Abstract
Background
Despite the interest from the scientific community and regulatory agencies, limited data are available on the association between health-related quality-of-life (QoL) results, outcome of efficacy and drug approvals.
Materials and methods
We updated the previously published meta-research study of phase III clinical trials in patients with solid tumours treated with systemic treatments, published from 2012 to 2021 in 11 selected journals. For the present analysis, we focused on studies conducted in the advanced setting. The primary outcome was the association of global QoL results with study primary endpoints (EP1), overall survival (OS) and progression-free survival (PFS), while a secondary outcome was the frequency of positive global QoL results among treatments approved by regulatory agencies [European Medicines Agency (EMA)/Food and Drug Administration (FDA)]. A descriptive analysis was carried out and the association between QoL results and characteristics of studies and of publications was tested.
Results
Five hundred and ninety-two eligible publications were identified from 2012 to 2021. The primary endpoint was OS in 298 clinical trials (50.3%) and PFS in 304 clinical trials (51.4%). A positive result in EP1 analysis was reported in 124 trials (41.6%) with OS as EP1 and in 182 trials (59.5%) with PFS as EP1. Among studies with positive OS and PFS, global QoL results were positive in 39 (31.5%) and 45 studies (24.7%), respectively. FDA and EMA approvals were available for 143 (24.2%) and 142 studies (24%), respectively. Among these, global QoL results were positive in 55 (38.5%) and 56 studies (39.4%), respectively. QoL results were available for most drugs approved by regulatory agencies, but the proportion of approvals with positive global QoL results was not significantly increased from 2012-2016 to 2017-2021.
Conclusions
Despite QoL data being available for most cancer treatments recently approved by regulatory agencies, QoL improvement has been demonstrated in a minority of studies with positive results in the primary endpoint.
Key words: quality of life, overall survival, progression-free survival, drug approvals
Graphical abstract
Highlights
-
•
Positive results in global QoL were identified in 31.5% and 24.7% of studies with positive OS and PFS, respectively.
-
•
Studies sponsored by industries and published in journals with high impact factor reported more frequently a QoL benefit.
-
•
Most trials corresponding to approval by regulatory agencies have available QoL results.
-
•
Less than 40% of trials leading to regulatory approval have reported an improvement in global QoL.
Introduction
In recent years, the scientific community has emphasized the role of health-related quality of life (QoL) in oncology. Patient-reported outcomes (PROs) provide the patient’s perspective on the impact of cancer symptoms and treatments, implementing the conventional investigator-assessed measures of efficacy and toxicity with a subjective point of view.
Defining the appropriate endpoints for research questions and disease settings is a crucial step in statistical design of clinical trials, leading to a methodologically correct structure of primary hypothesis and so to a proper measure of the benefit of the experimental therapeutic strategy. Overall survival (OS) and QoL are considered the most relevant endpoints in oncology clinical trials.1,2 QoL has a complementary role in the assessment of treatment efficacy, especially when progression-free survival (PFS), which is a measure of instrumental benefit, is identified as the primary endpoint.2,3 Similarly, the availability of QoL data within non-inferiority studies is crucial for interpretation of results, guiding clinical decisions in favour of treatment with the greatest benefit on QoL.3,4 The European Medicines Agency (EMA) and the Food and Drug Administration (FDA) encouraged the use of PROs in oncology clinical trials with dedicated recommendations.5,6
Despite the interest from the scientific community and regulatory agencies, several systematic reviews have shown that the adoption of QoL among the endpoints in oncology clinical trials and the reporting of QoL results are still suboptimal.7, 8, 9, 10, 11, 12 In a 10-year analysis, the adoption of QoL in the endpoints has increased in recent years, especially in sponsored trials. Nevertheless, this systematic review showed a decrease through time in reporting QoL results in primary publications.11
Recently, a systematic review of 45 phase III trials published in 2019 reported an improvement in QoL in only a quarter of the trials that tested oncology drugs in the advanced setting.10 In this analysis, a QoL benefit was more frequently associated with an OS improvement (P = 0.04), instead of PFS (P = 0.87). Nearly half of the trials that demonstrated a greater efficacy of experimental arm compared to standard of care in terms of PFS superiority did not show improvement in OS or QoL. This study also emphasizes the importance of a careful interpretation of QoL results, especially for articles that report favourable presentation of QoL data for experimental treatment with the absence of statistically significant difference or worsening of QoL between arms.10 Two systematic reviews published in 2018 and 2019 failed to show a significant association between PFS prolongation and QoL improvement.13,14 Unfortunately, the assumption that patients who live longer without disease progression will experience better QoL is not always true, and PFS is not a valid surrogate for extrapolating QoL. A retrospective cohort study of EMA drug approvals from 2009 to 2013 showed that 57% of approved cancer treatments were not associated with an improvement of OS or QoL.15
Limited data are available in literature about the presence of positive QoL in studies with positive efficacy outcomes associated with experimental treatments, and the available case series regard few clinical studies in a very selected time period. Even less data are available on the presence of positive QoL among treatments approved in the last decade.
With the aim of assessing the association of QoL results with study primary endpoints and with EMA/FDA approvals in clinical trials in advanced cancer setting in a decade, we updated the previously published meta-research study of phase III randomized clinical trials in patients with solid neoplasms treated with systemic therapy, published from 2012 to 2021.11
Materials and methods
Search strategy and selection criteria
For the present retrospective cohort study, we used the database of a previously published meta-research study,11 updating the records with new details about primary endpoints, QoL results and regulatory approvals. The present analysis was focused on studies conducted in patients with locally advanced and metastatic disease. Inclusion criteria, for the exception of the focus on advanced setting, were the same as the previous analysis: phase III randomized controlled trials testing anticancer treatments in adults affected by solid tumour (≥18 years) and published in English in 11 selected journals from 2012 to 2021 were included. Non-pharmacological approaches, supportive care and haematological neoplasms were excluded.
Data were collected from the previously selected articles. For the list of records, we included only primary publications of the clinical trials. Secondary QoL publications were searched on PubMed or on major search engines, using the name or acronym of the clinical trial, Clinicaltrials.gov identifier or authors’ names of the primary publication. In addition, any updates in QoL data presentations were collected from the European Society for Medical Oncology and American Society of Clinical Oncology conference websites for the studies included in the analysis.
The study was conducted following the recommendations of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) (Supplementary Figure S1, available at https://doi.org/10.1016/j.esmoop.2024.103654).
Data analysis
All variables were categorized. According to the Journal Citation Reports and the year of publication, we conventionally categorized the articles into three impact factor (IF) categories: low (<15), intermediate (15-30) and high (>30). Experimental treatments were classified into four categories: chemotherapy, targeted therapies, hormonal therapies and immunotherapy. Endpoint details and QoL data were collected from the paper, protocol and supplementary material.
Studies were classified into two groups according to the primary endpoint (OS or PFS). Primary endpoint results were considered positive in case of a significant advantage for experimental treatment compared to the control arm. The QoL results were obtained from the primary and/or secondary publications (and meeting presentations) and were divided into four categories: absent (if not available), negative (statistically significant worse QoL result in the experimental arm), positive (statistically significant better QoL in the experimental arm) and not different QoL (without statistically significant differences between the arms). For the main analysis, results in terms of global QoL/global health status were considered. In addition, we adopted a ‘broader’ definition of QoL positivity: QoL was considered positive for the experimental arm in the presence of positive statistically significant differences in the global QoL or—even in the case of no difference in global QoL—in the presence of significant differences in some items or scales.
Treatment toxicity was classified into four main categories according to the rate of adverse events of the experimental treatment: more toxic, less toxic, without statistically significant differences compared to the control arm and with a different toxicity profile (more favourable for some adverse events and more toxic for others). The definition of toxicity also took into account the comments and general message of the authors.
In order to improve the quality of the database, reducing the risk of bias in data collection and of random errors, quality control was assured by a double reading by a second investigator, in a random sample of 200 records.
Our main aim was to describe QoL results among randomized phase III trials conducted in patients with advanced/metastatic cancer that demonstrated a superiority of experimental treatment, based on a statistically significant result in the primary endpoint (OS or PFS). Secondary outcomes were: the association between QoL results and characteristics of studies and publications among trials with positive results in the primary endpoint; the frequency of positive QoL results within regulatory decisions by EMA/FDA, with a time-trend analysis; and the association between QoL results and treatment toxicity.
A descriptive analysis was conducted reporting the percentage of positive clinical trials and drug approvals according to QoL results. Association between characteristics of studies and publication and QoL results among positive trials was tested with the chi-square test (χ2). A P value < 0.05 was considered statistically significant. Considering the exploratory nature of the analysis, no correction for multiplicity was applied. All statistical analyses were carried out using IBM SPSS Statistics software for Windows, version 29.0.1.0.
Results
Overall, out of the 834 studies published from 2012 to 2021 globally included in the database, 592 publications were conducted in patients with advanced disease and were eligible for this analysis (Supplementary Figure S1, available at https://doi.org/10.1016/j.esmoop.2024.103654). The two 5-year periods reported a similar distribution of included trials: 322 (54.4%) studies were published in 2012-2016 and 270 (45.6%) in 2017-2021. Included studies were published in journals with high IF in 265 articles (44.8%) and had a pharmaceutical sponsorship in 381 (64.4%) articles. The majority of trials were conducted in patients with gastrointestinal cancers (25.5%), thoracic cancers (23.3%), genitourinary cancers (13.3%) and breast cancer (12%). The main characteristics of the included publications are reported in Table 1.
Table 1.
Main characteristics of the 592 included publications
| Main characteristics | N (%) |
|---|---|
| Year of primary publication | |
| 2012-2016 | 322 (54.4) |
| 2017-2021 | 270 (45.6) |
| Primary manuscript journal | |
| Annals of Oncology | 77 (13) |
| British Journal of Cancer | 10 (1.7) |
| Cancer | 6 (1) |
| European Journal of Cancer | 28 (4.7) |
| JAMA | 8 (1.4) |
| JAMA Oncology | 14 (2.4) |
| Journal of Clinical Oncology | 158 (26.7) |
| Journal of the National Cancer Institute | 2 (0.3) |
| Lancet | 45 (7.6) |
| Lancet Oncology | 155 (26.2) |
| New England Journal of Medicine | 89 (15) |
| IF | |
| High IF (>30) | 265 (44.8) |
| Intermediate IF (15-30) | 222 (37.5) |
| Low IF (<15) | 105 (17.7) |
| Sponsorship | |
| Industry-sponsored | 381 (64.4) |
| Academic | 211 (35.6) |
| Study design | |
| Superiority | 548 (92.6) |
| Non-inferiority | 44 (7.4) |
| Masking | |
| Open label | 378 (63.9) |
| Blinded | 214 (36.1) |
| Tumour type | |
| Breast | 71 (12) |
| GI | 151 (25.5) |
| GU | 79 (13.3) |
| Thoracic | 138 (23.3) |
| Others | 153 (25.8) |
| Type of experimental treatmenta | |
| Chemotherapy | 322 (54.4) |
| Targeted therapy | 331 (55.9) |
| Hormonal therapy | 52 (8.8) |
| Immunotherapy | 94 (15.9) |
GI, gastrointestinal; GU, genitourinary; IF, impact factor.
Non-mutually exclusive categories.
The quality control showed a high concordance rate between readers for all the items. In only 16 cases (8%), investigators reported a different interpretation, mostly for a different judgement on some positive QoL items in the absence of statistically significant differences, for transcription errors or for updated results still not available at the first lecture. The rate of discordance for the analysis of primary endpoints and QoL results was <2%. High concordance was found for the analysis of frequency of QoL-positive results among drugs approved by regulatory agencies.
The primary endpoint was OS in 298 clinical trials (50.3%) and PFS in 304 clinical trials (51.4%), with 79 studies (13.3%) with multiple primary endpoints.
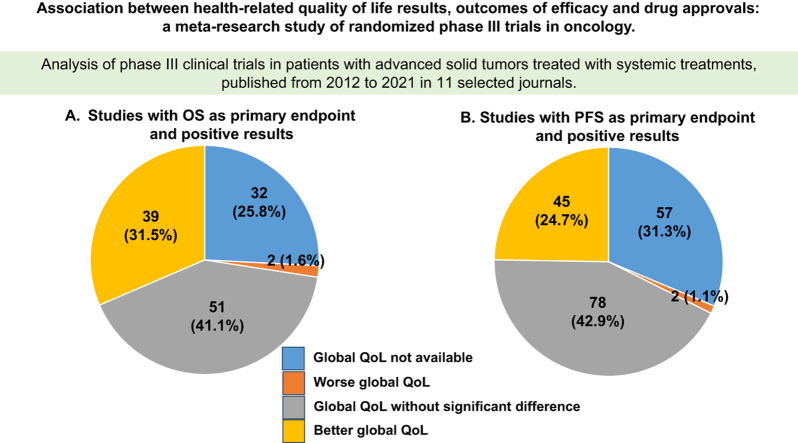
Among trials with OS as primary endpoint, a positive OS result was reported in 124 trials (41.6%) (Table 2). Among these, experimental treatment was associated with a positive QoL result in 39 studies (31.5%), unfavourable QoL results in 2 (1.6%), not statistically significant different QoL results in 51 (41.1%) and not available QoL results in 32 (25.8%) (Figure 1A). Applying a broader definition of QoL positivity (positivity in single items even in the absence of positive global QoL), experimental treatment was associated with a positive QoL result in 75 (60.5%) studies.
Table 2.
Characteristics of studies with OS as primary endpoint and positive results
| Trials with positive OS (EP1) (N = 124) |
QoL results (global QoL), n (%) |
||||
|---|---|---|---|---|---|
| Not available | Without statistically significant difference | Unfavourable | Positive | Chi-square test | |
| 32 (25.8) | 51 (41.1) | 2 (1.6) | 39 (31.5) | — | |
| Journal IF | |||||
| Journals with high IF | 16 (18.6) | 38 (44.2) | 1 (1.2) | 31 (36.0) | P = 0.036 |
| Journals with low/intermediate IF | 16 (42.1) | 13 (34.2) | 1 (2.6) | 8 (21.1) | |
| Years of primary publication | |||||
| Studies published in 2012-2016 | 15 (28.3) | 18 (34.0) | 1 (1.9) | 19 (35.8) | P = 0.574 |
| Studies published in 2017-2021 | 17 (23.9) | 33 (46.5) | 1 (1.4) | 20 (28.2) | |
| Masking | |||||
| Open label | 25 (29.4) | 31 (36.5) | 1 (1.2) | 28 (32.9) | P = 0.348 |
| Blinded | 7 (17.9) | 20 (51.3) | 1 (2.6) | 11 (28.2) | |
| Sponsorship | |||||
| Industry-sponsored | 18 (20.5) | 35 (39.8) | 1 (1.1) | 34 (38.6) | P = 0.031 |
| Academic | 14 (38.9) | 16 (44.4) | 1 (2.8) | 5 (13.9) | |
| Type of tumour | |||||
| Breast | 2 (20) | 4 (40.0) | 0 (0) | 4 (40.0) | P = 0.445 |
| GI | 17 (39.5) | 15 (34.9) | 0 (0) | 11 (25.6) | |
| GU | 2 (9.5) | 11 (52.4) | 0 (0) | 8 (38.1) | |
| Thoracic | 8 (27.6) | 11 (37.9) | 1 (3.4) | 9 (31.0) | |
| Other | 3 (14.3) | 10 (47.6) | 1 (4.8) | 7 (33.3) | |
| Type of experimental treatment | |||||
| Chemotherapy ± other | 15 (42.9) | 11 (31.4) | 1 (2.9) | 8 (22.9) | P = 0.245 |
| Targeted therapy ± other | 8 (24.2) | 16 (48.5) | 0 (0) | 9 (27.3) | |
| Hormonal therapy ± other | 1 (9.1) | 6 (54.5) | 0 (0) | 4 (36.4) | |
| Immunotherapy ± other | 7 (15.9) | 18 (40.9) | 1 (2.3) | 18 (40.9) | |
EP1, primary endpoint; GI, gastrointestinal; GU, genitourinary; IF, impact factor; OS, overall survival; QoL, quality of life.
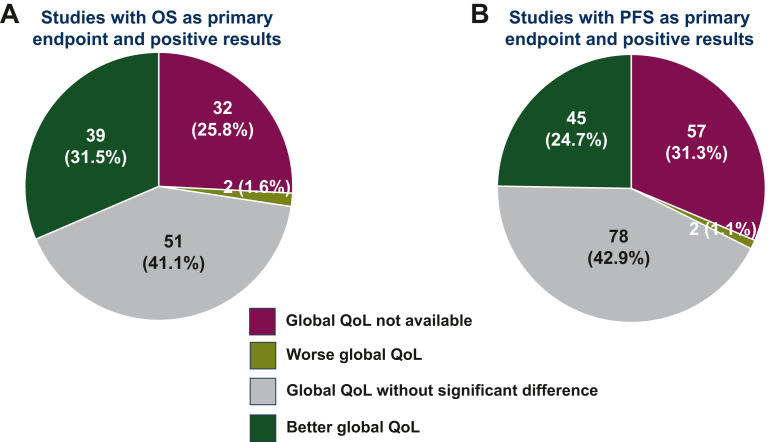
Figure 1.
Health-related QoL results in studies with positive results in the primary endpoint. (A) Studies with OS as primary endpoint and positive results; (B) Studies with PFS as primary endpoint and positive results. OS, overall survival; PFS, progression-free survival; QoL, quality of life.
Among trials with PFS as primary endpoint, positive PFS results were reported in 182 trials (59.5%) (Table 3). Among these, QoL analysis for the experimental arm was positive in 45 studies (24.7%), without a statistically significant difference in 78 (42.9%), unfavourable in 2 (1.1%) and not available in 57 (31.3%) (Figure 1B). Applying the broader definition of QoL positivity, experimental treatment was associated with a positive QoL result in 91 (50.0%) trials. Among the 182 trials with positive results in the primary endpoint of PFS, a positive result in OS was available in 65 (35.7%). Among the remaining 117 without positive OS results, global QoL was significantly better in the experimental arm in 24 (20.5%) trials, worse in 1 (0.9%) trial, without significant differences in 49 (41.9%) trials and not available in 43 (36.8%) trials.
Table 3.
Characteristics of studies with PFS as primary endpoint and positive results
| Trials with positive PFS (EP1) (N = 182) |
QoL results (global QoL), n (%) |
||||
|---|---|---|---|---|---|
| Not available | Without statistically significant difference | Unfavourable | Positive | Chi-square test | |
| Studies with positive PFS | 57 (31.3) | 78 (42.9) | 2 (1.1) | 45 (24.7) | — |
| Journal IF | |||||
| Journals with high IF | 28 (23.1) | 56 (46.3) | 2 (1.7) | 35 (28.9) | P = 0.006 |
| Journals with low/intermediate IF | 29 (47.5) | 22 (36.1) | 0 (0) | 10 (16.4) | |
| Years of primary publication | |||||
| Studies published in 2012-2016 | 26 (32.9) | 33 (41.8) | 0 (0) | 20 (25.3) | P = 0.636 |
| Studies published in 2017-2021 | 31 (30.1) | 45 (43.7) | 2 (1.9) | 25 (24.3) | |
| Masking | |||||
| Open label | 35 (35.0) | 34 (34.0) | 1 (1.0) | 30 (30.0) | P = 0.057 |
| Blinded | 22 (26.8) | 44 (53.7) | 1 (1.2) | 15 (18.3) | |
| Sponsorship | |||||
| Industry-sponsored | 38 (25.7) | 66 (44.6) | 1 (0.7) | 43 (29.1) | P = 0.001 |
| Academic | 19 (55.9) | 12 (35.3) | 1 (2.9) | 2 (5.9) | |
| Type of tumour | |||||
| Breast | 14 (36.8) | 14 (36.8) | 0 (0) | 10 (26.3) | P = 0.494 |
| GI | 10 (34.5) | 16 (55.2) | 0 (0) | 3 (10.3) | |
| GU | 3 (16.7) | 9 (50.0) | 0 (0) | 6 (33.3) | |
| Thoracic | 13 (28.3) | 16 (34.8) | 1 (2.2) | 16 (34.8) | |
| Other | 17 (33.3) | 23 (45.1) | 1 (2.0) | 10 (19.6) | |
| Type of experimental treatment | |||||
| Chemotherapy ± other | 16 (61.5) | 8 (30.8) | 0 (0) | 2 (7.7) | P = 0.051 |
| Targeted therapy ± other | 29 (28.7) | 43 (42.6) | 2 (2.0) | 27 (26.7) | |
| Hormonal therapy ± other | 5 (22.7) | 12 (54.5) | 0 (0) | 5 (22.7) | |
| Immunotherapy ± other | 7 (21.2) | 15 (45.5) | 0 (0) | 11 (33.3) | |
EP1, primary endpoint; GI, gastrointestinal; GU, genitourinary; IF, impact factor; PFS, progression-free survival; QoL, quality of life.
Both in studies with improved primary endpoint of OS and in studies with improved primary endpoint of PFS, positive results in global QoL for the experimental arm were more frequent in journals with high IF (P = 0.036 and 0.006, respectively). Positive global QoL results were more frequent in industry-sponsored trials, both in studies with a positive primary endpoint of OS (P = 0.031) and in studies with a positive primary endpoint of PFS (P = 0.001). Irrespective of the positive primary endpoint, no significant association was detected between QoL results and the distribution between the two 5-year period, primary tumour, blind masking and type of treatment.
Despite the majority of experimental treatments (55.1%) being associated with a more toxic profile compared to the control arm, a positive global QoL result was globally reported in 99 studies (16.7%). Positive results in global QoL occurred in different categories of toxicity: namely, positive global QoL results were reported in 14/116 studies (12.1%) without statistically significant differences in toxicity between arms, in 44/326 studies (13.5%) with more toxic experimental treatment, in 25/66 (37.9%) studies with less toxic experimental treatment and in 16/84 (19.0%) studies with different toxicity profile between arms (Table 4). Nevertheless, there was a statistically significant association between the toxicity and QoL results: a better toxicity profile is associated with a higher probability of positive QoL results (P < 0.001).
Table 4.
Association between toxicity of experimental treatment and global QoL results
| Toxicity | QoL results (global QoL), n (%) |
||||
|---|---|---|---|---|---|
| Not available | Without statistically significant difference | Unfavourable | Positive | Chi-square test | |
| Without statistically significant difference between arms (N = 116, 19.6%) | 61 (52.6) | 36 (31.0) | 5 (4.3) | 14 (12.1) | P < 0.001 |
| More toxic experimental arm (N = 326, 55.1%) | 151 (46.3) | 114 (35.0) | 17 (5.2) | 44 (13.5) | |
| Less toxic experimental arm (N = 66, 11.1%) | 20 (30.3) | 19 (28.8) | 2 (3.0) | 25 (37.9) | |
| Different toxicity profile (N = 84, 14.2%) | 42 (50.0) | 26 (31.0) | 0 (0) | 16 (19.0) | |
QoL, quality of life.
FDA drug approvals were available for 143 clinical trials (24.2%): namely, experimental treatments were approved due to an improvement in OS in 69 studies (48.2%) and PFS in 91 studies (63.6%). Among the studies with FDA drug approvals, the experimental arm was associated with positive results in global QoL in 55 studies (38.5%), without statistically significant difference in QoL in 63 (44.1%) or not available in 24 (16.8%).
Similarly, EMA approvals were available for 142 studies (24%) with an OS benefit for 68 studies (47.8%) and PFS benefit for 90 (63.3%) for experimental strategy. Among EMA drug approvals, the experimental arm was associated with positive results in global QoL in 56 studies (39.4%), without a statistically significant difference in 64 studies (45.1%) or results not available in 21 studies (14.8%).
As shown in Supplementary Table S1, available at https://doi.org/10.1016/j.esmoop.2024.103654, QoL data were available for the majority of treatments approved by the FDA and EMA both in the 2012-2016 and in the 2017-2021 period. However, no time-trend of positive global QoL results for treatments approved by the FDA and EMA was found. Namely, FDA approvals associated with positive QoL data have moved from 40.3% to 37.0% (P = 0.793), and EMA approvals from 40.0% to 39.0% (P = 0.722), in the two 5-year periods, respectively.
Discussion
In this study, we evaluated the association between the outcomes in terms of primary endpoints (OS and PFS) within randomized phase III trials of anticancer treatments and QoL results. We found a demonstration of improvement in patients’ global QoL with experimental treatment only in 31.5% of the studies with an OS benefit. Of note, positive QoL results were available in only 24.7% of studies with a superiority of experimental treatment in terms of PFS improvement.
Despite the well-known limitations of PFS, it is chosen as the primary endpoint with a similar frequency as OS in the studies conducted in patients with advanced disease. There is a tendency in choosing PFS as the primary endpoint even in settings where it is not fully validated as a surrogate endpoint. In this context, the availability of QoL data has a complementary and significant role, leading to a proper interpretation of the clinical relevance of an experimental drug. Of note, focusing on the trials with positive results in the primary endpoint of PFS but without demonstration of improvement in OS, only 20.5% of trials have a demonstrated improvement in global QoL with the experimental treatment. This finding could challenge, at least in some cases, the clinical relevance of the PFS benefit.
Among studies with a positive primary endpoint, studies sponsored by pharmaceutical industries and published in journals with high IF reported more frequently a QoL benefit compared to academic studies and journals with lower IF. However, even if the inclusion of QoL among endpoints has increased in the last 5 years in for-profit studies, a decreased rate of reporting QoL results in the primary publication has been observed in the same period.11 Even for well-designed and high-quality studies, presentation of QoL data remains often suboptimal for several issues, especially for academic research: poor compliance to PROs, complex statistical analysis and attention focused by authors and editors on primary endpoints.
In studies where immunotherapy, targeted therapies and hormonal therapy were more effective than standard treatment in terms of OS or PFS improvements, the presence of improved QoL was reported more frequently than in studies testing chemotherapy, but those differences were not statistically significant. Although a systematic review conducted on a smaller cohort of studies reported conflicting results,10 this finding confirms clinicians’ perceptions about the burden of chemotherapy on the patient and their potential preference for chemo-free treatments. However, a high percentage of studies testing immunotherapy included QoL among the endpoints, but the presentation of results in the primary publication is often lacking.11
We found a variable association of treatment toxicity with QoL. A favourable safety profile is not always synonymous with good QoL, because QoL is the result of a dynamic and multidimensional balance. Conversely, more toxic treatments are more likely to compromise patients’ mental and physical well-being and QoL. Of note, 44 trials (13.5% of those with a more toxic experimental arm) showed a positive result in global QoL. In most cases, those trials tested an effective treatment compared to placebo or best supportive care, or tested the addition of a drug to the previous standard (e.g. chemotherapy + immunotherapy versus chemotherapy alone). Despite this kind of comparison producing more toxicity with the experimental arm, the improvement in global QoL can be related to better disease control. Our study supports the need for QoL results in addition to investigator-assessed endpoints.
The absence of QoL results prevents a comprehensive evaluation of the clinical value of experimental drugs from the scientific community. Nonetheless, we reported the presence of a QoL analysis in a high proportion of studies in advanced settings, irrespective of the primary endpoints, and in a high proportion of trials leading to approval by regulatory agencies. Despite the limitations, this testifies the growing interest and awareness among the scientific community and regulatory agencies regarding the critical role of QoL in clinical decision making.
Efficacy and QoL data should guide clinical decisions and drug approvals. About one-quarter of the publications included in our analysis correspond to EMA and FDA drug approvals. Approved drugs are associated with improvement in global QoL as well as improved primary endpoint in less than one third of cases. The assessment of drug approvals was limited to the available published data in literature, not directly considering the dossier analysed by regulatory agencies. This is a potential limitation of our analysis and the rate of approvals with QoL data could be underestimated.
We acknowledge that the definition of QoL positivity may be affected by some limitations. Firstly, in most studies QoL was a secondary or exploratory endpoint, without a formal hypothesis to be satisfied for the definition of positivity. Secondly, minimal clinically important difference was not considered, so the clinical relevance of some positive results is not guaranteed. Thirdly, for the primary analysis presented in this paper, also following comments by reviewers, in order to define the positivity of QoL we considered the global QoL/global health status. We had also explored a ‘broader’ definition of QoL positivity, based on single items even in the absence of better global QoL: this broader definition permits to record any type of QoL benefit reported in the publications, regardless of the domains or functional spheres involved. This analysis was previously reported.16 There are limitations in both approaches: considering only global QoL could ignore important differences in some other scales or domains, while the broader definition could definitely overestimate the rate of QoL positivity. However, in order to limit subjectivity related to the interpretation of the results, quality control was carried out in a dedicated second lecture by a second investigator, reporting a high concordance rate in the definition of QoL positivity. Lastly, there is an inherent risk of false-positive results due to multiplicity of statistical tests for the different QoL scales and domains analysed within each study.
We also have to acknowledge that the proportion of studies with QoL data, especially if negative or without significant differences, may have been underestimated due to publication bias. However, we attempted to limit the risk of reporting bias by extending the search for QoL data to secondary publications and/or presentations not only in PubMed but also at the major oncology conferences. Similarly, as already discussed for the original study,7 including studies from the screening of 11 selected scientific journals, we may have excluded some relevant clinical trials.
To our knowledge, this is the first analysis with a very large sample size that evaluated the association between outcomes of efficacy, QoL results and drug approvals through a decade.
Conclusions
In conclusion, our data could be useful for the scientific debate and the growing interest about QoL analysis in oncology. The proportion of treatments approved by EMA and FDA with available QoL data is high, although an improvement in global QoL in addition to the positive results in the primary endpoints is demonstrated only in a minority of treatments.
Acknowledgements
The authors thank Federica Trastu, Eleonora Ghisoni, Pasquale Lombardi, Annapaola Mariniello, Maria Lucia Reale, Giacomo Aimar, Marco Audisio, Maristella Bungaro, Raimondo Di Liello, Piera Gargiulo, Alessandro Rossi, Valentina Tuninetti, Fabio Turco, Anna La Salvia, Cristina Sonetto, Emmanuele De Luca, Daniele Pignataro, Rosario Francesco Di Stefano, Elena Trevisi, Gianmarco Leone, Leonardo Muratori and Maddalena Marcato for their contribution to the original database.7,11
Funding
None declared.
Disclosure
MDM reports honoraria from AstraZeneca, Boehringer Ingelheim, Janssen, Merck Sharp & Dohme (MSD), Novartis, Pfizer, Roche, GlaxoSmithKline, Amgen, Merck, Takeda for consultancy or participation to advisory boards and direct research funding from Tesaro/GlaxoSmithKline, institutional funding for work in clinical trials/contracted research from Beigene, Exelixis, MSD, Pfizer and Roche. FP reports honoraria from Roche, Bayer, AstraZeneca, Pfizer, Incyte, Tesaro/GSK, Merck for institutional financial support for clinical trials and from Bayer, Pierre Fabre, AstraZeneca, Incyte, Ipsen, Clovis, Astellas, Sanofi, Roche and Pfizer for consultancy. LM reports honoraria from Merck and Gilead for presentations and from Janssen as support for attending meetings. All other authors have declared no conflicts of interest.
Supplementary data
References
- 1.Booth C.M., Tannock I. Reflections on medical oncology: 25 years of clinical trials - where have we come and where are we going? J Clin Oncol. 2008;26(1):6–8. doi: 10.1200/JCO.2007.13.8156. [DOI] [PubMed] [Google Scholar]
- 2.Wilson M.K., Karakasis K., Oza A.M. Outcomes and endpoints in trials of cancer treatment: the past, present, and future. Lancet Oncol. 2015;16(1):e32–e42. doi: 10.1016/S1470-2045(14)70375-4. [DOI] [PubMed] [Google Scholar]
- 3.Di Maio M., Perrone F. Lessons from clinical trials on quality-of-life assessment in ovarian cancer trials. Ann Oncol. 2016;27(6):961–962. doi: 10.1093/annonc/mdw153. [DOI] [PubMed] [Google Scholar]
- 4.Notarnicola S., Zumstein L., Paparo J., Marandino L., Perrone F., Di Maio M. Systematic review of adoption, reporting and impact of health-related quality of life in phase III non-inferiority trials of systemic oncology treatments. Eur J Cancer. 2023;195 doi: 10.1016/j.ejca.2023.113374. [DOI] [PubMed] [Google Scholar]
- 5.U.S. Food and Drug Administration Core Patient-Reported Outcomes in Cancer Clinical Trials (Draft Guidance for Industry). Rockville: U.S. Department of Health and Human Services, Food and Drug Administration. 2021. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/core-patient-reported-outcomes-cancer-clinical-trials Available at.
- 6.European Medicines Agency . European Medicines Agency; Amsterdam: 2016. Appendix 2 to the Guideline on the Evaluation of Anticancer Medicinal Products in Man - the Use of Patient-Reported Outcome (PRO) Measures in Oncology Studies. [Google Scholar]
- 7.Marandino L., La Salvia A., Sonetto C., et al. Deficiencies in health-related quality-of-life assessment and reporting: a systematic review of oncology randomized phase III trials published between 2012 and 2016. Ann Oncol. 2018;29(12):2288–2295. doi: 10.1093/annonc/mdy449. [DOI] [PubMed] [Google Scholar]
- 8.Marandino L., De Luca E., Zichi C., et al. Quality-of-life assessment and reporting in prostate cancer: systematic review of phase 3 trials testing anticancer drugs published between 2012 and 2018. Clin Genitourin Cancer. 2019;17(5):332–347. doi: 10.1016/j.clgc.2019.07.007. [DOI] [PubMed] [Google Scholar]
- 9.Lombardi P., Marandino L., De Luca E., et al. Quality of life assessment and reporting in colorectal cancer: a systematic review of phase III trials published between 2012 and 2018. Crit Rev Oncol Hematol. 2020;146 doi: 10.1016/j.critrevonc.2020.102877. [DOI] [PubMed] [Google Scholar]
- 10.Samuel J.N., Booth C.M., Eisenhauer E., Brundage M., Berry S.R., Gyawali B. Association of quality-of-life outcomes in cancer drug trials with survival outcomes and drug class. JAMA Oncol. 2022;8(6):879–886. doi: 10.1001/jamaoncol.2022.0864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marandino L., Trastu F., Ghisoni E., et al. Time trends in health-related quality of life assessment and reporting within publications of oncology randomised phase III trials: a meta-research study. BMJ Oncol. 2023;2(1) [Google Scholar]
- 12.Di Maio M., Basch E., Denis F., et al. The role of patient-reported outcome measures in the continuum of cancer clinical care: ESMO clinical practice guideline. Ann Oncol. 2022;33(9):878–892. doi: 10.1016/j.annonc.2022.04.007. [DOI] [PubMed] [Google Scholar]
- 13.Kovic B., Jin X., Kennedy S.A., et al. Evaluating progression-free survival as a surrogate outcome for health-related quality of life in oncology: a systematic review and quantitative analysis. JAMA Int Med. 2018;178(12):1586–1596. doi: 10.1001/jamainternmed.2018.4710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hwang T.J., Gyawali B. Association between progression-free survival and patients’ quality of life in cancer clinical trials. Int J Cancer. 2019;144(7):1746–1751. doi: 10.1002/ijc.31957. [DOI] [PubMed] [Google Scholar]
- 15.Davis C., Naci H., Gurpinar E., Poplavska E., Pinto A., Aggarwal A. Availability of evidence of benefits on overall survival and quality of life of cancer drugs approved by European Medicines Agency: retrospective cohort study of drug approvals 2009-13. BMJ. 2017;359 doi: 10.1136/bmj.j4530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Paratore C., Schiavone R., Zichi C., et al. Health-related quality of life (QoL) in randomized phase III trials in oncology: association between results of QoL, results of primary endpoint and drug approval. J Clin Oncol. 2024;42:11109. doi: 10.1016/j.esmoop.2024.103654. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.