Abstract
Parkinson's disease (PD) is a neurodegenerative disorder caused by the selective destruction of dopaminergic neurons (DA-nergic). Clinically, PD is diagnosed based on developing signs and symptoms. A neurological and physical examination and sometimes medical and family history also help in the diagnosis of PD. However, most of these features are visible when more than 80% of the dopaminergic neurons have degenerated. An understanding of the selective degeneration process at the cellular and molecular level and the development of new biomarkers are required for effective PD management. Several studies have been carried out using a selected set of miRNAs/ mRNAs and proteins to develop biomarkers of PD; however, an unbiased and combined miRNA–protein profiling study was required to identify the markers of progressive and selected degeneration of dopaminergic neurons in PD patients. In the present study, we have carried out global protein profiling through LC–MS/MS and miRNA profiling by using a “brain-specific” miRNA array panel of 112 miRNAs in PD patients and healthy controls to find the unprejudiced group of proteins and miRNAs that are deregulating in PD. In the whole blood samples of PD patients compared to healthy controls, the expression of 23 miRNAs and 289 proteins was significantly increased, whereas the expression of 4 miRNAs and 132 proteins was considerably downregulated. Network analysis, functional enrichment, annotation, and analysis of miRNA–protein interactions were also performed as part of the bioinformatics investigation of the discovered miRNAs and proteins revealing several pathways that lead to PD development and pathogenesis. Based on the analysis of miRNA and protein profiling, we have identified four miRNAs (hsa-miR-186-5p, miR-29b, miR-139 & has-miR-150-5p) and four proteins (YWHAZ, PSMA4, HYOU1, & SERPINA1), which can be targeted for the development of new biomarkers of PD. In vitro studies have identified the role of miR-186-5p in regulating the levels of the YWHAZ/YWHAB & CALM2 gene, which has shown maximum downregulation in PD patients and is known for its role in neuroprotection from apoptotic cell death & calcium regulation. In conclusion, our research has identified a group of miRNA–proteins that can be developed as PD biomarkers; however, future studies on the release of these miRNAs and proteins in extracellular vesicles circulating in the blood of PD patients can further validate these as specific biomarkers of PD.
Graphical Abstract
Supplementary Information
The online version contains supplementary material available at 10.1007/s10571-023-01362-4.
Keywords: Parkinson’s disease, MicroRNA, OpenArray, Proteomics, Biomarker
Introduction
Parkinson’s disease (PD) is the second most prevalent age-associated neurodegenerative disorder after Alzheimer’s disease, which selectively affects the motor-associated functions of the human body. Epidemiological studies have established a positive correlation between the occurrence of PD and increasing age and have reported the occurrence of PD up to 1% of the population, which has crossed the age of 60 years (Tysnes and Storstein 2017). Characteristic symptoms of PD are motor system impairment, such as bradykinesia, tremor at rest, muscular rigidity, postural instability, and non-motor symptoms like disturbed sleep, disturbed autonomic function, psychiatric problems, constipation, and even urinary disturbances in later stages (Sveinbjornsdottir 2016). Despite a large number of studies, PD pathology and etiology have remained unclear; however, it is clear from earlier studies that both genetic and environmental factors are involved in the etiology of PD either individually or in an interplay manner (Warner and Schapira 2003). Pathologically, PD is specified by the loss of dopaminergic (DA-nergic) neurons in the substantia nigra pars compacta (SNpc) and the development of fibrillar α-synuclein aggregates neuronal inclusions, termed Lewy bodies (Dickson 2018). DA-nergic neuronal loss in the SNpc is accountable for the dysfunction of motor systems and other symptoms that appear in PD (Meder et al. 2019). Due to the complex etiology and lack of any biochemical diagnostic tests, usually PD is detected very late and aging of the PD patients further deteriorates their conditions. Different preventive strategies such as drugs, advanced therapies (surgery and infusional therapies), and physical therapies may provide improvement in the motor complications of PD but PD itself is as yet incurable (Kulisevsky et al. 2018). The motor symptoms are the primary determinant of the clinical diagnosis of PD, which first manifest when the DA-nergic neurons of the SNpc are destroyed by more than 80%. (Sveinbjornsdottir 2016). Relatively low clinical diagnostic accuracy (75.3%) is reported in patients diagnosed by neuropathological examination (Joutsa et al. 2014). In general, prevention and monitoring of the disease are lagging far behind its appearance. As a result, in recent years, there has been an increase in fundamental research aimed at developing a reliable diagnostic biomarker for the early detection of PD. (Parnetti et al. 2019). Omics approaches such as proteomics and genomics-based molecular biomarkers are the recent advancement in biomarker research and have been under investigation for a potential biomarker for early diagnosis of PD have been the subject of a potential biomarker investigation for early diagnosing patients with PD (Miller and O'Callaghan 2015; Yadav et al. 2015).
MicroRNAs (miRNAs) as named are small RNA molecules, anneal with 3’-UTR of mRNAs with complementary sequences. The binding of single-stranded mature miRNAs with 3’-UTR of mRNAs triggers either the decay of bound mRNAs or inhibition of translation by the accumulation of bound mRNAs in stress bodies of cells (Wang et al. 2017; Zhang and Wang 2017; Bartel 2004). Similar to mRNAs, the expression of mature miRNAs has been detected in all body tissues and fluids including cerebrospinal fluid (CSF) and their expression is responsive to both pathological and physiological conditions of cells (Botta-Orfila et al. 2014; Serafin et al. 2014; Cao et al. 2017). Earlier studies from our laboratory, as well as other labs, have identified the deregulation of miRNAs in both developing and neurotoxicant-exposed neuronal cells (Pandey et al. 2015b, 2015a; Yadav et al. 2011; Srivastava et al. 2020; Jauhari et al. 2020, 2017). Using rotenone-exposed rats, our studies have identified the coordinated role of miR-146a and the Parkin gene in regulating rotenone-induced neurodegeneration (Jauhari et al. 2020).
In the peripheral blood, miRNAs are present in a highly stable form and can be easily detectible, making them perfect biomarkers. In addition to miRNA biomarkers, differentially expressed proteins (DEPs) have been proposed as biomarkers in various diseases, including PD (Chahine et al. 2014). Neuroproteomics is a revolutionary technique that quantifies thousands of proteins, providing a method for understanding the neuronal alteration associated with neurodegeneration, including PD (Pienaar et al. 2008; Kim et al. 2004). In addition, proteome mapping from various body fluids is being used in the discovery of biomarkers (Ahn and Simpson 2007). Global profiling of proteins and miRNAs in Parkinson’s disease patient’s blood samples can provide unbiased information about the molecular alterations that take place during the PD progression and help to identify the new biomarker in PD. In this context, our lab's research reported the combined role of miRNAs and proteome in ZnO nanoparticle-induced neurodegeneration and identified the role of proteasome complex, spliceosome complex, and mitochondrial protein complex in neurodegeneration (Srivastava et al. 2020). In a recent study using rotenone-induced rats as an animal model of PD, we demonstrated significant upregulation in 19 miRNAs and 96 proteins, while significant downregulation in levels of 22 proteins in rotenone-exposed rats blood samples (Yadav et al. 2022). These findings suggest that differentially expressed proteins and miRNAs in the PD can help to understand the molecular complexity of PD and can also help in the early detection and treatment of people with PD, even though many efforts are needed to find additional PD-related miRNAs and proteins and to validate the results at various levels.
In the present study, we have carried out global protein and miRNA profiling of blood samples collected from Parkinson’s disease patients and healthy controls by using high-throughput LC–MS/MS and OpenArray technologies. Furthermore, miRNAs as the regulator of protein expression are also under modulation and capable to modulate various biological pathways. In this regard, we performed miRNA and protein interaction and pathway enrichment analysis which provide a more extensive explanation of the functional roles of identified miRNAs and proteins in the pathogenesis of Parkinson’s disease (PD). Finally, the validation and regulation of candidate miRNA and proteins were also performed in the cellular model of PD.
Materials and Methods
Chemicals and Reagents
OpenArray Preparation
RNAlater™ Solution (cat# AM7020), RiboPure™- Blood (cat# AM1928), High-capacity cDNA reverse transcription kit (Cat# 4368813), TaqMan™ MicroRNA Reverse Transcription Kit (Cat# 4366597), TaqMan® Custom RT pool (Cat#A25630), TaqMan® Custom OpenArray PreAmp Pool (Cat#4485255), TaqMan™ PreAmp Master Mix (2X) (Cat# 4384266), Tris–EDTA buffer, pH 7.0, RNase-free (AM9861), TaqMan® OpenArray™ RT-PCR Plate Custom Format (P/N # 4470813), TaqMan® OpenArray® Real-Time PCR Master Mix (Cat#4462159), etc. were procured from Thermo Fisher Scientific, Waltham, MA, USA.
Real-Time PCR Preparation
Single-tube assay primers including miR-186 (Cat# 4427975, ID# 002285), miR-29b (Cat# 4427975, ID# 000413), miR-150 (Cat# 4427975, ID# 000473), and Maxima Probe/ROX QPCR Master Mix (2X) (Cat# 0233), PowerUP™ SYBR® Green Master Mix, and other RT-PCR-related reagents were procured from Thermo Fisher Scientific, Waltham, MA, USA.
Proteomics Preparation
Pierce™ Albumin depletion columns (Cat#85160), Pierce™ BCA Protein Assay Kit (Cat# 23227), Pierce® Detergent Removal Spin Columns (Cat#87776), Pierce™ C-18 Spin Columns (Cat# 89870), Pierce™ Quantitative Colorimetric Peptide Assay Kit (Cat# 23275), and NP-40 Surfact-Amps™ Detergent Solution (Cat# 85124) were procured from Thermo Fisher Scientific, Waltham, MA, USA. Dimethyl sulfoxide (DMSO) (Cat# 67685), Dithiothreitol (DTT) (Cat# 10197777001), Iodoacetamide (IAA) (Cat# I1149), and Protease Inhibitor Cocktail (Cat# P8340) were procured from Sigma-Aldrich, USA. RapiGest™ SF Surfactant (Cat#186001861) was procured from Waters, Milford, USA. Trypsin/Lys-C Mix, Mass Spec Grade (Cat# V5071) was procured from Promega, USA.
Cell Culture and Transfection Preparation
SH-SY5Y, a human neuroblastoma (Cat# SH-SY5Y CRL-2266) was procured from ATCC, USA. Media and reagents require for the SH-SY5Y culture including F12 Nutrient Media (Cat# 21700-075), Minimum Essential Media (Cat# 61100-01), Antibiotic/antimycotic solution (Ab/Am) (Cat# 5240062), fetal bovine serum (FBS) (Cat# 10270106), all-trans Retinoic Acid (RA) (Cat# R2625), Brain-Derived Neurotrophic Factor (BDNF) (Cat# 450–10), L-Glutamine supplement (200 mM) (Cat# 25030081), and Gibco sodium pyruvate (100 mM) (Cat# 11360-070), Gibco Opti-MEM Reduced Serum Medium (Cat# 31985-062), Trypan Blue Stain (0.4%) (Cat# 15250-061), Rotenone (Cat# R8875), etc. were procured from Thermo Fisher Scientific, Waltham, MA, USA. DharmaFECT 1 Transfection (Cat# T-2001-02) and Non-Target Control (NTC), 5X siRNA buffer (Cat# B-002000-UB-100) were procured from Dharmacon, USA.
Experimental Design
The experimental workflow for the expression profiling of miRNAs and proteomic study is shown in Fig. 1. Briefly, the miRNA profiling was carried out in total RNA isolated from whole blood, and the proteomic studies are performed in plasma samples. Total RNA was isolated from all the subject’s whole blood (n = 48 PD patients and n = 48 controls) reverse transcribed for cDNA synthesis, pre-amplified, and finally used for OpenArray-based real-time PCR profiling. For proteomics studies, albumin was depleted from blood plasma samples (n = 30 PD patients and n = 30 healthy controls) and then plasma protein samples were processed for peptide preparation. Peptide digest obtained by reduction, alkylation, and tryptic digestion was run on nano-LC–MS/MS for protein profiling. Label-free quantitative (LFQ) proteomics was employed for differential protein expression levels. Different bioinformatics tools and online databases were used to analyze the profiled miRNAs, differentially expressed proteins (DEPs), and associated cellular pathways. Finally, the validation of candidate miRNAs and their target genes was performed in rotenone-exposed differentiated SH-SY5Y cells using loss and gain of function studies.
Fig. 1.
Schematic diagram of the workflow involved in profiling studies of miRNA expression and protein levels
Clinical Samples
The study was approved by the Institutional Ethics committee of King George’s Medical University (KGMU) (Ref. code: 89th ECM II A/P7) and the Indian Institute of Toxicology Research (IITR) (Ref. No: CSIR-IITR/IHEC/JULY/2021/2). All the participants have signed and are provided with written informed consent. All the procedures were performed according to the relevant guidelines. We recruited participants at Neurology OPD of King George’s Medical University (U.P., India). A total of 48 male patients were recruited with PD according to the UK PD Society Brain Bank Diagnostic Criteria, with 59.56 ± 8.83 years average age. The patients with other neurological diseases, liver and lung diseases, renal dysfunction, and cerebrovascular & cardiovascular diseases were excluded from the study. In addition, we recruited 48 healthy controls, unrelated to the enrolled patients and matched by age (54.43 ± 8.62 years) and sex (Table 1 & supplementary Table 1).
Table 1.
General characteristics of the PD patients and controls
| Characteristics | PD Patients (n = 48) | Controls (n = 48) | P Value |
|---|---|---|---|
| Age, Years (min–max) | 59.56 ± 8.83 (43–84) | 54.43 ± 8.62 (41–70) | 0.0897 |
| Sex | M | M |
Data are presented as mean ± SD, and range
Blood and Plasma Collection
Two milliliters (ml) of peripheral blood sample were withdrawn from all the participants (n = 48 PD patients and n = 48 controls), which are distributed equally between tubes containing RNAlater solution (for RNA work) and CPD buffer (for protein work). Whole blood samples were collected in CPD buffer vials (n = 30 PD patients and n = 30 controls) and centrifuged 2000×g for 10 min at 4 °C and the upper layer containing plasma was collected in new micro-centrifuge tubes as described earlier (Yadav et al. 2022). The whole blood and plasma samples were then stored at −80 °C for subsequent analysis.
Total RNA Extraction from Whole Blood and Quality Control
RiboPure™- Blood kit (Thermo Fisher Scientific, USA) was utilized for the total RNAs (including small RNAs) extraction from the whole blood samples (n = 48 for PD patients and n = 48 for controls) using the manufacturer’s protocol. The quality & quantity of RNA were determined by using a BioSpectrometer (Eppendorf, Hamburg, Germany). All the RNA samples used in the study showed an absorbance ratio of 260/280 nm and 260/230 nm between 1.8 and 2. Further, before miRNA profiling, the integrity and DNA contamination of total RNA was checked by agarose gel electrophoresis. In addition, to remove any potential contamination of genomic DNA, the RNA samples were also processed with DNase-I provided along with RiboPure™- Blood kit according to the kit protocol.
Isolation of Total RNA from SH-SY5Y Cells and RT-PCR
The total RNA (including small RNAs) was isolated from control and treated SH-SY5Y cells (n = 3 controls and n = 3 treated SH-SY5Y group) by using miRVana™ miRNA Isolation Kit from Thermo Fisher as manufacturer protocol. The cDNA for normal genes and miRNAs was synthesized by a high-capacity reverse transcription kit and TaqMan™ MicroRNA Reverse Transcription Kit, respectively, from Thermo Fisher using manufacturer protocol. Real-time polymerase chain reaction (RT-PCR) of miRNAs (miR-186, miR-29b, & miR-150) was performed using TaqMan single-tube assays and genes (NeuN, TUBB3, NF-M, Nestin, SYN1, SYP, DJ1, PINK1, SNCA, TH, PARKIN, TP53, BCL2, BAX, YWHAZ, YWHAB, ACTG1 and CALM2) was performed using SYBR Green chemistry. Three experiments were performed with three technical triplicates of each biological group.
Reverse Transcription and Preamplification for miRNA
Reverse transcription (RT) reaction was carried out using a TaqMan miRNA Reverse Transcription kit (Thermo Fisher Scientific, Waltham, MA, USA). In brief, the 7.5 µl RT reaction mixture contained Custom RT primers for OpenArray as 1X, 2.0 mM deoxynucleotide triphosphates (dNTPs), 2 units of RNase inhibitor, 3 mM MgCl2, RT buffer in 1X, 75 units of MultiScribe reverse transcriptase, and 100 ng of total RNA. The thermal cycling program for RT reactions was 40 cycles of 16 °C for 2 min, 42 °C for 1 min, and 1 s at 50 °C. The reaction was terminated by incubating it at 85 °C for 5 min and immediately cooled at 4 °C. After that, the preamplification of the prepared RT product (cDNA) was achieved by using an OpenArray preamp pool of primers and TaqMan preamp master mixture (Thermo Fisher Scientific, USA). The preamplification reaction contained 12.5 µl of preamp master mix, 2.5 µl custom preamp primer pools, 2.5 µl of cDNA, and nuclease-free water to make up the final volume of 25 µl. The thermal cycling parameters used for preamplification were 95 °C for 10 min, 55 °C for 2 min, 72 °C for 2 min, and 12 cycles of 95 °C/15 s, 60 °C/4 min. At the end of the reaction, preamplification products were incubated at 99.9 °C for 10 min and immediate cooling at 4 °C. The preamplification products were diluted by 1:40 in 0.1X Tris–EDTA buffer before using it for real-time PCR.
Expression Profiling of miRNAs Using OpenArray
Around 2588 mature miRNA molecules have been predicted or identified in human samples (miRBase Release 21) (Ozdilek and Demircan 2020). For the identification of PD-regulated or PD-related miRNAs, we have used a “brain-specific” miRNA array panel of 112 miRNAs, which contains miRNAs involved in neural development, neurodegeneration, or expressed in substantially high amounts in brain tissues/ cells (based on earlier studies of our lab) and miRNAs reported by several studies for their role in PD or neurodegeneration (Yadav et al. 2015; Wang et al. 2017; Botta-Orfila et al. 2014; Serafin et al. 2014; Cao et al. 2017; Li et al. 2020; Lugli et al. 2015; Tatura et al. 2016). The OpenArray panel also contains one non-targeting control (NTC) (ath-miR-159a) and five endogenous controls (RNU38B, RNU58A, RNU48, RNU44 & U6 snRNA) for normalization (supplementary Table 2 & supplementary Fig. 1). The reverse transcription and preamplification were done as described above. The diluted preamplification product was mixed with 2X TaqMan OpenArray Real-Time PCR master mix and loaded on the OpenArray plates by OpenArray AccuFill System (Thermo Fisher Scientific, Waltham, MA, USA) to perform the real-time PCR. The raw data were analyzed using the Expression Suite software v1.3 and the relative expression of miRNAs was calculated using the comparative Ct method (2−ΔΔCt) with U6 snRNA as the normalizing control. The significance was given by the ExpressionSuite OpenArray analysis software by applying Student’s t-test. p < 0.05 is significant. The OpenArray data generated and discussed in the present study have been deposited in NCBI-Gene Expression Omnibus (GEO) and the accession no is GSE222480.
Albumin Depletion & In-Solution Tryptic Digestion for Proteomics Studies
Before tryptic digestion, plasma samples (n = 30 for PD patients and n = 30 for healthy controls) were depleted of albumin using Pierce™ Albumin Depletion Kit (Thermo Fisher Scientific, Waltham, MA, USA) as described in the manufacturer protocol. Albumin depletion was standardized by running the depleted sample on SDS-Phage followed by Coomassie staining. After that, in-solution tryptic digestion was carried out following our previous studies (Yadav et al. 2022). In brief, Pierce Detergent Removal Spin Columns (Thermo Fisher Scientific, USA) were used to clean the plasma and estimated the plasma protein using Pierce™ bicinchoninic acid (BCA) protein assay kit (Thermo Fisher Scientific, Waltham, MA, USA). Total 100 µg of plasma protein from every sample and mixed with 0.1% (v/v) surfactant RapiGest SF (Waters, Milford, USA). Reduction and alkylation were achieved by adding 5 mM dithiothreitol (DTT) and 15 mM Iodoacetamide (IAA). After reduction and alkylation, protein samples were overnight digested with Trypsin/Lys-C (Promega, USA) (1:30 (w/w) enzyme/protein ratio) at 37 °C. After overnight digestion, the reaction was quenched by adding formic acid (FA) and incubated for 60 min at 37 °C. The peptide digest was centrifuged at a speed of 20000 g for 10 min at 4 °C and the tryptic peptides in the supernatant were collected in a new tube. Further, tryptic peptides were passed through Pierce C18 Spin Columns for purification and concentration. Finally, the tryptic peptides passed through C18 columns and lyophilized. Lyophilized samples were reconstituted in LCMS grade water and estimated by Pierce Quantitative Colorimetric Peptide Assay Kit (Thermo Fisher Scientific, USA). Finally, the quantified peptide samples were loaded on nano-LC–MS/MS.
Label-Free Quantitative LC–MS/MS Proteomics
Proteomics experiment was performed by an LC–MS comprised of a nano-LC (EASY-nLC 1200; Thermo Fisher, USA)-coupled mass spectrometer (Q Exactive; Thermo Fisher, USA) through ESI source (Thermo Fisher Scientific) in HRMS facility- IITR as described earlier (Yadav et al. 2022). Briefly, the digested peptide samples with 0.1% FA were loaded on reverse-phase Trap Column and then eluted to the analytical column (Easy spray). The mobile phase consisting of (A): 0.1% v/v FA & 1% v/v acetonitrile (ACN) in LC–MS grade water, (B): 0.1% v/v FA & 80% v/v ACN in LC–MS grade water. A 105 min linear gradient of solvent B (5% for 0 min; 5% → 40% for 100 min; 40% → 90% for 5 min) at 300 nL/min flow rate was applied for the elution of loaded Peptides. The voltage 2200 V was applied for ionization and the capillary temp was set to 300 °C. Peptide identification was done by data-dependent acquisition (DDA) mode. The top 10 most prevalent precursors were used for MS data and dynamic exclusion was the 40 s. Full MS resolution was 70,000 with 350–1700 m/z scan range and AGC (automatic gain control) was 1 × 106 with 50 ms maximum injection time. The dd-MS2 resolution was 17,500 and the AGC was 5 × 104 with 100 ms maximum injection time. The accuracy of Peptide samples was checked by the full MS1 spectra of the base peak and total ion chromatogram (TIC).
Protein Identification and Quantitation
Proteome Discoverer software (Proteome Discoverer v 2.4, Thermo Fisher Scientific) was used to analyze the raw proteomics data from all the samples and the peptide lists were searched against the online Homo sapiens (SwissProtTaxID 9606) Uniprot database with N-terminal acetylation and methionine oxidation static modification while cysteine carbamidomethylation as fixed modification. The precursor mass tolerance of 10 ppm and fragment mass tolerance 0.1 Da was set for the database search and a maximum of two missed cleavage sites were allowed. A 1% FDR (false discovery rate) was used for peptide identification. The LC–MS/MS data generated and discussed in this study have been deposited in CCMS- Mass Spectrometry Interactive Virtual Environment (MassIVE) and the accession number is MSV000091013.
GO Analysis and KEGG Pathway Analysis
GO (Gene Ontology) and KEGG (Kyoto Encyclopedia of Genes and Genomes) pathway identification of altered miRNAs were performed by the online web tool DIANA-miRPath V3.0 (http://www.microrna.gr/miRPathv3) (Vlachos et al. 2015). GO terms and KEGG pathways of all significantly deregulated miRNAs were represented as a heat map (Benjamin-Hochberg false discovery rate (FDR) correction p value < 0.05) (Supplementary Table 6 & 7). Proteome discoverer 2.4 was used for the GO annotations including biological processes, cellular components, and molecular functions of differentially expressed proteins (DEPs). In addition, KEGG analysis of DEPs was performed using the online program DAVID (http://david.abcc.ncifcrf.gov/) and PANTHER v 17.0 (http://www.pantherdb.org) (Huang da et al. 2009; Mi and Thomas 2009) (p < 0.05 is significant, Supplementary Table 8).
Interactions Network Analysis
STRING version 11.5 (https://string-db.org/) was used to create the protein–protein interactions (PPIs) network of the identified differentially expressed proteins (DEPs) with interaction score ≥ 0.400 (medium confidence) (Szklarczyk et al. 2019). TargetScan Human release 7.2 web portal (http://www.targetscan.org/vert_72/) was used to obtain the potential targets of deregulated miRNAs in identified DEPs to create miRNA–protein interaction networks (Agarwal et al. 2015) (context + + score percentile ≥ 70).
Cell Culture, Neuronal Differentiation, Chemical Exposure, and Transfection Studies
SH-SY5Y cells (ATCC) were cultured in a mixture of Minimum Essential Media (MEM) and F12 Nutrient Media in a 1:1 ratio, which was supplemented with 1% antibiotic/antimycotic (Ab/Am) solution and 10% fetal bovine serum (FBS). The SH-SY5Y cells were grown at 37 ℃ temperature with 5% CO2 and 95% relative humidity. The differentiation of SH-SY5Y cells to mature neurons was achieved by the exposure of 10 µM of retinoic acid (RA) for 5 days with reducing serum followed by 3 days of brain-derived neurotrophic factor (BDNF) exposure without serum as reported in our previous lab studies (Jauhari et al. 2017). Every alternate day, the differentiating SH-SY5Y cells media was replaced with fresh media containing RA (10 µM) for up to 5 days or BDNF (100 ng/mL) for 3 days after RA exposure. The length of neurites of differentiating SH-SY5Y cells was measured by using a phase-contrast microscope with the software NIS Element BR of Nikon, USA, as reported in our previous study (Pandey et al. 2022). For studying the regulation of target genes by miR-168, cells were transfected with miR-186-mimic and miR-186-inhibitor by using DharmaFECT (Cat#T-2001-02) as described by the manufacturer. Before expression studies, the transfection efficiency of miR-186-mimic/ miR-186-inhibitor in RA + BDNF-induced differentiated SH-SY5Y cells was assessed by RT-PCR (supplementary Fig. 5). SH-SY5Y Cells were exposed to rotenone (2.5 µM, 24 h), a well-known neurotoxicant used for neurodegeneration. The concentration of rotenone was chosen from earlier studies (Xiong et al. 2013).
Statistical Analysis
GraphPad Prism v.9.0 software (Graph Pad Software) was used to perform statistical analysis and graph plot. The data are expressed as the mean ± standard deviation (SD). Relative expression ratio in clinical samples was performed using the formula, ratio = 2Ct(reference)–Ct(target). Mann–Whitney U-test was used to determine the statistical significance of variance for the normalized expression method used for clinical samples. Receiver operating characteristic (ROC) curve analysis was performed to evaluate the discriminative power of the candidate miRNA and protein for PD. The ROC curve analysis and calculation of AUC with a 95% confidence interval (CI) were performed by using GraphPad software. The fold change in the expression of miRNAs/mRNA was calculated by using the 2−ΔΔCt method. Statistical analysis was carried out using Student’s t-test among groups. The statistically significant p value was set at less than 0.05. The adequacy of the sample size was determined by the Power method as described in our earlier study (Yadav et al. 2022).
The raw data related to this article are available on NCBI-GEO (accession no GSE222480) & CCMS-MassIVE (accession no MSV000091013) online data repositories.
Results
Expression of miRNAs in the Blood Isolated from PD Patients and Matching Control Population
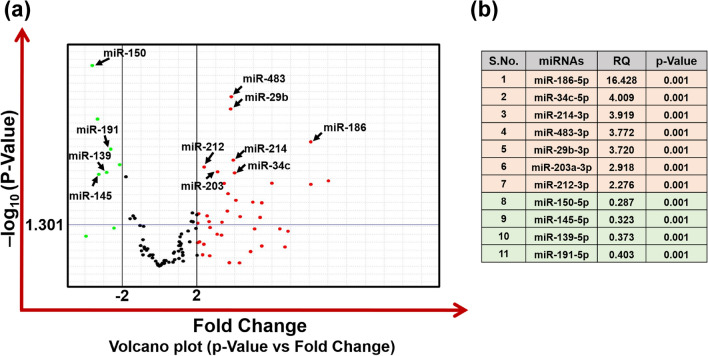
The expression of “brain-specific” miRNAs was examined in total RNA isolated from the whole blood of PD patients and matching controls, using real-time-based OpenArray Panel, which contains primers for 112 miRNAs as listed in supplementary Fig. 1 & Table 2. A Volcano plot analysis was carried out using twofold as threshold boundary for change in expression of miRNAs and p < 0.05 as significance threshold (Fig. 2a). Based on the Volcano plot analysis, 23 and 4 miRNAs were found to be significantly upregulated and downregulated simultaneously in the blood of PD patients in comparison with blood samples of control population (Fig. 2a). Top most significant deregulated miRNAs are listed in the Fig. 2b. All the miRNAs, which were significantly up or downregulated are listed in the supplementary Table 3. To further analyze the inter-individual differences in the expression of substantially up or downregulated miRNAs, we have plotted the scatter plot for the top most significant selected miRNAs (hsa-miR-186-5p, hsa-miR-29b-3p, hsa-miR-483-3p, hsa-miR-214-3p, hsa-miR-146b-5p, hsa-miR-34c-5p, hsa-miR-212-3p, hsa-miR-203a-3p, hsa-miR-150-5p, hsa-miR-139-5p, hsa-miR-145-5p, and hsa-miR-191-5p) using the expression of U6 snRNA as normalization gene (Fig. 3a & b).
Table 2.
List of Primer sequences used for the SYBR Green-based real-time PCR
| S.No | Gene Name | Primer Sequence 5′-3′ |
|---|---|---|
| 1 | SYN1 |
Forward- 5′-CCCCAATCACAAAGAAATGCTC-3’ Reverse- 5’-ATGTCCTGGAAGTCATGCTG-3′ |
| 2 | SYP |
Forward- 5′-AGACAGGGAACACATGCAAG-3′ Reverse- 5′-TCTCCTTAAACACGAACCACAG-3′ |
| 3 | NeuN |
Forward- 5′-GTAGAGGGACGGAAAATTGAGG-3′ Reverse- 5′-CATAGAATTCAGGCCCGTAGAC-3′ |
| 4 | NF-M |
Forward- 5′-CCACAACCACGACCTCAG-3′ Reverse- 5′-AGCGATTTCTATATCCAGAGCC-3′ |
| 5 | TUBB3 |
Forward- 5′-TTTGGACATCTCTTCAGGCC-3′ Reverse- 5′-TTTCACACTCCTTCCGCAC-3′ |
| 6 | Nestin |
Forward- 5′-TGCGGGCTACTGAAAAGTTC-3′ Reverse- 5′-GGCTGAGGGACATCTTGAG-3′ |
| 7 | SNCA |
Forward- 5′-CTGGAAGATATGCCTGTGGATC-3′ Reverse- 5′-AGCACTTGTACAGGATGGAAC-3′ |
| 8 | PINK1 |
Forward- 5′-GAGTATGGAGCAGTCACTTACAG-3′ Reverse- 5′-CAGCACATCAGGGTAGTCG-3′ |
| 9 | Parkin |
Forward- 5′-CGGGAAAACTCAGGGTACAG-3′ Reverse- 5′-AAATTCTGCACTAGTCCCAGG-3′ |
| 10 | DJ1 |
Forward- 5′-GCTGTGAAGGAGATACTGAAGG-3′ Reverse- 5′-TGTCTTTAGCAAGAGGGTGTG-3′ |
| 11 | TH |
Forward- 5′-TCCCAAGAAAAGTGTCAGAGC-3′ Reverse- 5′-AAGGCGATCTCAGCAATCAG-3′ |
| 12 | TP53 |
Forward -5′-GAAGACCCAGGTCCAGATGA -3′ Reverse -5′-CTCCGTCATGTGCTGTGACT-3′ |
| 13 | BAX |
Forward-5′-GACATGTTTTCTGACGGCAAC -3′ Reverse -5′-AAGTCCAATGTCCAGCCC-3′ |
| 14 | BCL2 |
Forward -5′-GTGGATGACTGAGTACCTGAAC-3′ Reverse -5′-GCCAGGAGAAATCAAACAGAGG-3′ |
| 15 | YWHAZ |
Forward- 5′-CTACCGTTACTTGGCTGAGG-3′ Reverse- 5′-CCAGTCTGATAGGATGTGTTGG-3′ |
| 16 | YWHAB |
Forward- 5′-TGCAGTTACTTAGGGACAATCTC-3′ Reverse- 5′-CAGATCACAAAGCACGAGAAAC-3′ |
| 17 | ACTG1 |
Forward-5′-CCGAGCCGTGTTTCCTTC-3′ Reverse-5′-GACGATGCCATGCTCAATG-3′ |
| 18 | CALM2 |
Forward-5′-GTAATGGCACAATTGACTTCCC-3′ Reverse-5′- TCACATGGCGAAGTTCTGC-3′ |
| 19 | ACTB |
Forward-5′- ACCTTCTACAATGAGCTGCG -3′ Reverse-5′- CCTGGATAGCAACGTACATGG-3′ |
Fig. 2.
Volcano plot analysis of miRNA expression data of 112 “brain-specific” miRNAs carried out in blood samples of 48 PD patients and matched 48 healthy controls using OpenArray panel. (a) Volcano plot of miRNAs expressed in PD patient's blood employing custom “brain-specific” miRNAs array with 112 miRNAs in an OpenArray plate format. The volcano plot is drawn between the p value and fold change. A horizontal line in the volcano plot denotes the threshold for the p value of the t-test, which is set as 0.05 (1.301 in –log10) while two additional vertical lines showing the cut-off boundary (± twofold) for the expression of downregulated and upregulated miRNAs. The green dots denote miRNAs that are downregulated, and the red dots denote miRNAs that are upregulated. List of the miRNAs that are most significantly deregulated in the blood samples of PD patients (b). RQ; Relative Quantification, (Two additional green dots in the significant downregulated section are for small-nucleolar RNAs; RNU44 & RNU38B housekeeping genes)
Fig. 3.
Scatter plot analysis of most significantly deregulated miRNAs identified through OpenArray panel studies in the blood samples of PD patient's and control population (n = 48 for PD patients and n = 48 for controls). Relative expression of 7 miRNAs showed significant upregulation (a) while 4 miRNAs showed significant downregulation (b) in the PD patients samples compared to controls. The expression of the miRNAs is normalized with the expression of U6 snRNA. The samples above the mean are indicated by the sample number in both the control and the patients group. The Mann–Whitney U-test was applied for statistical significance. significant p < 0.05
PD Predictive Capability of miRNAs Deregulated in PD Patient’s Samples
Receiver operating characteristic (ROC) curves were generated to assess the PD predictive capability of identified miRNAs (Fig. 4). The ROC curve analysis of upregulated miRNAs, i.e., hsa-miR-186-5p, hsa-miR-29b-3p, hsa-miR-483-3p, hsa-miR-214-3p, hsa-miR-34c-5p, hsa-miR-212-3p, and hsa-miR-203a-3p (Fig. 4a), and downregulated miRNAs, i.e., hsa-miR-150-5p, hsa-miR-139-5p, hsa-miR-145-5p, and hsa-miR-191-5p (Fig. 4b) showed the area AUC more than 0.65. Regulation of miR-150-5p, which was downregulated in PD patient’s blood samples, showed the highest area AUC of 0.8059; however, miRNA hsa-miR-186-5p, which was maximally upregulated in PD patients, showed the area AUC of 0.6957 (Fig. 4a & b).
Fig. 4.
Receiver operating characteristic (ROC) curves of all seven upregulated (a) and four downregulated (b) miRNAs in PD patient’s blood samples compared to controls. The discriminative ability of the upregulated miRNAs was shown by the ROC curves with 1-specificity (false-positive rate) at the x-axis and sensitivity (true-positive rate) at the y-axis
Functional Enrichment of Deregulated miRNAs
DIANA-miRPath v.3 program (http://www.microrna.gr/miRPathv3) was used to perform GO and KEGG pathway analysis of miRNAs significantly deregulated in PD patients. GO analysis revealed the association of a different number of miRNAs with cellular processes and cellular parts. GO analysis has identified a maximum number of miRNAs related to ion binding functions (24), followed by the cellular protein modification process (22) and cellular nitrogen compound metabolic process (21) (Supplementary Fig. 6). The results of KEGG pathway analysis revealed the involvement of significantly deregulated miRNAs in a wide range of biological processes (Fig. 5) like ECM-receptor interaction (5), amoebiasis (2), glycosphingolipid biosynthesis—lacto and neolacto series (5), glioma (8), TGF-beta signaling pathway (6), signaling pathways regulating pluripotency of stem cells (6), thyroid hormone synthesis (3), protein digestion and absorption (2), lysine degradation (5), focal adhesion (4), proteoglycans in cancer (6), estrogen signaling pathway (5), and PI3K-Akt signaling pathway (3). The dendrogram also showed the clustering of involved microRNA and targeted functions as the heat map (Fig. 5 and supplementary Fig. 6). Detail list of GO analysis and KEGG pathway analysis is provided in the supplementary Table 6 & 7.
Fig. 5.
KEGG pathway analysis of miRNAs significantly deregulated in PD patients' blood samples. The heat map shows the miRNAs involved in significant KEGG pathways. The color key at the right top bottom represents the significance level of the pathway: red represents high significance while yellow shows less or significance level (significant p < 0.05)
Global Profiling of Plasma Proteins in PD Patients and Control Population Using LC–MS/MS
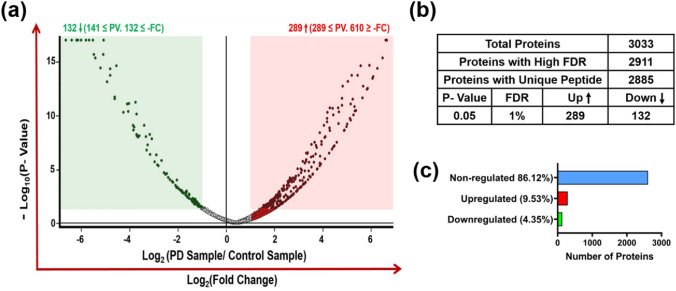
Plasma samples of Parkinson’s disease patients (n = 30) and matched healthy controls (n = 30) were analyzed using LC–MS/MS for the identification of the DEPs. The raw data generated from the mass spectrometry were submitted to Proteome Discoverer 2.4 (Thermo Fisher Scientific, MA) platform to search and matched against the Homo sapiens database. In blood plasma, a total of 3033 proteins were identified of which 2885 proteins consist of (a minimum of one distinct high-confidence peptide per protein) (Fig. 6b). A Volcano plot analysis of LC–MS/MS data was carried out by setting twofold as threshold limit and p < 0.05 as significant (Fig. 6a). 2612 (86.12%) proteins out of the total proteins did not meet the threshold criteria for a significant change (Fig. 6c). 132 (4.35%) proteins were significantly downregulated (abundance ratio < twofold) in the PD patient group compared to the control group, while a total of 289 (9.53%) proteins were significantly upregulated (abundance ratio > twofold) (Fig. 6a-c) (Supplementary Table 4). Table 3 provides a list of the top 25 proteins deregulated in the PD patient group. The scatter plot analysis of deregulated proteins showed a clear difference between the abundances of identified proteins in the control and PD group and identified significant and substantial downregulation in the levels of YWHAZ, PSMA4, TPI1, ACTG1, ACTN1, PFN1, SELENBP1, and CFL1 proteins (Fig. 7a) and upregulation in levels of HYOU1, SERPINA1, CDH1, C1RL, HEL-214, MCAM, and APOA4 proteins (Fig. 7b).
Fig. 6.
Proteomic analysis of the differentially expressed proteins in 30 PD patient’s blood plasma samples compared to 30 healthy controls. The volcano plot is drawn between –log10 p value at the y-axis versus fold change (log2) at the x-axis. The Red color box on the right side of the volcano plot denotes significantly upregulated proteins (as red dots) and on the left side, the green color box denotes significantly downregulated proteins (as green dots). The log2 fold change boundary is ± 1 (± twofold) with p value < 0.05 (1.301 in –log10) (a). Table showing the total number of proteins detected and number of proteins with unique peptides (b) and bar diagram showing the percent of proteins up or downregulated (c)
Table 3.
List of top 25 plasma proteins significantly deregulated in PD patients relative to the controls
| S.No | Description | Accession | Gene Symbol | MW [kDa] | Abundance Ratio (log2) (Fold Change) | p Value |
|---|---|---|---|---|---|---|
| 1 | Calmodulin | B2RDW0 | CALM2 | 16.8 | −6.64 | 1E-17 |
| 2 | Triosephosphate isomerase | B4DUI5 | TPI1 | 22.9 | −6.21 | 1E-17 |
| 3 | Hemoglobin delta-beta fusion protein (Fragment) | Q5XTR9 | HBB | 3.9 | −5.53 | 1E-17 |
| 4 | Actin, cytoplasmic 2 | P63261 | ACTG1 | 41.8 | −4.61 | 1.93E-12 |
| 5 | Osteonectin (Fragment) | F5GY03 | SPARC | 17.5 | −4.05 | 4.16E-11 |
| 6 | UV excision repair protein RAD23 | B4DDJ7 | RAD23A | 22.2 | −4.03 | 7.92E-12 |
| 7 | Proteasome subunit alpha type (Fragment) | H0YL69 | PSMA4 | 26.4 | −3.72 | 7.39E-11 |
| 8 | Ribonuclease inhibitor | A0A140VJT8 | RNH1 | 49.9 | −3.4 | 8.07E-10 |
| 9 | Carbonic anhydrase | V9HWE3 | HEL-S-11 | 28.9 | −3.16 | 4.42E-07 |
| 10 | Profilin-1 | P07737 | PFN1 | 15 | −2.96 | 2.12E-06 |
| 11 | Methanethiol oxidase | Q13228 | SELENBP1 | 52.4 | −2.89 | 4.79E-07 |
| 12 | cDNA FLJ58490, highly similar to Homo sapiens CCR4- NOT transcription complex, subunit 1 (CNOT1), transcript variant 1, mRNA | B4DYL7 | CNOT1 | 139.6 | −2.81 | 4.69E-06 |
| 13 | 14–3-3 protein zeta/delta, Epididymis luminal protein 4 | D0PNI1 | YWHAZ | 27.7 | −2.81 | 4.92E-06 |
| 14 | 14–3-3 protein beta/alpha | P31946 | YWHAB | 28.1 | −2.57 | 2.12E-05 |
| 15 | cDNA, FLJ92775, highly similar to Homo sapiens melanoma cell adhesion molecule (MCAM), mRNA | B2R642 | MCAM | 71.6 | 2.51 | 0.000318 |
| 16 | Complement C1r subcomponent-like protein | Q9NZP8 | C1RL | 53.5 | 2.6 | 0.004539 |
| 17 | APOA4 protein (Fragment) | Q13784 | APOA4 | 28.1 | 2.73 | 8.75E-05 |
| 18 | Protease (Fragment) | A8JS90 | N/A | 10.5 | 2.94 | 0.000953 |
| 19 | Alpha-1-antitrypsin (Fragment) | A0A0B4J278 | SERPINA1 | 15.7 | 4.09 | 1.10E-06 |
| 20 | cDNA FLJ56074, highly similar to 150 kDa oxygen- regulated protein (Orp150) | B7Z2N4 | HYOU1 | 109.6 | 3.66 | 3.16E-08 |
| 21 | Env polyprotein (Fragment) | Q9WIT4 | env | 19.6 | 4.29 | 1.93E-11 |
| 22 | Epididymis luminal protein 214 | V9HW68 | HEL-214 | 51.7 | 4.44 | 8.94E-08 |
| 23 | E-cadherin | Q9UII7 | CDH1 | 99.6 | 5.27 | 9.01E-11 |
| 24 | Alpha-1-antitrypsin short transcript variant 1C4 | A0A1L7B5J3 | SERPINA1 | 5.1 | 5.85 | 3.14E-13 |
| 25 | Virion infectivity factor | G1FP33 | vif | 22.7 | 6.64 | 1E-17 |
Fig. 7.
Scatter plot analysis of significantly deregulated proteins in all the tested blood plasma samples. The abundance of 8 proteins (YWHAZ, PSMA4, TPI1, ACTG1, ACTN1, PFN1, SELENBP1, & CFL1) showed significant downregulation (a) and abundance of 7 proteins (HYOU1, SERPINA1, CDH1, C1RL, HEL-214, MCAM, & APOA4) showed significant upregulation (b) in the 30 PD patients samples compared to 30 healthy controls. The samples above the mean are indicated by the sample number in both the control and patients group. The Mann–Whitney U-test was applied for statistical significance. Significant p < 0.05
PD Predictive Capability of Proteins Deregulated in PD Patient’s Samples
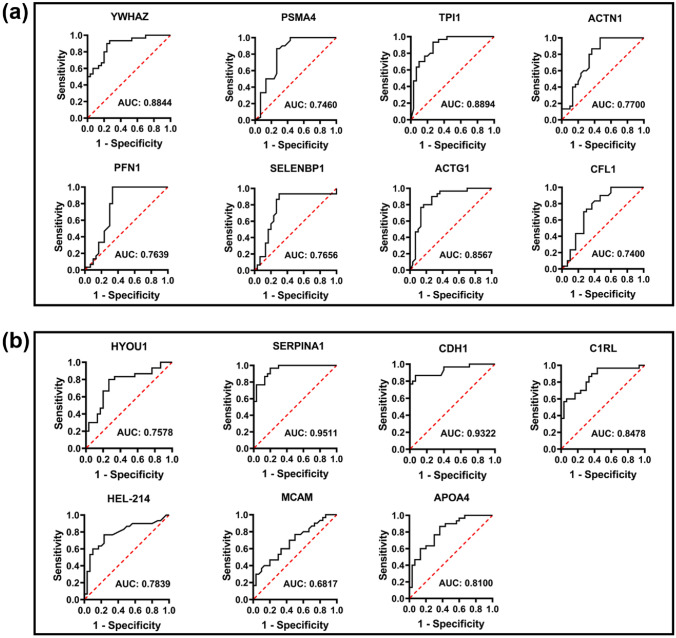
Receiver operating characteristic (ROC) curves of deregulated proteins were also generated to determine the PD predictive capability of identified proteins (Fig. 8). ROC curve analysis of maximally deregulated proteins identified through global protein profiling, which are upregulated (HYOU1, SERPINA1, CDH1, C1RL, HEL-214, MCAM, and APOA4; Fig. 8b) and downregulated (YWHAZ, PSMA4, TPI1, ACTG1, ACTN1, PFN1, SELENBP1, and CFL1; Fig. 8a) showed the area AUC more than 0.7. Maximally upregulated protein SERPINA1 showed the highest area AUC of 0.9511 (Fig. 8b) and the maximum downregulated protein YWHAZ showed an area AUC of 0.8844 (Fig. 8a).
Fig. 8.
Receiver operating characteristic (ROC) curves of eight downregulated and seven upregulated proteins in all the tested samples (n = 30 PD patients and n = 30 healthy controls). The discriminative ability of the downregulated proteins was shown by the ROC curves with 1-specificity (false-positive rate) at the x-axis and sensitivity (true-positive rate) at the y-axis (a & b). The AUC values of more than 0.65
Functional Enrichment of DEPs in Different Classes and Pathway
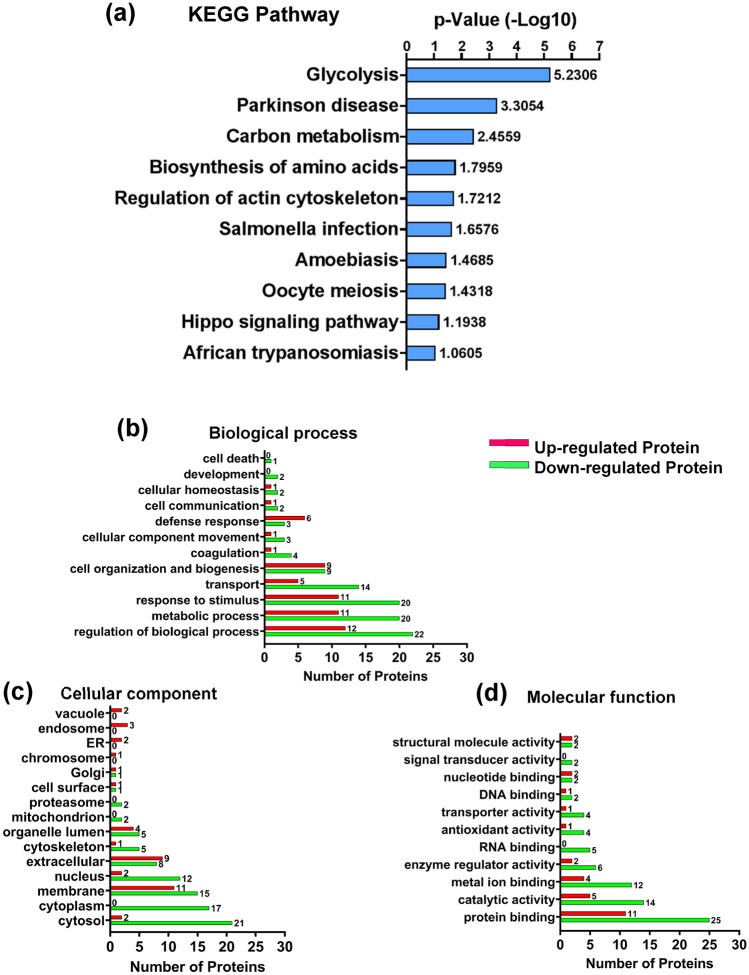
Differentially expressed proteins (DEPs) identified from our study were uploaded on the DAVID portal (http://david.abcc.ncifcrf.gov/) for the KEGG pathway enrichment analysis. The results of the KEGG pathway analysis showed that the proteins identified in our study are involved in major pathways like glycolysis (3), Parkinson’s disease (3), carbon metabolism (4), biosynthesis of amino acids (3), and regulation of actin cytoskeleton in which glycolysis and Parkinson’s disease pathway are the most significant (Fig. 9a and Supplementary Table 8). Additionally, GO analysis was carried out using the GO enrichments analysis tool by Proteome Discoverer 2.4 to form categorization clusters based on biological processes, cellular compartments, and molecular functioning. Numbers on the top of each red bar (upregulated) and green bar (downregulated) represent the number of proteins (Fig. 9b–d).
Fig. 9.
KEGG pathway analysis of the differentially expressed proteins (DEPs). PANTHER, KEGG, and DAVID were employed to functionally annotate regulatory pathways of proteins differentially expressed in the blood of PD patients. The DEPs involved in different biological pathways such as glycolysis, Parkinson’s disease, carbon metabolism, amino acid biosynthesis, regulation of actin cytoskeleton, etc. (a). Gene ontology (GO) analysis of proteins differentially expressed in PD patient’s blood using Proteome Discoverer 2.4 (b-d). The red bar represents the upregulated proteins while the green bar represents the downregulated proteins and the numbers present on the top of each bar denote the number of proteins (b-d). The DEPs were grouped into various functional groups using biological process (b), cellular component (c), and molecular function (d) (significant p < 0.05)
Protein–Protein Interactions (PPIs) Network Analysis
A protein–protein interaction network was generated employing the STRING algorithm (https://string-db.org/), which showed a complex and strong interaction network of all differentially expressed proteins at the medium confidence score of 0.4 (Fig. 10a). Further STRING analysis demonstrated that several proteins were dysregulated in clustered like post-transcriptional regulation of gene expression, actin cytoskeleton organization, ATP biosynthesis process, ubiquitin-dependent protein catabolic regulation, inflammatory response, cellular detoxification, gas/oxygen transport, autophagy, and regulation of localization as demonstrated in Fig. 10b.
Fig. 10.
Protein–protein and protein–miRNA interactions network analysis. The functional interaction network of DEPs in the blood plasma of PD patients in comparison with controls using STRING networks. PPIs of all DEPs show complex and strong interaction (a). Protein clusters drawn in the circle represent biological functions based on gene ontology annotations (b). TargetScan, a free online tool, was used to find correlations between discovered miRNAs and proteins. The downregulated proteins indicated as green bubbles were directly targeted by upregulated miRNAs represented as red triangles (through black arrows) (c)
Construction of Differentially Expressed Proteins and miRNAs Interaction Networks
To further investigate the interaction between the significantly deregulated miRNAs and proteins, the TargetScan web portal (http://targetscan.org) was used. As miRNAs are reported to negatively regulate the expression of their target genes by binding at 3’-UTR of protein-coding genes, potential binding sites of miRNAs were identified through the TargetScan web portal. A total of 20 miRNAs that have been significantly upregulated possess predicted binding sites at 3’UTR to the proteins (context + + score percentile ≥ 70), which are downregulated in PD patient’s blood as observed in our LC–MS/MS studies (Fig. 10c). The upregulated miRNAs (red triangle) target the downregulated proteins (green bubble) as represented by solid black arrows (Fig. 10c). Downregulated protein YWHAZ was targeted by the maximum number (11) of upregulated miRNAs including hsa-miR-186-5p, hsa-miR-384, hsa-miR-206, hsa-miR-503-5p, hsa-miR-29c-3p, hsa-miR-29b-3p, hsa-miR-214-3p, hsa-miR-192-5p, hsa-miR-204-5p, hsa-miR-155-5p, and hsa-miR-664a-3p. Other DEPs proteins that are potential targets of deregulated miRNAs are represented in Table 4. The proteins were predicted to play distinct roles in numerous biological pathways including post-transcriptional regulation of gene expression, actin cytoskeleton organization, ATP biosynthesis process, ubiquitin-dependent protein catabolic regulation, and regulation of localization, using STRING software (Fig. 10b).
Table 4.
List of miRNAs targeted Proteins interaction analyzed by TargetScan software
| S.No | Target Protein | miRNA | Position in the UTR | Seed match | Context + + score | Context + + score percentile |
|---|---|---|---|---|---|---|
| Upregulated miRNA- Downregulated protein interaction | ||||||
| 1 | CALM2 | hsa-miR-206 | 562–568 | 7mer-1A | −0.24 | 92 |
| hsa-miR-186-5p | 315–321 | 7mer-1A | −0.09 | 97 | ||
| 2 | YWHAZ | hsa-miR-186-5p | 1446–1452 | 7mer-m8 | −0.02 | 73 |
| hsa-miR-384 | 3704–3710 | 7mer-1A | −0.08 | 76 | ||
| hsa-miR-206 | 590–597 | 8mer | −0.35 | 97 | ||
| hsa-miR-503-5p | 3548–3554 | 7mer-1A | −0.15 | 89 | ||
| hsa-miR-29c-3p | 3612–3618 | 7mer-m8 | −0.18 | 80 | ||
| hsa-miR-29b-3p | 3612–3618 | 7mer-m8 | −0.18 | 80 | ||
| hsa-miR-214-3p | 613–619 | 7mer-m8 | −0.26 | 97 | ||
| hsa-miR-192-5p | 3657–3663 | 7mer-1A | −0.15 | 86 | ||
| hsa-miR-204-5p | 369–375 | 7mer-m8 | −0.2 | 92 | ||
| hsa-miR-155-5p | 1353–1360 | 8mer | −0.1 | 71 | ||
| hsa-miR-664a-3p | 712–718 | 7mer-m8 | −0.05 | 80 | ||
| 3 | YWHAB | hsa-miR-511-5p | 522–528 | 7mer-m8 | −0.04 | 81 |
| hsa-miR-186-5p | 621–627 | 7mer-m8 | −0.04 | 91 | ||
| hsa-miR-146b-5p | 1365–1372 | 8mer | −0.16 | 87 | ||
| hsa-miR-384 | 130–137 | 8mer | −0.33 | 98 | ||
| hsa-miR-143-3p | 1006–1012 | 7mer-m8 | −0.06 | 61 | ||
| hsa-miR-214-3p | 1671–1677 | 7mer-1A | −0.17 | 91 | ||
| hsa-miR-212-3p | 1156–1162 | 7mer-m8 | −0.18 | 93 | ||
| 4 | ENO1 | hsa-miR-143-3p | 282–288 | 7mer-m8 | −0.12 | 80 |
| 5 | ACTG1 | hsa-miR-186-5p | 610–616 | 7mer-m8 | −0.02 | 73 |
| hsa-miR-212-3p | 646–652 | 7mer-1A | −0.09 | 85 | ||
| 6 | ANXA3 | hsa-miR-143-3p | 170–176 | 7mer-1A | −0.21 | 93 |
| 7 | ANXA5 | hsa-miR-214-3p | 21–27 | 7mer-m8 | −0.16 | 89 |
| hsa-miR-34a-5p | 44–50 | 7mer-m8 | −0.3 | 91 | ||
| hsa-miR-34c-5p | 44–50 | 7mer-m8 | −0.29 | 90 | ||
| hsa-miR-511-3p | 129–135 | 7mer-1A | −0.21 | 93 | ||
| hsa-miR-29c-3p | 156–162 | 7mer-m8 | −0.31 | 93 | ||
| hsa-miR-29b-3p | 156–162 | 7mer-m8 | −0.31 | 92 | ||
| hsa-miR-384 | 651–657 | 7mer-1A | −0.09 | 76 | ||
| hsa-miR-186-5p | 1083–1089 | 7mer-m8 | −0.02 | 73 | ||
| 8 | C1RL | hsa-miR-511-5p | 1858–1864 | 7mer-m8 | −0.03 | 77 |
| hsa-miR-331-5p | 2395–2401 | 7mer-m8 | −0.27 | 93 | ||
| hsa-miR-206 | 993–999 | 7mer-m8 | −0.21 | 90 | ||
| hsa-miR-143-3p | 841–847 | 7mer-1A | −0.15 | 86 | ||
| hsa-miR-214-3p | 1291–1297 | 7mer-m8 | −0.19 | 93 | ||
| hsa-miR-204-5p | 1200–1206 | 7mer-m8 | −0.07 | 74 | ||
| 9 | FLNA | hsa-miR-503-5p | 210–216 | 7mer-m8 | −0.22 | 95 |
| hsa-miR-146b-5p | 275–281 | 7mer-m8 | −0.13 | 82 | ||
| 10 | CNOT1 | hsa-miR-9-5p | 403–409 | 7mer-1A | −0.08 | 81 |
| hsa-miR-206 | 389–395 | 7mer-m8 | −0.19 | 88 | ||
| hsa-miR-384 | 757–763 | 7mer-1A | −0.08 | 74 | ||
| hsa-miR-192-5p | 1149–1155 | 7mer-m8 | −0.1 | 77 | ||
| 11 | CFL1 | hsa-miR-511-5p | 509–516 | 8mer | −0.49 | 99 |
| hsa-miR-29c-3p | 598–605 | 8mer | −0.47 | 98 | ||
| hsa-miR-29b-3p | 598–605 | 8mer | −0.48 | 98 | ||
| 12 | SERPINB1 | hsa-miR-490-3p | 461–468 | 8mer | −0.3 | 98 |
| hsa-miR-214-3p | 711–717 | 7mer-1A | −0.15 | 88 | ||
| hsa-miR-212-3p | 394–400 | 7mer-m8 | −0.16 | 92 | ||
| 13 | SPARC | hsa-miR-29c-3p | 104–110 | 7mer-1A | −0.32 | 94 |
| hsa-miR-29b-3p | 104–110 | 7mer-1A | −0.32 | 94 | ||
| hsa-miR-146b-5p | 703–709 | 7mer-m8 | −0.12 | 81 | ||
| hsa-miR-384 | 2236–2242 | 7mer-m8 | −0.15 | 89 | ||
| hsa-miR-192-5p | 1836–1842 | 7mer-m8 | −0.18 | 90 | ||
| hsa-miR-204-5p | 441–448 | 8mer | −0.21 | 93 | ||
| 14 | PRDX2 | hsa-miR-143-3p | 1081–1087 | 7mer-m8 | −0.11 | 79 |
| hsa-miR-214-3p | 819–825 | 7mer-m8 | −0.12 | 83 | ||
| 15 | PGAM4 | hsa-miR-146b-5p | 219–225 | 7mer-1A | −0.08 | 71 |
| hsa-miR-206 | 432–438 | 7mer-1A | −0.07 | 70 | ||
| hsa-miR-214-3p | 39–45 | 7mer-m8 | −0.11 | 82 | ||
| 16 | PSMA4 | hsa-miR-511-5p | 1449–1455 | 7mer-m8 | −0.03 | 78 |
| hsa-miR-146b-5p | 29–36 | 8mer | −0.59 | 99 | ||
| hsa-miR-384 | 203–209 | 7mer-m8 | −0.14 | 89 | ||
| hsa-miR-29c-3p | 818–824 | 7mer-m8 | −0.17 | 79 | ||
| hsa-miR-29b-3p | 818–824 | 7mer-m8 | −0.17 | 78 | ||
| 17 | ESD | hsa-miR-146b-5p | 627–633 | 7mer-1A | −0.1 | 77 |
| hsa-miR-384 | 197–203 | 7mer-1A | −0.15 | 89 | ||
| hsa-miR-212-3p | 474–480 | 7mer-1A | −0.07 | 81 | ||
| hsa-miR-664a-3p | 515–521 | 7mer-m8 | −0.07 | 85 | ||
| 18 | RAD23A | hsa-miR-214-3p | 609–615 | 7mer-m8 | −0.15 | 88 |
| Downregulated miRNA- Upregulated protein interaction | ||||||
| 1 | SERPINA1 | hsa-miR-150-5p | 916–922 | 7mer-1A | −0.03 | 70 |
| 2 | LAMP2A | hsa-miR-145-5p | 695–701 | 7mer-m8 | −0.14 | 88 |
| 3 | CDH1 | hsa-miR-150-5p | 2490–2496 | 7mer-1A | −0.09 | 88 |
| hsa-miR-139-5p | 1899–1905 | 7mer-m8 | −0.15 | 90 | ||
Regulation of miRNAs and Proteins Identified in Dopaminergic SH-SY5Y Cells Exposed to Rotenone
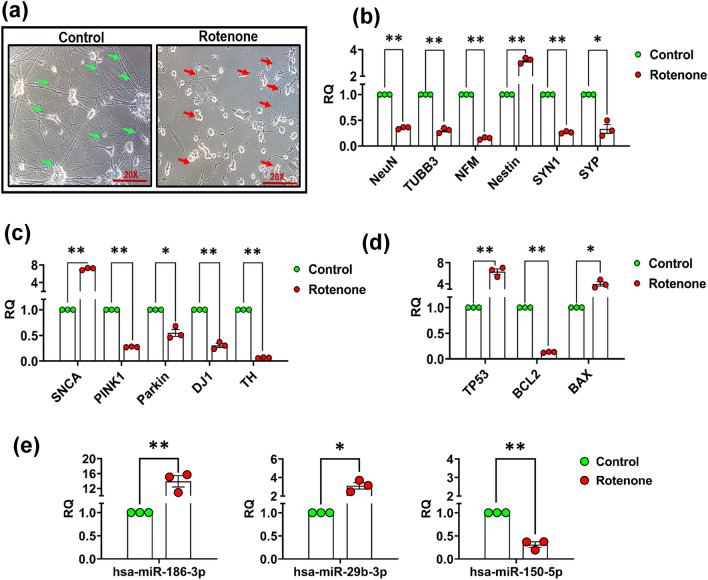
Rotenone exposure (2.5 µM, for 24 h) to dopaminergic SH-SY5Y cells (fully differentiated) induced fragmentation of neurites and promoted the degeneration of cells (Fig. 11a). The expression of neuronal (NeuN, NF-M, TUBB3) and synaptic marker (SYN1 & SYP) was substantially decreased, while the expression of immature neuronal marker Nestin was increased in rotenone-exposed differentiated SH-SY5Y cells (Fig. 11b). Moreover, expressions of PD- or dopamine-associated genes such as tyrosine hydroxylase (TH), DJ1, PARKIN, and PINK1 have been significantly downregulated, while the expression of the SNCA gene increased significantly in the rotenone-exposed differentiated SH-SY5Y cells (Fig. 11c). The expression studies of apoptosis gene (BAX and BCL2) and transcription factor TP53 has shown a significant downregulation in the expression of BCL2 gene and a significant upregulation in the expression of BAX and TP53 genes in the rotenone-exposed differentiated SH-SY5Y cells (Fig. 11d). A similar trend of expression in these genes was also observed in blood samples of PD patient’s (Supplementary Fig. 4a–c). Furthermore, the expression of the top three candidate miRNAs (miR-186, miR-29b & miR-150) was also verified by single-tube assay primer, and a similar trend of expression with some alteration was obtained in all three miRNAs as we found in the PD patient’s samples (Fig. 11e).
Fig. 11.
Phase-contrast image and expression of neuronal and PD-related genes and candidate miRNAs after exposure to rotenone in differentiated SH-SY5Y neuroblastoma cells. Images of control and rotenone-exposed SH-SY5Y cells were captured using a Nikon NIS microscope. The control group showed intact neurites (green arrows) while the rotenone group showed damaged/degraded neurites and floating dead cells (red arrows) (a). Expression of NeuN, TUBB3, NF-M, Nestin, SYN1, and SYP genes was carried out in total RNA isolated from rotenone-exposed differentiated SH-SY5Y cells compared to controls using real-time PCR (b). Expression of SNCA, PINK1, PARKIN, DJ1, and TH gene was determined through real-time PCR in rotenone-exposed differentiated SH-SY5Y cells compared to controls (c). Real-time PCR of expression of apoptosis gene (BAX and BCL2) and transcription factor TP53 (d). Real-time expression of candidate miRNAs (hsa-miR-186. hsa-miR-29b, and hsa-miR-150) in rotenone-exposed SH-SY5Y cells (e). Three independent experiments were carried out with three technical replicates of each sample group. The significance level was calculated using the Student’s t-test. Data are represented as mean ± SD. *p < 0.05, **p < 0.01, versus control. (RQ- Relative Quantification)
miR-186 Regulates the Expression of Potential Targets
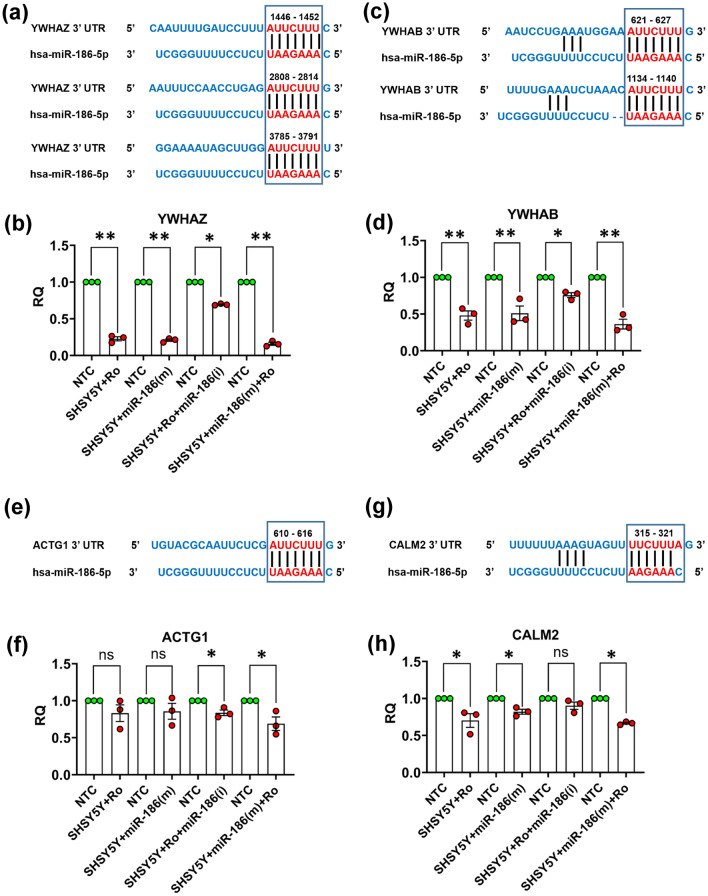
The TargetScan analysis of 3’-UTR of YWHAZ, YWHAB, ACTG1, and CALM2 genes has shown the binding sites of miR-186 in these proteins, which are identified through global proteome profiling in blood samples of PD patients (Fig. 12). YWHAZ has shown< maximum 3 binding sites (1446-1452, 2808-2814, and 3785-3791position at 3’-UTR) and YWHAB has shown two (621-627 & 1134-1140 position at 3’ UTR) while ACTG1 (610-616 position) and CALM2 (315-321 position) have shown single-single binding site at the 3’ UTR (Fig. 12a, c, e, & g). Real-time expression studies carried out in differentiated SH-SH5Y cells transfected with mimics of miR-186 have shown significant downregulation in the expression of YWHAZ and YWHAB genes as found in rotenone-exposed differentiated SH-SY5Y cells (Fig. 12b & d). The decreased expression of YWHAZ and YWHAB genes was recovered, when SH-SY5Y cells were transfected with inhibitors of miR-186. Similarly, when rotenone-pre-exposed SH-SY5Y cells were transfected with mimics of miR-186, a further decrease was observed in the expression of YWHAZ and YWHAB genes (Fig. 12b & d). Additionally, the expression of ACTG1 & CALM2 was found slightly regulated by rotenone exposure or miR-186 mimic/inhibitors (Fig. 12f & h). The expression of ACTG1 was not significantly changed by the rotenone or miR-186 mimic individually while rotenone with miR-186 mimic caused the downregulation of ACTG1 (Fig. 12f). Furthermore, the expression of CALM2 was significantly downregulated by the rotenone or miR-186 mimic individually or together in SH-SY5Y cells (Fig. 12h).
Fig. 12.
Role of miR-186 expression in the regulation of levels of YWHAZ, YWHAB, ACTG1,and CALM2 proteins. Scanning of 3’ UTR of YWHAZ, YWHAB, ACTG1, and CALM2 genes through TargetScan portal (a, c, e & g). Effect of transfection of miR-186(m) and miR-186 (i) on the expression of YWHAZ, YWHAB, ACTG1, and CALM2 genes in differentiated SH-SY5Y cells as measured through real-time PCR (b, d, f & h). Three individual experiments were performed using different passage SH-SY5Y cells. Data are represented as mean ± SD. *p < 0.05, **p < 0.01, versus control. RQ- Relative Quantification, NTC-non-target control, m-mimic, i-inhibitor, Ro- Rotenone, ns- not significant (p > 0.05)
Discussion
PD is an age-associated disorder, which is difficult to manage with the increasing age of patients (Peters et al. 2011). The share of aged people in the total population is increasing globally, which warrants us to look for a cure and early diagnosis of age-associated diseases and disorders like PD. The cause of PD is not fully identified; however, a combination of genetic and environmental factors is currently believed as the major causative agents (Jankovic and Tan 2020). The diagnosis of PD is based on the clinical symptoms, which are mostly motor features defined by UK Brain Bank Criteria, UPDRS, and Hoehn and Yahr scale (Jankovic 2008). The addition of any knowledge, which can be used for the early detection or monitoring of PD management, can be very crucial for PD patients. Keeping this in view, we have screened the expression of “brain-specific” miRNAs and performed global protein profiling in blood samples of PD patients and respective controls to identify the set of proteins and miRNAs, which can be used to diagnose or monitor the development of PD.
Label-free quantitative proteomics technique was employed to find the proteins that were differentially expressed, and the OpenArray platform, based on real-time PCR, was used for miRNA profiling. Herein, an endogenously customized miRNA OpenArray panel contained primers for 112 miRNAs, which are either related to neural development or neurodegeneration termed as “brain-specific” miRNAs array was used. Based on the analysis of “brain-specific miRNAs” array run in 48 patients and 48 control samples, significant upregulation in the expression of 23 miRNAs, and significant downregulation in the expression of 4 miRNAs was identified in PD patients. Similarly, in global protein profiling, 2885 proteins were identified through unique peptides, out of which, 289 proteins were significantly upregulated, while 132 proteins were significantly downregulated in the blood plasma of PD patients, compared to respective controls. Interestingly, we have observed substantial and significant upregulation and downregulation in levels of several fragments of immunoglobulin proteins (Supplementary Table 5). In the list of downregulated proteins, Calmodulin was maximally downregulated (6.64 folds) in the blood of PD patients (Supplementary Table 4). Calmodulin is a small, acidic, calcium-binding protein, which acts as a calcium sensor and relays signals to various calcium-sensitive enzymes, ion channels, and other proteins (Yaduvanshi et al. 2021). Calcium-induced excitotoxicity is one of the important mechanisms, which are considered responsible for the neurodegeneration of dopaminergic neurons; however, its role in the selective degeneration of dopaminergic neurons is not fully understood (Hurley et al. 2013). Studies by Hurley et al., (2013) have performed immunohistochemistry in PD brain tissues and have observed substantial downregulation in Calmodulin positive cells in tissue samples of PD brains (Hurley et al. 2013). However, to the best of our knowledge, we are the first to report dramatic downregulation of Calmodulin proteins in plasma samples of PD patients through unbiased global protein profiling. Other than the fragments of immunoglobulins, triosephosphate isomerase (TPI1) is another protein, which has shown substantial and significant downregulation in plasma samples of PD patients (Supplementary Table 4). Triosephosphate isomerase maintains rapid equilibration of the triosephosphate produced in glycolysis and its interconnection with lipid metabolism (Bonnet et al. 2004). TPI1 is an important enzyme of glycolysis, which catalyzes the isomerization of dihydroxyacetone phosphate (DHAP) to glyceraldehyde-3-phosphate (G3P) (Orosz et al. 2009). A study by Bonnet et al., (2004) has identified strong binding and interaction of triosephosphate isomerase with a chemical similar to MPTP+ (beta-carbolines, a natural endotoxin) (Bonnet et al. 2004). Unfortunately, the role of Triosephosphate isomerase has not been further studied extensively in PD development; however, studies on triosephosphate isomerase mutations have demonstrated some correlation between decreased levels of triosephosphate isomerase and protein misfolding (Bonnet et al. 2004). Interestingly, scatter plots and ROC curve analysis carried out for levels of TPI1 in the present study have shown high sensitivity and specificity (Figs. 7a, 8a).
Our OpenArray-based miRNA profiling identified maximum upregulation in the expression of hsa-miR-186-5p in most PD patients. Earlier studies have reported the prominent role of miR-186-5p in the promotion of apoptosis in various cell types (Wang et al. 2018; Xu et al. 2020). Earlier study by Niu et al. (2021) reported the decreased level of circRIMSs leads to the miR-186 overexpression that causes neuronal apoptosis via targeting BDNF (Niu et al. 2021). An increased rate of apoptosis is also the major mode of cell death in dopaminergic cells (Singh and Dikshit 2007). In the current study, we have observed the increased expression of the pro-apoptotic gene BAX and decreased expression of anti-apoptotic gene BCL2 in both PD patients (Supplementary Fig. 4a–c) & cellular model of degenerating dopaminergic cells. i.e., rotenone-exposed SH-SY5Y cells (Fig. 11d). Studies of Vila et al. (2001) has also reported dopaminergic neurodegeneration due to the upregulation of Bax and downregulation of BCL2 in MPTP-induced mice PD model (Vila et al. 2001). We are the first to identify the upregulation of miR-186-5p in PD patients; however, upregulation of miR-186-5p was identified in AD (Satoh et al. 2015; Lau et al. 2013). Satoh et al. (2015) performed RNA-seq-based sequencing and identified 27 differentially expressed miRNAs, in which miR-186-5p was one of the significantly upregulated miRNAs in the blood sample of AD patients (Satoh et al. 2015). Another study by Pierre Lau et al. (2013) has also reported the upregulation of miR-186-5p in the prefrontal cortex of AD patients (Lau et al. 2013). Moreover, bioinformatics analysis also showed the enrichment of maximum processes including cell death, post-translational protein modification, synaptic transmission, and various signaling pathways including TGF-beta signaling, thyroid hormone signaling, estrogen signaling, and axon guidance suggesting the role of miR-186-5p in PD pathology (Fig. 5 and Supplementary Fig. 6). Furthermore, the downstream target analysis of miR-186-5p by TargetScan revealed that many downregulated proteins have a binding site for the miR-186-5p. YWHAZ and YWHAB genes are identified as the most substantially and significantly regulated genes, which also have the site for binding of miR-186-5p and the same has been validated in SH-SY5Y cells through transfection of mimics and inhibitors of miR-186 (Fig. 12c & d). Individually, the expression of miR-186-5p showed high sensitivity and specificity to detect PD with an AUC value of 0.6957.
In addition to miR-186-5p, miRNA profiling studies have also shown statistically significant and substantial downregulation in the expression of miR-150-5p in PD patients, which is known for its anti-inflammatory and anti-apoptotic role (Ji et al. 2018; Deng et al. 2020; Zhu et al. 2020). Haiting Li et al. (2020) have identified significant downregulation in the expression of miR-150-5p in the serum samples of Parkinson’s patients and demonstrated a negative correlation between the expression of miR-150-5p and pro-inflammatory cytokines in PD patients (Li et al. 2020). Similar to PD, studies by Lugli et al. (2015) have reported a significant downregulation in the expression of miR-150-5p in AD patients (Lugli et al. 2015). Moreover, Liu and Wang (2019) reported that miR-150-5p negatively regulates the tumor suppressor gene TP53 to modulate cell proliferation and apoptosis, and a decreased expression of miR-150-5p or overexpression of TP53 leads to an increase in apoptosis (Liu and Di Wang 2019). In the present study, we have also observed the upregulation of TP53, which may regulate the expression level of apoptotic genes in PD patients as well as in rotenone-exposed SH-SY5Y cells (Fig. 11d and Supplementary Fig. 4a–c). The gene ontology study has identified the enrichment of miR-150-5p in the mitotic cell cycle, cellular protein modification, gene expression, fibroblast growth receptor, and neurotrophin TRK receptor signaling pathways. Out of the many downstream targets of miR-150-5p identified by TargetScan, one is the SERPIN1 gene, which is upregulated in the blood of PD patients. CDH1 is another gene that shows the interaction with miR-150 and also reported earlier by Wen et al. (2022) that supports our finding (Wen et al. 2022). Interestingly, miR-150-5p showed high sensitivity and specificity for PD detection with an AUC value of 0.8059 (Fig. 4b). Increased expression of miR-29b-3p, miR-146b-5p, and miR-214-3p has also been observed in the blood samples of PD. miR-29b-3p is a member of the miR-29 family, which includes miR-29a, miR-29b (miR-29b-1, miR-29b-2), and miR-29c and is well recognized for its involvement in aging and neurodegeneration (Yadav et al. 2022; Swahari et al. 2021). Tatura et al. (2016) reported the upregulation of miR-29b in the cingulate gyrus of the postmortem brain of PD patients through global profiling (Tatura et al. 2016). miR-29 family (miR-29a/b/c) has shown interaction with many downstream proteins (Table 4 & Fig. 10c) in which SPARC had reported in many studies similar to present study (Zhang et al. 2017; Qiu et al. 2015). Furthermore, miR-146b-5p was reported to be upregulated in neuroinflammation, a phenomenon that plays important role in PD pathogenesis (Parisi et al. 2013; Hirsch et al. 2013). Recent studies from our laboratory using different animal and cellular models of PD have also reported upregulation in the expression of miR-146a-5p and miR-214-3p and miR-29a (Jauhari et al. 2020; Yadav et al. 2022).
Global protein profiling has shown significant downregulation in the levels of two proteins of the 14-3-3 family of proteins, i.e., YWHAZ and YWHAB in blood samples of PD patients. YWHAZ is known for its role in neurogenesis, neuronal connectivity, and neurotransmission (Antón-Galindo et al. 2021) and their colocalization has also been observed in the Lewy bodies (LBs), which are the pathological hallmark of PD development (Berg et al. 2003). Another study by Jin Xu et al. (2002) has shown that decreased levels of 14-3-3 protein make cells more vulnerable to apoptosis in the Substantia nigra of PD patients (Xu et al. 2002) because 14-3-3 proteins protect the cells from apoptosis by binding to pro-apoptotic proteins like BAD and transcription factor like Forkhead (Yuan and Yankner 2000). Recently, a study using in vivo model of PD and a global proteomic approach has also shown the involvement of YWHAZ proteins (14–3-3ζ) in the apoptosis process, mitochondrial dysfunction, amyloid clearance, serine synthesis, and neuroprotection (Jiang et al. 2019). Meta-analysis of Networks carried out by Jack Kelly (2019) has also shown the downregulation of YWHAZ proteins in PD as we found from unbiased protein profiling studies of PD patients (Kelly et al. 2019). Our in silico enrichment analysis has also shown the enrichment of YWHAZ protein in the PD-associated pathways and post-transcriptional gene regulation (Figs. 9a & 10b). Furthermore, 14-3-3 ζ protein also acts as an activator of TH; an important enzyme for dopamine synthesis and its reduction is associated with a decrease in dopamine synthesis (Wang et al. 2009). Moreover, in silico analysis of the target genes using the TargetScan web portal has identified multiple upregulated miRNAs, which have the binding site on 3’-UTR of the YWHAZ gene, indicating its possible regulation by miRNAs. The studies of Ji et al. (2019) reported the interaction of miR-206/YWHAZ as we found in our miRNA & protein-integrated approach (Table 4 & Fig. 10c) (Ji et al. 2019). All the above finding shows the importance of the YWHAZ protein in the PD pathogenesis, which could be a potential PD biomarker.
Our LC–MS/MS-based global protein profiling has also identified SERPINA1 (alpha-1 antitrypsin) as another highly upregulated protein in the plasma samples of PD patients. SERPINA1 is an acute-phase protein, a member of the serpin superfamily and under normal conditions, its level is very low in most of the human tissues including all major brain regions, except the liver and blood (Gettins 2002; Ebbert et al. 2017). The upregulation of SERPINA1 has been detected in the brains of patients with AD (Gollin et al. 1992) and FTD (frontotemporal lobar degeneration), indicating that high expression of SERPINA1 may be detrimental to normal neuronal function (Higgins et al. 2021). Furthermore, many previous studies reported higher levels of SERPINA1 in PD, AD, PDD (Parkinson’s disease with dementia), and dementia with Lewy bodies compared to control (Halbgebauer et al. 2016; Puchades et al. 2003; Nielsen et al. 2007; Jesse et al. 2012), which correlates with our findings. Moreover, SERPINA1 is an anti-inflammatory protein, which prevents microglial activation and reduces neuroinflammation (Gold et al. 2014; Zhou et al. 2018) and is expected to be at higher levels during neuroinflammation. In the present study, string analysis has shown the involvement of SERPIN1 in inflammatory responses (Fig. 10b). Moreover, TargetScan analysis of SERPIN1 has revealed the binding site on its 3’-UTR regions for miR-150-5p. So, SERPIN1 might be upregulated due to the low expression of hsa-miR-150-5p in PD pathogenesis (Table 4).
In comparison with our previous study carried out in the rotenone-induced rat PD model, our present study has identified a similar trend of expression in microRNAs viz. miR-29c, miR-143, and miR-34a as reported in our previous studies (Yadav et al. 2022). These miRNAs were found to be upregulated in both rat PD models and human PD patients. Some common miRNAs family members such as 29 family (29a, 29b), 34 family (34b, 34c), and 146 family (146a, 146b) were also found upregulated in the human PD samples as we have found in the PD model. These miRNAs play a critical role in neurodegeneration and aging (Yadav et al. 2022; Swahari et al. 2021; Tatura et al. 2016; Jauhari et al. 2020; Parisi et al. 2013; Hirsch et al. 2013; Tufekci et al. 2021; Wang et al. 2022; Sun et al. 2020). Additionally, the proteomics study in the human PD patient has also identified some common deregulation of protein families such as cytoskeletal protein, apolipoproteins, complement proteins, and serpin family proteins as we found in the rat PD model study (Yadav et al. 2022). However, the maximum deregulated miRNAs and proteins reported in our rotenone-induced rat PD model study are not replicated in the human PD patient, probably due to rotenone-specific toxicity. Measuring the amount of identified miRNAs or proteins in brain-specific exosomes isolated from the blood of rat-based PD models and PD patients can probably provide better comparison and similarity.
In the pathway enrichment analysis, we have identified the glycolytic pathway as the most significantly enriched pathway from the downregulated proteins including TPI, ENO1, and PGAM4 which were important for ATP biosynthesis (Figs. 9a, 10b). Altered energy metabolism and ATP reduction may be due to impaired glycolysis, which is a common feature of PD pathogenesis (Cai et al. 2019). In support of this, we have observed dramatic downregulation of TPI1 in PD patients as discussed above. In PD, α-synuclein aggregation causes the impairment of spectrin and the actin cytoskeleton protein which leads to mitochondrial dysfunction and cell death (Ordonez et al. 2018). In the current study, downregulation was observed in the expression of actin cytoskeleton regulating proteins (ACTN1, ACTG1, PFN1, CFL1, FLNA, and TTN) which were also observed downregulated (Fig. 10b). Moreover, the individual abundance and ROC curve analysis of ACTG1, ACTN1, CFL1, and PFN1 showed high sensitivity and specificity with AUC values of 0.8567, 0.7700, 0.7400, and 0.7639, respectively (Figs. 7a, 8a). Besides the above pathways, identified deregulated proteins are also involved in ubiquitin-dependent protein catabolism, cellular detoxification, autophagy, localization, and transport (Fig. 10b). Impairment of the ubiquitin–proteasome clearance pathway is one of the major reasons for protein aggregation in PD pathogenesis (Betarbet et al. 2006). Recently, Yuan, Q. et al. (2020) have identified decreased level of PSMA4 & PSME4 in PD patients compared to controls (Yuan et al. 2020). In the present study, we have also observed decreased levels of PSMA4 in PD patients. SELENBP1, a protein that controls mitochondrial function and apoptosis and decreases in PD patients, was also downregulated in our studies with an AUC value of 0.7656 (Cabeza-Arvelaiz and Schiestl 2012). The aggregation of misfolded proteins in the lumen of the endoplasmic reticulum (ER) is a common characteristic of most neurodegenerative diseases including PD (Lindholm et al. 2006). HYOU1 (Hypoxia-upregulated 1) is a chaperone protein found in ER and overexpressed during ER stress (Rao et al. 2021). Multiple studies have reported the induction of ER stress markers in the cellular models and postmortem brains of PD and AD (Lindholm et al. 2006). In line with the previous studies, our study has also identified significantly increased levels of HYOU1 protein in PD patients.
In summary, our studies using unbiased profiling of proteins and miRNA OpenArray analysis in 48 PD and equally control samples suggest the use of selected miRNAs (miR-186-5p, miR-29b, miR-150-5p) and proteins (YWHAZ, PSMA4, HYOU1, SERPINA1) for the diagnosis and progression of the PD, which was correlated with PD through pathway enrichment and cell culture studies. Studies in our lab are in progress to monitor the regulation of these identified miRNAs and proteins in brain-derived exosomes of PD patients, which are supposed to show better ROC values and correlation with PD pathogenesis.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
Dr Sanjeev Kumar Yadav is grateful to CSIR, New Delhi, for providing JRF/SRF fellowship. We also thank Dr VK Khanna for the valuable suggestions. The CSIR-IITR communication reference number is IITR/SEC/MS/2023/01.
Author Contributions
SY, DP, RKG, and SP designed the study. SY guided the students in the protocols and new methods development. SKY, AJ, and NS performed the experiments. SKY collated the data, performed the data analysis, and write the first draft of the manuscript. AP and SS provided the technical assistance for the data analysis. All the authors reviewed and gave critical suggestions and approved the manuscript.
Funding
The funding for the present study has been provided by the CSIR Network project (miND) and Science and Engineering Research Board (SERB), New Delhi project (GAP-359; Grant Sanction no. EMR/2016/002965).
Data Availability
All the data generated or analyzed during the present study are included in this article and its supplementary information files.
Code Availability
NCBI-GEO accession number, GSE222480, and CCMS-MassIVE accession number, MSV000091013.
Declarations
Conflict of interest
The authors declare no conflict of interest with this article.
Ethical Approval
The study was approved by the Institutional Ethics committee of King George’s Medical University (KGMU) U.P., India (Ref. code: 89th ECM II A/P7) and the Institutional Human Ethics Committee of Indian Institute of Toxicology Research (IITR) U.P., India (Ref. No: CSIR-IITR/IHEC/JULY/2021/2).
Consent to Participate
All the patients or their family members are provided written informed consent for participation in this study.
Consent for Publication
Not applicable.
Footnotes
The raw data related to this article is available on NCBI-GEO (Accession No GSE222480) & CCMS-MassIVE (Accession No MSV000091013) online data repositories
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Abhishek Jauhari and Nishant Singh have contributed equally to this work.
Contributor Information
Devendra Parmar, Email: dparmar@iitr.res.in.
Sanjay Yadav, Email: syaiims@aiimsrbl.edu.in.
References
- Agarwal V, Bell GW, Nam JW, Bartel DP (2015) Predicting effective microRNA target sites in mammalian mRNAs. eLife. 10.7554/eLife.05005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn SM, Simpson RJ (2007) Body fluid proteomics: prospects for biomarker discovery. Proteomics Clin Appl 1(9):1004–1015. 10.1002/prca.200700217 [DOI] [PubMed] [Google Scholar]
- Antón-Galindo E, Dalla Vecchia E, Orlandi JG, Castro G, Gualda EJ, Young AM, Aguado F, Loza-Alvarez P, Cormand B, Norton WH (2021) Deficiency of the ywhaz gene, involved in neurodevelopmental disorders, alters brain activity and behaviour in zebrafish. BioRxiv. 10.1101/2021.06.30.450513 [DOI] [PubMed] [Google Scholar]
- Bartel DP (2004) MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 116(2):281–297. 10.1016/s0092-8674(04)00045-5 [DOI] [PubMed] [Google Scholar]
- Berg D, Riess O, Bornemann A (2003) Specification of 14–3-3 proteins in Lewy bodies. Ann Neurol 54(1):135. 10.1002/ana.10621 [DOI] [PubMed] [Google Scholar]
- Betarbet R, Canet-Aviles RM, Sherer TB, Mastroberardino PG, McLendon C, Kim JH, Lund S, Na HM, Taylor G, Bence NF, Kopito R, Seo BB, Yagi T, Yagi A, Klinefelter G, Cookson MR, Greenamyre JT (2006) Intersecting pathways to neurodegeneration in Parkinson’s disease: effects of the pesticide rotenone on DJ-1, alpha-synuclein, and the ubiquitin-proteasome system. Neurobiol Dis 22(2):404–420. 10.1016/j.nbd.2005.12.003 [DOI] [PubMed] [Google Scholar]
- Bonnet R, Pavlovic S, Lehmann J, Rommelspacher H (2004) The strong inhibition of triosephosphate isomerase by the natural beta-carbolines may explain their neurotoxic actions. Neuroscience 127(2):443–453. 10.1016/j.neuroscience.2004.05.002 [DOI] [PubMed] [Google Scholar]
- Botta-Orfila T, Morató X, Compta Y, Lozano JJ, Falgàs N, Valldeoriola F, Pont-Sunyer C, Vilas D, Mengual L, Fernández M, Molinuevo JL, Antonell A, Martí MJ, Fernández-Santiago R, Ezquerra M (2014) Identification of blood serum micro-RNAs associated with idiopathic and LRRK2 Parkinson’s disease. J Neurosci Res 92(8):1071–1077. 10.1002/jnr.23377 [DOI] [PubMed] [Google Scholar]
- Cabeza-Arvelaiz Y, Schiestl RH (2012) Transcriptome analysis of a rotenone model of parkinsonism reveals complex I-tied and -untied toxicity mechanisms common to neurodegenerative diseases. PLoS one 7(9):e44700. 10.1371/journal.pone.0044700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai R, Zhang Y, Simmering JE, Schultz JL, Li Y, Fernandez-Carasa I, Consiglio A, Raya A, Polgreen PM, Narayanan NS, Yuan Y, Chen Z, Su W, Han Y, Zhao C, Gao L, Ji X, Welsh MJ, Liu L (2019) Enhancing glycolysis attenuates Parkinson’s disease progression in models and clinical databases. J Clin Investig 129(10):4539–4549. 10.1172/jci129987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao XY, Lu JM, Zhao ZQ, Li MC, Lu T, An XS, Xue LJ (2017) MicroRNA biomarkers of Parkinson’s disease in serum exosome-like microvesicles. Neurosci Lett 644:94–99. 10.1016/j.neulet.2017.02.045 [DOI] [PubMed] [Google Scholar]
- Chahine LM, Stern MB, Chen-Plotkin A (2014) Blood-based biomarkers for Parkinson’s disease. Parkinsonism Relat Disord 20(1):S99-103. 10.1016/s1353-8020(13)70025-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Huang W, Sherman BT, Lempicki RA (2009) Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc 4(1):44–57. 10.1038/nprot.2008.211 [DOI] [PubMed] [Google Scholar]
- Deng X, Lin Z, Zuo C, Fu Y (2020) Upregulation of miR-150-5p alleviates LPS-induced inflammatory response and apoptosis of RAW2647 macrophages by targeting Notch1. Open Life Sci 15(1):544–552. 10.1515/biol-2020-0058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson DW (2018) Neuropathology of Parkinson disease. Parkinsonism Relat Disord 46(Suppl 1):S30–S33. 10.1016/j.parkreldis.2017.07.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebbert MTW, Ross CA, Pregent LJ, Lank RJ, Zhang C, Katzman RB, Jansen-West K, Song Y, da Rocha EL, Palmucci C, Desaro P, Robertson AE, Caputo AM, Dickson DW, Boylan KB, Rademakers R, Ordog T, Li H, Belzil VV (2017) Conserved DNA methylation combined with differential frontal cortex and cerebellar expression distinguishes C9orf72-associated and sporadic ALS, and implicates SERPINA1 in disease. Acta Neuropathol 134(5):715–728. 10.1007/s00401-017-1760-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gettins PG (2002) Serpin structure, mechanism, and function. Chem Rev 102(12):4751–4804. 10.1021/cr010170+ [DOI] [PubMed] [Google Scholar]
- Gold M, Dolga AM, Koepke J, Mengel D, Culmsee C, Dodel R, Koczulla AR, Bach JP (2014) α1-antitrypsin modulates microglial-mediated neuroinflammation and protects microglial cells from amyloid-β-induced toxicity. J Neuroinflammation 11:165. 10.1186/s12974-014-0165-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gollin PA, Kalaria RN, Eikelenboom P, Rozemuller A, Perry G (1992) Alpha 1-antitrypsin and alpha 1-antichymotrypsin are in the lesions of Alzheimer’s disease. NeuroReport 3(2):201–203. 10.1097/00001756-199202000-00020 [DOI] [PubMed] [Google Scholar]
- Halbgebauer S, Nagl M, Klafki H, Haußmann U, Steinacker P, Oeckl P, Kassubek J, Pinkhardt E, Ludolph AC, Soininen H, Herukka SK, Wiltfang J, Otto M (2016) Modified serpinA1 as risk marker for Parkinson’s disease dementia: analysis of baseline data. Sci Rep 6:26145. 10.1038/srep26145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins NR, Greenslade JE, Wu JJ, Miranda E, Galliciotti G, Monteiro MJ (2021) Serpin neuropathology in the P497S UBQLN2 mouse model of ALS/FTD. Brain Pathol (Zurich, Switzerland) 31(5):e12948. 10.1111/bpa.12948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch EC, Jenner P, Przedborski S (2013) Pathogenesis of Parkinson’s disease. Mov Disord 28(1):24–30. 10.1002/mds.25032 [DOI] [PubMed] [Google Scholar]
- Hurley MJ, Brandon B, Gentleman SM, Dexter DT (2013) Parkinson’s disease is associated with altered expression of CaV1 channels and calcium-binding proteins. Brain 136(Pt 7):2077–2097. 10.1093/brain/awt134 [DOI] [PubMed] [Google Scholar]
- Jankovic J (2008) Parkinson’s disease: clinical features and diagnosis. J Neurol Neurosurg Psychiatry 79(4):368–376. 10.1136/jnnp.2007.131045 [DOI] [PubMed] [Google Scholar]
- Jankovic J, Tan EK (2020) Parkinson’s disease: etiopathogenesis and treatment. J Neurol Neurosurg Psychiatry 91(8):795–808. 10.1136/jnnp-2019-322338 [DOI] [PubMed] [Google Scholar]
- Jauhari A, Singh T, Pandey A, Singh P, Singh N, Srivastava AK, Pant AB, Parmar D, Yadav S (2017) Differentiation induces dramatic changes in miRNA profile, where loss of dicer diverts differentiating SH-SY5Y cells toward senescence. Mol Neurobiol 54(7):4986–4995. 10.1007/s12035-016-0042-9 [DOI] [PubMed] [Google Scholar]
- Jauhari A, Singh T, Mishra S, Shankar J, Yadav S (2020) Coordinated action of miR-146a and parkin gene regulate rotenone-induced neurodegeneration. Toxicol Sci 176(2):433–445. 10.1093/toxsci/kfaa066 [DOI] [PubMed] [Google Scholar]
- Jesse S, Lehnert S, Jahn O, Parnetti L, Soininen H, Herukka SK, Steinacker P, Tawfik S, Tumani H, von Arnim CA, Neumann M, Kretzschmar HA, Kulaksiz H, Lenter M, Wiltfang J, Ferger B, Hengerer B, Otto M (2012) Differential sialylation of serpin A1 in the early diagnosis of Parkinson’s disease dementia. PLoS One 7(11):e48783. 10.1371/journal.pone.0048783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji LJ, Shi J, Lu JM, Huang QM (2018) MiR-150 alleviates neuropathic pain via inhibiting toll-like receptor 5. J Cell Biochem 119(1):1017–1026. 10.1002/jcb.26269 [DOI] [PubMed] [Google Scholar]
- Ji N, Wang Y, Bao G, Yan J, Ji S (2019) LncRNA SNHG14 promotes the progression of cervical cancer by regulating miR-206/YWHAZ. Pathol Res Pract 215(4):668–675. 10.1016/j.prp.2018.12.026 [DOI] [PubMed] [Google Scholar]
- Jiang H, Yu Y, Liu S, Zhu M, Dong X, Wu J, Zhang Z, Zhang M, Zhang Y (2019) Proteomic study of a Parkinson’s disease model of undifferentiated SH-SY5Y cells induced by a proteasome inhibitor. Int J Med Sci 16(1):84–92. 10.7150/ijms.28595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joutsa J, Gardberg M, Röyttä M, Kaasinen V (2014) Diagnostic accuracy of Parkinsonism syndromes by general neurologists. Parkinsonism Relat Disord 20(8):840–844. 10.1016/j.parkreldis.2014.04.019 [DOI] [PubMed] [Google Scholar]
- Kelly J, Moyeed R, Carroll C, Albani D, Li X (2019) Gene expression meta-analysis of Parkinson’s disease and its relationship with Alzheimer’s disease. Mol Brain 12(1):16. 10.1186/s13041-019-0436-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SI, Voshol H, van Oostrum J, Hastings TG, Cascio M, Glucksman MJ (2004) Neuroproteomics: expression profiling of the brain’s proteomes in health and disease. Neurochem Res 29(6):1317–1331. 10.1023/b:nere.0000023618.35579.7c [DOI] [PubMed] [Google Scholar]
- Kulisevsky J, Oliveira L, Fox SH (2018) Update in therapeutic strategies for Parkinson’s disease. Curr Opin Neurol 31(4):439–447. 10.1097/wco.0000000000000579 [DOI] [PubMed] [Google Scholar]
- Lau P, Bossers K, Janky R, Salta E, Frigerio CS, Barbash S, Rothman R, Sierksma AS, Thathiah A, Greenberg D, Papadopoulou AS, Achsel T, Ayoubi T, Soreq H, Verhaagen J, Swaab DF, Aerts S, De Strooper B (2013) Alteration of the microRNA network during the progression of Alzheimer’s disease. EMBO Mol Med 5(10):1613–1634. 10.1002/emmm.201201974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Yu L, Li M, Chen X, Tian Q, Jiang Y, Li N (2020) MicroRNA-150 serves as a diagnostic biomarker and is involved in the inflammatory pathogenesis of Parkinson’s disease. Mol Genet Genom Med 8(4):e1189. 10.1002/mgg3.1189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindholm D, Wootz H, Korhonen L (2006) ER stress and neurodegenerative diseases. Cell Death Differ 13(3):385–392. 10.1038/sj.cdd.4401778 [DOI] [PubMed] [Google Scholar]
- Liu F, Di Wang X (2019) miR-150-5p represses TP53 tumor suppressor gene to promote proliferation of colon adenocarcinoma. Sci Rep 9(1):6740. 10.1038/s41598-019-43231-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugli G, Cohen AM, Bennett DA, Shah RC, Fields CJ, Hernandez AG, Smalheiser NR (2015) Plasma exosomal miRNAs in Persons with and without Alzheimer disease: altered expression and prospects for biomarkers. PLoS One 10(10):e0139233. 10.1371/journal.pone.0139233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meder D, Herz DM, Rowe JB, Lehéricy S, Siebner HR (2019) The role of dopamine in the brain—lessons learned from Parkinson’s disease. Neuroimage 190:79–93. 10.1016/j.neuroimage.2018.11.021 [DOI] [PubMed] [Google Scholar]
- Mi H, Thomas P (2009) PANTHER pathway: an ontology-based pathway database coupled with data analysis tools. Methods Mol Biol (clifton, NJ) 563:123–140. 10.1007/978-1-60761-175-2_7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller DB, O’Callaghan JP (2015) Biomarkers of Parkinson’s disease: present and future. Metabolism 64(3 Suppl 1):S40-46. 10.1016/j.metabol.2014.10.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen HM, Minthon L, Londos E, Blennow K, Miranda E, Perez J, Crowther DC, Lomas DA, Janciauskiene SM (2007) Plasma and CSF serpins in Alzheimer disease and dementia with Lewy bodies. Neurology 69(16):1569–1579. 10.1212/01.wnl.0000271077.82508.a0 [DOI] [PubMed] [Google Scholar]
- Niu Y, Wan C, Zhang J, Zhang S, Zhao Z, Zhu L, Wang X, Ren X, Wang J, Lei P (2021) Aerobic exercise improves VCI through circRIMS2/miR-186/BDNF-mediated neuronal apoptosis. Mol Med (Cambridge, Mass) 27(1):4. 10.1186/s10020-020-00258-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ordonez DG, Lee MK, Feany MB (2018) α-synuclein Induces mitochondrial dysfunction through spectrin and the actin cytoskeleton. Neuron 97(1):108-124.e106. 10.1016/j.neuron.2017.11.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orosz F, Oláh J, Ovádi J (2009) Triosephosphate isomerase deficiency: new insights into an enigmatic disease. Biochem Biophys Acta 1792(12):1168–1174. 10.1016/j.bbadis.2009.09.012 [DOI] [PubMed] [Google Scholar]
- Ozdilek B, Demircan B (2020) Serum microRNA expression levels in Turkish patients with Parkinson’s disease. Int J Neurosci. 10.1080/00207454.2020.1784165 [DOI] [PubMed] [Google Scholar]
- Pandey A, Jauhari A, Singh T, Singh P, Singh N, Srivastava AK, Khan F, Pant AB, Parmar D, Yadav S (2015) Transactivation of P53 by cypermethrin induced miR-200 and apoptosis in neuronal cells. Toxicol Res 4(6):1578–1586 [Google Scholar]
- Pandey A, Singh P, Jauhari A, Singh T, Khan F, Pant AB, Parmar D, Yadav S (2015b) Critical role of the miR-200 family in regulating differentiation and proliferation of neurons. J Neurochem 133(5):640–652. 10.1111/jnc.13089 [DOI] [PubMed] [Google Scholar]
- Pandey A, Sarkar S, Yadav SK, Yadav SS, Srikrishna S, Siddiqui MH, Parmar D, Yadav S (2022) Studies on regulation of global protein profile and cellular bioenergetics of differentiating SH-SY5Y cells. Mol Neurobiol. 10.1007/s12035-021-02667-5 [DOI] [PubMed] [Google Scholar]
- Parisi C, Arisi I, D’Ambrosi N, Storti AE, Brandi R, D’Onofrio M, Volonté C (2013) Dysregulated microRNAs in amyotrophic lateral sclerosis microglia modulate genes linked to neuroinflammation. Cell Death Dis 4(12):e959. 10.1038/cddis.2013.491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parnetti L, Gaetani L, Eusebi P, Paciotti S, Hansson O, El-Agnaf O, Mollenhauer B, Blennow K, Calabresi P (2019) CSF and blood biomarkers for Parkinson’s disease. Lancet Neurol 18(6):573–586. 10.1016/s1474-4422(19)30024-9 [DOI] [PubMed] [Google Scholar]
- Peters M, Fitzpatrick R, Doll H, Playford D, Jenkinson C (2011) Does self-reported well-being of patients with Parkinson’s disease influence caregiver strain and quality of life? Parkinsonism Relat Disord 17(5):348–352. 10.1016/j.parkreldis.2011.02.009 [DOI] [PubMed] [Google Scholar]
- Pienaar IS, Daniels WM, Götz J (2008) Neuroproteomics as a promising tool in Parkinson’s disease research. J Neural Transmission (Vienna, Austria: 1996) 115(10):1413–1430. 10.1007/s00702-008-0070-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puchades M, Hansson SF, Nilsson CL, Andreasen N, Blennow K, Davidsson P (2003) Proteomic studies of potential cerebrospinal fluid protein markers for Alzheimer’s disease. Brain Res Mol Brain Res 118(1–2):140–146. 10.1016/j.molbrainres.2003.08.005 [DOI] [PubMed] [Google Scholar]
- Qiu F, Sun R, Deng N, Guo T, Cao Y, Yu Y, Wang X, Zou B, Zhang S, Jing T, Ling T, Xie J, Zhang Q (2015) miR-29a/b enhances cell migration and invasion in nasopharyngeal carcinoma progression by regulating SPARC and COL3A1 gene expression. PloS One 10(3):e0120969. 10.1371/journal.pone.0120969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao S, Oyang L, Liang J, Yi P, Han Y, Luo X, Xia L, Lin J, Tan S, Hu J, Wang H, Tang L, Pan Q, Tang Y, Zhou Y, Liao Q (2021) Biological function of HYOU1 in tumors and other diseases. Onco Targets Ther 14:1727–1735. 10.2147/ott.S297332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh J, Kino Y, Niida S (2015) MicroRNA-seq data analysis pipeline to identify blood biomarkers for Alzheimer’s disease from public data. Biomarker Insights 10:21–31. 10.4137/bmi.S25132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serafin A, Foco L, Blankenburg H, Picard A, Zanigni S, Zanon A, Pramstaller PP, Hicks AA, Schwienbacher C (2014) Identification of a set of endogenous reference genes for miRNA expression studies in Parkinson’s disease blood samples. BMC Res Notes 7:715. 10.1186/1756-0500-7-715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh S, Dikshit M (2007) Apoptotic neuronal death in Parkinson’s disease: involvement of nitric oxide. Brain Res Rev 54(2):233–250. 10.1016/j.brainresrev.2007.02.001 [DOI] [PubMed] [Google Scholar]
- Srivastava AK, Yadav SS, Mishra S, Yadav SK, Parmar D, Yadav S (2020) A combined microRNA and proteome profiling to investigate the effect of ZnO nanoparticles on neuronal cells. Nanotoxicology 14(6):757–773. 10.1080/17435390.2020.1759726 [DOI] [PubMed] [Google Scholar]
- Sun C, Jia N, Li R, Zhang Z, Zhong Y, Han K (2020) miR-143-3p inhibition promotes neuronal survival in an Alzheimer’s disease cell model by targeting neuregulin-1. Folia Neuropathol 58(1):10–21. 10.5114/fn.2020.94002 [DOI] [PubMed] [Google Scholar]
- Sveinbjornsdottir S (2016) The clinical symptoms of Parkinson’s disease. J Neurochem 139(Suppl 1):318–324. 10.1111/jnc.13691 [DOI] [PubMed] [Google Scholar]
- Swahari V, Nakamura A, Hollville E, Stroud H, Simon JM, Ptacek TS, Beck MV, Flowers C, Guo J, Plestant C, Liang J, Kurtz CL, Kanke M, Hammond SM, He YW, Anton ES, Sethupathy P, Moy SS, Greenberg ME, Deshmukh M (2021) MicroRNA-29 is an essential regulator of brain maturation through regulation of CH methylation. Cell Rep 35(1):108946. 10.1016/j.celrep.2021.108946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szklarczyk D, Gable AL, Lyon D, Junge A, Wyder S, Huerta-Cepas J, Simonovic M, Doncheva NT, Morris JH, Bork P, Jensen LJ, Mering CV (2019) STRING v11: protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res 47(D1):D607-d613. 10.1093/nar/gky1131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatura R, Kraus T, Giese A, Arzberger T, Buchholz M, Höglinger G, Müller U (2016) Parkinson’s disease: SNCA-, PARK2-, and LRRK2- targeting microRNAs elevated in cingulate gyrus. Parkinsonism Relat Disord 33:115–121. 10.1016/j.parkreldis.2016.09.028 [DOI] [PubMed] [Google Scholar]
- Tufekci KU, Alural B, Tarakcioglu E, San T, Genc S (2021) Lithium inhibits oxidative stress-induced neuronal senescence through miR-34a. Mol Biol Rep 48(5):4171–4180. 10.1007/s11033-021-06430-w [DOI] [PubMed] [Google Scholar]
- Tysnes OB, Storstein A (2017) Epidemiology of Parkinson’s disease. J Neural Transmission (Vienna, Austria: 1996) 124(8):901–905. 10.1007/s00702-017-1686-y [DOI] [PubMed] [Google Scholar]
- Vila M, Jackson-Lewis V, Vukosavic S, Djaldetti R, Liberatore G, Offen D, Korsmeyer SJ, Przedborski S (2001) Bax ablation prevents dopaminergic neurodegeneration in the 1-methyl- 4-phenyl-1,2,3,6-tetrahydropyridine mouse model of Parkinson’s disease. Proc Natl Acad Sci USA 98(5):2837–2842. 10.1073/pnas.051633998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlachos IS, Zagganas K, Paraskevopoulou MD, Georgakilas G, Karagkouni D, Vergoulis T, Dalamagas T, Hatzigeorgiou AG (2015) DIANA-miRPath v3.0: deciphering microRNA function with experimental support. Nucleic Acids Res 43(W1):W460-466. 10.1093/nar/gkv403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Lou H, Pedersen CJ, Smith AD, Perez RG (2009) 14–3-3zeta contributes to tyrosine hydroxylase activity in MN9D cells: localization of dopamine regulatory proteins to mitochondria. J Biol Chem 284(21):14011–14019. 10.1074/jbc.M901310200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Yang Z, Le W (2017) Tiny but mighty: promising roles of micrornas in the diagnosis and treatment of Parkinson’s disease. Neurosci Bull 33(5):543–551. 10.1007/s12264-017-0160-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R, Bao H, Zhang S, Li R, Chen L, Zhu Y (2018) miR-186-5p promotes apoptosis by targeting IGF-1 in SH-SY5Y OGD/R model. Int J Biol Sci 14(13):1791–1799. 10.7150/ijbs.25352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Jia Q, Guo X, Li K, Chen W, Shen Q, Xu C, Fu Y (2022) microRNA-34 family: from mechanism to potential applications. Int J Biochem Cell Biol 144:106168. 10.1016/j.biocel.2022.106168 [DOI] [PubMed] [Google Scholar]
- Warner TT, Schapira AH (2003) Genetic and environmental factors in the cause of Parkinson’s disease. Ann Neurol 53(3):S16-23. 10.1002/ana.10487 [DOI] [PubMed] [Google Scholar]
- Wen JY, Chen G, Li JD, Luo JY, He J, Wang RS, Qin LT (2022) Downregulated miR-150-5p in the tissue of nasopharyngeal carcinoma. Genet Res 2022:2485055. 10.1155/2022/2485055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong N, Xiong J, Jia M, Liu L, Zhang X, Chen Z, Huang J, Zhang Z, Hou L, Luo Z, Ghoorah D, Lin Z, Wang T (2013) The role of autophagy in Parkinson’s disease: rotenone-based modeling. Behav Brain Funct 9:13. 10.1186/1744-9081-9-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Kao SY, Lee FJ, Song W, Jin LW, Yankner BA (2002) Dopamine-dependent neurotoxicity of alpha-synuclein: a mechanism for selective neurodegeneration in Parkinson disease. Nat Med 8(6):600–606. 10.1038/nm0602-600 [DOI] [PubMed] [Google Scholar]
- Xu YF, Liu J, Wang J, Guo YC, Shen YZ (2020) MiR-186 promotes the apoptosis of glioma U87 cells by down-regulating the expression of Smad6. Eur Rev Med Pharmacol Sci 24(14):7681–7689. 10.26355/eurrev_202007_22269 [DOI] [PubMed] [Google Scholar]
- Yadav S, Pandey A, Shukla A, Talwelkar SS, Kumar A, Pant AB, Parmar D (2011) miR-497 and miR-302b regulate ethanol-induced neuronal cell death through BCL2 protein and cyclin D2. J Biol Chem 286(43):37347–37357. 10.1074/jbc.M111.235531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav S, Jauhari A, Singh N, Singh T, Srivastav AK, Singh P, Pant A, Parmar D (2015) MicroRNAs are emerging as most potential molecular biomarkers. Biochem Anal Biochem. 10.4172/2161-1009.1000191 [Google Scholar]
- Yadav SK, Pandey A, Sarkar S, Yadav SS, Parmar D, Yadav S (2022) Identification of altered blood microRNAs and plasma proteins in a rat model of Parkinson’s disease. Mol Neurobiol. 10.1007/s12035-021-02636-y [DOI] [PubMed] [Google Scholar]
- Yaduvanshi S, Ero R, Kumar V (2021) The mechanism of complex formation between calmodulin and voltage gated calcium channels revealed by molecular dynamics. PloS one 16(10):e0258112. 10.1371/journal.pone.0258112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan J, Yankner BA (2000) Apoptosis in the nervous system. Nature 407(6805):802–809. 10.1038/35037739 [DOI] [PubMed] [Google Scholar]
- Yuan Q, Zhang S, Li J, Xiao J, Li X, Yang J, Lu D, Wang Y (2020) Comprehensive analysis of core genes and key pathways in Parkinson’s disease. Am J Transl Res 12(9):5630–5639 [PMC free article] [PubMed] [Google Scholar]
- Zhang F, Wang D (2017) The pattern of microRNA binding site distribution. Genes. 10.3390/genes8110296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Jin J, Tian X, Wu L (2017) hsa-miR-29c-3p regulates biological function of colorectal cancer by targeting SPARC. Oncotarget 8(61):104508–104524. 10.18632/oncotarget.22356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou T, Huang Z, Zhu X, Sun X, Liu Y, Cheng B, Li M, Liu Y, He C, Liu X (2018) Alpha-1 antitrypsin attenuates M1 microglia-mediated neuroinflammation in retinal degeneration. Front Immunol 9:1202. 10.3389/fimmu.2018.01202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu XG, Zhang TN, Wen R, Liu CF (2020) Overexpression of miR-150-5p alleviates apoptosis in sepsis-induced myocardial depression. Biomed Res Int 2020:3023186. 10.1155/2020/3023186 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All the data generated or analyzed during the present study are included in this article and its supplementary information files.
NCBI-GEO accession number, GSE222480, and CCMS-MassIVE accession number, MSV000091013.