Abstract
Clinical trials often demonstrate treatment efficacy through change in forced expiratory volume in one second (FEV1), comparing single FEV1 measurements from post-versus pre-treatment timepoints. Day-to-day variation in measured FEV1 is common for reasons such as diurnal variation and intermittent health changes, relative to a stable, monthly average. This variation can alter estimation of associations between change in FEV1 and baseline in predictable ways, through a phenomenon called regression to the mean. We quantify and explain day-to-day variation in percent-predicted FEV1 (ppFEV1) from 4 previous trials, and we present a statistical, data-driven explanation for potential bias in ceiling and floor effects due to commonly observed amounts of variation. We recommend accounting for variation when assessing associations between baseline value and change in CF outcomes in single-arm trials, and we consider possible impact of variation on conventional standards for study eligibility.
Keywords: Clinical Trials, measurement error, regression to the mean, eligibility, FEV
Rationale
Ceiling and floor effects for changes in lung function are frequently reported in the literature for people with cystic fibrosis (pwCF). Described ceiling effects include reduced improvement with therapeutic interventions for pwCF having high pre-treatment ppFEV1; and floor effects include diminished decline in pwCF with low baseline FEV1, a consideration which is most relevant for observational studies (1, 2, 3, 4). We demonstrate here conceptually that individual day-to-day variation in ppFEV1 has the potential to influence assessments of ceiling and floor effects, and we particularly focus on ceiling effects which are relevant to clinical trial eligibility. Concepts described are relevant to continuous outcomes (such as ppFEV1) when assessing the association between baseline value as a covariate and change as an outcome; for example, evaluation of the association between pre-treatment ppFEV1 and treatment effect based on change from a single pre-treatment ppFEV1 to a single post-treatment ppFEV1.
Methods
We evaluated multiple studies involving the collection of repeat spirometry over a short window and assessed the degree of variation present when measuring ppFEV1 (see footnote to Table 1 for descriptions) (5, 6, 7, 8, 9). We considered windows no longer than 30 days, to identify a steady state period in which the natural decline of ppFEV1 would be minimal. We selected 30 days as a steady state period, in which any potential natural decline in ppFEV1 would be anticipated to be very small, only ~1/12th as high as the recorded annual decline of ~1.5% per year; with decline likely further diminished with modulator use (10, 11). Spirometry measurements for all studies excluded pulmonary exacerbations (footnote to Table 1). To determine the relative amount of day-to-day variation, we performed variance components analysis (12). With a single linear mixed effects model incorporating only an intercept and a random term for individual, and using the R package ‘lme4’ and function ‘VarCorr’, we estimated between person and within-person, or day-to-day variation (13).
Table 1.
Estimates of variation in ppFEV1 from several studies, when periods of exacerbations are excluded.
| Study | Collection | Persons | Window (days) |
Total measures/Possible (%) |
Measures per person median (range) |
|||
|---|---|---|---|---|---|---|---|---|
| Thornton (5) | Home | 13 | 14 | 151/182 (83%) | 12 (5, 14) | 6.3 | 20.0 | 9.8% |
| Thornton (5) | Home | 13 | 30 | 314/390 (81%) | 25 (11, 30) | 5.9 | 19.5 | 9.1% |
| e-ICE* (7) | Home | 133 | 30 | 901/1140 (79%) | 8 (1, 12) | 8.7 | 21.6 | 16.3% |
| AZM-0001 (8) | Clinic | 185 | 14 | 370/370 (100%) | 2 (2, 2) | 3.5 | 22.3 | 2.5% |
| PROMISE (6) | Home | 202 | 30 | 404/404 (100%) | 2 (2,2) | 4.1 | 20.0 | 4.2% |
Studies: Thornton conducted a long-term study of the airway microbiome in 13 persons. e-ICE (Early Intervention in Cystic Fibrosis Exacerbation) studied whether home spirometry would speed detection of exacerbations. AZM-0001 was a RCT of azithromycin treatment for chronic Pseudomonas infection. And PROMISE is an ongoing observational study of changes with CFTR modulator initiation. Window is the maximum time between all included measures per person, = between person standard deviation, = within person or day-to-day standard deviation. * indicates those persons randomized to do frequent home spirometry (early intervention arm). Study-specific definitions of exacerbations were use of intravenous antibiotics (e-ICE, AZM-0001) and clinical decision (Thornton, PROMISE).
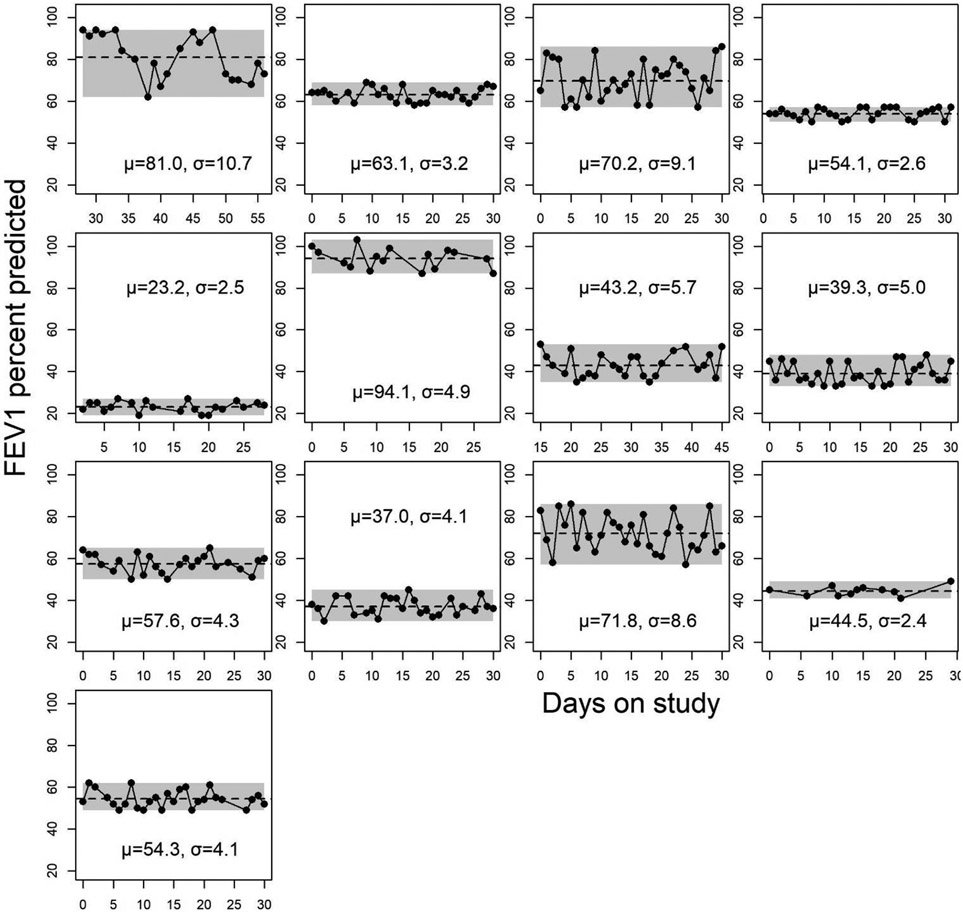
Within one study of 13 participants with especially frequent, near-daily home spirometry, we also graphed ppFEV1 during 30 contiguous days of observation (5, 9).
Results
A. Individual day-to-day variability is observed in CF home spirometry
Figure 1 shows all 13 participant’s measurements from the Thornton study over ~30 contiguous study days, along with their steady state means and day-to-day standard deviations about that mean (5, 9). Participants contributed a mean of 25 measurements each (total 314 measurements), and individual level standard deviations averaged 5.2 (range 2.4 to 10.7). Over all 4 studies, the day-to-day variation from home spirometry was estimated at 4.2% to 16.3% as high as between-person variation (Table 1). Day-to-day variation was lower using spirometry from the clinic (2.5%).
Figure 1.
The first 30 days (excluding exacerbations) from the 13 pwCF who participated in Thornton’s study of near-daily home spirometry, along with their individual-level steady state means and standard deviations . The best measurement from each day was used for the analyses, and spirometry collected during and 14 days before and after episodic antibiotic treatment for pulmonary exacerbation was excluded. Shaded areas show range of min and max ppFEV1 over the interval. Average within-person standard deviation was 5.2 ppFEV1 and ranged from 2.4 to 10.7.
B. Change to next measure is associated with current value
Figure 2a-c shows the day-to-day differences in ppFEV1 from a single study participant from the above study, where differences were computed from current ppFEV1 to the subsequent ppFEV1. Overall, (Figure 2a) the average of the day-to-day differences depicted by the arrows is −0.9, close to zero. When only the ppFEV1 measures above the mean of 94.1 are selected (filled circles), the differences from current to the subsequent ppFEV1 average −6.0, indicating that, on average, values above the mean tend to decline (Figure 2b). Similarly, when only the ppFEV1 measures below the mean of 94.1 are selected (open circles), the differences to the subsequent ppFEV1 average +5.0, tending to increase. This statistical phenomenon, long observed, is called regression to the mean, where extreme values tend to resolve (14, 15, 16).
Figure 2.
Using spirometry from a single individual in figure 1, an example of how change from current to next is associated with current level. This is way of visualizing regression to the mean.
C. Measurements above a fixed lung function threshold are more likely to be above individual-level steady state
Next, we evaluated the 314 ppFEV1 measurements comprising the first 30 days of all 13 participants and compared to a fixed ppFEV1 threshold of 70. This threshold is often used to dichotomize baseline FEV1 levels when examining their association with treatment (17, 18). Of the 67 measurements above the ppFEV1 threshold of 70, 72% were also above the individual steady state mean for that person. In contrast, of the 247 measurements below 70, only 43% were above the individual steady state means. We repeated the computation at the 90% threshold (a common clinical trial cut point) and found very similar results to the 70% threshold.
D. Combining these concepts, an apparent ceiling effect is anticipated
In the presence of day-to-day variation in ppFEV1 (paragraph A), persons having a high single ppFEV1 measurement are likely to be above their individual steady state (paragraph C). In the absence of a change in health or an intervention, the subsequent ppFEV1 measure is likely to be lower due to the inherent variability of lung function which leads to regression to the mean (paragraph B).
Now imagine the context of an effective therapeutic intervention, in which ppFEV1 is measured once before and once after treatment, a common procedure in trials. An individual with a high pre-treatment ppFEV1 is likely to experience these opposing influences over the subsequent treatment period: a) the treatment benefit (ppFEV1 increase) as well as b) the regression to steady state (ppFEV1 decrease). A high pre-treatment ppFEV1 in the context of day-to-day variation results in a diminished estimated treatment effect, an apparent ceiling effect. This flattening is observed regardless of biological association between current lung health and treatment benefit and is a consequence of the associations previously described.
E. Does day-to-day variation impact study findings?
In related work (not shown here), some of us recently completed a simulation study to investigate the degree to which day-to-day variation impacts measures of association with the known variation described above (19). That simulation addresses potential bias in tests for association between baseline value and the outcome of change in any continuous measure of CF-related health (e.g., ppFEV1, sweat chloride, etc.). The findings are relevant for uncontrolled study designs (single-arm, or observational), but not controlled studies, as the comparison between arms nullifies the variation-related bias in each. Even for ratios as low as 3% , we found the potential for ceiling and floor effects to be exaggerated. Potential amelioratory approaches include averaging multiple baseline and follow-up FEV1 measurements, as well as regression approaches (by Yanez et al. and Chambless et al.) that account for variation and remove bias (14, 20, 21).
F. Limitations
While ppFEV1 is less variable in clinic than at home, both home and in-clinic spirometry data demonstrate that day-to-day variation in ppFEV1 is ubiquitous. Measured variation is the result of a combination of factors, only some of which can be addressed in study design and are especially influential in-home spirometry: testing environment, device, coaching, diurnal variation, recency of treatment. Other sources of variation are pervasive and include sleep, exercise, effort, and mild infections. We know theoretically that we can reduce day-to-day variation by gathering more frequent samples pre- and post-intervention, as the standard deviation of the average of samples is . However, clinical trials to date have not included frequent, repeated FEV1 measurements over multiple days, either at home or in clinic. Therefore, we have not been able to directly test whether computing steady state ppFEV1 over a longer sampling period, both before and after treatment, results in a diminishment of apparent ceiling effect.
G. Summary
PwCF with high ppFEV1 values have historically been excluded from clinical trials of therapeutic interventions, with the anticipation that they have little room for improvement even if exposed to a drug with high effectiveness. Their exclusion has been founded, in part, on perceived ceiling effects that may have some basis in biological mechanisms, but which may also be influenced by the statistical phenomenon of regression to the mean. Should treatment benefit be, in truth, more equitable across baseline health, these eligibility exclusions may be unjustified. These trial exclusions for high ppFEV1 may also increasingly limit study enrollment, as highly effective CFTR modulators improve lung function in pwCF. Caution and examination of day-to-day variation is needed when ascribing a biological foundation for ceiling effects. Bias-corrective measures for day-to-day variation are available and should be implemented (19, 20, 21).
Highlights.
Ceiling effects in therapeutic benefit are commonly understood to impact cystic fibrosis.
Day-to-day variation in measured FEV1 is common.
Floor and ceiling effects can be exaggerated in the presence of measurement error.
Estimation of ceiling effects should account for biasing effects of measurement error.
Acknowledgments:
The authors wish to thank study investigators, staff and participants from the Thornton study (PI John J. LiPuma), e-ICE, AZM-0001 and PROMISE for their contributions, particularly Dave Nichols for permitting the use of accruing PROMISE data. Funding was provided by the grants from the Cystic Fibrosis Foundation, primarily GOSS22Y0 and CAVERL20Y5, and by NIH grants 5R01-HL-136647.
Footnotes
Credit Author Statement
Amalia S. Magaret: data curation, formal analysis, methodology, writing-original draft. Ellen Graham: methodology, writing-review and editing. Lindsay J. Caverly: funding acquisition, investigation, writing-review and editing. Elizabeth A. Cromwell: writing-review and editing. Alex Paynter: conceptualization, methodology, writing-review and editing. Margaret Rosenfeld: investigation, writing-review and editing. Christina S. Thornton: data curation, funding acquisition, investigation, writing-review and editing. Christopher H. Goss: funding acquisition, writing-review and editing.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:
Related to other projects, CHG has received consultancy, honoraria and/or travel fees from Enterprise Therapeutics, Gilead Sciences, and Vertex Pharmaceuticals. LJC, EAC, EG, ASM, AP, MR, CST report only CFF and/or NIH grants related to the work.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rowe SM, Heltshe SL, Gonska T, Donaldson SH, Borowitz D, Gelfond D, et al. Clinical mechanism of the cystic fibrosis transmembrane conductance regulator potentiator ivacaftor in G551D-mediated cystic fibrosis. Am J Respir Crit Care Med. 2014;190(2):175–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Szczesniak R, Heltshe SL, Stanojevic S, Mayer-Hamblett N. Use of FEV1 in cystic fibrosis epidemiologic studies and clinical trials: A statistical perspective for the clinical researcher. J Cyst Fibros. 2017;16(3):318–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harun SN, Wainwright C, Klein K, Hennig S. A systematic review of studies examining the rate of lung function decline in patients with cystic fibrosis. Paediatr Respir Rev. 2016;20:55–66. [DOI] [PubMed] [Google Scholar]
- 4.Konstan MW, Wagener JS, Vandevanter DR, Pasta DJ, Yegin A, Rasouliyan L, et al. Risk factors for rate of decline in FEV1 in adults with cystic fibrosis. J Cyst Fibros. 2012;11(5):405–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thornton C, Magaret A, Carmodya L, Kalikina L, Simon R, LiPuma J, et al. Quantifying variation in home spirometry in people with cystic fibrosis during baseline health, and associations with clinical outcomes. Journal of Cystic Fibrosis. 2023;in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nichols DP, Rowe SM. Spirometry data from ongoing PROMISE study (NCT04038047). 2023. [Google Scholar]
- 7.Paynter A, Khan U, Heltshe SL, Goss CH, Lechtzin N, Hamblett NM. A comparison of clinic and home spirometry as longtudinal outcomes in cystic fibrosis. J Cyst Fibros. 2022;21(1):78–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saiman L, Marshall BC, Mayer-Hamblett N, Burns JL, Quittner AL, Cibene DA, et al. Azithromycin in patients with cystic fibrosis chronically infected with Pseudomonas aeruginosa: a randomized controlled trial. JAMA. 2003;290(13):1749–56. [DOI] [PubMed] [Google Scholar]
- 9.Widder S, Carmody LA, Opron K, Kalikin LM, Caverly LJ, LiPuma JJ. Microbial community organization designates distinct pulmonary exacerbation types and predicts treatment outcome in cystic fibrosis. Nat Commun. 2024;15(1):4889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Szczesniak R, Andrinopoulou ER, Su WJ, Afonso PM, Burgel PR, Cromwell E, et al. Lung Function Decline in Cystic Fibrosis Impact of Data Availability and Modeling Strategies on Clinical Interpretations. Annals of the American Thoracic Society. 2023;20(7):958–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Muilwijk D, Zomer-van Ommen DD, Gulmans VAM, Eijkemans MJC, van der Ent CK, Dutch Cystic Fibrosis Registry Steering G. Long-term effectiveness of dual CFTR modulator treatment of cystic fibrosis. ERJ Open Res. 2022;8(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Neter J, Wasserman W, Kutner MH. Applied linear statistical models : regression, analysis of variance, and experimental designs. 3rd ed. Homewood, IL: Irwin; 1990. xvi, 1181 pages p. [Google Scholar]
- 13.Bates D, Mächler M, Bolker BM, Walker SC. Fitting Linear Mixed-Effects Models Using lme4. Journal of Statistical Software. 2015;67(1):1–48. [Google Scholar]
- 14.Barnett AG, van der Pols JC, Dobson AJ. Regression to the mean: what it is and how to deal with it. Int J Epidemiol. 2005;34(1):215–20. [DOI] [PubMed] [Google Scholar]
- 15.Stigler SM. Regression towards the mean, historically considered. Stat Methods Med Res. 1997;6(2):103–14. [DOI] [PubMed] [Google Scholar]
- 16.Stanojevic S, Filipow N, Ratjen F. Paediatric reproducibility limits for the forced expiratory volume in 1 s. Thorax. 2020;75(10):891–6. [DOI] [PubMed] [Google Scholar]
- 17.Heijerman HGM, McKone EF, Downey DG. Efficacy and safety of the elexacaftor plus tezacaftor plus ivacaftor combination regimen in people with cystic fibrosis homozygous for the F508del mutation: a double-blind, randomised, phase 3 trial. Lancet. 2019;394:1940–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Middleton PG, Mall MA, Drevinek P. Elexacaftor–Tezacaftor–lvacaftor for Cystic Fibrosis with a Single Phe508del Allele. New Engl J Med. 2019;381(19):1809–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Graham E, Magaret A. Baseline-dependent improvement in cystic fibrosis studies, plausibility of bias. Contemporary Clinical Trials Communications; 2024; in press. [Google Scholar]
- 20.Chambless LE, Davis V. Analysis of associations with change in a multivariate outcome variable when baseline is subject to measurement error. Statistics in Medicine. 2003;22(7):1041–67. [DOI] [PubMed] [Google Scholar]
- 21.Yanez ND 3rd, Kronmal RA, Shemanski LR. The effects of measurement error in response variables and tests of association of explanatory variables in change models. Stat Med. 1998;17(22):2597–606. [DOI] [PubMed] [Google Scholar]