Abstract
The nuclear receptor (NR) superfamily of transcription factors encodes expression of 48 human genes that are important for maintaining cellular homeostasis and in pathophysiology and this has been observed for all sub families including orphan receptors for which endogenous ligands have not yet been identified. The orphan NR4A1 (Nur77, TR3) and other members of this sub-family (NR4A2 and NR4A3) are immediate early genes induced by diverse stressors and these receptors play an important role in the immune function and are upregulated in some inflammatory diseases including solid tumors. Although endogenous ligands for NR4A have not been identified, several different classes of compounds have been characterized as NR4A1 ligands that bind the receptor. These compounds include cytosporone B and structurally related analogs, bis-indole derived (CDIM) compounds, the triterpenoid celastrol and a number of other chemicals including polyunsaturated fatty acids. NR4A1 ligands bind different regions/surfaces of NR4A1 and exhibit selective NR4A1 modulator (SNR4AM) activities that are dependent on ligand structure and cell/tissue context. NR4A1 ligands exhibit pharmacologic activities in studies on cancer, endometriosis metabolic and inflammatory diseases and are promising agents with clinical potential for treating multiple diseases.
Keywords: NR4A1, ligands, agonists, antagonists
Nuclear Receptors (NRs): Introduction
Humans and mice encode 48 and 49 nuclear receptor genes respectively which are classified based on their common structural domains which include N-terminal A/B and C-terminal E/F domains, a DNA binding domain (C) and a hinge region (D) (1, 2). These receptors are broadly classified as ligand-activated transcription factors although there is evidence that some NRs exhibit extranuclear functions and ligand-independent activities. The NR superfamily and their receptor variants play a critical role in maintaining cellular homeostasis associated with development, reproduction and metabolism, and they also are important in multiple disease processes. The glucocorticoid receptor (GR) and estrogen receptor α (ERα) were the first NRs identified (3, 4) and are part of the endocrine receptor subfamily which also include the progesterone, androgen and mineralocorticoid receptors. Other sub-classes of the NR family include the heterodimeric receptors, adopted orphan receptors (lipid sensors and enigmatic orphans) and the orphan receptors (1). Endogenous ligands have been identified for all but the orphan receptors. The activities of NRs are dependent on cell context where tissue/cell-specific expression (or lack thereof) of nuclear cofactors including coregulators and corepressors and chromatin structure are essential elements for NR function (5–8).
For most NRs their endogenous ligands such as 17β-estradiol (E2) for ERα are also critical drivers of NR functions (9). Initial studies on ERα defined the high affinity interactions of E2 with ERα and for other high affinity NR ligands and their cognate receptors; however, it has been demonstrated that ERα and other NRs bind structurally diverse ligands usually with lower affinity and many of these compounds have important endogenous functions or are associated with adverse side-effects (10–12). For example, diethylstilbestrol binds with high affinity to ERα and use of this pharmacologic agent by pregnant women resulting in serious health problems in their male and female offspring (13, 14). Tamoxifen is a synthetic drug that exhibits modest binding affinity for ERα and has been invaluable as an antiestrogen for treating early stage ER-positive breast cancer in women (15). In contrast, other synthetic ligands such as bisphenol-A that bind ERα with modest-low affinity are classified as endocrine disruptors and it has been hypothesized that they may be linked to multiple adverse health effects (16, 17). Thus, the structure of receptor ligands can dramatically modulate the functions of ligand-mediated responses and this review will focus on NR4A1, a member of the NR4A sub-family of orphan nuclear receptors and the effects of ligands on receptor-mediated activity (18–20).
NR4A subfamily of orphan NRs.
NR4A1 (Nur77, TR3), NR4A2 (Nurr1) and NR4A3 (Nor1) are early immediate genes that are induced by diverse physiological and physical stimuli and play a role in adaptation to cellular stress and in pathophysiology (20–25). These genes exhibit similarities in their C-terminal (58–65%) (AF2) and DNA binding (94–95%) domains whereas sequence conservation in their N-terminal (AF1) domains was 26–28% and AF-1 dependent difference in transactivation by NR4As has been reported (26–28). There are also differences in NR4A null mutant mice; NR4A1−/− mice are viable, NR4A2 mice die soon after birth due to deficits in the dopaminergic system. NR4A3−/− mice exhibit inner ear deficits and one study also reported embryo lethality in NR4A3−/− mice due to a failure to complete gastrulation (29–34). Endogenous ligands for NR4A1, NR4A2 and NR4A3 have not been identified and X-ray crystallographic analysis of the LBD of NR4A2 indicates that bulky amino acid side chains may preclude their interactions with ligands (35). Ongoing studies demonstrate a role for NR4A1 in multiple diseases (18–20, 36–38) that could potentially be targeted by ligands and this is now paralleled by studies on the development and potential applications of structurally diverse NR4A1 ligands (18–20).
Cytosporone B and related compounds
Wu and coworkers pioneered the identification and subsequent applications of NR4A1 ligands by their screening of a natural products library which identified cytosporone B (Csn-B) (39) an octaketide fungal metabolite as an NR4A1 ligand (Fig. 1A). Csn-B but not structurally related Csn-C induced NR4A1-dependent transactivation in human gastric BGC-823 cells and directly bound the ligand binding domain (LBD) of NR4A1 with a KD value of 7.4 × 10−7 M. Moreover, Csn-B quenched the fluorescence of Tyr453 which is conserved in the ligand binding pocket of many nuclear receptors (40–43) whereas fluorescence was not quenched in the binding of Csn-B to the mutant NR4A1 (LBD)-Y453A. The results of Csn-B induced transactivation assays in BGC-823 cells implies a nuclear function for NR4A1 however, Csn-B also induces nuclear export of approximately 70% of cellular NR4A1 where it forms a pro-apoptotic bcl2-NR4A1 complex and induced cell death in cancer cell lines and inhibited tumor growth in vivo. At doses of 50 mg/kg Csn-B acts as an NR4A1 agonist and also enhances blood glucose levels and hepatic expression of gluconeogenic genes and NR4A1 in mouse models (39). Subsequent structure-activity studies identified synthetic analogs of Csn-B that were also NR4A1 ligands and these compounds enhanced expression of NR4A1, activated nuclear NR4A1 and also nuclear export of the receptor (44). Another paper also developed a synthesis of Csn-B and showed the importance of the 3-hydroxyl group for maintaining NR4A1 binding activity (45). Wu and coworkers recently reported the crystal structure of the NR4A1 (LBD) – Csn-B complex and showed that the ligand bridges the LBDs of an NR4A1 homodimer. Essential amino acids for this novel interaction include residues Asp481, GIn571 and Arg572 (46). These results were obtained in a study on the role of the NR4A1 homodimer as an inhibitor of breast cancer progression through suppression of genes involved in fatty acid uptake into cancer cells. This paper also demonstrated that loss of NR4A1 from genetic and carcinogen-induced mouse models of breast cancer resulted in enhanced tumorigenesis (46) and this was in contrast to other studies showing a pro-oncogenic role for NR4A1 in some breast cancer cell lines (47, 48). Csn-B has been extensively used to investigate the role of NR4A1 in the presence or absence of ligand in multiple inflammatory diseases in mouse models (49–53).
Figure 1.
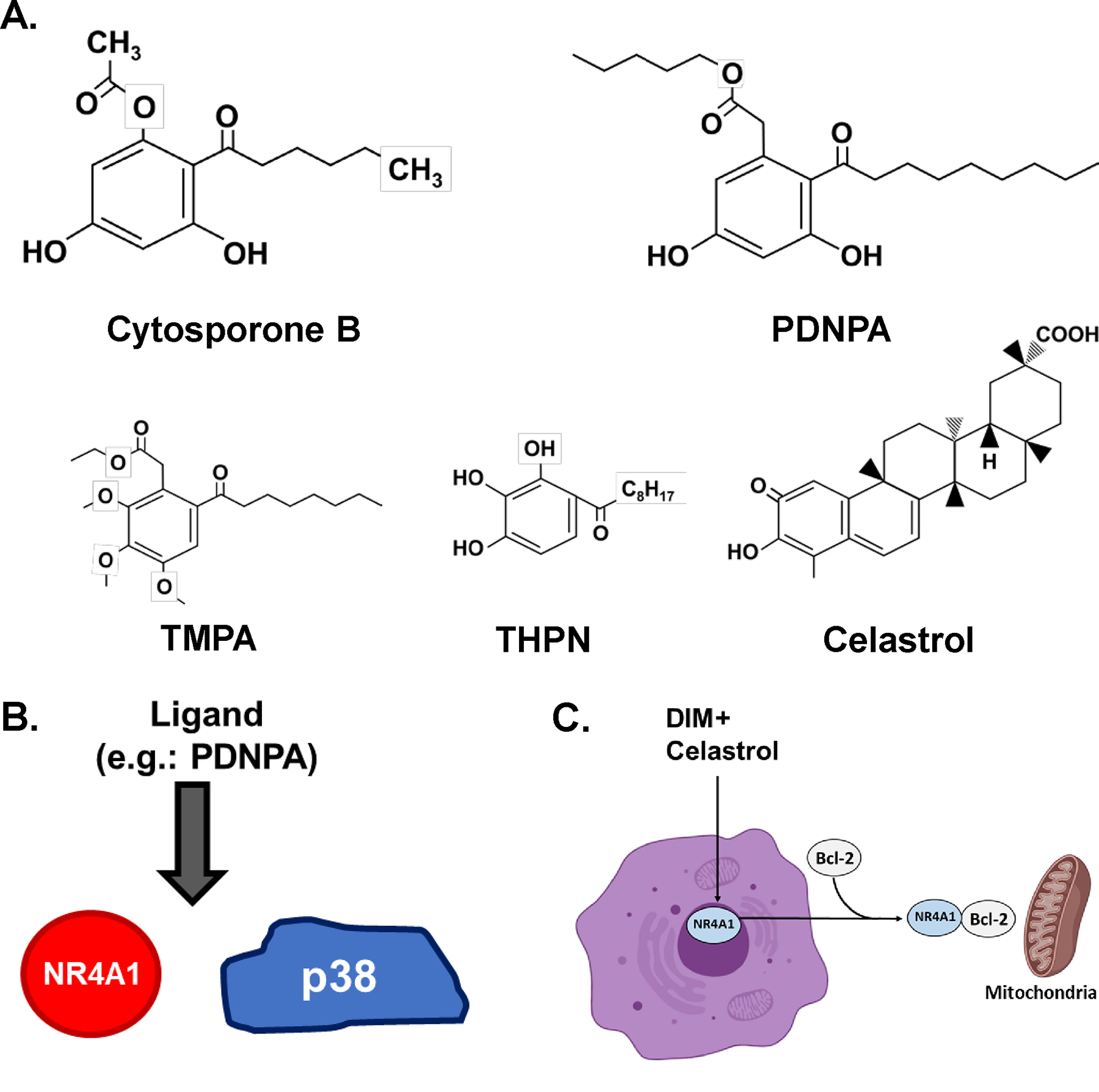
A. Structures of CsnB and related compounds and celastrol B. Mechanisms associated with PDNPA inducing dissociation of NR4A1 from p38 (51) and (B) effects of celastrol and oxidized DIM compounds inducing nuclear export of NR4A1 which forms a pro-apoptotic NR4A1-bcl2 complex that target mitochondria (54).
n-Pentyl 2-[3,5-dihydroxy-2-(1-nonanoyl)phenyl acetate (PDNPA) is another NR4A1 ligand that has structural similarities to Csn-B and PDNPA competes with p38 for binding to the LBD of NR4A1 (54). Modeling and binding studies show that amino acids Leu437, Ser441 and Asp549 are important for PDNPA-NR4A1 (LBD) binding and by inhibiting NR4A1-p38 interaction PDNPA (50 mg/kg) decreased LPS induced inflammation. However, the effects of PDNPA are specific for inhibiting NR4A1-p38 binding and it does not interact with the canonical binding pocket of NR4A1 (Fig. 1B). Interestingly, PDNPA also binds NR4A2 and NR4A3 but these interactions were not accompanied by phenotypic effects.
Ethyl 2-[2,3,4-trimethoxy-6-(1-octanoyl)phenyl acetate (TMPA) is another Csn-B – like compound which inhibits NR4A1-LKB1 interactions resulting in nuclear export of LKB1 and activation (phosphorylation) of AMPK (55). This process results in the inhibition of gluconeogenesis and TMPA decreased blood glucose levels in mouse models of insulin resistant and these effects were in direct contrast to Csn-B – dependent increase in blood glucose levels (39). X-ray crystallographic analysis of TMPA-NR4A1 (LBD) interactions shows interactions of the ligand with side chains of several amino acids (Arg515, Glu445, Thr595, His372, Arg450, Tyr453, Leu492 and Val498) and the ligand primarily binds close to the surface of the LBD and not deep within the binding pocket. Mutational analysis confirmed that Thr595 is necessary for NR4A1 – LKB1 interactions whereas Cys566 is required for TMPA binding to NR4A1. Differences in the binding of PDNPA and TMPA to NR4A1 correlated with their functional differences; PDNPA but not TMPA rescued mice from a lethal LPS challenge whereas TMPA but not PDNPA decreased blood glucose levels in diabetic mice (54). Presumably this is due to antagonist (TMPA) and agonist (PDNPA) activities.
Wu and coworkers also identified another Csn-B analog, 1-(3,4,5-trihydroxyphenyl)nonan-1-one (THPN) (56) which bound NR4A1 and induced autophagic cell death via activation of mitochondria in some melanoma cell lines. THPN did not induce nuclear export of NR4A1 but induce targeting of cytosolic NR4A1 (found in melanoma cells) to mitochondria. THPN bind surface residues Arg563 and Ser553 around the binding cavity and these sites were necessary for interaction of the THPN-bound NR4A1 with the mitochondrial Nix protein which plays a role in activation of autophagy. Thus, although THPN did not directly bind Nix, interaction of this ligand with NR4A1 was required for NR4A1-Nix binding and subsequent activation of autophagic cell death through specific mitochondrial interactions. Thus, Csn-B and related analogs exhibit structure-dependent interactions with different amino acids in the ligand binding AF2 domain of NR4A1 and it is possible that this variability may contribute to their diverse agonist and antagonist activities. These differences may also be related to other NR4A1 interactants (e.g.: NR4A1-Nix) where ligand-induced responses are due to both receptor binding and parallel interactions with other factors.
Celastrol and related compounds
Celastrol is a naturally-occuring triterpenoid (Fig. 1A) with anticancer activity; this compound bound NR4A1 with a KD value of 0.29 μM (57, 58) and celastrol inhibited NR4A1-dependent transactivation. Molecular modeling studies showed that celastrol interacted with a region on the surface near the LBD of NR4A1 as previously described for TMPA (55) and like TMPA, celastrol inhibited high fat diet-induced chronic inflammation and weight gain. Celastrol induces nuclear export of NR4A1 where it interacts with mitochondrial tumor necrosis factor receptor-associated factor 2 (TRAF2) and this triggers anti-inflammatory responses (57) (Fig. 1C). Modeling of celastrol-NR4A1 binding showed interactions with GIn547 and Asp499, and the KD value was 0.32 μM. Structure activity studies (58) identified the two hydroxyl groups on the A ring as important binding determinants and addition of substituents (other than H) at C-6 in the B ring also resulted in the loss of activity as an NR4A1 ligand.
Bis-indole derived (CDIM) ligands
In solid tumors NR4A1 is overexpressed and for breast, colon, lung and ovarian tumors NR4A1 is a negative prognostic factor for patient survival or recurrence (rev. in 20). Several studies show that in most solid tumor-derived cell lines knockdown of NR4A1 results in decreased growth, survival, migration and invasion, and associated genes (Fig. 2) demonstrating that NR4A1 is a pro-oncogenic factor. Moreover, in alveolar rhabdomyosarcoma (ARMS), a devastating pediatric cancer, NR4A1 not only regulates multiple pro-oncogenic pathways/genes as illustrated in Figure 2 but also the PAX3-FOX01 fusion gene that is the major transcriptional driver of this tumor (59). Bis-indole derived (CDIM) compounds were initially identified as PPARγ ligands (60). Structure-induction study of a series of ten 4-substituted phenyl CDIM analogs showed that the 4-trifluoromethyl, 4-methoxy and 4-H analogs were most active as NR4A1 agonists whereas the 4-trifluoromethyl, 4-bromo- and 4-tbutyl analogs were most active NR4A2 agonists in pancreatic cancer cells (61, 62). However, subsequent structure-transactivation screening using GAL4-receptor chimeras identified a group of C-DIMs that not only bound NR4A1 but inhibited NR4A1-dependent transactivation in multiple cancer cell lines (20). Moreover, NR4A1-active CDIMs inhibited most of the pro-oncogenic pathways and associated genes illustrated in Figure 2 in colon, pancreatic, lung, breast, rhabdomyosarcoma, kidney and endometrial cancer cell lines (63–70). Thus, knockdown of NR4A1 or inhibition of NR4A1 by CDIMs gave comparable effects in cancer cell lines and the CDIMs were classified as NR4A1 antagonists even though array or RNAseq analysis show that both NR4A1 silencing or antagonism results in both induction and inhibition of gene expression (64). The designation of CDIMs as NR4A1 antagonists is based on their antagonism of the functional pro-oncogenic properties of NR4A1.
Figure 2.
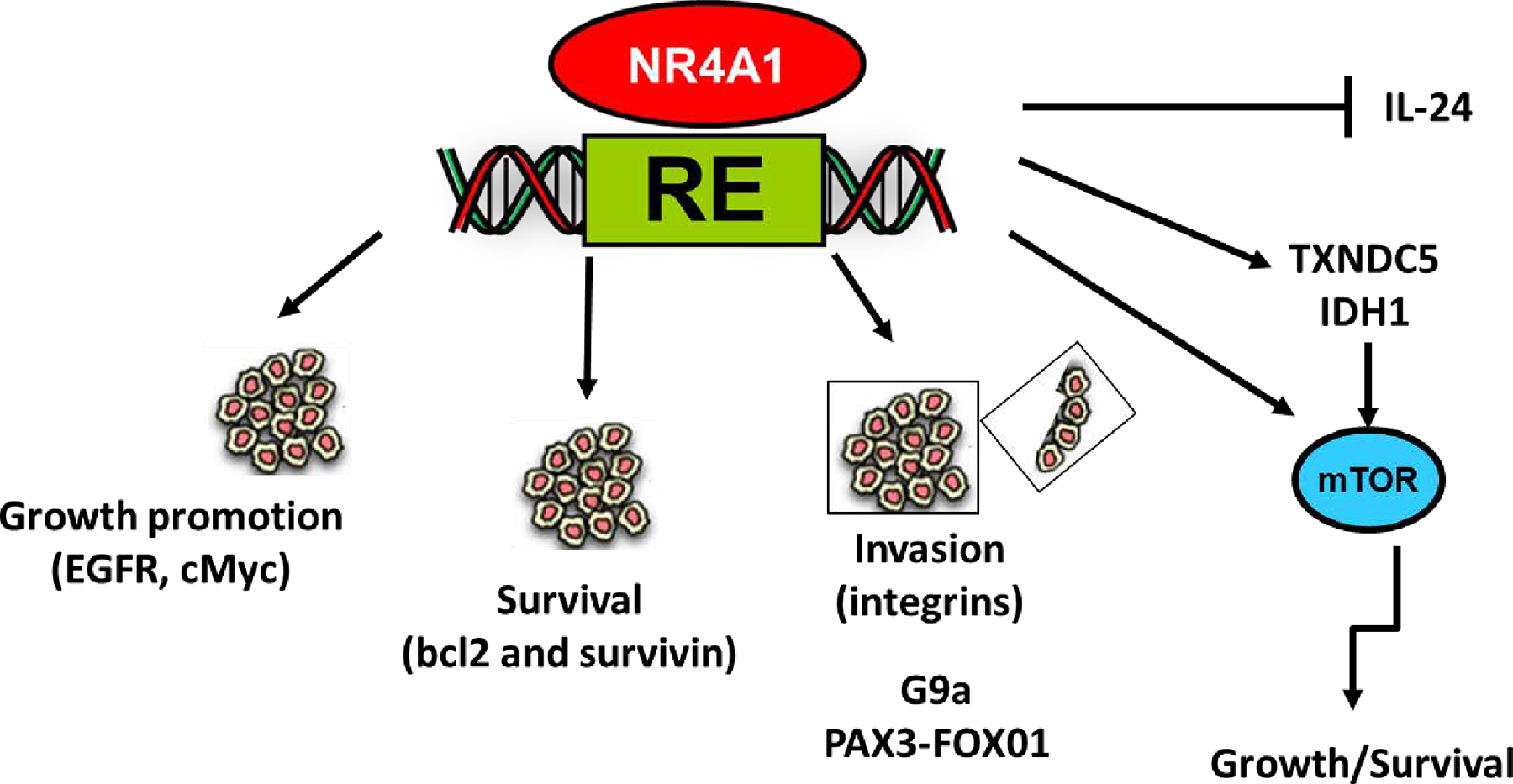
Multiple pro-oncogenic pathways/gene regulated by NR4A1 in solid tumors that are inhibited by CDIM/NR4A1 antagonists. Results of NR4A1 knockdown and subsequent functional and genomic analysis have identified several pathways and genes responsible for cancer cell proliferation, survival, migration and invasion (rev. in 20). These pathways have been primarily determined in receptor knockdown studies and similar responses are observed after treatment with CDIM/NR4A1 antagonists.
Initial studies on NR4A1-active CDIMs focused on the 1,1-bis(3΄-indolyl)-1-(p-hydroxyphenyl)methane (DIM-C-pPhOH or CDIM8) and the corresponding p-carboxymethyl analog (DIM-C-pPhCO2Me) (70) (Fig. 3). CDIM8 inhibits cancer cell and tumor growth (20–40 mg/kg/d) in athymic nude mouse xenograft models, however, pharmacokinetic studies show that this compound is rapidly metabolized and blood levels are low (71). Recent studies show that addition of substituents ortho to the 4-hydroxyl at C-3 and C-5 in the phenyl ring results in a buttressing effect which decreases metabolic conjugation of the hydroxyl group and enhances potency of tumor growth inhibition in athymic nude mouse xenograft (breast cancer cells) models by approximately 10-fold (72). Direct binding of CDIM8 to NR4A1 has been reported using a fluorescence quenching assay and by circular dichroism and modeling studies have identified Asp594, His556, Arg515 and Glu445 as key amino acids interacting with CDIM8 (70). Oxidized analogs of several 4-substitutedphenyl CDIMs have also been reported (73, 74) (Fig. 3) and these compounds are also potent inhibitors of cancer cell growth and appear to be more active than their parent precursors in several cancer cell lines and in vivo (prostate cancer). The oxidized mesylate derivative of DIM-C-pPhCF3 (4-trifluoromethylphenyl) bound NR4A1 and key interactions with side chains of His372 and Tyr453 located in helices 1 and 5 respectively were key binding determinants.
Figure 3.
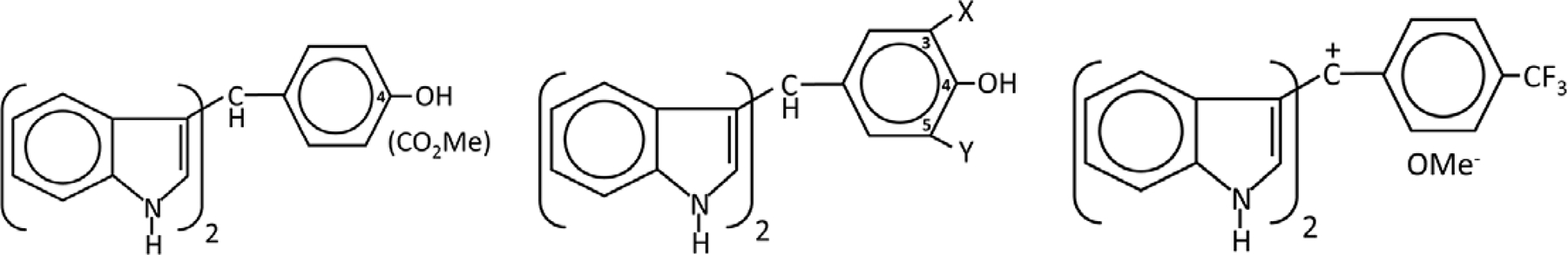
Structures of CDIM8 and buttressed analogs substituted at X and Y, and oxidized CDIMs (20, 68, 69). CDIM8 contains a 4-hydroxyl group on the phenyl ring and has been used extensively as a prototypical NR4A1 ligand and the 3,5-substituted buttressed analogs of CDIM8 are more potent as NR4A1 antagonists in both cell culture and in vivo studies (68, 72). The 4-carboxymethyl analogs was also active as an NR4A1 ligand. The oxidized CDIMs are prepared from the corresponding CDIMs and induced NR4A1-dependent anticancer activity and induce nuclear export or NR4A1 (73, 74).
The mechanisms associated with CDIM modulation of gene expression involve direct interactions with nuclear NR4A1 which bind cognate NBRE and NuRE sequences as a monomers and dimers respectively as previously described (75–77) (Figs. 4A and 4B). Another mechanism of NR4A1-dependent transactivation involves NR4A1-RXR complexes (Fig. 4C) that interact with a DRE motif (78) and recent studies have identified that NR4A2 interactions with novel promoter sequences that may also bind NR4A1 (79). Genomic analysis of NR4A1 regulated genes shows that many of these genes such as survivin and epidermal growth factor receptor (EGFR) have previously been characterized as specificity protein 1 (Sp1)-regulated genes. NR4A1 knockdown or treatment with CDIM/NR4A1 antagonists decreased expression of survivin in pancreatic cancer cells and subsequent analysis showed that levels of survivin are dependent not only on Sp1 but also NR4A1 and p300 (63). Moreover, individual knockdown of Sp1, NR4A1 and p300 decreased survivin expression and indicating that NR4A1 is acting as a nuclear cofactor and there is extensive evidence showing that many nuclear receptors are cofactors for Sp1-regulated genes (80). Subsequent studies have shown that NR4A1/Sp1 and/or NR4A1/Sp4 regulate multiple genes through their NR4A1/Sp interactions with GC-rich promoters and these include PAX3-FOX01, PD-L1, G9a and several integrins (63–65, 81, 82) (Fig. 4D). TGFβ plays a key role in invasion of breast and lung cancer cells and this involves phosphorylation and subsequent export of nuclear NR4A1 which interacts with a proteasome complex that degrades inhibitory SMAD7 (83, 84). CDIM/NR4A1 interacts with NR4A1 to inhibit nuclear export and this is accompanied by decreased degradation of SMAD-7 and TGFβ-induced invasion (47, 48) (Fig. 4E). CDIM NR4A1 antagonists enhance apoptosis in RD embryonal rhabdomyosarcoma (ERMS) cells by interacting with constitutive cytosolic NR4A1 which results in apoptosis (83) (Fig. 4F). The oxidized CDIM+ compounds induce nuclear export of NR4A1 which forms a pro-apoptotic NR4A1-bcl2 complex as described for celastrol (Fig. 1C) and this pathway is also activated by other pro-apoptotic agents that do not directly bind NR4A1 (rev. in (20, 84)).
Figure 4.
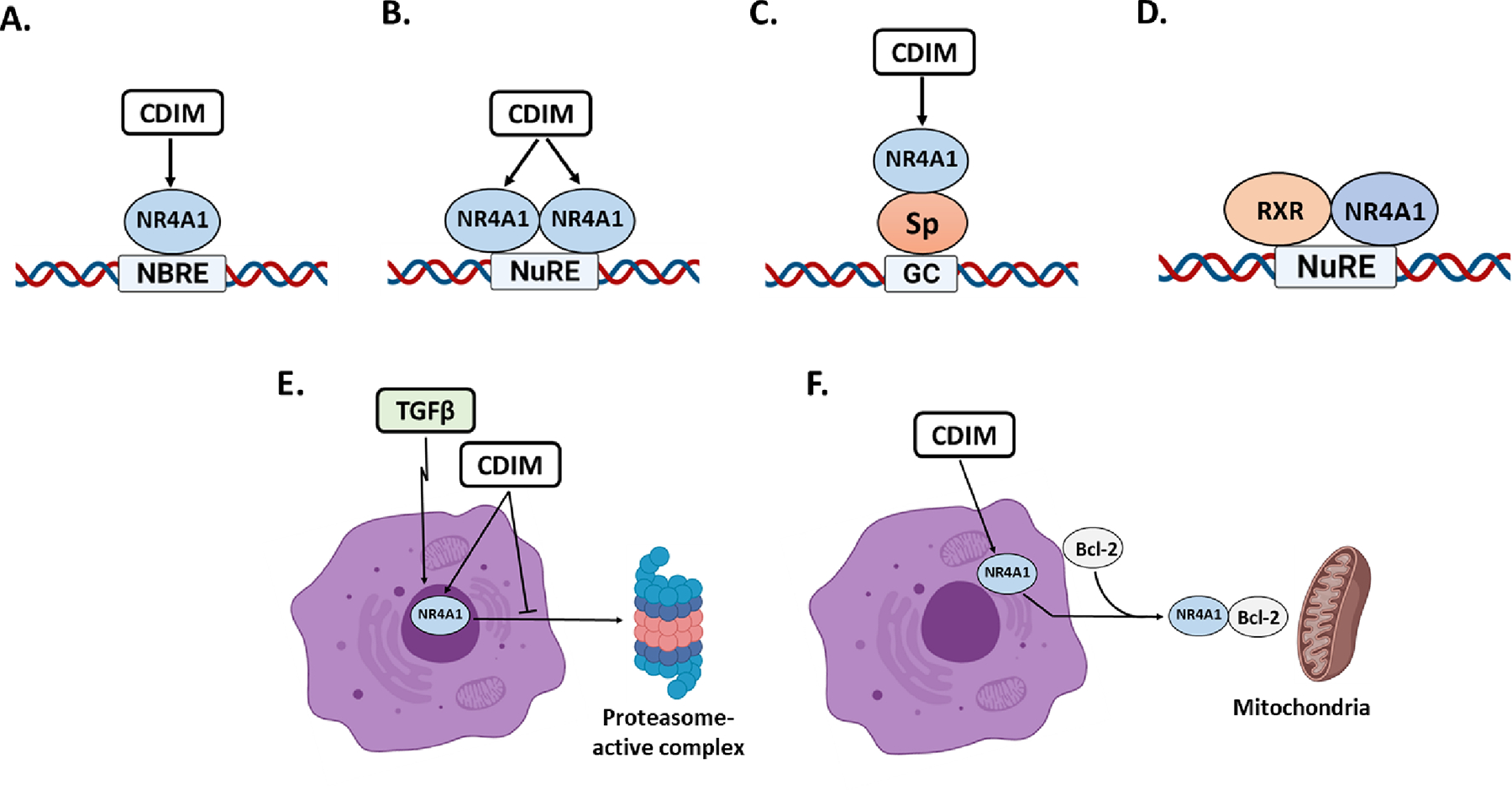
Transactivation mechanisms associated with CDIM/NR4A1 ligands including activation of NR4A1 monomer (A), homodimer (B) and NR4A1/Sp (C) and NR4A1/RXR heterodimer (D); It has also been demonstrated that TGFβ-induced invasion induces nuclear export of NR4A1 in breast cancer cells (48) which can be blocked by CDIMs (E) and CDIMs also activate cytosolic NR4A1 in RD rhabdomyosarcoma cells to bind bcl2 (F) to form a pro-apoptotic complex which interacts with bcl-2 (83).
Other NR4A1 ligands:
A survey of several lipids as NR4A1 ligands identified unsaturated fatty acids arachidonic and docosahexaenoic acids as compounds that bound NR4A1 whereas this was not observed for saturated fatty acids (85). Binding and receptor stabilization studies suggested that the unsaturated fatty acids might play a role in stabilizing NR4A1 oligomer complex formation. Prostaglandin A2, another lipophilic endogenous compound also binds NR4A1 and forms a covalent adduct at Cys566 of NR4A1 via Michael addition associated with the cyclopentenone ring (86). PGA2 also induces NR4A1-dependent transactivation in normal human bronchial epithelial cells. Interestingly, PGA2 also binds NR4A3 (87) and is thus a dual receptor ligand. Computation based modeling studies identified 2-imino-6-methoxy-2H-chromene-3-carbothioamide (IMCA) as an NR4A1 ligand and functional studies indicated that this compound acts via induction of nuclear export of NR4A1 in medullary thyroid cancer cells (88). A recent study on a series of putative NR4A ligands (89) showed that cytosporone B also bound NR4A2; many of the compounds exhibited NR4A2-independent activities and this may also be true for some of the NR4A1 ligands described in this review.
Summary and Conclusions
The important immune cell functions of NR4A1 and its expression in multiple inflammatory cell types make this receptor an attractive drug target for treating multiple diseases. This review outlines several structural classes of NR4A1 ligands that differentially bind and modulate NR4A1-dependent responses and genes. For example, Csn-B and structurally related TMPA both bind NR4A1, however, in in vivo models of metabolic disease Csn-B increased and TMPA decreased blood glucose levels. There are conflicting and unresolved issues in breast cancer where there is evidence that NR4A1 is both a tumor promoter and inhibitor (46–48, 72). However, both Csn-B and CDIM/NR4A1 ligands inhibit mammary tumorgenesis despite their different modes of action. NR4A1 exhibits pro-oncogenic functions in solid tumors, however, NR4A1 is a tumor suppressor in most blood derived cancers and proposed receptor-derived therapies for leukemia include agents that induce NR4A1 expression (rev. in (20, 90)). Thus, development of pharmacologic agents targeting NR4A1 may require some disease-specificity. However, studies with CDIMs suggest that these compounds may be selective NR4A1 modulators since NR4A1-active CDIMs inhibited growth of solid tumor derived cancers, enhanced glucose uptake in muscle cells, decreased neuronal inflammation in models of Parkinson’s disease, inhibited endometriosis and enhanced learning and memory in mouse models (20, 91–96).
Summary Points.
NR4A1 regulates both cellular homeostasis and pathophysiology.
Structurally-diverse NR4A1 ligands have been characterized.
NR4A1 ligands are promising pharmacologic agents for treating multiple diseases.
Acknowledgements
The financial support of AgriLife Research, the National Institutes of Health (P30-ES029067), the Syd Kyle Chair endowment and Systems Oncology is gratefully acknowledged.
Footnotes
Competing Interest: S. Safe has a licensing agreement for the CDIM compounds from System Oncology, Scottsdale AZ; there are no other competing interest.
References
- 1.Sonoda J, Pei L, Evans RM. Nuclear receptors: decoding metabolic disease. FEBS letters. 2008;582(1):2–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McKenna NJ, Cooney AJ, DeMayo FJ, Downes M, Glass CK, Lanz RB, et al. Minireview: Evolution of NURSA, the Nuclear Receptor Signaling Atlas. Molecular endocrinology. 2009;23(6):740–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hollenberg SM, Weinberger C, Ong ES, Cerelli G, Oro A, Lebo R, et al. Primary structure and expression of a functional human glucocorticoid receptor cDNA. Nature. 1985;318(6047):635–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Walter P, Green S, Greene G, Krust A, Bornert JM, Jeltsch JM, et al. Cloning of the human estrogen receptor cDNA. Proc Natl Acad Sci U S A. 1985;82(23):7889–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gallastegui N, Mackinnon JA, Fletterick RJ, Estebanez-Perpina E. Advances in our structural understanding of orphan nuclear receptors. Trends Biochem Sci. 2015;40(1):25–35. [DOI] [PubMed] [Google Scholar]
- 6.Helsen C, Kerkhofs S, Clinckemalie L, Spans L, Laurent M, Boonen S, et al. Structural basis for nuclear hormone receptor DNA binding. Molecular and cellular endocrinology. 2012;348(2):411–7. [DOI] [PubMed] [Google Scholar]
- 7.Shibata H, Spencer TE, Onate SA, Jenster G, Tsai SY, Tsai MJ, et al. Role of co-activators and co-repressors in the mechanism of steroid/thyroid receptor action. Recent Prog Horm Res. 1997;52:141–64; discussion 64–5. [PubMed] [Google Scholar]
- 8.Billas I, Moras D. Allosteric controls of nuclear receptor function in the regulation of transcription. J Mol Biol. 2013;425(13):2317–29. [DOI] [PubMed] [Google Scholar]
- 9.Tao LJ, Seo DE, Jackson B, Ivanova NB, Santori FR. Nuclear Hormone Receptors and Their Ligands: Metabolites in Control of Transcription. Cells. 2020;9(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kuiper GG, Lemmen JG, Carlsson B, Corton JC, Safe SH, van der Saag PT, et al. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor beta. Endocrinology. 1998;139(10):4252–63. [DOI] [PubMed] [Google Scholar]
- 11.Safe SH, Pallaroni L, Yoon K, Gaido K, Ross S, McDonnell D. Problems for risk assessment of endocrine-active estrogenic compounds. Environmental health perspectives. 2002;110 Suppl 6:925–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.vom Saal FS, Akingbemi BT, Belcher SM, Birnbaum LS, Crain DA, Eriksen M, et al. Chapel Hill bisphenol A expert panel consensus statement: integration of mechanisms, effects in animals and potential to impact human health at current levels of exposure. Reproductive toxicology. 2007;24(2):131–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Giusti RM, Iwamoto K, Hatch EE. Diethylstilbestrol revisited: a review of the long-term health effects. Ann Intern Med. 1995;122(10):778–88. [DOI] [PubMed] [Google Scholar]
- 14.Al Jishi T, Sergi C. Current perspective of diethylstilbestrol (DES) exposure in mothers and offspring. Reproductive toxicology. 2017;71:71–7. [DOI] [PubMed] [Google Scholar]
- 15.Jordan VC. Tamoxifen: a most unlikely pioneering medicine. Nat Rev Drug Discov. 2003;2(3):205–13. [DOI] [PubMed] [Google Scholar]
- 16.Gore AC, Chappell VA, Fenton SE, Flaws JA, Nadal A, Prins GS, et al. EDC-2: The Endocrine Society’s Second Scientific Statement on Endocrine-Disrupting Chemicals. Endocr Rev. 2015;36(6):E1–E150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.La Merrill MA, Vandenberg LN, Smith MT, Goodson W, Browne P, Patisaul HB, et al. Consensus on the key characteristics of endocrine-disrupting chemicals as a basis for hazard identification. Nat Rev Endocrinol. 2020;16(1):45–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu L, Chen L. Characteristics of Nur77 and its ligands as potential anticancer compounds (Review). Mol Med Rep. 2018;18(6):4793–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang L, Wang Q, Liu W, Liu F, Ji A, Li Y. The Orphan Nuclear Receptor 4A1: A Potential New Therapeutic Target for Metabolic Diseases. J Diabetes Res. 2018;2018:9363461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Safe S, Karki K. The Paradoxical Roles of Orphan Nuclear Receptor 4A (NR4A) in Cancer. Molecular cancer research : MCR. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maxwell MA, Muscat GE. The NR4A subgroup: immediate early response genes with pleiotropic physiological roles. Nucl Recept Signal. 2006;4:e002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pearen MA, Muscat GE. Minireview: Nuclear hormone receptor 4A signaling: implications for metabolic disease. Molecular endocrinology. 2010;24(10):1891–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen L, Fan F, Wu L, Zhao Y. The nuclear receptor 4A family members: mediators in human disease and autophagy. Cell Mol Biol Lett. 2020;25(1):48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Crean D, Murphy EP. Targeting NR4A Nuclear Receptors to Control Stromal Cell Inflammation, Metabolism, Angiogenesis, and Tumorigenesis. Front Cell Dev Biol. 2021;9:589770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Odagiu L, May J, Boulet S, Baldwin TA, Labrecque N. Role of the Orphan Nuclear Receptor NR4A Family in T-Cell Biology. Front Endocrinol (Lausanne). 2020;11:624122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wansa KD, Harris JM, Yan G, Ordentlich P, Muscat GE. The AF-1 domain of the orphan nuclear receptor NOR-1 mediates trans-activation, coactivator recruitment, and activation by the purine anti-metabolite 6-mercaptopurine. The Journal of biological chemistry. 2003;278(27):24776–90. [DOI] [PubMed] [Google Scholar]
- 27.Maira M, Martens C, Batsche E, Gauthier Y, Drouin J. Dimer-specific potentiation of NGFI-B (Nur77) transcriptional activity by the protein kinase A pathway and AF-1-dependent coactivator recruitment. Molecular and cellular biology. 2003;23(3):763–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wansa KD, Harris JM, Muscat GE. The activation function-1 domain of Nur77/NR4A1 mediates trans-activation, cell specificity, and coactivator recruitment. The Journal of biological chemistry. 2002;277(36):33001–11. [DOI] [PubMed] [Google Scholar]
- 29.Saucedo-Cardenas O, Quintana-Hau JD, Le WD, Smidt MP, Cox JJ, De Mayo F, et al. Nurr1 is essential for the induction of the dopaminergic phenotype and the survival of ventral mesencephalic late dopaminergic precursor neurons. Proc Natl Acad Sci U S A. 1998;95(7):4013–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.DeYoung RA, Baker JC, Cado D, Winoto A. The orphan steroid receptor Nur77 family member Nor-1 is essential for early mouse embryogenesis. The Journal of biological chemistry. 2003;278(47):47104–9. [DOI] [PubMed] [Google Scholar]
- 31.Cheng LE, Chan FK, Cado D, Winoto A. Functional redundancy of the Nur77 and Nor-1 orphan steroid receptors in T-cell apoptosis. EMBO J. 1997;16(8):1865–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ponnio T, Burton Q, Pereira FA, Wu DK, Conneely OM. The nuclear receptor Nor-1 is essential for proliferation of the semicircular canals of the mouse inner ear. Molecular and cellular biology. 2002;22(3):935–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee SL, Wesselschmidt RL, Linette GP, Kanagawa O, Russell JH, Milbrandt J. Unimpaired thymic and peripheral T cell death in mice lacking the nuclear receptor NGFI-B (Nur77). Science. 1995;269(5223):532–5. [DOI] [PubMed] [Google Scholar]
- 34.Zetterstrom RH, Solomin L, Jansson L, Hoffer BJ, Olson L, Perlmann T. Dopamine neuron agenesis in Nurr1-deficient mice. Science. 1997;276(5310):248–50. [DOI] [PubMed] [Google Scholar]
- 35.Wang Z, Benoit G, Liu J, Prasad S, Aarnisalo P, Liu X, et al. Structure and function of Nurr1 identifies a class of ligand-independent nuclear receptors. Nature. 2003;423(6939):555–60. [DOI] [PubMed] [Google Scholar]
- 36.Ando M, Ito M, Srirat T, Kondo T, Yoshimura A. Memory T cell, exhaustion, and tumor immunity. Immunol Med. 2020;43(1):1–9. [DOI] [PubMed] [Google Scholar]
- 37.Lith SC, van Os BW, Seijkens TTP, de Vries CJM. ‘Nur’turing tumor T cell tolerance and exhaustion: novel function for Nuclear Receptor Nur77 in immunity. Eur J Immunol. 2020;50(11):1643–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Safe S, Jin UH, Morpurgo B, Abudayyeh A, Singh M, Tjalkens RB. Nuclear receptor 4A (NR4A) family - orphans no more. The Journal of steroid biochemistry and molecular biology. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhan Y, Du X, Chen H, Liu J, Zhao B, Huang D, et al. Cytosporone B is an agonist for nuclear orphan receptor Nur77. Nat Chem Biol. 2008;4(9):548–56. [DOI] [PubMed] [Google Scholar]
- 40.Agostini M, Gurnell M, Savage DB, Wood EM, Smith AG, Rajanayagam O, et al. Tyrosine agonists reverse the molecular defects associated with dominant-negative mutations in human peroxisome proliferator-activated receptor gamma. Endocrinology. 2004;145(4):1527–38. [DOI] [PubMed] [Google Scholar]
- 41.Koehler KF, Helguero LA, Haldosen LA, Warner M, Gustafsson JA. Reflections on the discovery and significance of estrogen receptor beta. Endocr Rev. 2005;26(3):465–78. [DOI] [PubMed] [Google Scholar]
- 42.Nolte RT, Wisely GB, Westin S, Cobb JE, Lambert MH, Kurokawa R, et al. Ligand binding and co-activator assembly of the peroxisome proliferator-activated receptor-gamma. Nature. 1998;395(6698):137–43. [DOI] [PubMed] [Google Scholar]
- 43.Ray DW, Suen CS, Brass A, Soden J, White A. Structure/function of the human glucocorticoid receptor: tyrosine 735 is important for transactivation. Molecular endocrinology. 1999;13(11):1855–63. [DOI] [PubMed] [Google Scholar]
- 44.Liu JJ, Zeng HN, Zhang LR, Zhan YY, Chen Y, Wang Y, et al. A unique pharmacophore for activation of the nuclear orphan receptor Nur77 in vivo and in vitro. Cancer research. 2010;70(9):3628–37. [DOI] [PubMed] [Google Scholar]
- 45.Xia Z, Cao X, Rico-Bautista E, Yu J, Chen L, Chen J, et al. Relative impact of 3- and 5-hydroxyl groups of cytosporone B on cancer cell viability. Medchemcomm. 2013;4(2):332–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yang PB, Hou PP, Liu FY, Hong WB, Chen HZ, Sun XY, et al. Blocking PPARgamma interaction facilitates Nur77 interdiction of fatty acid uptake and suppresses breast cancer progression. Proc Natl Acad Sci U S A. 2020;117(44):27412–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhou F, Drabsch Y, Dekker TJ, de Vinuesa AG, Li Y, Hawinkels LJ, et al. Nuclear receptor NR4A1 promotes breast cancer invasion and metastasis by activating TGF-beta signalling. Nat Commun. 2014;5:3388. [DOI] [PubMed] [Google Scholar]
- 48.Hedrick E, Safe S. Transforming Growth Factor beta/NR4A1-Inducible Breast Cancer Cell Migration and Epithelial-to-Mesenchymal Transition Is p38alpha (Mitogen-Activated Protein Kinase 14) Dependent. Molecular and cellular biology. 2017;37(18). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jiang Y, Zeng Y, Huang X, Qin Y, Luo W, Xiang S, et al. Nur77 attenuates endothelin-1 expression via downregulation of NF-kappaB and p38 MAPK in A549 cells and in an ARDS rat model. Am J Physiol Lung Cell Mol Physiol. 2016;311(6):L1023–L35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Brunet A, LeBel M, Egarnes B, Paquet-Bouchard C, Lessard AJ, Brown JP, et al. NR4A1-dependent Ly6C(low) monocytes contribute to reducing joint inflammation in arthritic mice through Treg cells. Eur J Immunol. 2016;46(12):2789–800. [DOI] [PubMed] [Google Scholar]
- 51.Xiong Y, Ran J, Xu L, Tong Z, Adel Abdo MS, Ma C, et al. Reactivation of NR4A1 Restrains Chondrocyte Inflammation and Ameliorates Osteoarthritis in Rats. Front Cell Dev Biol. 2020;8:158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liang H, Xie X, Song X, Huang M, Su T, Chang X, et al. Orphan nuclear receptor NR4A1 suppresses hyperhomocysteinemia-induced hepatic steatosis in vitro and in vivo. FEBS letters. 2019;593(10):1061–71. [DOI] [PubMed] [Google Scholar]
- 53.Hiwatashi N, Mukudai S, Bing R, Branski RC. The effects of cytosporone-B, a novel antifibrotic agent, on vocal fold fibroblasts. Laryngoscope. 2018;128(12):E425–E8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li L, Liu Y, Chen HZ, Li FW, Wu JF, Zhang HK, et al. Impeding the interaction between Nur77 and p38 reduces LPS-induced inflammation. Nat Chem Biol. 2015;11(5):339–46. [DOI] [PubMed] [Google Scholar]
- 55.Zhan YY, Chen Y, Zhang Q, Zhuang JJ, Tian M, Chen HZ, et al. The orphan nuclear receptor Nur77 regulates LKB1 localization and activates AMPK. Nat Chem Biol. 2012;8(11):897–904. [DOI] [PubMed] [Google Scholar]
- 56.Wang WJ, Wang Y, Chen HZ, Xing YZ, Li FW, Zhang Q, et al. Orphan nuclear receptor TR3 acts in autophagic cell death via mitochondrial signaling pathway. Nat Chem Biol. 2014;10(2):133–40. [DOI] [PubMed] [Google Scholar]
- 57.Hu M, Luo Q, Alitongbieke G, Chong S, Xu C, Xie L, et al. Celastrol-Induced Nur77 Interaction with TRAF2 Alleviates Inflammation by Promoting Mitochondrial Ubiquitination and Autophagy. Mol Cell. 2017;66(1):141–53 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chen Z, Zhang D, Yan S, Hu C, Huang Z, Li Z, et al. SAR study of celastrol analogs targeting Nur77-mediated inflammatory pathway. Eur J Med Chem. 2019;177:171–87. [DOI] [PubMed] [Google Scholar]
- 59.Lacey A, Hedrick E, Li X, Patel K, Doddapaneni R, Singh M, et al. Nuclear receptor 4A1 (NR4A1) as a drug target for treating rhabdomyosarcoma (RMS). Oncotarget. 2016;7(21):31257–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Qin C, Morrow D, Stewart J, Spencer K, Porter W, Smith R 3rd, et al. A new class of peroxisome proliferator-activated receptor gamma (PPARgamma) agonists that inhibit growth of breast cancer cells: 1,1-Bis(3’-indolyl)-1-(p-substituted phenyl)methanes. Molecular cancer therapeutics. 2004;3(3):247–60. [PubMed] [Google Scholar]
- 61.Chintharlapalli S, Burghardt R, Papineni S, Ramaiah S, Yoon K, Safe S. Activation of Nur77 by selected 1,1-Bis(3’-indolyl)-1-(p-substituted phenyl)methanes induces apoptosis through nuclear pathways. The Journal of biological chemistry. 2005;280(26):24903–14. [DOI] [PubMed] [Google Scholar]
- 62.Li X, Lee SO, Safe S. Structure-dependent activation of NR4A2 (Nurr1) by 1,1-bis(3’-indolyl)-1-(aromatic)methane analogs in pancreatic cancer cells. Biochemical pharmacology. 2012;83(10):1445–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lee SO, Abdelrahim M, Yoon K, Chintharlapalli S, Papineni S, Kim K, et al. Inactivation of the orphan nuclear receptor TR3/Nur77 inhibits pancreatic cancer cell and tumor growth. Cancer research. 2010;70(17):6824–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lacey A, Rodrigues-Hoffman A, Safe S. PAX3-FOXO1A Expression in Rhabdomyosarcoma Is Driven by the Targetable Nuclear Receptor NR4A1. Cancer research. 2017;77(3):732–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hedrick E, Li X, Safe S. Penfluridol Represses Integrin Expression in Breast Cancer through Induction of Reactive Oxygen Species and Downregulation of Sp Transcription Factors. Molecular cancer therapeutics. 2017;16(1):205–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lee SO, Andey T, Jin UH, Kim K, Singh M, Safe S. The nuclear receptor TR3 regulates mTORC1 signaling in lung cancer cells expressing wild-type p53. Oncogene. 2012;31(27):3265–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hedrick E, Lee SO, Doddapaneni R, Singh M, Safe S. Nuclear receptor 4A1 (NR4A1) as a drug target for breast cancer chemotherapy. Endocrine-related cancer. 2015. [DOI] [PubMed] [Google Scholar]
- 68.Mohankumar K, Li X, Sridharan S, Karki K, Safe S. Nuclear receptor 4A1 (NR4A1) antagonists induce ROS-dependent inhibition of mTOR signaling in endometrial cancer. Gynecol Oncol. 2019;154(1):218–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hedrick E, Lee SO, Kim G, Abdelrahim M, Jin UH, Safe S, et al. Nuclear receptor 4A1 (NR4A1) as a drug target for renal cell adenocarcinoma. PloS one. 2015;10(6):e0128308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lee SO, Li X, Hedrick E, Jin UH, Tjalkens RB, Backos DS, et al. Diindolylmethane analogs bind NR4A1 and are NR4A1 antagonists in colon cancer cells. Molecular endocrinology. 2014;28(10):1729–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.De Miranda BR, Miller JA, Hansen RJ, Lunghofer PJ, Safe S, Gustafson DL, et al. Neuroprotective efficacy and pharmacokinetic behavior of novel anti-inflammatory para-phenyl substituted diindolylmethanes in a mouse model of Parkinson’s disease. The Journal of pharmacology and experimental therapeutics. 2013;345(1):125–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hedrick E, Li X, Cheng Y, Lacey A, Mohankumar K, Zarei M, et al. Potent inhibition of breast cancer by bis-indole-derived nuclear receptor 4A1 (NR4A1) antagonists. Breast cancer research and treatment. 2019;177(1):29–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sanchez M, Xia Z, Rico-Bautista E, Cao X, Cuddy M, Castro DJ, et al. Oxidized analogs of Di(1H-indol-3-yl)methyl-4-substituted benzenes are NR4A1-dependent UPR inducers with potent and safe anti-cancer activity. Oncotarget. 2018;9(38):25057–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chen X, Cao X, Tu X, Alitongbieke G, Xia Z, Li X, et al. BI1071, a Novel Nur77 Modulator, Induces Apoptosis of Cancer Cells by Activating the Nur77-Bcl-2 Apoptotic Pathway. Molecular cancer therapeutics. 2019;18(5):886–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wilson TE, Fahrner TJ, Johnston M, Milbrandt J. Identification of the DNA binding site for NGFI-B by genetic selection in yeast. Science. 1991;252(5010):1296–300. [DOI] [PubMed] [Google Scholar]
- 76.Maira M, Martens C, Philips A, Drouin J. Heterodimerization between members of the Nur subfamily of orphan nuclear receptors as a novel mechanism for gene activation. Molecular and cellular biology. 1999;19(11):7549–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Philips A, Lesage S, Gingras R, Maira MH, Gauthier Y, Hugo P, et al. Novel dimeric Nur77 signaling mechanism in endocrine and lymphoid cells. Molecular and cellular biology. 1997;17(10):5946–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zetterstrom RH, Solomin L, Mitsiadis T, Olson L, Perlmann T. Retinoid X receptor heterodimerization and developmental expression distinguish the orphan nuclear receptors NGFI-B, Nurr1, and Nor1. Molecular endocrinology. 1996;10(12):1656–66. [DOI] [PubMed] [Google Scholar]
- 79.Jiang L, Dai S, Li J, Liang X, Qu L, Chen X, et al. Structural basis of binding of homodimers of the nuclear receptor NR4A2 to selective Nur-responsive DNA elements. The Journal of biological chemistry. 2019;294(51):19795–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Safe S, Kim K. Non-classical genomic estrogen receptor (ER)/specificity protein and ER/activating protein-1 signaling pathways. Journal of molecular endocrinology. 2008;41(5):263–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Karki K, Wright GA, Mohankumar K, Jin UH, Zhang XH, Safe S. A Bis-Indole-Derived NR4A1 Antagonist Induces PD-L1 Degradation and Enhances Antitumor Immunity. Cancer research. 2020;80(5):1011–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Shrestha R, Mohankumar K, Jin UH, Martin GG, Safe S. The Histone Methyltransferase Gene G9a Is Regulated by Nuclear Receptor 4a1 (Nr4a1) in Alveolar Rhabdomyosarcoma Cells. Molecular cancer therapeutics. 2020; 20, 612–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Shrestha R, Mohankumar K, Safe S. Bis-indole derived nuclear receptor 4A1 (NR4A1) antagonists inhibit TGFbeta-induced invasion of embryonal rhabdomyosarcoma cells. Am J Cancer Res. 2020;10(8):2495–509. [PMC free article] [PubMed] [Google Scholar]
- 84.Zhang XK. Targeting Nur77 translocation. Expert opinion on therapeutic targets. 2007;11(1):69–79. [DOI] [PubMed] [Google Scholar]
- 85.Vinayavekhin N, Saghatelian A. Discovery of a protein-metabolite interaction between unsaturated fatty acids and the nuclear receptor Nur77 using a metabolomics approach. J Am Chem Soc. 2011;133(43):17168–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lakshmi SP, Reddy AT, Banno A, Reddy RC. Molecular, chemical, and structural characterization of prostaglandin A2 as a novel agonist for Nur77. The Biochemical journal. 2019;476(19):2757–67. [DOI] [PubMed] [Google Scholar]
- 87.Kagaya S, Ohkura N, Tsukada T, Miyagawa M, Sugita Y, Tsujimoto G, et al. Prostaglandin A2 acts as a transactivator for NOR1 (NR4A3) within the nuclear receptor superfamily. Biol Pharm Bull. 2005;28(9):1603–7. [DOI] [PubMed] [Google Scholar]
- 88.Zhang L, Liu W, Wang Q, Li Q, Wang H, Wang J, et al. New Drug Candidate Targeting the 4A1 Orphan Nuclear Receptor for Medullary Thyroid Cancer Therapy. Molecules. 2018;23(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Munoz-Tello P, Lin H, Khan P, de Vera IMS, Kamenecka TM, Kojetin DJ. Assessment of NR4A Ligands That Directly Bind and Modulate the Orphan Nuclear Receptor Nurr1. J Med Chem. 2020;63(24):15639–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Boudreaux SP, Duren RP, Call SG, Nguyen L, Freire PR, Narayanan P, et al. Drug targeting of NR4A nuclear receptors for treatment of acute myeloid leukemia. Leukemia. 2019;33(1):52–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.De Miranda BR, Popichak KA, Hammond SL, Jorgensen BA, Phillips AT, Safe S, et al. The Nurr1 Activator 1,1-Bis(3’-Indolyl)-1-(p-Chlorophenyl)Methane Blocks Inflammatory Gene Expression in BV-2 Microglial Cells by Inhibiting Nuclear Factor kappaB. Molecular pharmacology. 2015;87(6):1021–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.De Miranda BR, Popichak KA, Hammond SL, Miller JA, Safe S, Tjalkens RB. Novel para-phenyl substituted diindolylmethanes protect against MPTP neurotoxicity and suppress glial activation in a mouse model of Parkinson’s disease. Toxicological sciences : an official journal of the Society of Toxicology. 2015;143(2):360–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chatterjee S, Walsh EN, Yan AL, Giese KP, Safe S, Abel T. Pharmacological activation of Nr4a rescues age-associated memory decline. Neurobiol Aging. 2020;85:140–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bridi MS, Hawk JD, Chatterjee S, Safe S, Abel T. Pharmacological Activators of the NR4A Nuclear Receptors Enhance LTP in a CREB/CBP-Dependent Manner. Neuropsychopharmacology. 2017;42(6):1243–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mohankumar K, Li X, Sung N, Cho YJ, Han SJ, Safe S. Bis-Indole-Derived Nuclear Receptor 4A1 (NR4A1, Nur77) Ligands as Inhibitors of Endometriosis. Endocrinology. 2020;161(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mohankumar K, Lee J, Wu CS, Sun Y, Safe S. Bis-Indole-Derived NR4A1 Ligands and Metformin Exhibit NR4A1-Dependent Glucose Metabolism and Uptake in C2C12 Cells. Endocrinology. 2018;159(5):1950–63. [DOI] [PMC free article] [PubMed] [Google Scholar]


