Abstract
Introduction
The aim of this study was to investigate the role of circulating regulatory T cells (Tregs) as a novel marker associated with liver metastases and treatment response to transarterial embolization (TAE) in patients with gastroenteropancreatic neuroendocrine tumors (GEP-NETs).
Methods
Circulating Tregs, defined as the CD4+CD25+CD127low/− population, were examined by flow cytometry in peripheral blood mononuclear cells from patients with GEP-NETs. Clinicopathological parameters, radiologic response, and hepatic progression-free survival (hPFS) data were collected.
Results
The association between circulating Tregs and clinicopathological parameters was analyzed in 139 GEP-NET patients. Higher Treg levels were significantly associated with more progressive clinical features, including a higher WHO grade, more advanced TNM stage, and the presence of liver metastases. A Treg level ≥8.015% distinguished between patients with and without liver metastases. Among a cohort of 51 GEP-NET patients who were subjected to TAE for reducing liver metastasis burden, patients with higher Treg levels depicted unfavorable responses and significantly reduced hPFS after TAE treatment. We also revealed that patients with Treghigh (≥8.975%) displayed significantly shorter median hPFS than patients with Treglow (<8.975%). Additionally, after adjusting for other confounding clinical parameters, the association between Tregs and treatment response as well as hPFS remained significant, suggesting that Tregs may have a strong and independent prognostic impact in GEP-NETs.
Conclusion
Our data suggest that circulating Tregs are a novel immunological marker associated with liver metastases and treatment response to TAE in patients with GEP-NETs.
Keywords: Circulating regulatory T cell, Gastroenteropancreatic neuroendocrine tumors, Liver metastasis, Transarterial embolization
Introduction
Gastroenteropancreatic neuroendocrine tumors (GEP-NETs) are a heterogeneous group of tumors derived from neuroendocrine cells along the gastro-intestinal tract and pancreas, comprising a heterogeneous family of neoplasms with a wide and complex spectrum of clinical behavior [1]. Although GEP-NETs are a group of rare neoplasms, their incidence has increased more than six-fold between 1997 and 2012 [2], which may be related to improvements in pathological and imaging diagnostic techniques.
The clinical behavior of GEP-NETs is highly unpredictable in terms of prognosis and response to treatment, making their management a challenging clinical task. GEP-NETs are typically characterized by an indolent nature, but metastases from NETs are very prevalent, with approximately 40–50% of NET patients presenting with distant metastases at initial diagnosis [3]. Metastases are most commonly located in the liver and/or lymph nodes, with the peritoneum, bone, lung, brain, and other relatively rare sites being less frequent locations. The presence of liver metastases is regarded as one of the most significant poor prognostic factors for long-term survival among patients with NETs and leads to significant detriments in quantity and quality of life [4, 5]. The molecular mechanisms underlying liver metastasis in GEP-NETs are poorly understood.
For patients who are not candidates for hepatic resection due to either the extent of hepatic tumor burden or performance status, transarterial embolization (TAE), one of the liver-directed therapies, has been applied as an effective approach to reduce hepatic tumor burden and significantly improve the survival outcomes of GEP-NET patients [6]. However, the response to TAE treatment in GEP-NET patients varies widely. Identification of patients who are most likely to benefit from TAE remains a largely unmet need. In previous studies, several prognostic biomarkers of response to TAE in patients with NETs have been identified, including Ki-67 index; hepatic tumor burden; bone metastasis, etc [6]. Further exploration for additional reliable prognostic markers is highly beneficial for better clinical management.
Regulatory T cells (Tregs) constitute a distinct lineage of CD4+ T cells known for their potent immunosuppressive functions [7]. They play a critical role in maintaining immune homeostasis and preventing autoimmune disease [8]. However, Tregs can also impede beneficial responses by suppressing sterilizing immunity and dampening anti-tumor immunity. They achieve this by secreting anti-inflammatory cytokines such as IL-10, IL-35, and TGF-β, mediating cytolysis via perforin and granzyme A and/or granzyme B, or by inhibiting the maturation or function of antigen-presenting cells, such as dendritic cells [9]. In this context, tumor cells exploit Tregs as a protective shield against anti-tumor immune response, thereby promoting tumor progression. Increased Treg levels have been associated with metastatic disease, poor prognosis, and resistance to therapy in various cancer patients, including those with lung, ovarian, gastric, breast, pancreatic cancers, and melanoma [10–16]. However, investigations related to Tregs in GEP-NET patients are scarce.
In this study, our aim was to investigate the clinical relevance of circulating CD4+CD25+CD127low/− Tregs in GEP-NETs. Our study identified the role of circulating Tregs as a marker associated with liver metastases and assessed their potential as a prognostic biomarker for treatment response to TAE in patients with GEP-NETs.
Materials and Methods
Patients
This was a single-institution study conducted at the First Affiliated Hospital, Sun Yat-sen University, Guangzhou, China. Eligible participants included patients with pathologically confirmed diagnoses of GEP-NETs. Patients with a history of other cancer types were excluded. Peripheral blood samples were collected from 139 patients with GEP-NETs between May 2021 and December 2022. Data on clinical parameters were extracted from electronic patient medical records, including age, gender, functionality, primary site, tumor grade, hepatic tumor burden, stage, metastatic sites, and previous treatments. The tumor pathology of each case was reviewed by pathologists in accordance with the 5th edition of the World Health Organization (WHO) classification of tumors of the digestive system [17]. The hepatic tumor burden was assessed using computed tomography (CT) imaging scans selected at the level showing the most significant disease involvement. It was categorized as <25%, 25–50%, or >50% based on the relative area of the tumor to the normal liver [6]. Tumor-node-metastasis (TNM) staging was performed following the 8th edition of the AJCC Cancer Staging Manual [18]. The presence of metastases was determined using cross-sectional CT imaging, and somatostatin receptor scintigraphy with Gallium-68 (68Ga) DOTANOC positron emission tomography/CT. All patients had peripheral blood samples collected for Treg detection.
For the analysis of Treg association with the treatment response to TAE, a total of 51 GEP-NET patients with liver metastases who underwent TAE to reduce hepatic tumor burden were selected for the association analysis. All of these patients received octreotide LAR (Sandostatin-LAR; Novartis) as systemic anticancer therapy. Exclusion criteria included patients who (1) had previously undergone embolotherapy at other hospitals; (2) were lost to follow-up after TAE treatment; (3) received concurrent treatment with other anticancer medications such as tyrosine kinase inhibitors, mammalian target of rapamycin inhibitors, or chemotherapy during TAE sessions. The TAE procedures were performed in patients who met the following criteria: (1) predominant liver disease not eligible for ablation or resection; (2) ECOG score ≤2; (3) Child-Pugh score A or B; and (4) absence of biliary duct dilation or cholangitis. Peripheral blood samples were collected at baseline (within 1 week before TAE) for Treg detection. Ethical approval was obtained from the Hospital Research Ethics Committee, and written informed consent was obtained from all participants and from parents/legal guardians for all participants aged under 18.
TAE Procedure and Response Evaluation
The TAE procedures followed our standard protocol, which has been detailed in our previous studies [6, 19]. In brief, TAE was performed under local anesthesia using the traditional femoral approach. To mitigate the potential risk of a carcinoid crisis during TAE, patients received continuous intravenous infusion of octreotide before the procedure. The femoral artery was accessed using the Seldinger’s technique, and a 5F catheter was guided to the celiac trunk and superior mesenteric artery for angiography, allowing visualization of hepatic arterial anatomy, tumor blood supply, and portal vein patency. Further catheterization was undertaken to access tumor-involved lobar or segmental arterial branches, where 40–120 µm Embosphere (Merit Medical) and 100 µm polyvinyl alcohol (Cook) were sequentially injected into the microcatheter under fluoroscopy. Embolization was performed until stasis, defined as the absence of contrast flow visually observed after two to five heartbeats. Successful embolization was confirmed when no contrast staining in the tumor was detected on post-embolization angiography. In cases where hepatic lesions could not be completely targeted in one TAE session, two or more sessions were conducted to achieve a more favorable outcome. Multiphase contrast CT of the upper abdomen was performed at baseline and 1 month after each TAE session. The response to TAE was categorized as follows: complete response, partial response (PR), stable disease (SD), or progressive disease (PD), in accordance with the response evaluation criteria in solid tumor version 1.1 (RECIST 1.1) [20]. After successful procedures, all patients underwent close follow-up with contrast CT until March 2023. We employed the best response achieved across multiple TAE sessions as the evaluation endpoint for statistical analysis. Hepatic progression-free survival (hPFS), defined as the duration from the initial TAE to the first identification of hepatic disease progression, was assessed and compared in stratified groups of patients with high or low Treg levels.
Immunofluorescence Staining and Flow Cytometric Gating Strategy for Tregs
Blood samples were collected in EDTA-anticoagulant and processed within 24 h of collection. After incubation with monoclonal antibodies of Treg surface markers (CD4-FITC, CD25-APC, and CD127-PE, all from Cellgene Biotech Co., Ltd.) for 20 min at room temperature, erythrocytes were lysed with ammonium chloride (PharmLyse, BD Biosciences, San Diego, CA, USA) at room temperature for 10 min using a standard lyse/wash technique. An eight-color flow cytometry analysis was performed on an FACScanto Plus flow cytometer (BD Biosciences), which underwent daily standardization using CS&T beads. Data were analyzed using Kaluza Analysis Software. Instrument alignments, sensitivities, and spectral compensation were verified by standards, calibrators, procedural controls, and normal peripheral blood samples before processing patient samples. The gating strategy for Tregs (CD4+CD25+CD127−/low) is shown in Figure 1a.
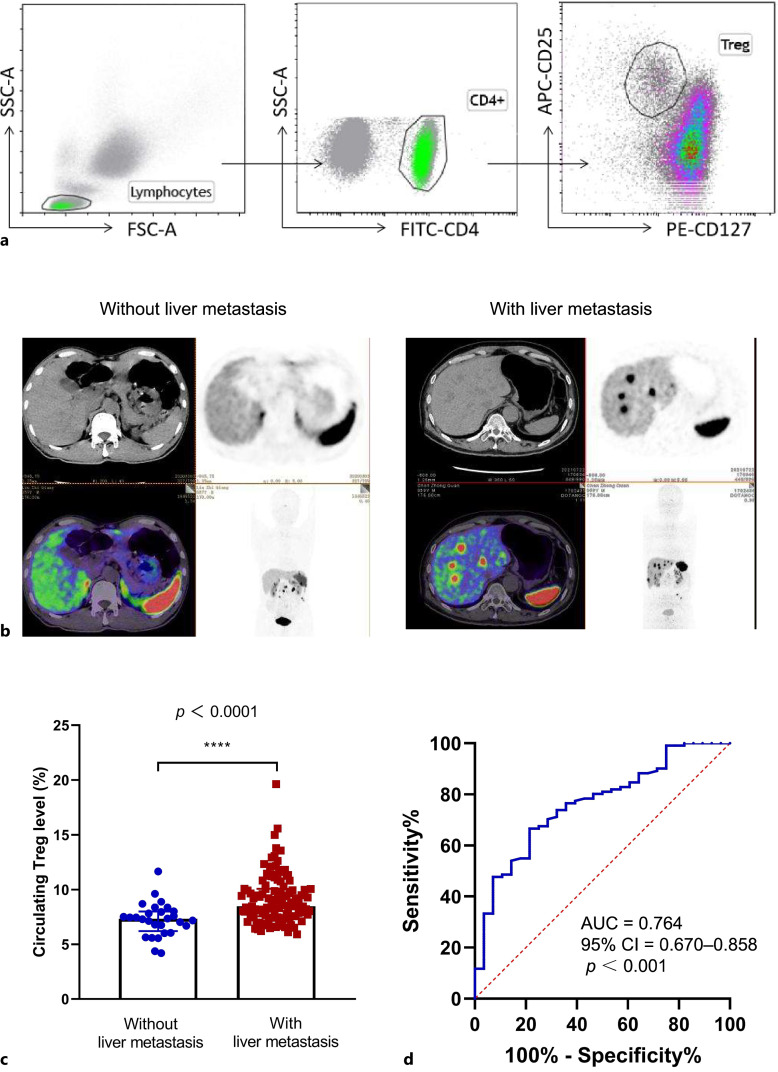
Fig. 1.
Correlation between circulating treg levels and liver metastasis in GEP-NET patients. a Gating strategy for Tregs identification: the gating strategy outlines the sequential identification of Tregs within peripheral blood, involving lymphocytes, CD4+ T cells, and CD4+CD25+CD127−/low Tregs. b Representative examples of liver metastasis: PET/CT images illustrate representative cases of GEP-NET patients with and without liver metastases as determined by 68Ga-DOTANOC PET/CT. c Treg Levels in patients with and without liver metastases: Comparison analysis of Treg levels in patients with liver metastases (n = 111) and those without liver metastases (n = 28) using Mann-Whitney U tests. Data are presented as medians with interquartile ranges. ***p < 0.001. d Receiver operating characteristic (ROC) curve: an ROC curve was constructed to identify the optimal cut-off value of Tregs that can distinguish between those GEP-NET patients with and without liver metastases. PET/CT, positron emission tomography/computer tomography.
Statistical Analyses
Statistical analyses were conducted using SPSS software (Version 25.0; IBM Corp.), and statistical figures were generated using GraphPad Prism version 8 software (GraphPad, Inc., La Jolla, CA, USA). Continuous variables were described using the median and interquartile range (IQR; 25th–75th percentile) and compared using the Mann-Whitney U test. To identify the optimal cut-off value of Tregs that can distinguish between those GEP-NET patients with and without liver metastases, receiver operating characteristics (ROC) curves were plotted, and the area under the ROC curve (AUC) was calculated. The optimal cut-off value was determined using Youden’s Index. Univariate and multivariate logistic regression models were used to analyze factors associated with liver metastases and factors affecting treatment response. Additionally, univariate and multivariate proportional hazards Cox regression models were applied to analyze factors affecting hPFS. In the multivariate regression models, we employed the “Enter” method to handle variables. The multivariable regression models were adjusted for the following variables: gender (female/male), age (continuous, years), functionality (yes/no), primary sites (pancreas/GI tract), grade (G1/G2/G3), and Treg level (high/low). Classification and regression tree (CART) analysis was employed to identify the optimal cutoff point for Tregs in stratifying patients into high and low Treg levels. This cutoff point was utilized to dichotomize the Treg variable for the Cox regression analysis. To mitigate potential overfitting, pruning was conducted based on the best cost-complexity parameter (CP) values. The Kaplan-Meier method was employed to analyze hPFS data, and the comparison between patients with Treghigh and Treglow was performed using the log-rank test. Differences with p < 0.05 were considered statistically significant.
Results
Clinical and Pathologic Features of Cases
A total of 139 patients with GEP-NETs were recruited, including 32 patients with pancreatic NETs (pNETs) and 107 patients with gastrointestinal NETs (GI-NETs). The demographics and clinical characteristics of the patients are detailed in Table 1. The majority of patients had grade-2 tumors (72.7%), with 18.7% having grade-1 and 8.6% having grade-3 tumors. Most patients (87.1%) had nonfunctional NETs. Stage IV tumors accounted for 84.2% of cases, with the majority of distant metastases occurring in the liver (79.9%). At the time of enrolment, 10.8% of patients were treatment-naive. The primary tumor had been resected in 60 patients (43.2%). The most commonly used treatments included somatostatin analogs (71.2%) and liver-targeted therapy (51.5%), followed by chemotherapy (28.1%) and molecular targeted therapy such as tyrosine kinase inhibitors (18.0%) and mammalian target of rapamycin inhibitors (11.5%).
Table 1.
Demographic and clinical characteristics of patients with GEP-NETs
| Characteristic | N | Percentage |
|---|---|---|
| Age, median (range), years | 52 (14–76) | |
| Gender | ||
| Female | 71 | 51.1% |
| Male | 68 | 48.9% |
| Functionality | ||
| No | 121 | 87.1% |
| Yes | 18 | 12.9% |
| Carcinoid syndrome | 8 | 5.8% |
| Cushing syndrome | 1 | 0.7% |
| Insulinoma | 2 | 1.4% |
| Glucagonoma | 1 | 0.7% |
| Gastrinoma | 6 | 4.3% |
| Primary sites | ||
| Pancreas | 32 | 23.0% |
| Stomach | 15 | 10.8% |
| Duodenum | 12 | 8.6% |
| Small intestine | 14 | 10.1% |
| Colon | 3 | 2.2% |
| Rectum | 63 | 45.3% |
| Grade | ||
| G1 | 26 | 18.7% |
| G2 | 101 | 72.7% |
| G3 | 12 | 8.6% |
| AJCC stage | ||
| I | 17 | 12.2% |
| II | 3 | 2.2% |
| III | 2 | 1.4% |
| IV | 117 | 84.2% |
| Metastatic sites | ||
| Lymph nodes | 83 | 59.7% |
| Liver | 111 | 79.9% |
| Bone | 35 | 25.2% |
| Lung | 6 | 4.3% |
| Others | 26 | 18.7% |
The Association between Circulating Treg Levels and Clinicopathologic Characteristics
Circulating Treg levels ranged from 4.19% to 19.12% in patients with GEP-NETs, and more than half of the patients (91/139, 65.5%) had elevated Treg levels in their peripheral blood (normal range: 2.86–7.74%). The association between circulating Treg levels and clinicopathological characteristics is presented in Table 2. Significant associations were observed between Treg levels and functionality, primary sites, tumor grade, TNM stage, and liver metastasis. Functional GEP-NETs had significantly higher levels of Tregs compared to non-functional GEP-NETs (9.4% vs. 8.09%, p = 0.0053). PNETs displayed significantly higher Treg levels than GI-NETs (9.405% vs. 8.02%, p = 0.0008). Treg levels increased with increasing NET grade. The median Treg levels for grade-1 NETs was 7.660%, for grade-2 NETs was 8.300%, and for grade-3 NETs was 9.695%. The difference in Treg levels between grade-1 and grade-3 NETs was statistically significant (p = 0.0190). Although patients with a hepatic tumor burden >50% had slightly higher Treg levels compared to those with a tumor burden ≤50%, no statistical significance was achieved between the groups. GEP-NETs in advanced stages (III/IV) had significantly higher Treg levels than those in early stages (I/II) (8.460% vs. 7.390%, p = 0.0003). A marginal association was noted between lymph node metastasis and Treg levels; patients with lymph node metastases tended to have higher Treg levels than those without lymph node metastases (8.370% vs. 8.015%, p = 0.0576). No association was found between bone or lung metastasis and Treg levels. However, a significant association was observed between liver metastasis and Treg levels; GEP-NETs with liver metastases had significantly higher Treg levels than those without liver metastases (8.480% vs. 7.310%, p < 0.0001) (Fig. 1b, c).
Table 2.
Association between circulating Treg levels and clinical characteristics in patients with GEP-NETs
| Characteristic | Treg level (%) | p value | |
|---|---|---|---|
| median | IQR | ||
| Gender | |||
| Female | 8.140 | 7.160–9.930 | 0.4718 |
| Male | 8.420 | 7.353–9.823 | |
| Age | |||
| 0–50 years | 8.470 | 7.450–9.905 | 0.1444 |
| Over 50 years | 8.040 | 7.040–9.870 | |
| Functionality | |||
| No | 8.090 | 7.165–9.600 | 0.0053 |
| Yes | 9.400 | 8.290–12.96 | |
| Primary sites | |||
| Pancreas | 9.405 | 8.115–11.15 | 0.0008 |
| GI tract | 8.020 | 7.020–9.480 | |
| Grade | |||
| G1 | 7.660 | 7.058–8.535 | 0.1293 |
| G2 | 8.300 | 7.240–9.935 | 0.0860 |
| G3 | 9.695 | 7.868–11.71 | 0.0190 |
| Burden of liver metastases | |||
| <25% | 8.250 | 7.103–9.620 | 0.9410 |
| 25–50% | 8.165 | 7.413–9.633 | 0.5460 |
| >50% | 9.070 | 7.310–10.38 | 0.3228 |
| TNM stage | |||
| I/II | 7.390 | 6.703–7.995 | 0.0003 |
| III/IV | 8.460 | 7.450–10.06 | |
| Lymph node metastases, n | |||
| No | 8.015 | 7.148–9.305 | 0.0576 |
| Yes | 8.370 | 7.390–10.74 | |
| Lung metastases, n | |||
| No | 8.250 | 7.165–9.775 | 0.4148 |
| Yes | 8.705 | 7.788–11.36 | |
| Liver metastases, n | |||
| No | 7.310 | 6.195–7.995 | <0.0001 |
| Yes | 8.480 | 7.550–10.07 | |
| Bone metastases, n | |||
| No | 8.195 | 7.288–9.508 | 0.1477 |
| Yes | 9.200 | 7.160–11.11 | |
| Other metastases, n | |||
| No | 8.210 | 7.135–9.645 | 0.1279 |
| Yes | 8.855 | 7.640–11.32 | |
Other metastases include the brain, kidney, ovary, enterocoelia, pelvic cavity, etc.
The Association between Circulating Treg Level and Liver Metastasis
To identify the optimal cut-off value of Tregs that can distinguish between those GEP-NET patients with and without liver metastases, we conducted ROC curve analysis. A Treg level ≥8.015% distinguished between patients with and without liver metastases with a sensitivity of 66.7% and a specificity of 78.6%, yielding an AUC of 0.764 (95% CI = 0.670–0.858, p < 0.001) (Fig. 1d). In univariate logistic regression analyses, factors such as the primary site (pancreas: OR 4.815, 95% CI: 1.076–21.535, p = 0.040), higher grade (G2/G3: OR 6.533, 95% CI: 2.548–16.749, p < 0.001), and Treg level ≥8.015% (OR 6.769, 95% CI: 2.532–18.094, p < 0.001) were significantly associated with increased odds of liver metastases. In a multivariate model adjusting for clinical variables, including age, gender, functionality, and primary sites, only higher grade and Treg level ≥8.015% remained significantly associated with liver metastasis (Table 3). Therefore, both Treg level and tumor grade are independent factors that correlate with liver metastasis in GEP-NETs.
Table 3.
Univariate and multivariate logistic regression model for metastasis to liver
| Variable | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p value | OR | 95% CI | p value | |
| Gender | ||||||
| Female | 1 | 0.768 | 1 | 0.912 | ||
| Male | 1.133 | 0.494–2.601 | 1.058 | 0.393–2.846 | ||
| Age, years | ||||||
| 0–50 | 1 | 0.639 | 1 | 0.938 | ||
| Over 50 | 1.220 | 0.532–2.797 | 1.043 | 0.361–3.012 | ||
| Functionality | ||||||
| No | 1 | 0.694 | 1 | 0.424 | ||
| Yes | 1.302 | 0.349–4.852 | 0.531 | 0.112–2.509 | ||
| Primary sites | ||||||
| GI tract | 1 | 0.040 | 1 | 0.201 | ||
| Pancreas | 4.815 | 1.076–21.535 | 2.810 | 0.577–13.694 | ||
| Grade | ||||||
| G1 | 1 | <0.001 | 1 | 0.002 | ||
| G2/G3 | 6.533 | 2.548–16.749 | 5.937 | 1.949–18.086 | ||
| Treg level | ||||||
| <8.015% | 1 | <0.001 | 1 | 0.001 | ||
| ≥8.015% | 6.769 | 2.532–18.094 | 6.337 | 2.078–19.328 | ||
Circulating Treg Level Is Associated with Treatment Response to TAE and Correlates with hPFS in Patients with GEP-NETs
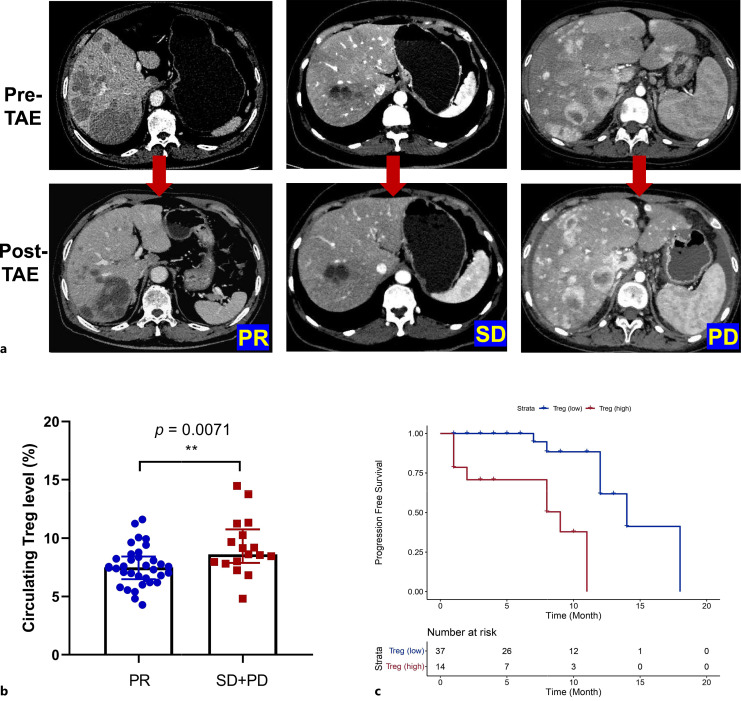
Among all GEP-NET patients who underwent TAE to reduce hepatic tumor burden (n = 51), 34 patients achieved favorable responses (PR = 34, complete response = 0), while 17 patients did not respond well to TAE (SD = 13, PD = 4). Representative CT images of patients who achieved PR, SD, or PD after TAE treatment are shown in Figure 2a. Non-responders had significantly higher baseline levels of Tregs compared to responders (8.620% vs. 7.505%, p = 0.0071) (Fig. 2b). In univariate logistic regression analyses, an increase in Treg levels (OR 1.542, 95% CI: 1.091–2.179, p = 0.014) was a negative factor for treatment response. In a multivariate model adjusting for clinical variables, including age, gender, functionality, primary sites, and tumor grade, an increase in Treg levels remained significantly associated with unfavorable responses to TAE treatment (OR 1.653, 95% CI: 1.002–2.728, p = 0.049) (Table 4). Therefore, Tregs are an independent factor that correlates with the treatment response to TAE in GEP-NET patients.
Fig. 2.
Correlation between circulating Treg levels, treatment response, and hPFS in GEP-NET patients receiving TAE treatment. a Representative CT images: CT images illustrate representative cases of patients achieving partial response (PR), stable disease (SD), or progressive disease (PD) after TAE treatment. b Treg levels in responders versus nonresponders: comparison of Treg levels in patients with favorable responses (PR, n = 34) and unfavorable responses (SD + PD, n = 17) to TAE. Data are presented as medians with interquartile ranges. ***p < 0.001. Kaplan-Meier Survival Curves: Kaplan-Meier curves depicting hepatic progression-free survival (PFS) in GEP-NET patients with high (n = 14) or low (n = 37) levels of Tregs. The stratification is based on the cutoff value of 8.975%, as determined by CART analysis.
Table 4.
Univariate and multivariate logistic regression analyses for treatment response of TAE in GEP-NET patients
| Variable | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p value | OR | 95% CI | p value | |
| Gender | ||||||
| Female | 1 | 0.158 | 1 | 0.669 | ||
| Male | 0.390 | 0.105–1.443 | 0.671 | 0.108–4.182 | ||
| Age, years | ||||||
| 0–50 | 1 | 0.001 | 1 | 0.037 | ||
| Over 50 | 0.111 | 0.029–0.429 | 0.182 | 0.037–0.902 | ||
| Functionality | ||||||
| No | 1 | 0.203 | 1 | 0.720 | ||
| Yes | 3.429 | 0.515–22.837 | 1.732 | 0.086–34.805 | ||
| Primary sites | ||||||
| Pancreas | 1 | 0.824 | 1 | 0.409 | ||
| GI tract | 0.864 | 0.237–3.145 | 0.470 | 0.079–2.817 | ||
| Grade | ||||||
| G1 | 1 | 0.165 | 1 | 0.133 | ||
| G2 | 0.315 | 0.062–1.607 | 0.202 | 0.025–1.631 | ||
| Treg level | 1.542 | 1.091–2.179 | 0.014 | 1.653 | 1.002–2.728 | 0.049 |
Next, we conducted univariate Cox proportional hazard models to assess the association between circulating Treg levels and hPFS in GEP-NET patients receiving TAE treatment. HPFS improved as the circulating Treg levels decreased (HR 1.782, 95% CI: 1.312–2.422, p < 0.001). In a multivariate model adjusting for clinical variables, including age, gender, functionality, primary sites, and tumor grade, Treg levels remained significantly correlated with hPFS (HR 1.703, 95% CI: 1.214–2.388, p = 0.002) (Table 5). Therefore, Tregs are an independent factor that correlates with hPFS in GEP-NET patients receiving TAE treatment.
Table 5.
Univariate and multivariate Cox proportional hazards models for hepatic progression-free survival
| Variable | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p value | HR | 95% CI | p value | |
| Gender | ||||||
| Female | 1 | 0.341 | 1 | 0.873 | ||
| Male | 0.571 | 0.180–1.810 | 0.893 | 0.224–3.555 | ||
| Age, years | ||||||
| 0–50 | 1 | 0.022 | 1 | 0.200 | ||
| Over 50 | 0.256 | 0.080–0.823 | 0.419 | 0.111–1.587 | ||
| Functionality | ||||||
| No | 1 | 0.469 | 1 | 0.704 | ||
| Yes | 1.746 | 0.386–7.887 | 0.662 | 0.079–5.553 | ||
| Primary sites | ||||||
| Pancreas | 1 | 0.641 | 1 | 0.873 | ||
| GI tract | 1.359 | 0.373–4.950 | 0.888 | 0.208–3.787 | ||
| Grade | ||||||
| G1 | 1 | 0.243 | 1 | 0.429 | ||
| G2 | 0.391 | 0.081–1.894 | 0.447 | 0.061–3.290 | ||
| Treg level | 1.782 | 1.312–2.422 | <0.001 | 1.703 | 1.214–2.388 | 0.002 |
Using the CART method, we identified a cut-off value of 8.975% to stratify patients into Treghigh or Treglow level in this cohort. Kaplan-Meier analysis revealed that patients with Treghigh (≥8.975%) displayed a significantly shorter median hPFS compared to patients in the Treglow group (<8.975%) (9-month vs. 14-month, p < 0.0001) (Fig. 2c). This underscores the prognostic value of Tregs in GEP-NET patients receiving TAE treatment.
Discussion
GEP-NETs frequently metastasize to the liver, with approximately 28–77% of patients developing neuroendocrine liver metastases during their lifetime [3, 21]. The presence of liver metastases is one of the most significant negative prognostic factors associated with compromised survival among patients with GEP-NETs. Therefore, NETs that colonize the liver may be considered an aggressive subtype. Understanding the molecular mechanisms of liver metastases may enhance our ability to manage these cases and develop therapeutic interventions to improve prognosis.
Complex interactions take place among the cellular components of the tumor microenvironment (TME) and contribute to the biological diversity observed in NETs [22]. The components of the TME include surrounding blood vessels, immune cells, fibroblasts, signaling molecules, and the extracellular matrix, collectively determining tumor biology. As one of the most predominant immunosuppressive cells in the TME, Tregs participate in tumor growth, invasion, dissemination, and influence treatment responses [7]. To date, only a handful of small studies have examined Tregs in the NET population. In a cohort of patients with pNETs, a high number of tumor-infiltrating Tregs were significantly associated with poorer overall survival [23]. Increased frequencies of circulating Tregs were also found in a cohort of midgut carcinoid patients [24]. More studies are still required to further explore the role of Treg in NETs from a more comprehensive perspective. In this study, we investigated the clinical relevance of circulating CD4+CD25+CD127low/− Tregs and explored their associations with liver metastases in patients with GEP-NETs.
In line with previously published data, circulating Treg levels were strongly associated with advanced tumor stage, distant metastasis, and higher tumor grade [10, 25–27]. We observed that Treg levels were significantly higher in advanced stage (III/IV) GEP-NETs compared to early stage (I/II), and Treg levels increased with the grade of GEP-NETs. Treg levels significantly correlated with liver involvement, while no significant association was found with other metastatic sites, including lung, bone, and lymph nodes. We also found that a Treg level ≥8.015% distinguished between GEP-NET patients with and without liver metastases. Multivariate analysis identified Tregs as an independent factor that correlates with liver metastasis, suggesting a potential role of Tregs in the process of liver metastases in GEP-NETs. Increased Treg frequencies have been reported to be associated with a greater risk of metastasis in multiple cancer types [27]. For instance, in intrahepatic cholangiocarcinoma, Tregs induce a suppressive immune milieu and promote lymph node metastasis [28]. Similarly, in colorectal cancer, patients with liver metastases have elevated levels of functionally immune suppressive CD4+CD25+CD127dim/− Tregs in their peripheral blood [29]. In cutaneous squamous cell carcinoma, OX40+ Tregs suppress effector T-cell responses and are associated with increased metastatic potential [30]. The mechanisms underlying Treg-mediated cancer metastasis are variable and complicated. By disturbing both innate and adaptive anti-tumor immune responses, Tregs can inhibit the function of cytotoxic T lymphocytes (CTLs) and/or NK cells and shield malignant cells from immune attack, thereby allowing malignant cells to survive, proliferate, and acquire characteristics that facilitate dissemination [27]. Additionally, besides suppressing innate and adaptive immunity, Tregs may facilitate cancer progression by releasing angiogenic factors like vascular endothelial growth factor (VEGF) and directly promoting tumor cell angiogenesis and invasion [31]. However, the exact mechanisms involved in Treg-mediated liver metastasis in GEP-NETs are unclear and require further exploration.
TAE, as a classical liver-directed embolotherapy, has shown remarkable efficacy in reducing intrahepatic tumor burden in patients with GEP-NETs [6, 19]. However, the responses to TAE are variable, and reliable markers to identify patients who will respond positively are lacking. Currently, accumulating studies are revealing that multiple locoregional therapies, including embolotherapies, have the potential for immune modulation by inducing antigen and pro-inflammatory cytokine release, and activated immune responses are associated with clinical response to locoregional anti-tumor therapies [32–34]. Hence, immunological biomarkers have been developed to reflect individual immune status and predict the efficacy of locoregional treatments. For instance, in hepatocellular carcinoma, higher percentages of PD-1-expressing and Tim-3-expressing CD8+ T cells were found in sustained responders to Yttrium-90-radioembolisation treatment [35], and high baseline neutrophil-to-lymphocyte ratios (NLRs) and platelet-to-lymphocyte ratios (PLRs) predicted poorer tumor response and shorter PFS to transarterial chemoembolization (TACE) treatment [36]. As an immunological biomarker, Tregs have also been found to predict sensitivity to anticancer treatments [37]. However, in the context of GEP-NET treated by TAE, the role of Tregs has not been fully clarified. In this study, we investigated the prognostic value of pretreatment Treg levels, measured in peripheral blood samples, as biomarkers for clinical outcome after TAE treatment in patients with GEP-NETs.
The results presented here demonstrate that GEP-NET patients with higher Treg levels displayed unfavorable responses and reduced hPFS after TAE treatment. We revealed that patients with Treghigh (≥8.975%) had a significantly shorter median hPFS than patients with Treglow (<8.975%). Additionally, after adjusting for other confounding clinical parameters, the association between Tregs and treatment response, as well as hPFS, remained significant, suggesting that Tregs have an independent prognostic impact in GEP-NETs. To the best of our knowledge, this is the first study showing the link between circulating Treg levels and the therapeutic efficacy of TAE among GEP-NET patients. Apart from its prognostic significance, the finding of a correlation between high Treg levels and poor response to TAE treatment may have promising therapeutic implication in GEP-NETs. This finding suggests that immunosuppression plays an important role in tumor progression in GEP-NET patients undergoing TAE treatment, and targeting Tregs could be beneficial in overcoming Treg-mediated tumor resistance and improving the clinical response in GEP-NET patients. Tregs can be targeted through the use of antibodies against their surface marker CD25 [38], or by interrupting the stability of their transcription factor, forkhead box protein P3 (FOXP3), using FOXP3 inhibitors such as AZD8701 [39]. Other feasible approaches include targeting pathways that positively regulate Treg stability, function, and survival, such as the phosphatidylinositol 3-kinase (PI3K) signaling pathway [40], or targeting chemokine or their receptors associated with Treg chemotaxis through neutralizing antibodies or antagonists [41, 42]. Immunotherapeutic interventions targeting Tregs in combination with TAE may reprogram the suppressive immune landscape and result in improved tumor control, while such combination therapy warrants further investigations in GEP-NETs.
Our research may also have broader implications for investigating the role of Tregs in association with other treatment modalities in NET patients, such as immune checkpoint inhibitors (ICIs), somatostatin analogs (SSAs), etc. ICIs have shown promising results in some types of cancer but may not be as effective in NETs compared to other cancer types [43]. The resistance mechanisms of ICIs involve low tumor mutational burden (TMB), dysfunctional antigen presentation machinery, and an immunosuppressive TME characterized by the presence of Tregs, myeloid-derived suppressor cells (MDSCs), and other immune cells that hinder the anti-tumor immune response [44]. Understanding these resistance mechanisms is crucial for developing strategies to overcome immunotherapy resistance in NETs. Our study showed that a substantial proportion of patients (65.5%) with GEP-NETs harbored elevated Treg levels in their peripheral blood, suggesting that Treg-induced immunosuppression does, indeed, exist in GEP-NETs and may cause resistance to ICIs. Octreotide long-acting release (LAR), a widely used SSA to treat functional and/or metastatic NETs, has been found to induce a decreased level and functional impairment of circulating Tregs together with MDSCs in patients with metastatic GEP-NETs and functioning lung-NETs [45]. Both Tregs and MDSCs are key immunosuppressive cell types, which collectively promote tumor escape from immune attack [45]. The effect of Octreotide LAR on Tregs and MDSCs highlights the potential role of SSAs in restoring active immune immunosurveillance and overcoming immunotherapy resistance, providing readily translatable combination strategies to empower immunotherapy in NET patients.
The potential correlation between immunotherapy efficacy and TAE is also an interesting hypothesis. TAE is a procedure used to treat liver tumors by blocking the blood supply to the tumor, which can lead to local inflammation [32]. This inflammation could theoretically alter the TME, potentially making it more responsive to immunotherapy. However, the specific correlation between TAE-induced local inflammatory patterns and the subsequent response to immunotherapy in NET patients is not well-established. Research in this area would involve studying the immune response in the TME post-TAE, and how it interacts with subsequent immunotherapeutic approaches.
Our study represents a pioneering investigation into the clinical implications of Tregs and their correlation with the response to TAE in patients with GEP-NETs. However, it is essential to acknowledge certain limitations within our research. First, the study was conducted at a single center, resulting in a limited sample size, which could introduce potential biases. It is imperative to conduct multi-center studies and validate our findings in a more expansive patient cohort to affirm the robustness of the results. Second, due to the relatively low incidence and slow progression of GEP-NETs, evaluating the prognostic significance of Tregs for patient overall survival remains challenging. Prolonged follow-up to ascertain subsequent survival status is warranted. Third, variability in the technique employed for TAE might exist across the patient cohort due to individualized treatment plans based on the clinician’s judgment and patient-specific conditions. This variation might introduce variability in the treatment response assessment, which is an inherent limitation of retrospective studies. Fourth, given the retrospective nature of this study, determining the predictive value of Tregs was not feasible. A meticulously designed prospective clinical study is imperative to comprehensively investigate the role of Tregs in GEP-NET patients. Finally, the precise biological effects of Tregs on liver metastasis and their influence on the response to TAE in GEP-NETs remain unclear. Future laboratory research efforts are essential to elucidate the underlying mechanisms involved.
In conclusion, this study demonstrates the role of circulating Tregs as both a marker associated with liver metastasis and a promising prognostic biomarker associated with treatment response to TAE in GEP-NET patients. This immunological marker holds the potential to guide treatment strategies and enhance our understanding of disease progression, with the aim of improving clinical management of GEP-NET patients.
Acknowledgments
We thank the patients and their families, nurses, and pathologists who participated in this study. We thank Dr. Luohai Chen for statistical analysis.
Statement of Ethics
The study was approved by the Institutional Ethics Committee (IEC) for Clinical Research of the First Affiliated Hospital of Sun Yat-sen University (approval number [2021] 775). Written informed consent was obtained from participants and from parents/legal guardians for all participants aged under 18.
Conflict of Interest Statement
The authors have no financial conflicts of interest.
Funding Sources
This study was funded by the National Natural Science Foundation of China (Grant No.: 82002502) and the Natural Science Foundation of Guangdong Province (Grant No.: 2019A1515012027).
Author Contributions
Collection and assembly of data: Man Liu, Hang Yu, and Haikuan Liu. Data analysis and interpretation: Man Liu, Haikuan Liu, Hang Yu, and Luohai Chen. Manuscript writing: Man Liu and Yu Wang. Manuscript revision: Ning Zhang and Yu Wang. Technical or material support: Luohai Chen, Dequan Yang, Juan Ouyang, Jiang Zhang, Xu Yan, Yanji Luo, Yuan Lin, Qiao He, and Minhu Chen. Study supervision: Ning Zhang and Yu Wang. All authors reviewed iterations of the report and approved the final version for submission.
Funding Statement
This study was funded by the National Natural Science Foundation of China (Grant No.: 82002502) and the Natural Science Foundation of Guangdong Province (Grant No.: 2019A1515012027).
Data Availability Statement
All data generated or analyzed during this study are included in this article. Further inquiries can be directed to the corresponding author.
References
- 1. Pavel M, Öberg K, Falconi M, Krenning EP, Sundin A, Perren A, et al. Gastroenteropancreatic neuroendocrine neoplasms: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2020;31(7):844–60. [DOI] [PubMed] [Google Scholar]
- 2. Dasari A, Shen C, Halperin D, Zhao B, Zhou S, Xu Y, et al. Trends in the incidence, prevalence, and survival outcomes in patients with neuroendocrine tumors in the United States. JAMA Oncol. 2017;3(10):1335–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pavel M, Baudin E, Couvelard A, Krenning E, Öberg K, Steinmüller T, et al. ENETS Consensus Guidelines for the management of patients with liver and other distant metastases from neuroendocrine neoplasms of foregut, midgut, hindgut, and unknown primary. Neuroendocrinology. 2012;95(2):157–76. [DOI] [PubMed] [Google Scholar]
- 4. Frilling A, Modlin IM, Kidd M, Russell C, Breitenstein S, Salem R, et al. Recommendations for management of patients with neuroendocrine liver metastases. Lancet Oncol. 2014;15(1):e8–21. [DOI] [PubMed] [Google Scholar]
- 5. Cloyd JM, Ejaz A, Konda B, Makary MS, Pawlik TM. Neuroendocrine liver metastases: a contemporary review of treatment strategies. Hepatobiliary Surg Nutr. 2020;9(4):440–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Liu Y, Liu H, Chen W, Yu H, Yao W, Fan W, et al. Prolonged progression-free survival achieved by octreotide LAR plus transarterial embolization in low-to-intermediate grade neuroendocrine tumor liver metastases with high hepatic tumor burden. Cancer Med. 2022;11(13):2588–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Li C, Jiang P, Wei S, Xu X, Wang J. Regulatory T cells in tumor microenvironment: new mechanisms, potential therapeutic strategies and future prospects. Mol Cancer. 2020;19(1):116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sakaguchi S, Mikami N, Wing JB, Tanaka A, Ichiyama K, Ohkura N. Regulatory T cells and human disease. Annu Rev Immunol. 2020;38:541–66. [DOI] [PubMed] [Google Scholar]
- 9. Vignali DAA, Collison LW, Workman CJ. How regulatory T cells work. Nat Rev Immunol. 2008;8(7):523–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Saleh R, Elkord E. FoxP3+ T regulatory cells in cancer: prognostic biomarkers and therapeutic targets. Cancer Lett. 2020;490:174–85. [DOI] [PubMed] [Google Scholar]
- 11. Song N-Y, Li X, Ma B, Willette-Brown J, Zhu F, Jiang C, et al. IKKα-deficient lung adenocarcinomas generate an immunosuppressive microenvironment by overproducing Treg-inducing cytokines. Proc Natl Acad Sci U S A. 2022;119(6):e2120956119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhou J, Li X, Wu X, Zhang T, Zhu Q, Wang X, et al. Exosomes released from tumor-associated macrophages transfer miRNAs that induce a Treg/Th17 cell imbalance in epithelial ovarian cancer. Cancer Immunol Res. 2018;6(12):1578–92. [DOI] [PubMed] [Google Scholar]
- 13. Ji L, Qian W, Gui L, Ji Z, Yin P, Lin GN, et al. Blockade of β-catenin-induced CCL28 suppresses gastric cancer progression via inhibition of Treg cell infiltration. Cancer Res. 2020;80(10):2004–16. [DOI] [PubMed] [Google Scholar]
- 14. Plitas G, Konopacki C, Wu K, Bos PD, Morrow M, Putintseva EV, et al. Regulatory T cells exhibit distinct features in human breast cancer. Immunity. 2016;45(5):1122–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Huang H, Wang Z, Zhang Y, Pradhan RN, Ganguly D, Chandra R, et al. Mesothelial cell-derived antigen-presenting cancer-associated fibroblasts induce expansion of regulatory T cells in pancreatic cancer. Cancer Cell. 2022;40(6):656–73.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jacobs JFM, Nierkens S, Figdor CG, de Vries IJM, Adema GJ. Regulatory T cells in melanoma: the final hurdle towards effective immunotherapy? Lancet Oncol. 2012;13(1):e32–42. [DOI] [PubMed] [Google Scholar]
- 17. Nagtegaal ID, Odze RD, Klimstra D, Paradis V, Rugge M, Schirmacher P, et al. The 2019 WHO classification of tumours of the digestive system. Histopathology. 2020;76(2):182–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Amin MB, Greene FL, Edge SB, Compton CC, Gershenwald JE, Brookland RK, et al. The Eighth Edition AJCC Cancer Staging Manual: continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J Clin. 2017;67(2):93–9. [DOI] [PubMed] [Google Scholar]
- 19. Liu Y, Chen W, Cui W, Liu H, Zhou X, Chen L, et al. Quantitative pretreatment CT parameters as predictors of tumor response of neuroendocrine tumor liver metastasis to transcatheter arterial bland embolization. Neuroendocrinology. 2020;110(7–8):697–704. [DOI] [PubMed] [Google Scholar]
- 20. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz L, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228–47. [DOI] [PubMed] [Google Scholar]
- 21. Yao JC, Hassan M, Phan A, Dagohoy C, Leary C, Mares JE, et al. One hundred years after “carcinoid”: epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol. 2008;26(18):3063–72. [DOI] [PubMed] [Google Scholar]
- 22. Cives M, Pelle E, Quaresmini D, Rizzo FM, Tucci M, Silvestris F. The tumor microenvironment in neuroendocrine tumors: biology and therapeutic implications. Neuroendocrinology. 2019;109(2):83–99. [DOI] [PubMed] [Google Scholar]
- 23. de Reuver PR, Mehta S, Gill P, Andrici J, D’Urso L, Clarkson A, et al. Immunoregulatory forkhead box protein p3-positive lymphocytes are associated with overall survival in patients with pancreatic neuroendocrine tumors. J Am Coll Surg. 2016;222(3):281–7. [DOI] [PubMed] [Google Scholar]
- 24. Vikman S, Sommaggio R, De La Torre M, Oberg K, Essand M, Giandomenico V, et al. Midgut carcinoid patients display increased numbers of regulatory T cells in peripheral blood with infiltration into tumor tissue. Acta Oncol. 2009;48(3):391–400. [DOI] [PubMed] [Google Scholar]
- 25. Hiraoka N, Onozato K, Kosuge T, Hirohashi S. Prevalence of FOXP3+ regulatory T cells increases during the progression of pancreatic ductal adenocarcinoma and its premalignant lesions. Clin Cancer Res. 2006;12(18):5423–34. [DOI] [PubMed] [Google Scholar]
- 26. Li F, Sun Y, Huang J, Xu W, Liu J, Yuan Z. CD4/CD8 + T cells, DC subsets, Foxp3, and Ido expression are predictive indictors of gastric cancer prognosis. Cancer Med. 2019;8(17):7330–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Halvorsen EC, Mahmoud SM, Bennewith KL. Emerging roles of regulatory T cells in tumour progression and metastasis. Cancer Metastasis Rev. 2014;33(4):1025–41. [DOI] [PubMed] [Google Scholar]
- 28. Konishi D, Umeda Y, Yoshida K, Shigeyasu K, Yano S, Toji T, et al. Regulatory T cells induce a suppressive immune milieu and promote lymph node metastasis in intrahepatic cholangiocarcinoma. Br J Cancer. 2022;127(4):757–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Brudvik KW, Henjum K, Aandahl EM, Bjørnbeth BA, Taskén K. Regulatory T-cell-mediated inhibition of antitumor immune responses is associated with clinical outcome in patients with liver metastasis from colorectal cancer. Cancer Immunol Immunother. 2012;61(7):1045–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lai C, August S, Albibas A, Behar R, Cho SY, Polak ME, et al. OX40+ regulatory T cells in cutaneous squamous cell carcinoma suppress effector T-cell responses and associate with metastatic potential. Clin Cancer Res. 2016;22(16):4236–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Facciabene A, Peng X, Hagemann IS, Balint K, Barchetti A, Wang LP, et al. Tumour hypoxia promotes tolerance and angiogenesis via CCL28 and T (reg) cells. Nature. 2011;475(7355):226–30. [DOI] [PubMed] [Google Scholar]
- 32. Tischfield DJ, Gurevich A, Johnson O, Gatmaytan I, Nadolski GJ, Soulen MC, et al. Transarterial embolization modulates the immune response within target and nontarget hepatocellular carcinomas in a rat model. Radiology. 2022;303(1):215–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Llovet JM, De Baere T, Kulik L, Haber PK, Greten TF, Meyer T, et al. Locoregional therapies in the era of molecular and immune treatments for hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. 2021;18(5):293–313. [DOI] [PubMed] [Google Scholar]
- 34. Ayaru L, Pereira SP, Alisa A, Pathan AA, Williams R, Davidson B, et al. Unmasking of alpha-fetoprotein-specific CD4 (+) T cell responses in hepatocellular carcinoma patients undergoing embolization. J Immunol. 2007;178(3):1914–22. [DOI] [PubMed] [Google Scholar]
- 35. Chew V, Lee YH, Pan L, Nasir NJM, Lim CJ, Chua C, et al. Immune activation underlies a sustained clinical response to Yttrium-90 radioembolisation in hepatocellular carcinoma. Gut. 2019;68(2):335–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Schobert IT, Savic LJ, Chapiro J, Bousabarah K, Chen E, Laage-Gaupp F, et al. Neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios as predictors of tumor response in hepatocellular carcinoma after DEB-TACE. Eur Radiol. 2020;30(10):5663–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kang DH, Chung C, Sun P, Lee DH, Lee SI, Park D, et al. Circulating regulatory T cells predict efficacy and atypical responses in lung cancer patients treated with PD-1/PD-L1 inhibitors. Cancer Immunol Immunother. 2022;71(3):579–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Onda M, Kobayashi K, Pastan I. Depletion of regulatory T cells in tumors with an anti-CD25 immunotoxin induces CD8 T cell-mediated systemic antitumor immunity. Proc Natl Acad Sci U S A. 2019;116(10):4575–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Revenko A, Carnevalli LS, Sinclair C, Johnson B, Peter A, Taylor M, et al. Direct targeting of FOXP3 in Tregs with AZD8701, a novel antisense oligonucleotide to relieve immunosuppression in cancer. J Immunother Cancer. 2022;10(4):e003892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ali K, Soond DR, Pineiro R, Hagemann T, Pearce W, Lim EL, et al. Inactivation of PI(3)K p110δ breaks regulatory T-cell-mediated immune tolerance to cancer. Nature. 2014;510(7505):407–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ni X, Langridge T, Duvic M. Depletion of regulatory T cells by targeting CC chemokine receptor type 4 with mogamulizumab. Oncoimmunology. 2015;4(7):e1011524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Jackson JJ, Ketcham JM, Younai A, Abraham B, Biannic B, Beck HP, et al. Discovery of a potent and selective CCR4 antagonist that inhibits Treg trafficking into the tumor microenvironment. J Med Chem. 2019;62(13):6190–213. [DOI] [PubMed] [Google Scholar]
- 43. Albertelli M, Dotto A, Nista F, Veresani A, Patti L, Gay S, et al. Present and future of immunotherapy in neuroendocrine tumors. Rev Endocr Metab Disord. 2021;22(3):615–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sharma P, Hu-Lieskovan S, Wargo JA, Ribas A. Primary, adaptive, and acquired resistance to cancer immunotherapy. Cell. 2017;168(4):707–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. von Arx C, Rea G, Napolitano M, Ottaiano A, Tatangelo F, Izzo F, et al. Effect of octreotide long-acting release on Tregs and MDSC cells in neuroendocrine tumour patients: a pivotal prospective study. Cancers. 2020;12(9):2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this article. Further inquiries can be directed to the corresponding author.