted, and hybridized with a 32P-labeled 16.0-kbp BamHI restriction fragment as a probe. (C) Viral growth in macrophage cell cultures. Primary swine macrophage cell cultures were infected (MOI = 5) with either E70 or BA71V recombinant virus, and at the indicated times, duplicate samples were collected and titrated. These data are the means and standard errors of two independent experiments.
Construction of recombinant ASFV Pr4 viruses containing deletions in MGF360 and MGF530 genes.
Gene deletion mutants Pr4Δ2AB and Pr4VΔ2AB were generated by homologous recombination between ASFV Pr4 genomes and recombination transfer vectors following infection and transfection of cell cultures (49). Flanking DNA fragments to the left (1.3 kbp) and right (1.6 kbp) of MGF360 ORFs 2A and 2B were amplified using primer sets each of which introduced a BamHI restriction site adjacent to the MGF360 ORFs and a BglII site (right flanking fragment) at the opposite end. The primer sets were as follows: left flank forward primer, 5′-TCCATGCTATGATGATTAAGTATT-3′; left flank reverse primer, 5′-ATATTGGATCCTAGTGATGTGCGT-3′; right flank forward primer, 5′-AACAACTTGATTGGATCCGTCTGG-3′; right flank reverse primer, 5′-TGTAGGAGATCTGATATTGATCAT-3′. The fragments were digested with the appropriate restriction enzymes and cloned into pCR2.1 to give pPr4-2AB. A reporter cassette, p72β-Gal, containing the β-galactosidase gene under the control of an ASFV late structural gene promoter, p72, was inserted into BamHI-digested pPr4-2AB to yield p72β-GalΔ2AB. Primary porcine macrophage or Vero cell cultures were infected with Pr4 or its cell culture-adapted variant, Pr4V, respectively (MOI = 10), and transfected with p72β-GalΔ2AB. Recombinant viruses were purified to homogeneity by plaque assay on macrophage or Vero cell cultures and characterized by Southern blot hybridization (see Fig. 9).
FIG. 9.
Characterization of ASFV MGF360 and MGF530 gene deletion mutants Pr4Δ2AB, Pr4Δ35, and Pr4VΔ2ABΔ35. (A) Diagram of the MGF360 and MGF530 gene regions in the parental Pr4 isolate and the deletion mutants. Transfer vectors and recombinants with genes deleted were constructed as described in Materials and Methods. LVR, left variable region; CVR, central variable region; RVR, right variable region. (B) Southern blot hybridization of EcoRI-digested viral DNAs from parental isolate Pr4 (lanes 1 and 4) and recombinants Pr4Δ2AB (lane 2), Pr4Δ35 (lane 5), and Pr4VΔ2ABΔ35 (lanes 3 and 6). Blots were probed with 32P-labeled 8.2-kbp (lanes 1 to 3) and 12.8-kbp (lanes 4 to 6) EcoRI fragments, respectively. DNA sizes are shown in kilobase pairs at the left. (C) Growth characteristics of ASFV isolate Pr4 and viruses Pr4Δ2AB, Pr4Δ35, and Pr4VΔ2ABΔ35 in swine macrophage cell cultures. Primary macrophage cell cultures were infected (MOI = 1), and at the indicated times postinfection, duplicate samples were titrated for virus yield. These data are the means and standard errors of two independent experiments. TCID50, 50% tissue culture-infective doses.
ASFV gene deletion mutants Pr4Δ35 and Pr4VΔ2ABΔ35 were constructed essentially as described above (see Fig. 9A). Recombination transfer vector p35, used to delete MGF360 ORFs 3CL, 3DL, 3EL, 3HL, 3IL, and 3LL and MGF530 ORFs 3FR and 3NR, was constructed by sequentially cloning PCR-derived DNA fragments mapping to the left (1.09 kbp) and right (1.15 kbp) of the desired deletion into pCR2.1. The p72GUS reporter gene cassette was then inserted into BamHI-digested p35. Primer sets for flanking fragments were as follows: left flank forward primer, 5′-TTGCTTAAGATCCTTTAGATCCTT-3′; left flank reverse primer, 5′-GGATCCGTTAAAAGATTATCATGC-3′; right flank forward primer, 5′-CCACCGGATCCAGAGACATTTGTA-3′; right flank reverse primer, 5′-CAAAAGATCTTTATGCTGATATTT-3′. The resulting construct, p72GUSΔ35, was then used with Pr4 viruses in transfection-infection experiments to construct deletion mutant viruses. Recombinants Pr4Δ35 and Pr4VΔ2ABΔ35 were purified and characterized as described above (see Fig. 9).
Nucleotide sequence accession numbers.
The E70 G7 sequences were assigned GenBank accession no. AF327839, and the MS16 M25 sequences were assigned GenBank accession no. AF327840.
RESULTS
Cell culture-adapted ASFVs MS16 and BA71V do not replicate in porcine macrophage cell cultures.
Growth characteristics of monkey cell culture-adapted MS16 and BA71V viruses were compared to those of pathogenic ASFV isolate E70. Primary porcine macrophage cell cultures and Vero cell cultures were infected with each virus, and virus titers were determined at various times postinfection (Fig. 1). As expected, E70 replicated in macrophages (Fig. 1A), reaching a maximum virus yield of approximately 107 50% tissue culture-infective doses per ml by 48 hpi. In contrast, MS16 and BA71V did not produce detectable infectious progeny. Both cell culture-adapted viruses replicated normally in Vero cell cultures (Fig. 1B), indicating that the growth defect was macrophage specific.
FIG. 1.
Growth characteristics of ASFV pathogenic isolate E70 and cell culture-adapted MS16 and BA71V viruses in primary porcine macrophage (A) and Vero (B) cell cultures. Cells were infected (MOI = 5) with the appropriate viruses, and at the indicated times postinfection, duplicate samples were collected and titrated for virus yield. These data are the means and standard errors of three independent experiments. TCID50, 50% tissue culture-infective doses.
Infection with MS16 and BA71V results in early macrophage cell death.
To define the MS16 and BA71V growth defect in macrophages, levels of viral protein expression and DNA replication were examined using immunoprecipitation and semiquantitative dot blot analysis. Monospecific rabbit antisera raised against ASFV early protein p30 and late protein p54 (Lu et al., unpublished data) specifically immunoprecipitated p30 at 3 to 6 hpi and p54 at 6 to 9 hpi (Fig. 2A) from E70-, MS16-, and BA71V-infected cultures at comparable levels. DNA dot blot hybridization showed comparable levels of ASFV DNA in each virus-infected culture at 8 hpi (Fig. 2B). These results indicate that the block in MS16 and BA71V replication is late in the infection cycle, occurring after DNA replication and late protein synthesis.
FIG. 2.
(A) Expression of early (p30) and late (p54) ASFV proteins in infected macrophage cell cultures. Immunoprecipitation of cell extracts from mock-infected macrophages (lane M) and macrophages infected with E70 (lanes 1 and 4), MS16 (lanes 2 and 5), or BA71V (lanes 3 and 6), radiolabeled from 3 to 6 or 6 to 9 hpi, was performed using a mixture of anti-p30 and anti-p54 monospecific rabbit antiserum as previously described (1). Sizes were estimated using Rainbow 14C-methylated protein molecular size markers (Amersham Life Science). (B) Viral DNA replication in E70 (lanes 1 and 4)-, MS16 (lanes 2 and 5)-, or BA71V (lanes 3 and 6)-infected or mock-infected (lane M) macrophage cell cultures. Cells were infected (MOI = 5), total cellular low-molecular-weight DNA was isolated at the indicated times postinfection, and twofold dilution sets of 10 μg of total DNA were blotted onto Zeta Probe membranes (Bio-Rad) and probed with a 32P-labeled E70 genomic DNA probe.
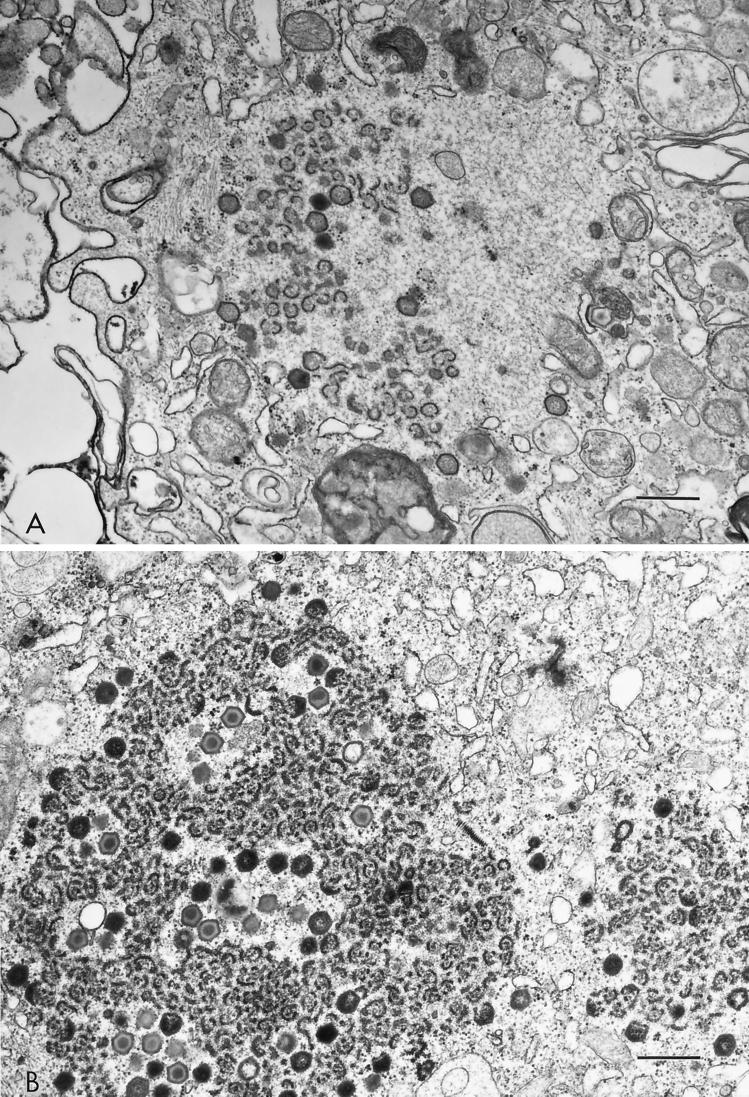
MS16- and BA71V-infected macrophages exhibited extensive early cytopathology (Fig. 3A) that was not observed in E70-infected macrophages (Fig. 3B). Ultrastructural analysis revealed loss of cellular organization, membrane disintegration, and marked nuclear chromatin condensation as early as 12 hpi in MS16- and BA71V-infected cells (Fig. 3A and data not shown). In both E70- and MS16-infected macrophages, nascent virus factories were present in the cell cytoplasm at 12 hpi (Fig. 4A and B). However, MS16-infected cells contained only incomplete polyhedral structures and immature virus particles without nucleoid cores (Fig. 4A), whereas E70 virus factories contained numerous virions (Fig. 4B).
FIG. 3.
Electron micrographs of ASFV-infected swine macrophages. Cell cultures were infected (MOI = 5) with MS16 (A) or E70 (B) virus and examined at 16 hpi. Note the extensive cytopathology in MS16-infected macrophages compared to those infected with E70. Virus factories (arrows) are present in the cell cytoplasm.
FIG. 4.
Morphology of virus factories in MS16 (A)- and E70 (B)-infected macrophages at 16 hpi. The size bar represents 0.5 μm. Note in the MS16-infected macrophage the incomplete and complete polyhedral virion structures, which lack the characteristic, centrally located nucleoid.
Time to death for MS16- and BA71V-infected macrophages was determined by using a quantitative cell viability assay. Macrophage cell cultures were infected with E70, MS16, or BA71V, and cell survival was assessed by trypan blue dye exclusion at various times postinfection. There was a significant difference between E70 and either virus strain MS16 or BA71V in infected-macrophage survival time (Fig. 5). More rapid cell death was observed for MS16 and BA71V virus-infected cells. Significant cell death, 40 to 60% of all cells, occurred between 10 and 16 hpi, and by 40 hpi, less than 5% of the infected cells were viable. In contrast, approximately 90% of E70-infected macrophages were viable at 20 hpi and more than 50% remained alive as late as 40 hpi. Thus, early cell death occurs in MS16- and BA71V-infected macrophages prior to infectious-progeny production.
FIG. 5.
ASFV-infected cell viability. Porcine macrophage cell cultures were infected (MOI = 5) with E70, MS16, or BA71V virus, and viable cells were assayed by trypan blue dye exclusion at various times postinfection. These data are the means and standard errors of four independent experiments.
MGF360 and MGF530 genes rescue MS16 and BA71V growth in swine macrophage cell cultures.
Marker rescue experiments were used to identify genomic regions capable of restoring MS16 and BA71V growth in macrophages. Rescued recombinants capable of replication in swine macrophage cell cultures were obtained following recombination between parental virus MS16 or BA71V and E70 genomic clones G7 and EP as described in Materials and Methods. The G7 cosmid clone, containing a 38-kbp DNA fragment, successfully rescued both the MS16 and BA71V viruses, while the 10.3-kbp DNA fragment-containing subclone EP was sufficient for rescue of BA71V. Four recombinants derived from either parental virus MS16 or BA71V were selected by limiting-dilution assay in macrophage cultures and verified as products of a double-crossover recombination event. Recombinant viruses MS16-C2 and BA71V-E5 were chosen for further analysis. Genomic DNAs from E70, the parental viruses, and the recombinants were digested with restriction endonucleases, gel electrophoresed, Southern blotted, and hybridized with 32P-labeled DNA probes.
In the MS16-C2 recombinant, the recombination event introduced a 20.2-kbp DNA segment from the left variable region of the E70 genome (Fig. 6A). Genomic DNAs from E70, MS16, and MS16-C2 were digested with the EcoRI (E) and BamHI (B) restriction endonucleases and hybridized with a mixture of 32P-labeled 10-kbp (B1/B2) and 15-kbp (B2/B3) BamHI fragments contained in clone G7 (Fig. 6B). As expected, restriction fragments with predicted sizes of 15.0 kbp (E4/B3), 4.8 kbp (E1/E2), 4.3 kbp (E3/B2), 0.9 kbp (B2/E4), and 0.7 kbp (E2/E3) were observed for E70 and a single 6.5-kbp fragment was detectable for MS16 (Fig. 6B, lanes 1 and 2, respectively). As a result of G7 insertion, MS16-C2 showed a pattern similar to that of the E70 viral genome in this region (Fig. 6B, lane 3).
FIG. 6.
Characterization of marker-rescued MS16-C2 and BA71V-E5 viruses. (A) Diagram of the left variable region in ASFV pathogenic isolate E70, cell culture-adapted MS16 and BA71V viruses, and rescued recombinant viruses MS16-C2 and BA71V-E5. (B and C) Southern blot analysis of E70 (lanes B1 and C1), MS16 (B, lane 2), MS16-C2 (B, lane 3), BA71V (C, lane 2), and BA71V-E5 (C, lane 3). Purified viral DNAs were digested with EcoRI/BamHI (B) or BamHI (C), electrophoresed, blotted, and hybridized with DNA probes including the deleted regions of MS16 and BA71V. Positions of molecular size markers are shown in kilobase pairs at the left.
The rescued recombinant BA71V-E5 contained an 8.2-kbp DNA segment from the E70 genome (Fig. 6A). Southern blot analysis using the EP clone as a probe confirmed the presence of novel DNA sequences in the rescued virus. In contrast to parental virus BA71V, where a BamHI fragment of 7.7 kbp was detected (Fig. 6C, lane 2), BA71V-E5 contained a larger fragment of 15.9 kbp (Fig. 6C, lane 3).
To analyze the genetic content of genomic regions involved in marker rescue of macrophage growth, the G7 clone was completely sequenced and compared with sequences of a cosmid clone (M25) of the MS16 left variable region (Lu et al., unpublished data) and sequences available for BA71V (46). Comparative sequence analysis revealed that both MS16 and BA71V had significant deletions in this region. Deleted regions contained multiple members of MGF110, MGF300, MGF360, and MGF530 (Fig. 6A). More precisely, a 20.2-kbp deletion was observed in the MS16 genome, comprising three ORFs of MGF110 (1VL, 1XL, and 1YL), three ORFs of MGF300 (2DL, 2EFR, and 2HL), nine ORFs of MGF360 (2AL, 2BL, 3BL, 3CL, 3DL, 3EL, 3HL, 3IL, and 3LL), and two ORFs of MGF530 (3FR and 3NR). Two deletions were identified in the BA71V genome within the region involved in the rescue. A 5.0-kbp region containing three ORFs of MGF110 (1VL, 1XL, and 1YL), and two ORFs of MGF360 (2AL and 2BL) was deleted, as was an 8.2-kbp DNA region which contained six ORFs of MGF360 (3CL, 3DL, 3EL, 3HL, 3IL, and 3LL) and one MGF530 ORF (3FR). Restoration of the entire 20.2-kbp deletion was necessary to rescue MS16 viral growth in porcine macrophages. The 8.2-kbp region within the EP clone alone was sufficient to restore BA71V viral growth in porcine macrophages. These data indicate that ASFV MGF360 and MGF530 genes play an essential role in determining viral host range in swine macrophages.
One-step growth curve experiments were performed to determine the growth characteristics of marker-rescued MS16-C2 and BA71V-E5 recombinants in swine macrophage cell cultures. Primary porcine macrophage cell cultures derived from either peripheral blood or lung thissue were infected (MOI = 5) with pathogenic ASFV isolate E70, cell culture-adapted viruses MS16 and BA71V, and marker-rescued recombinants MS16-C2 and BA71V-E5, and samples were titrated at various times postinfection. As observed before, cell culture-adapted viruses MS16 and BA71V failed to replicate in either peripheral blood or alveolar macrophage cell cultures (Fig. 7A and B). However, the growth kinetics and viral yields of MS16-C2 and BA71V-E5 recombinants were indistinguishable from those of E70 (Fig. 7A and B). Thus, E70 genomic regions contained within the cosmid clone (G7) and its subclone (EP) were capable of completely restoring the growth of the MS16 and BA71V viruses, respectively, in macrophages.
FIG. 7.
Growth characteristics of ASFV pathogenic isolate E70, the cell culture-adapted MS16 and BA71V parental viruses, and rescued recombinant viruses MS16-C2 and BA71V-E5. Porcine peripheral blood (A) and alveolar (B) macrophages were infected (MOI = 5), and at indicated times, duplicate samples were collected and titrated for virus yield. These data are the means and standard errors of two independent experiments. TCID50, 50% tissue culture-infective doses.
To further define specific determinants within the left variable region of the ASFV genome involved in macrophage host range determination, segments of the E70 EP clone were recombined into BA71V and recombinant viruses were tested for macrophage growth. To allow selection of putative recombinants by GUS-positive plaque assay on macrophage cell cultures, GUS-expressing BA71V variant BA71VG and BA71VG-E5 were constructed as described in Materials and Methods. Recombinant viruses BA71VG-A and BA71VG-B were constructed by homologous recombination between parental virus BA71VG and transfer vectors containing either a 5.3-kbp or a 3.8-kbp PCR fragment amplified from the EP clone (Fig. 8A). The 5.3-kbp fragment from the left side of EP contained promoters and ORFs of MGF360 (3CL, 3DL, and 3EL) and MGF530 (3FR); the 3.8-kbp insert from the right contained the other part of EP, including the MGF360 3HL, 3IL, and 3LL ORFs and promoter regions. The genomic structure of the recombinants was confirmed by Southern blot hybridization (Fig. 8B). As predicted, novel restriction fragments were seen in BA71VG-A and BA71VG-B (Fig. 8B) as a result of the insertion of the 5.3- and 3.8-kbp fragments, respectively. A parental 7.7-kbp fragment was not observed in either recombinant, suggesting that they were free of contaminating parental virus.
The growth characteristics of recombinants BA71VG-A and BA71VG-B in swine macrophage cell cultures were examined as described above and compared to those of E70, parental virus BA71VG, and its EP-rescued recombinant BA71VG-E5 (Fig. 8C). As expected, BA71VG failed to grow in primary macrophage cell cultures and the EP-rescued variant BA71VG-E5 showed growth kinetics and a yield similar to those of E70, suggesting that insertion of the p72GUS cassette into the viral genome did not alter the growth properties of BA71VG. Interestingly, both the BA71VG-A and BA71VG-B viral mutants exhibited growth in swine macrophages. Contrary to the parental virus, they formed distinct, visible blue plaques on macrophage cultures (not shown). One-step growth curve experiments revealed that approximately 104 50% tissue culture-infective doses of infectious virus per ml was produced by both recombinants (Fig. 8C). In two independent experiments, BA71VG-A and BA71VG-B virus yields were consistently 100- to 1,000-fold lower than those observed with E70. Thus, while the EP clone was sufficient to rescue the growth defect of BA71V or BA71VG completely, subclones representing genomic regions with fewer ORFs of MGF360 and MGF530 resulted in only partial rescue, suggesting that multiple family members are needed.
Deletion of MGF360 and MGF530 genes from ASFV Pr4 results in a macrophage growth defect.
To confirm the role of MGF360 and MGF530 genes in macrophage host range determination, deletion mutants of macrophage growth-competent virus Pr4 were constructed and analyzed. ASFV MGF360 and MGF530 gene deletion mutants Pr4Δ2AB, Pr4Δ35, and Pr4VΔ2ABΔ35 were generated from pathogenic African isolate Pr4 by homologous recombination between parental viral genomes and recombination transfer vectors as described in Materials and Methods. In Pr4Δ2AB and Pr4VΔ2ABΔ35, the introduced deletion removed 2,761 bases (Fig. 9A), which contained all but the carboxyl-terminal 104 nucleotides of the MGF360 2A ORF and the amino-terminal 250 nucleotides of the MGF360 2B ORF and inserted in their place a 3.6-kbp p72β-Gal reporter gene cassette. Deletion mutants Pr4Δ35 and Pr4VΔ2ABΔ35 were constructed by deleting a 10,163-bp region from Pr4 and Pr4Δ2AB, respectively (Fig. 9A). The deletion removed six MGF360 ORFs (3CL, 3DL, 3EL, 3HL, 3IL, and 3LL) and two MGF530 ORFs (3FR and 3NR) and inserted in their place a 2.4-kbp p72GUS reporter gene cassette. Genomic DNAs from parental virus Pr4 and deletion mutants Pr4Δ2AB, Pr4Δ35, and Pr4VΔ2ABΔ35 were analyzed by Southern blot hybridization as described above. A novel EcoRI fragment with the predicted size of 9.1 kbp was observed for both Pr4Δ2AB and Pr4VΔ2ABΔ35 (Fig. 9B, lanes 2 and 3), and as expected, an 8.2-kbp fragment was seen for the parental Pr4 virus (Fig. 9B, lane 1) when the filter was probed with a 32P-labeled genomic fragment containing the 2A and 2B ORFs and flanking sequences. The net 0.9-kbp size increase in the deletion mutants compared to the parental Pr4 virus resulted from the insertion of the p72β-Gal reporter gene cassette. The 12.8-kbp EcoRI fragment used as a genomic probe, containing the six MGF360 and two MGF530 ORFs, hybridized to this fragment of the parental virus (Fig. 9B, lane 4), and a predicted 2.6-kbp fragment was present in both the Pr4Δ35 and Pr4VΔ2ABΔ35 recombinants (Fig. 9B, lanes 5 and 6). These results verify the predicted genomic structure of the recombinant Pr4 viruses and show that the mutant stocks were free of any contaminating parental virus.
The growth kinetics and viral yields of ASFV Pr4 MGF360 and MGF530 gene deletion mutants were compared to those of the Pr4 parental virus by infection of primary macrophage cell cultures (MOI = 1) and then titration of infectious virus at various times postinfection (Fig. 9C). Recombinant virus Pr4Δ2AB showed unaltered growth characteristics compared with parental virus Pr4; however, Pr4Δ35 exhibited a 100- to 1,000-fold growth defect. Interestingly, like the MS16 and BA71V viruses, deletion mutant Pr4VΔ2ABΔ35 did not produce any detectable viral progeny in macrophage cell cultures. This mutant was constructed in Vero cell cultures by deletion of both the MGF360 2A and 2B ORFs and the 10.2-kbp region containing six MGF360 and two MGF530 ORFs. Notably, this deletion mutant had a genomic arrangement in the left variable region similar to that of BA71V. These data indicate that ASFV MGF360 and MGF530 genes perform essential macrophage host range functions and that multiple family members are likely involved.
DISCUSSION
Here, we have shown that ASFV MGF360 and MGF530 genes perform a macrophage host range-determining function by promoting infected-cell survival.
Cell culture-adapted, highly attenuated ASFV variants MS16 and BA71V (13, 38) failed to replicate in macrophage cell cultures. Infection with these viruses resulted in early cell death, which occurred prior to viral progeny production. ASFV terminal variable regions comprise the left 35-kbp and the right 15-kbp ends of the genome and contain at least five MGFs, i.e., MGF100, MGF110, MGF300, MGF360, and MGF530 (4, 5, 11, 18, 43, 47). Variations within these regions, including gene deletion events, are observed during ASFV adaptation to monkey cell lines (6, 38). The actual number of MGF genes present in a given virus isolate may vary substantially, with copy numbers in pathogenic isolates being higher (11, 18). The degree of variability that occurs within the terminal variable regions of the ASFV genome suggests that these ORFs, while not essential for growth in cell cultures, perform host range functions. The MS16 and BA71V viruses contain several deletions and insertions in these terminal regions (7, 38, 46).
Using marker rescue, we identified and characterized genomic sequences in the left variable region of the ASFV genome responsible for the macrophage growth defect of the MS16 and BA71V viruses. Comparative sequence analysis of genomic clones from the left variable region of E70 and MS16 (Lu et al., unpublished data) (46) indicated that both MS16 and BA71V had significant deletions in this genomic region. Restriction endonuclease analysis previously detected an approximately 15-kbp deletion in the left end of the MS16 genome (38), and DNA sequence comparisons of the BA71V genome with data available for ASFV isolates Malawi Lil-20/1 and LIS57 demonstrated deletions in terminal regions of the BA71V genome (43, 46, 47). Here, we have been able to map the locations and sizes of deletions associated with the macrophage growth defect for both cell culture-adapted viruses. Restoration of a 20.2-kbp deletion in the left end of MS16 genome was necessary to completely rescue growth. For rescue, a 38-kbp cosmid clone from E70 was necessary to achieve double-crossover recombination events by providing homologous flanking sequences upstream and downstream of the large deletion present in MS16. In BA71V, the EP clone from E70, which restored an 8.2-kbp deletion, completely rescued macrophage growth, indicating that this genomic region was sufficient for restoration of BA71V growth in macrophages. The 8.2-kbp region contained seven ASFV ORFs, representing multiple members of MGF360 and MGF530 that were deleted in BA71V. Our findings indicate that while MGF360 and MGF530 genes are dispensable in monkey cell lines, they are essential for viral growth in macrophages.
Multiple copies of MGF360 and MGF530 genes were required to completely restore the growth of MS16 and BA71V in macrophages. Moreover, multiple gene deletions from the Pr4 genome led to complete loss of growth on macrophages. Removal of individual MGF530 genes (Δ3FR and Δ3NR) or groups of MGF360 genes (Δ3CL3DL3EL and Δ3HL3IL3LL) from the left variable region of the Pr4 isolate did not alter viral growth in macrophage cell cultures (data not shown). Deletion of six MGF360 and two MGF530 ORFs from the left end of the Pr4 genome markedly reduced viral growth in primary macrophage cell cultures by 100- to 1,000-fold; removal of two additional MGF360 genes resulted in no growth at all. Although little is known about MGF gene function during virus replication, it is tempting to speculate that gene dosage is a factor in replication in macrophages. Alternatively, specific MGF members may function and/or interact with each other in a yet-to-be-identified cellular pathways(s).
MGF360 and MGF530 genes and their predicted protein products do not share significant similarity with other known genes or proteins; however, the amino-terminal regions of predicted MGF360 proteins do share similarity with comparable regions of MGF530 ORFs (47) (Lu et al., unpublished data). MGF360 and MGF530 proteins share 28% amino acid identity over the first 100 amino acids (Lu et al., unpublished data). Among members of these MGFs, amino acid similarity ranged from 23 to 88% for MGF360 and from 46 to 74% for MGF530 genes (47) (Lu et al., unpublished data). Given the lack of similarity of predicted MGF360 and MGF530 proteins to other known proteins, it is difficult to speculate about their function in virus-cell interactions. ASFV infection does induce apoptosis in primary swine macrophages in vitro at late times postinfection (27), and ASFV encodes a functional Bcl-2 homolog that may be an essential gene (2, 26). It is possible that transient modulation of infected macrophage survival by MGF360 and MGF530 proteins is also necessary for productive viral replication in this cell type. Consistent with a host range function, MGF360 and MGF530 genes are expressed early in infection (18, 33, 47).
In summary, the results reported here indicate that ASFV left variable region MGF360 and MGF530 genes perform an essential macrophage host range function(s) involving promotion of infected-cell survival. Given that macrophages are the primary target cells of ASFV in swine, it is likely that these genes are also of significance for viral pathogenesis and virulence in domestic swine. Future studies will examine this possibility.
ACKNOWLEDGMENTS
We thank Aniko Zsak, Adriene Ciupryk, and the PIADC animal care staff for excellent technical assistance.
REFERENCES
- 1.Afonso C L, Alcaraz C, Brun A, Sussman M D, Onisk D V, Escribano J M, Rock D L. Characterization of p30, a highly antigenic membrane and secreted protein of African swine fever virus. Virology. 1992;189:368–373. doi: 10.1016/0042-6822(92)90718-5. [DOI] [PubMed] [Google Scholar]
- 2.Afonso C L, Neilan J G, Kutish G F, Rock D L. An African swine fever virus bcl-2 homolog, 5-HL, suppresses apoptotic cell death. J Virol. 1996;70:4858–4863. doi: 10.1128/jvi.70.7.4858-4863.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Afonso C L, Zsak L, Carrillo C, Borca M V, Rock D L. African swine fever virus NL gene is not required for virus virulence. J Gen Virol. 1998;79:2543–2547. doi: 10.1099/0022-1317-79-10-2543. [DOI] [PubMed] [Google Scholar]
- 4.Almazán F, Rodríguez J M, Andrés G, Pérez R, Viñuela E, Rodríguez J F. Transcriptional analysis of multigene family 110 of African swine fever virus. J Virol. 1992;66:6655–6667. doi: 10.1128/jvi.66.11.6655-6667.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Almendral J M, Almazán F, Blasco R, Viñuela E. Multigene families in African swine fever virus: family 110. J Virol. 1990;64:2064–2072. doi: 10.1128/jvi.64.5.2064-2072.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blasco R, Agüero M, Almendral J M, Viñuela E. Variable and constant regions in African swine fever virus DNA. Virology. 1989;168:330–338. doi: 10.1016/0042-6822(89)90273-0. [DOI] [PubMed] [Google Scholar]
- 7.Blasco R, de la Vega I, Almazán F, Agüero A, Viñuela E. Genetic variation of African swine fever virus: variable regions near the ends of the viral DNA. Virology. 1989;173:251–257. doi: 10.1016/0042-6822(89)90241-9. [DOI] [PubMed] [Google Scholar]
- 8.Brown F. The classification and nomenclature of viruses: summary of results of meetings of the International Committee on Taxonomy of Viruses in Sendai, September 1984. Intervirology. 1986;25:141–143. doi: 10.1159/000150091. [DOI] [PubMed] [Google Scholar]
- 9.Colgrove G S, Haelterman E O, Coggins L. Pathogenesis of African swine fever in young pigs. Am J Vet Res. 1969;30:1343–1359. [PubMed] [Google Scholar]
- 10.Costa J V. African swine fever virus. In: Darai G, editor. Molecular biology of iridoviruses. Norwell, Mass: Kluwer Academic Publishers; 1990. pp. 247–270. [Google Scholar]
- 11.De la Vega I, Viñuela E, Blasco R. Genetic variation and multigene families in African swine fever virus. Virology. 1990;179:234–246. doi: 10.1016/0042-6822(90)90293-z. [DOI] [PubMed] [Google Scholar]
- 12.Dixon L K, Wilkinson P J. Genetic diversity of African swine fever virus isolates from soft ticks (Ornithodoros moubata) inhabiting warthog burrows in Zambia. J Gen Virol. 1988;69:2981–2993. doi: 10.1099/0022-1317-69-12-2981. [DOI] [PubMed] [Google Scholar]
- 13.Enjuanes L, Carrascosa A L, Moreno M A, Viñuela E. Titration of African swine fever (ASF) virus. J Gen Virol. 1976;32:471–477. doi: 10.1099/0022-1317-32-3-471. [DOI] [PubMed] [Google Scholar]
- 14.Ewing B, Green P. Base-calling of automated sequencer traces using Phred. II. Error probabilities. Genome Res. 1998;8:186–194. [PubMed] [Google Scholar]
- 15.Ewing B, Hillier L, Wendl M C, Green P. Base-calling of automated sequencer traces using Phred. I. Accuracy assessment. Genome Res. 1998;8:175–185. doi: 10.1101/gr.8.3.175. [DOI] [PubMed] [Google Scholar]
- 16.Genovesi E V, Villinger F, Gerstner D J, Whyard T C, Knudsen R C. Effect of macrophage-specific colony-stimulating factor (CSF-1) on swine monocyte/macrophage susceptibility to in vitro infection by African swine fever virus. Vet Microbiol. 1990;25:153–176. doi: 10.1016/0378-1135(90)90074-6. [DOI] [PubMed] [Google Scholar]
- 17.Goebel S J, Johnson G P, Perkus M E, Davis S W, Winslow J P, Paoletti E. The complete DNA sequence of vaccinia virus. Virology. 1990;179:247–266. doi: 10.1016/0042-6822(90)90294-2. [DOI] [PubMed] [Google Scholar]
- 18.González A, Calvo V, Almazan F, Almendral J M, Ramirez J C, de la Vega I, Blasco R, Viñuela E. Multigene families in African swine fever virus: family 360. J Virol. 1990;64:2073–2081. doi: 10.1128/jvi.64.5.2073-2081.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.González A, Talavera A, Almendral J M, Viñuela E. Hairpin loop structure of African swine fever virus DNA. Nucleic Acids Res. 1986;14:6835–6844. doi: 10.1093/nar/14.17.6835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kleiboeker S B, Kutish G F, Neilan J G, Lu Z, Zsak L, Rock D L. A conserved African swine fever virus right variable region gene, I11L, is nonessential for growth in vitro and virulence in domestic swine. J Gen Virol. 1998;79:1189–1195. doi: 10.1099/0022-1317-79-5-1189. [DOI] [PubMed] [Google Scholar]
- 21.Konno S, Taylor W D, Dardiri A H. Acute African swine fever. Proliferative phase in lymphoreticular tissue and the reticuloendothelial system. Cornell Vet. 1971;61:71–84. [PubMed] [Google Scholar]
- 22.Konno S, Taylor W D, Hess W R, Heuschele W P. Liver pathology in African swine fever. Cornell Vet. 1971;61:125–150. [PubMed] [Google Scholar]
- 23.Massung R F, Esposito J J, Liu L, Qi J, Utterback T R, Knight J C, Aubin L, Yuran T E, Parsons J M, Loparev V N, Selivanov N A, Cavallaro K F, Kerlavage A R, Mahy B W J, Venter J C. Potential virulence determinants in terminal regions of variola smallpox virus genome. Nature (London) 1993;366:748–751. doi: 10.1038/366748a0. [DOI] [PubMed] [Google Scholar]
- 24.Mebus C A. African swine fever. Adv Virus Res. 1988;35:251–269. doi: 10.1016/s0065-3527(08)60714-9. [DOI] [PubMed] [Google Scholar]
- 25.Moulton J, Coggins L. Comparison of lesions in acute and chronic African swine fever. Cornell Vet. 1968;58:364–388. [PubMed] [Google Scholar]
- 26.Neilan J G, Lu Z, Afonso C L, Kutish G F, Sussman M D, Rock D L. An African swine fever virus gene with similarity to the proto-oncogene bcl-2 and the Epstein-Barr virus gene BHRF1. J Virol. 1993;67:4391–4394. doi: 10.1128/jvi.67.7.4391-4394.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Neilan J G, Lu Z, Kutish G F, Zsak L, Burrage T G, Borca M V, Carrillo C, Rock D L. A BIR motif containing gene of African swine fever virus, 4CL, is nonessential for growth in vitro and viral virulence. Virology. 1997;230:252–264. doi: 10.1006/viro.1997.8481. [DOI] [PubMed] [Google Scholar]
- 28.Ortin J, Enjuanes L, Viñuela E. Cross-links in African swine fever virus DNA. J Virol. 1979;31:579–583. doi: 10.1128/jvi.31.3.579-583.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pearson W R. Rapid and sensitive sequence comparison with FASTP and FASTA. Methods Enzymol. 1990;183:63–98. doi: 10.1016/0076-6879(90)83007-v. [DOI] [PubMed] [Google Scholar]
- 30.Plowright W, Parker J, Pierce M A. African swine fever virus in ticks (Ornithodoros moubata, Murray) collected from animal burrows in Tanzania. Nature (London) 1969;221:1071–1073. doi: 10.1038/2211071a0. [DOI] [PubMed] [Google Scholar]
- 31.Plowright W, Parker J, Pierce M A. The epizootiology of African swine fever in Africa. Vet Rec. 1969;85:668–674. [PubMed] [Google Scholar]
- 32.Plowright W, Thomson G R, Neser J A. African swine fever. In: Coetzer J A W, Thomson G R, Tustin R C, editors. Infectious diseases in livestock with special reference to South Africa. Vol. 1. Oxford, United Kingdom: Oxford University Press; 1994. pp. 568–599. [Google Scholar]
- 33.Rodríguez J M, Yáñez R J, Pan R, Rodríguez J F, Salas M L, Viñuela E. Multigene families in African swine fever virus: family 505. J Virol. 1994;68:2746–2751. doi: 10.1128/jvi.68.4.2746-2751.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 35.Senkevich T G, Koonin E V, Bugert J J, Darai G, Moss B. The genome of molluscum contagiosum virus: analysis and comparison with other poxviruses. Virology. 1997;233:19–42. doi: 10.1006/viro.1997.8607. [DOI] [PubMed] [Google Scholar]
- 36.Sogo J M, Almendral J M, Talavera A, Viñuela E. Terminal and internal inverted repetitions in African swine fever virus DNA. Virology. 1984;133:271–275. doi: 10.1016/0042-6822(84)90394-5. [DOI] [PubMed] [Google Scholar]
- 37.Sumption K J, Hutchings G H, Wilkinson P J, Dixon L K. Variable regions on the genome of Malawi isolates of African swine fever virus. J Gen Virol. 1990;71:2331–2340. doi: 10.1099/0022-1317-71-10-2331. [DOI] [PubMed] [Google Scholar]
- 38.Tabarés E, Olivares I, Santurde G, Garcia M J, Martin E, Carnero M E. African swine fever virus DNA: deletions and additions during adaptation to growth in monkey kidney cells. Arch Virol. 1987;97:333–346. doi: 10.1007/BF01314431. [DOI] [PubMed] [Google Scholar]
- 39.Takezaki N, Rzhetsky A, Nei M. Phylogenetic test of the molecular clock and linearized trees. Mol Biol Evol. 1995;12:823–833. doi: 10.1093/oxfordjournals.molbev.a040259. [DOI] [PubMed] [Google Scholar]
- 40.Tamura K, Nei M. Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Mol Biol Evol. 1993;10:512–526. doi: 10.1093/oxfordjournals.molbev.a040023. [DOI] [PubMed] [Google Scholar]
- 41.Thomson G R, Gainaru M, Lewis A, Biggs H, Nevill E, Van Der Pypekamp M, Gerbes L, Esterhuysen J, Bengis R, Bezuidenhout D, Condy J. The relationship between ASFV, the warthog and Ornithodoros species in southern Africa. In: Wilkinson P J, editor. ASF, EUR 8466 EN, proceedings of CEC/FAO Research Seminar, Sardinia, Italy, September 1981. Brussels, Belgium: Commission of the European Communities; 1983. pp. 85–100. [Google Scholar]
- 42.Thomson G R, Gainaru M D, Dellen A F V. Experimental infection of warthog (Phacochoerus aethiopicus) with African swine fever virus. Onderstepoort J Vet Res. 1980;47:19–22. [PubMed] [Google Scholar]
- 43.Vydelingum S, Baylis S A, Bristow C, Smith G L, Dixon L K. Duplicated genes within the variable right end of the genome of a pathogenic isolate of African swine fever virus. J Gen Virol. 1993;74:2125–2130. doi: 10.1099/0022-1317-74-10-2125. [DOI] [PubMed] [Google Scholar]
- 44.Wesley R D, Tuthill A E. Genome relatedness among African swine fever virus field isolates by restriction endonuclease analysis. Prev Vet Med. 1984;2:53–62. [Google Scholar]
- 45.Wilkinson P J. African swine fever virus. In: Pensaert M B, editor. Virus infections of porcines. Amsterdam, The Netherlands: Elsevier Science Publishers; 1989. pp. 17–35. [Google Scholar]
- 46.Yáñez R J, Rodríguez J M, Nogal M L, Yuste L, Enríquez C, Rodriguez J F, Viñuela E. Analysis of the complete nucleotide sequence of African swine fever virus. Virology. 1995;208:249–278. doi: 10.1006/viro.1995.1149. [DOI] [PubMed] [Google Scholar]
- 47.Yozawa T, Kutish G F, Afonso C L, Lu Z, Rock D L. Two novel multigene families, 530 and 300, in the terminal variable regions of African swine fever virus genome. Virology. 1994;202:997–1002. doi: 10.1006/viro.1994.1426. [DOI] [PubMed] [Google Scholar]
- 48.Zsak L, Caler E, Lu Z, Kutish G F, Neilan J G, Rock D L. A nonessential African swine fever virus gene UK is a significant virulence determinant in domestic swine. J Virol. 1998;72:1028–1035. doi: 10.1128/jvi.72.2.1028-1035.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zsak L, Lu Z, Kutish G F, Neilan J G, Rock D L. An African swine fever virus virulence-associated gene NL-S with similarity to the herpes simplex virus ICP34.5 gene. J Virol. 1996;70:8865–8871. doi: 10.1128/jvi.70.12.8865-8871.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]