Abstract
Viruses are suspected but usually unproven triggering factors in autoimmunity. One favored mechanism to explain the role of viruses in the genesis of autoimmunity is molecular mimicry. An immunoinflammatory blinding lesion called herpetic stromal keratitis (HSK) that follows ocular infection with herpes simplex virus (HSV) is suggested to result from a CD4+ T-cell response to a UL6 peptide of HSV that cross-reacts with a corneal autopeptide shared with the immunoglobulin G2ab (IgG2ab) isotype. The present report reevaluates the molecular mimicry hypothesis to explain HSK pathogenesis. Our results failed to reveal cross-reactivity between the UL6 and IgG2ab peptides or between peptide reactive T cells and HSV antigens. More importantly, animals infected with HSV failed to develop responses that reacted with either peptide, and infection with a recombinant vaccinia UL6 vector failed to cause HSK, in spite of generating UL6 reactivity. Other lines of evidence also failed to support the molecular mimicry hypothesis, such as the failure to affect HSK severity upon tolerization of susceptible BALB/c and B-cell-deficient mice with IgG2ab or UL6 peptides. An additional study system revealed that HSK could be induced in mouse strains, such as the OT2 × RAG1−/− mice (T cell receptor transgenic recognizing OVA323–339) that were unable to produce CD4+ T-cell responses to any detectable HSV antigens. Our results cast doubt on the molecular mimicry hypothesis as an explanation for the pathogenesis of HSK and indicate that if autoimmunity is involved its likely proceeds via a bystander activation mechanism.
That autoimmune diseases result from virus infection is an attractive hypothesis, but it has yet to be proven, at least for any human autoimmune syndrome (39). However, several animal models clearly link viruses and autoimmunity (11, 19, 25, 38, 40), although the mechanisms by which viruses trigger autoreactivity remain uncertain. The explanations for this include molecular mimicry and bystander activation (12, 39). The latter represents a complex which could include the release of normally sequestered antigens from damaged cells that now become immunogenic, alteration of host protein structure (27), and the subversion of host cells, causing proinflammatory mediator production or the synthesis of abnormal products such as autoantibodies (39). The more simple and perhaps most appealing idea to explain the genesis of virus-induced autoimmunity is molecular mimicry (19). This suggests that causative viruses express epitopes that cross-react with a host protein and that the initial immune response to viruses carries over to include anti-host reactivity (40). Proving unequivocally the molecular mimicry hypothesis in any model has been difficult (39).
One model advocated to support the molecular mimicry hypothesis is a blinding inflammatory reaction in the cornea set off by infection with herpes simplex virus (HSV). This lesion, termed herpetic stromal keratitis (HSK), is orchestrated by CD4+ T cells that are suggested to recognize autoantigens in the cornea (31, 36). One such autoantigen may be a 16 amino acid peptide shared by the immunoglobulin G2ab (IgG2ab) isotype (2). A peptide of almost identical amino acid sequence is found in the UL6 protein of HSV, and this has been advocated as the molecular mimic which elicits the HSK syndrome (41). Supporting the corneal autoantigen hypothesis is the observation that mice such as C57BL/6 (B6) and CB.17, which express the Ig2ab isotype and so are immunologically tolerant to the autoantigen, are highly resistant to HSK development (2, 21, 35). In addition, viral mutants that lack the mimicking peptide fail to induce HSK in susceptible mouse strains and lesions can be adoptively transferred to virus-infected nude mice with UL6 and IgG2a immune T cells (2, 41). Accordingly, the case for HSK representing an autoimmune reaction set off by a virus that acts as a molecular mimic appears persuasive.
In the present report, we readdress the role of autoimmunity and molecular mimicry between IgG2a and UL6 peptides in HSK pathogenesis by using a mouse model involving different mice strains on the same genetic background as used in the previous reports and a strain of HSV type 1 (HSV-1) (strain RE) usually used for the induction of HSK lesions. Mice strains used in the study included susceptible BALB/c (B/c) mice, resistant B6 mice, and B6 μ-chain knockout (Bk/o) mice. The latter do not produce immunoglobulin (Ig) and therefore would lack tolerance to the corneal autoantigen shared with the IgG2ab isotype. Several observations failed to support the molecular mimicry hypothesis. First, analysis of in vivo delayed-type hypersensitivity (DTH) reactions and in vitro T-cell responses of draining lymph node and splenocytes revealed none of the expected cross-reactivities. Accordingly, mice ocularly infected with HSV failed to generate demonstrable in vivo and in vitro responses to either the UL6 or IgG2ab peptides. Second, although infection with recombinant vaccinia virus expressing the UL6 protein induced T cells that reacted with the UL6 peptide, neither cross-reactivity with the IgG2ab peptide nor cross-reactivity with HSV could be demonstrated. Moreover, mice immunized with either UL6 or IgG2ab peptides elicited T cells that reacted only with homologous peptides. Third, attempts to change the susceptibility of B/c or Bk/o mice by tolerizing them to the UL6 or IgG2ab peptides prior to HSV infection failed to significantly affect their HSK susceptibility status. Fourth, adoptive transfer of HSV immune CD4+ T cells to SCID mice later infected with replication-defective mutants, which were either UL6 positive or negative, both failed to yield HSK. Finally, we demonstrated that OT2 × RAG1−/− (OT2xRAG1−/−) mice, which are T cell transgenic to recognize an OVA peptide, still develop HSK; yet such mice failed to recognize any HSV antigens. Although our results cannot exclude autoreactivity as being involved in the pathogenesis of HSK, taken together, they provide no support that the UL6 protein or any other protein of HSV provides a molecular mimic which induced molecular mimicry. Rather, our results indicate that HSV represents an immunoinflammatory reaction which, if in part autoimmune, involves a bystander activation mechanism.
MATERIALS AND METHODS
Mice.
B/c mice and B6 mice (4 to 6 weeks old) were purchased from Harlan Sprague-Dawley (Indianapolis, Ind.). B-cell-deficient mice (Bk/o, H-2b-background), made by targeted disruption of the membrane exon of the Ig μ-chain gene (13) were kindly provided by H. W. Virgin (Washington University School of Medicine, St. Louis, Mo.). OT2xRAG1−/− mice were kindly provided by J. Kapp (Emory University, Atlanta, Ga.) (22). BALB/c SCID mice (Taconic Farms, Germantown, N.Y.) were bred in the specific-pathogen-free facility. All manipulations involving the SCID and RAG1−/− mice were performed in a laminar flow hood. To prevent bacterial superinfections, all SCID and RAG1−/− mice received prophylactic treatment of a Sulfatrim pediatric suspension (Barre-National, Baltimore, Md.) at the rate of 5 ml per 200 ml of drinking water. All experimental procedures were in complete agreement with the Association for Research in Vision and Ophthalmology resolution on the use of animals in research.
Virus and reagents.
The HSV-1 RE and HSV-1 KOS strains were propagated and titrated on monolayers of Vero cells (ATCC CCL81) by using standard protocols (30). All virus stocks were separated into aliquots and stored at −80°C. Vaccinia virus expressing UL6 was kindly provided by A. H. Patel (Glasgow University, Glasgow, United Kingdom). The ICP8−/− HSV-1 KOS mutant was kindly provided by D. Knipe (Harvard University, Boston, Mass.), and the ICP 4−/− HSV-1 KOS mutant was from J. C. Glorioso (University of Pittsburgh, Pittsburgh, Pa.). UL6 (299–314), IgG2a (292–308), and hemagglutinin (HA) peptides were synthesized by Genemed Synthesis, Inc., South San Francisco, Calif.
Corneal HSV infections and clinical observations.
Corneal infections of all mice groups were conducted under deep anesthesia induced by the inhalant anesthetic methoxyfurane (Metofane; Pittman Moore, Mondelein, Ill.). Mice were scarified on their corneas with a 27-gauge needle, and a 4-μl drop containing the required viral dose was applied to the eye and gently massaged with the eyelids. The eyes were examined on different days postinfection with a slit lamp biomicroscope (Kowa Co., Nagoya, Japan), and the clinical severity of the keratitis of individually scored mice was recorded. The scoring system was as follows: +1, mild corneal haze; +2, moderate corneal opacity or scarring; +3, severe corneal opacity, but iris visible; +4, opaque cornea, iris not visible; and +5, necrotizing stromal keratitis.
Virus recovery and titrations.
At various time points postinfection, swabs of the corneal surface were obtained. The swabs were put into sterile tubes containing 500 μl of Dulbecco modified Eagle medium (DMEM) with 100 IU of penicillin and 100 μg of streptomycin (Life Technologies, Grand Island, N.Y.) per ml and then stored at −80°C. For the detection and quantification of HSV in the swabs, the samples were thawed and vortexed. Duplicate 200-μl aliquots of each sample of thawed swab media were plated on Vero cells grown to confluence in 24-well plates at 37°C in 5% CO2 for 1 h and 30 min. Medium was aspirated, and 1 ml of 2× DMEM containing 1% low-melting-point agarose was added to each well. Cultures were observed daily for the development of typical cytopathic effect. The titers were calculated as the PFU according to the standard protocol (30).
Analysis of viral expression of UL6 protein.
HEp-2 cells were infected with the indicated viruses at a multiplicity of infection (MOI) of 20.0 or were mock infected. At 12 h postinfection, total cell extracts were prepared, and proteins were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis in a 9% DATD (N, N′-diallyltartardiamide)-cross-linked sodium dodecyl sulfate-gel, transferred by electroblotting, and processed for immunoblotting using rabbit polyclonal anti-UL6 antibody (1:500 dilution) kindly provided by Joel Baines (33) and secondary antibody, followed by visualization as described previously (29).
DTH.
At day 10 post-virus infection on scarified corneas of mice or with peptide immunization in complete Freund adjuvant (CFA), test antigens in 20 μl of phosphate-buffered saline (PBS) were injected in the left ear pinna of anesthetized mice, and PBS diluent (peptide-specific DTH reaction) or Vero cell extract (HSV-specific DTH reaction) was injected in the right ear, respectively. Ear thickness was measured 48 h postinjection with a screw gauge meter (Oditest; H. C. Kroeplin GhBH, Schluechtern, Germany) as described elsewhere (14). The test antigens used were 20 μl of UV-inactivated HSV-1 KOS (105 PFU prior to UV inactivation) or 10 μg of peptides per ml. The mean increase between the thickness of the left and right ear was calculated and is expressed in 10−2 mm.
Lymphoproliferation assay.
To test antigen-specific T-cell responses, individual spleens and cervical and mandibular lymph nodes were used as responders for lymphoproliferation assays stimulated with enriched dendritic-cell populations obtained by the method of Nair et al. (18). Briefly, these responders were restimulated in vitro with irradiated syngeneic dendritic cells infected with UV HSV-1 KOS (MOI = 5.0) or pulsed with peptides at various concentrations or with irradiated naive dendritic cells and then incubated for 4 days at 37°C for peptide stimulation or for 5 days for HSV-1 stimulation. At 18 h before harvesting, [3H]thymidine (1.0 μCi/well) was added to all culture wells, and the plates were read using a β-scintillation counter (Trace 96; Inotech, Lansing, Mich.). The results were expressed as mean counts per minute (cpm) ± the standard deviation.
Quantification of IFN-γ production by ELISA.
To assay for gamma interferon (IFN-γ) production, 2 × 106 splenocytes per ml were stimulated in vitro with 5 μg of peptide or UV-inactivated virus-pulsed irradiated syngeneic antigen-presenting cells per ml. Supernatants were collected at 48 h for concanavalin A (ConA) stimulation (5 μg/ml), at 72 h for peptide stimulation (5 μg/ml), or at 96 h for UV-inactivated HSV-1 (MOI = 5.0) or vaccinia virus (MOI = 5.0) stimulation and then screened for the presence of IFN-γ by enzyme-linked immunosorbent assay (ELISA) as described previously (5).
Histopathology and immunohistochemical staining.
Eyes were enucleated and fixed in 10% buffered neutral formalin and embedded in paraffin as described previously (35). Sections (5 μm thick) were cut and stained with hematoxylin and eosin. For immunohistochemistry, eyes were frozen in optimum cutting temperature (OCT) compound (Miles, Elkart, Ind.). The sections (5 μm thick) were cut, air dried, and fixed in cold acetone for 5 min. The sections were then blocked with heat-inactivated rabbit serum (Sigma) and stained for the presence of HSV antigens by the use of rabbit anti-HSV antiserum (Dako Corp., Carpinteria, Calif.), which was followed by treatment with biotinylated anti-rabbit antibody (1/20 dilution; Biogenex, San Ramon, Calif.). Alternatively, sections were stained with biotinylated anti-CD4 or anti-KJ1-26.1 antibody (biotinylated anti-OVA-TCR, a kind gift from Jerold Woodward, University of Kentucky, Lexington, Ky.). Sections were treated with horseradish peroxidase-conjugated streptavidin (1/1,000 dilution; Jackson Immunoresearch Laboratories, Inc.) and 3,3′-diaminobenzidine substrate (Biogenex, San Ramon, Calif.) and then counterstained with hematoxylin. For OVA-TCR (KJ1-26.1) staining, sections were pretreated with a tyramide signal amplification kit (TSA Indirect; Dupont NEN, Boston, Mass.) before treatment with diaminobenzidine.
Flow cytometry.
Cervical lymph node cells were analyzed for the cell surface expression of activation markers. Viable cells were blocked with heat-inactivated fetal bovine serum and washed with flow cytometry buffer (1× PBS with 1% bovine serum albumin and 0.05% sodium azide). Cells were double stained with anti-CD4-FITC (Pharmingen) and anti-CD44-PE (Pharmingen) or with anti-CD62L-PE (Pharmingen) and anti-CD45RB-RPE (Calbiochem). Events were recorded with FACSCALIBUR (Becton Dickson, San Jose, Calif.) and analyzed using Cellquest 3.0 version (Becton Dickson).
Statistical analysis.
Wherever specified, the data obtained were analyzed for statistical significance by using the Student's t test.
RESULTS
Analyses of cross-reactivity of Ig peptide and UL6 peptide of HSV in B/c and B6 mice.
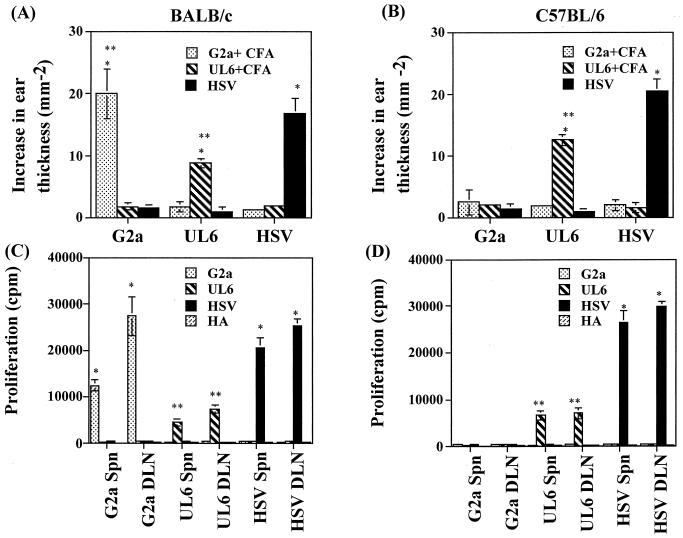
Previous reports indicate that HSK in a susceptible mouse strain represents an immune response against a peptide derived from the UL6 protein of HSV that cross-reacts with a corneal autoantigen that forms part of the IgG2ab (amino acids 292 to 308) Ig isotype encoded by the IgHb locus (2). This latter peptide is referred to as G2a. If the UL6 peptide acts as a molecular mimic of G2a and is indeed involved in the pathogenesis, then HSV ocular infection would be expected to induce T-cell responses in recipient animals that react with both the UL6 and the G2a peptides. To test these ideas, groups of susceptible B/c and resistant B6 mice were infected on scarified corneas with HSV or were immunized systemically with either the UL6 or G2a peptides emulsified in CFA. The resultant pattern of reactivity was measured. By 10 days postinfection HSV-infected B/c mice showed signs of HSK which gradually increased in severity over the next 2 weeks (data not shown). In contrast, under the same infection conditions B6 mice showed none or extremely mild signs of HSK (data not shown). However, with regard to antigen-specific immune reactivity, several points are recorded in Fig. 1 and Table 1. First, HSV infection of both mouse strains resulted in T-cell responses reactive to virus detectable in vivo by the DTH reaction (Fig. 1A and B) or in vitro in lymphoid tissues by specific lymphoproliferation (Fig. 1C and D) or cytokine release (Table 1). However, neither B/c nor B6 mice showed detectable cross-reactivity against either the UL6 or G2a peptides. It was evident, nevertheless, that both the UL6 and G2a peptides were immunogenic in B/c mice, but the responses detected were neither mutually cross-reactive nor reactive in vitro with HSV-1. Interestingly, the UL6 peptide was not as immunogenic as the G2a peptide in the B/c mice strain. Furthermore, as expected, the G2a peptide was not immunogenic in the B6 strain in which it is a self-peptide.
FIG. 1.
Analyses of cross-reactivity between G2a peptide, UL6 peptide, and HSV in lymphoid cells following peptide immunization or HSV-1 RE ocular infection. Groups of B/c and B6 mice were immunized subcutaneously with 100 μg of peptide in CFA at the base of neck or were infected with 5 × 105 PFU or 107 PFU of HSV-1 RE, respectively, on scarified corneas. (A and B) At day 15 postinfection, a DTH reaction was elicited in the ear pinnae of mice to 10 μg of peptides per ml or UV-inactivated HSV-1 (105 PFU) and 1× PBS or Vero extract in a 20-μl volume in the right and left ear pinnae, respectively. The increase in the ear thickness was measured after 48 h as described in Materials and Methods. The data are expressed as the difference in ear thickness ± the standard deviation (in mm−2) and represents the mean of two experiments, each including six to seven mice. Statistically significant differences between groups (∗∗, P < 0.001) and within groups (∗, P < 0.05) are indicated. (C and D) At days 9 to 15 post-peptide immunization and at days 15 to 18 post-HSV-1 ocular infection, mice (n = 4) were sacrificed, and cervical and submandibular DLN or spleen cells were used as responders in a lymphoproliferation assay as described in Materials and Methods. Responders were stimulated with irradiated syngeneic splenocytes pulsed with a range of peptide concentrations (10, 1.0, and 0.1 μg/ml), UV-irradiated virus (MOI = 5.0), or no stimulation. Data are represented only for peptide stimulations with a 10-μg/ml concentration. Polyclonal stimulator, ConA (2 μg/ml), was used as a positive control (data not shown). Results are the mean of three independent experiments. The experiment was repeated at least six times with similar results. Statistically significant differences between HSV-stimulated responders and peptide-stimulated responders (∗, P < 0.0001; ∗∗, P < 0.05) are indicated.
TABLE 1.
IFN-γ cytokine responses in peptide-immunized and HSV-infected micea
| Mouse type and treatment | Tissue | IFN-γ cytokine concn (mean ± SD [ng/ml])
|
|||||
|---|---|---|---|---|---|---|---|
| Unstimulated | ConA | G2a | UL6 | UV-HSV | HA | ||
| B/c | |||||||
| G2a plus CFA | Spleen | <0.3 | 32.8 ± 1.6 | 3.9 ± 0.8∗ | <0.3 | <0.3 | <0.3 |
| DLN | <0.3 | 38.1 ± 1.2 | 4.2 ± 1.2∗ | <0.3 | <0.3 | <0.3 | |
| UL6 plus CFA | Spleen | <0.3 | 36.4 ± 0.8 | <0.3 | 2.3 ± 0.5∗ | <0.3 | <0.3 |
| DLN | <0.3 | 35.8 ± 1.5 | <0.3 | 3.2 ± 0.6∗ | <0.3 | <0.3 | |
| HSV | Spleen | <0.3 | 32.8 ± 2.8 | <0.3 | <0.3 | 11.6 ± 1.1∗ | <0.3 |
| DLN | <0.3 | 29.8 ± 5.9 | <0.3 | <0.3 | 14.9 ± 2.3∗ | <0.3 | |
| B6 | |||||||
| G2a plus CFA | Spleen | <0.3 | <0.3 | 4.8 ± 0.7∗ | <0.3 | <0.3 | <0.3 |
| DLN | <0.3 | <0.3 | 5.6 ± 1.2∗ | <0.3 | <0.3 | <0.3 | |
| UL6 plus CFA | Spleen | <0.3 | 26.5 ± 1.4 | <0.3 | 2.5 ± 0.9∗ | <0.3 | <0.3 |
| DLN | <0.3 | 25.0 ± 1.5 | <0.3 | 3.2 ± 0.4∗ | <0.3 | <0.3 | |
| HSV | Spleen | <0.3 | 32.4 ± 1.8 | <0.3 | <0.3 | 8.8 ± 0.5∗ | <0.3 |
| DLN | <0.3 | 35.8 ± 4.2 | <0.3 | <0.3 | 10.4 ± 0.6∗ | <0.3 | |
Mice (n = 6) were immunized subcutaneously at the base of neck with 100 μg of peptide in CFA or else infected with HSV-1 RE on scarified corneas (B/c, 5 × 105 PFU; B6, 107 PFU). At days 9 to 15 postimmunization and days 15 to 18 post-HSV infection, splenocytes or cervival and submandibular DLN cells (2 × 106 cells/ml) were in vitro stimulated with a 1.5 MOI of UV-irradiated HSV-1 (UV-HSV) KOS, ConA (5 μg/106 cells/ml), or peptides (5 μg/ml). Uninfected splenocytes were used as negative controls. Culture supernatants from each group were collected and analyzed for cytokine production by ELISA assay as described in Materials and Methods. The data represent the means ± the standard deviations from two experiments, each of which included three individual mice. The limit of detection of the ELISA was 0.3 ng/ml. Statistically significant differences between stimulated groups (P < 0.5) are indicated by an asterisk.
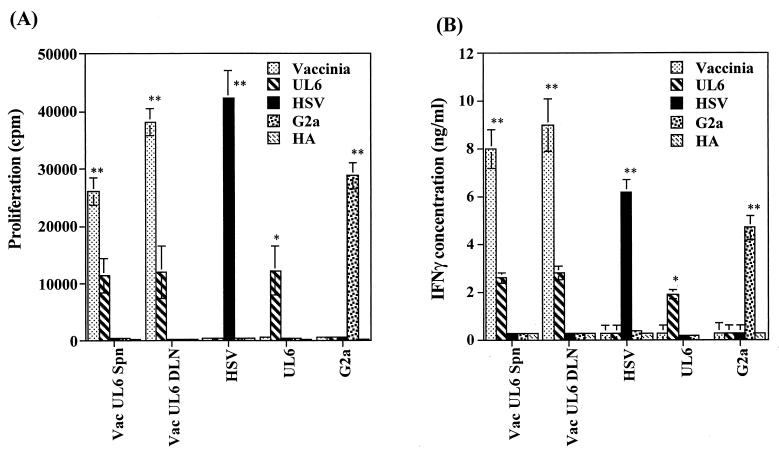
These results fail to support molecular mimicry and indicate that the UL6 protein of HSV fails to induce detectable UL6 peptide-specific responses in lymphoid tissue following ocular infection. However, to further examine a possible role of UL6 in HSK, B/c mice were ocularly infected with a recombinant vaccinia virus expressing the UL6 protein (kindly provided by A. H. Patel). This infection failed to cause lesions typical of HSK (data not shown), but analysis of DLN at 14 days postinfection revealed UL6 peptide-specific T cells detectable by proliferation (Fig. 2A) or IFN-γ cytokine release assay (Fig. 2B). Nevertheless, the same population failed to react with the G2a peptide or to stimulation with HSV antigen. Taken together, the data indicate that the UL6 protein in HSV infections is not a strong immunogen. Immune reactivity against UL6, however, can be detected only under ideal circumstances, such as when administered with adjuvant or in the form of vaccinia virus expressing UL6 protein.
FIG. 2.
Recombinant vaccinia virus expressing UL6 protein fails to induce reactivity to HSV or G2a peptide. Groups of B/c mice (n = 6) were infected with 2 × 106 PFU of vaccinia virus expressing UL6 (Vac UL6) on scarified corneas. At day 14 postinfection mice were sacrificed, and pooled popliteal lymph nodes and individual splenocytes were used as responders in a lymphoproliferation assay (A) and an IFN-γ cytokine ELISA (B) as described in Materials and Methods. Responders were stimulated with irradiated syngeneic splenocytes pulsed with UV-irradiated vaccinia virus (MOI = 5.0), UV-irradiated HSV (MOI = 5.0), and the UL6, G2a, and HA peptides (10, 1.0, and 0.1 μg/ml). The data represent peptide stimulation with a 10-μg/ml concentration. The polyclonal stimulator, ConA (2 μg/ml), was used as a positive control (data not shown). The results of two independent experiments are expressed as the means ± the standard deviations. Statistically significant differences within groups (∗∗, P < 0.001) and between groups (∗, P < 0.05) are indicated.
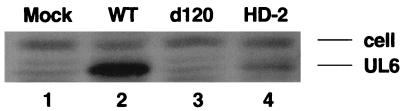
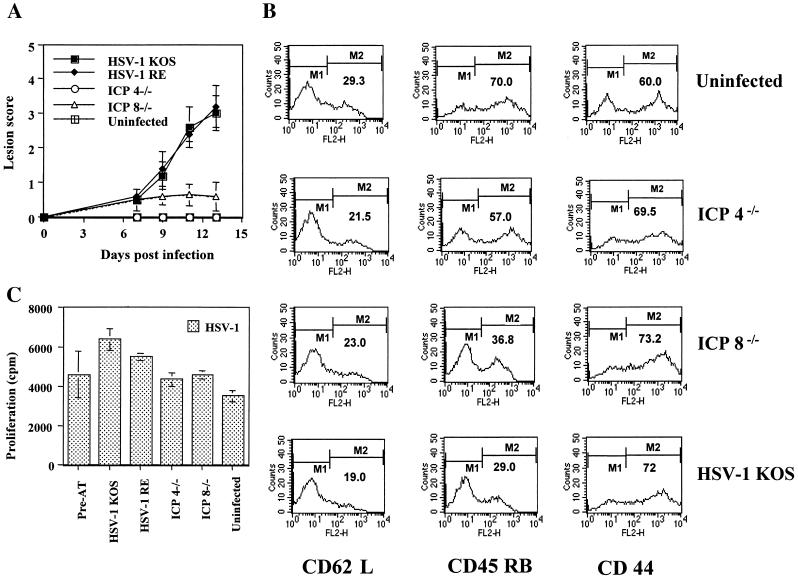
A further experiment to test the role of a peptide derived from the UL6 protein in the pathogenesis of HSK, was to analyze the susceptibility of SCID mice infected with HSV or mutant viruses and reconstituted with CD4+ T cells reactive to HSV. This reconstitution model results in HSK, provided the SCID mice are infected with wild-type HSV (16). However, when reconstituted SCID mice were repeatedly infected at days 0, 2, and 4 with either ICP4− mutant d120, which expressed little if any UL6, or an ICP8− mutant (which expressed UL6 at levels about one-third that of the wild-type virus) (Fig. 3), neither infection resulted in the expression of HSK (Fig. 4A). However, the infection procedure was assumed to be immunogenic, since the recipient mice developed lymphadenopathy, had an increased number of activated cells in the draining lymph nodes (DLN) (Fig. 4B), and showed enhanced HSV-specific lymphoproliferation responses compared to uninfected reconstituted SCID mice (Fig. 4C) in samples tested at day 11 postinfection.
FIG. 3.
Analysis of UL6 protein expression by various HSV strains. Infected cell lysates from Hep-2 cells infected with the indicated viruses were analyzed for UL6 expression by Western blotting as described in Materials and Methods. Shown in figure is the image of Western blotting. The top band provides a loading control, and the bottom band is the UL6 protein. Lanes: 1, mock infected; 2, KOS wild-type (WT) virus; 3, KOS d120 (ICP4−) virus; 4, KOS HD-2 (ICP8−) virus.
FIG. 4.
Infection with replication-defective HSV (UL6 positive or negative) fails to induce HSK in SCID mice reconstituted with HSV immune T cells. SCID mice (n = 5/group) were infected with ICP4−/−, ICP8−/−, and HSV-1 KOS (5 × 105 PFU) on scarified corneas at days 0, 2, and 4. At day 1 postinfection, SCID mice were reconstituted with 107 HSV immune splenocytes in a 400-μl volume of 1× PBS given i.v. The data represents the results from one of two independent experiments with similar results. (A) Mean clinical scores of SCID mice at days 7, 9, 11, and 12 postinfection. Mice were scored by using a slit-lamp microscope as described in Materials and Methods. (B and C) Mice were terminated at day 13 postinfection, and cervical and submandibular DLN cells were used as responders in an HSV-specific lymphoproliferation assay (C) or were stained for activation markers CD62L, CD45 RB, and CD44 by flow cytometry assay (B) as described in Materials and Methods. The percentage of cell surface expression of activation markers under marker M2 is indicated in the histograms. The data indicate the presence of activated HSV-specific CD4+ T cells in all of the wild-type and mutant virus-infected reconstituted SCID mice groups irrespective of the development of HSK lesions shown in panel A.
Role of the Igh-1b locus in affecting susceptibility to HSK.
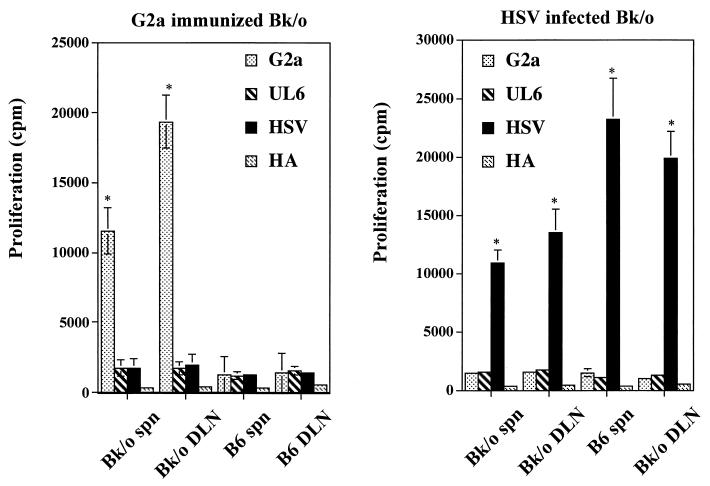
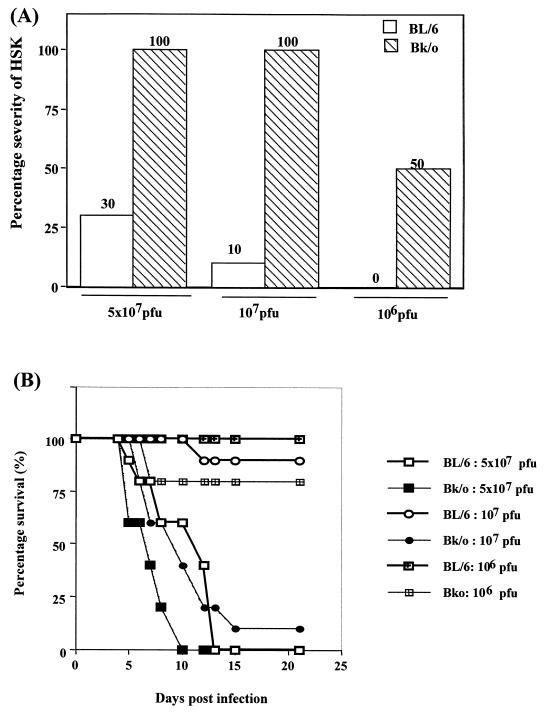
The autoimmune hypothesis explaining the pathogenesis of HSK finds support from the observation that the susceptible mouse strains develop CD4+ T cells which recognize the G2a peptide expressed in the damaged corneal stroma during HSK (2). Resistant strains such as CB17 and B6 do not recognize the G2a peptide, since in these strains the peptide is a self-component. Our data support the observation that HSK resistant B6 mice fail to respond to G2a peptide upon immunization in CFA (Fig. 5A). In contrast, mice of the B6 background that lack Ig expression because of an Ig μ-chain knockout did respond upon immunization with the G2a peptide (Fig. 5A). Furthermore, Bk/o mice were far more susceptible to ocular infection with HSV (Fig. 6). At doses of virus well tolerated by B6 mice, most Bk/o animals succumbed to viral encephalitis. At lower, nonlethal doses of HSV infection, most Bk/o mice expressed HSK, whereas the same virus dose failed to induce lesions in B6 mice. The observation of greater susceptibility combined with reactivity to the G2a peptide could appear as supportive of autoimmunity pathogenesis in HSK. However, the data in Fig. 5B cast doubt as to any role for the G2a peptide in HSK in Bk/o mice. Accordingly, following HSV infection of such mice, the animals failed to develop demonstrable reactivity in the DLN to the G2a peptide nor, in fact, to the UL6 peptide. Nevertheless, the mice did develop reactivity to HSV.
FIG. 5.
Peptide G2a immunization or HSV infection in Bk/o fails to generate cross-reactivity. Groups of Bk/o and B6 mice (n = 4) were infected with 107 PFU of HSV-1 RE on scarified corneas or were immunized with 100 μg of UL6, G2a, or HA peptides in CFA. Mice were terminated from days 9 to 15 post-peptide immunization or days 15 to 18 post-HSV infection. DLN and spleen cells were used in lymphoproliferation assays with various concentrations of G2a, UL6, and HA peptides (10, 1.0, or 0.1 μg/ml; the data shown here represent peptide stimulation with a 10-μg/ml concentration) or UV-irradiated HSV (MOI = 5.0) as described previously. The data are representative of one of three experiments with similar results. Statistically significant differences between groups are indicated (∗, P < 0.001).
FIG. 6.
Bk/o mice are highly susceptible to herpetic encephalitis and HSK. Bk/o and B6 mice (n = 10 to 12) were infected on scarified corneas with a range of infectious doses and then examined for survival and induction of HSK lesions. The data represent one of two similar experiments. (A) Lesions were scored using a slit-lamp microscope and were recorded as described in Materials and Methods. The data represent the percentage of mice developing lesion scores at day 15 postinfection of ≥3.0. (B) Mice were examined daily for signs of herpetic encephalitis. The results are expressed as the percent survival of mice per time point.
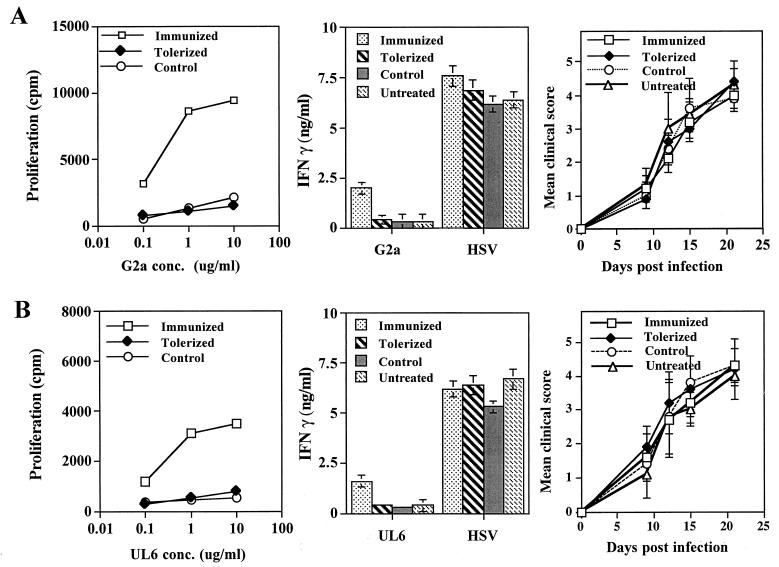
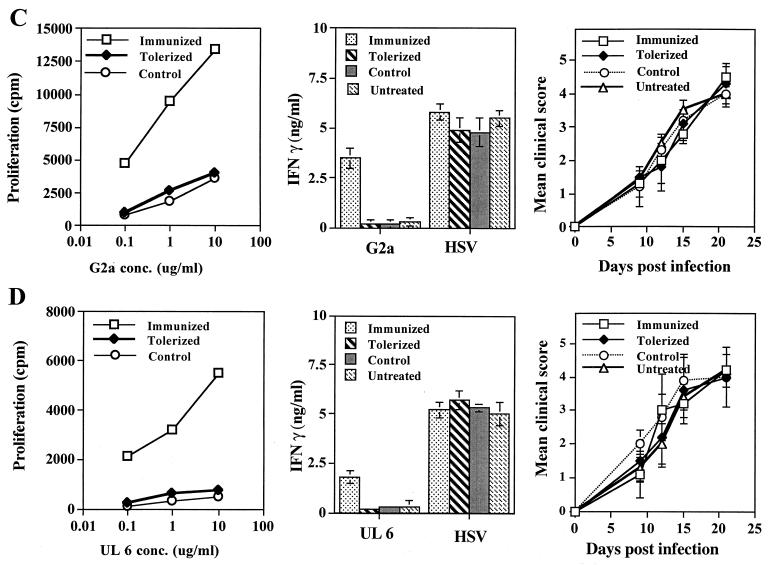
To further test the putative role of the G2a peptide as the autoantigen recognized by CD4+ T cells in HSK-susceptible mice, the effects of tolerization against the peptide on the subsequent susceptibility to ocular infection was investigated. Should the G2a peptide be a major target during HSK, tolerization was expected to diminish susceptibility to HSK, as was indicated to occur in susceptible CAL.20 mice tolerized with the G2ab protein (2). In our experiments, both B/c and Bk/o mice were tolerized by exposure to high amounts of the soluble peptide, as described by others (2, 41). Mice were judged to be tolerant based upon their subsequent response to immunization with the G2a or UL6 peptide in CFA. As shown in Fig. 7, tolerized mice had markedly diminished peptide-specific proliferation and cytokine responses compared to controls. Despite the apparent tolerization of both B/c and Bk/o mice, both failed to become more resistant to HSK compared to control animals. These data cast doubt regarding the contribution of the G2a peptide, at least in HSK-susceptible B/c and B6 Bk/o mice, to HSK pathogenesis.
FIG. 7.
Tolerization of peptides fails to induce resistance to HSK in susceptible Bk/o or B/c mice. Groups of Bk/o mice and B/c mice (n = 12) were tolerized i.v. with 50 μg of soluble UL6 or G2a peptides at days 0 and 7. At day 15 posttolerization, groups of peptide tolerized mice (n = 6) were infected with HSV-1 RE alone (tolerized) or were infected with HSV-1 RE (107 PFU for Bk/o mice and 106 PFU for B/c mice) on scarified corneas, as well as immunized with 100 μg of the same peptide in CFA (control). Groups of age- and sex-matched mice (n = 6) were immunized with 100 μg of the same peptide in CFA and then infected with HSV-1 RE (immunized) or infected with HSV-1 RE alone (untreated). Mice were examined for lesions by using a slit-lamp microscope, and the mean clinical scores at days 9, 12, 15, and 21 are shown. Mice were terminated at day 18 postinfection, and DLN cells were used in a lymphoproliferation assay to tolerogenic peptides and in an IFN-γ cytokine assay to peptides and UV-irradiated HSV (MOI = 5.0), as described previously. The data represent one of three experiments with similar results. (A) G2a-tolerized Bk/o mice (B) UL6-tolerized Bk/o mice (C) G2a-tolerized B/c mice. (D) UL6-tolerized B/c mice.
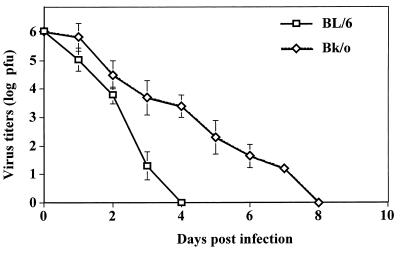
An explanation for the heightened susceptibility of Bk/o mice to HSK.
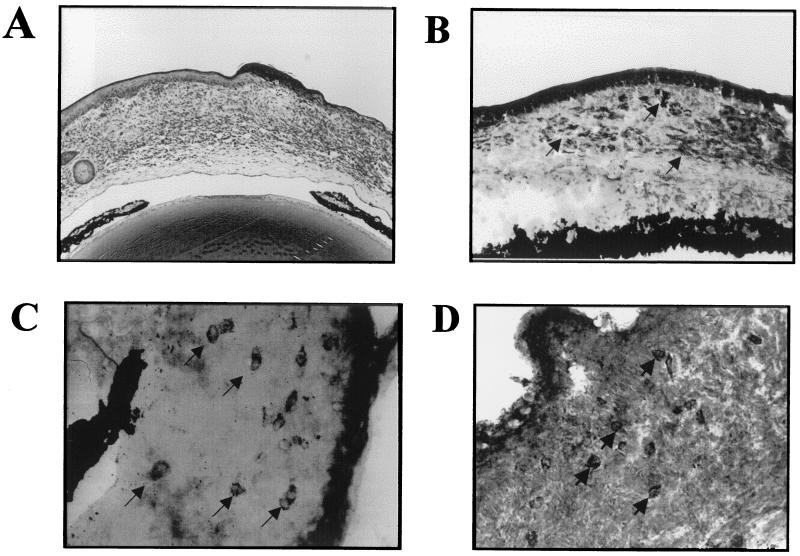
As indicated previously, Bk/o mice of the same genetic background as B6 mice, yet lacking B cells and the ability to produce antibody, were highly susceptible to ocular infection with HSV. Although viral clearance with respect to HSV infection is usually considered principally carried out by T-cell-mediated immunity (21), the inability to generate antibody may result in a more prolonged infection of the cornea. This was in fact the case, as shown in Fig. 8. The duration of detectable virus in ocular swabs was at least 4 days longer in Bk/o mice than in B6 mice. This prolonged presence of virus could bring into operation an additional mechanism of pathogenesis referred to as bystander activation (6, 7). Thus, as observed previously, T-cell-transgenic B/c mice backcrossed to SCID or RAG still develop HSK, even though they are unable to immunologically recognize HSV antigens (6, 7, 8). Support for the bystander mechanism to explain the HSK pathogenesis is shown in Fig. 8. The results show HSK following HSV infection of mice on a B6 background that were T cell transgenic, with their CD4+ T cells recognizing the OVA323–339 peptide. Such OT2 mice were backcrossed to RAG−/− and so were incapable of producing Ig and generated negative or minimal T-cell responses to antigens other that the OVA peptide (22). Upon HSV infection of scarified corneas, the animals developed typical HSK before dying of encephalitis at ca. day 13 postinfection. Animals failed to express detectable HSV-specific immune responses (Table 2) even if animals were repeatedly immunized with UV-inactivated HSV (data not shown). The presence of viral antigens in the corneal stroma of such mice evident at the site of inflammation likely provides the stimulus for bystander activation of T cells (Fig. 9B), resulting in HSK (Fig. 9A, C, and D). Since the OT2xRAG1−/− mice failed to develop detectable immune responses to HSV and yet still developed HSK, as was also observed in other strain combinations (7, 8), these data fail to support a role for any HSV protein as providing a molecular mimic of a corneal autoantigen involved in HSK.
FIG. 8.
Persistence of virus for longer durations in the absence of Ig in Bk/o mice. Groups of Bk/o and B6 mice (n = 6) were infected with 107 PFU on scarified corneas. Ocular swabs were taken on days 0 to 10 postinfection and tested for the presence of virus by the standard plaque assay described in Materials and Methods. The data are represented as the means ± the standard deviations.
TABLE 2.
Absence of reactivity to HSV following ocular infection of OT2xRAG1−/− mice
| Mouse typea | Mean clinical score (day 10) ± SD | Lymphoproliferation (mean cpm ± SD)b
|
Mean IFN-γ concn (ng/ml)c ± SD with:
|
||||
|---|---|---|---|---|---|---|---|
| Responders plus uninfected stimulators | Responders plus HSV stimulators | Responders plus OVA stimulators | UV-HSV | OVA | ConA | ||
| OT2xRAG1−/− | 2.4 ± 0.5 | 116 ± 90 | 110 ± 48 | 19,680 ± 1,450 | <1 | 6.8 ± 1.3 | 14.8 ± 2.0 |
| B6 | 0 | 270 ± 120 | 20,590 ± 1,120 | 280 ± 80 | 4.9 ± 0.5 | <1 | 16.5 ± 1.5 |
| C57BL/6 SCID | 2.9 ± 0.8 | 210 ± 110 | 30,897 ± 1,148 | 180 ± 50 | 8.8 ± 0.6 | <1 | 18.3 ± 1.4 |
Mice (n = 6) were infected with 2 × 106 PFU of HSV-1 RE on scarified corneas and then scored for clinical lesions using the slit-lamp microscope as described previously. The data are represented as the mean clinical scores ± the standard deviations. Ocularly infected C57BL/6 SCID mice reconstituted with B6 HSV immune splenocytes were used as positive control for inducing HSK.
Mice (n = 6) were terminated at day 10 postinfection, and spleen and lymph node cells were used in a lymphoproliferation assay as described previously. The data are represented as the means cpm incorporated ± the standard deviations.
Splenocytes (2 × 106 cells/ml) from mice were restimulated in vitro with UV-irradiated HSV (UV-HSV; MOI = 5.0), OVA (10 μg/ml), or ConA (2 μg/ml), and the supernatant was assayed for IFN-γ by ELISA.
FIG. 9.
Viral antigen and CD4+ KJ+ T cells in the corneal stroma of OT2xRAG1−/− HSV ocularly infected mice. OT2xRAG1−/− mice were infected with 2 × 106 PFU virus on scarified corneas. At days 10 to 13 postinfection, mice were sacrificed, and the eyes were snap-frozen in OCT compound. (A) Histopathology of infiltrating cells in the corneal stroma. (B) Immunohistochemistry for viral antigens in the corneal stroma (magnification, × 200). (C and D) Immunohistochemistry for CD4+ cells (magnification, × 400) (C) and for OVA323–339 TCR clonotypic antibody KJ1-26.1+ cells (magnification, ×400) (D).
DISCUSSION
Viruses are suspected as triggering agents in several autoimmune lesions with several mechanisms likely involved (39). A favored hypothesis is that viruses contain epitopes that cross-react with host proteins and that the immunity induced reacts both to virus and to self. This molecular mimicry hypothesis arouses enthusiasm (19), but it has been difficult to prove, especially in natural of autoimmune diseases. One animal disease model that supports molecular mimicry is HSK, a blinding immunoinflammatory reaction of the cornea caused by HSV infection (31, 36). Molecular mimicry in HSK is suggested to occur between the HSV-encoded protein UL6 and a corneal autoantigen that also forms part of the IgG2ab isotype of Ig (2, 41). The present report, however, raises doubt that molecular mimicry, at least with regard to the UL6 protein, explains the pathogenesis of HSK. Accordingly, if a peptide derived from an HSV protein was principally involved in driving an inflammatory reaction, either virus induced or autoreactive, it would likely be strongly immunogenic and induce a readily detectable immune response in infected animals. In our studies, we failed to detect UL6 peptide-specific T-cell responses in either the DLN or splenocytes of HSV-infected animals. Animals could process and respond to the UL6 peptide, however, since animals ocularly infected with a recombinant vaccinia virus vector that expressed the UL6 protein generated UL6 peptide-specific T cells. Also, contrary to the molecular mimicry idea, we were unable to detect cross-reactivity between the UL6 and G2a peptides when DLN cells from peptide-immunized mice were analyzed for lymphoproliferation or cytokine-producing responses in vitro. Additionally, tolerization of susceptible recipients to either UL6 or G2a peptides had no demonstrable effect on the nature of their HSK lesions, and when SCID mice, reconstituted with HSV immune CD4+ T cells, were infected with UL6+ or UL6− HSV mutants, neither type of infection resulted in HSK. Finally, HSK could still be induced in OT2xRAG1−/− mice that were unable to mount detectable immune responses against HSV antigens. Taken together, our results fail to support a role for molecular mimicry involving HSV proteins, and particularly UL6, in the pathogenesis of HSK. Although we cannot exclude autoimmune events as contributing to lesion expression, these would seem to involve mechanisms such as bystander activation rather than molecular mimicry.
Although it is likely that infectious agents are involved in the causation and expression of autoimmune diseases, it has been difficult to verify this notion, especially with human autoimmune diseases (39). The case for infectious agents as causes of some animal autoimmune diseases is stronger particularly for inflammatory demyelinating disorders. For example, both Theiler's virus and murine corona virus appear able to cause autoreactive lesions in the central nervous system (4, 17, 34). In these examples, the mechanisms involved remain unresolved, but they are unlikely to involve molecular mimicry (4, 17, 34). Another model, coxsackievirus myocarditis, it often assumed to be an autoimmune lesion resulting from molecular mimicry (12, 20), but since the virus persists (32) this model could represent a chronic antiviral response rather than autoreactivity (9, 23). Similarly, coxsackievirus has been incriminated in insulin-dependent diabetes mellitus by a molecular mimicry mechanism (1). However, this notion has been questioned by others based on the development of diabetes in TCR-transgenic mice that involves islet antigens that differ from those implicated in molecular mimicry (10).
Molecular mimicry as an inciting mechanism for autoimmunity has been suggested for several additional rodent autoimmune-disease models, but in almost all cases the evidence is circumstantial or unconfirmed (39). The basis for the view that HSK is an autoreactive T-cell-mediated lesion is also largely circumstantial and derives mainly from the observation that lesions persist and may even progress in the apparent absence of viral antigens (31, 36). The best evidence that HSK could be an autoimmune lesion involving molecular mimicry came from an analysis of the susceptibility status of two congenic strains of mice differing only in the IgH locus (encoding the IgG2a isotype of Ig) (2, 41). In such studies, corneal extracts from susceptible affected mice could stimulate T-cell clones reactive with a 16-amino-acid peptide of IgG2ab. Moreover, the G2a-specific T-cell clones could transfer disease to usually resistant HSV-infected athymic mice. In addition, susceptible CAL.20 mice became resistant if tolerized with the G2a peptide prior to infection. Subsequently, the molecular mimicry hypothesis was invoked since a peptide in the UL6 protein shared sequence similarity with G2a (41). In addition, lesions in susceptible mice were reported to occur only if animals were infected with UL6+ but not with UL6− HSV mutants. Taken together, such data make a strong case for molecular mimicry and autoimmunity in HSK.
In the present report using slightly different mouse strains but with the same IgH locus disparities, we failed to confirm a role for a UL6 epitope in HSK. We have no explanation for our discordant results. We reasoned that, as is noted to occur with other models that involve molecular mimicry (19, 39), if the UL6 peptide is involved as a molecular mimic in HSK pathogenesis it should be a potent immunogen when the virus was used for infection. Our studies failed to demonstrate any UL6 peptide-specific responsiveness in mice infected with HSV, although weak though undetectable responses remain a possibility. In addition, T cells reactive with UL6, taken from peptide-immunized mice, neither reacted with HSV-infected cells (not shown) or indeed the G2a peptide. Furthermore, if the UL6 peptide, cross-reacting with G2a, accounts for the HSK lesions, one might have expected lesions to result from ocular infection with a recombinant vaccinia virus expressing UL6 protein (which induced UL6 peptide reactivity). This did not occur. Finally, we consistently failed to induce HSK in reconstituted SCID or nude (not shown) mice repeatedly infected ocularly with mutant viruses. One of the compelling lines of evidence that supported molecular mimicry in a previous report was that lesions were only produced if reconstituted nude mice were subsequently infected with mutant virus that was UL6+ but not when infected with UL6− virus mutants. We and others (28) found that generating HSK requires infection with replication-competent virus (3). Recently, others have also questioned the role of UL6 in human HSK. Thus, reactivity to UL6 protein has not been observed with T-cell clones derived from human ocular herpetic keratitis (37), and no genetic variability in UL6 amino acids 299 to 314 has corresponded with the pathogenic patterns of recurrent HSK (15).
Our data do not formally exclude an involvement for autoreactivity in HSK. Indeed, as presented in detail elsewhere (6, 7, 8), substantial evidence indicates that HSK lesions can be caused by HSV as long as infected animals possess CD4+ T cells. Such T cells, however, do not need to recognize HSV-derived antigens. Accordingly, it was shown that DO11.10 T-cell transgenic mice backcrossed to RAG−/− or SCID failed to mount HSV-specific lesions (6, 7, 8) but still, unlike normal SCIDs, expressed HSK. Similar, although less-extensive, observations were also made in OT2xRAG1−/− mice in the present study. Such observations make a strong case that none of the HSV proteins provide molecular mimics to elicit HSK. However, since HSK occurs in transgenic mice unable to generate HSV-specific T cells, the lesions that do occur result from persisting virus in the cornea that causes chronic proinflammatory cytokine production and the activation of transgenic T cells (7, 8). Similarly, in the B-cell-deficient mice the severe HSK observed likely occurred as a consequence of persisting viral antigens in the corneal stroma. It remains to be seen if a similar bystander activation mechanism occurs also in normal immunocompetent mice in which virus infection of the cornea is rapidly contained. Conceivably, HSV or an immune response to it could damage cells and establish an autoreactive response involving the release of sequestered self-epitopes which, in turn, sustain an inflammatory reaction. Such a mechanism was recently proposed to occur in coxsackie B virus-induced insulin-dependent diabetes mellitus and dengue hemorrhagic fever (10, 24). We are currently attempting to determine if this mechanism is involved in the pathogenesis of HSK.
ACKNOWLEDGMENTS
We are grateful to Herbert Virgin (Washington University School of Medicine, St. Louis, Mo.) for providing the B-cell-deficient mice. We also thank Arvind Patel (Glasgow, United Kingdom) for the generous gift of the vaccinia virus expressing the UL6 protein.
B.T.R. is supported by National Institutes of Health grant EY05093. J.A.K. is supported by the Foundation Fighting Blindness, a core grant from the National Eye Institute (P30 EYO 06360), and the Jules and Doris Stein Professorship in Ophthalmology awarded by Research to Prevent Blindness.
REFERENCES
- 1.Atkinson M A, Bowman M A, Campbell L, Darrow B L, Kaufman D L, Maclaren N K. Cellular immunity to a determinant common to glutamate decarboxylase and coxsackie virus in insulin-dependent diabetes. J Clin Investig. 1994;94:2125–2129. doi: 10.1172/JCI117567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Avery A C, Zhao Z S, Rodriguez A, Bikoff E K, Soheilian M, Foster C S, Cantor H. Resistance to herpes stromal keratitis conferred by an IgG2a derived peptide. Nature. 1995;276:431–434. doi: 10.1038/376431a0. [DOI] [PubMed] [Google Scholar]
- 3.Babu J S, Thomas J, Kanangat S, Morrison L A, Knipe D M, Rouse B T. Viral replication is required for induction of ocular immunopathology by herpes simplex virus. J Virol. 1996;70:101–107. doi: 10.1128/jvi.70.1.101-107.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buchmeier M J, Lane T E. Viral induced neurodegenerative disease. Curr Opin Microbiol. 1999;2:398–402. doi: 10.1016/S1369-5274(99)80070-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chun S, Daheshia M, Kuklin N A, Rouse B T. Modulation of viral immunoinflammatory responses with cytokine DNA administered by different routes. J Virol. 1998;72:5545–5551. doi: 10.1128/jvi.72.7.5545-5551.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gangappa S, Babu J S, Thomas J, Daheshia M, Rouse B T. Virus-induced immunoinflammatory lesions in the absence of viral antigen recognition. J Immunol. 1998;161:4289–4300. [PubMed] [Google Scholar]
- 7.Gangappa S, Deshpande S P, Rouse B T. Bystander activation of CD4+ T cells can represent an exclusive means of immunopathology in a virus infection. Eur J Immunol. 2000;29:3674–3682. doi: 10.1002/(SICI)1521-4141(199911)29:11<3674::AID-IMMU3674>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 8.Gangappa S, Deshpande S P, Rouse B T. Bystander activation of CD4+ T cells accounts for herpetic ocular lesions. Investig Opthalmol Vis Sci. 2000;41:453–459. [PubMed] [Google Scholar]
- 9.Horwitz M S, Cava A L, Fine C, Rodriguez E, Ilic A, Sarvetnick N. Pancreatic expression of interferon γ protects mice from lethal coxsackie virus B3 infection and subsequent myocarditis. Nat Med. 2000;6:693–697. doi: 10.1038/76277. [DOI] [PubMed] [Google Scholar]
- 10.Horwitz M G, Sarvetnik N. Viruses, host responses and autoimmunity. Immunol Rev. 1999;169:141–153. doi: 10.1111/j.1600-065X.1999.tb01319.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Horwitz M S, Bradley L M, Harbertson J, Krahl T, Lee J, Sarvetnick N. Diabetes induced by coxsackie virus: initiation by bystander damage and not molecular mimicry. Nat Med. 1998;4:781–785. doi: 10.1038/nm0798-781. [DOI] [PubMed] [Google Scholar]
- 12.Huber S A, Lodge P A. Coxssackievirus B-3 myocarditis in BALB/c mice: evidence for autoimmunity to myocyte antigens. Am J Pathol. 1984;116:21–29. [PMC free article] [PubMed] [Google Scholar]
- 13.Kitamura D, Roes J, Kuhn R, Rajewsky K. A B cell deficient mouse by targeted disruption of the membrane exon of the immunoglobulin μ chain gene. Nature. 1991;250:423–427. doi: 10.1038/350423a0. [DOI] [PubMed] [Google Scholar]
- 14.Manickan E, Rouse R J D, Yu Z, Wire W S, Rouse B T. Genetic immunization against herpes simplex virus. Protection is mediated by CD4 T lymphocytes. J Immunol. 1995;155:259–265. [PubMed] [Google Scholar]
- 15.Margolis T P, Ellison A R, Yang L, Gordan Y J, Cevallos A V. Analysis of the HSV UL6 encoding region in patients with recurrent HSV keratitis. Investig Opthalmol Vis Sci. 2000;41:S945. [Google Scholar]
- 16.Mercadel C, Bouley D, De Staphano D, Rouse B T. Herpetic stromal keratitis in the reconstituted SCID mouse model. J Virol. 1993;67:3404–3408. doi: 10.1128/jvi.67.6.3404-3408.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miller S D, Vanderlugt C L, Begolka W S, Pao W, Yauch R L, Neville K L, Katz-Levy Y, Carrizosa A, Kim B S. Persistent infection with Theiler's virus leads to CNS autoimmunity via epitope spreading. Nat Med. 1997;3:1133–1136. doi: 10.1038/nm1097-1133. [DOI] [PubMed] [Google Scholar]
- 18.Nair S, Buiting A M J, Rouse R J D, van Rosijen N, Huang L, Rouse B T. Role of macrophages and dendritic cells in primary cytotoxic T lymphocyte responses. Int Immunol. 1995;7:679–688. doi: 10.1093/intimm/7.4.679. [DOI] [PubMed] [Google Scholar]
- 19.Oldstone M B A. Molecular mimicry and immune-mediated diseases. FASEB J. 1998;12:1255–1265. doi: 10.1096/fasebj.12.13.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oldstone M B A, Nerenberg M, Southern P, Price J, Lewicki H. Virus infection triggers insulin-dependent diabetes mellitus in a transgenic model. Cell. 1991;65:319–331. doi: 10.1016/0092-8674(91)90165-u. [DOI] [PubMed] [Google Scholar]
- 21.Opremcak E M, Wells P A, Thompson P, Daigle J A, Rice B A, Millin J, Foster C S. Immunogenetic influence of IgH-1 phenotype on experimental herpes simplex virus type-1 corneal infection. Investig Ophthalmol Vis Sci. 1988;29:749–754. [PubMed] [Google Scholar]
- 22.Robertson J M, Jensen P E, Evavold B D. D011.10 and OT-II T cells recognize a C-terminal ovalbumin 323–339 epitope. J Immunol. 2000;164:4706–4712. doi: 10.4049/jimmunol.164.9.4706. [DOI] [PubMed] [Google Scholar]
- 23.Rose N. Viral damage or ‘molecular mimicry’: placing the blame in myocarditis. Nat Med. 2000;6:631–632. doi: 10.1038/76199. [DOI] [PubMed] [Google Scholar]
- 24.Rothman A, Ennis F. Immunopathogenesis of dengue hemorrhagic fever. Virology. 1999;257:1–6. doi: 10.1006/viro.1999.9656. [DOI] [PubMed] [Google Scholar]
- 25.Rouse B T. Virus-induced immunopathology. Adv Virus Res. 1996;47:353–375. doi: 10.1016/S0065-3527(08)60739-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rouse B T, Daheshia M, Schmid S D. A balance of powers. In: Cunningham M, Fujinami R, editors. Effects of microbes on the immune system. Philadelphia, Pa: The Williams & Wilkins Co.; 1999. pp. 387–397. [Google Scholar]
- 27.Sercarz E E, Lehmann P V, Ametani A, Benichou G, Miller A, Moudgil K. Dominance and crypticity of T cell antigenic determinants. Annv Rev Immunol. 1993;11:729–766. doi: 10.1146/annurev.iy.11.040193.003501. [DOI] [PubMed] [Google Scholar]
- 28.Shimeld C, Hill T J, Blyth W A, Easty D L. Passive immunization protects the mouse eye from damage after herpes simplex virus infection by limiting spread of virus in the nervous system. J Gen Virol. 1990;71:681–687. doi: 10.1099/0022-1317-71-3-681. [DOI] [PubMed] [Google Scholar]
- 29.Song B, Liu J J, Yeh K C, Knipe D M. Herpes simplex virus infection blocks events in G1 phase of the cell cycle. Virology. 2000;267:326–331. doi: 10.1006/viro.1999.0146. [DOI] [PubMed] [Google Scholar]
- 30.Spear P G, Roizman B. Proteins specified by herpes simplex virus. V. Purification and structural proteins of the herpes virion. J Virol. 1972;9:143–159. doi: 10.1128/jvi.9.1.143-159.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Strielein J W, Reza Dana M, Ksander B R. Immunity causing blindness: five different paths to herpes stromal keratitis. Immunol Today. 1997;9:443–449. doi: 10.1016/s0167-5699(97)01114-6. [DOI] [PubMed] [Google Scholar]
- 32.Tam P E, Messner R P. Molecular mechanisms of Coxsackie persistence in chronic inflammatory myopathy: viral RNA persists through formation of a double-stranded complex without associated genomic mutations or evolution. J Virol. 2000;73:10113–10121. doi: 10.1128/jvi.73.12.10113-10121.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Taus N S, Salmon B, Baines J D. The herpes simplex virus 1 UL17 gene is required for localization of capsids and major and minor capsid proteins to intranuclear sites where viral DNA is cleaved and packaged. Virology. 1998;252:115–200. doi: 10.1006/viro.1998.9439. [DOI] [PubMed] [Google Scholar]
- 34.Ter Meulen V. Autoimmune reactions against myelin basic proteins induced by corona virus and measles virus. Ann N Y Acad Sci. 1988;540:202–209. doi: 10.1111/j.1749-6632.1988.tb27062.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thomas J, Rouse B T. Immunopathology of herpetic stromal keratitis: discordance in CD4+ T cell function between euthymic host and reconstituted SCID recipients. J Immunol. 1998;160:3965–3970. [PubMed] [Google Scholar]
- 36.Thomas J T, Rouse B T. Immunopathogenesis of herpetic ocular disease. Immunol Res. 1997;16/4:375. doi: 10.1007/BF02786400. [DOI] [PubMed] [Google Scholar]
- 37.Verjans-George M G M, Remeijer L, Mooy C M, Osterhaus O D. Herpes simplex virus specific T-cells infiltrate the cornea of patients with herpetic stromal keratitis (HSK): no evidence for intra-corneal autoreactive T-cells in human HSK. Investig Opthalmol Vis Sci. 2000;41:2607–2612. [PubMed] [Google Scholar]
- 38.Von Herranth M G, Oldstone M B A. Virus-induced autoimmune disease. Curr Opin Immunol. 1996;8:878–885. doi: 10.1016/S0952-7915(96)80019-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Whitton L J, Fujinami R S. Viruses as triggers of autoimmunity: facts and fantasies. Curr Opin Microbiol. 1999;2:392–397. doi: 10.1016/s1369-5274(99)80069-1. [DOI] [PubMed] [Google Scholar]
- 40.Wucherpfennig K W, Strominger J L. Molecular mimicry in T cell mediates autoimmunity: viral peptides activate human T cell clones specific for MBP. Cell. 1995;80:695–705. doi: 10.1016/0092-8674(95)90348-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhao Z, Granucci F, Yeh L, Schaffer P, Cantor H. Molecular mimicry by herpes simplex virus type-1: autoimmune disease after viral infection. Science. 1998;279:1344–1347. doi: 10.1126/science.279.5355.1344. [DOI] [PubMed] [Google Scholar]