Abstract
Tripartite motif (TRIM) protein superfamily is a group of E3 ubiquitin ligases characterized by the conserved RING domain, the B-box domain, and the coiled-coil domain (RBCC). It is widely involved in various physiological and pathological processes, such as intracellular signal transduction, cell cycle regulation, oncogenesis, and innate immune response. Central nervous system (CNS) diseases are composed of encephalopathy and spinal cord diseases, which have a high disability and mortality rate. Patients are often unable to take care of themselves and their life quality can be seriously declined. Initially, the function research of TRIM proteins mainly focused on cancer. However, in recent years, accumulating attention is paid to the roles they play in CNS diseases. In this review, we integrate the reported roles of TRIM proteins in the pathological process of CNS diseases and related signaling pathways, hoping to provide theoretical bases for further research in treating CNS diseases targeting TRIM proteins.
Graphical Abstract
TRIM proteins participated in CNS diseases. TRIM protein family is characterized by a highly conserved RBCC domain, referring to the RING domain, the B-box domain, and the coiled-coil domain. Recent research has discovered the relations between TRIM proteins and various CNS diseases, especially Alzheimer’s disease, Parkinson’s disease, and ischemic stroke.
Keywords: TRIM proteins, E3 ubiquitin ligases, CNS diseases, Signaling pathway
Introduction
Tripartite motif (TRIM) protein family is the largest subfamily of E3 ubiquitin ligases, which are crucial in the ubiquitination process. Ubiquitination is associated with not only protein degradation but also some non-degradation functions such as receptor internalization and protein trafficking (Hristova et al. 2020). Although ubiquitin modification has been reported to induce autophagy, in most cases, it is related with protein degradation by the proteosome (Chen et al. 2019; Hristova et al. 2020). Ubiquitin–proteasome system (UPS) plays a part in degrading abnormal proteins and short-lived proteins in cells to keep the homeostasis; it consists of ubiquitin, ubiquitin-activating enzyme (E1), ubiquitin-conjugating enzyme (E2), and ubiquitin-protein ligase (E3) and the proteosome (Lee et al. 2022; Zhang et al. 2020a, b, c). Among the three ubiquitination-related enzymes, only 2 E1 enzymes and 38 E2 enzymes have been found so far, while more than 600 E3 ligases have been identified (Zhang et al. 2020a, b, c).
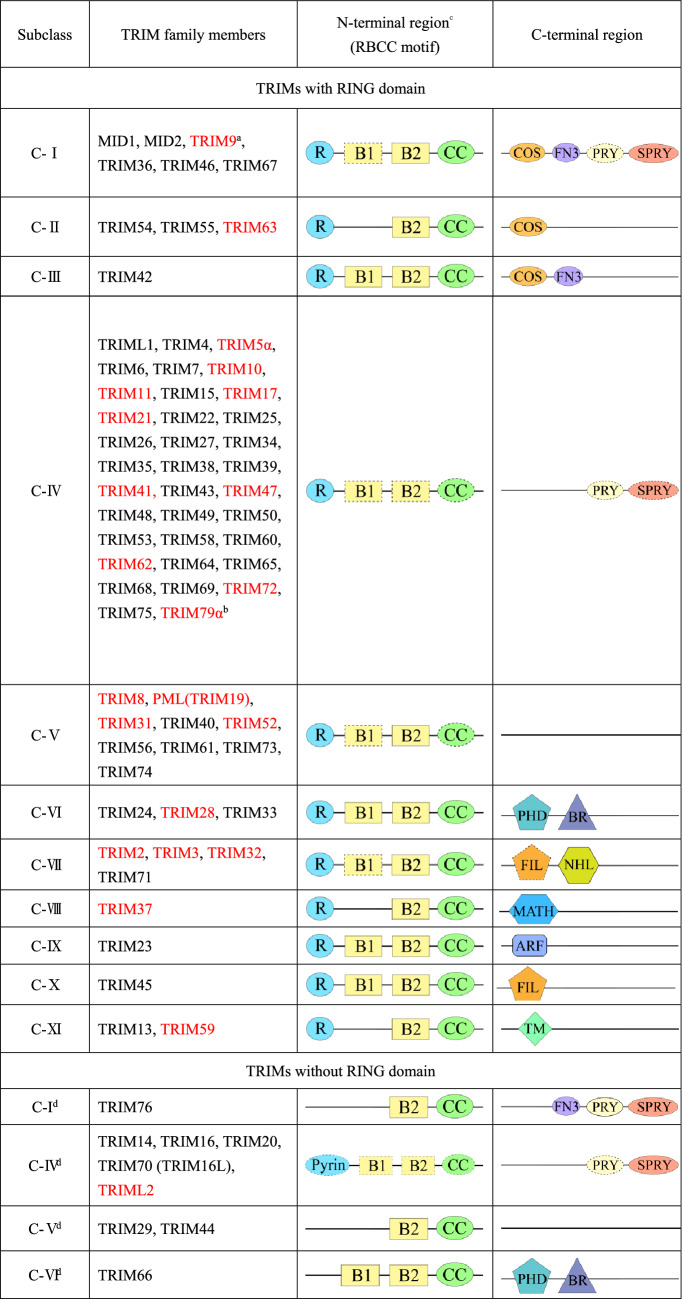
TRIM family proteins are characterized by a highly conserved RBCC domain, including the RING domain, the B-box domain, and the coiled-coil domain. RING domain, located within the 10–20 amino acids from the first methionine at the N-terminal, binds to zinc and has the activity of E3 ubiquitin ligase (Ozato et al. 2008); and its polymerization is linked to small ubiquitin-like modifier-ligase activities (SUMOylation) (Wang et al. 2018). B-box domain is also a zinc-bound motif with B-box1 and B-box2 domains. These two B-boxes share a part of consensus sequences, while the common sequences are different between TRIM protein members. One TRIM protein may have B-box2 only, while B-box1 always exists companied with B-box2 (Ozato et al. 2008). At present, the function of the B-box domain remains unclear, but some evidence implicates that it is related to enhancing E3 ligase activity and even gives the enzymatic activity to TRIM protein without the RING domain (Bell et al. 2012). Coiled-coil domain occurs in many protein families. In the TRIM family, it mediates the homodimeric interactions between members and heterodimeric interactions between members and other proteins (Napolitano and Meroni 2012). According to the different structures of the C-terminal domain, more than 70 TRIMs have been classified into 11 different subclasses (C-I to C-XI) while 10 TRIMs without RING domain are not classified officially yet (Hatakeyama 2017; Short and Cox 2006; Ozato et al. 2008; Zhang et al. 2020a, b, c) (Table 1). The variable C-terminal region of TRIM protein aids in cellular localization, constitutes the active unit that recognizes the substrate, and sometimes gets involved in transcriptional regulation (C-VI family) (Zhang et al. 2020a, b, c; Ozato et al. 2008).
Table 1.
Schematic structures of TRIM protein family members
ARF ADP ribosylation factor-like, B B-box, BR bromodomain, CC coiled-coil, COS C-terminal subgroup one signature, FN3 fibronectin type3, FIL filamin-type immunoglobulin, MATH meprin and tumor necrosis factor receptor-associated factor homology, MID midline, N-terminal amino-terminal, PHD plant homeodomain, PML promyelocytic leukemia, R RING finger, TM transmembrane
aTRIM members highlighted in red are TRIMs related to CNS diseases (Tanji et al. 2010; Zeng et al. 2019; von Grabowiecki et al. 2016; Nexo et al. 2013; Huang et al. 2019; Chen et al. 2018; Jabbari et al. 2018; Wang et al. 2019; Zhu et al. 2020; Lassot et al. 2018; Manocha et al. 2014; Yang et al. 2009; Hao et al. 2019; Liu et al. 2020; Schmidt et al. 2014; Guan et al. 2019; Yao et al. 2016; Yi et al. 2021; Taylor et al. 2011; Bai et al. 2020; Zhang et al. 2020a, b, c; Guo et al. 2014; Zeng et al. 2021; Fan et al. 2016; Rousseaux et al. 2016; Rousseaux et al. 2018; Balastik et al. 2008; Dong et al. 2020; Zhao et al. 2021; Wezyk et al. 2018a, b; Kang et al. 2016).
bTRIM79α is rodent-specific
cDotted outlines indicate domains that are not present in all TRIM family members in one subclass
dUnofficial classifications
Central nervous system (CNS) consists of the brain and spinal cord, which is the most vital part of the human nervous system and the structural basis of human senior activities (Chen et al. 2021). It accepts the afferent information from all over the body, then translates it into coordinated movement or stores it as the neural basis of learning and memory (Bai et al. 2021). Due to the importance and the complexity of CNS, its disturbance usually leads to serious clinical consequences, such as impairment of language ability, motor ability, memory, and so on, which bring great inconvenience to patients (Salter et al. 2017; Chen et al. 2021). Based on the etiology and pathological features, CNS diseases can generally be divided into cerebrovascular diseases, neurodegenerative diseases, infectious diseases, and traumatic diseases (Chen et al. 2021; Lambert et al. 2016). Millions of people suffer from CNS disorders worldwide every year, accounting for 6.3% of the global disease burden (Lambert et al. 2016). Most CNS diseases show high morbidity, complex pathogenesis, and long treatment cycle, especially in middle-aged and elderly people. Therefore, it is urgent to find new targets to develop drugs with better efficacy and fewer side effects. In CNS, TRIMs are generally expressed in both neurons and glial cells with different proportions in inhibitory neurons, excitatory neurons, astrocytes, oligodendrocytes, and microglia (Ponten et al. 2008). And they were reported to get involved in various CNS diseases. Therefore, they may have the potential to be developed as therapeutical targets. In this review, we summarize the roles TRIM proteins play in CNS diseases, hoping to provide theoretical bases for further therapy in treating these disorders.
TRIMs-related CNS Diseases
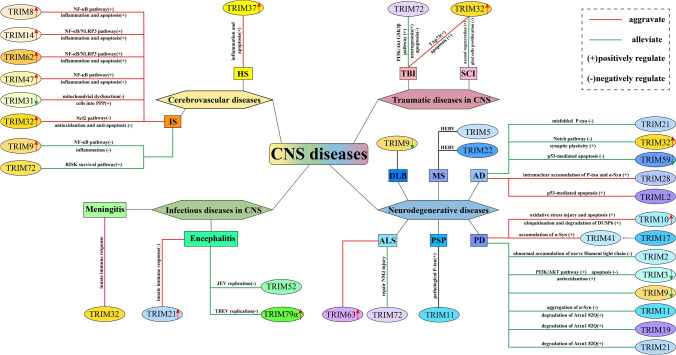
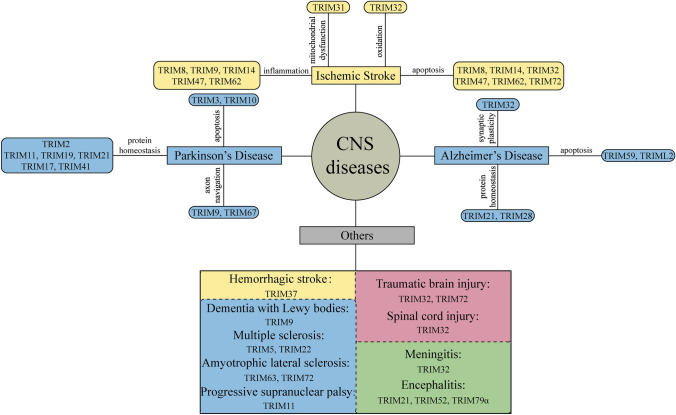
Many studies have shown that the TRIM protein family exerts critical functions in extensive fundamental life processes, such as intracellular signal transduction, cell cycle regulation, oncogenesis, and innate immune response, which lays the foundation for participating in the CNS disease process (Zhang et al. 2020a, b, c). In turn, when some CNS diseases attack, the expression levels of related TRIMs will also be altered (Fig. 1).
Fig. 1.
Expression changes of TRIM proteins and their roles in CNS diseases. Expression changes of proteins without the arrowhead mark are equivocal yet. CNS central nervous system, AD Alzheimer’ s disease, PD Parkinson’ s disease, DLB dementia with Lewy bodies, I progressive supranuclear palsy, ALS amyotrophic lateral sclerosis, MS multiple sclerosis, IS ischemic stroke, HS hemorrhagic stroke, TBI traumatic brain injury, SCI spinal cord injury, DUSP6 dual specificity phosphatase 6, PPP pentose phosphate pathway, RISK reperfusion injury salvage kinase, NMJ neuromuscular junction, HERV human endogenous retrovirus, JEV Japanese Encephalitis virus, TBEV tick-borne encephalitis virus, TRIM tripartite motif
Neurodegenerative Diseases
Alzheimer’s Disease
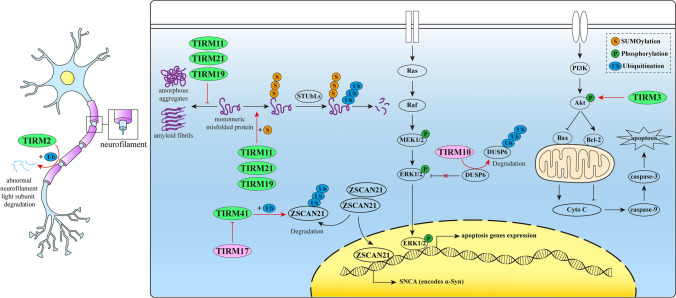
Alzheimer’s disease (AD) is a common neurodegenerative disease known for chronic and progressive cognitive dysfunction and memory impairment. The pathological features of AD include neuritis amyloid plaques, cerebrovascular amyloidosis, and neurofibrillary tangles caused by hyperphosphorylation of tau protein (P-tau), as well as severe loss of cholinergic neurons in the basal forebrain that innervate the hippocampus and neocortex (Selkoe 2004). In recent years, studies have shown that the TRIM protein family impacts AD development by participating in apoptosis, synaptic plasticity, and the regulation of α-synuclein (α-Syn) and P-tau (Fig. 2).
Fig. 2.
Partial mechanisms of TRIM proteins in AD. NICD notch intracellular domain, SUMO small ubiquitin-like modifier-ligase; CSL C protein binding factor 1/Suppressor of Hairless/Lag-1, Co-A co-activator
TRIM21 is a receptor that contributes to regulating antibody-mediated intracellular immunity (Mallery et al. 2010). It is extensively expressed in most tissues without specificity and has a high affinity with Fc, which makes it prone to bind with antibodies. AD is associated with the misfolded P-tau entering and then aggregating in the cytoplasm. It is generally believed that the development of AD is related to the diffusion of P-tau between the connecting regions of the brain (Braak et al. 1991), namely the propagation of misfolded P-tau seeds. Such transmission is similar to the viral infection, which relies on the physical transfer of P-tau assemblies between cells and thus may tendentiously be intercepted by antibodies. TRIM21 was shown to prevent misfolded P-tau from assembling to help avoid AD occurrence (McEwan et al. 2017). In detail, misfolded P-tau seeds entered the cell labeled with anti-tau antibody, and soon after recruited the cytoplasmic Fc receptor TRIM21 to bind with the antibody to activate proteasome and AAA ATPase P97/VCP and then triggered the tau seeds neutralization, so the further assemblies of misfolded P-tau were intercepted.
TRIM28, initially named Krüppel-associated box-domain interacting protein 1 (KAP1), was first discovered as a transcriptional and epigenetic regulator (Randolph et al. 2022). However, recent studies showed that TRIM28 was involved in AD with its E3 ligase activity. Rousseaux et al. (2016) reported that TRIM28 could regulate the steady-state level of α-Syn and P-tau in physiological conditions, while drive neurodegeneration by promoting their accumulation in the cell nucleus when AD occurred. The molecular mechanism may relate to the formation of a complex consisted of the E3 ligase domain of TRIM28. The complex could enhance the stability of α-Syn and P-tau proteins and promote their accumulation in the nucleus. Reductions in the level of both proteins were detected when mutating the E3 ligase domain of TRIM28. This suggested that TRIM28 might play the role in AD through SUMOylation or ubiquitination at the protein level, rather than through epigenetic modification or indirect binding with α-Syn and P-tau (Cheng et al. 2014). In 2018, the same research team confirmed that TRIM28 SUMOylated these two proteins (Rousseaux et al. 2018). Theoretically, the knockdown of Trim28 could decrease the incidence of neurodegenerative diseases, and heterozygote Trim28± was sufficient to reduce neurotoxicity caused by TRIM28 (Rousseaux et al. 2016). Remarkably, previous studies showed that complete knockout (KO) of Trim28 could lead to embryonic death (Cammas et al. 2000), and the specific KO of Trim28 in developing tissues could also cause many developmental defects (Trono 2015). However, the depletion of Trim28 did not affect adult survival or even exhibit pathological phenotypes (Rousseaux et al. 2018). These results indicated that in addition to the progress of neurodegenerative diseases, TRIM28 also played a vital role in tissue development, with no obvious effect in mature tissues. Therefore, Trim28 holds promise as a selective therapeutic target for neurodegenerative diseases in adults. Rousseaux et al. (2016) hypothesized two mechanisms by which TRIM28 increased the intranuclear aggregation of α-Syn and P-tau to promote neurodegeneration. The active aspect was that TRIM28 increased the toxicity of these two proteins, while the passive one was that TRIM28 blocked the natural degradation of these two proteins, thereby increasing their amounts and bioactivity.
TRIM32 is relevant to the synaptic plasticity in AD (Hillje et al. 2013; Ntim et al. 2020). The expression of TRIM32 is higher in the occipital lobe of AD patients than in that of healthy adults (Yokota et al. 2006). The activation of Notch signaling pathway is a contributor of AD pathophysiology (Perna et al. 2021). When TRIM32 was knocked down in mice, synaptic plasticity was impaired by activating this pathway (Ntim et al. 2020). Consistently, the blockage of Notch pathway was found to improve synaptic plasticity, as well as learning and memory ability of patients with brain injury (Zhang et al. 2018). Interestingly, the deletion of TRIM32 only disrupted long-term plasticity (LTP), while affecting neither short-term synaptic plasticity nor synaptic transmission efficiency. After TRIM32-KO, the neurotropic factor NGF expression in the brain was decreased; on the other hand, the HES1 expression was increased because of the activation of the Notch pathway. These two changes led to the up-regulation of NGN3, which reduced the expression of transcription factor mash1, thus causing cells to exit the cell cycle and initiate neuronal differentiation to the excitatory glutamatergic phenotype instead of the inhibitory GABAergic one. Such unbalanced neural differentiation resulted in excitotoxicity, which led to not only functional and structural changes in synaptic plasticity, but also a decrease in the number of neurons in the hippocampus and cortex, and finally came to impair the synaptic plasticity (Ntim et al. 2020).
TRIM59 is composed of 403 amino acid residues with a transmembrane domain except for the unique RBCC domain of the TRIM family. Its encoding gene is located on chromosome 3 and the protein mainly exists in the endoplasmic reticulum in cells (Chang et al. 2002). Epigenetic mechanisms play an important role in the development of various neurodegenerative diseases. Abnormal methylation of genes responsible for regulating transcription, DNA replication, and apoptosis is correlative with AD pathology. In general, DNA in cerebral cortical neurons is hypomethylated in AD patients (Chouliaras et al. 2013; Mendioroz Iriarte et al. 2015). However, low expression of TRIM59 caused by hypermethylation of Trim59 was found potentially accelerate the pathological process of AD (Wezyk et al. 2018a, b). In familial early-onset AD (FEOAD) patients, it was found that TRIM59 was down-regulated due to the hypermethylation of Trim59, and meanwhile, the activity of p53, a key regulator of aging, in fibroblasts and neurons was increased (Wezyk et al. 2018a, b). Generally speaking, the methylation status of TRIM59 and its promoter may be the molecular switch of physiological and pathological aging transformation. And the hypermethylation of TRIM59 facilitated the cell apoptosis, acting through the p53 pathway. Moreover, accumulating research reported that dysregulation of p53 might cause abnormal compensation reactions, which contribute to neurodegenerative diseases, including AD (Abate et al. 2020). Therefore, when Trim59 is hypermethylated, the expression of TRIM59 is inhibited, leading to excessive existence of p53 by reducing its ubiquitination and degradation (Zhou et al. 2014); thus, the symptoms of AD would deteriorate.
Protein TRIM-like2 (TRIML2) is located on the long arm of chromosome 4 (q35.2), which is linked to the onset of AD (Lee et al. 2015). Studies showed that the apoptosis process was partially regulated by p53 and there was a close interaction between some TRIM family proteins and the p53 signaling pathway (Lanni et al. 2007). TRIML2 facilitated p53-mediated apoptosis by enhancing p53 protein levels through SUMOylation and promoted the activation of proapoptotic target genes at the same time (Kung et al. 2015). Based on this, Kang et al. (2016) investigated the relationship between single nucleotide polymorphism (SNP) in the TRIML2 coding region and AD. Both coding SNPs (cSNPs) of allele C (rs79698746 and rs2279551) in the coding region of TRIML2 could increase the susceptibility to AD.
Parkinson’ s Disease
Parkinson’s disease (PD) is a general neurodegenerative disease, which is more common in the elderly, with an average onset age of about 60 years old, and relatively rare in young under 40. Most patients with PD are sporadic, and less than 10% have a family history. The major pathological changes of PD are the degeneration and death of dopaminergic neurons in the substantia nigra; these result in a significant decrease in dopamine content in the striatum (Trevisan et al. 2017; Wen et al. 2018). Clinical manifestations are motor dysfunction (such as postural instability, static tremor, rigidity) and cognitive impairment (Barrett et al. 2005; Buizza et al. 2012). The exact etiology of these pathological changes is still unclear. Genetic factors, environmental factors, aging, and oxidative stress can all be involved in the degeneration and death process of PD dopaminergic neurons. To date, accumulating studies have shown that the TRIM protein family is related to the pathogenesis of PD (Fig. 3).
Fig. 3.
Partial mechanisms of TRIM proteins in PD. SUMO small ubiquitin-like modifier-ligase, STUbL SUMO-targeted ubiquitin ligase; MEK mitogen-activated protein kinase, ERK extracellular regulated kinase, DUSP6 dual specificity phosphatase 6
For the sake of exploring the relationship between PD progression and the TRIM family, Nenasheva et al. (2017) used fibroblasts from skin explants of healthy subjects and PD patients and cells from PD patients with different mutation sites to detect their initial state and the changes of 15 Trim genes (Trim1, 2, 5α, 6, 9, 14, 16, 19, 24, 26, 27, 31, 32, 37, 71) during transformation. Ultimately, they concluded that Trim9, Trim6, and Trim24 may have significant effects on the PD process. TRIM9 was down-regulated at the early stage of neuronal differentiation when PD occurred. Trim6 and Trim24 appeared the greatest differences in transcription at almost all stages this research investigated in cells derived from PD patients, suggesting that these two genes are associated with the process of PD. Consistently, TRIM24 was found to be a potential risk factor for early-onset PD in Chinese population (Li et al. 2021). As for Trim27, it was dysregulated in PD, but no significant expression change was shown in the transcriptional analysis (Liu et al. 2014). So it can be inferred that the loss of Trim27 occurs at a later stage than the terminal differentiation of nerve cells.
TRIM2 is mainly expressed in the cytoplasm of hippocampal neurons and can promote neuronal axon outgrowth and specification (Lokapally et al. 2020). It participated in the process of neurodegeneration by affecting protein homeostasis, such as preventing the abnormal accumulation of nerve filament light (NFL) chain. TRIM2 could interact with NFL through its central region and help NFL ubiquitinate in the presence of UbcH5a E2 (Balastik et al. 2008).
TRIM3, whose gene is on chromosome 11P15.5, is a member of the TRIM family located on cytoplasmic filaments (Monti et al. 2018). It is a key protein involved in the molecular mechanism of PD by affecting intracellular vesicles, ubiquitin-protein ligases and binding activities of transition metal ions (Dong et al. 2019). Compared with healthy adults, TRIM3 expression was down-regulated in PD patients (Dong et al. 2020, 2019). What’s more, after deep brain stimulation surgery treatment, the expression of TRIM3 was increased but did not return to the original level (Dong et al. 2019). When PD occurred, the anti-apoptotic PI3K/AKT pathway was highly activated, which may be the compensatory response against excessive oxidative stress (Yalcinkaya et al. 2016). TRIM3 alleviated PD via activating PI3K/AKT signaling pathway, thus attenuating apoptosis of midbrain tissues. When PI3K inhibitors were used, apoptosis inhibition induced by TRIM3 up-regulation disappeared (Dong et al. 2020). Besides this, TRIM3 could also enhance the anti-oxidant capacity of the body, registering as the expression change of reactive oxygen species (ROS), glutathione (GSH), and superoxide dismutase (SOD). ROS is closely relevant to the oxidative stress response, which has been shown to facilitate the neurotoxicity of dopaminergic neurons (Hattingen et al. 2009). ROS can also breed protein misfolding, such as α-Syn, which promotes the neurodegenerative process of PD (Norris et al. 2015). GSH and SOD are two decisive members of the free radical scavenging system, which are critical for maintaining the balance between oxidation and anti-oxidation (Wang and Hai 2016; Sajadimajd et al. 2016; Khan et al. 2017). And one of the pathological features of PD is the decrease in GSH and SOD levels (Meng et al. 2017). After up-regulating TRIM3, ROS in PD mice were prominently reduced, while the levels of GSH and SOD were increased.
TRIM9 is a brain-specific E3 ligase that is mainly expressed in cortical neurons (Zeng et al. 2019; Tanji et al. 2010). Under normal conditions, higher levels of TRIM9 were shown in the cerebral cortex and the hippocampus, the region responsible for cognitive, learning, and memory functions, in mouse brains. When PD occurred, expression of TRIM9 in substantia nigra and PD affected brain regions was decreased significantly. Ubiquitination-proteasome degradation of TRIM9, which is mediated by itself cooperating with E2 ligase UbcH5b, plays an important role in neurodegenerative diseases. Moreover, TRIM9 was also found in Lewy bodies (LBs) of PD patients, suggesting TRIM9 not only participated in the regulation of neuronal function but also got involved in the formation or decomposition of abnormal inclusion bodies on the basis of its ligase activity (Tanji et al. 2010). Besides, TRIM9 and TRIM67 contributed to the axon guidance to explore the extracellular environment, whose abnormality would lead to PD onset (Kalaani et al. 2016; Boyer et al. 2020). In the process of axon navigation, TRIM9 competed with TRIM67 to interact with vasodilator-stimulated phosphoprotein (VASP) and regulated its ubiquitination. TRIM9 could ubiquitinate VASP while TRIM67 antagonist this process. And the loss of VASP is necessary for axons to response to the extracellular guidance cues (Menon et al. 2015). Therefore, this pair of TRIM proteins is important for the correct axon orientation (Kalaani et al. 2016).
TRIM10, one of the members of the TRIM protein family, has been pinpointed to have a genetic basis for influencing PD (Witoelar et al. 2017). What’s more, TRIM10 was reported to promote PD-induced apoptosis and ROS overproduction by inhibiting the protein dual specificity phosphatase 6 (DUSP6) in the mitogen-activated protein kinase (MAPK) pathway. The expression of TRIM10 was increased, while the level of DUSP was dropped after PD onset. On the contrary, when TRIM10 was silenced, the down-regulation of DUSP after PD was reversed (Huang et al. 2019). Excessive ROS in neurons could stimulate the expression of the extracellular regulated kinase (ERK) 1/2, which belongs to MAPKs that regulate a number of biological processes such as cell proliferation and apoptosis (Samanta et al. 1998). The activation of the MAPK signaling pathway could lead to neurotoxicity and it is controlled by bi-specific (Thr/Tyr) MAPK phosphatase (MKPs). DUSP6/MKP-3 is crucial in MAPK pathway as a specific inactivation protein of ERK (Owens et al. 2007). TRIM10 assisted the ubiquitination of DUSP6 and accelerated its proteasome degradation, which caused the disinhibition of ERK1/2, and thus activated the MAPK/ERK pathway. Consistently, when DUSP6 was over-expressed in cells, the apoptosis and ROS overproduction resulting from increased TRIM10 expression were alleviated (Huang et al. 2019).
TRIM11 is well known for its tumor-promoting effect in most cases (Wang et al. 2019). However, in 2018, TRIM11 was reported to stimulate proteasome activation by inhibiting proteasome-associated de-ubiquitination enzyme USP14 (Chen et al. 2018). And later in 2020, TRIM11 was discovered to get involved in PD, manifesting as reducing the loss of dopaminergic neurons, and improving motor impairments by inhibiting the α-Syn aggregation. Apart from that, TRIM11 was also recognized as a member of the protein quality control system (PQC). It has three kinds of activities, serving as the molecular chaperone, disaggregase, and SUMO ligase, to keep some proteins in a normal state. It could inhibit the formation of amorphous or fibrillar aggregates as a molecular chaperone in an ATP-independent manner, unlike canonical chaperones. On the other hand, TRIM11 dissolved pre-existing amorphous aggregates and preformed amyloid fibrils through its disaggregase activity. What’s more, it could also SUMOylate aberrant proteins in folding states. Interestingly, TRIM11 could recognize abnormal proteins depending on its RING domain and it was up-regulated under proteotoxic stress. Physiologically, TRIM11 mainly existed in the cytoplasm, whereas it translocated to the nuclear and accumulated in Atxn1 82Q inclusions in the presence of Atxn1 82Q, the pathogenic form of ataxin1 protein (Chen et al. 2017; Zhu et al. 2020). This characteristic of TRIM11 is similar to heat shock protein 70 (Hsp70), a traditional molecular chaperone. Furthermore, the structural study showed that the molecular chaperone and disaggregase activities of TRIM11 were structurally separate from its SUMO ligase activity, although they were all required for the effective removal of defective proteins (Zhu et al. 2020).
A variety of TRIM family proteins can act as ATP-independent molecular chaperones and depolymerase to control the protein quality in metazoan. Besides TRIM11, TRIM21 and TRIM19 also mediate the degradation of Atxn1 82Q, whose accumulation without the need for ATP is a characteristic of familial PD (Guo et al. 2014). TRIM21 is a cytoplasmic receptor with a PRY-SPRY/B30.2 region, similar to TRIM11 (Ozato et al. 2008; Keeble et al. 2008). Therefore, its function is also homologous with TRIM11. It could prevent some proteins, such as luciferase, from being inactivated, and also dissolve and re-activate the denatured luciferase. And it preferentially bound with the aberrant luciferase rather than the normal one. TRIM19 is a nuclear protein involved in a host of cellular processes (Bernardi et al. 2007). Unlike TRIM11 and TRIM21, TRIM19 does not contain a recognizable C-terminal domain (Ozato et al. 2008). Nevertheless, the purified recombinant TRIM19 protein could still re-activate heat-denatured green fluorescent protein (GFP) in cells. And TRIM19-KO accelerated the inactivation of nuclear loci luciferase during heat shock and delayed its re-activation, while the over-expression showed the opposite effect (Zhu et al. 2020). Besides, TRIM19 could interact with misfolded proteins such as Atxn1 82Q to form proteinaceous nuclear bodies (Bernardi et al. 2007; Guo et al. 2014). Also, TRIM19 degraded defective proteins through SUMOylation and then mediated the SUMO-targeted ubiquitin ligases (STUbL)-mediated ubiquitination (Guo et al. 2014).
TRIM17, also known as Terf, was found to be expressed at low levels in most adult tissues except the testis and some brain regions (Basu-Shrivastava et al. 2021). The first identified α-Syn encoding gene correlative with familial PD was SNCA. ZSCAN21 (also called Zipro1/RU49/ZNF38) could stimulate SNCA transcription by interacting with a consensus element located in intron 1 and so induce α-Syn expression. Lassot et al. (2018) indicated that TRIM41 and TRIM17 controlled α-Syn expression by regulating the stability of ZSCAN21. TRIM41 ubiquitinated ZSCAN21 as an E3 ubiquitin ligase and reduced α-Syn content. Previous studies demonstrated that TRIM17 initiated neuronal apoptosis by participating in the ubiquitination and degradation of the anti-apoptotic protein Mcl-1 (Lassot et al. 2010). In terms of its role in regulating α-Syn, TRIM17 was able to interact with both TRIM41 and ZSCAN21 and inhibited the degradation of ZSCAN21 by TRIM41, resulting in the accumulation of α-Syn. In addition, two variants of TRIM17 were found in autosomal dominant PD patients: TRIM17p. R78W and TRIM17p. T407N (Lassot et al. 2018). The mutation site R78W is located in the RBL Linker connecting TRIM17, namely the region of RING and B-box domains (Reymond et al. 2001), while T407N is situated in the PRY-SPRY region (Gushchina et al. 2018). Both of these two regions are vital for the function of TRIM proteins. Moreover, TRIM17 directly resisted the degradation of another transcription factor NFATc3, which is linked to the degeneration of dopaminergic neurons in the midbrain induced by α-Syn in PD patients (Mojsa et al. 2015; Luo et al. 2014; Caraveo et al. 2014).
Other Neurodegenerative Diseases
In addition to the familiar AD and PD, neurodegenerative diseases also include dementia with Lewy bodies (DLB), progressive supranuclear palsy (PSP), amyotrophic lateral sclerosis (ALS), multiple sclerosis (MS), and so on.
DLB is a group of neurodegenerative diseases overlapping between PD and AD in clinical and pathological manifestations, recognized by fluctuating cognitive dysfunction, visual hallucinations, and the appearance of LBs. Tanji et al. (2010) discerned that the immunoreactivity of TRIM9 was significantly reduced in the cytoplasm of hippocampal and temporal cortex neurons in DLB patients, and the levels of total TRIM9 protein and insoluble TRIM9 protein in DLB brain tissue were also decreased.
PSP, or Steel-Richardson-Olszewski syndrome, is an infrequent neurodegenerative disease manifesting as pseudobulbar palsy, vertical supranuclear ophthalmoplegia, extrapyramidal muscle rigidity, gait ataxia, and mild dementia, accompanied by symptoms of PD (Schrag et al. 1999). The Trim11 locus is a genetic modifier of PSP phenotype and has the potential as a target for disease modification and treatment (Jabbari et al. 2018). The intron variant rs564309 in Trim11, which is responsible for differences in clinical phenotypes of PSP, could lead to the low level of TRIM11 expression and might promote the neurofibrillary tangles (NFT) pathology and increase the burden of pathological P-tau in PSP (Valentino et al. 2020).
ALS is a fatal neuromuscular disease presented as progressive loss of motor neurons and muscle atrophy. It begins with the damage of cortical motor neurons and glial cells or neuromuscular junctions (NMJ) and then comes to progressive NMJ loss in early stages and eventually respiratory muscle weakness leading to death (Lepore et al. 2019; Boillee et al. 2006; Niedermeyer et al. 2019). The expression of TRIM63, an effect factor of muscular atrophy, was induced by the transcriptional activator TAp63 causing the aggravation of ALS (von Grabowiecki et al. 2016). Besides, TRIM72 is involved in the repair of NMJ injury (Yi et al. 2021). And dramatic intracellular TRIM72 aggregation was detected in the diaphragms of mice after establishing the ALS model and muscles of human ALS patients. However, when the rhTRIM72 protein was systematically administered to ALS mice, the diaphragm injury was reduced, the NMJ remained intact, and the progression of ALS was slowed down.
MS is the most common central nerve demyelinating disease, which belongs to autoimmune diseases. There are multiple inflammatory demyelinating spots in the white matter of the CNS in the acute active stage, and old lesions formed by calcified spots due to the proliferation of glial fibers. It has been proposed that MS is influenced by the human endogenous retrovirus (HERV) locus (Nexo et al. 2011; Hansen et al. 2011). Trims are one of the genes that have the effect of limiting retroviruses in humans. Trim5 and Trim22 are closely related to chromosome 11 and play different roles in regulating immune responses to retroviruses and other RNA viruses (Di Pietro et al. 2013). TRIM5α, the α isoform of TRIM5, is a typical example of TRIM protein acting as both a direct viral limiting factor and a pathogen recognition receptor (Pertel et al. 2011). Both Trim5 and Trim22 were proven to have a significant correlation with the onset of MS, providing evidence for the hypothesis that HERV may be involved in such diseases (Nexo et al. 2013). At the same time, the negative correlation between SNPs of Trim5 and Trim22 and the risk of MS may be linked to the change in immune response caused by HERV transcription, or even the reduction of HERV transcription (Morris et al. 2019).
Cerebrovascular Diseases
Cerebral stroke is an acute cerebrovascular disease caused by either a sudden rupture of cerebral vessels or a blockage that prevents blood from flowing to the brain. It consists of ischemic stroke (IS, 87%), hemorrhagic stroke (HS, 10%), and subarachnoid hemorrhage (SAH, 3%) (Tsao et al. 2022).
Ischemic Stroke
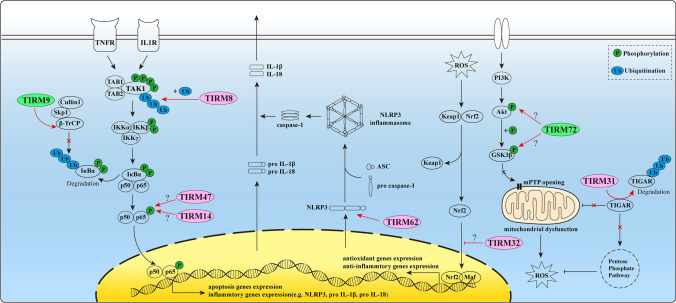
IS has the characteristics of high morbidity and recurrence, which can lead to disability and even death. The pathogenesis of IS is cerebral ischemia for multiple reasons, leading to functional disorders of the brain and necrosis of the brain tissue due to insufficient blood supply. What’s more, ischemia reperfusion (I/R) can also result in severe impairment of brain function. With the prolongation of I/R time, the content of excitatory transmitters will decrease, and the ultrastructure of brain tissue will be damaged, presenting irreversible injury. Several members of TRIM family proteins have been corroborated to be involved in the progression of IS (Fig. 4).
Fig. 4.
Partial mechanisms of TRIM proteins in IS. β-TrCP β-transducing repeat-containing protein, TNFR tumor necrosis factor-α receptor, IL1R interleukin 1 receptor, TAK transforming growth factor-β-activated kinase, TAB TAK binding protein, IKK IκB kinase, NLRP3 nucleotide-binding oligomerization domain-like receptor family pyrin domain-containing 3, ROS reactive oxygen species, mPTP mitochondrial permeability transition pore, TIGAR TP53-induced glycolysis and apoptosis regulator. ? The specific mechanism remains vague
TRIM8 was found to be significantly over-expressed in the peri-infarct cortex in mice. Besides, the down-regulation of TRIM8 showed neuroprotective effects, manifesting as reduced infarct size, decreased neurological deficit score, alleviated cognitive impairment, and reduction of hippocampal apoptosis after I/R (Bai et al. 2020). As for the further mechanism, TRIM8 regulated the tumor necrosis factor-α (TNF-α) and interleukin (IL)-1β-induced NF-κB activation by mediating K63-linked polyubiquitination of transforming growth factor-β-activated kinase 1 (TAK1), at the K158 residue (Li et al. 2011; Fan et al. 2010; Deng et al. 2019). It is worth noting that only when the cells were stimulated with TNF-α or IL-1β, such as when I/R injury occurred, did TRIM8 interact with TAK1 and then activate the NF-κB pathway to cause neuroinflammation and apoptosis, while this association was very weak under normal conditions (Li et al. 2011; Deng et al. 2019).
TRIM9 not only participates in PD and DLB process as mentioned above, but also relates to the protection against the IS damage. TRIM9 expression was up-regulated in the peri-infarct area after IS. Moreover, the anti-neuroinflammatory response mediated by TRIM9 conferred neuroprotective effects on IS mice (Zeng et al. 2019). Compared with wild-type (WT) mice, brain injury was aggravated and the infiltration of peripheral immune cells was increased in mice with TRIM9 deficiency. Mechanism studies showed that TRIM9 inhibited immune inflammatory response by dampening the activation of NF-κB pathway by interacting with β-transducing repeat-containing protein (β-TrCP) (Shi et al. 2014).
TRIM14 is important part of innate immunity(Nenasheva et al. 2021)Recently, the impact it exerts on I/R was reported. Interestingly, when I/R injury attacked, the expression of TRIM14 in hippocampal tissues was up-regulated and reached the peak at 24 h after reperfusion (Xie et al. 2021). And inhibition of TRIM14 alleviated I/R damage by weakening inflammatory response and reducing neuronal apoptosis, which registers as less dysfunction of learning and memory. Furthermore, TRIM14 might conduct these functions through NF-κB/nucleotide-binding oligomerization domain-like receptor family pyrin domain-containing 3 (NLRP3) pathway (Xie et al. 2021).
TRIM31 has been shown to be involved in a variety of pathological conditions by regulating the ubiquitination of corresponding proteins. It was down-regulated after cerebral ischemia. TRIM31 contributed to IS injury by mediating the ubiquitous degradation of TP53-induced glycolysis and apoptosis regulator (TIGAR) with its B-box domain (Zeng et al. 2021). TIGAR plays a critical role in pushing cells into pentose phosphate pathway (PPP) and preventing mitochondria from dysfunction after IS (Li et al. 2014; Zeng et al. 2021). During cerebral ischemia and hypoxia, cells lack oxygen and sufficient glucose supply, and cannot complete normal aerobic respiration to produce a large amount of energy ATP for physiological activities (Khoshnam et al. 2017). At this point, small amounts of glucose tend to be transferred to anaerobic respiration or PPP metabolism (Sun et al. 2017). Nonetheless, glycolysis will produce large amounts of ROS to further ruin the cells. In this case, the presence of TIGAR can reduce the level of fructose-2, 6-diphosphate in cells, resulting in the inhibition of glycolysis, and pushing glucose metabolism to PPP, which eventually leads to the overall decrease of intracellular ROS levels (Cao et al. 2015). Meanwhile, when cells were ischemic and hypoxic, oxidative stress caused mitochondrial dysfunction, which manifested as aberrant mitochondrial membrane potential, abnormal mitochondrial dynamics and less mitochondrial DNA (mtDNA) synthesis (Ong et al. 2015). When TRIM31 was knocked down in mice, the expression of TIGAR was increased, and thus alleviating the IS injury. Interestingly, when TIGAR was knocked down in TRIM31-deficient mice, cerebral ischemia damage was aggravated. This further demonstrated that the effect of TRIM31 on IS is TIGAR-dependent.
TRIM32 has been authenticated to play a potential role in neural regeneration (Fu et al. 2017). Wei et al. (2019) proved that TRIM32-KO was beneficial to the survival of primary hippocampal neurons in vitro under the condition of ischemia and hypoxia. The expression of TRIM32 was significantly increased in oxygen glucose deprivation/re-oxygenation (OGD/R)-treated hippocampal neurons. And siRNA interference with TRIM32 expression increased the cell viability of OGD/R-stimulated hippocampal neurons, the contents of SOD, and glutathione peroxidase, while it decreased the production of ROS. Inhibition of TRIM32 also increased the expression of bcl-2, while decreasing the expression of bax and the activity of caspase-3, which indicated that cell apoptosis was resisted. What’s more, down-regulation of TRIM32 enhanced the activation of the Nrf2 signaling pathway induced by OGD/R in hippocampal neurons. And the protective effect of TRIM32-KO on neurons can be removed after the interference of Nrf2. These results suggested that TRIM32-KO protected hippocampal neurons from OGD/R-induced oxidative damage by activating the Nrf2 signaling pathway.
TRIM47 is considered to take part in apoptosis and inflammation in many diseases (Ji et al. 2021). After IS injury, TRIM47 expression was significantly induced (Hao et al. 2019). And TRIM47-KO could alleviate the injury after middle cerebral artery occlusion (MCAO) in rats, and this was correlative with the inhibition of caspase-3 cleavage. In addition, decreasing TRIM47 expression significantly reduced the release of pro-inflammatory factors IL-6, TNF-α, and inducible nitric oxide synthase (iNOS) in the brain of MCAO rats, partly due to the blocking-up of NF-κB signaling pathway. Correspondingly, when TRIM47 was over-expressed, I/R pathological progress got accelerated by promoting apoptosis and inflammation.
TRIM62 is involved in innate immune responses as a modifier of inflammation (Schmidt et al. 2014). Recently, its functions in IS were discovered. It was significantly up-regulated in microglia treated with OGD, as well as in the peri-infarct region of WT mice after dealing with MCAO. By contrast, TRIM62 inhibition had a strong protective effect on IS by inhibiting NLRP3-regulated neuroinflammation. Furthermore, TRIM62 activated NLRP3 inflammasomes by interacting with them through its K63-polyubiquitin linkage, which augmented the neuroinflammatory response. Additionally, suppressing TRIM62-dependent NLRP3 activation could also inhibit NF-κB signaling, registering as the decreased transcriptional activities of IL-1β and IL-18 after I/R injury (Liu et al. 2020).
TRIM72, better known as MG53, is a muscle-specific TRIM family protein, which has a wide range of physiological and pathological effects, including regulating membrane repair mechanisms (Xie et al. 2020; Philouze et al. 2021). TRIM72 also exhibited the protective effect on mice suffering from I/R injury. Interestingly, it does not express in brain tissue, and skeletal muscle secreted TRIM72 cannot enter the brain through blood–brain barrier (BBB) under normal conditions. However, after IS occurred, both TRIM72 protein secreted from skeletal muscle and exogenous TRIM72 could directly reach the site injury in brain through BBB and play their roles in repairing cell membrane. As for mechanism studies, it was considered that the neuroprotective effect of rhTRIM72 was not only due to its protective effect on cell membrane integrity, but also linked to the inhibition of early cell apoptosis in cerebral infarction region mediated by Reperfusion Injury Salvage Kinase (RISK) signaling pathway activation via promoting the phosphorylation of Akt and GSK3β (Yao et al. 2016).
Hemorrhagic Stroke
The other subtype of stroke is HS, namely cerebral bleed, accounting for about 10% of stroke patients (Tsao et al. 2022). Risk factors proposed for HS include hypertension, diabetes, excessive alcohol consumption, smoking, and so on (Keep et al. 2012). HS has high mortality and even survivors are always left with severe neurological impairments (Gushchina et al. 2018).
TRIM37 was involved in the microglia apoptosis and IL-1β release by regulating peroxisome proliferator-activated receptor γ (PPARγ) ubiquitination after HS (Han et al. 2019). Compared with healthy controls, TRIM37 mRNA expression was up-regulated in peripheral blood mononuclear cells (PBMCs) of HS patients. In mouse microglia BV-2 cells, TRIM37 could bind to PPARγ, which played a neuroprotective role in HS, and enhance its ubiquitination. In general, thrombin was produced in the brain immediately after HS and meanwhile triggered the activation of microglia (Lee et al. 1996; Moller et al. 2000). And TRIM37 over-expression affected BV-2 cells similarly to thrombin. And treatment with PPARγ antagonists might eliminate such effect of TRIM37. Moreover, TRIM37 down-regulation could partially reverse thrombin-induced apoptosis and IL-1β release in BV-2 cells.
Traumatic Diseases in CNS
Traumatic Brain Injury
In addition to endogenous diseases, CNS injuries can also result from trauma to the brain or spinal cord. Traumatic brain injury (TBI), including primary brain injury and secondary brain injury, refers to a series of diseases and functional disorders caused by external damage. Primary brain injury is induced by direct or indirect compression, shearing, stretching, or torsion of the head. And subsequent events such as mitochondrial dysfunction, free radical damage, calcium overload, inflammation, and excitatory toxicity emerging after brain trauma are called secondary brain injury (Xiong et al. 2013; Chhor et al. 2017). Among TRIM family, TRIM32 and TRIM72 are the main members relevant to TBI research at the moment.
The inhibition of TRIM32 could enhance motor function repair after TBI by preventing apoptosis (Zhang et al. 2017). First, better neurological function was shown in TRIM32-KO TBI mice from day 1 to day 11 compared with WT mice after TBI. In addition, the expression of TRIM32 was significantly increased mainly in neurons at 7 days after TBI. Meanwhile, the number of apoptotic cells and the expression of protein 73 (p73) in TRIM32-KO mice were decreased. P73 is a member of the p53 superfamily with the function of inducing cell apoptosis (Jost et al. 1997; Kaghad et al. 1997). TAp73, the full-length p73, could combine with the Trim32 promoter and initiate its expression (Gonzalez-Cano et al. 2013; El Husseini et al. 2016). It was found that the down-regulation of TRIM32 might decrease TAp73 level through feedback regulation to reduce apoptosis, thus alleviating nerve injury after TBI.
TRIM72 could augment the survival rate and therapeutic effect of stem cells after TBI in mice (Guan et al. 2019). The recombinant human TRIM72 (rhTRIM72) protein was proven to protect human umbilical cord-derived mesenchymal stem cells (hUC-MSCs) from H2O2-induced oxidative damage and promoted their proliferation and migration. In the contusion brain injury model in mice, intravenous administration of TRIM72 protein helped transplanted hUC-MSCs survive and alleviated brain edema, neurological deficits, and anxiety and depression-like behaviors. Combined treatment with TRIM72 and hUC-MSCs promoted neurogenesis by improving PI3K/Akt-GSK3β signal transduction and reducing apoptosis. These results suggest that TRIM72 adjuvant therapy may provide a new strategy for alleviating brain injury and optimizing recovery.
Spinal Cord Injury
Spinal cord injury (SCI) is a disease caused by direct or indirect external factors, resulting in various motor, sensory, and sphincter dysfunction, muscle tone abnormalities, and pathological reflexes at the corresponding segment of the injury. SCI can lead to neuronal death, axon damage, and demyelination, eventually ending with permanent motor, sensory nerves, and autonomic nerves dysfunction (Johnson et al. 2010). Generally speaking, primary SCI refers to a traumatic impact on the spinal cord, resulting in immediate and irreversible tissue destruction and necrosis (McDonald et al. 2003). Secondary SCI makes the nearby surviving tissue becoming fragile, and then triggers a series of pathophysiological processes, including vascular biochemical reactions and inflammation. After the spinal cord trauma a few hours to several days, the tissue degeneration is further promoted, leading to neurological dysfunction (Lin et al. 2016).
At present, only TRIM32 has been revealed to be associated with SCI among the TRIM protein family. TRIM32 contains six repetitive NHL domains in addition to the iconic RBCC domain of the TRIM family, so it belongs to the TRIM-NHL protein, which is a conserved stem cell regulator (Schwamborn et al. 2009; Fu et al. 2015); it could promote the recovery of motor function after SCI by inhibiting the proliferation of glial cells (Fu et al. 2017). TRIM32 was rarely detected in normal glial cells, but was strongly expressed in astrocytes and microglia after SCI. In addition, the deletion of TRIM32 resulted in elevated astrocyte and microglia numbers, accompanied by increased cell proliferation and IL-1 and IL-10 secretion. After the SCI model was established in TRIM32−/− mice, the distance from corticospinal tract fibers to the site of injury was increased and axon initiation was decreased, causing delayed movement recovery. Furthermore, TRIM32 was unveiled to interact with ERK. And blocking epidermal growth factor receptor (EGFR) -ERK pathway could up-regulate TRIM32 expression and promote axonal regeneration in injured areas and functional recovery after SCI (Xue et al. 2020). TRIM32 could ubiquitinate c-Myc, an important protein in cell cycle regulation and proliferation, and regulate its degradation and promote the differentiation of NSCs into neurons, while ERK stabilized c-Myc expression and promoted cell proliferation (Sears et al. 2000; Ma et al. 2017; Alessi et al. 1995; Luscher et al. 1999).
Infectious Diseases in CNS
The major infectious diseases in CNS are encephalitis and meningitis. Meningitis is a diffuse inflammatory change caused by bacteria, viruses, fungi, spirochetes, protozoa, tumors, and leukemia or other biological pathogenic factors that invade the pia mater and spinal cord membrane. Encephalitis refers to inflammation of the brain resulting from exposure to pathogens. Encephalitis may occur in different genders and ages and always be acute or subacute. Clinically, it is characterized by high fever, headache, vomiting, coma, convulsion, and other symptoms, mostly accompanied by changes in cerebrospinal fluid components. TRIM proteins contribute to central infectious diseases mainly in two ways. One is mediating direct degradation of viral proteins with the E3 ligase activity. The other is promoting host innate immunity to exert the antiviral activity (Zhang et al. 2012; McNab et al. 2011; Fan et al. 2016).
TRIM32, which is involved in AD, TBI, and SCI, also participates in meningitis. Besides these, TRIM32 drove the pathological development of meningitis induced by Streptococcus suis via regulating the innate immune response (OuYang et al. 2020). TRIM32-KO significantly reduced bacteremia levels and pro-inflammatory cytokines production after severe S. suis infection, thus protecting mice from infection. Studies showed that TRIM32 helped S. suis survive, causing higher levels of bacteremia in the blood, and triggering an excessive pro-inflammatory immune response that led to an exacerbation of S. suis meningitis and higher mortality. Compared with infected WT control group, bacterial load level and cerebral hemorrhage indexes of infected Trim32−/− mice were reduced. In addition, TRIM32 deficiency increased the permeability of BBB and recruitment of inflammatory monocytes in the early stage of S. suis infection, thus limiting the development of S. suis meningitis.
Roles of TRIM21 in AD and PD have been described previously. As an immune modulator, it is also involved in Japanese Encephalitis virus (JEV)-induced encephalitis. JEV is a flavivirus with single-stranded RNA, which is the main cause of viral encephalitis in most Southeast Asian countries (Unni et al. 2011). The production of type I interferon (IFN) is essential for the antiviral response. Manocha et al. (2014) investigated the regulatory role of TRIM21 in the type I IFN pathway in human microglia infected with JEV. In this pathway, IFN regulatory factor 3 (IRF-3) plays a key role in viral infection response. Phosphorylation of IRF-3 leads to its activation, dimerization, and nuclear translocation, which in turn initiates the transcription of IFN-β. TRIM21 could interact with IRF-3, IRF-7, and IRF-8 and mediate their degradation through ubiquitination (Yang et al. 2009). Therefore, TRIM21 directly regulated type I IFN signal transduction by regulating upstream transcription factors. JEV infection up-regulated TRIM21 expression in human microglial cells (CHME3). Also, TRIM21 dampened IFN-β production by reducing IRF-3 phosphorylation and inhibited the process of natural immune responses.
Another TRIM family member, TRIM79α, also influences the course of encephalitis by affecting the type I IFN pathway. However, unlike TRIM21, TRIM79α is an IFN-stimulated gene (ISG) product that enhances the antiviral effect of type I IFN. It could inhibit the replication of tick-borne encephalitis virus (TBEV) by degrading viral RNA polymerase (Taylor et al. 2011). TBEV, also known as forest encephalitis virus, is a highly virulent neurotropic virus, mainly invading CNS, causing high mortality and disability rates. TRIM79α limited TBEV replication by mediating lysosomal degradation of flavivirus non-structural protein 5 (NS5), which is essential for viral replication. TRIM79α specifically recognized NS5 from TBEV but did not recognize NS5 from West Nile virus (WNV) or inhibit its replication. In the absence of TRIM79α, the antiviral effect of IFN-β was weakened in RAW 264.7 cells. In addition, TRIM79α degraded the NS5 of TBEV in a proteasome-independent way, but the turnover of TRIM79α was regulated by the proteasome.
TRIM52 is a noncanonical TRIM protein with a unique expanded RING domain (Fan et al. 2017). It is associated with various biological functions, including cell proliferation, regulation of inflammation,and antiviral effect (Mu et al. 2019; Liu et al. 2020; Fan et al. 2016). Unlike TRIM79α, TRIM52 resisted the replication of JEV by ubiquitinating and degrading virus NS2A in a proteasome-dependent manner. Structurally, the RING domain was crucial to the antiviral function of TRIM52 (Fan et al. 2016).
Conclusion and Perspective
At present, TRIM protein has been widely studied in the fields of antiviral and tumor, but not so much in CNS diseases. Herein, we summarize the research results of TRIM proteins in various CNS diseases in recent years, hoping to provide references for future related studies (Table 2).
Table 2.
Effects of TRIM protein members in CNS diseases
| Disease | Protein | Effect | References |
|---|---|---|---|
| AD | TRIM21 |
Delayed AD occurrence by preventing misfolded P-tau from assembling |
McEwan et al. (2017) |
| TRIM28 | Accelerated AD process by promoting the intranuclear accumulation of α-Syn and P-tau in cell nuclei | Rousseaux et al. (2016) | |
| Deletion of Trim28 had little effect on mature tissues | Rousseaux et al. (2018) | ||
| TRIM32 | Delayed AD occurrence by inhibiting the Notch pathway and preventing the damage of synaptic plasticity | Ntim et al. (2020) | |
| TRIM59 | Delayed the pathological process of AD by promoting the degradation of p53 | Wezyk et al. (2018a, b) | |
| TRIML2 | Accelerated AD process by promoting p53-mediated apoptosis | Kang et al. (2016) | |
| PD | TRIM2 | Delayed PD pathology by preventing the abnormal accumulation of nerve filament light chain | Balastik et al. (2008) |
| TRIM3 | Attenuated PD by inhibiting apoptosis via activating PI3K/AKT signal pathway, enhanced the anti-oxidant capacity | Dong et al. (2020) | |
| TRIM9 | Delayed the pathological process of PD by promoting the axon respond to the extracellular guidance cues | Tanji et al. (2010) | |
| TRIM67 | Accelerated the pathological process of PD by inhibiting the axon respond to the extracellular guidance cues | ||
| TRIM10 | Accelerated the PD process by increasing PD-induced apoptosis and ROS overproduction and accelerating ubiquitination and degradation of DUSP6 | Huang et al. (2019) | |
| TRIM11 TRIM21 TRIM19 | Attenuated PD by mediating degradation of Atxn1 82Q | Zhu et al. (2020) | |
|
TRIM17 TRIM41 |
TRIM17 promoted α-Syn accumulation by restraining the inhibitory effect of TRIM41 on ZSCAN21, which accelerated the PD process | Lassot et al. (2018) | |
| DLB | TRIM9 | Expressed less in DLB brain | Tanji et al. (2010) |
| PSP | TRIM11 | Increased the burden of pathological P-tau in PSP | Valentino et al. (2020) |
| ALS | TRIM63 | Aggravated ALS through being induced by TAp63 | Von Grabowiecki et al. (2016) |
| TRIM72 (MG53) | Attenuated ALS by repairing NMJ injury | Yi et al. (2021) | |
| MS | TRIM5α TRIM22 | Provided evidence for HERV involvement in MS | Nexo et al. (2013), Morris et al. (2019) |
| IS | TRIM8 | Aggravated IS by activating the NF-κB pathway and promoting inflammation and apoptosis | Bai et al. (2020), Zhang et al. (2020a, b, c) |
| TRIM9 | Alleviated IS by inhibiting the NF-κB pathway | Zeng et al. (2019) | |
| TRIM14 TRIM62 | Aggravated IS by activating NF-κB/NLRP3 pathway and promoting inflammation and apoptosis | Xie et al. (2021), Liu et al. (2020) | |
| TRIM31 | Aggravated IS by degrading TIGAR, inhibiting the mitochondrial dysfunction and promoting cells into PPP | Cho et al. (2013), Zeng et al. (2021) | |
| TRIM32 | Aggravated oxidative stress injury and apoptosis after OGD injury in hippocampal neurons by inhibiting the Nrf2 pathway | Wei et al. (2019) | |
| TRIM47 | Aggravated IS by promoting inflammation by activating NF-κB pathway and promoting apoptosis through Caspase-3 pathway | Hao et al. (2019) | |
| TRIM72 (MG53) | Alleviated IS injury by activating the RISK survival pathway | Yao et al. (2016) | |
| HS | TRIM37 | Aggravated HS by ubiquitinating PPARγ and promoting apoptosis and IL-1β release | Han et al. (2019) |
| TBI | TRIM32 | Up-regulated TAp73 through feedback regulation to promote apoptosis, aggravate nerve injury after TBI | Zhang et al. (2017) |
| TRIM72 (MG53) | Promoted neurogenesis by improving PI3K/Akt-GSK3β signal transduction, reduced apoptosis | Guan et al. (2019) | |
| SCI | TRIM32 | Promoted axonal regeneration and the recovery of motor function after SCI by inhibiting the proliferation of glial cells | Xue et al. (2020), Fu et al. (2017) |
| Meningitis | TRIM32 | Promoted the pathological development of meningitis caused by Streptococcus suis by regulating the innate immune response | OuYang et al. (2020) |
| Encephalitis | TRIM21 | Interfered the antiviral effect of type I IFN by reducing I/RF3 phosphorylation, inhibited the process of natural immune responses | Manocha et al. (2014) |
| TRIM79α | Enhanced the antiviral effect of type I IFN, resist TBEV replication by mediating lysosomal degradation of flavivirus NS5 | Taylor et al. (2011) | |
| TRIM52 | Resisted JEV replication by degrading virus NS2A | Fan et al. (2016) |
To date, several roles of TRIMs were found in CNS diseases. They mainly regulate the process of protein homeostasis, inflammation, oxidative stress, and apoptosis in CNS. Besides, they could also exert functions in the synaptic plasticity, neurogenesis, axonal guidance, glial proliferation, and mitochondrial dysfunction. TRIMs influence these processes mainly by the following mechanisms. Firstly, the most intuitive function of TRIMs is regulating the protein level by mediating the ubiquitin–proteasome degradation, such as TRIM2, TRIM9, TRIM10, TRIM41 in PD, TRIM31 in IS, TRIM37 in HS, TRIM32 in SCI, as well as TRIM21 and TRIM52 in encephalitis (Zeng et al. 2021; Xue et al. 2020; Tanji et al. 2010; Manocha et al. 2014; Lassot et al. 2018; Huang et al. 2019; Han et al. 2019; Fan et al. 2016; Balastik et al. 2008). To be more specific, for example, TRIM41 could ubiquitinate and mediate the degradation of ZSCAN21, decrease the amount of ZSCAN entering the nucleus, and thus reduce ZNSA encoding to α-Syn (Lassot et al. 2018). Secondly, TRIM protein can sometimes SUMOylate other proteins to affect their localization or stability. The case in point is TRIM28, TRIML2 in AD and TRIM11, TRIM19, and TRIM21 in PD (Rousseaux et al. 2016; Kang et al. 2016; Zhu et al. 2020). TRIM28 increased the intranuclear aggregation of α-Syn and P-tau by SUMOylating them (Rousseaux et al. 2018). TRIML2 SUMOylated p53 and increased its steady-state level (Kang et al. 2016). TRIM11, TRIM19 and TRIM21 SUMOylated defective proteins and made them ubiquitinated by STUbL and degraded (Zhu et al. 2020). Thirdly, some TRIMs serve as molecular chaperones or disaggragases in PQC to ensure proteins exert normal physiological functions. For example, TRIM11, TRIM19, and TRIM21 could not only prevent accumulation of anomalous proteins but dissolve pre-exist protein aggregation depending on their multiple functions (Zhu et al. 2020). In addition, some TRIMs get involved in innate immunity in CNS infectious diseases. TRIM32 activated excessive pro-inflammatory response and aggravated S. suis meningitis (OuYang et al. 2020). TRIM21 lessened the production of IFN-β and inhibited innate immunity (Manocha et al. 2014). Apart from these, TRIM79α restricted the replication of TBEV by mediating the lysosome-dependent degradation of NS5, while TRIM52 inhibited the replication of JEV by involving the degradation of NS2A in the ubiquitin-proteosome pathway (Fan et al. 2016; Taylor et al. 2011). In addition, TRIMs also play roles in regulating a series of signaling pathways. For instance, TRIM8 ubiquitinated and activated TAK1, thus triggering the NF-κB signaling pathway after the stimulation of TNF-α or IL-1β (Bai et al. 2020). Unlike TRIM8, the deep-seated mechanisms behind many other TRIMs associated with signaling pathways still remain unclear. For example, TRIM32 could dampen the activation of the Notch signaling pathway, preventing the synaptic plasticity and thus avoiding AD (Ntim et al. 2020); meanwhile, it could also activate the Nrf2 pathway to worsen the ischemia and hypoxia injury of hippocampal neurons (Wei et al. 2019). TRIM3 could activate PI3K/Akt pathway, leading to apoptosis inhibition in PD (Dong et al. 2020). TRIM9, TRIM14, and TRIM47 could influence IS through NF-κB pathway (Zeng et al. 2019; Xie et al. 2021; Hao et al. 2019; Bai et al. 2020), while TRIM14 and TRIM62 were involved in NLRP3 pathway (Xie et al. 2021; Liu et al. 2020). Besides, TRIM72 could alleviate IS injury by activating the RISK signaling pathway (Yao et al. 2016).
Collating the existing research results, it can be seen that different TRIM family members may have different effects on the same disease. What is noteworthy is that one TRIM protein may have different effects in different CNS diseases, which may be beneficial for one disease while causing the occurrence or aggravation of another. In the development of drugs targeting such proteins, attention should be paid to balancing the relationship between therapeutic effects and side effects to achieve the best therapeutic consequence. At the same time, this also provides an idea for further research that is whether the same protein expressed at different locations in the body has different effects. For example, some TRIM proteins distribute in not only CNS, but also some important organs, such as liver and kidney (Ponten et al. 2008). In this case, manipulating TRIMs to treat diseases may face with the risk of organ damage. If so, conditionally inhibiting or enhancing the expression of a protein at a specific site may achieve the purpose of precision therapy. Another point for developing medications for CNS diseases is that whether the complexes can pass through the BBB, which protects the brain from harmful substances in blood circulation while increasing the difficulty of drug treatment in brain disorders.
Recently, proteolysis-targeting chimeras (PROTACs) give novel insight into applications of TRIM proteins (Lu et al. 2022). PROTAC technology employs heterobifunctional molecules linking a ligand of protein of interest (POI) with a ligand of E3 ligases, to recruit the POI and the E3 ligase and push the endogenous target protein to get ubiquitinated and degraded (Farrell and Jarome 2021; Ma et al. 2021; Lu et al. 2022). This emerging method leads the way to develop new drugs with high efficiency. To date, more than 15 PROTAC molecules have entered clinical trials (Mullard 2021). In the field of treating CNS diseases, PROTACs have not been utilized in clinic, but some progress has been made in preclinical research (Farrell and Jarome 2021). For instance, PROTACs can be employed to degrade P-tau in AD and to target bromodomain-containing protein 4 (BRD4) treating IS (Jangampalli Adi et al. 2021; Wang et al. 2021; Liu et al. 2022). And the ability to pass through the BBB makes it possible to work on alleviating neurodegenerative diseases (Yang et al. 2022). However, one of the main challenges of this technique is the lack of E3 ligases for targeted degradation. As typical E3 ligases, it is feasible to take TRIM proteins as targets of PROTACs to design promising PROTAC drugs.
In summary, the mechanisms TRIM proteins get involved in CNS diseases are still in the primary stage. But their roles in treating these disorders are promising and preclinical studies have been carried out on some drugs targeting TRIM proteins. In future, much effort needs to be made on disclosing clearer principles of TRIM protein actions and regulating them precisely to get the best therapeutic efficacy.
Author contributions
MP contributed to conception of the study, acquisition of data and analysis, and drafting the article. XL contributed to conception of the study and revising article critically. GX and XT contributed to revising article critically. YL contributed to study supervision. WF contributed to review, revision of the manuscript, and study supervision. All authors approved the version to be published and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Funding
This work was supported by the Natural Science Foundation of Jiangsu Province of China (Program No. BK202013 28) and the Natural Science Foundation of China (Program No. 82073845, 82174051).
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Yunman Li, Email: liyunman@cpu.edu.cn.
Weirong Fang, Email: weirongfang@cpu.edu.cn.
References
- Abate G, Frisoni GB, Bourdon JC, Piccirella S, Memo M, Uberti D (2020) The pleiotropic role of p53 in functional/dysfunctional neurons: focus on pathogenesis and diagnosis of Alzheimer’s disease. Alzheimers Res Ther 12(1):160. 10.1186/s13195-020-00732-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alessi DR, Cuenda A, Cohen P, Dudley DT, Saltiel AR (1995) PD 098059 is a specific inhibitor of the activation of mitogen-activated protein kinase kinase in vitro and in vivo. J Biol Chem 270(46):27489–27494. 10.1074/jbc.270.46.27489 [DOI] [PubMed] [Google Scholar]
- Bai X, Zhang YL, Liu LN (2020) Inhibition of TRIM8 restrains ischemia-reperfusion-mediated cerebral injury by regulation of NF-kappaB activation associated inflammation and apoptosis. Exp Cell Res 388(2):111818. 10.1016/j.yexcr.2020.111818 [DOI] [PubMed] [Google Scholar]
- Bai X, Fu RJ, Zhang S, Yue SJ, Chen YY, Xu DQ, Tang YP (2021) Potential medicinal value of celastrol and its synthesized analogues for central nervous system diseases. Biomed Pharmacother 139:111551. 10.1016/j.biopha.2021.111551 [DOI] [PubMed] [Google Scholar]
- Balastik M, Ferraguti F, Pires-da Silva A, Lee TH, Alvarez-Bolado G, Lu KP, Gruss P (2008) Deficiency in ubiquitin ligase TRIM2 causes accumulation of neurofilament light chain and neurodegeneration. Proc Natl Acad Sci U S A 105(33):12016–12021. 10.1073/pnas.0802261105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett JC, Fry B, Maller J, Daly MJ (2005) Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics 21(2):263–265. 10.1093/bioinformatics/bth457 [DOI] [PubMed] [Google Scholar]
- Basu-Shrivastava M, Kozoriz A, Desagher S, Lassot I (2021) To ubiquitinate or not to ubiquitinate: TRIM17 in cell life and death. Cells 10(5):1235. 10.3390/cells10051235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell JL, Malyukova A, Holien JK, Koach J, Parker MW, Kavallaris M, Marshall GM, Cheung BB (2012) TRIM16 acts as an E3 ubiquitin ligase and can heterodimerize with other TRIM family members. PLoS ONE 7(5):e37470. 10.1371/journal.pone.0037470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernardi R, Pandolfi PP (2007) Structure, dynamics and functions of promyelocytic leukaemia nuclear bodies. Nat Rev Mol Cell Biol 8(12):1006–1016. 10.1038/nrm2277 [DOI] [PubMed] [Google Scholar]
- Boillee S, Vande Velde C, Cleveland DW (2006) ALS: a disease of motor neurons and their nonneuronal neighbors. Neuron 52(1):39–59. 10.1016/j.neuron.2006.09.018 [DOI] [PubMed] [Google Scholar]
- Boyer NP, McCormick LE, Menon S, Urbina FL, Gupton SL (2020) A pair of E3 ubiquitin ligases compete to regulate filopodial dynamics and axon guidance. J Cell Biol. 10.1083/jcb.201902088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak HEB (1991) Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol 82(4):239–259 [DOI] [PubMed] [Google Scholar]
- Buizza L, Cenini G, Lanni C, Ferrari-Toninelli G, Prandelli C, Govoni S, Buoso E, Racchi M, Barcikowska M, Styczynska M, Szybinska A, Butterfield DA, Memo M, Uberti D (2012) Conformational altered p53 as an early marker of oxidative stress in Alzheimer’s disease. PLoS ONE 7(1):e29789. 10.1371/journal.pone.0029789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cammas F, Mark M, Dolle P, Dierich A, Chambon P, Losson R (2000) Mice lacking the transcriptional corepressor TIF1beta are defective in early postimplantation development. Development 127(13):2955–2963. 10.1242/dev.127.13.2955 [DOI] [PubMed] [Google Scholar]
- Cao L, Chen J, Li M, Qin YY, Sun M, Sheng R, Han F, Wang G, Qin ZH (2015) Endogenous level of TIGAR in brain is associated with vulnerability of neurons to ischemic injury. Neurosci Bull 31(5):527–540. 10.1007/s12264-015-1538-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caraveo G, Auluck PK, Whitesell L, Chung CY, Baru V, Mosharov EV, Yan X, Ben-Johny M, Soste M, Picotti P, Kim H, Caldwell KA, Caldwell GA, Sulzer D, Yue DT, Lindquist S (2014) Calcineurin determines toxic versus beneficial responses to alpha-synuclein. Proc Natl Acad Sci U S A 111(34):E3544-3552. 10.1073/pnas.1413201111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang R, Xu X, Li MD (2002) Molecular cloning, mapping and characterization of a novel mouse RING finger gene, Mrf1. Gene 291(1–2):241–249. 10.1016/s0378-1119(02)00603-0 [DOI] [PubMed] [Google Scholar]
- Chen L, Brewer MD, Guo L, Wang R, Jiang P, Yang X (2017) Enhanced degradation of misfolded proteins promotes tumorigenesis. Cell Rep 18(13):3143–3154. 10.1016/j.celrep.2017.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Zhu G, Johns EM, Yang X (2018) TRIM11 activates the proteasome and promotes overall protein degradation by regulating USP14. Nat Commun 9(1):1223. 10.1038/s41467-018-03499-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen RH, Chen YH, Huang TY (2019) Ubiquitin-mediated regulation of autophagy. J Biomed Sci 26(1):80. 10.1186/s12929-019-0569-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Wu H, Zhang M (2021) Long non-coding RNA: An underlying bridge linking neuroinflammation and central nervous system diseases. Neurochem Int 148:105101. 10.1016/j.neuint.2021.105101 [DOI] [PubMed] [Google Scholar]
- Cheng CT, Kuo CY, Ann DK (2014) KAPtain in charge of multiple missions: emerging roles of KAP1. World J Biol Chem 5(3):308–320. 10.4331/wjbc.v5.i3.308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chhor V, Moretti R, Le Charpentier T, Sigaut S, Lebon S, Schwendimann L, Ore MV, Zuiani C, Milan V, Josserand J, Vontell R, Pansiot J, Degos V, Ikonomidou C, Titomanlio L, Hagberg H, Gressens P, Fleiss B (2017) Role of microglia in a mouse model of paediatric traumatic brain injury. Brain Behav Immun 63:197–209. 10.1016/j.bbi.2016.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho B, Choi SY, Cho HM, Kim HJ, Sun W (2013) Physiological and pathological significance of dynamin-related protein 1 (drp1)-dependent mitochondrial fission in the nervous system. Exp Neurobiol 22(3):149–157. 10.5607/en.2013.22.3.149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chouliaras L, Mastroeni D, Delvaux E, Grover A, Kenis G, Hof PR, Steinbusch HW, Coleman PD, Rutten BP, van den Hove DL (2013) Consistent decrease in global DNA methylation and hydroxymethylation in the hippocampus of Alzheimer’s disease patients. Neurobiol Aging 34(9):2091–2099. 10.1016/j.neurobiolaging.2013.02.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng Y, Chen D, Wang L, Gao F, Jin B, Lv H, Zhang G, Sun X, Liu L, Mo D, Ma N, Song L, Huo X, Yan T, Miao Z (2019) Silencing of long noncoding RNA nespas aggravates microglial cell death and neuroinflammation in ischemic stroke. Stroke 50(7):1850–1858. 10.1161/STROKEAHA.118.023376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Pietro A, Kajaste-Rudnitski A, Oteiza A, Nicora L, Towers GJ, Mechti N, Vicenzi E (2013) TRIM22 inhibits influenza A virus infection by targeting the viral nucleoprotein for degradation. J Virol 87(8):4523–4533. 10.1128/JVI.02548-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong W, Qiu C, Gong D, Jiang X, Liu W, Liu W, Zhang L, Zhang W (2019) Proteomics and bioinformatics approaches for the identification of plasma biomarkers to detect Parkinson’s disease. Exp Ther Med 18(4):2833–2842. 10.3892/etm.2019.7888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong W, Luo B, Qiu C, Jiang X, Shen B, Zhang L, Liu W, Zhang W (2020) TRIM3 attenuates apoptosis in Parkinson’s disease via activating PI3K/AKT signal pathway. Aging 13(1):735–749. 10.18632/aging.202181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Husseini N, Schlisser AE, Hales BF (2016) Editor’s highlight: hydroxyurea exposure activates the P53 signaling pathway in murine organogenesis-stage embryos. Toxicol Sci 152(2):297–308. 10.1093/toxsci/kfw089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Y, Yu Y, Shi Y, Sun W, Xie M, Ge N, Mao R, Chang A, Xu G, Schneider MD, Zhang H, Fu S, Qin J, Yang J (2010) Lysine 63-linked polyubiquitination of TAK1 at lysine 158 is required for tumor necrosis factor alpha- and interleukin-1beta-induced IKK/NF-kappaB and JNK/AP-1 activation. J Biol Chem 285(8):5347–5360. 10.1074/jbc.M109.076976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan W, Wu M, Qian S, Zhou Y, Chen H, Li X, Qian P (2016) TRIM52 inhibits Japanese Encephalitis Virus replication by degrading the viral NS2A. Sci Rep 6:33698. 10.1038/srep33698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan W, Liu T, Li X, Zhou Y, Wu M, Cui X, Chen H, Qian P (2017) TRIM52: A nuclear TRIM protein that positively regulates the nuclear factor-kappa B signaling pathway. Mol Immunol 82:114–122. 10.1016/j.molimm.2017.01.003 [DOI] [PubMed] [Google Scholar]
- Farrell K, Jarome TJ (2021) Is PROTAC technology really a game changer for central nervous system drug discovery? Expert Opin Drug Discov 16(8):833–840. 10.1080/17460441.2021.1915979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu B, Wang L, Ding H, Schwamborn JC, Li S, Dorf ME (2015) TRIM32 senses and restricts influenza a virus by ubiquitination of PB1 polymerase. PLoS Pathog 11(6):e1004960. 10.1371/journal.ppat.1004960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Q, Zou MM, Zhu JW, Zhang Y, Chen WJ, Cheng M, Liu CF, Ma QH, Xu RX (2017) TRIM32 affects the recovery of motor function following spinal cord injury through regulating proliferation of glia. Oncotarget 8(28):45380–45390. 10.18632/oncotarget.17492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Cano L, Hillje AL, Fuertes-Alvarez S, Marques MM, Blanch A, Ian RW, Irwin MS, Schwamborn JC, Marin MC (2013) Regulatory feedback loop between TP73 and TRIM32. Cell Death Dis 4:e704. 10.1038/cddis.2013.224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan F, Huang T, Wang X, Xing Q, Gumpper K, Li P, Song J, Tan T, Yang GL, Zang X, Zhang J, Wang Y, Yang Y, Liu Y, Zhang Y, Yang B, Ma J, Ma S (2019) The TRIM protein Mitsugumin 53 enhances survival and therapeutic efficacy of stem cells in murine traumatic brain injury. Stem Cell Res Ther 10(1):352. 10.1186/s13287-019-1433-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo L, Giasson BI, Glavis-Bloom A, Brewer MD, Shorter J, Gitler AD, Yang X (2014) A cellular system that degrades misfolded proteins and protects against neurodegeneration. Mol Cell 55(1):15–30. 10.1016/j.molcel.2014.04.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gushchina LV, Kwiatkowski TA, Bhattacharya S, Weisleder NL (2018) Conserved structural and functional aspects of the tripartite motif gene family point towards therapeutic applications in multiple diseases. Pharmacol Ther 185:12–25. 10.1016/j.pharmthera.2017.10.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han C, Xia X, Jiao S, Li G, Ran Q, Yao S (2019) Tripartite motif containing protein 37 involves in thrombin stimulated BV-2 microglial cell apoptosis and interleukin 1beta release. Biochem Biophys Res Commun 516(4):1252–1257. 10.1016/j.bbrc.2019.06.158 [DOI] [PubMed] [Google Scholar]
- Hansen B, Oturai AB, Harbo HF, Celius EG, Nissen KK, Laska MJ, Sondergaard HB, Petersen T, Nexo BA (2011) Genetic association of multiple sclerosis with the marker rs391745 near the endogenous retroviral locus HERV-Fc1: analysis of disease subtypes. PLoS ONE 6(10):e26438. 10.1371/journal.pone.0026438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao MQ, Xie LJ, Leng W, Xue RW (2019) Trim47 is a critical regulator of cerebral ischemia-reperfusion injury through regulating apoptosis and inflammation. Biochem Biophys Res Commun 515(4):651–657. 10.1016/j.bbrc.2019.05.065 [DOI] [PubMed] [Google Scholar]
- Hatakeyama S (2017) TRIM family proteins: roles in autophagy, immunity, and carcinogenesis. Trends Biochem Sci 42(4):297–311. 10.1016/j.tibs.2017.01.002 [DOI] [PubMed] [Google Scholar]
- Hattingen E, Magerkurth J, Pilatus U, Mozer A, Seifried C, Steinmetz H, Zanella F, Hilker R (2009) Phosphorus and proton magnetic resonance spectroscopy demonstrates mitochondrial dysfunction in early and advanced Parkinson’s disease. Brain 132(Pt 12):3285–3297. 10.1093/brain/awp293 [DOI] [PubMed] [Google Scholar]
- Hillje AL, Pavlou MA, Beckmann E, Worlitzer MM, Bahnassawy L, Lewejohann L, Palm T, Schwamborn JC (2013) TRIM32-dependent transcription in adult neural progenitor cells regulates neuronal differentiation. Cell Death Dis 4:e976. 10.1038/cddis.2013.487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hristova V, Sun S, Zhang H, Chan DW (2020) Proteomic analysis of degradation ubiquitin signaling by ubiquitin occupancy changes responding to 26S proteasome inhibition. Clin Proteomics 17:2. 10.1186/s12014-020-9265-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Q, Zhu X, Xu M (2019) Silencing of TRIM10 alleviates apoptosis in cellular model of Parkinson’s disease. Biochem Biophys Res Commun 518(3):451–458. 10.1016/j.bbrc.2019.08.041 [DOI] [PubMed] [Google Scholar]
- Jabbari E, Woodside J, Tan MMX, Shoai M, Pittman A, Ferrari R, Mok KY, Zhang D, Reynolds RH, de Silva R, Grimm MJ, Respondek G, Muller U, Al-Sarraj S, Gentleman SM, Lees AJ, Warner TT, Hardy J, Revesz T, Hoglinger GU, Holton JL, Ryten M, Morris HR (2018) Variation at the TRIM11 locus modifies progressive supranuclear palsy phenotype. Ann Neurol 84(4):485–496. 10.1002/ana.25308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jangampalli AP (1867) Reddy PH (2021) Phosphorylated tau targeted small-molecule PROTACs for the treatment of Alzheimer’s disease and tauopathies. Biochim Biophys Acta 8:166162. 10.1016/j.bbadis.2021.166162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji B, Liu L, Guo Y, Ming F, Jiang J, Li F, Zhao G, Wen J, Li N (2021) Upregulated tripartite motif 47 could facilitate glioma cell proliferation and metastasis as a tumorigenesis promoter. Comput Math Methods Med 2021:5594973. 10.1155/2021/5594973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson PJ, Parker SR, Sakiyama-Elbert SE (2010) Fibrin-based tissue engineering scaffolds enhance neural fiber sprouting and delay the accumulation of reactive astrocytes at the lesion in a subacute model of spinal cord injury. J Biomed Mater Res A 92(1):152–163. 10.1002/jbm.a.32343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jost CA, Marin MC, Kaelin WG Jr (1997) p73 is a simian [correction of human] p53-related protein that can induce apoptosis. Nature 389(6647):191–194. 10.1038/38298 [DOI] [PubMed] [Google Scholar]
- Kaghad M, Bonnet H, Yang A, Creancier L, Biscan JC, Valent A, Minty A, Chalon P, Lelias JM, Dumont X, Ferrara P, McKeon F, Caput D (1997) Monoallelically expressed gene related to p53 at 1p36, a region frequently deleted in neuroblastoma and other human cancers. Cell 90(4):809–819. 10.1016/s0092-8674(00)80540-1 [DOI] [PubMed] [Google Scholar]
- Kalaani J, Roche J, Hamade E, Badran B, Jaber M, Gaillard A, Prestoz L (2016) Axon guidance molecule expression after cell therapy in a mouse model of Parkinson’s disease. Restor Neurol Neurosci 34(6):877–895. 10.3233/RNN-150587 [DOI] [PubMed] [Google Scholar]
- Kang WS, Park JK, Kim YJ, Cho AR, Park HJ, Kim SK, Paik JW, Lee KJ, Na HR, Kim YY, Lim HK, Jeong HG, Kim JW (2016) Association of tripartite motif family-like 2 (TRIML2) polymorphisms with late-onset Alzheimer’s disease risk in a Korean population. Neurosci Lett 630:127–131. 10.1016/j.neulet.2016.07.046 [DOI] [PubMed] [Google Scholar]
- Keeble AH, Khan Z, Forster A, James LC (2008) TRIM21 is an IgG receptor that is structurally, thermodynamically, and kinetically conserved. Proc Natl Acad Sci U S A 105(16):6045–6050. 10.1073/pnas.0800159105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keep RF, Hua Y, Xi G (2012) Intracerebral haemorrhage: mechanisms of injury and therapeutic targets. Lancet Neurol 11(8):720–731. 10.1016/S1474-4422(12)70104-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan F, Khan TJ, Kalamegam G, Pushparaj PN, Chaudhary A, Abuzenadah A, Kumosani T, Barbour E, Al-Qahtani M (2017) Anti-cancer effects of Ajwa dates (Phoenix dactylifera L.) in diethylnitrosamine induced hepatocellular carcinoma in Wistar rats. BMC Complement Altern Med 17(1):418. 10.1186/s12906-017-1926-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoshnam SE, Winlow W, Farzaneh M, Farbood Y, Moghaddam HF (2017) Pathogenic mechanisms following ischemic stroke. Neurol Sci 38(7):1167–1186. 10.1007/s10072-017-2938-1 [DOI] [PubMed] [Google Scholar]
- Kung CP, Khaku S, Jennis M, Zhou Y, Murphy ME (2015) Identification of TRIML2, a novel p53 target, that enhances p53 SUMOylation and regulates the transactivation of proapoptotic genes. Mol Cancer Res 13(2):250–262. 10.1158/1541-7786.MCR-14-0385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert C, Cisternas P, Inestrosa NC (2016) Role of Wnt signaling in central nervous system injury. Mol Neurobiol 53(4):2297–2311. 10.1007/s12035-015-9138-x [DOI] [PubMed] [Google Scholar]
- Lanni C, Uberti D, Racchi M, Govoni S, Memo M (2007) Unfolded p53: a potential biomarker for Alzheimer’s disease. J Alzheimers Dis 12(1):93–99. 10.3233/jad-2007-12109 [DOI] [PubMed] [Google Scholar]
- Lassot I, Robbins I, Kristiansen M, Rahmeh R, Jaudon F, Magiera MM, Mora S, Vanhille L, Lipkin A, Pettmann B, Ham J, Desagher S (2010) Trim17, a novel E3 ubiquitin-ligase, initiates neuronal apoptosis. Cell Death Differ 17(12):1928–1941. 10.1038/cdd.2010.73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lassot I, Mora S, Lesage S, Zieba BA, Coque E, Condroyer C, Bossowski JP, Mojsa B, Marelli C, Soulet C, Tesson C, Carballo-Carbajal I, Laguna A, Mangone G, Vila M, Brice A, Desagher S (2018) The E3 ubiquitin ligases TRIM17 and TRIM41 modulate alpha-synuclein expression by regulating ZSCAN21. Cell Rep 25(9):2484–2496. 10.1016/j.celrep.2018.11.002 [DOI] [PubMed] [Google Scholar]
- Lee D, Hong JH (2022) Physiological overview of the potential link between the UPS and Ca(2+) signaling. Antioxidants (Basel) 11(5):997. 10.3390/antiox11050997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KR, Colon GP, Betz AL, Keep RF, Kim S, Hoff JT (1996) Edema from intracerebral hemorrhage: the role of thrombin. J Neurosurg 84(1):91–96. 10.3171/jns.1996.84.1.0091 [DOI] [PubMed] [Google Scholar]
- Lee JH, Cheng R, Vardarajan B, Lantigua R, Reyes-Dumeyer D, Ortmann W, Graham RR, Bhangale T, Behrens TW, Medrano M, Jimenez-Velazquez IZ, Mayeux R (2015) Genetic modifiers of age at onset in carriers of the G206A mutation in PSEN1 with familial Alzheimer disease among Caribbean Hispanics. JAMA Neurol 72(9):1043–1051. 10.1001/jamaneurol.2015.1424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepore E, Casola I, Dobrowolny G, Musaro A (2019) Neuromuscular junction as an entity of nerve-muscle communication. Cells 8(8):906. 10.3390/cells8080906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Yan J, Mao AP, Li C, Ran Y, Shu HB, Wang YY (2011) Tripartite motif 8 (TRIM8) modulates TNFalpha- and IL-1beta-triggered NF-kappaB activation by targeting TAK1 for K63-linked polyubiquitination. Proc Natl Acad Sci U S A 108(48):19341–19346. 10.1073/pnas.1110946108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Sun M, Cao L, Gu JH, Ge J, Chen J, Han R, Qin YY, Zhou ZP, Ding Y, Qin ZH (2014) A TIGAR-regulated metabolic pathway is critical for protection of brain ischemia. J Neurosci 34(22):7458–7471. 10.1523/JNEUROSCI.4655-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Ou R, Hou Y, Chen Y, Gu X, Wei Q, Cao B, Zhang L, Liu K, Chen X, Song W, Zhao B, Wu Y, Shang H (2021) Genetic analysis of TRIM family genes for early-onset Parkinson’s disease in Chinese population. Parkinsonism Relat Disord 90:105–113. 10.1016/j.parkreldis.2021.08.005 [DOI] [PubMed] [Google Scholar]
- Lin CW, Chen B, Huang KL, Dai YS, Teng HL (2016) Inhibition of autophagy by estradiol promotes locomotor recovery after spinal cord injury in rats. Neurosci Bull 32(2):137–144. 10.1007/s12264-016-0017-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Lei Q (2020) TRIM62 knockout protects against cerebral ischemic injury in mice by suppressing NLRP3-regulated neuroinflammation. Biochem Biophys Res Commun 529(2):140–147. 10.1016/j.bbrc.2020.06.014 [DOI] [PubMed] [Google Scholar]
- Liu Y, Zhu M, Lin L, Fan X, Piao Z, Jiang X (2014) Deficiency of Trim27 protects dopaminergic neurons from apoptosis in the neurotoxin model of Parkinson’s disease. Brain Res 1588:17–24. 10.1016/j.brainres.2014.09.018 [DOI] [PubMed] [Google Scholar]
- Liu P, Cui L, Shen L (2020) Knockdown of TRIM52 alleviates LPS-induced inflammatory injury in human periodontal ligament cells through the TLR4/NF-kappaB pathway. Biosci Rep 40(8):1223. 10.1042/BSR20201223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Yang C, Lavayen BP, Tishko RJ, Larochelle J, Candelario-Jalil E (2022) Targeted BRD4 protein degradation by dBET1 ameliorates acute ischemic brain injury and improves functional outcomes associated with reduced neuroinflammation and oxidative stress and preservation of blood–brain barrier integrity. J Neuroinflamm 19(1):168. 10.1186/s12974-022-02533-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lokapally A, Neuhaus H, Herfurth J, Hollemann T (2020) Interplay of TRIM2 E3 ubiquitin ligase and ALIX/ESCRT complex: control of developmental plasticity during early neurogenesis. Cells 9(7):1734. 10.3390/cells9071734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu K, Pan Y, Huang Z, Liang H, Ding ZY, Zhang B (2022) TRIM proteins in hepatocellular carcinoma. J Biomed Sci 29(1):69. 10.1186/s12929-022-00854-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo J, Sun L, Lin X, Liu G, Yu J, Parisiadou L, Xie C, Ding J, Cai H (2014) A calcineurin- and NFAT-dependent pathway is involved in alpha-synuclein-induced degeneration of midbrain dopaminergic neurons. Hum Mol Genet 23(24):6567–6574. 10.1093/hmg/ddu377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luscher B, Larsson LG (1999) The basic region/helix-loop-helix/leucine zipper domain of Myc proto-oncoproteins: function and regulation. Oncogene 18(19):2955–2966. 10.1038/sj.onc.1202750 [DOI] [PubMed] [Google Scholar]
- Ma Z, Zang T, Birnbaum SG, Wang Z, Johnson JE, Zhang CL, Parada LF (2017) TrkB dependent adult hippocampal progenitor differentiation mediates sustained ketamine antidepressant response. Nat Commun 8(1):1668. 10.1038/s41467-017-01709-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma K, Han XX, Yang XM, Zhou SL (2021) Proteolysis targeting chimera technology: a novel strategy for treating diseases of the central nervous system. Neural Regen Res 16(10):1944–1949. 10.4103/1673-5374.308075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallery DL, McEwan WA, Bidgood SR, Towers GJ, Johnson CM, James LC (2010) Antibodies mediate intracellular immunity through tripartite motif-containing 21 (TRIM21). Proc Natl Acad Sci U S A 107(46):19985–19990. 10.1073/pnas.1014074107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manocha GD, Mishra R, Sharma N, Kumawat KL, Basu A, Singh SK (2014) Regulatory role of TRIM21 in the type-I interferon pathway in Japanese encephalitis virus-infected human microglial cells. J Neuroinflamm 11:24. 10.1186/1742-2094-11-24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald JW, Becker D (2003) Spinal cord injury: promising interventions and realistic goals. Am J Phys Med Rehabil 82(10 Suppl):S38-49. 10.1097/01.PHM.0000086994.53716.17 [DOI] [PubMed] [Google Scholar]
- McEwan WA, Falcon B, Vaysburd M, Clift D, Oblak AL, Ghetti B, Goedert M, James LC (2017) Cytosolic Fc receptor TRIM21 inhibits seeded tau aggregation. Proc Natl Acad Sci U S A 114(3):574–579. 10.1073/pnas.1607215114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNab FW, Rajsbaum R, Stoye JP, O’Garra A (2011) Tripartite-motif proteins and innate immune regulation. Curr Opin Immunol 23(1):46–56. 10.1016/j.coi.2010.10.021 [DOI] [PubMed] [Google Scholar]
- Mendioroz Iriarte M, Pulido Fontes L, Mendez-Lopez I (2015) Neuroepigenetics: desoxyribonucleic acid methylation in Alzheimer’s disease and other dementias. Med Clin (barc) 144(10):457–464. 10.1016/j.medcli.2014.03.023 [DOI] [PubMed] [Google Scholar]
- Meng F, Wang J, Ding F, Xie Y, Zhang Y, Zhu J (2017) Neuroprotective effect of matrine on MPTP-induced Parkinson’s disease and on Nrf2 expression. Oncol Lett 13(1):296–300. 10.3892/ol.2016.5383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon S, Boyer NP, Winkle CC, McClain LM, Hanlin CC, Pandey D, Rothenfusser S, Taylor AM, Gupton SL (2015) The E3 ubiquitin ligase TRIM9 is a filopodia off switch required for netrin-dependent axon guidance. Dev Cell 35(6):698–712. 10.1016/j.devcel.2015.11.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mojsa B, Mora S, Bossowski JP, Lassot I, Desagher S (2015) Control of neuronal apoptosis by reciprocal regulation of NFATc3 and Trim17. Cell Death Differ 22(2):274–286. 10.1038/cdd.2014.141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moller T, Hanisch UK, Ransom BR (2000) Thrombin-induced activation of cultured rodent microglia. J Neurochem 75(4):1539–1547. 10.1046/j.1471-4159.2000.0751539.x [DOI] [PubMed] [Google Scholar]
- Monti C, Colugnat I, Lopiano L, Chio A, Alberio T (2018) Network analysis identifies disease-specific pathways for Parkinson’s disease. Mol Neurobiol 55(1):370–381. 10.1007/s12035-016-0326-0 [DOI] [PubMed] [Google Scholar]
- Morris G, Maes M, Murdjeva M, Puri BK (2019) Do human endogenous retroviruses contribute to multiple sclerosis, and if so, how? Mol Neurobiol 56(4):2590–2605. 10.1007/s12035-018-1255-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mu X, Li H, Zhou L, Xu W (2019) TRIM52 regulates the proliferation and invasiveness of lung cancer cells via the Wnt/betacatenin pathway. Oncol Rep 41(6):3325–3334. 10.3892/or.2019.7110 [DOI] [PubMed] [Google Scholar]
- Mullard A (2021) Targeted protein degraders crowd into the clinic. Nat Rev Drug Discov 20(4):247–250. 10.1038/d41573-021-00052-4 [DOI] [PubMed] [Google Scholar]
- Napolitano LM, Meroni G (2012) TRIM family: pleiotropy and diversification through homomultimer and heteromultimer formation. IUBMB Life 64(1):64–71. 10.1002/iub.580 [DOI] [PubMed] [Google Scholar]
- Nenasheva VV, Novosadova EV, Makarova IV, Lebedeva OS, Grefenshtein MA, Arsenyeva EL, Antonov SA, Grivennikov IA, Tarantul VZ (2017) The transcriptional changes of trim genes associated with Parkinson’s disease on a model of human induced pluripotent stem cells. Mol Neurobiol 54(9):7204–7211. 10.1007/s12035-016-0230-7 [DOI] [PubMed] [Google Scholar]
- Nenasheva VV, Nikitenko NA, Stepanenko EA, Makarova IV, Andreeva LE, Kovaleva GV, Lysenko AA, Tukhvatulin AI, Logunov DY, Tarantul VZ (2021) Human TRIM14 protects transgenic mice from influenza A viral infection without activation of other innate immunity pathways. Genes Immun 22(1):56–63. 10.1038/s41435-021-00128-6 [DOI] [PubMed] [Google Scholar]
- Nexo BA, Christensen T, Frederiksen J, Moller-Larsen A, Oturai AB, Villesen P, Hansen B, Nissen KK, Laska MJ, Petersen TS, Bonnesen S, Hedemand A, Wu T, Wang X, Zhang X, Brudek T, Maric R, Sondergaard HB, Sellebjerg F, Brusgaard K, Kjeldbjerg AL, Rasmussen HB, Nielsen AL, Nyegaard M, Petersen T, Borglum AD, Pedersen FS (2011) The etiology of multiple sclerosis: genetic evidence for the involvement of the human endogenous retrovirus HERV-Fc1. PLoS ONE 6(2):e16652. 10.1371/journal.pone.0016652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nexo BA, Hansen B, Nissen KK, Gundestrup L, Terkelsen T, Villesen P, Bahrami S, Petersen T, Pedersen FS, Laska MJ (2013) Restriction genes for retroviruses influence the risk of multiple sclerosis. PLoS ONE 8(9):e74063. 10.1371/journal.pone.0074063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niedermeyer S, Murn M, Choi PJ (2019) Respiratory failure in amyotrophic lateral sclerosis. Chest 155(2):401–408. 10.1016/j.chest.2018.06.035 [DOI] [PubMed] [Google Scholar]
- Norris KL, Hao R, Chen LF, Lai CH, Kapur M, Shaughnessy PJ, Chou D, Yan J, Taylor JP, Engelender S, West AE, Lim KL, Yao TP (2015) Convergence of parkin, PINK1, and alpha-synuclein on stress-induced mitochondrial morphological remodeling. J Biol Chem 290(22):13862–13874. 10.1074/jbc.M114.634063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ntim M, Li QF, Zhang Y, Liu XD, Li N, Sun HL, Zhang X, Khan B, Wang B, Wu Q, Wu XF, Walana W, Khan K, Ma QH, Zhao J, Li S (2020) TRIM32 deficiency impairs synaptic plasticity by excitatory-inhibitory imbalance via notch pathway. Cereb Cortex 30(8):4617–4632. 10.1093/cercor/bhaa064 [DOI] [PubMed] [Google Scholar]
- Ong SB, Samangouei P, Kalkhoran SB, Hausenloy DJ (2015) The mitochondrial permeability transition pore and its role in myocardial ischemia reperfusion injury. J Mol Cell Cardiol 78:23–34. 10.1016/j.yjmcc.2014.11.005 [DOI] [PubMed] [Google Scholar]
- OuYang X, Guo J, Lv Q, Jiang H, Zheng Y, Liu P, Zhao T, Kong D, Hao H, Jiang Y (2020) TRIM32 drives pathogenesis in streptococcal toxic shock-like syndrome and streptococcus suis meningitis by regulating innate immune responses. Infect Immun. 10.1128/IAI.00957-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens DM, Keyse SM (2007) Differential regulation of MAP kinase signalling by dual-specificity protein phosphatases. Oncogene 26(22):3203–3213. 10.1038/sj.onc.1210412 [DOI] [PubMed] [Google Scholar]
- Ozato K, Shin DM, Chang TH, Morse HC 3rd (2008) TRIM family proteins and their emerging roles in innate immunity. Nat Rev Immunol 8(11):849–860. 10.1038/nri2413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perna A, Marathe S, Dreos R, Falquet L, Akarsu Egger H, Auber LA (2021) Revealing NOTCH-dependencies in synaptic targets associated with Alzheimer’s disease. Mol Cell Neurosci 115:103657. 10.1016/j.mcn.2021.103657 [DOI] [PubMed] [Google Scholar]
- Pertel T, Hausmann S, Morger D, Zuger S, Guerra J, Lascano J, Reinhard C, Santoni FA, Uchil PD, Chatel L, Bisiaux A, Albert ML, Strambio-De-Castillia C, Mothes W, Pizzato M, Grutter MG, Luban J (2011) TRIM5 is an innate immune sensor for the retrovirus capsid lattice. Nature 472(7343):361–365. 10.1038/nature09976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philouze C, Turban S, Cremers B, Caliez A, Lamarche G, Bernard C, Provost N, Delerive P (2021) MG53 is not a critical regulator of insulin signaling pathway in skeletal muscle. PLoS ONE 16(2):e0245179. 10.1371/journal.pone.0245179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponten F, Jirstrom K, Uhlen M (2008) The Human Protein Atlas–a tool for pathology. J Pathol 216(4):387–393. 10.1002/path.2440.HumanProteinAtlasproteinatlas.org [DOI] [PubMed] [Google Scholar]
- Randolph K, Hyder U, D’Orso I (2022) KAP1/TRIM28: transcriptional activator and/or repressor of viral and cellular programs? Front Cell Infect Microbiol 12:834636. 10.3389/fcimb.2022.834636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reymond A, Meroni G, Fantozzi A, Merla G, Cairo S, Luzi L, Riganelli D, Zanaria E, Messali S, Cainarca S, Guffanti A, Minucci S, Pelicci PG, Ballabio A (2001) The tripartite motif family identifies cell compartments. EMBO J 20(9):2140–2151. 10.1093/emboj/20.9.2140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousseaux MW, de Haro M, Lasagna-Reeves CA, De Maio A, Park J, Jafar-Nejad P, Al-Ramahi I, Sharma A, See L, Lu N, Vilanova-Velez L, Klisch TJ, Westbrook TF, Troncoso JC, Botas J, Zoghbi HY (2016) TRIM28 regulates the nuclear accumulation and toxicity of both alpha-synuclein and tau. Elife. 10.7554/eLife.19809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousseaux MW, Revelli JP, Vazquez-Velez GE, Kim JY, Craigen E, Gonzales K, Beckinghausen J, Zoghbi HY (2018) Depleting Trim28 in adult mice is well tolerated and reduces levels of alpha-synuclein and tau. Elife. 10.7554/eLife.36768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sajadimajd S, Yazdanparast R, Roshanzamir F (2016) Augmentation of oxidative stress-induced apoptosis in MCF7 cells by ascorbate-tamoxifen and/or ascorbate-juglone treatments. In Vitro Cell Dev Biol Anim 52(2):193–203. 10.1007/s11626-015-9961-4 [DOI] [PubMed] [Google Scholar]
- Salter MW, Stevens B (2017) Microglia emerge as central players in brain disease. Nat Med 23(9):1018–1027. 10.1038/nm.4397 [DOI] [PubMed] [Google Scholar]
- Samanta S, Perkinton MS, Morgan M, Williams RJ (1998) Hydrogen peroxide enhances signal-responsive arachidonic acid release from neurons: role of mitogen-activated protein kinase. J Neurochem 70(5):2082–2090. 10.1046/j.1471-4159.1998.70052082.x [DOI] [PubMed] [Google Scholar]
- Schmidt F, Kny M, Zhu X, Wollersheim T, Persicke K, Langhans C, Lodka D, Kleber C, Weber-Carstens S, Fielitz J (2014) The E3 ubiquitin ligase TRIM62 and inflammation-induced skeletal muscle atrophy. Crit Care 18(5):545. 10.1186/s13054-014-0545-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrag A, Ben-Shlomo Y, Quinn NP (1999) Prevalence of progressive supranuclear palsy and multiple system atrophy: a cross-sectional study. Lancet 354(9192):1771–1775. 10.1016/s0140-6736(99)04137-9 [DOI] [PubMed] [Google Scholar]
- Schwamborn JC, Berezikov E, Knoblich JA (2009) The TRIM-NHL protein TRIM32 activates microRNAs and prevents self-renewal in mouse neural progenitors. Cell 136(5):913–925. 10.1016/j.cell.2008.12.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sears R, Nuckolls F, Haura E, Taya Y, Tamai K, Nevins JR (2000) Multiple Ras-dependent phosphorylation pathways regulate Myc protein stability. Genes Dev 14(19):2501–2514. 10.1101/gad.836800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selkoe DJ (2004) Cell biology of protein misfolding: the examples of Alzheimer’s and Parkinson’s diseases. Nat Cell Biol 6(11):1054–1061. 10.1038/ncb1104-1054 [DOI] [PubMed] [Google Scholar]
- Shi M, Cho H, Inn KS, Yang A, Zhao Z, Liang Q, Versteeg GA, Amini-Bavil-Olyaee S, Wong LY, Zlokovic BV, Park HS, Garcia-Sastre A, Jung JU (2014) Negative regulation of NF-kappaB activity by brain-specific TRIpartite Motif protein 9. Nat Commun 5:4820. 10.1038/ncomms5820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Short KM, Cox TC (2006) Subclassification of the RBCC/TRIM superfamily reveals a novel motif necessary for microtubule binding. J Biol Chem 281(13):8970–8980. 10.1074/jbc.M512755200 [DOI] [PubMed] [Google Scholar]
- Sun S, Hu F, Wu J, Zhang S (2017) Cannabidiol attenuates OGD/R-induced damage by enhancing mitochondrial bioenergetics and modulating glucose metabolism via pentose-phosphate pathway in hippocampal neurons. Redox Biol 11:577–585. 10.1016/j.redox.2016.12.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanji K, Kamitani T, Mori F, Kakita A, Takahashi H, Wakabayashi K (2010) TRIM9, a novel brain-specific E3 ubiquitin ligase, is repressed in the brain of Parkinson’s disease and dementia with Lewy bodies. Neurobiol Dis 38(2):210–218. 10.1016/j.nbd.2010.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor RT, Lubick KJ, Robertson SJ, Broughton JP, Bloom ME, Bresnahan WA, Best SM (2011) TRIM79alpha, an interferon-stimulated gene product, restricts tick-borne encephalitis virus replication by degrading the viral RNA polymerase. Cell Host Microbe 10(3):185–196. 10.1016/j.chom.2011.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trevisan AC, Ribeiro FB, Itikawa EN, Alexandre LS, Pitella FA, Santos AC, Simoes BP, Wichert-Ana L (2017) 18F-FDG PET/CT/MRI fusion images showing cranial and peripheral nerve involvement in neurolymphomatosis. Indian J Nucl Med 32(1):77–78. 10.4103/0972-3919.198502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trono D (2015) Transposable elements, polydactyl proteins, and the genesis of human-specific transcription networks. Cold Spring Harb Symp Quant Biol 80:281–288. 10.1101/sqb.2015.80.027573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsao CW, Aday AW, Almarzooq ZI, Alonso A, Beaton AZ, Bittencourt MS, Boehme AK, Buxton AE, Carson AP, Commodore-Mensah Y, Elkind MSV, Evenson KR, Eze-Nliam C, Ferguson JF, Generoso G, Ho JE, Kalani R, Khan SS, Kissela BM, Knutson KL, Levine DA, Lewis TT, Liu J, Loop MS, Ma J, Mussolino ME, Navaneethan SD, Perak AM, Poudel R, Rezk-Hanna M, Roth GA, Schroeder EB, Shah SH, Thacker EL, VanWagner LB, Virani SS, Voecks JH, Wang NY, Yaffe K, Martin SS (2022) Heart disease and stroke statistics-2022 update: A report from the American Heart Association. Circulation 145(8):e153–e639. 10.1161/CIR.0000000000001052 [DOI] [PubMed] [Google Scholar]
- Unni SK, Ruzek D, Chhatbar C, Mishra R, Johri MK, Singh SK (2011) Japanese encephalitis virus: from genome to infectome. Microbes Infect 13(4):312–321. 10.1016/j.micinf.2011.01.002 [DOI] [PubMed] [Google Scholar]
- Valentino RR, Koga S, Heckman MG, Brushaber DE, Diehl NN, Walton RL, Dickson DW, Ross OA (2020) Association of tripartite motif containing 11 rs564309 with tau pathology in progressive supranuclear palsy. Mov Disord 35(5):890–894. 10.1002/mds.28010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Grabowiecki Y, Abreu P, Blanchard O, Palamiuc L, Benosman S, Meriaux S, Devignot V, Gross I, Mellitzer G, Gonzalez de Aguilar JL, Gaiddon C (2016) Transcriptional activator TAp63 is upregulated in muscular atrophy during ALS and induces the pro-atrophic ubiquitin ligase Trim63. Elife. 10.7554/eLife.10528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Hai C (2016) Novel insights into redox system and the mechanism of redox regulation. Mol Biol Rep 43(7):607–628. 10.1007/s11033-016-4022-y [DOI] [PubMed] [Google Scholar]
- Wang P, Benhenda S, Wu H, Lallemand-Breitenbach V, Zhen T, Jollivet F, Peres L, Li Y, Chen SJ, Chen Z, de The H, Meng G (2018) RING tetramerization is required for nuclear body biogenesis and PML sumoylation. Nat Commun 9(1):1277. 10.1038/s41467-018-03498-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, Wang G, Wang Q, Zhu Z, Wang Y, Chen K, Yang H (2019) Silencing of TRIM11 suppresses the tumorigenicity of chordoma cells through improving the activity of PHLPP1/AKT. Cancer Cell Int 19:284. 10.1186/s12935-019-1007-7 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Wang W, Zhou Q, Jiang T, Li S, Ye J, Zheng J, Wang X, Liu Y, Deng M, Ke D, Wang Q, Wang Y, Wang JZ (2021) A novel small-molecule PROTAC selectively promotes tau clearance to improve cognitive functions in Alzheimer-like models. Theranostics 11(11):5279–5295. 10.7150/thno.55680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei L, Zhang JS, Ji SF, Xu H, Zhao ZH, Zhang L, Pang L, Zhang JF, Yang PB, Ma H (2019) Knockdown of TRIM32 protects hippocampal neurons from oxygen-glucose deprivation-induced injury. Neurochem Res 44(9):2182–2189. 10.1007/s11064-019-02857-7 [DOI] [PubMed] [Google Scholar]
- Wen Z, Zhang J, Tang P, Tu N, Wang K, Wu G (2018) Overexpression of miR185 inhibits autophagy and apoptosis of dopaminergic neurons by regulating the AMPK/mTOR signaling pathway in Parkinson’s disease. Mol Med Rep 17(1):131–137. 10.3892/mmr.2017.7897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wezyk M, Spolnicka M, Pospiech E, Peplonska B, Zbiec-Piekarska R, Ilkowski J, Styczynska M, Barczak A, Zboch M, Filipek-Gliszczynska A, Skrzypczak M, Ginalski K, Kabza M, Makalowska I, Barcikowska-Kotowicz M, Branicki W, Zekanowski C (2018a) Hypermethylation of TRIM59 and KLF14 influences cell death signaling in familial Alzheimer’s disease. Oxid Med Cell Longev 2018:6918797. 10.1155/2018/6918797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wezyk M, Szybinska A, Wojsiat J, Szczerba M, Day K, Ronnholm H, Kele M, Berdynski M, Peplonska B, Fichna JP, Ilkowski J, Styczynska M, Barczak A, Zboch M, Filipek-Gliszczynska A, Bojakowski K, Skrzypczak M, Ginalski K, Kabza M, Makalowska I, Barcikowska-Kotowicz M, Wojda U, Falk A, Zekanowski C (2018b) Overactive BRCA1 Affects Presenilin 1 in Induced Pluripotent Stem Cell-Derived Neurons in Alzheimer’s Disease. J Alzheimers Dis 62(1):175–202. 10.3233/JAD-170830 [DOI] [PubMed] [Google Scholar]
- Witoelar A, Jansen IE, Wang Y, Desikan RS, Gibbs JR, Blauwendraat C, Thompson WK, Hernandez DG, Djurovic S, Schork AJ, Bettella F, Ellinghaus D, Franke A, Lie BA, McEvoy LK, Karlsen TH, Lesage S, Morris HR, Brice A, Wood NW, Heutink P, Hardy J, Singleton AB, Dale AM, Gasser T, Andreassen OA, Sharma M, International Parkinson’s Disease Genomics Consortium NABEC, United Kingdom Brain Expression Consortium I (2017) Genome-wide pleiotropy between parkinson disease and autoimmune diseases. JAMA Neurol 74(7):780–792. 10.1001/jamaneurol.2017.0469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie H, Wang Y, Zhu T, Feng S, Yan Z, Zhu Z, Ni J, Ni J, Du R, Zhu J, Ding F, Liu S, Han H, Zhang H, Zhao J, Zhang R, Quan W, Yan X (2020) Serum MG53/TRIM72 is associated with the presence and severity of coronary artery disease and acute myocardial infarction. Front Physiol 11:617845. 10.3389/fphys.2020.617845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie X, Wang F, Li X (2021) Inhibition of TRIM14 protects cerebral ischemia/reperfusion injury through regulating NF-kappaB/NLRP3 pathway-mediated inflammation and apoptosis. J Recept Signal Transduct Res. 10.1080/10799893.2021.1887218 [DOI] [PubMed] [Google Scholar]
- Xiong Y, Mahmood A, Chopp M (2013) Animal models of traumatic brain injury. Nat Rev Neurosci 14(2):128–142. 10.1038/nrn3407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue W, Zhao Y, Xiao Z, Wu X, Ma D, Han J, Li X, Xue X, Yang Y, Fang Y, Fan C, Liu S, Xu B, Han S, Chen B, Zhang H, Fan Y, Liu W, Dong Q, Dai J (2020) Epidermal growth factor receptor-extracellular-regulated kinase blockade upregulates TRIM32 signaling cascade and promotes neurogenesis after spinal cord injury. Stem Cells 38(1):118–133. 10.1002/stem.3097 [DOI] [PubMed] [Google Scholar]
- Yalcinkaya N, Haytural H, Bilgic B, Ozdemir O, Hanagasi H, Kucukali CI, Ozbek Z, Akcan U, Idrisoglu HA, Gurvit H, Tuzun E (2016) Expression changes of genes associated with apoptosis and survival processes in Parkinson’s disease. Neurosci Lett 615:72–77. 10.1016/j.neulet.2016.01.029 [DOI] [PubMed] [Google Scholar]
- Yang K, Shi HX, Liu XY, Shan YF, Wei B, Chen S, Wang C (2009) TRIM21 is essential to sustain IFN regulatory factor 3 activation during antiviral response. J Immunol 182(6):3782–3792. 10.4049/jimmunol.0803126 [DOI] [PubMed] [Google Scholar]
- Yang T, Hu Y, Miao J, Chen J, Liu J, Cheng Y, Gao X (2022) A BRD4 PROTAC nanodrug for glioma therapy via the intervention of tumor cells proliferation, apoptosis and M2 macrophages polarization. Acta Pharm Sin B 12(6):2658–2671. 10.1016/j.apsb.2022.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao Y, Zhang B, Zhu H, Li H, Han Y, Chen K, Wang Z, Zeng J, Liu Y, Wang X, Li Y, He D, Lin P, Zhou X, Park KH, Bian Z, Chen Z, Gong N, Tan T, Zhou J, Zhang M, Ma J, Zeng C (2016) MG53 permeates through blood–brain barrier to protect ischemic brain injury. Oncotarget 7(16):22474–22485. 10.18632/oncotarget.7965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi J, Li A, Li X, Park K, Zhou X, Yi F, Xiao Y, Yoon D, Tan T, Ostrow LW, Ma J, Zhou J (2021) MG53 preserves neuromuscular junction integrity and alleviates ALS disease progression. Antioxidants (Basel) 10(10):1522. 10.3390/antiox10101522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokota T, Mishra M, Akatsu H, Tani Y, Miyauchi T, Yamamoto T, Kosaka K, Nagai Y, Sawada T, Heese K (2006) Brain site-specific gene expression analysis in Alzheimer’s disease patients. Eur J Clin Invest 36(11):820–830. 10.1111/j.1365-2362.2006.01722.x [DOI] [PubMed] [Google Scholar]
- Zeng J, Wang Y, Luo Z, Chang LC, Yoo JS, Yan H, Choi Y, Xie X, Deverman BE, Gradinaru V, Gupton SL, Zlokovic BV, Zhao Z, Jung JU (2019) TRIM9-mediated resolution of neuroinflammation confers neuroprotection upon ischemic stroke in mice. Cell Rep 27(2):549–560. 10.1016/j.celrep.2018.12.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng S, Zhao Z, Zheng S, Wu M, Song X, Li Y, Zheng Y, Liu B, Chen L, Gao C, Liu H (2021) The E3 ubiquitin ligase TRIM31 is involved in cerebral ischemic injury by promoting degradation of TIGAR. Redox Biol 45:102058. 10.1016/j.redox.2021.102058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Hu MM, Wang YY, Shu HB (2012) TRIM32 protein modulates type I interferon induction and cellular antiviral response by targeting MITA/STING protein for K63-linked ubiquitination. J Biol Chem 287(34):28646–28655. 10.1074/jbc.M112.362608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang ZB, Xiong LL, Lu BT, Zhang HX, Zhang P, Wang TH (2017) Suppression of Trim32 enhances motor function repair after traumatic brain injury associated with antiapoptosis. Cell Transplant 26(7):1276–1285. 10.1177/0963689717716510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang HM, Liu P, Jiang C, Jin XQ, Liu RN, Li SQ, Zhao Y (2018) Notch signaling inhibitor DAPT provides protection against acute craniocerebral injury. PLoS ONE 13(2):e0193037. 10.1371/journal.pone.0193037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang JR, Li XX, Hu WN, Li CY (2020a) Emerging role of TRIM family proteins in cardiovascular disease. Cardiology 145(6):390–400. 10.1159/000506150 [DOI] [PubMed] [Google Scholar]
- Zhang X, Feng Y, Li J, Zheng L, Shao Y, Zhu F, Sun X (2020b) MicroRNA-665–3p attenuates oxygen-glucose deprivation-evoked microglial cell apoptosis and inflammatory response by inhibiting NF-kappaB signaling via targeting TRIM8. Int Immunopharmacol 85:106650. 10.1016/j.intimp.2020.106650 [DOI] [PubMed] [Google Scholar]
- Zhang X, Pavlicev M, Jones HN, Muglia LJ (2020c) Eutherian-specific gene TRIML2 attenuates inflammation in the evolution of placentation. Mol Biol Evol 37(2):507–523. 10.1093/molbev/msz238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z, Ji Z, Wang Y, Li J, Cao H, Zhu HH, Gao WQ (2014) TRIM59 is up-regulated in gastric tumors, promoting ubiquitination and degradation of p53. Gastroenterology 147(5):1043–1054. 10.1053/j.gastro.2014.07.021 [DOI] [PubMed] [Google Scholar]
- Zhu G, Harischandra DS, Ghaisas S, Zhang P, Prall W, Huang L, Maghames C, Guo L, Luna E, Mack KL, Torrente MP, Luk KC, Shorter J, Yang X (2020) TRIM11 prevents and reverses protein aggregation and rescues a mouse model of Parkinson’s disease. Cell Rep 33(9):108418. 10.1016/j.celrep.2020.108418 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.