Abstract
The purpose of this study was to investigate early stage dynamic changes in relevant indicators in neurocritical patients to identify biomarkers that can predict a poor prognosis at an early stage (1–4 days after admission). This study retrospectively collected clinical data, inflammatory indicators, and nutritional indicators from 77 patients at the neurology intensive care unit. The 3-month modified Rankin scale score was used as the outcome indicator. A linear mixed model was used to analyze changes in inflammatory indicators and nutritional indicators in neurocritical patients over time from 1–4 days after admission. Logistic regression was used to determine the independent risk factors for a poor prognosis in neurocritical patients and to construct a predictive model. The predictive efficacy of the model was verified using leave-one-out cross-validation and decision curve analysis methods. The analysis results showed that 1–4 days after admission, the inflammatory indicators of white blood cell and absolute monocyte counts and the nutritional indicators of body cell mass(BCM), fat-free mass, body cell mass/phase angle (BCM/PA), intracellular water, extracellular water, and skeletal muscle index increased overall, while the nutritional indicators of albumin and visceral fat area decreased overall. The logistic multivariate regression model showed that the Charlson comorbidity index (CCI) (odds ratio (OR) = 2.526, 95% CI [1.202, 5.308]), hemoglobin (Hb)(on admission)-Hb(min) (OR = 1.049, 95% CI [1.015, 1.083), BCM(on admission) (OR = 0.794, 95% CI [0.662, 0.952]), and the change in BCM/PA 1–4 days after admission (OR = 1.157, 95% CI [1.070, 1.252]) were independent risk factors for a poor prognosis in neurocritical patients. The predictive analysis showed that the predictive power of Model 1 with BCM/PA (area under the curve (AUC) = 0.95, 95% CI (0.90, 0.99)) was 93%, 65%, 141%, and 133% higher than that of Model 2 without BCM/PA, the CCI, the APACHE II score, and the NRS2002 score (all P < 0.05), respectively. The CCI, Hb(on admission)-Hb(min), BCM(on admission), and an increase in BCM/PA 1–4 days after admission were independently associated with a poor prognosis in neurocritical patients. Of these variables, BCM/PA may be a valid indicator for early stage prediction of a poor prognosis in neurocritical patients.
Keywords: Biomarkers, Bioelectrical impedance, Systemic inflammatory response syndrome, Modified Rankin Scale, Neurocritical patient
Subject terms: Biomarkers, Neurology
Introduction
In recent years, the mortality and disability rates of neurocritical patients have increased yearly, which has resulted in substantial medical and social burdens1. A large number of studies have found that a persistent severe inflammatory response can lead to aggravation of the primary disease. It is an important cause of poor prognosis and even death in neurocritical patients2–6. Brain-body crosstalk is an important component of the pathophysiological process of systemic inflammatory response syndrome (SIRS) in neurocritical patients7,8. In related studies of patients with acute cerebral hemorrhage and status epilepticus, mortality and disability rates were higher in the patients with SIRS than in the non-SIRS group8,9. In a study of stroke patients, Vahidy10 found that after stroke, the spleen was activated and contracted, releasing a large number of immune cells into the bloodstream that migrate to the brain and infiltrate the brain parenchyma through the damaged blood–brain barrier. The number of immune cells in the brain reaches its peak within 1–4 days after stroke and further promotes the release of inflammatory factors in brain glial cells, causing an inflammatory cascade reaction that induces a large amount of neuronal cell necrosis, which affects the patient’s prognosis. Therefore, the severity of SIRS in neurocritical patients in the early stage (1–4 days after admission) may be the key to determining their prognosis, possibly related to neuronal cell necrosis due to inflammation. The pathogenesis is abnormal accumulation of misfolded/unfolded proteins within the endoplasmic reticulum of nerve cells11. Normally, DNA information transmission translates into polypeptide chains12–14, which are folded into functional proteins within the cellular endoplasmic reticulum, and protein folding is important for maintaining the balance of cellular homeostasis15–17. At the onset of neurocritical illness, abnormal accumulation of misfolded/unfolded proteins provokes endoplasmic reticulum stress in neuronal cells, which induces a cascade of cell death and inflammatory processes when endoplasmic reticulum stress is persistent and intense. The inflammatory cascade in turn aggravates endoplasmic reticulum stress, creating a vicious cycle that triggers neuronal cell death and further leading to the occurrence of a poor prognosis11.
Previous studies have suggested that the severity of SIRS is affected by nutritional status18.The main manifestation of malnutrition in neurocritical patients is protein-energy malnutrition (PEM)19. By damaging the immune system’s defense ability, PEM leads to a significant reduction in the body's resistance to infection20. Its pathophysiological mechanism manifests primarily as atrophy of thymus and lymph node immune tissues, impaired humoral and cellular immune functions, weakened leukocyte phagocytosis and reduced protein synthesis, which create favorable conditions for infection21. After infection, inflammatory cytokines act on the neuroendocrine system to stimulate the release of stress hormones (including cortisol and catecholamines) and increase catabolism, leading to further deterioration of immune system function in neurocritical patients, aggravating the degree of inflammatory response, accelerating death and leading to a poor prognosis22.f
Currently, the relevant indicators that can be used to predict adverse outcomes in neurocritical patients include neutrophil count, lymphocyte count, neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio, lymphocyte-to-monocyte ratio, albumin, and hemoglobin. However, the specificity of these indicators is not high23–26, and most studies are based on a one-time cutoff point and lack observations of dynamic change trends; thus, the predictive performance of these indicators is controversial, and they are not suitable as early stage and effective prognostic indicators for neurocritical patients. In recent years, studies have confirmed that relevant indicators measured by bioelectrical impedance analysis (BIA), such as phase angle (PA), body cell mass(BCM), and hydration status, are associated with inflammatory responses and a poor prognosis in patients who are elderly, have cancer, are undergoing hemodialysis, or are critically ill27–31. They are expected to become novel indicators for predicting patient prognosis, but their predictive efficacy needs to be further verified.
Therefore, this study continuously and dynamically collected the clinical data (demographic characteristics + disease-related indicators), inflammatory indicators, and nutritional indicators (biochemical and BIA indicators) of 77 neurocritical patients who were admitted to the neurology intensive care unit (NICU) from January to July 2021 in the early stage (1–4 days after admission). A retrospective analysis of the changes in the data was conducted to identify objective occurrence and development patterns and to identify novel and sensitive early stage indicators for the objective prediction of poor prognosis in neurocritical patients.
Methods
Study design and participants
This was a case–control study. The subjects were patients who were admitted to the NICU of the First Affiliated Hospital of Chongqing Medical University from January 2021 to July 2021. Medical case data during hospitalization were collected. The study was conducted in accordance with the Declaration of Helsinki and was approved by the Medical Ethics Committee of the First Affiliated Hospital of Chongqing Medical University. This study was registered with the China Clinical Trials Registry with registration number ChiCTR1800014324.
Inclusion criteria
(1) since the hospital is an adult hospital, the age of the included subjects was ≥ 18 years; (2) NICU admission ≥ 4 days; (3) patients or their guardians provided written informed consent; and (4) patients who can remain in a static supine position after sedation.
Exclusion criteria
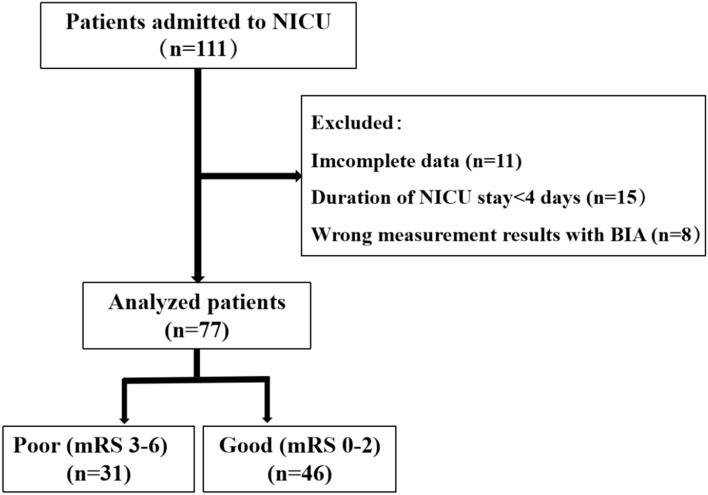
(1) patients with moderate or severe disability and no self-care in life before admission; (2) patients with agitation, those who were unable to undergo BIA treatment due to the implantation of metal devices (such as pacemakers or artificial femoral heads), and those with incorrect BIA values; (3) patients whose conditions relapsed during hospitalization and those who required two or more transfers to the NICU; (4) patients with unstable hemodynamics; (5) patients who required surgical treatment; and (6) patients with incomplete data; (7) patients who received a red blood cell transfusion (Fig. 1).
Fig. 1.
Flowchart of patient recruitment.
Outcome indicator
The outcome indicator was the 3-month modified Rankin scale (mRS) score. According to the mRS score, the patients were divided into two groups: the poor prognosis group (mRS 3–6) and the good prognosis group (mRS 0–2).
Data collection
In this study, a retrospective data collection method was used to continuously and dynamically collect the clinical data, inflammatory indicators, and nutritional indicators (including biochemical and BIA indicators) of 77 NICU patients over 1–4 days of admission. All data were collected and compiled by two dedicated research assistants using the electronic medical record system and the BIA instrument recording system, and data entry was performed separately. After data entry was completed, the patient’s name and medical record number were deleted, and the patient was given a unique study number. All data were reviewed by a dedicated study coordinator to confirm their accuracy and to manually verify inconsistent or outlier values. Prior to the statistical analysis, the dataset was validated and cleaned to prevent any further changes and ensure the consistency and completeness of the data in the statistical report and analysis. All researchers who collected and compiled the data were unaware of the contents of the study.
Clinical data:
Demographic characteristics
Sex, age, and body mass index (BMI).
Disease-related indicators
Acute Physiology and Chronic Health Evaluation (APACHE) II score, CCI, Nutrition Risk Screening 2002 (NRS 2002) results, disease diagnosis, and history of previous neurological diseases.
Inflammation-related indicators
White blood cell (WBC) count, absolute neutrophil count (ANC), absolute lymphocyte count (ALC), absolute monocyte count (AMC), platelet count (PLT), neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), and high-sensitivity C-reactive protein (hsCRP).
Nutrition-related indicators
Nutritional biochemical indicators
Albumin (Alb), Hb, and random blood glucose (Glu).
BIA indicators
BCM, PA, BCM/PA, intracellular water (ICW), extracellular water (ECW), total body water (TBW), fat-free mass (FFM), skeletal muscle index (SMI), whole-body protein (WBP), and visceral fat area (VFA).
Definition of relevant indicators
CCI32: The patient’s medical history was obtained through the electronic medical record system, and CCI scores were calculated according to the patient’s comorbidities. APACHE II score33: The assessment value within 24 h after admission was used. If there were multiple assessment values, the highest assessment value was selected. NRS2002 score34: The assessment value within the first day of admission was used.
All laboratory indicators were evaluated at the Department of Laboratory Medicine, the First Affiliated Hospital of Chongqing Medical University, and uploaded to the electronic medical record system. The patients’ test values 1–4 days after admission were collected based on the time blood specimens being taken. In this context, Hb(on admission) is the value detected within 24 h after admission and Hb(min) is the minimum value of Hb detected within 1–4 days after admission. In this study, the normal reference ranges for the relevant laboratory indicators were WBC (3.50–9.50*109/L), ANC (1.80–6.30*109/L), ALC (1.10–3.20*109/L), AMC (0.10–0.60*109/L), PLT (85–303*109/L), hsCRP (0–10 mg/ml), Alb (35–50 g/L), Hb (130–175 g/L), and Glu (< 11.1 mmol/L). The BIA indicators were measured by physicians of the Department of Nutrition of the First Affiliated Hospital of Chongqing Medical University using BIA according to a uniform and standardized method on days 1–4 of admission, and the measurement values were collected. The BIA model was InBody S10 from Biospace Co., Ltd., South Korea35. In this study, the normal reference ranges for BIA indicators were PA (> 3 degrees), TBW (0.36–0.39%), TBW/FFM (72.7–74.3%), SMI (men > 7 kg/m2; women > 5.5 kg/m2), and VFA (< 100 cm2).
The guidelines of the European Working Group on sarcopenia suggest that the BIA indicator skeletal muscle mass is closely related to height, and the composite index SMI, which is the combination of skeletal muscle and height, can accurately reflect the nutritional status of the human body36. Therefore, this study included the composite indicator SMI, which was calculated using the following formula: SMI = skeletal muscle mass/height2. Furthermore, the BIA measurement BCM is the sum of the number of metabolically active and functionally intact somatic cells in human lean body mass. PA is the cotangent value of reactance (Xc) and impedance (Z) generated by a current flowing through the human body. Its formula is sin(PA) = Reactance(Xc)/Impedance(Z), which reflects the integrity of the cell membrane. Relevant studies have pointed out that a decrease in PA reflects poor structure and low function of the cell membrane as a result of a decrease in BCM, and there is a strong interaction between BCM and PA37. Therefore, this study innovatively combined BCM and PA detection into one BIA measurement as the second-level indicator BCM/PA and calculated the ratio to determine the prognostic value of this second-level indicator.
In recent years, a large amount of evidence has shown that there is a strong interaction among inflammatory cytokines and between inflammatory cytokines and platelets in the pathophysiological development of systemic inflammatory responses38. Therefore, this study included the analysis of relevant composite inflammatory indicators, including NLR and PLR. The NLR and PLR values were calculated using the same blood specimen. The calculation formulas are NLR = ANC/ALC and PLR = PLT/ALC.
Statistical description
Normally distributed measurement data are described as the mean ± standard deviation, and two independent sample t tests were used for comparisons between groups. Measurement data with a skewed distribution are described as the median and interquartile range, and the Mann–Whitney U test was used for comparisons between groups. Count data are described as the number of cases and rate, and comparisons between groups were performed using the chi-square test or Fisher’s exact probability test. This study was reported based on the TRIPOD guidelines for prediction model development/validation38. A mixed linear model was used to calculate the slopes of the changes in inflammatory and nutritional indicators over time on days 1–4 of admission, and the slope for each patient was calculated using the random effects model. Variables with P < 0.05 in the univariate analysis were included in the multivariate analysis, and the variables were screened using the stepwise method. Multivariate logistic regression was used to investigate the risk factors associated with the prognosis at discharge. The area under the receiver operator characteristic (ROC) curve (AUC), accuracy, sensitivity, specificity, integrated discrimination improvement index (IDI), net reclassification improvement (NRI), and decision curve analysis (DCA) were used to compare the value of different indicators or models for predicting the prognosis at discharge. AUCs were compared using the Delong test. The leave-one-out cross-validation method was used for internal validation. The Delong test, IDI, NRI, and DCA were performed in the nsROC, PredictABEL, nricens, and rmda packages in R (version 4.0.0), respectively, and the other analyses were performed in SAS 9.4 (Copyright ©2016 SAS Institute, Inc., Cary, NC, USA).
Results
Baseline data analysis
A total of 77 neurocritical patients were included in this study. The analysis of their baseline data is shown in Table 1. There were 31 patients with a poor prognosis according to the mRS (3–6), of whom 16 died; 46 patients had a good prognosis according to the mRS (0–2). The analysis results showed that indicators within the first day of admission (age, APACHE II score, CCI, NRS2002, hsCPR, BCM/PA ratio, TBW) and Hb(on admission)-Hb(min) were higher in the poor prognosis group than in the good prognosis group, and the differences between the two groups were statistically significant (all P values < 0.05) (Table 1).
Table 1.
Comparison of baseline data between the poor prognosis group and the good prognosis group.
| Indicator | Group | χ2/t/Z | P value | |
|---|---|---|---|---|
| Good (mRS 0–2) (n = 46) | Poor (mRS 3–6) (n = 31) | |||
| Clinical data | ||||
| Sex | ||||
| Male | 33(71.74) | 16(51.61) | 3.242 | 0.072 |
| Female | 13(28.26) | 15(48.39) | ||
| Age, years | 59.13 ± 16.23 | 71.45 ± 13.77 | − 3.467 | 0.001 |
| BMI, kg/m2 | 24.43 ± 4.14 | 23.38 ± 3.85 | 1.087 | 0.281 |
| APACHE II | 9.61 ± 4.96 | 15 ± 5.2 | − 4.584 | < 0.001 |
| CCI | 0.43 ± 0.75 | 1.48 ± 1.36 | − 3.905 | < 0.001 |
| NRS2002 | 2.87 ± 2.01 | 5.13 ± 2 | − 4.856 | < 0.001 |
| Diagnosis (classification) | ||||
| Cerebrovascular diseases | 31(67.39) | 21(67.74) | / | > 0.999 |
| CNS infectious diseases | 5(10.87) | 3(9.68) | ||
| Neuromyelitis optica | 1(2.17) | 1(3.23) | ||
| Epilepsy | 7(15.22) | 4(12.90) | ||
| Metabolic encephalopathy | 1(2.17) | 1(3.23) | ||
| Degenerative diseases of the nervous system | 1(2.17) | 1(3.23) | ||
| Previous history of neurological diseases | ||||
| No | 41(89.13) | 28(90.32) | / | > 0.999 |
| Yes | 5(10.87) | 3(9.68) | ||
| Inflammatory indicators within the first day of admission | ||||
| WBC, *109/L | 9.63 ± 3.12 | 10.77 ± 4.72 | − 1.181 | 0.244 |
| ANC, *109/L | 7.78 ± 3.12 | 8.56 ± 3.87 | − 0.98 | 0.33 |
| ALC, *109/L | 1.22 ± 0.55 | 0.99 ± 0.51 | 1.806 | 0.075 |
| AMC, *109/L | 0.55 ± 0.23 | 0.61 ± 0.44 | − 0.766 | 0.448 |
| PLT, *109/L | 208.96 ± 71.23 | 184.45 ± 60.1 | 1.574 | 0.12 |
| NLR | 8.62 ± 7.44 | 10.57 ± 6.77 | − 1.171 | 0.245 |
| PLR | 196.58 ± 103.67 | 217.98 ± 106.03 | − 0.876 | 0.384 |
| hsCPR, mg/L | 5.22(2.46,10.87) | 14.91(6.21,20) | − 3.317 | 0.001 |
| Nutritional biochemical indicators | ||||
| Alb(on admission), g/L | 39.67 ± 5.02 | 37.32 ± 5.88 | 1.865 | 0.066 |
| Hb(on admission), g/L | 140.57 ± 16.48 | 127.29 ± 24.69 | 2.625 | 0.012 |
| Hb(on admission)-Hb(min), g/L | 9.5(5,20) | 35(6,59) | 3.166 | 0.002 |
| Glu(on admission), mmol/L | 6.5(5.4,8.9) | 7.9(6.5,8.7) | 1.369 | 0.171 |
| BIA indicators within the first day of admission | ||||
| BCM, kg | 30.54 ± 5.1 | 27.1 ± 5.8 | 2.659 | 0.01 |
| PA, degree | 5.57 ± 0.86 | 4.06 ± 0.93 | 7.077 | < 0.001 |
| BCM/PA, kg/degree | 5.53 ± 0.83 | 6.89 ± 1.64 | − 4.04 | < 0.001 |
| ICW, L | 21.33 ± 3.56 | 18.92 ± 4.06 | 2.663 | 0.01 |
| ECW, L | 13.27 ± 2.07 | 12.53 ± 2.41 | 1.396 | 0.167 |
| TBW, % | 0.38 ± 0.01 | 0.4 ± 0.01 | − 6.733 | < 0.001 |
| FFM, kg | 46.97 ± 7.57 | 42.57 ± 8.65 | 2.287 | 0.025 |
| TBW/FFM, % | 73.64 ± 0.38 | 73.79 ± 0.37 | − 1.722 | 0.089 |
| SMI, kg/m2 | 9.55 ± 1.32 | 8.53 ± 1.62 | 2.929 | 0.005 |
| WBP, kg | 9.21 ± 1.54 | 8.18 ± 1.75 | 2.649 | 0.01 |
| VFA, cm2 | 93.9 ± 49.57 | 118.33 ± 63.64 | − 1.834 | 0.071 |
| Outcome indicators | ||||
| Mortality rate within 3 months | ||||
| Survival | 46(100.00) | 15(48.39) | 29.969 | < 0.001 |
| Death | 0(0.00) | 16(51.61) | ||
| Mechanical ventilation time, days | 0(0,0) | 6(0,13) | 4.597 | < 0.001 |
| NICU hospital stay, days | 4(2,10) | 13(7,23) | 4.551 | < 0.001 |
| Total hospital stay, days | 15.5(13,25) | 25(13,37) | 1.763 | 0.078 |
| Hospitalization expenses, thousand yuan | 29.15(20.11,69.84) | 112.97(56.2,192.59) | 4.072 | < 0.001 |
Cerebrovascular diseases which include ischemic stroke, intracerebral hemorrhage; CNS infectious diseases = Central nervous system infection diseases, CNS infectious diseases which include N-methyl-D-aspartate receptors, tuberculous meningitis, viral encephalitis, toxoplasma encephalitis, purulent meningitis; Degenerative diseases of the nervous system which include parkinson's disease, Alzheimer's disease.
Within the first day of admission, the Hb, BCM, PA, ICW, FFM, SMI, and WBP of the poor prognosis group were all lower than those of the good prognosis group, and the differences between the two groups were statistically significant (all P < 0.05). Additionally, among the outcome indicators, the differences in mortality, mechanical ventilation time, length of NICU stay, and hospitalization expenses between the two groups were statistically significant (all P values < 0.05) (Table 1).
Analysis of the change trends in inflammatory and nutritional indicators 1–4 days after admission
The inflammatory and nutritional indicators of the 77 neurocritical patients were repeatedly measured (1–4 days after admission) and were treated as time-dependent variables. The analysis of the change trends in the indicators over time is shown in Table 2. Inflammation indicators (WBC, AMC) showed an overall upward trend over time from day 1 to day 4 after admission. The nutritional index albumin (Alb) decreased gradually with time; BIA indicators (BCM, BCM/PA, ICW, ECW, FFM, SMI) showed an overall upward trend over time from day 1 to day 4 after admission. However, the trend of BIA indicator (VFA) changes with time is gradually declining (Table 2).
Table 2.
Trend analysis of the change in indicators in neurocritical patients 1–4 days after admission.
| Variable | Day 1 | Day 2 | Day 3 | Day 4 | Coefficient | Standard error | t value | P value |
|---|---|---|---|---|---|---|---|---|
| Inflammatory indicators | ||||||||
| WBC, *109/L | 10.09 ± 3.85 | 10.98 ± 4.95 | 12.17 ± 5.47 | 11.50 ± 4.76 | 0.549 | 0.213 | 2.58 | 0.012 |
| ANC, *109/L | 8.09 ± 3.44 | 8.47 ± 3.67 | 9.68 ± 4.93 | 9.37 ± 4.48 | 0.397 | 0.203 | 1.96 | 0.055 |
| ALC, *109/L | 1.13 ± 0.54 | 1.20 ± 0.50 | 1.19 ± 0.54 | 1.18 ± 0.59 | 0.04 | 0.021 | 1.93 | 0.058 |
| AMC, *109/L | 0.57 ± 0.33 | 0.72 ± 0.27 | 0.87 ± 0.39 | 0.84 ± 0.42 | 0.099 | 0.018 | 5.41 | < .0001 |
| PLT, *109/L | 199.09 ± 67.65 | 183.24 ± 60.97 | 193.54 ± 74.38 | 189.85 ± 70.01 | − 1.516 | 2.322 | − 0.65 | 0.516 |
| NLR | 9.40 ± 7.20 | 8.27 ± 4.67 | 10.30 ± 8.14 | 9.81 ± 5.99 | 0.061 | 0.363 | 0.17 | 0.868 |
| PLR | 205.31 ± 104.47 | 177.82 ± 89.17 | 193.06 ± 111.96 | 187.92 ± 89.47 | − 7.504 | 4.572 | − 1.64 | 0.105 |
| Nutritional biochemical indicators | ||||||||
| Alb, g/L | 38.71 ± 5.47 | 36.56 ± 5.51 | 36.25 ± 5.21 | 35.75 ± 5.64 | − 0.984 | 0.227 | − 4.34 | < .0001 |
| BIA indicators BCM, kg | 29.22 ± 5.60 | 29.14 ± 5.76 | 29.53 ± 5.76 | 29.88 ± 5.49 | 0.119 | 0.059 | 2.02 | 0.047 |
| PA, degree | 4.99 ± 1.15 | 4.92 ± 1.28 | 8.18 ± 18.43 | 4.96 ± 1.30 | 0.159 | 0.511 | 0.31 | 0.757 |
| BCM/PA, kg/degree | 6.05 ± 1.37 | 6.19 ± 1.51 | 6.42 ± 2.17 | 6.31 ± 1.54 | 0.112 | 0.041 | 2.71 | 0.008 |
| ICW, L | 20.41 ± 3.91 | 20.35 ± 4.01 | 20.62 ± 4.03 | 20.88 ± 3.83 | 0.086 | 0.04 | 2.14 | 0.035 |
| ECW, L | 12.99 ± 2.22 | 13.02 ± 2.28 | 13.28 ± 2.22 | 13.35 ± 2.11 | 0.109 | 0.03 | 3.59 | 0.001 |
| TBW, % | 0.39 ± 0.01 | 0.39 ± 0.01 | 0.39 ± 0.01 | 0.46 ± 0.47 | 0.015 | 0.021 | 0.7 | 0.486 |
| FFM, kg | 45.28 ± 8.23 | 45.23 ± 8.41 | 45.90 ± 8.39 | 46.38 ± 7.95 | 0.244 | 0.095 | 2.58 | 0.012 |
| SMI, kg/m2 | 9.16 ± 1.52 | 9.15 ± 1.56 | 9.24 ± 1.52 | 9.41 ± 1.53 | 0.043 | 0.02 | 2.18 | 0.033 |
| WBP, kg | 8.82 ± 1.69 | 8.79 ± 1.75 | 8.91 ± 1.73 | 9.00 ± 1.67 | 0.032 | 0.019 | 1.71 | 0.091 |
| VFA, cm2 | 103.27 ± 56.25 | 102.13 ± 52.49 | 102.40 ± 51.29 | 95.80 ± 54.02 | − 1.879 | 0.711 | − 2.64 | 0.01 |
The results of the differential analysis based on time-dependent variables showed that the BCM/PA of the poor prognosis group had a higher change trend 1–4 days after admission than the good prognosis group (P < 0.05) (Table 3).
Table 3.
Differential analysis of the change trends in indicators between the two groups 1–4 days after admission.
| Indicator | Group | t | P value | |
|---|---|---|---|---|
| Good (mRS 0–2) (n = 46) | Poor (mRS 3–6) (n = 31) | |||
| Inflammatory indicators | ||||
| WBC slopes, *109/L/day | 0.46 ± 0.79 | 0.68 ± 0.91 | − 1.114 | 0.269 |
| AMC slopes, *109/L/day | 0.09 ± 0.07 | 0.11 ± 0.09 | − 0.936 | 0.352 |
| Nutritional biochemical indicators | ||||
| Alb slopes, g/L/day | − 0.88 ± 0.8 | − 1.14 ± 1.07 | 1.24 | 0.219 |
| BIA indicators | ||||
| BCM slopes, kg/day | 0.1 ± 0.17 | 0.14 ± 0.32 | − 0.616 | 0.541 |
| BCM/PA slopes, kg/degree/day | 0.04 ± 0.11 | 0.22 ± 0.21 | − 4.448 | < 0.001 |
| ICW slopes, L/day | 0.07 ± 0.11 | 0.11 ± 0.22 | − 0.767 | 0.448 |
| ECW slopes, L/day | 0.09 ± 0.1 | 0.14 ± 0.2 | − 1.195 | 0.239 |
| FFM slopes, kg/day | 0.2 ± 0.31 | 0.31 ± 0.57 | − 0.939 | 0.353 |
| SMI slopes, kg/m2/day | 0.04 ± 0.05 | 0.05 ± 0.1 | − 0.829 | 0.412 |
| VFA slopes, cm2/day | − 1.62 ± 3.17 | − 2.26 ± 3.81 | 0.793 | 0.43 |
Correlation analysis for poor prognosis in neurocritical patients
The above variables with P < 0.05, which included age, APACHE II, CCI, NRS2002, indicators within the first day of admission (hsCPR, Hb, BCM, PA, BCM/PA, ICW, TBW, FFM, SMI, WBP), Hb(on admission)-Hb(min) and change in BCM/PA 1–4 days after admission (Table 1), were included in multivariate analysisin.
The results showed that CCI (odds ratio (OR) = 2.526, 95% confidence interval (CI) [1.202, 5.308]), Hb(on admission)-Hb(min) (OR = 1.049, 95% CI [1.015, 1.083]) and change in BCM/PA 1–4 days after admission (OR = 1.157, 95% CI [1.070, 1.252]) were independent risk factors for poor prognosis in neurocritical patients. In contrast, higher BCM (on admission) (30.54 ± 5.1) was a protective factor (OR = 0.794, 95% CI [0.662, 0.952]) (Table 4).
Table 4.
Results of the multivariate logistic regression model analysis for poor prognosis in neurocritical patients.
| Variable | β | Standard error | χ2 | P | OR(95%CI) |
|---|---|---|---|---|---|
| CCI | 0.926 | 0.379 | 5.978 | 0.014 | 2.526(1.202,5.308) |
| Hb(on admission)-Hb(min), g/L | 0.047 | 0.017 | 8.041 | 0.005 | 1.049(1.015,1.083) |
| BCM (on admission), kg | − 0.231 | 0.093 | 6.179 | 0.013 | 0.794(0.662,0.952) |
| BCM/PA slopes, *10−2, kg/degree/day | 0.146 | 0.04 | 13.315 | < 0.001 | 1.157(1.070,1.252) |
Predictive analysis results
The variables that were significant in the multivariate analysis, including CCI, Hb(on admission)-Hb (min), and BCM(on admission), and the change in BCM/PA 1–4 days after admission were used to establish Model 1 and Model 2, respectively. The change in BCM/PA 1–4 days after admission was included in Model 1 but not in Model 2. The remaining predictors were the same in both Model 1 and Model 2. The results of the predictive analysis showed that the AUC of Model 1 (AUC = 0.95, 95% CI (0.90, 0.99)) was higher than that of Model 2 (AUC = 0.85, 95% CI (0.76, 0.95)) (P < 0.05). The accuracy, sensitivity, and specificity of Model 1 were all higher than those of Model 2, the CCI, the APACHE II score, and the NRS2002 score. The NRI indicator of IDI in Model 1 was higher than that in Model 2, CCI, APACHE II and NRS2002. That is, compared with Model 2, the CCI, the APACHE II score, and the NRS2002 score, the proportion of correct patient classifications by Model 1 increased by 20%, 43%, 40%, and 37%, respectively, and its predictive ability increased by 93%, 65%, 141% and 133%, respectively (all P < 0.05) (Table 5).
Table 5.
Comparison of the efficacy of different indicators for predicting the prognosis of neurocritical patients.
| Predictors | AUC(95%CI) | P | Accuracy% | Sensitivity% | Specificity% | IDI(95%CI) | P | NRI(95%CI) | P |
|---|---|---|---|---|---|---|---|---|---|
| Model 1 | 0.95(0.90,0.99) | 85.71 | 93.55 | 80.43 | |||||
| Model 2 | 0.85(0.76,0.95) | 0.024a | 81.82 | 80.65 | 82.61 | 0.93(0.57,0.93)a | < 0.001a | 0.20(0.09, 0.31)a | 0.003a |
| CCI | 0.74(0.63,0.95) | < 0.001a | 70.13 | 70.97 | 69.57 | 0.65(0.39,0.65)a | < 0.001a | 0.43(0.31,0.55)a | < 0.001a |
| APACHEII | 0.77(0.66,0.88) | 0.002a | 71.43 | 80.65 | 65.22 | 1.41(1.14,1.41)a | < 0.001a | 0.40(0.27,0.53)a | < 0.001a |
| NRS2002 | 0.79(0.68,0.89) | 0.003a | 72.73 | 80.65 | 67.39 | 1.33(1.01,1.33)a | < 0.001a | 0.37(0.23,0.51)a | < 0.001a |
Model 1 cannot be calculated at admission since the indicator (BCM/PA) is to be obtained upon continuous measurement within the first 4 days after admission.
Model 1: Includes BCM/PA slopes, CCI, Hb(on admission)-Hb(min), and BCM(on admission).
Model 2: Includes CCI, Hb(on admission)-Hb(min), and BCM(on admission).
a: Compared with Model 1.
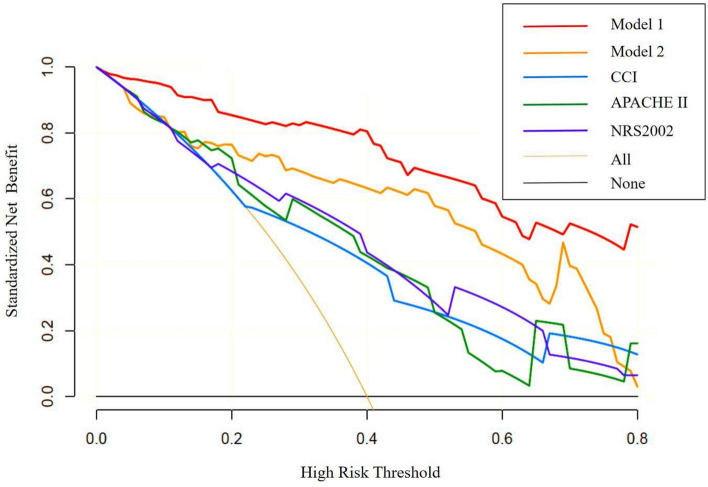
According to the results of the decision curve analysis, the standard net benefit values of Model 1 were all higher than those of Model 2, the CCI, the APACHE II score, and the NRS2002 (Fig. 2).
Fig. 2.
Decision curves of different indicators for predicting the prognosis of discharged patients.
Discussion
This was a case–control study that included 77 neurocritical patients. The study retrospectively analyzed the patients’ clinical data (demographic characteristics, disease-related indicators), inflammatory indicators, and nutritional indicators (nutritional biochemical indicators, BIA indicators). The results of the linear mixed model analysis showed that the inflammatory indicators (WBC and AMC) and BIA indicators (BCM, BCM/PA, ICW, ECW, FFM, and SMI) increased overall 1–4 days after admission, while the nutritional biochemical indicators (Alb) and BIA indicator (VFA) decreased overall. After adjusting for confounding factors using the logistic multivariate regression model, CCI, Hb(on admission)-Hb(min), BCM(on admission), and the change in BCM/PA 1–4 days after admission were independently correlated with a poor prognosis in neurocritical patients.
The CCI is an assessment scale that reflects patient comorbidity and can be used to predict patients’ mortality, disability rate, and risk of readmission39. Previous studies have shown that the CCI is independently associated with functional deterioration within 1 year and increased 30-day mortality in stroke patients32. This study also found that the CCI was independently associated with a 3-month poor prognosis in neurocritical patients. Furthermore, Arata26 et al. pointed out that decreased Hb during hospitalization is associated with a poor prognosis in neurocritical patients. Kellert40 et al. also found that the occurrence of anemia within 5 days after stroke was associated with an increase in the mortality rate of patients in the short term. Additionally, a decrease in Hb greater than 15 g/L during hospitalization in patients with acute stroke is an independent risk factor for poor prognosis at discharge26. This study found that the greater the decrease in Hb during hospitalization was, the worse the prognosis of neurocritical patients was; the decreasing values of Hb during hospitalization among the neurocritical patients was 35(6, 59) g/L, which was independently associated with the 3-month poor prognosis. Infection or inflammation may stimulate neutrophils to release the iron-binding protein lactoferrin. Lactoferrin is internalized by bacteria, sequestering iron and leading to iron deficiency anemia manifested by decreased hemoglobin. An increase in the hemoglobin D-value reflects worsening anemia in neurocritical patients and will accelerate the course of the disease and affect patient outcomes41.
BCM is an important indicator in the analysis of human body composition; it is defined as the total mass of metabolically active, viable, functional cells42. Increasing evidence shows that BCM is closely related to the inflammatory response in cancer patients, elderly patients, patients with chronic diseases, and hemodialysis patients, and it has an impact on patients’ clinical outcomes31,43–45. Toshimi46 et al. confirmed that low preoperative BCM (BCM ≤ 23 kg) was a risk factor for sepsis and death from infection after liver transplantation. This study also found that a low BCM (27.1 ± 5.8) kg at admission can affect the 3-month prognosis of neurocritical patients and can be used as an effective indicator for predicting poor prognosis and death.
At the same time, this study for the first time observed the relationship between the dynamic trend of BCM and other related indicators and the prognosis of neurocritical patients 1–4 days after admission. One to four days after admission is the peak period for the occurrence of SIRS in neurocritical patients, and the severity of the inflammatory response at this stage directly affects the patient’s prognosis. A study of the prognosis of neurocritical patients found that 43% of patients with status epilepticus developed SIRS at admission, which was independently related to their drug resistance and 30-day mortality9. SIRS occurred in 64% of patients with spontaneous intracerebral hemorrhage and in 78% of patients with spontaneous subarachnoid hemorrhage within 3 days of admission and was closely associated with increased cerebral hemorrhage, exacerbation of vasospasm, and delayed cerebral ischemia47–49. Fifty-six percent of patients with ischemic stroke will develop SIRS within 4 days of admission47. Moreover, the greater the severity of SIRS is, the higher the 3-month disability rate and mortality rate50. Severe brain injury can induce SIRS, and the occurrence of SIRS further aggravates the severity of brain injury and adversely affects patient prognosis. We further analyzed the trend and found that a gradual increase in BCM was an important factor in the poor prognosis of neurocritical patients. This relationship may be related to the pathological process of tissue cell degeneration and necrosis caused by acute inflammation in the SIRS state. Tissue and cell necrosis caused by acute inflammation specifically manifests as necroptosis, that is, cell swelling, cell membrane rupture, and the release of cell contents51–53. Therefore, cell swelling is an early stage manifestation of tissue cell programmed necrosis caused by acute inflammation, and the main reason for cell swelling is the increase in ICW content54. However, BIA estimates BCM by measuring ICW using the formula BCW = WBP + ICW55 . In this study, the WBP of neurocritical patients did not change significantly 1–4 days after admission, but both ICW and BCM gradually increased; that is, the more intracellular water there was, the more obvious the swelling was, which may reflect early stage changes in the acute inflammatory phase with reduced somatic cell function and programmed cell necrosis. However, in the multivariate analysis, we did not find that an increase in the BCM was independently associated with poor prognosis. The reason for this result may be related to inadequate consideration of the functional state of the cell membrane, i.e., its permeability56.
To improve the efficacy for predicting a poor prognosis, this study innovatively combined BCM and PA into the secondary index BCM/PA. PA is the cotangent value of the reactance (Xc) generated by the current flowing through the human cell membrane and the impedance (Z) generated by the water flowing inside and outside the cells. It can reflect the functional status of the cell membrane. The lower the PA value is, the more severe the damage to the cell membrane structure and the worse the cell’s functional status57. A number of previous studies have shown that a low PA value at a specific time point is an effective predictor of inflammatory status, adverse functional outcomes, and death in elderly, obese, cancer, and hemodialysis patients27–29,58,59. In this study, the PA of the patients in the poor prognosis group (4.06 ± 0.93) within the first day of admission was lower than that of the patients in the good prognosis group (5.57 ± 0.86), and the difference between the two groups was statistically significant (P < 0.05). Therefore, the gradual increase in BCM at low PA values may truly reflect the early stage changes in cell membrane damage, gradual swelling of cells, gradual deterioration of cell function, and programmed cell necrosis in the SIRS state. That is, the larger the BCM/PA value is, the more severe the degree of SIRS, and the worse the patient’s prognosis. Further analysis of the prediction model showed that the model that included the secondary index BCM/PA had significantly higher predictive value than the prediction model that did not include BCM/PA. Moreover, compared with the traditional prognostic scoring scales (CCI, APACHE II, and NRS2002), the sensitivity (93.55%) and specificity (80.43%) of the prediction model that included the secondary index BCM/PA were both higher, suggesting that the model has high predictive ability for the prognosis of neurocritical patients. Therefore, BCM/PA plays an important role in improving the predictive value of the model and can be used as an effective early stage predictive indicator of poor prognosis in neurocritical patients.
Limitations
1. This study is a single-center retrospective case–control study with a relatively small sample size. A larger sample size and prospective studies are needed in the future to verify the findings of this study. 2. Since the severity of SIRS in neurocritical patients in the early stage (1–4 days after admission) is the key to determining their prognosis, we focused on the correlation between the dynamic changes in relevant indicators during this time period and the 3-month prognosis of patients to identify effective indicators for the early stage prediction of patient prognosis. However, this study did not observe the impact of relevant indicators on the prognosis of neurocritical patients after 4 days of admission. Therefore, subsequent studies can explore the change in relevant indicators after 4 days of admission to more realistically depict the correlation between the pathological changes in SIRS and poor prognosis. 3. The prediction model that included BCM/PA was verified only internally, and the conclusion requires external verification. 4. BIA devices from different manufacturers are affected by age and geographical population, which may lead to differences in measurement results. Therefore, in clinical application, the use of BIA equipment that is appropriate for the research subjects should be carefully selected. The subjects in this study were all Asian individuals older than 18 years of age, which essentially ensured the homogeneity of the study samples and reduced the impact of measurement differences on the accuracy of human body composition indicators. 5. Indicators for the predictive model in this study can be directly measured by BIA tool. However, BCM/PA slope has to be calculated by using the statistical method in a specific program. In the future, collaborative efforts with BIA technology research team are needed to optimize software technology, so as to improve the accuracy and convenience of the predictive model in clinical application.
Conclusions
In summary, BCM/PA is a sensitive indicator of SIRS severity in the early stage and can effectively predict the 3-month prognosis of neurocritical patients. Its measurement is noninvasive and simple. Medical and nursing staff can complete the procedure and interpret the results within a few minutes after simple training. This method is promising and universally easy to use in the ICU, thus warranting further validation and promotion.
Abbreviations
- Alb
Albumin;
- ALC
Absolute lymphocyte count;
- AMC
Absolute monocyte count
- ANC
Absolute neutrophil count
- APACHE II
Acute Physiology and Chronic Health Evaluation II;
- AUC
Area under the receiver operator characteristic curve
- BCM
Body cell mass;
- BIA
Bioimpedance analysis;
- BMI
Body mass index;
- CCI
Charlson Comorbidity Index;
- CI
Confidence interval;
- CNS
Central nervous system;
- DCA
Decision curve analysis
- ECW
Extracellular water;
- FFM
Fat-free mass;
- Glu
Blood glucose
- Hb
Hemoglobin
- hsCRP
High-sensitivity C-reactive protein
- ICW
Intracellular water
- IDI
Integrated discrimination improvement index
- NICU
Neurology intensive care unit;
- NLR
Neutrophil-to-lymphocyte ratio
- NRI
Net reclassification improvement
- NRS2002
Nutrition Risk Screening 2002
- mRS
Modified Rankin scale
- OR
Odds ratios;
- PA
Phase angle;
- PEM
Protein-energy malnutrition
- PLR
Platelet-to-lymphocyte ratio
- PLT
Platelet count
- ROC
Receiver operator characteristic
- SIRS
Systemic inflammatory response syndrome
- SMI
Skeletal muscle index
- TBW
Total body water.
- WBC
White blood cell
- VFA
Visceral fat area
Author contributions
F.L. J.P. and Y.X. contributed to the conception and study design. J.P. Y.X. G.L. and S.L. performed data acquisition; F.L. J.P. Y.X. G.L. and S.L. participated in the interpretation of data; J.P. and Y.X. performed statistical analysis and drafted the manuscript. F.L. and J.P. contributed to revisions of the manuscript. All authors read and approved the final manuscript, and all authors agreed to be accountable for all aspects of the work.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Consent for publication
All the coauthors have approved this version of the manuscript and consent to publication.All the coauthors confirm that this manuscript has not been published elsewhere and is not under consideration by another journal.
Ethical approval/Informed consent
This retrospective study was approved by the institutions ethics committee.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Jingjing Peng and Yanling Xiang.
References
- 1.Global, regional, and national burden of epilepsy, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol.18(4), 357–375 (2019). [DOI] [PMC free article] [PubMed]
- 2.Sharma, R. et al. Infections after a traumatic brain injury: The complex interplay between the immune and neurological systems. Brain Behav. Immun.79, 63–74 (2019). [DOI] [PubMed] [Google Scholar]
- 3.Learoyd, A. E. et al. Infections Up to 76 Days After Stroke Increase Disability and Death[J]. Transl Stroke Res8(6), 541–548 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Badjatia, N. et al. Inflammation, negative nitrogen balance, and outcome after aneurysmal subarachnoid hemorrhage. Neurology84(7), 680–687 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heikinheimo, T. et al. Preceding and poststroke infections in young adults with first-ever ischemic stroke: Effect on short-term and long-term outcomes. Stroke44(12), 3331–3337 (2013). [DOI] [PubMed] [Google Scholar]
- 6.Wastfelt, M., Cao, Y. & Strom, J. O. Predictors of post-stroke fever and infections: A systematic review and meta-analysis. BMC Neurol.18(1), 49 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang, Y. et al. Crosstalk between Inflammation and the BBB in Stroke. Curr. Neuropharmacol.18(12), 1227–1236 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saand, A. R. et al. Systemic inflammation in hemorrhagic strokes-A novel neurological sign and therapeutic target?. J. Cereb. Blood Flow Metab.39(6), 959–988 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Szklener, S. et al. Systemic inflammatory response syndrome in the course of status epilepticus: 7-year, two-center observational study. Epilepsy Res.137, 53–55 (2017). [DOI] [PubMed] [Google Scholar]
- 10.Vahidy, F. S. et al. Acute splenic responses in patients with ischemic stroke and intracerebral hemorrhage. J. Cereb. Blood Flow Metab.36(6), 1012–1021 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shi, M. et al. Endoplasmic reticulum stress-associated neuronal death and innate immune response in neurological diseases. Front. Immunol.12, 794580 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marjani, N. et al. Evaluation of the binding effect and cytotoxicity assay of 2-Ethyl-5-(4-methylphenyl) pyramido pyrazole ophthalazine trione on calf thymus DNA: Spectroscopic, calorimetric, and molecular dynamics approaches. Luminescence37(2), 310–322 (2022). [DOI] [PubMed] [Google Scholar]
- 13.Zare-Feizabadi, N. et al. Determining the interaction behavior of calf thymus DNA with anastrozole in the presence of histone H1: Spectroscopies and cell viability of MCF-7 cell line investigations. DNA Cell Biol.40(8), 1039–1051 (2021). [DOI] [PubMed] [Google Scholar]
- 14.Dareini, M. et al. A novel view of the separate and simultaneous binding effects of docetaxel and anastrozole with calf thymus DNA: Experimental and in silico approaches. Spectrochim Acta A Mol. Biomol. Spectrosc.228, 117528 (2020). [DOI] [PubMed] [Google Scholar]
- 15.Chamani, J. et al. Cooperative alpha-helix formation of beta-lactoglobulin induced by sodium n-alkyl sulfates. J. Colloid Interface Sci.293(1), 52–60 (2006). [DOI] [PubMed] [Google Scholar]
- 16.Sadeghzadeh, F. et al. Characterizing the binding of angiotensin converting enzyme I inhibitory peptide to human hemoglobin: Influence of electromagnetic fields. Protein Pept. Lett.27(10), 1007–1021 (2020). [DOI] [PubMed] [Google Scholar]
- 17.Chamani, J. & Moosavi-Movahedi, A. A. Effect of n-alkyl trimethylammonium bromides on folding and stability of alkaline and acid-denatured cytochrome c: A spectroscopic approach. J. Colloid Interface Sci.297(2), 561–569 (2006). [DOI] [PubMed] [Google Scholar]
- 18.Anrather, J. & Iadecola, C. Inflammation and stroke: An overview. Neurotherapeutics13(4), 661–670 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smith, S. E. et al. Protein-energy malnutrition developing after global brain ischemia induces an atypical acute-phase response and hinders expression of GAP-43. PLoS One9(9), e107570 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bresnahan, K. A. & Tanumihardjo, S. A. Undernutrition, the acute phase response to infection, and its effects on micronutrient status indicators. Adv. Nutr.5(6), 702–711 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chandra, R. K. Protein-energy malnutrition and immunological responses. J. Nutr.122(3 Suppl), 597–600 (1992). [DOI] [PubMed] [Google Scholar]
- 22.Zhang, J. et al. Organ- and cell-specific immune responses are associated with the outcomes of intracerebral hemorrhage. FASEB J.32(1), 220–229 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhu, W., Guo, Z. & Yu, S. Higher neutrophil counts before thrombolysis for cerebral ischemia predict worse outcomes. Neurology86(11), 1077 (2016). [DOI] [PubMed] [Google Scholar]
- 24.Guldolf, K. et al. Neutrophil-to-lymphocyte ratio predicts delirium after stroke. Age Ageing50(5), 1626–1632 (2021). [DOI] [PubMed] [Google Scholar]
- 25.Soeters, P. B., Wolfe, R. R. & Shenkin, A. Hypoalbuminemia: Pathogenesis and clinical significance. JPEN J. Parenter. Enteral Nutr.43(2), 181–193 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abe, A. et al. Decline in hemoglobin during hospitalization may be associated with poor outcome in acute stroke patients. J. Stroke Cerebrovasc. Dis.27(6), 1646–1652 (2018). [DOI] [PubMed] [Google Scholar]
- 27.Matthews, L. et al. The use of bioelectrical impedance analysis to predict post-operative complications in adult patients having surgery for cancer: A systematic review. Clin. Nutr.40(5), 2914–2922 (2021). [DOI] [PubMed] [Google Scholar]
- 28.Garlini, L. M. et al. Phase angle and mortality: A systematic review. Eur. J. Clin. Nutr.73(4), 495–508 (2019). [DOI] [PubMed] [Google Scholar]
- 29.Tomeleri, C. M. et al. Phase angle is related with inflammatory and oxidative stress biomarkers in older women. Exp. Gerontol.102, 12–18 (2018). [DOI] [PubMed] [Google Scholar]
- 30.Samoni, S. et al. Impact of hyperhydration on the mortality risk in critically ill patients admitted in intensive care units: Comparison between bioelectrical impedance vector analysis and cumulative fluid balance recording. Crit. Care20, 95 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oliveira, T. et al. Low body cell mass index in hemodialysis patients: Association with clinical parameters and survival. Hemodial Int.24(2), 228–236 (2020). [DOI] [PubMed] [Google Scholar]
- 32.Schmidt, M. et al. Eighteen-year trends in stroke mortality and the prognostic influence of comorbidity. Neurology82(4), 340–350 (2014). [DOI] [PubMed] [Google Scholar]
- 33.Su, Y. Y. et al. Predicting hospital mortality using APACHE II scores in neurocritically ill patients: A prospective study. J. Neurol.256(9), 1427–1433 (2009). [DOI] [PubMed] [Google Scholar]
- 34.Majari, K. et al. Comparison of modified NUTRIC, NRS-2002, and MUST scores in Iranian critically Ill patients admitted to intensive care units: A prospective cohort study. JPEN J. Parenter. Enteral Nutr.45(7), 1504–1513 (2021). [DOI] [PubMed] [Google Scholar]
- 35.Kyle, U. G. et al. Bioelectrical impedance analysis–part I: Review of principles and methods. Clin. Nutr.23(5), 1226–1243 (2004). [DOI] [PubMed] [Google Scholar]
- 36.Cruz-Jentoft, A. J. et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing48(1), 16–31 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lukaski, H. C., Kyle, U. G. & Kondrup, J. Assessment of adult malnutrition and prognosis with bioelectrical impedance analysis: Phase angle and impedance ratio. Curr. Opin. Clin. Nutr. Metab. Care20(5), 330–339 (2017). [DOI] [PubMed] [Google Scholar]
- 38.Collins, G. S. et al. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): The TRIPOD statement. BMJ350, g7594 (2015). [DOI] [PubMed] [Google Scholar]
- 39.Roffman, C. E., Buchanan, J. & Allison, G. T. Charlson comorbidities index. J. Physiother.62(3), 171 (2016). [DOI] [PubMed] [Google Scholar]
- 40.Kellert, L. et al. The impact of low hemoglobin levels and transfusion on critical care patients with severe ischemic stroke: STroke: RelevAnt Impact of HemoGlobin, Hematocrit and Transfusion (STRAIGHT)–an observational study. J. Crit. Care29(2), 236–240 (2014). [DOI] [PubMed] [Google Scholar]
- 41.Fraenkel, P. G. Anemia of inflammation: A review. Med. Clin. North Am.101(2), 285–296 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moore, F. D. & Boyden, C. M. Body cell mass and limits of hydration of the fat-free body: Their relation to estimated skeletal weight. Ann. N. Y. Acad. Sci.110, 62–71 (1963). [DOI] [PubMed] [Google Scholar]
- 43.McMillan, D. C. et al. Relationship between weight loss, reduction of body cell mass and inflammatory response in patients with cancer. Br. J. Surg.81(7), 1011–1014 (1994). [DOI] [PubMed] [Google Scholar]
- 44.McMillan, D. C. et al. Albumin concentrations are primarily determined by the body cell mass and the systemic inflammatory response in cancer patients with weight loss. Nutr. Cancer39(2), 210–213 (2001). [DOI] [PubMed] [Google Scholar]
- 45.Rondanelli, M. et al. Beyond Body Mass Index. Is the Body Cell Mass Index (BCMI) a useful prognostic factor to describe nutritional, inflammation and muscle mass status in hospitalized elderly?: Body Cell Mass Index links in elderly. Clin. Nutr.37(3), 934–939 (2018). [DOI] [PubMed] [Google Scholar]
- 46.Kaido, T. et al. Pre- and perioperative factors affecting infection after living donor liver transplantation. Nutrition28(11–12), 1104–1108 (2012). [DOI] [PubMed] [Google Scholar]
- 47.Kalita, J. et al. Systemic inflammatory response syndrome predicts severity of stroke and outcome. J. Stroke Cerebrovasc. Dis.24(7), 1640–1648 (2015). [DOI] [PubMed] [Google Scholar]
- 48.Hagen, M. et al. Systemic inflammatory response syndrome and long-term outcome after intracerebral hemorrhage. Neurol. Neuroimmunol. Neuroinflamm.6(5), e588 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rass, V. et al. Systemic inflammatory response syndrome as predictor of poor outcome in nontraumatic subarachnoid hemorrhage patients. Crit. Care Med.46(12), e1152–e1159 (2018). [DOI] [PubMed] [Google Scholar]
- 50.Xu, X. et al. Systemic inflammatory response syndrome and outcomes in ischemic patients treated with endovascular treatment. Clin. Interv. Aging15, 2331–2340 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Heckmann, B. L., Tummers, B. & Green, D. R. Crashing the computer: Apoptosis vs. necroptosis in neuroinflammation. Cell Death Differ.26(1), 41–52 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yuan, J., Amin, P. & Ofengeim, D. Necroptosis and RIPK1-mediated neuroinflammation in CNS diseases. Nat. Rev. Neurosci.20(1), 19–33 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Duprez, L. et al. RIP kinase-dependent necrosis drives lethal systemic inflammatory response syndrome. Immunity35(6), 908–918 (2011). [DOI] [PubMed] [Google Scholar]
- 54.Lorenzo, I., Serra-Prat, M. & Yebenes, J. C. The role of water homeostasis in muscle function and frailty: A review. Nutrients11(8), 63 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Earthman, C. et al. Bioimpedance spectroscopy for clinical assessment of fluid distribution and body cell mass. Nutr. Clin. Pract.22(4), 389–405 (2007). [DOI] [PubMed] [Google Scholar]
- 56.Lang, F. Mechanisms and significance of cell volume regulation. J. Am. Coll. Nutr.26(5 Suppl), 613S-623S (2007). [DOI] [PubMed] [Google Scholar]
- 57.Norman, K. et al. Bioelectrical phase angle and impedance vector analysis–clinical relevance and applicability of impedance parameters. Clin. Nutr.31(6), 854–861 (2012). [DOI] [PubMed] [Google Scholar]
- 58.Barrea, L. et al. Phase angle as an easy diagnostic tool of meta-inflammation for the nutritionist. Nutrients13(5), 31 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wirth, R. et al. Bioelectric impedance phase angle is associated with hospital mortality of geriatric patients. Arch. Gerontol. Geriatr.51(3), 290–294 (2010). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.