Abstract
Background
Lesbian, gay, bisexual, transgender, and queer (LGBTQ) individuals with cancer have specific and unique health issues and needs. Reports persist of inequalities in the care provided for these patients, making it important to assess the attitudes and knowledge of LGBTQ needs among those who provide care.
Materials and methods
The European Society for Medical Oncology (ESMO) and the European Society for Paediatric Oncology (SIOP Europe) Adolescents and Young Adults Working Group designed this survey comprising 67 questions covering demographics, knowledge, and education of LGBTQ health needs, and attitudes regarding LGBTQ patients with cancer.
Results
Among the 672 respondents, a majority do not ask about sexual orientation and gender identity during first visit (64% and 58%, respectively). Only a minority of the respondents considered themselves well informed regarding gay/lesbian and transgender patients’ health (44% and 25%, respectively) and psychosocial needs (34%). There was high interest in receiving education regarding the unique health needs of LGBTQ patients (73%).
Conclusions
Survey respondents indicated a willingness to provide care to LGBTQ patients, but a lack of confidence in the knowledge of the health issues and needs of LGBTQ individuals. Lack of training provided in medical schools and postgraduate training programmes and strong interest for additional education on these issues were reported.
Key words: LGBTQ, cancer, specific care, education
Highlights
-
•
We report data from a global survey of oncology care providers’ attitudes when treating LGBTQ patients with cancer.
-
•
Most providers feel comfortable treating LGBTQ patients.
-
•
Respondents do not feel well-prepared by pre/postgraduate training programmes about the health care needs of LGBTQ patients.
-
•
The need for improved information gathering of gender identification and sexual orientation status was raised.
-
•
Strong interest for additional education on these issues was expressed.
Introduction
Individuals within the lesbian, gay, bisexual, transgender, and queer (LGBTQ) community have specific and unique health issues, including lower access to oncological screening, higher incidence of specific cancer types, and lower survival.1, 2, 3, 4, 5, 6, 7, 8, 9 Moreover, members of the LGBTQ community continue experiencing discrimination in health care settings in terms of communication and respect by health care providers10 that may create barriers to patient/provider relationship, holistic care, and support.11
As an increasing number of individuals identify as LGB or transgender, the European Society for Medical Oncology (ESMO) and the European Society for Paediatric Oncology (SIOP Europe) Adolescents and Young Adults Working Group (WG) became interested to assess the attitudes of cancer care providers (CCPs) toward treating LGBTQ patients with cancer, creating a survey to address the current knowledge and the need for further education in these topics. Furthermore, the importance of sexual orientation (SO)/gender identity (GI) data collection and the extent of this assessment in daily practice were explored.
Materials and methods
The survey was developed by the members of the WG. It was reviewed and approved by the ESMO Executive Board. A link to the survey was sent to ESMO (n = 23 856) and SIOP Europe members (n = 2500). Representatives of the LGBTQ community were not involved through specific enrichment invitation.
Sixty-seven questions were included regarding demographics (n = 15), practices (n = 4), education, and recommendations for further education (n = 10) related to health issues of LGBTQ patients with cancer. Questions also addressed attitudes regarding ability to treat LGBTQ patients (n = 12), knowledge of risk of certain cancers in the LGBTQ population, lifestyle risk factors (n = 14), and understanding of the needs of LGBTQ patients (n = 12). The attitudes and knowledge of LGB and transgender patients were addressed separately. Anonymity was preserved and answering was voluntary. Responses were collected from 15 December 2020 to 31 January 2021.
A five-point Likert scale was used: strongly disagree, disagree, neutral, agree, or strongly agree; respondents could choose “don’t know” or “don’t want to answer”. The responses were evaluated using a descriptive analysis and broken down according to the self-reported SO. The responses of those who self-identified as LGBTQ, ‘other’, and ‘would prefer not to say’ were compared to those who identified as heterosexual. A P value <0.05 was considered significant.
Demographics
In total, 672 responses were received from 75 countries. Almost half of the respondents (46.53%) were between 30 and 39 years of age, while 6.19% were aged ≥60 years.
Two hundred and eighty-six (53.76%) identified themselves as male, 238 (44.74%) as female, and 3 (0.56%) as transgender or other.
In terms of SO, 371 of 532 (69.74%) identified themselves as heterosexual, 14 (2.63%) as lesbian, 74 (13.91%) as gay, 26 (4.89%) as bisexual, 10 (1.88%) as not sure, 7 (1.32%) as other, and 30 (5.64%) did not want to specify. In terms of gender by SO group, 47.97% of heterosexuals identified as male, 51.76% as female, and 0.27% as transgender. In the lesbian group, 92.86% identified as female and 7.14% as transgender, while in the gay group 98.65% identified as male and 1.35% as transgender. In the bisexual group, more respondents (53.85%) identified as female compared to 46.15% as male (Supplementary Figure S1A, available at https://doi.org/10.1016/j.esmoop.2024.103618).
Most of the respondents were medical oncologists (68.61%), followed by paediatric haematologists/oncologists (9.59%). The majority (45.30%) worked in university hospitals and 25.38% in a cancer centre. The countries with most respondents are shown in Supplementary Figure S1B, available at https://doi.org/10.1016/j.esmoop.2024.103618.
Ability to treat LGBTQ patients with cancer
Of the 620 respondents, 555 (89.52%) indicated that they were comfortable treating lesbian and gay patients. Responses differed somewhat by SO: 84.28% of heterosexuals strongly agreed/agreed, compared to 94.34% of LGBTQ/other (P = 0.015).
Of the 618 respondents, 81.23% strongly agreed/agreed that they felt comfortable treating transgender patients. Per SO subgroup, 79.19% of heterosexuals strongly agreed/agreed and answered similarly in each SO subgroup (Supplementary Figure S1C, available at https://doi.org/10.1016/j.esmoop.2024.103618).
Few of the 573 respondents ‘find more difficult to treat LGBTQ patients’ (n = 43; 7.51%), while 74.69% disagreed/strongly disagreed, and 17.63% remained neutral. A statistically higher percentage of disagreement was found between heterosexuals and LGBTQ/other (70.54% versus 83.02%; P = 0.003) (Supplementary Figure S1D, available at https://doi.org/10.1016/j.esmoop.2024.103618).
Of the 573 respondents, 25.65% strongly agreed/agreed that LGBTQ patients are disadvantaged in accessing cancer care, while 56.37% disagreed/strongly disagreed, and 17.8% were neutral (Supplementary Figure S2A, available at https://doi.org/10.1016/j.esmoop.2024.103618). Interestingly, this was one of the questions where both the net percentage of agreement and that of disagreement were statistically significantly different between heterosexual respondents and LGBTQ/other: 22.16% versus 32.08% (P = 0.016) and 47.08% versus 60.27% (P = 0.016), respectively.
Another one involved the statement ‘I feel well informed about the health needs of lesbian and gay patients’. Of the 568 respondents, 250 (44.01%) strongly agreed/agreed, while 26.41% disagreed/strongly disagreed. However, a greater proportion of LGBTQ/other agreed compared to heterosexuals (67.72% versus 33.51%; P < 0.001). The percentages for disagreement were 18.99% and 29.46% (P = 0.013).
A quarter of the 570 respondents (147; 25.79%) indicated that they felt well informed about the health needs of transgender patients; 42.98% disagreed/strongly disagreed and 31.23% remained neutral or refused to answer. Responses to this question by SO revealed that 35.85% of LGBTQ/other strongly agreed/agreed, compared to 21.29% of heterosexuals (P < 0.001).
Respondents felt more uncertain about their understanding of the psychosocial needs of LGBTQ patients. One-third of the 568 respondents (34.21%) agreed/strongly agreed that they felt well informed about the psychosocial characteristics of this population; 37.02% disagreed and 28.42% remained neutral. The breakdown of responses by SO shows a different pattern, with the majority of LGBTQ/other reporting that they felt well informed, compared to 24.36% of heterosexuals (P < 0.001) (Supplementary Figure S2B, available at https://doi.org/10.1016/j.esmoop.2024.103618).
The majority of the 572 respondents (66.96%) agreed with being listed as an LGBTQ-friendly provider. Only 6.30% did not want to be listed as such. The percentage was higher for LGBTQ/other (83.63% versus 61.25%) than for heterosexuals (P < 0.001).
Knowledge about specific risk factors for the LGBTQ community
One-third of the 549 respondents (198; 36.07%) strongly agreed/agreed that ‘the risk of cancer in patients who identify as different gender is different from the risk estimated based on their sex at birth’. In relation to cancer risk and SO, 42.05% of the 547 respondents strongly agreed/agreed that cancer risk differs by SO, while 37.85% disagreed and 14.63% did not agree (Supplementary Figure S2C, available at https://doi.org/10.1016/j.esmoop.2024.103618).
There was some disagreement regarding specific risk factors. While 4.75% of the 547 respondents agreed that lesbian women are not at risk for human papillomavirus-associated cervical dysplasia, 81.54% disagreed/strongly disagreed; 13.71% did not know or were neutral (Supplementary Figure S2D, available at https://doi.org/10.1016/j.esmoop.2024.103618).
Very few believed that lesbian and bisexual women have a lower risk of breast and ovarian cancer (Supplementary Figure S3A, available at https://doi.org/10.1016/j.esmoop.2024.103618).
Of the 547 respondents, ∼14% believed that only human immunodeficiency virus (HIV)-positive gay and bisexual men are at increased risk of anal cancer, while almost 80% disagreed/strongly disagreed (Supplementary Figure S3B, available at https://doi.org/10.1016/j.esmoop.2024.103618).
The greatest degree of uncertainty was found regarding the utility of anal Pap testing. Of 545 respondents, just <34% agreed that anal Pap testing increases life expectancy, 28.26% answered ‘neutral’, and 26.61% stated ‘do not know’. Only 11.19% disagreed (Supplementary Figure S3C, available at https://doi.org/10.1016/j.esmoop.2024.103618).
There was uncertainty as to whether smoking is more common in this population. Neutral answers were given by 25.64% of the 546 respondents and 22.34% did not know. However, 24.36% strongly agreed/agreed that there is a higher prevalence, while 27.66% disagreed/strongly disagreed that LGBTQ people are more likely to smoke (Supplementary Figure S3D, available at https://doi.org/10.1016/j.esmoop.2024.103618).
Obtaining information about SO and GI status and importance of knowing SO/GI for optimal cancer care
Of 575 respondents, 40.87% assumed that a patient is heterosexual at the first visit; 30.26% were neutral, and 28.17% disagreed/strongly disagreed. Of note, more LGBTQ/other disagreed (41.25% versus 22.43% of heterosexuals; P < 0.001) (Supplementary Figure S4A, available at https://doi.org/10.1016/j.esmoop.2024.103618).
Obtaining information on SO or GI does not seem to be routine in practice. Few strongly agreed/agreed (81/573; 14.14%) that they ask about SO as part of patient’s history (Supplementary Figure S4B, available at https://doi.org/10.1016/j.esmoop.2024.103618). Similar responses were given in relation to whether they ask about GI (Supplementary Figure S4C, available at https://doi.org/10.1016/j.esmoop.2024.103618).
In contrast, more than half of the respondents thought that it is or could be important to know a patient’s SO (Supplementary Figure S5A, available at https://doi.org/10.1016/j.esmoop.2024.103618). Of the 594 respondents, 324 (54.55%) thought it was important to know the patient’s GI (Supplementary Figure S5B, available at https://doi.org/10.1016/j.esmoop.2024.103618), while more (n = 373; 62.79%) thought it was important to know the patient’s sex at birth (Supplementary Figure S5C, available at https://doi.org/10.1016/j.esmoop.2024.103618). This response pattern was fairly consistent across all SO subgroups.
Attitudes towards education about LGBTQ-specific health needs
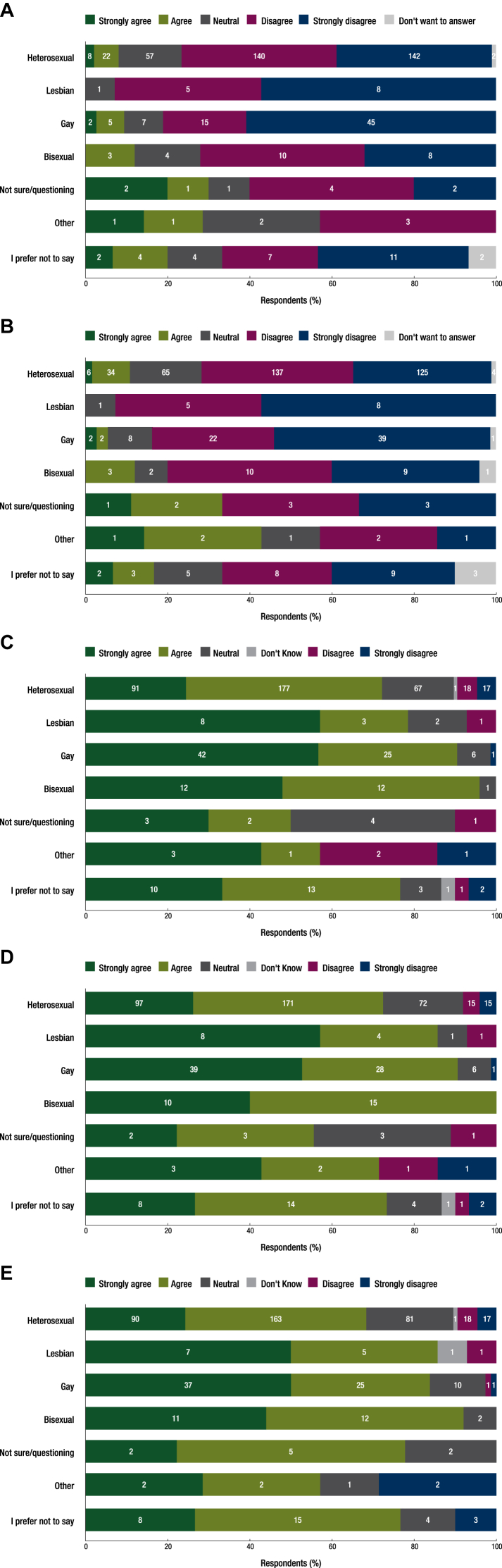
Although the majority felt ‘well informed’ about the health needs of LGBTQ patients, only 10.23% of the 567 respondents strongly agreed/agreed that this understanding was provided by the education at medical school (Figure 1A). Similarly, 10.78% of the 566 respondents agreed that their postgraduate training prepared them to address the needs of LGBTQ patients (Figure 1B).
Figure 1.
Respondent beliefs about education on the health care needs of LGBTQ patients. (A) Sexual orientation subgroups of respondents who believe that their education at medical school provided them with an understanding of the health care needs of LGBTQ patients. (B) Sexual orientation subgroups of respondents who believe that their postgraduate training in oncology provided them with an understanding of the health care needs of LGBTQ patients with cancer. (C) Sexual orientation subgroups of respondents who believe that education about the needs of LGBTQ patients should be included in medical school curricula. (D) Sexual orientation subgroups of respondents who believe that education about the needs of LGBTQ patients should be included in oncology postgraduate training curricula. (E) Sexual orientation subgroups of respondents who believe that all cancer specialists should have core education in LGBTQ cancer care.
LGBTQ, lesbian, gay, bisexual, transgender, and queer.
Most of the 538 respondents, 75.84%, strongly agreed/agreed that education about the needs of LGBTQ patients should be included in medical school curricula. Similarly, 76.68% strongly agreed/agreed that education about the needs of LGBTQ patients should be included in postgraduate oncology education curricula. Respondents agreed that all cancer specialists should have basic training in the treatment of LGBTQ patients (Figure 1C-E).
The respondents were overwhelmingly interested in further education about LGBTQ health issues; only 7.5% were not interested.
Discussion
This report represents the largest survey of CCPs’ attitude, knowledge, and education regarding LGBTQ patients with cancer and the very first, to our knowledge, to elicit information from 75 countries across continents, investigating the respondents’ SO and breaking down responses according to respondents’ SO.
Most of our respondents (67%) were willing to be listed as an LGBTQ-friendly provider, and 89% were comfortable treating LGBTQ patients, which is in accord with the 94% reported by Shetty et al.12 In our report, 90% and 81% of respondents were comfortable treating LGB and transgender patients, respectively, in accordance with other studies.13 Also the breakdown as per SO of the respondents was in accord with other studies, but showed a trend where lesbian and gay CCPs expressed more comfort and confidence in the health needs of this community.13
Although most respondents indicated that they were comfortable treating LGB patients, fewer (44%) expressed confidence in their knowledge of these patients’ health needs. This percentage differs from the Berner et al.’s14 survey in the UK, where only 8% felt confident of their knowledge of LGBTQ health care. Responses were quite divided according to SO; in the heterosexual subgroup, approximately two-thirds were confident of LGBTQ health needs, while in the lesbian and gay subgroups of CCPs, 79% were confident.
Our survey showed that CCPs were less comfortable treating transgender patients than individuals from the LGB community, perhaps due to the lower level of knowledge of their health needs. All subgroups of respondents, regardless of their SO, indicated that they did not feel well informed about the health needs of transgender patients.
Regrettably, few respondents (14%) reported a clear understanding of the psychosocial needs of LGBTQ individuals. Failure to identify LGBTQ patients may impair the therapeutic relationship, hindering open communication15 and possibly reducing therapeutic impact.16
The most compelling finding of our survey was the need for more education regarding LGBTQ health care; >90% of respondents expressed an interest in further education. Respondents were nearly unanimous in saying that their medical school and postgraduate training programme did not provide sufficient information regarding the needs of this community, with 72% stating that all cancer specialists should have a core training in LGBTQ cancer care.
Our survey has some limitations. It may not be representative of CCPs in general as it was sent to members of two medical societies only; it included relatively young CCPs and did not take into account differences in sociocultural factors and legislation affecting the LGBTQ community. Moreover, it focused on the issues of adult LGBTQ patients even if LGBTQ children and adolescents represent a group that face similar and some specific considerations. Many LGBTQ adolescents reported stigma in the health care setting, while many paediatricians experience difficulties in discussing SO and feel inadequately prepared to address issues pertaining to the health care needs of these adolescents.17,18 Future efforts in this population should address specific concerns, like parental-care dynamic and development as a barrier to disclosure.19
In conclusion, we recommend that education regarding the health issues specific to each LGBTQ subgroup should be incorporated into medical school and postgraduate curricula and that CCPs should be inclusive and sensitive for issues involving LGBTQ patients.
Acknowledgements
This is a project initiated by the ESMO/SIOPE Cancer in Adolescents and Young Adults Working Group. We thank ESMO and SIOP Europe leadership for their support in this manuscript, especially to ESMO Executive Board member—Director of Membership, Dr Evandro de Azambuja for his valuable comments on the manuscript. We also thank ESMO staff Mariya Radeva and Roberta Ferrandino for their help in survey conduction and analysis.
Funding
This work was supported by the European Society for Medical Oncology (ESMO) and the European Society for Paediatric Oncology (SIOP Europe) (no grant number).
Disclosure
ES reports receipt of honoraria for participation in Advisory Board from AstraZeneca A.E., AstraZeneca UK Ltd., Gilead Sciences Hellas, Merck, Sharp & Dohme, Pfizer Hellas; receipt of honoraria as invited speaker from Amgen Hellas, Pfizer Hellas; receipt of honoraria for providing expert testimony from Ipsen; and non-remunerated member of Board of Directors of Hellenic Oncology Research Group, Hellenic Society of Medical Oncology. IBS reports receipt of honoraria for participation in Advisory Board from Roche; receipt of honoraria as invited speaker from AstraZeneca, Novartis, Pfizer, Roche; receipt of personal and institutional financial interest as local principal investigator (PI) from Novartis, Roche; non-financial interest for serving as local PI in EORTC Breast Group, OncoDistinct studies; and non-remunerated advisory role in the Ethical Committee Serbia, National agency for drug registration Serbia, the Working Group for Oncology of the Ministry of Health Serbia. NG reports receipt of honoraria to institution for participation in Advisory Board from Y-mAbs Therapeutics; receipt of financial support to institution from Eisai for covering travel expenses and registration fees as invited speaker at international meeting for the presentation of the results from the studies as international PI; receipt of consultancy fee to institution from Ipsen; non-remunerated Co-chair of the Fostering Age inclusive Research (FAIR) trial group of ACCELERATE; non-remunerated member of the Executive Committee of EEC (Euro-Wing Consortium); non-financial interest for leadership role as Chair of the FOSTER Consortium (Fight Osteosarcoma Through European Research); and non-remunerated member of GO-AJA, SFCE, SIOP Europe. GM reports receipt of honoraria for participation in Advisory Board from BMS, Janssen, Roche, Takeda; and receipt of honoraria as invited speaker from Amgen, AstraZeneca, MSD, Novartis, Pfizer. SB reports receipt of honoraria for participation in Advisory Board from Bayer Healthcare, Boehringer Ingelheim, Eli Lilly, Hofmann La Roche, MAP-Biopharma; non-financial interest as PI of the Bayer’s larotrectinib study; and non-remunerated member of the European Musculoskeletal Oncology Society (EMSOS), German Pediatric Oncology Society (GPOH). DS reports receipt of honoraria for providing academic peer review for research programme from French INCa; non-financial interest for advisory role in the clinical reference group advising NHS England about cancer policy in children and young people; and non-remunerated membership of the Association of Cancer Physicians of the UK, SIOP Europe Adolescent Cancer Committee. AT reports receipt of honoraria for participation in Advisory Board from MSD, AstraZeneca, Pfizer, Eli Lilly; receipt of honoraria as invited speaker from Eli Lilly, Novartis; and non-remunerated member of AIOM. KS reports receipt of honoraria for participation in Advisory Board from Bayer, Novartis, Novo Nordisk, Roche; non-financial interest for leadership role in PanCare, SPOG, SSPHO; and non-remunerated member of Board of Directors of SIOP Europe. FP reports receipt of honoraria for participation in Advisory Board from Roche Diagnostics; receipt of honoraria for providing expert testimony from Ipsen, Merck; and non-financial interest for leadership role as Scientific Director of the European School of Oncology. All other authors have declared no conflicts of interest.
Footnotes
SIOP Europe Head Office, Clos Chapelle-aux-Champs 30, 1200 Brussels, Belgium. Tel: +32-2-880-62-82 E-mail: office@siope.eu
Supplementary data
References
- 1.Hunt R., Fish . The British Library; 2008. Prescription for Change: Lesbian and Bisexual Women’s Health Check 2008.https://www.stonewallcymru.org.uk/our-work/campaigns/2008-prescription-change-%e2%80%93-landmark-report-lesbian-and-bi-women%e2%80%99s-health [Google Scholar]
- 2.Peitzmeier S.M., Khullar K., Reisner S.L., Potter J. Pap test use is lower among female-to-male patients than non-transgender women. Am J Prev Med. 2014;47:808–812. doi: 10.1016/j.amepre.2014.07.031. [DOI] [PubMed] [Google Scholar]
- 3.Wilton T. Open University Press; Buckingham: 2000. Sexualities in Health and Social Care. [Google Scholar]
- 4.Cochran S.D., Mays V.M. Risk of breast cancer mortality among women cohabiting with same sex partners: findings from the national health interview survey, 1997–2003. J Womens Health. 2012;13(5):528–533. doi: 10.1089/jwh.2011.3134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blosnich J., Lee J.G., Horn K. A systematic review of the aetiology of tobacco disparities for sexual minorities. Tob Control. 2013;22:66–73. doi: 10.1136/tobaccocontrol-2011-050181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kamen C., Blosnich J.R., Lytle M., Janelsins M.C., Peppone L.J., Mustian K.M. Cigarette smoking disparities among sexual minority cancer survivors. Prev Med Rep. 2015;2:283–286. doi: 10.1016/j.pmedr.2015.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chin-Hong P.V., Vittinghoff E., Cranston R.D., et al. Age-related prevalence of anal cancer precursors in homosexual men: the EXPLORE study. J Natl Cancer Inst. 2005;97(12):896–905. doi: 10.1093/jnci/dji163. [DOI] [PubMed] [Google Scholar]
- 8.Quinn G.P., Sanchez J.A., Sutton S.K., et al. Cancer and lesbian, gay, bisexual, transgender/transsexual, and queer/questioning (LGBTQ) populations. CA Cancer J Clin. 2015;65(5):384–400. doi: 10.3322/caac.21288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chin-Hong P.V., Vittinghoff E., Cranston R.D., et al. Age-specific prevalence of anal human papillomavirus infection in HIV-negative sexually active men who have sex with men: the EXPLORE study. J Infect Dis. 2004;190(12):2070–2076. doi: 10.1086/425906. [DOI] [PubMed] [Google Scholar]
- 10.NHS England and Improvement . 2019. NHS Cancer Patient Experience Survey.https://www.england.nhs.uk/statistics/statistical-work-areas/cancer-patient-experience-survey/ [Google Scholar]
- 11.Lisy K., Peters M.D.J., Schofield P., Jefford M. Experiences and unmet needs of lesbian, gay, and bisexual people with cancer care: a systematic review and meta-synthesis. Psychooncology. 2018;27(6):1480–1489. doi: 10.1002/pon.4674. [DOI] [PubMed] [Google Scholar]
- 12.Shetty G., Sanchez J.A., Lancaster J.M., et al. Oncology healthcare providers’ knowledge, attitudes, and practice behaviors regarding LGBT health. Patient Educ Couns. 2016;99(10):1676–1684. doi: 10.1016/j.pec.2016.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schabath M.B., Blackburn C.A., Sutter M.E., et al. National survey of oncologists at National Cancer Institute-designated comprehensive cancer centers: attitudes, knowledge, and practice behaviors about LGBTQ patients with cancer. J Clin Oncol. 2019;37(7):547–558. doi: 10.1200/JCO.18.00551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Berner A.M., Hughes D.J., Tharmalingam H., et al. An evaluation of self-perceived knowledge, attitudes and behaviours of UK oncologists about LGBTQ patients with cancer. ESMO Open. 2020;5(6) doi: 10.1136/esmoopen-2020-000906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Banerjee S.C., Staley J.M., Alexander K., Walters C.B., Parker P.A. Encouraging patients to disclose their lesbian, gay, bisexual, or transgender (LGBT) status: oncology health care providers’ perspectives. Transl Behav Med. 2020;10(4):918–927. doi: 10.1093/tbm/iby105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fish J., Williamson I., Brown J. Disclosure in lesbian, gay and bisexual cancer care: towards a salutogenic healthcare environment. BMC Cancer. 2019;19:678. doi: 10.1186/s12885-019-5895-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lena S.M., Tannis W., Sara I., Jabbour M. Pediatricians’ knowledge, perceptions, and attitudes towards providing health care for lesbian, gay, and bisexual adolescents. Ann R Coll Physicians Surg Can. 2022;35(7):406–410. [PubMed] [Google Scholar]
- 18.Stern M. Perspectives of LGBTQ youth and pediatricians in the primary care setting: a systematic review. J Prim Care Community Health. 2021;12:1–5. doi: 10.1177/21501327211044357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gannon T., Phillips B., Saunders D., Berner A.M. Knowing to ask and feeling safe to tell - understanding the influences of HCP-patient interactions in cancer care for LGBTQ+ children and young people. Front Oncol. 2022;12 doi: 10.3389/fonc.2022.891874. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.