Abstract
Is the cerebrum involved in its own activation to states of attention or arousal? “Telencephalon” is a term borrowed from embryology to identify not only the cerebral hemispheres of the forebrain, but also the basal forebrain. We review a generally undercited literature that describes nucleus basalis of Meynert, located within the substantia innominata of the ventrobasal forebrain, as a telencephalic extension of the ascending reticular activating formation. Although that formation’s precise anatomical definition and localization have proven elusive over more than 70 years, a careful reading of sources reveals that there are histological features common to certain brainstem neurons and those of the nucleus basalis, and that a largely common dendritic architecture may be a morphological aspect that helps to define non-telencephalic structures of the ascending reticular activating formation (e.g., in brainstem) as well as those parts of the formation that are telencephalic and themselves responsible for cortical activation. We draw attention to a pattern of dendritic arborization described as “isodendritic,” a uniform (isos-) branching in which distal dendrite branches are significantly longer than proximal ones. Isodendritic neurons also differ from other morphological types based on their heterogeneous, rather than specific afferentation. References reviewed here are consistent in their descriptions of histology, particularly in studies of locales rich in cholinergic neurons. We discuss the therapeutic implications of a basal forebrain site that may activate cortex. Interventions that specifically target nucleus basalis and, especially, the survival of its constituent neurons may benefit afflictions in which higher cortical function is compromised due to disturbed arousal or attentiveness, including not only coma and related syndromes, but also conditions colloquially described as states of cognitive “fog” or of “long-haul” mental compromise.
Keywords: Ascending reticular activating system, Arousal, Attention, Dendrite, Isodendritic, Nucleus basalis
Introduction: The Problematic Anatomical Definition of the Ascending Reticular Activating System (ARAS)
Consensus regarding what structures—which specific nuclei or tracts; or which discrete locales, rather than others, of brainstem and/or diencephalon—contribute to the ARAS has been debated since at least the late 1940s. Using Frédéric Bremer’s encéphale isolé preparation (Bremer 1937), in which cat brainstems were transected in anesthetized animals at the caudal end of medulla just above the high cervical cord, Moruzzi and Magoun (1949) reported that electrical stimulation (50–300 discharges/sec) of the brainstem’s “central core,” extending from medial medulla through pontine and mesencephalic tegmentum to caudal diencephalon, resulted in electroencephalographic (EEG) changes that put the authors in mind of a transition from sleep to alertness and attention—viz., a change from high-voltage slow waves to low-voltage fast activity. An accompanying study of the effects of lesions (Lindsley et al. 1949) found that neither transection at C1 nor lesions of medulla, periaqueductal gray, and lateral mesencephalon changed EEG activation patterns in unanesthetized cats; alternatively, large areas of mesencephalic tegmental damage (as well as paramedian, basal diencephalic lesions) resulted in delta waves, other large amplitude bursts, and spindles—i.e., a transition quite away from desynchronized, fast EEG activity characteristic of aroused attention.
In the early 1950s, at the start of work examining “fine structure” of the ARAS in various species, Scheibel and Scheibel (1958) reviewed more than a dozen histological and physiological studies which, in the aggregate, indicated that afferent information of various types may converge, for example, on individual medullary reticular neurons and that collaterals “pour” into the brainstem’s reticular core from medial lemniscus, spinoreticular locales, brachium conjunctivum, the pyramids, trapezoid body, vestibular nuclei, inter alia. Characterization of the ARAS based on anatomical boundaries, the architecture of its truly component parts, and its unwieldy connectivity has wrestled with its inescapable reticularism, which makes description of the ARAS problematic. As J.A. Hobson (1980) wittily noted in a monograph to mark 20 years after a major 1957 symposium entitled Reticular Formation of the Brain (Jasper et al. 1958), ARAS could still easily seem like just so many arrows “shooting up and down the neuraxis.”
Contemporary study enlisting tractography posits a somewhat different view, that paths of ARAS arrows can be carefully traced. The paths in question represent primarily axonal projections. Two representative studies from the early 2010s share a consideration that connectivity is the sine qua non of the ARAS, but dendritic morphology per se is not addressed. In a study of 26 young, normal, healthy persons (Yeo et al. 2013), a portion of the ARAS was determined by selection of fibers that passed through a seed region of interest (ROI, in pons at the trigeminal nerve entry zone) and a target ROI located in the vicinity of intralaminar nuclei of thalamus (central lateral, centromedian/parafascicular, and paracentral nuclei). Reconstructed ARAS tracts originated from pons, ascended through mesencephalic tegmentum posterior to the red nucleus, and terminated on thalamic intralaminar nuclei. The study reveals, in a snapshot, a problem with tractographic analyses: ROIs identify two points of a connection without therefore elucidating the full extent of the ARAS, as the authors recognized.
Edlow et al. (2012), studying two autopsied brains and one living subject, chose a larger sample of seed ROIs, including cuneiform and subcuneiform nuclei of mesencephalon, ventral tegmental area, median and dorsal raphe nuclei, locus coeruleus, oral pontine reticular nucleus, and the parabrachial nuclear complex of pons (in the vicinity of the superior cerebellar peduncle); thalamic target ROIs included reticular nucleus of thalamus, central lateral nucleus, and centromedian/parafascicular nuclei—the latter two also chosen by Yeo et al. (2013). The ensuing tractography maps illustrated somewhat unexpected connections, including interesting bifurcations into ventral and dorsal tracts from midbrain extending to hypothalamus and thalamus (respectively, as has been described in animals), with further divisions of the ventral tract (in humans) that reach variously to diencephalon, subthalamus, globus pallidus, and basal forebrain. Noteworthy though these findings are, we notice a conundrum related, again, to the choice of ROIs. An inherent assumption is that the ROIs are already thought to be constituents of the ARAS, such that, for all the interest in specific connections, a definition regarding what other structures contribute to the ARAS (or which do not) remains either unanswered or not answerable given the constraints of what areas authors choose as their ROIs.
In counterpoint to study of axons (e.g., their targets or the morphology of terminal axonal arbors), this paper draws attention to the morphology of dendrites in neurons located in what Magoun (1949) described as “a corticopetal projection” of the ARAS. Since the study of the anatomical basis of an ascending projection (the ARAS) that begins somewhere caudal to the telencephalon is relevant to an understanding of various “higher” disorders of arousal or consciousness, it would be of interest to identify some criterion of inclusion, related perhaps specifically to dendritic morphology, to determine whether a neuron or a nucleus anywhere in the brain or brainstem is or is not anatomically part of the ARAS. Study of dendritic morphology is relevant to both physiological and pathologic processes: sculpting of both neuronal dendrites and axons by glia occurs in normal brain development; in addition, in normal aging and in neurodegenerations variously associated with higher cortical dysfunction, phagocytosis of neuronal elements (dendrites, axons, and synapses) has been studied in detail, and molecular signaling along a neuron’s surface that may drive phagocytosis has been elucidated, albeit not completely (reviewed in Vilalta and Brown 2017).
We think it is fitting to revisit the study of dendritic morphology specifically, because disturbances in input at the level of dendritic arbors may contribute to higher cognitive deficits that occur in myriad conditions, not all of which are neurodegenerative (as in acute traumatic brain injury or virus-associated disturbances of mentation). With support not only from our historical review, but also from recent research, we observe how the basal forebrain (specifically the nucleus basalis of Meynert) may represent a telencephalic extension of the ARAS, as has been articulated in studies of forebrain cholinergic nuclei. Many of those studies, however, have focused on the projection networks of neurons, rather than the study of neuronal (dendritic) morphology per se.
Early Morphological Studies
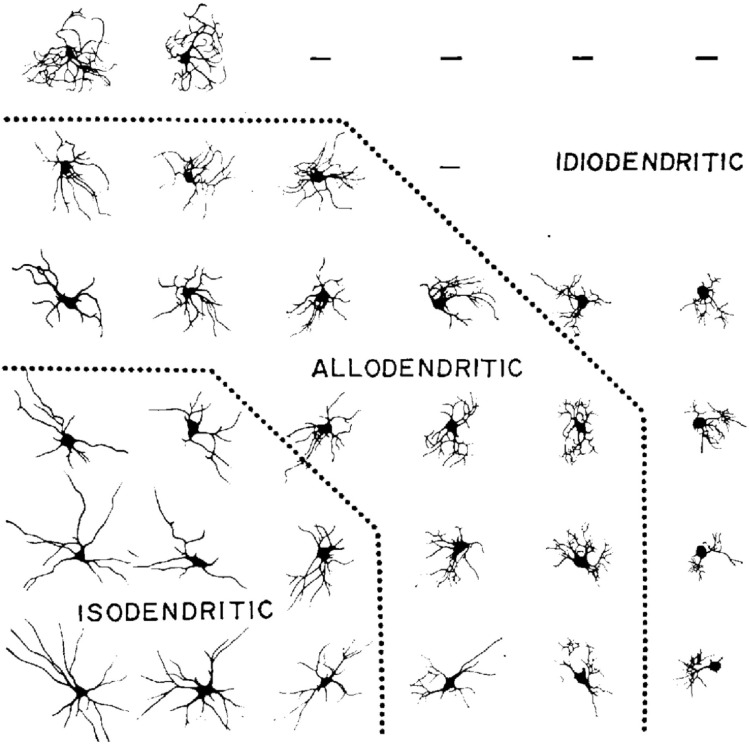
We first borrow and reintroduce the adjective “isodendritic” to describe a pattern of dendritic arborization in certain brainstem neurons rather than others. Although the characterization admits of limitations to be addressed, below, a feature of isodendritic morphology is the way in which the branching of dendrites differs from that of a tree: branches of distal dendrites, those furthest away from the cell body, tend to be longer than more proximal dendritic branches, and the “reach” of the distal dendrites can extend across extensive territories, as would occur in an ascending (or descending) system with extension to cortical (or spinal) locales. To our knowledge, the word “isodendritic” appeared first in Ramón-Moliner and Nauta (1966), although seminal work by Mannen (1960) also examined similar patterns of arborization. Inspired by Mannen’s work, Ramón–Moliner and Nauta distinguished a “uniform” appearance of a dendritic arbor (isos: unchanging, uniform) as opposed to “different” (allodendritic) or “peculiar” (idiodendritic) appearances, and they offered that the progression from iso- to allo- to idio- may have to do with increasing neuronal specialization in company with decreasing extent of arborization—both related to specific, as opposed to heterogeneous, afferentation of neurons (see Fig. 1). Ramón-Moliner and Nauta provide representative examples, of which we cite four: in nucleus propositus hypoglossi of the lower pons and upper medulla, large isodendritic arbors “mingle freely” within the interstices of passing fiber bundles of medial longitudinal fasciculus (see Fig. 3b; Fig. 3a and c are additional examples—of a medullary neuron and basal forebrain neurons, respectively); likewise, isodendritic arbors of the dorsal motor nucleus of vagus “overlap” with those of the solitary tract, with ramification as far as the area postrema, such that one could refer to an “apparatus in which the processes of motor, sensory, and internuncial neurons mingle closely.” By comparison, allodendritic arbors of inferior collicular neurons have comparatively small, tufted arbors, which remain within the confines of the colliculus. Idiodendritic arbors are the smallest and their individual dendritic branches are even more tufted, as can be observed within inferior olive.
Fig. 1.
Varieties of dendritic patterns of neurons, feline brainstem; reproduced from Ramón–Moliner and Nauta (1966) with permission. Distal dendritic extensions are longer than proximal stems in “isodendritic” arbors. Allo- and idiodendritic morphologies may relate either to higher degrees of neuronal specialization, or to specific rather than nonspecific neuronal afferentation, or to both
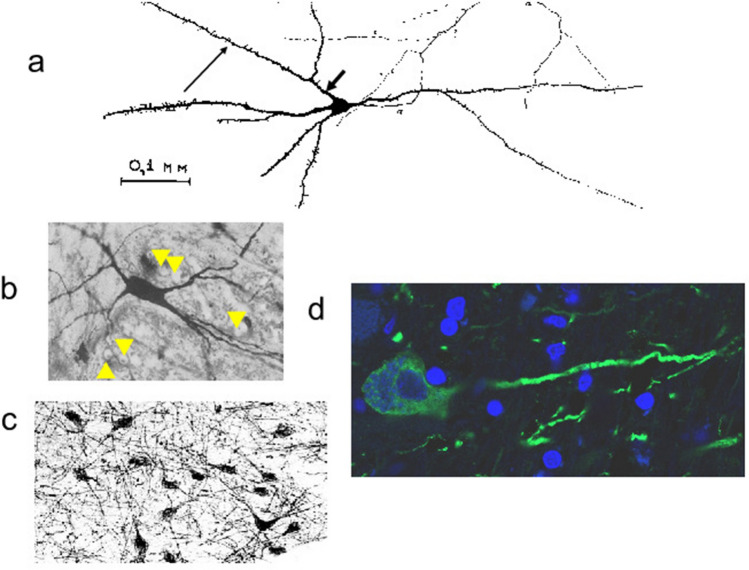
Fig. 3.
Isodendritic arbors of neurons in different locations. a Canine medulla oblongata, Golgi stain; reproduced from Leontovich and Zhukova (1963), with permission. Short, thick arrow indicates a proximate dendrite that is lengthwise shorter than a distal dendrite branch (longer, thin arrow). Leontovich and Zhukova (1963) describe long, straight, sparse ramification of dendrites, but axons (α) and cell bodies are also stained by the Golgi method; b. Nucleus prepositus hypoglossi (× 300), species unidentified, Golgi-Cox variant staining; reproduced from Ramón-Moliner and Nauta (1966) with permission. Dendrites freely intermingle with transversely cut fibers of the medial longitudinal fasciculus. Orthogonal views are indicated with yellow arrowheads. c. Human NB (× 134), nerve growth factor receptor (NGFr) immunostain, reproduced from Mesulam et al. (1989) with permission. Neurons in the field of view exhibit similar morphologies to the single neuron in b. d. Neuron from human NB (see boxed areas of interest in Fig. 2), confocal micrograph (× 630). Microtubule-associated protein 2 (MAP2) immunostaining highlights neuronal perikarya and dendrites (green stain); a counterstain with 4’,6 diamidino-2-phenylindole (DAPI), which binds DNA, is used to highlight nuclei (blue stain). Only a single elongated dendrite of the central neuron is seen. Full visualization of a dendritic arbor is often not possible in a single plane of section (see text for discussion). (Color figure online)
In his review of the above literature, Zahm (2006) specifically observed that cholinergic neurons projecting to cortex possess dendritic arbors “indistinguishable” from morphologies that are observed in brainstem locales: “dendrites are long and simple and innervated from multiple sources.... [S]imilar properties also characterize neurons that are prominent through the entire rostrocaudal extent of the lateral hypothalamus and preoptic region and in the basal forebrain magnocellular formation.” If isodendritic neuronal morphology exists rostral to brainstem and diencephalon, then what is the telencephalic extent of the ARAS? The question is of both contemporary and historical interest. Current investigations examining cognitive disturbances in diverse neuropsychiatric disorders (Liu et al. 2015), as well as those attempting to define nodes of a default-mode network in normal brains, are actively revisiting the basal forebrain as a core telencephalic locus or node involved in arousal and, more generally, in higher cortical function and dysfunction (Alves et al. 2019; Li et al. 2021).
An Historical Perspective Regarding Nucleus Basalis (NB)
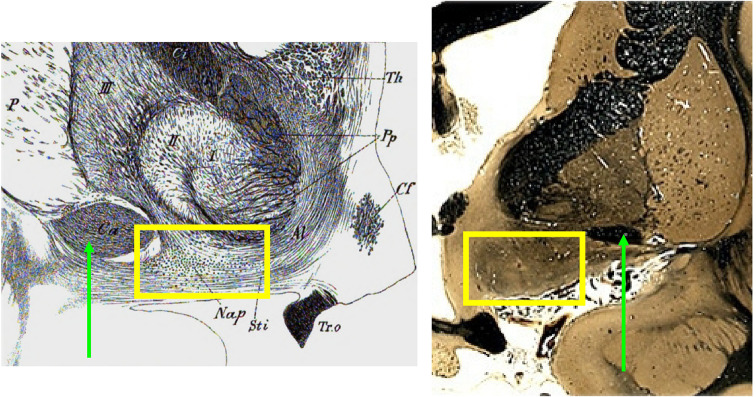
The term “basal nucleus” is Kölliker’s (1896), although he rightly credited Meynert (1872; 1884) with the original observation of a cluster of hyperchromatic, spindle-like neurons in close proximity to Gratiolet’s ansa peduncularis, a fibrous, handle-like loop that encircles the cerebral peduncle, passes anteriorly, and then blends with other white matter ventral and medial to Reil’s substantia innominata in the basal forebrain [for translations of Meynert and Kölliker, see Pearce (2003) and, especially, Engelhardt (2013)]. Figure 2 reproduces Kölliker’s 1896 illustration of relevant basal forebrain anatomy along with a comparison coronal section. Although Meynert did not illustrate NB neurons in his writing, he described them as 50 microns in length and 15 microns in width—measurements that put one in mind of a subsequent characterization (Ramón-Moliner 1962): a cylindrical neuron (along with its dendritic arbor) whose length is much greater than its diameter. A discussion of histology specific to ARAS neurons—and shared by NB neurons—has noteworthy history, which we now examine in greater detail.
Fig. 2.
Ventral basal forebrain, human, coronal sections. Black and white image on the left is from Kölliker (1896), without current copyright, public domain. The comparison image to the right (stained for fibers) is from The Human Brain Project (accessed 01 May 2022), part of the brain collections of the National Museum of Health and Medicine, Michigan State University, and the University of Wisconsin, used with permission. The area of interest (NB) in both images is boxed in yellow. For purpose of orientation, the green arrows indicate the location of the anterior commissure in both images. Abbreviations: anterior commissure (Ca), column of fornix (Cf), optic tract (Tr.O), Meynert’s NB (Nap); “Sti” refers to the anterior thalamic peduncle contiguous with ansa peduncularis (represented by Pp, the pes pedunculi) and fibers of the ansa lenticularis (Al). Roman numerals I, II, and III refer to components of the lentiform nucleus. “Th” represents the rostral tip of thalamus.
Studying Golgi, Nissl, and Weigert stains of brainstem preparations from rabbits, cats, and humans, Mannen (1960) wrote about the difficulty in knowing the true length of dendrites, since they cannot be followed to their complete extent in the majority of histological sections, but he nevertheless reported morphological differences among brainstem neurons related, first, to the length of their dendrites and, second, to the passage of dendrites beyond the limits of a discrete nucleus, such as the trochlear nucleus. Brainstem nuclei could be considered either “open” or “closed,” depending on the dendrites of their constituent neurons. Mannen characterized the reticular formation itself as a vast open system that contained relatively few closed nuclei (he did not argue that ARAS includes only so-called open nuclei). Quoting Lorente de Nó, Mannen fundamentally suggested that morphology of a dendritic arbor is a clue to a neuron’s function: “When two cells have similar axonal apparatus, but dendrites which are distributed differently, they belong to two different types, because they receive different impulses. When two cells have similar dendrites, but different ramified axons, they again belong to different types because they transmit their impulse in a different manner.”
The concept of an open nucleus introduces a question about what the boundaries of any nucleus might be—for example, historically, the anatomic boundaries of NB have been a matter of debate (Liu et al. 2015). Inspection of largely rectilinear neuronal dendrites in relationship to myelinated fiber bundles that pass nearby, especially in brainstem, finds that dendrites may or may not intermingle with passing fibers. In the former instance, when they do intermingle, one refers to openness of a nucleus; in the latter case, one refers to its closed or delimited quality. The majority of brainstem neurons possess dendrites that freely intermingle with passing fibers and are thus “open” in Mannen’s sense.
Cytoarchitectonic studies dating to the nineteenth century used the Nissl method to visualize cell bodies, but Leontovich and Zhukova (1963) maintained that histological criteria to assign a specific neuron to the ARAS required visualization well beyond that neuron’s cell body. Using the Golgi method as Mannen did, they identified a “reticular cell” (with a “neuronal form inherent to the brainstem reticular formation”) as having few, long, straight, poorly ramified dendrites and a small cell body. Reticular neurons, they observed, were not confined to ARAS as understood by other means [e.g., based on electroencephalographic activation, as in Moruzzi and Magoun (1949)], but reticular neurons differed histologically from cells of specific, presumably non-reticular nuclei at the same level of brainstem.
Roughly contemporaneously with Leontovich and Zhukova, Ramón-Moliner (1962) attempted a dendroarchitectonic classification of neurons in mouse, rat, cat, and monkey brains, using modifications of the Golgi method along with a Nissl counterstain to allow for visualization of cell body nuclei. Ramón-Moliner characterized dendritic arbors for diverse neurons of the cerebrum and cerebellum; his work specific to the ARAS will be discussed in what follows. Of interest in the 1962 paper is the description of a radiate pattern described above, in which distal dendritic branches are longer than more proximal stems. Ramón-Moliner speculated that the radiate dendritic pattern “may well be related to the heterogeneous origin and/or to the presence of relatively widely spaced afferent terminal fibers,” but he further discussed the relationship between a dendrite’s orientation in space relative to nearby fibers of other neurons. With specific reference to the brainstem reticular core, dendrites of those core neurons align in parallel to afferent terminal fibers, but perpendicular to passing fibers. Based on the study of brainstems of many mammals, including human infants, Scheibel and Scheibel (1958), whom Ramón-Moliner cites, also identify a longitudinal extent of the core that passes from cord to hypothalamus, thalamus, corpus striatum, septum, and cortex, but the Scheibels emphasized a lateral dispersion of connections especially in the brainstem, warning that “it is not possible to estimate the amount of dispersion, or to diagram the significant lateral circuitry without overindulging in assumptions.” Fig. 3d is a confocally imaged human NB neuron whose dendrites cannot be fully imaged in a single plane of section.
Conditions, Restrictions, and Utility of the Isodendritic Concept
Ramón-Moliner and Nauta (1966) eloquently discuss provisos to their observations. “The concept of a reticular formation,” they begin, “would lose in significance if its presumptive extent were to be amplified excessively.” If morphological criteria exist to identify an ARAS-component structure, one cannot therefore assume that physiology related to a locale in brainstem or elsewhere is attributable to common morphology of its neurons. Likewise, common physiology need not necessitate that there would be a uniform morphology. Yet, in the spirit of Mannen’s earlier description of dendrites openly intermingling with passing fibers, Ramón-Moliner and Nauta entertained that overlapping dendritic fields, with individual dendrites on average longer than 300 microns, may be a noteworthy feature of ARAS neurons, one consistent with a large functional territory or reticular continuum that ascends at least from medulla to mesencephalon, despite some degree of isodendritic morphological heterogeneity.
The above considerations notwithstanding, especially in light of a precedent that reticularism was first understood anatomically and microscopically (for example, by Golgi 1906) rather than physiologically, Ramón-Moliner and Nauta suggested that the term “isodendritic core” replace “reticular formation.” “The main advantage of the term,” they wrote, “is that the inclusion of a given cell territory in the family of isodendritic regions can be based on purely descriptive grounds: it is always relatively easy to determine whether a particular cell group displays generalized or specialized dendritic patterns.” Of note, among isodendritic regions, deeper layers of cerebral cortex, reticular, and some intralaminar nuclei of thalamus, and substantia innominata are additional examples. “Leontovich and Zhukova (’63) have attributed to this extensive territory,” Ramón–Moliner and Nauta wrote, “dendritic particularities similar to those of the reticular formation of the mesencephalon, pons, and medulla oblongata. On the basis of a cursory survey of Golgi material, we are inclined to agree with this point of view.” In his review of the above literature, Zahm (2006) specifically observed that cholinergic neurons projecting to cortex possess dendritic arbors “indistinguishable” from morphologies that are observed in brainstem locales: “dendrites are long and simple and innervated from multiple sources.... [S]imilar properties also characterize neurons that are prominent through the entire rostrocaudal extent of the lateral hypothalamus and preoptic region and in the basal forebrain magnocellular formation.”
Review of Citations to the Present
To ascertain how widely cited the concept of an isodendritic core has been since 1966, apart from textbook references (Nieuwenhuys 1985; Zaborsky et al., 2015), we searched PubMed as of September, 2022, to find just 17 articles (13 of relevance), based on the search terms “isodendritic” and “core.” By comparison, interrogation with “reticular formation” or ARAS yields up to 34,000 hits. The limited number of hits in our search reflects how methods to study both axonal and dendritic arbors have advanced over time, such that the concept of an isodendritic arbor has become much more complex, and the word “isodendritic” less in use. Yet dendritic morphology as described by Mannen, Ramón-Moliner, Nauta, Leontovich, and Zhukova remains surprisingly germane.
We tracked references, especially from Zaborsky et al. (2015), to increase the breadth our review, but among the 13 articles from the PubMed search, features of an isodendritic arbor remain consistent. Neurons of locus coeruleus, indoleaminergic nuclei of the raphe, dopaminergic neurons of the substantia nigra and ventral tegmental area, neurons in periaqueductal gray, and brainstem cholinergic nuclei share such features (Liu et al., 1976; Rossor 1981; Gebhart 1982; Kline and Felten 1985; Newman and Liu 1987; Theofilas et al. 2015; Bucci et al. 2017); we note additionally that at least locus coeruleus and midbrain dopamine neurons project directly to basal forebrain (Zaborsky et al., 2015). Catecholaminergic, indoleaminergic, and cholinergic systems innervate cortex and other sites rostral to brainstem: one study in our PubMed search (Newman and Liu 1987), with its particular attention to isodendritic neuronal morphology in the rat brain, found as many as 33 brainstem subnuclei, many of them catecholaminergic, which project to cortex; the neurons had variable cell body shapes (fusiform, triangular, or polygonal), but all had sparsely branching dendrites, characteristic of the reticular cell described by Leontovich and Zhukova (1963). Rossor (1981), citing evidence that both Parkinson’s and Alzheimer’s diseases (PD and AD, respectively) exhibit changes in the isodendritic core, argued that neuronal dysfunction precedes effects at the level of terminal fields of isodendritic neurons. Hyperphosphorylated tau in locus coeruleus and the dorsal raphe has been observed in early AD, specifically Braak-AD stage 0 or 1 (Ehrenberg et al. 2017), and variations in functional connectivity of locus coeruleus may predict memory disturbance even in normal aging (Jacobs et al 2018). Corticopetal progression of dysfunction or histopathology, from core to cortex, has been posited by other investigators who have observed decremental function of terminal, presumably axonal nerve endings of isodendritic neurons in AD, without comment about dendritic abnormality specifically (Benton et al. 1982; see below regarding dendritic spine abnormalities in AD described more recently). Neurofibrillary pathology prior to cortical AD pathology has been observed in the isodendritic core (Theofilas et al. 2015). Other papers argue that, as in AD and PD, schizophrenia may likewise manifest histopathology of the isodendritic core (Cadet 1984, 1988); periaqueductal and diencephalic periventricular glial fibrosis has been observed in autopsied schizophrenic brains (Holden 1989).
Pathology of isodendritic neurons is variable both in appearance and in location. Lees (2009) refers to the isodendritic core by name in relationship to PD, citing vulnerability of catecholaminergic, long-axon projection neurons; of note, the vulnerability is not specific to dopaminergic (catecholaminergic) neurons. Frank cell loss, reflected in pallor of substantia nigra and its nigrosomes and of locus coeruleus in PD, is not the only finding that one finds in the isodendritic core. In an abbreviated list, Lewy pathology may include Lewy bodies within surviving neuronal cell bodies of substantia nigra, so-called coiled bodies in oligodendrocytes rather than neurons, alpha-synuclein immunoreactive neuronal processes (Lewy neurites, LNs), and eosinophilic, immunoreactive fibrils in neuropil or in fiber tracts. In a dated series (Greenfield and Bosanquet 1953)—unique in that it included cases of post-encephalitic Parkinsonism—the authors concentrated on pigmented brainstem neurons that bear the burden of pathology. Examination of melanin-rich neurons as they degenerate has generally been the bias and teaching of PD neuropathological discussions for decades. But Lewy himself (1923) focused on NB rather than substantia nigra in his Parkinsonian brains to find his eponymic bull’s-eye-like, hyaline bodies (coined “corps de Lewy” by Trétiakoff), which are also observed in other isodendritic locations, including dorsal motor nucleus of vagus (as Lewy observed), pedunculopontine nucleus, raphe nuclei, and periaqueductal gray matter, among other brainstem sites, as has been corroborated and extended in more recent work (Halliday et al. 2012; Seidel et al. 2015).
With the advent of neurotransmitter-specific identification of neurons (e.g., Mesulam and Geula 1988; Mesulam et al. 1989; Duque et al. 2007) and genetically directed cell type-specific labeling techniques (e.g., Rotolo et al. 2008; Wu et al. 2014; Li et al. 2018), whole neurons, including their dendritic arbors, have been visualized selectively: with the use, for example, of a cell type-specific label, sparse numbers of neurons can be visualized in a field of view devoid of other, like neurons and their overlapping dendritic arbors. These types of investigations, with their attention centered on dendritic anatomy of selected neurons, differ from other studies that attend to diffuse or selective projections to cortex and other locales from isodendritic neurons (e.g., Bloem et al. 2014; Kim et al. 2016). The matter of truly identifying an anatomical extent of branching, despite the availability of specific stains to identify dendrites (Figs. 3), requires three-dimensional (3D) reconstruction of neurons as their dendrites ramify in space, as opposed to visualization of dendrites in just a two-dimensional plane of section (Ertürk et al. 2012). With directed attention to basal forebrain, single-cell 3D reconstruction of electrophysiologically identified cholinergic neurons has revealed that “relative straight primary dendrites bifurcate in an iterative fashion, and the sum of the lengths of the daughter branches is usually longer than that of the mother branch”—a pattern consistent with isodendritic morphology found in brainstem structures associated with ARAS (Zaborsky et al., 2015; Duque et al. 2007). In older-generation Golgi staining to visualize dendritic arbors (also using of 3D reconstructions), Arendt et al. (1995) have interestingly observed that sparsely ramified reticular neurons form a pool of pluripotent neurons that retain their neuronal plasticity in life (see below for further discussion).
Telencephalic Extent of the Isodendritic Core
Citations discussed, above, as well as investigations into the brain’s cholinergic nuclei—NB in particular—invite consideration of the ARAS’s extent into the forebrain or elsewhere in the telencephalon. Leontovich and Zhukova (1963) and Ramón-Moliner, and Nauta (1966) do not exclude the possibility that isodendritic morphology may exist in cortex. Ramón-Moliner and Nauta (1966) write emphatically that “not one single histological feature can be attributed to the reticular formation that cannot be found also in other regions of the nervous system.” A line of investigation beginning with Mesulam and Van Hoesen (1976) informs a “reassessment” (Fuller et al. 2011) in which ARAS’s contributions to the state of arousal—a state which, in turn, underpins higher cortical function—may be mediated at least in part, and perhaps mainly, by NB as a telencephalic extension of the isodendritic core. The purpose of this section’s discussion is not to cite the many non-cholinergic loci of the ARAS (we have mentioned just a few; for more comprehensive reviews, see Li et al. 2021; Jacobs et al. 2018; Parvisi and Damasio 2001). Nor do we address an extensive but mixed literature regarding cholinergic output to rostral sites, in terms of the diffusivity or selectivity of those outputs (for reviews, see Ballinger et al. 2016; Gielow and Zaborsky 2017). More conservatively, we revisit Mesulam and Van Hoesen (1976), Mesulam and Geula (1988), and Mesulam et al. (1989), three publications that encapsulate the history that we attempt to summarize in this paper. The authors of those three papers systematically described cholinergic loci with isodendritic morphology which extend from brainstem to septum and NB.
In a retrograde tracer study, Mesulam and Van Hoesen (1976) injected horseradish peroxidase (HRP) into Brodmann’s areas 4 and 6 (motor cortex and premotor/supplementary motor cortices); after several days, HRP reaction product was observed in NB (of substantia innominata), the medial septum, in the proximity of white-matter laminae within globus pallidus, and lateral hypothalamus. The same areas also avidly stained for acetylcholinesterase, suggesting that retrograde tracing had identified a cholinergic projection arising from basal forebrain, principally from NB. “On a morphological basis,” the authors remark, “the SI [substantia innominata] is a rostral extension of the reticular formation.” As reference for the statement, Mesulam and Van Hoesen cite only Ramón–Moliner and Nauta (1966). Do we conclude that the ARAS extends to (though it also projects to) telencephalon, or do we just say that ventral forebrain, and perhaps other cortical or diencephalic areas, simply exhibit isodendritic attributes? The latter view, corroborated by work dating to the 1940s as reviewed by Bishop (1958) (for a recent perspective see Geser 2021), does not obviate telencephalon as a locale to which the ARAS may extend.
In addition to cholinergic projection neurons in the vicinity of septum (medial septal nucleus or Ch1; vertical and horizontal-limb nuclei of the diagonal band of Broca or Ch2 and Ch3, respectively; and NB or Ch4), as reviewed in Mesulam and Geula (1988), the 1989 paper additionally identified a Ch5 complex with a “center of gravity” in the pedunculopontine nucleus at the pontomesencephalic border and a Ch6 complex whose neurons ramify into the periaqueductal gray, medial longitudinal fasciculus, and locus coeruleus, but which centers at the laterodorsal tegmental nucleus in rostral pons. Ch5 and Ch6, unlike the cortical destinations of Ch1–Ch4 neurons, seem to project largely to thalamus. Borrowing Mannen’s (1960) characterization of “openness” of nuclei, Mesulam et al. (1989) wrote that Ch4, Ch5, and Ch6 groups “fit the description of ‘open’ nuclei since their constituent neurons displayed considerable cytological heteromorphism, overlapping and nonspecialized dendritic branching patterns, and a propensity for extending into adjacent fiber bundles in the form of interstitial elements.” Despite subtle cytochemical differences in the Ch groups, although they are all characteristically cholinergic (as determined both by acetylcholinesterase histochemistry and by choline acetyltransferase immunohistochemistry), arguably all Ch groups—Ch4 (NB), Ch5, and Ch6 in particular—represent a broadened concept of the human reticular formation. The ARAS, characterized as a core with consistent isodendritic features, has telencephalic extension, the latter principally represented by NB but also by septal cholinergic groups Ch1-6.
A Telencephalic Reticular Arousal System: Research and Clinical Implications
Attention to isodendritic morphology of basal forebrain neurons may reveal morphological correlations for various disturbances of higher cortical function. Contemporary histological investigation has not neglected dendritic morphology in disease. For example, Arnold et al. (2013) have observed reduced density of dendritic spines in human frontal neocortical neurons in AD. But specific to arbors, Arendt et al. (1995) have observed that basal forebrain neurons are vulnerable to degeneration in AD, PD, and Korsakoff’s syndrome, but the dendritic arbors of those neurons appear to remodel—viz., to exhibit structural plasticity or a significant degree of pluripotency—in the context of ongoing pathology. The remodeling takes the form of distal dendritic lengthening that characterizes isodendritic arbors both in basal forebrain and in brainstem. Questions arise regarding the mechanisms of that remodeling; the authors suggest the possibility that the lengthening of dendrite branches, mediated by unspecified trophic factors among other mechanisms, may represent an attempt to compensate for neuronal loss (Arendt et al. 1995). Basal forebrain receives input (adrenergic, especially) from tightly packed brainstem locales (e.g., in midbrain or rostral pons) that are constituents of what Ramón-Moliner and Nauta called the “isodendritic core;” other subcortical sites, including various hypothalamic nuclei, corpus striatum (especially nucleus accumbens), and amygdala also project to basal forebrain. In the aggregate, brainstem and subcortical NB afferentation and its myriad associated neurotransmitters (norepinephrine, dopamine, glutamate, somatostatin, neuropeptide Y, among others, as reviewed in Zaborsky et al., 2015) have been invoked as influences on the arousing or alerting function of ARAS. We wonder whether modalities such as noninvasive (transcranial magnetic stimulation or stereotactically directed ultrasound pulsation) or invasive (deep brain stimulation) techniques targeting basal forebrain, which is more discretely accessible than bunched brainstem locales, may yield benefit based on isodendritic remodeling. As Hardenacke et al. (2013) maintain, NB remains “auspicious target” for alleviation of cognitive deficits in AD, at least.
Using contemporary techniques which create cell lines from patient-derived, induced pluripotent stem cells (iPSCs) (Lagomarsino et al. 2021), study of cholinergic basal forebrain neurons in vitro may allow future work to address many as-yet unanswered questions.
Relevant to therapeutics can individually derived, differentiated neurons be interrogated by drugs “in the dish” (Khurana et al. 2015) to ascertain likelihood of personal clinical response—for example, reduction of an individual’s cholinergic neuronal senescence (neflamapimod, for example, has been recently studied in preclinical and early clinical work [Jiang et al. 2022])? Beyond the context of neurodegenerative disorders, leveraging neuronal plasticity by increasing neuronal survival might find surprisingly wide application—for example, in categories of affliction in which the brain is affected by what has been informally termed a “fog” of compromised arousal or attentiveness.
Does an iPSC-derived cholinergic neuron have isodendritic morphology in vitro? If not, why not? What are the genetic mechanisms that govern the development of a specific arbor, even in reaction to pathophysiology elsewhere in an already mature brain? Can CRISPR-Cas9 genome editing, which has been used in vivo (Swiech et al. 2015), be employed in iPSC-derived cell lines to elucidate what drives maturation or subsequent plasticity of a cholinergic neuron? Pluripotent neurons in the area of mammalian dentate gyrus in hippocampus have been recognized for some time (Gage 2000), but NB isodendritic neurons may represent an alternative locale to study neuronal plasticity.
In addition to the above considerations, there are insights to be gleaned at the bedside, based on a notion that ARAS extends to telencephalon. It need not be the case that even extensive, cell-specific thalamic lesions, as opposed to cell-specific lesions of basal forebrain, must result in impaired behavioral or electrocortical arousal, as has been observed (Fuller et al. 2011). In light of such evidence, perhaps NB’s role itself in arousal is underestimated, whereas thalamocortical influences are overestimated. Also, the notion of ARAS as a projection system to either thalamus or to basal forebrain from cuneiform/subcuneiform nucleus in midbrain (Edlow et al. 2012), elsewhere in midbrain (e.g., ventral tegmental area [Li et al. 2021]), or pontine locations (Yeo et al. 2013; Edlow et al. 2012) might be revisited, as has been recently entertained (Alves et al. 2019). We disagree with Alves et al., however, that basal forebrain has been underappreciated as part of either the default-mode network or in terms of its connectivity within ARAS. Edlow et al. (2012) make a good case that connectivity study addressing arousal must address hypothalamus, basal forebrain, ventral thalamus, and even globus pallidus as component nodes. The concept of telencephalic arousal, with NB as an isodendritic ROI that might be associated with salutary, or, alternatively pathological, functional change in cortical networks, does not exclude the possibility of multiple ascending influences (some arising from brainstem are mentioned, above). Rather, the concept of an isodendritic core shifts focus to basal forebrain as an activating center in and of itself, in receipt of heterogeneous input, as is characteristic of all isodendritic neurons.
Summary
The concept of an isodendritic neuron, based on dendritic morphology, deserves renewed attention, especially with respect to the basal forebrain. NB (specifically, its isodendritic, cholinergic neurons) is a node relevant to cortical activation. We discuss the way in which attention to dendritic anatomy has advanced from an early, simple description of “branches of distal dendrites, those furthest away from the cell body, being longer than more proximal dendritic branches.” Study of changes to isodendritic neurons in diverse locales in brainstem and, more importantly, in telencephalic structures whose neurons exhibit isodendritic morphology, may lead, in time, to a greater understanding of the likely myriad forms of disturbances of the ARAS. Attention to dendritic anatomy may offer new insights, including those based on patient-derived iPSCs, regarding diverse conditions affecting cortical function that are not acknowledged as degenerative, as in so-called fatigue states or conditions described as kinds of “long-haul” mental compromise.
Acknowledgements
Umberto DeGirolami, M.D. (Mass General Brigham Department of Pathology and Harvard Medical School) helped with the translation of Mannen (1960); he corroborated, corrected, and then elaborated on the first author’s translation. The authors thank Drs. Vikram Khurana and Tracy Young-Pearse, both from Mass General Brigham Department of Neurology and Harvard Medical School, for discussions relevant to the uses of induced pluripotent stem cells.
Author Contributions
All authors contributed to the study conception. The literature review was performed by EKM, who also wrote the first draft of the manuscript. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Funding
E.K.M. reports no funding sources. S.B. receives support from the Judy Pauline Staples Aull Fund at Brigham and Women’s Hospital, Boston, MA USA and research support from Alexion Pharmaceuticals and National Institute of Health under award number OT2HL161847-01. M.T. is supported by the National Cancer Institute of the National Institutes of Health under award number F32CA257210. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Data Availability
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
Declarations
Conflict of interest
The authors have not disclosed any competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Alves PN, Foulon C, Karolis V, Bzdok D, Margulies DS, Volle E, Thiebaut de Schotten M (2019) An improved neuroanatomical model of the default-mode network reconciles previous neuroimaging and neuropathological findings. Commun Biol 2:370. 10.1038/s42003-019-0611-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arendt T, Brückner MK, Bigl V, Marcova L (1995) Dendritic reorganization in the basal forebrain under degenerative conditions and its defects in Alzheimer’s disease. 3. The basal forebrain compared with other subcortical areas. J Comp Neurol 351:223–246. 10.1002/cne.903510204 [DOI] [PubMed] [Google Scholar]
- Arnold SE, Louneva N, Cao K, Wang L-S, Han L-Y, Wolk DA, Negash S, Leurgans SE, Schneider JA, Buchman AS, Wilson RS, Bennett DA (2013) Cellular, synaptic and biochemical features of resilient cognition in Alzheimer’s disease. Neurobiol Aging 34:157–168. 10.1016/j.neurobiolaging.2012.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballinger EC, Ananth M, Talmage DA, Role LW (2016) Basal forebrain cholinergic circuits and signaling in cognition and cognitive decline. Neuron 91:1199–1218. 10.1016/j.neuron.2016.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton JS, Bowen DM, Allen SJ, Haan EA, Davison AN, Neary D, Murphy DRP, Snowden JS (1982) Alzheimer’s disease as a disorder of isodendritic core. Lancet 319:456. 10.1016/s0140-6736(82)91667-1 [DOI] [PubMed] [Google Scholar]
- Bishop GH (1958) The place of cortex in a reticular system. In: Jasper HH, Proctor LD, Knighton RS, Noshay WC, Costello RT (eds) Reticular Formation of the Brain. Little, Brown and Company, Boston, pp 413–421 [Google Scholar]
- Bloem B, Schoppink L, Rotaro DC, Faiz A, Hendriks P, Mansvelder HD, van de Berg WDJ, Wouterlood FG (2014) Topographic mapping between basal forebrain cholinergic neurons and the medial prefrontal cortex in mice. J Neurosci 34:16234–16246. 10.1523/JNEUROSCI.3011-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremer F (1937) L’activité cérébrale au cours du sommeil et de la narcose. Contribution à l’étude du mécanisme du sommeil. Bull Acad Roy Med Belgique 2:68–86 [Google Scholar]
- Bucci D, Busceti CL, Calierno MT, Di Pietro P, Madonna M, Biagioni F, Ryskalin L, Limanaqi F, Nicoletti F, Fornai F (2017) Systematic morphometry of catecholamine nuclei in the brainstem. Front Neuroanat 11:98. 10.3389/fnana.2017.00098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadet JL (1984) Disorders of the isodendritic core of the brainstem. Schizophr Bull 10:1–3. 10.1093/schbul/10.1.1 [DOI] [PubMed] [Google Scholar]
- Cadet JL (1988) A unifying theory of movement and madness: involvement of free radicals in disorders of the isodendritic core of the brainstem. Med Hypotheses 27:59–63. 10.1016/0306-9877(88)90085-0 [DOI] [PubMed] [Google Scholar]
- Duque A, Tepper JM, Detari L, Ascoli GA, Zaborsky L (2007) Morphological characterization of electrophysiologically and immunohistochemically identified basal forebrain cholinergic and neuropeptide Y-containing neurons. Brain Struct Funct 212:55–73. 10.1007/s00429-007-0143-3 [DOI] [PubMed] [Google Scholar]
- Edlow BL, Takahashi E, Wu O, Benner T, Dai G, Bu L, Grant PE, Greer DM, Greenberg SM, Kinney HC, Folkerth RD (2012) Neuroanatomic connectivity of the human ascending arousal system critical to consciousness and its disorders. J Neuropathol Exp Neuro 71:531–546. 10.1097/NEN.0b013e3182588293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrenberg AJ, Nguy AK, Theofilas P, Dunlop S, Suemoto CK, Di Lorenzo Alho AT, Leite RP, Diehl Rodriguez R, Mejia MB, Rüb U, Farfel JM, de Lucena Ferretti-Rebustini RE, Nascimento CF, Nitrini R, Pasquallucci CA, Jacob-Filho W, Miller B, Seeley WW, Heinsen H, Grinberg LT (2017) Quantifying the accretion of hyperphosphorylated tau in the locus coeruleus and dorsal raphe nucleus: the pathological building blocks of early Alzheimer’s disease. Neuropathol Appl Neurobiol 43:393–408. 10.1111/nan.12387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelhardt E (2013) Meynert and the basal nucleus. Dement Neuropsychol 7:435–438. 10.1590/S1980-57642013DN7400013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ertürk A, Becker K, Jährling N, Mauch CP, Hojer CD, Egen JG, Hellal F, Bradke F, Sheng M, Dodt H-U (2012) Three-dimensional imaging of solvent-cleared organs using 3DISCO. Nat Protoc 7:1983–1995. 10.1038/nprot.2012.119 [DOI] [PubMed] [Google Scholar]
- Fuller P, Sherman D, Pedersen NP, Saper CB, Lu J (2011) Reassessment of the structural basis of the ascending arousal system. J Comp Neurol 519:933–956. 10.1002/cne.22559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage FH (2000) Mammalian neural stem cells. Science 287:1433–1438. 10.1126/science.287.5457.1433 [DOI] [PubMed] [Google Scholar]
- Gebhart GF (1982) Opiate and opioid peptide effects on brain stem neurons: relevance to nociception and antinociception mechanisms. Pain 12:93–140. 10.1016/0304-3959(82)90189-0 [DOI] [PubMed] [Google Scholar]
- Geser F (2021) The dendroarchitectonics of E. Ramon-Moliner [abstract]. Eur J Neurol 28suppl1:920.
- Gielow MR, Zaborsky L (2017) The input-output relationship of the cholinergic basal forebrain. Cell Rep 18:1817–1830. 10.1016/j.celrep.2017.01.060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golgi C (1906) The neuron doctrine—theory and facts. www.nobelprize.org/prizes/medicine/1906/golgi/lecture/>. Accessed 30 April 2022.
- Greenfield JG, Bosanquet FD (1953) The brain-stem lesions in Parkinsonism. J Neurol Neurosurg Psychiat 16:213–226. 10.1136/jnnp.16.4.213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliday G, McCann H, Shepherd C (2012) Evaluation of the Braak hypothesis: how far can it explain the pathogenesis of Parkinson’s disease? Exp Rev Neurother 12:673–686. 10.1586/ern.12.47 [DOI] [PubMed] [Google Scholar]
- Hardenacke K, Shubina E, Bührle CP, Zapf A, Lenartz D, Klosterkötter J, Visser-Vandewalle V, Kuhn J (2013) Deep brain stimulation as a tool for improving cognitive functioning in Alzheimer’s dementia: a systematic review. Front Psychiatry 4:159. 10.3389/fpsyt.2013.00159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobson JA (1980) Toward a cellular neurophysiology of the reticular formation: conceptual and methodological milestones. In: Hobson JA, Brazier MAB (eds) The Reticular Formation Revisited. Raven Press, New York, pp 7–29 [Google Scholar]
- Holden TJ (1989) An integrated neuropathophysiological model of schizophrenia. Med Hypotheses 28:109–113. 10.1016/0306-9877(89)90023-6 [DOI] [PubMed] [Google Scholar]
- Jacobs HIL, Müller-Ehrenberg L, Priovoulos N, Roebroeck A (2018) Curvilinear locus coeruleus functional connectivity trajectories over the adult lifespan: a 7T MRI study. Neurobiol Aging 69:167–176. 10.1016/j.neurobiolaging.2018.05.021 [DOI] [PubMed] [Google Scholar]
- Jasper H, Proctor LD, Knighton RS, Noshay WC, Costello RT (1958) Reticular Formation of the Brain. Little Brown, Boston [Google Scholar]
- Jiang Y, Alam JJ, Gomperts SN, Maruff P, Lemstra AW, Germann UA, Stavrides PH, Darji S, Malampati S, Peddy J, Bleiwas C, Pawlik M, Pensalfini A, Yang D-S, Subbanna S, Basavarajappa BS, Smiley JF, Gardner A, Blackburn K, Chu H-M, Prins ND, Teunissen CE, Harrson JE, Scheltens P, Nixon RA (2022) Preclinical and randomized clinical evaluation of the p38α kinase inhibitor neflamapimod for basal forebrain cholinergic degeneration. Nature Comm 13:5308. 10.1038/s41467-022-32944-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khurana V, Tardiff DF, Chung CY, Lindquist S (2015) Toward stem cell-based phenotypic screens for neurodegenerative diseases. Nat Rev Neurol 11:339–350. 10.1038/nrneurol.2015.79 [DOI] [PubMed] [Google Scholar]
- Kim J-H, Jung A-H, Jeong D, Choi H, Kim K, Shin S, Kim SJ, Lee S-H (2016) Selectivity of neuromodulatory projections from the basal forebrain and locus ceruleus to primary sensory cortices. J Neurosci 36:5314–5327. 10.1523/JNEUROSCI.4333-15.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kline S, Felten DL (1985) Ventral tegmental area of the rabbit brain: a developmental Golgi study. Brain Res Bull 14:485–492. 10.1016/0361-9230(85)90027-9 [DOI] [PubMed] [Google Scholar]
- Koelliker A (1896) Ausstrahlungen des rothen Kernes. Basales Ganglion (Ganglion der Ansa peduncularis, Meynert). In: Koelliker A Handbuch der Gewebelehre des Menschen. Zweiter Band, Wilhelm Engelmann, Leipzig, pp. 454–458.
- Lagomarsino VN, Pearse RV, Liu L, Hsieh Y-C, Fernandez MA, Vinton EA, Pauli D, Felsky D, Tasaki S, Gaiteri C, Vardarajan B, Lee H, Muratore CR, Benoit CR, Chou V, Fancher SB, He A, Merchant JP, Duong DM, Martinez H, Zhou M, Bah F, Vicent MA, Stricker JMS, Xu J, Dammer EB, Levey AI, Chibnik LB, Menon V, Seyfried NT, De Jager PL, Noggie S, Selkoe DJ, Bennett DA, Young-Pearse TL (2021) Stem cell derived neurons reflect features of protein networks, neuropathology and cognitive outcome of their aged human donors. Neuron 109:3402-3420.e9. 10.1016/j.neuron.2021.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lees AJ (2009) The Parkinson chimera. Neurology 72(7):S2-11. 10.1212/WNL.0b013e318198daec [DOI] [PubMed] [Google Scholar]
- Leontovich TA, Zhukova GP (1963) The specificity of the neuronal structure and topography of the reticular formation in the brain and spinal cord of carnivora. J Comp Neurol 121:347–381. 10.1002/cne.901210305 [DOI] [PubMed] [Google Scholar]
- Lewy FH (1923) Spezielle pathologie der paralysis agitans. In: Lewy FH Die Lehre vom Tonus under der Bewegung Zugleich Systematische Untersuchungen zur Klinik, Physiologie, Pathologie und Pathogenese der Paralysis Agitans, Julius Springer, Berlin, pp. 171–316.
- Li X, Yu B, Sun Q, Zhang Y, Ren M, Zhang X, Li A, Yuan J, Madisen L, Luo Q, Zeng H, Gong H, Qiu Z (2018) Generation of a whole-brain atlas for the cholinergic system and mesoscopic projectome analysis of basal forebrain cholinergic neurons. PNAS 115:415–420. 10.1073/pnas.1703601115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Curley WH, Guerin B, Dougherty DD, Dalca AV, Fischl B, Horn A, Edlow BL (2021) Mapping the subcortical connectivity of the human default mode network. Neuroimage 245:118758. 10.1016/j.neuroimage.2021.118758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsley DB, Bowden JW, Magoun HW (1949) Effect upon the EEG of acute injury to the brain stem activating system. Electroenceph Clin Neurophysiol 1:475–486 [PubMed] [Google Scholar]
- Liu RP, Hamilton BL (1976) Intranuclear bodies in neurons of the periaqueductal gray matter in the cat. Am J Anat 147:139–145. 10.1002/aja.1001470115 [DOI] [PubMed] [Google Scholar]
- Liu AKL, Chang RCC, Pearce RKB, Gentleman SM (2015) Nucleus basalis of Meynert revisited: anatomy, history and differential involvement in Alzheimer’s and Parkinson’s disease. Acta Neuropathol 129:527–540. 10.1007/s00401-015-1392-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magoun HW (1964) Reticulo-cortical influences for wakefulness, orienting and attention. In: Charles C (ed) Magoun HW, The Waking Brain, 2nd. Thomas, Springfield, pp 74–97 [Google Scholar]
- Mannen H (1960) “Noyau fermé” et “noyau ouvert” Contribution à l’etude cytoarchitectonique du tronc cerebral envisage du point de vue du mode d’arborisation dendritique. Archiv Italiennes De Biologie 98:333–350 [Google Scholar]
- Mesulam MM, Geula C (1988) Nucleus basalis (Ch4) and cortical cholinergic innervation in the human brain: observations based on the distribution of acetylcholinesterase and choline acetyltransferase. J Comp Neurol 275:216–240. 10.1002/cne.902750205 [DOI] [PubMed] [Google Scholar]
- Mesulam MM, van Hoesen GW (1976) Acetylcholinesterase-rich projections from the basal forebrain of the rhesus monkey to neocortex. Brain Res 109:152–157. 10.1016/0006-8993(76)90385-1 [DOI] [PubMed] [Google Scholar]
- Mesulam MM, Geula C, Bothwell MA, Hersh LB (1989) Human reticular formation: cholinergic neurons of the pedunculopontine and laterodorsal tegmental nuclei and some cytochemical comparisons to forebrain cholinergic neurons. J Comp Neurol 281:611–633. 10.1002/cne.902830414 [DOI] [PubMed] [Google Scholar]
- Meynert T (1872) Vom Gehirne der Säugetiere. In: Stricker S (ed) Meynert T. Handbuch der Lehre von den Geweben des Menschen under der Thiere. Zweiter Band. Wilhelm Engelmann, Leipzig, pp 694–808 [Google Scholar]
- Meynert T (1884) Formen und Zusammenhang des Gehirnes. Meynert T Psychiatrie. Klinik der Erkankungen des Vorderhirns. Erste Hälfte. Wilhelm Braumüller, Wien, pp 1–125 [Google Scholar]
- Moruzzi G, Magoun HW (1949) Brain stem reticular formation and activation of the EEG. Electroenceph Clin Neurophysiol 1:455–473 [PubMed] [Google Scholar]
- Newman DB, Liu RP (1987) Nuclear origins of brainstem reticulocortical systems in the rat. Am J Anat 178:279–299. 10.1002/aja.1001780309 [DOI] [PubMed] [Google Scholar]
- Nieuwenhuys R (1985) Survey of chemically defined cell groups and pathways. Nieuwenhuys R Chemoarchitecture of the Brain, Springer-Verlag, Berlin, Heidelberg, Acetylcholine. In, pp 7–11 [Google Scholar]
- Parvisi J, Damasio A (2001) Consciousness and the brainstem. Cognition 79:135–159. 10.1016/s0010-0277(00)00127 [DOI] [PubMed] [Google Scholar]
- Pearce JMS (2003) The nucleus of Theodor Meynert (1833–1892). J Neurol Neurosurg Psychiatry 74:1358. 10.1136/jnnp.74.9.1358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramón-Moliner E (1962) An attempt at classifying nerve cells on the basis of their dendritic patterns. J Comp Neurol 119:211–227. 10.1002/cne.901190207 [DOI] [PubMed] [Google Scholar]
- Ramón-Moliner E, Nauta WJH (1966) The isodendritic core of the brain stem. J Comp Neurol 126:311–335. 10.1002/cne.901260301 [DOI] [PubMed] [Google Scholar]
- Rossor MN (1981) Parkinson’s disease and Alzheimer’s disease as disorders of the isodendritic core. Br Med J 283:1588–1590. 10.1136/bmj.283.6306.1588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotolo T, Smallwood PM, Williams J, Nathans J (2008) Genetically-directed, cell type-specific sparse labeling for the analysis of neuronal morphology. PLoS ONE 3:e4099. 10.1371/journal.pone.0004009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheibel ME, Scheibel AB (1958) Structural substrates for integrative patterns in the brain stem reticular core. In: Jasper HH, Proctor LD, Knighton RS, Noshay WC, Costello RT (eds) Reticular Formation of the Brain. Little, Brown and Company, Boston, Toronto, pp 31–68 [Google Scholar]
- Seidel K, Mahlke J, Siswanto S, Krüger R, Heinsen H, Auburger G, Bouzrou M, Grinberg LT, Wicht H, Korf H-W, den Dunnen W (2015) The brainstem pathologies of Parkinson’s disease and dementia with Lewy bodies. Brain Path 25:121–135. 10.1111/bpa.12168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swiech L, Heidenreich M, Banerjee A, Habib N, Li Y, Rombetta J, Sur M, Zhang F (2015) In vivo interrogation of gene function in the mammalian brain using CRISPR-Cas9. Nat Biotechnol 33:102–106. 10.1038/nbt.3055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Human Brain Project, https://brains.anatomy.msu.edu/brains/human/coronal/1680_fiber.html. Accessed 01 May 2022.
- Theofilas P, Dunlop S, Heinsen H, Grinberg LT (2015) Turning on the light within: subcortical nuclei of the isodendritic core and their role in Alzheimer’s disease pathogenesis. J Alzheimers Dis 46:17–34. 10.3233/JAD-142682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilalta A, Brown GC (2017) Neurophagy, the phagocytosis of live neurons and synapses by glia, contributes to brain development and disease. FEBS J 285:3541–3683. 10.1111/febs.14323 [DOI] [PubMed] [Google Scholar]
- Wu H, Williams J, Nathans J (2014) Complete morphologies of basal forebrain cholinergic neurons in the mouse. Elife 3:e02444. 10.7554/eLife.02444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo SS, Change PH, Jang SH (2013) The ascending reticular activating system from pontine reticular formation to the thalamus in the human brain. Front Hum Neurosci 7:416. 10.3389/fnhum.2013.00416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaborszky L, Duque A, Gielow M, Gombkoto P, Nadasdy Z, Somogyi J (2015) Organization of the basal forebrain cholinergic projection system: specific or diffuse? In: Paxinos G (ed) The Rat Nervous System. Elsevier, Amsterdam, pp 491–507 [Google Scholar]
- Zahm DS (2006) The evolving theory of basal forebrain functional-anatomical ‘macrosystems.’ Neurosci Biobehav Rev 30:148–172. 10.1016/jneubiorev.2005.06.003 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.