Abstract
As the final product of glycolysis, lactate features not only as an energy substrate, a metabolite, and a signaling molecule in a variety of diseases—such as cancer, inflammation, and sepsis—but also as a regulator of protein lactylation; this is a newly proposed epigenetic modification that is considered to be crucial for energy metabolism and signaling in brain tissues under both physiological and pathological conditions. In this review, evidence on lactylation from studies on lactate metabolism and disease has been summarized, revealing the function of lactate and its receptors in the regulation of brain function and summarizing the levels of lactylation expression in various brain diseases. Finally, the function of lactate and lactylation in the brain and the potential mechanisms of intervention in brain diseases are presented and discussed, providing optimal perspectives for future research on the role of lactylation in the brain.
Graphical Abstract
Keywords: Lactate, Brain diseases, Epigenetic modification, Protein lactylation
Introduction
As a newly proposed epigenetic modification, lactylation modifications of histone lysine residues have been found to directly stimulate gene transcription from chromatin. This modification process could be driven by lactate (Sharma and Pal 2021). Recent research has revealed that increasing lactylation levels require both exogenous and endogenous lactate syntheses and that inhibiting mitochondrial oxidative phosphorylation and increasing glycolysis under hypoxic settings increase both intracellular lactate and protein lactation levels, with pleiotropic consequences in several disorders via metabolic reorganization and epigenetic modifications (Zhang et al. 2019). A growing number of studies have concentrated on protein lactylation, and its expression level has been demonstrated to be correlated with the emergence of several disorders such as tumors, inflammation, infections, and cognitive impairment (Irizarry-Caro et al. 2020; Chu et al. 2021; Cui et al. 2021; Yu et al. 2021; Pan et al. 2022a, b), in which a potential connection between protein lactylation and brain function has been observed (Hagihara et al. 2021; Dai et al. 2022; Pan et al. 2022a, b). Herein, we summarize current developments in protein lactylation in brain diseases and provide a broad overview of the function of lactate and its derived lactylation in brain tissues and nerve cells (Fig. 1).
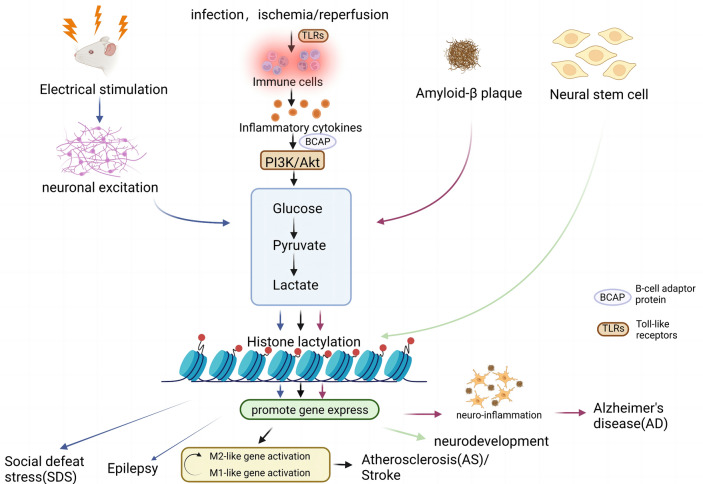
Fig. 1.
Lactate-derived lactylation in brain diseases. As a signaling molecule, metabolite, and energy substrate, lactate is produced by glycolysis and stimulates gene transcription through histone lysine lactylation. Factors such as the excitation of neurons by electrical stimulation; activation of PI3K/Akt pathway by infection, local ischemia, or reperfusion promoting the secretion of inflammatory factors; and deposition of amyloid-β(Aβ) plaques can affect the concentration of lactate and further influence the level of histone lactylation, which may have an impact on disease development (e.g., SDS, epilepsy, AD, AS, and stroke). SDS social defeat stress, Aβ amyloid-β, AD Alzheimer’s disease, AS atherosclerosis (Created with BioRender.com)
Lactate in the Brain
Lactate is usually considered to be a metabolite of glycolysis in the body under hypoxic conditions. Under normal physiological conditions, aerobic respiration occurs in the cytoplasmic matrix and mitochondria. The first stage of glycolysis generates pyruvate and a small amount of adenosine triphosphate (ATP). The second stage comprises the conversion of pyruvate to acetyl coenzyme A in the mitochondrial matrix and participation in the tricarboxylic acid (TCA) cycle. The third stage comprises oxidative phosphorylation to generate ATP with the assistance of NAHD+, H+, and FADH2 in the oxidized state in the mitochondrial inner membrane. In pathological hypoxic conditions, respiration occurs without the involvement of oxygen, and the resulting pyruvate is catalyzed by lactate dehydrogenase (LDH) to generate lactic acid, which then dissociates to lactate and H+, allowing lactate to accumulate in the body (Boveris and Boveris 2007; Hohnholt et al. 2017). Notably, cancer cells produce ATP and lactate through glycolysis even under fully aerobic conditions, a phenomenon known as the Warburg effect (Warburg et al. 1927). As a result, lactic acid/lactate, a byproduct of the Warburg effect of aerobic glycolysis, has been extensively studied in cancer cells (Warburg et al. 1927; Sharma and Pal 2021). However, Brandon et al. indicated that lactate acts not only as an end product of glycolysis but also as an important energy source (carrier) for energy supply and maintenance of tumor metabolism in vivo (Faubert et al. 2017). In mouse models of non-small cell lung cancer, it was demonstrated that lactate functions better than glucose as a fuel for the TCA cycle. Therefore, it is assumed that lactate is used by tumors as a fuel to increase the metabolic activity of tumors in cancer (Faubert et al. 2017).
As a chiral molecule, lactate has three isomeric structures: dl-lactate, d-lactate, and l-lactate. In the brain, l-Lactate predominates over d-lactate, which is hardly present (Gibbs and Hertz 2008), whereas glycolysis of glucose in astrocytes is considered to be the main source of l-lactate in the brain (Newman et al. 2011). During increased neuronal activity, l-lactate can serve as an energy substrate to provide ATP to active neurons while fulfilling the energy needs of astrocytes and can operate as a signaling molecule (Newman et al. 2011; Steinman et al. 2016), a concept known as the astrocyte–neuron lactate shuttle hypothesis (Pellerin and Magistretti 1994), which was proposed to shift the stereotype of lactate as a metabolic waste product and emphasize the critical function of lactate in brain metabolism. In addition, MCTs can be used to transport high blood lactate concentrations across the blood–brain barrier and into the brain (Aveseh et al. 2014). Based on the astrocyte–neuron lactate shuttle hypothesis, several studies in recent years have demonstrated that lactate, particularly l-lactate, has a significant function in improving memory impairment, increasing cerebral blood flow, enhancing cerebral energy metabolism, reducing neurological deficits, and encouraging neural regeneration (Yamanishi et al. 2006; Berthet et al. 2009, 2012; Yang et al. 2014; El Hayek et al. 2019; Lev-Vachnish et al. 2019; Zhai et al. 2020; Hu et al. 2021; Lambertus et al. 2021). Increased l-lactate in the brain was observed in both diabetes and Alzheimer's disease (AD) but not in neurons, which suggests that illness-induced neuronal underutilization of lactate is a significant contributor to cognitive impairment in both diabetic encephalopathy and AD (Zhang et al. 2018a, b; Zhao et al. 2018). Moreover, it is believed that lactate has a neuroprotective effect after a stroke. Ischemia-induced increases in lactate at low concentrations may have a neuroprotective effect on the brain (Berthet et al. 2009, 2012), whereas high lactate concentrations may also cause lactic acidosis, which exacerbates neurological damage (Shen et al. 2015). However, although it has been demonstrated that high concentrations of lactate can exacerbate neuronal damage, it has also been indicated that only high doses of l-lactate (20 mM) can reverse the decrease in ATP in primary cultured neurons and N2A cells to prevent cell death during oxygen glucose deprivation (OGD), whereas low doses of lactate cannot (Shen et al. 2015). The explanation for this difference in lactate effect may be that different concentrations of l-lactate may have different effects on the regulation of brain metabolism and function in different physiological metabolic states. In addition, their effects may be related to the time point of intervention or the type of neuronal cells. Moreover, a study has been performed to promote mitochondrial energy metabolism and elimination of the cytotoxic byproduct methylglyoxal (MGO) by regulating the interaction between d-LDH and d-lactate on the inner mitochondrial membrane, which is considered to alleviate the progression of AD (de Bari et al. 2019); intravenous d-lactate can also reduce the infarct volume in tMCAO mice (Cai et al. 2022). Thus, although the complex mechanisms of action of lactate in the brain have not been elucidated, the protective effects of lactate on the brain and nervous system under pathological conditions, either as an energy substrate or as a signaling molecule, have been demonstrated but need to be studied further.
MCTs—Lactate Transporters in the Brain
With the use of intracellular and intercellular shuttling, lactate is subjected to energy regulation and signaling. Concentration gradients, pH gradients, and redox states are involved in this process. Cell–cell lactate shuttling includes lactate exchange between white (glycolytic) and red (oxidative) fibers within the muscle bed and between skeletal muscles and organs (such as the brain and heart). Intracellular lactate is primarily transported through cytoplasmic–mitochondrial and cytoplasmic–peroxisomal shuttles (Brooks 2020). In addition, under the influence of intracellular lactate concentration and pH gradient, the entry of lactate across the plasma membrane is undertaken through several monocarboxylate transporters (MCTs).
According to the Human Genome Organisation nomenclature, MCTs belong to the solute carrier 16 (SLC16) gene family. The SLC16 gene family has 14 members (Halestrap 2013), three of which (SLC16A1, SLC16A3, and SLC16A7) encode monocarboxylate transporters (MCT1, MCT4, and MCT2, respectively) that have been observed to be widely expressed in the brain (Halestrap and Price 1999; Bergersen 2015). l-Lactate, the most abundant MCT substrate in the brain, is also transported by monocarboxylate transport proteins such as MCT1, MCT4, and MCT2. MCT4 promotes the efflux of lactate from the cell, whereas MCT1 and MCT2 promote the influx of lactate from outside the cell, where the lactate concentration gradient is a key factor in determining the direction of transport (Bergersen 2015; Pucino et al. 2018). Benjamin et al. modulated lactate transport through dual inhibition of MCT1 and MCT4 by drugs, which inhibited glycolysis and led to elevated intracellular lactate levels, resulting in the inhibition of the end product of LDH and concomitant inhibition of MCT2, further demonstrating that lactate is transmitted between cells via MCTs (Benjamin et al. 2018). Moreover, CD147, a chaperone protein of MCTs, promotes lactate input and oxidation in the mitochondrial reticulum together with MCT1 and LDH in mitochondria (Hashimoto et al. 2006), and promotes appropriate expression and localization of MCT1 and MCT4 on the cell surface (Wilson et al. 2005; Schneiderhan et al. 2009; Dana et al. 2020). Thus, under the influence of concentration and pH, lactate can undergo intracellular and intercellular shuttling across MCTs with the help of CD147 and can act on certain receptors (such as GPR81) (Payen et al. 2020). This suggests that MCTs have a significant function in lactate transport, both intracellularly and between cells and organs, and provides a basis for lactate to act as a signaling molecule to deliver metabolic information to neural tissues (Bergersen and Gjedde 2012). Notably, although MCT-mediated lactate transport is always influenced by the concentration gradient, it was observed that the intracellular lactate concentration is usually higher than the extracellular lactate concentration. This phenomenon is caused by the capture of low concentrations of extracellular substrates by high-affinity transport proteins when transport tends to saturate and is not dependent on lactate concentration (Bergersen 2015). For instance, MCT2 is a high-affinity transporter protein (Medel et al. 2022), MCT1 has a moderate-to-high affinity for lactate (Dalsgaard et al. 2004), and MCT4 was previously considered to be a low-affinity but high-throughput transporter protein (Brauchi et al. 2005). However, recent research indicated that MCT4 is a high-affinity transporter protein for lactate (Contreras-Baeza et al. 2019) and speculated that this result was caused by the omission of bicarbonate action from the experimental solution used to monitor the transport of monocarboxylates.
In addition, Mächler et al. indicated that lactate in blood is more readily taken up by astrocytes and neuronal cells and that lactate levels are higher in astrocytes, suggesting that lactate is transported from astrocytes to neurons and that this process is mediated through MCTs (Mächler et al. 2016). Moreover, the effect of MCT transport on the peripheral nervous system was demonstrated by deleting MCT1 and MCT4 from Schwann cells, which disrupted the maintenance of motor endplate innervation in the peripheral nervous system of model mice. Moreover, MCT4 was observed to be expressed in astrocytes; MCT1 in oligodendrocytes, microvascular endothelial cells, ventricular canal cells, and astrocytes; and MCT2 in neurons in the central nervous system (CNS) (Suzuki et al. 2011; Lee et al. 2012; Philips et al. 2021). These neurogliocytes appear to be metabolically coupled to neurons in the CNS, such as the spinal cord, cerebral cortex, and hippocampus, providing lactate that can be taken up by neurons that possess a high affinity for MCT2 (Debernardi et al. 2003). The results of Suzuki et al. indicated that memory formation in rats was associated with lactate release in the hippocampus, which blocked the formation of long-term memory by inhibiting the expression of MCT1 in astrocytes (Suzuki et al. 2011). This may be because glycolysis occurring in astrocytes produces and releases lactate, which enters neurons through transport by MCT1 and MCT4, to act as signaling molecules or energy substrates to form long-term memory. The astrocyte–neuron metabolic-coupling hypothesis is important for exploring the mechanisms of neurological disorders related to memory impairment and cognitive deficits such as AD (Suzuki et al. 2011; Ardanaz et al. 2022). A similar study altered the feeding behavior in rats by inhibiting the expression of MCT4, leading to abnormal expression of anorexigenic neuropeptides, and concluded that knockdown of MCT4 resulted in impaired lactate flux between glial cells (tanycytes)–neurons (anorexigenic neurons) and consequently anorexia (Elizondo-Vega et al. 2020). The significant function of MCTs and lactate in neural signaling was further demonstrated.
GPR81/HCAR1—a Lactate Receptor in the Brain
GPR81, a G protein–coupled receptor also known as hydroxycarboxylic acid receptor 1 (HCA1/HCAR1), is a currently recognized lactate receptor (Kuei et al. 2011). Normally, lactate is considered a metabolite and an energy substrate. However, studies have indicated that lactate can act as a signaling molecule to activate the receptor GPR81 and inhibit lipolysis in adipocytes by downregulating cAMP levels (Sun et al. 2017). In addition to adipose tissue, GPR81 is expressed in skeletal muscles, liver, kidneys, and other organs (Madaan et al. 2019; Brown and Ganapathy 2020) and mediates the function of l-lactate in various processes such as angiogenesis, neuroprotection, promotion of tumor metabolism, and anti-inflammatory effects (Shen et al. 2015; Feng et al. 2017; Madaan et al. 2017; Sun et al. 2017; Khatib-Massalha et al. 2020; Yang et al. 2020). Moreover, GPR81 has been shown to exist in the main neurons of the mammalian cerebral cortex and hippocampus, and is mostly concentrated in the synaptic membrane of excitatory synapses, showing postsynaptic dominance. GPR81 is also enriched at the blood–brain barrier (Lauritzen et al. 2014). Based on the significant role of lactate in brain function and brain metabolism, Chaudhari et al. found that lactate stimulation was beneficial for the increase in vascular endothelial growth factor, angiopoietin-1 and angiopoietin-2, platelet-derived growth factor, and other substances favorable for cerebral angiogenesis in the brain of mice with perinatal hypoxic/ischemic brain injury. Furthermore, the expression of thrombospondin-1 (TSP-1), which inhibits microangiogenesis, was decreased in neurons, and the infarct size of brain tissues was reduced. An opposite conclusion was reached in a mouse model lacking the lactate receptor GPR81, where injection of lactate into the lateral ventricles failed to alleviate the damage (Chaudhari et al. 2022). Moreover, exogenous l-lactate could upregulate GPR81 expression and facilitate neurological recovery in traumatic brain injury (TBI) rats (Zhai et al. 2020), cognitive impairment, and AD (Morland et al. 2015). Furthermore, l-lactate and its receptor GPR81 have significant functions in the development of retinal ganglion cells in the CNS and maintaining the integrity of the retinal thalamic pathway formed by the projection of cell axons to the thalamus (Laroche et al. 2021). The results of these studies suggest that lactate functions as a signaling molecule with its receptor GPR81 in a wide range of regions of the CNS and may contribute to linking synaptic function, regulating energy metabolism, and improving cerebral blood flow.
Protein Lactylation
Histone Lactylation
Histones are unique compounds composed of proteins in chromatin (nucleosome core) and DNA. They alter the properties of proteins by binding to specific amino acid residues through covalent bonds to form modification groups or by shearing off groups through protein hydrolysis, thereby regulating gene expression. This process is known as post-translational modifications (PTMs) (Sharma and Pal 2021). The classical forms of PTMs include phosphorylation, acetylation, glycosylation, and ubiquitination. In recent years, novel protein modifications, such as malonylation, succinylation, and glutarylation, have also been discovered, and they regulate cellular physiological functions by altering the spatial structure of proteins (Sabari et al. 2017). Zhang et al. found that lactylation of histone lysine residues induced by hypoxia and the Warburg effect can act as a novel PTM to stimulate gene transcription in chromatin, promote the expression of homeostatic genes such as arginase 1 (Arg1), and shift macrophages from M1 to M2 type (Zhang et al. 2019). Lactate can act as a precursor to stimulate histone lactylation and affect histone lysine lactylation (Kla) levels. Carlos et al. confirmed that l-lactate is a known precursor of the histone Kla process, and the process is most likely completed by the catalytic reaction of lactyl coenzyme A (Moreno-Yruela et al. 2022a, b), of which l-lactate is predominant in humans. Therefore, histone lactylation primarily involves lysine l-lactylation. Moreover, it has been demonstrated that MGO, the precursor of lysine d-lactylation, binds to glutathione in the presence of glyoxalase 1 to produce lactoyl glutathione (LGSH) and can be catabolized to d-lactate and glutathione catalyzed by glyoxalase 2 (Gaffney et al. 2020). However, studies have indicated that the occurrence of d-Kla originates from the non-enzymatic reaction between MGO and LGSH rather than d-lactate (Liu et al. 2022). Thus, protein lactylation can also be classified as either enzymatic or non-enzymatic lysine lactylation.
In addition, the processes of histone lactylation and delactylation can be regulated by the epigenetic activities of the writer, reader, and eraser enzymes (Hou et al. 2022; Yang et al. 2022a, b, c, d). Among them, p300, a multi-substrate histone acetyltransferase, can use different acyl-coAs as substrates for the acylation of multiple histone lysines (Sabari et al. 2017). Zhang et al. found that p300 can participate in the enzymatic reaction process of histone lactylation as a “writer enzyme” (Zhang et al. 2019). In addition, p300 and its homolog, CREB-binding protein C (CBP), have been shown to promote the lactylation of HMGB1 in macrophages, suggesting that p300/CBP may be one of the regulatory enzymes involved in histone lactylation (Yang et al. 2022a, b, c, d). In addition, in contrast to the facilitative role of the “writer enzyme,” Moreno-Yruela et al. demonstrated that Zn2+- and NAD+-dependent histone deacetylase (HDAC1-3) can be involved in the dynamic regulation of de-histone lactation as “eraser enzymes.” HDAC1 and HDAC3 showed more obvious de-L-lactylase activity in cells (Moreno-Yruela et al. 2022a, b). In addition, overall histone delactylation was detected in SIRT1-3, a member of the NAD+-dependent deacetylase family (Moreno-Yruela et al. 2022a, b). However, Jennings et al. found that the enzymatic efficiency of SIRT2 was much lower than that of HDAC1-3 (Jennings et al. 2021), demonstrating the significant role of HDAC1-3 as the main “eraser enzyme” for histone lactylation. In addition to the enzymatic reaction, other factors such as the expression of specific genes and neuronal excitation in the brain can influence the level of lactylation modification (Li et al. 2020; Hagihara et al. 2021) (Fig. 2).
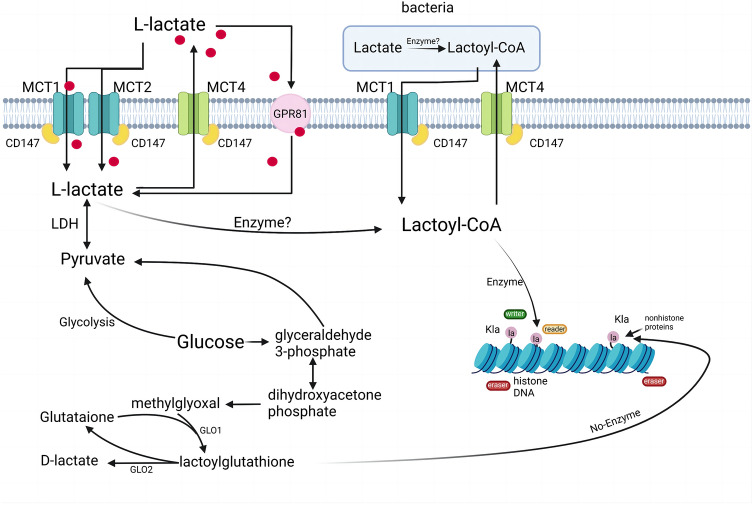
Fig. 2.
Pathways of lactate production and intracellular lactylation. Two different mechanisms of l-lactate and d-lactate production and enzymatic and non-enzymatic protein lactylation are described. In this case, l-lactate is converted to lactyl coenzyme A. Lactyl coenzyme A then transfers lactyl groups to lysine residues on histones in a specific enzymatic reaction to undergo histone lactylation, a process regulated by epigenetic writer, reader, and eraser enzymes (among the Kla writer enzymes identified are p300 and eraser enzymes including HDAC1–3 and SIRT1–3; the reader enzymes in Kla are not clear yet). Alternatively, the regulation of gene expression also depends on the non-enzymatic lactylation process from LGSH to lactoyl lysine. HDAC histone deacetylase, Kla lysine lactylation, LGSH lactoyl glutathione (Created with BioRender.com)
Non-enzymatic Lysine Lactylation
Although lysine residues of non-histone proteins are more difficult to undergo lactoyl substitution than histones, lactylation derived from the non-enzymatic reaction of lysine with LGSH has been demonstrated (Gaffney et al. 2020; Varner et al. 2020). In addition to core histones, other proteins distributed in the nucleus, cytoplasm, mitochondria, ribosomes, and cell membranes have been found to contain a large number of different Kla sites. While proposing the feedback mechanism of non-enzymatic lysine lactylation, Gaffney et al. identified 350 “LactoylLys” modifications on proteins in HEK293T cells, which were found to be abundant in high-glycolytic tissues and rich in glycolytic enzymes (Gaffney et al. 2020; Yang et al. 2022a, b, c, d). Furthermore, protein lactylation was found in ischemic brain tissues and eye tissues in high-altitude acclimatization (Hou et al. 2022; Yao et al. 2022), where lactylation modifications occurred on proteins with a molecular weight of 25 kDa in eye tissues of high-altitude acclimated mice (Hou et al. 2022). In addition to animal models, multiple lactylation protein modification sites have been observed in fungi, parasites, and plants, emphasizing the widespread presence of lactylation modifications in organisms (Gao et al. 2020; Meng et al. 2021; Zhang et al. 2021a, b).
Lactylation and Diseases
Protein lactylation has been detected in multiple disease models and has been widely used in the research on various cancers. The occurrence of histone lactylation in non-histone proteins has been observed in non-small cell lung cancer, clear cell renal cell carcinoma, colon cancer, gastric cancer, and other tumor tissues (Jiang et al. 2021; Yu et al. 2021; Pan et al. 2022a, b; Wang et al. 2022; Yang et al. 2022a, b, c, d). Lactate accumulation from metabolic reorganization in cancer patients can replenish the TCA cycle; in this process, lactate mediates the expression of the relevant genes through histone lactylation, which then regulates tumor cell proliferation and may be responsible for the immune escape and poor clinical outcome of the tumor microenvironment (TME) (Jin et al. 2022). It has been shown that lactate in tumor cells can act as a signaling molecule to induce vascular endothelial growth factor expression and M2-like polarization of macrophages mediated by hypoxia-inducible factor 1α (Palsson-McDermott et al. 2015). This communication between tumor cells and macrophages involves multiple signals; however, it is assumed that this signal involving the transition of macrophages from a pro-inflammatory state to an anti-inflammatory state may not be specific to tumor cells. In addition, the discovery that histone lactylation exhibits different temporal kinetics from histone acetylation during the polarization of M1 to M2 macrophages demonstrates the functional uniqueness of histone lactylation according to the unique characteristics of macrophages (Zhang et al. 2019).
Given the regulatory function of lactylation on macrophage phenotypic polarization, an association between macrophages and lactylation levels has also been observed in ulcerative colitis, inflammatory injury, cold stress, and sepsis, in addition to cancer (Irizarry-Caro et al. 2020; Sun et al. 2021; Lopez Krol et al. 2022; Lu et al. 2022; Yang et al. 2022a, b, c, d). In addition, lactylation may exert an anti-inflammatory effect of macrophages in patients with septic shock by affecting the expression of inflammatory cytokines (Chu et al. 2021). Moreover, the finding of elevated levels of histone lactylation in preeclamptic placentas has provided more insight into the mechanisms of placental dysfunction in preeclampsia. This demonstrates that lactylation modifications have significant functions in the mechanisms of multiple diseases (Li et al. 2022). In addition, lactylation modifications also have a unique function in the regulation of brain function and the mechanisms involved in the development of brain diseases (Hagihara et al. 2021; Pan et al. 2022a, b) (Table 1).
Table 1.
Modification of lactylation in diseases
| Disease | Enzyme regulation | Protein targets | Cell/tissue sample | Lactylated proteins (histone/non-histone) | References | |
|---|---|---|---|---|---|---|
| Histone | Ocular melanoma | YTHDF2 | OCM1/CRMM1/PIG1/92.1/OMM1/MUM2B/CRMM1/CRMM2/CM2005.1/MEL290/ocular melanoma tissues | H3 | Yu et al. (2021) | |
| AD | PKM2 | Macroglia/brain tissues | H3, H4 | Pan et al. (2022a, b) | ||
| Pulmonary fibrosis | p300 | Macrophage | – | Cui et al. (2021) | ||
| Intestinal inflammation | Macrophage/Th17 | H3 | Irizarry-Caro et al. (2020), Lopez Krol et al. (2022) | |||
| Non-small cell lung cancer | A549/H1299 | – | Jiang et al. (2021) | |||
| Social defeat stress | Brain tissues | H1 | Hagihara et al. (2021) | |||
| Liver cancer | HCCLM3-LCSCs/Hep3B-LCSCs | H3 | Pan et al. (2022a, b) | |||
| Septic shock | Arg1 | Peripheral blood mononuclear (PBMC) | H3 | Chu et al. (2021) | ||
| Clear cell renal cell carcinoma | PDGFRβ | HK2/786-O/A498/Caki-1/ccRCC tissues | H3 | Yang et al. (2022a, b, c, d) | ||
| Ulcerative colitis | Macrophage | H3 | (Sun et al. 2021) | |||
| Colorectal cancer | LINC00152 | HCT116/colon cancer tissues | H4 | Wang et al. (2022) | ||
| Placental dysfunction in preeclampsia | HTR-8/SVneo/TEV-1 | – | Li et al. (2022) | |||
| Cold stress | STAT6 | Macrophage | – | Lu et al. (2022) | ||
| Non-histone | Sepsis | p300 | Macrophage | HMGB1 | Yang et al. (2022a, b, c, d) | |
| Gastric cancer | AGS cells/HGC-27/MGC-803/GES-1 | – | Yang et al. (2022a, b, c, d) |
AD Alzheimer's disease, LCSCs liver cancer stem cells, PDGFRβ platelet-derived growth factor receptor β, Arg1 arginase 1, ccRCC clear cell renal cell carcinoma, YTHDF2 YTH N6-methyladenosine RNA-binding protein 2, PKM2 pyruvate kinase M2
Protein Lactylation Levels in the Regulation of Brain Function
Neuronal Excitation
Histone Kla has been shown to occur in brain tissue cells, and immunoreactivity for Kla has been detected in glutamatergic neurons, γ-aminobutyric acidergic neurons, astrocytes, and microglia (Hagihara et al. 2021). The level of lactate in the brain can be increased by neuronal excitatory stimuli (Díaz-García et al. 2017; Oses et al. 2019), and the dependence of the extent of Kla on the concentration of lactate has been demonstrated by previous studies (Zhang et al. 2019). Thus, in vitro neuronal cell culture showed that induction of neuronal depolarization process by high K state and electroconvulsive stimulation increased neuronal excitability, intracellular lactate concentration, and intracellular Kla levels. There was a positive correlation between the immunoreactivity of Kla and the expression of c-Fos (of which expression was positively correlated with the intensity of electrical stimulation) (Krukoff 1993). The result was also demonstrated in the brain cells of live mice in vivo, suggesting that neuronal excitation can induce neuronal Kla (Hagihara et al. 2021). Moreover, Kla occurring in the prefrontal cortex region of mice is considered to be associated with anxiety-like behavior in social defeat stress mice, and this association is caused by increased neural excitation due to stress. It is assumed that this regional occurrence of lactylation of brain tissues has potential implications for the function of the relevant neurological functional areas (Hagihara et al. 2021). Therefore, neuronal excitation can affect brain function by increasing the immunoreactivity of Kla through lactate, but the specific mechanism underlying the association between lactate and Kla (e.g., whether lactate can be a direct source of lactoyl) needs to be elucidated by research.
Inflammatory Regulation
Arg1 is considered to be associated with tissue repair and wound healing (Cai et al. 2019). Zhang et al. demonstrated that an increase in Kla resulted in increased Arg1 expression and that this alteration was correlated with the expression of a reparative macrophage phenotype (Zhang et al. 2019). Macrophages, which are important component cells of the body's intrinsic immunity, can recognize the corresponding injury and generate an inflammatory response, a process that activates toll-like receptors (TLRs) and produces pro-inflammatory cytokines such as tumor necrosis factor, interleukin (IL)-6, IL-12, and NF-κB to activate macrophages toward a pro-inflammatory phenotype. However, PI3K/Akt, a downstream signaling pathway of TLRs, can inhibit TLR-mediated activation of downstream transcription factors and the production of inflammatory factors (Miyazawa 2012). B-cell adaptor protein (BCAP) serves as a junctional protein for PI3K signaling by B cells (Inabe and Kurosaki 2002) and is an important protein that links TLR signaling to the PI3K/Akt pathway (Halabi et al. 2017). It has been shown that, in the absence of BCAP, the PI3K/Akt pathway cannot be activated, resulting in reduced aerobic glycolysis of cells, which further inhibits lactate production and lactylation modifications of histones and ultimately inhibits the shift of macrophages to the M2 phenotype associated with tissue repair (Irizarry-Caro et al. 2020). Thus, BCAP acts as an upstream bridging factor for aerobic glycolysis, enabling the production of lactate to promote lactylation of appropriate histones expressed by repair genes, which has a positive effect on the attenuation of inflammation.
Atherosclerosis (AS), a chronic inflammatory disease resulting from impaired inflammatory regression, is often considered an important cause of the development of cerebrovascular diseases (Yang et al. 2022a, b, c, d). Histone deacetylase 3 (HDAC3) is an epigenetic modifier, and overexpression of HDAC3 contributes to disease progression in AS. Carlos et al. demonstrated that HDAC3 can act as a deacetylase active protein to regulate the level of histone lactylation (sites of action include but are not limited to H4K5) (Moreno-Yruela et al. 2022a, b). Therefore, histone lactylation is a promising target for the treatment of AS and even cerebrovascular diseases because it participates in macrophage reprogramming and correction of the polarization direction of macrophages during AS (Jin et al. 2021).
Neuronal Development
Histone lactylation is dependent on the enzymatic promotion of lactate by lysine acetyltransferase enzymatic p300 and lactyl-CoA, and the level of lactylation is regulated by downstream writer and eraser enzymes (Moreno-Yruela et al. 2022a, b). Among them, the function of HDAC1–3 as an “eraser enzyme” for histone lactylation to regulate the level of Kla has been demonstrated (Dai et al. 2022; Moreno-Yruela et al. 2022a, b). Histone lactylation was observed to be widespread in the forebrain of E14.5 mice (the period of greatest neural stem cell enrichment), particularly in the N-terminal tail of H3 (Dai et al. 2022). Furthermore, it was found that, during the stages of neural development and differentiation in mice, a decrease in the levels of H3K18la and overall H3-Kla occurred with time, and the levels of H3K14la and H3K18la were increased by the inhibition of HDAC1-3, which may activate the transformation program of neural stem cells and facilitate the maturation and differentiation of neurons (Tang et al. 2019; Dai et al. 2022). However, the direct link between neural differentiation and changes in lactylation levels has not been demonstrated. The crosstalk of multiple epigenetic modifications might be one of the mechanisms.
Lactylation for Potential Application in Brain Diseases
AD
In recent years, it has been shown that the development of AD is associated with a metabolic defect resulting from the metabolic reprogramming of microglia (Guillot-Sestier et al. 2015; Baik et al. 2019), which act as intrinsic immune cells in the CNS; their immune function and metabolic processes can be inhibited by the long-term deposition of amyloid-β (Aβ), which is considered to be closely associated with neuronal deficits and cognitive impairment in AD (Baik et al. 2019). However, the exact mechanism of microglial dysfunction is currently unknown. It has been suggested that the microglial metabolic program shifts from oxidative phosphorylation to glycolysis in response to acute stress (e.g., exposure to Aβ) to produce large amounts of ATP to support the immune response of microglia (Pan et al. 2019; Hur et al. 2020). However, long-term sustained stimulation with Aβ has been shown to lead to metabolic defects in microglia that impair both glycolysis and OXPHOS, ultimately leading to microglial dysfunction, which favors the development of AD (Baik et al. 2019; Delizannis et al. 2021). Moreover, it has also been suggested that lactate production from sustained aerobic glycolysis negatively affects microglial function (McIntosh et al. 2019). Pan et al. found elevated levels of pan-lysine lactylation (Pan KLA) and H4K12LA in the prefrontal cortex and hippocampus of 5xFAD mice (a model of AD), where H4K12LA is enriched in the promoter of glycolytic genes and promotes transcription of glycolytic genes. These results are consistent with those observed in postmortem brain tissues of AD patients and suggest that the long-term persistent neuroinflammation in AD is mediated by a positive feedback loop of glycolysis/H4K12LA/pyruvate kinase M2; this suggests that the inhibition of the feedback loop of glycolysis–lactate-histone lactation–glycolysis may be an effective strategy for the treatment of AD (Pan et al. 2022a, b).
Stroke
After stroke, ischemic necrosis or softening of brain tissues at the lesion site occurs because of local ischemia and hypoxia, resulting in damage and death of brain cells (Yao et al. 2013). However, cerebral ischemia–reperfusion injury (CIRI) cannot be overlooked when improving the ischemia state and restoring blood flow reperfusion. CIRI can aggravate brain injury and accelerate the necrosis and apoptosis of nerve cells (Zhang et al. 2018a, b). As the research focus of stroke treatment, CIRI is believed to be associated with inflammatory response, blood–brain barrier damage, oxidative stress, intracellular Ca2+ overload, excitatory amino acid toxicity, free radical damage, and other mechanisms (Schurr et al. 1999; Takata et al. 2012; Zhou et al. 2013; Chu et al. 2014; Ma et al. 2016; Singh et al. 2019).
It has been shown that excitatory glutamate accumulated extracellularly after cerebral ischemia is neurotoxic and causes Ca2+ overload by mediating a large release of intracellular Ca2+ through a large Na+ inward flow (Kiewert et al. 2010). This can be linked to post-ischemic acidosis, as the occurrence of acidosis is considered to be closely related to the acid-sensing ion channels (Vick and Askwith 2015). ASIC1a, the most widespread class of subunits in brain tissues, has Ca2+ permeability activity that can cause intracellular Ca2+ overload and exacerbate neuronal cell necrosis and apoptosis by activating related proteases and generating nitric oxide or free radicals (Singh et al. 2019; Savic Azoulay et al. 2020). During cerebral ischemic acidosis, large amounts of lactate accumulate intracellularly (Bouzat and Oddo 2014; Cai et al. 2022) because stroke occurs in a state of ischemia and hypoxia in brain tissues and the mode of energy metabolism changes from aerobic to glycolytic processes to ensure the supply of ATP. This process depends on the activation of the glycolytic process in astrocytes through the uptake of increased extracellular glutamate and the combined effect of Na+ inward flow, which increases the concentration of lactate (Cai et al. 2022). Moreover, the damaged blood–brain barrier allows lactate from the blood to enter the brain, which may exacerbate the accumulation of lactate in the brain (Aveseh et al. 2014). Recent studies have indicated that lactate can act as a type of specific energy substrate for damaged neurons, especially l-lactate, during a specific time window after stroke onset (Berthet et al. 2012; Zhai et al. 2020; Zhang et al. 2021a, b). Yao et al. measured the level of protein lactylation in endothelial cells of CIRI rat brains and identified 49 upregulated proteins and 99 downregulated proteins, including 54 upregulated sites and 54 downregulated sites. They found that the main functions of proteins with high levels of lactylation in the brain are related to brain development, neuronal damage and regeneration, and energy transfer. Furthermore, after screening of protein interaction networks, it was concluded that lactate production and accumulation during cerebral ischemia and the lactylation occurring in Scl25a4 (K245), Slc25a5 (K96), and Vdac1 (K20 and K266), which are key proteins of the Ca2+ signaling pathway, may influence mitochondrial function and neuronal injury (Yao et al. 2022).
In addition, the damage-associated molecular patterns (DAMPs) released from damaged and apoptotic cells during stroke onset recognize and bind to toll-like receptors on the surface of microglia and activate the response of immune cells (such as macrophages) to induce the synthesis of inflammatory cytokines, such as tumor necrosis factor-α, IL-1β, and IL-6, leading to damage to the blood–brain barrier (Yao et al. 2013). As a result of the damaged blood–brain barrier, a variety of immune cells in peripheral blood reach the stroke area. Furthermore, a variety of immune cells, such as macrophages, accumulate locally in the lesion and cause the brain tissues at the damaged area to be infiltrated by immune cells, which can further aggravate brain injury (Shichita et al. 2012). Although the effect of histone lactylation on macrophage polarization (from pro-inflammatory M1 to reparative M2) has been demonstrated, this shift is accomplished by promoting Arg1 expression (Zhang et al. 2019). Moreover, higher concentrations of lactate increased the level of histone H3K18 lactylation in Th17 cells and reduced the production of the pro-inflammatory cytokine IL-17A, implying a potential link between lactate-induced histone lactylation and the transformation of T-cell phenotype (Lopez Krol et al. 2022). Therefore, a bold hypothesis is proposed here to link lactate-histone lactylation to stroke by interfering with the polarization of immune cells such as macrophages, which affects the homeostasis of the microenvironment in the stroke zone and participates in the repair of inflammatory damage and neurological function.
Epilepsy
Epilepsy, a neurological disorder characterized by neuronal hyperexcitability and sudden synchronous discharges, has been suggested in recent years to be associated with the generation and development of astrocyte dysfunction and disturbed glial metabolism in the brain (Boison and Steinhäuser 2018). Among them, astrocytes absorb glycogen from the blood and stimulate glycolysis to produce lactate and provide energy substrates to neurons via intercellular lactate shuttling, a process that requires the transport collaboration of MCTs (Mächler et al. 2016). MCT1 expression was demonstrated to be absent on microvessels and upregulated on astrocytes in the sclerosing epileptogenic hippocampal region of patients with drug-refractory temporal lobe epilepsy (Lauritzen et al. 2012a, b). Subsequently, a loss of MCT2 protein was seen in the astrocyte end-foot surrounding the vessels (Lauritzen et al. 2012a, b). This could also explain the phenomenon of elevated lactate concentrations in brain tissues extracellularly during epilepsy (van Hall et al. 2009). Due to the downregulation of cAMP levels via the lactate receptor GPR81, it is hypothesized that the downregulation of MCTs may be an adaptive response intended to maintain high levels of lactate in epileptic tissue and prevent seizures (Lauritzen et al. 2015). As a result, antiepileptic medication has been proposed to target lactate receptor signaling. Further research is still needed to determine whether the relevant proteins' increased lactylation is caused by the elevated lactate content.
Conclusion
In this study, it was found that by being involved in energy metabolism and signaling in several brain tissues in healthy and pathological conditions, lactate and protein lactylation had significant functions in the brain and may have potential applications. This review discussed how brain disorders, such as AD and ischemic brain damage, begin and progress. Lactate was found to control the expression of its downstream receptors and transporters in the brain, acting as both an energy substrate and a signaling agent. Furthermore, lactylation is a recently discovered epigenetic modification induced by lactate. There is mounting evidence for the potential association of lactate with neuroprotection and cognitive and memory aspects in the brain, although the specific mechanism is still unknown. Therefore, in-depth research into the mechanism of action of lactate and the related protein lactylation in the brain is warranted, particularly in the context of stroke and cognitive impairment, to provide new insights and ideas to examine the connection between protein lactylation and brain neurological function.
Acknowledgements
The figures from this article were created with BioRender.com. We are indebted to all individuals who participated in or helped with this research project.
Author Contributions
RL and YY are first authors, and contributed equally to this paper. RL and YY wrote the main manuscript text, HW, TZ, FD and KW prepared Figs. 1 and 2. SY and KX prepared Table 1. XJ and XS conceptualized the article and revised the final version. All authors read and approved the final manuscript.
Funding
This study was supported by funding from the National Nature Science Foundation of China (Grant No.82174261, No.81673865, and No.81503669), Heilongiiang Natural Science Foundation (LH2021H084 and H2015031), the outstanding Training Foundation of Heilongjiang University of Chinese Medicine (2019JC05), the outstanding Innovative Talents Support Plan of Heilongjiang University of Chinese Medicine (2018RCD11).
Data Availability
This article is a review article and does not contain relevant data.
Code Availability
This article is a review article and does not contain relevant code.
Declarations
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The authors have no relevant financial or non-financial interests to disclose.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Ruobing Li, Yi Yang, Xicheng Jiang, and Xiaowei Sun have contributed equally to this paper.
Contributor Information
Xicheng Jiang, Email: jiangxicheng5303@163.com.
Xiaowei Sun, Email: gemini19790530@163.com.
References
- Ardanaz CG, Ramírez MJ, Solas M (2022) Brain metabolic alterations in Alzheimer’s disease. Int J Mol Sci. 10.3390/ijms23073785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aveseh M, Nikooie R, Sheibani V et al (2014) Endurance training increases brain lactate uptake during hypoglycemia by up regulation of brain lactate transporters. Mol Cell Endocrinol 394:29–36. 10.1016/j.mce.2014.06.019 [DOI] [PubMed] [Google Scholar]
- Baik SH, Kang S, Lee W et al (2019) A breakdown in metabolic reprogramming causes microglia dysfunction in Alzheimer’s disease. Cell Metab 30:493-507.e496. 10.1016/j.cmet.2019.06.005 [DOI] [PubMed] [Google Scholar]
- Benjamin D, Robay D, Hindupur SK et al (2018) Dual inhibition of the lactate transporters MCT1 and MCT4 is synthetic lethal with metformin due to NAD+ depletion in cancer cells. Cell Rep 25:3047-3058.e3044. 10.1016/j.celrep.2018.11.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergersen LH (2015) Lactate transport and signaling in the brain: potential therapeutic targets and roles in body-brain interaction. J Cereb Blood Flow Metab 35:176–185. 10.1038/jcbfm.2014.206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergersen LH, Gjedde A (2012) Is lactate a volume transmitter of metabolic states of the brain? Front Neuroenergetics 4:5. 10.3389/fnene.2012.00005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthet C, Lei H, Thevenet J et al (2009) Neuroprotective role of lactate after cerebral ischemia. J Cereb Blood Flow Metab 29:1780–1789. 10.1038/jcbfm.2009.97 [DOI] [PubMed] [Google Scholar]
- Berthet C, Castillo X, Magistretti PJ et al (2012) New evidence of neuroprotection by lactate after transient focal cerebral ischaemia: extended benefit after intracerebroventricular injection and efficacy of intravenous administration. Cerebrovasc Dis 34:329–335. 10.1159/000343657 [DOI] [PubMed] [Google Scholar]
- Boison D, Steinhäuser C (2018) Epilepsy and astrocyte energy metabolism. Glia 66:1235–1243. 10.1002/glia.23247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouzat P, Oddo M (2014) Lactate and the injured brain: friend or foe? Curr Opin Crit Care 20:133–140. 10.1097/mcc.0000000000000072 [DOI] [PubMed] [Google Scholar]
- Boveris DL, Boveris A (2007) Oxygen delivery to the tissues and mitochondrial respiration. Front Biosci 12:1014–1023. 10.2741/2121 [DOI] [PubMed] [Google Scholar]
- Brauchi S, Rauch MC, Alfaro IE et al (2005) Kinetics, molecular basis, and differentiation of L-lactate transport in spermatogenic cells. Am J Physiol Cell Physiol 288:C523-534. 10.1152/ajpcell.00448.2003 [DOI] [PubMed] [Google Scholar]
- Brooks GA (2020) Lactate as a fulcrum of metabolism. Redox Biol 35:101454. 10.1016/j.redox.2020.101454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown TP, Ganapathy V (2020) Lactate/GPR81 signaling and proton motive force in cancer: role in angiogenesis, immune escape, nutrition, and Warburg phenomenon. Pharmacol Ther 206:107451. 10.1016/j.pharmthera.2019.107451 [DOI] [PubMed] [Google Scholar]
- Cai W, Dai X, Chen J et al (2019) STAT6/Arg1 promotes microglia/macrophage efferocytosis and inflammation resolution in stroke mice. JCI Insight. 10.1172/jci.insight.131355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai M, Wang H, Song H et al (2022) Lactate is answerable for brain function and treating brain diseases: energy substrates and signal molecule. Front Nutr 9:800901. 10.3389/fnut.2022.800901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhari P, Madaan A, Rivera JC et al (2022) Neuronal GPR81 regulates developmental brain angiogenesis and promotes brain recovery after a hypoxic ischemic insult. J Cereb Blood Flow Metab 42:1294–1308. 10.1177/0271678x221077499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu HX, Kim HA, Lee S et al (2014) Immune cell infiltration in malignant middle cerebral artery infarction: comparison with transient cerebral ischemia. J Cereb Blood Flow Metab 34:450–459. 10.1038/jcbfm.2013.217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu X, Di C, Chang P et al (2021) Lactylated histone H3K18 as a potential biomarker for the diagnosis and predicting the severity of septic shock. Front Immunol 12:786666. 10.3389/fimmu.2021.786666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contreras-Baeza Y, Sandoval PY, Alarcón R et al (2019) Monocarboxylate transporter 4 (MCT4) is a high affinity transporter capable of exporting lactate in high-lactate microenvironments. J Biol Chem 294:20135–20147. 10.1074/jbc.RA119.009093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui H, Xie N, Banerjee S et al (2021) Lung myofibroblasts promote macrophage profibrotic activity through lactate-induced histone lactylation. Am J Respir Cell Mol Biol 64:115–125. 10.1165/rcmb.2020-0360OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai SK, Liu PP, Li X et al (2022) Dynamic profiling and functional interpretation of histone lysine crotonylation and lactylation during neural development. Development. 10.1242/dev.200049 [DOI] [PubMed] [Google Scholar]
- Dalsgaard MK, Quistorff B, Danielsen ER et al (2004) A reduced cerebral metabolic ratio in exercise reflects metabolism and not accumulation of lactate within the human brain. J Physiol 554:571–578. 10.1113/jphysiol.2003.055053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dana P, Saisomboon S, Kariya R et al (2020) CD147 augmented monocarboxylate transporter-1/4 expression through modulation of the Akt-FoxO3-NF-κB pathway promotes cholangiocarcinoma migration and invasion. Cell Oncol (dordr) 43:211–222. 10.1007/s13402-019-00479-3 [DOI] [PubMed] [Google Scholar]
- de Bari L, Atlante A, Armeni T et al (2019) Synthesis and metabolism of methylglyoxal, S-D-lactoylglutathione and D-lactate in cancer and Alzheimer’s disease. Exploring the crossroad of eternal youth and premature aging. Ageing Res Rev 53:100915. 10.1016/j.arr.2019.100915 [DOI] [PubMed] [Google Scholar]
- Debernardi R, Pierre K, Lengacher S et al (2003) Cell-specific expression pattern of monocarboxylate transporters in astrocytes and neurons observed in different mouse brain cortical cell cultures. J Neurosci Res 73:141–155. 10.1002/jnr.10660 [DOI] [PubMed] [Google Scholar]
- Delizannis AT, Nonneman A, Tsering W et al (2021) Effects of microglial depletion and TREM2 deficiency on Aβ plaque burden and neuritic plaque tau pathology in 5XFAD mice. Acta Neuropathol Commun 9:150. 10.1186/s40478-021-01251-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Díaz-García CM, Mongeon R, Lahmann C et al (2017) Neuronal stimulation triggers neuronal glycolysis and not lactate uptake. Cell Metab 26:361-374.e364. 10.1016/j.cmet.2017.06.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Hayek L, Khalifeh M, Zibara V et al (2019) Lactate mediates the effects of exercise on learning and memory through SIRT1-dependent activation of hippocampal brain-derived neurotrophic factor (BDNF). J Neurosci 39:2369–2382. 10.1523/jneurosci.1661-18.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elizondo-Vega R, Oyarce K, Salgado M et al (2020) Inhibition of hypothalamic MCT4 and MCT1-MCT4 expressions affects food intake and alters orexigenic and anorexigenic neuropeptide expressions. Mol Neurobiol 57:896–909. 10.1007/s12035-019-01776-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faubert B, Li KY, Cai L et al (2017) Lactate metabolism in human lung tumors. Cell 171:358-371.e359. 10.1016/j.cell.2017.09.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng J, Yang H, Zhang Y et al (2017) Tumor cell-derived lactate induces TAZ-dependent upregulation of PD-L1 through GPR81 in human lung cancer cells. Oncogene 36:5829–5839. 10.1038/onc.2017.188 [DOI] [PubMed] [Google Scholar]
- Gaffney DO, Jennings EQ, Anderson CC et al (2020) Non-enzymatic lysine lactoylation of glycolytic enzymes. Cell Chem Biol 27:206-213.e206. 10.1016/j.chembiol.2019.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao M, Zhang N, Liang W (2020) Systematic analysis of lysine lactylation in the plant fungal pathogen botrytis cinerea. Front Microbiol 11:594743. 10.3389/fmicb.2020.594743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs ME, Hertz L (2008) Inhibition of astrocytic energy metabolism by D-lactate exposure impairs memory. Neurochem Int 52:1012–1018. 10.1016/j.neuint.2007.10.014 [DOI] [PubMed] [Google Scholar]
- Guillot-Sestier MV, Doty KR, Gate D et al (2015) Il10 deficiency rebalances innate immunity to mitigate Alzheimer-like pathology. Neuron 85:534–548. 10.1016/j.neuron.2014.12.068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagihara H, Shoji H, Otabi H et al (2021) Protein lactylation induced by neural excitation. Cell Rep 37:109820. 10.1016/j.celrep.2021.109820 [DOI] [PubMed] [Google Scholar]
- Halabi S, Sekine E, Verstak B et al (2017) Structure of the toll/interleukin-1 receptor (TIR) domain of the b-cell adaptor that links phosphoinositide metabolism with the negative regulation of the toll-like receptor (TLR) signalosome. J Biol Chem 292:652–660. 10.1074/jbc.M116.761528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halestrap AP (2013) The SLC16 gene family—structure, role and regulation in health and disease. Mol Aspects Med 34:337–349. 10.1016/j.mam.2012.05.003 [DOI] [PubMed] [Google Scholar]
- Halestrap AP, Price NT (1999) The proton-linked monocarboxylate transporter (MCT) family: structure, function and regulation. Biochem J 343(Pt 2):281–299 [PMC free article] [PubMed] [Google Scholar]
- Hashimoto T, Hussien R, Brooks GA (2006) Colocalization of MCT1, CD147, and LDH in mitochondrial inner membrane of L6 muscle cells: evidence of a mitochondrial lactate oxidation complex. Am J Physiol Endocrinol Metab 290:E1237-1244. 10.1152/ajpendo.00594.2005 [DOI] [PubMed] [Google Scholar]
- Hohnholt MC, Andersen VH, Bak LK et al (2017) Glucose, lactate and glutamine but not glutamate support depolarization-induced increased respiration in isolated nerve terminals. Neurochem Res 42:191–201. 10.1007/s11064-016-2036-4 [DOI] [PubMed] [Google Scholar]
- Hou J, Zheng D, Wen X et al (2022) Proteomic and morphological profiling of mice ocular tissue during high-altitude acclimatization process: an animal study at Lhasa. J Inflamm Res 15:2835–2853. 10.2147/jir.s361174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J, Cai M, Shang Q et al (2021) Elevated lactate by high-intensity interval training regulates the hippocampal BDNF expression and the mitochondrial quality control system. Front Physiol 12:629914. 10.3389/fphys.2021.629914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hur JY, Frost GR, Wu X et al (2020) The innate immunity protein IFITM3 modulates γ-secretase in Alzheimer’s disease. Nature 586:735–740. 10.1038/s41586-020-2681-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inabe K, Kurosaki T (2002) Tyrosine phosphorylation of B-cell adaptor for phosphoinositide 3-kinase is required for Akt activation in response to CD19 engagement. Blood 99:584–589. 10.1182/blood.v99.2.584 [DOI] [PubMed] [Google Scholar]
- Irizarry-Caro RA, McDaniel MM, Overcast GR et al (2020) TLR signaling adapter BCAP regulates inflammatory to reparatory macrophage transition by promoting histone lactylation. Proc Natl Acad Sci U S A 117:30628–30638. 10.1073/pnas.2009778117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings EQ, Ray JD, Zerio CJ et al (2021) Sirtuin 2 regulates protein LactoylLys modifications. ChemBioChem 22:2102–2106. 10.1002/cbic.202000883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang J, Huang D, Jiang Y et al (2021) Lactate modulates cellular metabolism through histone lactylation-mediated gene expression in non-small cell lung cancer. Front Oncol 11:647559. 10.3389/fonc.2021.647559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin F, Li J, Guo J et al (2021) Targeting epigenetic modifiers to reprogramme macrophages in non-resolving inflammation-driven atherosclerosis. Eur Heart J Open 1:oeab022. 10.1093/ehjopen/oeab022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin M, Cao W, Chen B et al (2022) Tumor-derived lactate creates a favorable niche for tumor via supplying energy source for tumor and modulating the tumor microenvironment. Front Cell Dev Biol 10:808859. 10.3389/fcell.2022.808859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khatib-Massalha E, Bhattacharya S, Massalha H et al (2020) Lactate released by inflammatory bone marrow neutrophils induces their mobilization via endothelial GPR81 signaling. Nat Commun 11:3547. 10.1038/s41467-020-17402-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiewert C, Mdzinarishvili A, Hartmann J et al (2010) Metabolic and transmitter changes in core and penumbra after middle cerebral artery occlusion in mice. Brain Res 1312:101–107. 10.1016/j.brainres.2009.11.068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krukoff TL (1993) Expression of c-fos in studies of central autonomic and sensory systems. Mol Neurobiol 7:247–263. 10.1007/bf02769178 [DOI] [PubMed] [Google Scholar]
- Kuei C, Yu J, Zhu J et al (2011) Study of GPR81, the lactate receptor, from distant species identifies residues and motifs critical for GPR81 functions. Mol Pharmacol 80:848–858. 10.1124/mol.111.074500 [DOI] [PubMed] [Google Scholar]
- Lambertus M, Øverberg LT, Andersson KA et al (2021) L-lactate induces neurogenesis in the mouse ventricular-subventricular zone via the lactate receptor HCA(1). Acta Physiol (oxf) 231:e13587. 10.1111/apha.13587 [DOI] [PubMed] [Google Scholar]
- Laroche S, Stil A, Germain P et al (2021) Participation of L-lactate and its receptor HCAR1/GPR81 in neurovisual development. Cells. 10.3390/cells10071640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauritzen F, Heuser K, de Lanerolle NC et al (2012a) Redistribution of monocarboxylate transporter 2 on the surface of astrocytes in the human epileptogenic hippocampus. Glia 60:1172–1181. 10.1002/glia.22344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauritzen F, Perez EL, Melillo ER et al (2012b) Altered expression of brain monocarboxylate transporter 1 in models of temporal lobe epilepsy. Neurobiol Dis 45:165–176. 10.1016/j.nbd.2011.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauritzen KH, Morland C, Puchades M et al (2014) Lactate receptor sites link neurotransmission, neurovascular coupling, and brain energy metabolism. Cereb Cortex 24:2784–2795. 10.1093/cercor/bht136 [DOI] [PubMed] [Google Scholar]
- Lauritzen F, Eid T, Bergersen LH (2015) Monocarboxylate transporters in temporal lobe epilepsy: roles of lactate and ketogenic diet. Brain Struct Funct 220:1–12. 10.1007/s00429-013-0672-x [DOI] [PubMed] [Google Scholar]
- Lee Y, Morrison BM, Li Y et al (2012) Oligodendroglia metabolically support axons and contribute to neurodegeneration. Nature 487:443–448. 10.1038/nature11314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lev-Vachnish Y, Cadury S, Rotter-Maskowitz A et al (2019) L-lactate promotes adult hippocampal neurogenesis. Front Neurosci 13:403. 10.3389/fnins.2019.00403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Chen K, Wang T et al (2020) Glis1 facilitates induction of pluripotency via an epigenome-metabolome-epigenome signalling cascade. Nat Metab 2:882–892. 10.1038/s42255-020-0267-9 [DOI] [PubMed] [Google Scholar]
- Li X, Yang N, Wu Y et al (2022) Hypoxia regulates fibrosis-related genes via histone lactylation in the placentas of patients with preeclampsia. J Hypertens 40:1189–1198. 10.1097/hjh.0000000000003129 [DOI] [PubMed] [Google Scholar]
- Liu X, Zhang Y, Li W et al (2022) Lactylation, an emerging hallmark of metabolic reprogramming: current progress and open challenges. Front Cell Dev Biol 10:972020. 10.3389/fcell.2022.972020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez Krol A, Nehring HP, Krause FF et al (2022) Lactate induces metabolic and epigenetic reprogramming of pro-inflammatory Th17 cells. EMBO Rep 23:e54685. 10.15252/embr.202254685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J, Fu S, Dai J et al (2022) Integrated metabolism and epigenetic modifications in the macrophages of mice in responses to cold stress. J Zhejiang Univ Sci B 23:461–480. 10.1631/jzus.B2101091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y, Yabluchanskiy A, Iyer RP et al (2016) Temporal neutrophil polarization following myocardial infarction. Cardiovasc Res 110:51–61. 10.1093/cvr/cvw024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mächler P, Wyss MT, Elsayed M et al (2016) In vivo evidence for a lactate gradient from astrocytes to neurons. Cell Metab 23:94–102. 10.1016/j.cmet.2015.10.010 [DOI] [PubMed] [Google Scholar]
- Madaan A, Nadeau-Vallée M, Rivera JC et al (2017) Lactate produced during labor modulates uterine inflammation via GPR81 (HCA(1)). Am J Obstet Gynecol 216:60.e61-60.e17. 10.1016/j.ajog.2016.09.072 [DOI] [PubMed] [Google Scholar]
- Madaan A, Chaudhari P, Nadeau-Vallée M et al (2019) Müller cell-localized g-protein-coupled receptor 81 (hydroxycarboxylic acid receptor 1) regulates inner retinal vasculature via Norrin/Wnt pathways. Am J Pathol 189:1878–1896. 10.1016/j.ajpath.2019.05.016 [DOI] [PubMed] [Google Scholar]
- McIntosh A, Mela V, Harty C et al (2019) Iron accumulation in microglia triggers a cascade of events that leads to altered metabolism and compromised function in APP/PS1 mice. Brain Pathol 29:606–621. 10.1111/bpa.12704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medel V, Crossley N, Gajardo I et al (2022) Whole-brain neuronal MCT2 lactate transporter expression links metabolism to human brain structure and function. Proc Natl Acad Sci U S A 119:e2204619119. 10.1073/pnas.2204619119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng X, Baine JM, Yan T et al (2021) Comprehensive analysis of lysine lactylation in rice (Oryza sativa) grains. J Agric Food Chem 69:8287–8297. 10.1021/acs.jafc.1c00760 [DOI] [PubMed] [Google Scholar]
- Miyazawa K (2012) A negative regulator or just an unconcerned passerby: phosphoinositide 3-kinase signalling in IL-12 production. J Biochem 152:497–499. 10.1093/jb/mvs122 [DOI] [PubMed] [Google Scholar]
- Moreno-Yruela C, Bæk M, Monda F et al (2022a) Chiral posttranslational modification to lysine ε-amino groups. Acc Chem Res 55:1456–1466. 10.1021/acs.accounts.2c00115 [DOI] [PubMed] [Google Scholar]
- Moreno-Yruela C, Zhang D, Wei W et al (2022b) Class I histone deacetylases (HDAC1–3) are histone lysine delactylases. Sci Adv 8:eabi0696. 10.1126/sciadv.abi6696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morland C, Lauritzen KH, Puchades M et al (2015) The lactate receptor, G-protein-coupled receptor 81/hydroxycarboxylic acid receptor 1: expression and action in brain. J Neurosci Res 93:1045–1055. 10.1002/jnr.23593 [DOI] [PubMed] [Google Scholar]
- Newman LA, Korol DL, Gold PE (2011) Lactate produced by glycogenolysis in astrocytes regulates memory processing. PLoS ONE 6:e28427. 10.1371/journal.pone.0028427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oses JP, Müller AP, Strogulski NR et al (2019) Sustained elevation of cerebrospinal fluid glucose and lactate after a single seizure does not parallel with mitochondria energy production. Epilepsy Res 152:35–41. 10.1016/j.eplepsyres.2019.03.007 [DOI] [PubMed] [Google Scholar]
- Palsson-McDermott EM, Curtis AM, Goel G et al (2015) Pyruvate kinase M2 regulates Hif-1α activity and IL-1β induction and is a critical determinant of the warburg effect in LPS-activated macrophages. Cell Metab 21:65–80. 10.1016/j.cmet.2014.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan RY, Ma J, Kong XX et al (2019) Sodium rutin ameliorates Alzheimer’s disease-like pathology by enhancing microglial amyloid-β clearance. Sci Adv 5:eaau6328. 10.1126/sciadv.aau6328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan L, Feng F, Wu J et al (2022a) Demethylzeylasteral targets lactate by inhibiting histone lactylation to suppress the tumorigenicity of liver cancer stem cells. Pharmacol Res 181:106270. 10.1016/j.phrs.2022.106270 [DOI] [PubMed] [Google Scholar]
- Pan RY, He L, Zhang J et al (2022b) Positive feedback regulation of microglial glucose metabolism by histone H4 lysine 12 lactylation in Alzheimer’s disease. Cell Metab 34:634-648.e636. 10.1016/j.cmet.2022.02.013 [DOI] [PubMed] [Google Scholar]
- Payen VL, Mina E, Van Hée VF et al (2020) Monocarboxylate transporters in cancer. Mol Metab 33:48–66. 10.1016/j.molmet.2019.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellerin L, Magistretti PJ (1994) Glutamate uptake into astrocytes stimulates aerobic glycolysis: a mechanism coupling neuronal activity to glucose utilization. Proc Natl Acad Sci U S A 91:10625–10629. 10.1073/pnas.91.22.10625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philips T, Mironova YA, Jouroukhin Y et al (2021) MCT1 deletion in oligodendrocyte lineage cells causes late-onset hypomyelination and axonal degeneration. Cell Rep 34:108610. 10.1016/j.celrep.2020.108610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pucino V, Cucchi D, Mauro C (2018) Lactate transporters as therapeutic targets in cancer and inflammatory diseases. Expert Opin Ther Targets 22:735–743. 10.1080/14728222.2018.1511706 [DOI] [PubMed] [Google Scholar]
- Sabari BR, Zhang D, Allis CD et al (2017) Metabolic regulation of gene expression through histone acylations. Nat Rev Mol Cell Biol 18:90–101. 10.1038/nrm.2016.140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savic Azoulay I, Liu F, Hu Q et al (2020) ASIC1a channels regulate mitochondrial ion signaling and energy homeostasis in neurons. J Neurochem 153:203–215. 10.1111/jnc.14971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneiderhan W, Scheler M, Holzmann KH et al (2009) CD147 silencing inhibits lactate transport and reduces malignant potential of pancreatic cancer cells in in vivo and in vitro models. Gut 58:1391–1398. 10.1136/gut.2009.181412 [DOI] [PubMed] [Google Scholar]
- Schurr A, Miller JJ, Payne RS et al (1999) An increase in lactate output by brain tissue serves to meet the energy needs of glutamate-activated neurons. J Neurosci 19:34–39. 10.1523/jneurosci.19-01-00034.1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma NK, Pal JK (2021) Metabolic ink lactate modulates epigenomic landscape: a concerted role of pro-tumor microenvironment and macroenvironment during carcinogenesis. Curr Mol Med 21:177–181. 10.2174/1566524020666200521075252 [DOI] [PubMed] [Google Scholar]
- Shen Z, Jiang L, Yuan Y et al (2015) Inhibition of G protein-coupled receptor 81 (GPR81) protects against ischemic brain injury. CNS Neurosci Ther 21:271–279. 10.1111/cns.12362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shichita T, Sakaguchi R, Suzuki M et al (2012) Post-ischemic inflammation in the brain. Front Immunol 3:132. 10.3389/fimmu.2012.00132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh V, Mishra VN, Chaurasia RN et al (2019) Modes of calcium regulation in ischemic neuron. Indian J Clin Biochem 34:246–253. 10.1007/s12291-019-00838-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman MQ, Gao V, Alberini CM (2016) The role of lactate-mediated metabolic coupling between astrocytes and neurons in long-term memory formation. Front Integr Neurosci 10:10. 10.3389/fnint.2016.00010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun S, Li H, Chen J et al (2017) Lactic acid: no longer an inert and end-product of glycolysis. Physiology (bethesda) 32:453–463. 10.1152/physiol.00016.2017 [DOI] [PubMed] [Google Scholar]
- Sun S, Xu X, Liang L et al (2021) Lactic acid-producing probiotic saccharomyces cerevisiae attenuates ulcerative colitis via suppressing macrophage pyroptosis and modulating gut microbiota. Front Immunol 12:777665. 10.3389/fimmu.2021.777665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki A, Stern SA, Bozdagi O et al (2011) Astrocyte-neuron lactate transport is required for long-term memory formation. Cell 144:810–823. 10.1016/j.cell.2011.02.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takata M, Nakagomi T, Kashiwamura S et al (2012) Glucocorticoid-induced TNF receptor-triggered T cells are key modulators for survival/death of neural stem/progenitor cells induced by ischemic stroke. Cell Death Differ 19:756–767. 10.1038/cdd.2011.145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang T, Zhang Y, Wang Y et al (2019) HDAC1 and HDAC2 regulate intermediate progenitor positioning to safeguard neocortical development. Neuron 101:1117-1133.e1115. 10.1016/j.neuron.2019.01.007 [DOI] [PubMed] [Google Scholar]
- van Hall G, Strømstad M, Rasmussen P et al (2009) Blood lactate is an important energy source for the human brain. J Cereb Blood Flow Metab 29:1121–1129. 10.1038/jcbfm.2009.35 [DOI] [PubMed] [Google Scholar]
- Varner EL, Trefely S, Bartee D et al (2020) Quantification of lactoyl-CoA (lactyl-CoA) by liquid chromatography mass spectrometry in mammalian cells and tissues. Open Biol 10:200187. 10.1098/rsob.200187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vick JS, Askwith CC (2015) ASICs and neuropeptides. Neuropharmacology 94:36–41. 10.1016/j.neuropharm.2014.12.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Liu Z, Xu Y et al (2022) Enterobacterial LPS-inducible LINC00152 is regulated by histone lactylation and promotes cancer cells invasion and migration. Front Cell Infect Microbiol 12:913815. 10.3389/fcimb.2022.913815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warburg O, Wind F, Negelein E (1927) The metabolism of tumors in the body. J Gen Physiol 8:519–530. 10.1085/jgp.8.6.519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson MC, Meredith D, Fox JE et al (2005) Basigin (CD147) is the target for organomercurial inhibition of monocarboxylate transporter isoforms 1 and 4: the ancillary protein for the insensitive MCT2 is EMBIGIN (gp70). J Biol Chem 280:27213–27221. 10.1074/jbc.M411950200 [DOI] [PubMed] [Google Scholar]
- Yamanishi S, Katsumura K, Kobayashi T et al (2006) Extracellular lactate as a dynamic vasoactive signal in the rat retinal microvasculature. Am J Physiol Heart Circ Physiol 290:H925-934. 10.1152/ajpheart.01012.2005 [DOI] [PubMed] [Google Scholar]
- Yang J, Ruchti E, Petit JM et al (2014) Lactate promotes plasticity gene expression by potentiating NMDA signaling in neurons. Proc Natl Acad Sci U S A 111:12228–12233. 10.1073/pnas.1322912111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang K, Xu J, Fan M et al (2020) Lactate suppresses macrophage pro-inflammatory response to LPS stimulation by inhibition of YAP and NF-κB activation via GPR81-mediated signaling. Front Immunol 11:587913. 10.3389/fimmu.2020.587913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang D, Yin J, Shan L et al (2022a) Identification of lysine-lactylated substrates in gastric cancer cells. Science 25:104630. 10.1016/j.isci.2022.104630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Sun Y, Li Q et al (2022b) Diverse epigenetic regulations of macrophages in atherosclerosis. Front Cardiovasc Med 9:868788. 10.3389/fcvm.2022.868788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Luo L, Zhao C et al (2022c) A positive feedback loop between inactive VHL-triggered histone lactylation and PDGFRβ signaling drives clear cell renal cell carcinoma progression. Int J Biol Sci 18:3470–3483. 10.7150/ijbs.73398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang K, Fan M, Wang X et al (2022d) Lactate promotes macrophage HMGB1 lactylation, acetylation, and exosomal release in polymicrobial sepsis. Cell Death Differ 29:133–146. 10.1038/s41418-021-00841-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao L, Kan EM, Lu J et al (2013) Toll-like receptor 4 mediates microglial activation and production of inflammatory mediators in neonatal rat brain following hypoxia: role of TLR4 in hypoxic microglia. J Neuroinflammation 10:23. 10.1186/1742-2094-10-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao Y, Bade R, Li G et al (2022) Global-scale profiling of differential expressed lysine-lactylated proteins in the cerebral endothelium of cerebral ischemia-reperfusion injury rats. Cell Mol Neurobiol. 10.1007/s10571-022-01277-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, Chai P, Xie M et al (2021) Histone lactylation drives oncogenesis by facilitating m(6)A reader protein YTHDF2 expression in ocular melanoma. Genome Biol 22:85. 10.1186/s13059-021-02308-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai X, Li J, Li L et al (2020) L-lactate preconditioning promotes plasticity-related proteins expression and reduces neurological deficits by potentiating GPR81 signaling in rat traumatic brain injury model. Brain Res 1746:146945. 10.1016/j.brainres.2020.146945 [DOI] [PubMed] [Google Scholar]
- Zhang H, Sun X, Xie Y et al (2018a) Isosteviol sodium inhibits astrogliosis after cerebral ischemia/reperfusion injury in rats. Biol Pharm Bull 41:575–584. 10.1248/bpb.b17-00921 [DOI] [PubMed] [Google Scholar]
- Zhang M, Cheng X, Dang R et al (2018b) Lactate deficit in an Alzheimer disease mouse model: the relationship with neuronal damage. J Neuropathol Exp Neurol 77:1163–1176. 10.1093/jnen/nly102 [DOI] [PubMed] [Google Scholar]
- Zhang D, Tang Z, Huang H et al (2019) Metabolic regulation of gene expression by histone lactylation. Nature 574:575–580. 10.1038/s41586-019-1678-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang N, Jiang N, Yu L et al (2021a) Protein lactylation critically regulates energy metabolism in the protozoan parasite Trypanosoma brucei. Front Cell Dev Biol 9:719720. 10.3389/fcell.2021.719720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Shang D, Shi H et al (2021b) Function of astrocytes in neuroprotection and repair after ischemic stroke. Eur Neurol 84:426–434. 10.1159/000517378 [DOI] [PubMed] [Google Scholar]
- Zhao L, Dong M, Ren M et al (2018) Metabolomic analysis identifies lactate as an important pathogenic factor in diabetes-associated cognitive decline rats. Mol Cell Proteomics 17:2335–2346. 10.1074/mcp.RA118.000690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou M, Wang CM, Yang WL et al (2013) Microglial CD14 activated by iNOS contributes to neuroinflammation in cerebral ischemia. Brain Res 1506:105–114. 10.1016/j.brainres.2013.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article is a review article and does not contain relevant data.
This article is a review article and does not contain relevant code.