Abstract
BACKGROUND:
Hepatitis C virus (HCV) infection, predominantly transmitted by exposure to infected blood, remains one of the major public health problems worldwide. This study aims to identify the risk factors of HCV transmission and its chronic complications among the study group.
MATERIALS AND METHODS:
This retrospective study was approved by the Research and Ethical Review and Approve Committee (RERAC) of Oman and conducted at a secondary-care hospital situated in the North Batinah region of Oman. The study population included all HCV cases confirmed by positive serology and reverse-transcription polymerase chain reaction tests during their presence at the hospital between January 2017 and December 2022. The relevant data of the study population were retrieved from the hospital electronic health record system. The data were analyzed using the Statistical Package for the Social Sciences (SPSS), Version 26.0.
RESULTS:
A total of 177 HCV confirmed cases were included in the study. HCV infection was predominant among males (74%) and individuals of the age group of 21–60 years (74.6%). Genotyping was possible only in 107 cases. Among HCV genotypes, genotype 3 (58.9%) was the most frequently identified, followed by genotype 1 (34.6%). Hemodialysis (21.5%), history of blood transfusion (16.4%), and injection drug use (11.9%) were the major risk factors for HCV infection, while cirrhosis (7.3%) and fatty liver disease (4%) were the most frequently observed chronic HCV complications. HCV infection in the spouse/partner (21.5%), alcohol use (7.3%), and co-infection with hepatitis B virus (2.3%) and human immunodeficiency virus (1.7%) were the other significant factors detected in our study population.
CONCLUSIONS:
HCV is a multi-factorial disease leading to severe chronic complications, thus representing a public health threat. This clearly emphasizes the cruciality of HCV community awareness campaigns and enhancement of Omani national guidelines for early screening of high-risk groups as well as effective management of HCV-infected cases to reduce the substantial burden of the disease on patients as well as the healthcare system.
Keywords: Alpha-interferon, blood transfusion, cirrhosis, genotype, reverse-transcriptase polymerase chain reaction
Introduction
Hepatitis C virus (HCV) infection remains a major global health problem despite recent development of safe and highly effective direct-acting antiviral agents. HCV is an enveloped positive-sense single-stranded RNA virus that belongs to Flaviviridae family within the genus Hepacivirus.[1] HCV is most often transmitted parenterally through exposure to infected blood from unsafe injection practices, unscreened blood transfusions, injection drug use (IDU), and unsafe medical procedures such as hemodialysis, surgery, and organ transplantation. Vertical transmission from mother to child and sexual contact are other well-known modes of transmission.[1] Moreover, HCV readily transmits, is up to 4 times more infectious than human immunodeficiency virus (HIV), and requires less exposure to cause infection.[2] Based on genome sequencing and phylogenetic analysis, HCV is classified into seven genotypes (1 to 7), each comprising multiple sub-types (1a, 1b, 1c, etc.). The influence of antiviral therapy and prognosis of hepatitis C depend on the genotype.[3] HCV is a hepatotropic virus, which primarily targets the liver. It is also notoriously known for undergoing high antigenic variation, resulting in the emergence of genetic variants within individual isolates termed as quasi-species. Thus, treating and developing a vaccine for HCV infection have proven difficult.[4,5] As per the World Health Organization (WHO) report, globally, around 58 million people have chronic HCV infection with about 1.5 million new infections occurring every year. The highest prevalence of chronic HCV was in the Eastern Mediterranean region (2.3%) including the Middle East, followed by the European region (1.5%) and African region (1%).[3] Furthermore, WHO estimated that in 2019, approximately 290,000 HCV-infected people died mostly from complications such as cirrhosis, liver failure, and liver cancer.[6] However, actual prevalence may be higher than what has been reported since many cases remain undiagnosed and risk factors go unrecognized.
HCV can cause both acute and chronic hepatitis, ranging in severity from mild to serious illness. Acute HCV infection becomes chronic in more than 50% of cases. Chronic HCV is often asymptomatic in many individuals for a prolonged period. Symptoms appear often in advanced stages of liver disease.[7] Chronic hepatitis C can lead to serious, even life-threatening liver problems such as cirrhosis, liver failure, and hepatocellular carcinoma (HCC). According to the Centers for Disease Control and Prevention (CDC) updates, 60–70% of people infected with HCV develop chronic liver disease. Of those with chronic HCV, 15–20% are at risk of developing cirrhosis within 20–30 years and about 1% of them will die from consequences of cirrhosis or HCC.[6,8,9] HCV infection can also result in extra-hepatic complications such as immune-mediated and metabolic disturbances, fatigue, and psychological disorders such as dementia and depression.[10,11] Although alcohol consumption, tobacco use, insulin resistance, and co-infection with hepatitis B virus (HBV) or HIV do not cause HCV infection, they enhance the risk of chronic inflammatory liver damage in chronic HCV patients.[12]
Public awareness about HCV is inadequate. As per the WHO report, the majority of HCV-infected people (>90%) are unaware of their infection, only 21% of people are diagnosed with HCV infection, and 62% of them receive antiviral therapy.[13,14] As such, prompt detection and treatment of infected patients will help to achieve a more meaningful impact on the morbidity and mortality of this disease. Acknowledging the serious impact on global health, WHO has proposed a strategy to eliminate hepatitis C by 2030.[13] Currently, only nine countries are right on track to achieve this target.[15]
To further stimulate the elimination of HCV in Oman, especially in the absence of an effective vaccine, a combination of tailored public awareness campaigns, screening and early treatment in hospital and community settings, and adhering to standard infection prevention strategies represent the cornerstone to reach this goal. Studies from Oman related to HCV prevalence, risk factors, and outcome of infection are scarce. So, more studies are necessary to generate reliable data on the clinical significance of HCV and its outcome among the Omani population to health planners and policy makers. In this context, our study aims to determine the frequency of HCV infection as well as risk factors and chronic complications among HCV patients presented during January 2017 to December 2022 at a secondary care hospital in the North Batinah region of Oman.
Materials and Methods
Study design and setting
This single-center retrospective study was conducted at a secondary-care hospital, situated in the northern region of Oman. The study was conducted for a period of 6 months.
Study participants and sampling
All HCV patients presented to Sohar hospital between January 2017 and December 2022 were included in the study. HCV infection was confirmed by positive anti-HCV antibody and HCV reverse-transcriptase polymerase chain reaction (RT-PCR) tests. Patients with incomplete data were excluded from the study. The relevant information such as demographic and clinical characteristics of the studied population, risk factors for HCV transmission, HCV genotypes, and chronic complications of HCV infection was retrieved from the hospital electronic health records.
Ethical consideration
This study was approved by the Research and Ethical Review and Approve Committee (RERAC), Ministry of Health, Oman [Approval no. MH/DGHS/NBG/18/2022].
Statistical analysis
Data were analyzed using the Statistical Package for the Social Sciences (SPSS), Version 26.0 (IBM Corp., Armonk, New York, USA). Results of categorical variables were expressed as numbers and percentages, and continuous variable results were expressed as means and standard deviations.
Results
In total, 177 patients diagnosed with HCV infection were included in the study. Table 1 depicts the demographic characteristics of the study population. HCV infection was predominant among males (74.0%) compared to females (36.0%). The majority of patients were Omanis (93.8%). Most of the patients (132, 74.6%) were adults of age between 20 and 60 years. Among HCV genotypes, genotype 3 (58.9%) was most frequent, followed by genotype 1 (34.6%), genotype 4 (7.5%), and genotype 2 (0.9%). Co-infection with other common blood-borne virus infections such as HBV (2.3%) and HIV (1.7%) was noticed among a few participants. Concerning the risk factors for HCV transmission, hemodialysis (21.5%), blood transfusion (16.4%), and injection drug use (11.9%) were most commonly noticed among the study population. Other risk factors such as cosmetic surgery (2.8%), tattooing (1.7%), and multiple sexual partners (2.3%) were less commonly noticed. Figure 1 reveals the frequency of HCV-associated complications among the study population. Cirrhosis (7.3%) and fatty liver disease (4%) were the most observed complications. Hepatic failure (1.1%), psychological disorders (1.7%) such as depression and dementia, and others (1.7%) such as end stage liver disease, hepatic encephalopathy, and ischemic liver disease were observed less frequently. History of alcohol consumption was noted in relatively less number (7.3%) of participants. Another interesting finding was the presence of HCV infection in 21.5% of spouses.
Table 1.
Demographic characteristics of the studied population
| Characteristic | Number (n) and percentage |
|---|---|
| Gender | |
| Male | 131 (74.0) |
| Female | 46 (36.0) |
| Nationality | |
| Omani | 166 (93.8) |
| Non-Omani | 11 (6.2) |
| Age distribution (years) | |
| 0-20 | 2 (1.1) |
| 21-40 | 76 (43.0) |
| 41-60 | 56 (31.6) |
| >60 | 43 (24.3) |
| HCV genotypes* | |
| Genotype 1 | 14 (13.1) |
| Genotype 1a | 16 (15.0) |
| Genotype 1b | 7 (6.5) |
| Genotype 2 | 1 (0.9) |
| Genotype 3 | 63 (58.9) |
| Genotype 4 | 8 (7.5) |
| Coinfection | |
| HIV | 3 (1.7) |
| Hepatitis B | 4 (2.3) |
| Risk factors | |
| Injection drug use | 21 (11.9) |
| Blood transfusion | 29 (16.4) |
| Hemodialysis | 38 (21.5) |
| Tattooing | 3 (1.7) |
| Cosmetic surgery | 5 (2.8) |
| Multiple sexual partner | 4 (2.3) |
| Spouse with HCV | 38 (21.5) |
| Alcohol consumption | 13 (7.3) |
*Genotype was determined in 107 cases and >1 genotype (mixed type) was identified in two patients
Figure 1.
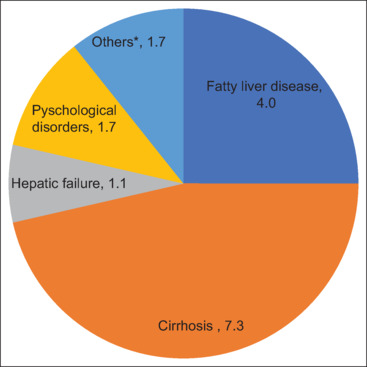
Frequency of HCV-associated complications among the studied population. *Others include hepatic encephalopathy, ischemic liver disease, and end stage liver disease
Discussion
HCV is a common blood-borne viral pathogen prevalent worldwide.[16] However, its incidence and genotype distribution vary considerably from one region to another. Due to non-availability of an effective vaccine, prevention of infection through public sensitization complemented by community-based screening programs targeting high-risk individuals and early initiation of antiviral therapy would play a pivotal role in reducing the burden of HCV. To the best of our knowledge, in Oman, there is a dearth in HCV screening and public awareness.
In the current study, HCV infection was more common among males of the age group of 20 to 60 years. This finding is consistent with the previous reports.[17,18] There are seven distinct HCV genotypes with many sub-types designated as a, b, c, and so on. The distribution of HCV genotypes differs worldwide according to epidemiological factors including differences in modes of transmission and ethnic and temporal factors. A previous report by Al-Busafi et al. revealed genotype 1 as the most prevalent (44.0%), followed by genotype 3 (35.1%), 4 (15.0%), 2 (3.7%), and mixed (2.2%) among the Omani population.[16] The prevalence of genotype 1 over other genotypes has been reported from other Middle-Eastern countries such as the United Arab Emirates (UAE), Turkey, and Bahrain.[19,20,21] However, reports from Saudi Arabia and Yemen showed predominance of genotype 4.[22,23] Contrastingly, in our study, genotype 3 was the most common compared to all other genotypes.
Before 1992, blood transfusion and organ transplantation were the major risk factors for HCV transmission worldwide. Subsequently, because of strict WHO guidelines for screening of blood samples for HCV infection and adherence to standard infection control practices, the incidence of HCV following blood transfusion and other health-care procedures has reduced gradually. However, it still remains the major risk factor in developing countries because of inadequate health-care facilities. In our study, we found the history of previous blood transfusion among 16.4% of participants. This finding is in line with another study from Oman by Al-Busafi et al., who reported a history of blood transfusion in one out of five HCV-infected participants.[16] In recent years, extensive use of recombinant erythropoietin to correct renal anemia in hemodialysis patients has significantly reduced blood transfusions. However, the literature suggests HCV infection may still occur in hemodialysis patients. Moreover, previous studies revealed that the duration of hemodialysis is an independent risk factor for HCV transmission among chronic hemodialysis patients. This might further increase the possibility of healthcare-associated HCV transmission from contaminated hemodialysis units.[24] In the present study, we found history of hemodialysis in 21.5% of participants.
High-risk behavior among sexually active adults such as injection drug use, tattooing and piercing, and unsafe sexual practices with multiple partners are regarded as major risk factors for HCV transmission in the modern world. Illicit drug use is one of major concerns among adults in Oman. Previous reports from many upper middle-income countries have suggested injection drug use is a leading risk factor for HCV transmission.[25,26] In our study, we found history of injection drug use and tattooing among 11.9% and 1.7% participants, respectively. However, the actual percentage of illicit drug use in Omani adults is likely inaccurate due to social stigma. High prevalence of HCV in illicit drug users mandates greater efforts to promote safe injection practices, provision for use of sterile instruments and equipment, and regular screening.
HCV can also spread through sex. The risk of transmission is even higher when a person has sexually transmitted diseases (STDs) such as HIV and who has indulged in unsafe sex or sex with multiple partners. In our study, there was a history of numerous sex partners (2.4%) and co-infection with HIV (1.7%) among a few participants. Therefore, awareness about safe sex practices is vitally important among the adult population. Vertical transmission from mother to child is another recognized mode of HCV transmission.[27] In our study, HCV infection was noted in 21.5% of spouses, thus being constantly at risk of conceiving. HCV infection in pregnant women is strongly associated with cholestasis and pre-term births. Furthermore, infection at an early age leads to a higher risk of subsequent development of chronic complications such as cirrhosis and hepatocellular carcinoma among the infected children. Hence, provision for antenatal screening of pregnant women, prenatal diagnosis of HCV infection, and early initiation of highly effective antiviral therapy has a dual benefit for mother and child.
Most HCV patients remain chronically infected, with an increased risk of fatty liver disease, cirrhosis, liver failure, and HCC. Less frequently, they may develop psychological problems.[8,11] In line with this, in our study population, cirrhosis (7.3%), fatty liver disease (4%), and psychological problems (1.7%) were noticed in a minority of the participants. The literature suggests alcohol consumption does not cause HCV transmission. However, excessive alcohol consumption is a well-known risk factor that leads to a more severe liver injury, promoting disease progression and increased risk of cirrhosis and HCC.[28] In our study participants, alcohol use was relatively uncommon. Out of 13 alcoholic patients, 4 developed cirrhosis and 2 developed end-stage renal disease.
Currently, interferon alfa and many antiviral drugs such as ribavirin and sofosbuvir remain mainstay in HCV treatment. These antiviral agents are associated with toxicities, but direct-acting antiviral therapies result in an initial cure rate of >95%.[29] Therefore, early diagnosis and early initiation therapy play a pivotal role in reducing the risk of HCV-related complications such as cirrhosis and HCC.[30] However, we could not analyze this fact because of lacunae in the antiviral therapy data.
Limitation and recommendation
This study had several potential limitations. First, the data were collected retrospectively from health records. Therefore, there is a paucity in relevant data such as the impact of antiviral therapy on the disease outcome, time of initial diagnosis, chronicity, and so on. Second, social stigma hinders proper disclosure and leads to an under-estimation of high-risk activities such as injection drug use, high-risk sexual relationships, and others. Third, the study did not analyze the association between risk factors and the outcome of infection due to a small sample size. Additionally, the study population was from a single center, and hence, the results of our study cannot be generalized. It is recommended to confirm our study findings via a multi-centric study with a large sample size.
Conclusion
Our study demonstrates that HCV risk is multi-factorial. Major risk factors for HCV transmission included hemodialysis, blood transfusion, and injection drug use, with genotypes 3 and 1 being the most common. However, cirrhosis and fatty liver disease were the most observed chronic HCV complications. There are obvious shortcomings in dealing with hepatitis C in Oman, as evidenced by the lack of basic disease information and public sensitization. The data presented in this study, even if limited, point to the fact that HCV is a major health issue compelling a greater focus in Oman. Prioritizing public awareness campaigns, escalation of early screening of at-risk individuals, and precise management of HCV cases are necessary to address this challenge. Furthermore, an intensified response by the Omani government is required through national policies implementing strict regulations on illicit drug use. Synergy between all these efforts is necessary if the HCV elimination goal is to become a reality in Oman.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgement
Authors express their sincere gratitude to Mr. Jayadev Prasad (IT manager, Sohar hospital) and the Microbiology laboratory staff of Sohar hospital for their assistance in data collection.
References
- 1.Sepulveda-Crespo D, Resino S, Martinez I. Hepatitis C virus vaccine design: Focus on the humoral immune response. J Biomed Sci. 2020;27:78. doi: 10.1186/s12929-020-00669-4. doi: 10.1186/s12929-020-00669-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Karoney MJ, Siika AM. Hepatitis C virus (HCV) infection in Africa: A review. Pan Afr Med J. 2013;14:44. doi: 10.11604/pamj.2013.14.44.2199. doi: 10.11604/pamj. 2013.14.44.2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rabaan AA, Al-Ahmed SH, Bazzi AM, Alfouzan WA, Alsuliman SA, Aldrazi FA, et al. Overview of hepatitis C infection, molecular biology, and new treatment. J Infect Public Health. 2020;13:773–83. doi: 10.1016/j.jiph.2019.11.015. [DOI] [PubMed] [Google Scholar]
- 4.Ayoub N, Hatab T, Bizri AR. Challenges facing viral hepatitis C elimination in Lebanon. Pathogens. 2023;12:432. doi: 10.3390/pathogens12030432. doi: 10.3390/pathogens12030432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Domingo E, Gomez J. Quasispecies and its impact on viral hepatitis. Virus Res. 2007;127:131–50. doi: 10.1016/j.virusres.2007.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.World Health Organization Hepatitis C. 2017 Available from: https://www.who.int/news-room/fact-sheets/detail/hepatitis-c . [Last accessed on 2023 Jul 18] [Google Scholar]
- 7.Abdo AA. Hepatitis C and poor quality of life: Is it the virus or the patient? Saudi J Gastroenterol. 2008;14:109–13. doi: 10.4103/1319-3767.41727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention Hepatitis C questions and answers for health professionals. 2020 Available from: https://www.cdc.gov/hepatitishcv/hcvfaq.htm . [Last accessed on 2023 Aug 15] [Google Scholar]
- 9.Petruzziello A, Marigliano S, Loquercio G, Cozzolino A, Cacciapuoti C. Global epidemiology of hepatitis C virus infection: An up-date of the distribution and circulation of hepatitis C virus genotypes. World J Gastroenterol. 2016;22:7824–40. doi: 10.3748/wjg.v22.i34.7824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Petta S. Hepatitis C virus and cardiovascular: A review. J Adv Res. 2017;8:161–8. doi: 10.1016/j.jare.2016.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dirks M, Haag K, Pflugrad H, Tryc AB, Schuppner R, Wedemeyer H, et al. Neuropsychiatric symptoms in hepatitis C patients resemble those of patients with autoimmune liver disease but are different from those in hepatitis B patients. J Viral Hepat. 2019;26:422–31. doi: 10.1111/jvh.12979. [DOI] [PubMed] [Google Scholar]
- 12.Alqahtani SA, Colombo M. Viral hepatitis as a risk factor for the development of hepatocellular carcinoma. Hepatoma Res. 2020;6:58. doi: 10.20517/2394-5079.2020.49. [Google Scholar]
- 13.Global health sector strategies 2022-2030. Available from: https://www.who.int/teams/global-hiv-hepatitis-and-stis-programmes/strategies/global-health-sector-strategies . [Last accessed on 2023 Aug] [Google Scholar]
- 14.Abdel-Gawad M, Nour M, El-Raey F, Nagdy H, Almansoury Y, El-Kassas M. Gender differences in prevalence of hepatitis C virus infection in Egypt: A systematic review and meta-analysis. Sci Rep. 2023;13:2499. doi: 10.1038/s41598-023-29262-z. doi: 10.1038/s41598-023-29262-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Polaris Observatory The authoritative resource for epidemiological data, modeling tools, training, and decision analytics to support global elimination of hepatitis B and C by 2030. Available from: http://www.hepbunited.org/assets/Webinar-Slides/8b54a78215/POLARIS-Brief-181217.pdf . [Google Scholar]
- 16.Al-Busafi SA, Al-Shuaili H, Omar H, Al-Zuhaibi H, Jeyaseelan L, Al-Naamani K. Epidemiology of chronic hepatitis C infections at a tertiary care centre in Oman. Sultan Qaboos Univ Med J. 2017;17:e404–10. doi: 10.18295/squmj.2017.17.04.005. doi: 10.18295/squmj.2017.17.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yousaf A, Ghafoor A, Fatima N, Danish M. Gender-specific frequency distribution of hepatitis C virus genotypes in Punjab province, Pakistan: A clinically significant descriptive cross-sectional study. Cureus. 2021;13:e17480. doi: 10.7759/cureus.17480. doi: 10.7759/cureus. 17480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Soliman G, Elzalabany MS, Hassanein T, Miller FD. Mass screening for hepatitis B and C in southern upper Egypt. BMC Public Health. 2019;19:1326. doi: 10.1186/s12889-019-7640-1. doi: 10.1186/s12889-019-7640-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abro AH, Al-Dabal L, Younis NJ. Distribution of hepatitis C virus genotypes in Dubai, United Arab Emirates. J Pak Med Assoc. 2010;60:987–90. [PubMed] [Google Scholar]
- 20.Bozdayi AM, Aslan N, Bozdayi G, Türkyilmaz AR, Sengezer T, Wend U, et al. Molecular epidemiology of hepatitis B, C and D viruses in Turkish patients. Arch Virol. 2004;149:2115–29. doi: 10.1007/s00705-004-0363-2. [DOI] [PubMed] [Google Scholar]
- 21.Ghaderi-Zefrehi H, Gholami-Fesharaki M, Sharafi H, Sadeghi F, Alavian SM. The distribution of hepatitis C virus genotypes in Middle Eastern countries: A systematic review and meta-analysis. Hepat Mon. 2016;16:e40357. doi: 10.5812/hepatmon.40357. doi: 10.5812/hepatmon. 40357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shobokshi OA, Serebour FE, Skakni L, Al-Saffy YH, Ahdal MN. Hepatitis C genotypes and subtypes in Saudi Arabia. J Med Virol. 1999;58:44–8. [PubMed] [Google Scholar]
- 23.Ohno T, Mizokami M, Saleh MG, Orito E, Ohba KI, Wu RR, et al. Usefulness and limitation of phylogenetic analysis for hepatitis C virus core region: Application to isolates from Egyptian and Yemeni patients. Arch Virol. 1996;141:1101–13. doi: 10.1007/BF01718613. [DOI] [PubMed] [Google Scholar]
- 24.Hinrichsen H, Leimenstoll G, Stegen G, Schrader H, Fölsch UR, Schmidt WE, et al. Prevalence and risk factors of hepatitis C virus infection in haemodialysis patients: A multicentre study in 2796 patients. Gut. 2002;51:429–33. doi: 10.1136/gut.51.3.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grassi A, Ballardini G. Hepatitis C in injection drug users: It is time to treat. World J Gastroenterol. 2017;23:3569–71. doi: 10.3748/wjg.v23.i20.3569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Trickey A, Fraser H, Lim AG, Peacock A, Colledge S, Walker JG, et al. The contribution of injection drug use to hepatitis C virus transmission globally, regionally, and at country level: A modelling study. Lancet Gastroenterol Hepatol. 2019;4:435–44. doi: 10.1016/S2468-1253(19)30085-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ragusa R, Corsaro LS, Frazzetto E, Bertino E, Bellia MA, Bertino G. Hepatitis C virus infection in children and pregnant women: An updated review of the literature on screening and treatments. AJP Rep. 2020;10:e121–7. doi: 10.1055/s-0040-1709185. doi: 10.1055/s-0040-1709185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schiff ER, Ozden N. Hepatitis C and alcohol. Alcohol Res Health. 2003;27:232–9. [PMC free article] [PubMed] [Google Scholar]
- 29.Fierer DS, Wyles DL. Re-treatment of hepatitis C infection after multiple failures of direct-acting antiviral therapy. Open Forum Infect Dis. 2020;7:ofaa095. doi: 10.1093/ofid/ofaa095. doi: 10.1093/ofid/ofaa095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Feeney ER, Chung RT. Antiviral treatment of hepatitis C. BMJ. 2014;348:g3308. doi: 10.1136/bmj.g3308. doi: 10.1136/bmj.g3308. [DOI] [PMC free article] [PubMed] [Google Scholar]


