Abstract
Tissue regeneration is a complex process that involves the recruitment of various types of cells for healing after injury; it is mediated by numerous precise interactions. However, the identification of effective targets for improving tissue regeneration remains a challenge. As an extracellular matrix protein, Agrin plays a critical role in neuromuscular junction formation. Furthermore, recent studies have revealed the role of Agrin in regulating tissue proliferation and regeneration, which contributes to the repair process of injured tissues. An in-depth understanding of the role of Agrin will therefore be of value. Given that repair and regeneration processes occur in various parts of the human body, the present systematic review focuses on the role of Agrin in typical tissue and highlights the potential signaling pathways that are involved in Agrin-induced repair and regeneration. This review offers important insight into novel strategies for the future clinical applications of Agrin-based therapies, which may represent a feasible treatment option for patients who require organ replacement or repair.
Key words: Agrin, repair, regeneration, mechanism
1. Introduction
Organ damage and loss are generally caused by congenital abnormalities or acquired disorders. The clinical application of regenerative medicine includes the use of biological products, stem cell therapy, tissue engineering, cellular reprogramming and gene therapy (1). Recently, a regenerative approach, in which the local milieu of the diseased tissue or organ is modulated into a regenerative environment to aid in the healing process, has attracted attention and provided new insights into 'translational medicine'. It is speculated that this approach will replace traditional transplantology in the near future. Thus, this new regenerative approach needs to be explored.
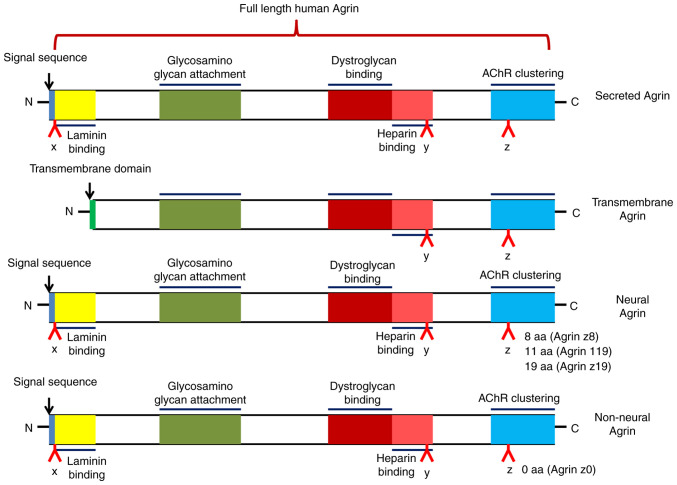
Agrin is an extracellular matrix protein that has heparan sulfate proteoglycan as the core protein. It is encoded by the AGRN gene and has a relative molecular weight of ~220 kDa, including nine protein kinase inhibitor domains, four epidermal growth factor-like domains and one adhesion molecule G homologous domain (2). The AGRN transcript can be spliced to produce different isoforms: Either a type II transmembrane protein or a secreted protein (3) (Fig. 1). This splicing determines both the localization and function of Agrin.
Figure 1.
Schematic showing different forms of Agrin. Referenced by Chakraborty et al (3) and recreated with BioRender.com. AChR, acetylcholine receptor.
Agrin was originally isolated from tissue with electrical activity. It binds to low-density lipoprotein receptor-related protein 4 (LRP4) and activates muscle-specific kinase (MuSK), thus forming a multiprotein complex at the neuromuscular junction (NMJ) (4). The function of Agrin in the formation and maintenance of the NMJ is relatively well known; however, it also has roles in proliferation, motility, cell adhesion and even the regulation of progression in certain types of cancer (5-7). In a previous study, the role of Agrin in regulating tissue proliferation and regeneration was investigated, revealing that Agrin exerts a pleiotropic therapeutic response, including intrinsic and extrinsic arms, which contributes to the repair process in injured animals (8). Agrin therapy has also been reported to promote both cardiac regeneration after myocardial infarction and the proliferation of human induced pluripotent stem cell-derived cardiomyocytes (9). However, the role of Agrin during tissue repair and regeneration remains largely unknown and investigations are needed to reveal whether Agrin employs a similar strategy to stimulate the proliferation of tissue and organs. In the subsequent sections of the present review, the roles and mechanisms of Agrin in regulating the repair of different tissues and organs were discussed, which may offer important insights into novel strategies for regenerative therapies. The search sequence (PRISMA flow diagram) is provided in Fig. S1.
2. Regulation of Agrin in neurogenesis
Possible mechanisms of Agrin in neurogenesis
Over the last several decades, Agrin has been identified to play a central role in the formation of skeletal neuromuscular synapses between presynaptic motor neurons and postsynaptic muscle fibers (10). During this process, Agrin is released by motor neurons and interacts with the transmembrane LRP4, which leads to acetylcholine receptor (AChR) aggregation via the activation of MuSK; this is important for the formation of a functional NMJ (4).
A previous study demonstrated that local Agrin stimulation induces the clustering of mitochondria and synaptic vesicles, two well-known presynaptic markers, and regulates vesicular trafficking (11). Furthermore, Gautam et al (12) reported that postsynaptic AChR aggregates are significantly reduced in number, size and density in the muscles of Agrin-deficient mutant mice, suggesting that mice lacking Agrin are unable to make synapses. This defect may be caused by the absence of a neuron-specific isoform, with an exon encoding only eight amino acids, which is a critical nerve-derived inducer of postsynaptic differentiation (13). However, two reports have indicated that synapses can form in the absence of Agrin (14,15). Cyclin-dependent kinase 5 is activated by ACh agonists, and is required for the ACh agonist-induced dispersion of AChR clusters that have not been stabilized by Agrin (14). Similarly, a study by Misgeld et al (15) demonstrated that the action of Agrin in vivo is critically dependent on cholinergic neurotransmission. Using double-mutant mice, these authors demonstrated that synapses can form in the absence of Agrin, provided that ACh is also absent. These results suggest that ACh causes instability in newly generated postsynaptic sites and indicate that a major physiological role of Agrin is to counteract this 'anti-synaptogenic' influence (15).
Of note, ectopic MuSK expression promotes ectopic synapse formation, and ectopic MuSK expression stimulates synapse formation in the absence of Agrin and rescues the lethality of AGRN-mutant mice. Furthermore, Agrin ablation is accompanied by a second gene transformation, leading to increased postsynaptic MuSK concentrations. These results further indicate that the postsynaptic cell, and particularly MuSK, has a crucial role in the regulation of synapse formation (16). Furthermore, studies suggest that Agrin may stabilize existing AChR aggregates in the presence of neurally derived dispersants (17,18). Although MuSK and the synapse-specific cytoplasmic protein rapsyn are required in the initial steps of postsynaptic differentiation and the formation of an end-plate band, Agrin is not essential. However, nerve-derived Agrin is required in the subsequent stages of synaptic growth and maintenance (17).
Neurogenesis occurs at every stage of life, including in adults. It has been reported that neurogenesis is associated with learning, memory and mood regulation, and its attenuation contributes to emotional and cognitive deficits during aging and neurological diseases such as Alzheimer's disease (19). In the adult hippocampus, neural stem/progenitor cells of the subgranular zone reorganize and proliferate to generate newborn neurons, which then integrate into the granule cell layer of the dentate gyrus (20). During this dynamic process, the activation and proliferation of quiescent stem cells, neuronal fate specification, cell migration and synaptic integration are all involved (21). In addition, Agrin mRNA levels are reportedly increased in the mouse hippocampus following exposure to an enriched environment, suggesting that Agrin expression is dependent on activity (22). Rather than as a key synaptic organizer, Agrin has been designated as a stabilizer that can induce postsynaptic differentiation in the absence of nerves (17,23). Another study reported that all three members of the Agrin-MuSK-LRP4 complex are involved in the induction of presynaptic differentiation (24), and particularly strong evidence supports the role of LRP4 (25).
During Agrin-induced neurogenesis, multiple types of cells and signaling pathways are involved (26). Transforming growth factor-β1 has been reported to enhance synaptogenesis via the upregulation of neuronal Agrin expression in Schwann cells, which is both sufficient and necessary for mediating synapse-promoting effects in the developing NMJ (27). In addition, Zhang et al (28) revealed that the combined treatment of Agrin and laminin in a co-culture system is able to enhance functional NMJ formation through a primarily neural mechanism, which has potential clinical importance for treating denervation injuries and creating functional neuromuscular constructs for muscle tissue. Zhang et al (22) have also reported that Agrin activates the receptor tyrosine kinase orphan receptor 2 (ROR2) through LRP4 in a mouse model, identifying a role for Agrin-LRP4-ROR2 signaling in adult neurogenesis. Similarly, Ma et al (29) determined the role of Agrin in botulinum neurotoxin type A-induced nerve sprouting in a rat model by regulating downstream MuSK and upstream microRNA (miR)-144, and confirmed that Agrin can regulate nerve sprouting via the miR-144-Agrin-MuSK signaling pathway. Yang et al (23) reported that the expression of the active Agrin isoforms B11 and B19 is upregulated in Schwann cells during nerve regeneration in adults. As well as reporting that neurons express active Agrin, they also noted that glial cells express active Agrin and play a role in inducing AChR clusters beneath perisynaptic Schwann cell sprouts (23), suggesting that Agrin may play an indispensable role in the repair of central nervous system injury.
Possible role of Agrin in nerve disease
Myasthenia gravis (MG) is an autoimmune disease in which antibodies against AChR, MuSK or other AChR-related proteins in the NMJ cause localized or general muscle weakness (30). In patients with MG, autoantibodies bind to the components of postsynaptic muscle endplates and destroy the structure and function of NMJ, thus leading to impaired neuromuscular transmission (31). Recent studies have revealed that antibodies against Agrin and its receptor LRP4 are both critical for NMJ formation and maintenance in patients with MG (32). Furthermore, Yu et al (33) revealed that anti-LRP4/Agrin antibodies in patients with MG are pathogenic; they impair the NMJ by interrupting Agrin-dependent LRP4-MuSK interactions. A multicenter study revealed that LRP4/Agrin antibody-positive patients with double-seronegative MG have more severe clinical disease than antibody-negative patients (34). These results suggest that Agrin-LRP4-MuSK signaling may be a potential therapeutic target for MG and other neuromuscular disorders (35). NT-1654 is a C-terminal fragment of mouse neural Agrin; it possesses the same mechanism of action as Agrin, by binding to LRP4 to activate MuSK protein Docking protein 7 signaling at the NMJ. It has also been demonstrated to induce AChR aggregation and alleviate a sarcopenia-like phenotype (36). Li et al (37) similarly reported that NT-1654 attenuates the clinical severity of this phenotype, effectively promotes AChR aggregation at the NMJ and attenuates the repair of NMJ transmission and the reduction of MuSK in rats with experimental autoimmune MG.
Agrin may also play an important role in other nerve-related diseases. Adult neurogenesis in the hippocampus may represent the plasticity of brain functions, including emotions, learning and memory. A decline in hippocampal neurogenesis is thought to cause emotional and cognitive deficits in aging and Alzheimer's disease. A recent study reported that neurogenesis in the brains of healthy individuals may be more conservative (38,39). Furthermore, Zhang et al (22) revealed that Agrin is upregulated in the hippocampus of mice stimulated by an enriched environment. The genetic deletion of AGRN in excitatory neurons decreases the proliferation of neural stem/progenitor cells and increases depressive-like behavior (22). A further analysis led to a working model in which Agrin activates ROR2 via LRP4 to promote adult neurogenesis.
Sepsis is another infection-induced neuromuscular dysfunction; it can induce denervation-like alterations in the NMJ, which may cause muscle weakness (40). Lv et al (41) reported that decreased Agrin expression may induce skeletal muscle dysfunction in sepsis, whereas exogenous Agrin alleviates neuromuscular dysfunction and downregulates γ- and α7-nicotinic AChR expression.
3. Role of Agrin in the tumor microenvironment
In the process of tumor progression, tumors make new capillaries by angiogenesis, and the targeting of angiogenesis contributes to tumor treatment (42). Localized benign tumors are surrounded by well-developed basement membranes, which limit tumors from migrating from the surrounding tissue into blood vessels. However, in malignant tumors, the tumor triggers an 'angiogenesis switch', thus tipping the balance between pro- and anti-angiogenic factors toward vascularization (43). Angiogenesis is a hallmark of cancer, and although multiple naturally occurring compounds have anti-angiogenic effects, these effects are not absolute (44,45). A recent study demonstrated that different protein modules within proteoglycans can enhance tumor cell plasticity and metastasis (46). However, the molecular mechanisms underlying their recruitment of blood vessels within the tumor niche remain largely elusive.
As a surface proteoglycan, the role of Agrin in promoting cancer angiogenesis has been demonstrated recently (7,47). He et al (47) reported a high expression of Agrin in cholangiocarcinoma tissue compared with that in adjacent non-tumor tissues; further analysis revealed that Agrin expression is associated with poorer prognosis, such as portal vein tumor thrombus and intrahepatic metastasis. Furthermore, forced Agrin expression in cholangiocarcinoma cells appears to promote tumor growth-related processes such as proliferation, colony formation, migration and invasion. In rectal cancer, Agrin expression has also been revealed to be markedly increased, and its upregulation is associated with poor prognosis. However, Agrin inhibition suppresses cell growth in rectal cancer, whereas Agrin overexpression prompts these behaviors in vitro (7). Mechanistically, multiple signaling pathways may participate in Agrin-regulated cancer progression. Agrin reportedly activates the Hippo signaling pathway and induces the translocation of yes-associated protein (YAP) to the nucleus in cholangiocarcinoma (47). However, Agrin also elevates Wnt pathway activity by increasing cyclin D1, c-Myc, phosphorylated glycogen synthase kinase-3β and phosphorylated β-catenin levels (7). These results indicate that Agrin may function as an oncogenic indicator of cancer progression through its activation of various pathways, which may be helpful for developing optimized therapies for cancer.
In the healthy liver, Agrin expression in hepatocytes is minimal and its expression is limited to certain regions surrounding blood vessels. However, during the transformation of liver cirrhosis and hepatocellular carcinoma, Agrin levels in hepatocytes are significantly increased (22). The role of Agrin in extracellular matrix sensing and mechanical conduction has been reported to integrate integrin and focal adhesion, as well as the activation of YAP/tafazzin (TAZ) to promote liver tumor growth (48,49). Furthermore, the depletion of Agrin in cancer cells reduces blood vessel infiltration and suppresses tumor growth, as well as the metastasis of hepatocellular carcinoma cells to mouse lungs. Strikingly, metastatic lesions that can colonize the lungs by Agrin deficiency lack the capacity to attract peripheral pulmonary vessels within these lesions (50).
Endothelial cell recruitment is critical for tumor vascularization. Njah et al (50) demonstrated that Agrin promotes the adherence of endothelial cells to tumor cells by recruiting blood vessels, and then facilitates tumor angiogenesis. Further analysis revealed that Agrin stabilizes vascular endothelial growth factor receptor 2 (VEGFR2) by enhancing its interactions with LRP4-integrin-b1-focal adhesion kinase (FAK) (Fig. 2). This suggests that tumor angiogenesis may be inhibited by targeting Agrin-induced VEGFR2 reductions. Furthermore, by inactivating the Hippo pathway, Agrin can promote extracellular matrix remodeling and stiffness by enhancing YAP/TAZ/transcriptional enhancer associated domain-dependent transcription, which then promotes tumorigenesis (49,51,52). Notably, consistent with other proteoglycans that affect endothelial cell migration, it has been reported that Agrin-either secreted by cancer cells or exogenously supplied - is essential for angiogenesis (53).
Figure 2.
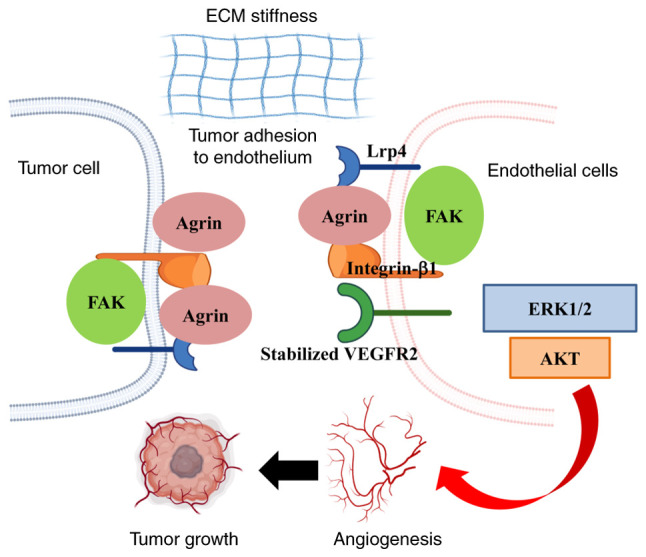
Agrin promotes adherence of endothelial cells to tumor cells and enhances tumor angiogenesis. The primary mechanism is extracellular matrix stiffness and Agrin stabilizes VEGFR2 by enhancing interactions with LRP4-Integrin-b1-FAK. Referenced by Njah et al (50) and recreated with BioRender.com. LRP4, low-density lipoprotein receptor-related protein 4, FAK, focal adhesion kinase; ECM, extracellular matrix.
In the context of oral squamous cell carcinoma progression, Agrin is upregulated in oral squamous cell carcinoma and promotes cell migration and adhesion, suggesting that Agrin also plays an oncogenic role in oral cancer (54). Agrin can be cleaved through protein hydrolysis to produce bioactive fragments (55). One of the cleavage products is the C-terminal fragment, which reportedly has a role in multiple pathological processes, including sarcopenia (56), renal dysfunction (57) and cancer (58). Rivera et al (59) reported that invasive oral carcinomas have higher Agrin expression than benign tissue. These results suggest that the presence of Agrin may promote cancer progression in the tumor microenvironment. In oral squamous cell carcinoma cells, Agrin can activate FAK by binding to integrins or dystroglycan complexes, and can then induce cell invasion and metastasis through growth factor receptor-bound protein 2, proto-oncogene tyrosine-protein kinase Src, extracellular regulated protein kinases and cyclin D (60,61). Nonetheless, the role of C-terminal fragment Agrin in angiogenesis needs to be further investigated in oral cancer, with a focus on tumor progression.
Other studies have reported that high Agrin expression is also associated with tumor progression and poor prognosis in hepatocellular carcinoma and lung adenocarcinoma (62,63). Agrin-positive staining can be used to identify patients with an increased risk of metastasis after surgery for lung adenocarcinoma, and may therefore be a valuable prognostic marker (62). Furthermore, the recurrence-free survival rate of Agrin-positive patients with hepatocellular carcinoma was reported to be significantly lower than that of Agrin-negative patients (63). The potential mechanism may be the result of Agrin-mediated tumor-related angiogenesis in the tumor microenvironment (53). Furthermore, Agrin knockdown in multiple human endothelial cell lines, including human umbilical vascular, human retinal, human dermal microvascular and human aortic endothelial cells, was observed to be associated with significantly reduced in vitro angiogenesis after treatment with soluble Agrin (50). However, in endothelial cell-specific Agrin knockout mice, normal endothelial and hematopoietic cell development was observed during embryogenesis (64). Of note, the growth of localized or metastatic cancer cells is not affected after their implantation into mice with Agrin-depleted endothelial cells. This finding suggests that Agrin may not play an important role in endothelial development during physiological and tumor-related angiogenesis; targeting endothelial-derived Agrin may therefore not be effective in inhibiting tumor angiogenesis.
Any discrepancies among these studies may be attributed to the availability of Agrin in vitro vs. in vivo, which may rescue the loss of Agrin in endothelial cells in AGRN-knockout mice during tumor angiogenesis. This suggests that in the early stages of tumor growth, endothelial cell recruitment does not require endothelial Agrin, as tumor-derived Agrin can compensate for the endothelial loss of Agrin. Thus, the profiles and mechanisms of Agrin in tumorigenesis need to be further studied.
4. Agrin-mediated cardiac regeneration
At present, heart disease is the leading cause of death world-wide, and repairing the damaged heart remains one of the most critical challenges in cardiovascular disease. In mammals, post-mitotic adult cardiomyocytes lose their proliferative capacity for replenishing damaged tissue (65). Upon injury, cardiomyocytes are replaced by fibrotic tissue, which usually has deleterious consequences. Hypertrophy therefore becomes responsible for most remaining heart growth. Notably, cardiomyocyte proliferation is sufficient for the repair of cardiac injury in neonatal mice; however, this ability is greatly diminished by 1 week after birth (66). In the past decade, however, researchers have questioned whether mature hearts truly lack the ability to produce new myocardium after injury; indeed, their findings suggest the possibility of a substantial endogenous regenerative capacity.
Studies have demonstrated that the proteoglycan Agrin can promote cardiomyocyte proliferation as an extracellular matrix component and is involved in neonatal heart repair (9,67). Of note, Agrin is reportedly enriched in the matrix on postnatal day 1 but decreased by postnatal day 7. Crucially, the administration of recombinant Agrin promotes cardiac regeneration in adult mice after myocardial infarction in vivo through the reactivation of cardiomyocyte proliferation. A porcine model of acute myocardial infarction, which is closely related to human cardiac physiology, has been used to demonstrate that recombinant human Agrin delivered to the infarcted heart can target the affected regions in an efficient and clinically relevant manner. These results indicate that recombinant Agrin may be a novel therapy for acute myocardial infarction and may prevent the onset of chronic heart failure (68). The data obtained from these mouse and porcine models highlight that Agrin treatment may strengthen pleiotropic effects and the broad cardiac repair process.
Agrin has been reported to bind and signal through α-dystroglycan (DAG1) (69) with C-terminus laminin G-like domains (LG1 and LG2) (60,70). In the heart, DAG1 serves as an Agrin receptor that is expressed by cardiomyocytes and mediates mild dedifferentiation and proliferation (9). The aforementioned reports emphasize the potential therapeutic role of Agrin in cardiac repair; this therapy is simple, safe and clinically relevant, and may be used in patients. However, further research is needed regarding the molecular mechanisms, effective dose ranges, toxicity and pharmacokinetics of Agrin.
The results from mouse (9) and porcine (68) myocardial infarction models that received local injections of recombinant Agrin into the damaged heart indicate that Agrin may have an overall cardioprotective effect. This was also accompanied by anti-inflammatory effects and blood vessel production, which adds to cardiomyocyte protection and induces proliferation. These findings suggest that Agrin has the potential to regulate cardiomyocyte cell proliferation. However, epicardial cells with multiple differentiation potential are also of great importance for cardiac regeneration (71). Normal development of the embryonic heart is reportedly regulated by epicardial cells through differentiation and proliferation (72,73). However, it remains unclear whether Agrin can promote the proliferation of embryonic epicardial cells. Jing et al (74) revealed that epicardial cell proliferation is promoted by Agrin through its regulation of YAP activity. Furthermore, YAP is key for the regulation of Hippo signaling (75), and Agrin has been reported to inhibit the Hippo signaling pathway and promote YAP activity (76). Although cardiac dysplasia can be induced by specific exfoliation of the embryonic epicardium (77), the abnormal epithelial-mesenchymal transition, migration and differentiation of epicardial cells can also be induced by proliferation disorders (78,79). The migration and epithelial-mesenchymal transition of epicardial cells are controlled by interactions between epicardial cells and the extracellular matrix (80,81). Thus, Agrin may play an important role in cardiac regeneration through its regulation of epicardial cells.
Considering the various risks and technical issues of current cardiac regenerative strategies, Agrin therapy has potential as a relatively safe and effective drug for repairing damaged hearts. However, caution is needed when performing this cardiac regenerative strategy because of the role of Agrin in tumorigenesis and angiogenesis. Furthermore, to evaluate the potential benefits of therapeutic Agrin, it should be considered whether Agrin can stimulate human cardiac fibroblast hyperproliferation. It would also be worthwhile to investigate whether Agrin has a similar mechanism of action for stimulating cardiomyocyte and cancer cell proliferation.
5. Agrin mediates cartilage formation
Cartilage loss leads to osteoarthritis; cartilage has a low turnover and often fails to repair after injury because it is devoid of blood vessels (82). Cartilage regeneration is therefore a priority in medicine. A study by Erickson et al (83) indicated that AGRN was initially upregulated throughout fracture healing, suggesting that Agrin may have a novel function in non-neural tissue, including cartilage. Consistent with a role for Agrin in skeletal development, a study by Hausser et al (84) demonstrated that Agrin is involved in postnatal skeletal development and endochondral bone formation in transgenic mice. Furthermore, chondrocytes have high Agrin expression in the growth plate, suggesting that the expression of Agrin in cartilage may have a critical role in normal skeletal growth (84).
Agrin is composed of a large N-terminal portion, which binds to components of the basal membrane, and a biologically active C-terminal portion, which contains three globular domains separated by epidermal growth factor-like repeats (85). Eldridge et al (86) reported that Agrin is expressed in a splice isoform without y and z motifs; they also identified Agrin as having strong therapeutic potential in cartilage regeneration. Agrin is expressed in normal cartilage but is progressively lost in osteoarthritis, and Agrin knockdown induces downregulation of the cartilage transcription factor SOX9, as well as other cartilage-specific extracellular matrix molecules. Conversely, cartilage differentiation in vitro and ectopic cartilage formation in vivo are supported by exogenous Agrin. These results suggest that Agrin plays an important role in chondrogenesis and the repair of osteochondral defects (87).
Reduced growth and impaired skeletal development have been observed in Agrin-null mice, thus indicating an important role for Agrin in chondrocyte biology (84). A study by Eldridge et al (87) also revealed that Agrin is upregulated in injured cartilage and induces chondrogenic differentiation in synovial membrane mesenchymal stem cells. A single intra-articular administration of Agrin induces the long-lasting regeneration of critical-size osteochondral defects by attracting joint-resident progenitor cells to the injury site. Furthermore, the simultaneous activation of cAMP responsive element-binding protein and the suppression of canonical Wnt signaling downstream of b-catenin are critical for inducing stable articular chondrocyte differentiation and the formation of stable articular cartilage. In addition, considering the close relationship between cartilage and bone, Agrin may be involved in the replacement of cartilage by bone; this process involves multiple steps and several components of the extracellular matrix (84,86,88,89).
Agrin plays an important role in chondrocyte biology and participates in multiple signal transduction pathways. The Wnt pathway has been well characterized, and is thus an attractive therapeutic target for bone repair and skeletal homeostasis (90). As described in the previous paragraph, β-catenin has various roles at different stages of bone repair and regulates the ratio of osteoblasts and chondrocytes in the callus, which arises from pluripotent mesenchymal stem cells in the early phases after injury (91). Later in the bone healing process, β-catenin induces osteoblast differentiation and osteoblastic matrix production (92). As the main receptor of Agrin, LRP4 is also involved in the modulation of Wnt signal transduction, which is an important pathway in osteogenic differentiation and bone formation (93-95). Souza et al (96) reported that Agrin and its receptors (LRP4 and DAG1) are expressed during the differentiation of osteoblasts from three different sources. Furthermore, Agrin disruption impairs the expression of its receptors, as well as osteoblast differentiation, and treatment with recombinant Agrin slightly improves this process. Agrin knockdown also downregulates the expression of genes related to Wnt and bone morphogenetic protein (BMP) signaling pathways. These results highlight the contribution of the Agrin-Wnt-BMP pathway to osteoblast differentiation and suggest that Agrin is a candidate target in the development of new therapeutic strategies for bone-related diseases and injuries (96).
Together, the aforementioned results indicate that Agrin may be an orchestrator of repair morphogenesis at the joint surface through its modulation of multiple signaling pathways. We therefore anticipate that it represents a unique therapeutic opportunity in the field of osteoarthritis, which opens new avenues of investigation for cartilage-regenerative medicine. However, in the clinic, cartilage defects are often associated with meniscal/ligament injury and are at times accompanied by osteoarthritis. Given that joint instability can be compromised in the presence of inflammation, it remains to be explored whether Agrin can induce cartilage regeneration under these conditions.
6. Role of Agrin in aging and disease
During aging, AChR clusters at the NMJ become fragmented and denervated. As the site of information exchange and storage between motor neurons and muscles, deleterious morphological, functional and molecular features are acquired in the NMJ with advancing age, and the NMJ ultimately degenerates (97). NMJ activity requires communication (through molecular mechanisms) among all three cellular components: The presynapse, postsynapse and postsynaptic currents. These three cellular components collaborate through synapse-associated molecules to regenerate adult NMJs following injuries that cause severed motor axons, postsynaptic current loss and muscle fiber atrophy (98). A study suggested that age-related NMJ decline is induced by compromised Agrin-LRP4-MuSK signaling (99). This highlights the interdependence between all three cellular components of the NMJ, as well as the roles of synapse-associated molecules, such as Agrin, LRP4 and MuSK receptors, in the maintenance and repair of adult NMJs. In this regard, a study has shown that Agrin deletion in a subset of adult motor neurons causes postsynaptic disintegration and motor axon degeneration, suggesting that synaptic molecules-including Agrin-remain essential in adult NMJs (100). This indicates that synaptic molecules that are essential for NMJ formation continue to play important functions in adulthood. Together, these findings suggest that age-related changes in NMJs may be mitigated by targeting these molecules. Studies that have assessed therapeutic potential following injury and in disease have demonstrated that Agrin and other integral components of the NMJ can repair age-related damage. Furthermore, following sciatic nerve crush surgery in young animals, administration of biologically active Agrin fragments into skeletal muscles can accelerate the rate of NMJ remodeling (36).
Sarcopenia is characterized by the loss of skeletal muscle mass, strength and function, and is a strong predictor of multiple adverse health outcomes, such as physical disability, hospitalization and mortality (101,102). During the neuromuscular remodeling process, the neuronal protease neurotrypsin proteolytically cleaves and inactivates AGRN, thus dissociating a 22-kDa C-terminal Agrin fragment (103). Recently, the C-terminal Agrin fragment concentrations in the circulation have emerged as a potential biomarker of skeletal muscle deterioration (104), which may signal the onset of sarcopenia.
Intriguingly, a study has demonstrated that appendicular lean mass, age and sex are significant explanatory factors for C-terminal Agrin fragment concentrations. In male individuals especially, there is a strong correlation between serum C-terminal Agrin concentrations and appendicular lean mass. Furthermore, vitamin D supplementation and physical exercise are significantly associated with lower C-terminal Agrin concentrations (105). This finding indicates that C-terminal Agrin fragments may be a potential marker for identifying sarcopenia in a subgroup of affected individuals in the future. Furthermore, the viability of the C-terminal Agrin fragment as a biomarker for sarcopenia has been confirmed in a variety of subpopulations (106). These results have also revealed that z-Agrin degradation during aging may contribute to NMJ pathology. Pratt et al (107) reported that plasma C-terminal Agrin fragment concentrations are significantly higher in sarcopenic individuals than in nonsarcopenic individuals, suggesting the potential relevance of C-terminal Agrin fragments as an accessible biomarker for skeletal muscle health. Together, these findings indicate that C-terminal Agrin fragments may be used for the diagnosis of sarcopenia, and may also have potential as an early indicator of denervation.
7. Functions of Agrin in other tissue and organs
In tissues such as the kidneys and lungs, Agrin plays a role in mechanotransduction by linking the cell cytoskeleton to other basement membrane components, including DAG1 and laminin-γ1 (108,109), through either direct binding or indirectly via integrins (60). Raats et al (110) concluded that the presence of Agrin in the glomerular capillary wall and its ultrastructural localization are involved in linking the podocyte cytoskeleton to the glomerular basement membrane. However, other studies have directly demonstrated the contribution of Agrin to glomerular basement membrane functionality in podocytes and have reported that the glomerular basement membrane shows normal renal function with no changes in glomerular architecture, suggesting that Agrin is not required for the establishment or maintenance of glomerular basement membrane architecture (111,112). Vestentoft et al (113) reported that the levels of extracellular matrix constituents, including Agrin, are significantly upregulated after rat liver injury, mainly in the second tier of defense. These findings suggest that Agrin may play an important role in the hepatic progenitor cell response process for tissue repair and indicate a potentially important biological role for Agrin in other tissues and organs; however, the detailed roles and mechanisms remain unclear.
Limbal stem cell deficiency is an ocular surface disorder that is caused by the decreased population of corneal epithelial stem or progenitor cells and their dysfunction, which leads to vision loss and corneal blindness (114-116). Corneal epithelium regeneration depends on limbal stem cells, which may contribute to maintaining corneal epithelium homeostasis (117). Hou et al (76) reported that Agrin promotes limbal stem cell proliferation in vitro and noted that Agrin accelerates the wound-healing rate of corneal epithelium by activating limbal stem-cell proliferation in vivo. Further analysis of the underlying mechanism revealed that Agrin can facilitate the nuclear translocation of YAP1 and the expression of cyclin D1 induced by YAP1 dephosphorylation, which subsequently promotes limbal stem cell proliferation (76).
Hematopoiesis is a dynamic process that refers to the production of all hematopoietic-lineage cells generated by multipotent hematopoietic stem cells (118). Among the extracellular matrix components, heparan sulfate proteoglycans reportedly play a crucial role in controlling the structural and functional organization of the bone marrow hematopoietic stem cell niche (119). Agrin is expressed by multipotent nonhematopoietic mesenchymal stem cells as well as by differentiated osteoblasts from the surface of the bone endometrium. Furthermore, Mazzon et al (69) reported that Agrin is a critical niche-derived signal that controls the survival and proliferation of hematopoietic stem cells, and demonstrated that, although Agrin-deficient mice display impaired hematopoiesis, this can be reverted by Agrin-sufficient stroma. These findings suggest that Agrin may play a crucial role in the hematopoietic niche and in the cross-talk between stromal and hematopoietic stem cells.
Wound healing represents a complex biological program for restoring damaged tissue architecture to a normal state (120). During this process, a major factor for effective wound healing following injury is the rate of deposition of new extracellular matrix and its components that favor the healing process, which may subsequently support keratinocyte proliferation, migration and angiogenesis (121). A clinical trial study by Chakraborty et al (122) revealed that the recombinant Agrin fragment has great potential to accelerate wound healing as a bio additive material, and enrichment of the proteoglycan Agrin occurs early in the wound microenvironment, which indicates that it is essential for healing. Importantly, Agrin enhances the actomyosin cables by sensing geometric stress and force after injury, and then completely alters the cytoskeletal structure. Furthermore, matrix metalloproteinase-12 (MMP12) has been identified as a downstream effector of Agrin (123), and the Agrin-MMP12 pathway integrates a broad range of mechanical stimuli to promote optimal mechanical biology for wound healing and the generation of pro-angiogenic parameters. Therefore, injury-triggered Agrin enrichment has been proposed to integrate a broad range of mechanical stimulation and enhanced mechanical sensation through MMP12 activity in keratinocytes, which ultimately favors wound healing.
8. Conclusions and perspectives
Although Agrin was initially identified as a factor that is critical for NMJ function, its function in other tissues-including cardiac regeneration, tumor growth and cartilage formation-implies a widespread role for this protein. Existing evidence suggests that Agrin function is associated with the regulation of tissue proliferation and regeneration. During this process, multiple fundamental signaling pathways that are modulated by Agrin are involved (3,9,22,74,76,86,87,122,124,125) (Table I), and different molecular mechanisms appear to play functional roles in different cell types. For instance, Agrin promotes epithelial-mesenchymal transition by decreasing β-catenin and promoting phosphorylated FAK localization at focal adhesions in human embryonic stem cell-derived epicardial-like cells, and enhances dystroglycan aggregation in the Golgi apparatus. The absence of Agrin leads to the dispersal of dystroglycan in vivo, thus disrupting basement membrane integrity and impairing epithelial-mesenchymal transition. Injury-triggered Agrin enrichment induces a broad range of signaling pathways in different tissues, which ultimately favors the development of an injury microenvironment. In summary, Agrin represents a unique therapeutic opportunity in regenerative medicine, and a new and exciting research avenue involves understanding how to achieve a high specificity of biological effects by regulating otherwise-pleiotropic signaling pathways. However, despite initial findings, the precise function of Agrin in repair and regeneration-at both the molecular and whole-organism levels-remains to be elucidated.
Table I.
Signaling network during Agrin-induced regeneration.
| Author(s), year | Cell type | Function | Targets | Mechanism | (Refs.) |
|---|---|---|---|---|---|
| Bassat et al, 2017 | Cardiomyocyte | Promotes division | DGC/YAP | Disassembly of the DGC, and Yap- and ERK-mediated signalling | (9) |
| Zhang et al, 2019 | NSPCs | Neurogenesis | LRP4/Ror2 | Promotes adult neurogenesis through proliferation and maturation of NSPCs | (22) |
| Jing et al, 2021 | Epicardial Cells | Promotes proliferation | YAP | Increases the expression of Ki67, pH3 and Aurora B through YAP | (74) |
| Hou et al, 2020 | Limbal stem cells | Promotes proliferation | YAP/Cyclin D1 | Facilitates the dephosphorylation of Yap1 and activates transcription of Cyclin D1 | (76) |
| Eldridge et al, 2016 | Chondrocyte | Differentiation | LRP4/DAG/DKK1 | Inhibition of WNT pathway and promotion of SOX9 expression | (86) |
| Eldridge et al, 2020 | Chondrocyte | Differentiation | LRP4/DAG/CaMKII | Inhibition of WNT signaling in a CREB-dependent manner | (87) |
| Chakraborty et al, 2021 | Keratinocytes | Enhances mechanically competent | MMP-12 | Overhauls cytoskeletal architecture via enhancing actomyosin cables | (122) |
| Chakraborty et al, 2018; Calvo et al, 2013 | Cancer cell | Carcinogenesis | MuSK | Inhibition of the Hippo pathway by promoting the FAK-ILK-PAK1 axis | (3,124) |
| Sun et al, 2021 | Epicardial cells | Regulator of EMT | β-catenin/pFAK | Enhances EMT by decreasing β-catenin and promoting pFAK localization and the aggregation of dystroglycan | (125) |
LRP4, low-density lipoprotein receptor-related protein 4, DKK1, dickkopf WNT signaling pathway inhibitor 1; DAG, dystroglycan; FAK-ILKPAK1, focal adhesion kinase-integrin linked kinase-p21 activated kinase; CaMKII, calcium/calmodulin-dependent protein kinase II; DGC, dystrophin-glycoprotein complex; Musk, muscle-associated receptor tyrosine kinase; NSPCs, neural stem/progenitor cells; YAP, yes-associated protein; EMT, epithelial-mesenchymal transition.
Supplementary Data
Acknowledgements
Not applicable.
Abbreviations
- LRP4
low density lipoprotein receptor-related protein 4
- MuSK
muscle-specific kinase
- NMJ
neuromuscular junction
- AChR
acetylcholine receptor
- NSPCs
neural stem/progenitor cells
- MG
myasthenia gravis
- DNMG
double-seronegative myasthenia gravis
- YAP
yes-associated protein
- VEGFR2
vascular endothelial growth factor receptor 2
- FAK
focal adhesion kinase
- ERK
extracellular regulated protein kinases
- CREB
cAMP responsive element binding protein
- BMP
bone morphogenetic protein
- MMP12
matrix metalloproteinase-12
Funding Statement
This work was supported by the Natural Science Foundation of Zhejiang Province (grant nos. LBY22H180006 and LY21H160048) and the Medical Science and Technology Project of Zhejiang Province (grant nos. 2021KY529 and 2022RC102).
Availability of data and materials
Not applicable.
Authors' contributions
YYM conceived the study. XL reviewed the literature and wrote the draft. YX interpreted the information and checked the manuscript. JXS and FG collected part of the information and produced the figures. YYM supervised the preparation of the study and revised the manuscript. All authors read and approved the final manuscript. Data authentication is not applicable.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Mao AS, Mooney DJ. Regenerative medicine: Current therapies and future directions. Proc Natl Acad Sci USA. 2015;112:14452–14459. doi: 10.1073/pnas.1508520112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tsim KW, Ruegg MA, Escher G, Kroger S, McMahan UJ. cDNA that encodes active agrin. Neuron. 1992;8:677–689. doi: 10.1016/0896-6273(92)90089-V. [DOI] [PubMed] [Google Scholar]
- 3.Chakraborty S, Hong W. Linking extracellular matrix agrin to the hippo pathway in liver cancer and beyond. Cancers (Basel) 2018;10:45. doi: 10.3390/cancers10020045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xie T, Xu G, Liu Y, Quade B, Lin W, Bai XC. Structural insights into the assembly of the agrin/LRP4/MuSK signaling complex. Proc Natl Acad Sci USA. 2023;120:e2300453120. doi: 10.1073/pnas.2300453120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Adamiok-Ostrowska A, Grzanka M, Czarnocka B. Agrin is a novel oncogenic protein in thyroid cancer. Oncol Lett. 2023;26:483. doi: 10.3892/ol.2023.14070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Han L, Shi H, Ma S, Luo Y, Sun W, Li S, Zhang N, Jiang X, Gao Y, Huang Z, et al. Agrin promotes non-small cell lung cancer progression and stimulates regulatory T cells via increasing IL-6 secretion through PI3K/AKT pathway. Front Oncol. 2022;11:804418. doi: 10.3389/fonc.2021.804418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang ZQ, Sun XL, Wang YL, Miao YL. Agrin promotes the proliferation, invasion and migration of rectal cancer cells via the WNT signaling pathway to contribute to rectal cancer progression. J Recept Signal Transduct Res. 2021;41:363–370. doi: 10.1080/10799893.2020.1811325. [DOI] [PubMed] [Google Scholar]
- 8.Sarig R, Rimmer R, Bassat E, Zhang L, Umansky KB, Lendengolts D, Perlmoter G, Yaniv K, Tzahor E. Transient p53-mediated regenerative senescence in the injured heart. Circulation. 2019;139:2491–2494. doi: 10.1161/CIRCULATIONAHA.119.040125. [DOI] [PubMed] [Google Scholar]
- 9.Bassat E, Mutlak YE, Genzelinakh A, Shadrin IY, Baruch Umansky K, Yifa O, Kain D, Rajchman D, Leach J, Riabov Bassat D, et al. The extracellular matrix protein agrin promotes heart regeneration in mice. Nature. 2017;547:179–184. doi: 10.1038/nature22978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li L, Xiong WC, Mei L. Neuromuscular junction formation, aging, and disorders. Annu Rev Physiol. 2018;80:159–188. doi: 10.1146/annurev-physiol-022516-034255. [DOI] [PubMed] [Google Scholar]
- 11.Oentaryo MJ, Tse AC, Lee CW. Neuronal MT1-MMP mediates ECM clearance and Lrp4 cleavage for agrin deposition and signaling in presynaptic development. J Cell Sci. 2020;133:jcs246710. doi: 10.1242/jcs.246710. [DOI] [PubMed] [Google Scholar]
- 12.Gautam M, Noakes PG, Moscoso L, Rupp F, Scheller RH, Merlie JP, Sanes JR. Defective neuromuscular synaptogenesis in agrin-deficient mutant mice. Cell. 1996;85:525–535. doi: 10.1016/S0092-8674(00)81253-2. [DOI] [PubMed] [Google Scholar]
- 13.Burgess RW, Nguyen QT, Son YJ, Lichtman JW, Sanes JR. Alternatively spliced isoforms of nerve- and muscle-derived agrin: Their roles at the neuromuscular junction. Neuron. 1999;23:33–44. doi: 10.1016/S0896-6273(00)80751-5. [DOI] [PubMed] [Google Scholar]
- 14.Lin W, Dominguez B, Yang J, Aryal P, Brandon EP, Gage FH, Lee KF. Neurotransmitter acetylcholine negatively regulates neuromuscular synapse formation by a Cdk5-dependent mechanism. Neuron. 2005;46:569–579. doi: 10.1016/j.neuron.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 15.Misgeld T, Kummer TT, Lichtman JW, Sanes JR. Agrin promotes synaptic differentiation by counteracting an inhibitory effect of neurotransmitter. Proc Natl Acad Sci USA. 2005;102:11088–11093. doi: 10.1073/pnas.0504806102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim N, Burden SJ. MuSK controls where motor axons grow and form synapses. Nat Neurosci. 2008;11:19–27. doi: 10.1038/nn2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lin W, Burgess RW, Dominguez B, Pfaff SL, Sanes JR, Lee KF. Distinct roles of nerve and muscle in postsynaptic differentiation of the neuromuscular synapse. Nature. 2001;410:1057–1064. doi: 10.1038/35074025. [DOI] [PubMed] [Google Scholar]
- 18.Yang X, Arber S, William C, Li L, Tanabe Y, Jessell TM, Birchmeier C, Burden SJ. Patterning of muscle acetylcholine receptor gene expression in the absence of motor innervation. Neuron. 2001;30:399–410. doi: 10.1016/S0896-6273(01)00287-2. [DOI] [PubMed] [Google Scholar]
- 19.Grabrucker S, Marizzoni M, Silajdzic E, Lopizzo N, Mombelli E, Nicolas S, Dohm-Hansen S, Scassellati C, Moretti DV, Rosa M, et al. Microbiota from Alzheimer's patients induce deficits in cognition and hippocampal neurogenesis. Brain. 2023;146:4916–4934. doi: 10.1093/brain/awad303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goncalves JT, Schafer ST, Gage FH. Adult neurogenesis in the hippocampus: From stem cells to behavior. Cell. 2016;167:897–914. doi: 10.1016/j.cell.2016.10.021. [DOI] [PubMed] [Google Scholar]
- 21.Ming GL, Song H. Adult neurogenesis in the mammalian brain: Significant answers and significant questions. Neuron. 2011;70:687–702. doi: 10.1016/j.neuron.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang H, Sathyamurthy A, Liu F, Li L, Zhang L, Dong Z, Cui W, Sun X, Zhao K, Wang H, et al. Agrin-Lrp4-Ror2 signaling regulates adult hippocampal neurogenesis in mice. Elife. 2019;8:e45303. doi: 10.7554/eLife.45303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang JF, Cao G, Koirala S, Reddy LV, Ko CP. Schwann cells express active agrin and enhance aggregation of acetylcholine receptors on muscle fibers. J Neurosci. 2001;21:9572–9584. doi: 10.1523/JNEUROSCI.21-24-09572.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yu J, Oentaryo MJ, Lee CW. Local protein synthesis of neuronal MT1-MMP for agrin-induced presynaptic development. Development. 2021;148:dev199000. doi: 10.1242/dev.199000. [DOI] [PubMed] [Google Scholar]
- 25.Uyen Dao TM, Barbeau S, Messeant J, Della-Gaspera B, Bouceba T, Semprez F, Legay C, Dobbertin A. The collagen ColQ binds to LRP4 and regulates the activation of the Muscle-Specific Kinase-LRP4 receptor complex by agrin at the neuromuscular junction. J Biol Chem. 2023;299:104962. doi: 10.1016/j.jbc.2023.104962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gao H, Zhao Z, Li J, Guo Z, Zhang F, Wang K, Bai X, Wang Q, Guan Y, Wang Y, et al. Platelet-rich plasma promotes skeletal muscle regeneration and neuromuscular functional reconstitution in a concentration-dependent manner in a rat laceration model. Biochem Biophys Res Commun. 2023;672:185–192. doi: 10.1016/j.bbrc.2023.05.123. [DOI] [PubMed] [Google Scholar]
- 27.Feng Z, Ko CP. Schwann cells promote synaptogenesis at the neuromuscular junction via transforming growth factor-beta1. J Neurosci. 2008;28:9599–9609. doi: 10.1523/JNEUROSCI.2589-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang BG, Quigley AF, Bourke JL, Nowell CJ, Myers DE, Choong PF, Kapsa RM. Combination of agrin and laminin increase acetylcholine receptor clustering and enhance functional neuromuscular junction formation In vitro. Dev Neurobiol. 2016;76:551–565. doi: 10.1002/dneu.22331. [DOI] [PubMed] [Google Scholar]
- 29.Ma L, Pan L, Liu W, Liu Y, Xiang X, Pan Y, Zhang X, Jin L. Agrin influences botulinum neurotoxin a-induced nerve sprouting via miR-144-agrin-MuSK signaling. Front Cell Dev Biol. 2020;8:15. doi: 10.3389/fcell.2020.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gilhus NE, Tzartos S, Evoli A, Palace J, Burns TM, Verschuuren JJGM. Myasthenia gravis. Nat Rev Dis Primers. 2019;5:30. doi: 10.1038/s41572-019-0079-y. [DOI] [PubMed] [Google Scholar]
- 31.Lazaridis K, Tzartos SJ. Autoantibody specificities in myasthenia gravis; implications for improved diagnostics and therapeutics. Front Immunol. 2020;11:212. doi: 10.3389/fimmu.2020.00212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yan M, Xing GL, Xiong WC, Mei L. Agrin and LRP4 antibodies as new biomarkers of myasthenia gravis. Ann N Y Acad Sci. 2018;1413:126–135. doi: 10.1111/nyas.13573. [DOI] [PubMed] [Google Scholar]
- 33.Yu Z, Zhang M, Jing H, Chen P, Cao R, Pan J, Luo B, Yu Y, Quarles BM, Xiong W, et al. Characterization of LRP4/agrin antibodies from a patient with myasthenia gravis. Neurology. 2021;97:e975–e987. doi: 10.1212/WNL.0000000000012463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rivner MH, Quarles BM, Pan JX, Yu Z, Howard JF, Jr, Corse A, Dimachkie MM, Jackson C, Vu T, Small G, et al. Clinical features of LRP4/agrin-antibody-positive myasthenia gravis: A multicenter study. Muscle Nerve. 2020;62:333–343. doi: 10.1002/mus.26985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ohno K, Ohkawara B, Ito M. Agrin-LRP4-MuSK signaling as a therapeutic target for myasthenia gravis and other neuromuscular disorders. Expert Opin Ther Targets. 2017;21:949–958. doi: 10.1080/14728222.2017.1369960. [DOI] [PubMed] [Google Scholar]
- 36.Hettwer S, Lin S, Kucsera S, Haubitz M, Oliveri F, Fariello RG, Ruegg MA, Vrijbloed JW. Injection of a soluble fragment of neural agrin (NT-1654) considerably improves the muscle pathology caused by the disassembly of the neuromuscular junction. PLoS One. 2014;9:e88739. doi: 10.1371/journal.pone.0088739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li Z, Li M, Wood K, Hettwer S, Muley SA, Shi FD, Liu Q, Ladha SS. Engineered agrin attenuates the severity of experimental autoimmune myasthenia gravis. Muscle Nerve. 2018;57:814–820. doi: 10.1002/mus.26025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kempermann G, Gage FH, Aigner L, Song H, Curtis MA, Thuret S, Kuhn HG, Jessberger S, Frankland PW, Cameron HA, et al. Human adult neurogenesis: Evidence and remaining questions. Cell Stem Cell. 2018;23:25–30. doi: 10.1016/j.stem.2018.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sorrells SF, Paredes MF, Cebrian-Silla A, Sandoval K, Qi D, Kelley KW, James D, Mayer S, Chang J, Auguste KI, et al. Human hippocampal neurogenesis drops sharply in children to undetectable levels in adults. Nature. 2018;555:377–381. doi: 10.1038/nature25975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li X, Sun B, Li J, Ye W, Li M, Guan F, Wu S, Luo X, Feng J, Jia J, et al. Sepsis leads to impaired mitochondrial calcium uptake and skeletal muscle weakness by reducing the micu1: Mcu protein ratio. Shock. 2023;60:698–706. doi: 10.1097/SHK.0000000000002221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lv B, Min S, Xie F, Yang J, Chen J. Alleviating sepsis-induced neuromuscular dysfunction linked with acetylcholine receptors by agrin. J Surg Res. 2019;241:308–316. doi: 10.1016/j.jss.2019.04.017. [DOI] [PubMed] [Google Scholar]
- 42.Abdalla A, Murali C, Amin A. Safranal inhibits angiogenesis via targeting HIF-1α/VEGF machinery: In vitro and Ex vivo insights. Front Oncol. 2022;11:789172. doi: 10.3389/fonc.2021.789172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hanahan D, Folkman J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell. 1996;86:353–364. doi: 10.1016/S0092-8674(00)80108-7. [DOI] [PubMed] [Google Scholar]
- 44.Abdalla Y, Abdalla A, Hamza AA, Amin A. Safranal prevents liver cancer through inhibiting oxidative stress and alleviating inflammation. Front Pharmacol. 2022;12:777500. doi: 10.3389/fphar.2021.777500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bouabdallah S, Al-Maktoum A, Amin A. Steroidal saponins: Naturally occurring compounds as inhibitors of the hallmarks of cancer. Cancers (Basel) 2023;15:3900. doi: 10.3390/cancers15153900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wiedmann L, De Angelis Rigotti F, Vaquero-Siguero N, Donato E, Espinet E, Moll I, Alsina-Sanchis E, Bohnenberger H, Fernandez-Florido E, Mulfarth R, et al. HAPLN1 potentiates peritoneal metastasis in pancreatic cancer. Nat Commun. 2023;14:2353. doi: 10.1038/s41467-023-38064-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.He M, Cheng C, Tu J, Ji SS, Lou D, Bai B. Agrin expression is correlated with tumor development and poor prognosis in cholangiocarcinoma. J Int Med Res. 2021;49:3000605211009722. doi: 10.1177/03000605211009722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chakraborty S, Lakshmanan M, Swa HL, Chen J, Zhang X, Ong YS, Loo LS, Akincilar SC, Gunaratne J, Tergaonkar V, et al. An oncogenic role of agrin in regulating focal adhesion integrity in hepatocellular carcinoma. Nat Commun. 2015;6:6184. doi: 10.1038/ncomms7184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chakraborty S, Njah K, Pobbati AV, Lim YB, Raju A, Lakshmanan M, Tergaonkar V, Lim CT, Hong W. Agrin as a mechanotransduction signal regulating YAP through the hippo pathway. Cell Rep. 2017;18:2464–2479. doi: 10.1016/j.celrep.2017.02.041. [DOI] [PubMed] [Google Scholar]
- 50.Njah K, Chakraborty S, Qiu B, Arumugam S, Raju A, Pobbati AV, Lakshmanan M, Tergaonkar V, Thibault G, Wang X, Hong W. A role of agrin in maintaining the stability of vascular endothelial growth factor receptor-2 during tumor angiogenesis. Cell Rep. 2019;28:949–965.e7. doi: 10.1016/j.celrep.2019.06.036. [DOI] [PubMed] [Google Scholar]
- 51.Bordeleau F, Mason BN, Lollis EM, Mazzola M, Zanotelli MR, Somasegar S, Califano JP, Montague C, LaValley DJ, Huynh J, et al. Matrix stiffening promotes a tumor vasculature phenotype. Proc Natl Acad Sci USA. 2017;114:492–497. doi: 10.1073/pnas.1613855114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Frye M, Taddei A, Dierkes C, Martinez-Corral I, Fielden M, Ortsater H, Kazenwadel J, Calado DP, Ostergaard P, Salminen M, et al. Matrix stiffness controls lymphatic vessel formation through regulation of a GATA2-dependent transcriptional program. Nat Commun. 2018;9:1511. doi: 10.1038/s41467-018-03959-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chakraborty S, Njah K, Hong W. Agrin mediates angiogenesis in the tumor microenvironment. Trends Cancer. 2020;6:81–85. doi: 10.1016/j.trecan.2019.12.002. [DOI] [PubMed] [Google Scholar]
- 54.Kawahara R, Granato DC, Carnielli CM, Cervigne NK, Oliveria CE, Rivera C, Yokoo S, Fonseca FP, Lopes M, Santos-Silva AR, et al. Agrin and perlecan mediate tumorigenic processes in oral squamous cell carcinoma. PLoS One. 2014;9:e115004. doi: 10.1371/journal.pone.0115004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Neill T, Schaefer L, Iozzo RV. Decoding the matrix: Instructive roles of proteoglycan receptors. Biochemistry. 2015;54:4583–4598. doi: 10.1021/acs.biochem.5b00653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Scherbakov N, Knops M, Ebner N, Valentova M, Sandek A, Grittner U, Dahinden P, Hettwer S, Schefold JC, von Haehling S, et al. Evaluation of C-terminal agrin fragment as a marker of muscle wasting in patients after acute stroke during early rehabilitation. J Cachexia Sarcopenia Muscle. 2016;7:60–67. doi: 10.1002/jcsm.12068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yu D, Li HX, Liu Y, Ying ZW, Guo JJ, Cao CY, Wang J, Li YF, Yang HR. The reference intervals for serum C-terminal agrin fragment in healthy individuals and as a biomarker for renal function in kidney transplant recipients. J Clin Lab Anal. 2017;31:e22059. doi: 10.1002/jcla.22059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sartori R, Hagg A, Zampieri S, Armani A, Winbanks CE, Viana LR, Haidar M, Watt KI, Qian H, Pezzini C, et al. Perturbed BMP signaling and denervation promote muscle wasting in cancer cachexia. Sci Transl Med. 2021;13:eaay9592. doi: 10.1126/scitranslmed.aay9592. [DOI] [PubMed] [Google Scholar]
- 59.Rivera C, Zandonadi FS, Sanchez-Romero C, Soares CD, Granato DC, Gonzalez-Arriagada WA, Paes Leme AF. Agrin has a pathological role in the progression of oral cancer. Br J Cancer. 2018;118:1628–1638. doi: 10.1038/s41416-018-0135-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bezakova G, Ruegg MA. New insights into the roles of agrin. Nat Rev Mol Cell Biol. 2003;4:295–308. doi: 10.1038/nrm1074. [DOI] [PubMed] [Google Scholar]
- 61.Sulzmaier FJ, Jean C, Schlaepfer DD. FAK in cancer: Mechanistic findings and clinical applications. Nat Rev Cancer. 2014;14:598–610. doi: 10.1038/nrc3792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Li D, Gu Q, Xie Z, Shen Q, Li H. Clinical significance of nuclear localisation of agrin in lung adenocarcinoma. Pol J Pathol. 2019;70:198–204. doi: 10.5114/pjp.2019.90396. [DOI] [PubMed] [Google Scholar]
- 63.Zhang QJ, Wan L, Xu HF. High expression of agrin is associated with tumor progression and poor prognosis in hepatocellular carcinoma. Math Biosci Eng. 2019;16:7375–7383. doi: 10.3934/mbe.2019368. [DOI] [PubMed] [Google Scholar]
- 64.Ye P, Fu Z, Chung JY, Cao X, Ko H, Tian XY, Tang PM, Lui KO. Endothelial agrin is dispensable for normal and tumor angiogenesis. Front Cardiovasc Med. 2022;8:810477. doi: 10.3389/fcvm.2021.810477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhu Y, Do VD, Richards AM, Foo R. What we know about cardiomyocyte dedifferentiation. J Mol Cell Cardiol. 2021;152:80–91. doi: 10.1016/j.yjmcc.2020.11.016. [DOI] [PubMed] [Google Scholar]
- 66.Porrello ER, Mahmoud AI, Simpson E, Hill JA, Richardson JA, Olson EN, Sadek HA. Transient regenerative potential of the neonatal mouse heart. Science. 2011;331:1078–1080. doi: 10.1126/science.1200708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zlatanova I, Sun F, Wu RS, Chen X, Lau BH, Colombier P, Sinha T, Celona B, Xu SM, Materna SC, et al. An injury-responsive mmp14b enhancer is required for heart regeneration. Sci Adv. 2023;9:eadh5313. doi: 10.1126/sciadv.adh5313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Baehr A, Umansky KB, Bassat E, Jurisch V, Klett K, Bozoglu T, Hornaschewitz N, Solyanik O, Kain D, Ferraro B, et al. Agrin promotes coordinated therapeutic processes leading to improved cardiac repair in pigs. Circulation. 2020;142:868–881. doi: 10.1161/CIRCULATIONAHA.119.045116. [DOI] [PubMed] [Google Scholar]
- 69.Mazzon C, Anselmo A, Cibella J, Soldani C, Destro A, Kim N, Roncalli M, Burden SJ, Dustin ML, Sarukhan A, Viola A. The critical role of agrin in the hematopoietic stem cell niche. Blood. 2011;118:2733–2742. doi: 10.1182/blood-2011-01-331272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Burgess RW, Dickman DK, Nunez L, Glass DJ, Sanes JR. Mapping sites responsible for interactions of agrin with neurons. J Neurochem. 2002;83:271–284. doi: 10.1046/j.1471-4159.2002.01102.x. [DOI] [PubMed] [Google Scholar]
- 71.Guadix JA, Orlova VV, Giacomelli E, Bellin M, Ribeiro MC, Mummery CL, Perez-Pomares JM, Passier R. Human pluripotent stem cell differentiation into functional epicardial progenitor cells. Stem Cell Reports. 2017;9:1754–1764. doi: 10.1016/j.stemcr.2017.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Germani A, Foglio E, Capogrossi MC, Russo MA, Limana F. Generation of cardiac progenitor cells through epicardial to mesenchymal transition. J Mol Med (Berl) 2015;93:735–748. doi: 10.1007/s00109-015-1290-2. [DOI] [PubMed] [Google Scholar]
- 73.Smits AM, Dronkers E, Goumans MJ. The epicardium as a source of multipotent adult cardiac progenitor cells: Their origin, role and fate. Pharmacol Res. 2018;127:129–140. doi: 10.1016/j.phrs.2017.07.020. [DOI] [PubMed] [Google Scholar]
- 74.Jing X, Liu B, Deng S, Du J, She Q. Agrin yes-associated protein promotes the proliferation of epicardial cells. J Cardiovasc Pharmacol. 2021;77:94–99. doi: 10.1097/FJC.0000000000000926. [DOI] [PubMed] [Google Scholar]
- 75.Sun K, Guo J, Guo Z, Hou L, Liu H, Hou Y, He J, Guo F, Ye Y. The roles of the hippo-YAP signalling pathway in cartilage and osteoarthritis. Ageing Res Rev. 2023;90:102015. doi: 10.1016/j.arr.2023.102015. [DOI] [PubMed] [Google Scholar]
- 76.Hou L, Fu W, Liu Y, Wang Q, Wang L, Huang Y. Agrin promotes limbal stem cell proliferation and corneal wound healing through hippo-yap signaling pathway. Invest Ophthalmol Vis Sci. 2020;61:7. doi: 10.1167/iovs.61.5.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Manner J, Schlueter J, Brand T. Experimental analyses of the function of the proepicardium using a new microsurgical procedure to induce loss-of-proepicardial-function in chick embryos. Dev Dyn. 2005;233:1454–1463. doi: 10.1002/dvdy.20487. [DOI] [PubMed] [Google Scholar]
- 78.Diman NY, Brooks G, Kruithof BP, Elemento O, Seidman JG, Seidman CE, Basson CT, Hatcher CJ. Tbx5 is required for avian and mammalian epicardial formation and coronary vasculogenesis. Circ Res. 2014;115:834–844. doi: 10.1161/CIRCRESAHA.115.304379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.van Wijk B, Gunst QD, Moorman AF, van den Hoff MJ. Cardiac regeneration from activated epicardium. PLoS One. 2012;7:e44692. doi: 10.1371/journal.pone.0044692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lan Y, Pan H, Li C, Banks KM, Sam J, Ding B, Elemento O, Goll MG, Evans T. TETs regulate proepicardial cell migration through extracellular matrix organization during zebrafish cardiogenesis. Cell Rep. 2019;26:720–732.e4. doi: 10.1016/j.celrep.2018.12.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Missinato MA, Tobita K, Romano N, Carroll JA, Tsang M. Extracellular component hyaluronic acid and its receptor Hmmr are required for epicardial EMT during heart regeneration. Cardiovasc Res. 2015;107:487–498. doi: 10.1093/cvr/cvv190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Verzijl N, DeGroot J, Thorpe SR, Bank RA, Shaw JN, Lyons TJ, Bijlsma JW, Lafeber FP, Baynes JW, TeKoppele JM. Effect of collagen turnover on the accumulation of advanced glycation end products. J Biol Chem. 2000;275:39027–39031. doi: 10.1074/jbc.M006700200. [DOI] [PubMed] [Google Scholar]
- 83.Erickson CB, Hill R, Pascablo D, Kazakia G, Hansen K, Bahney C. A timeseries analysis of the fracture callus extracellular matrix proteome during bone fracture healing. J Life Sci (Westlake Village) 2021;3:1–30. doi: 10.36069/JoLS/20220601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hausser HJ, Ruegg MA, Brenner RE, Ksiazek I. Agrin is highly expressed by chondrocytes and is required for normal growth. Histochem Cell Biol. 2007;127:363–374. doi: 10.1007/s00418-006-0258-2. [DOI] [PubMed] [Google Scholar]
- 85.Campanelli JT, Ferns M, Hoch W, Rupp F, von Zastrow M, Hall Z, Scheller RH. Agrin: A synaptic basal lamina protein that regulates development of the neuromuscular junction. Cold Spring Harb Symp Quant Biol. 1992;57:461–472. doi: 10.1101/SQB.1992.057.01.051. [DOI] [PubMed] [Google Scholar]
- 86.Eldridge S, Nalesso G, Ismail H, Vicente-Greco K, Kabouridis P, Ramachandran M, Niemeier A, Herz J, Pitzalis C, Perretti M, Dell'Accio F. Agrin mediates chondrocyte homeostasis and requires both LRP4 and α-dystroglycan to enhance cartilage formation in vitro and in vivo. Ann Rheum Dis. 2016;75:1228–1235. doi: 10.1136/annrheumdis-2015-207316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Eldridge SE, Barawi A, Wang H, Roelofs AJ, Kaneva M, Guan Z, Lydon H, Thomas BL, Thorup AS, Fernandez BF, et al. Agrin induces long-term osteochondral regeneration by supporting repair morphogenesis. Sci Transl Med. 2020;12:eaax9086. doi: 10.1126/scitranslmed.aax9086. [DOI] [PubMed] [Google Scholar]
- 88.Gentili C, Cancedda R. Cartilage and bone extracellular matrix. Curr Pharm Des. 2009;15:1334–1348. doi: 10.2174/138161209787846739. [DOI] [PubMed] [Google Scholar]
- 89.Grol MW, Lee BH. Gene therapy for repair and regeneration of bone and cartilage. Curr Opin Pharmacol. 2018;40:59–66. doi: 10.1016/j.coph.2018.03.005. [DOI] [PubMed] [Google Scholar]
- 90.Gomes KDN, Alves APNN, Dutra PGP, Viana GSB. Doxycycline induces bone repair and changes in Wnt signalling. Int J Oral Sci. 2017;9:158–166. doi: 10.1038/ijos.2017.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bao Q, Chen S, Qin H, Feng J, Liu H, Liu D, Li A, Shen Y, Zhao Y, Li J, Zong Z. An appropriate Wnt/β-catenin expression level during the remodeling phase is required for improved bone fracture healing in mice. Sci Rep. 2017;7:2695. doi: 10.1038/s41598-017-02705-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wang T, Zhang X, Bikle DD. Osteogenic Differentiation of Periosteal Cells During Fracture Healing. J Cell Physiol. 2017;232:913–921. doi: 10.1002/jcp.25641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ahn Y, Sims C, Murray MJ, Kuhlmann PK, Fuentes-Antras J, Weatherbee SD, Krumlauf R. Multiple modes of Lrp4 function in modulation of Wnt/β-catenin signaling during tooth development. Development. 2017;144:2824–2836. doi: 10.1242/dev.150680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Houschyar KS, Tapking C, Borrelli MR, Popp D, Duscher D, Maan ZN, Chelliah MP, Li J, Harati K, Wallner C, et al. Wnt pathway in bone repair and regeneration-what do we know so far. Front Cell Dev Biol. 2019;6:170. doi: 10.3389/fcell.2018.00170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Shen C, Xiong WC, Mei L. LRP4 in neuromuscular junction and bone development and diseases. Bone. 2015;80:101–108. doi: 10.1016/j.bone.2015.05.012. [DOI] [PubMed] [Google Scholar]
- 96.Souza ATP, Lopes HB, Oliveira FS, Weffort D, Freitas GP, Adolpho LF, Fernandes RR, Rosa AL, Beloti MM. The extracellular matrix protein Agrin is expressed by osteoblasts and contributes to their differentiation. Cell Tissue Res. 2021;386:335–347. doi: 10.1007/s00441-021-03494-9. [DOI] [PubMed] [Google Scholar]
- 97.Willadt S, Nash M, Slater C. Age-related changes in the structure and function of mammalian neuromuscular junctions. Ann N Y Acad Sci. 2018;1412:41–53. doi: 10.1111/nyas.13521. [DOI] [PubMed] [Google Scholar]
- 98.Taetzsch T, Tenga MJ, Valdez G. Muscle fibers secrete FGFBP1 to slow degeneration of neuromuscular synapses during aging and progression of ALS. J Neurosci. 2017;37:70–82. doi: 10.1523/JNEUROSCI.2992-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhao K, Shen C, Li L, Wu H, Xing G, Dong Z, Jing H, Chen W, Zhang H, Tan Z, et al. Sarcoglycan alpha mitigates neuromuscular junction decline in aged mice by stabilizing LRP4. J Neurosci. 2018;38:8860–8873. doi: 10.1523/JNEUROSCI.0860-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Samuel MA, Valdez G, Tapia JC, Lichtman JW, Sanes JR. Agrin and synaptic laminin are required to maintain adult neuromuscular junctions. PLoS One. 2012;7:e46663. doi: 10.1371/journal.pone.0046663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Benjumea AM, Curcio CL, Duque G, Gomez F. Dynapenia and sarcopenia as a risk factor for disability in a falls and fractures clinic in older persons. Open Access Maced J Med Sci. 2018;6:344–349. doi: 10.3889/oamjms.2018.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zhang X, Zhang W, Wang C, Tao W, Dou Q, Yang Y. Sarcopenia as a predictor of hospitalization among older people: A systematic review and meta-analysis. BMC Geriatr. 2018;18:188. doi: 10.1186/s12877-018-0878-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Stephan A, Mateos JM, Kozlov SV, Cinelli P, Kistler AD, Hettwer S, Rulicke T, Streit P, Kunz B, Sonderegger P. Neurotrypsin cleaves agrin locally at the synapse. FASEB J. 2008;22:1861–1873. doi: 10.1096/fj.07-100008. [DOI] [PubMed] [Google Scholar]
- 104.Kamiya K, Tachiki T, Sato Y, Kouda K, Kajita E, Tamaki J, Kagamimori S, Iki M. Association between the 110-kDa C-terminal agrin fragment and skeletal muscle decline among community-dwelling older women. J Cachexia Sarcopenia Muscle. 2023;14:2253–2263. doi: 10.1002/jcsm.13309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Drey M, Sieber CC, Bauer JM, Uter W, Dahinden P, Fariello RG, Vrijbloed JW, FiAT intervention group C-terminal Agrin Fragment as a potential marker for sarcopenia caused by degeneration of the neuromuscular junction. Exp Gerontol. 2013;48:76–80. doi: 10.1016/j.exger.2012.05.021. [DOI] [PubMed] [Google Scholar]
- 106.Racha P, Selvam S, Bose B, Bantwal G, Sambashivaiah S. Circulating C-terminal agrin fragment: A potential marker for sarcopenia among type 2 diabetes. Indian J Endocrinol Metab. 2022;26:334–340. doi: 10.4103/ijem.ijem_507_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Pratt J, De Vito G, Narici M, Segurado R, Pessanha L, Dolan J, Conroy J, Boreham C. Plasma C-terminal agrin fragment as an early biomarker for sarcopenia: Results from the GenoFit study. J Gerontol A Biol Sci Med Sci. 2021;76:2090–2096. doi: 10.1093/gerona/glab139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Denzer AJ, Brandenberger R, Gesemann M, Chiquet M, Ruegg MA. Agrin binds to the nerve-muscle basal lamina via laminin. J Cell Biol. 1997;137:671–683. doi: 10.1083/jcb.137.3.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Denzer AJ, Schulthess T, Fauser C, Schumacher B, Kammerer RA, Engel J, Ruegg MA. Electron microscopic structure of agrin and mapping of its binding site in laminin-1. EMBO J. 1998;17:335–343. doi: 10.1093/emboj/17.2.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Raats CJ, van den Born J, Bakker MA, Oppers-Walgreen B, Pisa BJ, Dijkman HB, Assmann KJ, Berden JH. Expression of agrin, dystroglycan, and utrophin in normal renal tissue and in experimental glomerulopathies. Am J Pathol. 2000;156:1749–1765. doi: 10.1016/S0002-9440(10)65046-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Goldberg S, Harvey SJ, Cunningham J, Tryggvason K, Miner JH. Glomerular filtration is normal in the absence of both agrin and perlecan-heparan sulfate from the glomerular basement membrane. Nephrol Dial Transplant. 2009;24:2044–2051. doi: 10.1093/ndt/gfn758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Harvey SJ, Jarad G, Cunningham J, Rops AL, van der Vlag J, Berden JH, Moeller MJ, Holzman LB, Burgess RW, Miner JH. Disruption of glomerular basement membrane charge through podocyte-specific mutation of agrin does not alter glomerular permselectivity. Am J Pathol. 2007;171:139–152. doi: 10.2353/ajpath.2007.061116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Vestentoft PS, Jelnes P, Andersen JB, Tran TA, Jorgensen T, Rasmussen M, Bornholdt J, Grovdal LM, Jensen CH, Vogel LK, et al. Molecular constituents of the extracellular matrix in rat liver mounting a hepatic progenitor cell response for tissue repair. Fibrogenesis Tissue Repair. 2013;6:21. doi: 10.1186/1755-1536-6-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Deng SX, Borderie V, Chan CC, Dana R, Figueiredo FC, Gomes JAP, Pellegrini G, Shimmura S, Kruse FE, The International Limbal Stem Cell Deficiency Working Group Global consensus on definition, classification, diagnosis, and staging of limbal stem cell deficiency. Cornea. 2019;38:364–375. doi: 10.1097/ICO.0000000000001820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kolli S, Ahmad S, Lako M, Figueiredo F. Successful clinical implementation of corneal epithelial stem cell therapy for treatment of unilateral limbal stem cell deficiency. Stem Cells. 2010;28:597–610. doi: 10.1002/stem.276. [DOI] [PubMed] [Google Scholar]
- 116.Whitcher JP, Srinivasan M, Upadhyay MP. Corneal blindness: A global perspective. Bull World Health Organ. 2001;79:214–221. [PMC free article] [PubMed] [Google Scholar]
- 117.Sacchetti M, Rama P, Bruscolini A, Lambiase A. Limbal stem cell transplantation: Clinical results, limits, and perspectives. Stem Cells Int. 2018;2018:8086269. doi: 10.1155/2018/8086269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ribatti D, d'Amati A. Hematopoiesis and Mast Cell Development. Int J Mol Sci. 2023;24:10679. doi: 10.3390/ijms241310679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Bruno E, Luikart SD, Long MW, Hoffman R. Marrow-derived heparan sulfate proteoglycan mediates the adhesion of hematopoietic progenitor cells to cytokines. Exp Hematol. 1995;23:1212–1217. [PubMed] [Google Scholar]
- 120.Sorg H, Sorg CGG. Skin wound healing: Of players, patterns, and processes. Eur Surg Res. 2023;64:141–157. doi: 10.1159/000528271. [DOI] [PubMed] [Google Scholar]
- 121.Xue M, Jackson CJ. Extracellular matrix reorganization during wound healing and its impact on abnormal scarring. Adv Wound Care (New Rochelle) 2015;4:119–136. doi: 10.1089/wound.2013.0485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Chakraborty S, Sampath D, Yu Lin MO, Bilton M, Huang CK, Nai MH, Njah K, Goy PA, Wang CC, Guccione E, et al. Agrin-matrix metalloproteinase-12 axis confers a mechanically competent microenvironment in skin wound healing. Nat Commun. 2021;12:6349. doi: 10.1038/s41467-021-26717-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Yu Lin MO, Sampath D, Bosykh DA, Wang C, Wang X, Subramaniam T, Han W, Hong W, Chakraborty S. YAP/TAZ drive agrin-matrix metalloproteinase-12 mediated diabetic skin wound healing. J Invest Dermatol. 2024 May 27; doi: 10.1016/j.jid.2024.05.005. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 124.Calvo F, Ege N, Grande-Garcia A, Hooper S, Jenkins RP, Chaudhry SI, Harrington K, Williamson P, Moeendarbary E, Charras G, Sahai E. Mechanotransduction and YAP-dependent matrix remodelling is required for the generation and maintenance of cancer-associated fibroblasts. Nat Cell Biol. 2013:637–646. doi: 10.1038/ncb2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Sun X, Malandraki-Miller S, Kennedy T, Bassat E, Klaourakis K, Zhao J, Gamen E, Vieira JM, Tzahor E, Riley PR. The extracellular matrix protein agrin is essential for epicardial epithelial-to-mesenchymal transition during heart development. Development. 2021;148:dev197525. doi: 10.1242/dev.197525. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.